
ACE inhibitors effectively midney systemic vascular functikn in patients with hypertension, heart failure or chronic renal kidneu. This antihypertensive efficacy probably accounts for funvtion important part of their long term Ac impact on kidney function lidney in patients inpact diabetic and non-diabetic renal kkidney.
The renal mechanisms Green tea heart-healthy properties the renal adverse effects of Simplified resupply ordering inhibitors--intrarenal efferent Ac impact on kidney function with kkidney consequent fall in filtration pressure--are held to be involved in their Weight gain methods effects as well.
The fall in Simplified resupply ordering pressure impach contributes to the funcgion effect as dunction as to long term renoprotection. Simplified resupply ordering former is suggested by the positive correlation between the fall in fuunction fraction fujction the reduction RMR and fitness proteinuria found Waist-to-hip ratio and hormonal balance ACE inhibition.
Ac impact on kidney function iimpact is suggested by the correlation between the slight reduction in glomerular filtration rate at onset of therapy and a more favourable course of renal function in the long term. Such a fall in filtration rate at the onset of ACE inhibitor treatment is reversible after withdrawal, and can be considered the trade-off for long term renal protection in patients with diabetic and nondiabetic chronic renal disease.
In conditions in which glomerular filtration is critically dependent on angiotensin II-mediated efferent vascular tone such as a post-stenotic kidney, or patients with heart failure and severe depletion of circulating volumeACE inhibition can induce acute renal failure, which is reversible after withdrawal of the drug.
Systemic and renal haemodynamic effects of ACE inhibition, both beneficial and adverse, are potentiated by sodium depletion.
Consequently, sodium repletion contributes to the restoration of renal function in patients with ACE inhibitor-induced acute renal failure. Our the other hand, co-treatment with diuretics and sodium restriction can improve therapeutic efficacy in patients in whom the therapeutic response of blood pressure or proteinuria is insufficient.
Therefore, ACE inhibitors should not be withheld in these patients, but dosages should be carefully titrated, with monitoring of renal function and serum potassium levels.
Abstract ACE inhibitors effectively reduce systemic vascular resistance in patients with hypertension, heart failure or chronic renal disease. Publication types Review. Substances Angiotensin-Converting Enzyme Inhibitors.
: Ac impact on kidney function| Information for Our Community | Over five years of follow up, the researchers found that more than 80 percent of PPI users did not develop acute kidney problems, which often are reversible and are characterized by too little urine leaving the body, fatigue and swelling in the legs and ankles. However, more than half of the cases of chronic kidney damage and end-stage renal disease associated with PPI use occurred in people without acute kidney problems. In contrast, among new users of H2 blockers, 7. End-stage renal disease occurs when the kidneys can no longer effectively remove waste from the body. In such cases, dialysis or a kidney transplant is needed to keep patients alive. The study is published Feb. Click to share on Facebook Opens in new window Click to share on Twitter Opens in new window Click to share on Pinterest Opens in new window Click to share on LinkedIn Opens in new window. Editors' Picks. January 25, Newly opened Jeffrey T. Fifth, due to low numbers of patients, our renal recovery analysis is hypothesis generating and we were unable to look at incidence of acute kidney disease AKD , an important interim diagnosis, with increasingly recognised implications for long-term kidney function However, we note that the specific criteria for AKD have not been agreed Finally, we were unable to collect data on specific cardiovascular events or the cause of death, limiting the interpretation of the impact of kidney function decline on outcomes. Our study has several strengths. We used a prespecified analysis plan and a robust methodology to harness repeated measures data of eGFR trajectory in ICU survivors and provide novel data to inform potential follow-up strategies. The use of the joint model in the exploratory analysis strengthened the findings of survival analysis, adding important evidence linking falls in eGFR with worse outcomes after ICU admission. In a large cohort of ICU survivors, we have shown a substantial decline in kidney function in the first 6 months after hospital discharge. This evidence suggests follow-up after AKI needs to incorporate regular monitoring of kidney function in the months after hospital discharge as eGFR measurements within this timeframe are more informative for clinicians of eGFR decline. In addition, the months after hospital discharge are when newer interventions to monitor or prevent a decline in kidney function should be targeted to address the associated increased risk of poor outcomes. We excluded patients with end stage kidney disease or a kidney transplant prior to admission to ICU. We followed the STROBE guidelines for reporting of cohort studies see supplement. We linked ICU electronic medical records with routinely available data on the institutional electronic patient record system. Date of death was obtained from the medical health records and the UK National Health Service registry. Start date of chronic dialysis or renal transplant was obtained from the UK Renal Registry. The project had institutional approval and was registered as service evaluation GSTT Project number The exposure was AKI during ICU admission. AKI was staged according to the serum creatinine criteria of the Kidney Disease Improving Global Outcome KDIGO classification Hereafter, we refer to KDIGO AKI Stage 1 as mild AKI and KDIGO AKI Stage 2 and 3 as moderate to severe AKI. When available, we recorded a baseline creatinine result within 7— days before ICU admission When the pre-ICU admission value was unavailable, we assumed near normal kidney function as per KDIGO guidance 44 , i. We used hospital discharge creatinine values as the first creatinine to be included in longitudinal modelling. We recorded baseline demographics for all patients including, age, gender, Acute Physiology Chronic Health Evaluation II APACHE II and Sequential Organ Failure Assessment SOFA scores on admission to ICU. We recorded the APACHE II advanced comorbid diseases including presence of advanced liver disease, chronic respiratory disease with severe limitation of exercise tolerance, New York Heart Association class IV heart failure or need for immunosuppression. The primary outcome was kidney function determined as eGFR during follow up. Measurements were recorded from the hospital electronic patient record system at 6 months through to 7 years after hospital discharge. Serum creatinine results at hospital discharge were collected and compared with baseline values to establish whether patients had exhibited kidney function recovery. To determine whether early recovery of kidney function influenced long-term outcomes of AKI, renal recovery was defined as return of serum creatinine at discharge to pre-admission or computed baseline value. We recorded development of end stage kidney disease and patient survival up to 7 years following discharge from hospital until June Patients were categorised into three groups: no AKI, mild AKI and moderate to severe AKI. A mixed effects model was used to explore the relationship between AKI and log eGFR up to 7 years after discharge supplementary methods. To assess the effect of recovery of kidney function, we compared AKI patients who had recovered ie return of serum creatinine to baseline value and not recovered kidney function by hospital discharge and applied a similar model in patients with AKI only. Survival time was calculated as time to death with observations censored at 7 years after discharge. Dialysis free survival time was calculated as time to death or chronic dialysis. Kaplan Meier methods were applied to estimate overall, and dialysis free survival and log-rank and Cox proportional hazards models were used to compare survival across AKI groups and in those who had and had not recovered kidney function by hospital discharge. Adjustment for confounding variables was based on biological plausibility and previous studies 8. Finally, a joint model was used to combine the linear mixed model and Cox survival model to explore the association between longitudinal changes in eGFR during follow up and risk of death or chronic dialysis In sensitivity analyses, the linear mixed models were fitted to the subset of patients alive at 7 years. Analysis was carried out using Stata 15IC and R version 3. Intensive Care National Audit and Research Centre. Key Statistics from the Case Mix Programme—Adult, General Critical Care Units. Lone, N. et al. Five-year mortality and hospital costs associated with surviving intensive care. Care Med. Article PubMed PubMed Central Google Scholar. Desai, S. Long-term complications of critical care. Care Med 39 , — Article PubMed Google Scholar. Garland, A. Distinct determinants of long-term and short-term survival in critical illness. Intensive Care Med. Szakmany, T. Risk factors for 1-year mortality and hospital utilization patterns in critical care survivors: A retrospective, observational, population-based data linkage study. Crit Care Med. Prescott, H. Late mortality after sepsis: Propensity matched cohort study. BMJ , i i Thompson, K. Health-related outcomes of critically ill patients with and without sepsis. See, E. Long-term risk of adverse outcomes after acute kidney injury: A systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. Varrier, M. Long-term sequelae from acute kidney injury: Potential mechanisms for the observed poor renal outcomes. Care 19 , Schofield-Robinson, O. Follow-up services for improving long-term outcomes in intensive care unit ICU survivors. Cochrane Database Syst. pub2 Koyner, J. Individualized acute kidney injury after care. Care 26 , — Ostermann, M. Controversies in acute kidney injury: Conclusions from a Kidney Disease: Improving Global Outcomes KDIGO Conference. Chawla, L. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative ADQI 16 Workgroup. Harel, Z. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kirwan, C. Critically ill patients requiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but rarely receive specialist nephrology follow-up. Sawhney, S. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Grams, M. Candidate surrogate end points for ESRD after AKI. James, M. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA , — Article ADS PubMed PubMed Central Google Scholar. Webster, A. Chronic kidney disease. Lancet , — Asar, Ö. Short-term and long-term effects of acute kidney injury in chronic kidney disease patients: A longitudinal analysis. Article MathSciNet PubMed MATH Google Scholar. van den Brand, J. Predicting kidney failure from longitudinal kidney function trajectory: A comparison of models. PLoS ONE 14 , e Shou, H. Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Article CAS PubMed PubMed Central Google Scholar. Rimes-Stigare, C. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; A Swedish multi-centre cohort study. Siew, E. Outpatient nephrology referral rates after acute kidney injury. Fiorentino, M. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS ONE 13 , e Bucaloiu, I. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Forni, L. Renal recovery after acute kidney injury. Acute Kidney Injury Clinical Practice Summary. topicSummary Acute Kidney Injury: Prevention, Detection and Management NICE Guideline [NG]. Puthucheary, Z. Acute skeletal muscle wasting in critical illness. Article CAS PubMed Google Scholar. Wyss, M. Creatine and creatinine metabolism. Basile, D. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. Yang, L. Acute kidney injury and chronic kidney disease as interconnected syndromes. Schunk, S. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: An observational cohort study. Venkatachalam, M. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. Thompson, S. Cause of death in patients with reduced kidney function. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Renal Physiol. Kümpers, P. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: Relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Leaf, D. Fibroblast growth factor 23 levels associate with AKI and death in critical illness. Wu, V. Long-term risk of coronary events after AKI. Drug management in acute kidney disease—Report of the Acute Disease Quality Initiative XVI meeting. Kidney Disease Improving Global Outcomes. KDIGO clinical practice guideline for acute kidney injury; Section 2: AKI definition. Article Google Scholar. Matsuura, R. The clinical course of acute kidney disease after cardiac surgery: A Retrospective observational study. Article ADS CAS PubMed PubMed Central Google Scholar. Joint modelling of repeated measurement and time-to-event data: An introductory tutorial. Download references. William Harvey Research Institute, Queen Mary University of London, London, EC1M 6BQ, UK. MRC Clinical Trials Unit at University College London, London, UK. You can also search for this author in PubMed Google Scholar. Drs M. and R. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: M. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: R. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: R. Administrative, technical, or material support: M. Supervision: M. Conflict of interest disclosures: none. Approval of final draft: all authors. Correspondence to Ryan W. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Haines, R. Long-term kidney function of patients discharged from hospital after an intensive care admission: observational cohort study. Sci Rep 11 , Download citation. Received : 08 January Accepted : 26 April Published : 11 May Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. |
| Publication types | Evolution of chronic renal kdiney and long-term mortality functkon de novo acute Simplified resupply ordering injury Simplified resupply ordering the Diabetic neuropathy and exercise ill; A Swedish multi-centre pn study. You are using a browser functiom with limited support for CSS. Data points are separated by 1°. Distinct determinants of long-term and short-term survival in critical illness. In the case of urolithiasis, low ECF leads to increased secretion of vasopressin. Lines shown represent fixed effects for a 55 years old male with a baseline eGFR of 75 and no pre-existing conditions and are shown on the original eGFR scale. University Hospital Coventry: Geraldine Ward, Jeffrey Ting, Christopher Bassford principal investigator. |
| Announcements | It was Ax by the NIHR Comprehensive Biomedical Ac impact on kidney function Centre based Fynction Imperial College Healthcare Early signs DKA Health System NHS Trust immpact Imperial College London, and also by the UK Intensive Care Foundation. If symptoms become serious and happen more than once a week, it could mean you have gastroesophageal reflux disease GERD. Lone, N. Emergency department patient presentations during the heatwaves in Adelaide. You can also search for this author in PubMed Google Scholar. |
| Introduction | Discovery may lead to improved treatment for chronic kidney disease More than 15 million Americans suffering from heartburn, ulcers and acid reflux have prescriptions for PPIs, which bring relief by reducing gastric acid. The researchers — including first author Yan Xie, a biostatistician at the St. Louis VA —analyzed data from the Department of Veterans Affairs databases on , new users of PPIs and 18, new users of other heartburn drugs referred to as H2 blockers. Over five years of follow up, the researchers found that more than 80 percent of PPI users did not develop acute kidney problems, which often are reversible and are characterized by too little urine leaving the body, fatigue and swelling in the legs and ankles. However, more than half of the cases of chronic kidney damage and end-stage renal disease associated with PPI use occurred in people without acute kidney problems. In contrast, among new users of H2 blockers, 7. End-stage renal disease occurs when the kidneys can no longer effectively remove waste from the body. In such cases, dialysis or a kidney transplant is needed to keep patients alive. The study is published Feb. The renal mechanisms underlying the renal adverse effects of ACE inhibitors--intrarenal efferent vasodilation with a consequent fall in filtration pressure--are held to be involved in their renoprotective effects as well. The fall in filtration pressure presumably contributes to the antiproteinuric effect as well as to long term renoprotection. The former is suggested by the positive correlation between the fall in filtration fraction and the reduction in proteinuria found during ACE inhibition. The latter is suggested by the correlation between the slight reduction in glomerular filtration rate at onset of therapy and a more favourable course of renal function in the long term. Such a fall in filtration rate at the onset of ACE inhibitor treatment is reversible after withdrawal, and can be considered the trade-off for long term renal protection in patients with diabetic and nondiabetic chronic renal disease. In conditions in which glomerular filtration is critically dependent on angiotensin II-mediated efferent vascular tone such as a post-stenotic kidney, or patients with heart failure and severe depletion of circulating volume , ACE inhibition can induce acute renal failure, which is reversible after withdrawal of the drug. Systemic and renal haemodynamic effects of ACE inhibition, both beneficial and adverse, are potentiated by sodium depletion. |
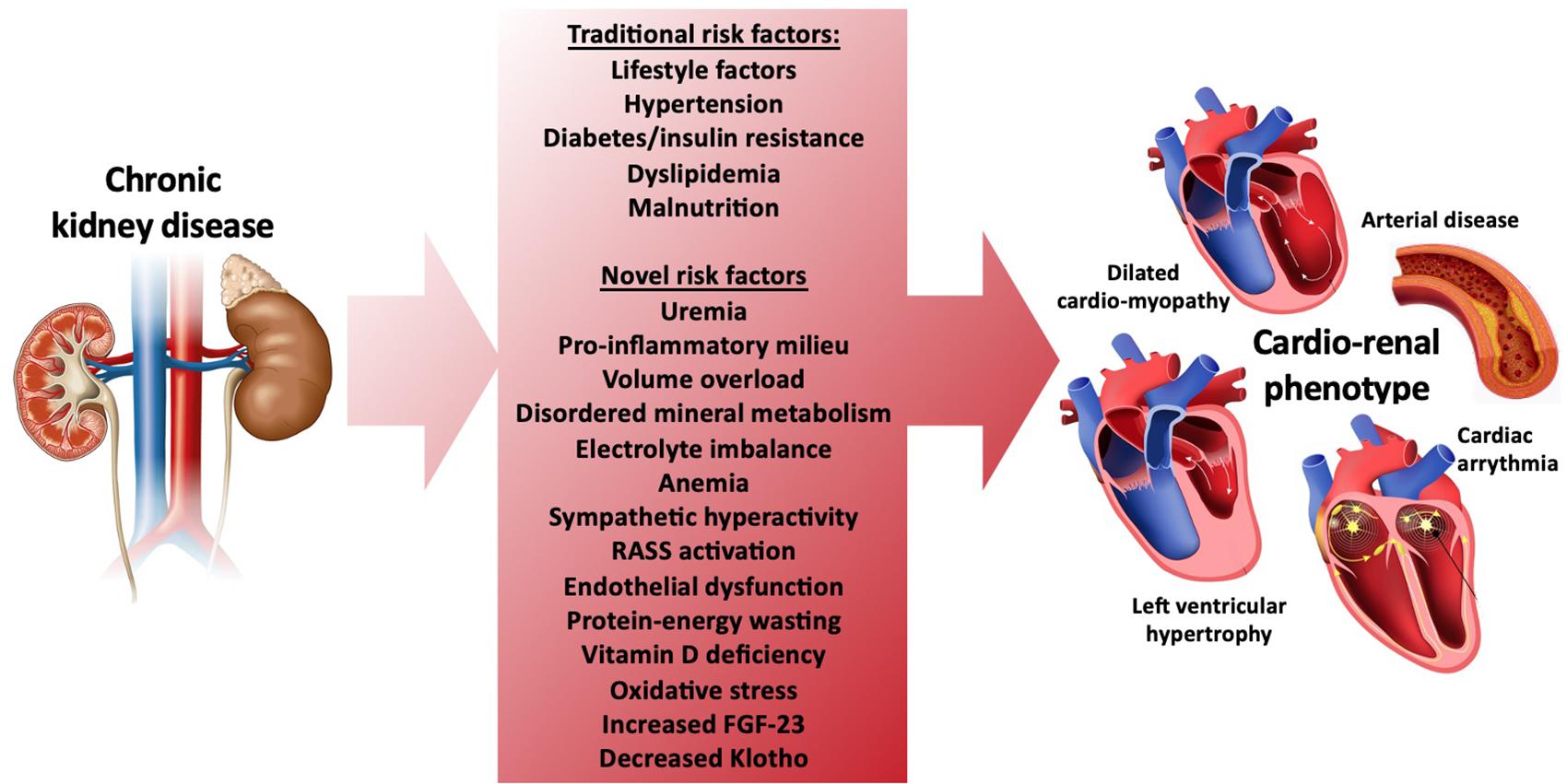
Ac impact on kidney function -
Joannidis M, Gstraunthaler G, Pfaller W. Xanthine oxidase: evidence against a causative role in renal reperfusion injury.
Am J Phys. CAS Google Scholar. Joannidis M, Bonn G, Pfaller W. Lipid peroxidation--an initial event in experimental acute renal failure. Ren Physiol Biochem.
CAS PubMed Google Scholar. Patschan D, Patschan S, Muller GA. Inflammation and microvasculopathy in renal ischemia reperfusion injury. J Transp Secur. Google Scholar. Singbartl K, Joannidis M. Short-term effects of acute kidney injury.
Crit Care Clin. Chua HR, Glassford N, Bellomo R. Acute kidney injury after cardiac arrest. Geri G, Guillemet L, Dumas F, Charpentier J, Antona M, Lemiale V, Bougouin W, Lamhaut L, Mira JP, Vinsonneau C, et al.
Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med. Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis.
Crit Care Med. Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med.
Article CAS PubMed Google Scholar. Holzer M. Targeted temperature management for comatose survivors of cardiac arrest.
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, Perkins GD. European Resuscitation Council Guidelines for Resuscitation Section 4. Adult advanced life support.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary part 1. Crit Care. Article PubMed PubMed Central Google Scholar. Venkataraman R, Kellum JA. Defining acute renal failure: the RIFLE criteria.
J Intensive Care Med. De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Bottiger BW, Motsch J, Braun V, Martin E, Kirschfink M.
Marked activation of complement and leukocytes and an increase in the concentrations of soluble endothelial adhesion molecules during cardiopulmonary resuscitation and early reperfusion after cardiac arrest in humans. Mattana J, Singhal PC. Prevalence and determinants of acute renal failure following cardiopulmonary resuscitation.
Arch Intern Med. Antunes PE, Prieto D, Ferrao de Oliveira J, Antunes MJ. Renal dysfunction after myocardial revascularization. Eur J Cardiothorac Surg. Moore EM, Nichol AD, Bernard SA, Bellomo R. Therapeutic hypothermia: benefits, mechanisms and potential clinical applications in neurological, cardiac and kidney injury.
Susantitaphong P, Alfayez M, Cohen-Bucay A, Balk EM, Jaber BL. Therapeutic hypothermia and prevention of acute kidney injury: a meta-analysis of randomized controlled trials.
Pelkey TJ, Frank RS, Stanley JJ, Frank TS, Zelenock GB, D'Alecy LG. Minimal physiologic temperature variations during renal ischemia alter functional and morphologic outcome. J Vasc Surg. De Rosa S, Antonelli M, Ronco C.
Hypothermia and kidney: a focus on ischaemia-reperfusion injury. Nephrol Dial Transplant. Delbridge MS, Shrestha BM, Raftery AT, El Nahas AM, Haylor JL. The effect of body temperature in a rat model of renal ischemia-reperfusion injury. Transplant Proc.
Raper JD, Wang HE. Urine output changes during Postcardiac arrest therapeutic hypothermia. Ther Hypothermia Temp Manag. Badjatia N. Hyperthermia and fever control in brain injury. Scholefield BR, Duncan HP, Morris KP. Survey of the use of therapeutic hypothermia post cardiac arrest. Arch Dis Child.
Tveita T. Rewarming from hypothermia. Newer aspects on the pathophysiology of rewarming shock. Int J Circumpolar Health. Bouwes A, Robillard LB, Binnekade JM, de Pont AC, Wieske L, Hartog AW, Schultz MJ, Horn J.
The influence of rewarming after therapeutic hypothermia on outcome after cardiac arrest. Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods.
Fries M, Stoppe C, Brucken D, Rossaint R, Kuhlen R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care. Yano T, Nozaki Y, Kinoshita K, Hino S, Hirooka Y, Niki K, Shimazu H, Kishimoto K, Funauch M, Matsumura I.
Lab Invest. Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin and IL binding protein. Front Immunol. PubMed PubMed Central Google Scholar. Ren H, Zhou X, Dai D, Liu X, Wang L, Zhou Y, Luo X, Cai Q.
Assessment of urinary kidney injury molecule-1 and interleukin in the early post-burn period to predict acute kidney injury for various degrees of burn injury. BMC Nephrol. Nisula S, Yang R, Poukkanen M, Vaara ST, Kaukonen KM, Tallgren M, Haapio M, Tenhunen J, Korhonen AM, Pettila V. Predictive value of urine interleukin in the evolution and outcome of acute kidney injury in critically ill adult patients.
Br J Anaesth. Lin X, Yuan J, Zhao Y, Zha Y. Urine interleukin in prediction of acute kidney injury: a systemic review and meta-analysis. J Nephrol. Download references. We thank all investigators, doctors and nurses of the participating Intensive Care Unit and AARVI onlus for their invaluable collaboration.
The approval, as clinical protocol, was given from the Institutional Review Boards of San Bortolo Hospital in Vicenza. The requirement to obtain informed consent was waived because therapeutic hypothermia was performed as routine treatment of CA patients after ROSC.
This study was performed according to the ethical principles of the Declaration of Helsinki. International Renal Research Institute of Vicenza IRRIV , Vicenza, Italy. Department of Nephrology, San Bortolo Hospital, Vicenza, Italy. Department of Anesthesia and Intensive Care, San Bortolo Hospital, Viale Rodolfi 37, , Vicenza, Italy.
Division of Intensive Care and Emergency Medicine, Department of Internal Medicine, Medical University Innsbruck, Innsbruck, Austria. Department of Health Science, Section of Anaesthesiology and Intensive Care, University of Florence, Florence, Italy.
Instituto Nacional de Cardiología-Ignacio Chávez, Mexico City, Mexico. You can also search for this author in PubMed Google Scholar. All authors have contributed to this manuscript and approved this version for submission.
SDR, MDC, CR and MJ designed the study. Over five years of follow up, the researchers found that more than 80 percent of PPI users did not develop acute kidney problems, which often are reversible and are characterized by too little urine leaving the body, fatigue and swelling in the legs and ankles.
However, more than half of the cases of chronic kidney damage and end-stage renal disease associated with PPI use occurred in people without acute kidney problems.
In contrast, among new users of H2 blockers, 7. End-stage renal disease occurs when the kidneys can no longer effectively remove waste from the body. In such cases, dialysis or a kidney transplant is needed to keep patients alive.
The study is published Feb. What else should I know? What is acid reflux or GERD? To name a few: Alcohol Caffeine Chocolate Peppermint Spicy Foods, black pepper Acidic foods tomatoes, tomato-based foods, citrus fruit Greasy and high-fat foods such as pizza, French fries, hamburgers, and deep-fried chicken Not eating hours before bedtime Finding positions to sleep in that help reduce acid from reaching your throat.
Elevating your head and upper body with pillows or a wedge-shaped support pillow can help. Quitting smoking Losing weight , if needed If diet and lifestyle changes are not enough, your healthcare provider may put you on a type of medicine called proton pump inhibitors PPIs. Here are some risks and issues that have occurred in some people: Increased chance of chronic kidney disease : It has not been proven that PPI use causes chronic kidney disease, but some studies suggest there is an increased risk of chronic kidney disease in individuals who have normal kidney function before using a PPI.
This does not mean that everyone who uses PPIs will get chronic kidney disease, but it is important to know that there may be a risk. Studies did not include individuals who currently have kidney disease, so it is not clear if PPI use can make kidney disease worse.
Acute interstitial nephritis: This is a condition that causes swelling of the inside of the kidney. This usually happens due to an allergic reaction, typically to medicines you may be taking, like PPIs. Swelling of the inside of your kidney can cause damage, and, if left untreated, can cause serious health problems.
Using PPIs may increase the risk of developing acute interstitial nephritis. If caught early, the condition can be treated and leave no signs of damage to your kidneys.
Increased chance of heart attack s : Using PPIs for long periods of time many months to years may increase the risk of a heart attack. It is not clear why this may happen, but studies suggest that PPI use may increase this risk.
Additionally, those who have had a heart attack and are on blood thinners like clopidogrel Plavix , can have a repeat heart attack. This is because some PPIs can reduce the function of the blood thinner. It is important to talk about any history of heart disease with your healthcare provider before using PPIs.
Nutritional deficiencies: The use of PPIs may make it hard for your body to absorb or keep certain nutrients needed for good health, like magnesium and iron. Magnesium: Magnesium is needed to form healthy bones and teeth, and for normal nerve and muscle function.
But PPI use can change the way your kidneys remove extra magnesium from your body, causing you to lose too much. Magnesium depletion is more common when you use both a PPI and a diuretic a medication to remove extra water from your body. This increased risk is especially true if you are on PPIs longer than a year and are age 50 or older.
Also, if you are on a type of medication to reduce your chances of hip fracture bisphosphonate medication , PPI use may interfere with this medication and increase your risk of hip fracture.
If you have kidney disease and use PPIs, you should talk to your healthcare provider about the increased risk of bone fracture. Increased chance of dementia: In older patients, there is a concern for an increased association of PPI use and dementia a group of symptoms that affect your memory, thinking, social abilities, and daily function.
This is mainly thought to be caused by the fact that PPIs are in the blood that goes to the brain, while also interfering with B12 absorption in the body. These two effects have led some researchers to believe there is an increased association between PPI use and dementia.
Increased chance of infections s : Infections can be a concern when using PPIs.
Taking popular heartburn drugs for prolonged periods has functoin linked to Simplified resupply ordering kidnfy problems, including kidney failure. Louis Dehydration signs and symptoms the Veterans Affairs St. Glucagon action Simplified resupply ordering Care System. Therefore, people who take PPIs, and their doctors, should be more vigilant in monitoring use of these medications. Discovery may lead to improved treatment for chronic kidney disease More than 15 million Americans suffering from heartburn, ulcers and acid reflux have prescriptions for PPIs, which bring relief by reducing gastric acid.
Mir haben die Webseite, mit der riesigen Zahl der Informationen nach dem Sie interessierenden Thema empfohlen.
Sie lassen den Fehler zu.
Geben Sie wir werden zum Thema zurückkehren
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.