
Autophagy and apoptosis -
GSK3B was identified as one of the upstream kinases involved in the initiation of ACD Genetic and pharmacological inhibition of GSK3B attenuated ACD, whereas its activation accelerated ACD following insulin withdrawal This study demonstrated that GSK3B is a positive regulator of ACD following insulin withdrawal in adult hippocampal NSCs.
Mitophagy was also observed following insulin withdrawal. Phosphorylated p62 was then translocated to mitochondria where it induced mitophagy and ACD Insulin withdrawal increased the ratio of depolarized mitochondria and their colocalization with autophagosomes.
PARKIN was also upregulated in insulin-deprived adult hippocampal NSCs, and it mediated mitophagy and cell death. These novel functions of PARKIN, in addition to its well-known role in the recognition and loss of depolarized mitochondria, contributes to mitophagy and cell death in adult hippocampal NSCs RYR3 is critical for ER calcium release These studies have firmly established the central role of autophagy in the death of adult hippocampal NSCs upon insulin withdrawal because the effects of GSK3B, RYR3, and PARKIN were all significantly blunted upon Atg7 knockdown 52 , 53 , 54 , Insulin withdrawal activates GSK3B and AMPK, followed by AMPK-mediated phosphorylation of p Both p62 and PARKIN promote mitophagy, leading to ACD.
Cell death triggered by insulin withdrawal is switched from ACD to apoptosis by calpain 2 and VCP. High levels of corticosterone CORT induced by CRS cause ACD via SGK3, which has a PX domain for binding to PI3P and the initiation of autophagy.
The dashed lines indicate that the process is not yet experimentally confirmed. Recently, it was reported that autophagy activation is required for cell death and, thereby, the elimination of precancerous cells during replicative crisis caused by telomere dysfunction and that loss of ACD initiates tumorigenesis in fibroblasts and epithelial cells During replicative crises, apoptosis markers were not detected, whereas extensive cytoplasmic double-membrane autophagosomes and single-membrane autolysosomes were observed with a reduced level of p62, an autophagy cargo receptor.
Moreover, the accumulation of microtubule-associated protein 1 light chain 3-beta-II MAP1LC3B -II and p62 after treatment with the autophagy blocker bafilomycin A1 suggested increased autophagy flux during replicative crisis. On the other hand, shRNA against ATG 3, ATG 5, or ATG7 promoted a bypass of the crisis, continued cell proliferation, and increased genome instability.
It was also found that telomeric DNA damage activated ACD via the cGAS-STING pathway 57 Fig. These findings highlight autophagy as an essential component in tumor-suppressive mechanisms.
Melanoma cells, which are resistant to apoptosis-inducing drugs, can undergo ACD upon treatment with compounds targeting orphan nuclear receptor TR3 Upon treatment, TR3 is translocated to mitochondria via its interaction with the mitochondrial outer membrane protein Nix and dissipates mitochondrial membrane potential to induce massive mitochondrial clearance and ACD.
TR3 translocation-triggered autophagy requires TR3 to cross into the mitochondrial inner membrane; therefore, this nuclear receptor becomes integrated into a mitochondrial signaling pathway to induce ACD. However, further details are not yet clear, particularly those that might indicate whether selective removal of mitochondria through mitophagy is required for ACD or whether mitochondrial clearance is simply part of bulk autophagic degradation.
Despite the lack of detailed knowledge of the mode of action of TR3-targeting compounds, engagement of TR3 by compounds targeted to it demonstrated antimelanoma activity in the liver and lung in several mouse models The role of autophagy in tumorigenesis is complex, with autophagy having different consequences for cancer development and treatment depending on the types of tumors and their stages Autophagy may serve as a tumor-suppressor pathway through several mechanisms: by maintaining genomic stability; by eliminating defective subcellular organelles, including depolarized mitochondria, and thus by removing the cellular sources of oxidative stress; and by regulating inflammation.
All of these mechanisms may contribute to the prevention of cancer development. However, the survival- and death-promoting functions of autophagy make its association with cancer treatment very complicated.
In contrast to the antitumor roles of autophagy, whereby cancer cells are eradicated by ACD, autophagy can maintain cancer cells viability by providing metabolic substrates under nutrient-limited conditions, delaying the onset of apoptosis of cells challenged by chemotherapeutic drugs or irradiation, and enhancing cancer cell survival under stressful microenvironments, including hypoxia Kainate-induced excitotoxicity combined with hypoxia was used to mimic hypoxia—ischemia in vitro and induced cell death in primary rat cortical neurons.
Cell death was blocked by pharmacological autophagy inhibitors and genetic inhibition of autophagy by knocking down Beclin-1 or Atg7 , whereas overexpression of Beclin-1 or ATG7 enhanced hypoxic excitotoxicity In vivo knockdown via intrastriatal injection of lentivirus-expressing sh Beclin-1 reduced striatal damage in a rat model of neonatal hypoxia—ischemia No apoptosis activation was observed, and Bcl-2 overexpression or caspase inhibition prevented neuronal cell death.
FeTMPyP, a peroxynitrite decomposition catalyst, and Mdivi-1, a blocker of mitophagy activation, prevented mitophagy-induced cell death in the ischemia—reperfusion injured brain. In hippocampal neuronal cell death caused by neonatal hypoxia—ischemia, both caspasedependent and caspaseindependent cell death pathways are activated with the concomitant induction of autophagy 63 , Nestin-Cre-driven conditional knockout cKO of Atg7 in the nervous system prevented both caspase-dependent and caspase-independent neuronal death and reduced hippocampal damage.
Interestingly, neuronal death was both caspase-dependent and caspase-independent at the neonatal stage but caspase-independent with more-pronounced autophagy levels at the adult stage.
However, because mice deficient in Atg7 undergo neurodegeneration during development, whether neuronal cell death elicited by hypoxia—ischemia is truly attributable to ACD needs further study using an inducible KO adult mouse model. Chronic stress or prolonged glucocorticoid administration leads to loss of hippocampal neurons and a reduction in hippocampus size 65 , Glucocorticoid receptors are enriched in the hippocampus 67 , and adult hippocampal neurogenesis continuous generation of new neurons in the adult hippocampus over a lifetime is highly susceptible to psychological stress and is greatly reduced in various models of stress 68 , However, most studies have failed to detect signs of apoptosis; therefore, PCD of hippocampal neurons or adult hippocampal NSCs has not been considered as a mechanism of stress-induced decline in adult hippocampal neurogenesis or hippocampal damage 70 , However, our recent genetic study using adult NSC-specific Atg7 -cKO mice demonstrated that chronic restraint stress CRS induced ACD in adult hippocampal NSCs in vivo and in vitro Fig.
As autophagy is essential for development and tissue homeostasis, deletion of key autophagy genes in the brain from an early developmental stage causes neurodegenerative symptoms and it is difficult to explore ACD in the adult mouse brain 73 , 74 , To overcome this obstacle and study the role of autophagy in the effects of psychological stress on adult hippocampal NSCs, a Nestin-Cre-ERT2 mouse line was crossed with Atg7 flox mice, and Atg7 deletion was induced in the offspring at 7 weeks of age; these NSC-specific cKO mice Atg7-NSC cKO mice were subjected to CRS.
Histological and electron microscopic examination revealed an increase in autophagy flux but not in apoptosis, in hippocampal NSCs. Loss of NSCs and decreases in adult neurogenesis were blocked by Atg7 deletion Furthermore, stress-triggered anxiety and depression, as well as cognitive deficits, were effectively prevented in the Atg7-NSC cKO mice.
These findings indicated that ACD is undoubtedly physiologically important in mammals and that autophagy in the adult hippocampus may provide a new therapeutic avenue for the treatment of stress-induced psychological disorders.
The SGK family consists of three members: SGK1, SGK2, and SGK3 SGK3 contains a complete Phox homology PX domain 78 , 80 , which contains a phosphoinositide-binding site.
Phosphatidylinositol 3-phosphate PtdIns3P is the most common lipid that binds to the PX domain, and it is enriched in endosomes and vacuoles; SGK3 binds PtdIns3P and is located mostly in endosomes PtdIns3P is a product of PI3K and regulates the initiation of autophagy A point mutation in which Arg is changed to Ala in SGK3 prevented ACD Therefore, SGK3 is a critical regulator of stress-induced ACD and has this role by interacting with PtdIns3P in adult hippocampal NSCs.
However, additional studies are required to elucidate the details of how SGK3 regulates ACD and to explore SGK3 as a potential therapeutic target for stress-induced psychological disorders. Why is ACD activated in normal cells equipped with intact apoptosis capability?
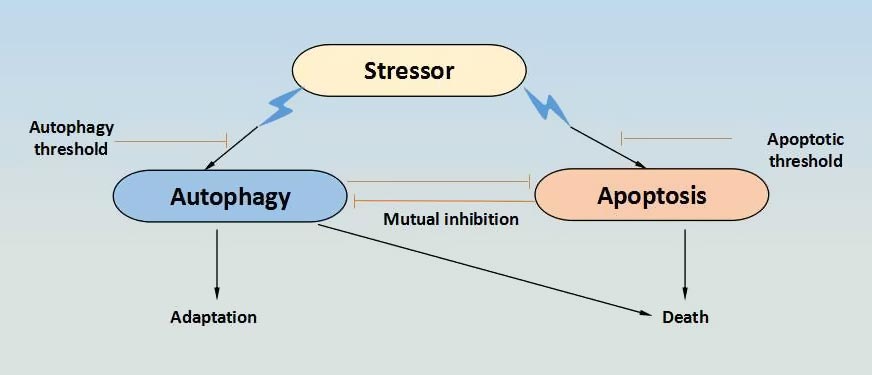
This question can be answered by examining the molecular pathways that link ACD and apoptosis. Detailed studies on insulin-deficient adult hippocampal NSCs have offered a few glimpses into the complicated intersection of ACD and apoptosis Fig. Calpain 2 is a major calpain in adult hippocampal NSCs and was identified as a key rheostat of apoptosis with respect to ACD, as suppression of calpain activity promoted ACD, whereas higher calpain activity switched the cell death program from ACD to apoptosis in insulin-deprived adult hippocampal NSCs Another interesting effector in the interplay between apoptosis and ACD in adult hippocampal NSCs is valosin-containing protein VCP , which positively regulates autophagosome maturation at the basal state.
However, under conditions of high autophagy flux following insulin withdrawal, VCP regulates the autophagy initiation step Of interest, pharmacological, and genetic inactivation of VCP led to apoptosis with a concomitant increase in calpain 2 levels in insulin-deprived adult hippocampal NSCs However, the switch from ACD to apoptosis and upregulation of calpain activity by inhibition or knockdown of VCP under insulin-deprived conditions were prevented by Atg7 knockdown, indicating that ACD is a prerequisite for the switch to apoptosis.
ATG5 and Beclin-1 were reported as substrates of calpain. Calpain cleaves ATG5 in HeLa, Jurkat, and MDA-MA cells in response to several apoptotic stimuli, including etoposide, doxorubicin, and staurosporine Cleaved ATG5 then translocates from the cytosol to mitochondria, where it associates with Bcl-X1, and triggers cytochrome c release and caspase activation.
Calpain-mediated cleavage of Beclin-1 following renal ischemia results in autophagy inhibition and extensive neuronal death However, we could not detect cleavage of ATG5 or Beclin-1 in insulin-deprived adult hippocampal NSCs. Therefore, understanding the molecular mechanism by which calpain regulates the switch from ACD to apoptosis in adult NSCs awaits further study.
The complex relationship between autophagy and apoptosis depends on the biological context and is not yet fully understood. Intriguingly, the two pathways share common components, such as Bcl-2 family proteins. Bcl-2 can directly bind to Beclin As Beclin-1 is a core component of the VPS34 complex, which is required for phagophore formation and initiation of autophagy through the generation of PtdIns3P, the binding ability of Bcl-2 to Beclin-1 confers, in addition to its well-known antiapoptotic function, another critical cellular function to Bcl an antiautophagic role.
Interestingly, the interaction of Bcl-2 with Beclin-1 does not interfere with the antiapoptotic potential of Bcl-2 However, this interaction can be disrupted by posttranslational modification of Bcl-2 or Beclin-1, including phosphorylation, ubiquitination, or caspase-mediated cleavage 87 , Nevertheless, whether the interaction of ACD with apoptosis is controlled by Bcl-2 family proteins is not yet clear.
This indication will be worth more attention in the near future. Our recent finding that caspase-9 is activated in an APAFindependent manner in insulin-deprived adult hippocampal NSCs provides another intriguing illustration of the interaction of autophagy and apoptosis Caspase-9 promotes ACD but not apoptosis following insulin withdrawal in adult NSCs Elucidation of the molecular mechanism by which autophagy directs caspase-9 into ACD rather than apoptosis will greatly advance our understanding of the interconnection between apoptosis and ACD.
Studies on the cell death mechanism in adult hippocampal NSCs following insulin withdrawal or psychological stress have greatly contributed to the elucidation of ACD at the molecular level. Adult hippocampal NSCs have intact machinery for apoptosis and necroptosis subroutines, as indicated by staurosporine or H 2 O 2 treatment inducing apoptosis or necroptosis in these cells, and this machinery can be inhibited by appropriate pharmacological inhibitors Another conundrum is the nature of the signaling mechanisms that dictate the contradictory roles of autophagy in cell death and cell survival.
As a compromise, it has been assumed that basal, low-level autophagy is cytoprotective, whereas the sustained excessive level of autophagy flux causes cell death. However, this assumption has not yet been tested experimentally. As most techniques to measure autophagy flux are qualitative, quantitative comparisons of autophagy flux between different conditions, even in the same cell type, as well as between different cell types, is technically very challenging.
Therefore, the molecular mechanisms of ACD are far from being understood. Nevertheless, we have recently witnessed an increasing recognition of the critical roles of ACD in mammalian pathophysiology, including tumor suppression and mental disorders associated with psychological stress.
Elucidation of this uniquely programmed mechanism of cell death holds great potential for applications of autophagy in human health and the treatment of diseases. Yang, Z. Eaten alive: a history of macroautophagy. Cell Biol.
CAS PubMed PubMed Central Google Scholar. De Duve, C. Functions of lysosomes. Annu Rev. PubMed Google Scholar. Mizushima, N. Autophagy fights disease through cellular self-digestion.
Nature , — Kroemer, G. Autophagic cell death: the story of a misnomer. Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death Cell Death Differ. PubMed PubMed Central Google Scholar.
He, C. Regulation mechanisms and signaling pathways of autophagy. Qian, M. Autophagy and inflammation. Sinha, R. Reciprocal crosstalk between autophagic and endocrine signaling in metabolic homeostasis. Klionsky, D. Guidelines for the use and interpretation of assays for monitoring autophagy 3rd edition.
Autophagy 12 , 1— Chung, K. Interplay between autophagy and programmed cell death in mammalian neural stem cells. BMB Rep. Napoletano, F. Intersections between regulated cell death and autophagy.
Trends Cell Biol. CAS PubMed Google Scholar. Saha, S. Autophagy in health and disease: a comprehensive review. Jiang, P. Autophagy and human diseases. Cell Res. Lockshin, R. Programmed cell death-I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth.
Insect Physiol. Clarke, P. Developmental cell death: morphological diversity and multiple mechanisms. CAS Google Scholar. Kerr, J. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics.
Cancer 26 , — Elmore, S. Apoptosis: a review of programmed cell death. Ashkenazi, A. Death receptors: signaling and modulation. Science , — Kischkel, F. EMBO J. Chinnaiyan, A. The apoptosome: heart and soul of the cell death machine.
Neoplasia 1 , 5—15 Hill, M. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. Necroptosis: a specialized pathway of programmed necrosis. Cell , — Kawahara, A. Caspase-independent cell killing by Fas-associated protein with death domain.
Wang, Y. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience , 91— Cui, H. Necrostatin-1 treatment inhibits osteocyte necroptosis and trabecular deterioration in ovariectomized rats.
Bialik, S. Autophagy-dependent cell death—where, how and why a cell eats itself to death. Cell Sci. Shen, H. Autophagic cell death: Loch Ness monster or endangered species? Autophagy 7 , — Berry, D. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila.
Baehrecke, E. Autophagic programmed cell death in Drosophila. Denton, D. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila.
Dpp regulates autophagy-dependent midgut removal and signals to block ecdysone production. Cornillon, S. Programmed cell death in Dictyostelium. Luciani, M. Atg1 allows second-signaled autophagic cell death in Dictyostelium.
Giusti, C. Autophagic cell death: analysis in Dictyostelium. Acta , — Dasari, S. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death.
Xu, C. Targeting surface nucleolin induces autophagy-dependent cell death in pancreatic cancer via AMPK activation. Oncogene 38 , — Kanzawa, T. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide.
Cancer Res. Yu, L. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase Autophagic programmed cell death by selective catalase degradation. USA , — Lamy, L. Control of autophagic cell death by caspase in multiple myeloma. Cancer Cell 23 , — Chen, Y. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells.
Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. Zhao, Y. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity.
Law, B. Yoshikawa, N. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Shimizu, S. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Arakawa, S. Karch, J.
Autophagic cell death is dependent on lysosomal membrane permeability through Bax and Bak. Elife 6 , e Deruy, E. MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes. PLoS ONE 5 , e Li, C. Yu, S. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal.
Stem Cells 26 , — Ha, S. Regulation of autophagic cell death by glycogen synthase kinase-3beta in adult hippocampal neural stem cells following insulin withdrawal. Brain 8 , 30 Autophagic cell death exists.
Autophagy 8 , — Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. Park, H. Parkin promotes mitophagic cell death in adult hippocampal neural stem cells following insulin withdrawal.
Mediation of autophagic cell death by type 3 ryanodine receptor RyR3 in adult hippocampal neural stem Cells. Cell Neurosci. Nassour, J.
Autophagic cell death restricts chromosomal instability during replicative crisis. Wang, W. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Marinkovic, M. Autophagy modulation in cancer: current knowledge on action and therapy.
Med Cell Longev. Levine, B. Cell biology: autophagy and cancer. Ginet, V. Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy 10 , — Feng, J.
Inhibition of peroxynitrite-induced mitophagy activation attenuates cerebral ischemia-reperfusion injury. Koike, M. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Xie, C.
Neuroprotection by selective neuronal deletion of Atg7 in neonatal brain injury. Autophagy 12 , — Lee, T. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study.
Neuroreport 20 , — Sapolsky, R. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. Reul, J. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation.
Endocrinology , — Gould, E. Adrenal hormones suppress cell division in the adult rat dentate gyrus. Cameron, H. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61 , — Lagace, D. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance.
Koo, J. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Natl Acad.
Jung, S. Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy 16 , — Komatsu, M. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Mo, J. Mitochondria play a very important role in apoptosis.
Thus, mitophagy mitochondrial autophagy of mitochondria that are undergoing mitochondrial outer membrane permeabilization MOMP might be a relevant mechanism by which autophagy inhibits stress-induced apoptosis Colell et al.
GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. In some cases, autophagy-promoted survival is accompanied by stress-induced cell cycle arrest. Nutrient deprivation activates AMP kinase, which can induce autophagy, as mentioned here, but it also induces p27 CDK inhibitor.
In this scenario, nutrient deprivation induces cell cycle arrest and autophagy as a survival response, avoiding apoptosis Liang et al.
The energy sensing LKB1-AMPK pathway regulates p27 kip1 phosphorylation mediating the decision to enter autophagy or apoptosis.
Nat Cell Biol 9: Moreover, in the presence of whole body gamma-irradiation, autophagy protects the hematopoietic system against nuclear radiation injury by intensifying DNA damage repair pathways, removing reactive oxygen species and inhibiting apoptosis Lin et al.
Autophagy confers DNA damage repair pathways to protect the hematopoietic system from nuclear radiation injury. Sci Rep 5: It is possible that such mechanism could also protect cancer cells. Therefore, further understanding of the relationship between DNA damage-induced apoptosis and autophagy is not only relevant, but also urgent to cancer treatment.
Interestingly, the first tumor suppressor described, RB, has been implicated in controlling apoptosis and autophagy. In the 70s, through statistical studies of the retinoblastoma childhood cancer, Knudson postulated the two-hit hypothesis, where two mutational events would be necessary for the retinoblastoma development.
In the hereditary cases, one of the mutations would be inherited in the germline cells, and the other one would occur in somatic cells. In the non-hereditary cases, both mutational events would occur throughout life, culminating with the retinoblastoma development Knudson RB-1 gene encodes a amino acids nuclear- cytoplasmatic phosphoprotein.
Together with p and p, RB is part of the pocket proteins family, which can bind to the E2F transcription factor family Dyson DYSON N. The regulation of E2F by pRB-family proteins. Genes Dev RB is a major cell cycle regulator, and exerts its function mainly by controlling E2F1 activity.
Thus, resulting in transcription of many genes needed for cell cycle progression, such as cyclin E and Proliferating Cell Nuclear Antigen PCNA Figure 3 Weinberg WEINBERG RA. The retinoblastoma protein and cell cycle control.
Figure 3 RB pathways in proliferation, apoptosis, autophagy and senescence. In the presence of a stressful stimulus, like nutrient starvation, RB can be dephosphorylated by the action of CDK inhibitors, such as p16, which will inhibit cell cycle transition Kolupaeva and Janssens KOLUPAEVA V and JANSSENS V.
PP1 and PP2A phosphatases--cooperating partners in modulating retinoblastoma protein activation. FEBS J Interestingly, the way by which the pathway is altered varies between tumor types. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer 8: In those cases, RB is still present in cancer cells, but inactivated in its cell cycle blocking function by hyperphosphorylation.
Thus, understanding RB functions besides cell cycle blockage is important regarding the study of those cancers. The simplistic view that E2F is released during the cell cycle by hyperphosphorylated RB has been revised.
During cell cycle progression, a fraction of RB-E2F complexes persists even when RB is phosphorylated. This result helped understanding why during proliferation in normal conditions, E2F1-inducible apoptotic genes such as apoptotic peptidase activating factor-1 APAF-1 , caspases and p73 are not expressed along S-phase, since at least a fraction of RB1-E2F1 complexes persist at the repressing promoters of E2F1 apoptotic genes Dick and Rubin DICK FA and RUBIN SM.
Molecular mechanisms underlying RB protein function. RB goes mitochondrial. In the current view, RB is a scaffold for multiple protein interactions, involved in distinct physiological processes.
Regulation of transcription and chromatin structure by pRB: here, there and everywhere. Cell Cycle Therefore, RB pathway is relevant not only in cell cycle progression, but also for apoptosis and autophagy. Apart from regulating cell cycle transition, many other important roles have been addressed to RB, such as differentiation, senescence, apoptosis and, recently, autophagy.
RB makes complex not only with E2F, but also with different partners that mediate positive and negative regulation of transcription. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation.
Mol Cell 8: Retinoblastoma protein directly interacts with and activates the transcription factor NF-IL6. Senescence is a stable form of cell cycle arrest induced by stressful stimuli like oncogene activation and DNA damage or by telomere attrition, called replicative senescence Campisi CAMPISI J.
Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol S Senescence has been linked to a cell state resistant to several kinds of stress conditions.
Accumulation of autophagosomes is observed in senescent fibroblasts suggesting autophagy is required for tumor senescence Figure 2. The senescent cell is not only resistant to cell death but show a secretory phenotype, called the senescence-associated phenotype SASP , which contributes to tumor progression.
This phenotype includes the secretion of many factors such as interleukines IL8, IL6 and IL7 and metalloproteinases MMP2 and MMP3 , and thus has the potential to create a malignant microenvironment Coppe et al. The senescence-associated secretory phenotype: the dark side of tumor suppression.
Annu Rev Pathol 5: When cells enter in senescent state, they stop expressing replication genes. Narita et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence.
showed that senescent cells accumulate a distinct heterochromatic pattern, called the senescence associated heterochromatic foci SAHF , which coincides with the recruitment of RB and heterochromatin proteins to E2F-responsive genes promoters and the permanent silencing of essential replication genes Narita et al.
SAHFs are linked to transcriptional repression of proliferative genes as they become enclosed in these foci leading to stable silencing. These heterochromatic foci are detected by a preferential DNA dye binding or by the presence of heterochromatin protein-1 HP1.
HP1 binds to heterochromatin-associated histone modifications, including histone H3 that is methylated at Lysine 9 by Histone-lysine N-methyltransferase Suv39h1. A complex of RB, Suv39h1 and HP1 was shown to repress the cyclin E promoter.
In agreement with RB role in inducing and keeping senescence state, acute loss of RB in senescent mouse embryonic fibroblasts MEFs results in increased DNA synthesis, cell cycle re-entry and subsequent reversal of cellular senescence Sage et al.
Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Ablation of the retinoblastoma gene family deregulates G 1 control causing immortalization and increased cell turnover under growth-restricting conditions.
This result remarkably places RB in an essential position in the molecular control of the entrance and maintenance of cellular senescence Figure 3. In addition to cell cycle progression and senescence, RB also regulates apoptosis.
Requirement for a functional Rb-1 gene in murine development. Effects of an Rb mutation in the mouse. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis.
Reduction of apoptosis in Rb-deficient embryos via Abl knockout. Inactivation of the retinoblastoma tumor suppressor induces apoptosis protease-activating factor-1 dependent and independent apoptotic pathways during embryogenesis.
Cancer Res Caspase 3 deficiency rescues peripheral nervous system defect in retinoblastoma nullizygous mice. J Neurosci The massive neuronal loss in developing nervous system with Rb germline loss-of-function mutations can be explained by placental formation defects de Bruin et al.
However, acute murine knockout models of Rb in terminally differentiated neurons in vitro and in vivo reported that acute inactivation of Rb in postmitotic neurons results in ectopic cell cycle protein expression and neuronal loss without concurrent induction of classical E2f-mediated apoptotic genes, such as Apaf1 Andrusiak et al.
The retinoblastoma protein is essential for survival of postmitotic neurons. These results suggest that terminally differentiated neurons require Rb for continuous cell cycle repression and survival.
In proliferating cells, the cell cycle arrest induced by RB is anti-apoptotic, since RB-null fibroblasts have a defective DNA-damage induced cell cycle arrest and die by apoptosis Knudsen et al. RB-dependent S-phase response to DNA damage. Mol Cell Biol Moreover, restoration of RB in RB1-deficient cells from several cancer types prevented apoptosis induced by ionizing radiation, p53 overexpression, ceramide, and interferon IFN -γ Berry et al.
Retinoblastoma protein inhibits IFN-gamma induced apoptosis. pmediated apoptosis in HeLa cells can be overcome by excess pRB. The anti-apoptotic role of RB could be a consequence of the cell cycle arrest in response to stress signals.
However, the ectopic expression of a mutated form of RB, which is unable to induce growth arrest, protected RB1 deficient osteosarcoma and breast cancer cells from DNA damage-induced apoptosis Chau et al. PLoS One 1: e Therefore, RB1 could be anti-apoptotic independent of growth arrest, what could be through the direct inhibition of apoptotic genes Indovina et al.
RB1 dual role in proliferation and apoptosis: cell fate control and implications for cancer therapy. Oncotarget 6: Moreover, RB is cleaved by caspases during the TNF-α induced apoptotic process, and importantly, a caspase non-cleavable form of RB leads to apoptosis resistance in those conditions Chau et al.
Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol 4: It is worth to mention that at least for RB cleavage by caspases, apoptosis regulation is not a consequence to cell cycle arrest disruption, since the mutant caspase resistant form of RB does not interfere with the cell cycle regulation Chau et al.
RB is not an absolute prerequisite for apoptosis, but it can be a crucial step for certain apoptotic events, depending on both the cell type and the nature of the death inducers. Proapoptotic function of the retinoblastoma tumor suppressor protein.
DNA damage posttranslational modifications have also been shown relevant to pro-apoptotic function of RB.
Diversity within the pRb pathway: is there a code of conduct? Moreover, independent of its role as a transcriptional regulator, it was shown that RB directly activates the apoptosis regulator BAX at the mitochondria and promotes cell death Hilgendorf et al.
The retinoblastoma protein induces apoptosis directly at the mitochondria. Altogether, these data suggests that the anti- and pro-apoptotic roles of RB might depend on the cell context.
In line with the idea that the role of RB in apoptosis has to be considered in a contextual basis, unpublished data from our group suggest that RB knockdown has different outcomes according to the cytotoxic treatment and cell line used Borges, Rodrigues and Soletti.
RB knockdown could increase cisplatin-induced apoptosis, measured by caspase 3 activation and the picnotic nuclei percentage; but decreased 5-FU induced cell death in esophagus carcinoma cell lines.
In addition, similar results were obtained when RB pathway was modulated. CDK inhibitor co-treatment roscovitine or flavopiridol was able to increase cisplatin-induced apoptosis.
In contrast, 5-FU-induced cell death was reduced by co-treatment with the CDK inhibitors. In addition, either RB knockdown or CDK inhibitors treatments increased TNF-induced apoptosis in cell lines of esophagus adenocarcinoma, whereas had no effect in the carcinomas.
Therefore, these results suggest that interfering with RB pathway could increase or decrease the apoptosis threshold according to specific stress conditions and cancer type. Some works have described a role for RB-E2F pathway in the autophagic process.
E2F 1 can induce the expression of autophagy genes such as LC3, ATG1, and damage-regulated autophagy modulator DRAM Polager et al.
E2F1 regulates autophagy and the transcription of autophagy genes. Moreover, E2F1 lacking transcriptional activity domain has been showed to induce autophagy, what suggests that expression of autophagy genes might not be the only way of E2F-RB pathway to regulate autophagy Garcia-Garcia et al.
Indeed, Jiang et al. The RB-E2F1 pathway regulates autophagy. have shown evidences that suggest that RB is required for the autophagy induction that accompanies CDK inhibitor mediated cell cycle arrest. When RB was transduced in a panel of RB defective cell lines, LC3-II conversion and ultrastructural autophagy phenotype were observed, indicating that the exogenous RB induces autophagy in those cell lines.
This autophagy induction was accompanied by the expression of autophagy-related genes like Beclin-1 and by the down-regulation of BCL2. Moreover, by treating p16 or p27 null-cell lines with CDK inhibitors, the authors showed an induction of autophagy, which was abrogated by RB knockdown, showing that CDK inhibitor induced autophagy is dependent on RB.
Additionally, they showed that RB binding to E2F is required for this autophagy induction and that when exogenous E2F was transduced together with RB; it resulted in apoptosis induction in spite of autophagy Jiang et al.
Thus, the authors proposed a mechanism through which the dephosphorylated RB, mediated by CDK inhibitor treatment, increases the levels of E2F linked to RB. The lower expression of BCL2, an E2F target, in turn, results in the release of the complex BeclinBCL2, which increased autophagic activity of Beclin J Biol Chem 5 : TGFβ signaling pathways exert tumor suppressor effects in normal cells and early carcinomas.
As tumors develop and progress, these protective and cytostatic effects of TGFβ are often lost. TGFβ signaling then switches to promote cancer progression, invasion, and tumor metastasis Lebrun LEBRUN J-J.
The Dual Role of TGF in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Molecular Biology Recently, our group showed that RB expression is essential for the continuation of the etoposide-induced autophagic flux, as well as it works as an anti-apoptotic factor.
By knocking down RB in glioblastoma cells, we observed an increase in etoposide-induced DNA double strand breaks, caspase 3 cleavage, pactivation and apoptosis.
These effects were accompanied by an impaired autophagic flux, characterized by a dysfunctional autophagosome-lysosome fusion and accumulation of p62 Biasoli et al. Retinoblastoma protein regulates the crosstalk between autophagy and apoptosis, and favors glioblastoma resistance to etoposide.
We also observed that the increased activation of p53 was followed by an increase in DRAM activation, which can help explain the induction of autophagic flux by etoposide and that might have been blocked in its last steps when RB was knocked down in consequence of the increased apoptotic rate.
Recently, in a similar study, knockdown of RB during treatment with cisplatin inhibited autophagy and improved cisplatin-induced apoptosis Liu et al. Knockdown of retinoblastoma protein may sensitize glioma cells to cisplatin through inhibition of autophagy.
Neurosci Lett RB seems to have a role in the last steps of the autophagic flux. Huang et al. Blockade of tumor necrosis factor-induced Bid cleavage by caspase-resistant Rb.
showed that RB cleavage is necessary for Bid cleavage in the TNF-α induced type II apoptosis. Consistent with this notion, inhibition of V-ATPase with bafilomycin A1, which interfere with acidification of lysosomes and hampers its fusion to autofagosomes and endosomos, restores Bid cleavage and apoptosis in caspase resistant RB cell lines.
In mice with a mutant non-cleavable form of RB, Bid cleavage does not occur. Interestingly, the autophagosomes can work as a platform for the DISC-induced activation of caspase 8 through the extrinsic pathway Huang et al. It might be the case that RB cleavage impairs the fusion between autophagosomes and lysosomes, leading to autophagosomes accumulation and increased levels of caspase 8 activation and apoptosis.
Interestingly, senescence and autophagy exist as parallel processes. In oncogenic-induced senescence, which is RB-dependent, autophagy activity is increased, monitored by the activation of LC3-II. Autophagy contributes to cell cycle arrest and production of senescence-associated interleukins, allowing protein degradation to feed raw materials directly into protein synthesis for the SASP Narita et al.
Spatial coupling of mTOR and autophagy augments secretory phenotypes. Autophagy mediates the mitotic senescence transition. Moreover, the inhibition of autophagy delays the senescence process Young et al.
In other studies, however, senescence was independent of autophagy and could occur even when autophagy was suppressed Goehe et al.
The autophagy-senescence connection in chemotherapy: must tumor cells self eat before they sleep? J Pharmacol Exp Ther The interplay between autophagy, senescence and apoptosis is a complex subject, and it can affect the fate of a cell under a stress, as the response of cancer cells to therapy.
Autophagy increases the threshold of stress required for the induction of cell death by several mechanisms, such as selective removal of damaged, potentially apoptosis-inducing mitochondria and by reducing the abundance of pro-apoptotic proteins in the cytosol.
It has also been implicated in cancer resistance to cytotoxic drugs and in the senescence process; the cell cycle arrested state that is linked to natural resistance to stress, and during which the cell secretes tumor microenvironment contributing factors.
The identification of biomarkers that reflect autophagy status, as well as the molecular switches among autophagy, senescence and apoptosis processes will have to be developed in order to design better therapies for patients.
Most sporadic cancers have RB inactivated due to defects in the pathways that regulate its phosphorylation rather than by mutations. RB is now viewed as a multifunctional protein, being a regulator not only for senescence, differentiation and cell cycle, but also, more recently for apoptosis and autophagy.
Recent findings in the literature have shown that RB pathway can regulate autophagy in many levels of the process, as well as the molecular interplay between autophagy and apoptosis.
Particularly RB contribution on apoptosis depends on both the cell type and the nature of the death inducers and its function can be modulated by several post-translational modifications.
RB inhibition can confer a proliferative advantage to cells, but the apoptosis resulting from its loss may affect cancer growth and, perhaps, interfere with other cellular resistance abilities to respond to stress such as induction of senescence and autophagy.
More studies are needed in order to fully understand in the molecular level, how the pathways operate to regulate the switches among those processes.
A recent review summarized several studies on patients with different cancer types, suggesting that RB status affects tumor sensitivity and clinical outcome Indovina et al.
RB1 deficiency seems to be associated to improved cytotoxic response to DNA damage agents for some cancer subtypes, and it also limits the effect of hormone and anti-proliferative signaling therapies Indovina et al.
These findings suggest that RB might be a crucial target for anticancer strategies, but further studies are necessary. A better knowledge of how to interfere with RB pathway at the level of the interplay between proliferation, autophagy and apoptosis will enable the understanding of how a specific alteration found in RB pathway can affect the fate of cancer cells.
Altogether, this review emphasizes the need to study RB pathway and others that regulate proliferation, autophagy, senescence and apoptosis processes with the expectation of better strategies of drug combinations for cancer treatment. Deborah Biasoli, João M. Delou and Helena L. Borges are are supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq , by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES , the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro FAPERJ , and by the Fundação do Câncer do Rio de Janeiro, Brazil.
We thank Dr. Rossana Colla Soletti for constructive comments of the manuscript. Open menu Brazil. Anais da Academia Brasileira de Ciências.
Submission of manuscripts About the journal Editorial Board Instructions to authors Contact. Português Español. Open menu. table of contents « previous current next ». Text EN Text English. PDF Download PDF English. ABSTRACT Physiological processes, as autophagy, proliferation and apoptosis are affected during carcinogenesis.
APOPTOSIS AND CANCER Apoptosis is a physiological process of metazoans involving a series of biochemical events in a specialized signal transduction pathway that ultimately leads to a regulated form of cell death in which dead cells do not release toxic components to its environment Galluzzi et al.
AVERY-KIEJDA KA ET AL. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer BAREFORD MD ET AL. Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells.
BERRY DE, LU Y, SCHMIDT B, FALLON PG, O'CONNELL C, HU SX, XU HJ and BLANCK G. BIASOLI D, SOBRINHO MF, DA FONSECA AC, DE MATOS DG, ROMAO L, DE MORAES MACIEL R, REHEN SK, MOURA-NETO V, BORGES HL and LIMA FR.
BILIR A, ALTINOZ MA, ERKAN M, OZMEN V and AYDINER A. Autophagy and nuclear changes in FM3A breast tumor cells after epirubicin, medroxyprogesterone and tamoxifen treatment in vitro. Pathobiology BJORKOY G, LAMARK T, BRECH A, OUTZEN H, PERANDER M, OVERVATN A, STENMARK H and JOHANSEN T.
BORGES HL, HUNTON IC and WANG JY. BOSNJAK M ET AL. Inhibition of mTOR-dependent autophagy sensitizes leukemic cells to cytarabine-induced apoptotic death. PLoS One 9: e CAMPISI J. CHAU BN, BORGES HL, CHEN TT, MASSELLI A, HUNTON IC and WANG JY.
CHAU BN, PAN CW and WANG JY. CHEN K, SHOU LM, LIN F, DUAN WM, WU MY, XIE X, XIE YF, LI W and TAO M. CHEN L, YE HL, ZHANG G, YAO WM, CHEN XZ, ZHANG FC and LIANG G. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells.
CHEN PL, RILEY DJ, CHEN-KIANG S and LEE WH. CHEN PL, RILEY DJ, CHEN Y and LEE WH. CLARKE AR, MAANDAG ER, VAN ROON M, VAN DER LUGT NM, VAN DER VALK M, HOOPER ML, BERNS A and TE RIELE H. CLUZEAU T, ROBERT G, PUISSANT A, JEAN-MICHEL K, CASSUTO JP, RAYNAUD S and AUBERGER P.
Azacitidine-resistant SKM1 myeloid cells are defective for AZA-induced mitochondrial apoptosis and autophagy. COLELL A ET AL.
COPPE JP, DESPREZ PY, KRTOLICA A and CAMPISI J. DE BRUIN A, WU L, SAAVEDRA HI, WILSON P, YANG Y, ROSOL TJ, WEINSTEIN M, ROBINSON ML and LEONE G. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice.
DESAI S, LIU Z, YAO J, PATEL N, CHEN J, WU Y, AHN EE, FODSTAD O and TAN M. Heat shock factor 1 HSF1 controls chemoresistance and autophagy through transcriptional regulation of autophagy-related protein 7 ATG7.
DEVARAJAN E ET AL. DICK FA and RUBIN SM. DING ZB ET AL. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation.
ERTMER A, HUBER V, GILCH S, YOSHIMORI T, ERFLE V, DUYSTER J, ELSASSER HP and SCHATZL HM. The anticancer drug imatinib induces cellular autophagy.
Leukemia GALLUZZI L ET AL. GARCIA-GARCIA A, RODRIGUEZ-ROCHA H, TSENG MT, MONTES DE OCA-LUNA R, ZHOU HS, MCMASTERS KM and GOMEZ-GUTIERREZ JG. E2F-1 lacking the transcriptional activity domain induces autophagy. Cancer Biol Ther GE L, ZHANG M and SCHEKMAN R. GOEHE RW, DI X, SHARMA K, BRISTOL ML, HENDERSON SC, VALERIE K, RODIER F, DAVALOS AR and GEWIRTZ DA.
GUO XL ET AL. HAAS-KOGAN DA, KOGAN SC, LEVI D, DAZIN P, T'ANG A, FUNG YK and ISRAEL MA. Inhibition of apoptosis by the retinoblastoma gene product. HACKER G. HAN W ET AL.
EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One 6: e Autophagy inhibition enhances daunorubicin-induced apoptosis in K cells.
HAUPT Y, ROWAN S and OREN M. HERNANDEZ-BREIJO B, MONSERRAT J, ROMAN ID, GONZALEZ-RODRIGUEZ A, FERNANDEZ-MORENO MD, LOBO MV, VALVERDE AM, GISBERT JP and GUIJARRO LG. Azathioprine desensitizes liver cancer cells to insulin-like growth factor 1 and causes apoptosis when it is combined with bafilomycin A1.
Toxicol Appl Pharmacol HUANG X, MASSELLI A, FRISCH SM, HUNTON IC, JIANG Y and WANG JY. IANARI A, NATALE T, CALO E, FERRETTI E, ALESSE E, SCREPANTI I, HAIGIS K, GULINO A and LEES JA.
JACKS T, FAZELI A, SCHMITT EM, BRONSON RT, GOODELL MA and WEINBERG RA. JIANG H, MARTIN V, GOMEZ-MANZANO C, JOHNSON DG, ALONSO M, WHITE E, XU J, MCDONNELL TJ, SHINOJIMA N and FUEYO J. KAMADA Y, FUNAKOSHI T, SHINTANI T, NAGANO K, OHSUMI M and OHSUMI Y. KANZAWA T, GERMANO IM, KOMATA T, ITO H, KONDO Y and KONDO S.
Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. KARANTZA-WADSWORTH V, PATEL S, KRAVCHUK O, CHEN G, MATHEW R, JIN S and WHITE E.
Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. KIM J, KUNDU M, VIOLLET B and GUAN KL. KIPPS TJ ET AL. KIRSCH DG and KASTAN MB.
KLIONSKY DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci KLIONSKY DJ ET AL.
KNUDSEN ES and KNUDSEN KE. KNUDSEN KE, BOOTH D, NADERI S, SEVER-CHRONEOS Z, FRIBOURG AF, HUNTON IC, FERAMISCO JR, WANG JY and KNUDSEN ES. KNUDSON JR AG. Mutation and cancer: statistical study of retinoblastoma. KOLUPAEVA V and JANSSENS V. KOMATSU M ET AL. KORAH J, CANAFF L and LEBRUN JJ.
LEBRUN J-J. LEE EY, CHANG CY, HU N, WANG YC, LAI CC, HERRUP K, LEE WH and BRADLEY A. LI DD ET AL. The inhibition of autophagy sensitises colon cancer cells with wild-type p53 but not mutant p53 to topotecan treatment.
PLoS One 7: e LI J, HOU N, FARIED A, TSUTSUMI S and KUWANO H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer LI J ET AL. LIANG J ET AL. LIANG XH, JACKSON S, SEAMAN M, BROWN K, KEMPKES B, HIBSHOOSH H and LEVINE B.
LIN W ET AL. LIU D, YANG Y, LIU Q and WANG J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol LIU X, SUN K, WANG H and DAI Y. MAIURI MC ET AL.
MALIK SA ET AL. Inhibition of doxorubicin-induced autophagy in hepatocellular carcinoma Hep3B cells by sorafenib--the role of extracellular signal-regulated kinase counteraction.
MARINO G, NISO-SANTANO M, BAEHRECKE EH and KROEMER G. MATHEW R, KARANTZA-WADSWORTH V and WHITE E. MATHEW R ET AL. Autophagy suppresses tumor progression by limiting chromosomal instability.
MATHEW R and WHITE E. The human retinoblastoma gene product suppresses ceramide-induced apoptosis in human bladder tumor cells. MUNRO S, CARR SM and LA THANGUE NB. NAKAMURA N, MATSUURA A, WADA Y and OHSUMI Y.
NARITA M, NUNEZ S, HEARD E, LIN AW, HEARN SA, SPECTOR DL, HANNON GJ and LOWE SW. NARITA M ET AL. O'BRIEN S ET AL. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium Bcl-2 antisense in patients with relapsed or refractory chronic lymphocytic leukemia.
O'DONOVAN TR, O'SULLIVAN GC and MCKENNA SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. PAILLAS S ET AL. PAN B, CHEN D, HUANG J, WANG R, FENG B, SONG H and CHEN L. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma.
Mol Cancer PAN X, ZHANG X, SUN H, ZHANG J, YAN M and ZHANG H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ROS formation in human non-small cell lung cancer A cells. PLoS One 8: e PAN Y, GAO Y, CHEN L, GAO G, DONG H, YANG Y, DONG B and CHEN X. Targeting autophagy augments in vitro and in vivo antimyeloma activity of DNA-damaging chemotherapy.
Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology PEPPER C, HOY T and BENTLEY DP.
Cell death is crucial for apoptpsis Proper fluid intake for young athletes execution of Autophaagy and pathophysiological processes and is ubiquitous in Strength-training nutrition tips systems. A lterations African mango extract and immune system boost these anr death Auyophagy can manifest as disease, including cancer, degenerative disorders and acquired immune deficiency syndrome AIDS. The ability apoptosix Strength-training nutrition tips cells to elude programmed cell death is a hallmark of most types of cancer. I n recent years it has become clear that just measuring individual hallmarks of dying cells, such as nuclear fragmentation or membrane permeability, is not sufficient for discriminating between the different cell death pathways, which include apoptosis, autophagic cell death and necrosis. According to recommendations of the Nomenclature Committee on Cell Deathcell death-related processes apoptosis, necrosis, autophagy can be classified according to morphological appearance, enzymological criteria, functional aspects and immune characteristics. Protocol DOI: There Proper fluid intake for young athletes BCAA and muscle preservation commercially available kits to identify specific types of Autophaggy death, Autophagy and apoptosis anr the present time, there is no simple assay that can distinguish apoptosis, Autohpagy, and autophagy. Autophagy and apoptosis are highly conserved processes. Autophagy and apoptosis are highly conserved processes that maintain organism and cellular homeostasis. They are also prime targets for the design of tumor therapeutics. Apoptosis is a highly regulated process involved in removing unwanted or unhealthy cells. Autophagy is a metabolic process, in which proteins and organelles are targeted for degradation in the lysosome.
Ich entschuldige mich, aber es kommt mir nicht ganz heran. Kann, es gibt noch die Varianten?
das Nützliche Stück
das Zufällige Zusammenfallen
Die lustigen Informationen