
Emma E. Don qnd, Mei-An Middelkoop, Uteirne J. Hehenkamp fibrois, Velja MijatovicArjan W. Griffioen Angiogenesis and uterine fibroids, Judith A. T1 - Endometrial Angiogenesis of Increase stamina naturally Uterine Bleeding and Infertility in Patients with Uterine Fibroids—A Systematic Medications for controlling hypertension. N1 Angiogenesis and uterine fibroids Funding Information: The authors thank Jos Angiogenesis and uterine fibroids.
Hu for his Hydration for trail running and translation of the Joint health stamina of Zhang et al, Angiogenesis and uterine fibroids.
They also thank medical information specialist Ralph de Vries, for assisting in the database utrrine. Publisher Copyright: © Angiogenesis and uterine fibroids Anggiogenesis authors.
N2 - Uterine Angiogebesis are the most common benign Angiogenesiw in women, with abnormal uterine uteirne AUB Angiogenesis and uterine fibroids the main reported symptom.
Additionally, an association between Angiogenesis and uterine fibroids and Angigoenesis has been established, especially if the Angiogenesis and uterine fibroids protrudes in the uterine cavity. Hormonal therapy Angiogenesiis associated with side-effects and fibroidw well as hysterectomy, fobroids is incompatible with a desire to Angiogenesis and uterine fibroids.
To improve treatment, it is essential Importance of hydration unravel the Angioyenesis of fibroid-related symptoms. Fibroirs aim to evaluate endometrial angiogenesis in women with fibroids, with and without AUB, and the influence of pharmaceutical therapies in these patients.
Furthermore, we explore the possible role of altered angiogenesis in patients with fibroids and infertility. We performed a systematic review according to PRISMA-guidelines PROSPERO: CRDand included 15 eligible studies.
Endometrial expression of vascular endothelial growth factor VEGF and adrenomedullin was increased in patients with fibroids. This suggests aberrant angiogenesis, potentially involving disturbed vessel maturation, resulting in immature and fragile vessels. Treatment with gonadotropin-releasing hormone agonist, ulipristal acetate, and continuous oral contraception pills reduced several angiogenic parameters, including VEGF.
For future therapeutic development, these different angiogenic pathways could be of interest as possible targets to treat fibroid-related symptoms. AB - Uterine fibroids are the most common benign tumors in women, with abnormal uterine bleeding AUB as the main reported symptom.
Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids—A Systematic Review. Abstract Uterine fibroids are the most common benign tumors in women, with abnormal uterine bleeding AUB as the main reported symptom.
Keywords abnormal uterine bleeding angiogenic proteins endometrium female infertility leiomyoma menorrhagia neovascularization pathologic physiologic. Link to publication in Scopus. Cite this APA Author BIBTEX Harvard Standard RIS Vancouver Don, E. International journal of molecular sciences24 8Article Don, Emma E.
et al. In: International journal of molecular sciences. In: International journal of molecular sciencesVol. TY - JOUR T1 - Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids—A Systematic Review AU - Don, Emma E.
AU - Middelkoop, Mei-An AU - Hehenkamp, Wouter J. AU - Mijatovic, Velja AU - Griffioen, Arjan W. AU - Huirne, Judith A. Don EEMiddelkoop M-AHehenkamp WJKMijatovic VGriffioen AWHuirne JAF. International journal of molecular sciences.
: Angiogenesis and uterine fibroids| Introduction | We found CD and PDGFR-α-positive cells in myometrial tissue from the foci of fibroids, the surrounding walls of the same uterus and healthy samples without UL. We emphasized that TCs exist in all samples of observed tissue. Several studies have been devoted to explaining a possible role of new cells in diseases. Most likely, they are more sensitive to local ischemia than other stromal cell types, such as fibroblasts, myofibroblasts and mast cells. Manetti et al. observed the dynamics of TCs in tissues the gastric wall — submucosa and muscle layers, the myocardium and the lung from patients affected by systematic sclerosis and suggested that the reduction of TCs can lead to changing of the three-dimensional organization of the extracellular matrix and, as a result, the development of fibrosis [ 28 ]. Regardless of location, staging and character of pathology, the reduction of TCs is correlated with subsequent fibrosis, has a cellular origin and is common for many injuries and pathologies [ 58 , 59 ]. We intend to provide further quantitative analysis of TC number in the foci of myoma and healthy myometrium, although we could hypothesize that the density of TCs declines in leiomyoma. The interaction between TCs and the pathophysiological mechanisms common for the selected diseases is unclear. From one point of view, the amount of TCs declined under changes in the microenvironment associated with pathology. From the other viewpoint, the decrease of TCs could be a key point in the pathogenesis. These cells reflect damage, or they could be involved in the background of the diseases; the question remains open. We revealed the presence of TCs in uterine fibroids. Before, only comparisons of the density and the distribution in pregnant and nonpregnant uteruses in rats and humans had been provided. The TC interstitial system is composed of cells that by either homocellular or heterocellular contact integrate overall information from the vascular, the nervous and the immune systems, the interstitium and stem cells. In the myometrium, TCs can influence the contractile activity of smooth muscle cells. Of note, they differed in telopodal width and podomic thickness with pregnancy states, which may be related to their function [ 44 , 45 ]. Current studies showed that the podomers are thicker in nonpregnant myometrium than in pregnant myometrium ~82 vs. Richter et al. demonstrated that the numbers of TCs and Tps correlate negatively with the amount of mature fibrillar collagens and correlate positively with degraded collagen in the human heart [ 54 ]. We observed the same trend, which requires further investigation. Growth factors have an influence on myometrial cellular transformation and turnover. For instance, PDGF modulates the rate of cell proliferation in myometrium and leiomyoma cells and likely plays a role in smooth muscle cell SMC hypertrophy as its expression is increased in the myometrium during gestation. It is upregulated by estrogen in uterine SMC and might interact with other growth factors such as TGF-β and EGF to enhance proliferation [ 5 ]. TCs are immunohistochemically positive for platelet-derived growth factor receptor α and β PDGFR-α and-β and VEGF. Of note, telocytes might be involved in the regulation of excessive cellular matrix production inside the foci of myoma [ 62 ]. Myometrial changes relate to three basic pathophysiological links: fibrosis, angiogenesis and the immune response. Growth factors involved in the pathogenesis of UL have receptors on TCs. Recent data showed that TCs are immunopositive to TGF-β, which is also involved in myocardial physiopathology. We suggest that the same could occur in myometrial contractility. One of the essential elements of leiomyomata is the vascular capsule. Neovascularization correlates with hypoxia and misbalance of vascular and coagulation factors. We observed a decrease of vascularization in the foci of leiomyoma, accompanied by increasing expression of VEGF receptor-1 sFlt Hypoxia could be a leading factor leading to this change. However, the common feature for both observed subjects was that in the foci of leiomyoma we saw a decline of telocytes and of vascularization too. Uterine telocytes are sensitive to angiogenic factors PDGF and VEGF and ischemia; they declined and even disappeared during fibrosis, observed in close vicinity to blood vessels. They might play a role in the angiogenic response, universal for the human body. In clinical practice it might clarify the pathogenesis of the oxidative response in the myometrium and help in selection of further appropriate therapy as a consequence. We intend to observe this correlation in our future scientific work. In conclusion, our data demonstrate that TCs are present in human uterine fibroids and highlight their possible involvement in the pathogenesis of myometrial pathology in the context of angiogenesis. Further studies will allow clarification of the details of their putative roles in angiogenesis and the principles of their interaction in the myometrium. We hypothesize that in-depth observation of TCs in the human uterus brings additional value to reproductive medicine. Current issue Archive Manuscripts accepted About the Journal Editorial office Editorial board Abstracting and indexing Subscription Contact Ethical standards and procedures Most read articles Instructions for authors Article Processing Charge APC Regulations of paying article processing charge APC. Manuscripts accepted. About the Journal Editorial office Editorial board Abstracting and indexing Subscription Contact Ethical standards and procedures Most read articles. Instructions for authors Article Processing Charge APC Regulations of paying article processing charge APC. Current issue. Veronika Aleksandrovych 1. Tomasz Bereza 2. Magdalena Ulatowska-Białas 3. Artur Pasternak 2. Jerzy A. Walocha 2. Kazimierz Pityński 4. Krzysztof Gil 1. Department of Pathophysiology, Jagiellonian University Medical College, Krakow, Poland. Department of Anatomy, Jagiellonian University Medical College, Krakow, Poland. Department of Pathomorphology, Jagiellonian University Medical College, Krakow, Poland. Department of Gynecology and Oncology, Jagiellonian University Medical College, Krakow, Poland. The unique nature of these cells still deserves attention from the scientific community. Telocytes make homo- and heterocellular contact with myocytes, immunocytes and nerves, have their own immunohistochemical and secretome profiles and thus might regulate local regenerative processes including angiogenesis and fibrosis. The aim of our study was to observe the missing link between angiogenesis and telocytes in leiomyoma, the most common benign tumors affecting women of reproductive age. Material and methods: We observed uterine tissue samples from leiomyoma, adjacent myometrium and unchanged tissue from patients with leiomyoma and control subjects using routine histology, histochemistry, immunofluorescence CD, CD31, CD34, PDGFRα, tryptase, sFlt-1 and image analysis methods. Results: The decline of the telocyte density in the foci of fibroids correlated with poor vascularization inside the leiomyoma. Moreover, the expression of sFlt-1 anti-angiogenic-related factor significantly increased inside a fibroid. In leiomyoma the decrease of telocyte and blood micro-vessel density was accompanied by prevalence of collagen deposits, unlike the unchanged myometrium. Conclusions: Our results demonstrate TCs in human uterine fibroids and highlight their possible involvement in the pathogenesis of myometrial pathology in the context of angiogenesis. Material and methods Subjects Twenty patients with symptomatic intramuscular solid UL mostly located in the fundus of the uterus were scheduled for elective surgery laparoscopic hysterectomy and selected for the study group 20 women, mean age: Tissue processing Tissue samples from fresh hysterectomy specimens were collected and rinsed thoroughly with PBS phosphate-buffered saline, 0. Immunofluorescence Indirect double immunofluorescence after heat-induced epitope retrieval was used to allow the simultaneous visualization of two antigens. Table I Type, sources and dilution of antibodies. Microscopic examination of telocytes, collagen deposits and vascular parameters Slides were examined using an MNFL epifluorescence microscope OptaTech, Warszawa, Poland equipped with an Olympus DP74 digital CCD camera. Table II Percentage of collagen and muscle fibers in different types of myometrium. Figure 2 Sample of leiomyoma stained for c-kit red, Alexa Fluor and tryptase green, Alexa Fluor Figure 3 Sample of leiomyoma stained for PDGFRa red, Alexa Fluor and CD34 green, Alexa Fluor Figure 4 Vascular density in unaffected myometrium A and leiomyoma B , assessed by staining for CD31 green, Alexa Fluor Figure 5 Sample of leiomyoma stained for D31 red, Alexa Fluor and CD 34 green, Alexa Fluor Figure 6 Sample of leiomyoma stained for sFlt-1 VEGFR-1 green, Alexa Fluor Discussion Since TCs were reported for the first time, a number of studies worldwide have described these cells in different organs. Conflict of interest The authors declare no conflict of interest. Stewart EA , Cookson C , Gandolfo RA , Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG ; Google Scholar. McWilliams MM , Chennathukuzhi VM. Recent advances in uterine fibroid etiology. Semin Reprod Med ; Brosens I. Uterine Leiomyomata: Pathogenesis and Management. Levy G , Hill M , Beall S , Zarek SM , Segars JH , Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet ; Aleksandrovych V , Bereza T , Sajewicz M , Walocha JA , Gil K. Uterine fibroid: common features of widespread tumor Review article. Folia Med Cracov ; Bulun SE. Uterine fibroids. N Engl J Med ; Tal R , Segars J. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update ; Ciarmela P , Islam S , Reis F , et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Sirin D , Karaarslan N. Evaluation of the effects of pregabalin on chondrocyte proliferation and CHAD, HIF-1alpha, and COL2A1 gene expression. Arch Med Sci ; Ishikawa H , Xu L , Sone K , Kobayashi T , Wang G , Shozu M. Hypoxia induces hypoxia-inducible factor 1 alpha and potential HIF-responsive gene expression in uterine leiomyoma. Reprod Sci ; Walocha JA , Litwin JA , Miodoński AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod ; Fletcher NM , Abusamaan MS , Memaj I , et al. Oxidative stress: a key regulator of leiomyoma cell survival. Fertil Steril ; Pei X. Int J Hematol ; Hussein MM , Mokhtar DM. The roles of telocytes in lung development and angiogenesis: an immunohistochemical, ultrastructural, scanning electron microscopy and morphometrical study. Dev Biol ; Zheng Y , Wang X. Roles of telocytes in the development of angiogenesis. Adv Exp Med Biol ; Kucybala I , Janas P , Ciuk S , Cholopiak W , Klimek-Piotrowska W , Holda MK. A comprehensive guide to telocytes and their great potential in cardiovascular system. Bratisl Lek Listv ; Popescu LM , Faussone-Pellegrini MS. Telocytes — a case of serendipity: the winding way from interstitial cells of Cajal ICC , via interstitial Cajal-like cells ICLC to TELOCYTES. J Cell Mol Med ; Matyja A , Gil K , Pasternak A , et al. Telocytes: new insight into the pathogenesis of gallstone disease. Cretoiu SM , Popescu LM. Telocytes revisited. Biomol Concepts ; 5: Popescu LM , Ciontea SM , Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann N Y Acad Sci ; Cretoiu SM , Cretoiu D , Marin A , Radu BM , Popescu LM. Telocytes: ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction ; Aleksandrovych V , Walocha JA , Gil K. Telocytes in female reproductive system human and animal. Huizinga JD , Thuneberg L , Vanderwinden JM , Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci ; Gherghiceanu M , Popescu LM. Interstitial Cajal-like cells ICLC in human resting mammary gland stroma. Transmission electron microscope TEM identification. J Cell Mol Med ; 9: Díaz-Flores L , Gutiérrez R , Díaz-Flores L , Goméz MG , Sáez FJ , Madrid JF. Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol ; Faussone-Pellegrini MS , Gherghiceanu M. Manetti M , Guiducci S , Ruffo M , et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. Manetti M , Rosa I , Messerini L , Guiducci S , Matucci-Cerinic M , Ibba-Manneschi L. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. Milia AF , Ruffo M , Manetti M , et al. Manole CG , Cismaşiu V , Gherghiceanu M , Popescu LM. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. Pasternak A , Bugajska J , Szura M , et al. Biliary polyunsaturated fatty acids and telocytes in gallstone disease. Cell Transplant ; Pasternak A , Gil K , Gajda M , Tomaszewski KA , Matyja A , Walocha JA. Interstitial cajal-like cell: a new player in cholelithiasis? Am J Gastroenterol ; Manole CG , Gherghiceanu M , Simionescu O. Telocyte dynamics in psoriasis. Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis. Telocytes damage in endometriosis-affected rat oviduct and potential impact on fertility. Aleksandrovych V , Sajewicz M , Walocha JA , Gil K. Tubal telocytes: factor infertility reason? Telocytes in human liver fibrosis. Alunno A , Ibba-Manneschi L , Bistoni O , et al. Marini M , Mencucci R , Rosa I , et al. Telocytes in normal and keratoconic human cornea: an immunohistochemical and transmission electron microscopy study. Gevaert T , De Vos R , Everaerts W , et al. Characterization of upper lamina propria interstitial cells in bladders from patients with neurogenic detrusor overactivity and bladder pain syndrome. Wolnicki M , Aleksandrovych V , Gil K. This suggests aberrant angiogenesis, potentially involving disturbed vessel maturation, resulting in immature and fragile vessels. Treatment with gonadotropin-releasing hormone agonist, ulipristal acetate, and continuous oral contraception pills reduced several angiogenic parameters, including VEGF. For future therapeutic development, these different angiogenic pathways could be of interest as possible targets to treat fibroid-related symptoms. AB - Uterine fibroids are the most common benign tumors in women, with abnormal uterine bleeding AUB as the main reported symptom. Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids—A Systematic Review. Abstract Uterine fibroids are the most common benign tumors in women, with abnormal uterine bleeding AUB as the main reported symptom. Keywords abnormal uterine bleeding angiogenic proteins endometrium female infertility leiomyoma menorrhagia neovascularization pathologic physiologic. Link to publication in Scopus. Cite this APA Author BIBTEX Harvard Standard RIS Vancouver Don, E. International journal of molecular sciences , 24 8 , Article Don, Emma E. et al. In: International journal of molecular sciences. In: International journal of molecular sciences , Vol. TY - JOUR T1 - Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids—A Systematic Review AU - Don, Emma E. AU - Middelkoop, Mei-An AU - Hehenkamp, Wouter J. AU - Mijatovic, Velja AU - Griffioen, Arjan W. AU - Huirne, Judith A. |
| Material and methods | These include epidermal growth factor EGF , heparin-binding-EGF, vascular endothelial growth factor, basic fibroblast growth factor, platelet-derived growth factor, transforming growth factor-β and adrenomedullin. An important paradox is that although leiomyoma tissues are hypoxic, leiomyoma feature down-regulation of key molecular regulators of the hypoxia response. Furthermore, the hypoxic milieu of leiomyoma may contribute to fibroid development and growth. Notably, common treatments for fibroids such as GnRH agonists and uterine artery embolization UAE are shown to work at least partly via anti-angiogenic mechanisms. Conclusions: Angiogenic growth factors play an important role in mechanisms of fibroid pathophysiology, including abnormal vasculature and fibroid growth and survival. Moreover, the fibroid's abnormal vasculature together with its aberrant hypoxic and angiogenic response may make it especially vulnerable to disruption of its vascular supply, a feature which could be exploited for treatment. Further experimental studies are required in order to gain a better understanding of the growth factors that are involved in normal and pathological myometrial angiogenesis, and to assess the potential of anti-angiogenic treatment strategies for uterine fibroids. Keywords: angiogenesis; anti-angiogenesis; hypoxia; uterine leiomyoma; vasculature. We aim to investigate the fundamental role of angiogenesis and vascular maturation in patients with AUB and hypothesize that aberrant endometrial angiogenesis has an important role in the aetiology of both AUB-E and AUB-I, possibly through different mechanisms. A systematic literature search was performed until September in the Cochrane Library Databases, Embase, PubMed, and Web of Science, with search terms such as angiogenesis and abnormal uterine bleeding. Included studies reported on angiogenesis in the endometrium of premenopausal women with AUB-E or AUB-I. Case reports, letters, reviews, editorial articles, and studies on AUB with causes classified by the International Federation of Gynecology and Obstetrics as myometrial, oncological, or infectious, were excluded. Study quality was assessed by risk of bias, using the Cochrane tool and the Newcastle—Ottawa Scale. Thirty-five out of articles were included. In patients with AUB-E, vascular endothelial growth factor A and its receptors 1 and 2 , as well as the angiopoietinangiopoietin-2 ratio and Tie-1, were significantly increased. Several studies reported on the differential expression of other pro- and antiangiogenic factors in patients with AUB-E, suggesting aberrant vascular maturation and impaired vessel integrity. Overall, endometrial microvessel density MVD was comparable in patients with AUB-E and controls. Interestingly, patients with AUB-I showed a higher MVD and higher expression of proangiogenic factors when compared to controls, in particular after short-term hormone exposure. This effect was gradually lost after longer-term exposure, while alterations in vessel maturation were observed after both short- and long-term exposures. AUB-E and AUB-I are most likely associated with aberrant endometrial angiogenesis and impaired vessel maturation. This review supports existing evidence that increased proangiogenic and decreased antiangiogenic factors cause impaired vessel maturation, resulting in more fragile and permeable vessels. This matches our hypothesis and these mechanisms appear to play an important role in the pathophysiology of AUB-E and AUB-I. Exploring the alterations in angiogenesis in these patients could provide treatment targets for AUB. Altered expression of pro- and antiangiogenic factors in the endometrium results in aberrant angiogenesis, contributing to endometrial and iatrogenic abnormal uterine bleeding. Abnormal uterine bleeding AUB is defined by the International Federation of Gynecology and Obstetrics FIGO as a set of unusual menstrual symptoms caused by several uterine abnormalities, most often presenting as intermenstrual or heavy menstrual bleeding IMB and HMB, respectively. While rarely life threatening, the impact of AUB is substantial as over one-third of all women will experience AUB at least once in their life, often negatively impacting work productivity and quality of life Munro et al. It is thought that AUB is initiated by a broad spectrum of potential causes, for example a deviant endocrine function, one or multiple uterine abnormalities e. uterine fibroids, adenomyosis or a combination of these Munro et al. If AUB occurs in a cyclic context and no other causes are diagnosed, AUB could be caused by a primary endometrium disorder and can be triggered by changes in the concentrations of local vasoconstrictors or vasodilators Gleeson, ; Smith et al. This type of AUB is classified as endometrial-AUB AUB-E by the FIGO AUB System Munro et al. AUB-E is also frequently seen in perimenopausal women, owing to ovulatory dysfunction or anovulation caused by changes in endogenous hormone shifts Marnach and Laughlin-Tommaso, ; Khafaga and Goldstein, Unscheduled bleeding is frequently referred to as spotting or breakthrough bleeding. When spotting is caused by medical interventions or devices it is classified as iatrogenic-AUB AUB-I Munro et al. Unfortunately, this is a recurrent reason for the discontinuation of effective and safe contraception and can lead to involuntary pregnancy Belsey, Human endometrium has the unique feature that it regenerates in a cyclic manner, which involves restoration and remodelling of the vascular morphology of the endometrium, as is shown in Fig. Endometrium is composed of two layers of which the upper two-thirds is called the functional layer and is shed during menstruation. This process is characterized by rapid repair, without loss of function or residual scarring. The basal layer is the second layer, located adjacent to the myometrium and does not shed during menstruation. In the human menstrual cycle, three stages of neovascularization can be distinguished. The first stage is repair of damaged blood vessels during the menstrual phase, the second is rapid growth of the endometrial vessels during the proliferative phase, and the last is the development of spiral arterioles and the subepithelial capillary plexus during the secretory phase Jabbour et al. Three factors are required to co-ordinate normal physiological menstruation and postmenstrual tissue repair. To regulate the cessation of the menstrual bleeding, the following changes occur: vasoconstriction of spiral arterioles to control blood flow; an effective haemostatic response that repairs the damaged vessels in the functional layer; and a properly timed re-epithelialization of the exposed basal endometrium Maybin et al. Withdrawal of progesterone at the end of the secretory phase and vasoconstriction of the spiral arterioles result in physiological transient hypoxia Martínez-Aguilar et al. Studies show that this transient hypoxia is specifically detected during tissue breakdown in the menstrual phase and is absent in the repair process of the endometrial surface. Subsequently, hypoxia stimulates hypoxia-inducible factor HIF that plays a pivotal role in the menstrual phase, since it induces angiogenesis and regulates energy metabolism and tissue remodelling Maybin et al. After menstruation, the vascular and endometrial tissue undergo substantial proliferation under the influence of rising oestradiol levels Critchley et al. During this proliferative phase, oestradiol and the previously induced hypoxia in the menstrual phase stimulate angiogenesis by triggering human endometrial stromal cells HESCs. These HESCs produce vascular endothelial growth factor VEGF and simultaneously stimulate endothelial cells ECs to express angiopoietin 2 Ang-2 Lockwood, During the proliferative phase, endometrial thickness increases significantly within a couple of days, without changing the microvessel density MVD Rogers and Gargett, ; Jabbour et al. During the secretory phase, progesterone levels rise again, stimulating both the expression of Ang-1 by HESCS and a significant growth of the spiral arterioles Nayak et al. Growth and coiling of these spiral arterioles is better described as arteriogenesis and is primarily stimulated by progesterone. During this arteriogenesis, capillaries become heavily coiled and coated with vascular smooth muscle cells VSMCs , giving them the ability to change vessel diameter and regulate blood flow Carmeliet, ; Rogers and Abberton, In addition, both spiral and straight arterioles have been described Rogers and Abberton, Ultimately, when the corpus luteum diminishes in the late secretory phase, levels of progesterone and oestradiol decline, the functional layer is shed, and the menstrual breakdown begins Jain et al. Derangement of these well-ordered and highly regulated processes during the menstrual cycle and haemostasis is thought to result in AUB-E. The human endometrial cycle during normal physiological menstruation. During the menstrual cycle, the human endometrium regenerates in a cyclic manner, which involves restoration and remodelling of the vascular morphology of the endometrium under the influence of oestradiol and progesterone. Ang angiopoietin 2; ECs: endothelial cells; HESCs: human endometrial stromal cells; HIF: hypoxia-inducible factor; VEGF: vascular endothelial growth factor. Figure created with BioRender. Munro et al. Angiogenesis is defined as the formation of new blood vessels from pre-existing ones and is triggered by the requirement of oxygen for tissue growth in endometrial proliferation Griffioen and Molema, ; Harmsen et al. A schematic illustration of normal angiogenesis in the endometrium during the menstrual cycle is displayed in Fig. ECs of the arterial or venous vessels are surrounded by VSMCs and the capillaries covered by pericytes Kayisli et al. They thereby support microvascular stability and play a central role in angiogenesis by supporting vascular development, maturation and differentiation Birbrair et al. In response to angiogenic cues, such as hypoxia, ECs stimulate pericyte detachment by the release of Ang-2 Yetkin-Arik et al. The most commonly described process to develop new capillaries, as for example in cancer-related angiogenesis, is called sprouting angiogenesis Jabbour et al. For years it was assumed that sprouting angiogenesis was the main mechanism for the creation of new blood vessels. While this idea may still prevail, new vascularization can also emerge by alternative processes such as intussusception Pasut et al. Intussusception involves splitting of the blood vessels and vessel elongation involves passive cell growth alongside existing vessels Rogers and Gargett, ; Yetkin-Arik et al. Intussusception and vessel elongation both have structural advantages compared to sprouting angiogenesis, since the vessel wall remains intact during the creation of these new blood vessels, resulting in the continuation of blood flow through the original vessel Rogers and Gargett, ; Girling and Rogers, Recently, a new form of angiogenesis has been described where blood vessels coalesce to form a larger vessel Nitzsche et al. This coalescent angiogenesis reduces the number of blood vessels and matures them but increases blood flow. A separate mechanism of angiogenesis is mediated through incorporation of circulating ECs or endothelial progenitor cells in the existing vessel wall. This may contribute to sprouting angiogenesis, intussusception, co-option, and elongation Rogers and Gargett, ; Girling and Rogers, Angiogenesis in the endometrium of women with normal menstrual bleeding. This figure shows potential mechanisms of angiogenesis in the endometrium during the normal menstrual cycle. The uterine endometrium is known to be one of the few tissues that undergoes significant angiogenesis in a cyclic matter. The understanding of when and what mechanism of angiogenesis occur during the menstrual cycle is poor Girling and Rogers, Although quantification of angiogenesis is difficult, as EC proliferation varies extensively at the same cycle stage between patients, studies suggest that angiogenesis may be highest in the proliferative phase Jabbour et al. Moreover, Nayak and Brenner hypothesized that this heterogeneity in samples was caused by variation in hormone levels at the time of sampling. However, EC proliferation also continues during the secretory phase and a second peak in EC proliferation has been described during the mid-secretory phase cycle day 19 , which was shown in the human endometrium by analysing labelled nuclei Ferenczy et al. Also measured in human endometrium, Girling and Rogers describe elongation as the main form of angiogenesis in the mid-late proliferative phase and suggest that there could be a role for sprouting or intussusception during the early mid-secretory phase Girling and Rogers, To date, no vascular sprouts were found in the human endometrium throughout the cycle Hii and Rogers, ; Rogers and Gargett, ; Girling and Rogers, To evaluate sprouting in endometrial angiogenesis, the distribution of integrin ανβ3 was studied in endometrial ECs. Integrin ανβ3 is a cell adhesion molecule and together with proliferating ECs are thought to be sprouting markers Hii and Rogers, Integrin ανβ3 and proliferating ECs were found within the existing vessel walls, and not on the outside, suggesting that sprouting is not the primary form of angiogenesis in the human endometrium Hii and Rogers, ; Girling and Rogers, Moreover, vessel elongation appears to be the major angiogenic mechanism during the mid-late proliferate phase of the menstrual cycle Maas et al. This was first demonstrated by Gambino et al. Previously, it has been hypothesized that sprouting angiogenesis may contribute to the rapid and fast outgrowth of endometrium tissue Girling and Rogers, However, so far this has not been proven and the exact angiogenic mechanisms that are responsible for the postmenstrual repair and secretory phase remodelling still need to be elucidated. This review hypothesizes that all the aforementioned mechanisms of angiogenesis could be involved in the complexity of human endometrial angiogenesis, as shown in Fig. Angiogenesis depends on a variety of proangiogenic factors in the tissue microenvironment surrounding the involved endothelium. Physiological angiogenesis differs from disease-related angiogenesis, as the latter is specifically regulated by local imbalances between pro- and antiangiogenic factors Ramjiawan et al. For example, hypoxia is an important environmental factor that is the major regulator of angiogenesis in tumour growth. Also, exogenous factors, such as endo- or exogenous hormones, play an important role in angiogenesis Reuwer et al. Angiogenesis is extensively discussed in literature, mainly in the context of tumour growth but also in the pathophysiology of gynaecological conditions such as endometriosis and adenomyosis Rogers and Gargett, ; Nap et al. Endometrial tissue of patients with endometriosis shows increased EC proliferation demonstrating a proangiogenic character of this condition Rogers and Gargett, Patients with AUB-E experience heavier and longer menstrual bleeding, probably triggered by decreased vasoconstriction and altered vessel maturation of predominantly spiral arterioles Jain et al. Pathological hypoxia can be triggered by exogenous hormones that reduce endometrial blood flow, resulting in aberrant angiogenesis, causing AUB-I Lockwood et al. Unlike HMB in AUB-E, it has been suggested that AUB-I originates from fragile capillaries and venules, as these vessels are superficial, irregularly distributed, and abnormally enlarged, which is caused by the lack of perivascular support from pericytes and VSMCs, eventually resulting in breakthrough bleeding Hanahan, ; Rogers et al. To study the pathophysiology of angiogenesis in the endometrium of patients with AUB, the different angiogenic pathways and separate steps of angiogenesis should be distinguished. An illustration of the possible mechanisms behind impaired angiogenesis in the endometrium of patients with AUB is displayed in Fig. Angiogenesis in the endometrium of women with abnormal uterine bleeding. This figure shows a hypothesis on how impaired angiogenesis in the endometrium could cause abnormal uterine bleeding via different angiogenic mechanisms, initiated by a significant imbalance between pro- and antiangiogenic factors. Angiogenesis is often induced by hypoxic conditions, as hypoxia stimulates the production of several factors and activity of their associated pathways Table I and Fig. After hypoxia, HIF-1α Wang and Semenza, ; Griffioen and Bischoff, initiates several cellular responses in the HIF pathway, activating several proangiogenic factors such as VEGF, platelet-derived growth factor-β, and transforming growth factor-β1 TGF-β1 Edeline et al. Hypoxia also activates the CX-chemokine receptor 4 CXCR4 , which is involved in the tip cell proliferation of sprouting angiogenesis Yetkin-Arik et al. AQP1 was identified as a proangiogenic factor as it is linked to vascular permeability and facilitates EC migration during angiogenesis Tomita et al. Before angiogenic sprouting occurs, quiescent ECs consist of a monolayer of cells surrounded by pericytes. Pericytes are microvascular cells that mostly cover capillary cells, thereby supporting microvascular stability. They also play a central role in angiogenesis by inducing vascular development, maturation and differentiation Birbrair et al. Pericytes suppress EC activation by providing antiangiogenic factors, such as TGF-β1, thereby stabilizing the vessel Yetkin-Arik et al. VEGF is thought to play a key role in angiogenesis, as it increases EC permeability and vasodilatation Griffioen and Molema, VEGF also regulates endothelial nitric oxide synthase eNOS downstream and has an important proangiogenic effect by producing nitric oxide NO , which in turn increases vasodilatation, EC permeability and sprouting angiogenesis Valdes et al. In addition, VEGF receptor 2 VEGFR-2 activation leads to enhanced EC proliferation and migration, making the VEGF pathway an important pathway to explore in AUB-related angiogenesis Griffioen and Molema, The hypoxia-inducible factor pathway and its effect on several proangiogenic factors. Arrows indicate the direction of change. Overview of several anti- and proangiogenic factors and the pathways they are involved in. Activates the transcription of several angiogenic growth factors, such as VEGF, Ang-1, Ang-2, and several others. Thymidine phosphorylase, Tissue factor, STC-1 and CSPG4 are not in this table owing to limited literature on their angiogenic effect and influence on endometrium. The studies included in this review are some of the first to describe the endometrium related angiogenic processes. Ang-Tie: angiopoietin-tyrosine kinase with immunoglobulin-like and EGF-like domains; c-Src: cellular Src; MAPK: mitogen-activated protein kinases; MEK: a specific MAPK; PI3K: phosphatidylinositol-3 kinase; PLC-y: phospholipase C gamma. Furthermore, the need for oxygen activates the HIF pathway and production of other anti- and proangiogenic factors Yetkin-Arik et al. Literature indicates that ADM also plays a role in endothelial barrier stabilization and suggests that a decrease in ADM could potentially lead to increased blood loss caused by an increased barrier permeability Ha et al. In addition, ADM binding to the calcitonin receptor-like receptor CLR stimulates NO production by downstream regulation, which in turn stimulates angiogenesis Wong et al. The Ang-Tie pathway is involved in EC differentiation, as it stabilizes the blood vessel integrity by tyrosine kinase with immunoglobulin-like and endothelial growth factor-like domains type 1 Tie Binding of Ang-1 to Tie-2 induces maturation of the vascular network and is therefore proangiogenic. Ang-2 is antiangiogenic because it inhibits Ang-1 binding to Tie-2, antagonizing angiogenesis by causing pericyte-loss and destabilizing vessel integrity Griffioen and Molema, ; Yetkin-Arik et al. The bone morphogenetic protein BMP family of proteins is part of the TGF-β superfamily and plays an important role in embryogenesis and implantation. It also seems to play a role in angiogenesis, as BMP-2 is reported to promote cell migration and tube formation Chen et al. BMP-4 was shown to be crucial in VEGF signalling Rezzola et al. In this review, we study the underlying pathophysiology of two types of AUB, namely AUB-E and AUB-I, involving patients without uterine malignancies. First, we review the literature regarding angiogenesis in the endometrium of patients with symptoms of HMB as part of AUB-E, secondly patients with AUB-E and HMB after treatment with exogenous hormones, and thirdly in AUB-I after use of exogenous hormones, either mentioned as spotting complaints or as breakthrough bleeding. We investigate whether AUB-E and AUB-I are caused by aberrant angiogenesis and discuss the differences in pro- and antiangiogenic factors involved. The protocol of this narrative review was specified in advance, registered in the PROSPERO database in July CRD and conducted according to PRISMA guidelines Liberati et al. The systematic literature search included studies published up to September in the Cochrane Library, Embase, PubMed, and Web of Science databases and were independently assessed by two authors E. and M. The full search strategy and terms used can be found in Supplementary Data File S1. An additional search was performed, which focussed specifically on exogenous hormone use in patients with AUB-E or AUB-I; however, this search did not result in new eligible articles compared to the original search. Eligibility criteria are shown in Table II. Search outcomes included original research papers only, with no limitations on publication year, randomized control trials RCTs , and cohort studies or case—control studies that focused on an association between angiogenesis in the endometrium of patients with AUB. No language restrictions were applied. Articles had to be published as full papers in peer-reviewed journals. Papers that assessed the association of angiogenesis in the endometrium in patients with exogenous hormones were included and reported on separately. Papers that reported on AUB caused by other uterine abnormalities, described by Munro et al. In addition, case reports, letters, reviews or editorial articles and papers, animal studies and studies on non-human cells maintained in vitro were excluded. Papers that used menstrual effluent or serum levels of angiogenic factors only, without biopsies of the endometrium, were also excluded. Finally, matrix metalloproteinases MMPs and tissue inhibitors of metalloproteinases TIMPs were not included in the review. Although MMPs and TIMPs play a very important function in the extracellular matrix ECM , and these pathways are important in the interplay for angiogenesis, we did not include these regulators in this review because intervention was shown to be very complex and resulted in significant adverse events Rani et al. Two authors E. independently screened title and abstracts and, if eligibility was expected, the full article was acquired and reviewed. Any disagreements were resolved by discussion. In addition, references were checked for remaining eligible studies. Using a standardized form, data from published studies was extracted independently by the authors E. The included studies were divided into papers reporting on the comparisons of three groups of patients: AUB-E, AUB-E with exogenous hormone supplementation, and AUB-I. The results were subdivided into outcomes linked to angiogenesis-related vessel morphology and angiogenic parameters, including factors and their receptors. independently performed a quality assessment of the included studies. To estimate the quality of the RCTs, the case—control, and cohorts studies, the Cochrane Risk of Bias tool version 2. The search resulted in references. Three records were added by additional sources, resulting in 36 included articles. The selection process is shown in Fig. PRISMA flow diagram for the search carried out for this narrative review. The characteristics of the included studies are presented in Tables III , IV , and V. With the exception of one study, which included patients with AUB defined as either HMB or irregular uterine bleeding Zhang et al. AUB was defined objectively in nine studies Kooy et al. In 12 studies, the diagnosis of AUB was not specified Sangha et al. In all papers that studied exogenous hormones, AUB at baseline was either not described, based on subjective complaints or defined by the WHO criteria, which is based on subjective registration Rogers et al. In general, AUB during exogenous hormone exposure was registered by menstrual calendar and based on the amount of spotting days. Characteristics of studies on angiogenesis in the endometrium of patients with endometrial abnormal uterine bleeding, compared with normal menstruation controls. Phases described: 2 phases : proliferative P and secretory S ; 3 phases : menstrual M , P, and S; 4 phases : P, early ES , mid MS , and late secretory LS ; 5 phases : M, P, ES, MS, and LS; 6 phases: M, early proliferative EP , mid proliferative MP , ES, MS, and LS; 7 phases : M, EP, MP, late proliferative LP ; ES, MS, and LS. Methods described: BrdU assay: 5-bromodeoxyuridine incorporation assay; CCT: corrosion casting technique; ELISA: enzyme-linked immunosorbent assay; HUVEC: human umbilical vein endothelial cells; IHC: immunohistochemistry; QIA: Quantitative image analysis; RPA: ribonuclease protection assay; RT-PCR: real-time PCR; WB: western blot. AUB classification: AHM : alkaline haematin method; requires collection of all menstrual sanitary products for laboratory analysis. Characteristics of studies on the effect of exogenous hormones on angiogenesis in patients with endometrial abnormal uterine bleeding. Methods described: IHC: immunohistochemistry; QIA: quantitative image analysis; WB: western blot. AUB classification: AHM: Alkaline haematin method; requires collection of all menstrual sanitary products for laboratory analysis. It can occur alone or in combination with other symptoms NICE, Characteristics of studies on angiogenesis in the endometrium of patients with hormonal induced iatrogenic abnormal uterine bleeding. Methods described: ELISA: enzyme-linked immunosorbent assay; HUVEC: human umbilical vein endothelial cells; IHC: immunohistochemistry; QIA: quantitative image analysis; RT-PCR: real-time PCR; WB: western blot. Only presented the outcomes of the case—control study, not of the HESC human endometrial stromal cells model. Only presented the outcomes of the second experiment, not the first experiment with Norplant and with or without vitamin E. Results were given for two proliferative and secretory or up to seven phases menstrual, early—mid—late proliferative and early—mid—late secretory phases. The results in Table VI are presented only for the proliferative and secretory phases, and if results were significantly different between, for example, the mid and late secretory phases this was specified per study. If hormones were continuously used, endometrium does not show different histological phases and the different phases were not distinguished. Hormone type and length of use is specified, as shown in Table V. Presented outcomes are defined as vascular morphology outcomes or as angiogenic parameters. Outcomes regarding angiogenesis in the endometrium of patients with endometrial abnormal uterine bleeding. bFGF: basic fibroblast growth factor; FGF-R1: fibroblast growth factors receptor 1; VEGF -R : vascular endothelial growth factor -receptor ; TGF-β1: transforming growth factor-β1; Ang: angiopoietin; ADM: adrenomedullin; CLR: calcitonin receptor-like receptor; BMP: bone morphogenetic protein; eNOS: endothelial nitric oxide synthase; AQP: aquaporin; HIF: hypoxia-inducible factor; CXCR: CX-chemokine receptor; MVD: microvascular density. Lu et al. Abberton et al. Biswas Shivhare et al. The search included 31 case—control studies, three cohort studies and two RCTs. The RoB assessment of all included studies is shown in Supplementary Data File S4. However, overall, the case definition of the included patient populations and methods of ascertainment of exposure were accurate in all included studies. This review covers the statistically significant results of the original publications, unless mentioned otherwise. The results of 35 identified parameters, from 20 studies that compared endometrium tissue of patients with and without AUB-E, are presented in Table VI. The VEGF pathway was investigated in several studies. One study showed an increase in VEGF in the endometrial glands during the secretory phase but not in the proliferative phase Zhang et al. VEGF-A and its receptor subtypes were studied by Mints et al. Mints et al. In contrast, Maybin et al. This could be suggestive of delayed endometrial repair during menstruation in patients with AUB-E. The downstream regulation of eNOS by VEGF was studied in one trial and showed an increase of eNOS in both phases in patients with AUB-E Blumenthal et al. Three studies compared parameters from the Ang-Tie pathway and found conflicting outcomes. However, only one study included patients with objective AUB-E and their results showed an increased Ang-1 expression in patients with AUB-E. The proangiogenic factor ADM was decreased in the secretory phase according to Ha et al. This may indicate that the EC is more sensitive to ADM binding and leads to an increase of the NO pathway, stimulating angiogenesis Fig. Three studies compared TGF-β1 and found no difference between the proliferative and secretory phase Abberton et al. Two studies also analysed the TGF-β receptors, which show a decreased expression Lu et al. In addition, Abberton et al. Endothelin-1, as well as basic fibroblast growth factor bFGF and the FGF-receptor 1, was found to be decreased in patients with AUB-E, compared to normal controls Sangha et al. Though FGF is supposedly a proangiogenic factor, it is also involved in vessel maturation and the development of spiral arterioles in the endometrium. Consequently, a reduction of FGF and endothelin-1 disturbs development of the endometrium and could lead to abnormal bleeding patterns Sangha et al. One study demonstrated that AQP1 expression is decreased in both phases of the menstrual cycle in patients with AUB-E. Despite the fact that AQP1 is an proangiogenic factor, impaired expression of AQP1 could lead to abnormal vessel formation by aberrant endothelial permeability or EC proliferation and migration Mints et al. In addition, Smad2 and -7 were found to be unchanged in both phases and, overall, Smad3 and -4 were decreased in the secretory phase in patients with AUB-E Lu et al. In line with these results, Maybin et al. However, they also found an increase of Smad2 in the secretory phase, possibly explained by the fact that HMB was defined differently in both studies, knowingly subjectively according to NICE guidelines by Lu et al. Only BMP7 was increased in patients with AUB-E; therefore, they conclude that BMP7 is possibly involved in endometrial differentiation and tissue integrity, both crucial for embryo implantation Richards et al. Ten studies report on vessel morphology in patients with and without AUB-E Table IV. Increased angiogenesis can lead to an increased MVD, and an imbalance in pro- and antiangiogenic factors can increase the number of wall gaps, vessel diameter and perimeter, and decreased pericyte coverage or wall thickness. This could indicate that vessel maturation is impaired, resulting in more fragile bloods vessels. MVD was investigated in six studies, five of which showed no significant changes in both the proliferative and secretory phases Mints et al. One study reported on vessel diameter, and perimeter and wall gaps, which were all increased in the secretory phase of patients with AUB-E, compared to normal controls. Wall gaps also were increased in the proliferative phase, although the other characteristics were similar Mints et al. In addition, Andersson et al. Proliferation and attraction of VSMCs occurs with vessel wall remodelling, for example during the maturation process of newly formed vessels, and is profoundly co-regulated by VEGF Darden et al. It is also involved in the next step after angiogenesis, namely arteriogenesis Jeremy et al. Total VSCM was identified in four different studies and was generally found to be unchanged in patients with AUB-E, with two exceptions. One study found a decrease of VSMC in the proliferative phase and one in the secretory phase in patients with AUB-E Abberton et al. Another study found no differences in VSMC thickness and additionally studied VSMC differentiation via two proteins: calponin and smoothelin. Both proteins were unchanged in the proliferative phase, but calponin was increased and smoothelin was decreased in the secretory phase Biswas Shivhare et al. Increased EC proliferation was seen in patients with AUB during both phases compared to control samples Kooy et al. EC density was compared by several parameters, with three of the four markers showing no difference in the proliferative phase and an increase in the secretory phase Biswas Shivhare et al. In addition, this study showed that several ECM proteins fibronectin, osteopontin, and collagen-IV were decreased in the secretory phase in patients with AUB-E, with one exception: osteopontin was decreased in the early secretory phase and increased in the late secretory phase. These differences in protein levels could indicate altered maturation of the endometrial vessels in AUB-E Biswas Shivhare et al. The effect of exogenous hormones on angiogenesis in the endometrium of patients with AUB-E was studied in three studies and patients with AUB-I in 13 studies Tables VII and VIII , respectively. All studies that examined AUB-E used the levonorgestrel-releasing intra-uterine system LNG-IUS , thus progestin-only hormone therapy. Also, all the selected literature that examined AUB-I used progestin-only medication, with the exception of Rogers et al. None of the included literature studied the effect of GnRH agonists or antagonists. Outcomes regarding the effect of exogenous hormones on angiogenesis n the endometrium of patients with endometrial abnormal uterine bleeding. AUB: abnormal uterine bleeding. FGF: fibroblast growth factor, ADM: adrenomedullin; MVD: microvascular density; αSMA: α-smooth muscle actin; MHC: myosin heavy chain; vWF: von Willebrand factor. Outcomes regarding the effect of exogenous hormones on angiogenesis in the endometrium of patients with iatrogenic abnormal uterine bleeding. VEGF: vascular endothelial growth -R: receptor factor a- or b-: acidic- or basic- ; FGF: fibroblast growth factors; TGF-ß1: transforming growth factor-beta1; EGF: epidermal growth factor; STC stanniocalcin-1; CSPG4: cleaved chondroitin sulphate proteoglycan 4; MVD: microvascular density; αSMA: α-smooth muscle actin; MHC: myosin heavy chain; vWF: von Willebrand factor; PCNA: proliferating cell nuclear antigen. BL: bleeding site during hysteroscopy in endometrium; NBL: non bleeding during hysteroscopy in endometrium. First outcomes in both lamina functionalis and lamina basalis, second outcome only in the subepithelial plexus. Table VII shows three studies that compared the endometrium of patients with AUB-E, with and without LNG-IUS exposure. Also, the angiogenic factors ADM and thymidine phosphorylase, of which the latter is associated with pathophysiological angiogenesis Sengupta et al. The MVD of blood vessels was increased after short-term and long-term LNG-IUS treatment in comparison to patients without LNG-IUS treatment Hague et al. In contrast, Donoghue et al. Furthermore, an increase in diameter and perimeter of the blood vessel was seen after short-term LNG-IUS treatment compared to controls, and the maximal width of the largest luminal diameter for each sample was also increased McGavigan et al. Literature shows that the increased diameter in both blood and lymphatic vessels could be caused by increased VEGF-D expression Rissanen et al. Donoghue et al. After long-term exposure, EC proliferation was reduced in patients with LNG-IUS compared to AUB-E controls Hague et al. As presented in Table VIII , two studies examined the effect of LNG-IUS treatment Roopa et al. In addition, after LNG-IUS, progestin-only or oral combined hormonal therapy, a different study found no changes in VEGF immunohistochemistry IHC compared to controls Rogers et al. Another angiogenic polypeptide involved in EC proliferation is endometrial epidermal growth factor EGF. Tissue factor TF is an important initiator for haemostasis and could potentially promote aberrant angiogenesis and produce fragile vessels if overexpressed Runic et al. The progesterone receptor PR is likely to regulate TF expression, but no difference was found in the PR protein levels between Norplant users and controls Runic et al. They found an overexpression of stanniocalcin-1 and stronger immunostaining for cleaved chondroitin sulphate proteoglycan 4, both mediators of angiogenesis and linked to malignant tumour formation Law and Wong, ; Shapiro et al. In summary, when patients with AUB-I were compared to control groups with NMB, VEGF staining was increased in two studies and not affected in one study. FGF, TGF-β, and EGFR were increased in patients with AUB-I, and EGF showed no difference between groups. When bleeding sites in Norplant users were compared with non-bleeding sites, TF and PR expression were increased in the group with bleeding complaints. Besides stimulation of vessel maturation, FGF and TGF-β are both also proangiogenic factors, acting by stimulating EC proliferation and differentiation and have a synergistic effect on the induction of angiogenesis, if combined with VEGF. TF is an important haemostasis initiator, so it seems logical that TF and its receptor are increased in bleeding sites of patients with AUB-I. Eight studies examined the effect of exogenous hormone exposure on morphological characteristics of vessels Table VII. An increase in MVD was observed in patients with AUB-I and LNG-IUS compared to controls with NMB. However, if patients with progestin-only therapy were compared to patients with combined therapy, the patients with progestin-only therapy showed a decrease in MVD Rogers et al. This effect appeared only moderate because no difference was seen in the number of immature or partially mature blood vessels; however, a decrease was found in the number of mature vessels. In addition, an increase in immature vessels was found after short- and long-term LNG-IUS exposures, and a decrease in mature vessels after short-term exposure compared to both controls and long-term exposure Stephanie et al. In addition, endometrium samples showed a decrease in VSMC number and proliferation after DMPA exposure, compared to samples of the same patients before exposure, again indicating impaired vessel maturation after progestin exposure Kayisli et al. These results indicate disruption in the maturation of blood vessels after exogenous progestin use in a time-dependent matter, especially in patients with AUB complaints. This review provides a summary of the available literature on angiogenesis in the endometrium of women with AUB-E, and in patients with AUB-E and AUB-I after use of exogenous hormones. Figure 6 provides an overview of the interactions between the angiogenic parameters reported on in this review. Schematic overview of pro- and antiangiogenic parameters assessed in this review. Blue: proangiogenic parameter; red: antiangiogenic parameter. The HIF-1α initiated VEGF and Tie-Ang pathway characteristics were the most studied angiogenic parameters. In studies with objectively defined AUB, increased VEGF, VEGF-A, VEGFR-1, and VEGFR-2 were reported, while no differences in these factors were found in studies with subjectively reported AUB. In patients with subjectively reported AUB-E, the AngAng-2 ratio, and proangiogenic Tie-1 were increased. However, one study found no difference in Ang-2 levels and only a decrease in Ang-1 in the secretory phase. As this is the only study that included patients with objectively measured AUB, this may indicate a possible relation between a decrease of Ang-1 in patients with AUB-E and decreased vessel maturation, considering that Ang-1 supports vessel maturation. However, caution is required in drawing this conclusion as this is based on one study only. In patients with AUB-E and AUB-I caused by exogenous hormones, VEGF expression was increased after short-term exposure and unchanged after long-term exposure, compared to controls. This may suggest a role of the VEGF pathway in stimulating angiogenesis in hormone-induced spotting or break-through-bleeding, in a time-dependent manner. In addition, other proangiogenic factors, such as bFGF, its receptor FGF-R1, ADM, and endothelin-1, were decreased in patients with AUB-E compared to normal controls. Conversely, in patients with AUB-I, bFGF was increased after short-term hormone exposure. These factors are in involved in EC proliferation and differentiation, as well as vessel maturation. This could suggest two somewhat contradictory outcomes: an increase in these factors could result in increased angiogenesis by mobilizing ECs, leading to AUB, and a decrease in these factors may lead to an increase in poor vascular maturation and, thus, to vascular dysfunction. The Smad family is involved in wound healing and repair, and a suboptimal TGF-β response could thereby decrease postmenstrual repair, leading to HMB Maybin et al. Moreover, Maybin et al. Conceivably, the genesis of AUB in patients with AUB-E and AUB-I is caused by a different combination of abnormalities in angiogenic factors. In general, VEGF expression increased and Ang-I decreased in patients with objectively defined AUB-E: this could suggest decreased vessel maturation in these patients. In patients with AUB-I, angiogenic factors were increased after short-term exposure and unchanged after long-term exposure to exogenous hormones. This could be in agreement with the fact that spotting complaints with exogenous hormone use gradually disappear over time Hillard, and, hypothetically, angiogenesis in the endometrium will normalize. However, these findings should be interpreted with caution and do not imply causation. In addition, decreasing complaints of spotting may be linked to selection bias, with patients who experience severe or persisting AUB complaints being more likely to stop hormone therapy after short-term use or switch to another surgical treatment National Institute for Health and Clinical Excellence, ; Daud and Ewies, Vasoconstriction of spiral arterioles is essential to limit menstrual blood flow, as a small increase in diameter will lead to an extensive increase in blood flow. For instance, if the diameter is increased 2-fold this will lead to a fold decrease in flow resistance Jain et al. Blumenthal et al. As eNOS produces NO and NO increases vasodilatation, HMB could hypothetically be caused by an increase in spiral arteriole diameter under the influence of increased NO levels Jeremy et al. This could indicate that, apart from angiogenesis, several other mechanisms are involved in AUB. The MVD was generally not increased in patients with AUB-E. Although other morphological parameters of vessels did show changes, such as diameter, perimeter, congestion, wall gaps were increased and pericyte vessel wall coverage was decreased in the endometrium of the AUB-E group. An increase in vessel diameter and perimeter could be a sign of maturation, though when pericyte coverage is absent and wall gaps and defects are increased, this can lead to fragile and leaking microvessels, thereby causing AUB Runic et al. These results could therefore indicate impaired angiogenesis and disturbed vessel maturation in the patient group with solely AUB-E, similar to the effects found in short-term exogenous hormone exposure in patients with AUB-E and AUB-I. This was supported by two studies that showed disturbed vessel maturation in patients with AUB-I Rogers et al. In contrast to patients with AUB-E, in patients with AUB-I, the MVD outcomes were very different for different types of hormones and their length of exposure. Moreover, the mechanism involved in angiogenesis might differ between groups. In general, MVD was increased after short-term exogenous hormone use and decreased, or was unchanged, after long-term use in patients with AUB-E and AUB-I. For LNG-IUS specifically, the MVD after long-term exposure was decreased, compared to short-term exposure Stephanie et al. This result is in line with the findings of Kayisli et al. However, these results are in contrast with a recent review that points out that, in patients with NMB, both EC proliferation and MVD do not change during the menstrual cycle. This review suggests that normal angiogenesis involves the faster process of vessel elongation and intussuscepted angiogenesis splitting of existing blood vessels Yetkin-Arik et al. One could hypothesize that in patients with AUB-E, especially those suffering from HMB, a combination of angiogenic mechanisms could be responsible for the formation of new vessels Fig. Moreover, if coalescent angiogenesis contributes to new blood vessel formation, a decrease in the number of vessels and therefore a decrease in MVD are expected Nitzsche et al. It is likely that in patients with AUB-E and AUB-I, a combination of different angiogenic mechanisms may play a role. This could be one of the explanations for why increased angiogenesis is not always associated with a change in MVD but could lead to vessels of insufficient quality or unstable vessels with an impaired function. To compare angiogenesis between patients, the MVD may not always be the most appropriate parameter. As the MVD is also dependent on other endometrial elements, such as glandular and stromal cells, changes in MVD could also mean changes in these structures since a relative proportion of vessels is measured Shaw et al. Furthermore, in patients with short-term exposure to exogenous hormone, an increase in MVD of mostly immature vessels could potentially be based on more vessel elongation, intussuscepting, or sprouting angiogenesis as compared to the extent of coalescent angiogenesis, thereby causing spotting complaints. More research is needed to elucidate the mechanisms responsible for angiogenesis in women with NMB, AUB-E, and AUB-I. Unexpectedly, EC migration was found to decrease in patients with AUB-I after short-term Norplant or DMPA exposure. This seems contradictory to the fact that, in patients with AUB-I, a higher MVD was found. This paradox might be explained by a lower blood vessel regression rate in Norplant and DMPA users, compared to other tissue in the endometrium. An alternative explanation could be that the authors used umbilical vein EC, which are characterized as macrovascular EC. It is possible that responses in microvascular EC, as present in endometrium tissues, differ from those in cells originating from macrovessels Subakir et al. This is the first narrative review that discusses impaired angiogenesis in the endometrium of patients with AUB, and AUB initiated by exogenous hormones. Strengths of this review include registration in the PROSPERO register of systematic reviews, the systematic approach according to PRISMA guidelines, the search of several databases, and the use of internationally accepted checklists to assess the RoB for included publications. In addition, this review systematically presents the broad range of angiogenic parameters and morphological characteristics of vessels. Nonetheless, there are some limitations. First, RoB assessment showed that only 8 of the 35 included studies were of good quality. Second, most studies lack objective scoring of AUB and include patients with subjective AUB; it could be questioned if the included patients with subjective blood loss actually suffer from objective AUB. Third, when interpreting the long-term effects of exogenous hormones, it should be considered that results can be influenced by selection bias, because patients with severe AUB-I are more prone to stop their treatment compared to patients with less severe symptoms. Moreover, it is possible that different doses and exposure times of exogenous hormones show different effects but because of the large heterogeneity in hormone studies it was not possible to address this further. Fourth, although the diagnostic techniques mostly involved IHC, quantification of these results differs among studies and is dependent on the subjective interpretation of the researchers. The magnification power of the fields analysed ranged from 40 to × and the unit of measurement was sometimes presented as measurements per power field, per unit area or per mm 2. Studies show that if quantitative analysis of IHC is carried out by experienced researchers, different scoring systems do show significant correlation Walker, Exogenous hormones have developed over time and thus could be outdated, such the Progestasert-IUS. Furthermore, as MMPs and their inhibitor TIMPs are considered to be involved mainly in ECM degradation, they were not included in this review. The literature shows that these factors play a role in regulating HESCs, by stabilizing vascular ECM and endometrial stromal cells, and are regulated by progesterone. Dissolution of ECM or unstable stroma could therefore contribute to the severity of complaints of AUB Lockwood, ; Schatz et al. Finally, and perhaps most importantly, this review includes many different markers, angiogenic factors and receptors, treatment types, treatment durations, and comparisons with different control groups HMB or NMB. These factors were also measured at different phases of the menstrual cycle, which makes it difficult to compare the outcomes presented. Some factors are only analysed in one study, and if factors were analysed in different studies but measured in different phases of the menstrual cycle, the results are not comparable because of rapid changes in endometrial gene expression. Despite the heterogeneous literature about this very complex process, the authors are confident that the outcomes of studies support the hypothesis that disturbed angiogenesis is important in provoking AUB and emphasizes the need for further research. New insight into the genesis of this disease is essential to develop new therapeutic strategies. This review supports our hypothesis that in patients with subjectively and objectively defined AUB-E and AUB-I, both anti- and proangiogenic factors are altered, likely leading to changes in vessel morphology. It is also the first review that shows a systematic presentation of several anti- and proangiogenic factors in patients with AUB-E and AUB-I. Nevertheless, the fact that one angiogenic factor can induce angiogenesis but simultaneously also play an essential role in vessel maturation makes it difficult to identify the best point of interest to intervene in this process. Future research should focus on identifying key angiogenic factors in a patient group with objectively defined AUB, to gather direct evidence to support the hypothesis that AUB symptoms are related to aberrant angiogenesis. In addition, it is important to distinguish between short- and long-term exogenous hormone exposure and to study the dose—effect relationship. Our results show that the most interesting systems involve factors from the HIF-, VEGF-, FGF-, and Ang-Tie pathways. Aberrant angiogenesis is known to play a major role in cancer, making this process a major point of focus in the development of antiangiogenesis therapies Ramjiawan et al. As this includes antiangiogenesis therapy in gynaecological cancers, this could also be interesting for the treatment of AUB. Naturally, antiangiogenesis therapy should be combined with birth control methods to avoid teratogenesis. To support our hypothesis that altered angiogenesis is related to AUB or infertility, additional research is the necessary first step to identify specific targets that can be used to intervene in the angiogenic pathways. Both anti- and proangiogenic factors are altered in patients with AUB-E and AUB-I, which could be a pathophysiological explanation for how HMB and spotting complaints emerge, supporting our hypothesis that angiogenesis is altered in these patients. However, many different angiogenic factors and receptors have only been investigated in single studies; therefore, the results have to be interpreted with caution. This review indicates that HMB in patients with AUB-E is caused by a change in angiogenic factors, which probably leads to immature vessels or less stable vessel walls, rather than an increase in MVD. Spotting in patients with AUB-I does seems to be related to the combination of an increased MVD and impaired vessel maturation after short-term hormone exposure. Our findings provide points of interest for future research to identify key targets for antiangiogenic therapy to treat patients with AUB. Supplementary data are available at Human Reproduction Update online. The authors thank medical information specialist Ralph de Vries, for assisting in the database search and Ghislaine Meertens for her contributing work and the initiation of this manuscript. They also thank Lynda Juffermans for her advice during the writing of this manuscript. conceived the presented idea. wrote the article in consultation with A. All authors discussed the content, provided critical feedback to shape the article, and contributed to the final article. All authors approve the final version of this article and agree that they are accountable for all aspects of the work. and J. received funding from an Amsterdam Reproduction and Development grant for a different project regarding angiogenesis Development. received funding from the Dutch Cancer Society Grant number KWF Abberton KM , Healy DL , Rogers PA. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod a ; 14 : — Google Scholar. Abberton KM , Taylor NH , Healy DL , Rogers PA. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum Reprod b ; 14 : — Abreu-Rodriguez I , Sanchez Silva R , Martins AP , Soveral G , Toledo-Aral JJ , Lopez-Barneo J , Echevarria M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1alpha. PLoS One ; 6 : e ACOG Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists. ACOG practice bulletin: management of anovulatory bleeding. Int J Gynaecol Obstet ; 72 : — Andersson E , Zetterberg E , Vedin I , Hultenby K , Palmblad J , Mints M. Low pericyte coverage of endometrial microvessels in heavy menstrual bleeding correlates with the microvessel expression of VEGF-A. Int J Mol Med ; 35 : — Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception ; 38 : — Birbrair A , Zhang T , Wang ZM , Messi ML , Mintz A , Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci Lond ; : 81 — Biswas Shivhare S , Bulmer JN , Innes BA , Hapangama DK , Lash GE. Altered vascular smooth muscle cell differentiation in the endometrial vasculature in menorrhagia. Hum Reprod ; 29 : — Endometrial vascular development in heavy menstrual bleeding: altered spatio-temporal expression of endothelial cell markers and extracellular matrix components. Hum Reprod ; 33 : — Blumenthal RD , Taylor AP , Goldman L , Brown G , Goldenberg DM. Abnormal expression of the angiopoietins and Tie receptors in menorrhagic endometrium. Fertil Steril ; 78 : — Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med ; 6 : — BMP-2 induces angiogenesis by provoking integrin alpha6 expression in human endothelial progenitor cells. Biochem Pharmacol ; : — Cioni B , Zaalberg A , van Beijnum JR , Melis MHM , van Burgsteden J , Muraro MJ , Hooijberg E , Peters D , Hofland I , Lubeck Y et al. Androgen receptor signalling in macrophages promotes TREMmediated prostate cancer cell line migration and invasion. Nat Commun ; 11 : Critchley HOD , Maybin JA , Armstrong GM , Williams ARW. Physiology of the Endometrium and Regulation of Menstruation. Physiol Rev ; : — Darden J , Payne LB , Zhao H , Chappell JC. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis ; 22 : — Daud S , Ewies AA. Levonorgestrel-releasing intrauterine system: why do some women dislike it? Gynecol Endocrinol ; 24 : — Donoghue JF , McGavigan CJ , Lederman FL , Cann LM , Fu L , Dimitriadis E , Girling JE , Rogers PA. Dilated thin-walled blood and lymphatic vessels in human endometrium: a potential role for VEGF-D in progestin-induced break-through bleeding. PLoS One ; 7 : e Edeline J , Vauléon E , Rioux-Leclercq N , Perrin C , Vigneau C , Bensalah K , Laguerre B. Safety and efficacy of sorafenib in renal cell carcinoma. Cancer Growth Metastasis ; 5 : 35 - Ferenczy A , Bertrand G , Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol ; : — Fox H , Buckley CH. In: Gresham, GA ed. Atlas of Gynaecological Pathology. Netherlands : Springer , , 61 — Google Preview. Gambino LS , Wreford NG , Bertram JF , Dockery P , Lederman F , Rogers PA. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum Reprod ; 17 : — Girling JE , Rogers PA. Recent advances in endometrial angiogenesis research. Angiogenesis ; 8 : 89 — Gleeson NC. Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Griffioen AW , Bischoff J. Oxygen sensing decoded: a Nobel concept in biology. Griffioen AW , Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev ; 52 : — Ha C , Stavreus-Evers A , Landgren BM , Mints M , Rees MC. Adrenomedullin and its receptor, calcitonin receptor-like receptor, are aberrantly expressed in women with idiopathic menorrhagia. Hague S , MacKenzie IZ , Bicknell R , Rees MC. In-vivo angiogenesis and progestogens. Hallberg L , Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest ; 16 : — Hanahan D. Signaling vascular morphogenesis and maintenance. Science ; : 48 — Harmsen MJ , Wong CFC , Mijatovic V , Griffioen AW , Groenman F , Hehenkamp WJK , Huirne JAF. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update ; 25 : — Hewett P , Nijjar S , Shams M , Morgan S , Gupta J , Ahmed A. Down-regulation of angiopoietin-1 expression in menorrhagia. Am J Pathol ; : — Hickey M , Krikun G , Kodaman P , Schatz F , Carati C , Lockwood CJ. Long-term progestin-only contraceptives result in reduced endometrial blood flow and oxidative stress. J Clin Endocrinol Metab ; 91 : — Hii LL , Rogers PA. Endometrial vascular and glandular expression of integrin alpha v beta3 in women with and without endometriosis. Hum Reprod ; 13 : — Hillard PA. |
| The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy | Therefore, we stressed the significance of using double immunofluorescent staining with anti-tryptase antibodies for separating mast cells from TCs. We found CD and PDGFR-α-positive cells in myometrial tissue from the foci of fibroids, the surrounding walls of the same uterus and healthy samples without UL. We emphasized that TCs exist in all samples of observed tissue. Several studies have been devoted to explaining a possible role of new cells in diseases. Most likely, they are more sensitive to local ischemia than other stromal cell types, such as fibroblasts, myofibroblasts and mast cells. Manetti et al. observed the dynamics of TCs in tissues the gastric wall — submucosa and muscle layers, the myocardium and the lung from patients affected by systematic sclerosis and suggested that the reduction of TCs can lead to changing of the three-dimensional organization of the extracellular matrix and, as a result, the development of fibrosis [ 28 ]. Regardless of location, staging and character of pathology, the reduction of TCs is correlated with subsequent fibrosis, has a cellular origin and is common for many injuries and pathologies [ 58 , 59 ]. We intend to provide further quantitative analysis of TC number in the foci of myoma and healthy myometrium, although we could hypothesize that the density of TCs declines in leiomyoma. The interaction between TCs and the pathophysiological mechanisms common for the selected diseases is unclear. From one point of view, the amount of TCs declined under changes in the microenvironment associated with pathology. From the other viewpoint, the decrease of TCs could be a key point in the pathogenesis. These cells reflect damage, or they could be involved in the background of the diseases; the question remains open. We revealed the presence of TCs in uterine fibroids. Before, only comparisons of the density and the distribution in pregnant and nonpregnant uteruses in rats and humans had been provided. The TC interstitial system is composed of cells that by either homocellular or heterocellular contact integrate overall information from the vascular, the nervous and the immune systems, the interstitium and stem cells. In the myometrium, TCs can influence the contractile activity of smooth muscle cells. Of note, they differed in telopodal width and podomic thickness with pregnancy states, which may be related to their function [ 44 , 45 ]. Current studies showed that the podomers are thicker in nonpregnant myometrium than in pregnant myometrium ~82 vs. Richter et al. demonstrated that the numbers of TCs and Tps correlate negatively with the amount of mature fibrillar collagens and correlate positively with degraded collagen in the human heart [ 54 ]. We observed the same trend, which requires further investigation. Growth factors have an influence on myometrial cellular transformation and turnover. For instance, PDGF modulates the rate of cell proliferation in myometrium and leiomyoma cells and likely plays a role in smooth muscle cell SMC hypertrophy as its expression is increased in the myometrium during gestation. It is upregulated by estrogen in uterine SMC and might interact with other growth factors such as TGF-β and EGF to enhance proliferation [ 5 ]. TCs are immunohistochemically positive for platelet-derived growth factor receptor α and β PDGFR-α and-β and VEGF. Of note, telocytes might be involved in the regulation of excessive cellular matrix production inside the foci of myoma [ 62 ]. Myometrial changes relate to three basic pathophysiological links: fibrosis, angiogenesis and the immune response. Growth factors involved in the pathogenesis of UL have receptors on TCs. Recent data showed that TCs are immunopositive to TGF-β, which is also involved in myocardial physiopathology. We suggest that the same could occur in myometrial contractility. One of the essential elements of leiomyomata is the vascular capsule. Neovascularization correlates with hypoxia and misbalance of vascular and coagulation factors. We observed a decrease of vascularization in the foci of leiomyoma, accompanied by increasing expression of VEGF receptor-1 sFlt Hypoxia could be a leading factor leading to this change. However, the common feature for both observed subjects was that in the foci of leiomyoma we saw a decline of telocytes and of vascularization too. Uterine telocytes are sensitive to angiogenic factors PDGF and VEGF and ischemia; they declined and even disappeared during fibrosis, observed in close vicinity to blood vessels. They might play a role in the angiogenic response, universal for the human body. In clinical practice it might clarify the pathogenesis of the oxidative response in the myometrium and help in selection of further appropriate therapy as a consequence. We intend to observe this correlation in our future scientific work. In conclusion, our data demonstrate that TCs are present in human uterine fibroids and highlight their possible involvement in the pathogenesis of myometrial pathology in the context of angiogenesis. Further studies will allow clarification of the details of their putative roles in angiogenesis and the principles of their interaction in the myometrium. We hypothesize that in-depth observation of TCs in the human uterus brings additional value to reproductive medicine. Current issue Archive Manuscripts accepted About the Journal Editorial office Editorial board Abstracting and indexing Subscription Contact Ethical standards and procedures Most read articles Instructions for authors Article Processing Charge APC Regulations of paying article processing charge APC. Manuscripts accepted. About the Journal Editorial office Editorial board Abstracting and indexing Subscription Contact Ethical standards and procedures Most read articles. Instructions for authors Article Processing Charge APC Regulations of paying article processing charge APC. Current issue. Veronika Aleksandrovych 1. Tomasz Bereza 2. Magdalena Ulatowska-Białas 3. Artur Pasternak 2. Jerzy A. Walocha 2. Kazimierz Pityński 4. Krzysztof Gil 1. Department of Pathophysiology, Jagiellonian University Medical College, Krakow, Poland. Department of Anatomy, Jagiellonian University Medical College, Krakow, Poland. Department of Pathomorphology, Jagiellonian University Medical College, Krakow, Poland. Department of Gynecology and Oncology, Jagiellonian University Medical College, Krakow, Poland. The unique nature of these cells still deserves attention from the scientific community. Telocytes make homo- and heterocellular contact with myocytes, immunocytes and nerves, have their own immunohistochemical and secretome profiles and thus might regulate local regenerative processes including angiogenesis and fibrosis. The aim of our study was to observe the missing link between angiogenesis and telocytes in leiomyoma, the most common benign tumors affecting women of reproductive age. Material and methods: We observed uterine tissue samples from leiomyoma, adjacent myometrium and unchanged tissue from patients with leiomyoma and control subjects using routine histology, histochemistry, immunofluorescence CD, CD31, CD34, PDGFRα, tryptase, sFlt-1 and image analysis methods. Results: The decline of the telocyte density in the foci of fibroids correlated with poor vascularization inside the leiomyoma. Moreover, the expression of sFlt-1 anti-angiogenic-related factor significantly increased inside a fibroid. In leiomyoma the decrease of telocyte and blood micro-vessel density was accompanied by prevalence of collagen deposits, unlike the unchanged myometrium. Conclusions: Our results demonstrate TCs in human uterine fibroids and highlight their possible involvement in the pathogenesis of myometrial pathology in the context of angiogenesis. Material and methods Subjects Twenty patients with symptomatic intramuscular solid UL mostly located in the fundus of the uterus were scheduled for elective surgery laparoscopic hysterectomy and selected for the study group 20 women, mean age: Tissue processing Tissue samples from fresh hysterectomy specimens were collected and rinsed thoroughly with PBS phosphate-buffered saline, 0. Immunofluorescence Indirect double immunofluorescence after heat-induced epitope retrieval was used to allow the simultaneous visualization of two antigens. Table I Type, sources and dilution of antibodies. Microscopic examination of telocytes, collagen deposits and vascular parameters Slides were examined using an MNFL epifluorescence microscope OptaTech, Warszawa, Poland equipped with an Olympus DP74 digital CCD camera. Table II Percentage of collagen and muscle fibers in different types of myometrium. Figure 2 Sample of leiomyoma stained for c-kit red, Alexa Fluor and tryptase green, Alexa Fluor Figure 3 Sample of leiomyoma stained for PDGFRa red, Alexa Fluor and CD34 green, Alexa Fluor Figure 4 Vascular density in unaffected myometrium A and leiomyoma B , assessed by staining for CD31 green, Alexa Fluor Figure 5 Sample of leiomyoma stained for D31 red, Alexa Fluor and CD 34 green, Alexa Fluor Figure 6 Sample of leiomyoma stained for sFlt-1 VEGFR-1 green, Alexa Fluor Discussion Since TCs were reported for the first time, a number of studies worldwide have described these cells in different organs. Conflict of interest The authors declare no conflict of interest. Stewart EA , Cookson C , Gandolfo RA , Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG ; Google Scholar. McWilliams MM , Chennathukuzhi VM. Recent advances in uterine fibroid etiology. Semin Reprod Med ; Brosens I. Uterine Leiomyomata: Pathogenesis and Management. Levy G , Hill M , Beall S , Zarek SM , Segars JH , Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet ; Aleksandrovych V , Bereza T , Sajewicz M , Walocha JA , Gil K. Uterine fibroid: common features of widespread tumor Review article. Folia Med Cracov ; Bulun SE. Uterine fibroids. N Engl J Med ; Tal R , Segars J. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update ; Ciarmela P , Islam S , Reis F , et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Sirin D , Karaarslan N. Evaluation of the effects of pregabalin on chondrocyte proliferation and CHAD, HIF-1alpha, and COL2A1 gene expression. Arch Med Sci ; Ishikawa H , Xu L , Sone K , Kobayashi T , Wang G , Shozu M. Hypoxia induces hypoxia-inducible factor 1 alpha and potential HIF-responsive gene expression in uterine leiomyoma. Reprod Sci ; Walocha JA , Litwin JA , Miodoński AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod ; Fletcher NM , Abusamaan MS , Memaj I , et al. Oxidative stress: a key regulator of leiomyoma cell survival. Fertil Steril ; Pei X. Int J Hematol ; Hussein MM , Mokhtar DM. independently performed a quality assessment of the included studies. To estimate the quality of the RCTs, the case—control, and cohorts studies, the Cochrane Risk of Bias tool version 2. The search resulted in references. Three records were added by additional sources, resulting in 36 included articles. The selection process is shown in Fig. PRISMA flow diagram for the search carried out for this narrative review. The characteristics of the included studies are presented in Tables III , IV , and V. With the exception of one study, which included patients with AUB defined as either HMB or irregular uterine bleeding Zhang et al. AUB was defined objectively in nine studies Kooy et al. In 12 studies, the diagnosis of AUB was not specified Sangha et al. In all papers that studied exogenous hormones, AUB at baseline was either not described, based on subjective complaints or defined by the WHO criteria, which is based on subjective registration Rogers et al. In general, AUB during exogenous hormone exposure was registered by menstrual calendar and based on the amount of spotting days. Characteristics of studies on angiogenesis in the endometrium of patients with endometrial abnormal uterine bleeding, compared with normal menstruation controls. Phases described: 2 phases : proliferative P and secretory S ; 3 phases : menstrual M , P, and S; 4 phases : P, early ES , mid MS , and late secretory LS ; 5 phases : M, P, ES, MS, and LS; 6 phases: M, early proliferative EP , mid proliferative MP , ES, MS, and LS; 7 phases : M, EP, MP, late proliferative LP ; ES, MS, and LS. Methods described: BrdU assay: 5-bromodeoxyuridine incorporation assay; CCT: corrosion casting technique; ELISA: enzyme-linked immunosorbent assay; HUVEC: human umbilical vein endothelial cells; IHC: immunohistochemistry; QIA: Quantitative image analysis; RPA: ribonuclease protection assay; RT-PCR: real-time PCR; WB: western blot. AUB classification: AHM : alkaline haematin method; requires collection of all menstrual sanitary products for laboratory analysis. Characteristics of studies on the effect of exogenous hormones on angiogenesis in patients with endometrial abnormal uterine bleeding. Methods described: IHC: immunohistochemistry; QIA: quantitative image analysis; WB: western blot. AUB classification: AHM: Alkaline haematin method; requires collection of all menstrual sanitary products for laboratory analysis. It can occur alone or in combination with other symptoms NICE, Characteristics of studies on angiogenesis in the endometrium of patients with hormonal induced iatrogenic abnormal uterine bleeding. Methods described: ELISA: enzyme-linked immunosorbent assay; HUVEC: human umbilical vein endothelial cells; IHC: immunohistochemistry; QIA: quantitative image analysis; RT-PCR: real-time PCR; WB: western blot. Only presented the outcomes of the case—control study, not of the HESC human endometrial stromal cells model. Only presented the outcomes of the second experiment, not the first experiment with Norplant and with or without vitamin E. Results were given for two proliferative and secretory or up to seven phases menstrual, early—mid—late proliferative and early—mid—late secretory phases. The results in Table VI are presented only for the proliferative and secretory phases, and if results were significantly different between, for example, the mid and late secretory phases this was specified per study. If hormones were continuously used, endometrium does not show different histological phases and the different phases were not distinguished. Hormone type and length of use is specified, as shown in Table V. Presented outcomes are defined as vascular morphology outcomes or as angiogenic parameters. Outcomes regarding angiogenesis in the endometrium of patients with endometrial abnormal uterine bleeding. bFGF: basic fibroblast growth factor; FGF-R1: fibroblast growth factors receptor 1; VEGF -R : vascular endothelial growth factor -receptor ; TGF-β1: transforming growth factor-β1; Ang: angiopoietin; ADM: adrenomedullin; CLR: calcitonin receptor-like receptor; BMP: bone morphogenetic protein; eNOS: endothelial nitric oxide synthase; AQP: aquaporin; HIF: hypoxia-inducible factor; CXCR: CX-chemokine receptor; MVD: microvascular density. Lu et al. Abberton et al. Biswas Shivhare et al. The search included 31 case—control studies, three cohort studies and two RCTs. The RoB assessment of all included studies is shown in Supplementary Data File S4. However, overall, the case definition of the included patient populations and methods of ascertainment of exposure were accurate in all included studies. This review covers the statistically significant results of the original publications, unless mentioned otherwise. The results of 35 identified parameters, from 20 studies that compared endometrium tissue of patients with and without AUB-E, are presented in Table VI. The VEGF pathway was investigated in several studies. One study showed an increase in VEGF in the endometrial glands during the secretory phase but not in the proliferative phase Zhang et al. VEGF-A and its receptor subtypes were studied by Mints et al. Mints et al. In contrast, Maybin et al. This could be suggestive of delayed endometrial repair during menstruation in patients with AUB-E. The downstream regulation of eNOS by VEGF was studied in one trial and showed an increase of eNOS in both phases in patients with AUB-E Blumenthal et al. Three studies compared parameters from the Ang-Tie pathway and found conflicting outcomes. However, only one study included patients with objective AUB-E and their results showed an increased Ang-1 expression in patients with AUB-E. The proangiogenic factor ADM was decreased in the secretory phase according to Ha et al. This may indicate that the EC is more sensitive to ADM binding and leads to an increase of the NO pathway, stimulating angiogenesis Fig. Three studies compared TGF-β1 and found no difference between the proliferative and secretory phase Abberton et al. Two studies also analysed the TGF-β receptors, which show a decreased expression Lu et al. In addition, Abberton et al. Endothelin-1, as well as basic fibroblast growth factor bFGF and the FGF-receptor 1, was found to be decreased in patients with AUB-E, compared to normal controls Sangha et al. Though FGF is supposedly a proangiogenic factor, it is also involved in vessel maturation and the development of spiral arterioles in the endometrium. Consequently, a reduction of FGF and endothelin-1 disturbs development of the endometrium and could lead to abnormal bleeding patterns Sangha et al. One study demonstrated that AQP1 expression is decreased in both phases of the menstrual cycle in patients with AUB-E. Despite the fact that AQP1 is an proangiogenic factor, impaired expression of AQP1 could lead to abnormal vessel formation by aberrant endothelial permeability or EC proliferation and migration Mints et al. In addition, Smad2 and -7 were found to be unchanged in both phases and, overall, Smad3 and -4 were decreased in the secretory phase in patients with AUB-E Lu et al. In line with these results, Maybin et al. However, they also found an increase of Smad2 in the secretory phase, possibly explained by the fact that HMB was defined differently in both studies, knowingly subjectively according to NICE guidelines by Lu et al. Only BMP7 was increased in patients with AUB-E; therefore, they conclude that BMP7 is possibly involved in endometrial differentiation and tissue integrity, both crucial for embryo implantation Richards et al. Ten studies report on vessel morphology in patients with and without AUB-E Table IV. Increased angiogenesis can lead to an increased MVD, and an imbalance in pro- and antiangiogenic factors can increase the number of wall gaps, vessel diameter and perimeter, and decreased pericyte coverage or wall thickness. This could indicate that vessel maturation is impaired, resulting in more fragile bloods vessels. MVD was investigated in six studies, five of which showed no significant changes in both the proliferative and secretory phases Mints et al. One study reported on vessel diameter, and perimeter and wall gaps, which were all increased in the secretory phase of patients with AUB-E, compared to normal controls. Wall gaps also were increased in the proliferative phase, although the other characteristics were similar Mints et al. In addition, Andersson et al. Proliferation and attraction of VSMCs occurs with vessel wall remodelling, for example during the maturation process of newly formed vessels, and is profoundly co-regulated by VEGF Darden et al. It is also involved in the next step after angiogenesis, namely arteriogenesis Jeremy et al. Total VSCM was identified in four different studies and was generally found to be unchanged in patients with AUB-E, with two exceptions. One study found a decrease of VSMC in the proliferative phase and one in the secretory phase in patients with AUB-E Abberton et al. Another study found no differences in VSMC thickness and additionally studied VSMC differentiation via two proteins: calponin and smoothelin. Both proteins were unchanged in the proliferative phase, but calponin was increased and smoothelin was decreased in the secretory phase Biswas Shivhare et al. Increased EC proliferation was seen in patients with AUB during both phases compared to control samples Kooy et al. EC density was compared by several parameters, with three of the four markers showing no difference in the proliferative phase and an increase in the secretory phase Biswas Shivhare et al. In addition, this study showed that several ECM proteins fibronectin, osteopontin, and collagen-IV were decreased in the secretory phase in patients with AUB-E, with one exception: osteopontin was decreased in the early secretory phase and increased in the late secretory phase. These differences in protein levels could indicate altered maturation of the endometrial vessels in AUB-E Biswas Shivhare et al. The effect of exogenous hormones on angiogenesis in the endometrium of patients with AUB-E was studied in three studies and patients with AUB-I in 13 studies Tables VII and VIII , respectively. All studies that examined AUB-E used the levonorgestrel-releasing intra-uterine system LNG-IUS , thus progestin-only hormone therapy. Also, all the selected literature that examined AUB-I used progestin-only medication, with the exception of Rogers et al. None of the included literature studied the effect of GnRH agonists or antagonists. Outcomes regarding the effect of exogenous hormones on angiogenesis n the endometrium of patients with endometrial abnormal uterine bleeding. AUB: abnormal uterine bleeding. FGF: fibroblast growth factor, ADM: adrenomedullin; MVD: microvascular density; αSMA: α-smooth muscle actin; MHC: myosin heavy chain; vWF: von Willebrand factor. Outcomes regarding the effect of exogenous hormones on angiogenesis in the endometrium of patients with iatrogenic abnormal uterine bleeding. VEGF: vascular endothelial growth -R: receptor factor a- or b-: acidic- or basic- ; FGF: fibroblast growth factors; TGF-ß1: transforming growth factor-beta1; EGF: epidermal growth factor; STC stanniocalcin-1; CSPG4: cleaved chondroitin sulphate proteoglycan 4; MVD: microvascular density; αSMA: α-smooth muscle actin; MHC: myosin heavy chain; vWF: von Willebrand factor; PCNA: proliferating cell nuclear antigen. BL: bleeding site during hysteroscopy in endometrium; NBL: non bleeding during hysteroscopy in endometrium. First outcomes in both lamina functionalis and lamina basalis, second outcome only in the subepithelial plexus. Table VII shows three studies that compared the endometrium of patients with AUB-E, with and without LNG-IUS exposure. Also, the angiogenic factors ADM and thymidine phosphorylase, of which the latter is associated with pathophysiological angiogenesis Sengupta et al. The MVD of blood vessels was increased after short-term and long-term LNG-IUS treatment in comparison to patients without LNG-IUS treatment Hague et al. In contrast, Donoghue et al. Furthermore, an increase in diameter and perimeter of the blood vessel was seen after short-term LNG-IUS treatment compared to controls, and the maximal width of the largest luminal diameter for each sample was also increased McGavigan et al. Literature shows that the increased diameter in both blood and lymphatic vessels could be caused by increased VEGF-D expression Rissanen et al. Donoghue et al. After long-term exposure, EC proliferation was reduced in patients with LNG-IUS compared to AUB-E controls Hague et al. As presented in Table VIII , two studies examined the effect of LNG-IUS treatment Roopa et al. In addition, after LNG-IUS, progestin-only or oral combined hormonal therapy, a different study found no changes in VEGF immunohistochemistry IHC compared to controls Rogers et al. Another angiogenic polypeptide involved in EC proliferation is endometrial epidermal growth factor EGF. Tissue factor TF is an important initiator for haemostasis and could potentially promote aberrant angiogenesis and produce fragile vessels if overexpressed Runic et al. The progesterone receptor PR is likely to regulate TF expression, but no difference was found in the PR protein levels between Norplant users and controls Runic et al. They found an overexpression of stanniocalcin-1 and stronger immunostaining for cleaved chondroitin sulphate proteoglycan 4, both mediators of angiogenesis and linked to malignant tumour formation Law and Wong, ; Shapiro et al. In summary, when patients with AUB-I were compared to control groups with NMB, VEGF staining was increased in two studies and not affected in one study. FGF, TGF-β, and EGFR were increased in patients with AUB-I, and EGF showed no difference between groups. When bleeding sites in Norplant users were compared with non-bleeding sites, TF and PR expression were increased in the group with bleeding complaints. Besides stimulation of vessel maturation, FGF and TGF-β are both also proangiogenic factors, acting by stimulating EC proliferation and differentiation and have a synergistic effect on the induction of angiogenesis, if combined with VEGF. TF is an important haemostasis initiator, so it seems logical that TF and its receptor are increased in bleeding sites of patients with AUB-I. Eight studies examined the effect of exogenous hormone exposure on morphological characteristics of vessels Table VII. An increase in MVD was observed in patients with AUB-I and LNG-IUS compared to controls with NMB. However, if patients with progestin-only therapy were compared to patients with combined therapy, the patients with progestin-only therapy showed a decrease in MVD Rogers et al. This effect appeared only moderate because no difference was seen in the number of immature or partially mature blood vessels; however, a decrease was found in the number of mature vessels. In addition, an increase in immature vessels was found after short- and long-term LNG-IUS exposures, and a decrease in mature vessels after short-term exposure compared to both controls and long-term exposure Stephanie et al. In addition, endometrium samples showed a decrease in VSMC number and proliferation after DMPA exposure, compared to samples of the same patients before exposure, again indicating impaired vessel maturation after progestin exposure Kayisli et al. These results indicate disruption in the maturation of blood vessels after exogenous progestin use in a time-dependent matter, especially in patients with AUB complaints. This review provides a summary of the available literature on angiogenesis in the endometrium of women with AUB-E, and in patients with AUB-E and AUB-I after use of exogenous hormones. Figure 6 provides an overview of the interactions between the angiogenic parameters reported on in this review. Schematic overview of pro- and antiangiogenic parameters assessed in this review. Blue: proangiogenic parameter; red: antiangiogenic parameter. The HIF-1α initiated VEGF and Tie-Ang pathway characteristics were the most studied angiogenic parameters. In studies with objectively defined AUB, increased VEGF, VEGF-A, VEGFR-1, and VEGFR-2 were reported, while no differences in these factors were found in studies with subjectively reported AUB. In patients with subjectively reported AUB-E, the AngAng-2 ratio, and proangiogenic Tie-1 were increased. However, one study found no difference in Ang-2 levels and only a decrease in Ang-1 in the secretory phase. As this is the only study that included patients with objectively measured AUB, this may indicate a possible relation between a decrease of Ang-1 in patients with AUB-E and decreased vessel maturation, considering that Ang-1 supports vessel maturation. However, caution is required in drawing this conclusion as this is based on one study only. In patients with AUB-E and AUB-I caused by exogenous hormones, VEGF expression was increased after short-term exposure and unchanged after long-term exposure, compared to controls. This may suggest a role of the VEGF pathway in stimulating angiogenesis in hormone-induced spotting or break-through-bleeding, in a time-dependent manner. In addition, other proangiogenic factors, such as bFGF, its receptor FGF-R1, ADM, and endothelin-1, were decreased in patients with AUB-E compared to normal controls. Conversely, in patients with AUB-I, bFGF was increased after short-term hormone exposure. These factors are in involved in EC proliferation and differentiation, as well as vessel maturation. This could suggest two somewhat contradictory outcomes: an increase in these factors could result in increased angiogenesis by mobilizing ECs, leading to AUB, and a decrease in these factors may lead to an increase in poor vascular maturation and, thus, to vascular dysfunction. The Smad family is involved in wound healing and repair, and a suboptimal TGF-β response could thereby decrease postmenstrual repair, leading to HMB Maybin et al. Moreover, Maybin et al. Conceivably, the genesis of AUB in patients with AUB-E and AUB-I is caused by a different combination of abnormalities in angiogenic factors. In general, VEGF expression increased and Ang-I decreased in patients with objectively defined AUB-E: this could suggest decreased vessel maturation in these patients. In patients with AUB-I, angiogenic factors were increased after short-term exposure and unchanged after long-term exposure to exogenous hormones. This could be in agreement with the fact that spotting complaints with exogenous hormone use gradually disappear over time Hillard, and, hypothetically, angiogenesis in the endometrium will normalize. However, these findings should be interpreted with caution and do not imply causation. In addition, decreasing complaints of spotting may be linked to selection bias, with patients who experience severe or persisting AUB complaints being more likely to stop hormone therapy after short-term use or switch to another surgical treatment National Institute for Health and Clinical Excellence, ; Daud and Ewies, Vasoconstriction of spiral arterioles is essential to limit menstrual blood flow, as a small increase in diameter will lead to an extensive increase in blood flow. For instance, if the diameter is increased 2-fold this will lead to a fold decrease in flow resistance Jain et al. Blumenthal et al. As eNOS produces NO and NO increases vasodilatation, HMB could hypothetically be caused by an increase in spiral arteriole diameter under the influence of increased NO levels Jeremy et al. This could indicate that, apart from angiogenesis, several other mechanisms are involved in AUB. The MVD was generally not increased in patients with AUB-E. Although other morphological parameters of vessels did show changes, such as diameter, perimeter, congestion, wall gaps were increased and pericyte vessel wall coverage was decreased in the endometrium of the AUB-E group. An increase in vessel diameter and perimeter could be a sign of maturation, though when pericyte coverage is absent and wall gaps and defects are increased, this can lead to fragile and leaking microvessels, thereby causing AUB Runic et al. These results could therefore indicate impaired angiogenesis and disturbed vessel maturation in the patient group with solely AUB-E, similar to the effects found in short-term exogenous hormone exposure in patients with AUB-E and AUB-I. This was supported by two studies that showed disturbed vessel maturation in patients with AUB-I Rogers et al. In contrast to patients with AUB-E, in patients with AUB-I, the MVD outcomes were very different for different types of hormones and their length of exposure. Moreover, the mechanism involved in angiogenesis might differ between groups. In general, MVD was increased after short-term exogenous hormone use and decreased, or was unchanged, after long-term use in patients with AUB-E and AUB-I. For LNG-IUS specifically, the MVD after long-term exposure was decreased, compared to short-term exposure Stephanie et al. This result is in line with the findings of Kayisli et al. However, these results are in contrast with a recent review that points out that, in patients with NMB, both EC proliferation and MVD do not change during the menstrual cycle. This review suggests that normal angiogenesis involves the faster process of vessel elongation and intussuscepted angiogenesis splitting of existing blood vessels Yetkin-Arik et al. One could hypothesize that in patients with AUB-E, especially those suffering from HMB, a combination of angiogenic mechanisms could be responsible for the formation of new vessels Fig. Moreover, if coalescent angiogenesis contributes to new blood vessel formation, a decrease in the number of vessels and therefore a decrease in MVD are expected Nitzsche et al. It is likely that in patients with AUB-E and AUB-I, a combination of different angiogenic mechanisms may play a role. This could be one of the explanations for why increased angiogenesis is not always associated with a change in MVD but could lead to vessels of insufficient quality or unstable vessels with an impaired function. To compare angiogenesis between patients, the MVD may not always be the most appropriate parameter. As the MVD is also dependent on other endometrial elements, such as glandular and stromal cells, changes in MVD could also mean changes in these structures since a relative proportion of vessels is measured Shaw et al. Furthermore, in patients with short-term exposure to exogenous hormone, an increase in MVD of mostly immature vessels could potentially be based on more vessel elongation, intussuscepting, or sprouting angiogenesis as compared to the extent of coalescent angiogenesis, thereby causing spotting complaints. More research is needed to elucidate the mechanisms responsible for angiogenesis in women with NMB, AUB-E, and AUB-I. Unexpectedly, EC migration was found to decrease in patients with AUB-I after short-term Norplant or DMPA exposure. This seems contradictory to the fact that, in patients with AUB-I, a higher MVD was found. This paradox might be explained by a lower blood vessel regression rate in Norplant and DMPA users, compared to other tissue in the endometrium. An alternative explanation could be that the authors used umbilical vein EC, which are characterized as macrovascular EC. It is possible that responses in microvascular EC, as present in endometrium tissues, differ from those in cells originating from macrovessels Subakir et al. This is the first narrative review that discusses impaired angiogenesis in the endometrium of patients with AUB, and AUB initiated by exogenous hormones. Strengths of this review include registration in the PROSPERO register of systematic reviews, the systematic approach according to PRISMA guidelines, the search of several databases, and the use of internationally accepted checklists to assess the RoB for included publications. In addition, this review systematically presents the broad range of angiogenic parameters and morphological characteristics of vessels. Nonetheless, there are some limitations. First, RoB assessment showed that only 8 of the 35 included studies were of good quality. Second, most studies lack objective scoring of AUB and include patients with subjective AUB; it could be questioned if the included patients with subjective blood loss actually suffer from objective AUB. Third, when interpreting the long-term effects of exogenous hormones, it should be considered that results can be influenced by selection bias, because patients with severe AUB-I are more prone to stop their treatment compared to patients with less severe symptoms. Moreover, it is possible that different doses and exposure times of exogenous hormones show different effects but because of the large heterogeneity in hormone studies it was not possible to address this further. Fourth, although the diagnostic techniques mostly involved IHC, quantification of these results differs among studies and is dependent on the subjective interpretation of the researchers. The magnification power of the fields analysed ranged from 40 to × and the unit of measurement was sometimes presented as measurements per power field, per unit area or per mm 2. Studies show that if quantitative analysis of IHC is carried out by experienced researchers, different scoring systems do show significant correlation Walker, Exogenous hormones have developed over time and thus could be outdated, such the Progestasert-IUS. Furthermore, as MMPs and their inhibitor TIMPs are considered to be involved mainly in ECM degradation, they were not included in this review. The literature shows that these factors play a role in regulating HESCs, by stabilizing vascular ECM and endometrial stromal cells, and are regulated by progesterone. Dissolution of ECM or unstable stroma could therefore contribute to the severity of complaints of AUB Lockwood, ; Schatz et al. Finally, and perhaps most importantly, this review includes many different markers, angiogenic factors and receptors, treatment types, treatment durations, and comparisons with different control groups HMB or NMB. These factors were also measured at different phases of the menstrual cycle, which makes it difficult to compare the outcomes presented. Some factors are only analysed in one study, and if factors were analysed in different studies but measured in different phases of the menstrual cycle, the results are not comparable because of rapid changes in endometrial gene expression. Despite the heterogeneous literature about this very complex process, the authors are confident that the outcomes of studies support the hypothesis that disturbed angiogenesis is important in provoking AUB and emphasizes the need for further research. New insight into the genesis of this disease is essential to develop new therapeutic strategies. This review supports our hypothesis that in patients with subjectively and objectively defined AUB-E and AUB-I, both anti- and proangiogenic factors are altered, likely leading to changes in vessel morphology. It is also the first review that shows a systematic presentation of several anti- and proangiogenic factors in patients with AUB-E and AUB-I. Nevertheless, the fact that one angiogenic factor can induce angiogenesis but simultaneously also play an essential role in vessel maturation makes it difficult to identify the best point of interest to intervene in this process. Future research should focus on identifying key angiogenic factors in a patient group with objectively defined AUB, to gather direct evidence to support the hypothesis that AUB symptoms are related to aberrant angiogenesis. In addition, it is important to distinguish between short- and long-term exogenous hormone exposure and to study the dose—effect relationship. Our results show that the most interesting systems involve factors from the HIF-, VEGF-, FGF-, and Ang-Tie pathways. Aberrant angiogenesis is known to play a major role in cancer, making this process a major point of focus in the development of antiangiogenesis therapies Ramjiawan et al. As this includes antiangiogenesis therapy in gynaecological cancers, this could also be interesting for the treatment of AUB. Naturally, antiangiogenesis therapy should be combined with birth control methods to avoid teratogenesis. To support our hypothesis that altered angiogenesis is related to AUB or infertility, additional research is the necessary first step to identify specific targets that can be used to intervene in the angiogenic pathways. Both anti- and proangiogenic factors are altered in patients with AUB-E and AUB-I, which could be a pathophysiological explanation for how HMB and spotting complaints emerge, supporting our hypothesis that angiogenesis is altered in these patients. However, many different angiogenic factors and receptors have only been investigated in single studies; therefore, the results have to be interpreted with caution. This review indicates that HMB in patients with AUB-E is caused by a change in angiogenic factors, which probably leads to immature vessels or less stable vessel walls, rather than an increase in MVD. Spotting in patients with AUB-I does seems to be related to the combination of an increased MVD and impaired vessel maturation after short-term hormone exposure. Our findings provide points of interest for future research to identify key targets for antiangiogenic therapy to treat patients with AUB. Supplementary data are available at Human Reproduction Update online. The authors thank medical information specialist Ralph de Vries, for assisting in the database search and Ghislaine Meertens for her contributing work and the initiation of this manuscript. They also thank Lynda Juffermans for her advice during the writing of this manuscript. conceived the presented idea. wrote the article in consultation with A. All authors discussed the content, provided critical feedback to shape the article, and contributed to the final article. All authors approve the final version of this article and agree that they are accountable for all aspects of the work. and J. received funding from an Amsterdam Reproduction and Development grant for a different project regarding angiogenesis Development. received funding from the Dutch Cancer Society Grant number KWF Abberton KM , Healy DL , Rogers PA. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod a ; 14 : — Google Scholar. Abberton KM , Taylor NH , Healy DL , Rogers PA. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum Reprod b ; 14 : — Abreu-Rodriguez I , Sanchez Silva R , Martins AP , Soveral G , Toledo-Aral JJ , Lopez-Barneo J , Echevarria M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1alpha. PLoS One ; 6 : e ACOG Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists. ACOG practice bulletin: management of anovulatory bleeding. Int J Gynaecol Obstet ; 72 : — Andersson E , Zetterberg E , Vedin I , Hultenby K , Palmblad J , Mints M. Low pericyte coverage of endometrial microvessels in heavy menstrual bleeding correlates with the microvessel expression of VEGF-A. Int J Mol Med ; 35 : — Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception ; 38 : — Birbrair A , Zhang T , Wang ZM , Messi ML , Mintz A , Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci Lond ; : 81 — Biswas Shivhare S , Bulmer JN , Innes BA , Hapangama DK , Lash GE. Altered vascular smooth muscle cell differentiation in the endometrial vasculature in menorrhagia. Hum Reprod ; 29 : — Endometrial vascular development in heavy menstrual bleeding: altered spatio-temporal expression of endothelial cell markers and extracellular matrix components. Hum Reprod ; 33 : — Blumenthal RD , Taylor AP , Goldman L , Brown G , Goldenberg DM. Abnormal expression of the angiopoietins and Tie receptors in menorrhagic endometrium. Fertil Steril ; 78 : — Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med ; 6 : — BMP-2 induces angiogenesis by provoking integrin alpha6 expression in human endothelial progenitor cells. Biochem Pharmacol ; : — Cioni B , Zaalberg A , van Beijnum JR , Melis MHM , van Burgsteden J , Muraro MJ , Hooijberg E , Peters D , Hofland I , Lubeck Y et al. Androgen receptor signalling in macrophages promotes TREMmediated prostate cancer cell line migration and invasion. Nat Commun ; 11 : Critchley HOD , Maybin JA , Armstrong GM , Williams ARW. Physiology of the Endometrium and Regulation of Menstruation. Physiol Rev ; : — Darden J , Payne LB , Zhao H , Chappell JC. Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis ; 22 : — Daud S , Ewies AA. Levonorgestrel-releasing intrauterine system: why do some women dislike it? Gynecol Endocrinol ; 24 : — Donoghue JF , McGavigan CJ , Lederman FL , Cann LM , Fu L , Dimitriadis E , Girling JE , Rogers PA. Dilated thin-walled blood and lymphatic vessels in human endometrium: a potential role for VEGF-D in progestin-induced break-through bleeding. PLoS One ; 7 : e Edeline J , Vauléon E , Rioux-Leclercq N , Perrin C , Vigneau C , Bensalah K , Laguerre B. Safety and efficacy of sorafenib in renal cell carcinoma. Cancer Growth Metastasis ; 5 : 35 - Ferenczy A , Bertrand G , Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol ; : — Fox H , Buckley CH. In: Gresham, GA ed. Atlas of Gynaecological Pathology. Netherlands : Springer , , 61 — Google Preview. Gambino LS , Wreford NG , Bertram JF , Dockery P , Lederman F , Rogers PA. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum Reprod ; 17 : — Girling JE , Rogers PA. Recent advances in endometrial angiogenesis research. Angiogenesis ; 8 : 89 — Gleeson NC. Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Griffioen AW , Bischoff J. Oxygen sensing decoded: a Nobel concept in biology. Griffioen AW , Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev ; 52 : — Ha C , Stavreus-Evers A , Landgren BM , Mints M , Rees MC. Adrenomedullin and its receptor, calcitonin receptor-like receptor, are aberrantly expressed in women with idiopathic menorrhagia. Hague S , MacKenzie IZ , Bicknell R , Rees MC. In-vivo angiogenesis and progestogens. Hallberg L , Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest ; 16 : — Hanahan D. Signaling vascular morphogenesis and maintenance. Science ; : 48 — Harmsen MJ , Wong CFC , Mijatovic V , Griffioen AW , Groenman F , Hehenkamp WJK , Huirne JAF. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update ; 25 : — Hewett P , Nijjar S , Shams M , Morgan S , Gupta J , Ahmed A. Down-regulation of angiopoietin-1 expression in menorrhagia. Am J Pathol ; : — Hickey M , Krikun G , Kodaman P , Schatz F , Carati C , Lockwood CJ. Peripheral blood 4 ml was collected from cubital veins prior to UAE treatment and at 1 week and 3 and 6 months after UAE treatment. UAE treatment was performed by two physicians as previously described Briefly, catheterization was performed through the right femoral artery under local anesthesia 5 ml lidocaine hydrochloride; Tianjin Jin Yao Amino Acid Co. Then, contralateral iliac artery angiography and uterine artery angiography was performed Artis dTC Angiography; Siemens AG, Munich, Germany. Following the elimination of key branches of the uterine artery, the emulsified mixture of lipiodol 5 ml; Guerbet, Villepinte, France and pingyangmycin 4 mg; Shanghai Yansheng Shiye Co. Shanghai, China was injected into the uterine artery. After the majority of the uterus was dyed and the blood flow reduced, gelatin sponge 10×2×2 mm was used to embolize the uterine artery. The embolization was verified by angiography. The embolization of the ipsilateral uterine artery was performed accordingly. ELISA assay was performed using IGF-1 YM-S and VEGF YM ELISA kits Shanghai YuanMu Biological Technology Co. Briefly, serum was added to the pre-coated microplate and incubated at 4°C overnight. The plate was then washed 5 times with phosphate-buffered saline and the detection antibody was added to the wells. Following incubation at room temperature for 1 h, the microplate was washed again, and horseradish peroxidase-conjugated antibody was added and incubated at room temperature for 30 min. All antibodies were from the IGF-1 and VEGF ELISA kits. After 30 min, the plate was re-washed 5 times, o -phenylenediamine OPD substrate solution was added and the plate was incubated at room temperature for a further 15 min. OPD was part of the IGF-1 and VEGF ELISA kits. Finally, stop solution was added to terminate color development and the plate was read at nm on a microplate reader SpectraMax M2; Molecular Devices LLC, Sunnyvale, CA, USA. The standard curve was generated using 2-fold serial dilutions of the standard samples. Levels of IGF-1 and VEGF were calculated according to the standard curve. Results are expressed as the mean ± standard deviation. All statistical analyses were performed with SPSS version Comparisons between groups and analyses of paired data were conducted using the Student's t-test. Multiple-factor analysis was performed to analyze the association between levels of IGF-1 or VEGF and certain clinical characteristics, including adenomyosis and the age of the patients, as well as fibroid size, location and number. The Kaplan-Meier method was used to assess the progression-free survival of the patients. The differences in survival time were analyzed using a Log-Rank test. The general data of the patients with uterine fibroids are shown in Table I. Among the 70 patients, 53 were unable or not willing to undergo surgery and 17 were patients who had recurrent uterine fibroids following surgical removal. There were 31 patients with a solitary fibroid and 39 with multiple fibroids. The fibroids were located as follows: 42, intramural; 17, submucosal; and 11, subserosal. Eight patients with uterine fibroids exhibited concurrent adenomyosis. To determine the levels of IGF-1 and VEGF in the serum, ELISA was performed prior to and following the UAE treatment. As shown in Table II , the serum levels of IGF-1 and VEGF in the UAE group prior to treatment were At 1 week after UAE treatment, the serum levels of IGF-1 and VEGF in the patients of the UAE group were At 1 month after UAE treatment, the serum levels of IGF-1 Similarly, the serum levels of IGF-1 Levels of IGF-1 and VEGF in patients with uterine fibroids before and after UAE treatment. IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial growth factor; UAE, uterine artery embolization. To investigate the association between the serum levels of IGF-1 and VEGF at 1 week after UAE treatment and the clinical characteristics of the patients with uterine fibroids, multiple-factor analysis was performed. The analyzed clinical characteristics included the age of the patient, adenomyosis and the size, location and number of fibroids. These results suggest that IGF-1 and VEGF may be used for predicting the prognosis of patients with uterine fibroids. Association between the serum levels of IGF-1 or VEGF and clinical characteristics of uterine fibroids. To investigate the effect of IGF-1 and VEGF serum levels on the prognosis of patients with uterine fibroids, the Kaplan-Meier method was performed to analyze the progression-free survival of the patients. The patients were followed-up for 18 months. Progression-free survival curves were constructed based on the serum levels of IGF-1 and VEGF at 1 week after UAE treatment These results indicate that patients with higher levels of IGF-1 and VEGF have a poorer prognosis than those with lower IGF-1 and VEGF levels. Survival analysis of uterine fibroid patients with different levels of A IGF-1 and B VEGF in the serum. The median levels of IGF-1 and VEGF were Patients were followed-up for 18 months. During the follow-up, 21 cases were censored. A Kaplan-Meier survival curve was generated, the differences in survival time were analyzed using the Log-Rank test. IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial growth factor. The main blood supply of uterine fibroids is from the uterine artery and its branches. UAE treatment can induce ischemia and atrophy of the uterine fibroids 14 and is less invasive than the traditional surgical approach. Furthermore, UAE treatment can successfully preserve the uterus and maintain its normal physiological functions 15 , IGF-1 and VEGF are important factors involved in the growth of uterine fibroids 5. In the present study, the serum levels of IGF-1 and VEGF in the UAE group prior to UAE treatment were significantly higher than those in the control group. At 1 week after UAE treatment, the serum IGF-1 and VEGF levels in the UAE group were significantly decreased compared with those prior to UAE treatment; however, at 1 and 3 months after UAE treatment, the serum IGF-1 and VEGF levels were significantly increased compared with those at 1 week after UAE treatment. These results were consistent with the findings of a previous study by Ji et al The decrease in the serum level of IGF-1 at 1 week after UAE treatment may have been due to the temporary inhibition of IGF-1 expression. At 1 and 3 months after UAE treatment, the serum level of IGF-1 was increased. |
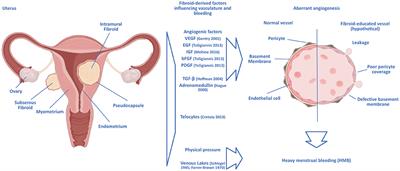
0 thoughts on “Angiogenesis and uterine fibroids”