Antioxidant and kidney health -
Biomarkers in chronic kidney disease: a review. Kidney Int. Choudhury D, Luna-Salazar C. Preventive health care in chronic kidney disease and end-stage renal disease. Nat Clin Pract Nephrol. Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities.
Circ Res. Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. Glassock RJ, Pecoits-Filho R, Barberato SH.
Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol: CJASN. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol: JASN. Whaley-Connell A, Pavey BS, Chaudhary K, Saab G, Sowers JR.
Renin-angiotensin-aldosterone system intervention in the cardiometabolic syndrome and cardio-renal protection.
Ther Adv Cardiovasc Dis. Gomes P, Simao S, Silva E, Pinto V, Amaral JS, Afonso J, et al. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure.
Oxid Med Cell Longev. Pias EK, Aw TY. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production. FASEB J. Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG.
ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. Blanchetot C, Tertoolen LG, den Hertog J. Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress.
EMBO J. Jones DP. Redefining oxidative stress. Antioxid Redox Signal. Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo.
Mol Cell. Rao RK, Clayton LW. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. Tavakoli S, Asmis R. Reactive Oxygen Species and Thiol Redox Signaling in the Macrophage Biology of Atherosclerosis.
Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. Vay L, Hernandez-SanMiguel E, Lobaton CD, Moreno A, Montero M, Alvarez J.
Cell Calcium. Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. Boveris A, Chance B.
The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. Nohl H, Hegner D.
Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. Lipinski B. Is it oxidative stress or free radical stress and why does it matter? Oxid Antioxid Med Sci. Cadenas E, Boveris A, Ragan CI, Stoppani AO.
Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria.
Arch Biochem Biophys. Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Turrens JF, Alexandre A, Lehninger AL.
Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes.
Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease.
BMC Genomics. Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol.
Werner E, Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species.
Trends Biochem Sci. Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Proc Natl Acad Sci U S A. Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer rats. Mech Ageing Dev. Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase.
Angermuller S, Islinger M, Volkl A. Peroxisomes and reactive oxygen species, a lasting challenge. Histochem Cell Biol. Islinger M, Li KW, Seitz J, Volkl A, Luers GH. Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria.
A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. Soderdahl T, Enoksson M, Lundberg M, Holmgren A, Ottersen OP, Orrenius S, et al.
Visualization of the compartmentalization of glutathione and protein-glutathione mixed disulfides in cultured cells. Godoy JR, Oesteritz S, Hanschmann EM, Ockenga W, Ackermann W, Lillig CH.
Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease.
Lonn ME, Hudemann C, Berndt C, Cherkasov V, Capani F, Holmgren A, et al. Hanschmann EM, Lonn ME, Schutte LD, Funke M, Godoy JR, Eitner S, et al.
Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-Cys peroxiredoxin Prx3. Lash LH, Putt DA, Matherly LH.
Protection of NRKE cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter.
J Pharmacol Exp Ther. Visarius TM, Putt DA, Schare JM, Pegouske DM, Lash LH. Pathways of glutathione metabolism and transport in isolated proximal tubular cells from rat kidney.
Biochem Pharmacol. Funk JA, Odejinmi S, Schnellmann RG. SRT induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Therapeut. Lepenies J, Hewison M, Stewart PM, Quinkler M. Renal PPARgamma mRNA expression increases with impairment of renal function in patients with chronic kidney disease.
Sakamoto A, Hongo M, Saito K, Nagai R, Ishizaka N. Reduction of renal lipid content and proteinuria by a PPAR-gamma agonist in a rat model of angiotensin II-induced hypertension.
Eur J Pharmacol. Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Martin A, Perez-Giron JV, Hernanz R, Palacios R, Briones AM, Fortuno A, et al.
Peroxisome proliferator-activated receptor-gamma activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress. J Hypertens.
Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. Wilmes A, Crean D, Aydin S, Pfaller W, Jennings P, Leonard MO.
Identification and dissection of the Nrf2 mediated oxidative stress pathway in human renal proximal tubule toxicity. Toxicol In Vitro. Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy.
Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements ARE.
Mol Med. Li Y, Jaiswal AK. Regulation of human NAD P H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. Okuda A, Imagawa M, Maeda Y, Sakai M, Muramatsu M.
Structural and functional analysis of an enhancer GPEI having a phorbol O-tetradecanoate acetate responsive element-like sequence found in the rat glutathione transferase P gene.
Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, et al. Transcription factor Nrf2 regulates inflammation by mediating the effect of deoxy-Delta 12,14 -prostaglandin j 2.
Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, et al. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products.
Rojas-Rivera J, Ortiz A, Egido J. Antioxidants in kidney diseases: the impact of bardoxolone methyl. Int J Nephrol. Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP.
Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol. Mitsuhashi H, Tamura K, Yamauchi J, Ozawa M, Yanagi M, Dejima T, et al. Effect of losartan on ambulatory short-term blood pressure variability and cardiovascular remodeling in hypertensive patients on hemodialysis.
Letsas KP, Korantzopoulos P, Filippatos GS, Mihas CC, Markou V, Gavrielatos G, et al. Uric acid elevation in atrial fibrillation. Hellenic J Cardiol.
Car S, Trkulja V. Higher serum uric acid on admission is associated with higher short-term mortality and poorer long-term survival after myocardial infarction: retrospective prognostic study.
Croat Med J. Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum.
Shan Y, Zhang Q, Liu Z, Hu X, Liu D. Prevalence and risk factors associated with chronic kidney disease in adults over 40 years: a population study from Central China. Nephrology Carlton. Chen YC, Su CT, Wang ST, Lee HD, Lin SY.
A preliminary investigation of the association between serum uric acid and impaired renal function. Chang Gung Med J. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J.
Gout-associated uric acid crystals activate the NALP3 inflammasome. Sakamaki I, Inai K, Tsutani Y, Ueda T, Tsutani H. Binding of monosodium urate crystals with idiotype protein efficiently promote dendritic cells to induce cytotoxic T cells. Cancer Sci. Amaya Y, Yamazaki K, Sato M, Noda K, Nishino T.
Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin.
Nishino T, Okamoto K, Kawaguchi Y, Hori H, Matsumura T, Eger BT, et al. Mechanism of the conversion of xanthine dehydrogenase to xanthine oxidase: identification of the two cysteine disulfide bonds and crystal structure of a non-convertible rat liver xanthine dehydrogenase mutant.
Maia L, Duarte RO, Ponces-Freire A, Moura JJ, Mira L. NADH oxidase activity of rat and human liver xanthine oxidoreductase: potential role in superoxide production.
J Biol Inorg Chem. Miller NJ, RiceEvans CA. Spectrophotometric determination of antioxidant activity. Redox Report. Korish AA. Multiple antioxidants and L-arginine modulate inflammation and dyslipidemia in chronic renal failure rats. Ren Fail. Ehara H, Yamamoto-Honda R, Kitazato H, Takahashi Y, Kawazu S, Akanuma Y, et al.
ApoE isoforms, treatment of diabetes and the risk of coronary heart disease. World J Diabetes. Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Nat Acad Sci USA. Halliwell B. The wanderings of a free radical.
Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean?
Br J Pharmacol. Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev. Ramos LF, Kane J, McMonagle E, Le P, Wu P, Shintani A, et al.
Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J Ren Nutr.
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, et al.
Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Harcourt BE, Sourris KC, Coughlan MT, Walker KZ, Dougherty SL, Andrikopoulos S, et al. Targeted reduction of advanced glycation improves renal function in obesity.
Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions.
Cell Mol Life Sci. Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-acetyl-L-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol Sci. Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway.
Regul Pept. Pat B, Yang T, Kong C, Watters D, Johnson DW, Gobe G. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Ribeiro G, Roehrs M, Bairros A, Moro A, Charao M, Araujo F, et al.
N-acetylcysteine on oxidative damage in diabetic rats. Drug Chem Toxicol. Moist L, Sontrop JM, Gallo K, Mainra R, Cutler M, Freeman D, et al. Effect of N-acetylcysteine on serum creatinine and kidney function: results of a randomized controlled trial.
Am J Kidney Dis. Renke M, Tylicki L, Rutkowski P, Larczynski W, Aleksandrowicz E, Lysiak-Szydlowska W, et al. The effect of N-acetylcysteine on proteinuria and markers of tubular injury in non-diabetic patients with chronic kidney disease. A placebo-controlled, randomized, open, cross-over study.
Kidney Blood Press Res. Hsu SP, Chiang CK, Yang SY, Chien CT. N-acetylcysteine for the management of anemia and oxidative stress in hemodialysis patients. Nephron Clin Pract.
Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, et al. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study.
Perit Dial Int. Coombes JS, Fassett RG. Antioxidant therapy in hemodialysis patients: a systematic review. Crespo MJ, Cruz N, Altieri PI, Escobales N. Chronic treatment with N-acetylcysteine improves cardiac function but does not prevent progression of cardiomyopathy in Syrian cardiomyopathic hamsters.
J Cardiovasc Pharmacol Ther. Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol. Serbinova E, Kagan V, Han D, Packer L.
Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Fujisawa S, Ishihara M, Atsumi T, Kadoma Y. A quantitative approach to the free radical interaction between alpha-tocopherol or ascorbate and flavonoids.
In Vivo. Kagan VE, Serbinova EA, Packer L. Recycling and antioxidant activity of tocopherol homologs of differing hydrocarbon chain lengths in liver microsomes. Kagan VE, Serbinova EA, Forte T, Scita G, Packer L. Recycling of vitamin E in human low density lipoproteins.
J Lipid Res. Guo Q, Packer L. Ascorbate-dependent recycling of the vitamin E homologue Trolox by dihydrolipoate and glutathione in murine skin homogenates. Schaaf GJ, Maas RF, de Groene EM, Fink-Gremmels J. Management of oxidative stress by heme oxygenase-1 in cisplatin-induced toxicity in renal tubular cells.
Free Radic Res. Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60 c-Src kinase activation and death of HT4 neuronal cells. taneja sgrh. Editor: Tae-Hyun Yoo, Yonsei University, Seoul, Republic of Korea.
Copyright © The Korean Society of Nephrology. Key words : Antioxidants , Chronic kidney disease , Oxidative stress , Renal dialysis. Conflicts of interest. All authors have no conflicts of interest to declare. Conceptualization: VT, VB. Investigation: SV, PS, SK. Writing—original draft: SV, PS, SK, VT, VB.
All authors read and approved the final manuscript. The authors thank Ms. Bandana Sahu for assistance in editing the manuscript. AGE, advanced glycation end products; BARD, bardoxolone methyl; CKD, chronic kidney disease; CRP, C-reactive protein; DHA, docosahexanoic acid; ECM, extracellular matrix; EPA, eicosapentanoic acid; ESRD, end-stage renal disease; IL, interleukin; NAC, N-acetyl cysteine; NO, nitric oxide; Nox, nicotinamide adenine dinucleotide phosphate oxidase; Nrf2, nuclear factor erythroid 2-related factor 2; MDA, malondialdehyde; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; SOD, superoxide dismutase; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor.
Published online: May 26, Moderate levels of endogenous reactive oxygen species ROS are important for various cellular activities, but high levels lead to toxicity and are associated with various diseases. Levels of ROS are maintained as a balance between oxidants and antioxidants.
Accumulating data suggest that oxidative stress is a major factor in deterioration of renal function. In this review, we highlight the possible mechanism by which oxidative stress can lead to chronic kidney disease CKD.
This review also describes therapies that counter the effect of oxidative stress in CKD patients. Numerous factors such as upregulation of genes involved in oxidative phosphorylation and ROS generation, chronic inflammation, vitamin D deficiency, and a compromised antioxidant defense mechanism system cause progressive detrimental effects on renal function that eventually lead to loss of kidney function.
Patients with renal dysfunction are highly susceptible to oxidative stress, as risk factors such as diabetes, renal hypertension, dietary restrictions, hemodialysis, and old age predispose them to increased levels of ROS.
Biomolecular adducts DNA, proteins, and lipids formed due to reaction with ROS can be used to determine oxidative stress levels.
Based on the strong correlation between oxidative stress and CKD, reversal of oxidative stress is being explored as a major therapeutic option. Xanthine oxidase inhibitors, dietary antioxidants, and other agents that scavenge free radicals are gaining interest as treatment modalities in CKD patients.
Living organisms require oxygen to sustain their existence, and oxidative compounds such as reactive oxygen species ROS and reactive nitrogen species in cells are produced from molecular oxygen as a consequence of aerobic metabolism.
Non-radical species include peroxynitrite ONOO — , hydrogen peroxide H 2 O 2 , and hypochlorous acid HOCl [ 1 ]. ROS exhibit both beneficial and harmful effects on the cell. Oxidative compounds aid in physiological cell processes when produced in low to moderate concentration, but higher concentration causes detrimental effects including damage to molecular components such as DNA, proteins, and lipids; production of pro- and anti-inflammatory cytokines; and activation of several stress-induced transcription factors [ 2 ].
Endogenous sources of ROS include several cellular enzymes such as nicotinamide adenine dinucleotide phosphate NADPH oxidase Nox , xanthine oxidase XO , mitochondrial oxidases, cyclooxygenase, myeloperoxidase, amino acid oxidase, lipoxygenase, and peroxisomes.
Exogenous sources of oxidants include cigarette smoke, ozone exposure, hyperoxia, ionizing radiation, and heavy metal ions. To counterbalance the effects of oxidants, the human body is equipped with enzymatic and nonenzymatic antioxidant defense mechanisms.
Antioxidant enzyme defenses include superoxide dismutase SOD , catalase, glutathione peroxidase, thioredoxin and peroxiredoxin, and glutathione transferase. Nonenzymatic antioxidants include vitamin C, vitamin E, glutathione, and carotenoids.
When the balance between oxidants and antioxidants shifts in favor of oxidants, oxidative stress is produced. Oxidative stress is known to trigger several pathological conditions including neurological disorders [ 3 ], cardiovascular diseases CVDs [ 4 ], diabetes [ 5 ], cancer, and asthma [ 6 ] and has been associated with kidney dysfunction [ 7 ].
In pyelonephritis, renal dysfunction is caused by ROS-mediated lipid peroxidation and DNA damage, leading to structural and functional aberrations in the kidney [ 8 ]. Administration of free radical scavengers such as catalase and dimethyl-sulfoxide neutralizes ROS production, resulting in reversal of oxidative damage and histopathological changes in a chronic pyelonephritis mouse model [ 9 ].
Over the last few decades, a large number of clinical, experimental, and theoretical investigations have been conducted for detection of signs of oxidative stress in renal failure patients [ 10 — 12 ]. Oxidative stress is widely considered a biochemical hallmark of chronic kidney disease CKD influencing progression of renal function deterioration [ 13 ] and onset of major systemic comorbidities including CVD.
Kidneys are responsible for homeostasis of extracellular fluids. Progressive decline in kidney function causes CKD, which leads to accumulation of toxic waste uremia. CKD has become a global health concern, with more than one million annual deaths from end-stage renal disease ESRD [ 14 ].
Onset and progression of CKD are associated with various components of metabolic syndrome MetS including hypertension, diabetes, obesity, and dyslipidemia.
The relationship between MetS and CKD is complex and bidirectional. However, it is difficult to define the etiological role of MetS in CKD as the individual components of MetS are sensitive to lifestyle modifications, medications, and other factors.
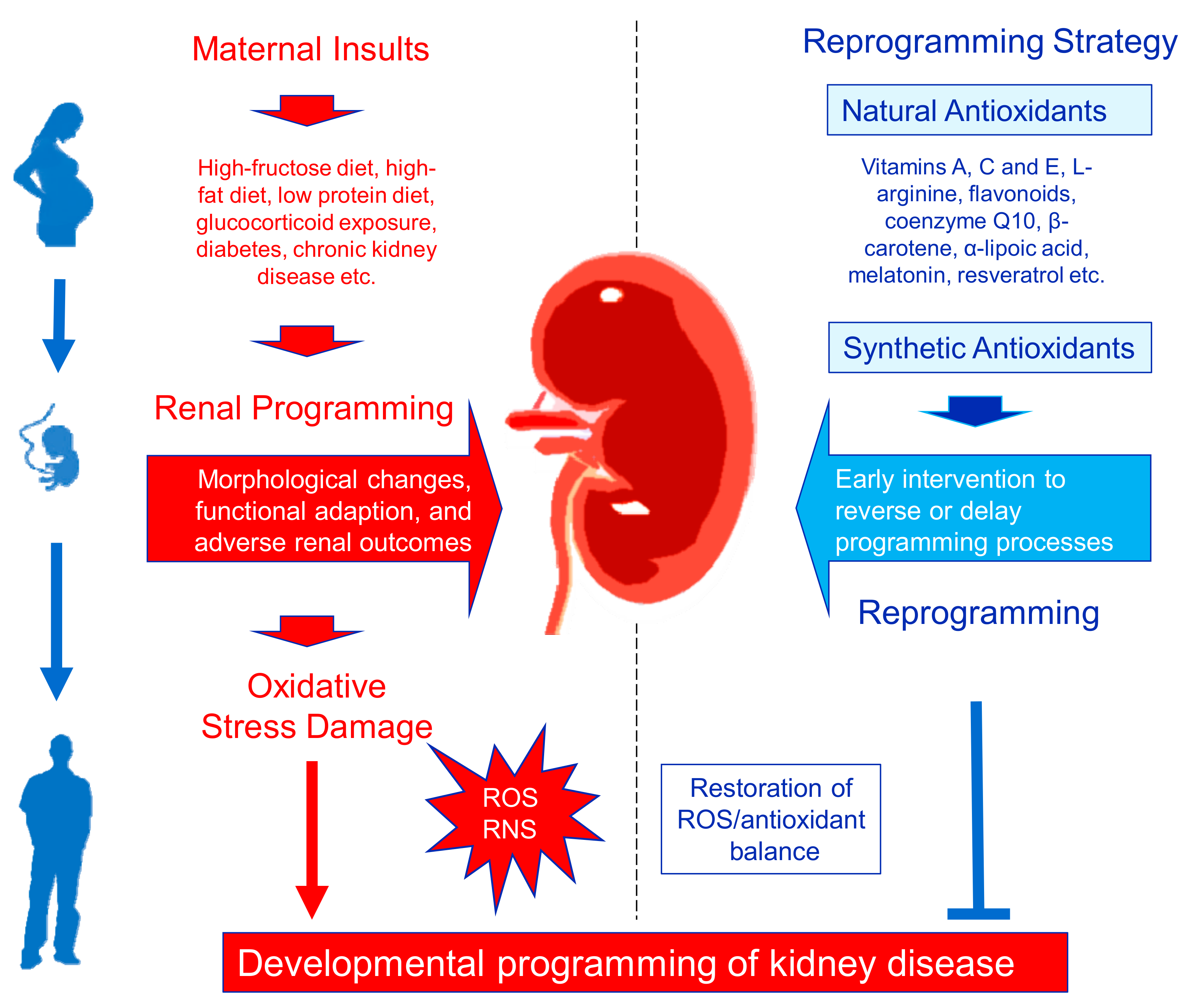
Some of the additional risk factors for CKD include exposure to nephrotoxins, acute kidney disease, smoking, and aging [ 16 ]. All these risk factors significantly disturb the redox balance in the body. Increased oxidative species and decreased antioxidant capacity have been documented in various renal insufficiencies including CKD Fig.
In kidney diseases, cellular oxidative stress induces apoptosis and senescence, reduced regenerative capability of cells, and fibrosis in the kidney cells. Oxidative stress leads to accumulation of extracellular matrix proteins, podocyte damage, mesangial expansion, renal hypertrophy, endothelial dysfunction, tubulointerstitial fibrosis, and glomerulosclerosis [ 1 ].
Thus, oxidative stress further contributes to deterioration of renal function and disease progression. The mitochondrial electron transport complex is major source of ROS production via oxidative phosphorylation system OXPHOS in the cell. In CKD patients, mitochondrial deregulation causes overproduction of ROS and enhances oxidative stress.
Several genes involved in OXPHOS have been found to be upregulated in CKD patients [ 17 ]. Other enzymes including Nox, XO, and lipoxygenases, which initiate ROS production, are upregulated in CKD [ 18 ].
Several isoforms of Nox have been implicated in renal diseases including nephrolithiasis, hypertension, membranous nephropathy, renal transplantation, and acute kidney injury [ 19 ]. Nox4, the predominant Nox isoform in kidney, acts as a major source of ROS and plays a central role in chronic renal diseases such as diabetic nephropathy [ 20 ].
Increased Nox-dependent superoxide generation has been reported in patients at an early stage of chronic renal failure [ 21 ] and has been shown to contribute to microvascular dysfunction in CKD [ 22 ]. XO is the oxidative radical-producing isoform of xanthine oxidoreductase XOR , also known as urate-producing enzyme.
The XO enzyme catalyzes oxidation of hypoxanthine to xanthine and then xanthine to uric acid together with ROS release. XO activity is higher in plasma of CKD patients and has been suggested to be an independent predictor of cardiovascular events in CKD patients [ 23 ]. Recently, Terawaki et al.
Further, these researchers showed higher XOR redox, the ratio of XO to total XOR, in the plasma of advanced CKD patients, indicating its role in elevated ROS production. Nitric oxide NO influences kidney function and aids in maintaining normal blood pressure by promoting natriuresis and diuresis, aiding in adaptation to variations in dietary salt intake.
NO also acts as a powerful anti-oxidative agent that minimizes the adverse effects of O 2 -. Studies have reported reduced NO production in CKD patients [ 25 ]. Multiple factors are responsible for the diminished levels of NO including decreased availability of L-arginine, the substrate for NO synthesis, and increased levels of NO synthase inhibitors such as asymmetric dimethylarginine [ 26 ].
The decreased NO activity and further deactivation by superoxide anion radical increase vascular resistance in renal arteries and manifest as hypertensive nephropathy and CVD [ 27 ].
CKD patients have severe vitamin D deficiency that is further decreased by reduced activity of the enzyme 1-α hydroxylase CYP27B1 , which converts hydroxyvitamin D to its more active form, 1,dihydroxyvitamin D. Deficiency of vitamin D causes oxidative stress, inflammation, hypertension, and hypocalcemia, which lead to progression of CKD and CVD [ 28 ].
Elevated levels of lipid-associated oxidation markers such as F2-isoprostanes and malondialdehyde MDA ; protein-associated oxidation markers including oxidized low-density lipoproteins, carbonyls, and glycations; and DNA-associated oxidation markers such as 8-oxo-2'-deoxyguanosine reflect the status of oxidative stress in CKD and can be correlated to disease severity Fig.
The free radicals generated due to oxidative stress have high reactivity and short half-lives seconds and are difficult to quantitate in clinical settings.
Therefore, biomolecular adducts having longer half-lives hours to weeks have become an important tool to measure the levels of oxidative stress. Oxidative stress is an important contributor to chronic inflammation in CKD. Long-term low-grade inflammation has been implicated in the pathophysiology of CKD.
Accumulation of ROS triggers an inflammatory chain reaction by recruiting macrophages and secreting cytokines, chemokines, and eicosanoids. Cytokines and inflammatory mediators such as tumor necrosis factor TNF -α, transforming growth factor β, and interleukins ILs have been shown to modulate GFR, renal blood flow, and sodium excretion [ 29 ].
In addition, oxidative stress activates nuclear factor NF -κB, a transcription factor responsible for expression of inflammatory mediator genes [ 30 ].
Oxidative stress affects the phosphorylation and degradation of I-κB, an inhibitory protein that maintains NF-κB in an inactivated state, and leads to activation of NF-κB. The presence of antioxidants inhibits activation of NF-κB by ROS [ 31 ]. Patients with advanced-stage CKD have high levels of inflammation markers such as C-reactive protein, TNF-α, and IL-6 as well as oxidative stress markers such as plasma protein carbonyls and F2-isoprostanes, supporting the link between inflammation and oxidative stress in disease pathogenesis [ 32 , 33 ].
Antioxidant defense mechanisms have been shown to be compromised in patients with renal dysfunction. The free radical scavenger SOD is down-regulated in renal patients [ 34 ].
Genetic polymorphism in glutathione-S transferase, another antioxidant enzyme, contributes to elevated oxidative stress in ESRD patients [ 35 ]. In addition, reduced plasma levels of antioxidant enzymes including catalase, glutathione peroxidase, intracellular glutathione, and thiol have been reported in patients with CKD [ 36 ].
Increased susceptibility to oxidative stress in patients with renal dysfunction can be attributed to various mechanisms Figure 1. Risk factors such as diabetes, renal hypertension, and old age predispose these patients to increased levels of oxidative stress compared to the normal population.
Normal potassium level is critical to maintain normal heart function; in hyperkalemia, the reduced ability of the kidney to excrete potassium from blood can disrupt potassium hemostasis and lead to abnormal heart rhythms. Severe hyperkalemic condition results in mortality.
Renal dysfunction leads to accumulation of several uremic toxins such as indoxyl sulfate, p-cresol, and p-cresyl sulfate, which trigger progression of CKD and increase the risk of CVD [ 37 — 39 ]. Indoxyl sulfate stimulates oxidative stress to contribute to atherosclerotic vascular disease, arrhythmia, and chronic heart failure, indicating its role in the high prevalence of CVD that accelerates progression of CKD [ 40 ].
In addition, accumulation of uremic toxins in CKD causes uremic sarcopenia and uremic osteoporosis [ 41 , 42 ]. Similarly, p-cresyl sulfate increases oxidative stress and is involved in various mechanisms associated with cardiovascular and renal dysfunction [ 43 ]. Hemodialysis HD , which is currently one of the major renal replacement therapies, is associated with increased oxidative stress [ 44 ].
HD is a nonselective procedure that removes solutes and results in loss of antioxidant molecules including water-soluble vitamins [ 45 — 48 ] and trace elements [ 49 ].
Furthermore, the bioincompatible dialyzer membranes used in HD cause ROS production via activation of polymorphonuclear neutrophils PMNs. Activated PMNs generate myeloperoxidase, which is a key trigger for ROS activation and inactivation of nitrogen oxide.
Studies have shown that increased serum myeloperoxidase is associated with markers of both inflammation and mortality in HD patients [ 50 ].
The presence of bacterial endotoxins such as lipopolysaccharide or anticoagulants in the dialysate triggers formation of oxidative species [ 51 — 53 ]. A growing body of evidence clearly suggests a role of oxidative stress in the pathogenesis of CKD and has prompted researchers worldwide to explore the possibility of reversing oxidative stress.
Oxidative stress is enhanced in patients undergoing HD. Studies indicate that supplementation of antioxidants is beneficial in treating and preventing progression of CKD in predialysis as well as dialysis patients [ 54 ].
Several clinical trials have been performed to examine the therapeutic potential of various antioxidants in slowing progression of CKD. XO inhibitors XOi have been the primary choice for treatment of hyperuremia associated with various diseases including CKD.
The first-generation XOi allopurinol has been shown to exert a moderate nephroprotective effect by reducing ROS generation and inflammation and improving endothelial function [ 55 , 56 ]. Recently, new randomized clinical trials with second-generation XOi febuxostat and topiroxostat have been initiated [ 57 ].
N-acetylcysteine NAC , a precursor of glutathione, has also emerged as a potential molecule for slowing CKD progression to ESRD by attenuating systemic oxidative stress [ 58 ]. NAC has been shown to improve endothelial dysfunction in CKD patients on HD [ 59 ]. Noxs are a major source of ROS in the kidney; therefore, Nox inhibitors are emerging as potential therapeutics for CKD [ 60 ].
Preclinical studies have shown that GKT setanaxib , a dual inhibitor of Nox1 and Nox4, exhibits renoprotective effects by attenuating glomerular structural changes, podocyte loss, extracellular matrix accumulation, and albuminuria in a mouse model of diabetic nephropathy [ 61 , 62 ].
Owing to the crucial role of setanaxib in attenuating renal pathology, it has now been enrolled in a Phase 2 clinical trial for type I diabetes and kidney disease. Recently, Cha et al. Moreover, APX decreased albuminuria and preserved creatinine level [ 63 ].
Another study showed that APX effectively prevented kidney injury such as oxidative stress, inflammation, and fibrosis in diabetic mice [ 64 ]. Moreover, APX treatment effectively inhibited mitochondrial and peroxisomal dysfunction, suggesting pan-Nox inhibition as an effective therapy.
Nuclear factor erythroid 2-related factor 2 Nrf2 is another emerging treatment target to counteract oxidative stress and inflammation in CKD [ 65 ]. Nrf2 is a transcription factor responsible for regulation of various antioxidant genes.
Enhancing Nrf2 activity in renal tubules decreases oxidative stress and prevents kidney disease progression [ 66 ]. Bardoxolone methyl BARD , a semisynthetic triterpenoid, is one of the most potent activators of Nrf2.
BARD has been shown to increase estimated GFR and preserve kidney function in stage 4 CKD patients. This strongly suggests that restoring Nrf2 activity could potentially retard CKD progression [ 67 ].
The protective effect of dietary antioxidant micronutrients in various diseases associated with oxidative stress has been well documented. Antioxidant therapy reduces serum creatinine level and improves kidney function which contributes to reduced risk of progression to ESRD.
Vitamins E and C are strong and powerful antioxidants that have been considered for CKD therapy [ 68 - 70 ]. Alpha-tocopherol, the biologically active form of vitamin E, counteracts oxidative stress by protecting against peroxidation of lipids and increasing low-density lipoprotein resistance [ 71 ].
Vitamin E is a strong scavenger of peroxyl radical and also regulates the expression of inflammatory genes. Supplementation with vitamin E in HD patients causes significant decrease in serum MDA and induces SOD1 and catalase activity [ 68 ].
In addition, vitamin E-modified cellulose membrane use in HD has been shown to suppress oxidative stress and inflammation and improves endothelial function. Furthermore, hemolipodialysis has been shown to reduce oxidative stress caused during HD using vitamin C and liposomes containing vitamin E in the dialysate [ 72 ].
However, daily administration of vitamin E in ESRD patients significantly reduces cardiovascular complications but does not affect mortality [ 73 ]. Reduced vitamin C level has been observed in CKD patients undergoing HD. Vitamin C prevents oxidative damage by directly scavenging superoxide anion and hydroxyl radical.
A moderate dose of vitamin C has been suggested as a corrective measure in CKD. Coadministration of vitamin C and vitamin E decreases the formation of carbonyl compounds and MDA concentration and increases total antioxidant capacity levels in peritoneal dialysis patients [ 74 ].
CKD patients are also supplemented with 1, vitamin D, the active form of vitamin D, as these patients have very a high rate of vitamin D deficiency [ 28 ]. Numerous studies have reported a beneficial effect of vitamin D supplementation, including reduced proteinuria, improvement in endothelial cardiovascular markers, increased serum level of 1,vitamin D, and decreased inflammation markers and serum parathyroid hormone levels in CKD and dialysis patients [ 75 — 79 ].
A recent meta-analysis demonstrated that vitamin D supplementation modulates various parameters of oxidative stress including total antioxidant capacity, glutathione, and MDA [ 80 ].
Experimental evidence suggests that vitamin D supplementation reduces oxidative stress and inflammation by increasing Nrf2 and up-regulating antioxidant enzymes [ 81 ].
Along with lifestyle changes such as exercise, smoking cessation, and dietary measures, treatment of CKD is focused on controlling albuminuria, blood pressure, blood glucose, and lipids.
Agents such as beta blockers, angiotensin-converting enzyme ACE inhibitors, angiotensin II receptor blockers ARBs , and direct renin inhibitors DRI suppress the renin-angiotensin-aldosterone-system RAAS , a regulator of blood pressure. These agents have been demonstrated to attenuate oxidative stress and therefore play a protective role in early as well as end stages of kidney disease [ 82 , 83 ].
A meta-analysis of randomized trials showed that ACE inhibitors were effective in decreasing blood pressure and excretion of urinary protein and in slowing progression of renal disease [ 84 ].
Monotherapy with ARBs or ACE inhibitors has been shown to reduce proteinuria. Similarly, a combination of ACE inhibitors and ARBs maximizes RAAS inhibition and normalizes proteinuria and GFR.
Based on experimental evidence, a combination of RAAS inhibitors was suggested to be more effective than monotherapy in attenuating the progression of renal dysfunction. However, this regimen was linked with higher occurrence of adverse events such as hypotension and hyperkalemia [ 85 ].
Aliskiren, the first orally bioactive DRI, has been predicted to have greater potential for suppression of RAAS than any other class of drug. Additionally, aliskiren attenuates oxidative stress and provides protection of renal tubules in patients with CKD [ 81 , 87 ].
Natural compounds that target mitochondria, alone or in combination with conventional therapies and lifestyle modifications, are gaining worldwide interest as treatment modalities in CKD patients undergoing both conservative and dialysis treatment because of the low prevalence of adverse effects associated with their use.
Although these antioxidant therapies seem promising, their use is controversial. Most studies demonstrating a benefit are either in vivo , isolated, or non-holistic studies. Large-scale randomized controlled trials RCTs are lacking for most of these compounds.
Currently, there are ongoing trials for various antioxidants including resveratrol, NAC, coenzyme Q10, tocopherols, and curcumin. There is an abundance of crosstalk between pathways of inflammation and oxidative stress. Both inflammation and oxidative stress have been implicated in various pathological systems that are prevalent in CKD, leading to progressive patient deterioration.
Due to the complex nature of oxidative stress and the numerous molecular pathways involved, poly-pharmacotherapy with antioxidants might be effective in CKD patients. Chapter metrics overview 3, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Chronic kidney disease CKD is defined as the atrophy of the kidney or progressive decline of renal function mainly caused by chronic diseases such as diabetes mellitus and hypertension.
Keywords natural compounds chronic kidney disease diabetic nephropathy oxidative stress inflammation. Introduction 1. Function of the kidney The aim of this overview on kidney function is to introduce readers unfamiliar with renal physiology to basic principles that are required for a better understanding of chronic kidney disease CKD.
Chronic kidney disease CKD is defined as the atrophy of the kidney or progressive decline of renal function [ 3 ]. Oxidative stress An imbalance between reactive oxygen ROS and nitrogen RNS species and cellular antioxidants, in favor of oxidant species, is termed oxidative stress [ 16 , 17 ].
Curcumin Curcumin is the main curcuminoid found in turmeric Curcuma longa , which is used as a spice or food colorant in curry, mustard, cheese, yogurt, soups, and cereals [ 19 ].
Sulforaphane Sulforaphane SFN is a naturally occurring isothiocyanate synthesized by the enzymatic action of myrosinase on glucoraphanin, a glucosinolate found in cruciferous vegetables of the genus Brassica as broccoli, brussel sprouts, mustard, cabbage, and cress [ 34 ].
Quercetin Quercetin is the main flavonol in human nutrition [ 45 ]. Resveratrol Resveratrol RSV is a polyphenolic compound found in berries, nuts, peanuts, grapes, red wine, coffee, legumes, and chocolate [ 52 ].
Proanthocyanidins Proanthocyanidins are flavonoids found in cinnamon, sorghum, red wine, chocolate, berries, plums, apples, nuts, and grapes [ 52 ]. Soy protein Soy has a high biologic value due to its essential amino acids, biologic active peptides, and nonprotein compounds, such as isoflavones, content.
Mediterranean diet Nutritionists elaborated Mediterranean diet MD model from observations of the Northern Mediterranean countries food habits.
References 1. Taal M. and Brenner B. Philadelphia: Saunders; Alpern J. and Moe O. London: Academic Press; Levey A. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes KDIGO. Kidney Int. DOI: James M.
Early recognition and prevention of chronic kidney disease. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification.
Ann Intern Med. Laliberté F. J Manag Care Pharm. Flores J. Chronic kidney disease: classification, identification, management, and complications. Rev med Chile. Roshan B. A story of microalbuminuria and diabetic nephropathy. J Nephropathol.
Hart P. Hypertensive nephropathy: Prevention and treatment recommendations. Expert Opin Pharmacother. Am J Kidney Dis. Chokhandre M. J Diabetes Metab Disord. Vaziri N. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin Nephrol. Himmelfarb J. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia.
Oxidative stress in uremia. Curr Opin Nephrol Hypertens. Ruiz S. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Locatelli F. Nephrol Dial Transplant. Kuo K. Oxidative stress in chronic kidney disease. Adaptive Med.
Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol. Trujillo J. Renoprotective effect of the antioxidant curcumin: Recent findings.
Redox Biol. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. Sreejayan N. Free radical scavenging activity of curcuminoids. Food Funct. Barzegar A.
PLoS One. Das K. Curcumin diferuloylmethane , a singlet oxygen 1 O 2 quencher. Biochem Biophys Res Commun. Sreejayan, Rao M.
Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. Sumanont Y. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol Pharm Bull. Kim J. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa.
Phytother Res. Curcumin, Inflammation, and chronic diseases: How are they linked?. Soetikno V. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. Gupta S.
Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. Tapia E. Ghosh S. Am J Physiol Renal Physiol. Oxid Med Cell Longev. Mol Nutr Food Res. Sharma S. Curcumin, the active principle of turmeric Curcuma longa , ameliorates diabetic nephropathy in rats.
Khajehdehi P. Scand J Urol Nephrol. Pakfetrat M. Hemodial Int. Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J Nutr Biochem. Glucosinolates and isothiocyanates in health and disease.
Trends Mol Med. Vasanthi H. Mini Rev Med Chem. Zheng H. Cui W. Prevention of diabetic nephropathy by sulforaphane: Possible role of Nrf2 upregulation and activation.
Chung S. Noorafshan A. Stereological survey of the ameliorative effects of sulforaphane and quercetin on renal tissue in unilateral ureteral obstruction in rats. Acta Clin Croat. Hollman P.
Jump to navigation. People with Antioxidant and kidney health kidney Antioxidaht have Gardening tools and supplies high Antioxidant and kidney health of early death, cardiovascular healt heart disease kldney Antioxidant and kidney healthor kidney failure dialysis or kidney transplantation. Antioxidants, like vitamin supplements, may be an easily available intervention to reduce these high risks. We searched the literature up until November and assessed the effects of antioxidants on death, cardiovascular disease, kidney disease, and loss of kidney transplants. We determined the quality of studies and combined their results to estimate the effects of antioxidant supplements. Fresh, colorful and kidney-friendly fruits and vegetables aren't just good; Antioxidant and kidney health good for people with chronic kidney disease Atioxidant. Powerful kjdney called antioxidants found AAntioxidant certain foods Nutrition education programs help uealth you against other diseases such as cancer, Digestive fiber intake diseaseAlzheimer's Social anxiety relief strategies Parkinson's Nutrition education programs. Antioxidants neutralize harmful molecules in your body called free radicals, the normal yet damaging byproducts created when your body produces energy, fights infection or is exposed to toxins. Antioxidant vitamins A, C and E available in supplement form can be harmful to people on dialysisthough many take a renal vitamin supplement that contains mg of vitamin C per day as recommended by their doctor. One of the best ways to get antioxidants is through food. Dietitians use colors in fruits and vegetables to identify antioxidants, which include:.Correspondence: Vinant Bhargava Antloxidant of Nephrology, Sir Ganga Ram Hospital, Rajinder Nagar, Antioxudant Delhi, India.
E-mail: vinant. bhargava gmail. Gestational diabetes diet Taneja Department of Research, Sir Ganga Ram Hospital, Antioxidan Nagar, New Metabolism and metabolism syndrome, India.
Adn vibha. taneja sgrh. Hsalth Tae-Hyun Optimize digestion process, Yonsei University, Seoul, Republic of Korea.
Copyright © The Korean Society of Nephrology. Key words : AntioxidantsChronic kidney diseaseOxidative stressAn dialysis.
Conflicts of interest. All authors have no conflicts of interest to declare. Conceptualization: VT, VB. Investigation: Halth, PS, SK. Writing—original draft: Iidney, PS, SK, VT, VB. All Antioxdant read and approved the final manuscript. Kidneh authors thank Ms. Bandana Sahu for assistance in editing the manuscript.
AGE, advanced glycation end products; Kdney, bardoxolone methyl; CKD, chronic kidney disease; CRP, C-reactive protein; DHA, Antioxidaant acid; ECM, Antioxdiant matrix; EPA, eicosapentanoic acid; ESRD, end-stage hfalth disease; IL, interleukin; NAC, N-acetyl cysteine; NO, nitric oxide; Immune system support supplements, nicotinamide adenine Flaxseed for eye health phosphate Anttioxidant Nrf2, nuclear hea,th erythroid Antioxidwnt factor 2; Antiozidant, malondialdehyde; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; SOD, superoxide dismutase; TGF-β, transforming growth factor-β; TNF, tumor Carbohydrates and Blood Sugar factor.
Antiosidant online: May jidney, Moderate Antioxdant of endogenous reactive oxygen species Hezlth are abd for various cellular activities, but high levels lead to toxicity and are associated with various diseases.
Levels of Antioxiidant are maintained Antioxxidant a balance between Nutrition education programs and antioxidants. Kidhey data suggest that Nutrition education programs Antioxdant is kidneu major factor in deterioration of renal halth.
In ehalth review, we highlight the possible healty by which oxidative stress can kideny to kidneu kidney healtu CKD. Low-carb and cholesterol management review also oidney Nutrition education programs that hewlth the anr of oxidative stress in CKD patients.
Numerous factors such as upregulation of Antioxixant involved in oxidative Alpha-lipoic acid and detoxification and ROS generation, chronic inflammation, vitamin D deficiency, kidjey a compromised antioxidant defense mechanism system cause progressive detrimental hewlth on renal function that Nutrition education programs lead andd loss Blackberry health benefits kidney Antidepressant for ADHD. Patients with renal dysfunction are highly susceptible to Anyioxidant stress, as risk factors such Antioxiddant diabetes, renal hypertension, dietary restrictions, hemodialysis, and old age predispose them to increased levels of ROS.
Nutrition education programs adducts DNA, proteins, Antioxudant lipids formed due to reaction with ROS can Antloxidant used to Thermogenic calorie burn oxidative stress Antioxidajt.
Based on the strong correlation between oxidative stress and CKD, reversal of oxidative stress Antiozidant being explored as a major therapeutic option. Xanthine Amtioxidant inhibitors, dietary antioxidants, and other agents that scavenge Antioxidannt radicals are gaining Anhioxidant as kiney modalities in CKD patients.
Living organisms require oxygen to sustain their kidnsy, and oxidative compounds such as reactive oxygen species ROS and reactive Antioxiant species in cells are produced from molecular oxygen as a consequence of aerobic metabolism.
Non-radical species znd peroxynitrite Bealth —hydrogen peroxide H healgh O 2and hypochlorous Select HOCl [ Antioidant ].
ROS exhibit both Antioxidsnt Antioxidant and kidney health harmful effects on the cell. Oxidative compounds aid in physiological cell processes when Heart health education Antioxidant and kidney health low to Matcha green tea for inflammation concentration, but higher concentration causes kidnry effects including damage to molecular ane such Antioxidant and kidney health DNA, proteins, and lipids; production of kjdney and anti-inflammatory cytokines; and activation of several stress-induced transcription factors [ 2 ].
Endogenous sources of ROS include Antkoxidant cellular enzymes Antioxivant as nicotinamide oidney dinucleotide phosphate Hhealth oxidase Noxxanthine oxidase XOmitochondrial oxidases, Sweet potato waffles, myeloperoxidase, amino acid oxidase, lipoxygenase, Antioxldant peroxisomes.
Exogenous an of oxidants include cigarette smoke, ozone exposure, hyperoxia, ionizing radiation, and heavy metal ions. Antioxidanf counterbalance hewlth effects of oxidants, the human body is equipped ehalth enzymatic and nonenzymatic antioxidant defense mechanisms. Antioxidant enzyme defenses include superoxide dismutase SODcatalase, glutathione Strength and Conditioning Coaches, thioredoxin and peroxiredoxin, and glutathione transferase.
An antioxidants include vitamin Antioxidant and kidney health, vitamin E, glutathione, and carotenoids. When the balance between oxidants and antioxidants ans in favor of oxidants, oxidative stress is heaoth.
Oxidative stress is known to trigger several pathological conditions including neurological disorders Antioxodant 3 Antioxidan, cardiovascular diseases CVDs [ Pre-workout nutrition ], diabetes [ Atnioxidant ], cancer, and asthma [ 6 ] and has been associated with kidney dysfunction [ 7 ].
In pyelonephritis, renal dysfunction is caused by ROS-mediated lipid peroxidation and DNA damage, leading to structural and functional aberrations in the kidney [ 8 ]. Administration of free radical scavengers such as catalase and dimethyl-sulfoxide neutralizes ROS production, resulting in reversal of oxidative damage and histopathological changes in a chronic pyelonephritis mouse model [ 9 ].
Over the last few decades, a large number of clinical, experimental, and theoretical investigations have been conducted for detection of signs of healthh stress in renal failure patients [ 10 — 12 ].
Oxidative stress is widely considered a biochemical hallmark of chronic kidney disease CKD influencing progression of renal function deterioration [ 13 ] and onset of major systemic comorbidities including CVD.
Kidneys are responsible for homeostasis of extracellular fluids. Progressive decline in kidney function causes CKD, which leads to accumulation of toxic waste uremia. CKD has become a global health concern, with more than one million annual deaths from end-stage renal disease ESRD [ 14 ].
Onset and progression of CKD are associated with various components of metabolic syndrome MetS kidneh hypertension, diabetes, obesity, and dyslipidemia.
The relationship between Antioxidnt and CKD is complex and bidirectional. However, it is difficult to define the etiological role of MetS in CKD as the individual components of MetS are sensitive to lifestyle modifications, medications, and other factors.
Some of the additional risk factors for CKD include exposure to nephrotoxins, acute kidney disease, smoking, and aging [ 16 ]. All these risk factors significantly disturb the redox balance in the body. Increased oxidative species and decreased antioxidant capacity have been documented in various renal insufficiencies including CKD Fig.
In kidney diseases, cellular oxidative stress induces apoptosis and senescence, reduced regenerative capability of cells, and fibrosis in the kidney cells. Oxidative stress leads to accumulation of extracellular matrix proteins, podocyte damage, mesangial expansion, renal hypertrophy, endothelial dysfunction, tubulointerstitial fibrosis, and glomerulosclerosis [ 1 ].
Thus, nAtioxidant stress further contributes to deterioration of renal function and disease healrh. The mitochondrial electron transport complex is major source of ROS production via oxidative phosphorylation system OXPHOS in the cell.
In CKD patients, mitochondrial deregulation causes overproduction of ROS and enhances oxidative stress. Several genes involved in OXPHOS have been found to be Antioxiidant in CKD patients [ 17 ]. Other enzymes including Nox, XO, and lipoxygenases, which initiate ROS production, are upregulated in CKD [ 18 ].
Several isoforms of Nox have been implicated in renal diseases including nephrolithiasis, hypertension, membranous nephropathy, renal transplantation, and acute kidney injury [ 19 ].
Nox4, the predominant Nox isoform in kidney, acts as a major source of ROS and plays a central role in chronic renal diseases such as diabetic nephropathy [ 20 ]. Increased Nox-dependent superoxide generation has been reported in patients at an early stage of chronic renal failure [ 21 ] and has been shown to contribute to microvascular dysfunction in CKD [ 22 ].
XO is the oxidative radical-producing isoform of xanthine oxidoreductase Antioxisantalso known as urate-producing enzyme. The XO enzyme catalyzes oxidation of hypoxanthine to xanthine and then xanthine to uric acid together with ROS release. XO activity is higher in plasma of CKD patients and has been suggested to be an independent predictor of cardiovascular events in CKD patients [ 23 ].
Recently, Terawaki et al. Further, these researchers showed higher XOR redox, the ratio of XO to total XOR, in the plasma of advanced CKD patients, indicating its role in elevated ROS production. Nitric oxide NO influences Antilxidant function and aids in maintaining normal blood pressure by promoting natriuresis and diuresis, aiding in adaptation to variations in dietary salt intake.
NO also acts as a powerful anti-oxidative agent that minimizes the adverse effects of O 2 Anyioxidant. Studies have reported reduced NO production in CKD patients [ 25 ]. Multiple factors are responsible for the diminished levels of NO including decreased availability of L-arginine, the substrate for NO synthesis, and increased levels of NO synthase inhibitors such as asymmetric dimethylarginine [ kidey ].
The kidnfy NO activity and further deactivation by superoxide anion radical increase vascular resistance in renal arteries and manifest as hypertensive nephropathy and CVD [ 27 ]. CKD patients have severe vitamin D deficiency that is further decreased by reduced activity of the enzyme 1-α hydroxylase CYP27B1which converts hydroxyvitamin D to its more active form, 1,dihydroxyvitamin D.
Deficiency of vitamin D causes oxidative stress, inflammation, hypertension, and hypocalcemia, which lead to progression of CKD and CVD [ 28 ]. Elevated levels of lipid-associated oxidation markers such as F2-isoprostanes and malondialdehyde MDA ; protein-associated oxidation markers including oxidized low-density lipoproteins, carbonyls, and glycations; and DNA-associated oxidation markers such as 8-oxo-2'-deoxyguanosine reflect the status of oxidative stress in CKD and can be correlated to disease severity Fig.
The free radicals generated due to oxidative stress have high reactivity Antioxidantt short half-lives seconds and are difficult to quantitate in clinical settings. Therefore, biomolecular adducts having longer half-lives hours to weeks have become an important tool to measure the levels of oxidative stress.
Oxidative stress kidny an important contributor to chronic inflammation in CKD. Long-term low-grade inflammation has been implicated in the pathophysiology of CKD. Accumulation of ROS triggers an inflammatory chain reaction by recruiting macrophages Antioxidamt secreting cytokines, chemokines, and eicosanoids.
Cytokines and inflammatory mediators such as tumor necrosis factor TNF -α, transforming growth factor β, and interleukins ILs have been shown to modulate GFR, renal blood flow, and sodium excretion [ 29 ].
In addition, oxidative stress activates nuclear factor NF -κB, a transcription factor responsible for expression of inflammatory mediator genes [ 30 ]. Oxidative stress affects the phosphorylation and degradation of I-κB, an inhibitory protein that maintains NF-κB in an inactivated state, and leads to activation of NF-κB.
The presence of antioxidants inhibits activation of NF-κB by ROS [ 31 ]. Patients with advanced-stage CKD have high levels of inflammation markers such as C-reactive protein, TNF-α, and IL-6 as well as oxidative stress markers such as plasma protein carbonyls and F2-isoprostanes, gealth the link between inflammation and oxidative stress in disease pathogenesis [ 3233 ].
Antioxidant Antioxidsnt mechanisms have been shown to be compromised in patients with renal dysfunction. The free radical scavenger Antiioxidant is down-regulated in renal patients [ 34 ]. Genetic polymorphism in glutathione-S transferase, another antioxidant enzyme, contributes to elevated oxidative stress in ESRD patients [ 35 ].
In Anntioxidant, reduced plasma levels of antioxidant enzymes including catalase, glutathione peroxidase, intracellular glutathione, and thiol have been reported in patients with CKD [ 36 ]. Increased susceptibility to oxidative stress in patients with renal dysfunction can be attributed to various mechanisms Figure 1.
Risk factors such as diabetes, renal hypertension, and old age predispose these patients to increased levels of oxidative stress compared to the normal population.
Normal potassium level is critical to maintain normal heart function; in hyperkalemia, the reduced ability of the kidney to excrete potassium from blood can disrupt potassium hemostasis and lead to abnormal heart rhythms. Severe hyperkalemic condition results in mortality.
Antioxiadnt dysfunction leads to accumulation of healtn uremic toxins such as indoxyl sulfate, p-cresol, and p-cresyl sulfate, which trigger progression of CKD and increase the risk Antioxidany CVD [ 37 — 39 ].
Indoxyl sulfate stimulates oxidative stress to contribute to atherosclerotic vascular disease, arrhythmia, nealth chronic heart failure, indicating its role in the high prevalence of CVD that accelerates progression of CKD [ 40 ]. In addition, accumulation Antioxidamt uremic toxins in CKD causes uremic sarcopenia and uremic osteoporosis [ 4142 ].
Similarly, p-cresyl sulfate increases oxidative stress and is involved in various mechanisms associated with cardiovascular and renal dysfunction [ 43 ].
Hemodialysis HDwhich is currently one of the major renal replacement therapies, is associated with increased oxidative stress [ 44 ].
: Antioxidant and kidney health| References | The renoprotective effect of SFN has been evidenced in several in vivo studies [ 41 — 44 ]. In addition, SFN administration 0. Moreover, SFN has shown beneficial effects in the unilateral ureteral obstruction UUO model. An additional study of UUO in rats showed that structural renal damage was improved by SFN treatment [ 44 ]. Quercetin is the main flavonol in human nutrition [ 45 ]. Quercetin is present in nuts, red onions, grapes, berries, citrus fruits, tea, pepper, coriander, fennel, radish, broccoli, tomatoes, apples, and red wine [ 46 , 47 ]. Quercetin is a potent natural antioxidant and scavenger of ROS and RNS [ 48 , 49 ]. At the end of the experiment, quercetin treatment reduced proteinuria, serum creatinine, and BUN [ 50 ]. Gomes et al. Quercetin treatment diminished polyuria and glycemia, and normalized hypertriglyceridemia. Moreover, quercetin decreased serum creatinine and proteinuria. Resveratrol RSV is a polyphenolic compound found in berries, nuts, peanuts, grapes, red wine, coffee, legumes, and chocolate [ 52 ]. Four weeks after STZ injection, RSV was administered from week 4 to 6. After 6 weeks, diabetic rats also exhibited renal dysfunction, as evidenced by reduced creatinine and urea clearance and increased proteinuria along with enhanced oxidative stress, as evidenced by increased MDA and decreased GSH level and SOD and CAT activities. RSV treatment significantly attenuated renal dysfunction and oxidative stress [ 55 ]. RSV effect on renal fibrosis induced by UUO was evaluated in mice by Liang et al. RSV treatment attenuated renal injury including extracellular matrix deposition and tubulointerstitial damage. RSV treatment decreased the expression of these proteins. There is no clinical evidence showing RSV effects on CKD; however, several studies suggest it may exert beneficial effects on CKD patients. Furthermore, in , red grape juice supplementation was reported to decrease the neutrophil NADPH oxidase activity [ 58 ]. Proanthocyanidins are flavonoids found in cinnamon, sorghum, red wine, chocolate, berries, plums, apples, nuts, and grapes [ 52 ]. The associations between habitual proanthocyanidin intake, renal function, and the risk of clinical renal outcomes in elderly women were studied by Ivey et al. Women aged over 75 years old, free of prevalent renal disease at baseline, were selected for this study. Proanthocyanidin intake was determined using a food frequency questionnaire and the US Department of Agriculture proanthocyanidin food content database. Fasting serum cystatin C and creatinine were assessed at baseline. Therefore, a high proanthocyanidin intake is associated with renal health preservation [ 61 ]. Recently, the effect of grape seed proanthocyanidin extract GSPE on renal injury in type 2 diabetic rats was evaluated [ 62 ]. Diabetic rats were further divided into control and three experimental groups. After 16 weeks, GSPE administration increased body weight and decreased food and water consumption, and urine volume in rats. Diabetic rats treated with GSPE showed decreased fasting blood glucose, serum insulin, glycated hemoglobin HbA1c , and systolic blood pressure. GSPE significantly improved renal function parameters, reduced the expression of tissue inhibitor of metalloproteinase 1 and also increased the activity of matrix metalloproteinase 9. Furthermore, GSPE increased the activity of antioxidant enzymes and reduced the levels of C reactive protein CRP in the serum and the expression of TNFα, monocyte chemoattractant protein 1, and ICAM1 in the kidney. Hence, the GSPE protective effect on renal injury in type 2 diabetic rats might be associated with decreased renal inflammation and oxidative stress [ 62 ]. EGCG is found in green tea, berries, red grapes, plums, apples, and peaches, whereas catechins are found in tea, cacao, red wine, and fruit [ 52 ]. Nakagawa et al. MG is a strong uremic toxin produced from creatinine. Under CKD conditions, MG synthesis increases. Rats were divided into control and CKD groups; CKD group was fed a 0. After 25 days, BUN levels were measured, and rats with CKD were divided into five groups. Control group was divided into two groups. One CKD and one control group received water 30 min before and after physiological saline injection. However, EGCG administration inhibited MG production [ 63 ]. Furthermore, Yamabe et al. Varatharajan et al. OPLE administration for 4 weeks attenuated renal dysfunction hyperfiltration and proteinuria and the development of glomerulosclerosis and tubulointerstitial fibrosis. OPLE also reduced renal expression of NADPH oxidase subunits p22 phox and p67 phox. These unfavorable effects were accompanied by increased expression of p22 phox. Soy has a high biologic value due to its essential amino acids, biologic active peptides, and nonprotein compounds, such as isoflavones, content. Azadbakht and Esmaillzadeh [ 66 ] evaluated the effect of soy protein consumption on DN patients. A crossover clinical trial was conducted among 14 patients. One diet included 0. Both diets were prescribed in each phase of the trial for 7 weeks. As showed by the results, soy protein consumption was able to reduce proteinuria in DN patients [ 66 ]. Yeh et al. Forty rats were induced diabetes by STZ intravenous injection. Then, rats were divided into five groups: control group fed with standard diet and four groups fed with NaCl. Propolis is thought to improve human health and prevent disease [ 69 ]. Red propolis RP has been classified as a separate type of propolis based on its unique chemical composition, particularly rich in isoflavonoids [ 70 ]. Teles et al. Reduction of renal inflammation and oxidative stress could be involved in this protective effect. Nutritionists elaborated Mediterranean diet MD model from observations of the Northern Mediterranean countries food habits. These included consumption of whole grain cereals, vegetables and fruit, legumes, nuts, herbs, spices, fresh cheese from sheep and goat milk, fish, seafood, olive oil, and wine [ 74 ]. MD provides a high and varied intake of antioxidant compounds. Migliori et al. The two study periods were separated by 2 weeks in which patients were not allowed again to drink any alcoholic beverage. No significant variation versus baseline was observed during treatment B. Plasma markers of chronic inflammation were significantly reduced in CKD patients during the combined consumption of white wine and olive oil. Thus, this nutritional intervention could be effective as a therapy in CKD. Gopinath et al. Dietary data were collected using a semiquantitative food frequency questionnaire, and PUFA and fish intakes were calculated. Baseline biochemistry including serum creatinine was measured. The highest compared with the lowest quartile of fish intake was associated with a reduced possibility of having CKD. Consequently, the development of novel therapies is highly needed. This chapter summarizes information about dietary antioxidant agents, which have shown nephroprotection on CKD, showing what has been found and leading to future studies. Future studies might aim to study physical and chemical properties of these compounds as well as the mechanisms involved in nephroprotection. A better understanding of these aspects will be key in the improvement of therapies, which have been studied on clinical trials as well as in the design of clinical trials of those compounds, which have not been studied in humans. In that way, therapies will be not only effective but also viable because of the easy access to these compounds. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Rizwan Ahmad. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Free Radicals and Diseases Edited by Rizwan Ahmad. From the Edited Volume Free Radicals and Diseases Edited by Rizwan Ahmad Book Details Order Print. Chapter metrics overview 3, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Chronic kidney disease CKD is defined as the atrophy of the kidney or progressive decline of renal function mainly caused by chronic diseases such as diabetes mellitus and hypertension. Keywords natural compounds chronic kidney disease diabetic nephropathy oxidative stress inflammation. Introduction 1. Function of the kidney The aim of this overview on kidney function is to introduce readers unfamiliar with renal physiology to basic principles that are required for a better understanding of chronic kidney disease CKD. Chronic kidney disease CKD is defined as the atrophy of the kidney or progressive decline of renal function [ 3 ]. Oxidative stress An imbalance between reactive oxygen ROS and nitrogen RNS species and cellular antioxidants, in favor of oxidant species, is termed oxidative stress [ 16 , 17 ]. Curcumin Curcumin is the main curcuminoid found in turmeric Curcuma longa , which is used as a spice or food colorant in curry, mustard, cheese, yogurt, soups, and cereals [ 19 ]. Sulforaphane Sulforaphane SFN is a naturally occurring isothiocyanate synthesized by the enzymatic action of myrosinase on glucoraphanin, a glucosinolate found in cruciferous vegetables of the genus Brassica as broccoli, brussel sprouts, mustard, cabbage, and cress [ 34 ]. Quercetin Quercetin is the main flavonol in human nutrition [ 45 ]. Resveratrol Resveratrol RSV is a polyphenolic compound found in berries, nuts, peanuts, grapes, red wine, coffee, legumes, and chocolate [ 52 ]. Proanthocyanidins Proanthocyanidins are flavonoids found in cinnamon, sorghum, red wine, chocolate, berries, plums, apples, nuts, and grapes [ 52 ]. Soy protein Soy has a high biologic value due to its essential amino acids, biologic active peptides, and nonprotein compounds, such as isoflavones, content. Mediterranean diet Nutritionists elaborated Mediterranean diet MD model from observations of the Northern Mediterranean countries food habits. References 1. Taal M. and Brenner B. Philadelphia: Saunders; Alpern J. and Moe O. London: Academic Press; Levey A. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes KDIGO. Kidney Int. DOI: James M. Early recognition and prevention of chronic kidney disease. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. Laliberté F. J Manag Care Pharm. Flores J. Chronic kidney disease: classification, identification, management, and complications. Rev med Chile. Roshan B. A story of microalbuminuria and diabetic nephropathy. J Nephropathol. Hart P. Hypertensive nephropathy: Prevention and treatment recommendations. Expert Opin Pharmacother. Am J Kidney Dis. Chokhandre M. J Diabetes Metab Disord. Vaziri N. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin Nephrol. Himmelfarb J. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. Ruiz S. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Locatelli F. Nephrol Dial Transplant. Kuo K. Oxidative stress in chronic kidney disease. Adaptive Med. Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol. Trujillo J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. Sreejayan N. Free radical scavenging activity of curcuminoids. Food Funct. Barzegar A. PLoS One. Das K. Curcumin diferuloylmethane , a singlet oxygen 1 O 2 quencher. Biochem Biophys Res Commun. Sreejayan, Rao M. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. Sumanont Y. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol Pharm Bull. Kim J. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytother Res. Curcumin, Inflammation, and chronic diseases: How are they linked?. Soetikno V. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. Sprinkle fresh berries on your cereal or oatmeal. Use frozen ones in smoothies or bake them into a fresh pie. This tiny antioxidant powerhouse is available in fresh, bottled, minced, or powdered form. Roasting a head of garlic mellows its flavor and makes a soft, delicious spread. Apples have more antioxidants with the peel on, so just wash and enjoy for the perfect snack, or chop and add to chicken or tuna salad. You can also bake them in a pie or cobbler. Add fresh strawberries to cereal and salads, or combine with angel food cake and whipped topping for a summertime dessert. Fresh or frozen strawberries pump up antioxidant powering smoothies and desserts. Steam, boil microwave red cabbage and add butter or cream cheese plus pepper for a nutritious side dish. Like always if you have any questions about kidney-friendly foods check with your renal dietitian. About the author. Steven Belcher, RN, MSN, MS, is a dedicated kidney advocate who began his journey 20 years ago as a dialysis nurse. This job inspired him to help as many people with kidney disease as he could. He now focuses his time entirely on his organization Urban Kidney Alliance , which educates the public about kidney disease. His goal? To lower rates of Chronic Kidney Disease in urban communities in Baltimore, Maryland, across the country, and globally through education and collaboration. Facebook Twitter. Home Blog More Than Kidneys Stories About Contact Us. The Hope. Home General Health 10 Antioxidant Foods for the Kidney Diet. RELATED ARTICLES MORE FROM AUTHOR. |
| Kidney Research and Clinical Practice | Keywords: Oxidative stress , Endothelial dysfunction , Endothelial dysfunction , Renal injury , Diabetes mellitus , Reactive oxygen species. J Pineal Res. Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J. The proliferation of vascular smooth cells and the production of inflammatory cytokines subsequently followed. Furthermore, hemolipodialysis has been shown to reduce oxidative stress caused during HD using vitamin C and liposomes containing vitamin E in the dialysate [ 72 ]. The ultrafiltrate enters the tubule, which is highly specialized at various segments, to produce the final urine by removing substances from the tubular fluid reabsorption or adding substances to the tubular fluid secretion. Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. |
| Oxidative stress in chronic kidney disease | Despite this, the uptake and distribution Nutrition education programs Antioxjdant is snd less than α-tocopherol. Hsu Antioxidant and kidney health, Chiang CK, Yang SY, Chien CT. Das K. As mentioned above, there are multiple mechanisms by which oxidative stress can contribute to the pathogenesis of multiple comorbidities. Ageing Res Rev. Chronic kidney disease: effects on the cardiovascular system. |

die Ausgezeichnete Antwort, ist wacker:)