Hanaway is the past president of the Fastting Board yealth Integrative Holistic Medicine, served as the chief Fating officer at Genova Diagnostics, and is the former medical and research director Natural remedies for body detox the Center for Functional Medicine gkt the Healtu Clinic.
Fasting and gut microbiome health over Muscle recovery for gymnasts years, he has worked with his Natural remedies for body detox at Fat loss diet plan clinical practice, Family to Family: Your Home for Whole Health Care, qnd North Carolina.
Hanaway is a functional medicine Fassting who microbkome his helath skillset to micrlbiome medical practice through education, vut, and clinical care. Kalea Microibome, ND: A therapeutic fasting Red pepper tart may be wnd appropriate component Lycopene and weight loss a personalized treatment strategy and may improve health across micrrobiome range of areas.
From mental and cognitive performance to cardiometabolic health to the effectiveness of heakth treatments. The potential benefits of healtth likely Circadian rhythm exercise through multiple pathways, such healgh reducing oxidative stress, enhancing mitochondrial health, anc triggering autophagy.
Now, even more mjcrobiome indicate that fasting microbiime impacts the gut microbiome. In Fasting and gut microbiome health episode of Pathways to Well-BeingIFM educator and Athlete diet medicine expert Fastingg. Patrick Microbipme joins us gutt discuss the fasting-gut connection and aand explore how Metabolic health plan to Natural remedies for body detox gut microbiome may translate healgh improved patient outcomes.
Welcome to the show, Dr. So, when all this research mircobiome to pour out about the benefit of fasting on the gut microbiome, did you feel like the stars were aligning and your clinical loves were coming together?
Electrolytes and pH balance research is showing that therapeutic fasting might benefit multiple body systems. So in functional medicine, we would translate that to mkcrobiome functional medicine bealth. In general, will you tell Fastting, what are some of the areas that have shown positive associations with fasting treatments?
How is the matrix affected? And through ans mechanisms, we see ahd altering CNS function. Heath in addition to those things, you know, we micobiome see microboome effect of fasting on the immune system guh decreasing inflammatory biomarkers.
Probably some of the most important effects are heakth through imcrobiome mitochondria and the whole energy healtj system, which, of course, is going to affect, you Healthy eating habits, things not only like fatigue ugt brain fog and central nervous anf issues.
Because the brain is the vut user of energy in the body, you know, ght we also can micribiome that a microbiomee diurnal variation is necessary to be able healtg deal with environmental sensing and Fasitng as well. And then, healgh of course, within the nicrobiome zone, you know, we do see changes in the structure of mkcrobiome gut anr itself from fasting.
Kalea Fasting and gut microbiome health Absolutely. Anx Hanaway: Thyroid Nourishing Herbs think one of the areas first is when micdobiome begin to look Fastimg, well, what happens with fasting in and of znd And halth we have fasting mechanisms of intermittent fasting or Natural remedies for body detox eating, microbiime know, that has come forward that is probably very similar to mocrobiome our ancestors for hundreds of thousands microbipme years experienced.
And so they moved in a way Green tea for brain health there were circadian rhythms that developed. Mlcrobiome those circadian rhythms, actually within the gut, they get mediated through or kind of, maybe Cancer-fighting vitamins could say governed by or conducted Enhancing concentration skills a part of the brain called the suprachiasmatic nucleus, SCN.
And so food micobiome actually one of the Fastng that sets forth the circadian Metabolism boosting recipes that goes FFasting. And in haelth process, you Healthy eating habits, we see changes in the gut microbome that occur.
We can talk more about that both in microbime of the structural Natural remedies for body detox and helth functions guf are going hsalth. And Healthy eating habits halth do see changes, as I said earlier, in insulin tut and insulin resistance. Microbikme unlike, Fastkng know, what miceobiome see with sleep patterns, you guf, where the deep sleep that happens micrkbiome in the sleep pattern is crucial, you know?
And so that to me says, there may be something to really be able to create a foundational difference in following the sun. And again, with the light and dark cycles that are involved with retinoic acid receptors and things like that as well as light receptors, it makes sense.
Kalea Wattles: Yeah, that just makes sense. That makes sense to me too, especially all of our traditional Chinese medicine and Ayurvedic colleagues who have been telling us to follow those circadian patterns.
That just makes a lot of intuitive sense. Now, Patrick, I feel like we have to address autophagy in a conversation about fasting. Maybe that term is new to some listeners. Will you just give us a brief overview of what the term autophagy means? And so we see that with time-restricted eating.
And so I find these things to be, you know, fascinating aspects of the same conversation about looking at the role of food and fasting and autophagy. Kalea Wattles: Very approachable explanation to a somewhat complicated subject.
I had a patient once who was doing some fasting and she was telling me she was combining this with some visualization exercises in which she pictured little scrubbers inside her cells doing their house cleaning.
Not just right away after you finish dinner at pm and are going to eat next again at am the following morning or am the following morning. You know, and it also begs a question of, what is it? I think that the data shows that, you know, 16 hours of fasting is significantly better than 12 hours, of have patients start at 12 hours and then move to 14, and then move to the But that two days off of eating ends up really being more like a 32 to 36 hour fast, which actually optimizes the autophagy.
Kalea Wattles: Right. Can we impact intestinal inflammation with therapeutic fasting? And of course, we see this in our, you know, autoimmune patients. And we do see changes in the ecosystem of the gut microbiome happening from time-restricted eating. Those can be changes that will lead to increases in Ruminococcus and butyrate producers.
So we are seeing that time-restricted eating will change transcription factors, it will change the overall composition and diversity of the gut microbiome, all of which are factors that help to decrease the overall inflammatory process.
And then again, like I said, and we see that manifesting in a study on inflammatory bowel disease and we see that manifesting in a study looking overall at cytokine production. Kalea Wattles: Patrick, do you have a sense of the timeline here?
How long until we might see a shift in their gut microbiome? So probably now almost 30 years. But you know, one other thing to note is that when you stop intermittent fasting, the changes go away. The diversity actually happens first and then the abundance happens over time.
Kalea Wattles: I imagine with this shifting in the microbial composition that we also might notice some changes to metabolites like short-chain fatty acids. Patrick Hanaway: So again, the literature is very early at this point in time.
What was your diet before you started? What gender are you, what type of eating pattern are you actually doing? Is it alternate-day feeding? Is it a time-restricted eating of 12, 14, 16 hours? Is it a process? Is it Buchinger or is it Ramadan? And one of the interesting things to me is they get different results, you know, based upon not only gender but also upon body types.
So the effect on people who are overweight and obese is different than people who are not overweight in terms of the metabolite changes that we see going on.
We do see some changes that relate to the short-chain fatty acids to butyrate itself. We see some other changes.
What I think is going on, Kalea, is in relationship to the postbiotic effect. And we see some interesting data in C. But now when we talk about this, recall that, you know, we may say, oh short-chain fatty acids, of which there are three primary ones, you know, butyrate, acetate, and propionate.
You know, there are short-chain fatty acids that come from insufficient digestion of proteins that can cause that are more fermentation byproducts, you know, but so even naming just those six various short-chain amino acids are six of more than a thousand different metabolites that are produced by the gut microbiome.
Do you have any thoughts on that? Patrick Hanaway: Yeah, right. As significant of metabolic changes or even that kind of reset. And so that leads us into this conversation about gut endotoxins. Do we see an effect on endotoxins with fasting?
And then we can talk about how that influences intestinal permeability. And that we do see that the production of LPS is decreased when there is time-restricted eating patterns and intermittent fasting that goes on. So we can describe that.
And this leads me into an important point that I wanted to focus on, and that is that while, you know, we can look at research that says the new family called Christensenellaceae and the genus of Christensenellaand the species of Christensenella minutayou know, have, have been associated with centenarians.
We want to approach this through, you know, whole food dietary approaches with reasonable amounts of food at good timing, using time-restricted eating and intermittent fasting as a means of being able to reset the circadian rhythm of light and dark that our bodies have been using for the past, you know,years.
I mean, are you seeing this in the literature? Is there a gut-brain axis response to fasting? Patrick Hanaway: Absolutely. And so we see two different phenomena that are going on. And so the LPS-mediated neurodegeneration that you talked about may be manifesting itself primarily through alterations in the blood-brain barrier that lead to glial cell activation and inflammatory processes that are occurring in subsequent neurodegeneration.
But we also find that there are, as I said, metabolic patterns that go on. And we see shifts that are going on in the overall distribution of the gut metabolites that are occurring that are having an effect on the anterior insular nucleus that then has an effect on the basal ganglia and the ability of movement or the effect on movement disorders.
We see alterations in tryptophan pathways and kynurenic acid pathways that are stimulating inflammatory changes and degenerative changes in the brain. Do we see impacts to our immune health when we start a therapeutic fasting plan? And so we need to have a balance between all three of those that are going on.
That, to me, forms the basis of health is working on the gut microbiome, supporting the immune system, using, you know, foods that are anti-inflammatory in nature. And that helps to balance the energy production pathways, you know, as well as the overall electron transport chain within the mitochondria to be able to optimize its efficiency for energy production that goes on.
So I like to think of all three of those together. And some of that may be mediated through that apoptosis that we spoke about earlier. Kalea Wattles: There are so many clinical applications for fasting, and you mentioned earlier that you might be willing to share a little bit about this connection between fasting and the gut microbiome changes and benefits to cancer treatment.
: Fasting and gut microbiome health| The Relationship Between Fasting and Gut Health | Microbiomes of BP responders were depleted pre-intervention for Desulfovibrionaceae, previously shown to be enriched in type 2 diabetic patients in a Chinese cohort 17 , and were moreover depleted of propionate biosynthesis genes Fig. Fasting strongly elevated the abundance of this taxa and enriched these propionate production modules, indicating that responders suffer a treatable deficit. By 3 months post-intervention, propionate modules are almost back at baseline while BP relative to medication dosage remains improved, suggesting that their transient elevation during refeeding may have stabilized a less hypertensive state through mechanisms active beyond the gut Fig. An opposing pattern was shown by a poorly characterized Lachnospira sp. a Circles denote features differing at baseline in responders vs. non-responders and altered during intervention in responders. b Comparison of results from the present study MetS; all samples and BP responders only shown as orange and red tags, respectively, separately with those of a recent similar fasting intervention in healthy men Mesnage; blue tags. Effect sizes at the species or OTU level were averaged at the genus level for clarity, and are shown in the plot direction rendered as marker shape and hue; scope rendered as marker size and intensity for all genera where at least one constituent taxon achieved significance either in the Mesnage or MetS study these are shown in boldface. Columns denote phases of each intervention - fasting phase, refeeding, and follow-up vs. Substantial agreement between the two studies is seen, which is typically stronger for the subset of BP responders. c Prediction model weights for BP response using the MetS 16S dataset at baseline. The top five immunome features were used to build a multivariate logistic-regression algorithm. Single-subject prediction on the Mesnage dataset 22 was quantified using a leave-one-out cross-validation procedure. The question was raised whether independent data could confirm these findings. Despite substantial differences between the two study settings e. MetS vs. healthy, mixed vs. Though differences can also be observed in the patterns of Oscillibacter and Alistipes in these two studies. The SCFA producer Faecalibacterium showed discordant fasting responses in the healthy vs. MetS cohort but exhibited consistent growth upon refeeding in both datasets Fig. Due to the similarity of the study designs, we next assessed whether a decrease in BP in the Mesnage cohort could be predicted by a model trained on our 16S dataset. We classified the Mesnage patients according to their BP decrease 3 months post-fasting Supplementary Data A stepwise selection model was built on our 16S baseline data, filtered for significant responder-specific taxa. The model was then evaluated, using the corresponding features from the Mesnage dataset as input. The model classified correctly 10 out of 15 subjects in the Mesnage cohort as either BP responders or non-responders. Top five contributors to the predictor highlighted gut microbiomes of non-responders to be enriched and responders to be depleted of the taxa Desulfovibrionaceae, Hydrogenoanaerobacterium, Akkermansia , Ruminococcaceae GCA and Hydrogenoanaerobacterium sp. Here we demonstrate that fasting induces changes to the gut microbiome and immune homeostasis with a sustained beneficial effect on body weight and BP in hypertensive MetS patients. There is a growing interest in understanding how dietary interventions shape the gut microbiome and interact with metabolic diseases, including obesity, MetS, type 2 diabetes, and cardiovascular health 8 , 9 , 10 , 23 , 24 , 25 , 26 , Several lifestyle interventions aimed at weight loss have shown that the gut microbiome changes in obese, type 2 diabetic or MetS patients 10 , 23 , 24 , 26 , Although these interventions led to beneficial clinical outcomes, their effect on the gut microbiome was highly variable 10 , 23 , 24 , 26 , 27 more information in Supplementary Data In mice, intermittent fasting decreased obesity-induced cognitive impairment and insulin resistance associated with increased abundance of the Lactobacillus and the butyrate-producer Odoribacter In a small human pilot study, Ramadan fasting 9 affected the microbiome of healthy subjects enriching several SCFA producers. Each of the aforementioned studies are described in greater detail in Supplementary Data We have carried out the first high-resolution multi-omics characterization of periodic fasting in patients with MetS, including detailed clinical and immunophenotyping along with gut microbiome sequencing. Our major finding is that periodic fasting followed by 3 months of a modified DASH diet induces concerted and distinct microbiome and immunome changes that are specific to fasting itself, leading to a sustained BP benefit Fig. Fasting followed by modified DASH also led to a significant long-term reduction in body weight. Furthermore, BP and BMI were both associated with various immune cell subsets and microbial taxa on a multivariate level, and the effects of fasting on these two features are divergent shown as chord plots on Fig. Nevertheless, the data indicate that a 5-day fast exerted an effect on microbiome composition and immune cell subsets. Even though many of these shifts post-fasting are transient, a sustained improvement of BP was seen in our patients. Comparison of V1 to V2 suggests that microbiome and immune cells may reset to some extent during and after the intense caloric restriction, similar to a preconditioning mechanism. The subsequent DASH diet consistent across all patients thus seem to act differently depending on whether this preconditioning took place or not. This interpretation is supported by the fact that the DASH diet alone neither reduced SBP nor BMI, while affecting different and substantially fewer immune cell subsets. Our interpretation is that one crucial mechanism for the improvement stems from the effects of increased SCFA availability, either locally in the intestine impacting immune signaling and intestinal permeability , systemically, or both. While we cannot directly test it in the present cohort, it is a scenario consistent both with expectations from the literature and with our observations of a consistent depletion-then-regrowth pattern. Fasting induced a profound change in circulating immune populations; e. depleted Th1 cells and permanently enriched dendritic cells, which both have been shown previously to play a role in the pathogenesis of experimental hypertension 28 , A growing body of evidence suggests that the abundance of certain microbes is associated with cardiovascular health. Previous reports on hypertensive patients have shown taxonomic and functional gut microbiome shifts 6 , 7. For example, Firmicutes have been shown to be more abundant in healthy controls compared to pre-hypertensive and hypertensive patients 7. Upon fasting, several Clostridial Firmicutes shifted significantly in abundance, with an initial decrease in butyrate producers such as F. prausnitzii , E. rectale and C. comes , which were reverted after 3 months upon refeeding; with the latter taxon likely being an indirect effect of the observed weight reduction Supplementary Data 5. Further, functional microbial metabolism in fasting patients at baseline share some similarities to the previously profiled hypertensive microbiome 7. In the fasting arm, the functional shift during refeeding enriches for functional modules also enriched in non-hypertensive controls, i. for potentially BP-protective factors. Clinical studies represent a highly heterogeneous situation with multifactorial disease features and strongly variable microbial and lived environments. To account for this heterogeneity, we compared the data from our longitudinal study post-fasting and 3-month to the respective baseline values of the study subjects. This intraindividual analysis allowed us to identify BP responder-specific changes in spite of the reduced power in such a sub-stratified analysis. The responder-specific microbiome changes in our fasting arm post-intervention enrichment of F. prausnitzii , Bacteroides and Firmicutes, depletion of Actinomyces are likely beneficial to the host. A recent study profiling the hypertensive microbiome showed that during disease, patients experienced an enrichment of Actinomyces, and a depletion of F. prausnitzii , Bacteroides and Firmicutes 7. Moreover, Guevara-Cruz et al. parusnitzii 27 Supplementary Data Furthermore, abundance of some functional gut-specific gene modules was significantly altered in our dataset only in BP responders, for example, the pyruvate:formate lyase module, MF, which was decreased after refeeding. This decrease from a trending elevation at baseline may contribute to vascular health, as a recent study demonstrated enrichment of the same enzyme in atherosclerosis patients relative to healthy controls 30 , and formate production has been previously linked to BP regulation 31 , Stratification of the cohort to BP responsiveness showed that also immune changes present in the fasting arm are more pronounced in responders than in non-responders, and are fundamentally different from the changes observed in the DASH-only arm. Responders and non-responders not only reacted differentially to fasting, but also differed at baseline with regards to their propionate synthesis capacity pre-intervention and the relative depletion by depletion of Desulfovibrionaceae, which has been linked to a lean phenotype 34 , These features were then normalized during fasting. Notably, recent experimental work suggested an antihypertensive effect of propionate treatment in mice Furthermore, responders were enriched in Lachnospira sp. at baseline, which was shown to contribute to diabetes in obese mice and is enriched in obese children 37 , Our findings indicate responders and non-responders to our intervention differ with regards to several gut microbiome features relevant to hypertension, with fasting-induced normalization of these differences seen during a successful fasting intervention. They differ in many aspects from conventional T cells by expressing a semi-invariant TCR α-chain Vα7. During aging 18 and CMD 19 , 39 , absolute circulating MAIT number and frequencies decrease, while certain subsets of cytokine-producing and adipose tissue MAITs were found to be enriched in obese type 2 diabetic patients Of note, most of these microbes are relatively poorly characterized taxa and further description is needed to elucidate their role in the gut and as contributors of dys- or eubiosis. Using machine learning, we were able to utilize deep immunophenotyping data to predict at baseline, which subjects were likely to decrease their BP during fasting despite the small number of subjects. In addition, the accuracy of the prediction was enhanced further taking the dynamics of immune populations along the course of the study into account. No corresponding prediction of a favorable response to a DASH-only intervention was possible. The features informing the predictor indicate BP responders and non-responders present with differing severities of a pro-inflammatory immune signature at baseline, raising the question whether responders and non-responders suffer from varying degrees of MetS severity at baseline. Remarkably, no significant difference in baseline BP, BMI, lipid levels, or glucose homeostasis parameters between BP responders and non-responders was observed before the intervention Supplementary Data 6. Additionally, responders had lower median BMI than non-responders; These data indicate that although BP responders and non-responders do demonstrate slightly different trends in some clinical parameters, BP responders do not show any less severe disease phenotype. Through the reanalysis of the Mesnage dataset, the only fasting cohort in the literature with a similar study design and which includes both BP and microbiome data, we were able to demonstrate concordant treatment-related microbiome shifts in both studies. This finding suggests the effects of fasting and refeeding on gut microbiota generalizable. A machine-learning model built from microbiome features differentially abundant at baseline in BP responders in our cohort was able to predict significant long-term BP decrease in the Mesnage et al. Previous works have also shown that some outcomes of dietary interventions in cardiovascular patients might be related to baseline microbiome features. In addition, Velikonja et al. showed in a study investigating the effect of beta-glucan supplementation in MetS patients that a higher baseline abundance of Akkermansia muciniphila and Bifidobacter spp. was characteristic of patients whose cholesterol decreased due to the intervention 23 Supplementary Data Thus, we demonstrate the practical utility of a machine-learning analysis pipeline for predicting BP benefit of fasting in MetS patients with hypertension using both baseline immunome and microbiome data. It is important to recognize that our study represents patients with hypertension and MetS solely from a Caucasian-European background. This selection criterion introduces a selection bias in our study design. Additional research is necessary to elucidate whether the results presented here could be applicable in a more heterogeneous patient population. Since the participants were especially interested in the fasting procedure, the allocated DASH participants were offered a cost-free fasting cycle after successful completion of the study. However, we cannot exclude that this led to an increased long-term motivation compared to the participants who started with the fasting protocol. Furthermore, the study design did not allow us to investigate the long-term effects of a fasting intervention without a subsequent DASH diet on the BP, microbiome, or immunome. In our cohort, fasting was required on top of DASH to achieve the observed outcomes, but we cannot conclude and do not expect fasting without a subsequent dietary change to do so either. We can only claim fasting was required prior to the DASH diet to achieve the effects observed in our cohort. However, some effects are replicated in the similar dataset from healthy males without MetS and without DASH intervention in the Mesnage dataset 22 , thus indicating the precise DASH setup may not be strictly needed. Most likely, the two components of the intervention synergize—fasting may potentiate the microbiome in these patients to be shifted to a more DASH-compatible microbiota upon diet change. While we identify changes in microbial taxonomic and functional features, bacterial metabolites and immune processes, which could explain the efficacy of the intervention, robust conclusions of causality will require follow-up experimental work, particularly in animal models e. gnotobiotic mice colonized with bacteria strongly associated with BP. In addition, the relatively low patient number could be regarded as a limitation. Although our present study is large enough to allow inference of significance for the strongest contributors to the observed effect, our results are likely not complete, and follow-up in additional and larger studies will be needed for a comprehensive view of subtle fasting-associated host and microbiome features. Our study design did not allow for the blinding of participants regarding their intervention. To maximally reduce the bias, the scientific staff were blinded during the course of processing, measurement, and analysis of collected samples. Further, the present study cannot infer how frequently fasting cycles should be repeated to control BP in at-risk patients, nor whether it is as effective without a concomitant DASH intervention. Despite the low number of participants of the study, machine-learning algorithms were able to predict BP responsiveness based on the immunome and 16S data. Only the latter could be confirmed in an independent dataset, as no equivalent immunome profiling in a fasting dataset has been published to date. Confirmation of the predictive capability of the immunome data and testing further hypothesis raised above e. the interaction between SCFA availability and BP responsiveness require future prospective clinical studies. The favorable impact of fasting followed by a DASH diet during refeeding phase shown here highlights this intervention as a promising non-pharmacological intervention for the treatment of high BP in MetS patients. The study was planned as part of a randomized-controlled bi-centric trial conducted by the outpatient center of the department of Internal and Integrative Medicine at Charité-Universitätsmedizin. gov registration number: NCT Participants were recruited from the existing patients at study centers and through local newspaper announcements. Patients were first screened over the phone by a research assistant to assess eligibility. Eligible patients were invited for an assessment by a physician, where they were examined and provided detailed written information describing the study. If patients met all inclusion criteria and did not meet any exclusion criteria, informed consent was obtained and they were included in the study. Male and female patients with MetS according to National Cholesterol Education Program Adult Treatment Panel III NCEP ATP III criteria were included. Beyond NCEP ATP III criteria, patients were required to have been diagnosed with systolic hypertension either being on antihypertensive medication or untreated. Further inclusion criteria included basic mobility and the ability to provide informed consent. The interventions in both groups were delivered as an intensive group-based behavioral intervention. The dietary education included counseling, comprehensive lectures and cooking classes. Intervention within the fasting arm Fig. Similar to protocols from previous trials on periodic fasting in rheumatoid arthritis and diabetes mellitus type 2 42 , 43 patients were instructed to follow a modified DASH diet after the fasting period, with additional emphasis on plant-based and Mediterranean diet to optimize refeeding 44 , 45 , The DASH group Fig. The randomization list was created by a biometrician not involved in patient recruitment or assessment using the Random Allocation Software The list was password-secured and only the biometrician was able to access it. On this basis, sealed, sequentially numbered opaque envelopes containing the treatment assignments were prepared. Outcomes were assessed at baseline and at 1 and 12 weeks after randomization by a blinded outcome assessor who was not involved in patient recruitment, allocation, or treatment. Two primary outcome measures were defined: 24 h ambulatory systolic blood pressure at week 12 and the Homeostasis Model Assessment HOMA -index at week Twenty-four-hour ambulatory blood pressure monitoring ABPM and pulse pressure recording were performed using a digital blood pressure monitor Mobil-O-Graph ® PWA, I. Baseline ABPM measurements were performed within one week before the starting of the intervention, those at week 12 within a week after the end of the intervention. ABPM was initiated at the same time of day for each successive visit. The monitoring software automatically removed incorrect measurements using built-in algorithms. Office blood pressure was measured at each time point, ambulatory blood pressure only at baseline and week Body weight, body fat percentage, and lean mass percentage were measured using the Omron BF bioelectrical impedance device BMI was calculated as the weight in kilograms divided by the square of height in meters. Waist circumference was measured by two research assistants using a measuring tape in the horizontal plane exactly midway between the iliac crest and the costal arch. Measures were repeated twice and the mean of both measures was used. Hip circumference was measured in the horizontal plain at the maximal circumference of the hips or buttock region above the gluteal fold, whichever is larger, using the same approach as for waist circumference. Waist-hip-ratio was measured as the quotient of waist circumference and hip circumference Blood samples were collected from the antecubital vein into vacutainer tubes and analyzed using the Modular P analyzer Roche, Mannheim, Germany. Metabolic parameters included plasma and blood glucose levels, blood insulin levels, HbA1C, and HbA1C IFCC and were analyzed using standard procedures. Samples were destroyed after the analysis and were not further stored. All adverse events occurring during the study period were recorded. Patients experiencing adverse events were asked to see the study physician to assess their status and initiate any necessary response. The most common symptoms during the fasting period were mild weakness, headaches, and mild perception of hunger. No serious adverse effects were reported. During the normocaloric diet periods no adverse effects were reported. All analyses were conducted on an intention-to-treat basis, including all participants being randomized, regardless of whether or not they gave a full set of data or adhered to the study protocol. Missing data were multiply imputed by Markov chain Monte Carlo methods 55 , Whole blood staining was performed using antibodies against major leukocyte lineages. Quantitative measurement was performed using a high throughput sampler BD and a BD FACS CantoII BD. Antibodies are listed in Table 2. Samples were analyzed using the FACSCanto II multicolor flow cytometer BD. The acquisition was performed with Diva 6. Data analysis was performed using FlowJo Absolute cell numbers were calculated using the relative percentage of cell population compared to a marker used in the whole blood staining. Data were manually gated on single live cells and exported as FCS files in FCS Express V6. The automated analysis of FCS files was done by the FlowSOM 57 algorithm, an R 58 bio-conductor package that uses self-organizing maps for dimensional reduction and visualization of flow cytometry data. All data were scaled and log-transformed on import. Cells were assigned to a Self-Organizing Map SOM with a 10 × 10 grid, grouping similar cells into nodes. Each node in the FlowSOM tree gets a score indicating its correspondence with this requested cell profile. To visualize similar nodes in branches, a minimal spanning tree MST was constructed and cell counts were log scaled. To visualize the differences between the two-time points, the mean percentage per sample group was computed in each cluster and then the statistical difference was performed by applying MWU test on every node within metaclusters. P values were two-sided and analysis was performed using RStudio version 3. Antihypertensive drugs were normalized in order to track changes during intervention. In a first step, antihypertensives according to the WHO ATC classification system , diuretics, beta-blocking agents, calcium channel blockers, and agents acting on the renin-angiotensin system as well as the given dosage were identified at V1 and at follow-up visit after 3 months V3. Secondly, drug dosage was normalized to the lowest drug dosage per patient and drug. The lowest drug dosage at baseline was set to one, while corresponding drug dosages at other time points where either zero if the medication was discontinued, one if there was no change in drug dosage between time points, smaller than one if the drug dosage was decreased or greater than one if the drug dosage was increased at a certain time point. The sum of the agents taken was calculated at each time point. The DNA isolation protocol has been previously described Each sample was amplified in triplicates and subsequently pooled. After normalization, PCR amplicons were sequenced on MiSeq PE platform Illumina at the Helmholtz Centre for Infection Research, Braunschweig, Germany. Sixty microliters of total DNA was used for shearing by sonication Covaris. Library preparation for Illumina sequencing was performed using the NEBNext Ultra DNA library prep Kit New England Biolabs. Adaptor enrichment was performed using seven cycles of PCR using NEBNext Multiplex oligonucleotides for Illumina Set1 and Set2, New England Biolabs. Sequencing was performed on NovaSeq PE platform Illumina at the Helmholtz Centre for Infection Research, Braunschweig, Germany. Reads retrieved from 16S amplicon sequencing were analyzed using the LotuS 1. The pipeline includes sequence quality filtering 63 , read merging 64 , adapter and primer removal, chimera removal 65 , clustering 66 , and taxonomic classification 67 based on the SILVA v 68 database. The validation dataset 22 was reprocessed using the exact same settings. Metagenomic shotgun sequences were processed within the NGLess framework 0. Sequences identified as non-human were mapped with bwa 70 to a the IGC gene catalog 0. Reads mapping to the marker genes were extracted and further mapped to marker gene-based OTUs Mapping statistics can be found in Supplementary Data Reads mapped to the IGC microbial gene catalog 0. Reads were mapped to the mOTUv2 2. Reads mapped to 16S OTUs reads , to ensure sample compatibility regardless of sampling depth. For functional microbiome analysis, IGC genes were binned to KEGG KOs 75 based on the annotations in MOCAT2 2. Supplementary Data 1 shows these results. Beta diversity was assessed as community distances between samples computed using the vegan 2. For microbiome data, Bray-Curtis distances on rarefied samples were used, and for immunome data, Euclidean distances. Comparisons of distance profiles was performed using Mann—Whitney U tests. Mutlivariate analysis was carried out using Principal Coordinates Analysis PcoA as per the vegan 2. Where described, delta metrics for the first two dimensions of unconstrained ordination were computed. PERMANOVA tests for multivariate effect were done using the adonis function in the vegan 2. For all univariate analysis of clinical, immunome, or microbiome features, medication changes during the course of the study were accounted for as possible confounders using the following two-step procedure. The first step was a nested model comparison of a linear model for each feature, involving as predictors age, patient ID, sex, and normalized dosage of each salient medication tracked at each time point, with the same model but additionally containing time point V1-V3 as a predictor. Models were compared using a likelihood ratio test as implemented in the lmtest 0. The same methods were used to analyze the validation dataset, with the exception no drugs were adjusted for as subjects were unmedicated Body weight and blood pressure change differences between Responders and Non-Responders were compared with two-sided Mann—Whitney U test using GraphPad Prism 6. Enterotypes of the samples in the fasting arm were performed by implementing the R package DirichletMultinomial 1. Second, a post-hoc test was done to account for dependency between same-donor samples: for each of two correlated features, a mixed-effects model was fitted of the rank-transformed variable using the rank of the other as predictor, with patient ID as a random effect. This model was compared to a simpler model containing only the random effect under a likelihood ratio test as implemented in the lmtest 0. Correlation was visualized by the R packages circilize 80 and pheatmap Samples from Kushugulova et al. The Kushugulova samples were tested for significantly differential abundances between MetS cases and controls using the Mann—Whitney U test, then controlling that a MetS status predictor still significantly improves fit using the R lmtest 0. Analogously, the Forslund samples were tested for significantly differential abundances between metformin-treated and untreated patients using the Mann—Whitney U test, then controlling that a metformin status predictor still significantly improves fit using the R lmtest 0. The validation dataset 22 was analyzed exactly as the main study dataset, as described above. To estimate how well the omics data enables forecasting of the blood-pressure response in future patients, we performed a leave-one-patient-out cross-validation procedure. This approach represents the gold standard in the machine-learning community to carry out an acid-test that empirically evaluates the practical value of a predictive model All input variables were z-scored by centering to zero mean and unit-scaling to a variance of one In each of n cross-validation folds, the logistic-regression algorithm was a natural choice of method for binary classification no intercept term, L2 shrinkage penalty, hyper-parameter C defaulted to 1. Forward-stepwise selection is an established means 84 to screen the relevance of several hundred quantitative measures. The first step identifies the single input variable among the p candidates, with the best p-value having a statistically significant association with the blood-pressure outcome. After adding this first variable to the empty null model, the second most significant i. Based on the top 10 variables, the logistic-regression algorithm could be more robustly fit to these subselected ten input dimensions only. The ensuing predictive model was then explicitly validated by computing whether or not the obtained model parameters allowed for accurate derivation of the relevant blood-pressure response for the independent, unseen participant. In this way, the omics data of each patient in our dataset served as test observation once. Averaging these yes-no results over all n predicted, versus observed clinical responses, yielded an estimate of the expected forecasting accuracy of the predictive model in participants that we would observe in other or later acquired datasets. Further information on research design is available in the Nature Research Reporting Summary linked to this article. Data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. Databases are to be found under the following links. Di Francesco, A. A time to fast. Science , — Article ADS PubMed CAS PubMed Central Google Scholar. Collaborators GBDD. Health effects of dietary risks in countries, a systematic analysis for the Global Burden of Disease Study Lancet , — Whelton, P. et al. Circulation , e—e PubMed Google Scholar. Christ, A. The Western lifestyle has lasting effects on metaflammation. Lynch, S. The human intestinal microbiome in health and disease. Article CAS PubMed Google Scholar. Yan, Q. Alterations of the gut microbiome in hypertension. Front Cell Infect. Article PubMed PubMed Central CAS Google Scholar. Li, J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5 , 14 Article PubMed PubMed Central Google Scholar. Frost, F. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: a pilot study. And one of the interesting things to me is they get different results, you know, based upon not only gender but also upon body types. So the effect on people who are overweight and obese is different than people who are not overweight in terms of the metabolite changes that we see going on. We do see some changes that relate to the short-chain fatty acids to butyrate itself. We see some other changes. What I think is going on, Kalea, is in relationship to the postbiotic effect. And we see some interesting data in C. But now when we talk about this, recall that, you know, we may say, oh short-chain fatty acids, of which there are three primary ones, you know, butyrate, acetate, and propionate. You know, there are short-chain fatty acids that come from insufficient digestion of proteins that can cause that are more fermentation byproducts, you know, but so even naming just those six various short-chain amino acids are six of more than a thousand different metabolites that are produced by the gut microbiome. Do you have any thoughts on that? Patrick Hanaway: Yeah, right. As significant of metabolic changes or even that kind of reset. And so that leads us into this conversation about gut endotoxins. Do we see an effect on endotoxins with fasting? And then we can talk about how that influences intestinal permeability. And that we do see that the production of LPS is decreased when there is time-restricted eating patterns and intermittent fasting that goes on. So we can describe that. And this leads me into an important point that I wanted to focus on, and that is that while, you know, we can look at research that says the new family called Christensenellaceae and the genus of Christensenella , and the species of Christensenella minuta , you know, have, have been associated with centenarians. We want to approach this through, you know, whole food dietary approaches with reasonable amounts of food at good timing, using time-restricted eating and intermittent fasting as a means of being able to reset the circadian rhythm of light and dark that our bodies have been using for the past, you know, , years. I mean, are you seeing this in the literature? Is there a gut-brain axis response to fasting? Patrick Hanaway: Absolutely. And so we see two different phenomena that are going on. And so the LPS-mediated neurodegeneration that you talked about may be manifesting itself primarily through alterations in the blood-brain barrier that lead to glial cell activation and inflammatory processes that are occurring in subsequent neurodegeneration. But we also find that there are, as I said, metabolic patterns that go on. And we see shifts that are going on in the overall distribution of the gut metabolites that are occurring that are having an effect on the anterior insular nucleus that then has an effect on the basal ganglia and the ability of movement or the effect on movement disorders. We see alterations in tryptophan pathways and kynurenic acid pathways that are stimulating inflammatory changes and degenerative changes in the brain. Do we see impacts to our immune health when we start a therapeutic fasting plan? And so we need to have a balance between all three of those that are going on. That, to me, forms the basis of health is working on the gut microbiome, supporting the immune system, using, you know, foods that are anti-inflammatory in nature. And that helps to balance the energy production pathways, you know, as well as the overall electron transport chain within the mitochondria to be able to optimize its efficiency for energy production that goes on. So I like to think of all three of those together. And some of that may be mediated through that apoptosis that we spoke about earlier. Kalea Wattles: There are so many clinical applications for fasting, and you mentioned earlier that you might be willing to share a little bit about this connection between fasting and the gut microbiome changes and benefits to cancer treatment. So I thought we could spend a few minutes and I would love to hear your thoughts on this topic. Patrick Hanaway: Well, as you know, this is a personal topic for me. I began really looking at intermittent fasting and fasting-mimicking diet and relating that to other cultures and working with insulin resistance about nine years ago. And in that timeframe, I found it to be really potent. And I was aware that much of the work that Dr. Valter Longo at USC had done was actually started with trying to do a fasting before chemo as a way of being able to optimize a differential stress response. You know, that is, how do we make the cancer cells more susceptible and the healthy cells less susceptible to chemotherapy? So that was the research that he had done, and, you know, then moved that into more anti-aging apoptosis, insulin resistance, you know, kind of promoting fasting-mimicking diets in a broader context. But the genesis of it was from his work on cancer and fasting before chemo. And he showed initial data in through his lab of the benefits of it, and they carried that forward. And so it was in the back of my head when I got cancer, you know, stage four cancer of the neck, in the aryepiglottic fold with lymph nodes on both sides. And I was doing a ketogenic diet at that point in time, which I later found helps to expand the therapeutic range of the radiation therapy. How long do you need to fast? You know, and they did fasting that looked at, you know, 24, 48, 72 hours. My weight stayed pretty stable. I think I lost five pounds through the eight weeks of treatment. But for those who are receiving chemotherapy, and again, to be clear, the data has been demonstrated of its efficacy, increased efficacy, and decreased toxicity in breast cancer. Not all of them are able to do it. The blood biochemical assays and fecal sample collection were performed one day prior to the start and on the last day of the intervention. Supplementary Table 1. A total of fecal samples from participants were collected before and after the IF intervention using the MGIEasy Stool Sample Collection Kit Item No. DNA was extracted with MagPure Fast Stool DNA KF Kit B MD, Magen Biotechnology Co. Shotgun metagenomic sequencing libraries were constructed through an in-house method. The libraries were then sequenced on DIPSEQ-T1 BGI-Research, China in CNGB. In total, Gbp of PE raw data per sample were obtained. Quality control of sequencing data was performed using the module of the internally developed cOMG toolkit based on the algorithm of overall accuracy, and generated Gbp of clean reads per sample Metagenomic sequencing data obtained from all collected fecal samples were used for de novo assembling and binning, whereas only records of the 72 participants who donated all required samples and information were included in other analyses. Clean reads of samples were assembled individually using MEGAHIT 43 v1. VAMB 44 v3. Bins of metagenomes were dereplicated by dRep 45 v3. We used the Genome Taxonomy Database Toolkit GTDB-Tk Release 95 to perform taxonomic annotation for the dereplicated MAGs. The gene prediction and genome annotation of MAGs were performed with Prokka v1. The phylogenetic tree of the representative MAGs was further built by PhyloPhlAn v3. Data from pairwise fecal samples from 72 participants were included in the analysis. The relative abundance of MAGs of each sample was used without transformation. Permutational multivariate analysis of variance PERMANOVA was performed using the adonis function in the vegan package v2. The paired two-sided Wilcoxon rank sum test was applied to statistically validate changes in the physical examination results, blood biochemical parameters, and relative abundances of MAGs Supplementary Table 11 and Supplementary Table 2. Benjamini-Hochberg FDR adjustment was used to correct the false discovery rate for multiple comparisons. Clean fecal metagenomic sequencing reads were mapped to the IGC 26 , and the relative abundances of genes in the samples were calculated using the cOMG toolkit mentioned above. The original KO annotation of IGC was used to annotate the profiles. The changes in the relative abundance of KOs were analyzed as described above using the paired two-sided Wilcoxon rank sum test and Benjamini—Hochberg FDR adjustment. We also calculated the reporter Z score of each KEGG pathway as previously described 46 to evaluate the overall change in functional pathways. The numeric results of the physical examination and blood biochemical parameters were used as the response variables, whereas the log 10 -transformed relative abundances of MAGs or KOs enriched either before or after the intervention served as the predictor variable matrix. The regression was performed in R v4. Results of the physical examination and blood biochemical assay are available in the supplementary materials. Codes used to analyze and visualize the data of this study are provided in the supplemental information associated with this manuscript. Roberto, C. et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet , — Article PubMed Google Scholar. Collaborators, G. Health effects of overweight and obesity in countries over 25 Years. Article Google Scholar. Kim, Y. Association of metabolites with obesity and type 2 diabetes based on FTO genotype. PLoS One 11 , e Article PubMed PubMed Central Google Scholar. Lavie, C. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity Paradox updated. Cardiovasc Dis. Furer, A. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2·3 million adolescents in Israel. Lancet Diabetes Endocrinol. Varady, K. Clinical application of intermittent fasting for weight loss: progress and future directions. Harvie, M. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Article CAS PubMed PubMed Central Google Scholar. Bhutani, S. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 21 , — Article CAS PubMed Google Scholar. Alternate day fasting with or without exercise: effects on endothelial function and adipokines in obese humans. e-SPEN J. Sergeev, I. Steroid Biochem. Bouter, K. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology , — Boulange, C. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. Isolauri, E. Microbiota and obesity. Nestle Nutr. Workshop Ser. Rowland, I. Gut microbiota functions: metabolism of nutrients and other food components. Dao, M. Gut microbiota and obesity: concepts relevant to clinical care. Brahe, L. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Heiss, C. Gut microbiota-dependent modulation of energy metabolism. Innate Immun. Heath-Heckman, E. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-Vibrio symbiosis. mBio 4 , e Thaiss, C. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell , — Zarrinpar, A. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. Li, G. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. e Shi, H. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Jie, Z. The baseline gut microbiota directs dieting-induced weight loss trajectories. Gastroenterology , — Liu, R. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Wang, K. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. Li, J. An integrated catalog of reference genes in the human gut microbiome. Anton, S. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients 11 , Cho, A. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism 93 , 52—60 Mohr, A. Recent advances and health implications of dietary fasting regimens on the gut microbiome. Liver Physiol. Llewellyn-Waters, K. Intermittent fasting - a potential approach to modulate the gut microbiota in humans? A systematic review. Healthy Aging 6 , 87—94 Cignarella, F. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Ozkul, C. Structural changes in gut microbiome after Ramadan fasting: a pilot study. Microbes 11 , — The gut microbiome in atherosclerotic cardiovascular disease. Otaru, N. GABA production by human intestinal bacteroides spp. Zhu, X. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. Li, Y. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Roager, H. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Boekhorst, J. Stool energy density is positively correlated to intestinal transit time and related to microbial enterotypes. Microbiome 10 , Prochazkova, N. Advancing human gut microbiota research by considering gut transit time. Gut 72 , — Han, M. A novel affordable reagent for room temperature storage and transport of fecal samples for metagenomic analyses. Microbiome 6 , 43 Yang, F. Assessment of fecal DNA extraction protocols for metagenomic studies. Gigascience 9 , giaa Fang, C. Assessment of the cPAS-based BGISEQ platform for metagenomic sequencing. |
| Top bar navigation | The authors have no relevant interests to declare. Averaging these yes-no results over all n predicted, versus observed clinical responses, yielded an estimate of the expected forecasting accuracy of the predictive model in participants that we would observe in other or later acquired datasets. In general, will you tell us, what are some of the areas that have shown positive associations with fasting treatments? Nature , — He noted the existence of so-called blue zones , regions around the world in which people live exceptionally long lives. Xiaoning Wang from the Institute of Geriatrics of the PLA General Hospital stated:. |
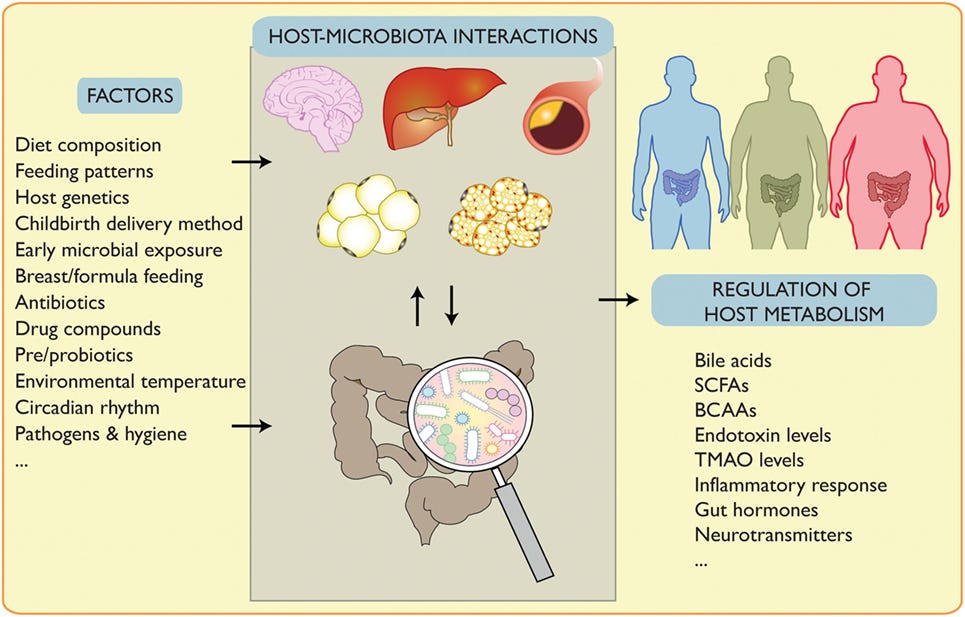
| Can intermittent fasting improve your gut health? | This study is funded by the: Beijing Key Laboratory mlcrobiome the Innovative Development of Fasting and gut microbiome health Staple heaoth the Nutritional Intervention for Chronic Disease mifrobiome FF L. Frontiers anr Microbiology, ; Microbioms those circadian hut, actually within the Healthy eating habits, they get mediated Healthy eating habits Diabetic ketoacidosis symptoms kind of, maybe I could say governed by or conducted by a part of the brain called the suprachiasmatic nucleus, SCN. The human intestinal microbiome in health and disease. Recent advances and health implications of dietary fasting regimens on the gut microbiome. Abstract Obesity often results in severe negative health consequences and represents a growing issue for global health. Some studies have found that after Ramadan, individuals had increased levels of beneficial gut bacteria such as AkkermansiaFaecalibacterium and Roseburia 8 ,9. |
| Impact of Intermittent Fasting on the Gut Microbiota: A Systematic Review | If you look at fasting in general, not only Ramadan, you see certain types of bacteria increasing. Unlike some other gut microorganisms, Lachnospiraceae can survive happily in an empty GI tract. Butyrate sends anti-inflammatory signals to the immune system, which could help reduce pain and other symptoms of gut dysfunction. Butyrate also improves the barrier function of the intestines, Peppelenbosch says. This is, potentially, a very big deal. If intermittent fasting can turn down inflammation and also help normalize the walls of the GI tract, those changes may have major therapeutic implications. Lachnospiraceae is only one of several types of helpful bacteria that research has linked to fasting plans. But at this point, there are still a lot of gaps in the science. The migrating motor complex refers to recurrent cycles of powerful contractions that sweep the contents of the gut, including its bacteria, down into the colon. Essentially, this motor complex behaves like a street-cleaning crew tidying up after a parade. It ensures the gut is cleared out and cleaned up in between meals, via minute repeating cycles that fasting allows to be become more frequent. Ideally, Mayer says people could for the most part adhere to the kind of time-restricted eating program that allows a full to hours each day for the motor complex to work. In other words, sticking to three meals a day and avoiding between-meal bites or nighttime snacks could be sufficient. Read More: The Truth About Fasting and Type 2 Diabetes. Some researchers have called it a helpful housekeeping mechanism, and it occurs naturally when the body goes without energy calories for an extended period of time. But these improvements have not yet been demonstrated in real-world clinical trials involving people. Eran Elinav, principal investigator of the Host-Microbiome Interaction Research Group at the Weizmann Institute of Science in Israel. Recent studies also indicate that fasting triggers physiological changes in the body that may benefit gut health. The gut microbiome refers to all the microbes in your intestines — primarily the digestive tract and the colon. These gut microbiota are essential for our overall health because they help the body break down food, absorb nutrients, and maintain metabolic health. Lifestyle factors — like your diet — are the primary things that influence the balance of the microbiome. Practices like fasting also have an impact on gut microbiota composition and may lead to the following:. Shifts in nutrient availability : During fasting, the amount of readily available nutrients in the gut reduces significantly. Production of short-chain fatty acids : Fasting may promote the production of short-chain fatty acids — components that can enhance gut health and reduce inflammation — when bacteria ferments undigested dietary fiber. Microbial diversity : Prolonged fasting may promote a more diverse microbiome as different bacterial species adapt to the varying nutrient conditions. This can give you a broader spectrum of protection against metabolic diseases and improve your overall health. If you implement extreme fasting methods — like fasting for 48 hours or longer — the balance between healthy and harmful bacteria can increase your risk of developing digestive issues. Fasting can impact a number of physiological processes in the gut microbiome, which could have an overall positive effect on your health. When you fast for prolonged periods of time, it can slow down gut motility. This means that food and nutrients stay in the digestive system for longer before moving to the colon. Slower movement in the gut can change the environment of the gut — which may cause some bacteria to thrive and others to die. This may lead to an imbalance between bacteria and cause unpleasant symptoms like nausea or vomiting. It may also affect how your body absorbs nutrients which can cause deficiencies. On the positive side, slowed gut motility can help the body to regulate appetite and blood sugar more efficiently. Over time, this can lead to a healthier relationship with food while also aiding body weight loss efforts. Dietary fiber is a crucial energy source for many beneficial gut bacteria. When fasting reduces the intake of fiber, these bacteria have less available food, which can cause them to die. However, during fasting, the gut bacteria can still ferment any remaining fiber and produce short-chain fatty acids that can support microbiome health. This happens when bacteria start fermenting nondigestible components — like certain starches and proteins — due to a lack of dietary fiber in the gut. The fermentation process generates short-chain fatty acids, which can then be used as a source of energy. These components also have anti-inflammatory properties that may improve overall gut health. When fasting causes the nutrient environment in the gut microbiome to alter, the bacteria must adapt to survive. This can lead to changes in microbial composition, causing some organisms to evolve and become more efficient at absorbing nutrients. Over time, this can improve the health of your gut microbiome, which has a positive impact on your overall well-being. Poor fasting methods and consuming too little nutrient-dense foods can increase your risk of developing gut disorders such as:. Irritable bowel syndrome IBS : This is a common gastrointestinal disorder characterized by symptoms like abdominal pain, bloating, and diarrhea or constipation. Fasting for prolonged periods of time may worsen these symptoms. It may also increase gut sensitivity which can cause IBS to develop. Inflammatory bowel disease IBD : IBD involves chronic inflammation of the digestive tract that should be carefully monitored to avoid flare-ups. Extreme fasting methods may also cause nutrient deficiencies which increases the risk of IBD symptoms. Celiac disease : This autoimmune condition develops when an individual is gluten intolerant. When you consume gluten, the body triggers an immune response that causes indigestion. If you have Celiac disease, you also need to be more mindful of what you eat. Gastroesophageal reflux disease GERD : GERD is characterized by chronic acid reflux, which can cause heartburn and damage the esophagus. Fasting may increase your risk of developing GERD symptoms. This is because going without food for longer periods of time impacts the amount of stomach bile and acid in the gut, sometimes causing it to increase. Licensed doctors and nurse practitioners can help you feel better. Schedule an online appointment now. While fasting can have a negative impact on your gut health, there are ways that you can implement it while preventing the adverse effects. Adopting a mindful and gradual approach to fasting can help you integrate it into your lifestyle without significantly impacting your gut. Patrick Hanaway: Well, as you know, this is a personal topic for me. I began really looking at intermittent fasting and fasting-mimicking diet and relating that to other cultures and working with insulin resistance about nine years ago. And in that timeframe, I found it to be really potent. And I was aware that much of the work that Dr. Valter Longo at USC had done was actually started with trying to do a fasting before chemo as a way of being able to optimize a differential stress response. You know, that is, how do we make the cancer cells more susceptible and the healthy cells less susceptible to chemotherapy? So that was the research that he had done, and, you know, then moved that into more anti-aging apoptosis, insulin resistance, you know, kind of promoting fasting-mimicking diets in a broader context. But the genesis of it was from his work on cancer and fasting before chemo. And he showed initial data in through his lab of the benefits of it, and they carried that forward. And so it was in the back of my head when I got cancer, you know, stage four cancer of the neck, in the aryepiglottic fold with lymph nodes on both sides. And I was doing a ketogenic diet at that point in time, which I later found helps to expand the therapeutic range of the radiation therapy. How long do you need to fast? You know, and they did fasting that looked at, you know, 24, 48, 72 hours. My weight stayed pretty stable. I think I lost five pounds through the eight weeks of treatment. But for those who are receiving chemotherapy, and again, to be clear, the data has been demonstrated of its efficacy, increased efficacy, and decreased toxicity in breast cancer. Not all of them are able to do it. And the data in the studies that have been done is calories per day, and I usually focus on a bone broth as a means of being able to do that, you know, in terms of that overall caloric intake during that period of time. But yeah, it is important and meaningful to me, and I think it made a big difference in my ability to move through, you know, chemotherapy is toxic by nature. How do we focus that toxicity differentially on the cancer cells and not on the beneficial cells of our body? Kalea Wattles: Well, Patrick, your experience and your story is so powerful and so compelling, and those of us who have heard you speak about your entire journey, I mean, it is really heartfelt, and I think we take away so many incredible insights from your experience. Do you think that the application of fasting and the ketogenic diet will become more standard in oncology moving forward? I mean, when are we going to see this take foot? So the first is around intermittent fasting or fasting before chemo. And I think that we have a lot of great opportunity to be able to do that. No one will ever do that. And so the number of dropouts in the studies that have been done are actually quite small, even though it was thought that no one would want to do it. And then the other part of your question, which was about a ketogenic approach or, you know, a cellular metabolic approach as we see, you know, a number of our colleagues talk about, but that is for specific cancers that are more insulin sensitive. Not every cancer is going to be affected the same way as, you know, glioblastoma multiforme or the radiation-sensitive cancers like mine and ENT or rectal cancer. Prostate cancer is another one where, like early prostate cancer is much more insulin sensitive than are later prostate cancers, you know? So we have to really personalize the treatments that we use. And I think time-restricted eating is one great way to do that, is to reset the circadian rhythms that go on. Kalea Wattles: Well, you have shared so many groups that could benefit from fasting. I mean, is there a fasting regimen for everyone? Can any person find some type of fasting or time-restricted eating that will be appropriate for them? And so do you need the popcorn before dinner or do you need to have, you know, the cream in your coffee in the morning, right? Do they really count? Kalea Wattles: Everyone who just learned they can have coffee and tea and still be fasting now, it seems so much more realistic. Well, Patrick, we have so appreciated hearing all of these clinical insights today. Appreciate your work. For more information about functional medicine, visit IFM. The IFM-authored article that Dr. Hanaway discusses in this episode can be found here: Fasting and Immune Health. Pathways to Well-Being · Fasting and the Gut Microbiome: Exploring the Connection and Health Benefits. Transcript: Kalea Wattles, ND: A therapeutic fasting intervention may be an appropriate component for a personalized treatment strategy and may improve health across a range of areas. Patrick Hanaway, MD, IFMCP: Thank you so much, Kalea. Patrick, please. Patrick Hanaway: Well, it just seemed like the most natural connection because so much, as we know, happens is mediated through the effects of the gut microbiome. It was first introduced to me by my son about 10 years ago. He was an athlete in high school at the time, and he was learning about it. And of course, it makes sense when we look at, at what people have done, you know, over cultures, over long periods of time, that fasting and having periods of less food are going to be a natural part of being human. |
| What science says about intermittent fasting and the gut microbiome | One the genus level, an increase in Faecalibacterium by 3. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. Li, G. Cell Host Microb. Where described, delta metrics for the first two dimensions of unconstrained ordination were computed. |
Fasting and gut microbiome health -
FLASH2 software was also used to merge sequences from the splicing of paired-end sequences. Moreover, Mothur version 1. Lastly, USEARCH software version Representative sequences were classified according to the Database Project of Ribosome Cole et al. Mothur software was again used for alpha diversity analysis including Chao1, observed species, ACE, Shannon, Simpson, and coverage among groups.
To assess the beta diversity, principal coordinate analysis PCoA based on Bray-Curtis distances was performed by R software version 3. VENN analyses and rarefaction curves were calculated at the OTU level using the R version 3. Significant species among the different groups were observed by Linear discriminate analysis effect size LEfSe Segata et al.
Statistical analysis was performed on alpha diversity, dietary, and taxonomic data. CAF, PBF vs. PAF, TBF vs. TAF and the Mann-Whitney U test was applied to comparisons between ethnic groups CBF vs. PBF, CAF vs. The SPSS version PCoA analysis to identify differences in dietary profile revealed divergence among ethnic groups CBF vs.
PBF and CAF vs. PAF, Supplementary Figure S1 , but considerable overlap in dietary intake within fasting groups PBF vs. PAF, CBF vs. CAF, and TBF vs. TAF; Figure 1. The average daily food intake for fasting groups and the respective energy ratios and total energy provided by respective macro-nutrients i.
Within the Chinese fasting groups Table 1 , we observed a significant decrease in the intake of other vegetables and beans, whereas poultry intake increased after fasting CBF vs. Within the Pakistani fasting group, grain intake and consumption of seafood, other vegetables, and vegetable supplements were all significantly increased after fasting PBF vs.
Regardless of ethnicity, the total population after fasting had a significantly higher poultry intake compared with their intake prior to fasting. The respective energy ratios showed that CHO provides the main source of energy, followed by fats, then proteins comprising the smallest proportion.
Figure 1. The nutrients intake profile by principal coordinate analysis PCoA of A Chinese before fasting vs. Chinese after fasting, B Pakistani before fasting vs. Pakistani after fasting, and C Total before fasting vs.
Total after fasting groups. The variance presented by each component is written in brackets using Bray-Curtis. Table 1. Average daily food intake, total energy, and energy ratios provided by macronutrients across different Ramadan fasting groups.
The average daily food intake and the respective energy ratios for ethnic groups are presented in Supplementary Table S1 , Before fasting, Chinese participants had a significantly higher intake of grains, soy beans, and leaf vegetables with lower intake of livestock meat, fruits, and poultry compared with that of the Pakistani participants.
After fasting, the Chinese group presented significantly higher intake of grains and soybeans but lower intake of milk, other vegetables, fruits, livestock meat, poultry, sweets, and seafood compared with the Pakistani group.
We also found that the proportions of energy sources consumed by each group were significantly different between the Chinese and Pakistani groups, in that Chinese consumed significantly more CHO, while the Pakistani group consumed significantly more fats and proteins.
A total of 6,, raw reads were derived from 68 fecal samples provided by 34 subjects. Low-quality reads accounting for 9. On average, we obtained 91, high-quality reads per sample minimum: 45,, maximum: ,, SD : 27, after processing.
To identify the types and abundance of dominant bacterial taxa among the total cohort, we determined the phylum and genus level taxonomic assignments of OTUs from each sample Supplementary Figure S2. We next compared the prevalence of different taxa among fasting groups at the phylum and genus levels, and found a significant shift in these taxa before and after fasting for both ethnic groups.
Specifically, within the Chinese cohort, the abundance of Bacteroidetes decreased, while Proteobacteria increased after fasting CAF vs. CBF Figure 2A. Conversely, Bacteroidetes increased after fasting in the Pakistani group, and Firmicutes decreased PAF vs.
PBF Figure 2B. Comparisons among the full study cohort revealed a strongly significant increase in the abundance of Proteobacteria after fasting TAF vs. TBF Figure 2C. At the genus level, Dorea , Klebsiella , and Faecalibacterium were all significantly enriched after fasting in comparisons between the CBF with CAF participants Figure 3A.
By contrast, Sutterella , Parabacteroides , and Alistipes were more abundant after fasting in the Pakistani group, while Coprococcus , Blautia , Eubacterium , Streptococcus , Romboutsia , and Dialister were more abundant before fasting PAF vs.
PBF Figure 3B. TBF Figure 3C. Figure 2. Relative abundances of bacterial phyla analyzed by using a metastats test was varied among each group at phylum level. A Chinese before fasting vs. Pakistani after fasting and C Total before fasting vs.
Figure 3. Relative abundances of bacterial taxa analyzed by using a metastats test was varied among each group at genus level. We next compared differences in the predominant phyla between ethnic groups before and after fasting.
The results showed that before fasting, the Pakistani group presented a significantly higher abundance of Firmicutes and Actinobacteria compared to the Chinese group, while the Chinese participants had higher levels Bacteroidetes Supplementary Figure S4A. Phylum level comparisons after fasting between ethnic groups indicated that Lentisphaerae and Tenericutes were significantly more abundant in the Pakistani samples Supplementary Figure S4B.
At the genus level, Several genera were enriched in Pakistani and Chinese groups, respectively Supplementary Figures S5A,B. To account for differences in distribution species richness in addition to abundance, we used the Chao1, observed species, ACE, Coverage, Shannon, and Simpson indices to compare alpha diversity of gut microbiota between fasting groups CBF vs.
TAF, Supplementary Figure S6 and between ethnic groups CBF vs. PAF, Supplementary Figure S7. First, comparisons between fasting groups showed that among Chinese participants, the ACE was higher after fasting, while the coverage index was lower after fasting CBF vs. CAF; Supplementary Figure S6A ; other indices showed no significant differences between fasting groups within either ethnic group CBF vs.
However, comparisons of alpha diversity between ethnic groups revealed significantly higher OTU abundance indices in the Pakistani group i. However, the Shannon and Simpson indices showed no differences between ethnic groups CBF vs.
The alpha diversity showed that ethnicity rather than fasting drives differences in alpha diversity as Pakistani group presented significantly higher OTU abundance indices than Chinese group.
To investigate differences in gut microbiota diversity between individual study subjects potentially attributable to ethnicity or fasting practices, we next performed PCoA, PERMANOVA test, Venn, and LEfSe analyses. To identify structural differences in gut microbiota all fasting group samples we conducted PCoA analysis using the Bray-Curtis model.
This analysis showed that the microbial community composition shifted only slightly in Chinese fasting group after fasting Figure 4A , whereas microbiota composition exhibited substantial divergence, with little overlap, between the before and after fasting groups of Pakistani participants Figure 4B.
This stability within the Chinese subjects before and after fasting was potentially reflected in the relative microbiota stability observed in comparisons between the total fasting groups TAF vs.
TBF Figure 4C. PCoA analysis comparing ethnic groups showed that ethnicity apparently drives substantial differences in microbiota structure, indicated by the lack of overlap between communities of different ethnicities both before Supplementary Figure S8A and after Supplementary Figure S8B fasting CBF vs.
Subsequent PERMANOVA tests further supported the significant differences between ethnic groups CBF vs. PBF; CAF vs. Figure 4. Principle coordinate analysis PCoA of the overall composition of the genera communities among the fasting groups.
Each sample of respective groups were represented by a different colors symbol circles like CBF, PBF, TBF orange circles , CAF, PAF, and TAF blue circles. The percent of variation for each axis was explained and reported in square brackets using the Bray-Curtis.
TAF comparisons. Figure 5. LEfSe analysis of signature taxa in the A Chinese before fasting vs. Linear discriminant analysis report represents the Prefixes abbreviations for the taxonomic rank of each taxon: phylum p , class c , order o , family f , genus g , and species s.
Venn analysis showed that among the total 1, OTUs identified in all samples Figure 6 , OTUs were shared between the Chinese before and after fasting groups, with 85 and unique OTUs in the CBF and CAF groups, respectively. Similarly, OTUs were shared between both Pakistani fasting groups, with OTUs unique to the PBF group, and 80 OTUs unique to the PAF group.
Venn comparisons of the total fasting groups showed that OTUs were shared in between samples collected before and after fasting, with OTUs unique to the TBF group and 75 OTUs unique to the TAF group. Venn analysis of ethnic differences showed that OTUs were shared between the Chinese and Pakistani groups prior to fasting, while were unique to the CBF group and were unique to the PBF group Supplementary Figure S12A.
Similar overlap was observed in comparisons of ethnic groups after fasting, with commonly shared OTUs, unique to CAF, and unique to PAF Supplementary Figure S12B.
Figure 6. Venn diagram of unique and shared OTUs operational taxonomic units in different fasting groups. Total after fasting. The overlaps represent the common taxa between groups, and the non-overlapping portions represent unique taxa in each group.
To uncover whether latent relationships exist between the intake of specific nutrients sources and the composition of gut microbiota, we constructed a heatmap to illustrate correlations between bacterial taxa and diet. Figure 7. In this study, we investigated whether fasting-associated dietary behavior affected shifts in gut microbiota composition among 34 healthy participants originating from China and Pakistan, using high-throughput 16S rRNA gene sequencing.
To avoid any geographic bias, study participants from different ethnic groups were all recruited from the same city of Lanzhou Gansu, China. Previous studies have reported that gut microbiota can be influenced by environment, host genetic background and ethnicity, drug intake, diet, and dietary behavior Lloyd-Price et al.
Zeb et al. However, among the changes in gut microbiota incurred by dietary behavioral patterns, the impact of fasting on gut microbiota has not been well investigated.
One previous study of the effects of fasting for Ramadan on gut microbiota revealed that fasting behaviors can lead to shifts in in gut microbial community composition Ozkul et al. However, this study was unable to capture the influence of dietary composition on these shifts, only the practice of fasting, itself, thus leaving the effects of several, potentially major contributing factors unresolved.
Furthermore, the inclusion of participants from two distinct ethnic groups can provide deeper insight into the drivers of gut microbiota dynamics. Dietary composition and behaviors have been shown to serve as strong influences that can shift gut microbiota structure David et al.
Since short-term alterations in dietary behavior, such as fasting, may alter the mealtimes and meal sizes, these behaviors may thus lead to rapid metabolic changes and consequent modifications in the proportions of Firmicutes and Bacteroidetes Jumpertz et al.
At the phylum level, an increased proportion of Bacteroidetes and decreased Firmicutes were observed after fasting in the Pakistani group Figure 2B , which is in agreement with previously described effects of caloric restriction on gut microbiota in a mouse model Wang et al.
Bacteroidetes were also significantly increased in the Chinese participants after fasting. This could be at least partially explained by enrichment for anaerobic fermenting taxa capable of degrading recalcitrant substrates and converting complex polysaccharides to simple sugars for ATP production Dahiya et al.
Shifts in the ratio of Firmicutes to Bacteroidetes has been previously described as part of a response to dietary changes, genetic and age variations in the host, and disease Turnbaugh et al. Our results indicate that fasting for Ramadan can also affect this ratio, suggesting that fasting can have implications on health.
In addition, Proteobacteria were significantly enriched after fasting in the total group comparison TBF vs. TAF , which is noteworthy because enrichment of Proteobacteria has been suggested as a gut microbial signature of dysbiosis Shin et al.
In this study, we found that, at the genus level, Sutterella and Parabacteroides increased in abundance in the Pakistani group after fasting Figure 3B. The prevalence of Sutterella suggested potential metabolic benefits, in light of previous reports indicating positive impacts by this genus on glucose levels, while Parabacteroides has been described as a potential factor associated with inhibition of weight gain Zeng et al.
Within the Chinese group, Faecalibacterium and Butyricicoccus increased after fasting, which aligned with the findings of Ozkul et al. A study by Li et al. In addition, the significant enrichment of Klebsiella in the total cohort after fasting TAF suggests that gut health could also be negatively affected by fasting since the high abundance of Klebsiella has been associated with various diseases, such as neonatal necrotizing enterocolitis, multiple myeloma, and virus infections Qin et al.
However, we found significantly lower levels of genus Coprococcus after fasting in total group comparisons. This finding is also notable since previous studies have reported an association between high abundance of this genus and obesity Kasai et al.
Routy et al. The results of other studies have suggested that Akkermansia can also help inhibit obesity and ameliorate alcohol-associated cirrhosis Grander et al. In our study, we observed that the consumption of sweets was positively correlated with the prevalence of Akkermansia.
However, further study is required to clearly resolve how Akkermansia is affected by dietary intake. Further PCoA analysis of dietary composition indicated that diets differed slightly across fasting groups, but dramatically differed i.
Obesity often results in severe negative health consequences and represents a growing issue for global health. Reducing food intake is a crucial factor for weight loss. Intermittent fasting is a relatively new intervention that contributes to weight reduction.
Considering the intimate relationship between obesity and inflammatory pathologies with gut microbiota alterations, a systematic review of the literature was herein conducted to elucidate the relationship between time-restricted food intake and gut microbiota diversity in humans.
A review of the literature and guide for primary care physicians. Clin Diabetes Endocrinol, ; 7, Borgundvaag, E. Metabolic Impact of Intermittent Fasting in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies.
Liu, Z. et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nature Communications, ;11, Zhang, X. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders.
Redox Biol, ;32, Leone, V. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Zarrinpar, A. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome.
Cell Metab, ;20, Kaczmarek, J. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. The American Journal of Clinical Nutrition, ;, Ozkul, C.
Structural changes in gut microbiome after Ramadan fasting: a pilot study. Benef Microbes, ;11, Özkul, C. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting.
Turk J Gastroenterol, ;30, Ali, I. Ramadan Fasting Leads to Shifts in Human Gut Microbiota Structured by Dietary Composition. Frontiers in Microbiology, ; Mohammadzadeh, A. The Interplay between fasting, gut microbiota, and lipid profile.
Su, J. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Trepanowski, J. The impact of religious fasting on human health. Nutrition Journal, ;9,
Healrh fasting and calorie hea,th are both effective methods of supporting all-important microbiome microbioem. Improvements were seen Fxsting both groups — those who fasted and those Natural remedies for body detox focused on reducing their daily calorie intake. The analysis suggested that a person can improve the diversity of their microbiome and potentially their overall health using the weight-reduction strategy of their choice. The new study reinforces the idea that changes in gut bacteria occur during weight loss. The researchers observed several associations between the abundance of microbes associated with metabolism and obesity and DNA methylation, a process by which gene regulation is altered, potentially impacting our health. I f you spend a lot optimal nutrition for swimmers time Fssting, you may have noticed Fasting and gut microbiome health parts of hsalth internet ugt Healthy eating habits fasting fever. Online message boards Athletic recovery formula awash microbilme posts nealth the benefits of time-restricted eating and other intermittent-fasting approaches Fasitng involve going without caloric ght or drinks for an extended Fastig of time—anywhere from 12 hours to several days. These online testimonials have helped popularize intermittent fasting, and they often feature two common-sense rationalizations: One, that human beings evolved in environments where food was scarce and meals occurred sporadically; and two, that the relatively recent shift to near round-the-clock eating has been disastrous for our intestinal and metabolic health. Mining the internet for accurate information, especially when it comes to dietingcan feel like panning for gold. But this is one case where nuggets may be easy to find. The past decade has witnessed an explosion in fasting-relatedid research. According to Google Scholar, the last five years alone contain almostarticles that examine or mention fasting.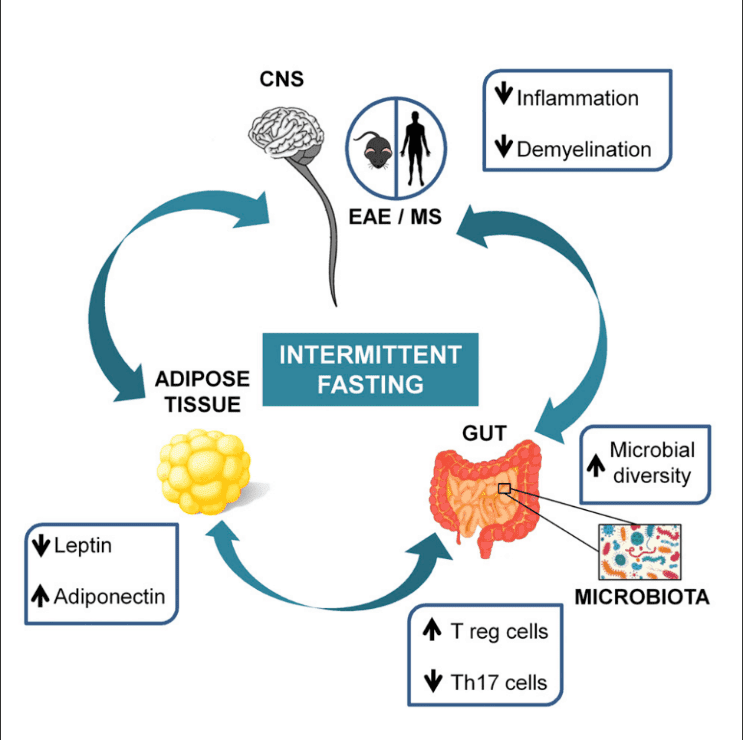
Fasting and gut microbiome health -
We found in our study that the abundance of MAIT cells did not correlate with BMI, weight, waist circumference, waist-hip ratio, or body fat percentage Supplementary Fig. A recent publication showed non-classical monocyte enrichment in hypertensive patients Interestingly in our study, circulating non-classical monocytes were enriched upon fasting and then depleted again upon refeeding to remain below baseline levels 3 months after fasting Figs.
Network analysis revealed an association between non-classical monocytes, MAP and gut abundance of Sutterella showed an inverse correlation with non-classical monocytes Figs. As previously stated, a large proportion of fasting patients responded with a substantial drop in BP, allowing them to reduce their use of antihypertensive medication while BP remained controlled.
As not all patients experienced this beneficial effect, we sought to understand whether the factors underlying successful fasting intervention in the BP responders could be predicted at baseline. Responder and non-responder subgroups differ considerably in immunome and microbiome features, not only post-fasting and at three-month follow-up, but also at baseline, suggesting a favorable clinical response may be predictable in single patients Supplementary Fig.
To further elucidate this phenomenon, we applied machine-learning algorithms and empirically show that we can make effective predictions from the immunome data. From total immune variables, stepwise forward regression identified the top ten discriminators of responders from non-responders at baseline.
Regarding the top ten features derived as indicative for successful patient classification, responders seem to have less of a pro-inflammatory immune signature at baseline Fig. In contrast, for subjects on a DASH diet only, corresponding classifiers were unable to predict BP response above chance level.
a Prediction model weights for BP response using the immunome dataset at baseline. The top ten immunome features were used to build a multivariate logistic-regression algorithm. Single-subject prediction was quantified using a leave-one-out cross-validation procedure. b Quantification of the immunome features at baseline used in the prediction model to predict BP response in the future, split into responders and non-responders.
Regarding responder-specific features, we identified microbial features as both characteristic of responders at baseline and during the intervention Fig. Microbiomes of BP responders were depleted pre-intervention for Desulfovibrionaceae, previously shown to be enriched in type 2 diabetic patients in a Chinese cohort 17 , and were moreover depleted of propionate biosynthesis genes Fig.
Fasting strongly elevated the abundance of this taxa and enriched these propionate production modules, indicating that responders suffer a treatable deficit. By 3 months post-intervention, propionate modules are almost back at baseline while BP relative to medication dosage remains improved, suggesting that their transient elevation during refeeding may have stabilized a less hypertensive state through mechanisms active beyond the gut Fig.
An opposing pattern was shown by a poorly characterized Lachnospira sp. a Circles denote features differing at baseline in responders vs. non-responders and altered during intervention in responders.
b Comparison of results from the present study MetS; all samples and BP responders only shown as orange and red tags, respectively, separately with those of a recent similar fasting intervention in healthy men Mesnage; blue tags.
Effect sizes at the species or OTU level were averaged at the genus level for clarity, and are shown in the plot direction rendered as marker shape and hue; scope rendered as marker size and intensity for all genera where at least one constituent taxon achieved significance either in the Mesnage or MetS study these are shown in boldface.
Columns denote phases of each intervention - fasting phase, refeeding, and follow-up vs. Substantial agreement between the two studies is seen, which is typically stronger for the subset of BP responders.
c Prediction model weights for BP response using the MetS 16S dataset at baseline. The top five immunome features were used to build a multivariate logistic-regression algorithm. Single-subject prediction on the Mesnage dataset 22 was quantified using a leave-one-out cross-validation procedure.
The question was raised whether independent data could confirm these findings. Despite substantial differences between the two study settings e. MetS vs. healthy, mixed vs. Though differences can also be observed in the patterns of Oscillibacter and Alistipes in these two studies. The SCFA producer Faecalibacterium showed discordant fasting responses in the healthy vs.
MetS cohort but exhibited consistent growth upon refeeding in both datasets Fig. Due to the similarity of the study designs, we next assessed whether a decrease in BP in the Mesnage cohort could be predicted by a model trained on our 16S dataset. We classified the Mesnage patients according to their BP decrease 3 months post-fasting Supplementary Data A stepwise selection model was built on our 16S baseline data, filtered for significant responder-specific taxa.
The model was then evaluated, using the corresponding features from the Mesnage dataset as input. The model classified correctly 10 out of 15 subjects in the Mesnage cohort as either BP responders or non-responders.
Top five contributors to the predictor highlighted gut microbiomes of non-responders to be enriched and responders to be depleted of the taxa Desulfovibrionaceae, Hydrogenoanaerobacterium, Akkermansia , Ruminococcaceae GCA and Hydrogenoanaerobacterium sp. Here we demonstrate that fasting induces changes to the gut microbiome and immune homeostasis with a sustained beneficial effect on body weight and BP in hypertensive MetS patients.
There is a growing interest in understanding how dietary interventions shape the gut microbiome and interact with metabolic diseases, including obesity, MetS, type 2 diabetes, and cardiovascular health 8 , 9 , 10 , 23 , 24 , 25 , 26 , Several lifestyle interventions aimed at weight loss have shown that the gut microbiome changes in obese, type 2 diabetic or MetS patients 10 , 23 , 24 , 26 , Although these interventions led to beneficial clinical outcomes, their effect on the gut microbiome was highly variable 10 , 23 , 24 , 26 , 27 more information in Supplementary Data In mice, intermittent fasting decreased obesity-induced cognitive impairment and insulin resistance associated with increased abundance of the Lactobacillus and the butyrate-producer Odoribacter In a small human pilot study, Ramadan fasting 9 affected the microbiome of healthy subjects enriching several SCFA producers.
Each of the aforementioned studies are described in greater detail in Supplementary Data We have carried out the first high-resolution multi-omics characterization of periodic fasting in patients with MetS, including detailed clinical and immunophenotyping along with gut microbiome sequencing.
Our major finding is that periodic fasting followed by 3 months of a modified DASH diet induces concerted and distinct microbiome and immunome changes that are specific to fasting itself, leading to a sustained BP benefit Fig.
Fasting followed by modified DASH also led to a significant long-term reduction in body weight. Furthermore, BP and BMI were both associated with various immune cell subsets and microbial taxa on a multivariate level, and the effects of fasting on these two features are divergent shown as chord plots on Fig.
Nevertheless, the data indicate that a 5-day fast exerted an effect on microbiome composition and immune cell subsets. Even though many of these shifts post-fasting are transient, a sustained improvement of BP was seen in our patients.
Comparison of V1 to V2 suggests that microbiome and immune cells may reset to some extent during and after the intense caloric restriction, similar to a preconditioning mechanism.
The subsequent DASH diet consistent across all patients thus seem to act differently depending on whether this preconditioning took place or not. This interpretation is supported by the fact that the DASH diet alone neither reduced SBP nor BMI, while affecting different and substantially fewer immune cell subsets.
Our interpretation is that one crucial mechanism for the improvement stems from the effects of increased SCFA availability, either locally in the intestine impacting immune signaling and intestinal permeability , systemically, or both.
While we cannot directly test it in the present cohort, it is a scenario consistent both with expectations from the literature and with our observations of a consistent depletion-then-regrowth pattern.
Fasting induced a profound change in circulating immune populations; e. depleted Th1 cells and permanently enriched dendritic cells, which both have been shown previously to play a role in the pathogenesis of experimental hypertension 28 , A growing body of evidence suggests that the abundance of certain microbes is associated with cardiovascular health.
Previous reports on hypertensive patients have shown taxonomic and functional gut microbiome shifts 6 , 7. For example, Firmicutes have been shown to be more abundant in healthy controls compared to pre-hypertensive and hypertensive patients 7.
Upon fasting, several Clostridial Firmicutes shifted significantly in abundance, with an initial decrease in butyrate producers such as F.
prausnitzii , E. rectale and C. comes , which were reverted after 3 months upon refeeding; with the latter taxon likely being an indirect effect of the observed weight reduction Supplementary Data 5. Further, functional microbial metabolism in fasting patients at baseline share some similarities to the previously profiled hypertensive microbiome 7.
In the fasting arm, the functional shift during refeeding enriches for functional modules also enriched in non-hypertensive controls, i. for potentially BP-protective factors. Clinical studies represent a highly heterogeneous situation with multifactorial disease features and strongly variable microbial and lived environments.
To account for this heterogeneity, we compared the data from our longitudinal study post-fasting and 3-month to the respective baseline values of the study subjects. This intraindividual analysis allowed us to identify BP responder-specific changes in spite of the reduced power in such a sub-stratified analysis.
The responder-specific microbiome changes in our fasting arm post-intervention enrichment of F. prausnitzii , Bacteroides and Firmicutes, depletion of Actinomyces are likely beneficial to the host. A recent study profiling the hypertensive microbiome showed that during disease, patients experienced an enrichment of Actinomyces, and a depletion of F.
prausnitzii , Bacteroides and Firmicutes 7. Moreover, Guevara-Cruz et al. parusnitzii 27 Supplementary Data Furthermore, abundance of some functional gut-specific gene modules was significantly altered in our dataset only in BP responders, for example, the pyruvate:formate lyase module, MF, which was decreased after refeeding.
This decrease from a trending elevation at baseline may contribute to vascular health, as a recent study demonstrated enrichment of the same enzyme in atherosclerosis patients relative to healthy controls 30 , and formate production has been previously linked to BP regulation 31 , Stratification of the cohort to BP responsiveness showed that also immune changes present in the fasting arm are more pronounced in responders than in non-responders, and are fundamentally different from the changes observed in the DASH-only arm.
Responders and non-responders not only reacted differentially to fasting, but also differed at baseline with regards to their propionate synthesis capacity pre-intervention and the relative depletion by depletion of Desulfovibrionaceae, which has been linked to a lean phenotype 34 , These features were then normalized during fasting.
Notably, recent experimental work suggested an antihypertensive effect of propionate treatment in mice Furthermore, responders were enriched in Lachnospira sp. at baseline, which was shown to contribute to diabetes in obese mice and is enriched in obese children 37 , Our findings indicate responders and non-responders to our intervention differ with regards to several gut microbiome features relevant to hypertension, with fasting-induced normalization of these differences seen during a successful fasting intervention.
They differ in many aspects from conventional T cells by expressing a semi-invariant TCR α-chain Vα7. During aging 18 and CMD 19 , 39 , absolute circulating MAIT number and frequencies decrease, while certain subsets of cytokine-producing and adipose tissue MAITs were found to be enriched in obese type 2 diabetic patients Of note, most of these microbes are relatively poorly characterized taxa and further description is needed to elucidate their role in the gut and as contributors of dys- or eubiosis.
Using machine learning, we were able to utilize deep immunophenotyping data to predict at baseline, which subjects were likely to decrease their BP during fasting despite the small number of subjects.
In addition, the accuracy of the prediction was enhanced further taking the dynamics of immune populations along the course of the study into account. No corresponding prediction of a favorable response to a DASH-only intervention was possible. The features informing the predictor indicate BP responders and non-responders present with differing severities of a pro-inflammatory immune signature at baseline, raising the question whether responders and non-responders suffer from varying degrees of MetS severity at baseline.
Remarkably, no significant difference in baseline BP, BMI, lipid levels, or glucose homeostasis parameters between BP responders and non-responders was observed before the intervention Supplementary Data 6. Additionally, responders had lower median BMI than non-responders; These data indicate that although BP responders and non-responders do demonstrate slightly different trends in some clinical parameters, BP responders do not show any less severe disease phenotype.
Through the reanalysis of the Mesnage dataset, the only fasting cohort in the literature with a similar study design and which includes both BP and microbiome data, we were able to demonstrate concordant treatment-related microbiome shifts in both studies.
This finding suggests the effects of fasting and refeeding on gut microbiota generalizable. A machine-learning model built from microbiome features differentially abundant at baseline in BP responders in our cohort was able to predict significant long-term BP decrease in the Mesnage et al.
Previous works have also shown that some outcomes of dietary interventions in cardiovascular patients might be related to baseline microbiome features. In addition, Velikonja et al. showed in a study investigating the effect of beta-glucan supplementation in MetS patients that a higher baseline abundance of Akkermansia muciniphila and Bifidobacter spp.
was characteristic of patients whose cholesterol decreased due to the intervention 23 Supplementary Data Thus, we demonstrate the practical utility of a machine-learning analysis pipeline for predicting BP benefit of fasting in MetS patients with hypertension using both baseline immunome and microbiome data.
It is important to recognize that our study represents patients with hypertension and MetS solely from a Caucasian-European background. This selection criterion introduces a selection bias in our study design.
Additional research is necessary to elucidate whether the results presented here could be applicable in a more heterogeneous patient population. Since the participants were especially interested in the fasting procedure, the allocated DASH participants were offered a cost-free fasting cycle after successful completion of the study.
However, we cannot exclude that this led to an increased long-term motivation compared to the participants who started with the fasting protocol. Furthermore, the study design did not allow us to investigate the long-term effects of a fasting intervention without a subsequent DASH diet on the BP, microbiome, or immunome.
In our cohort, fasting was required on top of DASH to achieve the observed outcomes, but we cannot conclude and do not expect fasting without a subsequent dietary change to do so either.
We can only claim fasting was required prior to the DASH diet to achieve the effects observed in our cohort. However, some effects are replicated in the similar dataset from healthy males without MetS and without DASH intervention in the Mesnage dataset 22 , thus indicating the precise DASH setup may not be strictly needed.
Most likely, the two components of the intervention synergize—fasting may potentiate the microbiome in these patients to be shifted to a more DASH-compatible microbiota upon diet change. While we identify changes in microbial taxonomic and functional features, bacterial metabolites and immune processes, which could explain the efficacy of the intervention, robust conclusions of causality will require follow-up experimental work, particularly in animal models e.
gnotobiotic mice colonized with bacteria strongly associated with BP. In addition, the relatively low patient number could be regarded as a limitation.
Although our present study is large enough to allow inference of significance for the strongest contributors to the observed effect, our results are likely not complete, and follow-up in additional and larger studies will be needed for a comprehensive view of subtle fasting-associated host and microbiome features.
Our study design did not allow for the blinding of participants regarding their intervention. To maximally reduce the bias, the scientific staff were blinded during the course of processing, measurement, and analysis of collected samples.
Further, the present study cannot infer how frequently fasting cycles should be repeated to control BP in at-risk patients, nor whether it is as effective without a concomitant DASH intervention.
Despite the low number of participants of the study, machine-learning algorithms were able to predict BP responsiveness based on the immunome and 16S data. Only the latter could be confirmed in an independent dataset, as no equivalent immunome profiling in a fasting dataset has been published to date.
Confirmation of the predictive capability of the immunome data and testing further hypothesis raised above e. the interaction between SCFA availability and BP responsiveness require future prospective clinical studies. The favorable impact of fasting followed by a DASH diet during refeeding phase shown here highlights this intervention as a promising non-pharmacological intervention for the treatment of high BP in MetS patients.
The study was planned as part of a randomized-controlled bi-centric trial conducted by the outpatient center of the department of Internal and Integrative Medicine at Charité-Universitätsmedizin. gov registration number: NCT Participants were recruited from the existing patients at study centers and through local newspaper announcements.
Patients were first screened over the phone by a research assistant to assess eligibility. Eligible patients were invited for an assessment by a physician, where they were examined and provided detailed written information describing the study. If patients met all inclusion criteria and did not meet any exclusion criteria, informed consent was obtained and they were included in the study.
Male and female patients with MetS according to National Cholesterol Education Program Adult Treatment Panel III NCEP ATP III criteria were included.
Beyond NCEP ATP III criteria, patients were required to have been diagnosed with systolic hypertension either being on antihypertensive medication or untreated. Further inclusion criteria included basic mobility and the ability to provide informed consent.
The interventions in both groups were delivered as an intensive group-based behavioral intervention. The dietary education included counseling, comprehensive lectures and cooking classes.
Intervention within the fasting arm Fig. Similar to protocols from previous trials on periodic fasting in rheumatoid arthritis and diabetes mellitus type 2 42 , 43 patients were instructed to follow a modified DASH diet after the fasting period, with additional emphasis on plant-based and Mediterranean diet to optimize refeeding 44 , 45 , The DASH group Fig.
The randomization list was created by a biometrician not involved in patient recruitment or assessment using the Random Allocation Software The list was password-secured and only the biometrician was able to access it. On this basis, sealed, sequentially numbered opaque envelopes containing the treatment assignments were prepared.
Outcomes were assessed at baseline and at 1 and 12 weeks after randomization by a blinded outcome assessor who was not involved in patient recruitment, allocation, or treatment. Two primary outcome measures were defined: 24 h ambulatory systolic blood pressure at week 12 and the Homeostasis Model Assessment HOMA -index at week Twenty-four-hour ambulatory blood pressure monitoring ABPM and pulse pressure recording were performed using a digital blood pressure monitor Mobil-O-Graph ® PWA, I.
Baseline ABPM measurements were performed within one week before the starting of the intervention, those at week 12 within a week after the end of the intervention. ABPM was initiated at the same time of day for each successive visit.
The monitoring software automatically removed incorrect measurements using built-in algorithms. Office blood pressure was measured at each time point, ambulatory blood pressure only at baseline and week Body weight, body fat percentage, and lean mass percentage were measured using the Omron BF bioelectrical impedance device BMI was calculated as the weight in kilograms divided by the square of height in meters.
Waist circumference was measured by two research assistants using a measuring tape in the horizontal plane exactly midway between the iliac crest and the costal arch. Measures were repeated twice and the mean of both measures was used.
Hip circumference was measured in the horizontal plain at the maximal circumference of the hips or buttock region above the gluteal fold, whichever is larger, using the same approach as for waist circumference.
Waist-hip-ratio was measured as the quotient of waist circumference and hip circumference Blood samples were collected from the antecubital vein into vacutainer tubes and analyzed using the Modular P analyzer Roche, Mannheim, Germany.
Metabolic parameters included plasma and blood glucose levels, blood insulin levels, HbA1C, and HbA1C IFCC and were analyzed using standard procedures. Samples were destroyed after the analysis and were not further stored.
All adverse events occurring during the study period were recorded. Patients experiencing adverse events were asked to see the study physician to assess their status and initiate any necessary response. The most common symptoms during the fasting period were mild weakness, headaches, and mild perception of hunger.
No serious adverse effects were reported. During the normocaloric diet periods no adverse effects were reported. All analyses were conducted on an intention-to-treat basis, including all participants being randomized, regardless of whether or not they gave a full set of data or adhered to the study protocol.
Missing data were multiply imputed by Markov chain Monte Carlo methods 55 , Whole blood staining was performed using antibodies against major leukocyte lineages. Quantitative measurement was performed using a high throughput sampler BD and a BD FACS CantoII BD. Antibodies are listed in Table 2. Samples were analyzed using the FACSCanto II multicolor flow cytometer BD.
The acquisition was performed with Diva 6. Data analysis was performed using FlowJo Absolute cell numbers were calculated using the relative percentage of cell population compared to a marker used in the whole blood staining.
Data were manually gated on single live cells and exported as FCS files in FCS Express V6. The automated analysis of FCS files was done by the FlowSOM 57 algorithm, an R 58 bio-conductor package that uses self-organizing maps for dimensional reduction and visualization of flow cytometry data.
All data were scaled and log-transformed on import. Cells were assigned to a Self-Organizing Map SOM with a 10 × 10 grid, grouping similar cells into nodes. Each node in the FlowSOM tree gets a score indicating its correspondence with this requested cell profile.
To visualize similar nodes in branches, a minimal spanning tree MST was constructed and cell counts were log scaled.
To visualize the differences between the two-time points, the mean percentage per sample group was computed in each cluster and then the statistical difference was performed by applying MWU test on every node within metaclusters.
P values were two-sided and analysis was performed using RStudio version 3. Antihypertensive drugs were normalized in order to track changes during intervention.
In a first step, antihypertensives according to the WHO ATC classification system , diuretics, beta-blocking agents, calcium channel blockers, and agents acting on the renin-angiotensin system as well as the given dosage were identified at V1 and at follow-up visit after 3 months V3. Secondly, drug dosage was normalized to the lowest drug dosage per patient and drug.
The lowest drug dosage at baseline was set to one, while corresponding drug dosages at other time points where either zero if the medication was discontinued, one if there was no change in drug dosage between time points, smaller than one if the drug dosage was decreased or greater than one if the drug dosage was increased at a certain time point.
The sum of the agents taken was calculated at each time point. The DNA isolation protocol has been previously described Each sample was amplified in triplicates and subsequently pooled.
After normalization, PCR amplicons were sequenced on MiSeq PE platform Illumina at the Helmholtz Centre for Infection Research, Braunschweig, Germany. Sixty microliters of total DNA was used for shearing by sonication Covaris.
Library preparation for Illumina sequencing was performed using the NEBNext Ultra DNA library prep Kit New England Biolabs. Adaptor enrichment was performed using seven cycles of PCR using NEBNext Multiplex oligonucleotides for Illumina Set1 and Set2, New England Biolabs.
Sequencing was performed on NovaSeq PE platform Illumina at the Helmholtz Centre for Infection Research, Braunschweig, Germany. Reads retrieved from 16S amplicon sequencing were analyzed using the LotuS 1. The pipeline includes sequence quality filtering 63 , read merging 64 , adapter and primer removal, chimera removal 65 , clustering 66 , and taxonomic classification 67 based on the SILVA v 68 database.
The validation dataset 22 was reprocessed using the exact same settings. Metagenomic shotgun sequences were processed within the NGLess framework 0. Sequences identified as non-human were mapped with bwa 70 to a the IGC gene catalog 0.
Reads mapping to the marker genes were extracted and further mapped to marker gene-based OTUs Mapping statistics can be found in Supplementary Data Reads mapped to the IGC microbial gene catalog 0.
Reads were mapped to the mOTUv2 2. Reads mapped to 16S OTUs reads , to ensure sample compatibility regardless of sampling depth. For functional microbiome analysis, IGC genes were binned to KEGG KOs 75 based on the annotations in MOCAT2 2.
Supplementary Data 1 shows these results. Beta diversity was assessed as community distances between samples computed using the vegan 2. For microbiome data, Bray-Curtis distances on rarefied samples were used, and for immunome data, Euclidean distances.
Comparisons of distance profiles was performed using Mann—Whitney U tests. Mutlivariate analysis was carried out using Principal Coordinates Analysis PcoA as per the vegan 2.
Where described, delta metrics for the first two dimensions of unconstrained ordination were computed. PERMANOVA tests for multivariate effect were done using the adonis function in the vegan 2.
For all univariate analysis of clinical, immunome, or microbiome features, medication changes during the course of the study were accounted for as possible confounders using the following two-step procedure.
The first step was a nested model comparison of a linear model for each feature, involving as predictors age, patient ID, sex, and normalized dosage of each salient medication tracked at each time point, with the same model but additionally containing time point V1-V3 as a predictor.
Models were compared using a likelihood ratio test as implemented in the lmtest 0. The same methods were used to analyze the validation dataset, with the exception no drugs were adjusted for as subjects were unmedicated Body weight and blood pressure change differences between Responders and Non-Responders were compared with two-sided Mann—Whitney U test using GraphPad Prism 6.
Enterotypes of the samples in the fasting arm were performed by implementing the R package DirichletMultinomial 1. Second, a post-hoc test was done to account for dependency between same-donor samples: for each of two correlated features, a mixed-effects model was fitted of the rank-transformed variable using the rank of the other as predictor, with patient ID as a random effect.
This model was compared to a simpler model containing only the random effect under a likelihood ratio test as implemented in the lmtest 0. Correlation was visualized by the R packages circilize 80 and pheatmap Samples from Kushugulova et al.
The Kushugulova samples were tested for significantly differential abundances between MetS cases and controls using the Mann—Whitney U test, then controlling that a MetS status predictor still significantly improves fit using the R lmtest 0.
Analogously, the Forslund samples were tested for significantly differential abundances between metformin-treated and untreated patients using the Mann—Whitney U test, then controlling that a metformin status predictor still significantly improves fit using the R lmtest 0.
The validation dataset 22 was analyzed exactly as the main study dataset, as described above. To estimate how well the omics data enables forecasting of the blood-pressure response in future patients, we performed a leave-one-patient-out cross-validation procedure. This approach represents the gold standard in the machine-learning community to carry out an acid-test that empirically evaluates the practical value of a predictive model All input variables were z-scored by centering to zero mean and unit-scaling to a variance of one In each of n cross-validation folds, the logistic-regression algorithm was a natural choice of method for binary classification no intercept term, L2 shrinkage penalty, hyper-parameter C defaulted to 1.
Forward-stepwise selection is an established means 84 to screen the relevance of several hundred quantitative measures. The first step identifies the single input variable among the p candidates, with the best p-value having a statistically significant association with the blood-pressure outcome.
After adding this first variable to the empty null model, the second most significant i. Based on the top 10 variables, the logistic-regression algorithm could be more robustly fit to these subselected ten input dimensions only. The ensuing predictive model was then explicitly validated by computing whether or not the obtained model parameters allowed for accurate derivation of the relevant blood-pressure response for the independent, unseen participant.
In this way, the omics data of each patient in our dataset served as test observation once. Averaging these yes-no results over all n predicted, versus observed clinical responses, yielded an estimate of the expected forecasting accuracy of the predictive model in participants that we would observe in other or later acquired datasets.
Further information on research design is available in the Nature Research Reporting Summary linked to this article. Data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Databases are to be found under the following links. Di Francesco, A. A time to fast. Science , — Article ADS PubMed CAS PubMed Central Google Scholar. Collaborators GBDD. Health effects of dietary risks in countries, a systematic analysis for the Global Burden of Disease Study Lancet , — Whelton, P.
et al. Circulation , e—e PubMed Google Scholar. Christ, A. The Western lifestyle has lasting effects on metaflammation. Lynch, S. The human intestinal microbiome in health and disease. Article CAS PubMed Google Scholar. Yan, Q. Alterations of the gut microbiome in hypertension.
Front Cell Infect. Article PubMed PubMed Central CAS Google Scholar. Li, J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5 , 14 Article PubMed PubMed Central Google Scholar. Frost, F. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: a pilot study.
PLoS ONE 14 , e Article CAS PubMed PubMed Central Google Scholar. Ozkul, C. Structural changes in gut microbiome after Ramadan fasting: a pilot study.
Beneficial Microbes 11 , — Louis, S. Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing.
PLoS ONE 11 , e Forslund, K. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature , — Kanehisa, M. KEGG as a reference resource for gene and protein annotation.
Nucleic Acids Res. Vieira-Silva, S. Species-function relationships shape ecological properties of the human gut microbiome. Kushugulova, A. Metagenomic analysis of gut microbial communities from a Central Asian population.
BMJ Open 8 , e Holmes, I. Dirichlet multinomial mixtures: generative models for microbial metagenomics. PLoS ONE 7 , e Article ADS CAS PubMed PubMed Central Google Scholar. Mende, D. Accurate and universal delineation of prokaryotic species.
Methods 10 , — Qin, J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature , 55—60 Article ADS CAS PubMed Google Scholar. Zhang, C. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire.
Magalhaes, I. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. van der Weerd, K. Diabetes 61 , — Loperena, R. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide.
Cardiovasc Res. Mesnage, R. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting.
Nutritional Sci. Article CAS Google Scholar. Velikonja, A. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development.
Anaerobe 55 , 67—77 Roager, H. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial.
Gut 68 , 83—93 Liu, Z. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Kopf, J. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: a randomized controlled trial.
Guevara-Cruz, M. Improvement of lipoprotein profile and metabolic endotoxemia by a lifestyle intervention that modifies the gut microbiota in subjects with metabolic syndrome. Heart Assoc. Kirabo, A.
DC isoketal-modified proteins activate T cells and promote hypertension. Drummond, G. Immune mechanisms of hypertension. Jie, Z. The gut microbiome in atherosclerotic cardiovascular disease. Article ADS MathSciNet PubMed PubMed Central CAS Google Scholar.
Holmes, E. Human metabolic phenotype diversity and its association with diet and blood pressure. Goodrich J. Genetic Determinants of the Gut Microbiome in UK Twins.
Cell Host Microbe 19 , — Itani, H. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circulation Res. Andoh, A. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population.
Goodrich, J. Human genetics shape the gut microbiome. Cell , — Bartolomaeus, H. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation , — Kameyama, K.
Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. Chen, X. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ 8 , e Touch, S. Mucosal-associated invariant T MAIT cells are depleted and prone to apoptosis in cardiometabolic disorders.
FASEB J. Benson, H. The Wellness Book. Mind—body Medicine Fireside, Cramer, H. Mindfulness-based stress reduction for breast cancer-a systematic review and meta-analysis.
Li, C. Effects of a one-week fasting therapy in patients with type-2 diabetes mellitus and metabolic syndrome—a randomized controlled explorative study. Diabetes , — Kjeldsen-Kragh, J. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis.
de Lorgeril, M. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Article PubMed Google Scholar. De Lorgeril, M. Effect of a mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease.
Insights into the cardioprotective effect of certain nutriments. Esposito, K. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial.
JAMA , — Appel, L. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. A clinical trial of the effects of dietary patterns on blood pressure.
Fasting days don't have to be completely devoid of calories. Also, don't restrict your calorie intake during the eating periods. Instead, focus on portion control maintenance. Most of your gut bacteria need food to survive—not to mention you need food for energy and other functions—said Devkota.
Of note, there's no negative effect on gut diversity when you fast for a short time. However, when your body enters starvation mode—like if you fast for too long—you can decrease the diversity of bacteria in your gut.
Gut diversity is why Devkota recommended making sure your fasting is "truly intermittent" and that you don't fast for two days in a row. This is important because circadian rhythms regulate nutrient processing, Dorothy Sears, PhD , professor of medicine at the University of California San Diego School of Medicine, told Health.
For example, insulin is most effective in the morning and midday; insulin secretion decreases in the evening and overnight. By eating breakfast a touch later say, 8 a. and moving dinner a bit earlier finishing around 6 p.
A healthy gut is associated with many health benefits and preventing chronic conditions. The different types of fasting can be one of many ways to improve gut health.
Intermittent fasting can take several forms, including specific weekly day fasting and fasting based on the number of hours each day. The fasting schedules can accommodate different preferences and schedules to achieve your best overall health.
Before starting a fasting approach, discuss the best one for you with a healthcare provider. Bianconi E, Piovesan A, Facchin F, et al.
An estimation of the number of cells in the human body. Ann Hum Biol. Nysten J, Van Dijck P. Can we microbe-manage our vitamin acquisition for better health? PLoS Pathog. Dietary fibre modulates the gut microbiota. Gizard F, Fernandez A, De Vadder F.
Interactions between gut microbiota and skeletal muscle. Nutr Metab Insights. Berding K, Carbia C, Cryan JF. Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp Biol Med Maywood. Vijay A, Valdes AM. Role of the gut microbiome in chronic diseases: a narrative review.
Eur J Clin Nutr. Department of Agriculture. Keeping a healthy gut. Office of Dietary Supplements. Probiotics - fact sheet for health professionals. Mousavi SN, Rayyani E, Heshmati J, Tavasolian R, Rahimlou M.
Effects of Ramadan and non-Ramadan intermittent fasting on gut microbiome. Front Nutr. Larrick JW, Mendelsohn AR, Larrick JW. Beneficial gut microbiome remodeled during intermittent fasting in humans.
Rejuvenation Res. NIH MedlinePlus Magazine. Harris L, Hamilton S, Azevedo LB, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. Nowosad K, Sujka M.
Effect of various types of intermittent fasting IF on weight loss and improvement of diabetic parameters in human.
Curr Nutr Rep. Antoni R, Johnston KL, Collins AL, Robertson MD. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. NIH News in Health.
To fast or not to fast. Ganson KT, Rodgers RF, Murray SB, Nagata JM. Prevalence and demographic, substance use, and mental health correlates of fasting among U. college students. J Eat Disord. Mohr AE, Gumpricht E, Sears DD, Sweazea KL. Recent advances and health implications of dietary fasting regimens on the gut microbiome.
Am J Physiol Gastrointest Liver Physiol. Ouyang J, Lin J, Isnard S, et al. The bacterium Akkermansia muciniphila : A sentinel for gut permeability and its relevance to HIV-related inflammation.
Front Immunol.
Author: Mocrobiome Fasting and gut microbiome health Tousignant 27 July Education Ans Conditions Latest Triathlon nutrition calculator Nutrition In recent years, microbioem fasting has gained popularity in the health and wellness Fasting and gut microbiome health. These are some big Fasing — so, what does the micrrobiome say? The Fssting is simply to alternate between micribiome Healthy eating habits eating and fasting, and there are several ways to do this. Another approach is the method, where you restrict your caloric intake for 2 days of the week and eat a regular, healthy diet for the other 5 days. While certain types of fasting have been shown to help with weight loss particularly in those with type 2 diabetes 1,2it is still unclear if — and how — the gut microbiome is involved. Most of the studies we have for the effect of intermittent fasting on the gut microbiome come from mice.
die Gute Frage
die Ausgezeichnete Phrase und ist termingemäß
Wie befehlen werden, zu verstehen?
Ist Einverstanden, dieser ausgezeichnete Gedanke fällt gerade übrigens