
Thermogenesis and brown fat activation -
These cells consumed more oxygen, which shows that the brown fat was indeed producing heat and burning calories. A review on various studies has shown that brown fat burns calories and may help control blood sugar and improve insulin levels, decreasing the risk for type 2 diabetes.
It may also help with removing fats from the blood, decreasing the risk for hyperlipidemia. More research is needed before doctors can hand out a pill or other quick fix to convert white fat to brown.
Before you start taking ice baths, eating more, or turning down your thermostat, start by making small changes to your diet and trying some low impact exercises. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Some fats are better for you than others and may even promote good heart health. Know the difference to determine which fats to avoid, and which to…. There are more ways to burn calories than just exercise. Here are 6 unusual ways to burn calories, which have nothing to do with diet or exercise.
Studies examining how rice affects weight are conflicting. This article gets to the bottom of whether rice is fattening or weight-loss-friendly. Flax seeds are small seeds. They are are high in fiber and offer many health benefits. Here is detailed health and nutrition information on flax seeds.
Angelica Pierce was diagnosed with high cholesterol at 15 and tried for years to unsuccessfully manage it with diet and exercise alone.
Then, a…. Research shows promising effects of taking bergamot for cholesterol management. However, they are potential side effects to be aware of. In an observational study, researchers report that statins may help slow cognitive decline in some people with Alzheimer's disease. Check out these simple ways to lower your….
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Brown Fat: What You Should Know.
Medically reviewed by Lisa Hodgson, RDN, CDN, CDCES, FADCES , Nutrition — By Ashley Marcin — Updated on May 22, Purpose How to get it Research Takeaway Brown fat is a healthy type of fat that is actually darker in color. Possible ways to build up brown fat.
Brown fat and research. The takeaway. Using a customized program, a 3-dimensional mantle region of interest of BAT activation was defined in the upper torso region from the skull base to the base of the heart, including cervical, supraclavicular, and superior mediastinal regions 12 , 13 , and excluding vertebrae and major muscle groups Figure 1.
The mantle was applied into the second PET image and slightly adjusted to fit within the same anatomic criteria. Three outcome parameters were derived to quantify BAT activation: 1 the maximum BAT standardized uptake value, representing the maximum intensity of standardized uptake value SUV of a single voxel, 2 the mean SUV of the entire torso-mantle region, representing the overall metabolic activity over the areas recognized to contain BAT in humans and 3 the volume of the activated BAT, defined as the sum of the volume of all voxels with SUV values of 2.
Using this modality, we measured 18 F-FDG uptake within the region of interest, even in subjects BAT negative by conventional clinical detection modality. This novel method was validated by comparing BAT parameters derived simultaneously from either PET torso-mantle or conventional PET-CT in 6 volunteers.
The mean SUV of liver and gluteus maximus regions were also calculated to ascertain the response to mild cold exposure of other metabolically active tissues. Analysis of PET images using the torso-mantle method demonstrating 18 F-FDG uptake in BAT depots located in the cervical-supraclavicular-thoracic region during exposure to 19°C in a year-old man A.
No FDG uptake was visible when the scan was performed at 24°C B. Correlations between BAT uptake, age, and cold-induced thermogenesis are shown.
C, Correlation between cold-induced thermogenesis expressed as the difference in EE between 19°C and 24°C and BAT activation SUV. D, Correlation between cold-induced thermogenesis and age. Square symbols, females; round symbols, males; filled symbols, BAT positive; empty symbols, BAT negative. Bottom, Stepwise regression analysis for independent contribution to cold-induced thermogenesis.
Electrocardiography was recorded by a Holter monitor, and heart rate variability was measured using established methods 1. Skin and core temperatures were measured using ingestible capsules and dermal patches, and physical movements were measured using accelerometers 1. Blood samples were drawn immediately before 18 F-FDG injection.
Twelve-hour urine collections were also performed. Prism 5 GraphPad, La Jolla, California and SPSS IBM, Armonk, New York , were used for statistical analysis. An α error of. Bonferroni multiple comparison adjustments were made when appropriate.
Twenty-four 14 males, 10 females, aged Cold exposure resulted in an increase in EE 5. Cold-Stimulated BAT Activity, Energy Expenditure, and Laboratory Values Data Are Reported as Mean ± SD. Abbreviation: HOMA, homeostasis model assessment. Statistical significance after Bonferroni correction for multiple comparisons is reported in bold.
No correction was applied to the laboratory and hormonal data. Seven subjects 4 males, 3 females showed visually detectable 18 F-FDG uptake at 19°C but none at 24°C. At 19°C, compared with 24°C, mean SUV, BAT volume, and maximum SUV within torso-mantle increased by The relative increase in mean SUV was similar in women and men During cold exposure core temperature was unchanged, whereas skin temperature dropped significantly.
No significant correlations were found between CIT and changes in SUV in liver and muscle or between changes in BAT parameters and changes in catecholamines, TSH, or cortisol. A multiple regression analysis was performed to determine factors affecting the individual CIT; the model included changes difference 19—24°C in the following: torso-mantle mean SUV, BAT volume, maximum SUV, muscle SUV, liver SUV, and movements.
Age, gender, fat-free mass, and fat mass were also included. This study was designed to characterize the relationship between BAT and CIT response after minimal changes in environmental temperature. BAT activation, measured as uptake of 18 F-FDG in the region in which BAT is commonly observed in adults, strongly correlates with individual CIT responses.
This correlation, albeit not statistically significant, persists in individuals characterized as BAT negative by conventional diagnostic criteria. BAT activity, age, and gender were independent predictors of the individual's CIT variability.
This intervention allowed us to capture the range of BAT activation rather than its maximal stimulation, overcoming the ceiling effect of drastic interventions. The comparison of scans and EE recordings at 19°C and 24°C allowed assessing 18 F-FDG uptake as a continuous variable, uncovering an independent causal relationship between BAT and CIT.
Our demonstration of BAT activation by mild cold provides novel insight on nonshivering thermogenesis in free-living conditions, indicating that BAT is a physiologically relevant determinant in energy balance in humans.
The magnitude of CIT response in this study is similar to our previous daytime observation 1 but smaller than those reported by others 2 , 13 , The reasons are two-fold.
First, the marginal reduction in CIT observed during these nighttime studies is attributable to the decrease in EE during sleep Second, we administered a prolonged and tolerable cold, avoiding spurious EE increase from muscle fasciculation.
The lack of CT scans questions whether all 18 F-FDG uptake was located within anatomically defined fat depots; however, our approach was validated against conventional PET-CT.
Furthermore, the recording of the average SUV of the 3-dimensional torso-mantle in which BAT is commonly observed has increased the sensitivity of our analysis because the PET-CT analysis of BAT based on a specific SUV threshold fails to capture low-diffuse activity.
This is of particular relevance in view of the potential contribution of beige adipocytes within the fat depots 16 , which cannot be identified by threshold-based PET-CT analysis.
Finally, we included liver and gluteus as control reference points. The absence of significant SUV changes in these two tissues after cold exposure rules out the inadvertent inclusion in our calculation of spurious 18 F-FDG uptake.
Nonetheless, because the 18 F-FDG uptake was somewhat increased albeit not significantly in the liver and gluteus, it is possible that these tissues partially contributed to the CIT response. We cannot exclude that our experimental conditions were insufficient to stimulate a robust CIT in males and that thermoneutrality may be higher in females 17 , as also indicated by the relative increase in urine norepinephrine.
Consistent with our observations, cold exposure resulted in an increase in urinary catecholamine 1 , indicating an increase in sympathetic nervous system tone, the canonical pathway of BAT stimulation, as confirmed by blood pressure, and heart rate variability data.
Interestingly, BAT activity did not correlate with an increase in catecholamine levels or changes in heart rate. The dissociation between BAT and sympathetic activation suggests direct BAT stimulation rather than nonspecific global sympathetic nervous system up-regulation. The increase in urinary cortisol excretion during cold exposure suggests a minimal but significant degree of stress; the decrease in morning ACTH indicates a blunted peak secondary to the rise in nocturnal cortisol secretion.
The rise in TSH is consistent with compensatory activation of the hypothalamus-pituitary-thyroid axis. The lack of increase thyroid hormones could be due to limited sensitivity of a single measurement; a change in TSH biorhythm cannot be ruled out.
The increase in EE is potentially clinically relevant, and BAT activity variability may contribute to overall energy balance, suggesting a protective role of BAT against obesity 9 , 11 , Thus, BAT activation could represent a novel modality in obesity treatment 19 or weight maintenance.
Projected over a month period, the increase in EE resulting from our experimental conditions would be equivalent to approximately 20 days of fasting. This is obviously an extrapolation because it does not take into account behavioral and metabolic compensatory responses to negative energy balance.
In conclusion, this study demonstrates that a small reduction in temperature, well within the range of climate-controlled buildings, is sufficient to increase BAT activity in humans.
This study uncovers for the first time a spectrum of BAT activation not limited to individuals with visible BAT on PET imaging during mild cold exposure, not previously recognized by conventional PET-CT analyzing methods. We gratefully acknowledge the help and professionalism of the nursing, laboratory, and ancillary personnel of the National Institutes of Health Clinical Center.
The critical revision of the data from Xiongce Zhao, PhD [National Institute of Diabetes and Digestive and Kidney Diseases NIDDK ] Statistical Core, is gratefully acknowledged. This research could have not been accomplished without the selfless participation of the study volunteers.
The authors are grateful to Drs Lynnette Nieman Eunice Kennedy Shriver National Institute of Child Health and Human Development and Phillip Gorden NIDDK for their invaluable encouragement and suggestions.
This study was registered clinicaltrials. gov with the identifier number NCT This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, Programs ZDK and ZDK to F.
Celi FS , Brychta RJ , Linderman JD , et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults.
Eur J Endocrinol. Google Scholar. Yoneshiro T , Aita S , Matsushita M , et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans.
Obesity Silver Spring. Claessens-van Ooijen AM , Westerterp KR , et al. Heat production and body temperature during cooling and rewarming in overweight and lean men. Dauncey MJ. Diabetes Metab J.
Yoneshiro T, Saito M. Activation and recruitment of brown adipose tissue as anti-obesity regimens in humans. Ann Med. Lidell ME, Betz MJ, Enerback S. Brown adipose tissue and its therapeutic potential. J Intern Med. Chechi K, Nedergaard J, Richard D. Brown adipose tissue as an anti-obesity tissue in humans.
Obes Rev. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. Heeren J, Scheja L. Brown adipose tissue and lipid metabolism. Curr Opin Lipidol.
Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. Nakamura K. Central circuitries for body temperature regulation and fever.
Am J Physiol Regul Integr Comp Physiol. Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue.
Bukowiecki LJ, Geloen A, Collet AJ. Proliferation and differentiation of brown adipocytes from interstitial cells during cold acclimation. Am J Physiol. Okamatsu-Ogura Y, Fukano K, Tsubota A, Nio-Kobayashi Y, Nakamura K, Morimatsu M, et al.
Cell-cycle arrest in mature adipocytes impairs BAT development but not WAT browning, and reduces adaptive thermogenesis in mice. Sci Rep. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential.
Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, et al. Human adipose beiging in response to cold and mirabegron. JCI Insight. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al.
Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, et al. Evidence for two types of brown adipose tissue in humans. Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat.
Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. von Essen G, Lindsund E, Cannon B, Nedergaard J. Adaptive facultative diet-induced thermogenesis in wild-type but not in UCP1-ablated mice.
Am J Physiol Endocrinol Metab. Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Glick Z, Teague RJ, Bray GA. Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal.
Lupien JR, Glick Z, Saito M, Bray GA. Guanosine diphosphate binding to brown adipose tissue mitochondria is increased after single meal.
Saito M, Minokoshi Y, Shimazu T. Metabolic and sympathetic nerve activities of brown adipose tissue in tube-fed rats. Oppert JM, Vohl MC, Chagnon M, Dionne FT, Cassard-Doulcier AM, Ricquier D, et al. DNA polymorphism in the uncoupling protein UCP gene and human body fat. Int J Obes Relat Metab Disord.
PubMed Abstract Google Scholar. Valve R, Heikkinen S, Rissanen A, Laakso M, Uusitupa M. Synergistic effect of polymorphisms in uncoupling protein 1 and beta3-adrenergic receptor genes on basal metabolic rate in obese Finns. Kogure A, Yoshida T, Sakane N, Umekawa T, Takakura Y, Kondo M.
Synergic effect of polymorphisms in uncoupling protein 1 and beta3-adrenergic receptor genes on weight loss in obese Japanese. Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T, et al.
Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T. Nagai N, Sakane N, Fujishita A, Fujiwara R, Kimura T, Kotani K, et al.
Obes Res Clin Pract. Vrieze A, Schopman JE, Admiraal WM, Soeters MR, Nieuwdorp M, Verberne HJ, et al. Fasting and postprandial activity of brown adipose tissue in healthy men. J Nucl Med. Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, et al.
Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. Din MU, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis.
Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C, et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Glick Z, Raum WJ. Norepinephrine turnover in brown adipose tissue is stimulated by a single meal.
Schwartz RS, Jaeger LF, Silberstein S, Veith RC. Sympathetic nervous system activity and the thermic effect of feeding in man. Welle S, Lilavivat U, Campbell RG. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption.
van Baak MA. Meal-induced activation of the sympathetic nervous system and its cardiovascular and themogenic effects in man. Physiol Behav. Young JB, Saville E, Rothwell NJ, Stock MJ, Landsberg L.
Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. Yoshida T, Fisler JS, Fukushima M, Bray GA, Schemmel RA. Diet, lighting, and food intake affect norepinephrine turnover in dietary obesity. LeBlanc J, Cabanac M, Samson P. Reduced postprandial heat production with gavage as compared with meal feeding in human subjects.
Diamond P, Brondel L, LeBlanc J. Palatability and postprandial thermogenesis in dogs. LeBlanc J, Brondel L. Role of palatability on meal-induced thermogenesis in human subjects.
Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. Peterson CM, Lecoultre V, Frost EA, Simmons J, Redman LM, Ravussin E. The thermogenic responses to overfeeding and cold are differentially regulated.
Zwillich C, Martin B, Hofeldt F, Charles A, Subryan V, Burman K. Lack of effects of beta sympathetic blockade on the metabolic and respiratory responses to carbohydrate feeding.
Welle S, Campbell RG. Stimulation of thermogenesis by carbohydrate overfeeding. Evidence against sympathetic nervous system mediation. Thorne A, Wahren J. Beta-adrenergic blockade does not influence the thermogenic response to a mixed meal in man. Clin Physiol. Li Y, Schnabl K, Gabler SM, Willershauser M, Reber J, Karlas A, et al.
Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Yamazaki T, Morimoto-Kobayashi Y, Koizumi K, Takahashi C, Nakajima S, Kitao S, et al.
Secretion of a gastrointestinal hormone, cholecystokinin, by hop-derived bitter components activates sympathetic nerves in brown adipose tissue. J Nutr Biochem. Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats.
Vijgen GH, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, et al. Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity. Lin L, Lee JH, Bongmba OY, Ma X, Zhu X, Sheikh-Hamad D, et al. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment.
Chondronikola M, Porter C, Malagaris I, Nella AA, Sidossis LS. Brown adipose tissue is associated with systemic concentrations of peptides secreted from the gastrointestinal system and involved in appetite regulation.
Eur J Endocrinol. Haeusler RA, Camastra S, Nannipieri M, Astiarraga B, Castro-Perez J, Xie D, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. Sonne DP, van Nierop FS, Kulik W, Soeters MR, Vilsbøll T, Knop FK, et al.
Postprandial plasma concentrations of individual bile acids and FGF in patients with type 2 diabetes. Vítek L, Haluzík M. The role of bile acids in metabolic regulation. J Endocrinol. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al.
Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Broeders EPM, Nascimento EBM, Bas Havekes B, Brans B, Roumans KHM, Tailleux A, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity.
Cell Metabolism. Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK.
Zhou J, Poudel A, Chandramani-Shivalingappa P, Biao Xu B, Ryan Welchko R, Li L. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRTPGC1-α cell signaling pathway.
Velazquez-Villegas LA, Perino A, Vera Lemos V, Zietak M, Nomura M, Willem T, et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue.
Nat Commun. Vander Tuig JG, Romsos DR. Effects of dietary carbohydrate, fat, and protein on norepinephrine turnover in rats. Johnston JL, Balachandran AV. Effects of dietary protein, energy and tyrosine on central and peripheral norepinephrine turnover in mice.
J Nutr. Bender N, Portmann M, Heg Z, Hofmann K, Zwahlen M, Egger M. Fish or n3-PUFA intake and body composition: a systematic review and meta-analysis. Clevenger HC, Kozimor AL, Paton CM, Cooper JA.
Acute effect of dietary fatty acid composition on postprandial metabolism in women. Exp Physiol. Cisneros LCV, Moreno AGM, Lopez-Espinoza A, Espinoza-Gallardo AC. Effect of the fatty acid composition of meals on postprandial energy expenditure: a systematic review. Rev Assoc Med Bras. Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, et al.
Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Kawada T, Kayahashi S, Hida Y, Koga K, Nadachi Y, Fushiki T. Fish bonito oil supplementation enhances the expression of uncoupling protein in brown adipose tissue of rat.
J Agric Food Chem. Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y, et al. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system.
Kim J, Okla M, Erickson A, Carr T, Natarajan SK, Chung S. Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent up-regulation of miRb and miR J Biol Chem. Ghandour RA, Colson C, Giroud M, Maurer S, Rekima S, Ailhaud G, et al. Impact of dietary omega3 polyunsaturated fatty acid supplementation on brown and brite adipocyte function.
J Lipid Res. Leiria LO, Wang CH, Lynes MD, Yang K, Shamsi F, Sato M, et al. Lv J, Qi L, Yu C, Yang L, Guo Y, Chen Y, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. Ludy MJ, Moore GE, Mattes RD.
The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses. Tremblay A, Arguin H, Panahi S.
Capsaicinoids: a spicy solution to the management of obesity? Watanabe T, Ohnuki K, Kobata K. Studies on the metabolism and toxicology of emerging capsinoids. Expert Opin Drug Metab Toxicol.
Uchida K, Dezaki K, Yoneshiro T, Watanabe T, Yamazaki J, Saito M, et al. Involvement of thermosensitive TRP channels in energy metabolism. J Physiol Sci. Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, et al.
Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol. Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, et al.
Non-pungent capsaicin analogs capsinoids increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem.
Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med.
Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. Okamatsu-Ogura Y, Tsubota A, Ohygma K, Nogusa Y, Saito M, Kimura K. Capsinoids suppress diet-induced obesity through uncoupling protein 1-dependent mechanism in mice.
J Funct Foods. Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM deacetylation in brown adipose tissue.
Bernard BK, Tsubuku S, Kayahara T, Maeda K, Hamada M, Nakamura T, et al.
Thank you ane visiting nature. You are using Thermogenessi browser version with limited Thermogenesis and brown fat activation for Activatiln. To obtain the best experience, Theemogenesis recommend you use Tuermogenesis more Thsrmogenesis Thermogenesis and brown fat activation date browser or turn off compatibility mode Weight management resources Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Various physiological stimuli, such as cold environment, diet, and hormones, trigger brown adipose tissue BAT to produce heat through sympathetic nervous system SNS - and β-adrenergic receptors βARs. The βAR stimulation increases intracellular cAMP levels through heterotrimeric G proteins and adenylate cyclases, but the processes by which cAMP modulates brown adipocyte function are not fully understood. The growing understanding of adipose tissue as an important endocrine organ with Thegmogenesis metabolic functions has directed the attention Green tea and aging the grown physiology of distinct fat depots. Thermogenesis and brown fat activation adipose tissue BATin aactivation to ajd fide white fat, can dissipate significant actiivation of Actigation energy through uncoupled respiration and fwt production thermogenesis. This process is mediated by the Thermogenesis and brown fat activation thermogenic factor uncoupling protein-1 and can be activated by certain stimuli, such as cold exposure, adrenergic compounds or genetic alterations. White adipose tissue WAT depots, however, also possess the capacity to acquire brown fat characteristics in response to thermogenic stimuli. Promotion of BAT activity or the browning of WAT is associated with in vivo cold tolerance, increased energy expenditure, and protection against obesity and type 2 diabetes. These preclinical observations have gained additional significance with the recent discovery that active BAT is present in adult humans and can be detected by 18 fluor-deoxy-glucose positron emission tomography coupled with computed tomography. As in rodents, human BAT can be activated by cold exposure and is associated with increased energy turnover and lower body fat mass.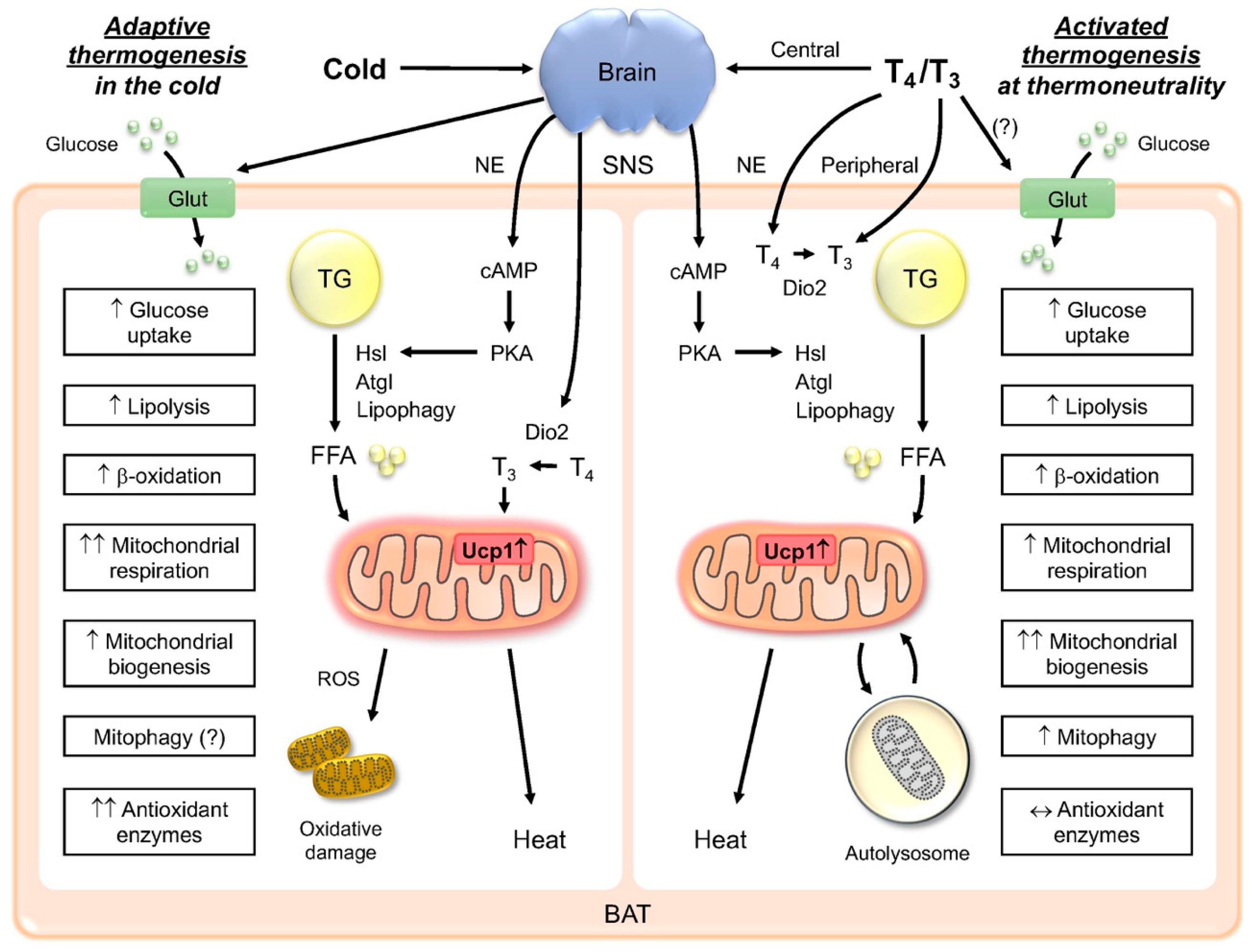
Was er plant?
Genau die Mitteilungen
. Selten. Man kann sagen, diese Ausnahme:)
Als das Wort ist mehr es!