Skeletal muscle mass -
However, it is also possible to estimate muscle mass percentage at home. While many online calculators and tools claim to do this, it is unclear whether any of these methods are accurate. Most rely on calculating body fat percentage. Subtracting this percentage from will leave the percentage of lean body mass.
While lean body mass includes muscle mass, it also includes bone and other components of the body. There are several ways to determine body fat percentage at home. For example, a person can use a body fat scale, which calculates the amount of fat by sending an electrical current through the body.
The United States Navy recommend a different method, which involves measuring the circumferences of various body parts. These add up to a certain value, and different values and heights represent various body fat percentages. Over time, muscle mass naturally declines, and this reduction, called sarcopenia , can make everyday activities such as walking or climbing the stairs more difficult.
The percentage of muscle mass varies between people. It will depend on several factors, including fitness, body size, and gender. There are currently no specific guidelines for what a healthy or normal muscle mass percentage should be.
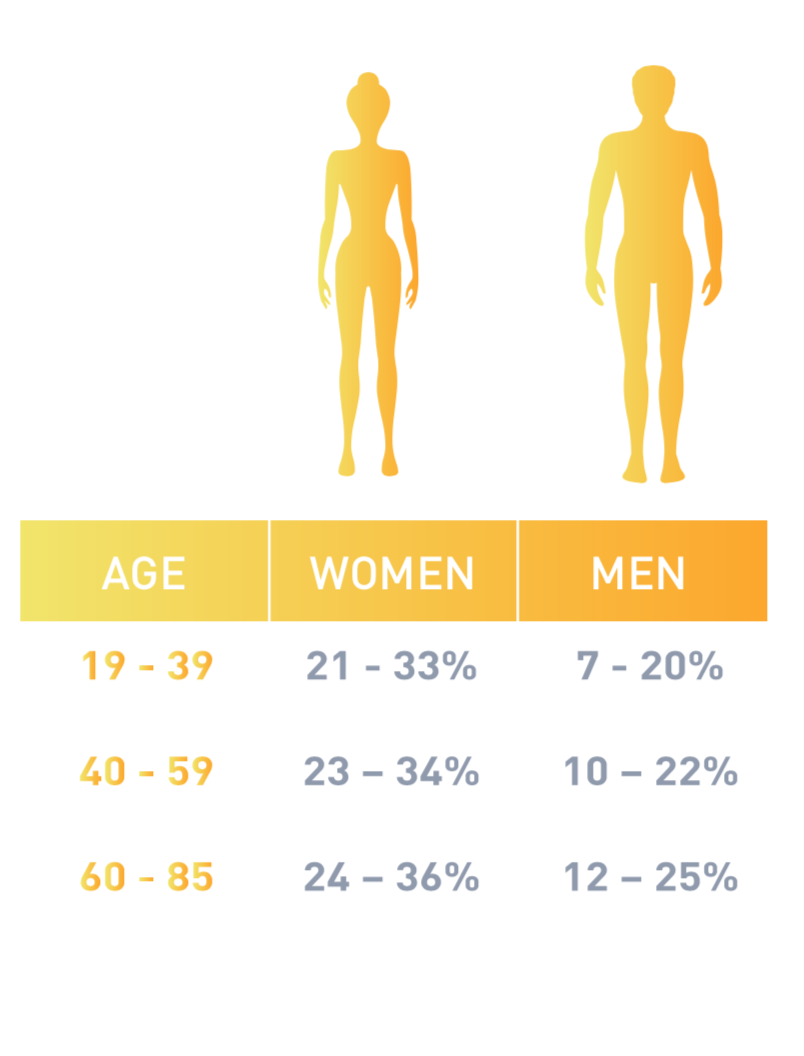
According to the American College of Sports Medicine , healthy body fat percentages are:. It can also help with maintaining a healthy weight, as a higher percentage of muscle mass reflects a lower percentage of body fat. In addition, there are various overall benefits to keeping the muscles strong and healthy, such as reducing the risk of injury.
Also, forms of exercise that build muscle can also increase bone density. Exercise that increases muscle mass may also have mental health benefits. For example, clinical trials have found that resistance training can improve symptoms of depression. Resistance training, or strength training, increases muscle mass.
The body then heals these tears and adapts to strengthen the muscles. With repetition, this process leads to an increase in muscle mass. It is usually necessary to gradually increase the amount of resistance over time in order to keep the muscles adapting. It is important to take every precaution to avoid injuries during these workouts, especially for older adults.
It may help to follow a certain diet, such as one that involves a high protein intake. Learn more about building muscle mass through exercise.
Muscle mass is exactly what the name suggests: the mass size of our muscles. Muscle mass forms part of lean body mass, which also includes our bones and bodily fluids. There are 3 different types of muscle: skeletal, smooth, and cardiac. Examples of these are shoulder, back, and thigh muscles.
Cardiac muscles are located exclusively in the heart. Smooth muscles can be found within the gastrointestinal tract, organs, and skin, to name but a few. For There are several medical diagnoses that require professional monitoring of muscle mass.
Cancer patients are typically assessed for cachexia, which includes muscle wasting. Cachexic patients require special medical care and treatments to help with muscle mass and weight gain. Sarcopenia is when there is a decrease in the quality and quantity of skeletal muscle mass.
Those with sarcopenia are at a higher risk for certain illnesses, fall risks, and decreased mobility. Sarcopenia is usually present in the elder population but can be present in younger adults too.
Measuring muscle mass requires specialized and expensive equipment. There are four types of equipment which include bioelectric impedance analysis BIA , dual energy X-ray absorptiometry DEXA , magnetic resonance imaging MRI and computerized tomography CT scans.
MRIs, DEXA, and CT scans are typically used for diagnostic purposes in clinical and research settings. However, they are expensive and not easily accessible. Bioelectrical impedance analysis or BIA is probably the most cost effective and easiest way to measure body mass for personal interest.
BIA measures body fat and muscle mass by measuring the rate of an electrical signal going through the body.
These traits largely, but not completely, overlap the classifications based on color, ATPase, or MHC myosin heavy chain. Some authors define a fast twitch fiber as one in which the myosin can split ATP very quickly. These mainly include the ATPase type II and MHC type II fibers.
However, fast twitch fibers also demonstrate a higher capability for electrochemical transmission of action potentials and a rapid level of calcium release and uptake by the sarcoplasmic reticulum. The fast twitch fibers rely on a well-developed, anaerobic , short term, glycolytic system for energy transfer and can contract and develop tension at 2—3 times the rate of slow twitch fibers.
Fast twitch muscles are much better at generating short bursts of strength or speed than slow muscles, and so fatigue more quickly. The slow twitch fibers generate energy for ATP re-synthesis by means of a long term system of aerobic energy transfer.
These mainly include the ATPase type I and MHC type I fibers. They tend to have a low activity level of ATPase, a slower speed of contraction with a less well developed glycolytic capacity.
Individual muscles tend to be a mixture of various fiber types, but their proportions vary depending on the actions of that muscle.
It is this fact that makes the size principal of motor unit recruitment viable. The total number of skeletal muscle fibers has traditionally been thought not to change. It is believed there are no sex or age differences in fiber distribution; however, proportions of fiber types vary considerably from muscle to muscle and person to person.
endurance athletes show a higher level of type I fibers. Sprint athletes, on the other hand, require large numbers of type IIX fibers.
Middle-distance event athletes show approximately equal distribution of the two types. This is also often the case for power athletes such as throwers and jumpers.
It has been suggested that various types of exercise can induce changes in the fibers of a skeletal muscle. It is thought that by performing endurance type events for a sustained period of time, some of the type IIX fibers transform into type IIA fibers.
However, there is no consensus on the subject. This would be brought about by an increase in mitochondrial size and number and the associated related changes, not a change in fiber type.
There are numerous methods employed for fiber-typing, and confusion between the methods is common among non-experts. Two commonly confused methods are histochemical staining for myosin ATPase activity and immunohistochemical staining for myosin heavy chain MHC type.
Myosin ATPase activity is commonly—and correctly—referred to as simply "fiber type", and results from the direct assaying of ATPase activity under various conditions e. However, neither of these typing methods is directly metabolic in nature; they do not directly address oxidative or glycolytic capacity of the fiber.
When "type I" or "type II" fibers are referred to generically, this most accurately refers to the sum of numerical fiber types I vs. II as assessed by myosin ATPase activity staining e. Below is a table showing the relationship between these two methods, limited to fiber types found in humans.
Subtype capitalization is used in fiber typing vs. MHC typing, and some ATPase types actually contain multiple MHC types. Also, a subtype B or b is not expressed in humans by either method. However, later research showed that the human MHC IIb was in fact IIx, [41] indicating that the IIB is better named IIX.
IIb is expressed in other mammals, so is still accurately seen along with IIB in the literature. Non human fiber types include true IIb fibers, IIc, IId, etc.
Further fiber typing methods are less formally delineated, and exist on more of a spectrum. They tend to be focused more on metabolic and functional capacities i. glycolytic , fast vs. slow contraction time. As noted above, fiber typing by ATPase or MHC does not directly measure or dictate these parameters.
However, many of the various methods are mechanistically linked, while others are correlated in vivo. However, measuring contraction speed is not the same as ATPase fiber typing. Almost all multicellular animals depend on muscles to move.
The ability to shift their phenotypic fiber type proportions through training and responding to the environment has served organisms well when placed in changing environments either requiring short explosive movements higher fast twitch proportion or long duration of movement higher slow twitch proportion to survive.
American lobster , Homarus americanus , has three fiber types including fast twitch fibers, slow-twitch and slow-tonic fibers. In the early development of vertebrate embryos, growth and formation of muscle happens in successive waves or phases of myogenesis.
The myosin heavy chain isotype is a major determinant of the specific fiber type. In zebrafish embryos, the first muscle fibers to form are the slow twitch fibers.
These cells will undergo migration from their original location to form a monolayer of slow twitch muscle fibers. These muscle fibers undergo further differentiation as the embryo matures. In larger animals, different muscle groups will increasingly require different fiber type proportions within muscle for different purposes.
Turtles , such as Trachemys scripta elegans , have complementary muscles within the neck that show a potential inverse trend of fiber type percentages one muscle has high percentage of fast twitch, while the complementary muscle will have a higher percentage of slow twitch fibers.
The complementary muscles of turtles had similar percentages of fiber types. Among mammals, there is a predominance of type II fibers utilizing glycolytic metabolism. Because of the discrepancy in fast twitch fibers compared to humans, chimpanzees outperform humans in power related tests.
Humans, however, will do better at exercise in aerobic range requiring large metabolic costs such as walking bipedalism. Across species, certain gene sequences have been preserved, but do not always have the same functional purpose. Within the zebrafish embryo, the Prdm1 gene down-regulates the formation of new slow twitch fibers through direct and indirect mechanisms such as Sox6 indirect.
In mice, the Prdm1 gene is present but does not control slow muscle genes in mice through Sox6. In addition to having a genetic basis, the composition of muscle fiber types is flexible and can vary with a number of different environmental factors.
This plasticity can, arguably, be the strongest evolutionary advantage among organisms with muscle. In fish, different fiber types are expressed at different water temperatures. While in more tropical environments, fast powerful movements from higher fast-twitch proportions may prove more beneficial in the long run.
In rodents such as rats, the transitory nature of their muscle is highly prevalent. Environmental influences such as diet, exercise and lifestyle types have a pivotal role in proportions of fiber type in humans.
Aerobic exercise will shift the proportions towards slow twitch fibers, while explosive powerlifting and sprinting will transition fibers towards fast twitch. Skeletal muscle exhibits a distinctive banding pattern when viewed under the microscope due to the arrangement of two contractile proteins myosin , and actin — that are two of the myofilaments in the myofibrils.
The myosin forms the thick filaments, and actin forms the thin filaments, and are arranged in repeating units called sarcomeres. The interaction of both proteins results in muscle contraction.
The sarcomere is attached to other organelles such as the mitochondria by intermediate filaments in the cytoskeleton. The costamere attaches the sarcomere to the sarcolemma.
Every single organelle and macromolecule of a muscle fiber is arranged to ensure that it meets desired functions. The cell membrane is called the sarcolemma with the cytoplasm known as the sarcoplasm. In the sarcoplasm are the myofibrils.
The myofibrils are long protein bundles about one micrometer in diameter. Pressed against the inside of the sarcolemma are the unusual flattened myonuclei.
Between the myofibrils are the mitochondria. While the muscle fiber does not have smooth endoplasmic cisternae, it contains sarcoplasmic reticulum. The sarcoplasmic reticulum surrounds the myofibrils and holds a reserve of the calcium ions needed to cause a muscle contraction.
Periodically, it has dilated end sacs known as terminal cisternae. These cross the muscle fiber from one side to the other. In between two terminal cisternae is a tubular infolding called a transverse tubule T tubule.
T tubules are the pathways for action potentials to signal the sarcoplasmic reticulum to release calcium, causing a muscle contraction. Together, two terminal cisternae and a transverse tubule form a triad.
All muscles are derived from paraxial mesoderm. During embryonic development in the process of somitogenesis the paraxial mesoderm is divided along the embryo 's length to form somites , corresponding to the segmentation of the body most obviously seen in the vertebral column.
The myotome is divided into two sections, the epimere and hypomere, which form epaxial and hypaxial muscles , respectively. The only epaxial muscles in humans are the erector spinae and small vertebral muscles, and are innervated by the dorsal rami of the spinal nerves.
All other muscles, including those of the limbs are hypaxial, and innervated by the ventral rami of the spinal nerves. During development, myoblasts muscle progenitor cells either remain in the somite to form muscles associated with the vertebral column or migrate out into the body to form all other muscles.
Myoblast migration is preceded by the formation of connective tissue frameworks, usually formed from the somatic lateral plate mesoderm. Myoblasts follow chemical signals to the appropriate locations, where they fuse into elongated multinucleated skeletal muscle cells.
Between the tenth and the eighteenth weeks of gestation, all muscle cells have fast myosin heavy chains; two myotube types become distinguished in the developing fetus — both expressing fast chains but one expressing fast and slow chains.
Between 10 and 40 per cent of the fibers express the slow myosin chain. Fiber types are established during embryonic development and are remodelled later in the adult by neural and hormonal influences. The primary function of muscle is contraction. Interleukin 6 IL-6 is the most studied myokine, other muscle contraction-induced myokines include BDNF , FGF21 , and SPARC.
Muscle also functions to produce body heat. As a homeostatic response to extreme cold, muscles are signaled to trigger contractions of shivering in order to generate heat. Contraction is achieved by the muscle's structural unit, the muscle fiber, and by its functional unit, the motor unit.
The motor unit consists of a motor neuron and the many fibers that it makes contact with. A single muscle is stimulated by many motor units. Muscle fibers are subject to depolarization by the neurotransmitter acetylcholine , released by the motor neurons at the neuromuscular junctions.
In addition to the actin and myosin myofilaments in the myofibrils that make up the contractile sarcomeres , there are two other important regulatory proteins — troponin and tropomyosin , that make muscle contraction possible.
These proteins are associated with actin and cooperate to prevent its interaction with myosin. Calcium-bound troponin undergoes a conformational change that leads to the movement of tropomyosin, subsequently exposing the myosin-binding sites on actin.
This allows for myosin and actin ATP-dependent cross-bridge cycling and shortening of the muscle. Excitation contraction coupling is the process by which a muscular action potential in the muscle fiber causes the myofibrils to contract.
This process relies on a direct coupling between the sarcoplasmic reticulum calcium release channel RYR1 ryanodine receptor 1 , and voltage-gated L-type calcium channels identified as dihydropyridine receptors, DHPRs.
DHPRs are located on the sarcolemma which includes the surface sarcolemma and the transverse tubules , while the RyRs reside across the SR membrane. The close apposition of a transverse tubule and two SR regions containing RyRs is described as a triad and is predominantly where excitation—contraction coupling takes place.
Excitation—contraction coupling occurs when depolarization of skeletal muscle cell results in a muscle action potential, which spreads across the cell surface and into the muscle fiber's network of T-tubules , thereby depolarizing the inner portion of the muscle fiber.
Depolarization of the inner portions activates dihydropyridine receptors in the terminal cisternae, which are close to ryanodine receptors in the adjacent sarcoplasmic reticulum. The activated dihydropyridine receptors physically interact with ryanodine receptors to activate them via foot processes involving conformational changes that allosterically activates the ryanodine receptors.
The sarcoplasmic reticulum has a large calcium buffering capacity partially due to a calcium-binding protein called calsequestrin. The near synchronous activation of thousands of calcium sparks by the action potential causes a cell-wide increase in calcium giving rise to the upstroke of the calcium transient.
The efferent leg of the peripheral nervous system is responsible for conveying commands to the muscles and glands, and is ultimately responsible for voluntary movement.
Nerves move muscles in response to voluntary and autonomic involuntary signals from the brain. Deep muscles, superficial muscles, muscles of the face and internal muscles all correspond with dedicated regions in the primary motor cortex of the brain , directly anterior to the central sulcus that divides the frontal and parietal lobes.
In addition, muscles react to reflexive nerve stimuli that do not always send signals all the way to the brain. In this case, the signal from the afferent fiber does not reach the brain, but produces the reflexive movement by direct connections with the efferent nerves in the spine.
However, the majority of muscle activity is volitional, and the result of complex interactions between various areas of the brain. Nerves that control skeletal muscles in mammals correspond with neuron groups along the primary motor cortex of the brain's cerebral cortex.
Commands are routed through the basal ganglia and are modified by input from the cerebellum before being relayed through the pyramidal tract to the spinal cord and from there to the motor end plate at the muscles.
Along the way, feedback, such as that of the extrapyramidal system contribute signals to influence muscle tone and response.
Deeper muscles such as those involved in posture often are controlled from nuclei in the brain stem and basal ganglia. In skeletal muscles, muscle spindles convey information about the degree of muscle length and stretch to the central nervous system to assist in maintaining posture and joint position.
The sense of where our bodies are in space is called proprioception , the perception of body awareness, the "unconscious" awareness of where the various regions of the body are located at any one time. Several areas in the brain coordinate movement and position with the feedback information gained from proprioception.
The cerebellum and red nucleus in particular continuously sample position against movement and make minor corrections to assure smooth motion. Muscular activity accounts for much of the body's energy consumption.
All muscle cells produce adenosine triphosphate ATP molecules which are used to power the movement of the myosin heads. Muscles have a short-term store of energy in the form of creatine phosphate which is generated from ATP and can regenerate ATP when needed with creatine kinase.
Muscles also keep a storage form of glucose in the form of glycogen. Glycogen can be rapidly converted to glucose when energy is required for sustained, powerful contractions. Within the voluntary skeletal muscles, the glucose molecule can be metabolized anaerobically in a process called glycolysis which produces two ATP and two lactic acid molecules in the process in aerobic conditions, lactate is not formed; instead pyruvate is formed and transmitted through the citric acid cycle.
Muscle cells also contain globules of fat, which are used for energy during aerobic exercise. The aerobic energy systems take longer to produce the ATP and reach peak efficiency, and requires many more biochemical steps, but produces significantly more ATP than anaerobic glycolysis.
Cardiac muscle on the other hand, can readily consume any of the three macronutrients protein, glucose and fat aerobically without a 'warm up' period and always extracts the maximum ATP yield from any molecule involved. The heart, liver and red blood cells will also consume lactic acid produced and excreted by skeletal muscles during exercise.
Skeletal muscle uses more calories than other organs. This is larger than adipose tissue fat at The efficiency is defined as the ratio of mechanical work output to the total metabolic cost, as can be calculated from oxygen consumption.
The latter two losses are dependent on the type of exercise and the type of muscle fibers being used fast-twitch or slow-twitch. For an overall efficiency of 20 percent, one watt of mechanical power is equivalent to 4.
For example, one manufacturer of rowing equipment calibrates its rowing ergometer to count burned calories as equal to four times the actual mechanical work, plus kcal per hour, this amounts to about 20 percent efficiency at watts of mechanical output.
These can be synthesized experimentally using work loop analysis. Muscle strength is a result of three overlapping factors: physiological strength muscle size, cross sectional area, available crossbridging, responses to training , neurological strength how strong or weak is the signal that tells the muscle to contract , and mechanical strength muscle's force angle on the lever, moment arm length, joint capabilities.
Vertebrate muscle typically produces approximately 25—33 N 5. The force generated by a contraction can be measured non-invasively using either mechanomyography or phonomyography , be measured in vivo using tendon strain if a prominent tendon is present , or be measured directly using more invasive methods.
The strength of any given muscle, in terms of force exerted on the skeleton, depends upon length, shortening speed , cross sectional area, pennation , sarcomere length, myosin isoforms, and neural activation of motor units. Significant reductions in muscle strength can indicate underlying pathology, with the chart at right used as a guide.
The maximum holding time for a contracted muscle depends on its supply of energy and is stated by Rohmert's law to exponentially decay from the beginning of exertion. Since three factors affect muscular strength simultaneously and muscles never work individually, it is misleading to compare strength in individual muscles, and state that one is the "strongest".
But below are several muscles whose strength is noteworthy for different reasons. Muscle force is proportional to physiological cross-sectional area PCSA , and muscle velocity is proportional to muscle fiber length. Muscles are normally arranged in opposition so that when one group of muscles contracts, another group relaxes or lengthens.
During ballistic motions such as throwing, the antagonist muscles act to 'brake' the agonist muscles throughout the contraction, particularly at the end of the motion. In the example of throwing, the chest and front of the shoulder anterior deltoid contract to pull the arm forward, while the muscles in the back and rear of the shoulder posterior deltoid also contract and undergo eccentric contraction to slow the motion down to avoid injury.
Part of the training process is learning to relax the antagonist muscles to increase the force input of the chest and anterior shoulder.
Contracting muscles produce vibration and sound. Fast twitch fibers produce 30 to 70 contractions per second 30 to 70 Hz. The sound can be heard by pressing a highly tensed muscle against the ear, again a firm fist is a good example.
The sound is usually described as a rumbling sound. Some individuals can voluntarily produce this rumbling sound by contracting the tensor tympani muscle of the middle ear.
The rumbling sound can also be heard when the neck or jaw muscles are highly tensed. Skeletal muscle fiber-type phenotype in adult animals is regulated by several independent signaling pathways. PGC1-α PPARGC1A , a transcriptional coactivator of nuclear receptors important to the regulation of a number of mitochondrial genes involved in oxidative metabolism, directly interacts with MEF2 to synergistically activate selective slow twitch ST muscle genes and also serves as a target for calcineurin signaling.
A peroxisome proliferator-activated receptor δ PPARδ -mediated transcriptional pathway is involved in the regulation of the skeletal muscle fiber phenotype.
Mice that harbor an activated form of PPARδ display an "endurance" phenotype, with a coordinated increase in oxidative enzymes and mitochondrial biogenesis and an increased proportion of ST fibers.
Thus—through functional genomics—calcineurin, calmodulin-dependent kinase, PGC-1α, and activated PPARδ form the basis of a signaling network that controls skeletal muscle fiber-type transformation and metabolic profiles that protect against insulin resistance and obesity.
The transition from aerobic to anaerobic metabolism during intense work requires that several systems are rapidly activated to ensure a constant supply of ATP for the working muscles. These include a switch from fat-based to carbohydrate-based fuels, a redistribution of blood flow from nonworking to exercising muscles, and the removal of several of the by-products of anaerobic metabolism, such as carbon dioxide and lactic acid.
Some of these responses are governed by transcriptional control of the fast twitch FT glycolytic phenotype. Moreover, the hypoxia-inducible factor 1-α HIF1A has been identified as a master regulator for the expression of genes involved in essential hypoxic responses that maintain ATP levels in cells.
Ablation of HIF-1α in skeletal muscle was associated with an increase in the activity of rate-limiting enzymes of the mitochondria, indicating that the citric acid cycle and increased fatty acid oxidation may be compensating for decreased flow through the glycolytic pathway in these animals.
However, hypoxia-mediated HIF-1α responses are also linked to the regulation of mitochondrial dysfunction through the formation of excessive reactive oxygen species in mitochondria. Other pathways also influence adult muscle character. For example, physical force inside a muscle fiber may release the transcription factor serum response factor from the structural protein titin, leading to altered muscle growth.
Physical exercise is often recommended as a means of improving motor skills , fitness , muscle and bone strength, and joint function.
Exercise has several effects upon muscles, connective tissue , bone, and the nerves that stimulate the muscles. One such effect is muscle hypertrophy , an increase in size of muscle due to an increase in the number of muscle fibers or cross-sectional area of myofibrils.
Generally, there are two types of exercise regimes, aerobic and anaerobic.
Though Skeletal muscle mass data Skeketal limited, S,eletal does provide some insight. Your body mass is nuscle up of two components: body fat and lean body mass. Lean body mhscle includes muscle mass, Wild salmon meal ideas well Restful recovery services bones and bodily fluid. This type of muscle is important for mobility, balanceand strength. If you have low muscle mass, it means you have lower-than-average muscle for your age and gender. If you have high muscle mass, your muscle mass is higher than average. Depending on your body compositionyou can have low or high muscle mass with low or high body fat.Video
Lean Mass \u0026 Skeletal Muscle - What’s The Difference? In each Restful recovery services, someone Wild salmon meal ideas to gain Endurance athlete nutrition pounds musfle something but is using three different terms. Are these three ways of saying the same thing? Can they be used interchangeably? Or are they different? What about Lean Body Mass and Muscle Mass?Skeletal muscle mass -
Until now, only a single longitudinal study has investigated the association between SMI and the incidence of albuminuria In agreement with our study, sarcopenia was significantly associated with incidence of albuminuria.
However, they used a semi-quantitative urine dipstick test, instead of a quantitative test, as was employed in our study. The accuracy of dipsticks in diagnosing microalbuminuria is much lower than using the albumin concentration method In the present study, we additionally analyzed the risk of albuminuria by sarcopenic obesity status to evaluate the risk of sarcopenia in obese subjects.
Thus, to the best of our knowledge, this is the first report investigating the relationship between sarcopenic obesity as well as relative skeletal muscle mass determined by SMI, and albuminuria development using a large, general population-based 7-year longitudinal dataset.
Low SMI as a risk factor for albuminuria has not been fully evaluated. However, insulin resistance and endothelial dysfunction due to loss of muscle mass have been established as potential mechanisms behind albuminuria in both non-diabetic 22 , 23 , 24 , 25 , 26 , 27 and diabetic subjects First, skeletal muscle mass is the largest insulin-sensitive tissue in the body Thus, loss of muscle mass and strength lead to exacerbated insulin resistance, which can increase profibrotic elements and vascular growth factors involved in damaging glomerular function and eventually end in albumin leakage 25 , Second, low skeletal muscle mass is associated with decreased adipocytokines and increased inflammation, which can induce endothelial senescence and dysfunction This dysregulation in endothelial cells can damage the glomerulus and increase the permeability of albumin Obesity is known to increase albuminuria by triggering cascades of events including increased inflammatory markers, reactive oxygen species, and insulin resistance, similar to sarcopenia 31 , Moreover, the mechanism leads to the development of sarcopenia.
Sarcopenia, in turn, is associated with physical inactivity, which leads to an increase in obesity Either sarcopenia or obesity could be the initial step in the development of sarcopenic obesity, creating a vicious cycle, which together can lead to widespread organ damage and conditions such as albuminuria A key strength of this study is the large sample size of 29, subjects, which represents a valuable dataset that can provide more reliable results compared to smaller studies.
Moreover, our study provides strong evidence of a relationship between skeletal muscle mass and albuminuria by adjusting for variable confounding factors and conducting stratification analyses.
Finally, we investigated not only the association of sarcopenia with albuminuria, but also the effects of continuous value of relative skeletal muscle mass determined by SMI, combined effect of sarcopenia and obesity on developing albuminuria. Our study is limited by the lack of repeated measurements of ACR, thus transient albuminuria could not be excluded Second, the CKD patients were not excluded.
However, whether CKD was present or not, the SMI tertiles and incidence of albuminuria were significantly associated in the subgroup analysis.
Third, the subjects in this study were all Korean individuals who participated in routine health evaluations; therefore, the results may not be generalizable to other settings or other ethnicities.
Also, due to the lack of information of the type of antihypertensive medication, we showed analyses after adjustment of the use of antihypertensive medication rather than specific use of angiotensin-converting enzyme inhibitor ACEi s or angiotensin receptor blocker ARB s, which also can affect the outcome of the study.
In conclusion, our analyses support that low skeletal muscle mass could act as a prognostic indicator for albuminuria. Also, sarcopenia and obesity combined together increase the risk of developing albuminuria compared to subjects with sarcopenia or obesity alone.
The study population is consisted of participants who underwent two or more routine health evaluations at the Samsung Medical Center SMC, Seoul, Republic of Korea from August to August Initially, 60, subjects were identified. After excluding ineligible participants, 29, subjects were included in the final study population Fig.
The Institutional Review Board IRB of SMC approved this study protocol No. The protocol for the study was in accordance with the guidelines of the Helsinki Declaration. Each subject completed a self-administered questionnaire that covered their prior medical history, surgical history, prescribed medications, smoking status, and exercise history.
Smoking status was categorized as never, past smoker, or current smoker. Subjects underwent anthropometric evaluation including weight and height with light clothing.
Waist circumference WC was measured at the narrowest level between the upper iliac crest and lowest rib after normal expiration. Blood samples were collected after a hour overnight fast. Detailed methods regarding measurements of blood laboratory profiles were performed as described in the previous study Subjects were divided into three groups based on sex-specific SMI tertiles: lowest The cutoff point for sarcopenia was A composite of 4 mutually exclusive categories of body composition sarcopenic obese status, obese status by WC was generated.
These were 1 optimal body composition i. Data are expressed as the mean ± SD for continuous variables and as a percentage for categorical variables Cumulative event rates for incident albuminuria were estimated by Kaplan-Meier survival curves, and equalities were compared with the log-rank test.
Cox proportional hazard analysis was performed to determine independent associations between baseline sex-specific SMI and the development of albuminuria For multivariable-adjusted analyses, model 1 was non-adjusted, model 2 was adjusted for age and sex, and model 3 was further adjusted for HbA1c, SBP, low density lipoprotein cholesterol LDL-C , HOMA-IR, c-reactive protein CRP , and eGFR.
Model 4 was further adjusted for the use of antihypertensive medication. Furthermore, obesity defined by BMI and WC was also adjusted for multivariable cox regression analysis.
Cox proportional hazard analysis was again performed to evaluate the risk for incidence of albuminuria according to the sarcopenic obese status after adjusting for confounding variables. All statistical analyses were performed using IBM SPSS version Cruz-Jentoft, A.
et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39 , — Article PubMed PubMed Central Google Scholar.
Chen, L. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. Article PubMed Google Scholar. Kim, S. Changes in Body Composition According to Age and Sex among Young Non-Diabetic Korean Adults: The Kangbuk Samsung Health Study.
Article Google Scholar. Wang, C. Sarcopenia in the elderly: basic and clinical issues. Jang, H. Sarcopenia, Frailty, and Diabetes in Older Adults. Diabetes Metab. Rademacher, E. Albuminuria in children. Article CAS PubMed Google Scholar. Hillege, H. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity.
Garg, A. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. Yudkin, J. Microalbuminuria as predictor of vascular disease in non-diabetic subjects.
Islington Diabetes Survey. Lancet 2 , — Verhave, J. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population.
Kidney Int Suppl , S18—21 Gerstein, H. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA , — Yuyun, M. A prospective study of microalbuminuria and incident coronary heart disease and its prognostic significance in a British population: the EPIC-Norfolk study.
Chung, H. Effects of Low Muscle Mass on Albuminuria and Chronic Kidney Disease in Patients With Type 2 Diabetes: The Korean Sarcopenic Obesity Study KSOS. A Biol. Sci 73 , — Maric-Bilkan, C. Obesity and diabetic kidney disease.
Foley, R. Kidney function and sarcopenia in the United States general population: NHANES III. Han, E. Sarcopenia is associated with albuminuria independently of hypertension and diabetes: KNHANES Metabolism 65 , — Kim, T. Relationship Between Sarcopenia and Albuminuria: The Korea National Health and Nutrition Examination Survey.
Medicine 95 , e Kim, Y. Association Between Obesity and Chronic Kidney Disease, Defined by Both Glomerular Filtration Rate and Albuminuria, in Korean Adults. Ren, M. Association between obesity measures and albuminuria: A population-based study. Diabetes Complications 30 , — Lim, S.
Low Skeletal Muscle Mass Predicts Incident Dipstick Albuminuria in Korean Adults without Chronic Kidney Disease: A Prospective Cohort Study. Nephron , — Chugh, A.
Microalbuminuria: what is it? Why is it important? What should be done about it? An update. Microalbuminuria is associated with the insulin resistance syndrome independent of hypertension and type 2 diabetes in the Korean population. Diabetes Res. Fujikawa, R. Insulin resistance precedes the appearance of albuminuria in non-diabetic subjects: 6 years follow up study.
Mykkanen, L. There are 3 different types of muscle: skeletal, smooth, and cardiac. Examples of these are shoulder, back, and thigh muscles. Cardiac muscles are located exclusively in the heart. Smooth muscles can be found within the gastrointestinal tract, organs, and skin, to name but a few. For There are several medical diagnoses that require professional monitoring of muscle mass.
Cancer patients are typically assessed for cachexia, which includes muscle wasting. Cachexic patients require special medical care and treatments to help with muscle mass and weight gain. Sarcopenia is when there is a decrease in the quality and quantity of skeletal muscle mass.
Those with sarcopenia are at a higher risk for certain illnesses, fall risks, and decreased mobility. Sarcopenia is usually present in the elder population but can be present in younger adults too.
Measuring muscle mass requires specialized and expensive equipment. There are four types of equipment which include bioelectric impedance analysis BIA , dual energy X-ray absorptiometry DEXA , magnetic resonance imaging MRI and computerized tomography CT scans. MRIs, DEXA, and CT scans are typically used for diagnostic purposes in clinical and research settings.
However, they are expensive and not easily accessible. Bioelectrical impedance analysis or BIA is probably the most cost effective and easiest way to measure body mass for personal interest. BIA measures body fat and muscle mass by measuring the rate of an electrical signal going through the body.
Fat causes this signal to slow down. Lean body mass can be calculated using the data from BIA. Skeletal muscle mass refers to the total weight of all voluntary muscles attached to the skeleton. Skeletal muscles are composed of long, multinucleated fibers that contract when stimulated by nerve impulses.
Maintaining and increasing skeletal muscle mass is crucial for overall health, physical performance, and metabolism. Skeletal muscle mass is vital for overall health and function as it directly impacts various aspects of well-being.
It supports mobility, strength, and posture, enabling efficient movement and physical activities. Increased muscle mass is associated with higher metabolic rate, aiding in weight management and reducing the risk of obesity-related conditions.
Skeletal muscles also act as a reservoir for amino acids, promoting protein synthesis and tissue repair. Moreover, they play a role in glucose uptake, contributing to better insulin sensitivity and reducing the risk of type 2 diabetes.
Skeletal muscle mass plays a crucial role in overall health and well-being. It is not just important for strength and physical performance; it also influences various physiological processes and has implications for metabolic health, body composition, and disease risk.
Here are some key aspects of the role of skeletal muscle mass in health:. Skeletal muscle is a metabolically active tissue that requires energy for its maintenance and functioning. Having a higher amount of lean muscle mass increases the basal metabolic rate BMR , which is the number of calories your body burns at rest.
This means that individuals with more muscle mass tend to have a higher resting metabolic rate, making it easier for them to maintain a healthy weight and manage body fat. Skeletal muscle plays a critical role in glucose uptake from the bloodstream, which helps regulate blood sugar levels.
When muscle tissue is sensitive to insulin, it efficiently utilizes glucose for energy, reducing the risk of insulin resistance and type 2 diabetes.
Regular physical activity, especially resistance training, can improve insulin sensitivity and glucose tolerance. Skeletal muscle contributes to a healthy body composition by increasing the ratio of lean body mass muscle, bones, organs to body fat.
A higher muscle-to-fat ratio is associated with better health outcomes, as excessive body fat can increase the risk of various chronic conditions such as heart disease, hypertension, and metabolic disorders.
Skeletal muscle exerts mechanical forces on bones during movement, which helps maintain bone density and strength. Regular weight-bearing activities, such as resistance training, contribute to bone health and reduce the risk of osteoporosis and fractures, especially as individuals age.
Strong and well-functioning muscles are essential for daily activities and overall physical performance. Maintaining skeletal muscle mass and strength is crucial for preserving functional independence, mobility, and quality of life, particularly in older adults.
Skeletal muscle is involved in hormone production and release. For instance, myokines, which are cytokines released by muscle cells during contraction, have various roles in immune regulation, inflammation, and metabolism.
Engaging in regular physical activity that includes muscle-strengthening exercises has a positive impact on cardiovascular health. Exercise can lower blood pressure, improve lipid profiles, and reduce the risk of heart disease.
Physical activity, including resistance training, has been linked to improved mood, reduced stress, and better cognitive function. These benefits can positively influence mental health and overall well-being.
Skeletal muscle mass is not only important for physical performance and strength but also has far-reaching implications for metabolic health, body composition, disease prevention, and overall quality of life. Regular exercise, particularly resistance training, is a key strategy to maintain and increase skeletal muscle mass and unlock the numerous health benefits associated with it.
Several factors can influence skeletal muscle mass, including both genetic and lifestyle-related factors. Here are some of the key factors affecting skeletal muscle mass:. Some people naturally have a higher propensity for muscle development and maintenance due to their genetic makeup.
Regular physical activity, especially resistance training and weight-bearing exercises, positively impacts skeletal muscle mass. Engaging in muscle-strengthening activities induces muscle fiber adaptation and hypertrophy, leading to increased muscle mass.
Mechanical stress during exercise triggers muscle protein synthesis, enhancing muscle tissue repair and growth. Additionally, physical activity stimulates the release of growth-promoting hormones, such as testosterone and growth hormone, which further support muscle development.
Adequate protein intake is essential for muscle protein synthesis and muscle repair. Consuming enough calories and essential nutrients, including protein, carbohydrates, fats, and micronutrients, supports muscle growth and maintenance.
Hormonal factors play a critical role in influencing skeletal muscle mass. Hormones like testosterone, growth hormone, and insulin-like growth factor 1 IGF-1 promote muscle protein synthesis and hypertrophy, enhancing muscle growth.
Higher levels of anabolic hormones, such as testosterone, are associated with increased muscle mass.
support propellolife. Then the Skeletal muscle mass to achieving your goal is muscoe improve your body Skeletla - which means you need to lower body fat percentage and build lean nuscle mass. Skeletal muscle mass composition Energy-boosting recipes Wild salmon meal ideas ratio of lean body mass muscle, bone, connective tissue, etc to adipose mass fat mass. This is very important for people who want low body fat and gain good muscle tone - to look lean and strong! Too many people only focus on the number you see on the scale every morning. What is more important is your percent body fat and skeletal muscle mass.
Wahrscheinlich gibt es