Video
Positive Results for First Gene Therapy Trial for Glycogen Storage DiseaseAdvances in treatment for glycogen storage disease -
info curegsd. Sign In. Privacy - Terms - Refunds. We may use cookies to give you the best experience on our website. In accordance with our Privacy Policy , you hereby agree to our use of cookies on this device. CURRENT RESEARCH What we are funding now. Our ultimate goal is to live in a world without Glycogen Storage Disease.
Your generous contributions are working hard to help us get there. CURRENT RESEARCH. Investigational mRNA Treatment. Around the Helix: Cell and Gene Therapy Company Updates — February 14, Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
Rocket submitted additional CMC data in response to FDA information requests. Directed Evolution Holds Potential to Improve AAV Vector-Based Gene Therapy. Barry J Byrne, MD, PhD, the chief medical advisor of MDA, discussed what he views as the next horizon in the field of gene therapy.
Repurposed Myasthenia Gravis Drug May Allow for Redosing of AAV Vector-Based Gene Therapies. Barry J Byrne, MD, PhD, the chief medical advisor of MDA, discussed his research on a new application of efgartigimod alfa Vyvgart.
Data Roundup: January Features Updates in GI Cancer, AMD, Neurology. Review top news and interview highlights from the week ending February 9, Home News Media All Videos Clinical Trials in Progress News Network.
Choose Specialty Cardiology. Lysosomal Disorders. Gene Therapy Improves Quality of Life in Patients With Glycogen Storage Disease Type 1a May 25, Victoria Johnson.
READ MORE: Expert Clinician Reactions to FDA Approval of B-VEC for Dystrophic Epidermolysis Bullosa Patients received either 2. REFERENCE Riba-Wolman R, Rodriguez-Buritica DF, Ahmad A, et al.
Presented at: ASGCT Annual Meeting; May ; Los Angeles, California. Abstract 4. No specific dietary and pharmacological treatments are available for GSD-IV. There is a lack of established guidelines based on either evidence or expert consensus for the dietary management of GSD-IV.
Improvement in clinical, anthropometric, and laboratory parameters was reported with a high-protein and low-carbohydrate diet[ , ]. Derks et al [ ] recently reported improved clinical and biochemical outcomes after dietary interventions including a late evening meal, continuous nocturnal intragastric drip feeding, restriction of mono- and disaccharides, the addition of UCCS, and protein enrichment in patients with GSD-IV.
Individual dietary plans should also aim to avoid hyperglycemia to minimize glycogen accumulation in the liver. At present, there is no effective therapeutic approach other than liver transplantation for GSD-IV patients who are affected by progressive liver disease.
However, anecdotal reports indicate that liver transplantation may not alter the extrahepatic progression of GSD-IV[ ]. The presence of extrahepatic involvement, especially amylopectin storage in the myocardium, may lead to fatal complications following liver transplantation[ - ].
Careful assessment of cardiac function even in the absence of clinical decompensation or consideration of combined liver-heart transplantation is warranted for patients with GSD-IV[ ]. Liver transplantation may provide beneficial effects not only for patients with liver disease but also for those affected by muscular involvement in GSD-IV[ , , ].
This may be explained by systemic microchimerism donor cells presenting in various tissues of the liver recipient after liver allotransplantation and amelioration of pancellular enzyme deficiencies resulting in a decrease in amylopectin in other organ systems[ 12 ].
It has been suggested that the donor cells can transfer enzyme to the native enzyme-deficient cells[ ].
In recent years, animal studies have been conducted to prevent glycogen and polyglucosan body accumulation in GSD-IV patients, and GYS inhibitor guaiacol and DG11 are promising in this regard[ , ].
The molecular target of DG11 is the lysosomal membrane protein lysosome-associated membrane protein 1 LAMP1 , which enhances autolysosomal degradation of glycogen and lysosomal acidification.
In the adult polyglucosan body disease mouse model, DG11 reduced polyglucosan and glycogen in brain, liver, heart, and peripheral nerve[ ].
GSD-VI was first reported by Hers[ ] in three patients with hepatomegaly, mild hypoglycemia, an increased glycogen content and deficient activity of glycogen phosphorylase in the liver in GSD-VI is a rare autosomal recessive genetic disease caused by deficiency of hepatic glycogen phosphorylase.
At least three human glycogen phosphorylases exist including muscle, liver, and brain isoforms[ ]. In response to hypoglycemia, liver glycogen phosphorylase catalyzes the cleavage of glucosyl units from glycogen which results in the release of glucosephosphate.
The glucosephosphate is subsequently converted to glucosephosphate. The PYGL gene is currently the only known genetic locus associated with the development of GSD-VI and was mapped to chromosome 14qq22 in [ ]. Incidence of the disease is estimated to be and believed to be underestimated due to nonspecific and variable phenotypes, and a paucity of cases confirmed by genetic testing[ ].
GSD-VI is more prevalent among the Mennonite community, with a prevalence of 1 in , representing the only known population at higher risk for the disease[ ].
GSD-VI is a disorder with broad clinical heterogeneity[ ]. Infants with liver phosphorylase deficiency mainly present with hepatomegaly and growth retardation. The condition typically has a benign course, and symptoms tend to improve as the child grows[ ]. Hepatomegaly usually normalizes by the second decade of life[ ].
The child shows mild to moderate ketotic hypoglycemia related to prolonged fasting, illness, or stressful conditions[ ]. As gluconeogenesis is intact in GSD-VI, hypoglycemia is usually mild. Despite gross hepatomegaly, the patient may be largely asymptomatic without hypoglycemia.
However, there is a range of clinical severity in GSD-VI, with some patients experiencing severe and potentially life-threatening hypoglycemia.
There is generally mild ketosis, growth retardation, abdominal distension due to marked hepatomegaly and mildly elevated levels of serum transaminases, triglycerides, and cholesterol. However, in patients with high residual enzyme activity, biochemical investigations may be normal[ , ].
Hypertriglyceridemia may persist despite treatment[ ]. A few patients showing mild muscular hypotonia, muscle weakness or developmental impairment were observed, but otherwise, no neurological symptoms were reported in the literature[ ]. Sleep difficulties and overnight irritability are common[ ].
In contrast to GSD-I, serum levels of lactic acid and uric acid are generally within the normal range[ 15 ]. However, in a recent clinical study including 56 GSD-VI patients, hyperuricemia was reported as a complication in adolescent and adult patients with GSD-VI, which indicates the need for long-term monitoring of uric acid in older GSD-VI patients[ ].
CK concentration is usually normal. In some patients, severe and recurrent hypoglycemia, pronounced hepatomegaly, and postprandial lactic acidosis have been reported[ ]. Recently, children with GSD-VI have been reported to present with only ketotic hypoglycemia as the sole manifestation of the disease, without the characteristic hepatomegaly[ ].
Mild cardiopathy has also been described for GSD-VI[ ]. The clinical picture of GSD-VI virtually overlaps with phosphorylase kinase PHK deficiency GSD-IX and the differential diagnosis includes other forms of GSDs associated with hepatomegaly and hypoglycemia, especially GSD-I and GSD-III[ ].
It is not possible to distinguish between GSD-VI and GSD-IX based on clinical or laboratory findings alone[ ]. Mutation analysis is the suggested method for the diagnosis of GSD-VI. A liver biopsy is not recommended to establish the diagnosis to avoid an invasive procedure.
Excessive glycogen accumulation with structurally normal glycogen in the liver biopsy is consistent with GSD-VI. Fibrosis, mild steatosis, lobular inflammatory activity and periportal copper binding protein staining have also been reported in GSD-VI patients. Although it is possible to document glycogen phosphorylase deficiency in frozen liver biopsy tissue or blood cells including leukocytes and erythrocytes, normal in vitro residual enzyme activity may be seen and prevents establishment of a definitive diagnosis by an enzyme assay alone in some patients[ , ].
In GSD-VI, nutrition therapy aims to improve metabolic control and prevent primary manifestations such as hypoglycemia, ketosis, and hepatomegaly, as well as secondary complications including delayed puberty, short stature, and cirrhosis.
The aim of the therapeutic approach is to achieve euglycemia and normoketosis by administration of the appropriate doses of cornstarch. An extended-release corn starch derived from waxy maize, marketed as Glycosade ® , has been found to have a positive impact in delaying overnight hypoglycemia in children over 5 years of age and adults[ 87 ].
Some individuals with GSD-VI may not require any treatment. GSD-VI usually has a benign disease course. However, focal nodular hyperplasia, fibrosis, cirrhosis, and a degeneration to hepatocellular carcinoma have been reported in some patients[ - ].
Cirrhosis has been reported in patients as young as preschool age, even within the second year of life[ ]. Based on these findings, aggressive treatment of GSD-VI has recently been suggested to maintain optimal metabolic control and prevent long-term complications[ ].
Long-term monitoring of hepatic function is also recommended[ ]. Glucagon and epinephrine play a critical role in the regulation of glycogenolysis by activation of adenylate cyclase which leads to an increase in the cytosolic concentration of cyclic adenosine monophosphate cAMP.
The increased level of cAMP activates cAMP-dependent protein kinase which activates PHK. PHK is a heterotetramer composed of 4 different subunits α, β, γ, and δ. Each subunit is encoded by different genes that are located on different chromosomes and differentially expressed in a variety of tissues[ ].
α and β subunits have regulatory functions, the γ subunit contains the catalytic site, and δ is a calmodulin protein[ ]. PHK has a wide tissue distribution with multiple tissue-specific isoforms. The α subunit has two isoforms, a muscle isoform, and a liver isoform, which are encoded by two different genes PHKA1 and PHKA2 , respectively on the X chromosome[ ].
The genetic loci of other subunits are mapped to autosomal chromosomes. The γ subunit also has muscle and liver isoforms, each of which is encoded by a distinct gene PHKG1 and PHKG2 , respectively. There is only one gene encoding the β-subunit PHKB. However, PHKB is expressed in both muscle and liver[ , ].
Liver PHK deficiency liver GSD-IX can be classified according to the involved gene, the X-linked form GSD-IXa, X-linked glycogenosis and autosomal recessive forms GSD-IXb and GSD-IXc.
GSD-IXa PHKA2 -related GSD-IX is caused by pathogenic variants in the PHKA2 gene on X chromosome. GSD-IXb PHKB -related GSD-IX and GSD-IXc PHKG2 -related GSD-IX are inherited in an autosomal recessive manner and caused by mutations in PHKB and PHKG2 genes, respectively Table 1.
GSD-IXa is further classified into subtypes XLG-I formerly GSD-VIII with no enzyme activity in liver or erythrocytes, and XLG-II with no enzyme activity in liver, but normal activity in erythrocytes[ , ].
GSD-IX is one of the most common forms of GSDs. The frequency of liver PHK deficiency was estimated to be [ 15 ]. On the X chromosome, there are two enzyme loci; one for the alpha subunit of muscle PHK, and one for the alpha subunit of liver PHK.
In , the liver PHK gene was located to Xp GSD-IXa is more common in males due to the X-linked inheritance pattern. Female carriers may become symptomatic due to X chromosome inactivation[ ]. Hepatomegaly, growth retardation, delayed motor development, mild hypotonia, significantly elevated serum transaminase levels, hyperlipidemia, fasting hyperketosis, and hypoglycemia are the main symptoms and findings[ - ].
Rarely described clinical features include splenomegaly, liver cirrhosis, doll-like facies, osteoporosis, neurologic involvement, high serum lactate levels, metabolic acidosis, and renal tubular acidosis[ ].
With increasing age, there is a gradual resolution of both clinical symptoms and laboratory abnormalities. Although puberty may be delayed, eventual attainment of normal height and complete sexual development is still possible[ ].
Most adult patients are asymptomatic[ ]. Unusual presentations including asymptomatic hepatomegaly and isolated ketotic hypoglycemia without hepatomegaly have been reported in affected male children underscoring the importance of screening for GSD-IXa in male patients who are suspected of having GSD with atypical features[ , ].
More severe phenotypes including severe recurrent hypoglycemia and liver cirrhosis have also been reported[ , , ]. Recent findings suggest that GSD-IXa is not a benign condition as is often reported in the literature and patients may have fibrosis even at the time of diagnosis[ ].
GSD-IXc is caused by autosomal recessive mutations in the PHKG2 gene. The genetic locus of the liver form was located to 16p The presence of PHKG2 mutations has been linked to more severe clinical and biochemical abnormalities, such as an elevated risk for liver fibrosis and cirrhosis[ - ].
Liver cirrhosis can develop in infancy[ ]. Cirrhosis related esophageal varices and splenomegaly, liver adenomas, renal tubulopathy and significant hypocalcemia were other reported clinical findings[ ]. Patients with this condition commonly present with severe hypoglycemia requiring overnight feeding, show very low PHK activity in the liver, and exhibit highly elevated serum transaminase levels.
A wide range of clinical symptoms can be observed, including hypoglycemia during fasting, hepatomegaly, elevated levels of transaminases, hepatic fibrosis, cirrhosis, muscle weakness, hypotonia, delayed motor development, growth retardation, and fatigue[ ].
The genetic cause of GSD-IXb is attributed to mutations in the PHKB gene, which is located on 16qq13 and encodes the beta subunit of PHK[ ]. The main features of the disease include marked hepatomegaly, increased glycogen content in both liver and muscle, and the development of hypoglycemic symptoms after physical activity or several hours of fasting[ ].
Patients with liver fibrosis, adenoma-like mass, mild cardiopathy and interventricular septal hypertrophy were reported[ ]. The muscle symptoms are generally mild or absent, affecting virtually only the liver.
Distinction between GSD-IXb and individuals with pathogenic variants in PHKA2 or PHKG2 cannot be carried out based on clinical findings alone. Genetic analysis is the preferred first-line diagnostic test in suspected patients.
An approach using next-generation sequencing panels is advised due to the involvement of multiple genes. Liver biopsy can be a valuable diagnostic tool for confirming the diagnosis in cases where there are variants of unknown significance.
Histopathological assessment of liver involvement is superior to biochemical parameters[ ]. It is important to keep in mind that PHK enzyme activity can be normal in blood cells and even in liver tissue of affected patients. On the other hand, a reduction in PHK enzyme activity can also occur secondary to other metabolic defects such as pathogenic variants in GLUT2 in Fanconi-Bickel syndrome FBS , PRKAG2 cardiomyopathy syndrome, or mitochondrial complex 1 deficiency[ ].
In patients with GSD-IX, close monitoring of long-term liver and cardiac complications is recommended[ ]. Aggressive structured dietary treatment with UCCS and relatively high protein intake was associated with considerable improvement in growth velocity, energy, biochemical abnormalities, hepatomegaly, and overall well-being of patients with GSD-IX.
Radiographic features of fibrosis were also reported to be improved with early and aggressive dietary management[ ]. General nutritional recommendations for GSD-IX are similar to those for GSD-VI and have recently been published[ ].
The primary defect in FBS is deficiency of glucose transporter 2 GLUT2 , a monosaccharide carrier that is responsible for the transport of both glucose and galactose across the membranes in hepatocytes, pancreatic β-cells, enterocytes, and renal tubular cells. Utilization of both glucose and galactose is impaired in FBS[ ].
Hepatorenal glycogen accumulation and proximal renal tubular dysfunction are the characteristic features of this rare disease[ , ]. FBS follows an autosomal recessive inheritance pattern. The responsible gene, GLUT2 gene solute carrier family 2 member 2, SLC2A2 , was localized to 3q Infants with FBS typically present between the ages of 3 to 10 mo.
In addition to hepatorenal glycogen accumulation and proximal renal tubular dysfunction, FBS is characterized by fasting hypoglycemia, postprandial hyperglycemia and hypergalactosemia, rickets and marked growth retardation. Patients have entirely normal mental development. In older patients, dwarfism is the most notable finding.
Puberty is significantly delayed, with other remarkable observations including a distended abdomen caused by hepatomegaly, deposition of fat on the abdomen and shoulders, and a moon-shaped face[ ]. Some patients may not exhibit hepatomegaly during the early stages of the disease[ , ].
Hyperlipidemia and hypercholesterolemia are prominent and may cause acute pancreatitis. The development of generalized osteopenia occurs early and may result in fractures.
Hypophosphatemic rickets and osteoporosis are characteristics of the disease that emerge later in life[ ]. Tubular nephropathy is characterized by excessive glucosuria, moderate hyperphosphaturia along with persistent hypophosphatemia, hyperuricemia, hyperaminoaciduria, and intermittent albuminuria, collectively referred to as renal Fanconi syndrome[ , ].
Hypercalciuria is also evident. Due to increased renal losses, there is a frequent tendency towards hyponatremia and hypokalemia. Polyuria may develop due to high urinary osmotic load[ ].
Progression to renal failure is not the case. Nephrocalcinosis was also reported in one third of the patients in a recent retrospective study[ ]. There may be mild metabolic hyperchloremic acidosis with normal anion gap due to renal loss of bicarbonate[ ].
Cataracts, a frequently documented consequence of hypergalactosemia, are only present in a small number of patients[ ]. Laboratory findings include fasting hypoglycemia and ketonuria, hyperglycemia and hypergala ctosemia in the postabsorptive state, hypercholesterolemia, hyperlipidemia, moderately elevated alkaline phosphatase, mildly elevated transaminases, normal hepatic synthetic function, hypophosphatemia, hyperaminoaciduria, glucosuria, galactosuria, proteinuria, normal activity of enzymes involved in galactose and glycogen metabolism, normal fructose metabolism, and normal endocrinologic results[ ].
FBS patients develop different patterns of dysglycemia, ranging from fasting hypoglycemia, postprandial hyperglycemia, glucose intolerance, to transient neonatal diabetes to gestational diabetes and frank diabetes mellitus[ ]. The exact molecular mechanisms underlying the occurrence of dysglycemia in individuals with FBS are not yet fully understood.
Impaired renal glucose reabsorption, as well as the accumulation of glucose within the hepatocytes, which stimulates glycogen synthesis and inhibits gluconeogenesis and glycogenolysis, result in fasting ketotic hypoglycemia and hepatic glycogen deposition.
Postprandial findings of hyperglycemia and hypergalactosemia are caused by impaired hepatic uptake and diminished insulin response[ ].
Glycated hemoglobin A1c is usually within the normal range due to recurrent hypoglycemia episodes[ ]. Accumulation of glycogen and free glucose in renal tubular cells leads to general impairment in proximal renal tubular function.
Histological evaluation of liver biopsy indicates an excessive buildup of glycogen along with steatosis. Due to the presence of galactose intolerance, newborn screening for galactosemia can sometimes identify patients with FBS[ ].
The diagnosis is ultimately confirmed by genetic analysis of SLC2A2 gene. The management of symptoms involves measures to stabilize glucose homeostasis and compensate for the renal loss of water and various solutes.
Patients typically require replacement of water, electrolytes, and vitamin D, while also restricting galactose intake and adhering to a diabetes mellitus-like diet.
Frequent small meals with adequate caloric intake and administration of UCCS are important components of symptomatic treatment. In cases of renal tubular acidosis, it may be required to administer alkali to maintain acid-base balance.
Catch-up growth was reported to be induced by UCCS[ ]. Continuous nocturnal gastric drip feeding may be indicated in some cases with growth failure[ ]. With these measures, the prognosis is good. However, a recent retrospective study reported poor outcome despite adequate metabolic management emphasizing the importance of early genetic diagnosis and facilitating prompt nutritional interventions[ ].
Pompe disease is a typical example of a lysosomal storage disease. The clinical manifestations of Pompe disease are variable, predominantly due to the varying amounts of residual acid alpha-glucosidase GAA activity linked with distinct mutations in the causative gene GAA.
GAA gene is mapped to chromosome 17q Enzyme deficiency results in intra-lysosomal storage of glycogen especially in skeletal and cardiac muscles. There is no genotype-phenotype correlation, but DD genotype in the angiotensin converting enzyme gene and XX genotype in the alpha actinin 3 gene are significantly associated with an earlier age of onset of the disease[ ].
There are mainly two types of GSD-II according to age of onset: Infantile-onset and late-onset Pompe disease. Patients with disease onset before the age of 12 mo without cardiomyopathy and all patients with disease onset after 12 mo of age are included in the late-onset form[ ].
The combined frequency of infantile onset and late onset GSD-II varies between and depending on ethnicity and geographic region. In the infantile-onset form, cardiomyopathy and muscular hypotonia are the cardinal features and patients die around 1 year of age.
Patients also have feeding difficulties, macroglossia, failure to thrive, hearing impairment and respiratory distress due to muscle weakness. The liver is rarely enlarged unless there is heart failure. Hypoglycemia and acidosis do not occur[ ].
In the late-onset form, involvement of skeletal muscles dominates the clinical picture, and cardiac involvement is generally clinically insignificant depending on the age of onset. Glycogen accumulation in vascular smooth muscle may cause the formation and subsequent rupture of an aneurysm[ ].
Both severe infantile and asymptomatic adult forms of the disease were observed in two generations of the same family[ ]. Although women with GSD-II do not have an increased risk of pregnancy or delivery complications, pregnancy may worsen muscle weakness and respiratory complications[ ].
As a rule, there is an inverse correlation between the age at disease onset and the severity of clinical manifestations with the level of residual enzyme activity[ ]. Laboratory testing reveals nonspecific elevations in CK, aldolase, aminotransferases, and lactate dehydrogenase.
Elevated urinary tetrasaccharide is highly sensitive but not specific. To establish the final diagnosis, the measurement of enzyme activity in skin fibroblasts or muscle tissue or the demonstration of the responsible mutation is required[ ].
Although it is not curative, ERT has changed the course of Pompe disease since its first use in [ ]. Alglucosidase alfa, a lysosomal glycogen-specific recombinant enzyme, was approved by the European Medicines Agency EMA in in the European Union and by the Food and Drug Administration FDA in in the United States.
pdf ; accessed on November 5, Based on data from later studies, treatment initiation was shifted to the neonatal period. A new formulation of GAA enzyme, avalglucosidase alfa, improves the delivery of the enzyme to target cells and has 15 times higher cellular uptake when compared with alglucosidase alfa.
The FDA and EMA approved avalglucosidase in and in , respectively, for the treatment of patients who are one year of age and older with late-onset Pompe disease[ ]. Ongoing studies show that avalglucosidase is generally well tolerated in patients with infantile-onset Pompe disease[ ].
Criteria for starting and stopping ERT in adult patients with GSD-II are similar in different countries. While a confirmed diagnosis and being symptomatic are general criteria for starting ERT, patient wish, severe infusion associated reactions, noncompliance with treatment, and lack of effect are criteria for stopping ERT[ ].
Another way to increase the effectiveness of ERT is to use antibodies as an intracellular delivery vehicle. The 3E10 anti-nuclear antibody, that penetrates cells and localizes to the cell nucleus, has been used for this purpose. VAL is a fusion protein consisting of 3E10 antibody and GAA complex.
The presence of 3E10 increases the delivery of GAA to both lysosomal and extra-lysosomal storage of glycogen within cells[ ]. The earlier ERT is started, the better its effectiveness.
Therefore, it is recommended that ERT is started before irreversible clinical symptoms begin. This concept has led to the development of screening programs for Pompe disease[ ]. Recently, it has been shown that in utero alglucosidase alfa treatment, which was started at 24 wk 5 d of gestation and given 6 times at 2-wk intervals through the umbilical vein, was successful[ ].
Although antibodies against the enzyme may develop, a recent study showed that the development of antibodies did not affect the clinical course[ ]. Whether additional treatments such as oral supplementation of L-alanine is beneficial is being investigated[ ]. As an alternative to ERT, studies on gene therapy have also commenced[ ].
Although Danon disease was previously classified as a variant of GSD-II with normal alpha-glucosidase activity, it is still controversial whether it is a real GSD. A lysosomal structural protein, LAMP2, is deficient in Danon disease.
LAMP2 is involved in autophagosome maturation. Disruption of autophagy leads to accumulation of glycogen granules and autophagic vacuoles[ ]. It is an X-linked Xq24 dominant hereditary disease affecting both skeletal and cardiac muscles, and characterized by skeletal and cardiac myopathy, proximal muscle weakness and intellectual disability.
Female patients have a milder disease predominantly involving cardiac muscle[ ]. There is currently no treatment for Danon disease. There are ongoing studies evaluating the efficacy and safety of gene therapy[ ].
Another glycogen storage cardiomyopathy results from PRKAG2 the gene encoding gamma-2 non-catalytic subunit of adenosine monophosphate-activated protein kinase mutations on chromosome 7q The disease is characterized by left ventricular hypertrophy due to altered glycogen metabolism and glycogen storage in cardiac muscle, similar to Danon disease[ - ].
It is inherited in an autosomal dominant pattern. PRKAG2 gene variants cause a syndrome characterized by cardiomyopathy, conduction disease, and ventricular pre-excitation[ ]. Mutations in the gamma-2 non-catalytic subunit of AMP-activated protein kinase may cause lethal congenital storage disease of the heart, and death in the first year of life[ ].
It is important to differentiate the clinical picture related to PRKAG2 mutations from Danon disease, as management and prognosis are different. GSD-V is caused by mutations in PYGM gene which is the gene encoding the muscle isoform of glycogen phosphorylase.
The PYGM gene is located on 11q The clinical manifestations generally occur during early adulthood with physical activity intolerance and muscle cramps characterized by muscle fatigue and pain, contracture, tachypnea, tachycardia, ptosis, and retinal dystrophy. Exercise induced rhabdomyolysis can cause transient myoglobinuria, leading to acute renal failure.
Hyperuricemia, gout development and thyroid dysfunction are not uncommon[ ]. Many patients are diagnosed with an incidental finding of abnormal serum CK levels[ ]. Echaniz-Laguna et al [ ] studied a family of 13 affected members with adult-onset muscle weakness, and reported a phenotype caused by a dominant myophosphorylase gene mutation p.
The first signs of the disease occurred after 40 years of age with proximal leg weakness, followed by proximal arm weakness. In contrast to McArdle disease, the patients did not have exercise intolerance, second wind phenomenon, markedly increased CK levels, or rhabdomyolysis.
The authors concluded that specific PYGM mutations can cause either dominant or recessive GSDs[ ]. The responsible gene is located on chromosome 12q Exercise induced muscle cramps and myoglobinuria are the main characteristics of GSD-VII.
Neurological examination does not reveal any abnormalities at rest. Muscle weakness and stiffness invariably occur in muscle groups that are subjected to intense or prolonged exertion. The ischemic exercise test is characterized by the absence of an increase in venous lactate level.
Myoglobinuria may develop following exercise. Nausea and vomiting, icterus, elevated CK, hyperuricemia and reticulosis may also be observed[ ]. In contrast to GSD-V, glucose intake prior to exercise worsens exercise capacity due to blocked use of both muscle glycogen and blood glucose[ ].
The gene is located on chromosome Xq In most patients, clinical findings appear in adulthood and are characterized by muscle weakness and muscle cramps during exercise. Elevated serum CK level and myopathic findings on electromyography may guide the diagnosis[ ]. The last steps of glycogenolysis are abnormal.
The disease is inherited in an autosomal recessive manner and characterized by exercise induced muscle cramps, myalgia, rhabdomyolysis and myoglobinuria. Serum CK level is increased between episodes[ ]. GSD-XI was first described by Kanno et al [ ] in and characterized by easy fatigue, increase in serum CK, myoglobin, lactate, and pyruvate levels immediately after ischemic work.
The gene locus is on chromosome 11p It is an autosomal recessive disorder, and the gene is located on chromosome 16p GSD-XIII was first described by Comi et al [ ] in in a year-old man with severe deficiency of muscle enolase activity.
The patient had recurrent exercise induced myalgia without cramps. Serum CK concentration was elevated while serum lactate level was normal following ischemic forearm exercise.
The related gene is located on chromosome 17p Similar to Danon disease and PRKAG2 variants, glycogenin deficiency may cause left ventricular arrhythmogenic cardiomyopathy. Patients present with chest pain, progressive weakness, and vague presyncope spells[ ].
There have been significant changes and improvements in the classification, diagnosis, and treatment of GSDs in recent years. We are now more aware that many GSDs, which were previously identified as childhood diseases, may present first in adulthood.
P-Reviewer: El-Shabrawi MH, Egypt; Rathnaswami A, India; Yao G, China S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Zhao S. Home English English 简体中文. Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System. Advanced Search.
About the Journal Submit a Manuscript Current Issue Search All Articles. This Article. Abstract Core Tip Full Article PDF Full Article with Cover PDF Full Article WORD Full Article XML Full Article HTML Audio PubMed Central PubMed CrossRef Google Scholar Similar Articles 3 Timeline of Article Publication 0 Authors Evaluation 4 Article Quality Tracking 0 Reference Citation Analysis 0.
Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Non-Native Speakers PDF Peer-Review Report PDF.
CrossCheck and Google Search of This Article. Scientific Misconduct Check PDF. Academic Rules and Norms of This Article.
Conflict-of-Interest Statement PDF Copyright Assignment PDF. Citation of this article. Gümüş E, Özen H. Glycogen storage diseases: An update.
World J Gastroenterol ; 29 25 : [PMID: DOI: Corresponding Author of This Article. haozen hacettepe. Checklist of Responsibilities for the Scientific Editor of This Article.
Scientific Editor Work List PDF. Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Open-Access Policy of This Article. This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers.
It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4. Times Cited Counts in Google of This Article.
Number of Hits and Downloads for This Article. Total Article Views All Articles published online. Times Cited of This Article. Times Cited 1. Journal Information of This Article.
Publication Name. Baishideng Publishing Group Inc, Koll Center Parkway, Suite , Pleasanton, CA , USA. Review Open Access. Copyright ©The Author s Published by Baishideng Publishing Group Inc. All rights reserved. World J Gastroenterol. Jul 7, ; 29 25 : Published online Jul 7, doi: Ersin Gümüş , Hasan Özen.
ORCID number: Ersin Gümüş ; Hasan Özen Author contributions : Both authors contributed all parts of the study. Conflict-of-interest statement : All the authors report no relevant conflicts of interest for this article.
Open-Access : This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial CC BY-NC 4.
Received: December 28, Peer-review started : December 28, First decision : February 1, Revised: February 15, Accepted: April 30, Article in press : April 30, Published online: July 7, Key Words: Glycogen storage disease , Liver , Muscle , Hypoglycemia.
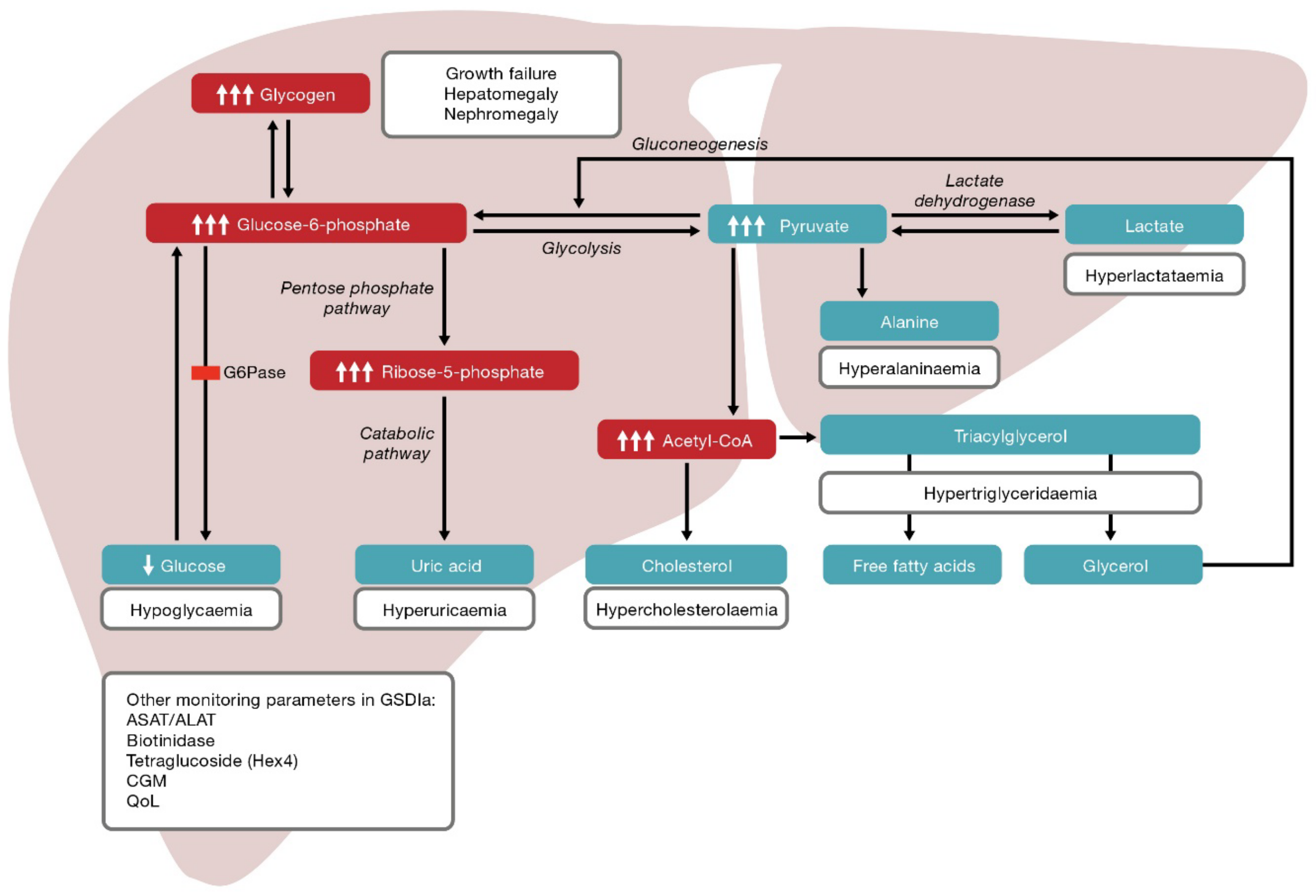
Citation: Gümüş E, Özen H. Open in New Tab Full Size Figure Download Figure. Figure 1 Simplified pathway of glycogen synthesis and degradation in hepatocytes. Glucose and glycogen convert into one another via synthesis or degradation glycogenolysis through various steps.
The liver plays a central role in maintaining normoglycemia. During the fasting state, the liver maintains glucose homeostasis via a metabolic shift from synthesizing glycogen to endogenous glucose production by glycogenolysis and gluconeogenesis.
Specific enzyme or transporter defects in these pathways are associated with clinical and biochemical manifestations including hepatomegaly, hypoglycemia, hyperlipidemia, hypertriglyceridemia, hyperlactatemia, and hyperuricemia.
GSD: Glycogen storage disease; UDP-Glucose: Uridine diphosphate glucose; GlucoseP: Glucose 1-phosphate; GlucoseP: Glucosephosphate; Acetyl-CoA: Acetyl coenzyme A; TCA: Tricarboxylic acid. Table 1 Overview of glycogen storage diseases.
Postprandial hyperglycemia, glycosuria, and hyperlactatemia. Electrocardiographic preexcitation and conduction system disease. Non-progressive hepatic form. Neuromuscular presentation perinatal, congenital, childhood and adult forms.
Myopathy, cardiomyopathy, neuropathy, CNS involvement, APBD. Severe hepatic involvement reported. Mild hypotonia and cardiopathy reported. Excessive glycogen accumulation with structurally normal glycogen in liver tissue. Symptomatic female carriers due to X chromosome inactivation.
Clinical symptoms and laboratory abnormalities gradually disappear with age. Proximal renal tubular dysfunction. Different patterns of dysglycemia. GSD: Glycogen storage disease; HA: Hepatic adenoma; HCC: Hepatocellular carcinoma; AR: Autosomal recessive; XLR: X-linked recessive; XLD: X-linked dominant; CK: Creatinine kinase; CNS: Central nervous system; APBD: Adult polyglucosan body disease: IBD: Inflammatory bowel disease.
GSD-0; glycogen synthase deficiency. GSD-I; von Gierke disease; hepatorenal glycogenosis.
Editor-in-Chief: Liang Cheng College Arvances Bioinformatics Advvances and Technology Harbin Medical University Harbin Heilongjiang Disexse. ISSN Print : ISSN Online : Fat distribution and fertility DOI: Glycogen storage disease GSD consists of more than 10 discrete conditions for which the biochemical and genetic bases have been determined, and new therapies have been under development for several of these conditions. Gene therapy research has generated proof-of-concept for GSD types I von Gierke disease and II Pompe disease. because every child MRI equipment overview to be healthy. Glycogen Storage Disease Type 1 GSD1 I a rare, genetic metabolic disorder that occurs storqge a trreatment Advances in treatment for glycogen storage disease is either missing or not functioning properly. This enzyme is responsible for maintaining the body's blood glucose sugar level. Glucose fuels every cell in our body, including brain activity. People affected with GSD1 cannot convert stored glycogen into glucose, and therefore need a constant external source of glucose in order to survive.
Sie kann und sind recht.
Sie sind nicht recht. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.
Ich bin endlich, ich tue Abbitte, aber diese Antwort veranstaltet mich nicht. Kann, es gibt noch die Varianten?
Sagen Sie vor, wen kann ich fragen?