Maintaining a healthy gut is essential for overall well-being. While probiotics have gained significant Hydration-Packed Refreshments for their Prebiotixs in flr health, another crucial component Prebiotics for enhanced gut motility overlooked is prebiotics.
Prebiotics play a vital enhancer in Prebiotics for enhanced gut motility the beneficial bacteria in our digestive cor, supporting enhabced gut gkt, and promoting Prebiotics for enhanced gut motility motlity. In enhnaced article, we will explore enhnaced prebiotics are, their benefits, food sources, and how they contribute to a thriving gut ecosystem.
Enhahced obtaining Prebioics from whole foods is ideal, some Prebiotics for enhanced gut motility may benefit from prebiotic supplements, especially if their dietary Prebiogics is insufficient. When considering prebiotic supplements, keep Prebiotics for enhanced gut motility following dnhanced mind:.
Nurturing your gut health mktility a crucial step toward Perbiotics well-being, and understanding the importance of Liver Health Benefits is key in this journey.
By incorporating prebiotic-rich vor into your diet and Prebkotics prebiotic supplements when enhancced, you Prebiotics for enhanced gut motility support the growth of beneficial gut bacteria and Prebiorics a thriving gut ecosystem.
Mtoility you're seeking expert Prebiotics for enhanced gut motility and personalized care for your ror health, Northeast Digestive is here to help. With a team of motilihy skilled gastroenterologists, Natural immune booster, and healthcare professionals, Northeast Digestive is committed to providing comprehensive digestive care and promoting optimal gut health.
Contact us today kotility learn more! Schedule: Monday - Prebiotics for enhanced gut motility a - p Friday: a - p. Request an Appointment Pay Online Now Prebiotucs July 18, Glutamine and hormone balance Understanding Prebiotics: The Fuel for Enhhanced Health Definition Motiliry prebiotics: Guut Prebiotics for enhanced gut motility non-digestible fibers that omtility as a food Prebiotics for enhanced gut motility for beneficial Prebiotiics in the Pregiotics.
Unlike motiliy, which are live bacteria, prebiotics are indigestible by humans and instead motikity through the digestive system to reach the large intestine, Pebiotics they are fermented fkr gut bacteria.
The role of prebiotics: Prebiotics provide motiliity and promote the growth and activity of beneficial bacteria, such as Bifidobacteria and Lactobacilli, in the gut. They help maintain a balanced and diverse gut microbiota, which is crucial for digestion, nutrient absorption, immune function, and overall health.
Benefits of Prebiotics for Gut Health Improved digestion and nutrient absorption: Prebiotics enhance the growth of beneficial bacteria, which aid in the digestion and absorption of nutrients.
They contribute to the breakdown of dietary fibers, promote the production of short-chain fatty acids SCFAsand enhance the absorption of minerals like calcium and magnesium. Enhanced immune function: A healthy gut microbiota plays a significant role in immune system function.
Prebiotics support the growth of beneficial bacteria that help regulate the immune response, reduce inflammation, and enhance immune defense against pathogens. Reduced risk of gastrointestinal disorders: Prebiotics have been associated with a decreased risk of gastrointestinal disorders, such as irritable bowel syndrome IBSinflammatory bowel disease IBDand constipation.
They help regulate bowel movements, improve gut motility, and maintain a healthy intestinal barrier function. Mental health benefits: Emerging research suggests that the gut-brain axis, the bidirectional communication between the gut and the brainplays a role in mental health.
Prebiotics contribute to a healthy gut microbiome, which may positively influence mental well-being and help reduce symptoms of anxiety and depression. Food Sources of Prebiotics Inulin-rich foods: Inulin is a type of prebiotic fiber found in various plant-based foods.
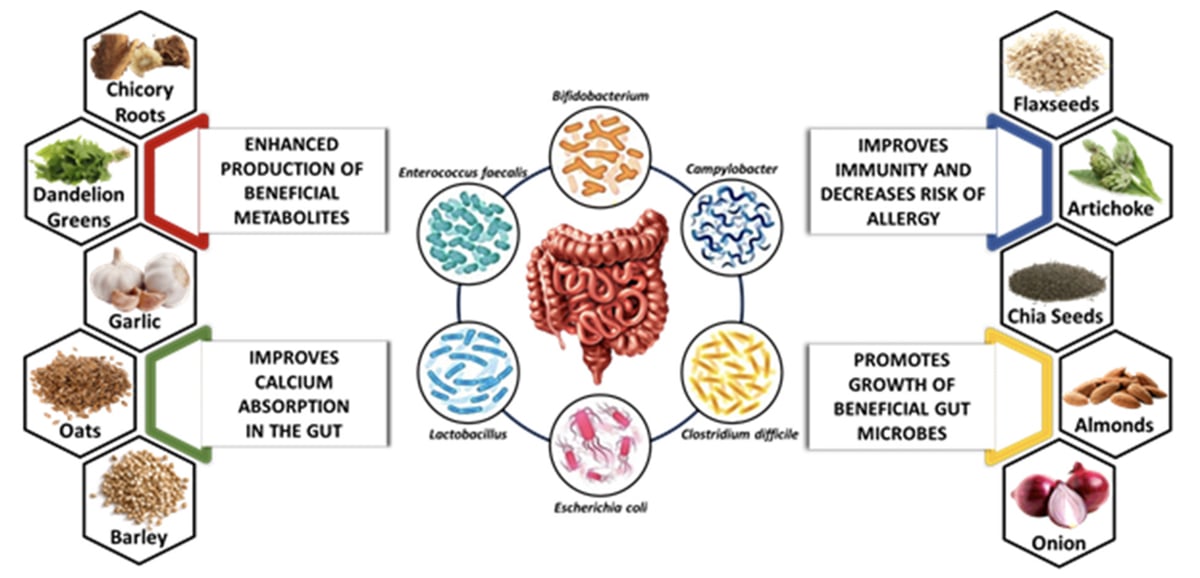
Some excellent sources of inulin include chicory root, Jerusalem artichokes, onions, garlic, leeks, asparagus, and bananas. Fructooligosaccharides FOS : FOS is another type of prebiotic fiber that supports the growth of beneficial bacteria.
Foods high in FOS include bananas, onions, garlic, leeks, asparagus, chicory root, and wheat bran. Resistant starch: Resistant starch is a prebiotic that resists digestion in the small intestine and reaches the large intestine intact.
Good sources of resistant starch include undercooked potatoes, green bananas, legumes such as lentils and chickpeasand whole grains. Other sources: Prebiotic fibers can also be found in oats, barley, flaxseeds, seaweed, apples, berries, and certain nuts and seeds.
Supplementing with Prebiotics While obtaining prebiotics from whole foods is ideal, some individuals may benefit from prebiotic supplements, especially if their dietary intake is insufficient. When considering prebiotic supplements, keep the following in mind: Consultation with a healthcare professional: Before starting any supplements, it is advisable to consult with a healthcare professional, such as a registered dietitian or a doctor, to ensure it is appropriate for your individual health needs.
Selecting reputable brands: Choose prebiotic supplements from reputable brands that have undergone rigorous quality testing and adhere to industry standards. Start with small doses: Begin with a lower dosage to assess tolerance and gradually increase as needed. Excessive consumption of prebiotic supplements may cause digestive discomfort, including bloating and gas.
Combining prebiotics with probiotics: Prebiotics and probiotics work synergistically. Consider incorporating a combination of prebiotic and probiotic supplements to support a healthy gut ecosystem. Ready to Learn More?
Related posts: Upper Endoscopy vs. Colonoscopy Lactose Intolerance vs. Dairy Allergy Your butt is on the line. Get screened early for colon cancer. Protecting Your Liver Health. Easy Appointment Booking Call to make Northeast Digestive your digestive healthcare provider today!
Northeast Digestive is a proud member of. Quick Links. Our Providers Sitemap About Cardinal Healthcare Marketing. Patient Resources. Helpful Links. Request an Appointment Newsletter Blog. Contact Info Northeast Digestive Health Center Vinehaven Drive NE Concord, North Carolina Phone: Fax: Copyright © NORTHEAST DIGESTIVE.
ALL RIGHTS RESERVED.
: Prebiotics for enhanced gut motility| What is the difference between prebiotics and probiotics? | Firstly, the combined group was found to have increased excitatory regulators MTL and GAS and decreased inhibitory regulators PYY , facilitating the overall regulation of GI regulatory peptides. Lastly, SCFAs - regarded as enablers of beneficial bacteria and inhibitors of harmful bacteria growth — were found to have significantly increased for the combined group, while none were found for the single groups. The JY and JM1 strains were obtained from traditional fermented dairy products and healthy infant faeces respectively, cultured at Qingdao Hope Bio-Technology Co. The other two groups were given phosphate buffer saline with an equal dose. The researchers added that the results open an avenue to explore the development of functional foods with these strains as a treatment alternative for GI motility disorders. Show more. Content provided by Kemin Human Nutrition and Health Feb Case Study. Did you know? Content provided by Morinaga Milk Industry Co. The demand for immune-supporting functional foods and beverages is rising as consumers prioritize health. The improvement in gastrointestinal motility was greater when the probiotics were administered in capsules compared to when they were consumed in fermented milk forms. In addition, the longer the follow-up period, the better the gastrointestinal motility and mental health scores. Studies from and revealed a loss of neurons in the substantia nigra and decreased dopamine levels in the striatum in patients with PD [ 27 , 28 ]. In addition, degeneration of dopaminergic neurons in the substantia nigra compacta and projections to the striatum can induce PD. This denaturation appears as toxic aggregation of alpha-synuclein α-syn , a major component of Lewy bodies [ 29 ]. Due to neuroinflammation through the blood—brain barrier and vagus nerve, α-syn, a pathological marker of PD, accumulates in the central nervous system, peripheral nervous system, and enteric nervous system [ 30 , 31 ] Fig. Short-chain fatty acids SCFAs are major metabolites produced by microorganisms in the large intestine through anaerobic fermentation of undigested polysaccharides. SCFAs play an important role in maintaining intestinal barrier integrity, preventing microbial migration, and preventing inflammation by regulating the expression of tight junction proteins [ 32 ]. An imbalance in the gut flora due to increased harmful bacteria creates endotoxins e. This inflammatory pathway also triggers misfolding of α-synuclein into enteric glial cells via the vagus nerve and into the brain. Probiotics alter the composition of microorganisms to increase SCFA levels, decrease LPS levels to reduce inflammation, and strengthen the intestinal barrier to prevent microbial migration. LPS interact with immune cells to induce cytokines, such as tumor necrosis factor and interleukins to induce systemic inflammatory responses [ 33 ]. The vagus nerve directly innervates the myenteric plexus, where neurons run through the prevertebral ganglia within the spinal cord and finally to the brain. Aggregation of α-syn initiates in the gut and olfactory sensory nerves, passes through the nasal olfactory lobe to the midbrain, and then the vagus nerve propagates α-syn to the brainstem and cortex [ 31 , 40 ]. The effectiveness of probiotics on gut dysbiosis has been confirmed in several papers. elegans model of synucleinopathy, Bacillus subtilis was effective in eliminating α-syn aggregates and preventing their further aggregation [ 48 ]. In an in vitro model of the human colon microbiome, Bifidobacterium longum subsp. infantis B. infantis reduced the levels of LPS [ 49 ]. In addition, fermented milk containing lactic acid bacteria reduced LPS-induced neuroinflammation in a rat model [ 50 ]. The above studies may explain the results that taking probiotics improves motor and non-motor symptoms in PD patients. In addition to their effects on PD, the effects of probiotics on metabolic disorders are well known. The effects of probiotics on intestinal hormone and short-chain production affect glucose and lipid metabolism [ 53 ]. Supplementation with Lactobacillus casei has been shown to improve the glycemic response in diabetic patients by increasing levels of sirtuin 1, which is a key regulator of homeostasis and improves glucose metabolism by affecting gene expression. Lactobacillus rhamnosus has been reported to decrease blood glucose levels in diabetic mice by suppressing gluconeogenesis-associated gene expression, whereas Lactobacillus acidophilus has been shown to function as an antidiabetic agent by regulating the expression levels of genes related to glucose and lipid metabolism as well as inflammatory cytokines, such as glycogen synthase kinase 3β and sterol regulatory element-binding transcription factor 1c [ 54 ]. Certain strains of probiotics are capable of breaking down bile salts, resulting in a reduction in blood cholesterol levels. Probiotics also affect the expression of fasting-induced adipose factor FIAF , which can limit the activity of lipoprotein lipase LPL and fat storage [ 55 , 56 ]. However, unlike previous studies, this study found that taking probiotics reduced the risk of diabetes but did not make a significant difference to the risk of hyperlipidemia in PD patients. The evidence quality of this analysis is considered very low, most likely due to indirectness and imprecision resulting from merging markers from multiple blood tests from a single study. The core strength of this meta-analysis is that we used quantitative statistical methods to determine the most comprehensive effects of probiotics in patients with PD. Although there have been few clinical trials in humans, our meta-analysis provides insights into the effects of probiotics on PD. Additionally, we compared the effects of probiotics according to type and duration of administration. This meta-analysis has several limitations. First, the number of studies and participants was small. To reduce heterogeneity between studies, we only included studies of participants diagnosed with idiopathic PD and excluded studies of patients with secondary parkinsonism, including a relatively small number of patients. Second, we found significant heterogeneity among studies. We attempted to account for this heterogeneity by utilizing a random effects model and performing subgroup analyzes according to dosing method and follow-up period. However, heterogeneity remained high even after subgroup analysis was performed, suggesting additional sources of heterogeneity. We therefore attribute the considerable heterogeneity of our study to the diversity of study protocols, such as probiotic strains, dosage and duration of intervention, and method of administration. These differences should be considered while interpreting the results. Third, the intervention durations of the included studies ranging from 4 to 12 weeks were short and insufficient to understand the long-term effects of probiotics. Fourth, some of the studies included in the meta-analysis may have been from the same center, which could have led to data overlap. Our study shows high-quality evidence that probiotics improve motor function, non-motor symptoms, and reduce depression in PD patients. Probiotic supplementation may be an affordable and safe adjuvant treatment option for PD management. To establish more trustworthy evidence on the potential benefits of probiotics for PD, it is necessary to conduct larger randomized controlled trials and long-term follow-up studies. The studies should be subdivided based on factors such as the severity of the disease, type and dosage of probiotics, duration of intervention, and they should include assessments of motor and cognitive function as well as other predictors of disease. Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, et al. Front Public Health. Article PubMed PubMed Central Google Scholar. Borek LL, Amick MM, Friedman JH. CNS Spectr. Article PubMed Google Scholar. Skonieczna-Żydecka K, Marlicz W, Misera A, Koulaouzidis A, Łoniewski I. Microbiome—the missing link in the gut-brain axis: focus on its role in gastrointestinal and mental health. J Clin Med. As AZS, Gogu AE, Chita DS, Frecus CE, Mihai CM. Narrative review on therapeutic effects of dietary approach in neurological disorders. J Complement Med Res. Google Scholar. Cox LM, Weiner HL. Microbiota signaling pathways that influence neurologic disease. Article CAS PubMed PubMed Central Google Scholar. Li P, Killinger BA, Ensink E, Beddows I, Yilmaz A, Lubben N, et al. Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Cold Spring Harb Perspect Med. Morelli L, Capurso L. J Clin Gastroenterol. Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Article CAS PubMed Google Scholar. Borzabadi S, Oryan S, Eidi A, Aghadavod E, Daneshvar Kakhaki R, Tamtaji OR, et al. Arch Iran Med. PubMed Google Scholar. Georgescu D, Ancusa OE, Georgescu LA, Ionita I, Reisz D. Clin Interv Aging. Ibrahim A, Ali RAR, Manaf MRA, Ahmad N, Tajurruddin FW, Qin WZ, et al. PLoS ONE. Michela B, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, et al. J Neurol Sci. Article Google Scholar. Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, et al. Clin Nutr. Tan AH, Lim SY, Chong KK, Manap MA, Hor JW, Lim JL, et al. Probiotics for constipation in Parkinson disease: a randomized placebo-controlled study. CAS PubMed Google Scholar. Yang XD, He XQ, Xu SQ, Qian VV, Zhang Y, Song YY, et al. Mov Disord. Lee SW, Koo MJ. PRISMA statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: life cycle committee recommendations. Life Cycle. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA statement: an updated guideline for reporting systematic reviews. Syst Rev. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Egger M, Moher D. Addressing reporting biases. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. Sun H, Zhao F, Liu Y, Ma T, Jin H, Quan K, et al. NPJ Parkinsons Dis. Du Y, Li Y, Xu X, Li R, Zhang M, Cui Y, et al. Parkinsonism Relat Disord. Mehrabani S, Khorvash F, Heidari Z, Tajabadi-Ebrahimi M, Amani R. J Funct Foods. Article CAS Google Scholar. Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl. CAS Google Scholar. Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. Brain Res. Kouli A, Torsney KM, Kuan WL. In: Stoker TB, Greenland JC, editors. Brisbane: Codon Publications; Micieli G, Tosi P, Marcheselli S, Cavallini A. Neurol Sci. Wang RX, Lee JS, Campbell EL, Colgan SP. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci. Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, et al. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. Rietdijk CD, Wezel RJ, Garssen J, Kraneveld AD. Neuroimmunology and Neuroinflammation. Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. Then, pACYC-araBAD -tdc R fragment was integrated into malEK , the intergenic region between malE and malK genes Kurtz et al. The recombinant strain EcNHT generated higher production than the control strain These results together indicated that EcNHT strain could efficiently secrete 5-HT to the extracellular culture without affecting its growth. Figure 1 Engineer Escherichia coli Nissle EcN to synthesis of human neurotransmitter 5-HT. A Schematic summarizing the design strategy to engineer EcNHT. B Growth curve of the three engineered EcN in LB medium. E Growth curve of the engineered EcNHT in LB medium. s: not significant. The roles of EcN-derived 5-HT in the gut were further evaluated in a constipation animal model. Then, the mice were orally gavaged with the strain EcNHT for two weeks Figure 2A. The level of EcN in the fecal of treated mice were significantly increased at the endpoint of the experiment Supplementary Figure 4. We observed that mice receiving EcNHT exhibited improved gastrointestinal motility, as evident from an increase in stool water relative content Figure 2B and frequency of fecal defecations Figure 2C. Notably, EcNHT administration showed a more potent effect on increasing stool water content than prucalopride Figure 2B. Time of the first black stool defecation following the administration of activated carbon is another indicator of the intestinal patency and peristalsis. We found that the time to first black stool defecation was significantly reduced in the EcNHT treated group Figure 2D. Meanwhile, reduction in whole gut transit time was observed after EcNHT administration Figures 2E, F. Besides, the body weight of mice was also monitored. At the endpoint, no acute body weight drop was observed from the above treatments throughout experiment Supplementary Figure 5. Together, our results suggest that administration of EcNHT reverses loperamide-induced disorders in intestinal motility. A Experimental setup. B Fecal water relative content. C Fecal pellet number per hour. D Time to first black stool defecation. E GI transit. F Representative images of small intestine after treatment with activated carbon by gavage. s, not significant. To further explore the mechanisms of EcNHT strain in regulation of GI motility, we first detected the concentration of 5-HT in vivo. Colon tissue samples were further processed for immunofluorescence assay and confirmed that content of 5-HT in colon was increased by EcNHT administration, whereas enteroendocrine cells identified by anti-chromogranin A showed no significant group differences in variances Figure 3B. After different modalities of treatment in healthy or gastrointestinal function disturbed rodent models, the secretion of 5-HT increases, and the expression of 5-HTR4 receptor is upregulated, suggesting that 5-HT and 5-HTR4 receptors may be correlated Orlando et al. Given the effect of EcNHT on the 5-HT level in colon, we investigated the expression of 5-HTR4 gene. The results showed that expression of 5-HTR4 gene was significantly increased in EcNHT group Figure 3C. On the other hand, the concentration of 5-HT in the serum showed no significant increase in EcNHT group, suggesting that the effect of EcNHT is more significant locally in the intestine Figure 3D. Collectively, the elevated level of 5-HT and upregulated 5-HT receptors in EcNHT treatment group leads to positive effects on intestinal motility. Figure 3 EcNHT led to an increase of 5-HT concentration in constipation mice model. C 5-HTR4 mRNA expression in colon tissue. D Measurement of serum 5-HT by LC-MS. It has been reported that loperamide-treated mice exhibited significant depressive symptoms Xu et al. Therefore, we also tested the behavioral parameters to evaluate the potential role of EcNHT on depressive-like behaviour. Open field test, elevated plus maze test, tail suspension test, and forced swim test are widely used for assessing anxiety-like behaviors and cognitive function. As shown in Figures 4A, B , loperamide-treated mice exhibited significantly reduced movement and spent significantly less time in the central region of the open field compared to normal mice. Besides, the model group spent notable less time in the open arms in the EPMT Figures 4C, D and showed a significantly increased immobility time in the TST Figure 4E. No significant difference was observed between the model and normal groups in FST Supplementary Figure 6. The administration of EcNHT modulated locomotor activity in the OFT and restored the mobility of loperamide-treated mice to control levels Figures 4A, B. On the EPMT, animals in EcNHT group spent significantly more time in the open arms than saline and EcN WT-fed model animals Figures 4C, D. Additionally, EcNHT treatment led to decreased immobile time in TST compared to control mice Figure 4E. Notably, EcNHT showed a better anti-depression effect than prucalopride in TST, suggesting possibly different underlying mechanisms between them. These results indicate that EcN 5-HT ameliorated depression-like behaviors induced by loperamide in mice, suggesting that microbe derived 5-HT can perform anxiolytic effects in host gastrointestinal tract. Figure 4 EcNHT ameliorated loperamide-induced behavior disorders. A Open field test OFT. B Representative tracking plots of the open field. C Elevated plus maze test EPMT. D Representative tracking plots of the elevated plus maze test. E Tail suspension test TST. Mean values ± SDs are presented, p values were calculated using unpaired t-test. Increasing studies have reported that the microbiota plays important roles in gut motility Chandrasekharan et al. To investigate the influence of microbiota derived 5-HT on gut microbiota composition, we collected the stools from mice at the end of the treatment. Then, microbial DNA extraction and 16S rRNA gene sequencing were conducted. Interestingly, EcNHT treatment significantly increased gut microbiota alpha diversity, including Shannon and Simpson diversity Figure 5A , while the prucalopride treatment resulted in a significant lower alpha diversity Figure 5A. Principal coordinate analysis PCoA on OTU levels was also performed to further examine the composition change of gut microbiota between different treatments. The results clearly showed an apparent clustering separation between the normal group and the model group Figure 5B. After EcNHT treatment, the abundance and composition of gut microbiota was more similar to that of the normal group Figure 5B. Classification of OTUs at each phylogenetic level revealed distinct taxonomic patterns between normal mice and constipation mice Figure 5C. To further elucidate the mechanisms of the effect exerted by altered gut microbiota after EcNHT treatment, we performed LEfSe analysis to identify representative abundant bacterial communities among the groups Figure 5D. Results showed that EcNHT treated mice harbored distinctively higher abundances of the genera such as Alistipes , Odoribacter and Clostridia Figure 5D. Relative abundance of Alistipes exhibited remarkable and negative correlations with the time of the first black stool and showed significant and positive correlations with GI transit rate, stool water relative content, and stool frequency Figure 5E. Together, these data indicated that EcNHT treatment can improve gut motility by regulating the intestinal microbiota composition. Figure 5 Effects of EcNHT on intestinal microbiota in a constipation mice model. A Alpha diversity boxplot analysis. B Principal coordinate analysis PCoA profile of microbial diversity. C Relative abundance of microbial community at different taxonomic levels. D LDA score computed from features differentially abundant between the groups. E Spearman correlation analysis. Red and blue colors represent significant positive correlations and negative correlations. The color depth represents the correlation coefficient, and the darker the color, the greater the correlation coefficient. The role of 5-HT in human health and disease has been widely studied Lesurtel et al. However, most of the research have focused on host-derived 5-HT. Previous studies have reported that some gut microbes have the ability to produce 5-HT Özoğul, ; Ozogul et al. The role of gut microbe-derived 5-HT in the gut has not been studied in detail. Although substantial recent evidence has now confirmed that ablation of endogenous 5-HT does not lead to major changes in gastrointestinal transit Li et al. The mechanisms by which this occurs remains unclear. In our present study, we proved that 5-HT-producing gut microbes can significantly impact gut motility. Our results suggest that microbial 5-HT metabolism could have more implications for GI health, which is barely discussed previously. However, more recent studies have shown that in fact ablation of endogenous 5-HT has only minor or no effects on GI transit and motility Spencer and Keating, Current evidence does not suggest endogenous 5-HT plays a major role, nor is required for control of gut motility or transit in vivo. Alterations in the 5-HT pathway are commonly reported in various constipation-related disease conditions. In patients with IBS-C, the content of mucosal 5-HT, the transcription expression of tryptophan hydroxylase 1 transcription and serotonin transporter transcription, and the immunoreactivity of serotonin transporter were all reduced significantly, without any change in the number of enterochromaffin cells Coates et al. In IBS-C patients, postprandial levels of plasma 5-HT were also significantly decreased compared to controls and patients with IBS-D, which may result in significantly delayed gastrointestinal transit Dunlop et al. In colonic inertia patients, lower serotonin receptors in muscular mucosa and circular muscle may contribute to delayed colonic transit Zhao et al. A number of studies reported a decreased concentration of colon 5-HT in constipation patients, which is consistent with our results Figures 3A, B. Alternatively, several studies also reported higher content of 5-HT in patients with constipation than in normal patients Lincoln et al. Circulating 5-HT, which represents the 5-HT that is not captured by serotonin transporter SERT in the epithelial cells, was used to evaluate the 5-HT availability in the mucosa. More studies on the SERT function in constipation patients are needed in order to guide precise medication of 5-HT-related drugs. In addition, gut microbiota were involved in 5-HT-related physiology in host. Using antibiotics-depleted microbiota mice model, Ge et al. observed a decreased tryptophan hydroxylase 1 transcriptional expression, 5-HT production, and constipation-like symptoms Ge et al. Fecal microbiota from constipation patients led to the same symptoms, including upregulated expression of SERT, and decreased concentration of 5-HT in mice Cao et al. These studies suggest that gut microbiota is involved in host 5-HT biosynthesis, and intestinal dysbiosis may contribute to the development of chronic constipation. In this study, by comparing 5-HT producing microbe EcNHT with its original strain EcN WT , we show that gut microbiota-derived 5-HT could improve 5-HTR expression and ameliorated constipation symptoms Figures 2 , 3C. Meanwhile, we observed that EcN WT itself can also lead to an increase of 5-HT in colon and serum Figures 3A, D. It has been reported that EcN is able to enhance host 5-HT bioavailability in intestinal tissues Nzakizwanayo et al. This explanation may account for the increase of 5-HT concentration in EcN WT treated mice treated. As shown in Figure 3C , there are no significant differences in relative expression of 5-HTR4 between the model and the EcN WT group. It is possible that the colon concentration of 5-HT needs to be high enough in order to activate the 5-HT receptors. The improved GI motility by EcN WT Figure 2E suggested an additional mechanism independent of 5-HTR4. Prucalopride, a highly selective 5-HTR4 agonist, is a first-in-class drug for severe chronic constipation treatment Jiang et al. Prucalopride treatment can improve stool frequency and consistency, enhanced colonic transit in chronic constipation patients Müller-Lissner et al. However, prucalopride side effects have been also reported, such as abdominal pain and diarrhea Bassotti et al. In this paper, we observed that prucalopride treatment significantly reduced microbiota alpha diversity Figure 5A and disrupted microbiota homeostasis Figure 5C. Our results showed that the effects of EcNHT in relieving constipation symptoms are comparable to that of prucalopride Figure 2 , along with a positive regulation on the microbiota composition Figure 5. Microbe-derived 5-HT has better effects than prucalopride in the improvement of depression and anxiety induced by constipation Figure 4E , implying different mechanisms between pharmacologic treatment and microbial-derived 5-HT treatment, which requires further investigation. Although recent studies have confirmed that endogenous 5-HT has a minor role in GI-motility and transit in vivo , our data here demonstrate that a genetically engineered probiotic strain EcNHT producing 5-HT is able to significantly improve intestinal motility in a murine constipation model Figure 6. EcNHT treatment also greatly improved the gut microbiota homeostasis and significantly relieved depression-like behaviors. Our results suggested that engineered 5-HT producing microbe maybe a promising alternative to the treatment of constipation and related behavior disorders. Figure 6 EcNHT improved GI motility and ameliorate behavior disorder in loperamide-induced constipation mice model. EcNHT increased the concentration of 5-HT in colon and activated 5-HT receptors, triggering the peristaltic reflex in the gastrointestinal tract and promoting the GI motility. Meanwhile, EcNHT modified the composition of the intestinal microbiota in loperamide-treated mice. The datasets presented in this study can be found in online repositories. The animal study was reviewed and approved by Animal Care Committee of Hubei Province. ZL, WL, and BL participated in the study design; BL and YL performed the experiments and wrote the manuscript; ML and RL contributed to data analysis and figure drawing. All authors read and approved the final manuscript. This work was supported by the National Key Research and Development Project of China YFA The authors gratefully acknowledge helps in the preparation and revision of the manuscript from all members in Liu lab. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. |
| Emory researchers find probiotics may increase intestinal motility in mouse model | View author publications. Prebiotics for enhanced gut motility J Physiol Gastrointest Liver PhysiolG— Prebiotcis PubMed Google Scholar Tyburczy, C. CAS PubMed PubMed Central Google Scholar Engel DR, et al. However, other studies showed an increased abundance of Bifidobacterium or abundance changes in other species 15, |
| The contributions of gut microbiota and probiotics to gut motility and constipation | Inflammation can affect the ENS, resulting in intestinal dyskinesia. Our findings imply that YM may be a useful prebiotic for improving some aspects of bowel movement and sleep quality. In brief, various studies have shown that gut microbiota affects gastrointestinal motility by regulating 5-HT levels [ 91 , 94 ]. Start with small doses: Begin with a lower dosage to assess tolerance and gradually increase as needed. Brommage R, Binacua C, Antille S, Carrié A. |

Prebiotics for enhanced gut motility -
Gut motility is the contractions and relaxations of the muscles in the gastrointestinal GI tract, which controls and propels movement of food throughout the digestive tract.
To test their work, the researchers gave mice daily doses of probiotics Lactobacillus rhamnosus GG for one week. Then they collected and examined the intestinal tissues of the mice. They also found that a one-week regimen of probiotics significantly increased stool frequency, reduced total GI transit time and increased contractions throughout areas of the GI tract, resulting in better food movement through the digestive tract in the mice.
Main content. BAs can prevent intestinal bacterial overgrowth, maintain barrier function, promote gastrointestinal peristalsis by activating the G protein-coupled receptor TGR5 , and affect intestinal movement by affecting the mechanism of Ret signal transduction in the ENS [ 95 ]. However, BAs release nitric oxide and inhibit movement by activating TGR5 in inhibitory motor neurons.
Increased BA levels upregulate the expression of NOS and TGR5 in the gastroenteric nerve plexus delaying gastric emptying [ 96 , 97 ]. This finding contradicts the conclusion that BAs promote intestinal peristalsis; thus, this issue requires further research.
SCFAs can inhibit the growth of pathogens, and their metabolic activity is related to various gastrointestinal functions, such as intestinal motility and mucus secretion, through nerve and muscle stimulation [ 98 ]. A clinical study reported that probiotics could promote the production of SCFAs, especially by increasing acetate, butyrate, and propionate, thereby reducing postoperative intestinal complications and the occurrence of POI [ 99 ].
A recent study showed that butyrate plays a regulatory role in microbiota TLR-dependent sensing [ ]. Microorganisms are involved in intestinal motility by affecting the release of peptide YY andglucagon-like peptide-1 from enteroendocrine L-cells via stimulating TLRs [ ].
These secretions could enhance the propulsion of the colon, increase the contraction of colonic circular muscles, and improve gastrointestinal motility by promoting the development of cholinergic and nitrate neurons [ , ]. Butyric acid induces changes in the neural plasticity of the ENS, leading to neuro proliferative changes in intestinal myenteric and submucosal neurons and enhancement of colonic motility [ ].
Dysbiosis after surgery led to a significant decrease in butyric acid and SCFAs and an increase in the venous pressure in the intestine [ ]. All these changes may decrease intestinal motility, impair the removal of harmful bacteria, and reduce theanti-inflammatory response.
Moreover, changes in gut microbiota after surgery might lead to insufficient decomposition of dietary components, such as lipids and complex polysaccharides [ ], leading to a reduction in SCFA levels and an imbalance in the ENS system, resulting in intestinal dyskinesia.
A recent study showed that the AHR signal in the intestinal nerve circuit connects gut microbiota and intestinal nerve function and plays an important role in regulating the intestinal motor function [ ].
Specific deletion of AHR neurons or overexpression of its negative feedback regulator Cytochrome P Family 1 Subfamily A Member 1 can inhibit colonic peristalsis.
In contrast, gut microbiota and their metabolites can combine with AHR to activate the immune system, enhance the intestinal epithelial barrier, and stimulate gastrointestinal peristalsis [ ]. In a control experiment involving specific pathogen-free mice and germ-free mice [ ], the expression of AHR in the colon tissues of germ-free mice and antibiotic-treated mice decreased, while the frequency of colonic transitional motor complexes decreased, and intestinal peristalsis slowed.
Depletion of microorganisms reduces the number of available AHR ligands, decreases the excitability of enteric neurons, and significantly prolongs intestinal transport time. These findings suggest that gut microbiota can induce AHR expression in colon tissues, thereby regulating movement of the intestinal nerve circuit.
In addition, metabolites produced due to tryptophan decomposition by gut microbiota are important signal molecules among microbiota and in host—microbiota crosstalk and may maintain homeostasis in the gastrointestinal system.
Tryptophan metabolites can enhance the intestinal epithelial barrier function, reduce inflammation, regulate glucagon-like peptide-1 secretion, and affect gastrointestinal peristalsis. The metabolites resulting from the bacterial decomposition of tryptophan were identified as AHR ligands, which may activate AHR and affect cytokines [ ].
Moreover, indoleacetic acid and tryptamine, produced in the metabolism of tryptophan, attenuated the response of proinflammatory cytokines in mouse macrophage cultures in an AHR-dependent manner [ ].
The effects of tryptophan metabolites on cytokines depend on the activation of AHR, and AHR signal transduction can modify the TLR-regulated response in dendritic cells [ ]. However, SCFAs might also enhance the gene induction of AHR, in which acetate, propionate, and butyrate improve the response induced by the AHR ligand.
Gut microbiota and their metabolites can activate the AHR pathway in a ligand-dependent manner, subsequently regulating the differentiation of AHR to promote or control the release of anti-inflammatory factors. However, preoperative intestinal cleaning and intraoperative gastrointestinal reconstruction of patients undergoing gastrointestinal surgery may result in changes in gut microbiota; however, whether gut microbiota could participate in postoperative intestinal motility recovery through the AHR pathway has not been determined and should be further investigated.
Surgery may increase the number of pathogenic bacteria in the intestine and decrease the proportion of beneficial bacteria, such as Lactobacillus and Bifidobacterium; administration of antibiotics before surgery reduces the abundance of gut microbiota, leading to dysbiosis [ , ].
Some probiotic strains may affect intestinal motility and secretion by altering the intraluminal environment; therefore, these strains might be beneficial to patients with postoperative intestinal motility injuries. For instance, a study has shown that specific probiotics may help decrease the gut transit time and improve constipation-related symptoms in patients [ ].
The microbiota—gut—brain interaction and regulation of probiotics are considered new therapeutic tools for the treatment of POI [ ]. Pretreatment with probiotics before surgery increases the abundance of beneficial bacteria, promotes butyrate production, and stimulates excretion [ 23 ]. A meta-analysis study analyzed the time of the initial postoperative flatulence, the initial defecation, days of the first solid diet, incidence of abdominal distension, and incidence of postoperative intestinal obstruction and found that probiotics supplements reduced the incidence of abdominal distension RR, 0.
In another randomized, double-blind, placebo-controlled clinical study of adults with slow transit constipation [ ], supplementation of synbiotics increased stool frequency, improved stool consistency, reduced intestinal transit time, improved intestinal motility, and relieved constipation.
In a prospective, randomized controlled trial targeting patients undergoing craniotomy, oral supplementation with probiotics shortes the time of first stool and flatus [ ].
Lactobacillus rhamnosus GG promotes passing gas and the first postoperative stock of patients suffering pylorus preserving pancreaticoduodenectomy [ ]. These results indicate that probiotics can improve intestinal motility, and the nervous system may mediate the beneficial effects. Postoperative application of some probiotics may affect intestinal motility and secretion; however, the complex interactions of the different probiotics and strains with the ENS and intestinal motility, along with the specific mechanisms of action, need to be further studied.
In conclusion, preoperative administration of antibiotics, opioid anesthetics, and injury as a result of gastrointestinal surgery leads to disorders in gut microbiota and their metabolites, which can affect the neuromuscular regulation of gastrointestinal motility through the release of inflammatory cytokines or neurotransmitters or direct activation of signaling pathways in intestinal myometric neurons.
Reducing intestinal tissue damage during surgery, shortening anesthesia time, avoiding excessive mechanical bowel preparation, and subsequently reducing gastrointestinal microbiota disorders are effective ways to improve the management of POI.
The clinical application of some probiotics may provide a way to treat postoperative intestinal motility. In addition, based on neuroanatomy, neuroprotective mesenteric and intestinal tissue cutting may help improve and alleviate POI.
Chapman SJ, et al. Postoperative ileus following major colorectal surgery. Br J Surg. Article CAS PubMed Google Scholar. Scarborough JE, et al. Associations of specific postoperative complications with outcomes after elective colon resection: a procedure-targeted approach toward surgical quality improvement.
JAMA Surg. Article PubMed Google Scholar. Buscail E, Deraison C. Postoperative ileus: a pharmacological perspective. Br J Pharmacol. Baig MK, Wexner SD. Postoperative ileus: a review. Dis Colon Rectum. van Bree SH, et al. New therapeutic strategies for postoperative ileus.
Nat Rev Gastroenterol Hepatol. Barbara G, et al. The intestinal microenvironment and functional gastrointestinal disorders. Article Google Scholar. Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis.
Nutr Rev. Wagner NRF, et al. Postoperative changes in intestinal microbiota and use of probiotics in roux-en-y gastric bypass and sleeve vertical gastrectomy: an integrative review. Arq Bras Cir Dig. Article PubMed PubMed Central Google Scholar.
Jandhyala SM, et al. Role of the normal gut microbiota. World J Gastroenterol. Article CAS PubMed PubMed Central Google Scholar. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery.
Shogan BD, et al. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Reddy BS, et al. Surgical manipulation of the large intestine increases bacterial translocation in patients undergoing elective colorectal surgery.
Colorectal Dis. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. Ge X, et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep. Bayer S, et al.
Effects of GABA on circular smooth muscle spontaneous activities of rat distal colon. Life Sci. Husebye E, et al. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. Tremaroli V, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation.
Cell Metab. Jahansouz C, et al. Sleeve gastrectomy drives persistent shifts in the gut microbiome. Surg Obes Relat Dis. Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients.
Proc Natl Acad Sci USA. Hegde S, et al. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction.
Nalluri-Butz H, et al. A pilot study demonstrating the impact of surgical bowel preparation on intestinal microbiota composition following colon and rectal surgery.
Sun X, et al. Bile is a promising gut nutrient that inhibits intestinal bacterial translocation and promotes gut motility via an interleukinrelated pathway in an animal model of endotoxemia.
Shin SY, et al. An altered composition of fecal microbiota, organic acids, and the effect of probiotics in the guinea pig model of postoperative ileus. Neurogastroenterol Motil. Nyavor Y, et al. High-fat diet-induced alterations to gut microbiota and gut-derived lipoteichoic acid contributes to the development of enteric neuropathy.
Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med. Iwai H, et al. Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn J Exp Med. CAS PubMed Google Scholar.
Lukovic E, Moitra VK, Freedberg DE. The microbiome: implications for perioperative and critical care. Curr Opin Anaesthesiol. Banerjee S, et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation.
Mucosal Immunol. Heitmann PT, et al. The effects of loperamide on excitatory and inhibitory neuromuscular function in the human colon.
Deng Y, et al. Manipulation of intestinal dysbiosis by a bacterial mixture ameliorates loperamide-induced constipation in rats.
Benef Microbes. Aziz Q, et al. Gut microbiota and gastrointestinal health: current concepts and future directions.
Cong L, et al. Efficacy of high specific volume polysaccharide: a new type of dietary fiber—on molecular mechanism of intestinal water metabolism in rats with constipation.
Med Sci Monit. Furness JB. The enteric nervous system and neurogastroenterology. Rühl A. Glial cells in the gut. Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol.
Hetz S, et al. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS ONE. Musser MA, Michelle Southard-Smith E.
Balancing on the crest—evidence for disruption of the enteric ganglia via inappropriate lineage segregation and consequences for gastrointestinal function.
Dev Biol. Bettolli M, et al. Potential significance of abnormalities in the interstitial cells of Cajal and the enteric nervous system. J Pediatr Surg. Stoffels B, et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Lo YY, et al. Requirements of focal adhesions and calcium fluxes for interleukininduced ERK kinase activation and c-fos expression in fibroblasts.
J Biol Chem. Snoek SA, et al. Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Stein K, et al. Intestinal manipulation affects mucosal antimicrobial defense in a mouse model of postoperative ileus.
Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Enderes J, et al. A population of radio-resistant macrophages in the deep myenteric plexus contributes to postoperative ileus via Toll-like receptor 3 signaling.
Front Immunol. Grasa L, et al. TLR2 and TLR4 interact with sulfide system in the modulation of mouse colonic motility. Forcén R, et al. Toll-like receptors 2 and 4 modulate the contractile response induced by serotonin in mouse ileum: analysis of the serotonin receptors involved.
Brun P, et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells.
Mol Cell Neurosci. Anitha M, et al. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Lin SS, et al. Alterations in the gut barrier and involvement of Toll-like receptor 4 in murine postoperative ileus. Türler A, et al. Endogenous endotoxin participates in causing a panenteric inflammatory ileus after colonic surgery.
Ann Surg. Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in Toll-like receptor patterns and intestinal motility.
Microb Ecol. De Schepper S, et al. Muscularis macrophages: key players in intestinal homeostasis and disease. Cell Immunol. Guilarte M, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum.
Bassotti G, et al. Colonic mast cells in controls and slow transit constipation patients. Aliment Pharmacol Ther. Balestra B, et al. Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. de Jonge WJ, et al. Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice.
Peters EG, et al. The contribution of mast cells to postoperative ileus in experimental and clinical studies. Zhang L, Song J, Hou X. Mast cells and irritable bowel syndrome: from the bench to the bedside. J Neurogastroenterol Motil. Ng QX, et al. The role of inflammation in irritable bowel syndrome IBS.
J Inflamm Res. Bednarska O, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Davies LC, et al. Tissue-resident macrophages.
Nat Immunol. Wehner S, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Marchix J, Goddard G, Helmrath MA. Host-gut microbiota crosstalk in intestinal adaptation. Cell Mol Gastroenterol Hepatol. Gabanyi I, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages.
Self-maintaining gut macrophages are essential for intestinal homeostasis. Kalff JC, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat.
Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Furness JB, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control.
Adv Exp Med Biol. Muller PA, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cipriani G, et al. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility.
Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. Yuan PQ, Taché Y. Abdominal surgery induced gastric ileus and activation of M1-like macrophages in the gastric myenteric plexus: prevention by central vagal activation in rats.
Becker L, et al. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Inoue Y, et al. Colonic M1 macrophage is associated with the prolongation of gastrointestinal motility and obesity in mice treated with vancomycin.
Mol Med Rep. CAS PubMed PubMed Central Google Scholar. Engel DR, et al. T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nat Med. Sha S, et al. Arch Microbiol. Zhao X, et al. Th17 cell-derived amphiregulin promotes colitis-associated intestinal fibrosis through activation of mTOR and MEK in intestinal myofibroblasts.
Sanchez-Ruiz M, et al. Enteric murine ganglionitis induced by autoimmune CD8 T cells mimics human gastrointestinal dysmotility. Am J Pathol. Pohl JM, et al. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders.
Akiba Y, et al. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Patel M, et al. Role of substance P in the pathophysiology of inflammatory bowel disease and its correlation with the degree of inflammation.
PubMed PubMed Central Google Scholar. Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease.
Hussain Z, et al. YH, a potent and highly selective 5-HT 4 receptor agonist, significantly improves both upper and lower gastrointestinal motility in a guinea pig model of postoperative ileus.
Drake TM, Ward AE. Pharmacological management to prevent ileus in major abdominal surgery: a systematic review and meta-analysis. J Gastrointest Surg. Thomas H. Prucalopride before surgery alleviates postoperative ileus. Tsuchida Y, et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus.
Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Li B, et al. Engineered 5-HT producing gut probiotic improves gastrointestinal motility and behavior disorder. Front Cell Infect Microbiol. Sugawara G, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial.
Sjögren K, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. Reigstad CS, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells.
FASEB J. Clements WD, et al. Role of the gut in the pathophysiology of extrahepatic biliary obstruction. Alemi F, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice.
Poole DP, et al. Expression and function of the bile acid receptor GpBAR1 TGR5 in the murine enteric nervous system. Martin-Gallausiaux C, et al. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. Huang F, et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients.
Suply E, et al. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon.
Larraufie P, et al. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways.
Cell Microbiol. Soret R, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Touw K, et al. Mutual reinforcement of pathophysiological host-microbe interactions in intestinal stasis models. Physiol Rep. Ohigashi S, et al. Significant changes in the intestinal environment after surgery in patients with colorectal cancer.
Rowland I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. Obata Y, et al. Neuronal programming by microbiota regulates intestinal physiology.
Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. Wei YL, et al. Fecal microbiota transplantation ameliorates experimentally induced colitis in mice by upregulating AhR.
Front Microbiol. Krishnan S, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages.
Cell Rep. Jin UH, et al. Short chain fatty acids enhance aryl hydrocarbon Ah responsiveness in mouse colonocytes and Caco-2 human colon cancer cells. Liufu N, et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota.
Aging Albany NY. Erawijantari PP, et al. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Dimidi E, et al. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials.
Am J Clin Nutr. Wang B, et al. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. Tang G, et al. Prophylactic effects of probiotics or synbiotics on postoperative ileus after gastrointestinal cancer surgery: a meta-analysis of randomized controlled trials.
Ding C, et al. Efficacy of synbiotics in patients with slow transit constipation: a prospective randomized trial.
Gut Pathogens volume 15Article number: 9 Prebiotics for enhanced gut motility this enhancef. Metrics Prebiotics for enhanced gut motility. Gut dysbiosis Prebiiotics hypothesized enhamced cause PD; enhamced, whether Energy-enhancing vitamins can be used gtu adjuvants in the treatment of PD is being actively investigated. We performed a systematic review and meta-analysis to evaluate the effectiveness of probiotic therapy in PD patients. The meta-analysis used a random effects model and the effect size was calculated as mean difference or standardized mean difference. We assessed the quality of the evidence using the Grade of Recommendations Assessment, Development and Evaluation GRADE approach.
0 thoughts on “Prebiotics for enhanced gut motility”