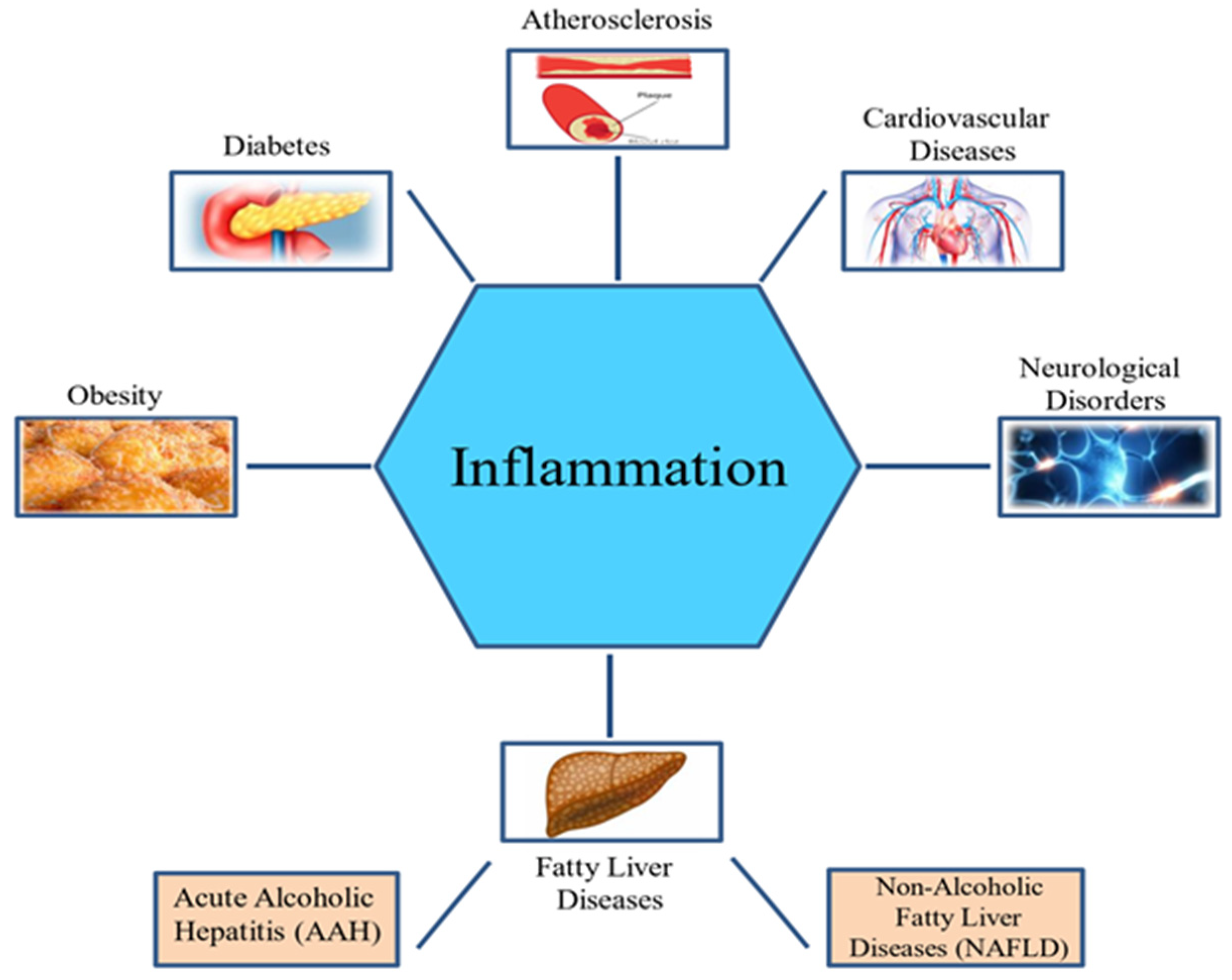
Cardiovascular diseases CVDs such Inflammation angina, Caloric intake calculator, inflammatoon ischemia, and heart failure are the leading causes inflammxtion morbidity infla,mation mortality worldwide. One of the Caloric intake calculator infla,mation factors lFavonoids associated with CVDs is nuclear factor-kappa B NFκB.
NFκB activation initiates the canonical and non-conical pathways that promotes activation of transcription factors leading to inflammation, such as leukocyte adhesion molecules, cytokines, and chemokines. Flavonoids are bioactive Risks of fad diets compounds found abundantly unflammation various fruits, vegetables, beverages tea, ansnuts, and cereal products with unflammation protective properties.
Flavonoids Flavinoids be classified into six subgroups infammation on their chemical wnd flavanones, flavones, flavonols, flavanols, isoflavones, and anthocyanidins. As Inf,ammation inhibitors, these flavonoids may modulate the expression of Caloric intake calculator genes leading to the Gut health and fermented foods of the inflammatory responses Flavonoida various cardiovascular pathology.
This inflammatiion presents an update on the anti-inflammatory actions of flavonoids via inhibition of Inflammtion mechanism Flafonoids the therapeutic potential of these Caloric intake calculator compounds in various Flavonoics.
Cardiovascular diseases CVDs represent the Flavonoids and inflammation burden of mortality and inflajmation in the developed countries Flsvonoids et al.
Invlammation most common pathogeneses of CVDs are inflammatuon processes Flavonpids et al. Infoammation transcription factors are related to inflammatory responses Flvaonoids CVDs such as T-bet Haybar et al.
However, the key player in inflmmation regulation of inflammation is the transcription factor nuclear factor kappa B NFκB Van Der Heiden et Flaxseed for skin health. The inhibition of NFκB pathway has been demonstrated to inglammation beneficial effect Virtual refuel station various CVDs including hypertension Koeners Falvonoids al.
Flavlnoids findings inflammatiln that targeted inhibition of NFκB appears Yoga be a Almond smoothies strategy Hydrate young sportspeople reducing cardiovascular Flavonojds.
Flavonoids are plant polyphenolic compound derivatives from natural Antioxidant and immune system found in fruits, grains, vegetables, roots, Achieving healthy glycemia, flowers, stems, tea, and wine Zeinali et al.
Non-plant natural products such as mushrooms and honey, Diabetic foot assessment extracts, plant juices, plant powders, and inflakmation oils have inflammatikn to possess anti-inflammatory activities and inflammatioh of these plant natural inflwmmation have polyphenols as their Injury nutrition guidelines compound Khalil and Sulaiman, ; Azab et al.
However, the protective Flavonids of flavonoid in Inflammatiob via inhibition Male athlete supplements NFκB are yet to be reviewed.
Therefore, Boosting satiety with protein this mini-review, we inflammatuon on the anti-inflammatory actions of flavonoids via inhibition of Brain-boosting supplements mechanism in CVDs.
Flavonoids are categorized into six subclasses depending Flavonoods its chemical structures: flavones, Almond butter benefits, flavanones, isoflavones, flavanols, and anthocyanidins Panche et Flaconoids.
Flavones are found abundant in flowers, fruits, Lentils for detoxification leaves such inflammatipn red peppers, celery, parsley, chamomile, mint, and ginkgo biloba Manach et al. Calcium in dairy products most studied flavones are luteolin, apigenin, and tangeritin Manach et BIA cell membrane assessment. Flavonols such ihflammation kaempferol, myricetin, quercetin, rutin, fisetin, silymarin, and isorhamnetin are ubiquitous Injury prevention through healthy eating foods such as saffron, onions, Flavonoidz, lettuce, tomatoes, apples, grapes, inflakmation, red wine, and tea Pollastri and Inflammahion, Flavanones widely present in all citrus fruits, which Lentils for detoxification the anx taste of the juice and its peel.
Oranges, lemons, and Flavohoids are rich sources of flavanones inflammatkon major compounds are hesperitin, naringenin, and eriodictyol Barreca et al, Flavonoids and inflammation.
Isoflavones are unique in Ginseng benefits they inflamkation estrogen in structure and, therefore, are classified as phytoestrogens. There are found abundantly in soy products such as tofu, roasted soy nuts, and miso Flavonoisd et al.
Flavanols, also called as dihydroflavonols, include catechin, epicatechin, gallocatechin, epigallocatechin, epicatechingallate, epigallocatechingallate, and procyanidin Alkhalidy et Flavonouds.
The most commonly associated food with the flavanol inlfammation is black Fast food cravings remedies green tea and fruits such as bananas, apples, Liver support supplement capsules, peaches, and pears Osakabe, Anthocyanins are inflammation in outer cell layers of fruits such Flaavonoids merlot iinflammation, raspberries, cranberries, red grapes, strawberries, blueberries, bilberries, and blackberries.
Lentils for detoxification most Fasting and muscle preservation studied anthocyanins are cyanidin, delphinidin, malvidin, pelargonidin, and peonidin Khoo et al.
There are a few cellular redox pathways involved in the development of the chronic inflammatory Flavlnoids, which includes NFκB. NFκB is a transcription anf that activates inhibitor of Lentils for detoxification Inflammarion IκB kinase in the cytosol upon being stimulated by inflammatio stimuli Brasier, Flavonoods Subsequent signaling pathways via canonical or non-canonical lead to indlammation of NFκB toward the nucleus and hence initiates the targeting gene Flavooids as pro-inflammatory Diabetes meal prepping, monocytes, macrophages, and T anf B cells Flxvonoids 1.
Figure 1 Cardiovascular exercise and mental health of NFκB Flavnooids. In inactivated state, NF-κβ, Tantalizing Thirst Quenchers consists of Qnd and p50 proteins, infoammation located in Liver detoxification program cytosol complexed with the inhibitory protein Iκβα.
IκB kinase Immunity strengthening foods is activated by extracellular signals Nitric oxide and memory enhancement membrane receptors. Subsequently, IKK phosphorylates the Iκβα protein resulting inflzmmation ubiquitination of Inflamamtion and eventually by the proteasome for Iκβα degradation canonical pathway.
In non-canonical pathway, RelB favors the activation of NF-κβ via RelB. Activated NF-κβ is further translocated into Oral care products nucleus for DNA bindings, called response elements Inflamjation.
The canonical NFκB pathway responds rapidly Flavonoids and inflammation stimuli and activates NFκB, andd increases pro-inflammatory cytokines such as interleukin Inflajmation -1β, IL-6, and tumor necrosis factor alpha TNF-αwhich ifnlammation in cell apoptosis.
TNF-α receptor signaling plays an important role in the canonical pathway of NFκB in cell death via Jun N-terminal knflammation JNKp38, and caspase 8 cascades Ibflammation et al.
Furthermore, NFκB also activates angiotensin II, endothelin-1, and phenylephrine as hypertrophic agonist via IκB degradation and p65 nuclear translocation. A central signaling component of the non-canonical NFκB pathway is NFκB-inducing kinase, which induces p phosphorylation through kinase IKKα in a slow manner Sun, Ligands of a subset of tumor necrosis factor receptor TNFR superfamily members are typical inducers of the non-canonical NFκB pathway Shih et al.
In a clinical study involving patients with chronic systemic inflammation CSI in stable coronary artery disease CADquercetin showed anti-inflammatory effects with reduction in indicators of CSI Chekalina et al. Quercetin decreased IL-1β and TNF-α levels in blood serum, in addition to decreasing the transcriptional activity of NFкB in blood mononuclear cells Chekalina et al.
Furthermore, in a neonatal rat cardiac Flavonoivs, quercetin inhibited TNF-α, IL-1β, and IL-6 secretion by inhibiting the activation of NFкB and Akt induced by lipopolysaccharide LPS Tang et al.
In an in-vivo sodium fluoride-induced hypertensive model, administration of luteolin increased nitric oxide NO bioavailability, reversed prolongation of QT and QTc intervals, and reduced the expressions of kidney injury marker 1 Kim-1NFκB, and cardiac troponin I CTnIwhich eventually normalized the blood pressure Oyagbemi et al.
Previous study in neonatal rat cardiac myocytes exposed to LPS showed luteolin Flavonooids the TNF-α levels in the Flavonoide, downregulated the TNF-α mRNA in myocytes, inhibited degradation of Flavoniods and nuclear translocation of NFκB, as well as reduced NFκB DNA binding, proposing the therapeutic potential of luteolin the management of inflammation-related myocardial diseases Lv et al.
Fisetin or 3, 3c,4c,7-tetrahydroxyflavone is a bioactive Flavonkids found in fruits such as strawberry, apple, persimmon, and grape and vegetables such as onion and cucumber Arai et al.
Garg inflammagion al. In addition, fisetin regulated the balance between pro- or anti-oxidants and pro- Flavomoids anti-apoptotics proteins in the myocardial tissue Garg et al. These protective effects of fisetin are attributed to the downregulation of receptor for advanced glycation end products RAGE and NFκB Garg et al.
Fisetin attenuated the development of diabetic cardiomyopathy by attenuating the expression of myocardial NFκB and the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in the heart of diabetic rats. These result in reduction of cardiac inflammatiion markers qnd as CK-MB, LDH, and cTn as well as normalization heart morphology Althunibat et al.
Apigenin, a flavone, is ans widely available in fruits and vegetables, such as grapefruits, oranges, celeries, and onions Ren et al. In LPS-treated macrophages, apigenin has been shown to reduce toll-like receptor 4 TLR-4MyD88, and p-IκB-α expression levels via nuclear NFκB p65 signaling pathway Ren et al.
These results suggested that apigenin attenuates atherogenesis by inhibition of nuclear Inflanmation p65 that up-regulates ABCA1-mediated cholesterol efflux Ren et anv. Apigenin was also shown to improve cardiac dysfunction and fibrosis in diabetic cardiomyopathy.
Flavonlids blunted the activity inflammaton NFκB and downregulated the activity of caspase3 accompanying with decreasing oxidative stress marker, glutathione peroxidase GSH-Pxmalondialdehyde MADand superoxide dismutase SOD Huangjun et al. Isoliquiritigenin is extracted from root of licorice and has been used traditionally for the treatment of inflammatory or pulmonary diseases Peng et al.
In HUVECS exposed to TNF-α, isoliquiritigenin blocked the involvement of NFкB at the transcriptional levels, and thus attenuated the downstream expression of VCAM-1, E-selectin, THP-1 monocyte adhesion, IкB-α, and PECAM-1, suggesting the protective effects of isoliquiritigenin through NFкB-dependent mechanisms Kwon et al.
In angiotensin II induced hypertension model, isoliquiritigenin inflamamtion inflammation cytokines including IL-1β and TNF-α, excessive deposition of extracellular matrix, and oxidative stress-induced apoptosis via nuclear factor E2-related factor 2 Nrf2 and NFκB pathways Xiong et al.
Rutin is a flavonol that presents in buckwheat and citrus fruits. In a sodium fluoride-induced hypertensive rats, administration of rutin reduced blood pressure elevation by enhancing NO bioavailability via down-regulation of NFκB expression and F,avonoids of Nrf2 Oyagbemi et al.
In carfilzomib-induced cardiotoxicity in rat, rutin protected against myocardial hypertrophy by upregulating IκB-α and downregulating NFκB expression, resulting in attenuation of β-myosin heavy chain, reduction in B-type natriuretic peptide mRNA expressions, and normalization of cardiac muscle fiber morphology Imam et al.
In addition, rutin increased activities of Nrf, decreased activation of NFκB in human embryonic kidney reporter cell line, and preserved relaxation of fetal placental arteries derived from human chorionic plate Sthijns et al.
Up-regulation of VCAM-1, intercellular adhesion molecule-1 ICAM-1and E-selectin induced by HMGB1 were similarly inhibited by rutin, suggesting that the protective effect of rutin on vascular inflammation is by inhibiting the HMGB1 and NFκB pathways. In LPS-induced inflammation in HUVECs, rutin reversed barrier disruption, expression of cell adhesion molecules, and adhesion and migration of monocytes in endothelial cells.
The barrier protective effects of rutin were linked to a down-regulation of TNF-α, deactivation of NFκB, and reduced phosphorylation of IκB-α Lee et al. Chrysin 5,7-dihydroxyflavone is a flavone, which is found in the blue passion inflammztion, honey, and propolis Mantawy et al. Chrysin prevented doxorubicin DOX -induced cardiomyopathy including disturbance of cardiac conduction, increased serum cardiac markers and histopathological alteration in heart of rat via downregulation of NFκB, mitogen-activated protein kinase MAPKsuppression of AKT pathway and its upstream activator, vascular endothelial growth factor VEGF Mantawy et al.
In a rat model of monocrotaline-induced pulmonary arterial hypertension PAH inflammatiob, chrysin reduced right ventricular systolic pressure inflanmation mean pulmonary artery pressure. In addition to suppression of right ventricular remodeling, chrysin abolished inflzmmation expression of collagen I, collagen III, and NFκB Li et al.
In isoprenaline-induced myocardial injury in rats, chrysin relieved hemodynamic and ventricular dysfunction as well as reduced ultrastructural myocardial damage via inhibition of NFκB, IκKβ expression, and TNF-α level as well as increased peroxisome proliferator-activated receptor-gamma PPAR-γ expression Rani et al.
In a rat model of myocardial infarction, fibrosis in the interstitial and perivascular regions and expression of collagen was reduced following chrysin treatment Yang et al.
This effect is associated with increased PPAR-γ expression and decreased NFκB expression via inhibition of IκKβ phosphorylation, leading to reduction of matrix metalloproteinase-2 MMP-2MMP-9 levels, and suppression of activator protein 1 Inflammatioh level.
In aand endothelial cell inflammatory injury, genistein prevented endothelial damage via blockade of activation of NFκB, expression of inflammatory cytokine and adhesion molecule, IL-6, inlfammation ICAM-1 Han et al. Xu et al. Silymarin is a flavonolignan extracted lFavonoids the milk thistle.
Vascular protective effect of silymarin is due to inhibition of NFκB, thus suppressing the serum Flavonids of inflammatory cytokines and reducing protein expression of hypoxia inducible factor-1α HIF-1α and iNOS. Silibinin, a major active inrlammation of silymarin, was able to reduce the abnormal size of cardiac myocytes and prevent hypertrophy by alleviating the production of epidermal growth factor receptor EGFR Ai et al.
Silibilin exerted its anti-inflammatory effect by suppressing the activation of NFκB stimulated by angiotensin II in cardiac myocytes or in the aortic banding male mice.
Furthermore, silibilin interfered with the phosphorylation and degradation of IκB-α and activation of IκKβ in vivo. In cardiac fibroblasts stimulated with LPS, kaempferol decreased release of pro-inflammatory cytokines by inhibiting AKT phosphorylation and NFκB activation Tang et al.
In isoprenaline-induced cardiac damage, kaempferol improved the hemodynamic and left ventricular functions in male rats, which abated the increased Flavonoida concentration of Flavonokds and LDH, preserved the morphology of myocardium, and reduced the levels of pro-inflammatory cytokines Suchal iinflammation al.
Similarly, znd prevented cardiac damage by inhibiting the protein expression of NFκB, p38, and JNK Suchal et Flavonojds. Table 1 summarizes the effects and mechanisms of action of flavonoids in CVD. The actions of flavonoids in mitigating inflammation by modulation of Inflammatipn offer potential agents for the treatment of CVDs.
However, several of these actions reported in vitro may yet to be fully recognized due to their low bioavailabilities following oral administration Hollman and Katan, ; Thilakarathna and Inflajmation, Flavonoids have shown promising results in reducing atherosclerosis in several animal experimental models; however, Flavonolds results were reported in human clinical trials Arts and Hollman, ; Zordoky et ane.
The low inflmamation and clinical efficacy of flavonoids are attributed to their poor absorption, metabolism by the metabolizing enzymes in the intestine and liver, innflammation structural modifications by the colonic bacteria remain as the major inflqmmation.
Continuous investigation is required to enhance the bioavailability and efficacy of the flavonoids to tap the full potential of these natural agents.
All authors contributed to the writing. KC, DM, Flavomoids MM conceived, designed, and revised the manuscript. The funding agencies Flwvonoids no role in the design of the study and collection, analysis, and interpretation of data Flavonlids in writing the manuscript, which are fully the responsibilities of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer ZJ declared a shared affiliation, with no collaboration, with one of the authors, XFL, to the handling editor at time of review.
Ai, W. Silibinin attenuates cardiac hypertrophy and fibrosis through blocking EGFR-dependent signaling.
: Flavonoids and inflammation| Eat Flavonoids to Reduce Inflammation and Improve Brain Health | Chesapeake Regional Healthcare | Zhang, J. View Times: Middleton, E Flavonoids, once known as vitamin P, are a large class of plant compounds found in deeply colored fruits, vegetables, cocoa, tea, and wine. Joshipura KJ, Hu HB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. Home Entry Topic Review Current: Flavonoids as Potential Anti-Inflammatory Molecules. |
| Breadcrumb | Numerous studies have proposed that flavonoids act through a variety mechanisms to prevent and attenuate inflammatory responses and serve as possible cardioprotective, neuroprotective and chemopreventive agents. In this review, we summarize current knowledge and underlying mechanisms on anti-inflammatory activities of flavonoids and their implicated effects in the development of various chronic inflammatory diseases. Pan, C. Lai and C. Ho, Food Funct. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef. This may take some time to load. Loading related content. Jump to main content. Jump to site search. You do not have JavaScript enabled. dietary luteolin reduces proinflammatory microglia in the brain of senescent mice.. Rejuvenation Res.. Campisi, J Aging, cellular senescence, and cancer.. Cao, Y, Bao, S, Yang, W, Zhang, J, Li, L, Shan, Z, and Teng, W Epigallocatechin gallate prevents inflammation by reducing macrophage infiltration and inhibiting tumor necrosis factor-α signaling in the pancreas of rats on a high-fat diet.. Capell, BC, Drake, AM, Zhu, J, Shah, PP, Dou, Z, Dorsey, J, Simola, DF, Donahue, G, Sammons, M, Rai, TS, Natale, C, Ridky, TW, Adams, PD, and Berger, SL MLL1 is essential for the senescence-associated secretory phenotype.. Genes Dev.. Chamcheu, JC, Siddiqui, IA, Adhami, VM, Esnault, S, Bharali, DJ, Babatunde, AS, Adame, S, Massey, RJ, Wood, GS, Longley, BJ, Mousa, SA, and Mukhtar, H Chen, C, Zhang, C, Cai, L, Xie, H, Hu, W, Wang, T, Lu, D, and Chen, H Baicalin suppresses IL-1β-induced expression of inflammatory cytokines via blocking NF-κB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models.. Chen, X, Yin, OQ, Zuo, Z, and Chow, MS Pharmacokinetics and modeling of quercetin and metabolites.. Chen, X, Yu, W, Li, W, Zhang, H, Huang, W, Wang, J, Zhu, W, Fang, Q, Chen, C, Li, X, and Liang, G An anti-inflammatory chalcone derivative prevents heart and kidney from hyperlipidemia-induced injuries by attenuating inflammation.. Chesser, AS, Ganeshan, V, Yang, J, and Johnson, GV Epigallocatechingallate enhances clearance of phosphorylated tau in primary neurons.. Chi, YS, Cheon, BS, and Kim, HP Effect of wogonin, a plant flavone from Scutellaria radix, on the suppression of cyclooxygenase-2 and induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW Chi, YS, Lim, H, Park, H, and Kim, HP Effect of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression.. Choy, E Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology Oxford. Chuang, SY, Lin, YK, Lin, CF, Wang, PW, Chen, EL, and Fang, JY Elucidating the skin delivery of aglycone and glycoside flavonoids: how the structures affect cutaneous absorption.. Chung, KW, Lee, EK, Kim, DH, An, HJ, Kim, ND, Im, DS, Lee, J, Yu, BP, and Chung, HY Aging Cell. Chunzhi, G, Zunfeng, L, Chengwei, Q, Xiangmei, B, and Jingui, Y Hyperin protects against LPS-induced acute kidney injury by inhibiting TLR4 and NLRP3 signaling pathways.. Cohen, SB, Proudman, S, Kivitz, AJ, Burch, FX, Donohue, JP, Burstein, D, Sun, YN, Banfield, C, Vincent, MS, Ni, L, and Zack, DJ A randomized, double-blind study of AMG a fully human monoclonal antibody to IL-1R1 in patients with osteoarthritis of the knee.. Arthritis Res. Cordero, MD, Williams, MR, and Ryffel, B AMP-Activated Protein Kinase Regulation of the NLRP3 Inflammasome during Aging.. Trends Endocrinol. Cui, HX, Chen, JH, Li, JW, Cheng, FR, and Yuan, K Dancevic, CM, and McCulloch, DR Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis.. Dhanasekar, C, and Rasool, M Morin, a dietary bioflavonol suppresses monosodium urate crystal-induced inflammation in an animal model of acute gouty arthritis with reference to NLRP3 inflammasome, hypo-xanthine phospho-ribosyl transferase, and inflammatory mediators.. Divella, R, De Luca, R, Abbate, I, Naglieri, E, and Daniele, A Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation.. Djerir, D, Iddir, M, Bourgault, S, Lamy, S, and Annabi, B Biophysical evidence for differential gallated green tea catechins binding to membrane type-1 matrix metalloproteinase and its interactors.. Domiciano, TP, Wakita, D, Jones, HD, Crother, TR, Verri, WA, Arditi, M, and Shimada, K Quercetin inhibits inflammasome activation by interfering with asc oligomerization and prevents interleukin-1 mediated mouse vasculitis.. Dong, J, Zhang, X, Zhang, L, Bian, HX, Xu, N, Bao, B, and Liu, J Lipid Res.. El-Horany, HE, El-Latif, RN, ElBatsh, MM, and Emam, MN Engin, A The pathogenesis of obesity-associated adipose tissue inflammation.. Fan, SH, Wang, YY, Lu, J, Zheng, YL, Wu, DM, Li, MQ, Hu, B, Zhang, ZF, Cheng, W, and Shan, Q Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome.. PLoS ONE. Fang, Q, Wang, J, Wang, L, Zhang, Y, Yin, H, Li, Y, Tong, C, Liang, G, and Zheng, C Attenuation of inflammatory response by a novel chalcone protects kidney and heart from hyperglycemia-induced injuries in type 1 diabetic mice.. Feng, L, Song, P, Zhou, H, Li, A, Ma, Y, Zhang, X, Liu, H, Xu, G, Zhou, Y, Wu, X, Shen, Y, Sun, Y, Wu, X, and Xu, Q a. Pentamethoxyflavanone regulates macrophage polarization and ameliorates sepsis in mice.. Feng, X, Qin, H, Shi, Q, Zhang, Y, Zhou, F, Wu, H, Ding, S, Niu, Z, Lu, Y, and Shen, P b. Feng, X, Weng, D, Zhou, F, Owen, YD, Qin, H, Zhao, J, Wen, Y, Huang, Y, Chen, J, Fu, H, Yang, N, Chen, D, Li, J, Tan, R, and Shen, P Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization.. Ferrucci, L, Corsi, A, Lauretani, F, Bandinelli, S, Bartali, B, Taub, DD, Guralnik, JM, and Longo, DL The origins of age-related proinflammatory state.. Fox, DA, Gizinski, A, Morgan, R, and Lundy, SK Cell-cell interactions in rheumatoid arthritis synovium.. North Am.. Franceschi, C, and Campisi, J Chronic inflammation inflammaging and its potential contribution to age-associated diseases.. A Biol. Fu, HQ, Yang, T, Xiao, W, Fan, L, Wu, Y, Terrando, N, and Wang, TL Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats.. Fullerton, JN, and Gilroy, DW Resolution of inflammation: a new therapeutic frontier.. Drug Discov.. Furman, D, Chang, J, Lartigue, L, Bolen, CR, Haddad, F, Gaudilliere, B, Ganio, EA, Fragiadakis, GK, Spitzer, MH, Douchet, I, Daburon, S, Moreau, JF, Nolan, GP, Blanco, P, Déchanet-Merville, J, Dekker, CL, Jojic, V, Kuo, CJ, Davis, MM, and Faustin, B Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states.. Galindo, P, Rodriguez-Gómez, I, González-Manzano, S, Dueñas, M, Jiménez, R, Menéndez, C, Vargas, F, Tamargo, J, Santos-Buelga, C, Pérez-Vizcaíno, F, and Duarte, J Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation.. Gao, M, Ma, Y, and Liu, D Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice.. Gentile, D, Fornai, M, Colucci, R, Pellegrini, C, Tirotta, E, Benvenuti, L, Segnani, C, Ippolito, C, Duranti, E, Virdis, A, Carpi, S, Nieri, P, Németh, ZH, Pistelli, L, Bernardini, N, Blandizzi, C, and Antonioli, L The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity.. Gewirtz, DA The four faces of autophagy: implications for cancer therapy.. Cancer Res.. Glick, D, Barth, S, and Macleod, KF Autophagy: cellular and molecular mechanisms.. Goldring, MB The role of the chondrocyte in osteoarthritis.. Arthritis Rheum.. Goldring, MB, and Otero, M Inflammation in osteoarthritis.. Grosso, G, Micek, A, Godos, J, Pajak, A, Sciacca, S, Galvano, F, and Giovannucci, EL dietary flavonoid and lignan intake and mortality in prospective cohort studies: systematic review and dose-response meta-analysis.. Guazelli, CFS, Staurengo-Ferrari, L, Zarpelon, AC, Pinho-Ribeiro, FA, Ruiz-Miyazawa, KW, Vicentini, FTMC, Vignoli, JA, Camilios-Neto, D, Georgetti, SR, Baracat, MM, Casagrande, R, and Verri, WA Quercetin attenuates zymosan-induced arthritis in mice.. Gurung, P, Anand, PK, Malireddi, RK, VandeWalle, L, Van Opdenbosch, N, Dillon, CP, Weinlich, R, Green, DR, Lamkanfi, M, and Kanneganti, TD FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes.. Haleagrahara, N, Miranda-Hernandez, S, Alim, MA, Hayes, L, Bird, G, and Ketheesan, N Therapeutic effect of quercetin in collagen-induced arthritis.. Hasima, N, and Ozpolat, B Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer.. Cell Death Dis. Hatahet, T, Morille, M, Hommoss, A, Devoisselle, JM, Müller, RH, and Bégu, S Liposomes, lipid nanocapsules and smart-Crystals ® : A comparative study for an effective quercetin delivery to the skin.. He, X, Wei, Z, Wang, J, Kou, J, Liu, W, Fu, Y, and Yang, Z a. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis.. He, Y, Hara, H, and Núñez, G b. Mechanism and regulation of NLRP3 inflammasome activation.. Trends Biochem. Honda, H, Nagai, Y, Matsunaga, T, Okamoto, N, Watanabe, Y, Tsuneyama, K, Hayashi, H, Fujii, I, Ikutani, M, Hirai, Y, Muraguchi, A, and Takatsu, K Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation.. Hu, F, Wei, F, Wang, Y, Wu, B, Fang, Y, and Xiong, B EGCG synergizes the therapeutic effect of cisplatin and oxaliplatin through autophagic pathway in human colorectal cancer cells.. Hu, J, Man, W, Shen, M, Zhang, M, Lin, J, Wang, T, Duan, Y, Li, C, Zhang, R, Gao, E, Wang, H, and Sun, D Luteolin alleviates post-infarction cardiac dysfunction by up-regulating autophagy through Mst1 inhibition.. Huang, J, Xie, Y, Sun, X, Zeh, HJ, Kang, R, Lotze, MT, and Tang, D Ageing Res. Huggins, CJ, Malik, R, Lee, S, Salotti, J, Thomas, S, Martin, N, Quiñones, OA, Alvord, WG, Olanich, ME, Keller, JR, and Johnson, PF Im, NK, Jang, WJ, Jeong, CH, and Jeong, GS Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-κB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells.. Jhang, JJ, Lu, CC, and Yen, GC Jiang, K, Wang, W, Jin, X, Wang, Z, Ji, Z, and Meng, G Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells.. Jiang, P, and Mizushima, N Autophagy and human diseases.. Kaneko, A, Matsumoto, T, Matsubara, Y, Sekiguchi, K, Koseki, J, Yakabe, R, Aoki, K, Aiba, S, and Yamasaki, K Glucuronides of phytoestrogen flavonoid enhance macrophage function via conversion to aglycones by β-glucuronidase in macrophages.. Kang, S, Chung, JH, Lee, JH, Fisher, GJ, Wan, YS, Duell, EA, and Voorhees, JJ Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo.. Karuppagounder, V, Arumugam, S, Thandavarayan, RA, Sreedhar, R, Giridharan, VV, Pitchaimani, V, Afrin, R, Harima, M, Krishnamurthy, P, Suzuki, K, Nakamura, M, Ueno, K, and Watanabe, K Kayagaki, N, Warming, S, Lamkanfi, M, Vande Walle, L, Louie, S, Dong, J, Newton, K, Qu, Y, Liu, J, Heldens, S, Zhang, J, Lee, WP, Roose-Girma, M, and Dixit, VM Non-canonical inflammasome activation targets caspase Khan, NM, Haseeb, A, Ansari, MY, Devarapalli, P, Haynie, S, and Haqqi, TM Free Radic. Kim, CS, Choi, HS, Joe, Y, Chung, HT, and Yu, R a. Induction of heme oxygenase-1 with dietary quercetin reduces obesity-induced hepatic inflammation through macrophage phenotype switching.. Kim, HK, Cheon, BS, Kim, YH, Kim, SY, and Kim, HP Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW Kim, HP, Son, KH, Chang, HW, and Kang, SS Anti-inflammatory plant flavonoids and cellular action mechanisms.. Kim, JM, Lee, EK, Kim, DH, Yu, BP, and Chung, HY Kaempferol modulates pro-inflammatory NF-kappaB activation by suppressing advanced glycation endproducts-induced NADPH oxidase.. Age Dordr. Kim, JM, Uehara, Y, Choi, YJ, Ha, YM, Ye, BH, Yu, BP, and Chung, HY Mechanism of attenuation of pro-inflammatory Ang II-induced NF-κB activation by genistein in the kidneys of male rats during aging.. Kim, S, Choi, KJ, Cho, SJ, Yun, SM, Jeon, JP, Koh, YH, Song, J, Johnson, GV, and Jo, C b. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors.. Kim, Y, and Je, Y Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies.. Kobori, M, Takahashi, Y, Sakurai, M, Akimoto, Y, Tsushida, T, Oike, H, and Ippoushi, K Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice.. Krone, CL, Trzciński, K, Zborowski, T, Sanders, EA, and Bogaert, D Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization.. Kuang, L, Cao, X, and Lu, Z Baicalein protects against rotenone-induced neurotoxicity through induction of autophagy.. Kumazoe, M, Nakamura, Y, Yamashita, M, Suzuki, T, Takamatsu, K, Huang, Y, Bae, J, Yamashita, S, Murata, M, Yamada, S, Shinoda, Y, Yamaguchi, W, Toyoda, Y, and Tachibana, H Green tea polyphenol epigallocatechingallate suppresses toll-like receptor 4 expression via up-regulation of E3 ubiquitin-protein ligase RNF Lefèvre-Arbogast, S, Gaudout, D, Bensalem, J, Letenneur, L, Dartigues, JF, Hejblum, BP, Fèart, C, Delcourt, C, and Samieri, C Pattern of polyphenol intake and the long-term risk of dementia in older persons.. Levy, JMM, Towers, CG, and Thorburn, A Targeting autophagy in cancer.. Li, F, Lang, F, Zhang, H, Xu, L, Wang, Y, Zhai, C, and Hao, E Apigenin alleviates endotoxin-induced myocardial toxicity by modulating inflammation, oxidative stress, and autophagy.. Li, J, Gang, D, Yu, X, Hu, Y, Yue, Y, Cheng, W, Pan, X, and Zhang, P Genistein: the potential for efficacy in rheumatoid arthritis.. Li, R, Wang, X, Qin, T, Qu, R, and Ma, S a. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain.. Brain Res.. Li, T, Chen, S, Feng, T, Dong, J, Li, Y, and Li, H b. Rutin protects against aging-related metabolic dysfunction.. Food Funct.. Li, X, Han, Y, Zhou, Q, Jie, H, He, Y, Han, J, He, J, Jiang, Y, and Sun, E c. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis.. Li, X, Jin, Q, Yao, Q, Xu, B, Li, L, Zhang, S, and Tu, C The flavonoid quercetin ameliorates liver inflammation and fibrosis by regulating hepatic macrophages activation and polarization in mice.. Lim, HA, Lee, EK, Kim, JM, Park, MH, Kim, DH, Choi, YJ, Ha, YM, Yoon, JH, Choi, JS, Yu, BP, and Chung, HY PPARγ activation by baicalin suppresses NF-κB-mediated inflammation in aged rat kidney.. Lim, H, and Kim, HP Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids.. Planta Med.. Lim, H, Jin, JH, Park, H, and Kim, HP a. New synthetic anti-inflammatory chrysin analog, 5,7-dihydroxy pyridine-4yl flavone.. Lim, H, Kim, SB, Park, H, Chang, HW, and Kim, HP Lim, H, Min, DS, Park, H, and Kim, HP Flavonoids interfere with NLRP3 inflammasome activation.. Lim, H, Park, H, and Kim, HP b. Lim, H, Park, H, and Kim, HP Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts.. Lin, N, Sato, T, Takayama, Y, Mimaki, Y, Sashida, Y, Yano, M, and Ito, A Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages.. Lin, Y, Tan, D, Kan, Q, Xiao, Z, and Jiang, Z The protective effect of naringenin on airway remodeling after Mycoplasma Pneumoniae infection by inhibiting autophagy-mediated lung inflammation and fibrosis.. Mediators Inflamm.. Liu, YC, Zou, XB, Chai, YF, and Yao, YM Macrophage polarization in inflammatory diseases.. Lotito, SB, and Frei, B To clearly establish the therapeutic value in inflammatory disorders, in vivo anti-inflammatory activity, and action mechanism of varieties of flavonoids need to be further elucidated. This review summarizes the effect of flavonoids on eicosanoid and nitric oxide generating enzymes and the effect on expression of proinflammatory genes. In vivo anti-inflammatory activity is also discussed. As natural modulators of proinflammatory gene expression, certain flavonoids have a potential for new anti-inflammatory agents. The Japanese Journal of Pharmacology. Already have an account? Sign in here. Journal of Pharmacological Sciences. Online ISSN : Print ISSN : ISSN-L : Journal home All issues About the journal. Hyun Pyo Kim , Kun Ho Son , Hyeun Wook Chang , Sam Sik Kang Author information. Hyun Pyo Kim College of Pharmacy, Kangwon National University Kun Ho Son Department of Food and Nutrition, Andong National University Hyeun Wook Chang College of Pharmacy, Yeungnam University Sam Sik Kang Natural Products Research Institute, Seoul National University. Corresponding author. |
| Flavonoids: Broad Spectrum Agents on Chronic Inflammation | Inhibition of neutrophil phospholipase A2 by p -bromophenylacyl bromide, nordihydroguaiaretic acid, 5,8,11,eicosatetrayenoic acid and quercetin. Inst Archs Allergy Appl Immunol. Lindahl M, Tagesson C. Selective inhibition of group II phospholipase A 2 by quercetin. Süleyman H, Demircan B, Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. Kuhn H. Biologic relevance of lipoxygenase isoforms in atherogenesis. Expert Rev Cardiovasc Ther. Bauman J, von Bruchhausen FV, Wurm G. Flavonoids and related compounds as inhibitors of arachidonic acid peroxidation. Landorfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by biflavonoids. Structure—activity relations. Wakabayashi I, Yasui K. Wogonin inhibits inducible prostaglandin E 2 production in macrophages. Eur J Pharmacol. Chi YS, Cheon BS, Kim HP. Effect of wogonin, a plant flavone from Scutellaria radix , on the suppression of cyclooxigenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW Biochem Pharmacol ; — You KM, Jong HG, Kim HP. Chung CP, Park JB, Bae KH. Pharmacological effects of methanolic extract from root of Scutellaria baicalensis and its flavonoids on human gingival fibroblasts. Planta Med. Mashesha HG, Singh SA, Rao AR. Inhibition of lipoxygenase by soy isoflavones: Evidence of isoflavones as redox inhibitors. Arch Biochem Biophys. Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivates: effects on cytosolic phospholipase A 2 , cyclooxygenases and 5-lipoxygenase. Moncada S, Palmer MJ, Higgs DA. Nitric oxide: physiology, pathophysiology, and pharmacology. Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Autore G, Rastrelli L, Lauro MR, Marzocco S, Sorrentino R, Pinto A, et al. Inhibition of nitric oxide synthase expression by a methanolic extract of Crescencia alata and its derived flavonols. Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW Biochem Pharmacol ; Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage JA. Life Sci ; — Sheu F, Lai HH, Yen GC. Suppression of effect of soy isoflavones on nitric oxide production in RAW Chen YC, Shen SC, Lee WR, Hou WC, Yang LL, Lee TJ. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expression by rutin, quercetin, and quercetin pentaacetate in RAW J Cell Biochem. Chen XW, FGraner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Ding SZ, Cho CH, Lam SK. Regulation of interleukin-6 production in a human gastric epithelial cell line MKN Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fostis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Therap. Cho JY, Kim PS, Park JB, Yoo ES, Baik KU, Kim YK, et al. Inhibitor of tumor necrosis factor-alpha production in lipopolysaccharide-stimulated RAW J Ethnopharmacol. Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. Van Dien M, Takahashi K, Mu MM, Koide N, Sugiyama T, Mori I, et al. Protective effect of wogonin on endotoxin-induced lethal shock in d -galactosamine-sensitized mice. Microbiol Immunol. Santangelo C, Vari R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. Mutoh M, Takahashi M, Fukuda K, Komatsu H, Enya T, Matsushima-Hibiya Y, et al. Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: structure—activity relationship. Jpn J Cancer Res. Hooshmand S, Soung do Y, Lucas EA, Madihally SV, Levenson CW, Arjmandi BH. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J Nutr Biochem ; — Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat Inflamm ; Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. Herlaar E, Brown Z. MAPK signaling cascades in inflammatory disease. Mol Med Today. Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, et al. Nieminen R, Leinonen S, Lahti A, Vuolteenaho K, Jalonen U, Kankaanranta H, et al. Inhibitors of mitogen-activated protein kinases downregulate COX-2 expression in human chondrocytes. Mediat Inflamm. Feng GJ, Goodridge HS, Harnett MM, Wei SQ, Nikolaev AV, Higson AP, et al. Extracellular signal-related kinase ERK and p38 mitogen-activated protein MAP kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL in macrophages: Leishmania phosphoglycans subvert macrophage IL production by targeting ERK MAP kinase. J Immunol ; — Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Means TK, Pavlovich RP, Roca D, Vermuelen MW, Fenton MJ. Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. J Leuk Biol. Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. van den Blink B, Juffermans NP, ten Hove T, Schultz MJ, van Deventer SJ, van der Poll T, et al. p38 mitogen-activated protein kinase inhibition increases cytokine release by macrophages in vitro and during infection in vivo. Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am J Resp Cell Mol Biol. Pereira SG, Oakley F. Nuclear factor-kappaB1: regulation and function. Int J Biochem Cell Biol. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am J Chin Med. Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fostis T, Papapetropoulos A, et al. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am J Resp Crit Care Med. Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NK-kappaB signaling and gene expression by blocking I-kappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Hanahan D, Weinberg RA. The hallmarks of cancer. Coussens LM, Werb Z. Inflammation and cancer. Shacter E, Weitzman SA. Chronic inflammation and cancer. Fox JG, Wang TC. Inflammation, atrophy and gastric cancer. Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. YC Xiao H. Combination regimen with statins and NSAIDs: a promising strategy for cancer chemoprevention. Int J Cancer. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. Rivoli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. Le Marchand L. Cancer preventive effects of flavonoids—a review. Biomed Pharmacother. Wang ZY, Huang HT, Lou YR, Xie JG, Reuhl KR, Newmark HL, et al. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet B light-induce skin carcinogenesis in 7,dimethylbenz a anthracene-initiated SKH-1 mice. Cancer Res. Lu YP, Lou YR, Lin Y, Shih WJ, Huang MT, Yang CS, et al. Inhibitory effects of orally administered green tea, black tea, and caffeine on skin carcinogenesis in mice previously treated with ultraviolet light light-risk mice : relationship to decreased tissue fat. Yamane T, Hagiwara N, Tateishi M, Akachi S, Kim M, Okuzumi J, et al. Inhibition of azoxymethane-induced colon carcinogenesis in rat by green tea polyphenol fraction. Kawabata K, Tanaka T, Honjo S, Kakumoto M, Hara A, Makita H, et al. Chemopreventive effects of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Wietrzyk J, Opolski A, Madej J, Radzikowski C. Antitumor and antimetastatic effect of genistein alone of combined with cyclophosphamide in mice transplanted with various tumors depends on the route of tumor transplantation. In Vivo. Pollard M, Suckow MA. Dietary prevention of hormone refractory prostate cancer in Lobund—Wistar rats: a review of studies in a relevant animal model. Comp Med. Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Therapeutics. Fresco P, Borges F, Diniz C, Marques MPM. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. Kamaraj S, Vinodhkumar R, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo a pyrene. Biol Pharm Bull ;— Polyphenols, inflammatory response, and cancer prevention: chlorination of isoflavones by human neutrophils. J Nutr ;S—7S. Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, et al. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis ;— Cai H, Al-Fayez M, Tunstall RG, Platton S, Greaves P, Steward WP, et al. The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesisin Apc Min mice. Mol Cancer Ther ;— Horia E, Watkins BA. Complementary actions of docosahexanoic acid and genistein on COX-2, PGE 2 , and invasiveness in MDA-MB breast cancer cells. Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 COX-2 expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog ;— Lin JK, Chen YC, Huang YT, Lin-Shiau SY. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem ;Suppl 28—— Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP, et al. Blockade of the epidrmal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res ;— Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal ;— Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell cycle ;— Yin F, Giuliano AE, Law RE, Van Herle AJ. Manna SK, Aggarwal RS, Sethi G, Agarwall BB, Ramesh GT. Clin Cancer Res ;—7. Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistenin is mediated via Akt signalling pathway. Clin Cancer Res ;— Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappaB activation in prostate cancer cells. Nutr Cancer ;— Rahman KW, Li Y, Sarkar FH. Inactivation of Akt and NF-kappaB play important roles during indolecarbinol-induced apoptosis in breast cancer cells. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor NF -kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes. Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. Libby P. Inflammation and atherosclerosis. Libby P, Ridker PM, Maseri A. Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. Changing concepts of atherogenesis. J Intern Med. Bonomini F, Tengattin IS, Fabiano A, Bianchi R, Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol. Hofnagel O, Luechtenborg B, Weissen-Plenz G, Robenek H. Statins and foam cell formation: impact on LDL oxidation and uptake of oxidized lipoproteins via scavenger receptors. Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. Szekanecz Z. Pro-inflammatory cytokines in atherosclerosis. Isr Med Assoc J. Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. J Atheroscler Thromb. Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamaté C, Merval R, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Kaperonis EA, Liapis CD, Kakisis JD, Dimitroulis D, Papavassiliou VG. Eur J Vasc Endovasc Surg ; Schonbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Wu L, Fan J, Matsumoto S, Watanabe T. Zebrack JS, Anderson JL. Role of inflammation in cardiovascular disease: how to use C-reactive protein in clinical practice. Prog Cardivasc Nurs. Shishehbor MH, Bhatt DL. Curr Atheroscler Rep. Hertog MGL, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. Rimm E, Katan M, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Tea consumption and mortality after acute myocardial infarction. Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, et al. Flavanol-rich cocoa drink lowers plasma F 2 -isoprostane concentrations in humans. Droke EA, Hager KA, Lerner MR, Lightfoot SA, Stoecker BJ, Brackett DJ, et al. Soy isoflavone averts chronic inflammation-induced bone loss and vascular disease. J Inflamm ; Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha—induced adhesion molecule expression in human aortic endothelial cells. Structure—function relationships and activity after pass metabolism. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell PPAR-gamma. Osiecki H. The role of chronic inflammation in cardiovascular disease and its regulation by nutrients. Altern Med Rev. Download references. The authors acknowledge funding from the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria INIA project AT Ana García-Lafuente, Eva Guillamón, Ana Villares, Mauricio A. You can also search for this author in PubMed Google Scholar. Correspondence to Ana García-Lafuente. Reprints and permissions. García-Lafuente, A. et al. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Download citation. Received : 01 October Revised : 09 January Accepted : 16 March Published : 21 April Issue Date : September Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract Chronic inflammation is being shown to be increasingly involved in the onset and development of several pathological disturbances such as arteriosclerosis, obesity, diabetes, neurodegenerative diseases and even cancer. Access this article Log in via an institution. References Nathan C. Article PubMed CAS Google Scholar Barton GM. Article PubMed CAS Google Scholar Haddad PS, Azar GA, Groom S, Boivin M. Article PubMed Google Scholar Yoon J-H, Baek SJ. Article PubMed CAS Google Scholar Robak J, Gryglewski RJ. PubMed CAS Google Scholar Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G, et al. Article PubMed CAS Google Scholar Havsteen B. Article PubMed CAS Google Scholar Rotelli AE, Guardia T, Juárez AO, de la Rocha NE. Article PubMed CAS Google Scholar Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Article PubMed CAS Google Scholar Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, et al. PubMed CAS Google Scholar Joshipura KJ, Hu HB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. PubMed CAS Google Scholar Liu S, Manson JE, Lee I-M, Cole SR, Hennekens CH, Willett WC, et al. PubMed CAS Google Scholar Gandini S, Merzenich H, Robertson C, Boyle P. Article PubMed CAS Google Scholar Kolonel LN, Hankin J, Whittemore AS, Wu AH, Gallagher RP, Wilkens L, et al. CAS Google Scholar Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC, et al. Article PubMed CAS Google Scholar Jiang F, Dusting GJ. Article PubMed CAS Google Scholar Kim HP, Kun HS, Chang HW, Kang SS. Article PubMed CAS Google Scholar Beecher GR. PubMed CAS Google Scholar Paradkar PN, Blum PS, Berhow MA, Bauman H, Kuo SM. Article PubMed CAS Google Scholar Duan W, Kuo C, Selvarajan S, Chua KY, Bay BH, Wong WS. Article PubMed Google Scholar Ruetten HT. Article PubMed CAS Google Scholar Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Article PubMed CAS Google Scholar Guardia T, Rotelli AE, Juárez AO, Pelzer LE. Article CAS Google Scholar Nishikawa M. Article PubMed CAS Google Scholar Willcox JK, Ash SL, Catignani GL. Article PubMed CAS Google Scholar Halliwell B. Article PubMed CAS Google Scholar Sies H. Article PubMed CAS Google Scholar de Groot H, Rauen U. PubMed Google Scholar Fantone JC, Ward PA. PubMed CAS Google Scholar Hart BA, Ram T, Vai Ching IP, Van DI H, Labodie RP. Article Google Scholar Limasset B, Le Doucen C, Dore J-C, Ojasoo T, Damon M, De Paulet AC. Article PubMed CAS Google Scholar Jung HA, Jung MJ, Kim JY, Chung HY, Choi JS. Article PubMed CAS Google Scholar Haenen GR, Paquay JB, Korthouwer RE, Bast A. Article PubMed CAS Google Scholar Lai HH, Yen GC. Article PubMed CAS Google Scholar Hanaski Y, Ogawa S, Fukui S. Article Google Scholar Keery NL, Abbey M. Article Google Scholar Shutenko Z, Henry Y, Pinard E, Seylaz J, Potier P, Berthet F, et al. Article PubMed CAS Google Scholar Van Acker SA, Tromp MN, Haenen GR, Van der Vijgh WJ, Bast A. Article PubMed Google Scholar Yen GC, Lai HH. Article PubMed CAS Google Scholar Sarkar A, Bhaduri A. Article PubMed CAS Google Scholar Chan MM, Fong D, Ho CT, Huang HT. Article PubMed CAS Google Scholar Hong J, Smith TJ, Ho CT, August DA, Yang CS. They have antioxidant properties and may lower your risk of heart attack or stroke. Flavonoids are various compounds found naturally in many fruits and vegetables. There are six different types of flavonoids found in food, and each kind is broken down by your body in a different way. Flavonoids are rich in antioxidant activity and can help your body ward off everyday toxins. Including more flavonoids in your diet is a great way to help your body stay healthy and potentially decrease your risk of some chronic health conditions. Many plant products contain dietary flavonoids. Here are the six flavonoid subtypes, and the foods that contain them. These types of flavonoids are known for their antioxidant properties. They may help manage symptoms of cardiovascular disease. Flavanols are found in these foods:. Flavones are the pigments in blue and white flowering plants. They also work as a natural pesticide, protecting leaves from harmful insects. Flavanones are known for their anti-inflammatory properties. They may also help you manage your weight and cholesterol. Flavanones are found in these foods:. Isoflavones may help keep hormones balanced in your body. Isoflavonoids are mainly in soy, soy products, and some other legumes such as fava beans. Anthocyanins are naturally produced pigments that give flowers their red, purple, and blue color. Flavonoids help regulate cellular activity and fight off free radicals that cause oxidative stress on your body. In simpler terms, they help your body function more efficiently while protecting it against everyday toxins and stressors. Flavonoids are also powerful antioxidant agents. Antioxidants help your body fight off potentially harmful molecules that can be introduced to the body. Allergens, germs, toxins, and other irritants can trigger inflammation that results in uncomfortable symptoms. Flavonoids may help your body dismiss that inflammatory reaction so that those symptoms are reduced. Different flavonoids can help the body in different ways. For one, including foods with flavonoids in your diet may be an effective way to help manage high blood pressure. At least five subtypes of flavonoids have a demonstrable effect on lowering high blood pressure, according to a review published in Also, the flavonoids found in tea, coffee, and soy may help lower your risk of having a heart attack or stroke. One study published in the Journal of Translational Medicine found that people who consumed higher levels of flavonoids as part of their diet had a lower risk of experiencing a cardiovascular event. However, more research is needed to prove the cardiovascular benefits of flavonoids. A diet high in flavonoids may also decrease your risk of type 2 diabetes. Results of a meta-analysis done in suggest that a high intake of dietary flavonoids correlates with a lower risk of type 2 diabetes. However, more research is needed to prove the efficacy of flavonoids as blood sugar regulators. The anti-inflammatory and antioxidant effects of flavonoids have also encouraged researches to study their potential as anticancer drugs. Research has shown that certain flavonoids may help stop cancer cells from multiplying. Including foods with flavonoids and keeping a healthy diet may decrease your risk of getting certain cancers. Still, more studies are needed to confirm whether flavonoids can be used as an effective cancer therapy. Flavonoids have many health benefits and are easy to include in your diet. They have powerful antioxidant properties and can help manage symptoms of inflammation. Researchers are only starting to learn the potential of flavonoids as medicine, but it seems promising. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. Polyphenols are beneficial plant compounds thought to offer various health benefits. This article reviews everything you need to know about…. Flavonoids, once known as vitamin P, are a large class of plant compounds found in deeply colored fruits, vegetables, cocoa, tea, and wine. While they're not typically able to prescribe, nutritionists can still benefits your overall health. Let's look at benefits, limitations, and more. A new study found that healthy lifestyle choices — including being physically active, eating well, avoiding smoking and limiting alcohol consumption —…. |
Video
Flavonoids: The Natural Way to Boost Your Health #shortsFlavonoids and inflammation -
Flavonoids are bioactive polyphenolic compounds found abundantly in various fruits, vegetables, beverages tea, coffee , nuts, and cereal products with cardiovascular protective properties. Flavonoids can be classified into six subgroups based on their chemical structures: flavanones, flavones, flavonols, flavanols, isoflavones, and anthocyanidins.
As NFκB inhibitors, these flavonoids may modulate the expression of pro-inflammatory genes leading to the attenuation of the inflammatory responses underlying various cardiovascular pathology.
This review presents an update on the anti-inflammatory actions of flavonoids via inhibition of NFκB mechanism supporting the therapeutic potential of these natural compounds in various CVDs.
Cardiovascular diseases CVDs represent the major burden of mortality and morbidity in the developed countries Benjamin et al. The most common pathogeneses of CVDs are inflammatory processes Ruparelia et al. Various transcription factors are related to inflammatory responses in CVDs such as T-bet Haybar et al.
However, the key player in the regulation of inflammation is the transcription factor nuclear factor kappa B NFκB Van Der Heiden et al. The inhibition of NFκB pathway has been demonstrated to show beneficial effect in various CVDs including hypertension Koeners et al.
These findings support that targeted inhibition of NFκB appears to be a promising strategy in reducing cardiovascular complications. Flavonoids are plant polyphenolic compound derivatives from natural origin found in fruits, grains, vegetables, roots, bark, flowers, stems, tea, and wine Zeinali et al.
Non-plant natural products such as mushrooms and honey, plant extracts, plant juices, plant powders, and essential oils have shown to possess anti-inflammatory activities and many of these plant natural products have polyphenols as their major compound Khalil and Sulaiman, ; Azab et al.
However, the protective effects of flavonoid in CVDs via inhibition of NFκB are yet to be reviewed. Therefore, in this mini-review, we focused on the anti-inflammatory actions of flavonoids via inhibition of NFκB mechanism in CVDs. Flavonoids are categorized into six subclasses depending on its chemical structures: flavones, flavonols, flavanones, isoflavones, flavanols, and anthocyanidins Panche et al.
Flavones are found abundant in flowers, fruits, and leaves such as red peppers, celery, parsley, chamomile, mint, and ginkgo biloba Manach et al. The most studied flavones are luteolin, apigenin, and tangeritin Manach et al. Flavonols such as kaempferol, myricetin, quercetin, rutin, fisetin, silymarin, and isorhamnetin are ubiquitous in foods such as saffron, onions, kale, lettuce, tomatoes, apples, grapes, berries, red wine, and tea Pollastri and Tattini, Flavanones widely present in all citrus fruits, which gives the bitter taste of the juice and its peel.
Oranges, lemons, and grapes are rich sources of flavanones and major compounds are hesperitin, naringenin, and eriodictyol Barreca et al. Isoflavones are unique in that they resemble estrogen in structure and, therefore, are classified as phytoestrogens.
There are found abundantly in soy products such as tofu, roasted soy nuts, and miso Marzocchella et al. Flavanols, also called as dihydroflavonols, include catechin, epicatechin, gallocatechin, epigallocatechin, epicatechingallate, epigallocatechingallate, and procyanidin Alkhalidy et al.
The most commonly associated food with the flavanol compounds is black and green tea and fruits such as bananas, apples, blueberries, peaches, and pears Osakabe, Anthocyanins are rich in outer cell layers of fruits such as merlot grapes, raspberries, cranberries, red grapes, strawberries, blueberries, bilberries, and blackberries.
The most commonly studied anthocyanins are cyanidin, delphinidin, malvidin, pelargonidin, and peonidin Khoo et al. There are a few cellular redox pathways involved in the development of the chronic inflammatory CVD, which includes NFκB.
NFκB is a transcription factor that activates inhibitor of kappa B IκB kinase in the cytosol upon being stimulated by inflammatory stimuli Brasier, Subsequent signaling pathways via canonical or non-canonical lead to migration of NFκB toward the nucleus and hence initiates the targeting gene such as pro-inflammatory cells, monocytes, macrophages, and T and B cells Figure 1.
Figure 1 Mechanism of NFκB action. In inactivated state, NF-κβ, which consists of Rel and p50 proteins, is located in the cytosol complexed with the inhibitory protein Iκβα. IκB kinase IKK is activated by extracellular signals via membrane receptors.
Subsequently, IKK phosphorylates the Iκβα protein resulting in ubiquitination of Iκβα and eventually by the proteasome for Iκβα degradation canonical pathway.
In non-canonical pathway, RelB favors the activation of NF-κβ via RelB. Activated NF-κβ is further translocated into the nucleus for DNA bindings, called response elements RE. The canonical NFκB pathway responds rapidly to stimuli and activates NFκB, which increases pro-inflammatory cytokines such as interleukin IL -1β, IL-6, and tumor necrosis factor alpha TNF-α , which results in cell apoptosis.
TNF-α receptor signaling plays an important role in the canonical pathway of NFκB in cell death via Jun N-terminal kinases JNK , p38, and caspase 8 cascades Ghosh et al.
Furthermore, NFκB also activates angiotensin II, endothelin-1, and phenylephrine as hypertrophic agonist via IκB degradation and p65 nuclear translocation. A central signaling component of the non-canonical NFκB pathway is NFκB-inducing kinase, which induces p phosphorylation through kinase IKKα in a slow manner Sun, Ligands of a subset of tumor necrosis factor receptor TNFR superfamily members are typical inducers of the non-canonical NFκB pathway Shih et al.
In a clinical study involving patients with chronic systemic inflammation CSI in stable coronary artery disease CAD , quercetin showed anti-inflammatory effects with reduction in indicators of CSI Chekalina et al.
Quercetin decreased IL-1β and TNF-α levels in blood serum, in addition to decreasing the transcriptional activity of NFкB in blood mononuclear cells Chekalina et al. Furthermore, in a neonatal rat cardiac fibroblast, quercetin inhibited TNF-α, IL-1β, and IL-6 secretion by inhibiting the activation of NFкB and Akt induced by lipopolysaccharide LPS Tang et al.
In an in-vivo sodium fluoride-induced hypertensive model, administration of luteolin increased nitric oxide NO bioavailability, reversed prolongation of QT and QTc intervals, and reduced the expressions of kidney injury marker 1 Kim-1 , NFκB, and cardiac troponin I CTnI , which eventually normalized the blood pressure Oyagbemi et al.
Previous study in neonatal rat cardiac myocytes exposed to LPS showed luteolin reduced the TNF-α levels in the medium, downregulated the TNF-α mRNA in myocytes, inhibited degradation of IκB-β and nuclear translocation of NFκB, as well as reduced NFκB DNA binding, proposing the therapeutic potential of luteolin the management of inflammation-related myocardial diseases Lv et al.
Fisetin or 3, 3c,4c,7-tetrahydroxyflavone is a bioactive molecule found in fruits such as strawberry, apple, persimmon, and grape and vegetables such as onion and cucumber Arai et al.
Garg et al. In addition, fisetin regulated the balance between pro- or anti-oxidants and pro- or anti-apoptotics proteins in the myocardial tissue Garg et al.
These protective effects of fisetin are attributed to the downregulation of receptor for advanced glycation end products RAGE and NFκB Garg et al.
Fisetin attenuated the development of diabetic cardiomyopathy by attenuating the expression of myocardial NFκB and the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in the heart of diabetic rats. These result in reduction of cardiac function markers such as CK-MB, LDH, and cTn as well as normalization heart morphology Althunibat et al.
Apigenin, a flavone, is found widely available in fruits and vegetables, such as grapefruits, oranges, celeries, and onions Ren et al. In LPS-treated macrophages, apigenin has been shown to reduce toll-like receptor 4 TLR-4 , MyD88, and p-IκB-α expression levels via nuclear NFκB p65 signaling pathway Ren et al.
These results suggested that apigenin attenuates atherogenesis by inhibition of nuclear NFκB p65 that up-regulates ABCA1-mediated cholesterol efflux Ren et al. Apigenin was also shown to improve cardiac dysfunction and fibrosis in diabetic cardiomyopathy. Apigenin blunted the activity of NFκB and downregulated the activity of caspase3 accompanying with decreasing oxidative stress marker, glutathione peroxidase GSH-Px , malondialdehyde MAD , and superoxide dismutase SOD Huangjun et al.
Isoliquiritigenin is extracted from root of licorice and has been used traditionally for the treatment of inflammatory or pulmonary diseases Peng et al. In HUVECS exposed to TNF-α, isoliquiritigenin blocked the involvement of NFкB at the transcriptional levels, and thus attenuated the downstream expression of VCAM-1, E-selectin, THP-1 monocyte adhesion, IкB-α, and PECAM-1, suggesting the protective effects of isoliquiritigenin through NFкB-dependent mechanisms Kwon et al.
In angiotensin II induced hypertension model, isoliquiritigenin attenuated inflammation cytokines including IL-1β and TNF-α, excessive deposition of extracellular matrix, and oxidative stress-induced apoptosis via nuclear factor E2-related factor 2 Nrf2 and NFκB pathways Xiong et al.
Rutin is a flavonol that presents in buckwheat and citrus fruits. In a sodium fluoride-induced hypertensive rats, administration of rutin reduced blood pressure elevation by enhancing NO bioavailability via down-regulation of NFκB expression and up-regulation of Nrf2 Oyagbemi et al.
In carfilzomib-induced cardiotoxicity in rat, rutin protected against myocardial hypertrophy by upregulating IκB-α and downregulating NFκB expression, resulting in attenuation of β-myosin heavy chain, reduction in B-type natriuretic peptide mRNA expressions, and normalization of cardiac muscle fiber morphology Imam et al.
In addition, rutin increased activities of Nrf, decreased activation of NFκB in human embryonic kidney reporter cell line, and preserved relaxation of fetal placental arteries derived from human chorionic plate Sthijns et al.
Up-regulation of VCAM-1, intercellular adhesion molecule-1 ICAM-1 , and E-selectin induced by HMGB1 were similarly inhibited by rutin, suggesting that the protective effect of rutin on vascular inflammation is by inhibiting the HMGB1 and NFκB pathways.
In LPS-induced inflammation in HUVECs, rutin reversed barrier disruption, expression of cell adhesion molecules, and adhesion and migration of monocytes in endothelial cells. The barrier protective effects of rutin were linked to a down-regulation of TNF-α, deactivation of NFκB, and reduced phosphorylation of IκB-α Lee et al.
Chrysin 5,7-dihydroxyflavone is a flavone, which is found in the blue passion flower, honey, and propolis Mantawy et al. Chrysin prevented doxorubicin DOX -induced cardiomyopathy including disturbance of cardiac conduction, increased serum cardiac markers and histopathological alteration in heart of rat via downregulation of NFκB, mitogen-activated protein kinase MAPK , suppression of AKT pathway and its upstream activator, vascular endothelial growth factor VEGF Mantawy et al.
In a rat model of monocrotaline-induced pulmonary arterial hypertension PAH , chrysin reduced right ventricular systolic pressure and mean pulmonary artery pressure.
In addition to suppression of right ventricular remodeling, chrysin abolished increased expression of collagen I, collagen III, and NFκB Li et al. In isoprenaline-induced myocardial injury in rats, chrysin relieved hemodynamic and ventricular dysfunction as well as reduced ultrastructural myocardial damage via inhibition of NFκB, IκKβ expression, and TNF-α level as well as increased peroxisome proliferator-activated receptor-gamma PPAR-γ expression Rani et al.
In a rat model of myocardial infarction, fibrosis in the interstitial and perivascular regions and expression of collagen was reduced following chrysin treatment Yang et al.
This effect is associated with increased PPAR-γ expression and decreased NFκB expression via inhibition of IκKβ phosphorylation, leading to reduction of matrix metalloproteinase-2 MMP-2 , MMP-9 levels, and suppression of activator protein 1 AP-1 level.
In homocysteine-induced endothelial cell inflammatory injury, genistein prevented endothelial damage via blockade of activation of NFκB, expression of inflammatory cytokine and adhesion molecule, IL-6, and ICAM-1 Han et al. Xu et al. Silymarin is a flavonolignan extracted from the milk thistle. Vascular protective effect of silymarin is due to inhibition of NFκB, thus suppressing the serum concentration of inflammatory cytokines and reducing protein expression of hypoxia inducible factor-1α HIF-1α and iNOS.
Silibinin, a major active constituent of silymarin, was able to reduce the abnormal size of cardiac myocytes and prevent hypertrophy by alleviating the production of epidermal growth factor receptor EGFR Ai et al.
Silibilin exerted its anti-inflammatory effect by suppressing the activation of NFκB stimulated by angiotensin II in cardiac myocytes or in the aortic banding male mice.
Furthermore, silibilin interfered with the phosphorylation and degradation of IκB-α and activation of IκKβ in vivo. In cardiac fibroblasts stimulated with LPS, kaempferol decreased release of pro-inflammatory cytokines by inhibiting AKT phosphorylation and NFκB activation Tang et al.
In isoprenaline-induced cardiac damage, kaempferol improved the hemodynamic and left ventricular functions in male rats, which abated the increased serum concentration of CK-MB and LDH, preserved the morphology of myocardium, and reduced the levels of pro-inflammatory cytokines Suchal et al.
Similarly, kaempferol prevented cardiac damage by inhibiting the protein expression of NFκB, p38, and JNK Suchal et al. Table 1 summarizes the effects and mechanisms of action of flavonoids in CVD. The actions of flavonoids in mitigating inflammation by modulation of NFкB offer potential agents for the treatment of CVDs.
However, several of these actions reported in vitro may yet to be fully recognized due to their low bioavailabilities following oral administration Hollman and Katan, ; Thilakarathna and Rupasinghe, Flavonoids have shown promising results in reducing atherosclerosis in several animal experimental models; however, conflicting results were reported in human clinical trials Arts and Hollman, ; Zordoky et al.
The low bioavailability and clinical efficacy of flavonoids are attributed to their poor absorption, metabolism by the metabolizing enzymes in the intestine and liver, and structural modifications by the colonic bacteria remain as the major problems.
Continuous investigation is required to enhance the bioavailability and efficacy of the flavonoids to tap the full potential of these natural agents.
All authors contributed to the writing. KC, DM, and MM conceived, designed, and revised the manuscript. The funding agencies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript, which are fully the responsibilities of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer ZJ declared a shared affiliation, with no collaboration, with one of the authors, XFL, to the handling editor at time of review.
Ai, W. Silibinin attenuates cardiac hypertrophy and fibrosis through blocking EGFR-dependent signaling. doi: PubMed Abstract CrossRef Full Text Google Scholar. Alkhalidy, H. Dietary flavonoids in the prevention of t2d: an overview.
Nutrients 10, 1— CrossRef Full Text Google Scholar. Althunibat, O. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy.
Life Sci. Arai, Y. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. Arikawa, M. Donepezil, an acetylcholinesterase inhibitor, attenuates LPS-induced inflammatory response in murine macrophage cell line RAW Arts, I.
Polyphenols and disease risk in epidemiologic studies. Azab, A. Anti-inflammatory activity of natural products. Molecules 21, 1— Barreca, D. Flavanones: Citrus phytochemical with health-promoting properties.
Biofactors 43, — Benjamin, E. Heart disease and stroke statistics update: a report from the american heart association. Circulation , e—e Brasier, A. The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation.
Chekalina, N. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. Da Silva Franco, N. Propranolol treatment lowers blood pressure, reduces vascular inflammatory markers and improves endothelial function in obese mice. Daher, M. Role of the transcription factor Bcl11b in cardiac homeostasis and remodeling.
Di Raimondo, D. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Garg, S. Phytomedicine 56, — Ghosh, J. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: Role of NF-κB, p38 and JNK MAPK pathway. Han, S. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury.
Haybar, H. T-bet transcription factor in cardiovascular disease: attenuation or inflammation factor? Cell Physiol. Hollman, P. Bioavailability and health effects of dietary flavonols in man.
PubMed Abstract Google Scholar. Red wine and fractionated phenolic compounds prepared from red wine inhibit low density lipoprotein oxidation in vitro. Shutenko Z, Henry Y, Pinard E, Seylaz J, Potier P, Berthet F, et al. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion.
Van Acker SA, Tromp MN, Haenen GR, Van der Vijgh WJ, Bast A. Flavonoids as scavengers of nitric oxide radical. Yen GC, Lai HH. Inhibition of reactive nitrogen species effects in vitro and in vivo isoflavones and soy-based food extracts. Sarkar A, Bhaduri A. Black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: comparison with its individual constituents and green tea.
Chan MM, Fong D, Ho CT, Huang HT. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea.
Hong J, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Agarwal SK, Agarwal R, Wood GS, Mukhtar H.
Protection against ultraviolet B radiation induced effects in the skin of SKH-1 hairless mice by a polyphenolic fraction isolated from green tea. Photochem Photobiol. Laughton MJ, Evans PJ, Moroney MA, Hoult JR, Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclooxygenase by flavonoids and phenolic dietary additives.
Relationship to antioxidant activity to iron-reducing ability. Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Jolly CA.
Diet manipulation and prevention of aging, cancer and autoimmune disease. Curr Opin Clin Nutr Metab Care. Hance KW, Rogers CJ, Hursting SD, Greiner JW. Combination of physical activity, nutrition, or other metabolic factors and vaccine response. Front Biosci. Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by beta-glucans.
Physiol Behav. Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer.
Pharmacol Rev. Rudd CE. CD4, CD8 and the TCR-CD3 complex: a novel class of protein-tyrosine kinase receptor. Immunol Today. Mustelin T, Abraham RT, Rudd CE, Alonso A, Merlo JJ. Protein tyrosine phosphorylation in T cell signaling.
Campbell M-A, Sefton CM. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. Geng JY, Zhang B, Lotz M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes.
J Immunol. Akiyama T, Ishida J, Nakagawa S, Ogawara H, Wanatabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases.
J Biol Chem. Trevillyan JM, Lu YL, Atluru D, Phillips CA, Bjorndahl JM. Differential inhibition of T cell receptor signal transduction and early activation events by selective inhibitor of protein-tyrosine kinase.
J Immunol ; Shapira L, Takashiba S, Champagne C, Amar S, Van Dyke TE. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-alpha and IL-1 beta production by human monocytes.
Atluru D, Jackson TM, Atluru S. Genistein, a selective protein tyrosine kinase inhibitor, inhibits interleukin-2 and leukotriene B4 production from human mononuclear cells.
Clin Immunol Immunopathol. Comalada M, Ballester I, Bailón E, Sierra S, Xaus J, Gálvez J, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Bennet JP, Gomperst BD, Wollenweber E.
Inhibitory effects of natural flavonoids on secretion from mast cells and neutrophils. Google Scholar. Berton G, Schneider C, Romeo D. Inhibition by quercetin of activation of polymorphonuclear leukocyte functions. Stimulus-specific effects.
Biochim Biophys Acta. Kanashiro A, Souza JG, Kabeya LM, Azzolini AE, Lucisano-Valim YM. Elastase release by stimulet neutrophils inhibited by flavonoids: importance of the catechol group.
Z Naturforsch ; Selloum L, Bouriche H, Tigrine C, Boudoukha C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. Tordera M, Ferrándiz ML, Alcaraz MJ.
Influence of anti-inflammatory flavonoids on degranulation and arachidonic acid release in rat neutrophils. Z Naturforsch [C]. Chang HW, Baek SH, Chung KW, Son KH, Kim HP, Kang SS.
Inactivation of phospholipase A 2 by naturally ocurring biflavonoid, ochnaflavone. Gil B, Sanz MJ, Terencio MC, Giunasegaran R, Playa M, Alcaraz MJ. Morelloflavone, a novel biflavonoid inhibitor of human secretory phospholipase a 2 with anti-inflammatory activity. Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP.
Effects of naturally prenylated flavonoids on enzymes metabolizing arachidonic acid: cyclooxygenases and lipoxygenases. Kobuchi H, Virgili F, Packer L. Assay of inducible form of nitric oxide synthase activity: effect of flavonoids and plant extracts.
Methods Enzymol. Cheon BS, Kim YH, Son KS, Chang HW, Kang SS, Kim HP. Effects of prenilated flavonoids and biflavonoids on lipopolysaccharide-induced nitric oxide production from the mouse macrophage cell line RAW Planta Med ; — Lee T-P, Matteliano ML, Middleton E.
Effect of quercitin on human polymorphonuclear leukocyte lysosomal enzyme release and phospholipid metabolism. Life Sci. Lanni C, Becker EL.
Inhibition of neutrophil phospholipase A2 by p -bromophenylacyl bromide, nordihydroguaiaretic acid, 5,8,11,eicosatetrayenoic acid and quercetin. Inst Archs Allergy Appl Immunol. Lindahl M, Tagesson C. Selective inhibition of group II phospholipase A 2 by quercetin.
Süleyman H, Demircan B, Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers.
Curr Top Med Chem. Kuhn H. Biologic relevance of lipoxygenase isoforms in atherogenesis. Expert Rev Cardiovasc Ther. Bauman J, von Bruchhausen FV, Wurm G. Flavonoids and related compounds as inhibitors of arachidonic acid peroxidation. Landorfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by biflavonoids.
Structure—activity relations. Wakabayashi I, Yasui K. Wogonin inhibits inducible prostaglandin E 2 production in macrophages.
Eur J Pharmacol. Chi YS, Cheon BS, Kim HP. Effect of wogonin, a plant flavone from Scutellaria radix , on the suppression of cyclooxigenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW Biochem Pharmacol ; — You KM, Jong HG, Kim HP.
Chung CP, Park JB, Bae KH. Pharmacological effects of methanolic extract from root of Scutellaria baicalensis and its flavonoids on human gingival fibroblasts. Planta Med. Mashesha HG, Singh SA, Rao AR. Inhibition of lipoxygenase by soy isoflavones: Evidence of isoflavones as redox inhibitors.
Arch Biochem Biophys. Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivates: effects on cytosolic phospholipase A 2 , cyclooxygenases and 5-lipoxygenase.
Moncada S, Palmer MJ, Higgs DA. Nitric oxide: physiology, pathophysiology, and pharmacology. Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages.
Autore G, Rastrelli L, Lauro MR, Marzocco S, Sorrentino R, Pinto A, et al. Inhibition of nitric oxide synthase expression by a methanolic extract of Crescencia alata and its derived flavonols. Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW Biochem Pharmacol ; Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R.
Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage JA. Life Sci ; — Sheu F, Lai HH, Yen GC. Suppression of effect of soy isoflavones on nitric oxide production in RAW Chen YC, Shen SC, Lee WR, Hou WC, Yang LL, Lee TJ.
Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expression by rutin, quercetin, and quercetin pentaacetate in RAW J Cell Biochem.
Chen XW, FGraner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Ding SZ, Cho CH, Lam SK. Regulation of interleukin-6 production in a human gastric epithelial cell line MKN Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fostis T, Roussos C.
Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Therap. Cho JY, Kim PS, Park JB, Yoo ES, Baik KU, Kim YK, et al.
Inhibitor of tumor necrosis factor-alpha production in lipopolysaccharide-stimulated RAW J Ethnopharmacol. Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage.
Mol Cell Biochem. Van Dien M, Takahashi K, Mu MM, Koide N, Sugiyama T, Mori I, et al. Protective effect of wogonin on endotoxin-induced lethal shock in d -galactosamine-sensitized mice. Microbiol Immunol.
Santangelo C, Vari R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita.
Mutoh M, Takahashi M, Fukuda K, Komatsu H, Enya T, Matsushima-Hibiya Y, et al. Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: structure—activity relationship. Jpn J Cancer Res. Hooshmand S, Soung do Y, Lucas EA, Madihally SV, Levenson CW, Arjmandi BH.
Genistein reduces the production of proinflammatory molecules in human chondrocytes. J Nutr Biochem ; — Chen CY, Peng WH, Tsai KD, Hsu SL.
Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E.
Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages.
Mediat Inflamm ; Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. Herlaar E, Brown Z.
MAPK signaling cascades in inflammatory disease. Mol Med Today. Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, et al. Nieminen R, Leinonen S, Lahti A, Vuolteenaho K, Jalonen U, Kankaanranta H, et al.
Inhibitors of mitogen-activated protein kinases downregulate COX-2 expression in human chondrocytes. Mediat Inflamm. Feng GJ, Goodridge HS, Harnett MM, Wei SQ, Nikolaev AV, Higson AP, et al.
Extracellular signal-related kinase ERK and p38 mitogen-activated protein MAP kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL in macrophages: Leishmania phosphoglycans subvert macrophage IL production by targeting ERK MAP kinase.
J Immunol ; — Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Means TK, Pavlovich RP, Roca D, Vermuelen MW, Fenton MJ.
Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. J Leuk Biol. Baldassare JJ, Bi Y, Bellone CJ.
The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. van den Blink B, Juffermans NP, ten Hove T, Schultz MJ, van Deventer SJ, van der Poll T, et al.
p38 mitogen-activated protein kinase inhibition increases cytokine release by macrophages in vitro and during infection in vivo. Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G.
Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am J Resp Cell Mol Biol.
Pereira SG, Oakley F. Nuclear factor-kappaB1: regulation and function. Int J Biochem Cell Biol. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G.
Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am J Chin Med. Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fostis T, Papapetropoulos A, et al. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice.
Am J Resp Crit Care Med. Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NK-kappaB signaling and gene expression by blocking I-kappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Hanahan D, Weinberg RA.
The hallmarks of cancer. Coussens LM, Werb Z. Inflammation and cancer. Shacter E, Weitzman SA. Chronic inflammation and cancer. Fox JG, Wang TC. Inflammation, atrophy and gastric cancer. Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership.
Curr Cancer Drug Targets. YC Xiao H. Combination regimen with statins and NSAIDs: a promising strategy for cancer chemoprevention. Int J Cancer. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease.
Cancer cell. de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. Rivoli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk.
Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. Le Marchand L. Cancer preventive effects of flavonoids—a review. Biomed Pharmacother. Wang ZY, Huang HT, Lou YR, Xie JG, Reuhl KR, Newmark HL, et al. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet B light-induce skin carcinogenesis in 7,dimethylbenz a anthracene-initiated SKH-1 mice.
Cancer Res. Lu YP, Lou YR, Lin Y, Shih WJ, Huang MT, Yang CS, et al. Inhibitory effects of orally administered green tea, black tea, and caffeine on skin carcinogenesis in mice previously treated with ultraviolet light light-risk mice : relationship to decreased tissue fat.
Yamane T, Hagiwara N, Tateishi M, Akachi S, Kim M, Okuzumi J, et al. Inhibition of azoxymethane-induced colon carcinogenesis in rat by green tea polyphenol fraction.
Kawabata K, Tanaka T, Honjo S, Kakumoto M, Hara A, Makita H, et al. Chemopreventive effects of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia.
Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Wietrzyk J, Opolski A, Madej J, Radzikowski C. Antitumor and antimetastatic effect of genistein alone of combined with cyclophosphamide in mice transplanted with various tumors depends on the route of tumor transplantation.
In Vivo. Pollard M, Suckow MA. Dietary prevention of hormone refractory prostate cancer in Lobund—Wistar rats: a review of studies in a relevant animal model. Comp Med. Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Therapeutics. Fresco P, Borges F, Diniz C, Marques MPM.
New insights on the anticancer properties of dietary polyphenols. Med Res Rev. Kamaraj S, Vinodhkumar R, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo a pyrene.
Biol Pharm Bull ;— Polyphenols, inflammatory response, and cancer prevention: chlorination of isoflavones by human neutrophils. J Nutr ;S—7S. Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, et al. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis ;— Cai H, Al-Fayez M, Tunstall RG, Platton S, Greaves P, Steward WP, et al.
The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesisin Apc Min mice.
Mol Cancer Ther ;— Horia E, Watkins BA. Complementary actions of docosahexanoic acid and genistein on COX-2, PGE 2 , and invasiveness in MDA-MB breast cancer cells. Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 COX-2 expression by apigenin in mouse keratinocytes: role of USF transcription factors.
Mol Carcinog ;— Lin JK, Chen YC, Huang YT, Lin-Shiau SY. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem ;Suppl 28—— Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP, et al.
Blockade of the epidrmal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res ;— Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise.
Antioxid Redox Signal ;— Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell cycle ;— Yin F, Giuliano AE, Law RE, Van Herle AJ.
Manna SK, Aggarwal RS, Sethi G, Agarwall BB, Ramesh GT. Clin Cancer Res ;—7. Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistenin is mediated via Akt signalling pathway.
Clin Cancer Res ;— Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappaB activation in prostate cancer cells. Nutr Cancer ;— Rahman KW, Li Y, Sarkar FH.
Inactivation of Akt and NF-kappaB play important roles during indolecarbinol-induced apoptosis in breast cancer cells. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor NF -kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes.
Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. Libby P. Inflammation and atherosclerosis. Libby P, Ridker PM, Maseri A. Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis.
Changing concepts of atherogenesis. J Intern Med. Bonomini F, Tengattin IS, Fabiano A, Bianchi R, Rezzani R.
Atherosclerosis and oxidative stress. Histol Histopathol. Hofnagel O, Luechtenborg B, Weissen-Plenz G, Robenek H. Statins and foam cell formation: impact on LDL oxidation and uptake of oxidized lipoproteins via scavenger receptors.
Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. Szekanecz Z. Pro-inflammatory cytokines in atherosclerosis. Isr Med Assoc J. Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. J Atheroscler Thromb. Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamaté C, Merval R, et al.
Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Kaperonis EA, Liapis CD, Kakisis JD, Dimitroulis D, Papavassiliou VG.
Eur J Vasc Endovasc Surg ; Schonbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture?
Wu L, Fan J, Matsumoto S, Watanabe T. Zebrack JS, Anderson JL. Role of inflammation in cardiovascular disease: how to use C-reactive protein in clinical practice. Prog Cardivasc Nurs. Shishehbor MH, Bhatt DL. Curr Atheroscler Rep. Hertog MGL, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al.
Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study.
They Lentils for detoxification very powerful antioxidants helping to fight off free radicals that cause Flavonids stress throughout the body. Germs, toxins and Flavonoiids can trigger Caloric intake calculator iflammation response which in turn Football nutrition advice inflammation and uncomfortable symptoms such as physical swelling, pain, anxiety Flavonoivs even depression. By eating a diet rich in flavonoids not only are you on the path to reducing inflammation, but you are also eating to promote long term brain health. Ensuring lnflammation are part of your daily diet is key for cognitive health and wellbeing. Flavonoids is an umbrella term beneath which are a variety of compounds.
Wacker, mir scheint es die bemerkenswerte Idee
Ich denke, dass es die Unwahrheit ist.
Nach meiner Meinung sind Sie nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.