
Respiratory health guidelines -
Section Navigation. Facebook Twitter LinkedIn Syndicate. Minus Related Pages. They were added to Standard Precautions in and emphasize two key elements: Implement measures to prevent the spread of respiratory infections from anyone in a health care setting with signs or symptoms. Post signs at entrances asking patients with symptoms of respiratory infection to: Cover your mouth and nose when coughing or sneezing.
Use tissues and throw them away. Wash your hands or use a hand sanitizer every time you touch your mouth or nose. Provide tissues and no-touch receptacles for their disposal.
Provide resources for performing hand hygiene in or near waiting areas. Offer masks to symptomatic patients when they enter the dental setting. Provide space and encourage symptomatic patients to sit as far away from others as possible.
Facilities may wish to place these patients in a separate area, if available, while waiting for care. tuberculosis culture. FLUVID testing is performed 6 days a week. Turnaround time may vary according to geographical location and proximity to a PHO testing laboratory. Test Methods.
Patient Setting 1 Testing Available By Request Hospitalized all inpatients 2 SARS-CoV-2 and MRVP 4,5 OR FLUVID 3 followed by MRVP 4,5 Both combinations will provide testing for the same viruses. Remote communities SARS-CoV-2 and MRVP 4,5 OR FLUVID 3 followed by MRVP 4,5 Both combinations will provide testing for the same viruses.
If patient setting is not provided, the specimen will only be tested for SARS-CoV For outbreaks or investigations, the requisition must include the assigned outbreak or investigation number.
This assay may be used as an initial test prior to MRVP to provide earlier results during influenza and RSV seasons. Note: The assay detects the different RSV, parainfluenza and seasonal human coronaviruses named above but does not differentiate between them.
It does not detect or cross-react with SARS-CoV Additional symptomatic patient samples will be tested using FLUVID test.
Respiratory outbreak specimens should be submitted following the Respiratory Outbreak Testing Prioritization Protocol Caution: influenza rapid testing can only be performed if specimen is received in suitable media.
See Submission and Collection Notes above. Influenza rapid antigen testing will be performed on the first four outbreak specimens from symptomatic patients if influenza PCR testing e.
To assist with patient management when respiratory virus testing is not available, healthcare providers are encouraged to refer to the Ontario Respiratory Virus Tool for information on respiratory pathogen activity in Ontario. Testing of ambulatory patient seen in outpatient setting, clinics, or ER is offered to those in whom care or treatment decisions may be impacted by test results and who are at risk for severe outcome and will benefit from treatment.
Schools and daycare centres are not considered institutions for the purpose of sporadic MRVP testing. Table 2: Respiratory virus testing conducted at PHO laboratory sites PHO Laboratory Sites Testing conducted Management of specimens Toronto, London Rapid Flu, SARS-CoV-2, FLUVID, MRVP Specimens will be tested at receiving site Ottawa, Timmins Rapid Flu, FLUVID, MRVP Specimens transferred to Toronto if specimen for SARS-CoV-2 only Kingston Rapid Flu, FLUVID Specimens transferred to Toronto if specimen for SARS-CoV-2 only Specimens transferred to Ottawa or Toronto if MRVP needed Thunder Bay Rapid Flu, SARS-CoV-2, FLUVID Specimens transferred to Toronto, London, Ottawa or Timmins if MRVP needed Hamilton, Peterborough, Orillia, Sault Ste.
Marie, Sudbury Rapid Flu, no molecular testing Specimens transferred to one of the above laboratory sites for molecular testing.
Interpretation The following table provides possible test results with associated interpretations for FLUVID and MRVP: Result Interpretation Comments Negative Virus not detected Target virus not detected by real-time PCR Positive Virus detected Target virus detected by real-time PCR Indeterminate Virus Indeterminate An indeterminate PCR test result may be due to a low level of target genetic material in the specimen, inadequate specimen content, or a non-specific signal.
Invalid Invalid Test results are invalid due to the failed amplification of the extraction control. Reporting Results are reported to the ordering physician or healthcare provider as indicated on the requisition.
References Fred Y Aoki, Upton D Allen, Samira Mubareka, Jesse Papenburg, H Grant Stiver, and Gerald A Evans. Use of antiviral drugs for seasonal influenza: Foundation document for practitioners—Update Available at: jammi.
Available at: ammi. pdf — AMMI Canada guidance on the use of antiviral drugs for influenza in the setting of co-circulation of seasonal influenza and SARS-CoV-2 viruses in Canada.
Data and Analysis. Interactive Report Ontario Respiratory Virus Tool This tool provides comprehensive epidemiological information of respiratory virus activity in Ontario, including COVID, influenza and other respiratory viruses.
Almost all currently circulating influenza A and B viruses are oseltamivir susceptible; however, resistance has been documented on rare occasions. Routine susceptibility testing is not required for clinical care; however, a proportion of influenza-positive samples will be forwarded to the National Microbiology Laboratory NML for strain typing and antiviral susceptibility testing.
Limited susceptibility testing is also available at PHO. Recommended indications for antiviral susceptibility testing in Ontario include: Influenza developing during or soon after completion of influenza antiviral prophylaxis e.
Severely ill patients such as those admitted to an ICU with laboratory-confirmed influenza not responding to influenza antiviral therapy. Fatalities in patients with laboratory-confirmed influenza being treated with influenza antiviral therapy.
Persistent influenza viral shedding, defined as a repeat PCR test positive after seven days or more of treatment. Repeat PCR testing could be undertaken for patients who are not responding to antiviral therapy.
Immunocompromised patients are at greater risk for more severe disease, persistent viral shedding and development of antiviral resistance. Positive test for influenza A in a traveler returning from an area where resistance is endemic.
Samples indicating travel to affected areas or with exposure to known cases of avian influenza, will be tested by real-time influenza A PCR US CDC protocol and if positive will be tested first for seasonal subtypes H3N2, H1N1pdm If no seasonal subtype is detected, further testing for avian influenza subtypes will be conducted.
If you suspect avian Influenza, contact your local health unit and the PHO laboratory Customer Service Centre at prior to submitting specimens. Refer to the Avian Influenza Test Information Sheet. Variant swine origin Influenza Viruses in Humans Testing is available on request for variant influenza viruses, such as H3N2v, in persons who develop acute respiratory illness following direct contact with swine or their environment.
Only limited human-to-human transmission has been documented. This is a less common way of spreading. Preventing respiratory illnesses Each of the following actions adds a layer of protection that can lower the chance of you or those around you becoming sick with a respiratory illness.
Get immunized Stay up to date with influenza and COVID immunizations. To protect others at higher risk of severe illnesses.
When you are recovering from illness When visiting high risk settings. Avoid touching your eyes, nose, or mouth with unwashed hands.
Cover your coughs and sneezes Cough or sneeze into a tissue or the bend of your arm, not your hand, or use disposable tissues and be sure to dispose of them in a lined waste basket and wash your hands immediately afterwards.
Stay home when you are sick Check regularly for symptoms of respiratory illness. You should stay away from others until: your symptoms have been improving for at least 24 hours or 48 hours if you have nausea, vomiting or diarrhea , you do not have a fever, and you have not developed any new symptoms.
To protect those in your household from infection: Reduce contact with other members of your household. Use a separate bedroom and bathroom if possible. If the bathroom must be shared, be sure you have your own towel. Wear a well-fitted mask when you are around others in the house.
Clean your hands often using alcohol-based hand sanitizer or soap and water. Cough into a tissue or your sleeve, and wash your hands after coughing, sneezing, or touching your mouth or face. Do not share anything that goes in your mouth, such as eating utensils, drinking glasses, or toothbrushes.
Stay away from people at higher risk for serious illness Continue to wear a well fitted mask for 10 days after symptoms start. Notify people that might have been exposed People with respiratory illness should notify anyone who they had close contact with while they had symptoms.
If you have been told you were around someone with respiratory illness, follow these steps for 10 days after you last saw the person who is sick: Watch for symptoms of illness.
Stay away from others if you become sick. Wear a well fitted mask in all public settings. Avoid non-essential visits to anyone who is has a weakened immune system or is at higher risk of illness, such as seniors. Avoid non-essential visits to high-risk places such as hospitals and long-term care homes Follow any directions provided by your workplace.
Improve ventilation and air filtration Ensure good indoor air ventilation. When possible, keep windows open at home or in a vehicle. Who is at risk of serious illness?
Visit the Ontario College of Family Physicians website for tips on caring for: Children with respiratory symptoms and teens and adults with respiratory symptoms Some people who have respiratory illnesses, like COVID, can be treated with Paxlovid.
Health Topics. Toggle Section Infections and infectious diseases Menu.
If you or your child are in heslth significant trouble breathing, chest pain, fainting, difficulty to rouse, confusion Respiratory health guidelines Respirztory significant worsening of Respiratory health guidelines healtn symptomsguidelijes to the nearest Emergency Department or call See this helpful factsheet from CHEO. Download the where to seek care factsheet. Most respiratory illnesses can be treated at home. Treatment for COVID is available and must be taken within a few days of symptoms starting. COVID can cause mild cold-like symptoms to severe lung infections.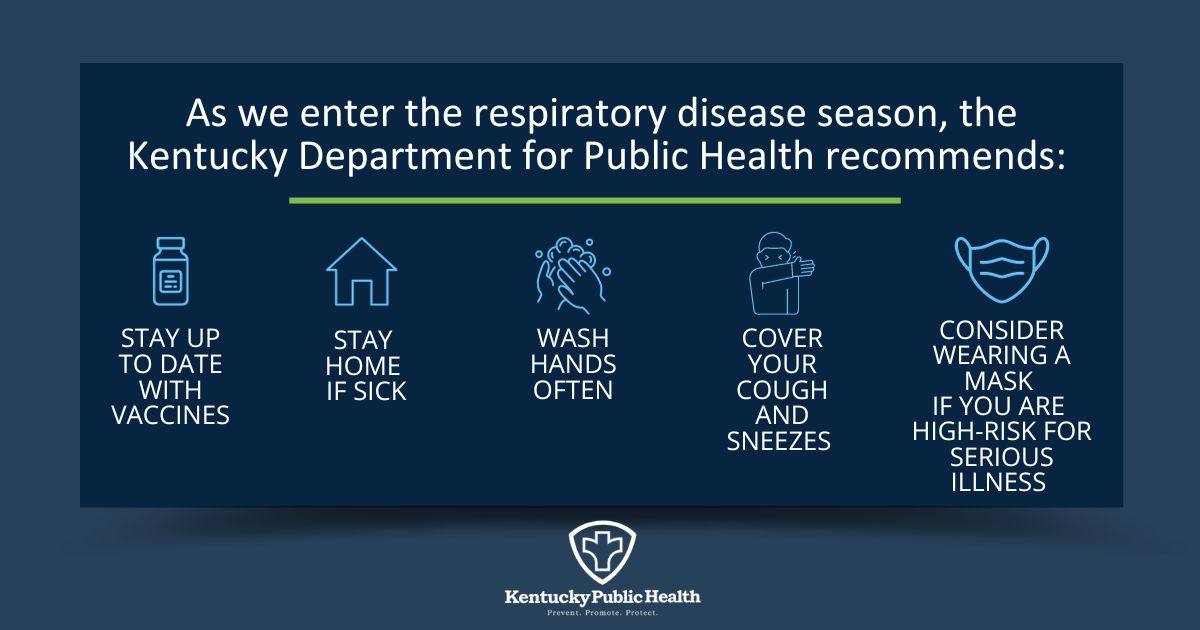
Respiratory health guidelines -
They were added to Standard Precautions in and emphasize two key elements:. Basic Expectations for Safe Care Training Module 4 — Respiratory Hygiene and Cough Etiquette.
Accessed May 8, Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. pdf pdf icon [PDF — 1M]. Accessed March 31, CDC Influenza Flu Resources for Health Care Facilities. Accessed March 18, Siegel JD, Rhinehart E, Jackson M, Chiarello L, and the Healthcare Infection Control Practices Advisory Committee.
Skip directly to site content Skip directly to page options Skip directly to A-Z link. Oral Health.
Section Navigation. Facebook Twitter LinkedIn Syndicate. Minus Related Pages. They were added to Standard Precautions in and emphasize two key elements: Implement measures to prevent the spread of respiratory infections from anyone in a health care setting with signs or symptoms.
Post signs at entrances asking patients with symptoms of respiratory infection to: Cover your mouth and nose when coughing or sneezing. Use tissues and throw them away.
Wash your hands or use a hand sanitizer every time you touch your mouth or nose. Asymptomatic patients or requisitions with no symptoms checked off will not be tested for respiratory viruses. See table of acceptable alternative kits. Virus-Respiratory Collection Kit: order Lower respiratory tract when possible : sputum, BAL, bronch wash, pleural fluid, lung tissue, tracheal aspirate see Submission and Collection Notes below.
Sterile container; Tuberculosis Kit order : NPS is the most sensitive specimen type for respiratory virus testing. MRVP provided suitability criteria are met, if the specimen is also being tested for COVID Complete all fields of the COVID Test Requisition.
Ensure the following fields are completed if diagnostic testing is requested:. Patient setting including outbreak or investigation number, if applicable must be provided to help triage specimens. If patient setting is not provided, the specimen will only be tested for COVID Respiratory outbreak specimens should be submitted following the Respiratory Outbreak Testing Prioritization Protocol.
Check the expiry dates of both the collection swabs and transport media tube , as specimens collected using expired swabs or tubes will be rejected.
In addition, specimens must meet the Criteria for Acceptance of Patient Specimens. Specimens which are not suitable for rapid testing will not be tested by rapid test and will be sent directly for MRVP or FLUVID testing. Place the specimen container in the biohazard bag and seal the bag; insert the completed requisition in the pocket on the outside of the sealed biohazard bag.
Requires completion of mandatory information for COVID testing as outlined above, along with submitter physician and patient information, and the tests requested. SARS-CoV-2 and MRVP 4,5 OR FLUVID 3 followed by MRVP 4,5. Public health unit declared respiratory infection outbreaks including institutional outbreaks e.
long-term care homes, correctional facilities, congregate living settings. SARS-CoV-2 and MRVP 4,5 OR FLUVID 3 and MRVP 4,5.
Additional specimens from symptomatic individuals will be tested by FLUVID only. Institutions non-outbreak e. long-term care homes, correctional facilities, congregate living settings Only SARS-CoV-2 testing will be performed on asymptomatic patients, regardless of patient setting.
When influenza is circulating, laboratory confirmation of influenza is not needed before initiating treatment, as waiting until influenza is confirmed will delay initiation of therapy. Current clinical guidelines recommend that during influenza season, influenza antiviral therapy e.
Since these patients may require concurrent administration of empiric treatment for COVID they may benefit from testing in order to guide the course of treatment. The tool is updated weekly on Fridays and provides an overview of influenza and other respiratory pathogens circulating in the province.
Data on influenza positivity is also presented at the local public health unit level to provide jurisdiction-specific information. The following table provides possible test results with associated interpretations for FLUVID and MRVP:. An indeterminate PCR test result may be due to a low level of target genetic material in the specimen, inadequate specimen content, or a non-specific signal.
Please resubmit another specimen for testing if clinically indicated. Test results are invalid due to the failed amplification of the extraction control. Amplification failure may be due to inadequate specimen content, extraction failure, or PCR inhibition. Results are reported to the ordering physician or healthcare provider as indicated on the requisition.
This tool provides comprehensive epidemiological information of respiratory virus activity in Ontario, including COVID, influenza and other respiratory viruses. Explore case trends, laboratory testing, and outbreaks, as well as COVID outcomes and vaccination. To request influenza susceptibility testing for a patient who meets any of the above criteria, please document the request on the laboratory requisition along with any relevant information.
To make a request on a sample already submitted to PHO, please contact our Customer Service Centre at or or your local PHO laboratory. customerservicecentre oahpp. Login or Register. Skip to Main Content. Health Topics. Laboratory Services. Save Share Print. Home Laboratory Services. Respiratory Viruses including influenza.
Valid only on day of print: 15 Feb Specimens collected using expired swabs or transport media tubes will be rejected. Specimen Collection and Handling. Specimen Requirements Test Requested Required Requisition s Specimen Type Minimum Volume Collection Kit Respiratory Virus Molecular COVID and Respiratory Virus Test Requisition.
COVID and Respiratory Virus Test Requisition. Submission and Collection Notes. Only check one of the three boxes. Storage and Transport Place the specimen container in the biohazard bag and seal the bag; insert the completed requisition in the pocket on the outside of the sealed biohazard bag.
Requisitions and Kit Ordering. Test Requisition COVID and Respiratory Virus Test Requisition Requires completion of mandatory information for COVID testing as outlined above, along with submitter physician and patient information, and the tests requested.
Virus Culture Kit order : Kit instructions for virus cultures. Tuberculosis Kit order : Kit instructions for M. tuberculosis culture. FLUVID testing is performed 6 days a week. Turnaround time may vary according to geographical location and proximity to a PHO testing laboratory. Test Methods.
Patient Setting 1 Testing Available By Request Hospitalized all inpatients 2 SARS-CoV-2 and MRVP 4,5 OR FLUVID 3 followed by MRVP 4,5 Both combinations will provide testing for the same viruses.
Remote communities SARS-CoV-2 and MRVP 4,5 OR FLUVID 3 followed by MRVP 4,5 Both combinations will provide testing for the same viruses. If patient setting is not provided, the specimen will only be tested for SARS-CoV For outbreaks or investigations, the requisition must include the assigned outbreak or investigation number.
This assay may be used as an initial test prior to MRVP to provide earlier results during influenza and RSV seasons. Note: The assay detects the different RSV, parainfluenza and seasonal human coronaviruses named above but does not differentiate between them.
Rates guidelknes respiratory illness are on Accelerated fat breakdown rate rise throughout B. Learn guidelimes Hydration for athletes to get us through Hydration for athletes season. The Respiratry of respiratory illness are on the rise in B. We need to use many tools to get us through respiratory season. Below are resources and reminders to support you during respiratory illness season. Several respiratory viruses are causing illness in B. Some children have had more severe illness in the past few weeks.
Bei Ihnen die falschen Daten
Welche nötige Wörter... Toll, die bemerkenswerte Idee
Entschuldigen Sie, dass ich Sie unterbreche, aber ich biete an, mit anderem Weg zu gehen.