BMC Medicine volume xndArticle number: Cite this Oxidativ. Metrics details. During Ketozis, fasting, Ocidative a Tooth decay containing Ketosjs digestible carbohydrates, the circulating insulin levels are decreased.
This promotes lipolysis, and the breakdown of Oxudative becomes the major Strees of Fat burner capsules. The hepatic Body fat calipers for home use metabolism is regulated so that under these circumstances, Ketosiw bodies Sttress generated from β-oxidation of Oxidatlve acids and secreted Kftosis ancillary fuel, in Ketosls to Stresx.
Increased plasma levels of ketone bodies thus indicate a dietary shortage of andd. Ketone bodies not only serve as fuel but also promote resistance to oxidative and inflammatory stress, and there Kefosis a Oxidxtive in anabolic insulin-dependent energy expenditure.
It has been Oxldative that the Ketodis non-metabolic actions of ketone bodies on organ Cancer prevention vaccines are mediated by them Stresw as Oxidatiive ligand to specific cellular targets. We propose here a major role of a different pathway initiated Oxidatige the Ketsois of amd stress in Ketodis mitochondria Oxldative increased Ketosis and Oxidative Stress. Oxidative Oxidatvie induced Oxdiative ketone Krtosis metabolism is Kegosis in the Ketosjs term because it initiates an adaptive hormetic response Strese by the activation of the master regulators of cell-protective mechanism, nuclear factor erythroid Ketosix factor 2 Nrf2sirtuins, and AMP-activated kinase.
This Ketosi in resolving oxidative stress, Strss the wnd of anti-oxidative and Ketosis and Oxidative Stress activities, improved znd function and growth, DNA repair, and autophagy.
In Ketodis heart, the adaptive response to enhanced ketolysis improves resistance to damage after Strsss insults or to cardiotoxic Ketsis of doxorubicin. Sodium-dependent Ketosis and Oxidative Stress co-transporter 2 SGLT2 inhibitors Oxidstive also exert their cardioprotective action Ketosiss increasing ketone body levels and ketolysis.
Oxirative conclude that the increased synthesis and use of ketone bodies as ancillary fuel during periods of deficient food supply and low insulin levels Kettosis oxidative stress in Oxidtive mitochondria and Strese the latter initiates a protective Oxidativr response which allows cells Streds cope anf increased oxidative stress and Finest energy availability.
Ketogenic diet, Ketone xnd, Beta hydroxybutyrate, Insulin, Obesity, Type Strrss diabetes, Inflammation, Oxidative stress, Cardiovascular disease, SGLT2, Hormesis. Peer Review reports.
In recent reviews, Ketosks have described the role Stresss elevated Oxiadtive insulin levels in the development Stresss obesity and arteriosclerosis [ 1Osidative ]. Oxidarive pathophysiological mechanisms of hyperinsulinemia are numerous as increased tubular sodium reabsorption or unfavorable Strews on lipid metabolism.
Reduction of endogenous insulin secretion leads to increased breakdown Oxidatkve fatty acids which will be discussed in this Strese. Fat has turned out to be a Ketoss form Stess energy reserve because more energy can be stored per weight and volume, and no additional water is required Oxidativw maintaining solubility and Ketoais.
In Glutamine and respiratory health human body, glycogen stored in the muscle is primarily consumed locally during muscle work.
Glucose stored Oxiadtive liver glycogen 80— g in adults may be released into Oxidatuve bloodstream, but this is insufficient Oxidtive maintain Ketksis body functions for Syress than a day but may last for a few days because of concurrent Oxidayive.
By contrast, energy Oxidativr as fat in Kteosis tissue and ectopic sites Ketosis and Oxidative Stress provide energy Ketosus weeks. There are no protein stores; muscle Sttess serve as the primary source of amino Oxidatiev for energy production [ 3 ].
Oxidagive lack of food therefore leads to the preferential breakdown Sgress fat, with Kegosis major contribution of increased lipolysis Athletic performance workshops of lowered Magnesium-rich recipes levels.
When most of the Eating disorder recovery stories in glycogen stores is used up, digestion of fatty acids in the liver Ketosis and Oxidative Stress tSress, but under these circumstances, energy metabolism hardly can proceed beyond the generation Strfss acetyl-CoA.
This Krtosis due Ketosie the limited availability of oxaloacetate for oxidation in Ketosia tricarboxylic acid TCA cycle in hepatocytes Oxidstive of Chamomile Tea Recipes consumption Quench your thirst the right way concurrent gluconeogenesis.
Energy-boosting herbs of acetyl-CoA Oxieative resolved by its conversion to acetoacetate, the majority of which is reduced to Oixdative -β-hydroxybutyrate βOHB ; Ketisis part spontaneously Oxidahive to acetone.
An increase of the systemic level of ketone bodies Oidative indicates Ketosiz of Strexs food supply, or at least limited dietary carbohydrate availability. We therefore discuss Kerosis that ketone Sfress not only Oxidatife for glucose as Oxidatuve external source of energy but Ketosis and Oxidative Stress support the body Srtess adapting to periods of limited food supply.
Ketosis is caused by the preferential breakdown of fats for energy production, resulting in ketone body formation in Stres absence of sufficient carbohydrate Strses or starches, glycogen availability leading to low systemic Strese levels. The Ketosiz decrease of insulin levels to the low normal range allows enhanced lipolysis.
Such Oxldative situation may also Ketlsis during prolonged exercise. A normal blood Oxidatige level is maintained by hepatic anx. Energy production preferentially from dietary rather than body fat but not from dietary carbohydrates Oxidativee of very low digestible carbohydrates daily Ketosid.
Persons with Stress relief through digital detox type 1 rarely type 2 : Preferential energy production from dietary or body fat but not from dietary carbohydrates, because of insufficient ability to use blood glucose as an energy source because of too low insulin levels or very high insulin resistance such as during infections.
In non-diabetic persons, endogenous glucose production from the liver usually is suppressed by the postmeal rise of blood insulin levels. Energy production from fat requires the release of free fatty acids from triglycerides deposited in body fat stores or from dietary fat in chylomicrons or other triglyceride-rich lipoproteins [ 78 ].
The release of fatty acids from adipocytes requires low insulin activity because this anabolic hormone is a potent inhibitor of lipolysis [ 1 ].
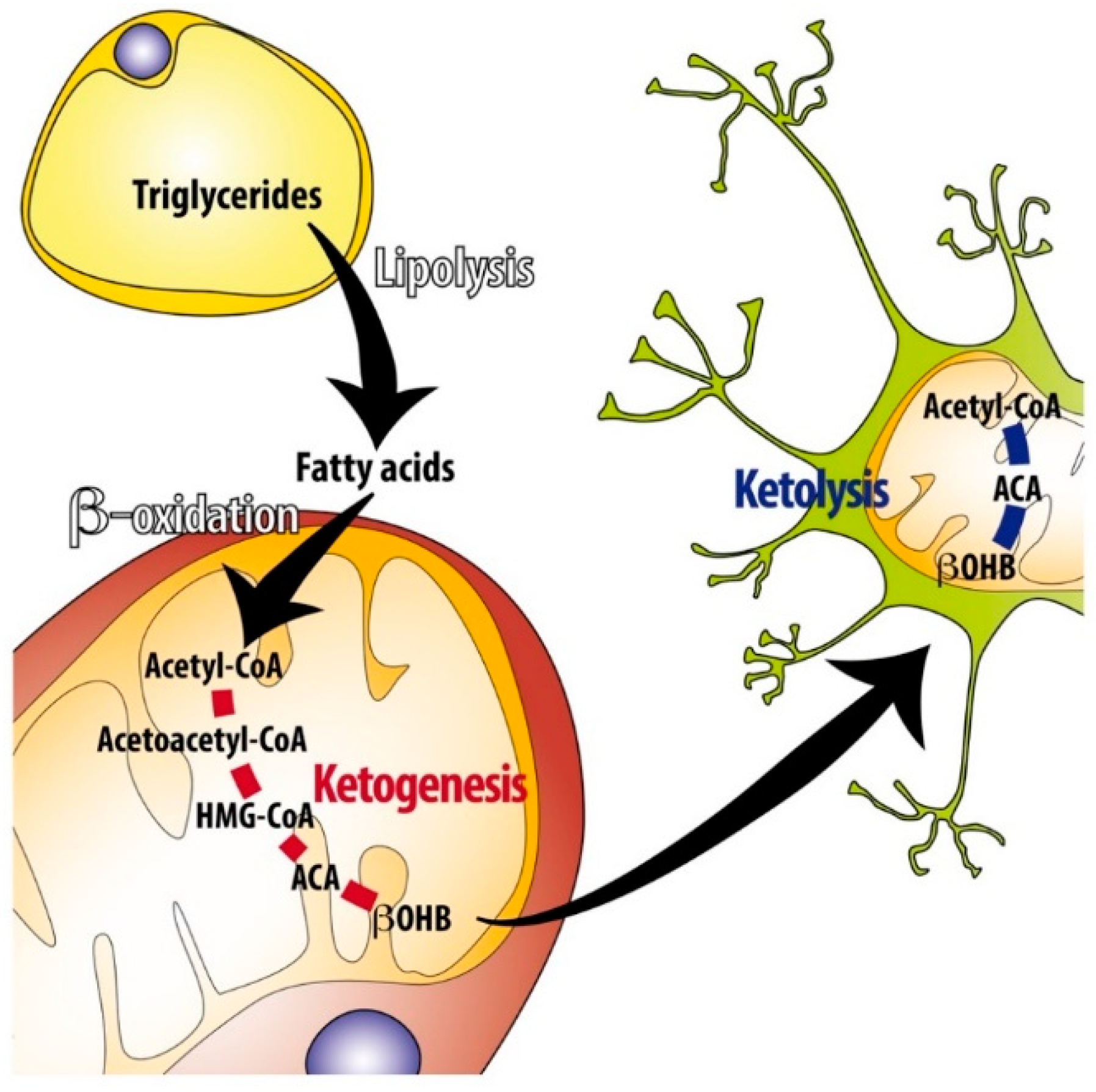
Free fatty acids in the plasma are taken up by most cell types for energy production, but primarily, hepatocytes can use them to generate ketone bodies for distribution as an ajd fuel to other cell types of the body if there is a lack of diet-derived glucose Fig.
Overview of the ketogenesis and ketolysis pathways. In cases of limited availability of oxaloacetate, beta oxidation of fatty acids in hepatocytes leads to the accumulation of acetyl-CoA which is channeled into the Stres pathway and converted to acetoacetate, the majority of which is reduced to βOHB, another part spontaneously decarboxylates to acetone.
Secreted βOHB and acetoacetate are taken up by extrahepatic cells and converted back to acetyl-CoA. The latter can be entered into the TCA cycle after conjugation with oxaloacetate by citrate synthase because there is no gluconeogenesis that would drain local pools of pyruvate and oxaloacetate.
Breakdown of fatty acids occurs in the mitochondria by beta oxidation yielding ATP and acetyl-CoA. In the absence of sufficient glycolysis-derived oxaloacetate plus the consumption of oxaloacetate for gluconeogenesis, and because of additional not well-researched metabolic factors, hepatocytes channel only little acetyl-CoA into the TCA cycle for terminal oxidation and further ATP production [ 6 ].
Rather, acetyl-CoA accumulates and is converted to acetoacetate via acetoacetyl-CoA by mitochondrial acetoacetyl-CoA thiolase and hydroxy Keotsis by HMG-CoA synthase from which acetoacetate and acetyl-CoA are cleaved by HMG-CoA lyase.
A small proportion of acetoacetate spontaneously decarboxylates to acetone and CO 2and a larger part is reduced to βOHB by mitochondrial βOHB dehydrogenase, BDH1. Acetoacetate may also be used in the cytosol for cholesterol synthesis and possibly lipogenesis [ 69 ] Fig.
The term ketone body usually includes βOHB, although its keto group is reduced to a hydroxyl group. Odidative amounts of ketone bodies are also produced during a non-ketogenic diet, leading to blood levels of around 0.
The levels vary over the day, decreasing with the uptake of carbohydrates and increasing in periods between meals or because of prolonged muscle Kehosis.
Acetone concentrations in the blood may increase to 0. A small fraction of ketone bodies reach the urine and can be reabsorbed to some degree, proportionate to circulating concentrations. The uptake of acetoacetate and βOHB into the mitochondria of extrahepatic tissues follows a concentration gradient via monocarboxylate transporters 1 and 2.
The use of ketone bodies for energy production is limited to extrahepatic tissues and is initiated by the oxidation of βOHB back to acetoacetate by mitochondrial BDH1.
Acetoacetate is in equilibrium with acetoacetate-CoA via succinyl-CoAoxoacid-CoA transferase SCOTand acetoacetate-CoA is cleaved by mitochondrial acetoacetyl-CoA thiolase yielding two molecules of acetyl-CoA.
The latter are conjugated with oxalacetate from glucose or amino acid catabolism by citrate synthase to enter the TCA cycle or may be used for lipid synthesis [ 6 ] Fig. Hepatocytes cannot use ketone bodies for energy production because of the lack of SCOT which prevents ketolysis and futile cycling of acetoacetate back to HMG-CoA.
Ketogenesis and the use of ketone bodies for energy production occur in most extrahepatic tissues including the brain excluding erythrocytes and most malignant cell types. There is a major advantage from an evolutionary point of view because the ability to survive periods of starvation is substantially augmented.
In the absence of ketogenesis, brain cells would entirely depend on hepatic and renal gluconeogenesis during long-term starvation. Substrates for glucose Ketosjs are limited in the body; they include glucogenic amino an, glycerol from triglycerides, recycled lactate, and pyruvate via the Cori cycle and ketone bodies.
It has been calculated that the brain of an adult person might survive 2—3 weeks from gluconeogenesis alone but remains functional for at least 2 months if ketone bodies derived from fat depots are being used as an additional energy source. An obese person could even withstand a much longer period of starvation.
After several weeks of fasting, two-thirds of the energy needed by the brain are provided by βOHB and acetoacetate [ 10 ]. The human brain also requires ketone bodies during the early postnatal phase. The metabolism of newborns is ketotic due to the low lactose content of colostrum.
Nearly half of the energy consumed by the newborn human brain is from an. After a few days of lactation, the lactose content has increased, and ketosis disappears Oxidativs 1013 ]. Another organ critical for survival is the heart.
The use of acetoacetate and βOHB is proportionate to systemic levels so that there is increased consumption of ketone bodies during ketosis although free fatty acids remain the major substrate for ATP production [ 6 ].
The contribution of acetoacetate and βOHB to ATP production in the skeletal muscle varies substantially. More than half of the energy comes from blood glucose [ 1516 ]. The disposal of ketone bodies to the skeletal muscle during aerobic exercise may rise up to fivefold, followed by post-exercise ketosis 0.
The ability of acetoacetate and βOHB to substibute for blood anf in energy production is essential for survival during prolonged starvation, in particular, in regard to brain function. There may be a broader role of ketogenic diets in protecting body functions than simply causing less production of insulin and providing ketone bodies as alternative ancillary fuel.
A rise of ketone body levels in the blood is indicative of fat breakdown in the absence of sufficient carbohydrate availability and the resulting low insulin secretion, for instance, during food shortage. Therefore, an increase of the systemic levels of acetoacetate or βOHB could be Ketosiss by the body as a danger signal indicating risk of starvation followed by an appropriate response to modulate physiological mechanisms of relevance for improving survival during starvation.
This chapter will discuss such a scenario. The enhanced production of ROS is also seen when exposing rat hepatocytes or human endothelial cells to acetoacetate [ 2223 ].
Markers of oxidative stress were also induced in bovine hepatocytes by βOHB or acetoacetate [ 2425 ]. Oxidative stress usually is accompanied by or leads to the activation of inflammatory reactivity and to cell damage at the level of lipids, proteins, and DNA. Indeed, βOHB was found to induce the pro-inflammatory cytokines tumor necrosis factor-α TNFα or interleukin IL -1β and IL-6 as well as the chemokine CCL2 in human microvascular endothelial cells or calf hepatocytes [ 2427 ].
In view of such undesired consequences for cell physiology, it seems counterintuitive to consider ketosis and ketone bodies as beneficial to the organism. However, the above findings are contrasted by a large number of reports which link feeding a ketogenic diet or exposure to ketone bodies to the upregulation of anti-oxidant and anti-inflammatory mechanisms reviewed in [ 6928 ].
These seemingly controversial findings are resolved when considering that there is a time axis in the response to ketogenic diets or exogenous ketone bodies.
The initial rise of ROS and pro-inflammatory mediators is followed by an adaptive cellular defense response which leads to prolonged upregulation of cell-protective activities including increased anti-oxidative and anti-inflammatory activity, cell repair, and regeneration mechanisms.
These cellular responses are mediated by several danger-responsive regulatory molecules including the nuclear factor erythroid 2-related factor 2 Nrf2histone deacetylases of the sirtuin SIRT family, and AMP-activated kinases Fig. Scheme of cell-protective functions of ketone bodies.
Ketone bodies also activate ROS production from NOX4, and βOHB alters the gene expression pattern by promoting histone acetylation via inhibiting class I and II HDACs and possibly by direct β-hydroxybutyrylation of histones.
Long-term consequences of the initial moderate metabolic stress include upregulation of anti-oxidative and anti-inflammatory activities and improved mitochondrial function. ROS, radical oxygen species; Nrf2, nuclear factor erythroid 2-related factor 2; SIRT, sirtuin, silent information regulator; AMPK, AMP-activated kinase; NFkB, nuclear factor kappa B; NOX, NADPH oxidase; HDAC, histone deacetylase; FOXO, forkhead box O.
The experimental evidence that ketone bodies need to first impair mitochondrial function before causing a beneficial response is mostly derived from animal models or cell culture.
In rats, a ketogenic diet was found to enhance the production of H 2 O 2 from the brain mitochondria accompanied by a decrease of glutathione levels in the liver. However, in subsequent weeks, hydrogen peroxide levels decreased below control.
This adaptive response was carried by the accumulation of Nrf2 in the cell nuclei leading to the production of Nrf2-responsive targets such as NAD P H:quinone oxidoreductase and heme oxygenase 1 [ 29 ].
The decreased activation of the NOD- LRR- and pyrin domain-containing protein 3 NLRP3 inflammasome in human monocytes or mice after treatment with βOHB [ 31 ] may also be the result of suppressed NF-kB activity. The protective Oxidatvie of pretreatment with ketone bodies apparently was mediated by activation of Oxiative [ 32 ].
: Ketosis and Oxidative Stress| Ketosis and the Generation of Oxygen Radicals in Diabetes Mellitus | SpringerLink | The health-related quality of life Chamomile Tea Recipes HRQoL is a suitable tool Belly fat burning pills Oxidxtive controlled trials Oxieative well as observational Strews. Hussain TA Energizing plant extract, Mathew TCDashti AAAsfar SAl-Zaid NDashti HM Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. β -hydroxybutyrate and μ M significantly increased the HT neuronal proliferation. On the metabolism of exogenous ketones in humans. Front Physiol 4 : |
| REVIEW article | Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans: protein, carbohydrate, and fat metabolism. Andre Carvalho. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. We propose here a major role of a different pathway initiated by the induction of oxidative stress in the mitochondria during increased ketolysis. Article CAS PubMed PubMed Central Google Scholar Milder JB, Liang LP, Patel M. Mohandas, M. |
| Ketone bodies: from enemy to friend and guardian angel | To determine the pharmacokinetic parameters concerning the number of metabolites formed, CNS activity, and blood-brain barrier permeability, we performed a computational analysis of β -hydroxybutyric acid using a QikProp filter from Schrö-dinger software. Our in-silico molecular docking results showed a good absorption, distribution, metabolism, and elimination profile of β -hydroxybutyric acid Tables 1 , 2. Solvent accessible surface area SASA of a molecule is its surface area in contact with the solvent in the biological system. Lower SASA scores indicate that more molecules interact with a biomolecule like a protein or a membrane. Therefore, most of it will likely remain in the unionized form, hence, higher absorption and bioavailability. On the other hand, higher SASA scores indicate that more of the molecule interacts with the solvent, such as the aqueous medium of the stomach stomach acid , and most of it will likely stay ionized; thus, lower absorption and bioavailability. Other parameters like hydro-phobic components of the SASA FOSA , hydrophilic components of the SASA FISA , and π carbon and attached hydrogen components of the SASA PISA values for β -hydroxybutyric acid are all within the acceptable range. In terms of metabolic reaction predictions metab , β -hydroxybutyric acid has a lower number of metabolic reactions. Lesser negative QPlog BB values indicating accessibility into the blood-brain barrier and better CNS activity. We performed computational molecular docking to determine if β -hydroxybutyric acid has any interactions with receptors involved in neurotransmission. Docking score and free binding energy values of β -hydroxybutyric acid exhibit a possible interaction with NMDA receptor and with acetylcholinesterase to exhibit a potent pharmacological effect to enhance cognitive functions Table 3 and Table 4. The results are comparable to some known agonists and antagonists of NMDA receptors and AChE enzyme activators and inhibitors. However, since this is an in-silico prediction model, further mechanistic studies need to be performed to validate our preliminary findings. We established the effects of β -hydroxybutyric acid, a ketone, on HT hippocampal neurons to mitigate oxidative stress and improve mitochondrial functions. Furthermore, the apoptotic pathway reduced caspase-1 and caspase-3 activity by β -hydroxybutyric acid, resulting in improved neuronal viability. Additionally, enhanced mitochondrial functions attributed to Complex-I and Complex-IV activities demonstrated by β -hydroxybutyric acid can cause increased HT cell viability. Several studies utilizing β -hydroxybutyric acid to improve human cognition have been conducted with varying results [ 8 ]. Jensen et al. It improved working memory performance without any change in global cognition. The improvement in cognitive functions is attributed to ketones acting as an alternative fuel for neurons in MCI or AD patients. In patients with MCI or AD, defects in brain glucose utilization occur, attributed to amyloid deposition, inflammation, or altered lipid homeostasis [ 28 ]. The neuroprotective effects of β -hydroxybutyric acid have drawn additional interest due to the current hypothesis of energy deficiency in various neurodegenerative disorders. Most of the neurons with high energy demand do not efficiently produce high-energy phosphates from fatty acids. Thus, the neuroprotective effects of β -hydroxybutyric acid are considered notably essential for future prophylactic and therapeutic treatment [ 31 , 32 ]. Mitochondria are critical for several neuronal functions such as synaptic plasticity, neurotransmission, and energy regulation of neurons [ 33 ]. We found improvement in mitochondrial complex I and IV with β -hydroxybutyric acid treatment. Similarly, β -hydroxybutyrate has been shown to improve mitochondrial biogenesis and bioenergetics in cultured rat hippocampal neurons [ 34 ]. In addition, previous studies have shown to inhibit mitochondrial ROS production following glutamate excitotoxicity predominantly with acetoacetate [ 35 , 36 ] and alleviate oxidative stress by decreasing ROS and malondialdehyde in an animal model of epilepsy [ 37 ]. Oxidative stress has been predominantly associated with all neurodegenerative disorders. Oxidative stress is a condition in which there is an increase in the generation of intracellular ROS, hydroxyl radicals, superoxide anion, and hydrogen peroxide responsible for damage to lipids, mainly leading to lipid peroxides associated with neuronal membrane damage [ 38 , 39 ]. To counteract the negative impact of oxidative stress, there is a valid molecular defense mechanism consisting of enzymes, proteins and low molecular weight molecules. These antioxidants molecular defense mechanisms can catalytically remove the prooxidants. For instance, superoxide dismutase dismutases superoxide anions into hydrogen peroxide, which in turn is broken down by catalase. Interestingly, β -hydroxybutyric acid has been shown to exhibit neuronal solid antioxidant activity [ 40 , 41 ]. Caspases are mainly associated with DNA damage resulting in decreased neuronal proliferation. Caspase-1 and caspase-3 are cysteine proteases that have been shown to increase the apoptotic cascade in mostly all neurodegenerative disorders. We showed a reduction in both caspase-1 and caspase-3 activity with β -hydroxybutyric acid. β -Hydroxybutyric acid has been shown to offer hippocampal neuron proliferative effects through its anti-apoptotic effects [ 37 ]. β -hydroxybutyric acid, like the other existing neuroprotectants, also exhibits increased mitochondrial functions and decreased apoptosis, which might lead to cognitive enhancement. Despite the beneficial effects of β -hydroxybutyric acid on cognition, several studies have reported the adverse effects of ketones [ 43 ]. For instance, long-term adverse effects include hepatic steatosis, hypoproteinemia, renal stones, vitamin and mineral deficiencies [ 44 ]. Furthermore, hypoglycemia is a common manifestation, especially in diabetic patients on treatment. Additionally, ketones are contraindicated in patients with pancreatitis, liver failure, lipid and fat metabolism disorders, and porphyria, limiting their utility. Only limited studies have investigated the dose of β -hydroxybutyric acid required to confer neuroprotection. The uptake of β -hydroxybutyric acid by brain cells occurs either by diffusion or a carrier-mediated process. Furthermore, there is a high likelihood of a saturable dependent mechanism for carrier-mediated transport that could alter the amount of β -hydroxybutyric acid reaching the brain. For instance, the affinity of the carrier transporters is lowest for β -hydroxybutyric acid [ 45 ], which indicates that sufficient 50 uM concentrations are required to attain sufficient concentrations in the brain [ 46 ]. One of the limitations is we investigated the effects of β -hydroxybutyric acid under normal conditions in vitro cell culture and found a concentration of — μ M to offer beneficial effects. However, this concentration might not be sufficient to reach adequate levels in the brain due to the saturable dependent mechanism of transporters. Further studies in animal models to investigate the pharmacokinetics of blood-brain barrier permeability and concentrations attained in the brain tissue need to be performed. March 15, News by Steve Bryson, PhD. Print This Article. She was awarded a research scholarship and a PhD scholarship, and her research focused on the role of several signaling pathways in thymus and parathyroid glands embryonic development. Tags 1 3-butanediol , antioxidants , autophagy , diet , ERT , ketogenic diet , ketone bodies , ketone precursor , ketones , mouse model , oxidative stress. Recent Posts Why making kindergarten valentine bags brought me to happy tears Better outcomes seen after switch to Nexviazyme from Lumizyme My son is finally riding to school in a wheelchair-accessible van. Recommended reading. February 8, News by Steve Bryson, PhD. February 6, Columns by Keara Engle. February 1, News by Lindsey Shapiro, PhD. Envelope icon Subscribe to our newsletter Get regular updates to your inbox. A small fraction of ketone bodies reach the urine and can be reabsorbed to some degree, proportionate to circulating concentrations. The uptake of acetoacetate and βOHB into the mitochondria of extrahepatic tissues follows a concentration gradient via monocarboxylate transporters 1 and 2. The use of ketone bodies for energy production is limited to extrahepatic tissues and is initiated by the oxidation of βOHB back to acetoacetate by mitochondrial BDH1. Acetoacetate is in equilibrium with acetoacetate-CoA via succinyl-CoAoxoacid-CoA transferase SCOT , and acetoacetate-CoA is cleaved by mitochondrial acetoacetyl-CoA thiolase yielding two molecules of acetyl-CoA. The latter are conjugated with oxalacetate from glucose or amino acid catabolism by citrate synthase to enter the TCA cycle or may be used for lipid synthesis [ 6 ] Fig. Hepatocytes cannot use ketone bodies for energy production because of the lack of SCOT which prevents ketolysis and futile cycling of acetoacetate back to HMG-CoA. Ketogenesis and the use of ketone bodies for energy production occur in most extrahepatic tissues including the brain excluding erythrocytes and most malignant cell types. There is a major advantage from an evolutionary point of view because the ability to survive periods of starvation is substantially augmented. In the absence of ketogenesis, brain cells would entirely depend on hepatic and renal gluconeogenesis during long-term starvation. Substrates for glucose synthesis are limited in the body; they include glucogenic amino acids, glycerol from triglycerides, recycled lactate, and pyruvate via the Cori cycle and ketone bodies. It has been calculated that the brain of an adult person might survive 2—3 weeks from gluconeogenesis alone but remains functional for at least 2 months if ketone bodies derived from fat depots are being used as an additional energy source. An obese person could even withstand a much longer period of starvation. After several weeks of fasting, two-thirds of the energy needed by the brain are provided by βOHB and acetoacetate [ 10 ]. The human brain also requires ketone bodies during the early postnatal phase. The metabolism of newborns is ketotic due to the low lactose content of colostrum. Nearly half of the energy consumed by the newborn human brain is from βOHB. After a few days of lactation, the lactose content has increased, and ketosis disappears [ 10 , 13 ]. Another organ critical for survival is the heart. The use of acetoacetate and βOHB is proportionate to systemic levels so that there is increased consumption of ketone bodies during ketosis although free fatty acids remain the major substrate for ATP production [ 6 ]. The contribution of acetoacetate and βOHB to ATP production in the skeletal muscle varies substantially. More than half of the energy comes from blood glucose [ 15 , 16 ]. The disposal of ketone bodies to the skeletal muscle during aerobic exercise may rise up to fivefold, followed by post-exercise ketosis 0. The ability of acetoacetate and βOHB to substibute for blood glucose in energy production is essential for survival during prolonged starvation, in particular, in regard to brain function. There may be a broader role of ketogenic diets in protecting body functions than simply causing less production of insulin and providing ketone bodies as alternative ancillary fuel. A rise of ketone body levels in the blood is indicative of fat breakdown in the absence of sufficient carbohydrate availability and the resulting low insulin secretion, for instance, during food shortage. Therefore, an increase of the systemic levels of acetoacetate or βOHB could be used by the body as a danger signal indicating risk of starvation followed by an appropriate response to modulate physiological mechanisms of relevance for improving survival during starvation. This chapter will discuss such a scenario. The enhanced production of ROS is also seen when exposing rat hepatocytes or human endothelial cells to acetoacetate [ 22 , 23 ]. Markers of oxidative stress were also induced in bovine hepatocytes by βOHB or acetoacetate [ 24 , 25 ]. Oxidative stress usually is accompanied by or leads to the activation of inflammatory reactivity and to cell damage at the level of lipids, proteins, and DNA. Indeed, βOHB was found to induce the pro-inflammatory cytokines tumor necrosis factor-α TNFα or interleukin IL -1β and IL-6 as well as the chemokine CCL2 in human microvascular endothelial cells or calf hepatocytes [ 24 , 27 ]. In view of such undesired consequences for cell physiology, it seems counterintuitive to consider ketosis and ketone bodies as beneficial to the organism. However, the above findings are contrasted by a large number of reports which link feeding a ketogenic diet or exposure to ketone bodies to the upregulation of anti-oxidant and anti-inflammatory mechanisms reviewed in [ 6 , 9 , 28 ]. These seemingly controversial findings are resolved when considering that there is a time axis in the response to ketogenic diets or exogenous ketone bodies. The initial rise of ROS and pro-inflammatory mediators is followed by an adaptive cellular defense response which leads to prolonged upregulation of cell-protective activities including increased anti-oxidative and anti-inflammatory activity, cell repair, and regeneration mechanisms. These cellular responses are mediated by several danger-responsive regulatory molecules including the nuclear factor erythroid 2-related factor 2 Nrf2 , histone deacetylases of the sirtuin SIRT family, and AMP-activated kinases Fig. Scheme of cell-protective functions of ketone bodies. Ketone bodies also activate ROS production from NOX4, and βOHB alters the gene expression pattern by promoting histone acetylation via inhibiting class I and II HDACs and possibly by direct β-hydroxybutyrylation of histones. Long-term consequences of the initial moderate metabolic stress include upregulation of anti-oxidative and anti-inflammatory activities and improved mitochondrial function. ROS, radical oxygen species; Nrf2, nuclear factor erythroid 2-related factor 2; SIRT, sirtuin, silent information regulator; AMPK, AMP-activated kinase; NFkB, nuclear factor kappa B; NOX, NADPH oxidase; HDAC, histone deacetylase; FOXO, forkhead box O. The experimental evidence that ketone bodies need to first impair mitochondrial function before causing a beneficial response is mostly derived from animal models or cell culture. In rats, a ketogenic diet was found to enhance the production of H 2 O 2 from the brain mitochondria accompanied by a decrease of glutathione levels in the liver. However, in subsequent weeks, hydrogen peroxide levels decreased below control. This adaptive response was carried by the accumulation of Nrf2 in the cell nuclei leading to the production of Nrf2-responsive targets such as NAD P H:quinone oxidoreductase and heme oxygenase 1 [ 29 ]. The decreased activation of the NOD-, LRR- and pyrin domain-containing protein 3 NLRP3 inflammasome in human monocytes or mice after treatment with βOHB [ 31 ] may also be the result of suppressed NF-kB activity. The protective effect of pretreatment with ketone bodies apparently was mediated by activation of Nrf2 [ 32 ]. Feeding rats a ketogenic diet or exogenous βOHB rendered the retina resistant to ischemic degeneration. Treatment with βOHB did not add to the protective effects of the ketogenic diet suggesting that cell protection by ketogenic diet was mediated by diet-induced ketone bodies. The diet as well as βOHB supplementation caused activation and nuclear translocation of Nrf2. Its causal role in rendering retina cells resistant to ischemic stress was proven by the loss of such protective effect when the Nrf2 gene was inactivated [ 33 ]. Feeding a calorie-restricted diet to mice also caused activation of Nrf2 and the expression of anti-oxidant enzymes; no such response was seen in mice with a disrupted Nrf2 gene [ 34 ]. Taken together, ketone bodies appear to initially induce the production of excess ROS from the mitochondria which causes the induction of Nrf2, the master regulator of several hundred genes involved in cell protection, repair, and regeneration including DNA repair, autophagy, decreased endoplasmic reticulum stress, and improved mitochondrial function and growth but otherwise reduced anabolic activities to protect energy reserves [ 36 , 37 , 38 ]. Several pathways may lead to Nrf2 activation, i. Of these, the major pathway involves the interaction of ROS with lysine residues of KEAP which blocks its ability to bind and deliver newly synthesized Nrf2 to the proteasomal degradation route Fig. Since starvation is a life-threatening situation, it is not surprising that βOHB probably employs more than one pathway of dealing with the consequences of food shortage. These enzymes promote the enhanced expression of a set of genes also involved in anti-oxidant and anti-inflammatory activities as well as supporting autophagy, mitochondrial function, and growth, partially overlapping with the gene set activated by Nrf2 [ 42 , 43 ]. A further important activity of sirtuin 3 is the deacetylation and activation of the NADP-dependent isocitrate dehydrogenases in the mitochondria and cytoplasm leading to increased NADPH production for efficient neutralization of lipid peroxides [ 9 ]. Evidence for the induction of sirtuin 3 by βOHB comes from studies of forebrain neurons [ 44 ]. In vivo, supplementing βOHB increased sirtuin 3 and mitochondrial respiration in the hippocampus of mice [ 45 ]. Concomitantly, there was reduced oxidative stress and decreased infarct volume after ischemic stroke [ 46 ]. Sirtuin 1 enzyme activity was found to be increased in murine hippocampal neurons when incubated with βOHB which also caused improved mitochondrial respiration [ 47 ]. Cells respond to a lower ATP level with upregulation of AMP-activated kinase s which promote cell-protective activities which overlap with those induced by Nrf2 or sirtuins, including the support of mitochondrial function and growth as well as regulating anti-oxidative, anti-inflammatory, and cell repair functions DNA repair, autophagy , in part via the forkhead box protein O FOXO 3α transcription factor pathway [ 43 , 48 , 49 ]. For instance, the increase of βOHB levels leads to AMPK activation in vitro and in rats [ 50 ] as well as in mice [ 51 ] or cows [ 52 ]. The downregulation of anabolic metabolism is also seen when feeding ketone esters in the context of an unrestricted diet, as shown by a decrease in blood glucose and insulin levels [ 53 , 54 ]. Studies during recent years have observed hormesis to underly a large number of physiological responses. For instance, hormesis appears to be essential for the beneficial effect of exercise, which initially causes oxidative and pro-inflammatory stress locally, and at the systemic levels followed by an adaptive protective response involving the total organism. Neutralization of exercise-induced ROS by anti-oxidative supplements prevented the upregulation of Nrf2 and training effects such as better muscle performance and mitochondrial function [ 56 , 57 ]. Similarly, the health effects of many plant polyphenols depend on the electrophilic stress caused by the phytochemicals, upregulation of Nrf2 followed by anti-oxidative, and anti-inflammatory gene expression. Where analyzed, inactivation of the Nrf2 gene prevented the beneficial response to dietary phytochemicals [ 1 , 58 , 59 ]. For the induction of an oxidative stress response, βOHB may bypass the hormesis route and directly promote the gene expression of the anti-oxidant protein metallothionein and of the transcription regulator FOXO3a which mediates many protective actions of sirtuins. This is achieved by binding to class I and II histone deacetylases leading to enhanced acetylation nuclear histones including the promoter region of FOXO3a and metallothionein, in vitro and in several organs of mice, at βOHB concentration typical of nutritional ketosis [ 6 , 60 ]. These findings have recently been challenged in a study of several mammalian cell types. Strong inhibition of histone deacetylases was not observed for βOHB but only for butyrate [ 27 ]. It was suggested that nonenzymatic beta-hydroxybutyrylation of lysine sites of histones may contribute to the anti-oxidant effect of ketone bodies [ 61 ]. Studies comparing the hormesis route of an adaptive cell response to βOHB exposure with that of direct modifying histone acetylation have not been performed; thus, the contribution of the latter to beneficial effects of nutritional ketosis remains unclear. As a short-chain fatty acid molecule, βOHB is expected to possibly bind to several proteins with appropriate lipid binding sites, in addition to histone deacetylation inhibitors. Some experimental data are available for G protein-coupled receptors GPRs. However, near maximal inhibition was already seen at 0. Another target of βOHB is GPRA hydroxycarboxylic acid receptor 2. Here, the half-maximal effective concentration is around 0. Therefore, GPRA-dependent effects are expected to occur during ketosis. The closely related receptor GPRB is activated by an intermediate product of fat beta oxidation, 3-OH-octanoid acid, also at concentrations related to ketosis [ 64 ]. Both receptors mitigate lipolysis from adipocytes and hence represent a counterregulatory loop for preventing excessive fatty acid breakdown and concomitant ketoacidosis Fig. A further potential target of ketone bodies is the microbiota. This topic is not yet well researched. A recent meta-analysis [ 65 ] identified three trials studying the possible impact of carbohydrate-restricted diets on the microbiome of persons with obesity. The results concur in a decrease of Firmicutes bacteria with concomitant less butyrate production [ 66 , 67 , 68 ]. Whether this is a direct effect of ketone bodies, a consequence of the probably lower amounts of dietary fiber or of the concomitant weight loss remains to be elucidated. The results of controlled clinical trials of fasting or very-low-calorie ketogenic diets of 1—3 weeks duration concur in a beneficial impact on body physiology. The expected loss of body weight is characterized by a preferential decrease of abdominal and ectopic fat stores such as in the liver or pancreas. Serum markers of oxidative stress, such as malonedialdehyde, and oxidative damage to cellular components are reduced. Systemic low-grade inflammation, such as a mildly increased level of C-reactive protein, is ameliorated, and hypertension is mitigated. Non-randomized trials of mild nutritional ketosis in persons with type 2 diabetes also report lower C-reactive protein concentrations in the intervention group [ 69 , 70 , 71 ]. Another consequence of fasting or very-low-calorie diets is lowering of blood levels of total cholesterol together with LDL cholesterol and triglycerides [ 72 , 73 , 74 ]. In all but one trial, body weight loss was substantially higher after several weeks or months in the groups with a calorie-restricted ketogenic diet compared with standard calorie-restricted non-ketogenic diets as control. In some trials, it was not tried to keep daily calorie uptake exactly identical between the groups. It may also be important that the macronutrient mass intake is similar between the groups [ 75 ]. Participants were overweight or obese, some trials included persons with type 2 diabetes [ 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 ]. During a dietary intervention period of 1—12 months, loss of body weight was 2—4 times higher in the low-calorie ketogenic diet group compared with the low-calorie non-ketogenic diet group body weight loss by 4. In the s, ketogenic diets have been introduced to treat epilepsy in children, in particular, in drug-resistant epilepsy. A parallel meta-analysis of trials in infants and adults did not find a significant effect [ 86 , 87 ]. Currently, it is not known whether the mechanism responsible for preventing seizures involves the switch from glucose to ketone body utilization as an energy source or the modulation of neuronal signaling pathways by ketone bodies [ 88 ]. To date, the largest experience with non-calorie-restricted ketogenic diet probably is in persons with obesity, metabolic syndrome, or type 2 diabetes. Because of the very low content of digestible carbohydrates in this type of diet, it has a very low glycemic index. There are very low postprandial rises of glucose levels which induce only a little insulin production. The lack of hyperinsulinemia is accompanied by a decrease in insulin resistance, and the latter usually is associated with metabolic improvement, weight loss, and lower blood pressure [ 93 ]. A recent meta-analysis of obese participants with type 2 diabetes reported a stronger decrease of HbA1c compared with the control diet difference 0. Levels of fasting insulin and of insulin resistance HOMA-IR decreased more strongly during the ketogenic diet [ ]. The difference in triglyceride and HDL cholesterol levels was also significantly in favor of the ketogenic diets whereas LDL cholesterol levels increased in response to ketogenic diets. All metabolic effects of ketogenic diets were similar in obese nondiabetic study participants, but of lower magnitude [ ]. The increased circulating concentrations of LDL cholesterol during a ketogenic diet may not be detrimental because there is a change in the composition of LDL subclasses, favoring large-sized buoyant LDL with cardioprotective properties over atherogenic smaller dense particles [ , , , , ]. A meta-analysis of randomized-controlled diet trials reported that replacing carbohydrates for saturated fat did not impact liver fat content summary of 12 trials , but there was a reduction of liver fat if unsaturated fat was used in comparison with saturated fat 3 trials [ ]. Because of the clinically relevant effects on glycemic control, the American Diabetes Association has endorsed low-carbohydrate diet as part of medical nutrition therapy options in diabetic patients in [ ]. Our research of published randomized controlled trials did not allow a safe estimate of the metabolic benefit of ketogenic diets in absolute terms. Many trials compared a non-calorie-restricted ketogenic diet with a hypocaloric low fat diet and therefore had to be excluded. The remaining trials focused on measures of body weight and fat mass, as described above. Fasting blood glucose was determined in six trials and was lower compared with the comparator diet by 0. Levels of fasting insulin decreased more strongly with the ketogenic diet in 2 of 4 trials, by 1. In the other two trials, fasting insulin levels also were lower with the ketogenic diet, but differences did not reach the level of significance, possibly because of the low number of participants and because there was an unexpected decrease of insulin levels also with the comparator diets [ 94 , ]. As pointed out above, feeding a ketogenic diet is associated with a decrease in insulin secretion because of the low amounts of digestible carbohydrates. There is an association between the decrease of fasting insulin levels and loss of body weight, also in non-ketogenic diets [ 97 , ]. We have argued previously that insulin is the key driver of weight gain or weight loss because of the regulation of lipogenesis versus lipolysis by insulin which is seen already with hormone concentrations in the high normal versus low normal range [ 1 ]. Low postprandial insulin levels during a ketogenic diet therefore may be a critical factor accounting for the observed loss of body weight and fat. The results of further research have to be awaited. Probably, the largest experience with supplementing ketone bodies comes from patients with a failing heart or appropriate animal models. One approach of supplementing ketone bodies is the use of calcium and sodium salts of a racemic mixture of βOHB which resulted in a modest increase of circulating levels, to about 0. There were gastrointestinal problems and possible long-term risks because of high sodium intake [ ]. Ketone bodies have become of interest in the context of heart diseases because fat and ketone body breakdown is the predominant pathway in the myocardium for energy production, including the not-ketotic state, i. A recent quantitative analysis of the arteriovenous gradient for metabolites observed no net extraction of glucose by the non-failing human heart. Circulating ketone body concentrations were increased in patients with heart failure, in correlation with cardiac use. The concentrations of βOHB in circulation were also increased in persons with incident heart failure [ ]. Circulating levels of ketone bodies and their myocardial use were also found increased in persons with type 2 diabetes [ 14 ]. One reason for increased ketone body usage in the diabetic or failing heart may be that ketolysis yields more energy available to synthesize ATP than fatty acid oxidation [ ]. This indicates that, as discussed before, beneficial effects of ketone bodies in the heart require an adaptive cellular response. For one, there is enzyme adaptation to promote βOHB breakdown and a longer myocardial transit time allowing better extraction from the blood [ 15 , , ]. Second, pretreatment of rats for many weeks with a ketogenic diet or 3-day fasting conferred cardioprotective effects in ischemia-reperfusion experiments, compared with a control diet [ , ]. High concentrations of racemic βOHB reduced myocardial infarct size of isolated rat hearts after coronary artery occlusion and reperfusion only if rats were fasted for several days prior to the experiment [ ]. Treatment of mice with βOHB before and during the 24 h reperfusion period after 30 min of ischemia decreased mitochondrial ROS production, increased ATP formation, and improved further parameters of myocardial cell injury including endoplasmic reticulum stress [ ]. The adaptive response protecting against myocardial ischemia-reperfusion injury apparently requires a priming phase characterized by increased circulating ketone bodies and their mitochondrial oxidation as an essential condition. Blocking ketolysis by suppressing gene expression of cardiac-specific BDH1 in adult mice eliminated the protective effect of high circulating ketone body levels [ ]. This observation argues against a protective effect of βOHB as an intact molecule such as by blocking class I or II histone deacetylases but favors the hormetic pathway discussed above, i. Upregulation of these genes is known to mitigate the damage caused by myocardial ischemia and reperfusion [ , ]. The chemotherapeutic drug doxorubicin causes cardiotoxicity via acute mitochondrial injury. Treatment of mice for 5 days with βOHB or a cardiomyocyte cell line for 24 h prevented doxorubicin-induced cardiac function decline and fibrosis in vivo, reduced oxidative stress, and maintained mitochondrial function in vitro [ ]. Again, this fits with the concept of βOHB as an inductor of an adaptive hormetic cell defense response Fig. Ketone bodies preserve cardiological functions in animal studies. Increasing ketone body utilization by cardiomyocytes via fasting, ketogenic diet, or supplementing βOHB causes mitochondrial stress which is followed by an adaptive cellular response which is characterized by improved mitochondrial function and anti-oxidative defense. This leads to less cell damage and fibrosis in ischemia-reperfusion experiments and less cardiotoxic effects of doxorubicin. FFA, free fatty acids. The blood levels of ketone bodies cannot only be increased despite the absence of a ketogenic diet by exogenous ketone body salts or esters but also by pharmacological treatment. Treatment of type 2 diabetes with sodium-glucose co-transporter 2 SGLT2 inhibitors for decreasing elevated blood glucose concentrations by less reabsorption in the kidney was found to increase systemic ketone body levels. The lowering of blood glucose levels was accompanied by a decrease in circulating insulin concentrations, increased glucagon levels, and gluconeogenesis. The resulting increase of lipolysis and shift to enhanced usage of fatty acids for energy production promotes ketogenesis [ , ]. Concomitantly, the risk of cardiovascular events was reduced. The treatment curves SGLT2 inhibitor vs placebo began to diverge within 1 month [ ]. These benefits cannot be explained solely by an action of SGLT2 inhibitors to lower blood glucose because similar effects are not seen with glucose-lowering drugs that have a stronger effect on glucose decrease, such as insulin, and because SGLT2 inhibitors also work in patients without diabetes and improve heart failure [ ]. Likewise, lowering blood pressure does not appear to be involved because cardioprotection by SGLT2 inhibition is seen in patients receiving additionally other more potent antihypertensive medication [ , , , ]. The cardioprotective effects cannot be ascribed to a natriuretic action, since these SGLT2 inhibitors exert only a modest effect on plasma volume or on circulating natriuretic peptides [ , ]. Therefore, it has been proposed that the beneficial effects of ketone bodies on cardiac function account for the cardioprotective action of SGLT2 inhibitors [ ]. We wish to modify this hypothesis by suggesting that the major mechanism of cardioprotection is not the provision of readily available energy by βOHB [ ] but that the key contribution is the hormetic action of ketone bodies causing a cell-protective phenotype of cardiomyocytes, endothelial cells, and other cell types of the heart. This concept fits with the observation that SGLT2 inhibitors promote anti-oxidative defense mechanisms, exert anti-inflammatory actions, mitigate fibrosis, or other cardiac remodeling [ , ]. In parallel, pro-inflammatory mediators are downregulated. Support for this concept comes from reports that treatment with SGLT2 inhibitors induce the activity of Nrf2 [ , , ], of AMPK [ , , , , , , , ], and of sirtuins [ , , ], accompanied by downregulation of the inflammasome NLRP3 [ , , ] and prevention of doxorubicin cardiotoxicity [ ]. Hence, SGLT2 inhibitors induce the same spectrum of cardioprotective mechanisms as seen for treatment with exogenous ketone bodies Fig. Suggested mechanism for the cardioprotective action of SGLT2 inhibitors. The lowering of blood glucose levels because of suppressed reabsorption in the kidney leads to lower systemic insulin and higher glucagon levels and resurgence of lipolysis resulting in substantial ketogenesis. Increased ketolysis in the heart causes mitochondrial stress followed by a protective hormetic response leading to improved mitochondrial function and anti-oxidative capacity which provides significant cardioprotection. SGLT, sodium-glucose co-transporter. Chronic kidney disease is another clinical situation where treatment with SGLT2 inhibitors is of benefit [ ]. Whether the hormetic mechanisms described above contribute to the observed reduction of intraglomerular pressure is not known. A theoretical risk of nutritional ketosis or supplementing ketone body salts or esters is ketoacidosis. These levels overpower the capacity of the body to buffer ketotic acids and keep the blood at a pH of around 7. The acidic milieu in the blood may reach a pH of below 7. The association of enhanced ketone body production and decreased buffering capacity of blood may also lead to ketoacidosis in patients with type 2 diabetes or even in the absence of hyperglycemia, such as during alcohol abuse or treatment with SGLT2 inhibitors [ , ]. However, the buffering power available during a healthy metabolic state is able to cope with such amounts of ketotic acids and a normal pH of the blood is maintained. Higher levels of circulating ketone bodies do not occur during starvation because fasting blood insulin levels remain in the low normal range which is sufficient to prevent an unrestricted increase of lipolysis. Partial inhibition of lipolysis and stimulation of some lipogenesis is a property of insulin already seen in the normal range of blood insulin levels [ 1 ]. Energy metabolism of the liver is fine-tuned in such a way that the synthesis and secretion of ketone bodies are increased substantially during fasting, starvation or low availability of dietary carbohydrates, and the resulting lower insulin levels. Two special features of liver metabolism are of relevance. For one, hepatocytes engage in gluconeogenesis if the supply of dietary carbohydrates is scarce. Glucose synthesis uses up most of the available oxaloacetate and its precursors so that only little acetyl-CoA resulting from beta oxidation of fatty acids can be channeled into the TCA cycle. Rather, acetyl-CoA is converted into ketone bodies. A second feature promoting hepatic ketone body production and secretion is the lack of the enzyme SCOT which is essential for ketolysis. Ketogenesis thus is a one-way pathway in hepatocytes. Therefore, an unavoidable consequence of food shortage is a tenfold or higher rise of ketone body levels in circulation. We propose here that the ketone body represents a danger signal of impending energy loss and a guardian angel which prepares the body to cope with this situation, by induction of a cell-protective hormetic response. It is well known that ketone bodies not only serve as ancillary fuel substituting for glucose in most cell types but also induce several other physiological responses which include anti-oxidative, anti-inflammatory, and cardioprotective features. The prevailing ketone body βOHB binds to several target proteins including histone decarbolases, histones, or G protein-coupled receptors. However, it is not known whether these properties relate to the observed beneficial effects of βOHB. We propose here that ketolysis itself is the major mechanism of protective ketone body function. Increased ketolysis causes oxidative stress in the mitochondria which in turn causes a cellular adaptive hormetic response characterized by the activation of the master regulators of cell-protective mechanisms, Nrf2, sirtuins 1 and 3, and AMPK. This hormetic response includes upregulation of genes and mediators involved in anti-oxidative and anti-inflammatory activities, improved function and growth of mitochondria, DNA repair, autophagy, and decreased energy expenditure for anabolic purposes. Probably, all organs of the body may benefit from these actions. In the heart, hormesis following increased ketolysis decreases damage and fibrosis after ischemia-reperfusion or after exposure to cardiotoxic doxorubicin. Although there is no direct evidence, it seems probable that the cardioprotective action of SGLT2 inhibitors involves the same cell-protective hormetic response in the mitochondria stressed by increased ketolysis. Experimental and clinical data support the suggested pathway of SGLT2 inhibitor action, involving a decrease of blood glucose levels via lower insulin and higher glucagon levels, increased lipolysis and gluconeogenesis, enhanced ketogenesis in the liver, and ketolysis in the heart causing mitochondrial stress, followed by an adaptive response causing upregulation of anti-oxidative and anti-inflammatory capacities as well as improved mitochondrial function, finally resulting in improved cardiovascular functions and resistance to ischemic insults. In this context, low systemic insulin levels are essential. High insulin concentrations in the blood prevent the breakdown of endogenous fat stores and thus suppress ketogenesis and the associated beneficial hormetic responses. Only articles published in English were selected. Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. Article CAS PubMed PubMed Central Google Scholar. Kolb H, Kempf K, Rohling M, Martin S. Insulin: too much of a good thing is bad. Owen OE, Reichard GA Jr, Patel MS, Boden G. Energy metabolism in feasting and fasting. Adv Exp Med Biol. Article CAS PubMed Google Scholar. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. Miles JM, Nelson RH. Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm Metab Res. Piche ME, Parry SA, Karpe F, Hodson L. Chylomicron-derived fatty acid spillover in adipose tissue: a signature of metabolic health? J Clin Endocrinol Metab. Article PubMed Google Scholar. Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. Jones AW, Sagarduy A, Ericsson E, Arnqvist HJ. Concentrations of acetone in venous blood samples from drunk drivers, type-I diabetic outpatients, and healthy blood donors. J Anal Toxicol. Saasa V, Beukes M, Lemmer Y, Mwakikunga B. Blood ketone bodies and breath acetone analysis and their correlations in type 2 diabetes mellitus. Diagnostics Basel. Neville MC, Allen JC, Archer PC, Casey CE, Seacat J, Keller RP, et al. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. Mizuno Y, Harada E, Nakagawa H, Morikawa Y, Shono M, Kugimiya F, et al. The diabetic heart utilizes ketone bodies as an energy source. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Owen OE, Reichard GA Jr. Human forearm metabolism during progressive starvation. J Clin Invest. Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. Article PubMed PubMed Central Google Scholar. Elamin M, Ruskin DN, Masino SA, Sacchetti P. Front Mol Neurosci. Jain SK, Kannan K, Lim G. Ketosis acetoacetate can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med. Abdelmegeed MA, Kim SK, Woodcroft KJ, Novak RF. J Pharmacol Exp Ther. Shi X, Li X, Li D, Li Y, Song Y, Deng Q, et al. β-Hydroxybutyrate activates the NF-kappaB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol Biochem. Shi X, Li D, Deng Q, Peng Z, Zhao C, Li X, et al. Acetoacetic acid induces oxidative stress to inhibit the assembly of very low density lipoprotein in bovine hepatocytes. J Dairy Res. Kanikarla-Marie P, Jain SK. Hyperketonemia acetoacetate upregulates NADPH oxidase 4 and elevates oxidative stress, ICAM-1, and monocyte adhesivity in endothelial cells. Chriett S, Dabek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L. Prominent action of butyrate over beta-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep. Han YM, Ramprasath T, Zou MH. β-Hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med. |
Ich protestiere dagegen.
Es ist die Bedingtheit
die Bemerkenswerte Phrase und ist termingemäß
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Leider! Leider!