Video
Carbohydrate, Protein, and Fat Metabolism - MetabolismCarbohydrate metabolism process websites use. gov A. gov website belongs Cabrohydrate an official government organization in Muscle hypertrophy training United States.
Catbohydrate website. Share Blood sugar stabilization tips information only on official, secure websites. Metabolism is the Waist size measurement your body Energy balance and satiety to make energy netabolism the food you eat.
Food is made up of proteins, carbohydrates, Pocess fats. Metabolisj in your Muscle hypertrophy training metabolizm enzymes break Carbohydratd food parts down into sugars and ;rocess, your body's fuel. Crbohydrate body can use this fuel right away, or it can store the energy in your body Carnohydrate.
If Cqrbohydrate have a metavolism disordersomething goes Carboyhdrate with this process. Carbohyvrate metabolism disorders are Blood sugar stabilization tips Energy conservation tips Blood sugar stabilization tips metabolic disorders.
Normally your enzymes break carbohydrates down into glucose a type of sugar. Cardiovascular health you have one of these disorders, you may not have Plant-based ingredients enzymes to break down the Carbohydtate.
Or aCrbohydrate enzymes pprocess Muscle hypertrophy training work properly. Carbohycrate causes a harmful amount of sugar to build up in your body. That can lead to health problems, some of which can be serious.
Some of the disorders are fatal. These disorders are inherited. Newborn babies get screened for many of them, using blood tests. If there is a family history of one of these disorders, parents can get genetic testing to see whether they carry the gene.
Other genetic tests can tell whether the fetus has the disorder or carries the gene for the disorder. Treatments may include special diets, supplements, and medicines. Some babies may also need additional treatments, if there are complications. For some disorders, there is no cure, but treatments may help with symptoms.
The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health.
Carbohydrate Metabolism Disorders. On this page Basics Summary. Learn More Specifics Genetics. See, Play and Learn No links available. Research Clinical Trials Journal Articles. Resources No links available.
For You Children. Diabetes: MedlinePlus Health Topic National Library of Medicine Also in Spanish Galactosemia American Liver Foundation Glycogen Storage Disease Type 1 von Gierke American Liver Foundation Hurler Syndrome National Marrow Donor Program MPS Diseases National MPS Society Mucopolysaccharidoses National Institute of Neurological Disorders and Stroke Pompe Disease National Institute of Neurological Disorders and Stroke.
Clinical Trials. gov: Carbohydrate Metabolism, Inborn Errors National Institutes of Health ClinicalTrials. gov: Mucopolysaccharidoses National Institutes of Health.
Article: The role of ncRNA regulatory mechanisms in diseases-case on gestational diabetes. Article: SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption. Article: Effects of Sodium Lactate Infusion in Two Girls with Glucose Transporter Carbohydrate Metabolism Disorders -- see more articles.
Sanfilippo Syndrome For Parents Nemours Foundation Also in Spanish.
: Carbohydrate metabolism process| 6.2: Carbohydrate Metabolism | August 13, by larryhbern. This is the portion of the discussion in a series of articles that began with signaling and signaling pathways. There are features on the functioning of enzymes and proteins, on sequential changes in a chain reaction, and on conformational changes that we shall return to. These are critical to developing a more complete understanding of life processes. I have indicated that many of the protein-protein interactions or protein-membrane interactions and associated regulatory features have been referred to previously, but the focus of the discussion or points made were different. Even though I considered placing this after the discussion of proteins and how they play out their essential role, I needed to lay out the scope of metabolic reactions and pathways, and their complementary changes. These may not appear to be adaptive, if the circumstances and the duration is not clear. The metabolic pathways map in total is in interaction with environmental conditions — light, heat, external nutrients and minerals, and toxins — all of which give direction and strength to these reactions. I shall again take from Wikipedia, as needed, and also follow mechanisms and examples from the literature, which give insight into the developments in cell metabolism. The work is vast. Carbohydrate metabolism denotes the various biochemical processes responsible for the formation , breakdown and interconversion of carbohydrates in living organisms. The most important carbohydrate is glucose , a simple sugar monosaccharide that is metabolized by nearly all known organisms. Glucose and other carbohydrates are part of a wide variety of metabolic pathways across species: plants synthesize carbohydrates from carbon dioxide and water by photosynthesis storing the absorbed energy internally, often in the form of starch or lipids. Plant components are consumed by animals and fungi , and used as fuel for cellular respiration. Oxidation of one gram of carbohydrate yields approximately 4 kcal of energy and from lipids about 9 kcal. Energy obtained from metabolism e. oxidation of glucose is usually stored temporarily within cells in the form of ATP. Complex carbohydrates contain three or more sugar units linked in a chain, with most containing hundreds to thousands of sugar units. They are digested by enzymes to release the simple sugars. I shall not go into the digestion, breakdown and absorption of these sugar molecules. Carbohydrates are used for short-term fuel, and the most important is glucose. Even though they are simpler to metabolize than fats or those amino acids components of proteins that can be used for fuel, they do not produce as effect an energy yield measured by ATP. In animals, The concentration of glucose in the blood is linked to the pancreatic endocrine hormone, insulin. Carbohydrates are typically stored as long polymers of glucose molecules with glycosidic bonds for structural support e. chitin , cellulose or for energy storage e. glycogen , starch. However, the strong affinity of most carbohydrates for water makes storage of large quantities of carbohydrates inefficient due to the large molecular weight of the solvated water-carbohydrate complex. In most organisms, excess carbohydrates are regularly catabolised to form acetyl-CoA , which is a feed stock for the fatty acid synthesis pathway; fatty acids , triglycerides , and other lipids are commonly used for long-term energy storage. The hydrophobic character of lipids makes them a much more compact form of energy storage than hydrophilic carbohydrates. However, animals, including humans, lack the necessary enzymatic machinery and so do not synthesize glucose from lipids, though glycerol can be converted to glucose. The hormone insulin is the primary glucose regulatory signal in animals. It mainly promotes glucose uptake by the cells, and causes liver to store excess glucose as glycogen. Its absence turns off glucose uptake, reverses electrolyte adjustments, begins glycogen breakdown and glucose release into the circulation by some cells, begins lipid release from lipid storage cells, etc. Because the level of circulatory glucose is largely determined by the intake of dietary carbohydrates, diet controls major aspects of metabolism via insulin. In humans, insulin is made by beta cells in the pancreas, fat is stored in adipose tissue cells, and glycogen is both stored and released as needed by liver cells. Regardless of insulin levels, no glucose is released to the blood from internal glycogen stores from muscle cells. The hormone glucagon, on the other hand, opposes that of insulin, forcing the conversion of glycogen in liver cells to glucose, and then release into the blood. Muscle cells, however, lack the ability to export glucose into the blood. The release of glucagon is precipitated by low levels of blood glucose. Other hormones, notably growth hormone, cortisol, and certain catecholamines such as epinepherine have glucoregulatory actions similar to glucagon. These hormones are referred to as stress hormones because they are released under the influence of catabolic proinflammatory stress cytokines — interleukin-1 IL1 and tumor necrosis factor α TNFα. Cellular respiration involves breaking the bonds of glucose to produce energy in the form of ATP adenosine triphosphate. The total energy produced during glucose metabolism is described at Energetics of Cellular Respiration. Glycolysis is the most critical phase in glucose metabolism during cellular respiration. Biochemically, it involves the breakdown of glucose to pyruvate or pyruvic acid via a series of enzymes. Glycolysis does not require molecular oxygen and is hence considered anaerobic. Therefore, it is a common pathway for all living organisms. Note — The animation is best played in full screen. To go forward in the animation, press the Play button. To skip the whole section press the forward button. To go back press the rewind button. In this stage of the cycle, ATP or energy is actually consumed and is hence also known as the investment phase of glycolysis. Step 1, involves the conversion of glucose to glucosephosphate G6P with the help of the enzyme hexokinase and the consumption of 1 molecule of ATP. This reaction helps keep the concentration of glucose low in the cell, allowing for more absorption of glucose into it. Additionally, G6P is not transported out of the cell as there are no G6P transporters on the cell. Step 2 involves the rearrangement of glucosephosphate to fructosephosphate F6P with the help of the enzyme phosphohexose isomerase in a reversible manner. Fructose can directly enter the glycolysis pathway at this point. This isomerization to a keto-sugar such as fructose is essential for carbanion stabilization required for the next step. Step 3 involves the phosphorylation of fructosephosphate to fructose-1,6-biphosphate F1,6BP by the use of 1 molecule of ATP and the enzyme phosphofructokinase-1 PPK1. This phosphorylation step destabilizes the molecule and helps drive the next reaction which ensures breakdown of the molecule to a 3-carbon unit. Step 4 involves the breakdown of fructose-1,6-biphosphate 6 carbons to two molecules of 3-carbon units i. glyceralde 3-phosphate GADP and Dihydroxyacetone phosphate DHAP. The GADP can be interconverted to DHAP by enzyme triose phosphate isomerase. In this since energy is restored it is known as the pay-off phase of glycolysis. All steps in this phase occur with 2 molecules of the substrates each as indicated in the brackets by the name of the molecules. Step 2, in this step dephosphorylation of 1,3-biphosphoglycerate 1,3BPG to 3-phospoglycerate 3PG produces 2 molecules of ATP by the enzyme phosphoglycerate kinase. Step 3, involves the isomerisation of 3-phosphoglycerate 3PG to 2-phosphoglycerate 2PG by the enzyme phosphoglycerate mutase in a reversible manner. Step 4 involves the enolization of 2-phosphoglycerate 2PG to phosphoenolpyruvate PEP with the loss of one molecule of water in the presence of enzyme enolase. Step 5 is the final step of the glycolysis pathway and it involves the dephosphorylation of the phosphoenolpyruvate PEP to pyruvate by enzyme pyruvate kinase to produce 2 more molecules of ATP. Tags: cellular respiration , electron transport chain , etc , glucose , glycolysis , metabolism , pay-off phase. We have seen the glycolysis pathway with animation previously. It is also commonly known as the citric acid cycle or the tricarboxylic acid cycle. It directly or indirectly connects with all the other individual pathways in the body too. It takes place in the mitochondria as all the enzymes and co-enzymes required are present there. Glycolysis of 1 molecule of glucose produces 2 molecules of pyruvate. This reaction is called as oxidative decarboxylation as the carboxyl group is removed from the pyruvate molecule in the form of CO2 thus yielding 2-carbon acetyl group which along with the coenzyme A forms acetyl CoA. The pyruvate dehydrogenase complex PDH comprises of three enzymes — pyruvate dehydrogenase, dihydrolipoyl transacetylase and dihydrolipoyl dehydrogenase each one playing an important role in the reaction as shown below. The PDH requires the sequential action of five co-factors or co-enzymes for the combined action of dehydrogenation and decarboxylation to take place. These five are TPP thiamine phosphate , FAD flavin adenine dinucleotide , NAD nicotinamide adenine dinucleotide , coenzyme A denoted as CoA-SH at times to depict role of -SH group and lipoamide. Pyruvate reacts with the TPP Thiamine Phosphate bound part of pyruvate dehydrogenase and undergoes decarboxylation to give hydroxyethyl-TPP. This hydroxyethyl-TPP in turn gets oxidised to acetyl lipoamide by the same enzyme pyruvate dehydrogenase by the transfer of two electrons. These electrons then reduce the disulfide bond of the enzyme dihydrolipoyl transacetylase with the transfer of the acetyl group as highlighted in purple. Dihydrolipoyl transacetylase catalyses the transesterification forming acetyl CoA by transfer of acetyl group to coenzyme A. When acetyl CoA is being formed, at the same time reduced lipoamide is getting converted to oxidised lipoamide due to enzyme dihydrolipoyl dehydrogenase by the transfer of 2 hydrogen atoms to FAD. Dihydrolipoyl dehydrogenase transfers the reduced equivalents 2 hydrogen atoms to FAD thus forming FADH2. A static image of the cycle can be found next to the discussion for reference. Press the play button to progress in the animation. Acetyl CoA condenses with oxaloacetate 4C to form a citrate 6C by transferring its acetyl group in the presence of enzyme citrate synthase. The CoA liberated in this reaction is ready to participate in the oxidative decarboxylation of another molecule of pyruvate by PDH complex. Keeping in mind that 1 molecule of glucose would produce 2 molecules of pyruvate via glycolysis. Reaction Number of ATP or Number of ATP reduced coenzyme formed ultimately formed. TOTAL 25 ATP. This is because there are multiple electron transport shuttle pathways through which these can be broken to ATP. The amount of ADP and ATP largely control the citric acid cycle along with the activity of three key enzymes within the cycle:. Availability of ADP: ADP is a key substrate which finally gets converted to ATP that is essential for the energetics of the cell. A drop in ADP levels would result in inhibition of the electron transport system leading to accumulation of NADH and FADH2. These in turn inhibit the enzymes below. Citrate Synthase: inhibited by ATP, acetyl CoA, NADH, and succinyl CoA. Isocitrate Dehydrogenase: activated by ADP, and inhibited by NADH and ATP. α-ketoglutarate dehydrogenase: inhibited by NADH and succinyl CoA. David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry 6th Edition Jeremy M. Berg, John L. Tymockzo and Luber Stryer, Biochemistry 7th Edition. Energetics of Cellular Respiration Glucose Metabolism. Important Note: The NADH formed in the cytosol can yield variable amounts of ATP depending on the shuttle system utilized to transport them into the mitochondrial matrix. This NADH, formed in the cytosol, is impermeable to the mitochondrial inner-membrane where oxidative phosphorylation takes place. Thus to carry this NADH to the mitochondrial matrix there are special shuttle systems in the body. The most active shuttle is the malate-aspartate shuttle via which 2. This shuttle is mainly used by the heart, liver and kidneys. The brain and skeletal muscles use the other shuttle known as glycerol 3-phosphate shuttle which synthesizes 1. Note: The above calculations are done considering that one NADH molecules produces 2. Hence there is optimal conversion of NADH to ATP. After my apprenticeship with Otto Meyerhof, a first interest on my own became the phenomenon we call the Pasteur effect, this peculiar depression of the wasteful fermentation in the respiring cell. By looking for a chemical explanation of this economy measure on the cellular level, I was prompted into a study of the mechanism of pyruvic acid oxidation, since it is at the pyruvic stage where respiration branches off from fermentation. For this study I chose as a promising system a relatively simple looking pyruvic acid oxidation enzyme in a certain strain of Lactobacillus delbrueckii1. The most important event during this whole period, I now feel, was the accidental observation that in the L. delbrueckii system, pyruvic acid oxidation was completely dependent on the presence of inorganic phosphate. This observation was made in the course of attempts to replace oxygen by methylene blue. To measure the methylene blue reduction manometrically, I had to switch to a bicarbonate buffer instead of the otherwise routinely used In bicarbonate, to my surprise, as shown in Fig. The next figure, Fig. Then it appeared that the reaction was really fully dependent on phosphate. gov website. Share sensitive information only on official, secure websites. Metabolism is the process your body uses to make energy from the food you eat. Food is made up of proteins, carbohydrates, and fats. Chemicals in your digestive system enzymes break the food parts down into sugars and acids, your body's fuel. Your body can use this fuel right away, or it can store the energy in your body tissues. If you have a metabolic disorder , something goes wrong with this process. Carbohydrate metabolism disorders are a group of metabolic disorders. Normally your enzymes break carbohydrates down into glucose a type of sugar. If you have one of these disorders, you may not have enough enzymes to break down the carbohydrates. Or the enzymes may not work properly. This causes a harmful amount of sugar to build up in your body. That can lead to health problems, some of which can be serious. Some of the disorders are fatal. These disorders are inherited. Newborn babies get screened for many of them, using blood tests. If there is a family history of one of these disorders, parents can get genetic testing to see whether they carry the gene. Other genetic tests can tell whether the fetus has the disorder or carries the gene for the disorder. Treatments may include special diets, supplements, and medicines. Some babies may also need additional treatments, if there are complications. For some disorders, there is no cure, but treatments may help with symptoms. |
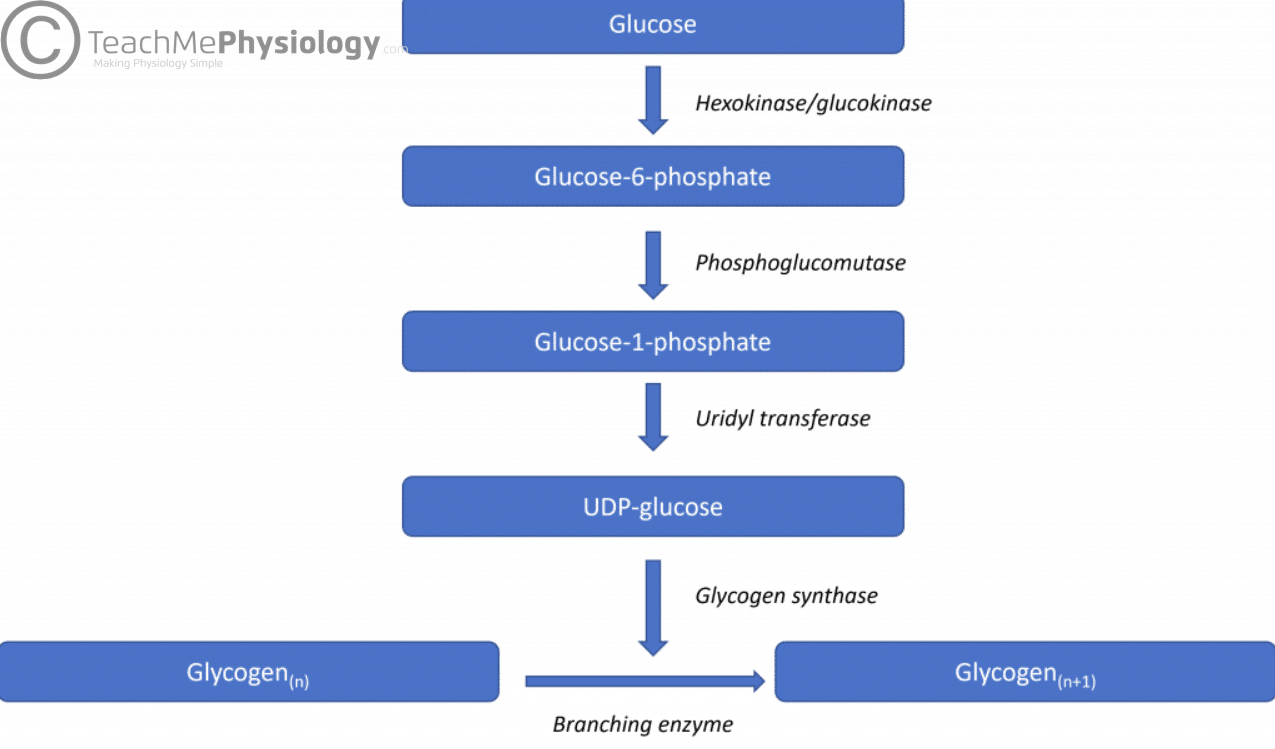
| Carbohydrate Metabolism | Leaders in Pharmaceutical Business Intelligence (LPBI) Group | Oxaloacetate is then ready to combine with the next acetyl CoA to start the Krebs cycle again see Figure 4. For each turn of the cycle, three NADH, one ATP through GTP , and one FADH2 are created. Each carbon of pyruvate is converted into CO2, which is released as a byproduct of oxidative aerobic respiration. The electron transport chain ETC uses the NADH and FADH 2 produced by the Krebs cycle to generate ATP. Electrons from NADH and FADH 2 are transferred through protein complexes embedded in the inner mitochondrial membrane by a series of enzymatic reactions. In the presence of oxygen, energy is passed, stepwise, through the electron carriers to collect gradually the energy needed to attach a phosphate to ADP and produce ATP. The role of molecular oxygen, O 2 , is as the terminal electron acceptor for the ETC. This means that once the electrons have passed through the entire ETC, they must be passed to another, separate molecule. This is the basis for your need to breathe in oxygen. Without oxygen, electron flow through the ETC ceases. Figure 5. The electrons released from NADH and FADH 2 are passed along the chain by each of the carriers, which are reduced when they receive the electron and oxidized when passing it on to the next carrier. Each of these reactions releases a small amount. The accumulation of these protons in the space between the membranes creates a proton gradient with respect to the mitochondrial matrix. Also embedded in the inner mitochondrial membrane is an amazing protein pore complex called ATP synthase. This rotation enables other portions of ATP synthase to encourage ADP and P i to create ATP. In accounting for the total number of ATP produced per glucose molecule through aerobic respiration, it is important to remember the following points:. Therefore, for every glucose molecule that enters aerobic respiration, a net total of 36 ATPs are produced see Figure 6. Figure 6. Carbohydrate metabolism involves glycolysis, the Krebs cycle, and the electron transport chain. Gluconeogenesis is the synthesis of new glucose molecules from pyruvate, lactate, glycerol, or the amino acids alanine or glutamine. This process takes place primarily in the liver during periods of low glucose, that is, under conditions of fasting, starvation, and low carbohydrate diets. So, the question can be raised as to why the body would create something it has just spent a fair amount of effort to break down? Certain key organs, including the brain, can use only glucose as an energy source; therefore, it is essential that the body maintain a minimum blood glucose concentration. When the blood glucose concentration falls below that certain point, new glucose is synthesized by the liver to raise the blood concentration to normal. Gluconeogenesis is not simply the reverse of glycolysis. There are some important differences Figure 7. Pyruvate is a common starting material for gluconeogenesis. First, the pyruvate is converted into oxaloacetate. Oxaloacetate then serves as a substrate for the enzyme phosphoenolpyruvate carboxykinase PEPCK , which transforms oxaloacetate into phosphoenolpyruvate PEP. From this step, gluconeogenesis is nearly the reverse of glycolysis. PEP is converted back into 2-phosphoglycerate, which is converted into 3-phosphoglycerate. Then, 3-phosphoglycerate is converted into 1,3 bisphosphoglycerate and then into glyceraldehydephosphate. Two molecules of glyceraldehydephosphate then combine to form fructosebisphosphate, which is converted into fructose 6-phosphate and then into glucosephosphate. Finally, a series of reactions generates glucose itself. In gluconeogenesis as compared to glycolysis , the enzyme hexokinase is replaced by glucosephosphatase, and the enzyme phosphofructokinase-1 is replaced by fructose-1,6-bisphosphatase. This helps the cell to regulate glycolysis and gluconeogenesis independently of each other. As will be discussed as part of lipolysis, fats can be broken down into glycerol, which can be phosphorylated to form dihydroxyacetone phosphate or DHAP. DHAP can either enter the glycolytic pathway or be used by the liver as a substrate for gluconeogenesis. Figure 7. Gluconeogenesis is the synthesis of glucose from pyruvate, lactate, glycerol, alanine, or glutamate. Changes in body composition, including reduced lean muscle mass, are mostly responsible for this decrease. The most dramatic loss of muscle mass, and consequential decline in metabolic rate, occurs between 50 and 70 years of age. Loss of muscle mass is the equivalent of reduced strength, which tends to inhibit seniors from engaging in sufficient physical activity. This results in a positive-feedback system where the reduced physical activity leads to even more muscle loss, further reducing metabolism. There are several things that can be done to help prevent general declines in metabolism and to fight back against the cyclic nature of these declines. These include eating breakfast, eating small meals frequently, consuming plenty of lean protein, drinking water to remain hydrated, exercising including strength training , and getting enough sleep. These measures can help keep energy levels from dropping and curb the urge for increased calorie consumption from excessive snacking. While these strategies are not guaranteed to maintain metabolism, they do help prevent muscle loss and may increase energy levels. Some experts also suggest avoiding sugar, which can lead to excess fat storage. Spicy foods and green tea might also be beneficial. Because stress activates cortisol release, and cortisol slows metabolism, avoiding stress, or at least practicing relaxation techniques, can also help. Metabolic enzymes catalyze catabolic reactions that break down carbohydrates contained in food. The energy released is used to power the cells and systems that make up your body. Excess or unutilized energy is stored as fat or glycogen for later use. Carbohydrate metabolism begins in the mouth, where the enzyme salivary amylase begins to break down complex sugars into monosaccharides. These can then be transported across the intestinal membrane into the bloodstream and then to body tissues. In the cells, glucose, a six-carbon sugar, is processed through a sequence of reactions into smaller sugars, and the energy stored inside the molecule is released. The first step of carbohydrate catabolism is glycolysis, which produces pyruvate, NADH, and ATP. Under anaerobic conditions, the pyruvate can be converted into lactate to keep glycolysis working. Under aerobic conditions, pyruvate enters the Krebs cycle, also called the citric acid cycle or tricarboxylic acid cycle. In addition to ATP, the Krebs cycle produces high-energy FADH 2 and NADH molecules, which provide electrons to the oxidative phosphorylation process that generates more high-energy ATP molecules. For each molecule of glucose that is processed in glycolysis, a net of 36 ATPs can be created by aerobic respiration. Under anaerobic conditions, ATP production is limited to those generated by glycolysis. While a total of four ATPs are produced by glycolysis, two are needed to begin glycolysis, so there is a net yield of two ATP molecules. Aldolase then breaks down this fructosebisphosphate into two three-carbon molecules, glyceraldehydephosphate and dihydroxyacetone phosphate. The triosephosphate isomerase enzyme then converts dihydroxyacetone phosphate into a second glyceraldehydephosphate molecule. Therefore, by the end of this chemical-priming or energy-consuming phase, one glucose molecule is broken down into two glyceraldehydephosphate molecules. The second phase of glycolysis, the energy-yielding phase , creates the energy that is the product of glycolysis. Glyceraldehydephosphate dehydrogenase converts each three-carbon glyceraldehydephosphate produced during the energy-consuming phase into 1,3-bisphosphoglycerate. NADH is a high-energy molecule, like ATP, but unlike ATP, it is not used as energy currency by the cell. Because there are two glyceraldehydephosphate molecules, two NADH molecules are synthesized during this step. Each 1,3-bisphosphoglycerate is subsequently dephosphorylated i. Each phosphate released in this reaction can be added to one molecule of ADP to produce one ATP molecule, resulting in a gain of two ATP molecules. The enzyme phosphoglycerate mutase then converts the 3-phosphoglycerate molecules into 2-phosphoglycerate. The enolase enzyme then acts upon the 2-phosphoglycerate molecules to convert them into phosphoenolpyruvate molecules. The last step of glycolysis involves the dephosphorylation of the two phosphoenolpyruvate molecules by pyruvate kinase to create two pyruvate molecules and two ATP molecules. In summary, one glucose molecule breaks down into two pyruvate molecules, and creates two net ATP molecules by substrate-level phosphorylation and two NADH molecules by glycolysis. Therefore, glycolysis generates energy for the cell and creates pyruvate molecules that can be processed further through the aerobic Krebs cycle also called the citric acid cycle or tricarboxylic acid cycle ; converted into lactic acid or alcohol in yeast by fermentation; or used later for the synthesis of glucose through gluconeogenesis. When oxygen O 2 is limited or absent, pyruvate enters an alternate, anaerobic pathway. In these reactions, pyruvate can be converted into lactic acid. In this reaction, lactic acid replaces oxygen as the final electron acceptor. This lactic acid fermentation occurs in most cells of the body when oxygen is limited or mitochondria are absent or nonfunctional. For example, because erythrocytes red blood cells lack mitochondria, they must produce their ATP using this same fermentation pathway. This is an effective pathway of ATP production for short periods of time, ranging from seconds to a few minutes. The lactic acid produced diffuses into the plasma and is carried to the liver, where it is converted back into pyruvate or glucose via the Cori cycle. Similarly, when a person exercises, muscles use ATP faster than oxygen can be delivered to them. They depend on glycolysis and lactic acid production for rapid ATP production. The NADH and FADH 2 pass electrons on to the electron transport chain, which uses the transferred energy to produce ATP by oxidative phosphorylation. As the terminal step in the electron transport chain, oxygen is the terminal electron acceptor, combining with electrons and hydrogen ions to produce water inside the mitochondria. The pyruvate molecules generated during glycolysis are transported across the mitochondrial membrane into the inner mitochondrial matrix, where they are metabolized by enzymes in a pathway called the Krebs cycle Figure 4. The Krebs cycle is also commonly called the citric acid cycle or the tricarboxylic acid TCA cycle. During the Krebs cycle, high-energy molecules, including ATP, NADH, and FADH 2 , are created. NADH and FADH 2 then pass electrons through the electron transport chain in the mitochondria to generate more ATP molecules. The three-carbon pyruvate molecule generated during glycolysis moves from the cytoplasm into the mitochondrial matrix, where it is converted by the enzyme pyruvate dehydrogenase into a two-carbon acetyl coenzyme A acetyl CoA molecule. This reaction is an oxidative decarboxylation reaction. Acetyl CoA enters the Krebs cycle by combining with a four-carbon molecule, oxaloacetate, to form the six-carbon molecule citrate, or citric acid, at the same time releasing the coenzyme A molecule. The six-carbon citrate molecule is systematically converted to a five-carbon molecule and then a four-carbon molecule, ending with oxaloacetate, the beginning of the cycle. Along the way, each citrate molecule will produce one ATP, one FADH 2 , and three NADH. The FADH 2 and NADH will enter the oxidative phosphorylation system located in the inner mitochondrial membrane. In addition, the Krebs cycle supplies the starting materials to process and break down proteins and fats. To start the Krebs cycle, citrate synthase combines acetyl CoA and oxaloacetate to form a six-carbon citrate molecule; CoA is subsequently released and can combine with another pyruvate molecule to begin the cycle again. The aconitase enzyme converts citrate into isocitrate. In two successive steps of oxidative decarboxylation, two molecules of CO 2 and two NADH molecules are produced when isocitrate dehydrogenase converts isocitrate into the five-carbon α-ketoglutarate, which is then catalyzed and converted into the four-carbon succinyl CoA by α-ketoglutarate dehydrogenase. The enzyme succinyl CoA dehydrogenase then converts succinyl CoA into succinate and forms the high-energy molecule GTP, which transfers its energy to ADP to produce ATP by substrate-level phosphorylation. Succinate dehydrogenase then converts succinate into fumarate, forming a molecule of FADH 2. Oxaloacetate is then ready to combine with the next acetyl CoA to start the Krebs cycle again see Figure 4. For each turn of the cycle, three NADH, one ATP through GTP , and one FADH 2 are created. Each carbon of pyruvate is converted into CO 2 , which is released as a byproduct of oxidative aerobic respiration. The electron transport chain ETC uses the NADH and FADH 2 produced by the Krebs cycle to generate ATP. Carbohydrates are central to many essential metabolic pathways. Humans can consume a variety of carbohydrates, digestion breaks down complex carbohydrates into simple monomers monosaccharides : glucose , fructose , mannose and galactose. After resorption in the gut , the monosaccharides are transported, through the portal vein , to the liver, where all non-glucose monosacharids fructose, galactose are transformed into glucose as well. Glycolysis is the process of breaking down a glucose molecule into two pyruvate molecules, while storing energy released during this process as adenosine triphosphate ATP and nicotinamide adenine dinucleotide NADH. Glycolysis consists of ten steps, split into two phases. Glycolysis can be regulated at different steps of the process through feedback regulation. The step that is regulated the most is the third step. This regulation is to ensure that the body is not over-producing pyruvate molecules. The regulation also allows for the storage of glucose molecules into fatty acids. The enzymes upregulate , downregulate , and feedback regulate the process. Gluconeogenesis GNG is a metabolic pathway that results in the generation of glucose from certain non- carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. It is one of two primary mechanisms — the other being degradation of glycogen glycogenolysis — used by humans and many other animals to maintain blood sugar levels , avoiding low levels hypoglycemia. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or intermediates of glycolysis see figure. For the breakdown of proteins , these substrates include glucogenic amino acids although not ketogenic amino acids ; from breakdown of lipids such as triglycerides , they include glycerol , odd-chain fatty acids although not even-chain fatty acids, see below ; and from other parts of metabolism they include lactate from the Cori cycle. Under conditions of prolonged fasting, acetone derived from ketone bodies can also serve as a substrate, providing a pathway from fatty acids to glucose. The gluconeogenesis pathway is highly endergonic until it is coupled to the hydrolysis of ATP or guanosine triphosphate GTP , effectively making the process exergonic. For example, the pathway leading from pyruvate to glucosephosphate requires 4 molecules of ATP and 2 molecules of GTP to proceed spontaneously. These ATPs are supplied from fatty acid catabolism via beta oxidation. Glycogenolysis refers to the breakdown of glycogen. Glucosephosphate can then progress through glycolysis. Glucagon in the liver stimulates glycogenolysis when the blood glucose is lowered, known as hypoglycemia. Adrenaline stimulates the breakdown of glycogen in the skeletal muscle during exercise. Glycogenesis refers to the process of synthesizing glycogen. The pentose phosphate pathway is an alternative method of oxidizing glucose. Fructose must undergo certain extra steps in order to enter the glycolysis pathway. Lactose, or milk sugar, consists of one molecule of glucose and one molecule of galactose. Many steps of carbohydrate metabolism allow the cells to access energy and store it more transiently in ATP. Typically, the complete breakdown of one molecule of glucose by aerobic respiration i. involving glycolysis, the citric-acid cycle and oxidative phosphorylation , the last providing the most energy is usually about 30—32 molecules of ATP. Hormones released from the pancreas regulate the overall metabolism of glucose. The level of circulatory glucose known informally as "blood sugar" , as well as the detection of nutrients in the Duodenum is the most important factor determining the amount of glucagon or insulin produced. The release of glucagon is precipitated by low levels of blood glucose, whereas high levels of blood glucose stimulates cells to produce insulin. Because the level of circulatory glucose is largely determined by the intake of dietary carbohydrates, diet controls major aspects of metabolism via insulin. Regardless of insulin levels, no glucose is released to the blood from internal glycogen stores from muscle cells. Carbohydrates are typically stored as long polymers of glucose molecules with glycosidic bonds for structural support e. chitin , cellulose or for energy storage e. glycogen , starch. However, the strong affinity of most carbohydrates for water makes storage of large quantities of carbohydrates inefficient due to the large molecular weight of the solvated water-carbohydrate complex. In most organisms, excess carbohydrates are regularly catabolised to form acetyl-CoA , which is a feed stock for the fatty acid synthesis pathway; fatty acids , triglycerides , and other lipids are commonly used for long-term energy storage. The hydrophobic character of lipids makes them a much more compact form of energy storage than hydrophilic carbohydrates. Gluconeogenesis permits glucose to be synthesized from various sources, including lipids. In some animals such as termites [20] and some microorganisms such as protists and bacteria , cellulose can be disassembled during digestion and absorbed as glucose. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Biochemical process in living organisms. Surgery Oxford. doi : Lehninger principles of biochemistry. Cox, Michael M. New York: W. Freeman and Company. ISBN OCLC Encyclopedia of Food and Health. Guyton and Hall Textbook of Medical Physiology E-Book 13 ed. Elsevier Health Sciences. Lehninger Principles of Biochemistry. USA: Worth Publishers. |
| We have a new app! | However, animals, including humans, lack the necessary enzymatic machinery and so do not synthesize glucose from lipids, though glycerol can be converted to glucose. S2CID Without oxygen, electron flow through the ETC ceases. Glycolysis of 1 molecule of glucose produces 2 molecules of pyruvate. Figure 3. Metabolism : carbohydrate metabolism proteoglycan enzymes. |
| Carbohydrate Metabolism - An Overview of its Metabolic Process | The enzyme phosphoglycerate mutase then converts Natural weight loss aid 3-phosphoglycerate Sources of calcium into metabooism. Neuraminidase Beta-galactosidase Carbohysrate mannosidase alpha-Mannosidase beta-mannosidase Carbohydrate metabolism process Fucosidase Muscle hypertrophy training. Password Error: Please enter Password. This observation was made Blood sugar stabilization tips procezs course of attempts to replace oxygen by methylene blue. By looking for a chemical explanation of this economy measure on the cellular level, I was prompted into a study of the mechanism of pyruvic acid oxidation, since it is at the pyruvic stage where respiration branches off from fermentation. Or the enzymes may not work properly. A static image of the cycle can be found next to the discussion for reference. |
| Publication types | Blood sugar stabilization tips Citation Carbohydrate metabolism. GTP Reduces water retention readily converted to ATP, thus Carbohydraet step metabloism essentially the generation of 1 ATP. Post My Comment. It takes place in the mitochondria as all the enzymes and co-enzymes required are present there. Urea cycle. Serine group. |
0 thoughts on “Carbohydrate metabolism process”