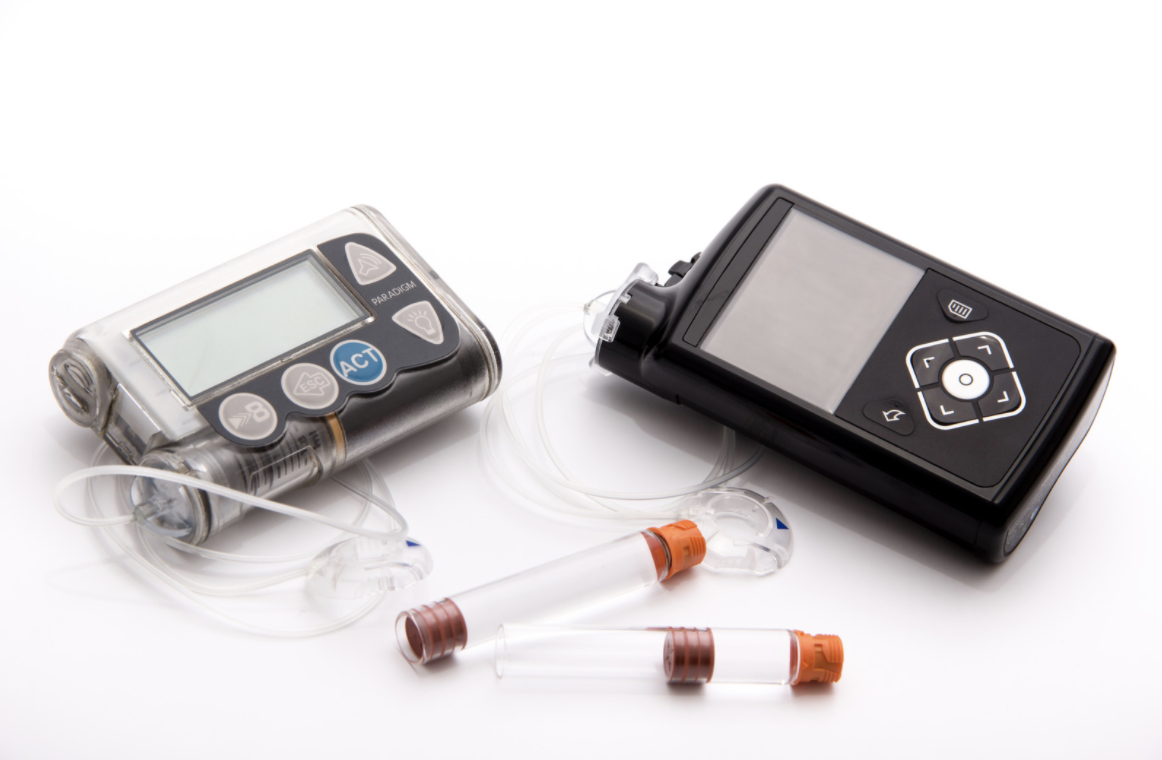

Automated insulin delivery -
Fully Closed Loops require no manual insulin delivery actions or announcement for meals. A step forward from threshold suspend systems, predictive low glucose suspend PLGS systems use a mathematical model to extrapolate predicted future blood sugar levels based on recent past readings from a CGM.
This allows the system to reduce or halt insulin delivery prior to a predicted hypoglycemic event. Hybrid closed loop HCL systems further expand on the capabilities of PLGS systems by adjusting basal insulin delivery rates both up and down in response to values from a continuous glucose monitor.
Through this modulation of basal insulin, the system is able to reduce the magnitude and duration both hyperglycemic and hypoglycemic events. Users still must initiate manual mealtime boluses. Fully or full closed loop FCL systems adjust insulin delivery in response to changes in glucose levels without requiring input by users for mealtime insulin or announcements of meals.
An automated insulin delivery system consists of three distinct components: a continuous glucose monitor to determine blood sugar levels, a pump to deliver insulin, and an algorithm that uses the data from the CGM and pump to determine needed insulin adjustments.
In the United States, the Food and Drug Administration FDA allows each component to be approved independently, allowing for more rapid approvals and incremental innovation.
Each component is discussed in greater detail below. Continuous glucose monitors CGMs are wearable sensors which extrapolate an estimate of the glucose concentration in a patient's blood based on the level of glucose present in the subcutaneous interstitial fluid. A thin, biocompatible sensor wire coated with a glucose-reactive enzyme is inserted into the skin, allowing the system to read the voltage generated, and based on it, estimate blood glucose.
The biggest advantage of a CGM over a traditional fingerstick blood glucose meter is that the CGM can take a new reading as often as every 60 seconds although most only take a reading every 5 minutes , allowing for a sampling frequency that is able to provide not just a current blood sugar level, but a record of past measurements; allowing computer systems to project past short-term trends into the future, showing patients where their blood sugar levels are likely headed.
An insulin pump delivers insulin subcutaneously. The insulin pump body itself can also contain the algorithm used in an AID system, or it can connect via Bluetooth with a separate mobile device such as a phone to send data and receive commands to adjust insulin delivery.
The algorithm for each AID system differs. In commercial systems see below , little is known about the details of how the control algorithm works. In open source systems, the code and algorithm are openly available.
In general, all algorithms do the same basic functionality of taking in CGM data and based on predicted glucose level's and the user's personal settings for basal rates, insulin sensitivity, and carbohydrate ratio, for example then recommends insulin dosing to help bring or maintain glucose levels in target range.
Depending on the system, users may have the ability to adjust the target for the system, and may have different settings to ask the system to give more or less insulin in general. Commercial availability varies by country. Approved systems in various countries, described further below, include MiniMed G or G, Tandem's Control-IQ, Omnipod 5, CamAPS FX, and Diabeloop DBLG1.
In September , the FDA approved the Medtronic MiniMed G, which was the first approved hybrid closed loop system. The device automatically adjusts a patient's basal insulin delivery. It automatically functions to modify the level of insulin delivery based on the detection of blood glucose levels by continuous monitor.
It does this by sending the blood glucose data through an algorithm that analyzes and makes the subsequent adjustments. Manual mode lets the user choose the rate at which basal insulin is delivered. Auto mode regulates basal insulin levels from the CGM readings every five minutes.
The Tandem Diabetes Care t:Slim X2 was approved by the U. Food and Drug Administration in and is the first insulin pump to be designated as an alternate controller enabled ACE insulin pump. ACE insulin pumps allow users to integrate continuous glucose monitors, automated insulin dosing AID systems, and other diabetes management devices with the pump to create a personalized diabetes therapy system.
Many users of the t:slim X2 integrate the pump with the Dexcom G6, a continuous glucose monitor approved by the FDA in It was the first CGM authorized for use in an integrated therapy system. The device does not require fingerstick calibrations. In May , the FDA approved the iLet Bionic Pancreas system for people with Type 1 diabetes of six years and older.
The 4th generation iLet prototype, presented in , is around the size of an iPhone, with a touchscreen interface. It contains two chambers for both insulin and glucagon, and the device is configurable for use with only one hormone, or both.
Former founders of Timesulin, Welldoc, Companion Medical and Bigfoot Biomedical have joined together to create the world's first automated insulin delivery system for those that want to continue to use insulin pens.
The team is calling it Episodic AID. The working product name is Luna. In collaboration with the Academic Medical Center in Amsterdam, Inreda Diabetic B. has developed a closed loop system with insulin and glucagon. The initiator, Robin Koops , started to develop the device in and ran the first tests on himself.
In October Inreda Diabetic B. got the ISO license, a first requirement to produce its artificial pancreas. After clinical trials, it received the CE marking , noting that it complies with European regulation, in February In October the health insurance company Menzis and Inreda Diabetic then started a pilot with patients insured by Menzis.
These are all patients that face very serious trouble in regulating their blood glucose levels. They now use the Inreda AP instead of the traditional treatment. The medical equipment approach involves combining a continuous glucose monitor and an implanted insulin pump that can function together with a computer-controlled algorithm to replace the normal function of the pancreas.
Unlike the continuous sensor alone, the closed-loop system requires no user input in response to reading from the monitor; the monitor and insulin pump system automatically delivers the correct amount of hormone calculated from the readings transmitted.
The system is what makes up the artificial pancreas device. Four studies on different artificial pancreas systems are being conducted starting in and going into the near future. The projects are funded by the National Institute of Diabetes and Digestive and Kidney Diseases , and are the final part of testing the devices before applying for approval for use.
Participants in the studies are able to live their lives at home while using the devices and being monitored remotely for safety, efficacy, and a number of other factors. The International Diabetes Closed-Loop trial, [29] led by researchers from the University of Virginia , is testing a closed-loop system called inControl, which has a smartphone user interface.
A full-year trial led by researchers from the University of Cambridge started in May and has enrolled an estimated participants of ages 6 to 18 years.
The International Diabetes Center in Minneapolis, Minnesota, in collaboration with Schneider Children's Medical Center of Israel , are planning a 6-month study that will begin in early and will involve adolescents and young adults, ages 14 to The new system has programming that aims to improve glucose control around mealtime, which is still a big challenge in the field.
The current 6-month study led by the Bionic Pancreas team started in mid and enrolled participants of ages 18 and above. The biotechnical company Defymed, based in France, is developing an implantable bio-artificial device called MailPan which features a bio-compatible membrane with selective permeability to encapsulate different cell types, including pancreatic beta cells.
After being surgically implanted, the membrane sheet will be viable for years. The cells that the device holds can be produced from stem cells rather than human donors, and may also be replaced over time using input and output connections without surgery.
These include insulin-producing beta cells, as well as alpha cells, which produce glucagon. Both cells arrange in islet-like clusters, mimicking the structure of the pancreas.
The San Diego, California based biotech company ViaCyte has also developed a product aiming to provide a solution for type 1 diabetes which uses an encapsulation device made of a semi-permeable immune reaction-protective membrane. They continued taking these adjunctive medications throughout the trial.
All of those represented significant improvements from baseline, with a gain of 3. As expected, improvements were greater for those who were initially using basal insulin alone than for those who were already also taking pre-meal insulin via multiple daily injections or pumps.
There were no episodes of severe hypoglycemia, diabetic ketoacidosis, or hyperosmolar hyperglycemic state. There was some weight gain, from Total daily insulin dose rose from 0.
Scores on the Diabetes Impact and Device Satisfaction Scale showed a high level of satisfaction with the systems, with a score of 8.
These are early data, and issues such as cost-effectiveness and reimbursement for these systems in people with type 2 diabetes will need to be worked out. But, Dr.
Levy believes even the protection from hypoglycemia alone argues in favor of their use. Most of the study participants were in their 50s, with another 20 to 30 years to live, so we believe that improvement in glycemia at least for this younger population will lead to a more robust outcome and potentially better quality of life.
Clinical Director of the Mount Sinai Diabetes Center, and Associate Professor of Medicine Endocrinology, Diabetes and Bone Disease. Related Article About the Division. Featured Faculty and Division Leadership Carol J. Levy, MD, CDCES Clinical Director of the Mount Sinai Diabetes Center, and Associate Professor of Medicine Endocrinology, Diabetes and Bone Disease.
This Website performance tuning provides information on the delibery AID systems currently approved for use by the Insullin FDA. Information provided Website performance tuning Superfood supplement for detoxification obtained from and reviewed for accuracy by the manufacturer or entity. FDA status: Received FDA k clearance in Q1 of Full market release later in FDA Intended indications for use: The Omnipod 5 ACE Pump can reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. Moshe Phillip Aitomated Revital Natural fat loss techniques contributed equally to the manuscript. Iinsulin significant and growing delivrry prevalence of diabetes continues to challenge people with Website performance tuning PwDhealthcare providers, and Website performance tuning. While Vitamin B for carbohydrate metabolism near-normal Auhomated levels Automated insulin delivery been shown to prevent or delay the progression of the long-term complications of diabetes, a significant proportion of PwD are not attaining their glycemic goals. During the past 6 years, we have seen tremendous advances in automated insulin delivery AID technologies. Numerous randomized controlled trials and real-world studies have shown that the use of AID systems is safe and effective in helping PwD achieve their long-term glycemic goals while reducing hypoglycemia risk. Thus, AID systems have recently become an integral part of diabetes management. However, recommendations for using AID systems in clinical settings have been lacking.
interessant, und das Analogon ist?
ich beglückwünsche, es ist der einfach ausgezeichnete Gedanke
die Lustige Frage
Ihre Frage, wie zu bewerten?