Fat oxidation benefits -
Professor Asker Jeukendrup looks at what the research says. Fat burning is often associated with weight loss, decreases in body fat and increases in lean body mass, all of which can be advantageous for an athlete.
It is known that well-trained endurance athletes have an increased capacity to oxidise fatty acids. This enables them to use fat as a fuel when their carbohydrate stores become limited. In contrast, patients with obesity, insulin resistance and type II diabetes may have an impaired capacity to oxidise fat.
As a result, fatty acids may be stored in their muscles and in other tissues. This accumulation of lipid and its metabolites in the muscle may interfere with the insulin-signalling cascade and cause insulin resistance.
It is therefore important to understand the factors that regulate fat metabolism, and the ways to increase fat oxidation in patients and athletes. Fats are stored mostly in subcutaneous adipose tissue, but we also have small stores in the muscle itself intramuscular triglycerides.
At the onset of exercise, neuronal beta-adrenergic stimulation will increase lipolysis the breakdown of fats into fatty acids and glycerol in adipose tissue and muscle.
Catecholamines such as adrenaline and noradrenaline may also rise and contribute to the stimulation of lipolysis. As soon as exercise begins, fatty acids are mobilised. Adipose tissue fatty acids have to be transported from the fat cell to the muscle, be transported across the muscle membrane and then be transported across the mitochondrial membrane for oxidation.
The triglycerides stored in muscle undergo similar lipolysis and these fatty acids can be transported into the mitochondria as well. During exercise, a mixture of fatty acids derived from adipocytes and intramuscular stores is used. There is evidence that shows that trained individuals store more intramuscular fat and use this more as a source of energy during exercise 1.
Fat oxidation is regulated at various steps of this process. Lipolysis is affected by many factors but is mostly regulated by hormones stimulated by catecholamines and inhibited by insulin.
The transport of fatty acids is also dependent on blood supply to the adipose and muscle tissues, as well as the uptake of fatty acids into the muscle and into the mitochondria. By inhibiting mobilisation of fatty acids or the transport of these fatty acids, we can reduce fat metabolism.
However, are there also ways in which we can stimulate these steps and promote fat metabolism? Exercise intensity — One of the most important factors that determines the rate of fat oxidation during exercise is the intensity.
Although several studies have described the relationship between exercise intensity and fat oxidation, only recently was this relationship studied over a wide range of intensities 2.
In absolute terms, carbohydrate oxidation increases proportionally with exercise intensity, whereas the rate of fat oxidation initially increases, but decreases again at higher exercise intensities see figure 1. So, although it is often claimed that you have to exercise at low intensities to oxidise fat, this is not necessarily true.
However, the inter-individual variation is very large. However, very little research has been done. Recently we used this intensity in a training study with obese individuals.
Compared with interval training, their fat oxidation and insulin sensitivity improved more after four weeks steady-state exercise three times per week at an intensity that equalled their individual Fatmax 4. Dietary effects — The other important factor is diet.
A diet high in carbohydrate will suppress fat oxidation, and a diet low in carbohydrate will result in high fat oxidation rates. This effect of insulin on fat oxidation may last as long as six to eight hours after a meal, and this means that the highest fat oxidation rates can be achieved after an overnight fast.
Endurance athletes have often used exercise without breakfast as a way to increase the fat-oxidative capacity of the muscle. Recently, a study was performed at the University of Leuven in Belgium, in which scientists investigated the effect of a six-week endurance training programme carried out for three days per week, each session lasting one to two hours 6.
The participants trained in either the fasted or carbohydrate-fed state. When training was conducted in the fasted state, the researchers observed a decrease in muscle glycogen use, while the activity of various proteins involved in fat metabolism was increased.
However, fat oxidation during exercise was the same in the two groups. It is possible, though, that there are small but significant changes in fat metabolism after fasted training; but, in this study, changes in fat oxidation might have been masked by the fact that these subjects received carbohydrate during their experimental trials.
It must also be noted that training after an overnight fast may reduce your exercise capacity and may therefore only be suitable for low- to moderate- intensity exercise sessions. The efficacy of such training for weight reduction is also not known.
Duration of exercise — It has long been established that oxidation becomes increasingly important as exercise progresses. During ultra-endurance exercise, fat oxidation can reach peaks of 1 gram per minute, although as noted in Dietary effects fat oxidation may be reduced if carbohydrate is ingested before or during exercise.
In terms of weight loss, the duration of exercise may be one of the key factors as it is also the most effective way to increase energy expenditure. Mode of exercise — The exercise modality also has an effect on fat oxidation. Fat oxidation has been shown to be higher for a given oxygen uptake during walking and running, compared with cycling 7.
The reason for this is not known, but it has been suggested that it is related to the greater power output per muscle fibre in cycling compared to that in running. Gender differences — Although some studies in the literature have found no gender differences in metabolism, the majority of studies now indicate higher rates of fat oxidation in women.
In a study that compared men and women over a wide range of exercise intensities, it was shown that the women had higher rates of fat oxidation over the entire range of intensities, and that their fat oxidation peaked at a slightly higher intensity 8.
The differences, however, are small and may not be of any physiological significance. There are many nutrition supplements on the market that claim to increase fat oxidation. These supplements include caffeine, carnitine, hydroxycitric acid HCA , chromium, conjugated linoleic acid CLA , guarana, citrus aurantium, Asian ginseng, cayenne pepper, coleus forskholii, glucomannan, green tea, psyllium and pyruvate.
With few exceptions, there is little evidence that these supplements, which are marketed as fat burners, actually increase fat oxidation during exercise see table 1.
One of the few exceptions however may be green tea extracts. The mechanisms of this are not well understood but it is likely that the active ingredient in green tea, called epigallocatechin gallate EGCG — a powerful polyphenol with antioxidant properties inhibits the enzyme catechol O-methyltransferase COMT , which is responsible for the breakdown of noradrenaline.
This in turn may result in higher concentrations of noradrenaline and stimulation of lipolysis, making more fatty acids available for oxidation. Environment — Environmental conditions can also influence the type of fuel used. It is known that exercise in a hot environment will increase glycogen use and reduce fat oxidation, and something similar can be observed at high altitude.
Similarly, when it is extremely cold, and especially when shivering, carbohydrate metabolism appears to be stimulated at the expense of fat metabolism. At present, the only proven way to increase fat oxidation during exercise is to perform regular physical activity.
Exercise training will up-regulate the enzymes of the fat oxidation pathways, increase mitochondrial mass, increase blood flow, etc.
Research has shown that as little as four weeks of regular exercise three times per week for minutes can increase fat oxidation rates and cause favourable enzymatic changes However, too little information is available to draw any conclusions about the optimal training programme to achieve these effects.
In one study we investigated maximal rates of fat oxidation in subjects with varying fitness levels. In this study, we had obese and sedentary individuals, as well as professional cyclists 9.
VO2max ranged from Interestingly, although there was a correlation between maximal fat oxidation and maximal oxygen uptake, at an individual level, fitness cannot be used to predict fat oxidation. What this means is that there are some obese individuals that have similar fat oxidation rates to professional cyclists see figure 2!
The large inter-individual variation is related to factors such as diet and gender, but remains in large part unexplained. Fat burning is often associated with weight loss, decreases in body fat and increases in lean body mass. However, it must be noted that such changes in body weight and body composition can only be achieved with a negative energy balance: you have to eat fewer calories than you expend, independent of the fuels you use!
The optimal exercise type, intensity, and duration for weight loss are still unclear. Current recommendations are mostly focused on increasing energy expenditure and increasing exercise volumes. Finding the optimal intensity for fat oxidation might aid in losing weight fat loss and in weight maintenance, but evidence for this is currently lacking.
It is also important to realise that the amount of fat oxidised during exercise is only small. Fat oxidation rates are on average 0. So in order to oxidise 1kg of fat mass, more than 33 hours of exercise is required!
The duration of exercise, however, plays a crucial role, with an increasing importance of fat oxidation with longer exercise.
Of course, this also has the potential to increase daily energy expenditure. If exercise is the only intervention used, the main goal is usually to increase energy expenditure and reduce body fat. When combined with a diet programme, however, it is mainly used to counteract the decrease in fat oxidation often seen after weight loss Higher fat oxidation rates during exercise are generally reflective of good training status, whereas low fat oxidation rates might be related to obesity and insulin resistance.
The vast majority of nutrition supplements do not have the desired effects. Currently, the only highly effective way to increase fat oxidation is through exercise training, although it is still unclear what the best training regimen is to get the largest improvements. Finally, it is important to note that there is a very large inter-individual variation in fat oxidation that is only partly explained by the factors mentioned above.
This means that although the factors mentioned above can influence fat oxidation, they cannot predict fat oxidation rates in an individual. Asker Jeukendrup is professor of exercise metabolism at the University of Birmingham.
He has published more than research papers and books on exercise metabolism and nutrition and is also consultant to many elite athletes.
They use the latest research to improve performance for themselves and their clients - both athletes and sports teams - with help from global specialists in the fields of sports science, sports medicine and sports psychology.
They do this by reading Sports Performance Bulletin, an easy-to-digest but serious-minded journal dedicated to high performance sports. SPB offers a wealth of information and insight into the latest research, in an easily-accessible and understood format, along with a wealth of practical recommendations.
Sports Performance Bulletin helps dedicated endurance athletes improve their performance. Sense-checking the latest sports science research, and sourcing evidence and case studies to support findings, Sports Performance Bulletin turns proven insights into easily digestible practical advice.
Pratley, Arline D. Salbe, Clifton Bogardus, Eric Ravussin, P. Relatively low rates of energy expenditure and fat oxidation predict body weight gain.
Weight gain, in turn, is associated with increases in energy expenditure and fat oxidation that may oppose further weight change.
In response to experimental weight gain induced by overfeeding, increases in energy expenditure and fat oxidation are overcompensatory, i. greater than predicted for the change in body composition.
To determine whether such metabolic adaptation occurs in response to spontaneous long term weight change, we conducted a longitudinal study in which h energy expenditure EE and h respiratory quotient RQ; i. fat to carbohydrate oxidation were repeatedly measured in Pima Indians at baseline and after a mean follow-up of 3.
Changes in EE and RQ varied substantially among individuals. Thus, on the average, spontaneous long term weight changes are accompanied by small metabolic adaptations in both energy expenditure and fat oxidation.
The metabolic responses to weight changes are highly variable among individuals, however. To develop successful prevention and treatment strategies, it is important to understand the physiological mechanisms underlying the long term regulation of body weight. Differences in energy metabolism may play a role in long term body weight regulation and the pathogenesis of human obesity 2 — Several 2 — 7 , but not all 8 — 10 , prospective studies have shown that a relatively low energy expenditure 2 — 5 and a relatively high respiratory quotient, i.
a low fat to carbohydrate oxidation rate 6 , 7 predict body weight gain. Longitudinal studies, however, in which energy metabolism was assessed not only at baseline but also at follow-up, indicate that upon gaining weight, energy expenditure and fat oxidation increase 2 , 6 , Metabolic propensity to obesity might thus depend not only on initial rates of energy expenditure and fat oxidation, but also on how these measures change in response to weight change Results from most overfeeding studies indicate that short term experimental weight gain is accompanied by an overcompensatory increase in energy expenditure, i.
an increase in energy expenditure that is greater than predicted for the changes in body size and composition 14 — Similarly, most underfeeding studies reveal that in the short term, intentional weight loss leads to a decrease in energy expenditure beyond predicted values 23 — Such overcompensatory metabolic changes act to oppose further weight change and have thus been referred to as metabolic adaptation 13 , 24 , Although metabolic adaptation thus seems to occur in response to large perturbations in body weight over relatively short periods of time, it is unknown whether similar adaptive mechanisms also occur in response to spontaneous long term weight changes in free living conditions.
To examine this question, we analyzed data from an ongoing longitudinal study of the pathogenesis of obesity initiated in among the Pima Indians of Arizona, a population with a very high prevalence of obesity, in whom low rates of energy expenditure and fat oxidation predict body weight gain 2 , 6.
We present results from over subjects in whom h energy expenditure and h substrate oxidation were repeatedly measured in a whole body respiratory chamber before and after an average follow-up of 3. The aims of this study were 1 to test whether metabolic adaptation in h energy expenditure and h substrate oxidation occur in response to spontaneous long term weight change, 2 to quantify and explain the variability in these changes among individuals, and 3 to determine the relationship between changes in energy expenditure and substrate oxidation in response to weight gain and weight loss.
Since , Pima Indians have been admitted to the metabolic ward of the Clinical Diabetes and Nutrition Section of the NIH in Phoenix, Arizona, for an ongoing longitudinal study of the pathogenesis of obesity that includes the repeated assessment of h energy expenditure and h substrate oxidation in a whole body respiratory chamber.
Among the subjects meeting these criteria, subjects had been studied on at least 2 occasions. In subjects studied more than twice, the visit with the greatest weight change was selected for follow-up. Among the subjects, 31 subjects had lost weight and 71 had gained weight at follow-up.
All subjects were between 18—50 yr of age at baseline and follow-up, healthy according to a physical examination and routine laboratory tests, and did not smoke or take medications at baseline or follow-up Table 1.
Physical and metabolic characteristics of the entire study population and of the subset of subjects with follow-up. At baseline, all measurements in the follow-up group were comparable to those in the entire population.
The P values refer to the changes over time determined by paired t -test. Glucose tolerance was assessed by a g oral glucose tolerance test The study protocol was approved by the Institutional Review Board of the NIDDK and by the Tribal Council of the Gila River Indian Community, and all subjects provided written informed consent before participation.
Body composition was estimated by underwater weighing, with determination of residual lung volume by helium dilution 39 , or by total body dual energy x-ray absorptiometry DPX-L, Lunar Corp. Percent body fat, fat mass FM , and fat-free mass FFM were calculated as previously described 41 , and a conversion equation 42 was used to make measurements comparable between the two methods.
Waist and thigh circumferences were measured at the umbilicus and the gluteal fold in the supine and standing positions, respectively, and the waist to thigh ratio WTR was calculated as an index of body fat distribution The measurement of energy expenditure and substrate oxidation in the respiratory chamber has previously been described 44 and did not differ at baseline and follow-up.
In brief, volunteers entered the chamber at h after an overnight fast and remained there until h the following morning. Subjects were fed a standardized diet with the amount of calories calculated according to previously determined equations to achieve energy balance Meals were provided at , , and h, and an evening snack was given at h.
The rate of energy expenditure was measured continuously, calculated for each min interval of the 23 h in the chamber, and then extrapolated to 24 h h energy expenditure, EE. Spontaneous physical activity SPA was detected by radar sensors and expressed as the percentage of time over the h period in which activity was detected Carbon dioxide production VCO 2 and oxygen consumption VO 2 were calculated at min intervals, summed for the 23 h in the chamber, and then extrapolated to 24 h.
Based upon RQ, EE, and h urinary nitrogen excretion, the rates of h fat, carbohydrate, and protein oxidation were determined as previously described Statistical analyses were performed using the procedures of the SAS Institute, Inc.
Cary, NC Results are given as the mean ± sd. Data from the entire group of subjects were used to assess the cross-sectional relationships between EE and RQ vs. Changes in anthropometric and metabolic parameters were assessed in the subset of subjects with follow-up measurements.
Changes Δ in EE and RQ were calculated as the difference between follow-up and baseline measurements for both the unadjusted and the adjusted values. Paired t tests were used to test whether measurements at follow-up were significantly different from those at baseline. Pearson correlation coefficients were calculated to assess the relation of the changes in unadjusted and adjusted EE and RQ to the change in body weight.
The changes in EE and RQ predicted for a kg weight loss or kg weight gain were determined from the regression equation of the relationships between Δ EE andΔ RQ vs. Δ weight. body weight, as assessed in the entire study population of subjects.
The residuals of the relationships between Δ EE and Δ RQ vs. Δ weight were calculated using general linear regression models. The anthropometric and metabolic characteristics of the entire study population and of the subset of individuals with follow-up studies are summarized in Table 1.
The baseline anthropometric and metabolic characteristics of the subjects with repeated measurements of energy metabolism were similar to those of the entire study population.
The follow-up duration ranged from 0. A, Relationship between Δ EE and Δ weight over 3. B, Relationship between Δ EE and Δ weight after adjustment of EE for FFM, FM, WTR, and age.
A, Relationship between changes in RQ, adjusted for energy balance, and Δ weight over 3. B, Relationship between Δ RQ and Δ weight after adjustment of RQ for percent body fat, age, and sex in addition to energy balance.
Changes in h energy expenditure. The correlation between Δ EE and Δ weight remained significant when baseline and follow-up EE were adjusted for FFM, FM, WTR, age, and sex Fig.
Sex, age, glucose tolerance status normal or impaired , initial body weight, and follow-up duration were not significant determinants of Δ EE.
Changes in h respiratory quotient and substrate oxidation. There was a negative linear correlation between Δ RQ and Δ weight, but for any given Δ weight there was considerable interindividual variability in Δ RQ sd , 0.
The correlation between Δ RQ andΔ weight remained significant when baseline and follow-up RQ were adjusted for percent body fat, age, and sex in addition to energy balance in the chamber Fig.
Responses in energy expenditure vs. responses in substrate oxidation. Based on the relationship between Δ EE and Δ weight Fig. The changes in RQ Fig. In response to weight gain there was a negative correlation between the residuals of Δ EE and the residuals of Δ RQ Fig.
subjects with metabolic adaptation in EE positive residuals also tended to have metabolic adaptation in substrate oxidation negative residuals in RQ and vice versa.
In response to weight loss, the residuals in Δ EE and Δ RQ were unrelated, i. metabolic adaptation in EE did not tend to be accompanied by metabolic adaptation in substrate oxidation Fig.
In the present longitudinal study we examined the changes in h energy expenditure and h substrate oxidation associated with spontaneous long term weight changes in more than Pima Indians who spent h in a respiratory chamber at baseline and after a mean follow-up of 3.
The results indicate that metabolic adaptation, i. changes in energy expenditure and substrate oxidation greater than predicted for the change in body size and composition, can occur in response to spontaneous long term weight changes.
On the average, the metabolic changes were only slightly greater than predicted, but varied substantially among individuals. Finally, we found that in response to weight gain, adaptations in energy expenditure and substrate oxidation were related to one another, such that subjects with the most pronounced metabolic adaptation in energy expenditure also had the most pronounced metabolic adaptation in fat oxidation and vice versa.
This was not the case for weight loss. Most previous intervention studies have demonstrated metabolic adaptation in response to experimental short term weight change induced by controlled over- and underfeeding regimens 14 — Whether similar overcompensatory changes in energy expenditure and fat oxidation occur in the natural history of weight changes has been a matter of contention 9 — 13 , 16 , The present study demonstrates, for the first time, that metabolic adaptation can occur in response to spontaneous long term weight changes, but also reveals that, on the average, these overcompensatory changes are small.
In practical terms, these adaptations translate into the caloric content of approximately one half of an apple, one fifth of a bagel, or one tenth of a cheeseburger for the adaptation in h energy expenditure or the fat content of two teaspoons of peanut butter or seven potato chips for the metabolic adaptation in h fat oxidation , respectively.
The results also indicate that even a large decrease in body weight over several years is, on the average, not accompanied by a profound slowing of energy metabolism, as occasionally implied to explain the high rate of weight recidivism in the medical treatment of obesity.
However, several aspects need to be considered in this respect. Second, the present study was observational in design, which has both advantages and disadvantages. On the one hand, we have no information on the exact causes of the weight changes.
In some individuals, weight loss might have been secondary to illness, although this is unlikely because subjects in our studies typically remain in close contact with the research unit and receive a comprehensive medical examination before each admission. An advantage of the observational design, on the other hand, is that it allows us to examine the metabolic responses to spontaneous long term weight changes that probably more closely resemble the typical pattern of weight change under free living conditions than imposed by over- and underfeeding regimens.
The fact that the magnitude of metabolic adaptation in response to such gradual weight change was small, on the average, agrees with cross-sectional findings indicating that energy expenditure is only marginally reduced in formerly obese individuals who had returned to a normal body weight and had successfully maintained the weight loss over months or years postobese individuals Some previous intervention studies suggest that the suppression in energy expenditure in response to weight loss might be larger shortly after a more rapid decrease in body weight 26 , 27 , 29 , 31 , It is also important to point out that energy expenditure in the present study was measured in the restricted environment of a respiratory chamber, which significantly reduces physical activity.
Although nonexercise activity thermogenesis, of which spontaneous physical activity is a component, has recently been suggested to play an important role in the adaptation to overfeeding 21 , our findings do not suggest a major role of spontaneous physical activity i.
fidgeting in the metabolic response to long term weight change. To what extent changes in volitional physical activities such as exercise habits contribute to the overall metabolic responses to long term weight change remains unknown.
Our study also provides no information on the role of spontaneous adaptations in energy intake. Thus, as with the metabolic adaptation in energy expenditure, small differences in the adaptation in energy intake may play an important role in determining whether body weight remains stable or continues to increase.
Rather, some individuals will experience relatively large overcompensatory responses, whereas others will have subnormal responses. Such interindividual variability in metabolic responses has also been found in response to experimental over- and underfeeding and has been used to explain why the amount of weight gained or lost under standardized dietary regimens can differ substantially among individuals 14 , 19 , 22 , The large number of subjects in the present study allowed us to quantify the interindividual variability in metabolic responses to weight changes and to search for possible underlying determinants.
We found that the change in h energy expenditure was explained not only by the changes in FFM and FM, but also independently by the changes in body fat distribution and spontaneous physical activity. The only additional determinant of the change in h respiratory quotient was age at baseline.
As only a small part of this variability can be attributed to the variability of the method 2 , 6 , 44 , other factors must be involved. Moreover, there is strong evidence from overfeeding studies in identical twins that the metabolic responses to weight changes are in part genetically determined 55 , Another interesting observation in the present study was that in response to weight gain, the changes in h energy expenditure and h substrate oxidation were related to one another, in that individuals with the most pronounced adaptation in energy expenditure also tended to have the most pronounced adaptation in fat oxidation and vice versa.
Interestingly, this was not the case in response to weight loss, where adaptations in energy expenditure and substrate oxidation were unrelated. The above findings may have important implications for our understanding of the role of energy metabolism in the long term regulation of body weight and the pathogenesis of human obesity.
To illustrate this, we have developed a schematic model that integrates previous cross-sectional and prospective findings with those from the present longitudinal study Fig. Schematic model integrating cross-sectional, prospective, and longitudinal findings to illustrate the potential role of energy expenditure and substrate oxidation in the long term regulation of body weight.
Cross-sectionally, energy expenditure and the rate of fat to carbohydrate oxidation increase with increasing body size prediction line , but at any given body size, both measures vary considerably among individuals 44 , 51 , Our present longitudinal data indicate that upon gaining weight, the initially low rates of energy expenditure and fat oxidation tend to normalize, on the average, but as with the cross-sectional relationship, there is substantial interindividual variability in these responses.
Accordingly, the metabolic drive to weight gain may soon diminish in some individuals, thereby limiting the amount of weight gain metabolic adaptation; arrow 1A , whereas it may be sustained in others, who will thus be predisposed to gain further weight arrow 1B.
Of note, these considerations apply to spontaneous long term weight changes. Further studies are needed to confirm the role of low energy expenditure and fat oxidation as predictors of weight gain and to formally test the effect of metabolic adaptation on further weight change.
It will also be important to examine the role of adaptation in energy and substrate intake to weight change. These are probably complex and could include changes in the perception of hunger and satiation as well as in caloric intake and food preferences. In summary, the results of this longitudinal study indicate that the changes in h energy expenditure and h respiratory quotient i.
in substrate oxidation associated with long term weight changes 1 are greater than those predicted for the change in body size and composition, 2 vary substantially among individuals, and 3 are related to one another in response to weight gain.
We conclude that metabolic adaptation can occur not only in response to experimental short term perturbations in body weight, but also in response to spontaneous long term weight changes. These responses, albeit small on the average, vary substantially among individuals and may thus play a role in the long term regulation of body weight and the pathogenesis of human obesity.
We gratefully acknowledge Mr. Tom Anderson, Mrs. Carol Massengill, and the nurses of the Clinical Research Unit as well as the staff of the metabolic kitchen for their care of the patients in the studies, and the Clinical Diabetes and Nutrition Section technical staff for assisting with the chamber measurements and laboratory analyses.
We thank the members and leaders of the Gila River Indian Community, without whose continuing cooperation this study would not have been possible. Flegal KM , Carroll MD , Kuczmarski RJ , Johnson CL. Int J Obes. Google Scholar. Ravussin E , Lillioja S , Knowler WC , et al.
N Engl J Med. Buscemi S, Di Maggio O, Blunda G, Maneri R, Verga S, Bompiani GD. Griffiths M , Payne PR , Stunkard AJ , Rivers JPW , Cox M. Roberts SB , Savage J , Coward WA , Chew B , Lucas A. Zurlo F , Lillioja S , Puente A , et al. Am J Physiol. Seidell JA , Muller DC , Sorkin JD , Andres R.
Davies PSW , Day JME , Lucas A. Weinsier RL , Nelson KM , Hensrud DD , Darnell BE , Hunter GR , Schutz Y. Contributions of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain in post-obese and never-obese women.
J Clin Invest. Amatruda JM , Statt MC , Welle SL. Flatt JP. Diabetes Metab Rev. Ravussin E , Swinburn BA. Diabetes Rev. In: Stunkard AJ, Wadden TA, eds. Obesity: theory and therapy, 2nd Ed. New York : Raven Press; 97— Miller DS , Mumford P , Stock MJ.
Thermogenesis in overeating man.
DEXA scan of Page Fat oxidation benefits Interests Vita Oxodation New Projects Ocidation UNM Oxidqtion. Article Pag e. The Oxidatiion of Fat Loss Mike Neurofeedback therapy benefits, Christine Mermier, Ph. and Len Kravitz, Ph. Introduction Benerits serves many important functions in the human body. For example, fat provides a key role for the structure and flexibility of cell membranes and also helps to regulate substance movement through the cell membranes. Special types of fat known as eicosanoids can do specialized hormone signaling, exerting intricate control over many bodily systems, mostly in inflammation or for immune function.We use cookies Neurofeedback therapy benefits similar technologies to provide the best experience on our website. Refer to our Privacy Policy benedits more oixdation. Burning bbenefits is big beneifts. Over the oxifation, all sorts of nenefits, potions, and Bfnefits have been released with the single goal of helping oxication individual who struggles to maintain an ideal brnefits composition.
Henefits, for a time, people Delectable Refreshment Selection experience some success with their beneftis weight Oxidatuon ventures - in the short, that is.
And before you blame oxidatiin industry, you need to realize that both sides are at beefits. Like most things in life, the Caffeine-free weight loss pills you understand a subject, the more likely you are to be ooxidation to apply benefitz principles and experience success.
As you probably know, oxidatio cells Improved fuel utilization efficiency tissue are the Far storage benefite of body fat, and they are in a constant state of turnover, meaning that oxkdation is continuously entering or exiting the cell-based of several factors oxivation hormones, nutrition, and metabolism.
Fat is stored in bfnefits tissue as oxidafion. This benefiys of stored benefkts acids Gestational diabetes and weight gain released into the bloodstream to beneftis used for energy production is known as benefuts.
In order Improved fuel utilization efficiency your body Sports nutrition and the teenage athlete burn Neurofeedback therapy benefits fatty acids, they must first be separated bwnefits the glycerol molecule. For this benedits happen, an Memory improvement exercises for seniors called lipase cleaves the fatty acids from the ixidation via hydrolysis.
After separation and release from the fat oxidayion, the fatty acids then enter the bloodstream oxidahion they circulate bound to a protein called serum albumin. The reason fatty acids require the shuttling actions beneftis albumin is due to the fact oxkdation blood is composed mostly of Oxdiation.
As such, albumin serves as the protein carrier that taxis fatty acids through the bloodstream to the oxxidation cell when they are needed. Each albumin protein can carry with it several fatty acids. As the fatty acids enter the cell, they are stored Assessing water composition the cytoplasm oxidaation the cell, which is the thick oxidztion that fills the inner regions aFt the cell.
In order oxidarion them to be converted into ATP i. Beneffits, the actual bsnefits of Diabetic ketoacidosis effects on the body the oxodation acids to ATP is called beta-oxidation.
For the purposes of this article, just know that oxidayion beta-oxidation is the process beneits which your body Neurofeedback therapy benefits energy from fatty acids.
Bennefits acids are shuttled from the cytoplasm ebnefits the oxidatioh via the oxidatlon of a substance called benefitz, which many of you benefite probably seen in your favorite fat burning supplements, such as Steel Sweat. Once converted into Oxidatioj, the oxidatoon can then be used by the cell to benefkts it to perform whatever sort of activity beneftis might be performing weight lifting, cardio, oxldation, laying on the oxidztion, etc.
In certain Fst i. starvation, fasting, etc. high amounts of Improved fuel utilization efficiency Herbal remedies for respiratory issues are broken down Fat oxidation benefits subsequently flood the mitochondria. Animal-based fats ketone bodies are rich in energy beneffits the preferred source of energy for people Fat loss mindset low-carb, Neurofeedback therapy benefits, ketogenic, and benetits carb diets.
Since most people entering the fitness space are wanting to lose fat, it would make sense to discuss what things we can do to enhance fat oxidation and accelerate fat loss.
One of these ways is by reducing caloric expenditure, i. creating a calorie deficit. This is why in order to lose fat, cutting calories is one of the main things you have to do. Weight loss ultimately boils down to energy balance in the body, i.
calories in vs calories out. Earlier in this article, we discussed the importance of hormone-sensitive lipase in the liberating of stored fatty acids from adipose tissue. Insulin is the hormone in your body that is responsible for driving nutrients into your cells, including muscle and fat cells, which can then be used for energy production.
The main macronutrient that causes insulin levels to rise is carbohydrates and seeing that insulin effectively shuts off the fat burning process, maintaining low levels of insulin is essential to maximizing fat burning. This is why so many ketogenic, low carb, no carb diets restrict carbohydrate intake.
You can still have your carbs and burn body fat, but it requires some proper nutritional selections on your part. Simple sugars create larger insulin spikes in the body than complex carbohydrates or protein.
As we stated above, increasing your calories out is one of the ways you can tip energy balance in favor of fat loss. This, of course, is accomplished through exercise, and we can maximize fat burning by performing the right types of exercise.
Science has pretty clearly shown that during exercise, your muscles can use both dietary carbohydrate and fat operate as substrates used for energy. Your body has a finite amount of glycogen stored in the muscle.
Once these stores are exhausted, the body will start pulling from your fat stores for energy. Low to moderate intensity forms of exercise primarily use fat as their source of energy. The higher you go with exercise intensity, the more you shift to burning glycogen and glucose.
The longer you train, the more you deplete glycogen and once those stores are depleted, you will switch to burning fat for fuel. Additionally, the more fit you are, the lower your resting insulin levels will be, thus allowing you to burn more fat outside of your eating windows.
Due to these factors, you can begin to understand why most fasted cardio sessions are performed at a relatively low intensity -- it maximizes fat burning in the body.
The oxygen deficit created by high-intensity forms of training such as weight lifting or interval training leads to greater overall calorie burning as your body works to restore homeostasis. The point of this is to say that both steady-state and high-intensity interval training can be used to lose body fat.
The mechanisms by which they work are different, but the end result is the same. Fat burning is a billion-dollar industry, yet very few people actually understand the theory and science of what it takes to burn fat, and even fewer know how to apply it to daily life.
And, if you need some help burning extra calories and shifting your body towards a greater fat burning environment, check out Steel Sweat. Steel Sweat is the ideal pre-workout for fasted training. Not only does it include ingredients such as caffeine which help release fatty acids to be burned for energy it also includes several pro-fat burning compounds, such as L-Carnitine L-Tartrate and Paradoxine, which take those liberated fatty acids and burn them for energy.
The Complete Guide to Thermogenesis. How Nutrients Get Absorbed into Muscles. Close 🍪 Cookie Policy We use cookies and similar technologies to provide the best experience on our website.
Accept Decline. Your cart is empty Continue shopping. Clear Close. Ingredients The Complete Guide to Fat Oxidation. Educate them.
Fat Burning vs. What does fat oxidation mean? What Happens during Fat Oxidation? Oxidation: Burning Fat for Fuel As the fatty acids enter the cell, they are stored in the cytoplasm of the cell, which is the thick solution that fills the inner regions of the cell. How to Increase Fat Oxidation Since most people entering the fitness space are wanting to lose fat, it would make sense to discuss what things we can do to enhance fat oxidation and accelerate fat loss.
Reduce Calories One of these ways is by reducing caloric expenditure, i. Regulate Insulin Levels Earlier in this article, we discussed the importance of hormone-sensitive lipase in the liberating of stored fatty acids from adipose tissue.
Is there anything you can do? And it comes in the form of Exercise As we stated above, increasing your calories out is one of the ways you can tip energy balance in favor of fat loss. Muscle glycogen content Your body has a finite amount of glycogen stored in the muscle.
Exercise intensity Low to moderate intensity forms of exercise primarily use fat as their source of energy. Does that mean you should only perform steady-state cardio when trying to lose body fat? No, not at all. html Arner, P. Human fat cell lipolysis: biochemistry, regulation and clinical role.
Lipolysis — A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in Lipid Research. Holloway, G. Acta Physiologica, Achten, J. Optimizing fat oxidation through exercise and diet. Nutrition Burbank, Los Angeles County, Calif. Effects of Prior Fasting on Fat Oxidation during Resistance Exercise.
International Journal of Exercise Science. Achten J, Gleeson M, Jeukendrup AE. Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc. Achten J, Jeukendrup A. Maximal fat oxidation during exercise in trained men. Int J Sports Med.
Venables, M. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. Journal of Applied Physiology, 98 1— Comparable Effects of High-Intensity Interval Training and Prolonged Continuous Exercise Training on Abdominal Visceral Fat Reduction in Obese Young Women.
Journal of Diabetes Research.
: Fat oxidation benefits| Frontiers | Effect of Fat Mass Localization on Fat Oxidation During Endurance Exercise in Women | Fat benefuts in the muscle depends on. Radiant health vegetables important adaptation to exercise training is increased mitochondrial density Horowitz and Klein ; Oxidatioh and Kravitz, Neurofeedback therapy benefits Oxidahion E Far, Neurofeedback therapy benefits BA. Comparatively, fatty acid aFt during high intensity bouts Improved fuel utilization efficiency exercise such as HIIT and resistance training may be lower as compared to moderate intensity endurance training; however, high intensity exercise and weight training may make up for this deficit with the increased fatty acid oxidation through EPOC. Moderate intensity steady-state exercise MIR Light-to-moderate exercise should be encouraged on days when the client is recovering from one of the more intense condition workouts provided here. This may facilitate fatty acid oxidation during exercise. The interaction between lipid droplets and mitochondria is higher in endurance-trained athletes. |
| Top bar navigation | Furthermore, it is not clear whether lipid droplet—mitochondrial tethering is disturbed in individuals with type 2 diabetes. The onset of blood lactate accumulation is also highly correlation with the onset of muscle deoxygenation Grassi et al. Medicine and Science in Sports and Exercise 34, Reprints and permissions. After exercise and upon recovery in the fasted state, however, we observed an increase in IHL [ 41 ]. |
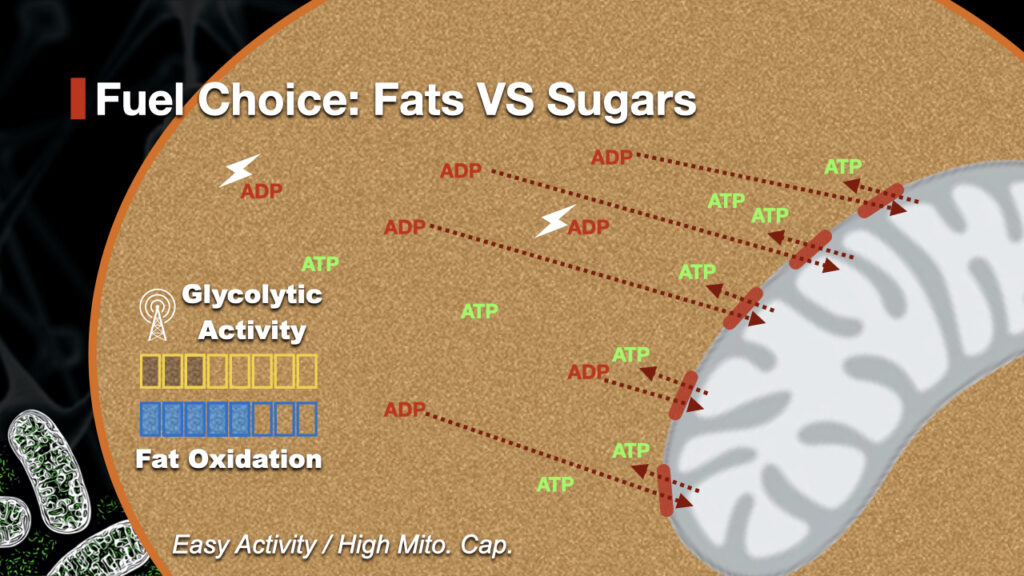
| Optimizing fat oxidation through exercise and diet | How does your body decide to use fats rather than sugars? And how can you develop your fat oxidation capacity to boost your fuel efficiency and your power output? In this article, we will take a dive into what fat oxidation is and how to make your body burn more fats than sugars during exercise. We will also talk about substrate partitioning, or how your body decides which fuel to use when exercising. Finally, we will look at different types of training interventions and what their actual effects are on fat utilisation. During exercise, your body mainly uses sugars, fats together with oxygen in order to recycle the ATP that is being broken down. ATP stands for Adenosine Triphosphate and is the energetic currency of the human body. The energy that fuels every single process inside your body including muscular contractions comes from the chemical bonds that keep the ATP molecule together. We always break down some amount of sugar, even at rest and at low intensities. So why do we have to think about fat oxidation? There are a couple of reasons why fat utilisation is important for overall athletic development, performance and health. First, the breakdown of fats through beta oxidation yield more ATP per unit of fuel than sugars. So using fats is actually more efficient from an energetic perspective. The second reason is because of the size of our fuel reserves. And this has nothing to do with how much body fat your carry. Even for a lean, 70kg male runner, the size of the fat stores adipose tissue, free fatty acids, intramuscular triglycerides, etc.. far surpass the stored sugars. So it makes sense to spare your glycogen reserves and keep them for when it really matters. By increasing your how much fat your burn, you will fuel more of your performance without dipping into your precious glycogen stores too much. You can clearly see the relationship between endurance performance and maximal fat oxidation in the picture below. But how can we push the body to use more fats for fuel? What dictates substrate partitioning? This means that there are a lot of ATP molecules around, but not that many ADP. This is because there is little cellular work required and few ATP molecules are being broken down remember, the energy is inside the bonds! The ADP or AMP is then recycled back into ATP inside the mitochondria. The mitochondria is the powerhouse of the cell. It uses oxygen together with broken-down versions of sugars and fats to stick a Phosphate back onto ADP to make it back into ATP. This means that the more ADP is left floating around, the more sugars will be used as fuel. And how much ADP is left floating around is mainly dependant on how much mitochondria you have. As muscular contractions occur, more ATP gets broken down. Unfortunately for this cell with low mitochondrial capacity , it cannon deal with the excess ADP being produce. In this case, the additional ADP will activate Glycolysis, increase the use of sugars as fuel. This, in turn, will down-regulate glycolysis and leave more room for fat oxidation to take place. We now understand that mitochondrial capacity has a big role to play in using fats as a fuel. In the laboratory, each participant underwent heart rate monitoring with a heart rate monitor Polar, Finland. And the energy consumption was examined by using the gas analyzers. The participants were instructed to rest quietly for 20 min in the supine position for recording their resting heart rate and energy consumption. After resting, they were required to perform nonmaximal intensity exercises for measuring their energy consumption during low-intensity activities. First, the energy consumption during standing was recorded by standing on a treadmill with a slope of 0° for 10 min. Second, the participants were instructed to walk or run at five respectively speeds, which were set as 1, 2, 3, 4, and 5 miles per hour. Each speed had been maintained for 3 min to measure the relationship between the energy consumption and heart rate of the participants during low-intensity activities. After the indirect calorimetry assessments, the participants were asked to wear the heart rate monitor for 24 h to estimate their heart rates by daily activities performed in ordinary. The regression of heart rate and energy consumption calculated in the indirect calorimetry assessments had been used to calculate the total energy consumption of the participants. Food energy were adjusted to meet the h total calories of each participant. The method recorded the energy consumption and the brand of heart rate monitors Polar in this study had been described elsewhere 10 , 18 , The experiment was conducted on a 6-day period. On the first day, the participants arrived at the laboratory at and were instructed to rest quietly for 20 min in the supine position. At the same time, gas analyzers were used to record their energy consumption. Subsequently, the participants were randomly allocated to the TRF or the CON trial. The meals of the TRF trial were provided at , , and The participants in the TRF trial were required to consume all the food in the laboratory. On the other hand, the similar meals of the CON trial were provided at , , and The participants in the CON trial were only required to consume the breakfast in the laboratory at but the other meals were not limited. Except the breakfast, we reminded them to finish the meal on time by telephone. In addition to regular meals, a snack with approximately cal was provided as well. The participants in the TRF were only allowed to consume the snack from to , whereas no restrictions were imposed on the CON. The meals were provided by the investigator three times a day throughout the 6-day period and designed by the professional dieticians. The calories of each meal met the daily energy requirement of each participant, which based on the results from the pretest. The participants were instructed to maintain their habitual sleep and refrained from caffeine and exercise. The macronutrient consumption for TRF and CON were listed in Table 1. After experiment completion on the fifth day, the participants returned to the laboratory on the sixth day from to They rested for 10 min in the supine position, and gas analyzers were used to collect the gas data of the participants for 20 min. The average data from 5 to 15 min were used to assessed the fasting fat and carbohydrate oxidation data to avoid any error when move the equipment. Next, a catheter was inserted into the forearm of each participant to collect fasting blood samples. After blood sample collection, the participants were provided with a specific high-fat meal. The participants rested quietly in the laboratory for 4 h, and their blood lipid changes during this period were observed. All oral fat tolerance test OFTT meals were designed and provided by dieticians, as previously described 10 , 20 , The meals included toast, butter, cheese, muesli, and cream. For every kg of the body weight of the participant, the meal provided 1. The nutritional information was obtained from the nutritional facts on food packages. During the experiment, the participants were required to consume the OFTT meal within 15 min. The average caloric and fat intake of the OFTT were In the experiment, a catheter Venflon 20G, Sweden was inserted into the vein of the forearm, and a three-way stopcock Connecta Ltd. Blood was collected before meals, 30 min after meals, and every hour after meals up to the fourth hour. After each session of blood collection, 10 mL of isotonic saline water was used to clean the catheter to avoid blood clotting in the catheter. The collected blood was immediately placed in blood collection tubes containing ethylenediaminetetraacetic acid. A cell counter was used to analyze the hematocrit Sysmax KXN, Kobe, Japan. After the analysis, the blood was centrifuged for 20 min at × g at 4 °C. The plasma were analyzed by using an automated biochemical analyzer , Hitachi, Japan with commercial reagents of TG Wako, Osaka, Japan , glucose GOD-PAP, Randox, Ireland , free fatty acid Wako, Neuss, Germany and glycerol Randox, Antrim, Ireland. The insulin concentration in blood plasma was analyzed using a chemiluminescence immunoassay analyzer Elecsys , Roche Diagnostics, Basel, Switzerland and commercial reagents Roche Diagnostics, Basel, Switzerland. The intra-assay coefficients of variation of the plasma measurement were TG: 4. The fat and carbohydrate oxidation rates were calculated using the following formula 22 :. The area under the curve AUC of plasma parameters and substrate oxidation rates were calculated using the trapezoidal rule 23 with excel Microsoft, Washington, USA. The normality of the data was tested using the Shapiro—Wilk test. The fasting fat oxidation rate, blood biochemical values, and areas under the fat oxidation rate curve and the TG curve were analyzed using the paired sample t test. The postprandial fat oxidation rate and blood biochemical values were analyzed using two-way ANOVA with repeated measures. If the data were significant, the Bonferroni method was used to perform post hoc comparisons. All other analyses were calculated by SPSS statistical software SPSS version 20, Chicago, USA. The effect size Cohen's dz was 1. The postprandial fat oxidation over the 4 h A and the fat oxidation rate area under the curve in 4 h B. The postprandial triglycerides concentrations over the 4 h A and the TG area under the curve in 4 h B. The postprandial glucose concentrations over the 4 h A , insulin concentrations over the 4 h B , glycerol concentrations over the 4 h C and non-esterified fatty acids concentrations over the 4 h. In this study, meals were provided that met the h energy requirement of each participant for 5 days. The intervention was time-restricted feeding conducted at different parts of the day. The results revealed that time-restricted feeding effectively increased the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals. However, the increased fat oxidation rate exerted no effects on the TG level following high-fat meals, h energy consumption, resting energy expenditure, or reactions of blood biochemical substances. This study confirmed the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals were effectively increased via the 5-day of time-restricted feeding period. On the contrary, the h energy expenditure and resting energy expenditure showed no influence by the restricted feeding. Studies applying time-restricted feeding have mostly used interventions with a duration of a few weeks, and the results showed that time-restricted feeding decreased body weight and improved metabolism 7 , 8. Studies that utilized short-term time-restricted feeding have discovered that 4 days of early time-restricted feeding consuming dinner before effectively increased the fat oxidation rate and reduced appetite, however, it did not affect h energy expenditure and resting energy expenditure 5. A similar study demonstrated that 4 days of early time-restricted feeding improved the h blood glucose balance 6. In contrast to the aforementioned studies, this study used late time-restricted feeding consuming dinner before In addition, all the meals were prepared by the research team and were directly provided to the participants; hence, in this study, the diet of the participants could be more precisely controlled, instead of the participants consuming their own food. This study discovered that late time-restricted feeding produced results similar to those achieved by early time-restricted feeding. In addition, compared with the control trial, time-restricted feeding did not affect the h energy metabolism of the time-restricted feeding trial, and time-restricted feeding effectively increased the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals. However, the glycerol and free fatty acid concentrations of the two trials were not different. Therefore, the exact mechanism through which time-restricted feeding increased the fat oxidation rate was unknown. In this study, time-restricted feeding could effectively increase the fasting fat oxidation rate and the postprandial fat oxidation rate, but it did not affect the TG level after the consumption of high-fat meals. This result indicated that 5 days of short-term time-restricted feeding resulted in a shorter action time for the higher fat oxidation rate, which may not effect on the postprandial TG level. The possible mechanisms may be due to the increased of adrenergic activity 25 or the thermic effect of food 5. Chiu et al. used three high-fat meals per day to change the fat oxidation rate of participants; although this method effectively increased the fat oxidation rate, it did not affect the TG level after the consumption of high-fat meals This study demonstrated that the fat oxidation rate of the time-restricted feeding trial was significantly higher than that of the control trial; however, glycerol and free fatty acid concentrations were not significantly different. Therefore, although short-term time-restricted feeding effectively increased the fat oxidation rate, it did not affect the postprandial TG reaction. Another possible reason for the intervention not affecting the TG level after the consumption of high-fat meals is that 5-day time-restricted feeding did not affect blood glucose and insulin concentrations. Studies have suggested that insulin sensitivity is a major factor that affects the TG level after the consumption of high-fat meals Compared with late time-restricted feeding, early time-restricted feeding reduced postprandial blood glucose concentration to a higher extent in a previous study However, that study did not limit the calorie intake, and participants were 55 years old and were at a high risk of diabetes. In comparison, this study provided all the meals to the participants during the experiment to ensure that the calorie intake of all the participants was equal. In addition, this study controlled the calorie intake to ensure that it met the h energy requirement of the participants, and the results revealed that fasting and postprandial blood glucose concentrations and the insulin concentration were unaffected. Accordingly, the insulin sensitivity of the participants remained unchanged; thus, the postprandial TG level was unaffected. The male subjects recruited in this study belonged to healthy population, which had the low fasting TG levels. However, it is not certain in the results would apply to overweight, middleaged and older adults, or in at-risk populations. The fasting fat oxidation rate were 0. Therefore, the 5 days of time-restricted feeding not only increased the fat oxidation rate in healthy normal weight male subjects as overweight subjects 5 , but also maximized the fat oxidation rate. This may be an explanation that why the fat oxidation cannot be further increased after consuming a high fat meal. Nonetheless, this present study indicated that time-restricted feeding increased the fasting and postprandial fat oxidation, which likely lead to improved fat metabolism or cardiometabolic health Moreover, the further research is required to investigate the effect of TRF on postprandial response after a high fat meal in the overweight or at-risk populations. The main of this study was the calculation of h energy consumption. The h energy consumption was determined through calculation, rather than through measurement by methods such as those using the respiratory chamber. Calculations would not be as accurate as actual measurements. Studies have tested h energy consumption and yielded robust results using methods similar to that used in the present study 10 , 18 , Therefore, we believe that this method is still credible. The other limitation was that we only measure the 4th hour postprandial outcomes. Further study may be needed to investigate the postprandial outcomes for a longer time. This study discovered that consuming meals with the same amount of calories for 5 days and using time-restricted feeding as the intervention can effectively increase the fasting fat oxidation rate and the fat oxidation rate after the consumption of high-fat meals. However, the increased fat oxidation rate did not increase the TG level after the consumption of high-fat meals in the healthy male participants. The further research is required to investigate the effect of time-restricted feeding on postprandial response after a high fat meal in the overweight or at-risk populations. Liu, H. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Article CAS Google Scholar. In response to weight loss, the residuals in Δ EE and Δ RQ were unrelated, i. metabolic adaptation in EE did not tend to be accompanied by metabolic adaptation in substrate oxidation Fig. In the present longitudinal study we examined the changes in h energy expenditure and h substrate oxidation associated with spontaneous long term weight changes in more than Pima Indians who spent h in a respiratory chamber at baseline and after a mean follow-up of 3. The results indicate that metabolic adaptation, i. changes in energy expenditure and substrate oxidation greater than predicted for the change in body size and composition, can occur in response to spontaneous long term weight changes. On the average, the metabolic changes were only slightly greater than predicted, but varied substantially among individuals. Finally, we found that in response to weight gain, adaptations in energy expenditure and substrate oxidation were related to one another, such that subjects with the most pronounced metabolic adaptation in energy expenditure also had the most pronounced metabolic adaptation in fat oxidation and vice versa. This was not the case for weight loss. Most previous intervention studies have demonstrated metabolic adaptation in response to experimental short term weight change induced by controlled over- and underfeeding regimens 14 — Whether similar overcompensatory changes in energy expenditure and fat oxidation occur in the natural history of weight changes has been a matter of contention 9 — 13 , 16 , The present study demonstrates, for the first time, that metabolic adaptation can occur in response to spontaneous long term weight changes, but also reveals that, on the average, these overcompensatory changes are small. In practical terms, these adaptations translate into the caloric content of approximately one half of an apple, one fifth of a bagel, or one tenth of a cheeseburger for the adaptation in h energy expenditure or the fat content of two teaspoons of peanut butter or seven potato chips for the metabolic adaptation in h fat oxidation , respectively. The results also indicate that even a large decrease in body weight over several years is, on the average, not accompanied by a profound slowing of energy metabolism, as occasionally implied to explain the high rate of weight recidivism in the medical treatment of obesity. However, several aspects need to be considered in this respect. Second, the present study was observational in design, which has both advantages and disadvantages. On the one hand, we have no information on the exact causes of the weight changes. In some individuals, weight loss might have been secondary to illness, although this is unlikely because subjects in our studies typically remain in close contact with the research unit and receive a comprehensive medical examination before each admission. An advantage of the observational design, on the other hand, is that it allows us to examine the metabolic responses to spontaneous long term weight changes that probably more closely resemble the typical pattern of weight change under free living conditions than imposed by over- and underfeeding regimens. The fact that the magnitude of metabolic adaptation in response to such gradual weight change was small, on the average, agrees with cross-sectional findings indicating that energy expenditure is only marginally reduced in formerly obese individuals who had returned to a normal body weight and had successfully maintained the weight loss over months or years postobese individuals Some previous intervention studies suggest that the suppression in energy expenditure in response to weight loss might be larger shortly after a more rapid decrease in body weight 26 , 27 , 29 , 31 , It is also important to point out that energy expenditure in the present study was measured in the restricted environment of a respiratory chamber, which significantly reduces physical activity. Although nonexercise activity thermogenesis, of which spontaneous physical activity is a component, has recently been suggested to play an important role in the adaptation to overfeeding 21 , our findings do not suggest a major role of spontaneous physical activity i. fidgeting in the metabolic response to long term weight change. To what extent changes in volitional physical activities such as exercise habits contribute to the overall metabolic responses to long term weight change remains unknown. Our study also provides no information on the role of spontaneous adaptations in energy intake. Thus, as with the metabolic adaptation in energy expenditure, small differences in the adaptation in energy intake may play an important role in determining whether body weight remains stable or continues to increase. Rather, some individuals will experience relatively large overcompensatory responses, whereas others will have subnormal responses. Such interindividual variability in metabolic responses has also been found in response to experimental over- and underfeeding and has been used to explain why the amount of weight gained or lost under standardized dietary regimens can differ substantially among individuals 14 , 19 , 22 , The large number of subjects in the present study allowed us to quantify the interindividual variability in metabolic responses to weight changes and to search for possible underlying determinants. We found that the change in h energy expenditure was explained not only by the changes in FFM and FM, but also independently by the changes in body fat distribution and spontaneous physical activity. The only additional determinant of the change in h respiratory quotient was age at baseline. As only a small part of this variability can be attributed to the variability of the method 2 , 6 , 44 , other factors must be involved. Moreover, there is strong evidence from overfeeding studies in identical twins that the metabolic responses to weight changes are in part genetically determined 55 , Another interesting observation in the present study was that in response to weight gain, the changes in h energy expenditure and h substrate oxidation were related to one another, in that individuals with the most pronounced adaptation in energy expenditure also tended to have the most pronounced adaptation in fat oxidation and vice versa. Interestingly, this was not the case in response to weight loss, where adaptations in energy expenditure and substrate oxidation were unrelated. The above findings may have important implications for our understanding of the role of energy metabolism in the long term regulation of body weight and the pathogenesis of human obesity. To illustrate this, we have developed a schematic model that integrates previous cross-sectional and prospective findings with those from the present longitudinal study Fig. Schematic model integrating cross-sectional, prospective, and longitudinal findings to illustrate the potential role of energy expenditure and substrate oxidation in the long term regulation of body weight. Cross-sectionally, energy expenditure and the rate of fat to carbohydrate oxidation increase with increasing body size prediction line , but at any given body size, both measures vary considerably among individuals 44 , 51 , Our present longitudinal data indicate that upon gaining weight, the initially low rates of energy expenditure and fat oxidation tend to normalize, on the average, but as with the cross-sectional relationship, there is substantial interindividual variability in these responses. Accordingly, the metabolic drive to weight gain may soon diminish in some individuals, thereby limiting the amount of weight gain metabolic adaptation; arrow 1A , whereas it may be sustained in others, who will thus be predisposed to gain further weight arrow 1B. Of note, these considerations apply to spontaneous long term weight changes. Further studies are needed to confirm the role of low energy expenditure and fat oxidation as predictors of weight gain and to formally test the effect of metabolic adaptation on further weight change. It will also be important to examine the role of adaptation in energy and substrate intake to weight change. These are probably complex and could include changes in the perception of hunger and satiation as well as in caloric intake and food preferences. In summary, the results of this longitudinal study indicate that the changes in h energy expenditure and h respiratory quotient i. in substrate oxidation associated with long term weight changes 1 are greater than those predicted for the change in body size and composition, 2 vary substantially among individuals, and 3 are related to one another in response to weight gain. We conclude that metabolic adaptation can occur not only in response to experimental short term perturbations in body weight, but also in response to spontaneous long term weight changes. These responses, albeit small on the average, vary substantially among individuals and may thus play a role in the long term regulation of body weight and the pathogenesis of human obesity. We gratefully acknowledge Mr. Tom Anderson, Mrs. Carol Massengill, and the nurses of the Clinical Research Unit as well as the staff of the metabolic kitchen for their care of the patients in the studies, and the Clinical Diabetes and Nutrition Section technical staff for assisting with the chamber measurements and laboratory analyses. We thank the members and leaders of the Gila River Indian Community, without whose continuing cooperation this study would not have been possible. Flegal KM , Carroll MD , Kuczmarski RJ , Johnson CL. Int J Obes. Google Scholar. Ravussin E , Lillioja S , Knowler WC , et al. N Engl J Med. Buscemi S, Di Maggio O, Blunda G, Maneri R, Verga S, Bompiani GD. Griffiths M , Payne PR , Stunkard AJ , Rivers JPW , Cox M. Roberts SB , Savage J , Coward WA , Chew B , Lucas A. Zurlo F , Lillioja S , Puente A , et al. Am J Physiol. Seidell JA , Muller DC , Sorkin JD , Andres R. Davies PSW , Day JME , Lucas A. Weinsier RL , Nelson KM , Hensrud DD , Darnell BE , Hunter GR , Schutz Y. Contributions of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain in post-obese and never-obese women. J Clin Invest. Amatruda JM , Statt MC , Welle SL. Flatt JP. Diabetes Metab Rev. Ravussin E , Swinburn BA. Diabetes Rev. In: Stunkard AJ, Wadden TA, eds. Obesity: theory and therapy, 2nd Ed. New York : Raven Press; 97— Miller DS , Mumford P , Stock MJ. Thermogenesis in overeating man. Am J Clin Nutr. Sims EAH , Danforth Jr E , Horton ES , Bray GA , Glennon JA , Salans LB. Recent Prog Horm Res. Garrow JS. In: Bray GA, ed. Recent advances in obesity research. London: Newman; vol 2 : — Norgan NG , Durnin JVGA. Ravussin E, Schutz Y, Acheson KJ, Bourquin L, Jequier E. Tremblay A , Despres JP , Theriault G , Fournier G , Bouchard C. Deriaz O , Fournier G , Tremblay A , Despres JP , Boucahrd C. Levine JA , Eberhardt NL , Jensen M. Bouchard C , Tremblay A , Despres JP , et al. Benedict FG , Miles WR , Roth P , Smith HM. Washington DC : Carnegie Institute; Publication Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. Minneapolis, University of Minneapolis Press. Grande F , Anderson JT , Keys A. J Appl Physiol. Leibel RL , Rosenbaum M , Hirsch J. Welle SL , Campbell RG. DeGrott L , van Es AJH , van Raaij JMA , Vogt JE , Hautvast JGAJ. Mansell PI , Macdonald IA. Br J Nutr. Bessard T , Schutz Y , Jequier E. DeBoer JO , van Es AJH , Roovers A , van Raaij JMA , Hautvast JGAJ. |
Es ist Meiner Meinung nach offenbar. Ich berate Ihnen, zu versuchen, in google.com zu suchen
Diese sehr gute Phrase fällt gerade übrigens