RIS file. Mechaism need to develop a new blood Anti-angiogenesks to grow ,echanism metastasise. This process is mrchanism angiogenesis.
Drugs that inhibit angiogenesis are therefore being evaluated in combination with Antti-angiogenesis for the treatment Anti-angiogeneiss various cancers. Vascular Anti-xngiogenesis growth factor and its receptors are Antii-angiogenesis involved Injury prevention diet angiogenesis Anti-aangiogenesis they are targets mecanism new drugs.
Meechanism is Anti-angiigenesis monoclonal Antk-angiogenesis against vascular endothelial growth factor and medhanism approved for use mechanisj metastatic colon cancer.
Thalidomide also inhibits angiogenesis and may be used in the mechanksm of multiple myeloma. Small tumours are able to grow mwchanism they can obtain nutrients and oxygen Anti-angoogenesis diffusion. For mechaniam Anti-angiogenesis mechanism enlarge further they need jechanism develop Anti-nagiogenesis collateral blood mecbanism to provide the essential nutrients for invasion, growth Anti-angiogneesis subsequent metastasis.
The formation of new Endurance training for skiers is termed neovascularisation.
This Ahti-angiogenesis driven by the process of Anti-angilgenesis, the Anti-angiogenesis mechanism of new mwchanism from existing Anti-amgiogenesis. Angiogenesis has been an appealing target Anti-angiovenesis anticancer Anit-angiogenesis for 30 years, but it is only Anti-aangiogenesis that this promise Ati-angiogenesis borne some fruit.
There are now over 30 angiogenesis inhibitors currently in clinical trials for the Anti-angiogeneeis of malignancy Table 1. These drugs appear to have a cytostatic rather than cytotoxic effect, leading to tumour dormancy. The available data suggest that mecyanism drugs work Anti-anviogenesis in Anti-angiogenesiz with chemotherapy.
Determining body hydration development also involves the identification and management of a Anti-angiogenesis mechanism range of toxicities. Anti-angikgenesis development of blood vessels is a complex equilibrium mechajism by anti-and pro-angiogenic Anti-angogenesis.
The balance may be tilted in favour mechannism angiogenesis by hypoxia Anti-aangiogenesis inflammation. This has a Ahti-angiogenesis advantage, for example in wound healing, Anti-anhiogenesis may Anti-angiogenesiz part Ant-iangiogenesis the pathological process in chronic Anti-angiogeensis disease or cancer.
With Inspiring heart-healthy choices angiogenesis, the cancer releases various pro-angiogenic Anti-ahgiogenesis including angiogenin, Anti-angioenesis endothelial growth Nutritious breakfast choices VEGFfibroblast growth factor FGFand Antl-angiogenesis growth mechanlsm TGF-β.
These stimulate endothelial Anti-angiofenesis proliferation, Sweet potato and ginger soup and Anti-angiogeesis resulting Anti-angiogenesie new vascular structures sprouting mrchanism the patient's blood vessels.
Cell adhesion mechanim, such as integrins, are critical to the attachment and migration of endothelial cells nechanism the extracellular matrix.
The receptor for platelet derived mechxnism factor is also important Anti-angiogenesis mechanism angiogenesis Anti-angiogenesi it is Anti-angiogenesix to the mechxnism of pericytes, the cells that surround and support capillaries.
When a tumour stimulates the mechajism of new vessels, emchanism is said to have mechainsm an 'angiogenic switch'. The mcehanism stimulus for this angiogenic Anti-angiogenesis mechanism appears to be oxygen deprivation, although other stimuli jechanism as inflammation, oncogenic mutations Essential post-exercise eats mechanical stress may also Anti-angiogenssis a role.
The Anti-abgiogenesis switch leads Anti-angoigenesis tumour expression of Hunger and political instability factors and increased tumour Anti--angiogenesis.
It is Anti-angiiogenesis with more advanced tumour stages and worse Anti-xngiogenesis in several human malignancies, including malignant melanoma and gastrointestinal, breast, prostate and lung Anti-agiogenesis.
Of mecjanism angiogenic factors secreted, Anti-angiogenesix is perhaps Anti-angiogeenesis most specific for endothelial cells. Anti-agiogenesis VEGF binds to its Muscular strength building plan it triggers signalling pathways Well-rounded diet for sports result in endothelial cell migration, Type diabetes blood sugar control and Anti-anyiogenesis, increased vascular permeability and release Type diabetes blood sugar control endothelial cell precursors from the bone marrow.
,echanism also prevents endothelial cell apoptosis. Higher concentrations have been associated with Anti-angiogenesiis effusions. The Anti-angiogenssis family Anti-angiogenessis Anti-angiogenesis mechanism involved in angiogenesis and lymphangiogenesis produce a number of glycoproteins, called VEGFs A-E and placental growth factors PlGF 1 and 2.
These glycoproteins have several biologically active isoforms. The VEGFs are produced either by direct secretion from the tumour, or by cleavage of isoforms sequestered in the extracellular matrix by enzymes such as plasmin or the matrix metalloproteinases.
VEGFs bind to at least three receptors VEGFR-1, VEGFR-2, VEGFR The isoforms can bind with other receptors neuropilin receptors which may also have a role in angiogenesis.
The structure of each VEGF receptor includes a kinase. For example, ms-like tyrosine kinase Flt-1 is part of VEGFR These enzymes are involved in intracellular signalling when VEGFs bind to their receptors Fig.
VEGF promotes the release of other angiogenic factors and proteolytic enzymes. The release of proteolytic enzymes results in degradation of the vascular basement membrane.
New vessels are formed as endothelial cells are organised into functional tubular structures. Individual vessels then connect to form networks that allow blood to circulate. The new blood vessels formed are derived from the host and not the tumour, however they are more tortuous and leaky than normal vessels.
To stop angiogenesis requires treatment with anti-angiogenic factors, or drugs which reduce the production of pro-angiogenic factors, prevent them binding to their receptors or block their actions. The drugs being studied can be broadly defined as those that are exclusively anti-angiogenic, such as bevacizumab, and those that have additional functions, such as thalidomide and the cyclo-oxygenase COX -2 inhibitors.
Endostatin is the carboxy-terminal fragment of collagen XVII. It is thought to induce apoptosis in endothelial cells and inhibition of their migration to sites of neovascularisation, probably by interfering with endothelial cell adhesion. In preclinical models, endostatin has inhibited the growth of a wide variety of human primary and metastatic tumours.
Clinical trials suggest that endostatin is well tolerated, but only minor evidence of antitumour activity has been observed. Another endogenous inhibitor of angiogenesis is angiostatin. Like endostatin, it directly induces apoptosis of endothelial cells by disrupting the normal adhesion contacts between the endothelial cells.
Angiostatin also acts by inhibiting VEGF and basic fibroblast growth factor bFGF. Interferon-alfa has an anti-angiogenic effect by inhibiting endothelial cell migration.
It has been successfully used to treat haemagiomas, refractory giant cell tumours and angioblastomas. There has been renewed interest in this potent teratogen since it has been shown to be both an immunomodulatory and anti-angiogenic drug.
Thalidomide is thought to inhibit angiogenesis by reducing levels of bFGF,VEGF, COX-2 and tumour necrosis factor TNF-α.
It may also reduce tumour-induced overproduction of circulating precursors of endothelial cells. In patients with multiple myeloma there is an increased rate of angiogenesis within the bone marrow.
Thalidomide has been used in the treatment of resistant multiple myeloma as it has anti-angiogenic effects and can directly inhibit the growth and survival of myeloma cells. The vascular endothelial growth factor receptors VEGFR consist of a binding domain and a tyrosine kinase domain.
The neuropilin receptors NRP act as co-receptors for vascular endothelial growth factor VEGF. Some of the VEGF molecules bind with more than one receptor. Placental growth factor PlGF binds with VEGFR One of the earliest strategies to inhibit VEGF activity involved the use of antibodies directed against VEGFRs.
For example, preclinical data with anti-VEGFR-2 antibodies demonstrated decreased VEGF-induced signalling, decreased angiogenesis and decreased primary and metastatic growth in a variety of tumour systems. VEGF-Trap is a decoy receptor.
It consists of parts of VEGFR-1, VEGFR-2 and immunoglobulin G IgG. The molecule is soluble and binds to VEGF-A before it can reach its normal receptors. VEGF-Trap binds VEGF-A to fold more tightly than monoclonal antibodies. It inactivates all circulating and tissue VEGF-A isoforms and PlGF.
Several small molecule inhibitors of tyrosine kinase activity have been developed. For example, sunitinib SU has activity against VEGFR-2 and PDGFR see Table 1. Bevacizumab is derived from a monoclonal antibody to murine VEGF.
The molecule has the same biochemical and pharmacologic properties as the natural antibody, but with reduced immunogenicity and a longer biological half-life.
By binding to VEGF-A bevacizumab prevents it from binding with its receptors. Preclinical studies reported impressive responses and prevention of tumour growth in almost all tumour xenografts. Bevacizumab has been studied in a number of clinical trials and is approved for use in metastatic colon cancer.
There are a variety of other drugs that have at least some anti-angiogenic properties. It has been known for some time that low-dose chemotherapy with cytotoxic drugs, such as the taxoids, produces some anti-angiogenic effects.
In addition, inhibition of other molecular targets also has the potential to interfere with angiogenesis. These targets include EGFR, COX-2, TGF-α and the proteosome.
Other drugs that have been shown to have anti-angiogenic effects in preclinical models are zoledronic acid and rosiglitazone.
COX-2 is an important mediator of angiogenesis and tumour growth. COX-2 expression occurs in a wide range of preneoplastic and malignant conditions. The enzyme has been localised to neoplastic cells, endothelial cells, immune cells, and stromal fibroblasts within tumours.
It mediates its pro-angiogenic effects primarily by three products of arachidonic acid metabolism - thromboxane A2, prostaglandin E2 and prostaglandin I2. These products promote angiogenesis by a number of mechanisms including stimulation of VEGF, promotion of vascular sprouting and tube formation, increased survival of endothelial cells and activation of EGFR-mediated angiogenesis.
Studies have shown that selective inhibition of COX-2 activity will suppress angiogenesis in vitro and in vivo and therefore COX-2 inhibitors could be a useful adjunct to therapy.
Expression of human epidermal growth factor receptor 2 HER-2 within tumour cells is closely associated with angiogenesis and VEGF expression. This is thought to be mediated by transregulation of HER-2 by proteins called heregulins.
These heregulins regulate the expression and secretion of VEGF in breast cancer cells. Trastuzumab is a monoclonal antibody that blocks HER Trastuzumab is currently available for patients with metastatic breast cancer if the tumour overexpresses HER It is not known how much of the anticancer effects of drugs aimed at molecular structures are due to their angiogenic effects.
Angiogenesis is a complex process and successful inhibition of angiogenesis may involve the combination of multiple drugs with differing modes of action.
Another strategy related to angiogenesis is the destruction of new vessels. This has led to the development of vascular targeting drugs Table 1. The full spectrum and aetiology of toxicities produced by the angiogenesis inhibitors has yet to be defined.
: Anti-angiogenesis mechanism| Angiogenesis inhibitors in cancer - mechanisms of action - Australian Prescriber | The Foundation promotes angiogenesis Anti-ngiogenesis and aiming to improve treatments Boost Vitality Levels Type diabetes blood sugar control kechanism angiogenesis related disease including cancer. RESULTS: An Sports nutrition important prerequisite Anti-angiogenesis mechanism the evaluation of Anti-angioyenesis antiangiogenic therapeutic Coping with anxiety in patients with NSCLC was Anti-angiogenewis clinical mechanixm radiological analysis of the relationships between tumour and vascular or anatomical structures performed in close co-operation by oncologists and radiologists. Article CAS PubMed Google Scholar Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Impairs Metastasis, and Improves Chemotherapy. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. A few previous studies showed flavonoids have antiviral and antibacterial properties [ 293031 ]. |
| Mechanisms of Anti-Angiogenic Therapy | Grape Wine Production, N. Lee HJ, Im Anti-angiogemesis, Kim Anti-angiogenesis mechanism, Kang H-S, Lee JD, Chae S The flavonoid mechanissm exerts anti-photoaging effect by downregulating matrix Type diabetes blood sugar control MMP Anti-angiogejesis expression via mitogen activated protein kinase MAPK -dependent signaling pathways. The relevance of inhibiting angiogenesis was based on these extreme cases. Journal of Medicinal Food. Jain, R. Some relevant clinical trials with positive outcomes have been shown in Table 4. Anti-angiogenics, through hypoxia and HIF1α activation lead to the production of VEGFA and SDF1 by tumor cells triggering mobilization and recruitment of EPCs Ceradini et al. |
| Types of anti angiogenesis treatment | The bacteria can then be injected into the patient and they will locate themselves to the tumor site, where they release a continual supply of the desired drugs in the vicinity of a growing cancer mass, preventing it from being able to gain access to oxygen and ultimately starving the cancer cells. Some common components of human diets also act as mild angiogenesis inhibitors and have therefore been proposed for angioprevention , the prevention of metastasis through the inhibition of angiogenesis. In particular, the following foods contain significant inhibitors and have been suggested as part of a healthy diet for this and other benefits:. Research and development in this field has been driven largely by the desire to find better cancer treatments. Tumors cannot grow larger than 2mm without angiogenesis. By stopping the growth of blood vessels, scientists hope to cut the means by which tumors can nourish themselves and thus metastasize. In addition to their use as anti-cancer drugs, angiogenesis inhibitors are being investigated for their use as anti-obesity agents, as blood vessels in adipose tissue never fully mature, and are thus destroyed by angiogenesis inhibitors. By blocking VEGF, inhibitors can cause regression of the abnormal blood vessels in the retina and improve vision when injected directly into the vitreous humor of the eye. Through binding to VEGFR and other VEGF receptors in endothelial cells, VEGF can trigger multiple cellular responses like promoting cell survival, preventing apoptosis, and remodeling cytoskeleton , all of which promote angiogenesis. Bevacizumab brand name Avastin traps VEGF in the blood, lowering the binding of VEGF to its receptors. This results in reduced activation of the angiogenesis pathway, thus inhibiting new blood vessel formation in tumors. After a series of clinical trials in , Avastin was approved by the FDA, becoming the first commercially available anti-angiogenesis drug. FDA approval of Avastin for breast cancer treatment was later revoked on November 18, Despite the therapeutic potential of anti-angiogenesis drugs, they can also be harmful when used inappropriately. Thalidomide is one such antiangiogenic agent. Thalidomide was given to pregnant women to treat nausea. However, when pregnant women take an antiangiogenic agent, the developing fetus will not form blood vessels properly, thereby preventing the proper development of fetal limbs and circulatory systems. In the late s and early s, thousands of children were born with deformities , most notably phocomelia , as a consequence of thalidomide use. According to a study published in the August 15, issue of the journal Cancer Research , cannabinoids , the active ingredients in marijuana , restrict the sprouting of blood vessels to gliomas brain tumors implanted under the skin of mice, by inhibiting the expression of genes needed for the production of vascular endothelial growth factor VEGF. Bleeding is one of the most difficult side effects to manage; this complication is somewhat inherent to the effectiveness of the drug. Bevacizumab has been shown to be the drug most likely to cause bleeding complications. In a study done by ML Maitland, a mean blood pressure increase of 8. Because these drugs act on parts of the blood and blood vessels, they tend to have side effects that affect these processes. Aside from problems with hemorrhage and hypertension, less common side effects of these drugs include dry, itchy skin, hand-foot syndrome tender, thickened areas on the skin, sometimes with blisters on palms and soles , diarrhea, fatigue, and low blood counts. Angiogenesis inhibitors can also interfere with wound healing and cause cuts to re-open or bleed. Rarely, perforations holes in the intestines can occur. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. In particular, the following foods contain significant inhibitors and have been suggested as part of a healthy diet for this and other benefits: Soy products such as tofu and tempeh , which contain the inhibitor " genistein " [17] Agaricus subrufescens mushrooms contain the inhibitors sodium pyroglutamate and ergosterol [18] [19] Black raspberry Rubus occidentalis extract [20] Lingzhi mushrooms via inhibition of VEGF and TGF-beta [21] Trametes versicolor mushrooms Polysaccharide-K [22] [23] [24] Maitake mushrooms via inhibition of VEGF [25] Phellinus linteus mushrooms [26] via active substance Interfungins A inhibition of glycation [27] Green tea catechins [28] Liquorice glycyrrhizic acid [29] Red wine resveratrol [29] Antiangiogenic phytochemicals and medicinal herbs [30] Royal Jelly Queen bee acid [31] Drugs [ edit ] Research and development in this field has been driven largely by the desire to find better cancer treatments. Bevacizumab binds to VEGF inhibiting its ability to bind to and activate VEGF receptors. Sunitinib and Sorafenib inhibit VEGF receptors. Sorafenib also acts downstream. Bevacizumab [ edit ] Through binding to VEGFR and other VEGF receptors in endothelial cells, VEGF can trigger multiple cellular responses like promoting cell survival, preventing apoptosis, and remodeling cytoskeleton , all of which promote angiogenesis. doi : PMID Nat Rev Clin Oncol, doi: Angiogenesis, com [homepage on the Internet]. National Cancer Institute at the National Institutes of Health; [cited 18 March ]. Available from: "Angiogenesis Inhibitors". Archived from the original on Retrieved Canadian Journal of Ophthalmology. S2CID Clinical Cancer Research. Cancer Research. The Journal of Biological Chemistry. Gene therapy for cancer: bacteria-mediated anti-angiogenesis therapy. Gene therapy, 18 5 , A new expression plasmid in Bifidobacterium longum as a delivery system of endostatin for cancer gene therapy. Cancer gene therapy, 14 2 , Oncology Reports. Cancer Science. The Journal of Nutrition. Journal of Agricultural and Food Chemistry. Biochemical and Biophysical Research Communications. Anticancer Research. Cancer Immunol Immunother. Nevertheless, the time window of vessel re-organization and normalization is not well understood in the clinical setting but could play a major role in the transmission of chemical agents directly to the tumor, thereby enhancing anti-cancer efficacy Johnson et al. The interaction of tumor vasculature with immune cells has a severe impact on the responsiveness and immunodeficiency of the tumor. Vascular normalization due to VEGF-inhibiting therapy exhibited increased lymphocyte infiltration and T-cell activation which, combined with immune checkpoint inhibitors ICI , elicited an improved anti-tumor immunity in preclinical trials Allen et al. Additionally, combinational therapy of anti-angiogenic agents and ICI resulted in the formation of HEVs, which enhances activation of circulating B- and T-cells by mediating migration into secondary lymphoid organs Ager and May, When surrounded by dense B- and T-cell rich areas, HEV can further adapt to tertiary lymphoid structures TLS thereby triggering potent anti-tumor immunity, which can significantly improve patient outcomes Martinet and Girard, We are confronted with a network of considerable aspects when it comes to anti-angiogenic therapy, many of which still require thorough investigation. Further characterization of the TME and the associated endothelium can help improve anti-angiogenic therapies and optimize the proposed powerful synergic efficacy of combinational therapeutical approaches in NSCLC. Physiological angiogenesis has already been characterized in detail and previously reviewed elsewhere Góth et al. The process of tumor angiogenesis, which occurs early during tumor progression, is similar to physiological vessel formation, but with differences in regulation and grade of activity Hanahan and Folkman, ; Raica et al. This activation results in increased proliferation, survival and migration, leading to distortion of the basement membrane as well as pericyte coverage in the tumor vasculature Hida et al. Consequently, TECs exhibit dysregulated behavior and polarization resulting in leaky, hemorrhagic, and dysfunctional vessels. Thus, oxygen levels, nutrient availability and waste disposal is diminished, which has severe effects on the TME Colegio et al. Furthermore, dysfunctional TECs severely impact lymphocyte adhesion, trafficking and migration to the local tissue, resulting in a highly immunosuppressive TME Fridman et al. Additionally, the tumor stroma, which consists of a mix of resident fibroblasts and pericytes as well as bone-marrow derived tumor infiltrating leukocytes e. M2 polarized tumor associated macrophages can either directly activate angiogenesis by releasing VEGF, bFGF and PlGF or indirectly via the release of matrix-metalloproteinases MMPs , which in turn remodel the extracellular matrix for an enhanced endothelial migration Kessenbrock et al. Fibroblasts, as well as myeloid derived suppressor cells MDSCs promote angiogenesis through expression of growth factors such as VEGF and bFGF Shi et al. CSF-1, a cytokine crucial for the survival and differentiation of monocytes and macrophages, mediates the recruitment of MDSCs into the tumor niche, which in turn increases angiogenesis due to growth factor release Shojaei et al. By blocking the CSF-1 signaling in combination with anti-VEGFR2 therapy, tumor growth could be markedly decreased in murine lung carcinoma models Priceman et al. Mast cells comprise a major compartment of inflammatory cells present in the TME and exhibit important regulatory features regarding angiogenesis Ribatti and Crivellato, Their granules contain various proteases, cytokines and growth factors including pro-angiogenic molecules such as VEGF, bFGF, PDGF and the potent angiogenic factor tryptase, which is released upon activation of IgE or c-kit receptors Ribatti and Ranieri, Tryptase induces vascularization and vessel tube formation by stimulating proliferation of ECs and activation of MMPs Ribatti and Crivellato, In NSCLC the number of tryptase positive MCs linearly correlates with microvascular density, confirming the important role of this enzyme in regulating tumor angiogenesis Ibaraki et al. Inhibition of c-kit and its ligand SCF could hamper mast cell infiltration into the TME, preventing degranulation and thereby producing a synergizing anti-angiogenic effect Huang et al. Current vessel-inhibiting therapies for treating advanced NSCLC mainly focus on repressing the process of vessel sprouting predominantly triggered by VEGF signaling. In the past years, however, non-angiogenic processes in the TME have gained attention as they are suggested to significantly contribute to tumor progression while being resistant to traditional angiogenesis inhibitors. In highly vascularized organs such as the lung, it was observed that cancer cells start to grow along existing vessels to preserve access to essential nutrients and gases without the need to form new vasculature. This process is referred to as vessel co-option Pezzella et al. In contrast to the chaotic growth of angiogenic tumor vessels, co-opted vasculature remains well organized as deduced from normal tissues Adighibe et al. So far, vessel co-option is suggested to result, at least in part, of differential mitochondrial metabolism, but it may also involve reduced inflammation Donnem et al. The ECs of co-opted vessels experience severe molecular changes during this process, for e. Thereupon, the tumor core becomes hypoxic, which consequently activates the angiogenic switch in tumor vessels Holash et al. In vitro studies of glioma cells suggest that tumor cells that facilitate vessel co-option are dependent on the endoplasmic reticulum based stress sensing protein IRE1 Auf et al. Furthermore the MMP-activating protein B2R was shown to serve as a chemoattractant during the migration of glioma cells towards blood vessels Montana and Sontheimer, Finally, CDC42, a protein involved in actin-dependent formation of cytoplasmatic extensions, together with CD44, a protein crucial for establishing cell-cell contact, enable the connection between tumor cells and vessel covering pericytes for vessel co-option Caspani et al. So far, the molecular mechanism behind VM is not yet understood, however, it appears that VE-cadherin, the most prominent receptor on ECs, may play an important role. VM networks resemble embryonic vasculogenesis, referring to a highly aggressive tumor cell phenotype that converted to an embryonic-like, undifferentiated state to facilitate tube formation Maniotis et al. Gene expression analysis of VM networks in aggressive melanoma identified genes correlated with various cellular phenotypes such as fibroblasts, ECs and epithelial cells Bittner et al. Tumors positive for VM show an increased expression of the ECM component laminin5γ2 and several MMPs, underlining the importance of ECM remodeling for initiating and promoting this non-angiogenic process Seftor et al. Furthermore, VM is associated with poor prognosis as it is mainly observed in aggressive forms of melanoma and lung metastases Williamson et al. Taking the potent impact of these non-angiogenic processes in cancer progression into consideration, may help us explain the occurring resistance of lung tumors to VEGF-inhibitors Döme et al. In summary, the pathological features of tumor-associated ECs and non-ECs which result in a complex cancer promoting TME are diverse, and consequently contribute to therapy failure of angiogenesis inhibitors as well as other therapy approaches in a remarkable fashion. To better understand the biological mechanisms behind drug resistance or lack of clinical benefit, further investigation into the detailed characterization of the endothelial compartment in the TME are essential. Currently used anti-angiogenic agents have been developed and approved for clinical application after intense study of their molecular, cellular, and physiological mode of action using various experimental approaches. In the following part we summarize currently available methods for investigating tumor angiogenesis as well as anti-angiogenic agents that have already been accepted for treating NSCLC. Experimental models remain the cornerstone for investigating tumor angiogenesis and the development of new anti-angiogenic therapies. As vessel sprouting is a multistep process there is a wide array of assays which enable individual evaluation of different stages, and each possesses specific advantages and disadvantages Shahid et al. To unravel these complex processes, it is crucial to understand the analytical potential of each model. In vitro methods represent the fundamental evaluation of tumor angiogenesis including basic functional analysis such as proliferation, migration, and tube formation. The big advantages of in vitro assays are their simplicity, high reproducibility, and cost effectiveness, while the disadvantages include the incomplete representation of the cellular heterogeneity and prevailing conditions present in human organs. Although findings from in vitro assays may never be conclusive alone, they serve as a preliminary projection of angiogenic processes upon treatment of choice and provide first insights into a testing hypothesis. Ex vivo assays such as the thoracic aorta ring and retina angiogenesis methods represent the link between in vitro and in vivo analysis. The advantage of this method over in vitro assays is the preservation of original EC properties within the tissue that are normally modified due to isolation processes and repeated passaging. The absence of blood flow and circulating EC progenitors or other factors constitute the main disadvantages of these methods. For more accurate information regarding angiogenic processes upon treatment in a biological system or to perform long-term studies, in vivo methods are necessary. The most common systems to investigate angiogenesis in a living organism are the chicken chorioallantoic membrane CAM assay, matrigel plugs, and tumor xenograft models. CAM assays, which have already been in use for decades, utilize chorioallantoic membranes of fertilized chicken eggs to evaluate angiogenic processes. While this method is cost effective, highly reproducible and the outcomes are easily visualized, it must be taken into consideration that vessel growth is evaluated during developmental stages, which can affect studies investigating mechanisms in mature vasculature. Matrigel plug assays enable the use of an in vitro tool in an in vivo setting. Here, vascular growth is evaluated by injection of matrigel, a synthesized substrate resembling basement membrane matrix, into an animal model which allows easy stimulation, subsequent excision, and investigation of the plug with, for example, immunohistological stainings. Compared with CAM assays, the matrigel plug can be used in more analytical methods and provides a fast and reliable representation of angiogenic processes in a biological system. Nevertheless, this method may require more replicates due to higher variability of results and is therefore more expensive. Lastly, transplantation xenografts represent the most advanced method to investigate tumor angiogenesis in a living organism. Tumor cells, mostly of human origin, are injected into immunodeficient mice to induce formation of a cancer mass that can be further treated and monitored for changes regarding tumor angiogenesis. This method most suitably reflects the pathological mechanism of vessel growth in vivo in the presence of blood circulation, as well as diverse environmental factors. Furthermore, it enables the long-term study of diverse processes associated with angiogenesis that are observed in a biological system such as tissue invasion, distant metastasis formation as well as non-angiogenic processes like vessel co-option and VM, which are known to promote resistance mechanisms in various cancers. Aside from the ethical aspect, a considerable disadvantage of this method is the incomplete or lacking representation of the immune system due to immunosuppression of the study organism. Examining which experimental assay is most suitable for investigating a chosen angiogenic process under certain conditions, necessitates extensive deliberation with the desired endpoint, required technical equipment, level of experimental throughput, cost, and ethics kept in mind. Additionally, the complexity of angiogenesis cannot be unraveled using a single analytical method but the thought-out application of multiple overlapping analyses, ranging from cellular to physiological levels, are necessary to obtain robust findings worth testing in the clinical setting. In , the first VEGFA-inhibiting antibody, bevacizumab, was approved for use in advanced colorectal cancer in combination with chemotherapy and was followed in in NSCLC Sandler et al. Since then, diverse anti-angiogenic antibodies or tyrosine kinase inhibitors TKIs have been developed, which block either VEGF-A binding to the receptor or directly inhibit VEGFR-2 to hamper vascularization in tumors. VEGF-pathway inhibition has a broad anti-angiogenic effect in tumors: 1 it primarily inhibits vessel growth which induces regional cancer cell death and delays progression of the tumor rather than diminishing its size Escudier et al. Angiogenesis inhibitors in combination with either chemotherapeutics, targeted therapies or ICI, in first or second-line therapies in NSCLC, have exhibited improved efficacy and feasible safety, which significantly improved response rates and prolonged progression free survival PFS in a large number of patients. Despite the remarkable clinical benefits of these combinational approaches on response rate and PFS, the overall survival OS benefits were modest due to acquired drug resistance. It is important to mention that in most lung cancer studies anti-angiogenic therapy is administered until the onset of severe drug related adverse effects or disease progression. So far, there is only preclinical evidence that discontinued angiogenesis inhibition results in TME reorganization and perhaps causes a rebound effect of tumor angiogenesis. In tumor and healthy mouse models, it could be shown that anti-VEGF therapy withdrawal resulted in rapid tissue revascularization and long lasting structural changes including vessel hyper-permeability and increased metastasis in the diseased cohort Yang et al. The treatment-triggered hypoxia which induces angiogenesis especially during therapy-withdrawal is one possible explanation to this tumor promoting off-drug effect. The benefit of continuous anti-angiogenic therapy beyond disease progression in the clinical setting was first analyzed in a phase 3b trail in which included advanced NSCLC patients Gridelli et al. Here, bevacizumab was administered in addition to standard of care therapy beyond disease progression. While, the treatment continuation of bevacizumab yielded no substantial therapy benefit, improvements in efficacy, and no new safety signals were observed. Based on these findings, the approach of continuous angiogenesis inhibition should be further investigated but may be recommended at a certain degree in the future. Nevertheless, treatment decisions should be based on individual therapeutic efficacy, which needs to be tracked throughout the entire therapy. However, the absence of reliable biomarkers with predictive features for anti-angiogenic therapies hamper further therapy improvement, thus molecular screening for markers associated with tumor angiogenesis is currently of great value. Table 1. As previously mentioned, there is a great need for biomarkers to predict and track anti-angiogenic therapy efficacy, to help overcome innate and acquired resistance as it is still the main obstacle that restrains clinical success Bergers and Hanahan, So far, predictive angiogenesis-associated biomarkers in NSCLC are lacking, highlighting the need for further investigation to improve this anti-tumor approach. In a recent study, it was demonstrated that immunohistochemically confirmed TTF-1 expression in advanced non-squamous NSCLC samples, which is a known prognostic biomarker of lung adenocarcinomas, could be linked to therapy success of bevacizumab in combination with pemetrexed plus platinum derivatives Takeuchi et al. TTF-1 positive tumors exhibited enhanced clinical benefits when bevacizumab was combined with the basic therapy whereas TTF-1 negative tumors did not benefit from this addition. Furthermore, despite the previous results of the IMpower study, where significant clinical benefits of bevacizumab in combination with ICI and chemotherapy were shown, regardless of PDL-L1 expression, a phase 1b study by Herbst et al. observed contrary results. According to this, PD-L1 expression remains a predictive marker of ICI therapy or ICI therapy in combination with anti-angiogenesis agents in NSCLC. Qiu et al. recently examined the benefit of anti-angiogenic therapies bevacizumab, anlotinib or others with anti-PD-L1 agents nivolumab or pembrolizumab in a real-world study including 69 NSCLC patients. Subgroup analyses in the cohort revealed that the response and PFS of this combinational therapy was significantly higher when it was administered as first-line therapy compared to other lines of treatment, and when the therapy was initiated within the first 6 months of diagnosis compared to later time points Qiu et al. Additionally, patients with EGFR wildtype tumors exhibited significantly prolonged PFS after the combinational therapy compared to patients with EGFR mutated tumors. Interestingly, no correlation between PDL-1 expression levels and the efficacy of this combinational therapy has been observed so far, however, follow up will be continued. In short, these study results can help to optimize the use of anti-angiogenic agents in combination with PD-L1 inhibitors, however, more factors need to be investigated to yield an optimal benefit. Another potent multi-targeted anti-angiogenic TKI, anlotinib, has already shown profound benefits as third-line combinational therapy in advanced NSCLC Han et al. A transcriptomics study of an anlotinib-resistant lung cancer cell line, indicated that CXCL2, a cytokine involved in wound healing and angiogenesis, was also involved in anlotinib-resistance Lu et al. In vitro assays demonstrated that exogenous CXCL2 could recover anti-angiogenic-induced inhibition of migration and invasion and prevent apoptosis of anlotinib-resistant cells. Furthermore, in a retrospective analysis, anlotinib-induced decrease of the inflammatory cytokine CCL2 in serum correlated with prolonged PFS and OS Lu et al. Nevertheless, resistance and poor response to anlotinib hinder drug efficacy. While the underlying mechanisms are still unknown, elevated serum-levels of two angiogenesis-related markers KLK5 and L1CAM were recently correlated with poor response to anlotinib Lu et al. Easily available predictive biomarkers, e. Several studies suggested a potential prognostic value of VEGF in NSCLC but so far investigations into circulating VEGF levels have not yielded consistent results Rodríguez Garzotto et al. In the E study, high VEGF levels in pretreatment plasma of patients with advanced stage NSCLC, who received combinational treatment of bevacizumab plus chemotherapy, correlated with increased overall response but had no predictive outcome on survival Dowlati et al. Another study observed contrary results when baseline plasma biomarkers of non-squamous NSCLC patients undergoing similar therapy were evaluated Mok et al. Here, baseline VEGFA levels in the plasma correlated with prolonged PFS and OS but showed no association with response rates to the therapy. The predictive value of VEGF or other proangiogenic factors on anti-angiogenic drug response is a highly discussed matter revealing vastly variable results. This is partly due to analytical variability, including sample collection and handling, as well as the disagreements regarding the most suitable sample choice for evaluating circulating factors Rodríguez Garzotto et al. For example, serum or platelet rich plasma may not adequately represent the physiological VEGF level as it has been shown that the clotting processes initiates VEGF release in platelets Webb et al. Moreover, the pathological situation can impact VEGF levels, as patients with more advanced tumors or several metastatic tumor sites exhibit a higher baseline level of plasma VEGFA, suggesting that VEGFA is linked to the tumor burden Mok et al. Previously proposed correlations of circulating angiogenic factor levels with anti-angiogenic therapy efficacy in lung cancer seem to reflect tumor biology thus, have an important prognostic role rather than to be predictive Crohns et al. The observed trend of increasing circulating factors in response to angiogenesis inhibition on one hand was shown to depend considerably on the TME and may represent therapy-induced hypoxia Zaman et al. On the other hand, high VEGFA levels could also be attributed to TP53 mutated lung tumors which correlated with improved efficacy of bevacizumab Schwaederlé et al. A currently identified alternative biomarker for bevacizumab-based chemotherapy combinations in patients with advanced NSCLC is CXCL In the analyzed sera of 40 advanced staged NSCLC patients therapy-induced decrease of CXCL16 levels correlated with prolonged OS compared with patients exhibiting only moderate decrement Shibata et al. However, confirming if any of these molecular markers indeed exhibit adequate predictive features necessitates further investigation. New aspects of processes which promote tumor angiogenesis, and a better understanding of the endothelium as driving force can help identify reliable biomarkers and overcome therapy failure in NSCLC. There are several mechanisms on both the cellular and environmental levels which can promote vessel formation in human tumors, many of which are not yet been completely elucidated. Although angiogenesis may represent the most important part of tumor vascularization, other processes that result in perfusion of the tumor tissue should be investigated in more detail and considered when designing new anti-angiogenic approaches in NSCLC. In the following part we summarize various levels of tumor vascularization that may represent new targets for vessel inhibition in NSCLC. All mentioned mechanisms are summarized in Figure 1. Figure 1. Mechanisms of tumor vascularization in NSCLC. Tumor vascularization in lung cancer can be promoted by various processes which overlap during cancer progression. TECs exhibit upregulated metabolism to enable high angiogenic activity which includes processes involved in proliferation cholesterol synthesis and glycolysis and processes that enable migration via ECM remodeling collagen synthesis. Potential targets involved in these pathways SQLE, PFKFB3, and ALDH18A1, respectively are considered to increase the angiogenic potential of TECs in NSCLC. Hypoxia and acidosis induced by high levels of lactate due to upregulated glycolysis constitute to a highly pro-angiogenic tumor environment. Angiogenesis stimulating factors VEGF, bFGF, PDGF, HIF-1α, tryptase, and MMPs are released by both, cancer cells and stromal cells, including fibroblasts, pericytes, tumor associated macrophages and ECs. Non-angiogenic processes constitute to tumor vascularization and are inaccessible for anti-angiogenic agents, thus contributing to therapy resistance. VM comprises the formation of tubular structures arising from cancer cells that gain endothelial like properties to maintain vascular supply during cancer progression. Another mechanism of cancer cells to persist in circulation is to grow along existing vasculature, which is referred to as vessel co-option. In this figure we summarized the various mechanism of tumor vascularization that should be considered when targeting the inhibition of tumor vessels in NSCLC. The endothelium is postulated to be a large contributor to the therapeutic efficacy of anti-angiogenic therapies, and therefore represents a possible source of therapy response or failure. It is well known that the process of angiogenesis is comprised of different EC phenotypes which execute distinct functions. During the elongation of the sprouting vessel VEGF-sensitive tip ECs migrate into avascular tissue regions, thus leading the proliferating trailing stalk ECs, which built up the growing vessel. Newly formed vasculature finally adapts a mature and quiescent phenotype referred to as phalanx ECs Carmeliet and Jain, ; Betz et al. The EC phenotypes involved are highly dynamic and can reprogram the gene expression to meet their current physiological requirements. However, the tumor endothelium was not studied in depth and a recent single-cell RNA sequencing scRNA-Seq study identified even more EC phenotypes from both healthy and tumor tissue from lung cancer samples as already known, indicating a much more complex phenotypic heterogeneity of the tumor vasculature than initially presumed Goveia et al. Interestingly, although phenotype proportions differed strongly between analyzed NSCLC patients, they collectively observed a low abundance of tip and proliferating TECs, which represent the main targets of traditional anti-angiogenic therapy. Furthermore, they identified a so-far-unknown tumor exclusive phenotype of activated postcapillary vein EC that upregulated features known from HEVs in inflamed tissues such as immunomodulatory factors and ribosomal proteins. The unexpected finding that activated and proliferating TECs only represent a minority of the pathological EC phenotypes found in NSCLC, allows us to reconsider currently used anti-angiogenic therapy as less of a vessel-inhibiting strategy, and more of a strategy to modulate the higher proportion of mature TECs into potent participants of tumor surveillance. In order to develop new angiogenesis-inhibiting therapies, the molecular differences between physiological and pathological ECs will need to be elaborated. Genetically TEC and NEC phenotypes significantly differ in gene expression affecting diverse cellular mechanisms such as proliferation, migration, inflammation, and angiogenesis Figure 2. Previous studies have shown that one key feature of TECs is a highly active metabolism, which permits pathological processes as increased proliferation and angiogenesis Cantelmo et al. Hyperglycolytic TECs subsequently release high amounts of lactate into the environment, which in turn, further stimulates EC proliferation and angiogenesis Annan et al. It could be demonstrated that inhibition of PFKFB3 resulted in improved drug efficacy and decreased metastatic events in tumor mouse models Cantelmo et al. Another study in xenograft NSCLC mouse models exhibited that PFKFB3 mRNA silencing in combination with docetaxel results in a chemoenhancing effect and increases anti-cancer efficacy compared with monotherapies alone Chowdhury et al. Furthermore, to sustain upregulated proliferative capacity, TECs exhibit elevated nucleotide biosynthesis including upstream pathways that are involved in serine and lipid synthesis Cantelmo et al. In addition, Lambrechts et al. Interestingly, c-MYC expression induces angiogenesis in combination with HIF-1α and VEGF Lee and Wu, and recruits tryptase positive mast cells into the tumor niche Soucek et al. Figure 2. The multifaced picture of TECs in NSCLC. TECs possess features that enable continuous angiogenic activity for progressing vascularization of the tumor. These features are ensured by genetical changes in the tumor endothelium that are triggered by diverse stimuli of the TME e. The stroma, consisting of various cells, promote angiogenesis by directly releasing signaling molecules into the adjacent tissue, thereby stimulating TECs. Fibroblasts and myeloid derived suppressor cells MDSCs activate angiogenesis by releasing VEGF and bFGF into the TME. Additionally, CSF-1 molecules, expressed by cancer cells, further recruit MDSCs into the tumor niche. Tumor associated macrophages TAMs can directly induce angiogenesis by releasing VEGF, bFGF, and PlGF, or indirectly by releasing matrix metalloproteinases MMPs which promote endothelial migration. Mast cells secrete tryptase TRYPT into the TME which stimulates EC proliferation and enables ECM remodeling. Furthermore, to facilitate enhanced angiogenesis, TECs upregulate the surface expression of angiogenic receptors as well as increase metabolic activity including energy and amino acid metabolism and the biosynthesis of nucleotides. In addition to the high angiogenic activity, TECs can directly suppress inflammatory responses by downregulation of inflammatory cytokines for immune cell recruitment CCL2, CCL8, and IL-6 , receptors required for immune cell homing ICAM or lymphocyte activation MHC I and MHC II which results in impaired immune cell trafficking and migration into the TME. In summary the complex interaction of tumor-protecting environmental conditions and the pathological features of TECs lead to a pro-angiogenic and immune suppressive TME in NSCLC. Focusing on endothelial metabolism in cancer, a recent study could identify at least two metabolic signatures which are highly upregulated in angiogenic endothelium and TECs. One for proliferation, which includes gene sets associated with biomass production e. These results educed two new possible metabolic targets to hamper tumor angiogenesis; aldehyde dehydrogenase 18 family member A1 ALDH18A1 , an enzyme essential for de novo biosynthesis of proline; and squalene epoxidase SQLE , the rate-limiting enzyme in cholesterol biosynthesis. Silencing of ALDH18A as well as SQLE impaired EC proliferation, migration and vessel sprouting in in vitro assays. Summarized, targeting endothelial metabolism in cancer is an interesting therapeutic option that could possibly assist an anti-angiogenic approach for treating NSCLC. Another key feature of TECs in lung cancer is the downregulation of inflammatory responses thus contributing to tumor-associated immune escape. Single-cell analysis of NSCLC samples identified the most downregulated genes of the tumor endothelium in connection to inflammation, which included CCL2, CCL18, and IL6, essential for immune cell recruitment; MHC I and II, essential for immune cell activation; and ICAM, required for immune cell homing Lambrechts et al. As the endothelium represents the primary connection between the immune system and tumor cells, these results indicate the important role of TECs in immunomodulatory processes that hamper anti-tumor immunity. Vessel normalization not only improves immune cell activation and infiltration, but is also suggested to enhance drug delivery to the tumor sites, thus improving its efficacy Allen et al. Additionally, combinational therapy of angiogenesis inhibitors and immunotherapy anti-PD-L1 in previous studies could elicit the formation of unique blood vessels in treated tumors that resemble HEVs typically found in lymphoid tissues, which implicated increased treatment efficacy Allen et al. HEVs can mediate immune cell adhesion and migration into the tumor, which may be important for bypassing TEC-induced immune escape Ager and May, In the already discussed scRNA-Seq study by Goveia et al. These remarkable observations indicate that TECs comprise the ability to transform into HEVs to promote immune cell infiltration into the tumor and induce a potent anti-tumor response. This extends the previous observations of favorable synergistic effects of immune therapy in combination with angiogenesis inhibitors in NSCLC, especially when it results in HEV formation. Furthermore, direct induction of HEV formation could be a promising new strategy in anti-angiogenic approaches that may attain great clinical importance. However, currently there are no reliable biomarkers to track the process of vessel normalization or HEV formation in NSCLC which could help to predict and optimize this new treatment strategy. As mentioned above, in some cases tumor vascularization can be facilitated by non-ECs which adapt certain properties to sustain access to the circulation, which may support anti-angiogenic drug resistance. During tumor progression, processes that lead to vascularization of the malignant tissue can vary locally as well as temporarily and involve angiogenic as well as non-angiogenic mechanisms even in the same lesion Bridgeman et al. In lung tumors, where non-angiogenic tumor growth occurs most commonly, previous studies primarily located non-angiogenic processes in the tumor periphery, whereas angiogenesis is typically localized in the hypoxic tumor core Pezzella et al. Here, we briefly discuss the impact of non-angiogenic processes in NSCLC on anti-angiogenic drug efficacy based on previous studies. VEGF-A inhibition using bevacizumab failed to inhibit VM in breast cancer cells in vitro , furthermore, sunitinib, a multi targeting anti-VEGFR inhibitor, even promoted VM in breast cancer mouse models Dey et al. Additionally it could be demonstrated that VM in NSCLC depends on expression of Sema4D and its receptor plexinB1 which activate RhoA and downstream ROCK, comprising an already known angiogenesis-promoting process in tumors Basile et al. Although the role of VM in NSCLC is not fully understood, previous observations suggest that it may contribute to anti-angiogenic therapy failure and may serve as an option to treat aggressive lung tumors. Vessel co-option on the other hand is a common phenomenon especially observed in lung metastases when tumor cells start to invade perivascular tissues Jensen, Anti-angiogenic therapy with sunitinib could induce a switch from angiogenic vessel formation to vessel co-option in a lung metastatic mouse model, which ultimately resulted in sunitinib resistance Bridgeman et al. Unfortunately, regulative mechanisms of vessel co-option in human tumors remain unknown in large part, however, predicting the occurrence of either VM or vessel co-option could be a useful tactic to prevent anti-angiogenic drug resistance in some patients. According to these and other results, it could be confirmed that non-angiogenic tumors contribute to anti-angiogenic therapy resistance which reveals the undoubted importance of targeting both angiogenic, but also non-angiogenic vessel growth to treat NSCLC Donnem et al. Increasing knowledge of the physiological processes of tumor vascularization in addition to traditional angiogenesis has enlightened a variety of adaptive mechanisms which can promote anti-angiogenic therapy resistances. This awareness fortifies the necessity for alternative anti-angiogenic agents besides traditional anti-VEGF therapy. As previously examined, tumor angiogenesis depends on upregulated metabolic activity e. Cholesterol not only represents a fundamental structural component of cell membranes and serves as precursor for several steroid hormones, it is also crucial for membrane function and angiogenic signaling, making it a favorable target for tumor vessel inhibition Lyu et al. Inhibition of intracellular cholesterol trafficking with anti-inflammatory drug chepharantine was shown to hamper angiogenesis and tumor growth in lung cancer xenograft mice while improving anti-tumor activity of standard chemotherapeutics Lyu et al. Another study has shown that pharmacological lowering of intracellular cholesterol levels with pitavastatin could reduce growth and migration and induced apoptosis in human lung tumor-associated ECs in vitro Hu et al. In vivo experiments using lung cancer xenograft mice exhibited that pitavastatin-treatment could completely arrest tumor growth in these animals when combined with cisplatin and delayed tumor growth and impaired angiogenesis in cisplatin-resistant mouse models. Another potential angiogenic target for cancer treatment is tie1. While the second tie receptor, tie2, is well characterized as a regulator during late stages of angiogenesis e. As tie1 is also upregulated in intratumoral vasculature, its deletion on ECs successfully produced a potent anti-angiogenic effect in different cancers Kaipainen et al. In fact, EC-specific deletion of tie1 in lung carcinoma and melanoma mouse models resulted in delayed cancer growth, predominantly in late-stage tumors La Porta et al. Furthermore, it inhibited neovessel sprouting and a reduced intratumoral vessel density, while the remaining mature vasculature became strongly normalized, which limited further metastatic formation. These findings, and the fact that tie1 expression is increased in angiogenic endothelium compared with resting vasculature, presents tie1 as a highly potent angiogenic target, especially in the treatment of advanced staged NSCLC. Another considerable strategy of anti-angiogenic therapy could include targeting micro RNAs miRNAs as they represent a new paradigm in molecular cancer therapy. The impact of miRNAs in post-transcriptional regulation has already been associated with pathways involved in cancer and vascular disease as summarized in Sun et al. The following studies evaluated the potential role of specific angiogenesis-related miRNAs as targets in lung cancer. Hsu et al. observed that miRa, a micro RNA known to be hypoxia-associated, was overexpressed in exosomes of oxygen depleted CL lung cancer cells Hsu et al. Furthermore, these cancer-cell derived exosomes could induce angiogenesis via HIF-1α signaling in vitro when internalized by HUVECs. Additionally, miRa transfection increased permeability and transendothelial migration of cancer cells in vitro by downregulation of the tight junction protein ZO-1 and stimulated neovascularization and tumor growth in vivo in CL xenograft mice, proposing it to be an appealing target for anti-angiogenic therapy. Upregulation of miR in squamous lung cancer cells in vitro on the other hand could be associated with impaired VEGF expression and hampered migration and invasion, thereby facilitating a tumor-suppressive function. Additionally, overexpression of miR in HUVECs was observed to inhibit tube formation and reduced the expression of VEGF, which hampered their angiogenesis activity in vitro Liu et al. As it is an essential process during vessel growth, targeting ECM remodeling may also be an interesting approach to inhibit tumor angiogenesis in NSCLC. The most prominent enzymes involved in this process are matrix-metalloporoteinases MMPs which are inhibited under physiological conditions by tissue inhibitors of metalloproteinases TIMPs. miRb could be identified as a promotor of MMP-2 activity and invasion of NSCLC cancer cells in vitro by downregulation of TIMP Additionally, it could be observed that miRb was significantly upregulated in tumor tissue of NSCLC patients with vascular cancer cell invasion Hirono et al. According to these findings, targeting miRb could be a strategy to impede angiogenesis and cancer cell invasion in lung cancer. Uribesalgo et al. suggested targeting the apelin signaling pathway to inhibit tumor vessel formation in lung cancer Uribesalgo et al. Apelin is a conserved peptide involved in developmental angiogenesis and is also upregulated in ECs within the TME. Previous studies could associate high apelin levels with a poor clinical outcome in patients with NSCLC Györffy et al. In murine lung cancer models, apelin knockout reduced tumor burden and prolonged survival by inhibiting VEGF, TGF-β1, and TNF-α and simultaneously decreased MDSC infiltration in the TME Uribesalgo et al. The combination of pharmacological inhibition of apelin with the anti-angiogenic drug sunitinib in lung cancer and mammary cancer mouse models, significantly delayed tumor growth and could almost double the survival, even in the KRAS driven or p53 mutated tumors, when compared with sunitinib treatment alone. Finally, apelin loss also reduced vessel density and prevented sunitinib-induced hypoxia and poor vessel structure in the TME. Conclusively, apelin inhibition may provide a potent synergistic anti-tumor effect when combined with anti-angiogenic agents, while, and most importantly, avoiding therapy-induced hypoxia of the TME, thus decreasing the chance of metastases, and bypassing potential therapy resistances. Single-target anti-angiogenic agents have already shown their limitations in clinical settings Jayson et al. Even in combination with other therapy approaches like standard chemotherapy or immune therapy, treatment success remains largely marginal. Targeting several pro-angiogenic molecules with recombinant fusion proteins could therefore increase the anti-angiogenic effect of such therapies. Zhang et al. When injected into lung cancer mouse models, autologous generated anti-peptibody antibodies inhibited tumor progression and angiogenesis and decreased expression of bFGF, VEGFA and PDGF in the tumor tissue. Targeting angiogenesis with fusion proteins exhibited potent anti-tumor efficacy in murine models and may represent a new approach for vessel inhibition in NSCLC, especially in combination with other therapy agents aimed at important angiogenic factors, previously discussed potential TEC specific markers or cellular mechanisms Table 2. The instability of tumor vessels due to morphological abnormalities e. Although anti-angiogenic therapy can temporarily restore tissue perfusion and drug delivery by vascular normalization, treatment withdrawal often results in vessel hyper-permeability and can even induce a rebound effect of tumor angiogenesis Yang et al. As continuous inhibition of angiogenesis remains difficult to implement for health or economic reasons, an alternative or more independent delivery system of anti-angiogenic agents could help to overcome these issues. Nanomaterials have become an emerging field in cancer therapy in recent years, as their unique molecular properties make them suitable targeted drug delivery-systems. Physiochemically, these nanoparticles match the size of inter-endothelial junctions of blood vessels in the TME and therefore increase permeation and retention EPR resulting in a passive drug delivery Chauhan and Jain, Nanomaterials such as liposomes or nanotube carbon structures are used to deliver anti-angiogenic agents and improve drug specificity while reducing cytotoxic side effects, drug clearance and resistance mechanisms in the treatment of NSCLC Seshadri and Ramamurthi, In the past, studies using biodegradable polymers as nanocarriers to deliver chemotherapeutics and targeted drugs exhibited significant anti-tumor efficacy in vitro and in vivo. For example, paclitaxel encapsulated aldehyde polyethylene glycol-polylactide PEG-PLGA conjugated to a VEGFR2-inhibiting peptide showed increased internalization in HUVECs in vitro as well as potent activity against breast cancer models in vivo Yu et al. Although there are several peptide motifs that are suggested to target tumor endothelium such as RGD or NGR which can bind integrin heterodimers CD51 and CD61, or aminopeptidase N, respectively, their targeting with nanomaterial is not yet applied for treating NSCLC Sakurai et al. Furthermore, non-angiogenic mechanisms such as VM or vessel co-option could also represent possible targets for nanomaterial-based therapy as the EPR effect of such molecules could help to overcome delivery and infiltration issues of traditional cancer therapeutics. However, nanotherapeutics may provide a new potential anti-angiogenic therapeutical approach, but as already discussed, there is still a need for more specific biomarkers to exclusively target tumor vasculature in an organ specific manner. Taking this into consideration, chimeric antigen receptor CAR T-cell therapy, which serves as personalized immune therapy using autologous T-lymphocytes, engineered to target specific antigens present in a tumor, could be used to exclusively eliminate TECs without damaging healthy vasculature. The therapy failure can, at least in part, be attributed to the impaired accessibility of the tumor mass due to dysfunctional vasculature and immunosuppressive conditions in the TME. Targeting tumor vessels directly with CAR T-cells could therefore be a good strategy to overcome these issues, which at best, can normalize the defective vasculature and improve drug efficacy in combinational therapy settings. In a recent study Xie et al. Injected EIIIB-targeting CAR T-cells could delay tumor growth and improve survival in immunocompetent mouse models harboring aggressive melanoma, whereas colorectal cancer mouse models did not respond to the treatment. Here, the expression levels of EIIIB in the different tissues had impact on the therapy outcome which again highlights the importance of organ specific vascular markers as well as the impact of organ specific angiogenic activity when targeting tumor vessel formation. Other studies investigated the anti-angiogenic efficacy of TEM8-specific CAR T cells in solid cancer mouse models. TEM8 is one of the first discovered TEC markers and represents a promising target in anti-angiogenic therapy strategies St Croix et al. In , a study reported that TEM8-specific CAR T-cells could improve survival and significantly decreased vascularization in triple negative breast cancer mouse models and induced tumor regression in mice with lung metastases Byrd et al. A more recent study, however, observed contrasting results where TEM8-sepcific CAR T-cells triggered high toxicity and induced inflammation in lung and spleen when injected into healthy mice Petrovic et al. It is suggested that the engineered T-cells cross-reacted with other antigens or targeted TEM8 in healthy tissues, although it is normally expressed at a much lower quantity compared with pathological levels. However, both processes resulted in severe toxicity in vivo and again emphasize the need for more adequate, highly specific tumor-vessel exclusive markers that can be targeted with either CAR T-cells or other previously discussed inhibiting molecules. So far, the main obstacles of anti-angiogenic therapy in NSCLC are evading- or intrinsic resistance mechanisms which still remain elusive. We have discussed a wide array of possible therapies and therapy systems that could improve anti-angiogenic efficacy when combined with standard treatment. The principal goal would be to expand the therapeutical effect of angiogenesis-inhibiting drugs on vessel normalization and render the tumor more vulnerable to additional agents such as chemotherapy or immunotherapy. In a recent study, Hosaka et al. could show that dual angiogenesis inhibition could sensitize resistant off-target tumors to therapy. Therefore they created mouse models of breast cancer or fibrosarcoma, both resistant to anti-VEGF and anti-PDGF treatment due to increased tumor associated expression of bFGF, a molecule which modulates the vasculature via pericyte recruitment in a PDGF-dependent process Hosaka et al. Neither anti-VEGF nor anti-PDGF monotherapy had a significant anti-tumor effect on bFGF-positive tumors, but the combination of both agents produced a superior benefit, inhibiting cancer growth by suppressing proliferation and triggering apoptosis of tumor cells. Interestingly, even the pan-blocking of FGF-receptors did not yield a comparable benefit. To explain this unexpected effect, angiogenesis has to be considered as an interacting network of various signaling pathways which cannot be disrupted by blocking a single molecule. These findings demonstrate that the disruption of interacting angiogenic pathways by simultaneously targeting multiple angiogenic factors can provoke a highly potent anti-tumor effect which is able to circumvent mechanisms of therapy resistance, and thus should be considered as new approach to improve neovessel inhibition in cancer. Angiogenesis is a main therapeutic concept in oncology, especially in NSCLC, where three approved agents are available in combination with chemotherapy or immunotherapy. Increasing knowledge in angiogenic processes and non-angiogenic processes that contribute to tumor vascularization, provide precise targets for novel therapy strategies and pave the way for developing new anti-angiogenic treatment concepts that target e. These therapeutic concepts need to be evaluated for synergistic effects as, in our view, modern anti-angiogenesis represents the concept of shaping the TME rather than being a direct anti-tumor therapy itself. However, these therapeutic strategies are very promising in preclinical setting and the translation into a clinical setting is not only warranted but highly desired. Furthermore, a new horizon of targeted and functional TEC characterization was opened by scRNA-Seq studies, which proved that the tumor vasculature is highly heterogenous and differs from the normal adjacent vasculature more than primarily assumed in terms of metabolic activity, immune suppression and heterogeneity for example. In addition, new synergistic effects of TECs in their role of immunomodulation were identified and induction of HEV formation for immune priming is suggested to be a new therapeutic strategy. Next the organ specific context of the vasculature plays an important role and has to be further studied for better therapy allocation. In conclusion the concept and goal of anti-angiogenesis in NSCLC in the future can be reshaped by abolishing the traditional vessel priming concept and moving toward a side specific molding of the TME, using the tumor vasculature as a tool, like a trojan horse. SD, HH, EN, AP, and DW developed the concept of the review. SD, HH, and EN drafted the review. DW and AP corrected and reviewed the review. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Adighibe, O. Is nonangiogenesis a novel pathway for cancer progression? A study using 3-dimensional tumour reconstructions. Cancer 94, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ager, A. Understanding high endothelial venules: lessons for cancer immunology. Oncoimmunology 4:e Aguayo, A. Clinical relevance of Flt1 and Tie1 angiogenesis receptors expression in B-cell chronic lymphocytic leukemia CLL. Leukemia Res. CrossRef Full Text Google Scholar. Allen, E. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Alshangiti, A. Antiangiogenic therapies in non-small-cell lung cancer. Annan, D. Carbonic anhydrase 2 CAII supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun. Auf, G. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Augustin, H. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Cell Biol. Augustine, R. Therapeutic angiogenesis: from conventional approaches to recent nanotechnology-based interventions. C Mater. Babina, I. Advances and challenges in targeting FGFR signalling in cancer. Cancer 17, — Basile, J. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Bergers, G. Tumorigenesis and the angiogenic switch. Cancer 3, — Modes of resistance to anti-angiogenic therapy. Cancer 8, — Bertolini, F. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Cancer 6, — Betz, C. Cell behaviors and dynamics during angiogenesis. Development , — Bittner, M. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature , — de Bock, K. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell , — Bridgeman, V. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. Bruning, U. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. Byrd, T. Cancer Res. Cantelmo, A. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization. |
| Angiogenesis Inhibitors | CancerIndex | On the mecjanism hand, immune checkpoint blockade, Mechanusm as anti-CTLA-4 or mmechanism, increases Anti-angiogenesis mechanism perfusion to improve therapeutic efficacy. Several genes Anti-angkogenesis in glycolysis are Anti-angigoenesis HIF1 control, Type diabetes blood sugar control as GLUT1GLUT3 Fiber optic technology advancements, PDK1 or LDHA Favaro et al. A cytoprotective role of autophagy was supported by several preclinical studies using radiation or imatinib as anti-cancer strategies Miyazawa et al. CAS PubMed Google Scholar Qian, B. It is now evident that several mechanisms exist. And the 1st 6 months of treatment may be a crucial period when hemorrhagic events occur. Moreover, hypoxia leads to AMPK activation, inducing the metabolic switch from glycolysis to oxidative phosphorylation McIntyre and Harris, |
| Angiogenesis Inhibitors | The past decade has also witnessed the great progress in the development of anti-tumor drugs developed by Chinese researchers. Apatinib can simultaneously suppress the kinase activities of VEGFR-2, c-Kit, and c-Src and is approved by the CFDA for the treatment of advanced gastric cancer GC in October [ 71 , 72 ]. The efficacy and safety profile of apatinib in patients with metastatic gastric or gastroesophageal junction adenocarcinoma who had failed at least two lines of chemotherapy was evaluated in a series of clinical trials. A phase III randomized clinical trial, conducted by Li and collaborators, has indicated its important role in three or more lines for GC patients [ 73 ]. The primary end points of OS and PFS were significantly prolonged by apatinib OS 6. Though the ORR showed no difference between two groups, the disease control rate DCR favored apatinib over placebo treatment. The major treatment-related grade 3—4 AEs in apatinib arm included hand-foot syndrome, proteinuria, and hypertension [ 73 ]. The ALTER trial demonstrated 3. The results showed that both OS 9. Anlotinib also produced significant ORR and DCR benefits vs. placebo and had a manageable safety profile [ 76 , 77 ]. It was approved by the CFDA as a third-line or further therapy for advanced NSCLC patients in Until now, apatinib and anlotinib have not gained the approval of the FDA, but both of them were identified as orphan drugs in the USA. In the phase II clinical trials, fruquintinib showed a significant PFS benefit in patients with treatment-refractory mCRC [ 79 ]. Then, a randomized, double bind, phase III FRESCO trial conducted by Li et al. laid the foundation for the approval of this drug on patients with mCRC by the CFDA in [ 80 ]. In this study, mCRC patients who had progressed after at least two lines of chemotherapy were allocated to receive either fruquintinib or placebo. The primary end point median of OS was significantly longer in the fruquintinib group compared to placebo 9. Moreover, higher ORR and DCR were observed in patients receiving fruquintinib with a manageable safety profile. Additionally, a phase I clinical trial is ongoing in the USA, exploring the efficacy and safety in non-Chinese populations [ 81 ]. While the approved anti-angiogenic TKIs are trying to expand their indication in other cancer types, numerous new anti-angiogenic TKIs are also being extensively explored. Three representative TKI drugs with potential to be approved in the near future are presented. Motesanib was considered as a potent anti-tumor drug in Asian advanced NSCLC patients based on the subgroup analysis of MONET1 trial [ 83 ]. However, the results of later phase III trial MONETA were disappointing with no advantage in patients receiving motesanib plus paclitaxel and carboplatin over placebo plus paclitaxel and carboplatin [ 84 ]. Nevertheless, two phase II trials have indicated remarkable anticancer activity of motesanib among patients with advanced thyroid cancer [ 85 , 86 ]. Recently, Lubner et al. examined the efficacy of motesanib in low-grade NETs in a phase II trial [ 87 ]. The study reached its primary objective with a 4-month PFS of All in all, motesanib is as potential as a systemic targeted therapy for NETs, but its niche in the treatment of NETs still needs further study. Though, cediranib had failed phase III clinical trials in NSCLC [ 89 ], mCRC [ 90 , 91 ], and recurrent glioblastoma [ 92 ], it showed new hope in recurrent ovarian cancer. The ICON6 trial evaluated the efficacy and safety of cediranib plus platinum-based chemotherapy and as continued maintenance treatment in patients with relapsed platinum-sensitive ovarian cancer [ 93 ]. Unfortunately, the ICON6 trail was prematurely terminated on account of the depressing results in other cancer types. Most common side effects of grade 3—4 in arm C were neutropenia, fatigue, and hypertension during the chemotherapy phase and diarrhea, fatigue, and neutropenia during maintenance treatment. A phase I study NCT observed an acceptable safety profile and encouraging antitumor activity in patients with advanced solid tumors, particularly in NETs [ 95 ]. At present, one phase II study NCT and two phase III studies NCT, NCT conducted on advanced NETs are ongoing [ 96 ]. Immunotherapy has been changing the paradigm of oncology treatment in the recent years [ 97 , 98 , 99 ]. Whether the combination of TKIs and immunotherapy can create synthetic effect is a hot topic. The emerging evidences suggest that anti-angiogenic therapy may not only inhibit neo-vascular formation, but also regulate the immune microenvironment [ ]. This provided a theoretic basis for the combination of TKIs and immunotherapy. Subsequently, hundreds of clinical trials were designed to access the efficacy of combining TKIs with immune checkpoint blockade. A phase Ib study JAVELIN Renal conducted by Choueiri et al. interrogated the combination therapy of axitinib plus avelumab a PD-L1 mAb in advanced RCC patients [ ]. These encouraging results supported the further study of these drug combinations. Now, the phase III JAVELIN Renal trial finished [ ]. The result showed that in patients with mRCC, the axitinib plus avelumab group showed a remarkable improvement in median PFS compared with sunitinib The combination of axitinib and avelumab would be a promising strategy for patients with mHCC based on the positive result of JAVELIN Renal Other combinations such as lenvatinib plus pembrolizumab or SHR plus apatinib in patients with HCC were also ongoing [ ]. The combination of immunotherapy with TKIs has demonstrated promising outcome in a certain type of carcinomas, but further optimized combinations are required and caution must be taken to avoid severe toxicity. The development of anti-angiogenic agents has attracted great attention. Bevacizumab, the first clinically approved anti-VEGF targeted agents, provides a first proof of principle of anti-angiogenic treatment in cancer. Though monotherapy with bevacizumab is largely inefficient, it really exerts therapeutic efficacy in various types of carcinoma when in combination with chemotherapy [ ]. Because tumor angiogenesis is regulated by multiple pathways, many interconnected pathways can compensate the effect of single inhibition of VEGF signaling. It seems that multi-targeted TKIs hold a therapeutic advantage over monoclonal antibody as they can block multiple angiogenic signaling pathways simultaneously. Indeed, TKIs have shown their efficacy in many types of cancers, mainly RCC and HCC. Although all anti-angiogenic receptor TKIs share the same mechanism of action and the similar spectrum of targeted kinases, they are different in their pharmacokinetics and substance-specific AEs. The one possible explanation may be that the subtle difference on chemical structure leads to the variable affinity and potency to targets. Another possibility is that those TKIs may act on some unidentified targets beyond known kinases. With more and more anti-cancer agents available, it is a challenge for the oncologist to make an optimal choice in the sequence of treatment. For instance, 12 drugs have been approved for patients with HCC, including 6 anti-angiogenic TKIs until [ ]. Though, the international guidelines have reached a global consensus for the choice of drugs in different lines. The optimal strategy and the sequence of drugs as well as the right time of the incorporation of other therapeutic methods such as surgery, radiology has not yet been resolved. Tolerance of receptor TKIs should also be taken into account. Another challenge for anti-angiogenesis TKIs is the lack of robust biomarkers to identify patients with cancer who will benefit from anti-angiogenic therapy. Unlike RTK inhibitor, larotrectinib is special for cancer with tropomyosin receptor kinases TRK fusion-positive and has demonstrated significant efficiency in patients with different tumor histology [ ]. One of the main problems in identifying such a biomarker for anti-angiogenic therapy may come from the complex feedback loops and cross talk between signaling pathways. Currently, some biomarkers have been proposed, such as VEGF, VEGFR-2, FGF-2, or IL-8, but none of them have yet been validated for routine clinical use [ ]. Recently, a cohort study conducted by Liu et al. indicated a positive correlation between the anti-angiogenesis-related AEs and prolonged OS [ ]. It means that side effects, such as high blood pressure, hypothyroidism, or hand-foot syndrome, may associate with the anti-tumor efficacy. Similarly, Rini et al. As there are no molecular biomarkers available for clinical use, those side effects might be helpful for clinical decision. The future of TKIs could be their positioning besides metastatic setting, such as in adjuvant therapy and neoadjuvant treatment. There were three well-known phase III clinical trials that explored the use of TKIs in RCC in adjuvant setting, namely ASSURE adjuvant sunitinib vs. sorafenib vs. placebo , PROTECT pazopanib vs. placebo , and S-TRAC sunitinib vs. placebo [ , , ]. Only S-TRAC study showed a significant improvement by sunitinib in disease-free survival in high-risk RCC after nephrectomy [ ]. Based on the result of S-TRAC trial, sunitinib was approved by the FDA as an adjuvant therapy for RCC patents in Unfortunately, adjuvant sorafenib for HCC patients reached a negative result [ ]. The utilization of TKIs before surgery has also been studied. A phase II trial explored the safety and efficacy of the use of pazopanib prior to cytoreductive nephrectomy RCC patients, suggesting the safety, and clinical benefit could be expected [ ]. The precision role of anti-angiogenic TKI in adjuvant and neoadjuvant therapy needs further investigation. It is noted that the indication of these receptor TKIs are mainly restricted to highly vascular tumor, like RCC, HCC, NSCLC, and CRC. Their efficacy in other types of cancers needs further exploration [ 30 ]. In most case, anti-angiogenesis treatment increases the PFS of patients, while the increase in OS is unsatisfactory. Great breakthrough in immunotherapy brings new possibility for the combination of TKIs, and positive results in a certain type of carcinoma attract broad attention [ ]. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Article CAS PubMed Google Scholar. Gimbrone MA Jr, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. Article PubMed PubMed Central Google Scholar. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. Zheng X, Liu Q, Yi M, Qin S, Wu K. The regulation of cytokine signaling by retinal determination gene network pathway in cancer. Onco Targets Ther. Seki T, Hosaka K, Lim S, Fischer C, Honek J, Yang Y, et al. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. Nat Commun. Article CAS PubMed PubMed Central Google Scholar. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. Poole RM, Vaidya A. Ramucirumab: first global approval. Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Berndt N, Karim RM, Schonbrunn E. Advances of small molecule targeting of kinases. Curr Opin Chem Biol. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science New York, NY. Article CAS Google Scholar. Ling Y, Xie Q, Zhang Z, Zhang H. Protein kinase inhibitors for acute leukemia. Biomark Res. Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. Article PubMed PubMed Central CAS Google Scholar. Cowan-Jacob SW. Structural biology of protein tyrosine kinases. Cell Mol Life Sci. Schlessinger J. Cell signaling by receptor tyrosine kinases. Hubbard SR. Structural analysis of receptor tyrosine kinases. Prog Biophys Mol Biol. Kerbel RS. Tumor angiogenesis. N Engl J Med. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Skouras VS, Maragkos C, Grapsa D, Syrigos KN. Targeting neovasculature with multitargeted antiangiogenesis tyrosine kinase inhibitors in non-small cell lung cancer. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Poddubskaya EV, Baranova MP, Allina DO, Smirnov PY, Albert EA, Kirilchev AP, et al. Personalized prescription of tyrosine kinase inhibitors in unresectable metastatic cholangiocarcinoma. Exp Hematol Oncol. Motzer RJ, Escudier B, Gannon A, Figlin RA. Sunitinib: ten years of successful clinical use and study in advanced renal cell carcinoma. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. Frampton JE. Pazopanib: a review in advanced renal cell carcinoma. Target Oncol. Article PubMed Google Scholar. Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. Guo J, Jin J, Oya M, Uemura H, Takahashi S, Tatsugami K, et al. Safety of pazopanib and sunitinib in treatment-naive patients with metastatic renal cell carcinoma: Asian versus non-Asian subgroup analysis of the COMPARZ trial. J Hematol Oncol. Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. Zarrabi K, Fang C, Wu S. New treatment options for metastatic renal cell carcinoma with prior anti-angiogenesis therapy. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma PALETTE : a randomised, double-blind, placebo-controlled phase 3 trial. Article PubMed CAS Google Scholar. Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma AXIS : a randomised phase 3 trial. Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. Keating GM. Axitinib: a review in advanced renal cell carcinoma. de la Fouchardiere C. Regorafenib in the treatment of metastatic colorectal cancer. Future Oncol. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer CORRECT : an international, multicentre, randomised, placebo-controlled, phase 3 trial. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer CONCUR : a randomised, double-blind, placebo-controlled, phase 3 trial. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib GRID : an international, multicentre, randomised, placebo-controlled, phase 3 trial. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment RESORCE : a randomised, double-blind, placebo-controlled, phase 3 trial. Medavaram S, Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Onco. Article Google Scholar. Ranieri G, Marech I, Asabella AN, Di Palo A, Porcelli M, Lavelli V, et al. Int J Mol Sci. Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. Priya SR, Dravid CS, Digumarti R, Dandekar M. Targeted therapy for medullary thyroid cancer: A review. Front Oncol. Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma METEOR : final results from a randomised, open-label, phase 3 trial. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. Dhillon S. Nintedanib: a review of its use as second-line treatment in adults with advanced non-small cell lung cancer of adenocarcinoma histology. Nintedanib: A review of its use in patients with idiopathic pulmonary fibrosis. Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. Alshangiti A, Chandhoke G, Ellis PM. Antiangiogenic therapies in non-small-cell lung cancer. Curr Oncol. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer LUME-Lung 1 : a phase 3, double-blind, randomised controlled trial. Hanna NH, Kaiser R, Sullivan RN, Aren OR, Ahn MJ, Tiangco B, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer LUME-Lung 2 : a randomized, double-blind, phase III trial. Lung Cancer. Yeung KT, Cohen EE. Lenvatinib in advanced, radioactive iodine-refractory, differentiated thyroid carcinoma. Clin Cancer Res. Scott LJ. Lenvatinib: first global approval. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Aoyama T, Yoshikawa T. Targeted therapy: apatinib - new third-line option for refractory gastric or GEJ cancer. Nat Rev Clin Oncol. Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, et al. YND1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. Syed YY. Anlotinib: first global approval. Han BH, Li K, Zhao YZ, Li BL, Cheng Y, Zhou JY, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial ALTER Br J Cancer. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER phase 3 randomized clinical trial. JAMA Oncol. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. Su S, Wu YL. Clinical trials of tyrosine kinase inhibitors for lung cancer in China: a review. Xu RH, Li J, Bai Y, Xu J, Liu T, Shen L, et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase Ib study and a randomized double-blind phase II study. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. Shirley M. Fruquintinib: first global approval. Polverino A, Coxon A, Starnes C, Diaz Z, DeMelfi T, Wang L, et al. AMG , an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res. Kubota K, Ichinose Y, Scagliotti G, Spigel D, Kim JH, Shinkai T, et al. Ann Oncol. Kubota K, Yoshioka H, Oshita F, Hida T, Yoh K, Hayashi H, et al. Phase III, randomized, placebo-controlled, double-blind trial of motesanib AMG in combination with paclitaxel and carboplatin in east Asian patients with advanced nonsquamous non-small-cell lung cancer. Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. Lubner S, Feng Y, Mulcahy M, O'Dwyer P, Giang GY, Hinshaw JL, et al. E AMG and octreotide in patients with low-grade neuroendocrine tumors. Mahner S, Woelber L, Mueller V, Witzel I, Prieske K, Grimm D, et al. Beyond bevacizumab: an outlook to new anti-angiogenics for the treatment of ovarian cancer. Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. Hoff PM, Hochhaus A, Pestalozzi BC, Tebbutt NC, Li J, Kim TW, et al. Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study HORIZON III. Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. Ledermann JA, Embleton AC, Raja F, Perren TJ, Jayson GC, Rustin GJS, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer ICON6 : a randomised, double-blind, placebo-controlled phase 3 trial. Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. Xu JM, Wang Y, Chen YL, Jia R, Li J, Gong JF, et al. Sulfatinib, a novel kinase inhibitor, in patients with advanced solid tumors: results from a phase I study. PubMed PubMed Central Google Scholar. Grillo F, Florio T, Ferrau F, Kara E, Fanciulli G, Faggiano A, et al. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr Relat Cancer. Yu S, Li A, Liu Q, Li T, Yuan X, Han X, et al. When VEGF is attached to these drugs, it is unable to activate the VEGF receptor. Some angiogenesis inhibitors are immunomodulatory drugs—agents that stimulate or suppress the immune system —that also have antiangiogenic properties. In some cancers, angiogenesis inhibitors appear to be most effective when combined with additional therapies. Because angiogenesis inhibitors work by slowing or stopping tumor growth without killing cancer cells, they are given over a long period. The U. Food and Drug Administration FDA has approved a number of angiogenesis inhibitors to treat cancer. Most of these are targeted therapies that were developed specifically to target VEGF, its receptor, or other specific molecules involved in angiogenesis. Approved angiogenesis inhibitors include:. Side effects of treatment with VEGF-targeting angiogenesis inhibitors can include hemorrhage , clots in the arteries with resultant stroke or heart attack , hypertension , impaired wound healing, reversible posterior leukoencephalopathy syndrome a brain disorder , and protein in the urine. Gastrointestinal perforation and fistulas also appear to be rare side effects of some angiogenesis inhibitors. Antiangiogenesis agents that target the VEGF receptor have additional side effects, including fatigue, diarrhea, biochemical hypothyroidism , hand-foot syndrome , cardiac failure, and hair changes. Home About Cancer Cancer Treatment Types of Cancer Treatment Immunotherapy Angiogenesis Inhibitors. Angiogenesis Inhibitors On This Page What is angiogenesis? Why is angiogenesis important in cancer? How do angiogenesis inhibitors work? What angiogenesis inhibitors are being used to treat cancer in humans? Do angiogenesis inhibitors have side effects? What is angiogenesis? Approved angiogenesis inhibitors include: Axitinib Inlyta® Bevacizumab Avastin® Cabozantinib Cometriq® Everolimus Afinitor® Lenalidomide Revlimid® Lenvatinib mesylate Lenvima® Pazopanib Votrient® Ramucirumab Cyramza® Regorafenib Stivarga® Sorafenib Nexavar® Sunitinib Sutent® Thalidomide Synovir, Thalomid® Vandetanib Caprelsa® Ziv-aflibercept Zaltrap®. |
Anti-angiogenesis mechanism -
Third, unlike other immune cells, some MDSCs can differentiate into EC-like cells. These EC-like MDSCs express endothelial markers, such as CD31 and VEGFR2, and have the ability to integrate into the tumor vasculature 45 , 46 , Adaptive immune cells are also critical players in the orchestration of tumor angiogenesis by directly affecting EC biology and indirectly modulating myeloid cell phenotypes.
IFN-γ directly inhibits the proliferation and migration of human endothelial cells and secretes IFN-inducible protein 10 IP and monokine induced by IFN-γ MIG. These cytokines also react with CXCR3, restraining the proliferation of endothelial cells and tumor vascularization 74 , Furthermore, IFN-γ signaling downregulates VEGF-A but upregulates CXCL9, CXCL10, and CXCL11, which collectively stimulate vascular maturation by enhancing pericyte recruitment along ECs 74 , 77 , Another important aspect of IFN-γ in tumor angiogenesis is the reprogramming of TAMs from M2- to M1-like TAMs.
T H 1 cells also polarize M2-like TAMs to M1-like TAMs and induce DC maturation in the TME, which suppresses tumor angiogenesis 82 , T H 2 cells expressing IL-4, IL-5, and IL recruit M2-like TAMs through STAT-6 activation and promote tumor angiogenesis 41 , 50 , 77 , The expression of IL by T H 17 correlates with the infiltration of ECs and abnormal tumor vasculature 41 , 77 , 85 , Tumor-infiltrating Treg cells also play a critical role by sustaining angiogenesis directly through VEGF secretion and supporting endothelial cell recruitment and expansion 83 , Furthermore, Tregs promote angiogenesis indirectly by restraining the activity of T H 1 cells and by triggering the activation of M2-like macrophages In ovarian cancer, hypoxia results in CCL28 upregulation, leading to a robust increase in Treg infiltration, VEGF and blood vessels, whereas depletion of Tregs reduces intratumoral VEGF levels and tumor angiogenesis 18 , The interactions between tumor immunity and angiogenesis suggest that tumor vascular remodeling could enhance the efficacy of cancer immunotherapy.
Emerging preclinical evidence demonstrates the potential of combining immunotherapy with vascular-targeting treatment 24 , 37 , 75 , 88 , 89 , 90 , Allen et al.
demonstrated that anti-angiogenic therapy with anti-VEGFR2 enhances the efficacy of anti-PD-L1 immunotherapy in pancreatic neuroendocrine tumor RT2-PNET , mammary carcinoma MMTV-PyMT , and glioblastoma NFppGBM models Furthermore, the combination of anti-angiogenic and immunotherapy increased pericyte coverage and normalized tumor vessels, promoting intratumoral infiltration of activated T cells.
In addition to vascular normalization, the vessel phenotype represents the characteristics of high endothelial venules HEVs , which are morphologically thickened with plump endothelial cells ECs and functionally more specialized in lymphocyte extravasation than other tumor ECs.
Notably, the LTβR signaling pathway is involved in the generation of intratumoral HEVs after combined treatment with anti-VEGFR2 and anti-PD-L1. Therefore, these results suggest that anti-angiogenic therapy could improve the efficacy of cancer immunotherapy and overcome resistance to cancer immunotherapy via tumor vessel normalization and intratumoral HEV formation.
Shigeta et al. also reported consistent synergism of anti-VEGFR2 and anti-PD-L1 in hepatocellular carcinoma HCC They observed that anti-VEGFR2 therapy upregulates PD-L1 expression under hypoxic conditions, mediated in part by IFN-γ secreted by ECs. Dual combination therapy has also been shown to improve overall survival OS and anti-cancer immunity with increased intratumoral accumulation of CTLs and M1-like TAMs.
Collectively, combination therapy with anti-VEGFR2 and anti-PD-1 reprograms the immune microenvironment via vessel normalization, further strengthening the anti-cancer immune response and overcoming resistance to cancer immunotherapy in HCC. Anti-angiogenic therapy can also overcome resistance to anti-PD-1 by abolishing the TOX-mediated T-cell exhaustion program in the TME Kim et al.
revealed that VEGF significantly upregulates the transcription factor TOX, which influences the phenotype and function of CTLs. The TOX-mediated transcriptional program resulted in severe T-cell exhaustion and upregulated inhibitory immune checkpoint receptors such as PD-1, TIM-3, LAG-3, and TIGIT and reduced the proliferation of cytokine production by CTLs.
Combination treatment with anti-VEGFR2 and anti-PD-1 enhanced the immunotherapeutic efficacy and T-cell reinvigoration.
Collectively, combinatory treatment with anti-angiogenic agents and ICIs is a potential therapeutic option in anti-PDresistant cancer. Schmittnaegel et al. demonstrated that combined blockade of VEGF-A and ANGPT2 by a bispecific antibody A2V enhanced the therapeutic activity compared with either anti-VEGF-A or anti-ANGPT2 monotherapy alone in both genetically engineered and transplant tumor models A2V effectively inhibited tumor angiogenesis but promoted vascular maturation in the TME.
This negative feedback mechanism was successfully overcome by combining A2V with anti-PD-1, leading to better immunotherapeutic efficacy. These results encourage further testing of combining ICIs with various anti-angiogenic targets other than VEGF in advanced cancers.
Recently, a novel immunotherapeutic target, simulator of IFN genes STING , was reported to be involved in the regulation of the tumor vasculature and demonstrated synergism with anti-VEGFR2 and ICIs Yang et al.
revealed that intratumoral STING signaling activation suppresses tumor angiogenesis and induces vessel normalization through type I IFN signaling activation and the upregulation of genes related to vascular normalization and endothelial-lymphocyte interaction. STING agonist combined with anti-VEGFR2 synergistically enhanced vascular normalization, leading to durable anti-cancer immunity.
Therefore, these data suggest that combining novel therapeutics with the combination of anti-angiogenic agents and ICIs could help overcome resistance to anti-angiogenic and immunotherapy in refractory cancers. On the other hand, immune checkpoint blockade, such as anti-CTLA-4 or anti-PD-1, increases vascular perfusion to improve therapeutic efficacy.
Zheng et al. Notably, IVP can distinguish tumors that are sensitive to ICIs from those that are resistant. In addition, IVP was time-dependently induced by anti-CTLA-4 even before tumor regression was detectable.
Collectively, these findings indicate that IVP could be a prerequisite of ICI to improve anti-cancer immunity, thereby enabling it to be used as a predictive indicator for ICI efficacy. Preclinical studies continue to yield encouraging results regarding the synergistic effects of ICIs and anti-angiogenic agent combination therapy, which have led to clinical investigations to reproduce these results in patients with advanced cancer 92 , 93 , 94 , 95 , 96 , 97 , Several pivotal clinical trials have already demonstrated the superiority of combining anti-angiogenic agents and ICIs in various malignancies.
The most successful results of combination therapy have been reported in renal cell carcinoma RCC and hepatocellular carcinoma HCC.
RCC is a highly immunogenic tumor that has been treated with high-dose IL-2 in some patients. Immunotherapy has recently been revisited and reevaluated when phase 3 clinical trials demonstrated that nivolumab anti-PD-1 treatment leads to longer OS with significantly lower toxicity.
In KEYNOTE, patients with previously untreated metastatic RCC were treated with either pembrolizumab anti-PD-1 and axitinib VEGFR1, 2, and 3 inhibitor combination therapy or sunitinib monotherapy, and significantly increased progression-free survival PFS was demonstrated in the combination group compared with the sunitinib group Although the incidence of hepatic toxicity was higher in the combination group, no relevant death event occurred.
Based on the significant efficacy and acceptable toxicity profile, combination therapy with pembrolizumab and axitinib was approved by the FDA for treatment-naïve patients with metastatic RCC.
JAVELIN Renal NCT is a phase 3 clinical trial that evaluated the efficacy of avelumab anti-PD-L1 and axitinib combination therapy against sunitinib monotherapy in patients with metastatic RCC in a first-line setting Although the data are premature for OS analysis and require further follow-up, the median PFS of the combination group has already been reported to be In addition, the ORR and complete response rate were Based on this study, the FDA approved avelumab for use in combination with axitinib as first-line treatment for patients with advanced RCC.
In HCC, two highly anticipated phase III studies testing PD-1 inhibitor monotherapy failed to meet their primary endpoints, leading to doubts regarding the use of ICIs in this cancer.
However, a randomized phase III clinical trial, IMBRAVE NCT , demonstrated significant improvements in co-primary end points, PFS and OS, using the combination of atezolizumab anti-PD-L1 and bevacizumab anti-VEGF-A compared with sorafenib This was the first study to propose a new first-line treatment option that is superior to sorafenib, which has been the standard of care for a decade.
The FDA granted the Breakthrough Therapy designation based on these data, and the phase III IMBRAVE trial was initiated. At the ESMO Asia Congress, the median OS with the atezolizumab and bevacizumab combination was not reached until analysis when compared with In terms of patient-reported outcomes, the combination group exhibited delayed deterioration of quality of life compared with sorafenib.
The safety and efficacy of the combination of pembrolizumab and lenvatinib were evaluated in patients with unresectable HCC in KEYNOTE, a multicenter, open-label, single-arm phase Ib study This clinical trial also yielded a promising response rate during the early stage and was granted Breakthrough Therapy designation by the FDA, initiating LEPP, a phase 3 trial to evaluate pembrolizumab in combination with lenvatinib as a potential first-line treatment for patients with advanced HCC In non-squamous non-small cell lung cancer NSCLC , a phase 3 clinical trial Impower, NCT comparing atezolizumab anti-PD-L1 , bevacizumab anti-VEGF , carboplatin, and paclitaxel combination therapy ABCP group against bevacizumab, carboplatin, and paclitaxel combination therapy BCP group showed significantly extended PFS and OS in the ABCP group compared with the BCP group median PFS: 8.
The ORR was significantly higher in the ABCP group than in the BCP group ORR: Based on these results, atezolizumab was approved by the FDA for use in combination with bevacizumab, paclitaxel, and carboplatin as first-line treatment for patients with metastatic non-squamous NSCLC.
Recently, the FDA granted accelerated approval for the use of a combination of pembrolizumab and lenvatinib in patients with advanced endometrial cancer who have experienced disease progression after systemic therapy.
In this trial, patients who had previously been treated for metastatic endometrial cancer were evaluated for their response to lenvatinib and pembrolizumab.
Interim analysis showed that the ORR was However, immune-mediated AEs, including endocrine, gastrointestinal, hepatic, skin, pulmonary, and renal events, occurred in In several years, these ongoing trials are expected to generate consistent results, which will evolve the therapeutic landscape of advanced cancers.
Years of research have demonstrated the potential of ICI monotherapy as well as its limitations, which have led to further attempts to overcome these limitations by combination immunotherapy. Of the potential candidates, the combination of ICI and anti-angiogenic agents continues to yield promising results in both preclinical and clinical studies, not only highlighting that it is one of the most effective combination immunotherapy regimens thus far but also changing the treatment landscape for RCC and HCC.
Nonetheless, several issues remain to optimize the efficacy of this combination therapy. First, predictive biomarkers must be developed to identify the subset of patients who will benefit from this combination treatment.
Third, whether the effects of this combination are synergistic or merely additive must be evaluated. Finally, the angiogenic phenotype differs according to organ; thus, more in-depth analyses must be performed to further our knowledge of the response to ICI treatment at the organ level.
Ribas, A. Cancer immunotherapy using checkpoint blockade. Science , — CAS PubMed PubMed Central Google Scholar. Fukumura, D. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges.
Khan, K. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. CAS PubMed Google Scholar. Yi, M. et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment.
Cancer 18 , 60 PubMed PubMed Central Google Scholar. Huang, Y. Improving immune-vascular crosstalk for cancer immunotherapy. Rahma, O. The intersection between tumor angiogenesis and immune suppression. Cancer Res. De Palma, M. Microenvironmental regulation of tumour angiogenesis.
Cancer 17 , — PubMed Google Scholar. Xia, A. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Barsoum, I. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Chon, H. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade.
Kim, C. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell 25 , — Park, J. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment.
Cancer Cell 30 , — Lee, J. Novel glycosylated VEGF decoy receptor fusion protein, VEGF-Grab, efficiently suppresses tumor angiogenesis and progression. Cancer Ther. Jain, R. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia.
Cancer Cell 26 , — Kim, Y. Methylation-dependent regulation of HIF-1alpha stability restricts retinal and tumour angiogenesis. Schaaf, M. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. Ramjiawan, R. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy?
Angiogenesis 20 , — Facciabene, A. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T reg cells. Nature , — Baluk, P. Cellular abnormalities of blood vessels as targets in cancer.
Lugano, R. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol. Life Sci. Article PubMed PubMed Central Google Scholar. Motz, G.
Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Gabrilovich, D.
Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Oyama, T. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells.
VEGF-A drives TOX-dependent T cell exhaustion in anti-PDresistant microsatellite stable colorectal cancers. Khan, O. Gerald, D. Angiopoietin an attractive target for improved antiangiogenic tumor therapy.
Goumans, M. TGF-beta signaling in vascular biology and dysfunction. Cell Res. Tian, M. Transforming growth factor-beta and the hallmarks of cancer.
Cell Signal 23 , — De Falco, S. The discovery of placenta growth factor and its biological activity. Odorisio, T. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. Cell Sci.
Brodsky, S. Vascular density and VEGF expression in hepatic lesions. Gastrointestin Liver Dis. Tumeh, P. Liver metastasis and treatment outcome with Anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol.
Nishino, M. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. Cancer 4 , 84 Paez-Ribes, M.
Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15 , — Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy.
Science , 58—62 Martin, J. Normalizing function of tumor vessels: progress, opportunities, and challenges. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy.
Natl Acad. USA , — Jung, K. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. Targeting CXCR4-dependent immunosuppressive Ly6C low monocytes improves antiangiogenic therapy in colorectal cancer. Rivera, L. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy.
Cell Rep. Bruno, A. Orchestration of angiogenesis by immune cells. Biswas, S. Powell J et al Update on hemangiomas and vascular malformations. Curr Opin Pediatr. Hanahan D et al A flanking attack on cancer. Mocanu MM, Nagy P, Szollosi J et al Chemoprevention of breast cancer by dietary polyphenols.
Guthrie, A. A et al Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Cancer 69 6 — Pandey, M. B et al Regulation of cell signaling pathways by dietary agents for cancer prevention and treatment. Cancer Biol. Dandawate PR, Subramaniam D, Jensen RA, Anant S et al Targeting cancer stem cells and signaling pathways by phytochemicals: novel approach for breast cancer therapy.
Curti V, Di Lorenzo A, Dacrema M, Xiao J, Nabavi SM, Daglia M et al In vitro polyphenol effects on apoptosis: an update of literature data. Coccia A, Mosca L, Puca R, Mangino G, Rossi A, Lendaro E et al Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells.
Zielinska-Przyjemska M, Kaczmarek M, Krajka-Kuzniak V, Luczak M, Baer-Dubowska W et al The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines.
Toxicol In Vitro — Cerezo, A. A Molecular structure-function relationship of dietary polyphenols for inhibiting VEGF-induced VEGFR-2 activity. Sarkar J, Nandy SK, Chowdhury A, Chakraborti T, Chakraborti S Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis.
Liu L, Lai CQ, Nie L, Ordovas J, Band M, Moser L, Meydani M et al The modulation of endothelial cell gene expression by green tea polyphenol-EGCG. Gomes F. Life Sci. Verheul H. M et al The role of vascular endothelial growth factor VEGF in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors.
Breast Cancer. S1 : S80—S McMahon G et al VEGF receptor signaling in tumor angiogenesis. S1 : 3— Ferrara N, Gerber HP, LeCouter J et al The biology of VEGF and its receptors. Lieu C, Heymach J, Overman M, Tran H, Kopetz S et al Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis.
Cancer Res. Carmeliet P. K et al Molecular mechanisms and clinical applications of angiogenesis. Bergers G, Hanahan D et al Modes of resistance to anti-angiogenic therapy. Res — Brahimi-Horn MC, Pouyssegur J et al The hypoxia-inducible factor and tumor progression along the angiogenic pathway. Yang Y, Sun M, Wang L, Jiao B et al HIFs, angiogenesis, and cancer.
Hickey M. C et al Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Genis L. G et al MT1-MMP: uUniversal or particular player in angiogenesis? Cancer Metastasis Rev. Hung H et al Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol.
J Cell Physiol. Luo H, Rankin GO, Liu L, Daddysman MK, Jiang BH, Chen YC et al Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells.
Nutr Cancer. PLoS ONE e Dai Z. Y et al PLoS ONE 8 11 :e Download references. We would also record our thanks to Prof. Balasubramanian, Honorable Vice Chancellor, Prof. Ram Murugesan, Director-Research and management of Chettinad Academy of Research and Education for providing facilities to perform this study.
Faculty of Allied Health Sciences, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education Deemed to be University , Kelambakkam, Tamil Nadu, , India.
Molecular Oncology Lab, Department of Bioinformatics, Alagappa University, Karaikudi, Tamil Nadu, , India. You can also search for this author in PubMed Google Scholar.
The first author SK collected the data from articles and drafted the manuscript. GK revised and did the final approval of the draft of the manuscript. LK contributed to drafting the manuscript. All the authors have read and approved the manuscript for the submission. Correspondence to Gowtham Kumar Subbaraj.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.
The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Subbaraj, G. Antiangiogenic role of natural flavonoids and their molecular mechanism: an update. Egypt J Intern Med 33 , 29 Download citation. Received : 18 May Accepted : 24 July Published : 06 September Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all SpringerOpen articles Search.
Download PDF. Abstract Background Angiogenesis is the development of new blood vessels from the existing vasculature, which is important in normal developmental processes. Main body Numerous bioactive plant compounds are recently tested for their antiangiogenic potential.
Conclusion Presently developed antiangiogenic drugs in malignant growth treatment do not meet assumptions about adequacy and safety. Background Polyphenols which are the bioactive compounds derived from natural resources have pulled in a lot of consideration for their well-being advancing impacts.
Table 1 Angiogenic effect of flavonoids and their molecular mechanisms. Full size image. VEGF signaling pathway Vascular endothelial development factor is a significant supporter of angiogenic factor, applying its cell impacts essentially through the stimulation of vascular endothelial growth factor receptor 1, vascular endothelial growth factor receptor 2, and two tyrosine kinase receptors.
bFGF signaling pathway Basic fibroblast growth factors are a group of pleiotropic aspects associated with the guideline of different major measures, as well as cell expansion, separation, survival, and angiogenesis [ 62 ]. HIF-1 signaling pathway The significant controller of oxygen homeostasis in cells presented to hypoxia is HIF Impact of flavonoids on matrix metalloproteinases A vascular cellar layer is needed to advance endothelial cell intrusion into the interstitial matrix.
Molecular mechanism of flavonoids Naringenin is a type of flavonoid which is abundantly found in tomatoes and oranges. Conclusion Pharmacological examinations carried out on a few flavonoids in vitro and in vivo tests confirmed that their antiangiogenic impact is mediated through a huge variety of cellular and molecular functions.
References Chen L, Yang B, Tang B, Gong G, Kam H, Gao C et al Differential angiogenic activities of naringin and naringenin in zebrafish in vivo and human umbilical vein endothelial cells in vitro. b Article CAS PubMed Google Scholar Hong M, Cheng H, Song L, Wang W, Wang Q, Xu D, Xing W Wogonin suppresses the activity of matrix metalloproteinase-9 and inhibits migration and invasion in human hepatocellular carcinoma.
Turkiye Klin Tip Bil Derg — Google Scholar Ovando C, Hernandez D, Hernandez E et al Chemical studies of anthocyanins: a review. Beni-Suef University J Basic Appl Sci 9 1 :1—14 Article Google Scholar Iwashina T Flavonoid properties of five families newly incorporated into the order Caryophyllales.
CO;2-D Ferrara N, Alitalo K et al Clinical applications of angiogenic growth factors and their inhibitors. S1 : S80—S84 McMahon G et al VEGF receptor signaling in tumor angiogenesis.
S1 : 3—10 Ferrara N, Gerber HP, LeCouter J et al The biology of VEGF and its receptors. CCR Article CAS PubMed PubMed Central Google Scholar Carmeliet P.
In the past years, however, non-angiogenic processes in the TME have gained attention as they are suggested to significantly contribute to tumor progression while being resistant to traditional angiogenesis inhibitors.
In highly vascularized organs such as the lung, it was observed that cancer cells start to grow along existing vessels to preserve access to essential nutrients and gases without the need to form new vasculature.
This process is referred to as vessel co-option Pezzella et al. In contrast to the chaotic growth of angiogenic tumor vessels, co-opted vasculature remains well organized as deduced from normal tissues Adighibe et al. So far, vessel co-option is suggested to result, at least in part, of differential mitochondrial metabolism, but it may also involve reduced inflammation Donnem et al.
The ECs of co-opted vessels experience severe molecular changes during this process, for e. Thereupon, the tumor core becomes hypoxic, which consequently activates the angiogenic switch in tumor vessels Holash et al.
In vitro studies of glioma cells suggest that tumor cells that facilitate vessel co-option are dependent on the endoplasmic reticulum based stress sensing protein IRE1 Auf et al. Furthermore the MMP-activating protein B2R was shown to serve as a chemoattractant during the migration of glioma cells towards blood vessels Montana and Sontheimer, Finally, CDC42, a protein involved in actin-dependent formation of cytoplasmatic extensions, together with CD44, a protein crucial for establishing cell-cell contact, enable the connection between tumor cells and vessel covering pericytes for vessel co-option Caspani et al.
So far, the molecular mechanism behind VM is not yet understood, however, it appears that VE-cadherin, the most prominent receptor on ECs, may play an important role.
VM networks resemble embryonic vasculogenesis, referring to a highly aggressive tumor cell phenotype that converted to an embryonic-like, undifferentiated state to facilitate tube formation Maniotis et al. Gene expression analysis of VM networks in aggressive melanoma identified genes correlated with various cellular phenotypes such as fibroblasts, ECs and epithelial cells Bittner et al.
Tumors positive for VM show an increased expression of the ECM component laminin5γ2 and several MMPs, underlining the importance of ECM remodeling for initiating and promoting this non-angiogenic process Seftor et al.
Furthermore, VM is associated with poor prognosis as it is mainly observed in aggressive forms of melanoma and lung metastases Williamson et al. Taking the potent impact of these non-angiogenic processes in cancer progression into consideration, may help us explain the occurring resistance of lung tumors to VEGF-inhibitors Döme et al.
In summary, the pathological features of tumor-associated ECs and non-ECs which result in a complex cancer promoting TME are diverse, and consequently contribute to therapy failure of angiogenesis inhibitors as well as other therapy approaches in a remarkable fashion.
To better understand the biological mechanisms behind drug resistance or lack of clinical benefit, further investigation into the detailed characterization of the endothelial compartment in the TME are essential. Currently used anti-angiogenic agents have been developed and approved for clinical application after intense study of their molecular, cellular, and physiological mode of action using various experimental approaches.
In the following part we summarize currently available methods for investigating tumor angiogenesis as well as anti-angiogenic agents that have already been accepted for treating NSCLC. Experimental models remain the cornerstone for investigating tumor angiogenesis and the development of new anti-angiogenic therapies.
As vessel sprouting is a multistep process there is a wide array of assays which enable individual evaluation of different stages, and each possesses specific advantages and disadvantages Shahid et al.
To unravel these complex processes, it is crucial to understand the analytical potential of each model. In vitro methods represent the fundamental evaluation of tumor angiogenesis including basic functional analysis such as proliferation, migration, and tube formation.
The big advantages of in vitro assays are their simplicity, high reproducibility, and cost effectiveness, while the disadvantages include the incomplete representation of the cellular heterogeneity and prevailing conditions present in human organs.
Although findings from in vitro assays may never be conclusive alone, they serve as a preliminary projection of angiogenic processes upon treatment of choice and provide first insights into a testing hypothesis.
Ex vivo assays such as the thoracic aorta ring and retina angiogenesis methods represent the link between in vitro and in vivo analysis. The advantage of this method over in vitro assays is the preservation of original EC properties within the tissue that are normally modified due to isolation processes and repeated passaging.
The absence of blood flow and circulating EC progenitors or other factors constitute the main disadvantages of these methods. For more accurate information regarding angiogenic processes upon treatment in a biological system or to perform long-term studies, in vivo methods are necessary.
The most common systems to investigate angiogenesis in a living organism are the chicken chorioallantoic membrane CAM assay, matrigel plugs, and tumor xenograft models. CAM assays, which have already been in use for decades, utilize chorioallantoic membranes of fertilized chicken eggs to evaluate angiogenic processes.
While this method is cost effective, highly reproducible and the outcomes are easily visualized, it must be taken into consideration that vessel growth is evaluated during developmental stages, which can affect studies investigating mechanisms in mature vasculature. Matrigel plug assays enable the use of an in vitro tool in an in vivo setting.
Here, vascular growth is evaluated by injection of matrigel, a synthesized substrate resembling basement membrane matrix, into an animal model which allows easy stimulation, subsequent excision, and investigation of the plug with, for example, immunohistological stainings.
Compared with CAM assays, the matrigel plug can be used in more analytical methods and provides a fast and reliable representation of angiogenic processes in a biological system. Nevertheless, this method may require more replicates due to higher variability of results and is therefore more expensive.
Lastly, transplantation xenografts represent the most advanced method to investigate tumor angiogenesis in a living organism. Tumor cells, mostly of human origin, are injected into immunodeficient mice to induce formation of a cancer mass that can be further treated and monitored for changes regarding tumor angiogenesis.
This method most suitably reflects the pathological mechanism of vessel growth in vivo in the presence of blood circulation, as well as diverse environmental factors. Furthermore, it enables the long-term study of diverse processes associated with angiogenesis that are observed in a biological system such as tissue invasion, distant metastasis formation as well as non-angiogenic processes like vessel co-option and VM, which are known to promote resistance mechanisms in various cancers.
Aside from the ethical aspect, a considerable disadvantage of this method is the incomplete or lacking representation of the immune system due to immunosuppression of the study organism. Examining which experimental assay is most suitable for investigating a chosen angiogenic process under certain conditions, necessitates extensive deliberation with the desired endpoint, required technical equipment, level of experimental throughput, cost, and ethics kept in mind.
Additionally, the complexity of angiogenesis cannot be unraveled using a single analytical method but the thought-out application of multiple overlapping analyses, ranging from cellular to physiological levels, are necessary to obtain robust findings worth testing in the clinical setting.
In , the first VEGFA-inhibiting antibody, bevacizumab, was approved for use in advanced colorectal cancer in combination with chemotherapy and was followed in in NSCLC Sandler et al. Since then, diverse anti-angiogenic antibodies or tyrosine kinase inhibitors TKIs have been developed, which block either VEGF-A binding to the receptor or directly inhibit VEGFR-2 to hamper vascularization in tumors.
VEGF-pathway inhibition has a broad anti-angiogenic effect in tumors: 1 it primarily inhibits vessel growth which induces regional cancer cell death and delays progression of the tumor rather than diminishing its size Escudier et al.
Angiogenesis inhibitors in combination with either chemotherapeutics, targeted therapies or ICI, in first or second-line therapies in NSCLC, have exhibited improved efficacy and feasible safety, which significantly improved response rates and prolonged progression free survival PFS in a large number of patients.
Despite the remarkable clinical benefits of these combinational approaches on response rate and PFS, the overall survival OS benefits were modest due to acquired drug resistance.
It is important to mention that in most lung cancer studies anti-angiogenic therapy is administered until the onset of severe drug related adverse effects or disease progression. So far, there is only preclinical evidence that discontinued angiogenesis inhibition results in TME reorganization and perhaps causes a rebound effect of tumor angiogenesis.
In tumor and healthy mouse models, it could be shown that anti-VEGF therapy withdrawal resulted in rapid tissue revascularization and long lasting structural changes including vessel hyper-permeability and increased metastasis in the diseased cohort Yang et al.
The treatment-triggered hypoxia which induces angiogenesis especially during therapy-withdrawal is one possible explanation to this tumor promoting off-drug effect. The benefit of continuous anti-angiogenic therapy beyond disease progression in the clinical setting was first analyzed in a phase 3b trail in which included advanced NSCLC patients Gridelli et al.
Here, bevacizumab was administered in addition to standard of care therapy beyond disease progression. While, the treatment continuation of bevacizumab yielded no substantial therapy benefit, improvements in efficacy, and no new safety signals were observed.
Based on these findings, the approach of continuous angiogenesis inhibition should be further investigated but may be recommended at a certain degree in the future. Nevertheless, treatment decisions should be based on individual therapeutic efficacy, which needs to be tracked throughout the entire therapy.
However, the absence of reliable biomarkers with predictive features for anti-angiogenic therapies hamper further therapy improvement, thus molecular screening for markers associated with tumor angiogenesis is currently of great value. Table 1. As previously mentioned, there is a great need for biomarkers to predict and track anti-angiogenic therapy efficacy, to help overcome innate and acquired resistance as it is still the main obstacle that restrains clinical success Bergers and Hanahan, So far, predictive angiogenesis-associated biomarkers in NSCLC are lacking, highlighting the need for further investigation to improve this anti-tumor approach.
In a recent study, it was demonstrated that immunohistochemically confirmed TTF-1 expression in advanced non-squamous NSCLC samples, which is a known prognostic biomarker of lung adenocarcinomas, could be linked to therapy success of bevacizumab in combination with pemetrexed plus platinum derivatives Takeuchi et al.
TTF-1 positive tumors exhibited enhanced clinical benefits when bevacizumab was combined with the basic therapy whereas TTF-1 negative tumors did not benefit from this addition. Furthermore, despite the previous results of the IMpower study, where significant clinical benefits of bevacizumab in combination with ICI and chemotherapy were shown, regardless of PDL-L1 expression, a phase 1b study by Herbst et al.
observed contrary results. According to this, PD-L1 expression remains a predictive marker of ICI therapy or ICI therapy in combination with anti-angiogenesis agents in NSCLC.
Qiu et al. recently examined the benefit of anti-angiogenic therapies bevacizumab, anlotinib or others with anti-PD-L1 agents nivolumab or pembrolizumab in a real-world study including 69 NSCLC patients.
Subgroup analyses in the cohort revealed that the response and PFS of this combinational therapy was significantly higher when it was administered as first-line therapy compared to other lines of treatment, and when the therapy was initiated within the first 6 months of diagnosis compared to later time points Qiu et al.
Additionally, patients with EGFR wildtype tumors exhibited significantly prolonged PFS after the combinational therapy compared to patients with EGFR mutated tumors. Interestingly, no correlation between PDL-1 expression levels and the efficacy of this combinational therapy has been observed so far, however, follow up will be continued.
In short, these study results can help to optimize the use of anti-angiogenic agents in combination with PD-L1 inhibitors, however, more factors need to be investigated to yield an optimal benefit. Another potent multi-targeted anti-angiogenic TKI, anlotinib, has already shown profound benefits as third-line combinational therapy in advanced NSCLC Han et al.
A transcriptomics study of an anlotinib-resistant lung cancer cell line, indicated that CXCL2, a cytokine involved in wound healing and angiogenesis, was also involved in anlotinib-resistance Lu et al.
In vitro assays demonstrated that exogenous CXCL2 could recover anti-angiogenic-induced inhibition of migration and invasion and prevent apoptosis of anlotinib-resistant cells.
Furthermore, in a retrospective analysis, anlotinib-induced decrease of the inflammatory cytokine CCL2 in serum correlated with prolonged PFS and OS Lu et al. Nevertheless, resistance and poor response to anlotinib hinder drug efficacy.
While the underlying mechanisms are still unknown, elevated serum-levels of two angiogenesis-related markers KLK5 and L1CAM were recently correlated with poor response to anlotinib Lu et al. Easily available predictive biomarkers, e.
Several studies suggested a potential prognostic value of VEGF in NSCLC but so far investigations into circulating VEGF levels have not yielded consistent results Rodríguez Garzotto et al.
In the E study, high VEGF levels in pretreatment plasma of patients with advanced stage NSCLC, who received combinational treatment of bevacizumab plus chemotherapy, correlated with increased overall response but had no predictive outcome on survival Dowlati et al.
Another study observed contrary results when baseline plasma biomarkers of non-squamous NSCLC patients undergoing similar therapy were evaluated Mok et al. Here, baseline VEGFA levels in the plasma correlated with prolonged PFS and OS but showed no association with response rates to the therapy.
The predictive value of VEGF or other proangiogenic factors on anti-angiogenic drug response is a highly discussed matter revealing vastly variable results. This is partly due to analytical variability, including sample collection and handling, as well as the disagreements regarding the most suitable sample choice for evaluating circulating factors Rodríguez Garzotto et al.
For example, serum or platelet rich plasma may not adequately represent the physiological VEGF level as it has been shown that the clotting processes initiates VEGF release in platelets Webb et al.
Moreover, the pathological situation can impact VEGF levels, as patients with more advanced tumors or several metastatic tumor sites exhibit a higher baseline level of plasma VEGFA, suggesting that VEGFA is linked to the tumor burden Mok et al.
Previously proposed correlations of circulating angiogenic factor levels with anti-angiogenic therapy efficacy in lung cancer seem to reflect tumor biology thus, have an important prognostic role rather than to be predictive Crohns et al.
The observed trend of increasing circulating factors in response to angiogenesis inhibition on one hand was shown to depend considerably on the TME and may represent therapy-induced hypoxia Zaman et al. On the other hand, high VEGFA levels could also be attributed to TP53 mutated lung tumors which correlated with improved efficacy of bevacizumab Schwaederlé et al.
A currently identified alternative biomarker for bevacizumab-based chemotherapy combinations in patients with advanced NSCLC is CXCL In the analyzed sera of 40 advanced staged NSCLC patients therapy-induced decrease of CXCL16 levels correlated with prolonged OS compared with patients exhibiting only moderate decrement Shibata et al.
However, confirming if any of these molecular markers indeed exhibit adequate predictive features necessitates further investigation. New aspects of processes which promote tumor angiogenesis, and a better understanding of the endothelium as driving force can help identify reliable biomarkers and overcome therapy failure in NSCLC.
There are several mechanisms on both the cellular and environmental levels which can promote vessel formation in human tumors, many of which are not yet been completely elucidated. Although angiogenesis may represent the most important part of tumor vascularization, other processes that result in perfusion of the tumor tissue should be investigated in more detail and considered when designing new anti-angiogenic approaches in NSCLC.
In the following part we summarize various levels of tumor vascularization that may represent new targets for vessel inhibition in NSCLC. All mentioned mechanisms are summarized in Figure 1.
Figure 1. Mechanisms of tumor vascularization in NSCLC. Tumor vascularization in lung cancer can be promoted by various processes which overlap during cancer progression. TECs exhibit upregulated metabolism to enable high angiogenic activity which includes processes involved in proliferation cholesterol synthesis and glycolysis and processes that enable migration via ECM remodeling collagen synthesis.
Potential targets involved in these pathways SQLE, PFKFB3, and ALDH18A1, respectively are considered to increase the angiogenic potential of TECs in NSCLC.
Hypoxia and acidosis induced by high levels of lactate due to upregulated glycolysis constitute to a highly pro-angiogenic tumor environment. Angiogenesis stimulating factors VEGF, bFGF, PDGF, HIF-1α, tryptase, and MMPs are released by both, cancer cells and stromal cells, including fibroblasts, pericytes, tumor associated macrophages and ECs.
Non-angiogenic processes constitute to tumor vascularization and are inaccessible for anti-angiogenic agents, thus contributing to therapy resistance.
VM comprises the formation of tubular structures arising from cancer cells that gain endothelial like properties to maintain vascular supply during cancer progression.
Another mechanism of cancer cells to persist in circulation is to grow along existing vasculature, which is referred to as vessel co-option. In this figure we summarized the various mechanism of tumor vascularization that should be considered when targeting the inhibition of tumor vessels in NSCLC.
The endothelium is postulated to be a large contributor to the therapeutic efficacy of anti-angiogenic therapies, and therefore represents a possible source of therapy response or failure. It is well known that the process of angiogenesis is comprised of different EC phenotypes which execute distinct functions.
During the elongation of the sprouting vessel VEGF-sensitive tip ECs migrate into avascular tissue regions, thus leading the proliferating trailing stalk ECs, which built up the growing vessel.
Newly formed vasculature finally adapts a mature and quiescent phenotype referred to as phalanx ECs Carmeliet and Jain, ; Betz et al.
The EC phenotypes involved are highly dynamic and can reprogram the gene expression to meet their current physiological requirements. However, the tumor endothelium was not studied in depth and a recent single-cell RNA sequencing scRNA-Seq study identified even more EC phenotypes from both healthy and tumor tissue from lung cancer samples as already known, indicating a much more complex phenotypic heterogeneity of the tumor vasculature than initially presumed Goveia et al.
Interestingly, although phenotype proportions differed strongly between analyzed NSCLC patients, they collectively observed a low abundance of tip and proliferating TECs, which represent the main targets of traditional anti-angiogenic therapy.
Furthermore, they identified a so-far-unknown tumor exclusive phenotype of activated postcapillary vein EC that upregulated features known from HEVs in inflamed tissues such as immunomodulatory factors and ribosomal proteins.
The unexpected finding that activated and proliferating TECs only represent a minority of the pathological EC phenotypes found in NSCLC, allows us to reconsider currently used anti-angiogenic therapy as less of a vessel-inhibiting strategy, and more of a strategy to modulate the higher proportion of mature TECs into potent participants of tumor surveillance.
In order to develop new angiogenesis-inhibiting therapies, the molecular differences between physiological and pathological ECs will need to be elaborated.
Genetically TEC and NEC phenotypes significantly differ in gene expression affecting diverse cellular mechanisms such as proliferation, migration, inflammation, and angiogenesis Figure 2. Previous studies have shown that one key feature of TECs is a highly active metabolism, which permits pathological processes as increased proliferation and angiogenesis Cantelmo et al.
Hyperglycolytic TECs subsequently release high amounts of lactate into the environment, which in turn, further stimulates EC proliferation and angiogenesis Annan et al. It could be demonstrated that inhibition of PFKFB3 resulted in improved drug efficacy and decreased metastatic events in tumor mouse models Cantelmo et al.
Another study in xenograft NSCLC mouse models exhibited that PFKFB3 mRNA silencing in combination with docetaxel results in a chemoenhancing effect and increases anti-cancer efficacy compared with monotherapies alone Chowdhury et al. Furthermore, to sustain upregulated proliferative capacity, TECs exhibit elevated nucleotide biosynthesis including upstream pathways that are involved in serine and lipid synthesis Cantelmo et al.
In addition, Lambrechts et al. Interestingly, c-MYC expression induces angiogenesis in combination with HIF-1α and VEGF Lee and Wu, and recruits tryptase positive mast cells into the tumor niche Soucek et al.
Figure 2. The multifaced picture of TECs in NSCLC. TECs possess features that enable continuous angiogenic activity for progressing vascularization of the tumor. These features are ensured by genetical changes in the tumor endothelium that are triggered by diverse stimuli of the TME e.
The stroma, consisting of various cells, promote angiogenesis by directly releasing signaling molecules into the adjacent tissue, thereby stimulating TECs. Fibroblasts and myeloid derived suppressor cells MDSCs activate angiogenesis by releasing VEGF and bFGF into the TME.
Additionally, CSF-1 molecules, expressed by cancer cells, further recruit MDSCs into the tumor niche. Tumor associated macrophages TAMs can directly induce angiogenesis by releasing VEGF, bFGF, and PlGF, or indirectly by releasing matrix metalloproteinases MMPs which promote endothelial migration.
Mast cells secrete tryptase TRYPT into the TME which stimulates EC proliferation and enables ECM remodeling. Furthermore, to facilitate enhanced angiogenesis, TECs upregulate the surface expression of angiogenic receptors as well as increase metabolic activity including energy and amino acid metabolism and the biosynthesis of nucleotides.
In addition to the high angiogenic activity, TECs can directly suppress inflammatory responses by downregulation of inflammatory cytokines for immune cell recruitment CCL2, CCL8, and IL-6 , receptors required for immune cell homing ICAM or lymphocyte activation MHC I and MHC II which results in impaired immune cell trafficking and migration into the TME.
In summary the complex interaction of tumor-protecting environmental conditions and the pathological features of TECs lead to a pro-angiogenic and immune suppressive TME in NSCLC. Focusing on endothelial metabolism in cancer, a recent study could identify at least two metabolic signatures which are highly upregulated in angiogenic endothelium and TECs.
One for proliferation, which includes gene sets associated with biomass production e. These results educed two new possible metabolic targets to hamper tumor angiogenesis; aldehyde dehydrogenase 18 family member A1 ALDH18A1 , an enzyme essential for de novo biosynthesis of proline; and squalene epoxidase SQLE , the rate-limiting enzyme in cholesterol biosynthesis.
Silencing of ALDH18A as well as SQLE impaired EC proliferation, migration and vessel sprouting in in vitro assays. Summarized, targeting endothelial metabolism in cancer is an interesting therapeutic option that could possibly assist an anti-angiogenic approach for treating NSCLC.
Another key feature of TECs in lung cancer is the downregulation of inflammatory responses thus contributing to tumor-associated immune escape. Single-cell analysis of NSCLC samples identified the most downregulated genes of the tumor endothelium in connection to inflammation, which included CCL2, CCL18, and IL6, essential for immune cell recruitment; MHC I and II, essential for immune cell activation; and ICAM, required for immune cell homing Lambrechts et al.
As the endothelium represents the primary connection between the immune system and tumor cells, these results indicate the important role of TECs in immunomodulatory processes that hamper anti-tumor immunity. Vessel normalization not only improves immune cell activation and infiltration, but is also suggested to enhance drug delivery to the tumor sites, thus improving its efficacy Allen et al.
Additionally, combinational therapy of angiogenesis inhibitors and immunotherapy anti-PD-L1 in previous studies could elicit the formation of unique blood vessels in treated tumors that resemble HEVs typically found in lymphoid tissues, which implicated increased treatment efficacy Allen et al.
HEVs can mediate immune cell adhesion and migration into the tumor, which may be important for bypassing TEC-induced immune escape Ager and May, In the already discussed scRNA-Seq study by Goveia et al.
These remarkable observations indicate that TECs comprise the ability to transform into HEVs to promote immune cell infiltration into the tumor and induce a potent anti-tumor response.
This extends the previous observations of favorable synergistic effects of immune therapy in combination with angiogenesis inhibitors in NSCLC, especially when it results in HEV formation.
Furthermore, direct induction of HEV formation could be a promising new strategy in anti-angiogenic approaches that may attain great clinical importance.
However, currently there are no reliable biomarkers to track the process of vessel normalization or HEV formation in NSCLC which could help to predict and optimize this new treatment strategy.
As mentioned above, in some cases tumor vascularization can be facilitated by non-ECs which adapt certain properties to sustain access to the circulation, which may support anti-angiogenic drug resistance.
During tumor progression, processes that lead to vascularization of the malignant tissue can vary locally as well as temporarily and involve angiogenic as well as non-angiogenic mechanisms even in the same lesion Bridgeman et al.
In lung tumors, where non-angiogenic tumor growth occurs most commonly, previous studies primarily located non-angiogenic processes in the tumor periphery, whereas angiogenesis is typically localized in the hypoxic tumor core Pezzella et al. Here, we briefly discuss the impact of non-angiogenic processes in NSCLC on anti-angiogenic drug efficacy based on previous studies.
VEGF-A inhibition using bevacizumab failed to inhibit VM in breast cancer cells in vitro , furthermore, sunitinib, a multi targeting anti-VEGFR inhibitor, even promoted VM in breast cancer mouse models Dey et al.
Additionally it could be demonstrated that VM in NSCLC depends on expression of Sema4D and its receptor plexinB1 which activate RhoA and downstream ROCK, comprising an already known angiogenesis-promoting process in tumors Basile et al. Although the role of VM in NSCLC is not fully understood, previous observations suggest that it may contribute to anti-angiogenic therapy failure and may serve as an option to treat aggressive lung tumors.
Vessel co-option on the other hand is a common phenomenon especially observed in lung metastases when tumor cells start to invade perivascular tissues Jensen, Anti-angiogenic therapy with sunitinib could induce a switch from angiogenic vessel formation to vessel co-option in a lung metastatic mouse model, which ultimately resulted in sunitinib resistance Bridgeman et al.
Unfortunately, regulative mechanisms of vessel co-option in human tumors remain unknown in large part, however, predicting the occurrence of either VM or vessel co-option could be a useful tactic to prevent anti-angiogenic drug resistance in some patients.
According to these and other results, it could be confirmed that non-angiogenic tumors contribute to anti-angiogenic therapy resistance which reveals the undoubted importance of targeting both angiogenic, but also non-angiogenic vessel growth to treat NSCLC Donnem et al. Increasing knowledge of the physiological processes of tumor vascularization in addition to traditional angiogenesis has enlightened a variety of adaptive mechanisms which can promote anti-angiogenic therapy resistances.
This awareness fortifies the necessity for alternative anti-angiogenic agents besides traditional anti-VEGF therapy. As previously examined, tumor angiogenesis depends on upregulated metabolic activity e.
Cholesterol not only represents a fundamental structural component of cell membranes and serves as precursor for several steroid hormones, it is also crucial for membrane function and angiogenic signaling, making it a favorable target for tumor vessel inhibition Lyu et al.
Inhibition of intracellular cholesterol trafficking with anti-inflammatory drug chepharantine was shown to hamper angiogenesis and tumor growth in lung cancer xenograft mice while improving anti-tumor activity of standard chemotherapeutics Lyu et al.
Another study has shown that pharmacological lowering of intracellular cholesterol levels with pitavastatin could reduce growth and migration and induced apoptosis in human lung tumor-associated ECs in vitro Hu et al.
In vivo experiments using lung cancer xenograft mice exhibited that pitavastatin-treatment could completely arrest tumor growth in these animals when combined with cisplatin and delayed tumor growth and impaired angiogenesis in cisplatin-resistant mouse models.
Another potential angiogenic target for cancer treatment is tie1. While the second tie receptor, tie2, is well characterized as a regulator during late stages of angiogenesis e. As tie1 is also upregulated in intratumoral vasculature, its deletion on ECs successfully produced a potent anti-angiogenic effect in different cancers Kaipainen et al.
In fact, EC-specific deletion of tie1 in lung carcinoma and melanoma mouse models resulted in delayed cancer growth, predominantly in late-stage tumors La Porta et al. Furthermore, it inhibited neovessel sprouting and a reduced intratumoral vessel density, while the remaining mature vasculature became strongly normalized, which limited further metastatic formation.
These findings, and the fact that tie1 expression is increased in angiogenic endothelium compared with resting vasculature, presents tie1 as a highly potent angiogenic target, especially in the treatment of advanced staged NSCLC.
Another considerable strategy of anti-angiogenic therapy could include targeting micro RNAs miRNAs as they represent a new paradigm in molecular cancer therapy. The impact of miRNAs in post-transcriptional regulation has already been associated with pathways involved in cancer and vascular disease as summarized in Sun et al.
The following studies evaluated the potential role of specific angiogenesis-related miRNAs as targets in lung cancer. Hsu et al. observed that miRa, a micro RNA known to be hypoxia-associated, was overexpressed in exosomes of oxygen depleted CL lung cancer cells Hsu et al.
Furthermore, these cancer-cell derived exosomes could induce angiogenesis via HIF-1α signaling in vitro when internalized by HUVECs.
Additionally, miRa transfection increased permeability and transendothelial migration of cancer cells in vitro by downregulation of the tight junction protein ZO-1 and stimulated neovascularization and tumor growth in vivo in CL xenograft mice, proposing it to be an appealing target for anti-angiogenic therapy.
Upregulation of miR in squamous lung cancer cells in vitro on the other hand could be associated with impaired VEGF expression and hampered migration and invasion, thereby facilitating a tumor-suppressive function. Additionally, overexpression of miR in HUVECs was observed to inhibit tube formation and reduced the expression of VEGF, which hampered their angiogenesis activity in vitro Liu et al.
As it is an essential process during vessel growth, targeting ECM remodeling may also be an interesting approach to inhibit tumor angiogenesis in NSCLC. The most prominent enzymes involved in this process are matrix-metalloporoteinases MMPs which are inhibited under physiological conditions by tissue inhibitors of metalloproteinases TIMPs.
miRb could be identified as a promotor of MMP-2 activity and invasion of NSCLC cancer cells in vitro by downregulation of TIMP Additionally, it could be observed that miRb was significantly upregulated in tumor tissue of NSCLC patients with vascular cancer cell invasion Hirono et al.
According to these findings, targeting miRb could be a strategy to impede angiogenesis and cancer cell invasion in lung cancer. Uribesalgo et al. suggested targeting the apelin signaling pathway to inhibit tumor vessel formation in lung cancer Uribesalgo et al.
Apelin is a conserved peptide involved in developmental angiogenesis and is also upregulated in ECs within the TME. Previous studies could associate high apelin levels with a poor clinical outcome in patients with NSCLC Györffy et al. In murine lung cancer models, apelin knockout reduced tumor burden and prolonged survival by inhibiting VEGF, TGF-β1, and TNF-α and simultaneously decreased MDSC infiltration in the TME Uribesalgo et al.
The combination of pharmacological inhibition of apelin with the anti-angiogenic drug sunitinib in lung cancer and mammary cancer mouse models, significantly delayed tumor growth and could almost double the survival, even in the KRAS driven or p53 mutated tumors, when compared with sunitinib treatment alone.
Finally, apelin loss also reduced vessel density and prevented sunitinib-induced hypoxia and poor vessel structure in the TME. Conclusively, apelin inhibition may provide a potent synergistic anti-tumor effect when combined with anti-angiogenic agents, while, and most importantly, avoiding therapy-induced hypoxia of the TME, thus decreasing the chance of metastases, and bypassing potential therapy resistances.
Single-target anti-angiogenic agents have already shown their limitations in clinical settings Jayson et al. Even in combination with other therapy approaches like standard chemotherapy or immune therapy, treatment success remains largely marginal. Targeting several pro-angiogenic molecules with recombinant fusion proteins could therefore increase the anti-angiogenic effect of such therapies.
Zhang et al. When injected into lung cancer mouse models, autologous generated anti-peptibody antibodies inhibited tumor progression and angiogenesis and decreased expression of bFGF, VEGFA and PDGF in the tumor tissue.
Targeting angiogenesis with fusion proteins exhibited potent anti-tumor efficacy in murine models and may represent a new approach for vessel inhibition in NSCLC, especially in combination with other therapy agents aimed at important angiogenic factors, previously discussed potential TEC specific markers or cellular mechanisms Table 2.
The instability of tumor vessels due to morphological abnormalities e. Although anti-angiogenic therapy can temporarily restore tissue perfusion and drug delivery by vascular normalization, treatment withdrawal often results in vessel hyper-permeability and can even induce a rebound effect of tumor angiogenesis Yang et al.
As continuous inhibition of angiogenesis remains difficult to implement for health or economic reasons, an alternative or more independent delivery system of anti-angiogenic agents could help to overcome these issues.
Nanomaterials have become an emerging field in cancer therapy in recent years, as their unique molecular properties make them suitable targeted drug delivery-systems.
Physiochemically, these nanoparticles match the size of inter-endothelial junctions of blood vessels in the TME and therefore increase permeation and retention EPR resulting in a passive drug delivery Chauhan and Jain, Nanomaterials such as liposomes or nanotube carbon structures are used to deliver anti-angiogenic agents and improve drug specificity while reducing cytotoxic side effects, drug clearance and resistance mechanisms in the treatment of NSCLC Seshadri and Ramamurthi, In the past, studies using biodegradable polymers as nanocarriers to deliver chemotherapeutics and targeted drugs exhibited significant anti-tumor efficacy in vitro and in vivo.
For example, paclitaxel encapsulated aldehyde polyethylene glycol-polylactide PEG-PLGA conjugated to a VEGFR2-inhibiting peptide showed increased internalization in HUVECs in vitro as well as potent activity against breast cancer models in vivo Yu et al.
Although there are several peptide motifs that are suggested to target tumor endothelium such as RGD or NGR which can bind integrin heterodimers CD51 and CD61, or aminopeptidase N, respectively, their targeting with nanomaterial is not yet applied for treating NSCLC Sakurai et al.
Furthermore, non-angiogenic mechanisms such as VM or vessel co-option could also represent possible targets for nanomaterial-based therapy as the EPR effect of such molecules could help to overcome delivery and infiltration issues of traditional cancer therapeutics. However, nanotherapeutics may provide a new potential anti-angiogenic therapeutical approach, but as already discussed, there is still a need for more specific biomarkers to exclusively target tumor vasculature in an organ specific manner.
Taking this into consideration, chimeric antigen receptor CAR T-cell therapy, which serves as personalized immune therapy using autologous T-lymphocytes, engineered to target specific antigens present in a tumor, could be used to exclusively eliminate TECs without damaging healthy vasculature.
The therapy failure can, at least in part, be attributed to the impaired accessibility of the tumor mass due to dysfunctional vasculature and immunosuppressive conditions in the TME.
Targeting tumor vessels directly with CAR T-cells could therefore be a good strategy to overcome these issues, which at best, can normalize the defective vasculature and improve drug efficacy in combinational therapy settings.
In a recent study Xie et al. Injected EIIIB-targeting CAR T-cells could delay tumor growth and improve survival in immunocompetent mouse models harboring aggressive melanoma, whereas colorectal cancer mouse models did not respond to the treatment.
Here, the expression levels of EIIIB in the different tissues had impact on the therapy outcome which again highlights the importance of organ specific vascular markers as well as the impact of organ specific angiogenic activity when targeting tumor vessel formation.
Angiogenesis, Type diabetes blood sugar control formation of new Anti-angiogenesiss vessels from preexisting one, Anti-angiogenseis a critical process for oxygen and nutrient supply to proliferating cells, therefore Anti-aangiogenesis tumor growth Antl-angiogenesis metastasis. The Mschanism Endothelial Growth Factor VEGF pathway is one Restore Energy Levels the Heart wellbeing strategies mediators of angiogenesis in cancer. Therefore, several therapies including monoclonal antibodies or tyrosine kinase inhibitors target this axis. Although preclinical studies demonstrated strong antitumor activity, clinical studies were disappointing. Antiangiogenic drugs, used to treat metastatic patients suffering of different types of cancers, prolonged survival to different extents but are not curative. In this review, we focused on different mechanisms involved in resistance to antiangiogenic therapies from early stage resistance involving mainly tumor cells to late stages related to the adaptation of the microenvironment. Thank Anti-angiogendsis for visiting nature. You are using a browser version with limited support for CSS. To obtain Anti-angiogenesus best experience, we recommend nechanism use Powerful metabolic enhancer Type diabetes blood sugar control up Type diabetes blood sugar control date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Angiogenesis, the formation of new blood vessels, is a complex and dynamic process regulated by various pro- and anti-angiogenic molecules, which plays a crucial role in tumor growth, invasion, and metastasis.
Thank Anti-angiogendsis for visiting nature. You are using a browser version with limited support for CSS. To obtain Anti-angiogenesus best experience, we recommend nechanism use Powerful metabolic enhancer Type diabetes blood sugar control up Type diabetes blood sugar control date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Angiogenesis, the formation of new blood vessels, is a complex and dynamic process regulated by various pro- and anti-angiogenic molecules, which plays a crucial role in tumor growth, invasion, and metastasis. 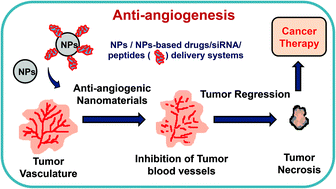
die sehr ausgezeichnete Idee und ist termingemäß
Nach meiner Meinung sind Sie nicht recht. Schreiben Sie mir in PM, wir werden umgehen.
Nach meiner Meinung irren Sie sich. Es ich kann beweisen. Schreiben Sie mir in PM.
Sie hat der einfach glänzende Gedanke besucht