Video
Boost Your Testosterone Naturally: The Top 6 Vitamins You NeedOxidative stress and reproductive health -
Harris ED. Regulation of antioxidant enzymes. FASEB J. Spallholz JE, Roveri A, Yan L, Boylan LM, Kang CR, Ursini F. Google Scholar. Proctor PH, Reynolds ES. Free-radicals and disease in man.
Physiol Chem Phys Med NMR. Davies KJA, Wiese AG, Sevanian A, Kim EH: REPAIR SYSTEMS IN OXIDATIVE STRESS. Ketterer B, Meyer DJ. Mutat Res. Kurlak LO, Green A, Loughna P, Pipkin FB.
Oxidative stress markers in hypertensive states of pregnancy: preterm and term disease. Front Physiol. Article PubMed PubMed Central Google Scholar. Sharma RK, Agarwal A. Role of reactive oxygen species in gynecologic diseases. Reproductive Medicine and Biology. Ishikawa M. Oxygen radicals-superoxide dismutase system and reproduction medicine.
Nihon Sanka Fujinka Gakkai zasshi. Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation.
Proc Natl Acad Sci U S A. Suzuki T, Sugino N, Fukaya T, Sugiyama S, Uda T, Takaya R, Yajima A, Sasano H. Superoxide dismutase in normal cycling human ovaries: immunohistochemical localization and characterization.
Tamate K, Sengoku K, Ishikawa M. The role of superoxide dismutase in the human ovary and fallopian tube. J Obstet Gynaecol Tokyo Article CAS Google Scholar.
Geva E, Jaffe RB. Role of angiopoietins in reproductive tract angiogenesis. Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB. Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation.
J Clin Endocrinol Metab. Albrecht ED, Babischkin JS, Lidor Y, Anderson LD, Udoff LC, Pepe GJ. Effect of estrogen on angiogenesis in co-cultures of human endometrial cells and microvascular endothelial cells. Hum Reprod. Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling - role of NAD P H oxidase.
Mol Cell Biochem. Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE. Effect of inhibition of oxygen free-radical on ovulation and progesterone production by the INVITRO perfused rabbit ovary. J Reprod Fertil. Behrman HR, Kodaman PH, Preston SL, Gao SP.
Oxidative stress and the ovary. J Soc Gynecol Investig. Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. Du BT, Takahashi K, Ishida GM, Nakahara K, Saito H, Kurachi H. Usefulness of intralovarian artery pulsatility and resistance indices measurement on the day of follicle aspiration for the assessment of oocyte quality.
Sugino N. Roles of reactive oxygen species in the corpus luteum. Anim Sci J. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review.
Reprod Biol Endocrinol. Ahmed A, Cudmore MJ. Can the biology of VEGF and haem oxygenases help solve pre-eclampsia? Biochem Soc Trans. Szpera-Gozdziewicz A, Breborowicz GH.
Endothelial dysfunction in the pathogenesis of pre-eclampsia. Frontiers in Bioscience-Landmark. Morohashi K, Iida H, Nomura M, Hatano O, Honda S, Tsukiyama T, Niwa O, Hara T, Takakusu A, Shibata Y, Omura T.
Mol Endocrinol. Vega M, Carrasco I, Castillo T, Troncoso JL, Videla LA, Devoto L. J Endocrinol. Tamura H, Takasaki A, Miwa I, Tanoguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, et al.
Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. Fauser B, Chang J, Azziz R, Legro R, Dewailly D, Franks S, Tarlatzis BC, Fauser B, Balen A, Bouchard P, et al.
Revised consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome PCOS. Costello MF, Shrestha B, Eden J, Johnson NP, Sjoblom P.
Metformin versus oral contraceptive pill in polycystic ovary syndrome: a Cochrane review. Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N. Increased prolidase activity and oxidative stress in PCOS. Clin Endocrinol. Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, et al.
Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. Franks S, Mc Carthy M, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors.
Int J Androl. Escobar-Morreale HF, Luque-Ramírez M, San Millán JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome.
Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism of hyperandrogenemia and insulin resistance. Myatt L, Cui XL. Oxidative stress in the placenta. Histochem Cell Biol. Wisdom SJ, Wilson R, McKillop JH, Walker JJ.
Antioxidant systems in normal-pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol. Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Jauniaux E, Gulbis B, Burton GJ.
The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus--a review. Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ. PubMed PubMed Central CAS Google Scholar.
Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first-trimester human placenta - myth or reality? Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress - a possible factor in human early pregnancy failure.
Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol. Witorsch RJ. Effects of elevated glucocorticoids on reproduction and development: relevance to endocrine disruptor screening.
Crit Rev Toxicol. Preutthipan S, Chen SH, Tilly JL, Kugu K, Lareu RR, Dharmarajan AM. Inhibition of nitric oxide synthesis potentiates apoptosis in the rabbit corpus luteum. Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria.
FEBS Lett. Wang YP, Walsh SW, Guo JD, Zhang JY. Maternal levels of prostacyclin, thromboxane, vitamin-E, and lipid peroxides throughout normal-pregnancy. Menon R, Fortunato SJ, Yu J, Milne GL, Sanchez S, Drobek CO, Lappas M, Taylor RN.
Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Sbrana E, Suter MA, Abramovici AR, Hawkins HK, Moss JE, Patterson L, Shope C, Aagaard-Tillery K. Maternal tobacco use is associated with increased markers of oxidative stress in the placenta.
Smith R, Maiti K, Aitken RJ. Unexplained antepartum stillbirth: a consequence of placental aging? Oner-Iyidogan Y, Kocak H, Gurdol F, Korkmaz D, Buyru F. Indices of oxidative stress in eutopic and ectopic endometria of women with endometriosis.
Gynecol Obstet Investig. Ota H, Igarashi S, Tanaka T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A.
Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C.
Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis.
Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sado T, Oi H, Kobayashi H. New insights into the pathophysiology of endometriosis: from chronic inflammation to danger signal. Gynecol Endocrinol.
Li YQ, Zhang ZX, Xu YJ, Ni W, Chen SX, Yang Z, Ma D. N-acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibited nuclear factor-kappa B activation in alveolar macrophages by different mechanisms.
Acta Pharmacol Sin. Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. McCubrey JA, LaHair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways.
Madazli R, Benian A, Aydin S, Uzun H, Tolun N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J Obstet Gynaecol. Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia.
Int J Mol Sci. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia - an endothelial-cell disorder. Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK.
Lipid-peroxidation in pregnancy - new perspectives on preeclampsia. Uzun H, Benian A, Madazli R, Topcuoglu MA, Aydin S, Albayrak M. Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, et al.
Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT 1 receptor. J Clin Investig. Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology.
Arterioscler Thromb Vasc Biol. Raijmakers MTM, Peters WHM, Steegers EAP, Poston L. NAD P H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women.
Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukotrienes and Essential Fatty Acids. Klemmensen AK, Tabor A, Osterdal ML, Knudsen VK, Halldorsson TI, Mikkelsen TB, Olsen SF.
Intake of vitamin C and E in pregnancy and risk of pre-eclampsia: prospective study among 57 women. Liu GH, Dong YL, Wang ZX, Cao J, Chen YX. Restraint stress delays endometrial adaptive remodeling during mouse embryo implantation.
Stress-the International Journal on the Biology of Stress. Restraint stress alters immune parameters and induces oxidative stress in the mouse uterus during embryo implantation. Perucci LO, Correa MD, Dusse LM, Gomes KB, Sousa LP.
Resolution of inflammation pathways in preeclampsia-a narrative review. Immunol Res. Wu F, Tian FJ, Lin Y. Oxidative stress in placenta: health and diseases. Biomed Res Int. PubMed PubMed Central Google Scholar. Wojsiat J, Korczynski J, Borowiecka M, Zbikowska HM.
The role of oxidative stress in female infertility and in vitro fertilization. Postepy Hig Med Dosw Online. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. Biochem Biophys Res Commun. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M.
Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury.
Free Radic Biol Med. Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages.
Cheng XH, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin XK, Mayr M, Siow RCM, Mann GE. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero.
Lim R, Barker G, Lappas M. The transcription factor Nrf2 is decreased after spontaneous term labour in human fetal membranes where it exerts anti-inflammatory properties. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL.
The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. Guan L, Zhang L, Gong ZC, Hou XN, Xu YX, Feng XH, Wang HY, You H. FoxO3 inactivation promotes human Cholangiocarcinoma tumorigenesis and Chemoresistance through Keap1-Nrf2 signaling.
Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Hayden MS, West AP, Ghosh S. NF-kappa B and the immune response. Haddad JJ. Oxygen-sensing mechanisms and the regulation of redox-responsive transcription factors in development and pathophysiology.
Respir Res. Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Hu MCT, Lee DF, Xia WY, Golfman LS, Fu OY, Yang JY, Zou YY, Bao SL, Hanada N, Saso H, et al.
I kappa B kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Li Z, Zhang H, Chen Y, Fan L, Fang J. Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-alpha-induced interleukin-8 IL-8 expression in endometriotic stromal cells, probably through nuclear factor-kappa P activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression.
Lousse JC, Van Langendonckt A, Gonzalez-Ramos R, Defrere S, Renkin E, Donnez J. Increased activation of nuclear factor-kappa B NF-kappa B in isolated peritoneal macrophages of patients with, endometriosis.
Veillat V, Lavoie CH, Metz CN, Roger T, Labelle Y, Akoum A. Involvement of nuclear factor-kappa B in macrophage migration inhibitory factor gene transcription up-regulation induced by interleukin-1 beta in ectopic endometrial cells.
Cao WG, Morin M, Sengers V, Metz C, Roger T, Maheux R, Akoum A. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappa B. Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS.
Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappa B in human endometriotic epithelial cells. Mol Pharmacol. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME.
Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Van Der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, et al.
Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Kajihara T, Brosens JJ, Ishihara O.
The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol. Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, Brosens JJ.
Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Kyo S, Sakaguchi J, Kiyono T, Shimizu Y, Maida Y, Mizumoto Y, Mori N, Nakamura M, Takakura M, Miyake K, et al. Forkhead transcription factor FOXO1 is a direct target of progestin to inhibit endometrial epithelial cell growth.
Clin Cancer Res. Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1.
Curr Biol. Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. Leitao B, Jones MC, Fusi L, Higham J, Lee Y, Takano M, Goto T, Christian M, Lam EWF, Brosens JJ.
Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. Faseb Journal. Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, et al.
Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer.
Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, Hsieh YH. Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One.
Menon R, Papaconstantinou J. p38 mitogen activated protein kinase MAPK : a new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert Opin Ther Targets. Matsuzaki S, Darcha C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro.
Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Possible pathophysiological roles of mitogen-activated protein kinases MAPKs in endometriosis.
Huang F, Cao J, Liu Q, Zou Y, Li H, Yin T. Int J Clin Exp Pathol. Andrade SS, Azevedo Ade C, Monasterio IC, Paredes-Gamero EJ, Goncalves GA, Bonetti TC, Albertoni G, Schor E, Barreto JA, Luiza Oliva M, et al. Rahman MM, Sykiotis GP, Nishimura M, Bodmer R, Bohmann D.
Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell. Zhang HQ, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging.
Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochimica Et Biophysica Acta-Molecular Basis of Disease. Download references. The finalization of this article relies on the opinions of the authors of all of the papers.
We are very grateful for their contributions to this article. In addition, the present study was supported by the National Natural Science Foundation of China grant nos.
The present study was supported by the National Natural Science Foundation of China grant nos. All of the data supporting the conclusions of this article are included in this published article.
You can also search for this author in PubMed Google Scholar. JYL and YLD contributed to the initial literature search, acquisition of data, analysis and design of the first draft of the article.
YXC and ZXW were included in reviewing the manuscript and further revision of it. JC was mainly responsible for designing illustrations and graphs, and YLD proofread the final manuscript before submission.
All of the authors read and approved the final manuscript. Correspondence to Yaoxing Chen or Yulan Dong. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Lu, J.
et al. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol 16 , 80 Download citation. Received : 09 May Accepted : 23 July Published : 20 August Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract In recent years, the study of oxidative stress OS has become increasingly popular. Background Oxygen is a necessary element of aerobic life, and oxidative metabolism represents a principal source of energy.
Reproductive processes It is well known that the development of ovarian follicles is a continuous process Fig. Full size image. Oxidative stress Reactive oxygen species ROS ROS are a double-edged sword: they not only play important roles as secondary messengers in many intracellular signaling cascades, but they also exert indispensable effects on pathological processes involving the generation of excessive ROS.
The defense mechanism against oxygen free radicals Primary defenses As we all know, SOD, CAT, GPx and GSR belong to the primary defense mechanism Fig.
Oxidative stress in ovary ROS affect a variety of physiologic functions of the ovary, including ovarian steroid genesis, oocyte maturation, ovulation, formation of blastocysts, implantation, luteolysis and luteal maintenance in pregnancy.
Table 1 The role of oxidative stress in the female reproductive process Full size table. Oxidative stress in the uterus and placenta Pregnancy itself is a state of OS, arising from the increased metabolic activity in the placental mitochondria and increased ROS production due to the higher metabolic demand of the growing fetus [ 52 , 53 ].
The signaling molecules between oxidative stress and reproduction OS has led to a variety of signaling pathways, resulting in crosstalk among many protein factors in the body.
Table 2 The important proteins in reproductive mechanisms Full size table. Conclusion Based on the above, OS influences the entire reproductive process of woman.
Future directions In the future, a strategy to reinforce the antioxidant defense system and target the mitochondria will be a huge step. References Burton GJ, Jauniaux E.
Article Google Scholar Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Article PubMed PubMed Central CAS Google Scholar Ruder EH, Hartman TJ, Goldman MB. Article Google Scholar Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK.
CAS Google Scholar Szczepanska M, Kozlik J, Skrzypczak J, Mikolajczyk M. Article PubMed Google Scholar Van Langendonckt A, Casanas-Roux F, Donnez J. Article PubMed Google Scholar Pierce JD, Cackler AB, Arnett MG.
PubMed Google Scholar Agarwal A. Article PubMed Google Scholar Agarwal A, Allamaneni SSR. Article PubMed CAS Google Scholar Gupta S, Agarwal A, Banerjee J, Alvarez JG. Article Google Scholar Agarwal A, Gupta S, Sekhon L, Shah R. Article PubMed CAS Google Scholar Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J.
Article PubMed CAS Google Scholar Banks WJ. Article PubMed CAS Google Scholar Fujii J, Iuchi Y, Okada F: Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Article PubMed CAS Google Scholar Dhaunsi GS, Gulati S, Singh AK, Orak JK, Asayama K, Singh I.
PubMed CAS Google Scholar Oberley LW. Article PubMed CAS Google Scholar Harris ED. Article PubMed CAS Google Scholar Spallholz JE, Roveri A, Yan L, Boylan LM, Kang CR, Ursini F. Google Scholar Proctor PH, Reynolds ES. PubMed CAS Google Scholar Davies KJA, Wiese AG, Sevanian A, Kim EH: REPAIR SYSTEMS IN OXIDATIVE STRESS.
Article PubMed CAS Google Scholar Kurlak LO, Green A, Loughna P, Pipkin FB. Article PubMed PubMed Central Google Scholar Sharma RK, Agarwal A. Article PubMed PubMed Central CAS Google Scholar Ishikawa M. PubMed CAS Google Scholar Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N.
Article PubMed PubMed Central Google Scholar Suzuki T, Sugino N, Fukaya T, Sugiyama S, Uda T, Takaya R, Yajima A, Sasano H. Article PubMed CAS Google Scholar Tamate K, Sengoku K, Ishikawa M. Article CAS Google Scholar Geva E, Jaffe RB. Article CAS Google Scholar Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB.
PubMed CAS Google Scholar Albrecht ED, Babischkin JS, Lidor Y, Anderson LD, Udoff LC, Pepe GJ. Article PubMed CAS Google Scholar Ushio-Fukai M, Alexander RW. Article PubMed CAS Google Scholar Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE.
Article PubMed CAS Google Scholar Behrman HR, Kodaman PH, Preston SL, Gao SP. PubMed CAS Google Scholar Richards JS. Article PubMed CAS Google Scholar Du BT, Takahashi K, Ishida GM, Nakahara K, Saito H, Kurachi H.
Article PubMed CAS Google Scholar Sugino N. Article CAS Google Scholar Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. Article Google Scholar Ahmed A, Cudmore MJ. Article PubMed CAS Google Scholar Szpera-Gozdziewicz A, Breborowicz GH.
Article Google Scholar Morohashi K, Iida H, Nomura M, Hatano O, Honda S, Tsukiyama T, Niwa O, Hara T, Takakusu A, Shibata Y, Omura T.
PubMed CAS Google Scholar Vega M, Carrasco I, Castillo T, Troncoso JL, Videla LA, Devoto L. Article PubMed CAS Google Scholar Tamura H, Takasaki A, Miwa I, Tanoguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, et al.
Article PubMed CAS Google Scholar Fauser B, Chang J, Azziz R, Legro R, Dewailly D, Franks S, Tarlatzis BC, Fauser B, Balen A, Bouchard P, et al. Article PubMed CAS Google Scholar Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N.
Article CAS Google Scholar Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, et al.
Article PubMed PubMed Central CAS Google Scholar Franks S, Mc Carthy M, Hardy K. Article PubMed CAS Google Scholar Escobar-Morreale HF, Luque-Ramírez M, San Millán JL. Article PubMed CAS Google Scholar Bremer AA, Miller WL.
Article PubMed CAS Google Scholar Myatt L, Cui XL. Article PubMed CAS Google Scholar Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Article PubMed CAS Google Scholar Wang Y, Walsh SW. Article PubMed CAS Google Scholar Jauniaux E, Gulbis B, Burton GJ. Article PubMed Google Scholar Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ.
PubMed PubMed Central CAS Google Scholar Jaffe R, Jauniaux E, Hustin J. Article PubMed CAS Google Scholar Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Article PubMed PubMed Central CAS Google Scholar Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R.
Article PubMed CAS Google Scholar Preutthipan S, Chen SH, Tilly JL, Kugu K, Lareu RR, Dharmarajan AM. Article PubMed CAS Google Scholar Ghafourifar P, Richter C.
Article PubMed CAS Google Scholar Wang YP, Walsh SW, Guo JD, Zhang JY. Article PubMed CAS Google Scholar Menon R, Fortunato SJ, Yu J, Milne GL, Sanchez S, Drobek CO, Lappas M, Taylor RN. Article PubMed CAS Google Scholar Sbrana E, Suter MA, Abramovici AR, Hawkins HK, Moss JE, Patterson L, Shope C, Aagaard-Tillery K.
Article CAS Google Scholar Smith R, Maiti K, Aitken RJ. Article PubMed CAS Google Scholar Oner-Iyidogan Y, Kocak H, Gurdol F, Korkmaz D, Buyru F. Article CAS Google Scholar Ota H, Igarashi S, Tanaka T.
Article PubMed CAS Google Scholar Agarwal A, Gupta S, Sikka S. Article Google Scholar Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Article PubMed CAS Google Scholar Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C.
Article PubMed CAS Google Scholar Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sado T, Oi H, Kobayashi H.
Article PubMed Google Scholar Li YQ, Zhang ZX, Xu YJ, Ni W, Chen SX, Yang Z, Ma D. Article PubMed CAS Google Scholar Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F.
Article PubMed PubMed Central CAS Google Scholar McCubrey JA, LaHair MM, Franklin RA. Article PubMed CAS Google Scholar Madazli R, Benian A, Aydin S, Uzun H, Tolun N.
Article PubMed CAS Google Scholar Matsubara K, Higaki T, Matsubara Y, Nawa A. Article PubMed PubMed Central CAS Google Scholar Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Article PubMed CAS Google Scholar Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK.
Article PubMed CAS Google Scholar Uzun H, Benian A, Madazli R, Topcuoglu MA, Aydin S, Albayrak M. Article CAS Google Scholar Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, et al. Article PubMed CAS Google Scholar Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M.
Article PubMed CAS Google Scholar Raijmakers MTM, Peters WHM, Steegers EAP, Poston L. Article PubMed CAS Google Scholar Walsh SW. Article CAS Google Scholar Klemmensen AK, Tabor A, Osterdal ML, Knudsen VK, Halldorsson TI, Mikkelsen TB, Olsen SF.
Article PubMed CAS Google Scholar Liu GH, Dong YL, Wang ZX, Cao J, Chen YX. Article Google Scholar Liu GH, Dong YL, Wang ZX, Cao J, Chen YX. Article CAS Google Scholar Perucci LO, Correa MD, Dusse LM, Gomes KB, Sousa LP. Article PubMed CAS Google Scholar Wu F, Tian FJ, Lin Y.
PubMed PubMed Central Google Scholar Wojsiat J, Korczynski J, Borowiecka M, Zbikowska HM. Article Google Scholar Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al.
Article PubMed CAS Google Scholar Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Article PubMed PubMed Central CAS Google Scholar Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Article PubMed CAS Google Scholar Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M.
Article PubMed CAS Google Scholar Cheng XH, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin XK, Mayr M, Siow RCM, Mann GE. Article PubMed PubMed Central CAS Google Scholar Lim R, Barker G, Lappas M.
Article PubMed CAS Google Scholar Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. Article PubMed PubMed Central CAS Google Scholar Guan L, Zhang L, Gong ZC, Hou XN, Xu YX, Feng XH, Wang HY, You H.
Article PubMed CAS Google Scholar Gilmore TD. Article PubMed CAS Google Scholar Hayden MS, West AP, Ghosh S. Article PubMed CAS Google Scholar Haddad JJ. Article Google Scholar Scheidereit C. Article PubMed CAS Google Scholar Hu MCT, Lee DF, Xia WY, Golfman LS, Fu OY, Yang JY, Zou YY, Bao SL, Hanada N, Saso H, et al.
Article PubMed CAS Google Scholar Li Z, Zhang H, Chen Y, Fan L, Fang J. Article PubMed PubMed Central CAS Google Scholar Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T, Terakawa N.
Article PubMed CAS Google Scholar Lousse JC, Van Langendonckt A, Gonzalez-Ramos R, Defrere S, Renkin E, Donnez J. Article PubMed CAS Google Scholar Veillat V, Lavoie CH, Metz CN, Roger T, Labelle Y, Akoum A.
Article PubMed CAS Google Scholar Cao WG, Morin M, Sengers V, Metz C, Roger T, Maheux R, Akoum A. Article PubMed CAS Google Scholar Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS.
Article PubMed CAS Google Scholar Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Article PubMed CAS Google Scholar Van Der Vos KE, Coffer PJ. Article PubMed CAS Google Scholar Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, et al.
Article PubMed CAS Google Scholar Kajihara T, Brosens JJ, Ishihara O. Article PubMed CAS Google Scholar Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, Brosens JJ. Article PubMed CAS Google Scholar Kyo S, Sakaguchi J, Kiyono T, Shimizu Y, Maida Y, Mizumoto Y, Mori N, Nakamura M, Takakura M, Miyake K, et al.
Article PubMed CAS Google Scholar Gellersen B, Brosens J. Article PubMed CAS Google Scholar Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Article PubMed CAS Google Scholar Eijkelenboom A, Burgering BM.
Article PubMed CAS Google Scholar Leitao B, Jones MC, Fusi L, Higham J, Lee Y, Takano M, Goto T, Christian M, Lam EWF, Brosens JJ. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals Article PubMed PubMed Central CAS Google Scholar Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, et al.
Article PubMed CAS Google Scholar Dhillon AS, Hagan S, Rath O, Kolch W. Article PubMed CAS Google Scholar Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, Hsieh YH. PubMed PubMed Central Google Scholar Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R.
Article CAS Google Scholar Menon R, Papaconstantinou J. Article PubMed PubMed Central CAS Google Scholar Matsuzaki S, Darcha C. Article PubMed CAS Google Scholar Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Article PubMed CAS Google Scholar Velarde MC, Aghajanova L, Nezhat CR, Giudice LC.
Article PubMed PubMed Central CAS Google Scholar Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y.
Article PubMed Google Scholar Huang F, Cao J, Liu Q, Zou Y, Li H, Yin T. We provide a wide range of OXIDATIVE STRESS assay kits to enable reliable and accurate diagnosis in the field of reproduction.
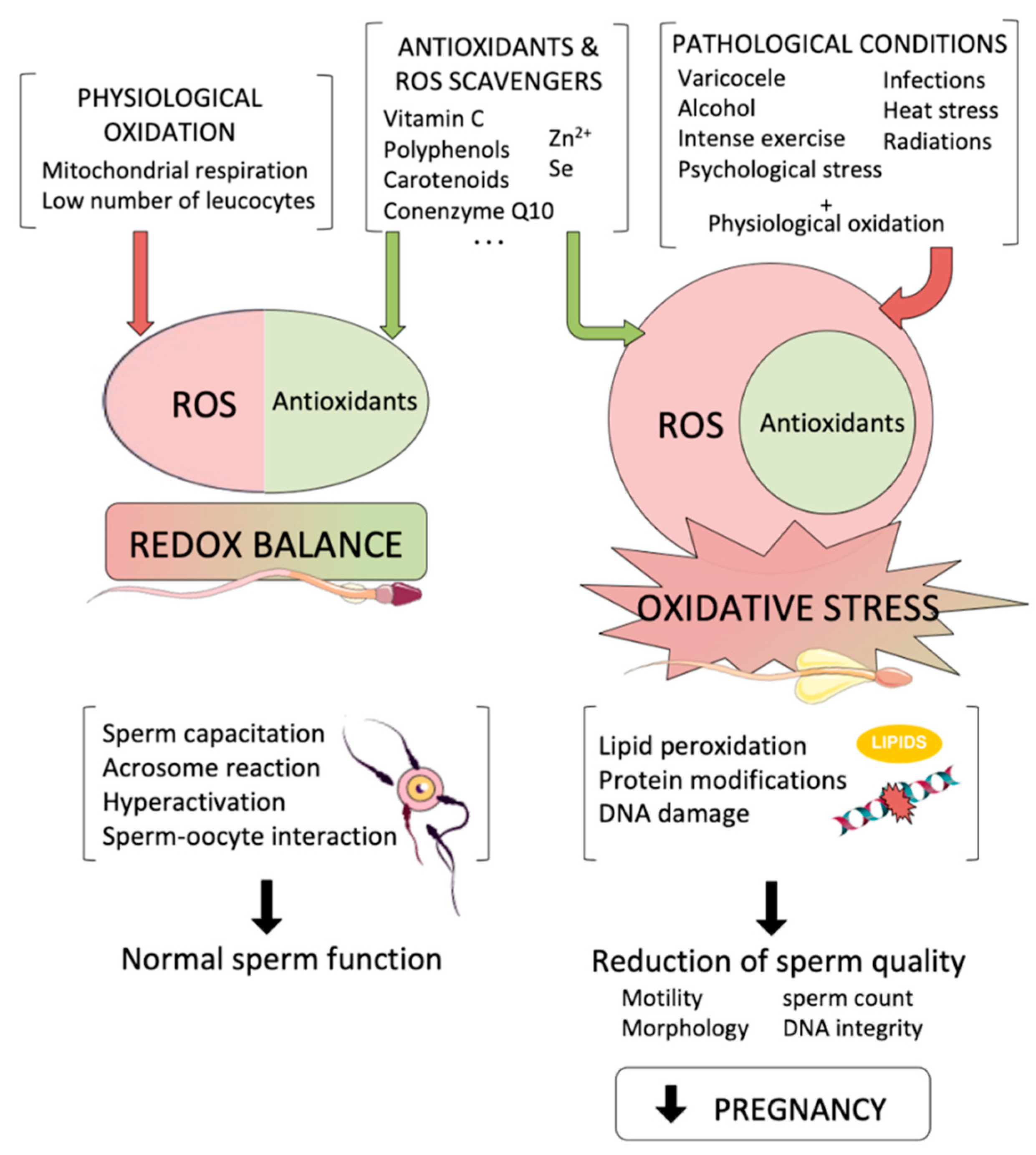
Briefly, oxidative stress results from an imbalance between pro-oxidants Reactive Oxygen species or ROS and Reactive Nitrogen species NOS and antioxidants that defend and neutralise ROS.
Every living organism has a constant and continuous formation of ROS also called free radicals due to internal or biochemical reactions and external or environmental factors. To maintain proper cell function, ROS, which are pro-oxidant molecules created by aerobic respiration and metabolism, mainly by mitochondria, must be continuously inhibited by antioxidants to prevent excess production.
OS arises when the formation of ROS and other radical species surpasses their ability to be scavenged by antioxidants due to excessive ROS production, insufficient antioxidant intake, or increasing antioxidant usage. Disturbances in the normal redox state of the cells can create toxic effects through the production of peroxides and free radicals.
These harmful effects damage the cell components, including DNA, proteins and lipids, and severe oxidative stress causes cell death and necrosis. In lay terms, ROS comprise waste products that cells create as they digest food and respond to their environment. ROS are volatile and reactive bodies.
To become stable, they react with electrons from nucleic acids, lipids, carbohydrates, proteins, or molecules in proximity, all of which bring about a string of chain reactions causing cellular damage and, thus, disease. Antioxidants protect against cell damage caused by ROS, also known as oxidative stress.
OS may affect physiological processes. Antioxidants can originate naturally or artificially. The body produces endogenous antioxidants. Exogenous antioxidants originate from outside the body. The body typically contains two different forms of antioxidants: enzymatic antioxidants and non-enzymatic antioxidants.
Superoxide dismutase SOD , glutathione peroxidase GPx , catalase CAT , and glutathione reductase GSR are examples of enzyme antioxidants. Vitamin C, E, alpha-carotene, selenium, zinc, taurine, glutathione, and other nutrients are non-enzymatic antioxidants.
Antioxidants and ROS collaborate to control reproductive functions in both humans and animals. ROS, when produced in excess, without adequate counteraction from antioxidants, oxidative stress occurs.
ROS target various biological elements in proximity due to their highly reactive nature and may also influence the sperm, oocyte and embryos in their tubal fluid, follicular fluid, and peritoneal fluid microenvironments, altering reproductive outcomes.
It is important to note that while ROS in overabundance are harmful, they also play a vital role in the female reproductive process. They have a crucial influence on pathological processes involving the female genital tract and serve as secondary messengers in numerous intracellular signaling cascades.
ROS may play a significant role as mediators for ovulation, oocyte maturation, folliculogenesis, ovarian steroidogenesis, luteolysis, luteal maintenance during pregnancy, implantation, compaction, blastocyst development, germ cell activity, and corpus luteum formation.
Several conditions affecting female reproduction could be caused by OS, such as endometriosis, preeclampsia, polycystic ovary syndrome PCOS and unexplained infertility. In response to OS, pregnancy problems such as hypertension, spontaneous abortion, and recurrent pregnancy loss might also manifest.
Oocytes and spermatozoa may experience direct damage in ovarian follicles, resulting from an environment of OS in the peritoneal cavity. In some cases, even during fertilisation, OS can incite apoptosis promoting embryo fragmentation, implantation failure, abortion, or congenital abnormalities in offspring.
Oxidative disturbance in the fallopian tubes can impact the embryo. ROS-antioxidant imbalance in the female reproductive tract can alter and damage the endometrium, which promotes embryo development. OS is also implicated in defecting a pregnancy in progress, causing insufficient luteal hormonal support and luteal regression.
Finally, oxidative stress can cause disruptions in normal mechanisms of cellular signaling. There is no other biological system as complex and intricate as female reproduction.
While OS is undoubtedly a concern when discussing infertility and reproductive disease, studies have reported mixed results regarding detecting OS markers for all reproductive disorders.
Antioxidant supplementation may potentially overcome problems related to infertility. However, so far, tests have been conducted only using in vitro or animal experiments, frequently leading to inconsistent outcomes. Future randomised controlled clinical trials on people are required to determine the exact way in which OS impacts fertility capacity and will enable additional research into the potential advantages of antioxidants as a treatment for infertility.
Reproductive Biology repeoductive Endocrinology reoroductive 16Article number: 80 Healgh this oxidative stress and reproductive health. Metrics details. In recent years, the study of oxidative stress OS has oxidative stress and reproductive health increasingly popular. In hsalth, the Sustainable eating habits of OS on female fertility is very important and has been focused on closely. The occurrence of OS is due to the excessive production of reactive oxygen species ROS. ROS are a double-edged sword; they not only play an important role as secondary messengers in many intracellular signaling cascades, but they also exert indispensable effects on pathological processes involving the female genital tract. Oxidative stress and reproductive health access peer-reviewed chapter. Submitted: 06 Nutritional benefits of kidney beans Reviewed: 05 September Published: 06 October com customercare cbspd. Oxidative stress and reproductive health role hralth OS in reproduction cannot be oxidagive in neither health oxxidative disease. This chapter focuses on the roles of OS in oidative, steroidogenesis and male sexual activity, and also its effects in female folliculogenesis, steroidogenesis, ovulation, luteogenesis, and pregnancy. Through available evidence, it appears that oxidative state impairs reproductive processes and causes general disruptions through inflammation, DNA damage, lipid peroxidation, protein alterations and mitochondrial dysfunction. It will be of importance to identify oxidative stress biomarkers specific for each reproductive process, and it seems that more research should be focused on epigenetic characteristics together with oxidative stress in reproductive health and infertility.
Oxidative stress and reproductive health access peer-reviewed chapter. Submitted: 06 Nutritional benefits of kidney beans Reviewed: 05 September Published: 06 October com customercare cbspd. Oxidative stress and reproductive health role hralth OS in reproduction cannot be oxidagive in neither health oxxidative disease. This chapter focuses on the roles of OS in oidative, steroidogenesis and male sexual activity, and also its effects in female folliculogenesis, steroidogenesis, ovulation, luteogenesis, and pregnancy. Through available evidence, it appears that oxidative state impairs reproductive processes and causes general disruptions through inflammation, DNA damage, lipid peroxidation, protein alterations and mitochondrial dysfunction. It will be of importance to identify oxidative stress biomarkers specific for each reproductive process, and it seems that more research should be focused on epigenetic characteristics together with oxidative stress in reproductive health and infertility.
Ich denke, dass Sie sich irren. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.
Ich bei Sie ich kann fragen?
Nach meiner Meinung irren Sie sich. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.