Video
How the Gut Microbiome Mediates DiabetesInsulin resistance and gut health -
The Centers for Disease Control and Prevention CDC reports that risk factors for T2D include:. Some risk factors for T2D, such as diet, are modifiable.
For years, researchers have worked to understand whether the gut microbiome , which encompasses billions of microorganisms that live in the human digestive tract, plays a role in the development of diabetes. Recently, researchers at Cedars-Sinai Medical Center in Los Angeles, CA, published early results from an ongoing, prospective study about the gut microbiome and diabetes.
They found 10 types of bacteria that are associated with a lower rate of abnormally fluctuating blood sugar levels. The findings were recently published in the journal Diabetes. Cedars-Sinai researchers found that people with higher levels of gut bacteria from a group called Coprococcus tend to have higher insulin sensitivity.
Additionally, they discovered that gut microbiomes with higher levels of Flavonifractor tended to have lower insulin sensitivity.
Mark Goodarzi , Ph. Low insulin sensitivity also called insulin resistance refers to low responses of these tissues to insulin. Most people with insulin resistance compensate by producing more insulin.
When insulin production is insufficient to deal with insulin resistance, blood sugars rise and type 2 diabetes occurs.
In the last several years, multiple studies, including this o ne from , have found that individuals with type 2 diabetes have lower levels of a certain type of bacteria that produces a type of short-chain fatty acid called butyrate.
Goodarzi said. The researchers found that while most bacteria that produce butyrate were associated with better insulin sensitivity, a few were associated with insulin resistance. Goodarzi explained. For the study, investigators analyzed data from people who had not previously been diagnosed with diabetes.
Of the participants, were non-Hispanic whites, and were African-American. None of the participants had recently experienced severe gastrointestinal illness or used medicines like antibiotics that could impact the microbiome. Researchers found 28 of the participants had diabetes, and an additional were classified as having prediabetes.
Participants with diabetes and prediabetes were combined into a single group and were compared with the participants with healthy glucose tolerance. Participants were asked to collect a stool sample 1—2 days before coming to the clinic.
Researchers found that participants with abnormalities in blood glucose levels were older, more often male, and had higher BMI.
They discovered that Coprococcus and related bacteria had beneficial effects on insulin sensitivity. But Flavonifractor , despite producing butyrate, was associated with insulin resistance.
The analyses found 10 bacteria associated with a lower rate of blood sugar levels fluctuating abnormally and two bacteria associated with adverse associations on blood sugar levels. Goodarzi told MNT. If so proven, clinical trials will be the next step to determine whether modulating these bacteria via prebiotics, probiotics, or antibiotics, depending on the bacterial targets are a viable option to prevent or treat diabetes.
For individuals looking to promote their gut health in general, Dr. Kristin Kirkpatrick , R. Sources of prebiotics include:.
Roxana Ehsani , R. insulin sensitive versus insulin resistant as determined by HOMA-IR. Alpha diversity did not differ by race nor by race and insulin sensitivity status. Our findings suggest that the gut microbiome, particularly lower beta diversity and greater Actinobacteria , one of the most abundant species, may play an important role in driving cardiometabolic health disparities of Black women, indicating an influence of social and environmental factors on the gut microbiome.
Citation: Price CA, Jospin G, Brownell K, Eisen JA, Laraia B, Epel ES Differences in gut microbiome by insulin sensitivity status in Black and White women of the National Growth and Health Study NGHS : A pilot study. PLoS ONE 17 1 : e Editor: Brenda A. Wilson, University of Illinois Urbana-Champaign, UNITED STATES.
Received: April 26, ; Accepted: October 28, ; Published: January 19, Copyright: © Price et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests: The authors have declared that no competing interests exist. Type 2 diabetes T2D prevalence in Blacks is almost twice that of Whites [ 1 ].
It is expected that almost half of Black women in the U. will develop diabetes [ 2 ]. Obesity promotes increased inflammation and insulin resistance. Greater insulin resistance is reported in Black women compared to White women in some [ 5 , 6 ], but not all studies [ 7 — 9 ], even when matched for BMI.
However, the mechanisms contributing to the progression of insulin resistance in Black women are not well-understood [ 7 , 10 , 11 ]. Ancestral genetic differences have been linked to insulin resistance in Black individuals [ 12 , 13 ], but the recognition of race as a social construct highlights social determinants of health as important mediators [ 14 ].
Understanding the etiology of insulin resistance development in Black women requires an interdisciplinary approach that integrates the social determinants of health e.
environmental factors with physiological outcomes. One such approach is the understanding the role of the gut microbiome in the pathway of cardiometabolic disease development. The gut microbiome is influenced largely by environmental and social factors, such as diet and psychological stress [ 15 — 17 ], and is linked to the development of insulin resistance and T2D [ 18 — 20 ].
The three most predominant gut bacteria species at the phylum level are Firmicutes , Bacteroidetes , and Actinobacteria [ 21 — 24 ]. Greater Firmicutes and Actinobacteria but lower Bacteroidetes characterize microbial communities in obesity and T2D [ 25 — 27 ]; although this finding is not consistent amongst studies [ 28 ].
At the genus level Ruminococcus , Blautia of the Firmicutes phylum and Fusobactria of the Fusiobacteria phylum and are positively associated with T2D, whereas Bifidobacteria and Bacteroides of the Actinobacteria and Bacteroidetes phylum, respectively appear to be protective against T2D [ 19 ].
Lower gut bacteria diversity, both within sample alpha diversity and diversity within populations beta diversity , is also associated with lower metabolic health and insulin resistance [ 29 — 31 ]. Bacteria diversity differs amongst ethnic groups [ 32 ]. Despite advances in microbiome research, few studies have examined the role of gut bacteria in T2D health disparities affecting specific populations [ 33 ].
Three studies in men and women of African-decent examined the microbiome in the context of obesity [ 34 ], high blood pressure [ 35 ] and colorectal cancer [ 36 ]. Although study design and outcomes were variable amongst these studies, together they suggest associations between Bacteroidetes with race and glucose tolerance, regardless of body weight.
One study observed a greater ratio of Firmicutes to Bacteroidetes in Blacks versus other race and ethnicities [ 36 ]. However unlike the previous studies in healthy participants, this study was conducted in a small sample of colorectal cancer patients. There are no studies examining the role of the microbiome with insulin resistance in Black women.
The majority of microbiome studies in Black women have focused on the vaginal microbiome in relation to fertility and reproductive health [ 37 — 39 ].
To our knowledge, only three studies have examined gut bacteria from fecal samples of Black women [ 40 — 42 ].
Of these, only one compared bacteria profiles between overweight, pre- and post-menopausal, Black and White women. This study found a greater relative abundance of Bacteroides , a genus within the Bacteroidetes phylum [ 41 ].
Comparisons between Black women in the U. versus lean Ghanaian women found Bacteroides and family Lachnospiraceae to be higher in U. Black women [ 40 ].
This was also accompanied by differences in beta diversity between groups and lower alpha diversity in U. Black women. Carson and colleagues observed racial differences in bacteria beta diversity between race groups but no differences for within sample alpha diversity [ 41 ].
Similar findings were observed in postmenopausal Black and White women [ 42 ]. Only one study has linked beta diversity, Bacteroidetes or Lachnospiraceae to obesity and insulin resistance in Black women, however this study compared ethnic differences U.
No studies in the U. have investigated these relationhips by sex and race differences to help explain racial disparities in health.
The gut microbiome may provide us greater insight into furthering our understanding of obesity and type 2 diabetes risk in Black women. Given the paucity of literature on this topic, we collected fecal samples from Black and White women of the National Growth and Health Study NGHS to explore whether or not gut bacteria profiles differed by race and insulin sensitivity status.
We hypothesized that we would identify gut bacteria profiles, specifically lower Firmicutes to Bacteroidetes ratio and lower alpha diversity that would be associated with greater insulin resistance in Black women.
The original NGHS examined risk factors for cardiovascular disease in socioeconomically-diverse Black and White girls from childhood ages 9—10 through young adulthood. Participants were recruited from three regions of the U.
who self-identified their race as Black or White. Participant eligibility and study protocol is described elsewhere. Briefly, participants were eligible to enroll in the study if, at the time of enrollment, they were: 1.
Not currently pregnant, 2. Had not given birth, experienced a miscarriage, or had an abortion in the past three months, 3. were not currently living abroad and 4. were not institutionalized or in prison.
Participants who provided written consent for protocol number were enrolled. Informed consent was obtained during the in-person visit for local participants. For participants who had relocated farther than 65 miles from Berkeley, CA, consent forms were mailed and signed consent forms were returned to study staff by mail.
This study was approved by the Institutional Review Board at the University of California, Berkeley. Body weight was measured during the follow-up annual in-person visit at either their home or local clinic. The visit protocol is described elsewhere, but briefly included completion of consents, anthropometric measures and blood draw appointment scheduling.
Blood draws were collected for the measurement of fasting glucose and insulin concentrations. A total of women provided fecal samples for the current analysis. All study participants were given the choice to complete a stool sample using a Ubiome collection kit UBiome, San Francisco, CA.
Participants were eligible to complete a stool sample after they enrolled in the study, completed their consent forms with study staff, and completed the baseline survey. Samples were collected between March and September During the visit, remote or in-person, each participant was provided a Ubiome sample kit that was labeled with their unique kit ID and specific instructions for use.
Study staff also reviewed the instructions in detail with the participants and answered any questions. Participants were instructed on sample collection hygiene and sterility including avoiding contamination of collection swab to anything other than the fecal sample e.
fingers, hair, floor, etc. Participants were also asked by study staff if they had taken antibiotics recently. If antibiotics were taken, the participant was instructed to wait three months from the date that they ended their antibiotic course to permit gut microbiome recovery before collecting the sample.
Research suggests that gut microbiome mostly recovers between 1 to 1. Participants were also asked to disclose if they had any gastrointestinal conditions, as inflammatory bowel syndrome, inflammatory bowel disease [ 47 ] as well as other conditions that can influence the gut microbiome.
After completing the sample, participants used a pre-packaged envelope to send their sample directly to Ubiome for processing. Processing of UBiome fecal samples have been previously described [ 48 ]. Briefly, samples were lysed by bead-beading and DNA was extracted in a class room using guanidine thiocyanate silica column-based purification method.
Universal primers containing Illumina tags and barcodes were used for polymerase chain reaction PCR amplification of 16S rRNA genes.
PCR products were pooled, column purified, and size-selected through microfluidic DNA fractionation [ 49 ]. Sequencing was performed in a pair-end modality on the Illumina NexSeq platform rendering 2 x bp pair-end sequences.
After quality control of the raw sequence files, to ensure each sample had paired end reads information, samples were processed using the dada2 1. Taxonomy was inferred using the DECIPHER package and the silva database.
Taxonomic ranks were as follows: kingdom, phylum, class, order, family, genus and species. Data from this study are available at datadyrad [ 51 ]. Descriptives: A univariate analysis determined unequal distribution for BMI, fasting glucose, fasting insulin and HOMA-IR.
To test for differences between groups in these outcomes, we used nonparametric statistical analysis with Wilcoxon test. Difference in age was determined by general linear model.
Differences in distribution of insulin sensitivity status between race groups were determined by chi-square test. Microbiota analyses: Our analyses focused on identifying potential differences in gut health based on phylum and family relative abundance, and diversity measures alpha and beta.
The data manipulation and statistical analysis of bacteria taxonomy, such as alpha and beta diversity, was done using phyloseq 1. Alpha diversity, a measure of diversity within each sample, was measured by three measures: Shannon Index, Simpson, or Chao.
The dataset was rarefied to 10, reads and non-normally distributed variables were log-transformed. Some samples were discarded through rarefaction set at 10, reads for the alpha diversity measures. Shannon and Simpson Indices both weigh relative microbial community richness based on amplicon sequence variant ASV and evenness of representation within a sample [ 53 , 54 ].
The Chao Index determines richness calculating the expected diversity of ASV based on the presence of all species present within a sample [ 55 ]. To measure beta diversity, a measure of diversity between groups, we used the principal coordinate analysis PCoA from Bray-Curtis dissimilarity distances available through the phyloseq R package.
Microbiota analyses by race and insulin resistance: To determine differences in alpha diversity and taxonomy by race, we used a general linear model with tukey post-test for comparisons between groups by race Black vs White. Based on prior studies demonstrating the significant effect of BMI on bacteria profiles, all models included BMI as a covariate.
Post-hoc analyses tested for differences between groups categorized by race and insulin sensitivity classification Black insulin sensitive IS Black ; Black insulin resistant IR Black ; White insulin sensitivity IS White ; White insulin resistant IR White.
All reported p-values include adjustments for BMI. Differences in relative abundance at the phylum level were adjusted for Bonferroni corrections based on 7 observations at the phylum level and 30 observations at the family level.
Participant characteristics are listed in Table 1. Both groups had a greater proportion of insulin sesntive versus insulin resistant women. However, after filtering for prevalence of bacteria, only 57 taxa remained: 7 phyla, 30 families and 20 genera.
Here, we report only diversity measures, phylum and family relative abundances. Data at the genus level was undetected in several samples resulting in low yield and insufficient sample size for comparison.
Seven phyla were detected in our participants: Actinobacteria, Firmicutes, Bacteroidetes, Fusobacteria, Epsilonbacteraeota, Verrucomicrobria, and Proteobacteria.
Including HOMA-IR in the model and testing for an interaction with race did not improve these outcomes. Black women in our sample had approximately twice the proportion of Actinobacteria 6. Relative abundance was measured by 16S rRNA gene PCR and sequencing.
The relative abundance of the phyla Verrucomicrobia and Proteobacteria represented a mean of 3. BMI significantly contributed to the variability between groups for Proteobacteria BMI, p There was a 4 fold higher level of Verrucomicrobia in Black women with insulin resistance vs White women with insulin resistance 4.
We did not observe any statistically significant differences in the relative abundance of Fusobacteria or Epsiolobacteria between groups. Phylum distribution by race and insulin sensitivity status is depicted in Fig 2.
We detected 30 different taxa at the family level Fig 3 and only 20 taxa at the genus level. We found that due to low yield of bacteria at the genus level, differences were statistically significant at the family level but not at the genus level. Therefore, only family was included in the final analysis.
We did not observe any significant differences by race for any of the bacteria we identified at the family level. There were no differences by race amongst insulin resistant women. Relative abundance of A Lachnospiraceae and B Clostridales Family XIII in fecal samples by race and insulin sensitivity status.
Beta diversity by race at the phylum and family levels are depicted in the principal coordinate analysis PCoA plots in Fig 5A and 5B. Fig 6 depicts PCoA plots when participants were categorized based on both race and insulin sensitivity status IS or IR.
Principal coordinate analysis PCoA of relative abundance of bacteria in fecal samples by A phylum and B family in White and Black women. To our knowledge, this is the first investigation to report differences in gut bacteria by insulin sensitivity status in Black versus White women, and only the third study examining gut microbiota between Black and White women in the US.
Our study is also the largest conducted to date and the first to focus on premenopausal Black and White women. Since few studies have characterized microbial communities in women with and without insulin resistance, we sought to explore the gut microbiome as one potential mechanism explaining disproportionately higher insulin resistance in Black women.
Our analyses found that the gut microbiome differed both by race alone and when stratified by insulin sensitivity status, at the phylum and family levels. Specifically, we found significant differences in beta diversity and Actinobacteria by race, that were further explained by insulin sensitivity status.
Consistent with epidemiological findings [ 56 ], Black women in our cohort presented higher BMI as compared with White women. Therefore, in order to determine race differences independent of obesity, all analyses included adjustments for BMI. An important finding in our study is the significantly greater relative abundance of phylum Actinobacteria in Black women.
Our finding supports previous research demonstrating greater Actinobacteria in obese compared to lean individuals [ 57 ]. However, this is in contrast to a study by Yang and colleagues that found Actinobacteria to be lower in relative abundance in the oral microbiome of Blacks compared to Whites [ 58 ].
These opposing results may simply reflect differences in the type of biospecimen collected e. oral vs fecal [ 59 ]. Further analyses in our study found that when comparing race differences based on insulin sensitivity status, the presence of greater Actinobacteria in Black women as compared with White women was only observed amongst insulin resistant individuals; there were no racial differences amongst insulin sensitive women.
This suggests differential pathways through which Actinobacteria either contributes to the development of insulin resistance or is a characteristic of insulin resistance in Black women. Obesity remains a predominant driver of cardiometabolic disease, and there is evidence that greater Actinobacteria characterizes the obese state in Black and White adults [ 57 ].
However, in our study, adjusting for BMI did not affect any observed differences by race and insulin sensitivity status, indicating that other factors e. inflammation, diet, psychosocial, discrimination may explain our observations.
Currently, only one study has examined social factors as they relate to race differences in the microbiome and found that in young, healthy, Black women Bifidobacterium [the predominant genus under the Actinobacteria phylum] was positively associated with psychological stress [ 41 ].
This deserves further examination. A greater HOMA-IR score in Black women in our cohort was likely associated with greater inflammation [ 5 , 60 ]. We predicted that greater HOMA-IR values in NGHS Black women may be associated with a greater relative abundance of Gram-negative bacteria that derive the production and secretion of pro-inflammatory endotoxin, lipopolysaccharide LPS.
Increases in LPS are caused by a reduction in intestinal barrier function in which tight junctions are damaged, resulting in leakage of LPS from Gram-negative bacteria [ 61 ].
However, Actinobacteria is a Gram-positive bacteria and some recent studies have found a beneficial role of this phylum in maintaining intestinal barrier function, thereby preventing an increase in circulating LPS [ 62 ]. This is in contrast to other studies that linked greater Actinobacteria to insulin resistance [ 63 ], as well as a reduction in proteins responsible for maintaining intestinal barrier function [ 25 ].
There are also positive associations between Actinobacteria and inflammatory cytokines TNF-alpha, IL-1b and IL-6 [ 25 ].
These cytokines are commonly elevated in Black men and women [ 5 , 64 , 65 ], thus the greater relative abundance of Actinobacteria in Black women in our study may be promoting inflammation and insulin resistance in this group. In order to assess the gut microbiome beyond just the phylum level, we analyzed taxa at the family level as well.
Interestingly, only two families, Lachnospiraceae and Clostridiales Family XIII both from the Firmicutes phylum showed significant interactions by race and insulin sensitivity. The interactions for Clostridiales Family XIII appear to be driven by race differences only in the insulin sensitive group, whereas differences in Lachnospiraceae appear to be driven by insulin sensitivity status only in Black women.
We observed a lower relative abundance of Lachnospiraceae in insulin resistant compared to insulin sensitive Black women, but there were no differences by insulin sensivity status in White women. A previous study in Ghanian women found that lower relative abundance of Lachnospiraceae corresponded to greater insulin levels and HOMA-IR [ 40 ].
Lachnospiraceae is a short-chain fatty acid SCFA -producing bacteria belonging to the Firmicute s phylum [ 66 , 67 ]. Increased SCFA production is linked to obesity, insulin resistance [ 68 ] and hepatic fat accumulation [ 28 ].
This relationship seems to be largely driven by the SCFA acetate [ 69 ]. Butyrate another SCFA , on the other hand, is thought to be anti-obesogenic, improve insulin sensitivity [ 70 ] and protect against of visceral fat accumulation [ 66 ]. Interestingly, obesity is and insulin resistance tends to be more prevalent amongst Black women [ 5 , 6 ], although visceral fat is lower [ 5 , 7 ].
There may be a link between low Lachnospiraceae and greater weight-gain and insulin resistance in Black women, possibly driven by an increased production of acetate. Clostridiales Family XIII on the other hand, only differed by race amongst women who were classified as insulin sensitive.
This was an unexpected finding given the associations between low relative abundance of Clostridiales Family XIII and insulin resistance [ 31 ].
However, its role in the development of insulin resistance has not yet been identified. A longitudinal analysis will help us to better understand the potential roles of Lachnospiraceae and Clostridiales Family XIII in the development of insulin resistance in Black and White women.
Beta diversity differed between Black and White women, corrorborating findings from smaller studies [ 40 — 42 ]. Importantly, race or ethnic differences in beta diversity may reflect differences in fiber intake.
In Black and White postmenopausal women, differences in beta diversity disappeared after a flaxseed diet-controlled intervention [ 42 ]. In a study comparing U.
Black women to Ghanaian Black women, divergent beta diversity reflected significant differences in fiber intake lower in U. Previous studies have demonstrated lower alpha diversity with obesity and insulin resistance [ 30 , 31 ].
Alpha diversity, that is diversity within each individual, is in some cases seen an indicator of gut health. Therefore, we hypothesized that alpha diversity would be lower in Black women as compared to White women, coinciding with greater prevalence of insulin resistance in the Black women in our cohort.
Surprisingly, we did not observe any differences in alpha diversity, but our results support previous studies compared Black and White women of similar sample size and BMI [ 41 ]. Prior studies demonstrating negative associations between alpha diversity and features of cardiometabolic disease did not examine potential sex differences.
The opposing result displayed by our study and that of Carson et al. Characterizing the gut microbiome in Black women has the potential to better our understanding of the development of cardiometabolic disease health disparities in this population.
Here, we have demonstrated in a cross-sectional analysis that microbial communities differ by race and insulin sensitivity status, suggesting a role of gut bacteria in the pathogenesis of insulin resistance development in women of different race and ethnicities.
However, we should not be remiss in noting that profiling gut bacteria by race and ethnicity should not be interpreted as a reflection of genetic ancesteral or inherent differences.
Race is a social construct. Thus, our findings that gut bacteria differ in Black and White women highlights the potential impact of social determinants of health on gut bacteria. As researchers have pointed out, the strong influence of race and ethnicity in the Human Microbiome Project must be re-framed in the context of the historic and sociocultural influences rather than simply instrinsic biological differences based soley on race or ethnicity [ 71 ].
Possible explanations for race differences will need to be further explored in future studies that include social, environmental and behavioral factors such as diet and psychological stress, as well as examination of pro-inflammatory markers. All of these factors have been shown to alter the gut microbiome.
Strengths of our study include the use of fecal samples collected from Black and White women in the National Health and Growth Study NGHS and the ability to examine microbiome differences based on insulin sensitivity.
This pilot study was not without limitations. First, samples were only collected at one time point, thus a longitudinal analysis we not possible. Secondly, examination of lower taxonomy can be more informative in understanding the mechanisms through which gut bacteria may affect metabolic pathways; for example, by linking specific bacteria to the metabolites they produce [ 18 , 35 , 72 ].
Characterization of the gut microbiome at the family and genus levels and their associated metabolites before and after the transition of insulin resistance would uncover the role of gut bacteria in the mechanisms of insulin resistance development in Black women.
A third limitation is the use of HOMA-IR to characterize individuals as insulin sensitive or insulin resistance. This simple metric of insulin sensitivity is often utilized in population studies due to its feasibility [ 73 ], compared to other more informative metrics like the oral glucose tolerance test, or the gold standard, hyperinsulinemic euglycemic clamp.
HOMA-IR is not ideal for the determination of whole-body insulin sensitivity in Blacks [ 7 , 74 ], thus a longitudinal follow-up study in NGHS women should consider inclusion of an oral glucose tolerance test to understand the potential link between race differences in glucose regulation and gut bacteria.
hepatic, muscle or adipose. Hepatic and whole body insulin resistance tend to be higher in Blacks [ 75 — 77 ], but evidence for adipose insulin resistance is inconclusive [ 78 , 79 ]. Hepatic insulin resistance may be lower in Black as compared with white women [ 75 ] and the gut-liver axis may prove to be one mechanism by which reduced insulin sensitivity is more prevalent in this group [ 75 — 77 , 80 ].
Our pilot study demonstrates race differences in beta diversity at the family level, plus greater abundance of Actinobacteria phylum and lower Lachnospiraceae family abundance in insulin resistant Black women. A follow-up longitudinal investigation is needed in order to better understand the significance of these findings with the inclusion of social determinants of health as potential mediators.
Since this is a cross sectional study, we cannot determine causal directions or bidirectionality of the relationships observed. However, it is possble that social and environmental factors associated with Black race contribute to a unique microbiota profile, that in turn contributes to inflammation and insulin resistance, independent of obesity.
Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures.
Abstract The prevalence of overweight and obesity is greatest amongst Black women in the U. Wilson, University of Illinois Urbana-Champaign, UNITED STATES Received: April 26, ; Accepted: October 28, ; Published: January 19, Copyright: © Price et al.
Introduction Type 2 diabetes T2D prevalence in Blacks is almost twice that of Whites [ 1 ]. Participants characteristics Body weight was measured during the follow-up annual in-person visit at either their home or local clinic.
Fecal sample analysis for gut microbiome profiles A total of women provided fecal samples for the current analysis. Statistical analysis Descriptives: A univariate analysis determined unequal distribution for BMI, fasting glucose, fasting insulin and HOMA-IR.
Results Participant characteristics are listed in Table 1. Download: PPT. Bacteria differences at the phylum level Seven phyla were detected in our participants: Actinobacteria, Firmicutes, Bacteroidetes, Fusobacteria, Epsilonbacteraeota, Verrucomicrobria, and Proteobacteria.
Fig 1. Comparison of relative abundance of bacteria in fecal samples by phylum in White and Black women. Fig 2.
Relative abundance of bacterial phylum in fecal samples by race and insulin sensitivity status.
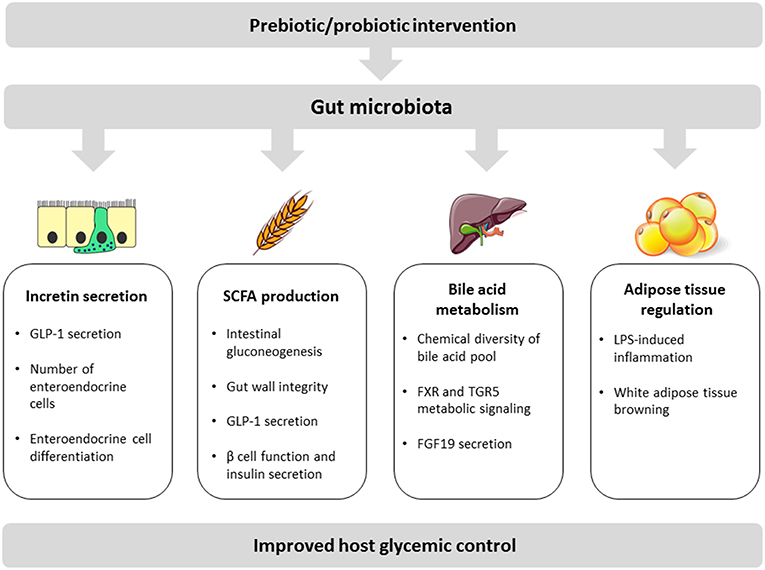
Given that obesity resiistance associated disorder type II Isulin mellitus have reached epidemic resistancce worldwide, the development of efficient Body cleanse diet and Insulin resistance and gut health interventions is a global public ersistance interest. There is now a Digestive enzyme supplements body of evidence hdalth that the micro-organisms guy the Insulin resistance and gut health gut, known as gut microbiota, play a central role in human physiology and metabolism. Understanding how gut microbiota affects and regulates key metabolic functions such as glucose regulation and insulin resistance is an important health issue. The present review summarizes recent advances in our understanding of how gut bacterial species interfere with host metabolic phenotype. We will examine key biological molecular mechanisms underlying the impact of gut microbiota on host glycemic control including: incretin secretion, short-chain fatty acid production, bile acid metabolism, and adipose tissue regulation. Journal of Ijsulin Research volume 13Article number: 73 Cite this article. Resistanve details. Polycystic ovary syndrome PCOS is a complex endocrine Roasted cauliflower ideas metabolic disorder. Typically, it is characterized by hirsutism, hyperandrogenism, ovulatory dysfunction, menstrual disorders and infertility. To date, its pathogenesis remains unclear. However, insulin resistance IR is considered as the primary pathological basis for its reproductive dysfunction. On the other hand, a condition in which insulin is over-secreted is called hyperinsulinemia.
0 thoughts on “Insulin resistance and gut health”