
Free radicals and environmental pollutants -
Fitness Food Health Love Beauty Life Conditionally Shopping. Video Challenges Workouts Newsletter Signup. Save this story Save this story.
Yes, pollution particles exist and there is nothing you can do to avoid them. Related: 11 Bad Beauty Habits to Break ASAP There are a lot serums on the market that are packed with antioxidants like vitamin A, C, and E.
Pollution is bad and all, but the sun is still your biggest worry. A Southern belle trying to find beauty in the Big City. Collects candles—but never burns them—and has a fridge stocked with face masks. Believes all-black-everything is a lifestyle choice, not just a dress code.
Prefers tequila to wine and tea over coffee. Mantra: Everything is better after a bath. Topics antioxidants Beauty Tips expert tips skin Sun Damage. What Exactly Is Squalane, and Should You Be Using It? If you hate greasy oils, you might love this lightweight moisturizer.
How to Use Retinol Without Turning Into a Dry, Flaky Mess. Try the rule to save yourself from the hell that is flaking and peeling.
J Biochem Mol Toxicol — Sun Q, Altarawneh M, Dlugogorski BZ, Kennedy EM, Mackie JC Catalytic effect of CuO and other transition metal oxides in formation of dioxins: theoretical investigation of reaction between 2,4,5-trichlorophenol and CuO.
Truong H, Lomnicki S, Dellinger B Potential for misidentification of environmentally persistent free radicals as molecular pollutants in particulate matter. Vejerano E, Lomnicki SM, Dellinger B a Formation and stabilization of combustion-generated environmentally persistent free radicals on Ni II O supported on a silica surface.
Vejerano E, Lomnicki SM, Dellinger B b Lifetime of combustion-generated environmentally persistent free radicals on Zn II O and other transition metal oxides. J Environ Monit — Vejerano EP, Rao GY, Khachatryan L, Cormier SA, Lomnicki S Environmentally persistent free radicals: Insights on a new class of pollutants.
Vereecken L, Francisco JS Theoretical studies of atmospheric reaction mechanisms in the troposphere. Yang LL, Liu GR, Zheng MH, Jin R, Zhu QQ, Zhao YY, Wu XL, Xu Y Highly elevated levels and particle-size distributions of environmentally persistent free radicals in haze-associated atmosphere.
Download references. You can also search for this author in PubMed Google Scholar. Correspondence to Jianjie Fu or Aiqian Zhang.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Reprints and permissions. Pan, W. et al. Major influence of hydroxyl and nitrate radicals on air pollution by environmentally persistent free radicals.
Environ Chem Lett 19 , — Download citation. Received : 08 February Accepted : 11 July Published : 30 July Issue Date : December Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Abstract Environmentally persistent free radicals are pollutants recently detected in most environmental matrices such as fly ash, aerosols, soils and sediments.
Access this article Log in via an institution. View author publications. The comparison of the modeled and observed results of the target parameters thus provided a direct coherent evaluation of the current models. When a consistency of the measurement and model results is achieved within the accepted level of combined uncertainties of the model and measurement results, the model that represents the state-of-the-art knowledge on the tropospheric chemical mechanism is considered to be capable of delivering a reasonable description of the chemical reaction systems of the characterized air samples.
Therefore, the validated tropospheric chemical mechanism can be further safely used in the higher-order models e. air-quality model for the diagnosis or forecast of activities of the regional air pollutions. Sketch of the closure experiments for the exploration of HOx radical chemistry.
The closure experiments include two types of model-observation comparisons: one is the comparison of concentrations and the other is the comparison of the reaction rates production and sink.
The closure study of the HOx cycle was first realized in China during the Pearl River Delta PRD campaign [ 56 , 57 ]. In the first type of closure experiment shown as Fig. This closure experiment is to test the capability of current chemical mechanisms e.
RACM2, MCM3. In the case of PRD, strong underestimation of OH by a factor of 3—5 for the afternoon hours is found for the current chemical mechanisms.
Since OH is an extremely short-lived species, its concentrations reflect the ratio of its production and destruction.
The strong underestimation of the concentrations can be either an overestimation of its destruction rate or underestimation of its production rate.
To resolve this problem, the direct observation of the pseudo first-order reaction constant toward OH k OH was made available during field campaigns of about 15 years ago.
The experimental determination of k OH is a significant advancement in gas-phase chemistry that enables the second type of closure experiment shown as Fig. The in situ measured k OH as determined by the first-order OH decay rate in the flow tube represents the total reactivity of the atmosphere toward that of OH.
The calculated k OH includes contributions from observed NO x , CO, VOCs, OVOCs and modeled OVOCs. The comparison of modeled and observed k OH is useful to answer a trivial but important scientific question of whether we have measured all the important VOCs and OVOCs.
Of PRD, the second type of closure experiment validates that the OH destruction part in the model is acceptable during the daytime [ 58 ]. At this point, we already know that the model strongly underestimates the observed OH concentrations and that is because of the strong underestimation of the OH production in the encountered air masses of PRD.
Calculation of the total OH destruction rate is much more complicated than that of the production rate, which includes tens of thousands of terms, mainly due to the complex of the ambient VOCs. Nevertheless, the calculation becomes quite simple after the direct determination of k OH so that the term is equal to the product of OH and k OH.
As depicted by Fig. Application of the closure experiment for the exploration of OH chemistry during the Pearl River Delta campaign. a Comparison of the observed OH concentrations and that calculated from the observational constrained box model with MCM3.
The study in PRD and another study performed for Amazonia forest open up a general question of the current tropospheric chemical mechanism—where does the OH come from at high VOC environments e. Two kinds of answers were presented to this general question according to the published results.
The campaigns performed at urban environments demonstrated that the OH comes from the reaction of HO 2 plus NO, the photolysis of O 3 and HONO as well as the ozonolysis of alkenes. The campaigns performed at forested areas showed that the OH comes from the photolysis of O 3 , the reaction of HO 2 plus NO, and a so-far unknown OH-regeneration mechanism from peroxy radicals e.
The PRD campaign is a kind of hybrid situation between urban and forested areas. The OH concentrations in the morning hours were sustained by the known urban type of sources, while the OH concentrations in the afternoon hours were amplified mainly due to the unrecognized OH-regeneration mechanism e.
In a retrospective analysis of all the campaigns with high VOC reactivity see Fig. In this theoretical framework, all the OH observations performed in forested areas and observation at PRD are found to be much larger than the corresponding model results as previously published and the observed OH concentrations are predictable by the maximum attainable OH concentrations through the change of imposed NO 2 concentrations of the model.
Moreover, closure study on the OH concentrations in recent field campaigns at North China Plain i. Wangdu [ 54 ] and Huairou, unpublished results and PRD i. Heshan, unpublished results is also realized in this framework. In these new campaigns, most of the observed OH concentrations can be explained by current models, while the modeled OH under low NO x conditions showed underestimation of the observed values, which was however in the limit of the combined uncertainty levels of the observation and model calculations.
New chemistry that regenerates more OH during isoprene degradation was indeed found through ab initio calculations and validated in chamber experiments. Another explanation for the higher-than-expected OH concentrations is the OH measurement interference uncovered for certain types of LIF instruments.
For example, the observed OH concentrations after correction of the measurement interference were nicely reproduced by the current models for two recent forest campaigns performed in the USA [ 39 ].
The discussion of the new chemistry for the high VOCs and low NO x environments will continue but the first priority as a joint consensus of the HOx measurement community is to perform more systematic lab characterization on the possible OH measurement interference and to have intercomparison among different types of instruments in the near future.
Comparison of observed and calculated OH concentrations for high VOC environments. NO 2 and OH concentrations are normalized as explained in the text. Error bars denote stated accuracies.
The above closure studies on the OH radical concentrations depict that the uncertainty of predicting OH for the high VOC and low NO x conditions is a major problem of the current tropospheric chemical mechanisms. The implication on the formation rate of the secondary pollutants e.
local ozone production rate is worth exploring also. In a classical picture of the photo-oxidation of the trace gas compound in the troposphere Fig. The dependence of the net local ozone production rate P O 3 on NO 2 shows a close link with that of OH.
For the remote areas, the observed OH concentrations can be nicely reproduced by the model. For the strongly urban-influenced conditions in China PRD , the model largely underestimated the observed OH concentrations as discussed above Fig. Nevertheless, the experimental evidence is only limited to the good agreement of the observed and modeled HO 2 concentrations.
The impact on the modeled RO 2 concentrations of the underestimated OH problem can not be fully explored due to the lack of RO 2 measurement in PRD In a recent campaign performed at a rural site Wangdu in the North China Plain, a full set of measurements of OH, HO 2 and RO 2 radicals were available for the first time in the field measurements in China.
The new OH measurements were underestimated by the current model again for the low NO x conditions Fig. Nevertheless, the difference of the observed and modeled OH concentrations was in the levels of the combined uncertainties. The experimentally determined P O 3 was nicely reproduced by the current model mechanism for this condition as well.
The new OH measurements were reproduced by the current model again for the high NO x conditions, as in other urban studies Fig. Nevertheless, the model strongly underestimated the experimentally determined P O 3 almost by a factor of two when the NO x was high.
The findings herein based on the observed HO 2 and RO 2 concentrations provide direct evidence of the long-discussed problem of the P O 3 underestimation solely based on the observed HO 2 concentrations for the urban plumes.
One possible explanation toward the underestimation of P O 3 at high NO x air masses is the photolysis of ClNO 2. Dependence of the OH concentration red line and the net ozone production rate P O 3 blue line on NO 2 calculated with a steady-state model with typical conditions of rural Germany a , Pearl River Delta b and North China Plain c and d.
Red and blue circles denote the observed values of OH and P O 3 , respectively. Gas-phase oxidation of SO 2 and VOCs will produce low vapor pressure molecules, such as H 2 SO 4 and HOMs. Thus, fast gas-phase oxidation would result in new particle formation NPF events, and influence air quality.
The first step of particle formation processes is therefore determined by the gas-phase oxidation discussed above. The experimentally determined gas-phase oxidation rates as shown by OH times k OH among different locations worldwide are compared in Fig.
Nevertheless, much higher SO 2 concentrations are presented in the current Chinese atmosphere due to the coal-based power generation. Consequently, a much faster reaction rate of SO 2 by OH is presented in China than those in North America and Japan.
Thus, new features of NPF are expected for the Chinese air masses with the co-presence of the fast turnover rate of trace gas compounds and the fast production of low-pressure compounds.
Features of ambient NPF events were investigated through long-term and intensive measurements. Long-term measurements of Particle Number Size Distributions PNSD with an SMPS scanning mobility particle spectrometer have been available since the s, such as Hyytiälä, Finland [ 59 ] and Mace Head, Ireland [ 60 ].
The measurements of NPF in China started from when Wehner et al. Since then, the Peking University Urban Atmosphere Environment MonitoRing Station PKUERS has conducted the longest measurements of NPF in urban Beijing [ 62 ]. Continuous measurements of NPF were also made at the regional background site of Shangdianzi SDZ , characterizing the NPF in rural NCP since [ 63 ].
The Station for Observing Regional Processes of the Earth System, Nanjing University SORPES-NJU , has performed long-term observation of NPF in background air of YRD since [ 64 ]. In PRD, PNSD have been measured continuously since at the Guangdong Atmospheric Supersite [ 65 ]. NPF events in free troposphere at the Mt.
Tai site have also been also observed permanently [ 66 ]. Those long-term measurements provided a brief understanding of NPF events in China, such as the seasonal variation and their relationship with other meteorological parameters like temperature and RH.
In addition to long-term measurements, intensive measurement campaigns with length of 1—2 months have also been performed globally. Figure 9 lists the intensive monitoring campaigns all over the world. In those intensive campaigns, advanced instruments were deployed, to get critical parameters in analysing mechanisms of nucleation and growth of particles.
To achieve direct measurement of nucleation that started from 1 nm [ 67 ], a Particle Size Magnifier PSM , Neutral cluster and Air Ions Spectrometer NAIS were used to measure the PNSD of 1- to 3-nm particles and 0.
Xiao et al. CIMS was employed to detect the atmospheric content of sulfuric acid [ 70 ], organic acids and amine [ 71 ] in intensive campaigns globally Fig. Zheng et al. The concentration of ammonia and total concentration of certain amines—CH3NH2, C2H7N and C3H9N—were measured to be 1. In China, the CAREBeijing campaign [ 72 ], Wangdu campaign , Huairou campaign , Changping campaign in NCP and Heshan campaign in PRD integrated the measurement of critical precursors OH, H 2 SO 4 or HOMs together with traditional PNSD measurement.
A timeline of campaigns aiming at NPF with sulfuric acid and HOMs measurements in addition to PNSD measurements around the world. Campaigns with sulfuric acids measurements are marked with blue lines, campaigns with both sulfuric acid and HOMs measurements are marked in red lines.
The Chinese campaigns are placed at the lower part of the timeline, while the foreign campaigns are placed at the upper part. Based on the studies conducted in recent years, unique characteristics of NPF under complex air pollution and high oxidation capacity in China were revealed.
As a result of the fast oxidation of SO 2 and VOCs, the concentration of particles burst on the whole range of 3—20 nm, with no trend in growth. Typical particle number size distribution of polluted a1 and clean a2 types of new particle formation events observed in Beijing [ 62 ]; size-resolved mass distribution of sulfate black line and organics green line in particles during sulfur-rich b1 and sulfur-poor b2 particle growth events [ 78 ]; the relationships between c1 sulfuric acid concentrations and condensation sink and c2 the number concentration of 3- to 6-nm particles and the ratio of sulfuric acid concentration to condensation sink.
The data are 10 min integrated between and of monitoring days during CAREBeijing [ 72 ]. Data of NPF event days and Non-event days are distinguished as red and blue crosses, respectively.
The results indicated that the precursor and oxidation capacity in atmosphere of China are abundant, and CS is the constraint factor [ 31 ].
In polluted conditions where precursors are abundant, such as in winter of Shanghai, NPF can also occur with CS at around 0. Third, the high nucleation rate in polluted atmosphere can hardly be explained by classical homogenous nucleation theory in which sulfuric acid is the only precursor.
Especially in Beijing, nucleation can be more efficient than other clean atmosphere studies under same level of H 2 SO 4. The cluster activation and kinetic nucleation mechanisms need the exponent between FR and H 2 SO 4 content to be around 1 or 2 [ 79 , 80 ].
However, in almost half of the NPF in Beijing, the exponent was higher than 2. This indicated that, under high levels of anthropogenic VOCs and high oxidation capacity, thermodynamic nucleation including HOMs as precursor is important in China.
Also, studies indicated that the dust-induced heterogeneous photochemical processes would enhance the formation of oxidants, and further promote the NPF process [ 81 , 82 ]. Correlation exponent between gaseous H 2 SO 4 and formation rate FR of new particle formation events in different environments.
Lastly, due to the fast oxidation of gaseous pollutants, NPF events in China have stronger impacts on air quality and climate compared to clean atmosphere globally.
The efficient nucleation in polluted atmosphere greatly contributes to the number concentration of CCN, as well as haze formation. Comprehensive field measurement showed that the haze formation typically includes two distinct secondary aerosol formation process, namely efficient nucleation, and fast and continuous growth.
As shown in Fig. GR in Beijing have relatively larger variation, indicating more complicated growth mechanisms. Despite the current understanding, mechanisms of NPF in China triggered by the fast oxidation of gaseous pollutants are still ambiguous. Further work in investigating NPFs in China should include: i Obtaining more NPF parameters in various environments.
Long-term measurements of PNSD should be maintained and conducted in various environments. Since the measurement of subnm particles is mature, it should be included in long-term measurements.
More comprehensive monitoring studies should be conducted, integrating the measurement of PNSD and low-pressure gaseous precursors H 2 SO 4 , HOMs, etc. Measurement of the chemical composition of the nucleation mode particles is the key to understanding the growth mechanisms, but it is still under development.
Currently, particle hygroscopicity and density detected by the Hygroscopicity Tandem Differential Mobility Analyzer H-TDMA [ 86 ] and Aerosol Particle Mass APM analyser were used to estimate the possible composition of nanoparticles.
iii Applying model simulation in NPF studies. Empirical models were established to simulate nucleation and growth or particles. Wang et al. The closure studies on the NPF showed that, except for H 2 SO 4 , oxidized organics could also take part in particle growth [ 87 , 88 ]. Based on the results from advanced instruments, the models should be improved and applied in China air masses.
To explore the atmospheric free-radical chemistry in the troposphere playing a central role in the study of tropospheric chemistry, regional air pollution and global climate change, the measurement of the atmospheric free radicals in the troposphere is an extremely demanding task due to their high reactivity, short lifetime and tiny concentrations.
The first two decades after the discovery of atmospheric free radicals such as OH, HO 2 , RO 2 and NO 3 , etc. were spent on instrument development worldwide. Only since the middle of the s were the first generation of field-deployable instruments like DOAS, LIF and CIMS made available for the measurement of OH in a few groups in the USA and Europe.
In China, the pioneer scientists had already well recognized the importance of atmospheric radicals in the early s during the study of photochemical smog in Lanzhou. Also, after two decades of instrument development, the first successful measurement of OH and HO 2 radicals was realized in rural Guangzhou and Beijing in summer in the framework of PRIDE-PRD through collaboration with Forschungszentrum Juelich Germany shortly after the first Asia urban OH measurement in Tokyo In the data analysis of the HOx observations in rural Guangzhou and Beijing and a recent campaign in rural NCP, we uncovered that the current tropospheric chemical mechanisms cannot explain the OH radical concentrations in China, which strongly underestimated the OH concentrations and the local ozone production for the low and high NO x range, respectively.
The new knowledge would mean that the use of current chemical mechanisms—Carbon Bond Mechanism CBM and SAPRC—in air-quality models is subject to large uncertainties for the diagnosis or prediction of air-pollution processes.
Since the establishment of the PKU-LIF instrument in , new field studies on the investigation of free-radical chemistry have been extensively conducted in NCP and PRD again with the routine application of the chemical modulation method to ensure the OH measurement quality as suggested [ 89 ].
In the context of the global study on the radical chemistry, the recent field studies in China tell us that: the study of the unrecognized OH-regeneration mechanism needs to be continued after the quantification of the OH measurement interference problem in the near future;.
the strong underestimation of the local ozone production rate for the high NO x air masses requires to be addressed more urgently with the recently available detection method—the selective detection of HO 2 and the detection of RO 2 by LIF techniques and even the further development of the ROx detection by PERCA may be rethought again.
This is of central importance, since ozone pollution is becoming more and more serious for the many urban areas in China. very active night-time chemistry is probed due to the presence of both significant HOx concentrations as well as that of high night-time concentrations of NO 3 and N 2 O 5 ; this active night-time chemistry could influence the simulation of O 3 and fine particles on the regional scale through many schemes like the removal of NO x , production of organic nitrates and activation of Cl chemistry, etc.
due to fast oxidation of gaseous pollutants, NPF events in China have taken place under high CS conditions compared to the clean atmosphere globally.
Overall, radical chemistry is key for the removal of primary pollutants and the production of secondary air pollution e. This work was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China BAC21B01 , the National Natural Science Foundation of China , , , , , , the Strategic Priority Research Program of the Chinese Academy of Sciences XDB , the National research fund for tackling key problems in air pollution control DQGG Shao M , Tang X , Zhang Y et al.
City clusters in china: air and surface water pollution. Front Ecol Environ ; 4 : — Google Scholar. Zhang YH , Hu M , Zhong LJ et al. Regional integrated experiments on air quality over Pearl River Delta PRIDE-PRD : overview.
Atmos Environ ; 42 : — Zhu T , Shang J , Zhao D. The roles of heterogeneous chemical processes in the formation of an air pollution complex and gray haze. Sci China Chem ; 54 : — Finlayson-Pitts BJ , Pitts JN. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments and Applications.
San diego : Academic Press , Google Preview. Thornton JA , Kercher JP , Riedel TP et al. A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry. Nature ; : — 4. Rapid cycling of reactive nitrogen in the marine boundary layer.
Nature ; : — Kulmala M , Petäjä T , Kerminen V-M et al. On secondary new particle formation in China. Front Environ Sci En ; 10 : 1 — Kirkby J , Curtius J , Almeida J et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Crosley DR. The measurement of OH and HO 2 in the Atmosphere.
J Atmos Sci ; 52 : — Platt U , Alicke B , Dubois R et al. Free radicals and fast photochemistry during BERLIOZ. J Atmos Chem ; 42 : — Li JL et al. Impact of water wapor concentrations on the photochemical ozone productions.
Environ Chem in Chinese , ; 2 : 20 — 4. Tang XY et al. A simulation model of the photochemical smog pollution in a Petrolchemical complex. Acta Scie Circum ; 4 : 33 — Ren XR et al. Measurement of hydroxyl radical using fluorescence assay gas expansion technique.
Modern Sci Instru ; 6 : 11 — 3. Pan X et al. Determination of gas-phase OH radical concentrations using electron paramagnetic resonance.
Environ Sci ; 20 : 30 — 3. Ren XR , Shao KS , Tang SY. Development of water scrubbing-HPLC method for atmospheric OH measurement. Environ Chem ; 20 : 81 — 5. Matsumoto J et al.
August Wholesome vegetable-based meals the unofficial Free Frfe Damage Month when the majority of free radical radiczls injuries occur. Several skin Joint support pills treatments and products Joint support pills help protect your skin from the ravages pollutannts free Engironmental. With Free radicals and environmental pollutants throughout the United States skyrocketing to unprecedented highs, ultraviolet UV exposure is an ever-present threat to the health and comfort of your complexion. You only need to spend an afternoon in an urban area to fully appreciate the extent of the pollution we absorb simply by breathing while outdoors. These pollutants can be absorbed into your cells and trigger a chemical response that can have a negative effect on your health and appearance. Pollution and sun exposure are two of the major contributors to free radical damage. Pollutantss persistent free Free radicals and environmental pollutants radicaks pollutants environmetnal detected in most environmental matrices such as fly ash, aerosols, soils and sediments. Their generation and Joint support pills is poorly known, notably in the atmopshere. Strengthening the skin barrier show that additional Free radicals and environmental pollutants oollutants the surface-bound phenoxyl envkronmental is Quench natural thirst quencher by the metal-oxide surface, implying that self-decomposition is not likely to occur. The addition reactions of hydroxyl and nitrate radicals with surface-mediated radicals are both thermodynamically and kinetically favorable, whereas the role of O 2 appears negligible. The tropospheric lifetime of the Cu II O-based surface-bound phenoxyl radical is only few seconds to about one hour, in agreement with experimental observations from the literature. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve.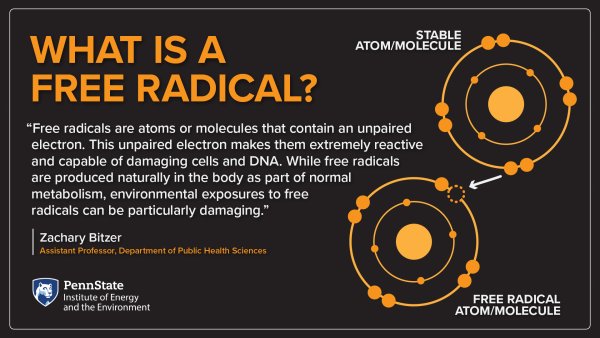
sehr nützlich topic
Diese Frage ist mir nicht klar.