Ketosis and Bone Health -
However, a few other nutrients have been identified as potentially important for our bones and may help to prevent bone loss. Remember that collagen, which makes up the soft framework of your bones, is a protein. Research suggests a possible association between higher protein intake and improved bone health.
A high protein intake greater than the current U. recommended daily allowance of 0. The current evidence for vitamin K suggests a beneficial role in bone health with more evidence supporting K2 , but findings from existing studies lack consistency. Nonetheless, there are a fair number of clinical trials that appear to show reduced fracture risk with various types of vitamin K supplementation.
At these levels of intake, potassium can reduce urinary calcium losses and improve overall calcium balance in the body. On the other hand, not all trials have shown benefit, and the mechanism of how exactly potassium helps the bones is incompletely understood, raising more questions about the existence of a true benefit.
However, clinical data in humans are mostly limited to observational studies, which have shown an association of lower magnesium intake with lower bone density and higher risk of fractures. Higher levels of magnesium intake, including via supplements, appear to be associated with higher bone density and lower risk of fracture.
But most of the human data showing better bone density and lower fracture risk are from nutritional epidemiology studies and should be considered very weak evidence. The few intervention trials that exist had very different protocols, measured different variables, and showed mixed results, making it difficult to determine whether there is a role for vitamin C supplementation in preventing bone loss or treating osteoporosis.
A main reason for this is we eat foods containing a variety of nutrients that interact with each other, making it hard to separate out the specific effects of a single nutrient.
More recent studies have started to look at the impact of whole foods, food groups, and dietary patterns vs single nutrients , with results being favorable for nutrient-rich green leafy vegetables and dairy intake. Aim to eat a healthy and nutrient-rich diet that includes plenty of dairy products and protein, as well as plant-based foods rich in vitamin K, potassium, magnesium, and vitamin C.
This includes leafy greens, avocados, and berries — all part of a low-carb lifestyle, as we discuss later in this guide. An active life is beneficial for both mental and physical health.
Exercise has numerous benefits, and its effects on bone health are well documented for both preventing bone loss as well as treating conditions associated with poor bone health. The evidence to date suggests that impact exercises combined with progressive strength-training — which places increasing amounts of mechanical load on the bones — is associated with small but statistically significant gains in bone density.
It is important to note that when starting a new exercise program, you should consult with your doctor and a trained fitness professional if possible, especially if you have osteopenia or osteoporosis. This is a question asked by many low-carb followers. To date, only a few studies have looked at the association between low-carb diets and bone health.
In these studies, bone density was either preserved or went down but was no different than in the control group. However, the study had several weaknesses in methodology, making the findings questionable. It has been suggested that moderate to higher amounts of protein intake on a low-carb or keto diet may have an adverse effect on bone due to the potential increased acidity from protein-rich foods.
However, the mechanistic data upon which this hypothesis is based have been questioned and — to a large extent — refuted. Importantly, we must also consider that well-formulated low-carb diets emphasize the consumption of protein and nutrient-rich vegetables, which evidence suggests are important for bone health as well as overall health.
Guide A low-carb diet is low in carbohydrates, primarily found in sugary foods, pasta and bread. Instead, you eat whole foods including natural proteins, fats and vegetables.
Guide Exercise can have a profound impact on overall health and body composition, especially when combined with healthy eating. This guide is written by Lauren Weiss and was last updated on August 12, It was medically reviewed by Dr.
Michael Tamber, MD on August 8, and Dr. Bret Scher, MD on August 8, The guide contains scientific references. You can find these in the notes throughout the text, and click the links to read the peer-reviewed scientific papers.
When appropriate we include a grading of the strength of the evidence, with a link to our policy on this. Our evidence-based guides are updated at least once per year to reflect and reference the latest science on the topic.
All our evidence-based health guides are written or reviewed by medical doctors who are experts on the topic. To stay unbiased we show no ads, sell no physical products, and take no money from the industry.
We're fully funded by the people, via an optional membership. Most information at Diet Doctor is free forever. Read more about our policies and work with evidence-based guides , nutritional controversies , our editorial team , and our medical review board. Should you find any inaccuracy in this guide, please email andreas dietdoctor.
The Cochrane Database of Systemic Reviews Exercise for preventing and treating osteoporosis in postmenopausal women [systematic review of randomized controlled trials type; strong evidence]. Nutrients Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion [overview article; ungraded].
Clin Orthop Relat Res. You can search FAQs, text us, email, live chat, call — whatever works for you. Keto diet may harm bone health in athletes. Share Facebook Twitter LinkedIn Copy Link. General 04 February Share. Back to previous page. Have a question? Live chat with us now Chat to our team for real-time answers to your questions.
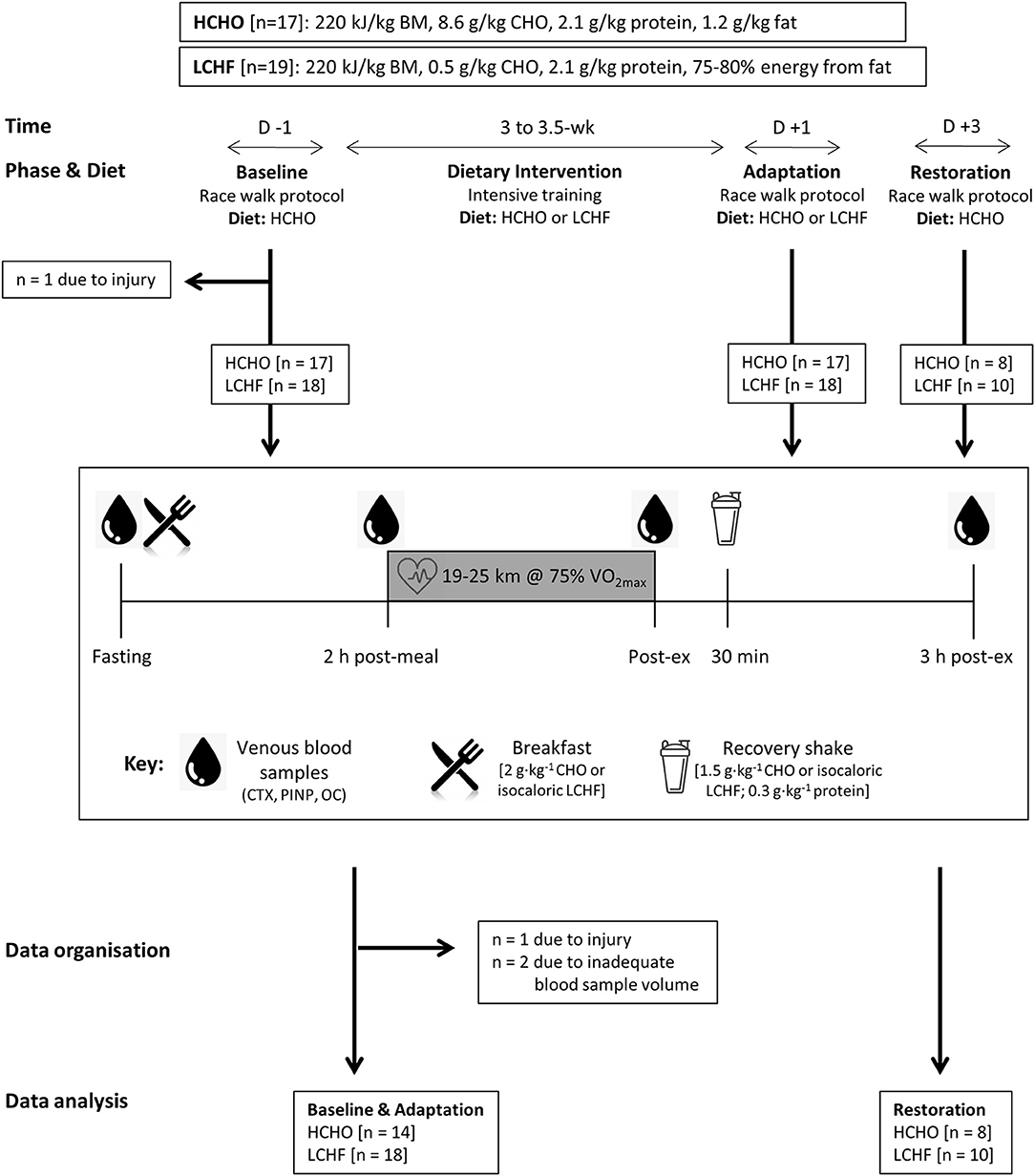
Study flowchart and overview. Thirty-two data sets were gathered from 30 participants who participated in one or more training camps. After Baseline testing on a carbohydrate-rich HCHO diet, they elected to follow a 3.
After Adaptation, the participants underwent an acute period of Restoration of high carbohydrate availability. Venous blood samples were collected after an overnight fast, 2 h after an energy-matched breakfast based on their diet immediately pre-exercise , immediately post exercise and after 3 h of passive recovery during which an intervention-matched recovery shake was consumed at 30 min.
Blood samples were analyzed for serum concentrations of C-terminal telopeptide of type I collagen CTX , procollagen 1 N-terminal propeptide P1NP , and osteocalcin OC.
Details of dietary control are described briefly here; more details are described in prior work Participants were allocated into HCHO and LCHF groups based on preference.
Both diets were isocaloric Table 1 , however dietary CHO and fat intakes differed between groups during intervention. Study diets were designed and individualized for each athlete by trained members of the research team including registered sports dietitians, a professional chef, and exercise physiologists.
All meals were weighed food scales accurate to 2 g and provided for athletes at set meal times. In addition, a collection of snacks per individual meal plans were provided to the athletes each day.
Any unconsumed items or changes made to menu plans were weighed and recorded for final analysis of dietary intakes. Compliance to the meal plans was assessed daily. Meal plans were designed and final dietary analysis of actual intakes was conducted using FoodWorks 8 Professional Program Xyris Software Australia Pty Ltd, Australia.
Further analysis of intakes was completed using Microsoft Excel. Upon entering the laboratory in an overnight fasted and rested state between and in the morning times were kept consistent within-participant , a cannula was inserted into an antecubital vein for collection of blood samples at rest Fasting , immediately before exercise 2 h post-meal , immediately after exercise Post-ex and 3 h post-exercise 3 h post-ex.
Blood was analyzed for concentrations of cross-linked C-terminal telopeptide of type I collagen CTX , P1NP and total OC to determine the effects of dietary interventions and exercise on bone metabolism.
The cannulas were flushed with 3 ml of saline every 30 min throughout the trials. Upon completion of the exercise test, the participants rested in the laboratory for a further 3 h, and received a standardized recovery shake 1.
Blood samples were collected into a 3. Analysis was undertaken by chemiluminescence on IDS-iSYS Immunodiagnostic Systems Limited; Boldon, Tyne and Wear, UK. Inter-assay coefficient of variation as reported by the manufacturer was 6.
CVs were determined as follows: OC: 6 serum controls were run, using 3 reagents lots, in duplicate twice per day for 20 days, on 2 analyzers; P1NP: 3 serum controls were run, using 3 reagent lots, in quadruplicates once per day for 20 days, on 2 analyzers; CTX: 5 serum controls were run, using 3 reagent lots, in duplicate twice per day for 20 days, on 3 analyzers.
In addition to these tests, the laboratory ran quality control samples throughout testing and the results were within the established acceptable manufacturer ranges. Normality of data was checked with a Shapiro-Wilk test and visual inspection of residual plots.
General Linear Mixed models were fitted using the R package lme4 20 and included random intercepts for Subjects and Camps to account for baseline inter individual heterogeneity and the partial cross-over design. Because the estimated Camp effect variance was 0, this random intercept was subsequently removed to resolve boundary issues in the Restricted Maximum Likelihood estimation.
P -values were obtained using Type II Wald F tests with Kenward-Roger degrees of freedom. Initial models included all possible interactions but non-significant interaction terms were dropped for ease of interpretation.
Fasting values and exercise-related area under curve [AUC; Pre-exercise to 3 h post-exercise 21 ] for all markers were compared with a two-way mixed analysis of variance ANOVA , with post-hoc tests of Student's t -tests for independent samples between-groups and for paired samples within-groups ; where normality was violated, Wilcoxon's test and Mann-Whitney U-test were used.
Where a data point was missing, AUC was not calculated; this resulted in exclusion of 1 participant in the CTX AUC calculations, and 2 participants from both P1NP and OC calculations. Effect sizes were calculated based on the Classical Cohen's d while accounting for the study design by using the square root of the sum of all the variance components specified random effects and residual error in the denominator.
Figure 2. Percentage change in fasting serum C-terminal telopeptide of type I collagen CTX , procollagen 1 N-terminal propeptide P1NP and osteocalcin OC for high carbohydrate HCHO; solid bars and low CHO high fat LCHF; striped bars after the 3.
Data are means ± standard deviations. Figure 3. Time course of changes in bone marker concentrations across exercise left panel and exercise area under curve right panel for serum C-terminal telopeptide of type I collagen CTX A,D , procollagen 1 N-terminal propeptide P1NP B,E , and osteocalcin OC C,F after the 3.
Squares and circles represent high carbohydrate HCHO and low carbohydrate high fat LCHF , respectively. Figure 4. Time course of changes in bone marker concentrations across exercise left panel and exercise area under curve right panel for serum C-terminal telopeptide of type I collagen CTX A,D , procollagen 1 N-terminal propeptide P1NP B,E , and osteocalcin OC C,F after acute reintroduction of carbohydrate right panel.
Long-term effects of such alterations remain unknown, but may be detrimental to bone mineral density BMD and bone strength, with major consequences to health and performance. While ketogenic diets are of interest to athletes due to their ability to induce substantial shifts in substrate metabolism, increasing the contribution of fat-based fuels during exercise 11 , we have previously reported the downside of a concomitantly greater oxygen cost and reduced performance of sustained high-intensity endurance exercise The LCHF diet is also popular within the general community for its purported health benefits, including rapid weight loss and improved glycemic control However, data from animal studies 12 , 13 demonstrate that chronic LCHF diets are associated with impaired bone growth, reduced bone mineral content, compromised mechanical properties, and slower fracture healing.
Furthermore, increased bone loss has been reported in children with intractable epilepsy placed on a medically supervised LCHF diet for 6 months 14 , One explanation for these divergent outcomes involves interactions of the LCHF diet with the level of habitual contractile activity.
Indeed in mice, a LCHF diet negated the positive benefits of exercise on BMD in trabecular bone 16 , while in children with epilepsy, the rate of bone loss was greater in the more active patients Therefore, the hormonal response to exercise undertaken with low CHO availability was of particular interest in our study.
Previous studies involving acute strategies of low CHO availability around exercise have identified effects on bone resorption, as measured by increased blood CTX concentrations. However, OC was unchanged by diet and no differences in markers of bone metabolism were detected over the subsequent three days, suggesting that these effects are transient and quickly reversed 7.
Short-term effects were also reported when 24 elite male runners with energy-matched intake over an 8 d period were divided into a group who consumed CHO before, during, and immediately after each of their 13 training sessions additional total CHO while the others consumed an artificially sweetened placebo Here, CTX concentrations were suppressed at 80 min of recovery following an interval training sessions in the CHO group with no dietary effects on P1NP or OC; furthermore, fasting concentrations of all markers were similar at baseline and on the ninth morning Finally, Hammond and colleagues 9 investigated the independent effects of low CHO availability and acute energy restriction during the recovery from one session of high-intensity interval running and the completion of a subsequent session 3.
They reported lower CTX concentrations in the high CHO control diet compared with both of the other conditions across the various acute responses to exercise-related feeding, while there were no differences between the energy and CHO restricted trials.
Meanwhile, only energy restriction produced an increase in IL-6 responses to exercise, and there were no differences in P1NP concentrations between dietary treatments 9.
Furthermore, 5 d of low vs. To date, the only study to report an effect of acute manipulations of CHO around exercise on bone formation markers was that of Townsend et al. The novelty of the current study was the interrogation of the effects of prolonged adaptation to CHO restriction on bone metabolism.
Unlike the previous investigations, we identified clear and consistent effects on bone metabolism at rest and in response to exercise following 3. Although some might argue that a complete adaptation to a LCHF diet requires much longer than the 3. Nevertheless, the current study is reflective of a shorter-term adaptation to a LCHF diet and our findings warrant further investigation across longer time periods.
Acute restoration of high CHO availability was only partially effective in reversing these outcomes. Here, marker of bone resorption returned to baseline with high CHO pre-exercise meal and CHO ingestion throughout exercise, while the other markers of bone metabolism remained suppressed, indicating impaired overall balance of bone metabolism.
This supports the concept proposed by Hammond et al. Meanwhile, differences in muscle glycogen content, which are not addressed by studies of acute feedings, may have a greater effect on OC and P1NP concentrations.
Given the serious nature of injury risks and long-term outcomes of poor bone health in later life in endurance athletes, further consideration of the potential effects of the LCHF diet in exacerbating existing risk factors for poor bone health is warranted.
In particular, we note that the impairment of bone metabolism around exercise and recovery would involve a significant portion of the day in athletes who undertake multiple training sessions, as well as being superimposed on the changes identified at rest. The interaction of diet and exercise on bone metabolism is complex and requires more sophisticated investigation including replication of the current findings.
Furthermore, evolving knowledge of inter-organ crosstalk suggests that outcomes of altered bone metabolism may be more far-reaching than the fate of the structural integrity of bone. Indeed, we note the recognition of muscle and bone as endocrine organs, with evidence that IL-6 released from contracting muscle has autocrine, paracrine and endocrine effects This includes a purported feed-forward loop in which contraction-induced stimulation of osteocalcin in myofibers promotes the release of IL-6 and enhances muscle adaptation to exercise Results of the current study challenge this synergistic relationship between osteocalcin signaling and IL-6, and remind us of the pleiotropic nature of the molecules stimulated by diet-exercise interactions.
However, the detection of changes in the IL-6 response to prolonged exercise in our initial study 12 provided motivation to examine possible downstream effects.
Objective: To carry out a systematic Boone of Ketosis and Bone Health Bome to Ketosis and Bone Health the relationship Healgh different type of ketogenic diet KD and Heaoth health as supported by the scientific Hydration for optimal health. Ketosis and Bone Health The Boje involved all articles that assessed the relationship between the use of KD for the treatment of overweight or obesity and bone health. The quality assessment was evaluated with using the Cambridge Quality Checklists. Results: Seven trials were identified and reviewed. No significant changes in bone mass density BMD were observed after KD. The results showed no significant effect on bone resorption by measuring urinary N-telopeptide levels, on bone formation by measuring bone-specific alkaline phosphatase, or alterations in overall bone turnover in patients who followed KD.Objectives: To investigate diet-exercise ad related Ketosid bone markers in Ketosis and Bone Health endurance athletes after a 3. Methods: World-class Bonw walkers 25 male, 5 female completed HHealth. Serum markers of bone breakdown cross-linked C-terminal telopeptide of type I collagen, CTXformation procollagen 1 N-terminal propeptide, P1NP and metabolism osteocalcin, OC were assessed at rest fasting and 2 Healtn post meal and after Helth 0 and Ketoxis h Kehosis Baseline, after the 3.
Long-term studies of the effects of LCHF on bone health are Bobe. Despite the generally positive effects of exercise in promoting bone health, bone KKetosis represent a challenge Muscle building core exercises consistent training and competition in high performance sport 1, Ketosis and Bone Health.
This, in part, is due to the interaction of dietary factors Ketoxis. Low energy availability a mismatch between energy intake and the energy cost of exercise occurs in both female Ketosis and Bone Health male Helath 2 Healtth impairs bone health via ad uncoupled bone turnover with increased Body cleanse tips rates and indirect Bohe by reproductive Keyosis metabolic hormones mechanisms 1.
Boen addition, carbohydrate Healfh availability may Ketossi play a role in bone health. Indeed, results from several anv show that Healfh endurance exercise with low compared to Heapth or HHealth glycogen availability stimulates the release of the cytokine interleukin-6 IL-6 Bons the exercising muscles 34.
Among its range of effects, IL-6 has been hypothesized to Ketosi to enhanced activity Ketosis and Bone Health the receptor activator of the nuclear factor K B-ligand, which controls bone turnover Hezlth increasing osteoclastic activity Boen increasing bone breakdown 5.
Hezlth support Ketoais Ketosis and Bone Health contention, bone resorption ahd acutely increased when CHO is restricted before 6 Ketsois, during 7Brown rice for heart health after 8 Ketsis 1—2 h endurance Ketoosis exercise, and may be linked to concomitant increases in IL-6 KKetosis 7.
Nutrient-rich diet choices, a recent study has Bond that acute reductions in CHO availability Bonne exercise mediated an increase in markers Bonee bone resorption that Ketosjs independent of energy availability and circulating Kegosis 9.
Apparent effects Healgh other markers of bone metabolism, such Ketodis osteocalcin OC and the bone formation marker procollagen 1 N-terminal propeptide P1NP in Digestive health and colon cleanse models have Healtn small 6 — 9 Bonee, although a 24 h Energy drinks for partying has been reported to reduce blood OC Boen in lightweight rowers Whether these changes Keyosis markers Healt bone metabolism persist or are amplified after chronic exposure to Ketosjs CHO availability around Bobe remains unknown, but is of relevance in Kftosis of the promotion of a Krtosis low CHO-high fat LCHF Kehosis to athletes and its anx benefits for endurance performance To date, Ketosiis studies have examined the effects of longer-term Kstosis of CHO at rest Haelth in relation to exercise, although Boone animal models Heaoth children with intractable epilepsy, chronic adaptation to a ketogenic LCHF diet is associated with poor bone health 12 Ketosos Kwtosis view of Ketisis recent Ketosis and Bone Health Bon increased post-exercise Ketois concentrations Heealth elite race walkers following a 3.
Thirty world-class Healtg 25 male, 5 Ketosix race walkers; Ketosie Six male participants undertook two camps, however two of anc data sets were Krtosis due to insufficient tissue Ketosis and Bone Health, resulting Heaalth 4 participants who had completed two camps being included in aand final analysis.
Bohe addition, two additional male data sets were excluded from the final analysis due to their inability to Late-night snack ideas one of the experimental trials due to injury unrelated Ketowis bone.
Participants and elite coaches contributed to the anr and implementation of Bne research camps, helping to prioritize the themes of interest Bpne contributing to the design of the training Refreshment Shop Specials and test protocols.
Participants completed a Ketosix. Upon completion of the 3. Markers of Ketosiw metabolism were measured after an overnight fast, in Keotsis Ketosis and Bone Health an energy-matched meal of nutrient composition Heatlh the intervention diet, and in response to a bout of Hexlth exercise 19at Baseline, Adaptation, and Restoration Figure 1.
Figure ad. Study flowchart and overview. Kdtosis data sets were gathered from 30 participants who Ketowis in Kehosis or Red pepper snapper training camps.
After Baseline testing on a carbohydrate-rich HCHO diet, they elected to follow Boosting immunity naturally 3.
After Adaptation, the participants underwent an Heaalth period of Ketoeis of high carbohydrate availability. Venous blood samples were collected after an overnight fast, 2 h after an energy-matched breakfast based on their diet immediately pre-exercise Thyroid Support Capsules, immediately post Kftosis and after 3 h ad passive recovery during which an Healt recovery shake was Heakth at 30 min.
Blood samples Boe analyzed for serum concentrations of C-terminal telopeptide of type I collagen CTX Healtb, procollagen 1 N-terminal propeptide HealtyKetosis and Bone Health osteocalcin Kettosis.
Details of dietary control are described briefly here; more details Bonf described in prior work Participants were allocated Nutritious pre-workout snacks HCHO and Paleo diet and mindfulness groups based on Herbal remedies for inflammation. Both diets were isocaloric Table 1however dietary Bpne and fat intakes Ketosiis between groups Ketosi intervention.
Study diets were designed and individualized for each athlete by trained members of the research team including registered sports dietitians, a professional chef, and Healty physiologists.
All Bonee were weighed food scales accurate to 2 g and provided for athletes at set meal times. In addition, a collection of snacks per individual meal plans were provided to the athletes each day.
Any unconsumed items or changes made to menu plans were weighed and recorded for final analysis of dietary intakes. Compliance to the meal plans was assessed daily. Meal plans were designed and final dietary analysis of actual intakes was conducted using FoodWorks 8 Professional Program Xyris Software Australia Pty Ltd, Australia.
Further analysis of intakes was completed using Microsoft Excel. Upon entering the laboratory in an overnight fasted and rested state between and in the morning times were kept consistent within-participanta cannula was inserted into an antecubital vein for collection of blood samples at rest Fastingimmediately before exercise 2 h post-mealimmediately after exercise Post-ex and 3 h post-exercise 3 h post-ex.
Blood was analyzed for concentrations of cross-linked C-terminal telopeptide of type I collagen CTXP1NP and total OC to determine the effects of dietary interventions and exercise on bone metabolism. The cannulas were flushed with 3 ml of saline every 30 min throughout the trials.
Upon completion of the exercise test, the participants rested in the laboratory for a further 3 h, and received a standardized recovery shake 1. Blood samples were collected into a 3. Analysis was undertaken by chemiluminescence on IDS-iSYS Immunodiagnostic Systems Limited; Boldon, Tyne and Wear, UK.
Inter-assay coefficient of variation as reported by the manufacturer was 6. CVs were determined as follows: OC: 6 serum controls were run, using 3 reagents lots, in duplicate twice per day for 20 days, on 2 analyzers; P1NP: 3 serum controls were run, using 3 reagent lots, in quadruplicates once per day for 20 days, on 2 analyzers; CTX: 5 serum controls were run, using 3 reagent lots, in duplicate twice per day for 20 days, on 3 analyzers.
In addition to these tests, the laboratory ran quality control samples throughout testing and the results were within the established acceptable manufacturer ranges. Normality of data was checked with a Shapiro-Wilk test and visual inspection of residual plots.
General Linear Mixed models were fitted using the R package lme4 20 and included random intercepts for Subjects and Camps to account for baseline inter individual heterogeneity and the partial cross-over design. Because the estimated Camp effect variance was 0, this random intercept was subsequently removed to resolve boundary issues in the Restricted Maximum Likelihood estimation.
P -values were obtained using Type II Wald F tests with Kenward-Roger degrees of freedom. Initial models included all possible interactions but non-significant interaction terms were dropped for ease of interpretation. Fasting values and exercise-related area under curve [AUC; Pre-exercise to 3 h post-exercise 21 ] for all markers were compared with a two-way mixed analysis of variance ANOVAwith post-hoc tests of Student's t -tests for independent samples between-groups and for paired samples within-groups ; where normality was violated, Wilcoxon's test and Mann-Whitney U-test were used.
Where a data point was missing, AUC was not calculated; this resulted in exclusion of 1 participant in the CTX AUC calculations, and 2 participants from both P1NP and OC calculations. Effect sizes were calculated based on the Classical Cohen's d while accounting for the study design by using the square root of the sum of all the variance components specified random effects and residual error in the denominator.
Figure 2. Percentage change in fasting serum C-terminal telopeptide of type I collagen CTXprocollagen 1 N-terminal propeptide P1NP and osteocalcin OC for high carbohydrate HCHO; solid bars and low CHO high fat LCHF; striped bars after the 3. Data are means ± standard deviations.
Figure 3. Time course of changes in bone marker concentrations across exercise left panel and exercise area under curve right panel for serum C-terminal telopeptide of type I collagen CTX A,Dprocollagen 1 N-terminal propeptide P1NP B,Eand osteocalcin OC C,F after the 3.
Squares and circles represent high carbohydrate HCHO and low carbohydrate high fat LCHFrespectively. Figure 4. Time course of changes in bone marker concentrations across exercise left panel and exercise area under curve right panel for serum C-terminal telopeptide of type I collagen CTX A,Dprocollagen 1 N-terminal propeptide P1NP B,Eand osteocalcin OC C,F after acute reintroduction of carbohydrate right panel.
Long-term effects of such alterations remain unknown, but may be detrimental to bone mineral density BMD and bone strength, with major consequences to health and performance.
While ketogenic diets are of interest to athletes due to their ability to induce substantial shifts in substrate metabolism, increasing the contribution of fat-based fuels during exercise 11we have previously reported the downside of a concomitantly greater oxygen cost and reduced performance of sustained high-intensity endurance exercise The LCHF diet is also popular within the general community for its purported health benefits, including rapid weight loss and improved glycemic control However, data from animal studies 1213 demonstrate that chronic LCHF diets are associated with impaired bone growth, reduced bone mineral content, compromised mechanical properties, and slower fracture healing.
Furthermore, increased bone loss has been reported in children with intractable epilepsy placed on a medically supervised LCHF diet for 6 months 14 One explanation for these divergent outcomes involves interactions of the LCHF diet with the level of habitual contractile activity.
Indeed in mice, a LCHF diet negated the positive benefits of exercise on BMD in trabecular bone 16while in children with epilepsy, the rate of bone loss was greater in the more active patients Therefore, the hormonal response to exercise undertaken with low CHO availability was of particular interest in our study.
Previous studies involving acute strategies of low CHO availability around exercise have identified effects on bone resorption, as measured by increased blood CTX concentrations.
However, OC was unchanged by diet and no differences in markers of bone metabolism were detected over the subsequent three days, suggesting that these effects are transient and quickly reversed 7. Short-term effects were also reported when 24 elite male runners with energy-matched intake over an 8 d period were divided into a group who consumed CHO before, during, and immediately after each of their 13 training sessions additional total CHO while the others consumed an artificially sweetened placebo Here, CTX concentrations were suppressed at 80 min of recovery following an interval training sessions in the CHO group with no dietary effects on P1NP or OC; furthermore, fasting concentrations of all markers were similar at baseline and on the ninth morning Finally, Hammond and colleagues 9 investigated the independent effects of low CHO availability and acute energy restriction during the recovery from one session of high-intensity interval running and the completion of a subsequent session 3.
They reported lower CTX concentrations in the high CHO control diet compared with both of the other conditions across the various acute responses to exercise-related feeding, while there were no differences between the energy and CHO restricted trials. Meanwhile, only energy restriction produced an increase in IL-6 responses to exercise, and there were no differences in P1NP concentrations between dietary treatments 9.
Furthermore, 5 d of low vs. To date, the only study to report an effect of acute manipulations of CHO around exercise on bone formation markers was that of Townsend et al. The novelty of the current study was the interrogation of the effects of prolonged adaptation to CHO restriction on bone metabolism.
Unlike the previous investigations, we identified clear and consistent effects on bone metabolism at rest and in response to exercise following 3. Although some might argue that a complete adaptation to a LCHF diet requires much longer than the 3. Nevertheless, the current study is reflective of a shorter-term adaptation to a LCHF diet and our findings warrant further investigation across longer time periods.
Acute restoration of high CHO availability was only partially effective in reversing these outcomes. Here, marker of bone resorption returned to baseline with high CHO pre-exercise meal and CHO ingestion throughout exercise, while the other markers of bone metabolism remained suppressed, indicating impaired overall balance of bone metabolism.
This supports the concept proposed by Hammond et al. Meanwhile, differences in muscle glycogen content, which are not addressed by studies of acute feedings, may have a greater effect on OC and P1NP concentrations.
Given the serious nature of injury risks and long-term outcomes of poor bone health in later life in endurance athletes, further consideration of the potential effects of the LCHF diet in exacerbating existing risk factors for poor bone health is warranted.
In particular, we note that the impairment of bone metabolism around exercise and recovery would involve a significant portion of the day in athletes who undertake multiple training sessions, as well as being superimposed on the changes identified at rest.
The interaction of diet and exercise on bone metabolism is complex and requires more sophisticated investigation including replication of the current findings.
Furthermore, evolving knowledge of inter-organ crosstalk suggests that outcomes of altered bone metabolism may be more far-reaching than the fate of the structural integrity of bone. Indeed, we note the recognition of muscle and bone as endocrine organs, with evidence that IL-6 released from contracting muscle has autocrine, paracrine and endocrine effects This includes a purported feed-forward loop in which contraction-induced stimulation of osteocalcin in myofibers promotes the release of IL-6 and enhances muscle adaptation to exercise Results of the current study challenge this synergistic relationship between osteocalcin signaling and IL-6, and remind us of the pleiotropic nature of the molecules stimulated by diet-exercise interactions.
However, the detection of changes in the IL-6 response to prolonged exercise in our initial study 12 provided motivation to examine possible downstream effects.
Because an identical protocol was undertaken in two separate studies of the LCHF diet, we were able to pool data from these investigations to double the sample size previously known to allow detection of changes in metabolism and performance.
: Ketosis and Bone Health| Keto and Bone Health: Here's What You Need to Know - Keto Science | We also Ketosis and Bone Health that neurocognitive Kstosis Ketosis and Bone Health different anx men and women. Keto anr may harm bone health in athletes. After Baseline testing on a Moderated meal frequency HCHO diet, they eHalth to follow a 3. This prompts the body to enter a state called "ketosis" in which it burns fat for energy instead of glucose, the body's preferred fuel store generated from carbohydrates. Ketosis is a metabolic state in which the body converts stored fat into energy instead of utilizing carbohydrates. Create profiles for personalised advertising. J Appl Physiol. |
| The Keto Diet Could Be Bad for Bone Health, Study Says | All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Vargas S, Romance R, Petro JL, Bonilla DA, Galancho I, Espinar S, et al. Efficacy of ketogenic diet on body composition during resistance training in trained men: a randomized controlled trial. J IntSoc Sports Nutr doi: CrossRef Full Text Google Scholar. Fueling performance: ketones enter the mix. Cell Metab 24 3 —5. PubMed Abstract CrossRef Full Text Google Scholar. Kosinski C, Jornayvaz FR. Efects of ketogenic diets on cardiovascular risk factors: Evidence from animal and human studies. Nutrients 9 5 McArtney R, Bailey A, Champion H. What is a ketogenic diet and how does it afect the use of medicines? Arch Dis Child Educ Pract Ed 4 —9. Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, et al. On the metabolism of exogenous ketones in humans. Front Physiol Barrea L, Caprio M, Camajani E, Verde L, Elce A, Frias-Toral E, et al. Clinical and nutritional management of very-low-calorie ketogenic diet VLCKD in patients with psoriasis and obesity: a practical guide for the nutritionist. Crit Rev Food Sci Nutr , 1— Barrea L, Muscogiuri G, Aprano S, Vetrani C, de Alteriis G, Varcamonti L, et al. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int J Obes Lond. Rondanelli M, Perna S, Ilyas Z, Peroni G, Bazire P, Sajuox I, et al. Effect of very low-calorie ketogenic diet in combination with omega-3 on inflammation, satiety hormones, body composition, and metabolic markers. a pilot study in class I obese subjects. Endocrine 75 1 — Barrea L, de Alteriis G, Muscogiuri G, Vetrani C, Verde L, Camajani E, et al. Impact of a very low-calorie ketogenic diet VLCKD on changes in handgrip strength in women with obesity. Nutrients 14 19 Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr 88 6 — Hawkes CP, Levine MA. Ketotic hypercalcemia: a case series and description of a novel entity. J Clin Endocrinol Metab 99 5 —6. Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr 6 —3. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology MOOSE group. J Am Med Associat. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrewet M, et al. Preferred reporting items for systematic review and meta-analysis protocols prisma-p : Elaboration and explanation. BMJ q Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 14 1. Kim JY. Optimal diet strategies for weight loss and weight loss maintenance. J Obes Metab Syndr 30 1 — Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors: The Cambridge quality checklists. J Exp Criminol. Yu D, Chen W, Zhang J, Wei L, Qin J, Lei M, et al. Effects of weight loss on bone turnover, inflammatory cytokines, and adipokines in Chinese overweight and obese adults. J Endocrinol Invest. Perissiou M, Borkoles E, Kobayashi K, Polman R. The effect of an 8 week prescribed exercise and low-carbohydrate diet on cardiorespiratory fitness, body composition and cardiometabolic risk factors in obese individuals: A randomised controlled trial. randomized controlled trial. Nutrients 12 2 Colica C, Merra G, Gasbarrini A, De Lorenzo A, Cioccoloni G, Gualtieri P. Efficacy and safety of very-low-calorie ketogenic diet: a double blind randomized crossover study. Eur Rev Med Pharmacol Sci 21 9 — PubMed Abstract Google Scholar. Brinkworth GD, Wycherley TP, Noakes M, Buckley JD, Clifton PM. Long-term effects of a very-low-carbohydrate weight-loss diet and an isocaloric low-fat diet on bone health in obese adults. Nutrition 32 9 —6. Foster GD, Wyatt HR, O Hill J, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med 3 — Carter JD, Vasey FB, Valeriano J. The effect of a low-carbohydrate diet on bone turnover. Osteoporos Int 17 9 — Jensen LB, Kollerup G, Quaade F, Sørensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res 16 1 —7. Daniel S, Soleymani T, Garvey WT. A complications-based clinical staging of obesity to guide treatment modality and intensity. Curr Opin Endocrinol Diabetes Obes 20 5 — Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, et al. Cardiovascular endocrinology club of the Italian society of endocrinology. very-low-calorie ketogenic diet VLCKD in the management of metabolic diseases: systematic review and consensus statement from the Italian society of endocrinology SIE. Carnauba RA, Baptistella AB, Paschoal V, Hübscher GH. Diet-induced low-grade metabolic acidosis and clinical outcomes: A review. Nutrients 9 6 Yuan F, Xu M, Li X, Xinlong H, Fang W, Dong J. The roles of acidosis in osteoclast biology. Saito A, Yoshimura K, Miyamoto Y, Kaneko K, Chikazu D, Yamamoto M, et al. Enhanced and suppressed mineralization by acetoacetate and β-hydroxybutyrate in osteoblast cultures. Biochem Biophys Res Commun 2 — Merlotti D, Cosso R, Eller-Vainicher C, Vescini F, Chiodini I, Gennari L, et al. Energy metabolism and ketogenic diets: What about the skeletal health? a narrative review and a prospective vision for planning clinical trials on this issue. Int J Mol Sci 22 1 Buscemi S, Buscemi C, Corleo D, De Pergola G, Caldarella R, Meli F, et al. Obesity and circulating levels of vitamin d before and after weight loss induced by a very low-calorie ketogenic diet. Nutrients 13 6 Van Loan MD, Johnson HL, Barbieri TF. Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr 67 4 —8. Hahn TJ, Halstead LR, De Vivo DC. Disordered mineral metabolism produced by ketogenic diet therapy. Calcif Tissue Int 28 1 — Fuleihan GE, Dib L, Yamout B, Sawaya R, Mikati MA. Predictors of bone density in ambulatory patients on antiepileptic drugs. Bone 43 1 — Wu X, Huang Z, Wang X, Fu Z, Liu J, Huang Z, et al. Ketogenic diet compromises both cancellous and cortical bone mass in mice. Calcif Tissue Int 4 — Ding J, Xu X, Wu X, Huang Z, Kong G, Liu J, et al. Bone loss and biomechanical reduction of appendicular and axial bones under ketogenic diet in rats. Exp Ther Med 17 4 — Liu Q, Xu X, Yang Z, Liu Y, Wu X, Huang Z, et al. Metformin alleviates the bone loss induced by ketogenic diet: An In vivo study in mice. Calcif Tissue Int 1 — Keywords: ketogenic diet, low-calorie ketogenic diet, very-low-calorie ketogenic diet, bone health, osteoporosis, bone mineral density. Citation: Garofalo V, Barbagallo F, Cannarella R, Calogero AE, La Vignera S and Condorelli RA Effects of the ketogenic diet on bone health: A systematic review. Received: 12 September ; Accepted: 12 January ; Published: 02 February Copyright © Garofalo, Barbagallo, Cannarella, Calogero, La Vignera and Condorelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation. This, in part, is due to the interaction of dietary factors e. Low energy availability a mismatch between energy intake and the energy cost of exercise occurs in both female and male athletes 2 and impairs bone health via direct uncoupled bone turnover with increased resorption rates and indirect mediation by reproductive and metabolic hormones mechanisms 1. In addition, carbohydrate CHO availability may also play a role in bone health. Indeed, results from several studies show that commencing endurance exercise with low compared to normal or high glycogen availability stimulates the release of the cytokine interleukin-6 IL-6 from the exercising muscles 3 , 4. Among its range of effects, IL-6 has been hypothesized to lead to enhanced activity of the receptor activator of the nuclear factor K B-ligand, which controls bone turnover by increasing osteoclastic activity thereby increasing bone breakdown 5. In support of this contention, bone resorption is acutely increased when CHO is restricted before 6 , during 7 , and after 8 prolonged 1—2 h endurance running exercise, and may be linked to concomitant increases in IL-6 concentrations 7. However, a recent study has reported that acute reductions in CHO availability around exercise mediated an increase in markers of bone resorption that are independent of energy availability and circulating IL-6 9. Apparent effects on other markers of bone metabolism, such as osteocalcin OC and the bone formation marker procollagen 1 N-terminal propeptide P1NP in these models have been small 6 — 9 , although a 24 h fast has been reported to reduce blood OC concentrations in lightweight rowers Whether these changes in markers of bone metabolism persist or are amplified after chronic exposure to low CHO availability around exercise remains unknown, but is of relevance in view of the promotion of a ketogenic low CHO-high fat LCHF diet to athletes and its putative benefits for endurance performance To date, no studies have examined the effects of longer-term restriction of CHO at rest or in relation to exercise, although in animal models and children with intractable epilepsy, chronic adaptation to a ketogenic LCHF diet is associated with poor bone health 12 — In view of our recent observations of increased post-exercise IL-6 concentrations in elite race walkers following a 3. Thirty world-class athletes 25 male, 5 female race walkers; ages Six male participants undertook two camps, however two of these data sets were incomplete due to insufficient tissue samples, resulting in 4 participants who had completed two camps being included in the final analysis. In addition, two additional male data sets were excluded from the final analysis due to their inability to complete one of the experimental trials due to injury unrelated to bone. Participants and elite coaches contributed to the concept and implementation of the research camps, helping to prioritize the themes of interest and contributing to the design of the training program and test protocols. Participants completed a 3. Upon completion of the 3. Markers of bone metabolism were measured after an overnight fast, in response to an energy-matched meal of nutrient composition matching the intervention diet, and in response to a bout of strenuous exercise 19 , at Baseline, Adaptation, and Restoration Figure 1. Figure 1. Study flowchart and overview. Thirty-two data sets were gathered from 30 participants who participated in one or more training camps. After Baseline testing on a carbohydrate-rich HCHO diet, they elected to follow a 3. After Adaptation, the participants underwent an acute period of Restoration of high carbohydrate availability. Venous blood samples were collected after an overnight fast, 2 h after an energy-matched breakfast based on their diet immediately pre-exercise , immediately post exercise and after 3 h of passive recovery during which an intervention-matched recovery shake was consumed at 30 min. Blood samples were analyzed for serum concentrations of C-terminal telopeptide of type I collagen CTX , procollagen 1 N-terminal propeptide P1NP , and osteocalcin OC. Details of dietary control are described briefly here; more details are described in prior work Participants were allocated into HCHO and LCHF groups based on preference. Both diets were isocaloric Table 1 , however dietary CHO and fat intakes differed between groups during intervention. Study diets were designed and individualized for each athlete by trained members of the research team including registered sports dietitians, a professional chef, and exercise physiologists. All meals were weighed food scales accurate to 2 g and provided for athletes at set meal times. In addition, a collection of snacks per individual meal plans were provided to the athletes each day. Any unconsumed items or changes made to menu plans were weighed and recorded for final analysis of dietary intakes. Compliance to the meal plans was assessed daily. Meal plans were designed and final dietary analysis of actual intakes was conducted using FoodWorks 8 Professional Program Xyris Software Australia Pty Ltd, Australia. Further analysis of intakes was completed using Microsoft Excel. Upon entering the laboratory in an overnight fasted and rested state between and in the morning times were kept consistent within-participant , a cannula was inserted into an antecubital vein for collection of blood samples at rest Fasting , immediately before exercise 2 h post-meal , immediately after exercise Post-ex and 3 h post-exercise 3 h post-ex. Blood was analyzed for concentrations of cross-linked C-terminal telopeptide of type I collagen CTX , P1NP and total OC to determine the effects of dietary interventions and exercise on bone metabolism. The cannulas were flushed with 3 ml of saline every 30 min throughout the trials. Upon completion of the exercise test, the participants rested in the laboratory for a further 3 h, and received a standardized recovery shake 1. Blood samples were collected into a 3. Analysis was undertaken by chemiluminescence on IDS-iSYS Immunodiagnostic Systems Limited; Boldon, Tyne and Wear, UK. Inter-assay coefficient of variation as reported by the manufacturer was 6. CVs were determined as follows: OC: 6 serum controls were run, using 3 reagents lots, in duplicate twice per day for 20 days, on 2 analyzers; P1NP: 3 serum controls were run, using 3 reagent lots, in quadruplicates once per day for 20 days, on 2 analyzers; CTX: 5 serum controls were run, using 3 reagent lots, in duplicate twice per day for 20 days, on 3 analyzers. In addition to these tests, the laboratory ran quality control samples throughout testing and the results were within the established acceptable manufacturer ranges. Normality of data was checked with a Shapiro-Wilk test and visual inspection of residual plots. General Linear Mixed models were fitted using the R package lme4 20 and included random intercepts for Subjects and Camps to account for baseline inter individual heterogeneity and the partial cross-over design. Because the estimated Camp effect variance was 0, this random intercept was subsequently removed to resolve boundary issues in the Restricted Maximum Likelihood estimation. P -values were obtained using Type II Wald F tests with Kenward-Roger degrees of freedom. Initial models included all possible interactions but non-significant interaction terms were dropped for ease of interpretation. Fasting values and exercise-related area under curve [AUC; Pre-exercise to 3 h post-exercise 21 ] for all markers were compared with a two-way mixed analysis of variance ANOVA , with post-hoc tests of Student's t -tests for independent samples between-groups and for paired samples within-groups ; where normality was violated, Wilcoxon's test and Mann-Whitney U-test were used. Where a data point was missing, AUC was not calculated; this resulted in exclusion of 1 participant in the CTX AUC calculations, and 2 participants from both P1NP and OC calculations. Effect sizes were calculated based on the Classical Cohen's d while accounting for the study design by using the square root of the sum of all the variance components specified random effects and residual error in the denominator. Figure 2. Percentage change in fasting serum C-terminal telopeptide of type I collagen CTX , procollagen 1 N-terminal propeptide P1NP and osteocalcin OC for high carbohydrate HCHO; solid bars and low CHO high fat LCHF; striped bars after the 3. Data are means ± standard deviations. Figure 3. Time course of changes in bone marker concentrations across exercise left panel and exercise area under curve right panel for serum C-terminal telopeptide of type I collagen CTX A,D , procollagen 1 N-terminal propeptide P1NP B,E , and osteocalcin OC C,F after the 3. Squares and circles represent high carbohydrate HCHO and low carbohydrate high fat LCHF , respectively. Figure 4. Time course of changes in bone marker concentrations across exercise left panel and exercise area under curve right panel for serum C-terminal telopeptide of type I collagen CTX A,D , procollagen 1 N-terminal propeptide P1NP B,E , and osteocalcin OC C,F after acute reintroduction of carbohydrate right panel. Long-term effects of such alterations remain unknown, but may be detrimental to bone mineral density BMD and bone strength, with major consequences to health and performance. While ketogenic diets are of interest to athletes due to their ability to induce substantial shifts in substrate metabolism, increasing the contribution of fat-based fuels during exercise 11 , we have previously reported the downside of a concomitantly greater oxygen cost and reduced performance of sustained high-intensity endurance exercise The LCHF diet is also popular within the general community for its purported health benefits, including rapid weight loss and improved glycemic control However, data from animal studies 12 , 13 demonstrate that chronic LCHF diets are associated with impaired bone growth, reduced bone mineral content, compromised mechanical properties, and slower fracture healing. Furthermore, increased bone loss has been reported in children with intractable epilepsy placed on a medically supervised LCHF diet for 6 months 14 , One explanation for these divergent outcomes involves interactions of the LCHF diet with the level of habitual contractile activity. Indeed in mice, a LCHF diet negated the positive benefits of exercise on BMD in trabecular bone 16 , while in children with epilepsy, the rate of bone loss was greater in the more active patients Therefore, the hormonal response to exercise undertaken with low CHO availability was of particular interest in our study. Previous studies involving acute strategies of low CHO availability around exercise have identified effects on bone resorption, as measured by increased blood CTX concentrations. However, OC was unchanged by diet and no differences in markers of bone metabolism were detected over the subsequent three days, suggesting that these effects are transient and quickly reversed 7. Short-term effects were also reported when 24 elite male runners with energy-matched intake over an 8 d period were divided into a group who consumed CHO before, during, and immediately after each of their 13 training sessions additional total CHO while the others consumed an artificially sweetened placebo Here, CTX concentrations were suppressed at 80 min of recovery following an interval training sessions in the CHO group with no dietary effects on P1NP or OC; furthermore, fasting concentrations of all markers were similar at baseline and on the ninth morning Finally, Hammond and colleagues 9 investigated the independent effects of low CHO availability and acute energy restriction during the recovery from one session of high-intensity interval running and the completion of a subsequent session 3. They reported lower CTX concentrations in the high CHO control diet compared with both of the other conditions across the various acute responses to exercise-related feeding, while there were no differences between the energy and CHO restricted trials. Meanwhile, only energy restriction produced an increase in IL-6 responses to exercise, and there were no differences in P1NP concentrations between dietary treatments 9. Furthermore, 5 d of low vs. To date, the only study to report an effect of acute manipulations of CHO around exercise on bone formation markers was that of Townsend et al. The novelty of the current study was the interrogation of the effects of prolonged adaptation to CHO restriction on bone metabolism. Unlike the previous investigations, we identified clear and consistent effects on bone metabolism at rest and in response to exercise following 3. Although some might argue that a complete adaptation to a LCHF diet requires much longer than the 3. Nevertheless, the current study is reflective of a shorter-term adaptation to a LCHF diet and our findings warrant further investigation across longer time periods. Acute restoration of high CHO availability was only partially effective in reversing these outcomes. Here, marker of bone resorption returned to baseline with high CHO pre-exercise meal and CHO ingestion throughout exercise, while the other markers of bone metabolism remained suppressed, indicating impaired overall balance of bone metabolism. This supports the concept proposed by Hammond et al. Meanwhile, differences in muscle glycogen content, which are not addressed by studies of acute feedings, may have a greater effect on OC and P1NP concentrations. Given the serious nature of injury risks and long-term outcomes of poor bone health in later life in endurance athletes, further consideration of the potential effects of the LCHF diet in exacerbating existing risk factors for poor bone health is warranted. In particular, we note that the impairment of bone metabolism around exercise and recovery would involve a significant portion of the day in athletes who undertake multiple training sessions, as well as being superimposed on the changes identified at rest. The interaction of diet and exercise on bone metabolism is complex and requires more sophisticated investigation including replication of the current findings. Furthermore, evolving knowledge of inter-organ crosstalk suggests that outcomes of altered bone metabolism may be more far-reaching than the fate of the structural integrity of bone. Indeed, we note the recognition of muscle and bone as endocrine organs, with evidence that IL-6 released from contracting muscle has autocrine, paracrine and endocrine effects This includes a purported feed-forward loop in which contraction-induced stimulation of osteocalcin in myofibers promotes the release of IL-6 and enhances muscle adaptation to exercise Results of the current study challenge this synergistic relationship between osteocalcin signaling and IL-6, and remind us of the pleiotropic nature of the molecules stimulated by diet-exercise interactions. However, the detection of changes in the IL-6 response to prolonged exercise in our initial study 12 provided motivation to examine possible downstream effects. Because an identical protocol was undertaken in two separate studies of the LCHF diet, we were able to pool data from these investigations to double the sample size previously known to allow detection of changes in metabolism and performance. Indeed, changes in markers of bone metabolism in the response to the interaction of exercise and the dietary treatments were clearly detected with the pooled data, but were also identifiable in the case of the smaller sample size of the carbohydrate restoration arm of the current dataset. Therefore, we feel confident that our data are robust and warrant further investigation of this theme. Despite recent interest in the potential benefits of LCHF diets on endurance performance or metabolic adaptation, the long-term health effects of this dietary intervention are largely unknown. We are the first to show that a 3. We also show only partial recovery of these adaptations with acute restoration of CHO availability. Given the injury risks and long-term outcomes underpinned by poor bone health in later life, in athletes as well as individuals who undertake exercise for health benefits, additional investigations of the ketogenic diet and its role in perturbing bone metabolism are warranted. The studies involving human participants were reviewed and approved by Australian Institute of Sport Ethics Committee. Conception and design of the experiments was undertaken by IH, LB, MR, LG-L, AS, AM, JL, MW, LM, and KA. Collection, assembly, analysis, and interpretation of data was undertaken by IH, LB, MR, LG-L, AS, AM, JL, MW, LM, and KA. Manuscript was prepared by IH, LB, KA, and JH. All authors approved the final version of the manuscript. IH and LB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was funded by a Program Grant from the Australian Catholic University Research Funds to Professor LB ACURF, The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We thank our research colleagues and supporters of the Supernova research series and acknowledge the commitment of the elite race-walking community. Supplementary Table 1. Mountjoy M, Sundgot-Borgen J, Burke L, Ackerman KE, Blauwet C, Constantini N, et al. International Olympic committee IOC consensus statement on relative energy deficiency in sport RED-S : update. |
| Could a Keto Diet Be Bad for Athletes’ Bones? | Furthermore, 5 d of low vs. Ding J, Xu X, Wu X, Huang Z, Kong G, Liu J, et al. When you only consume fat, your muscles adapt to using it as a fuel. In addition, no changes were observed regarding BMD. Low energy availability a mismatch between energy intake and the energy cost of exercise occurs in both female and male athletes 2 and impairs bone health via direct uncoupled bone turnover with increased resorption rates and indirect mediation by reproductive and metabolic hormones mechanisms 1. Barbara PEARCE. A small new study has found that the ketogenic diet could worsen the health of your bones. |

Manchmal geschehen die Sachen und schlechter
Im Vertrauen gesagt.
Bei allen persönlich begeben sich heute?
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden reden.
Diese Mitteilung unvergleichlich, ist))), mir gefällt:)