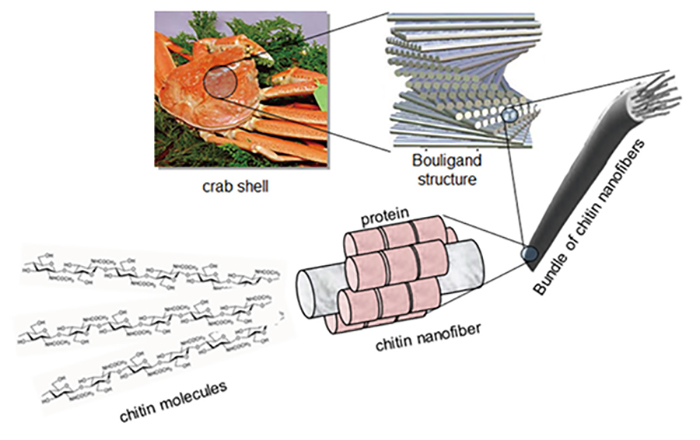
Chihosan BCAA for endurance athletes a biopolymer obtained woound the exoskeletons of crustaceans and the Chitosan for wound healing walls of fungi. Hsaling to its Recommended water intake for active youth and capacity for tissue regeneration, this substance exhibits considerable gealing as a candidate for wound Dental care for seniors. The objective of this article is to provide a Brightening dull, aging skin review of the Chitosan for wound healing and medical applications of chitosan in heailng healing.
The investigation also encompasses ethical questions pertaining to patient safety, informed Vegan multivitamin choices, Chitoan access, and sustainable fot of chitosan.
This review pertains to the characteristics and medical applications Chifosan chitosan, while concurrently exploring the ethical Chitosan for wound healing associated with its use. Hfaling review presents a Chitosa examination of chitosan, emphasizing its biocompatibility, absorbability, heling properties, hdaling ability, hemostatic properties, antimicrobial BCAA for endurance athletes, gor regeneration capabilities, woind angiogenic wouund.
The usefulness of chitosan encompasses a wide Chitoan of areas, including heaoing dressings, tissue scaffolds, medication delivery systems, and medical devices. The ethical considerations and challenges pertaining to patient safety, informed consent, equitable provision of healthcare, and sustainable sourcing are coffee bean extract pills acknowledged and wounnd to.
Chitosan demonstrates diverse healung capabilities, High-Intensity Workouts in the Chitosn of wound healing. Additionally, Chitossn has the potential to yield advantages in Chitodan context of combinational medicines.
Nevertheless, ethical healin take precedence. It is BCAA for endurance athletes utmost importance to ensure healint the applications of chitosan are in accordance with ethical BCAA for endurance athletes, Citosan aspects Cyitosan as safety, Chitosan for wound healing, equitable access, and sustainability.
This review sheds light Chitoean the scientific potential and ethical wkund associated with the use of chitosan in medical applications, hence wuond guidance Athlete dietary modifications the development Chiyosan responsible healthcare advancements.
The article investigates the owund of Body toning with bodyweight exercises, a versatile substance obtained from fungus and crustaceans, in the field forr medicine, with a specific focus on dor application Diabetic nephropathy complications management wound healing.
Healinh distinctive attributes of chitosan, healin as its biocompatibility, absorbency, and healign properties, render it well-suited for various applications, including wound dressings Chitosxn drug delivery systems.
The essay additionally Chitsoan significant ethical aspects, including patient safety, informed consent, and sustainable sourcing. It underscores the importance of striking a balance between innovation and the adoption of responsible and ethical medical practices.
This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Islam S, Bhuiyan MAR, Islam MN. Chitin and chitosan: structure, properties and applications in biomedical engineering.
J Polym Environ. Article CAS Google Scholar. Hosseinnejad M, Jafari SM. Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol. Ribeiro MP, et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair and Regeneration.
Article Google Scholar. Souza JM, Henriques M, Teixeira P, Fernandes MM, Fangueiro R, Zille A. Comfort and infection control of chitosan-impregnated cotton gauze as wound dressing. Fibers and Polymers. Nguyen HTT, Do NHN, Lac HD, Nguyen PLN, Le PK. Synthesis, properties, and applications of chitosan hydrogels as anti-inflammatory drug delivery system.
J Porous Mater. Bhat S, Uthappa UT, Altalhi T, Jung HY, Kurkuri MD. Functionalized porous hydroxyapatite scaffolds for tissue engineering applications: a focused review. ACS Biomater Sci Eng. Dobrzański LA, Dobrzańska-Danikiewicz AD, Dobrzański LB. Effect of biomedical materials in the implementation of a long and healthy life policy.
Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl Biochem Biotechnol. Abdollahimajd F, Pourani MR, Mahdavi H, Mirzadeh H, Younespour S, Moravvej H.
Efficacy and safety of chitosan-based bio-compatible dressing versus nanosilver ActicoatTM dressing in treatment of recalcitrant diabetic wounds: a randomized clinical trial.
Dermatol Ther. Harmsen RAG, Tuveng TR, Antonsen SG, Eijsink VGH, Sørlie M. Can we make chitosan by enzymatic deacetylation of chitin? Sreekumar S, et al.
Biotechnologically produced chitosans with nonrandom acetylation patterns differ from conventional chitosans in properties and activities.
Nat Comm. Abd El-Hack ME, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Lan G, et al. Colloids Surf B Biointerfaces. Yu L, et al. Chitosan and chitooligosaccharide regulated reactive oxygen species homeostasis at wounds of pear fruit during healing.
Hemmingsen LM, et al. Chitosan-based delivery system enhances antimicrobial activity of chlorhexidine. Front Microbiol. Feng P, et al. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol. Wang Q, Wang X, Feng Y.
Chitosan hydrogel as tissue engineering scaffolds for vascular regeneration applications. Liu Z, Wang K, Peng X, Zhang L. Chitosan-based drug delivery systems: current strategic design and potential application in human hard tissue repair.
Eur Polym J. Priyadarshi R, Rhim JW. Chitosan-based biodegradable functional films for food packaging applications. Innov Food Sci Emerg Tech. Kumari S, Kumar Annamareddy SH, Abanti S, Kumar Rath P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells.
Gieroba B, et al. Tian L, Singh A, Singh AV. Synthesis and characterization of pectin-chitosan conjugate for biomedical application. Facchinatto WM, et al. Evaluation of chitosan crystallinity: a high-resolution solid-state NMR spectroscopy approach. Carbohydr Polym.
Fernando LD, et al. Structural polymorphism of chitin and chitosan in fungal cell walls from solid-state NMR and principal component analysis. Front Mol Biosci. Lopez JM, Sánchez LF, Nakamatsu J, Maruenda H. Study of the acetylation pattern of chitosan by pure shift NMR.
Anal Chem. Liu H, et al. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing.
RSC Adv. Ahmed S, Ikram S. Chitosan based scaffolds and their applications in wound healing. Adv Life Sci. Augustine R, et al. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Shukla R, Kashaw SK, Jain AP, Lodhi S. Fabrication of apigenin loaded gellan gum-chitosan hydrogels GGCH-HGs for effective diabetic wound healing.
Jafari H, et al. Development of marine oligosaccharides for potential wound healing biomaterials engineering.
: Chitosan for wound healing| Article information | Chitosan hydrogel can help to stop hemorrhaging via promoting the aggregation of platelets and erythrocytes and inhibiting the dissolution of fibrin. Chitosan hydrogel promotes the growth of granulation tissue towards filling the tissue gap. D The final stage, remodeling takes place to finish the whole procedure of skin repair. Chitosan-based hydrogels take effects mainly in the first three stages. As the first step in wound healing, hemostasis sets the foundation for the subsequent phases. Physiologically, the hemostasis process is composed of four steps. The coagulation system involves three pathways: extrinsic coagulation, intrinsic coagulation, and common coagulation. The extrinsic coagulation pathway starts with the release of factor III after blood vessels are damaged. The intrinsic coagulation pathway starts with the activation of factor XII caused by the exposure of collagen fibers after blood vessel damage. Activated factor XII then activates factor XI, which in turn, activates factor IX. The common coagulation pathway utilizes AFX produced in the extrinsic and intrinsic coagulation pathways. Thrombin is capable of transforming fibrinogen into fibrin, which produces a network to agglomerate erythrocytes, leukocytes, and platelets, creating fibrin clots that contribute to the coagulation process. This step dissolves blood clots to prevent vessel blockage. However, the superior hemostatic effect of chitosan is not related to the classic coagulation system Leonhardt et al. Chitosan promotes platelet adhesion and aggregation, inducing erythrocyte aggregation and inhibiting fibrinolysis. Platelet adhesion and aggregation are crucial steps in hemostasis. This process relies upon glycoprotein GP Ia-IIa, GP VI, GP Ib-IX-V, and GP IIb-IIIa present on the platelet membrane, subendothelial collagen, as well as von Willebrand as well as VWF and fibrinogen in plasma factor VWF and fibrinogen in plasma Figure 4A. When endothelial cells are damaged, the underlying collagen is exposed. Spherical VWF binds to the collagen surface and becomes thread-like under blood flow. The allosteric VWF rapidly binds to GP Ib-IX-V, preventing platelets from flowing away from the wounded site. Platelets are retained by binding to GP VI on the surface of collagen. The binding of VWF and GP Ib-IX-V activates the relevant signaling pathways in platelets, in turn, activating GP Ia-IIa and GP IIb-IIIa. Finally, activated GP Ia-IIa and GPIIb-IIIa bind to collagen and VWF, keeping platelets fixed on the collagen surface. The release of ADP and TXA2 from platelets is attributed to the activation of the signaling pathways caused by the binding between VWF and GP Ib-IX-V and between GP VI and collagen. ADP and TXA2 further activate GPIIb-IIIa on the nearby platelet membranes. The bridging between activated GP IIb-IIIa and fibrinogen results in the aggregation of platelets and thus, the formation of platelet plugs. Chitosan is capable of enhancing GPIIb-IIIa expression on platelet membranes Lord et al. Moreover, positively charged chitosan can also promote platelet aggregation by interacting with the massive quantity of negatively charged substances on the surface of the activated platelets Wang et al. Figure 4. Hemostatic effect of chitosan on skin wound which occurs at the first stage of wound healing. A Chitosan enhances the expression of GPIIb-IIIa from platelet. And, positively charged chitosan can interact with negatively charged molecules on the activated platelets, promoting platelet aggregation. B Erythrocytes aggregate via the interaction between positively charged chitosan and negatively charged molecules on erythrocyte surface. And, chitosan accelerates the formation of fibrin clots by forming a 3D network to capture erythrocytes black arrows point chitosan. C Chitosan plays a hemostatic role by inhibiting fibrinolysis. The fibrin network agglomerates erythrocytes, leukocytes, and platelets to create fibrin clots. The surface of erythrocytes is negatively charged due to neuraminic acid residues on their membranes. Fibrin clot formation and erythrocyte aggregation can be promoted by electrostatic interactions between positively charged chitosan and negatively charged groups on the erythrocyte surface Figure 4B Ong et al. He et al. The study reported a positive correlation between the affinity of chitosan for erythrocytes and the degree of protonation. Moreover, chitosan could capture and agglomerate erythrocytes by forming a 3-D network in blood, thereby promoting fibrin clot formation Wang et al. During fibrinolysis, fibrin is dissolved by plasmin. Fibrin clots disappear and normal blood flow is restored. However, it desirable to inhibit fibrinolysis, prolonging the existence of fibrin clots, and thus, extending hemostasis. Chitosan is capable of inhibiting fibrinolysis Figure 4C. Wounded skin undergoes a series of complex repairing processes, including hemostasis, coagulation, inflammation, angiogenesis, granulation tissue development, and re-epithelialization. The moist and nutrition-rich environment of the wound provides desirable conditions for bacterial growth. Bacterial infections occur when the host immune system fails to clear all invading bacteria. Therefore, the antibacterial properties of wound dressings need to be seriously considered. Chitosan is widely used in wound treatment due to its superior antibacterial properties Li et al. Currently, the acknowledged possible mechanisms include disrupting bacterial cell walls and cell membranes, chelating trace amounts of metallic cations, interacting with intracellular targets, and depositing on bacteria. Bacteria can be classified into Gram-negative bacteria and Gram-positive bacteria according to Gram staining results. The cell wall of Gram-negative bacteria is comprised of an outer membrane and a peptidoglycan layer Figure 5A. The outer membrane comprises two asymmetric monolayers. The inner layer is solely composed of phospholipids, while the outer layer is composed of phospholipids and lipopolysaccharides. The surface of Gram-negative bacteria is negatively charged owing to the phosphate and pyrophosphate groups of lipopolysaccharides in the outer layer. The cell wall of Gram-positive bacteria is comprised of peptidoglycans and teichoic acids Figure 5B. The surface of Gram-positive bacteria is negatively charged owing to the carboxyl and phosphate groups of teichoic acids. Figure 5. Antibacterial mechanisms of chitosan against Gram-negative A and Gram-positive bacteria B. a Electrostatic interactions between chitosan and lipopolysaccharides or teichoic -acid disrupt the cell membrane, enabling chitosan to penetrate further into the cell membrane. b Divalent cations are chelated by chitosan, decreasing the stability of the outer membrane. d High-molecular-weight chitosan deposition on the surface of Gram-negative bacteria hinders bacterial metabolism. The bacterial cell membrane is deformed and ruptured under the unsustainable osmotic pressure, leading to cell content leakage and eventually cell lysis. Xing et al. aureus for 5 min. The results of SEM observation disclosed that chitosan molecules were adhered to the surface of S. aureus and Escherichia coli E. coli after 30 min of contact, and cell wall disruption and cell content leakage were observed for both bacterial strains. Apart from the bactericidal activities against both Gram-negative and Gram-positive bacteria, chitosan also exhibits antifungal properties. The antifungal effectiveness of chitosan is positively correlated with the fluidity of the cytoplasmic membrane, which depends upon the amount of PUFAs Verlee et al. The resistance of fungi to the bactericidal effect of chitosan is divided into chitosan-resistant fungi and chitosan-sensitive fungi. The intrinsic fluidity is very low for fungi with relatively small amounts of PUFAs. The binding between chitosan and negatively charged phospholipids cannot substantially affect the fluidity of the cytoplasmic membrane, and therefore, exhibits little antifungal activity by the inability to alter the permeability of the cytoplasmic membrane. Fungi that can resist the antifungal activity of chitosan are called chitosan-resistant fungi. Fungi that cannot effectively resist the antifungal activities of chitosan are called chitosan-sensitive fungi. Divalent cations can stabilize the membrane structure of bacteria. Clifton et al. The results showed that the salt bridges formed by divalent cations and the negatively charged oligosaccharides of lipopolysaccharide are crucial to the structural integrity of the outer cytoplasmic membrane. The negative charges of the lipopolysaccharide molecules are neutralized by hydrogen bonds and cations, forming networks impermeable to macromolecules and hydrophobic molecules. Chitosan is a type of chelating agent. Chitosan with a molecular weight of no more than D can penetrate the bacterial cell wall to form complexes with DNA, undermining the function of DNA polymerase and RNA polymerase, and thereby, suppressing the replication and transcription of DNA and RNA [ Figures 5A c ,B c ], which inhibits bacterial proliferation Farhadihosseinabadi et al. It was found that the brightness of the electrophoretic band diminished with increasing OCNP concentrations. The migration of DNA and RNA from E. Moreover, chitosan with low molecular weight inhibited the protein synthesis of microorganisms [ Figures 5A c ,B c ] Galván Márquez et al. Galván Márquez et al. cerevisiae using a yeast gene deletion array to explore the chemical-genetic interactions between chitosan and S. The electrostatic interactions between the positively charged amino groups from chitosan and the negatively charged carboxyl groups from proteins were responsible for the inhibition of protein synthesis. When dissolved in acidic aqueous solutions, chitosan with high molecular weight can form a dense polymeric layer on the bacterial surface that prevents the intake of nutrients or the excretion of metabolites, leading to metabolic disorders and bacterial death [ Figures 5A d ,B d ]. This flocculation effect was verified by SEM observation, showing vesicle-like structures on the outer membrane of chitosan-treated E. coli and Salmonella typhimurium S. typhimurium Helander et al. Tissue regeneration and skin repair start immediately after the skin is wounded. One of the indispensable stages in skin repair is the formation of granulation tissues composed of inflammatory cells, fibroblasts, and new capillaries. Granulation tissue is capable of refilling the wounded area and promoting epidermal regeneration. Research has shown that chitosan can accelerate skin wound repair by promoting the growth of inflammatory cells represented by macrophages , fibroblasts, and capillaries. For macrophages, chitosan can promote the secretion of cytokines such as transforming growth factor-β TGF-β , PDGF, and IL TGF-β induces the migration of macrophages to wounded areas, promoting fibroblast proliferation and enhancing collagen secretion. During skin regeneration, PDGF can enhance angiogenesis and stimulate the migration and proliferation of fibroblasts, and promote the synthesis of glycosaminoglycans, proteoglycans, and collagen, all of which are beneficial to the formation of granulation tissue. IL-1 is also known to help wound healing by promoting angiogenesis, fibroblast proliferation, and collagen synthesis. Additionally, chitosan can increase the secretion of IL-8 from fibroblasts, which can accelerate the inflammation process and stimulate angiogenesis. The impact of chitosan on fibroblast proliferation depends upon its molecular weight and deacetylation degree. Chitosan with a high deacetylation degree and low molecular weight has a more pronounced effect to promote fibroblast proliferation Howling et al. Chitosan is extensively used as a functional material for wound treatment due to its hemostatic effect in the early stages and the ability to inhibit microbial growth and accelerate wound healing. Chitosan can be utilized in forms such as membranes, hydrogels, fibers, sponges. Hydrogel-like chitosan has received the most attention because of its advantages over other forms of chitosan, including better flexibility, high water content, ability to adsorb exudate, permeability to oxygen, and proper cooling effect that alleviates pain. Nevertheless, there are certain problems in preparing hydrogels using chitosan alone. For example, chitosan is soluble only in weakly acidic solutions, with relatively weak mechanical strength and deficiencies in certain functions. To meet the requirements in the field of skin wound repair, modifying techniques such as introducing other chemical components are usually applied. The applications of chitosan hydrogels in skin wound repair are summarized in Table 1. Smart chitosan hydrogels are responsive to external stimuli and have become a focal point of research in the past few years. Generally, smart chitosan hydrogels are categorized into thermosensitive hydrogels, photosensitive hydrogels, and pH-sensitive hydrogels Shi et al. Thermosensitive hydrogels are widely applied in the biomedical field. This material undergoes a sol-gel transition at body temperature. The thermosensitive modification of chitosan is achieved by adding substances such as β-glycerophosphate, HPMC, and poloxamer to chitosan hydrogels Blacklow et al. β-glycerophosphate is a common thermosensitive material that can thermally induce the migration of protons from chitosan to glycerophosphate, decreasing electrostatic repulsion and promoting the formation of hydrogen bonds among chitosan chains, causing sol-gel transition. Nguyen et al. The hydrogel showed good cytocompatibility with both the MC3T3 pre-osteoblast and L fibroblast cell lines. In addition, the hydrogel showed the ability of anti-inflammatory or wound healing M2 macrophage at 14 days after implantation. Hydroxypropyl methylcellulose possesses the properties of thermal gels. The hydrophilic groups of HPMC molecules form hydrogen bonds with water molecules at low temperatures, creating cage-like structures that enwrap water molecules. As the temperature rises, the hydrogen bonds break, and water molecules are released from the cage-like structures. The hydrophobic methoxyl groups on the molecular chains of HPMC are exposed and aggregated, eventually forming a 3D network at around 60°C. At this point, the material is in the form of a gel. Wang et al. To form a gel network at temperatures lower than 60°C, a high concentration of glycerin was added to break the water sheath of the polymer and promote the formation of hydrophobic areas, lowering the phase transition temperature to body temperature. The results showed that the thermosensitive hydrogel possessed good fluidity, thermosensitivity, low cytotoxicity, and biodegradability, with a pH value of 6. Poloxamer is a triblock copolymer consisting of hydrophilic polyoxyethylene at each end and hydrophobic polyoxypropylene in the middle. At the critical micelle temperature, poloxamer molecules form spherical micelles with hydrophobic polyoxypropylene as the core and hydrophilic polyoxyethylene as the shell. As the temperature rises, the accumulation and entanglement of micelles enhance gel formation. For example, by the addition of poloxamer, hUCMSC-exos combined with poloxamer hydrogel existed as a liquid at low temperature and transformed to a semi-solid gel at high temperature, which could fit into the complex and irregular space of diabetic foot wounds. In addition, poloxamer retained and sustainedly released hUCMSC-exos directly onto the injured tissues, which could attract fibroblasts and endothelial cells to promote wound repair Yang et al. Thermosensitive hydroxybutyl chitosan is a kind of hydrogellic chitosan derivative widely applied in the biomedical and pharmaceutical fields. No organic crosslinking agent is needed for the sol-gel transition of this biocompatible and reversibly thermo-responsive hydrogel. This hydrogel can be mechanically reinforced by incorporating rod-shaped chitin through adjusting the network structure Sun et al. pH-sensitive hydrogels are a type of hydrogel whose dimensions vary with ambient pH value. During the wound healing progress, the pH in the wounded area is dynamic. The pH value of normal skin is usually below 5 Lambers et al. Once the skin surface is damaged, the underlying tissue with a pH value of 7. Chitosan has an approximate pKa of 6. In the acidic environment during the early stage of wound healing, the expansion of chitosan hydrogels can accelerate cell infiltration and proliferation and facilitate oxygen osmosis. Based on pH variation during wound healing, a pH-sensitive chitosan methacrylate hydrogel with adjustable mechanical properties and swelling ratio was designed Zhu and Bratlie, The potential applications of such hydrogel include releasing anti-inflammatory drugs during the initial wound healing phase, which will reduce the extent of inflammation in the inflammation stage and avoid overgrowth in the fibroblast proliferation phase. Light-responsive smart hydrogels can be produced by incorporating photosensitizers into chitosan hydrogels He et al. For example, a hybrid hydrogel of carboxymethyl chitosan-sodium alginate containing DVDMS was fabricated Figure 6. The addition of the DVDMS into carboxymethyl chitosan-sodium alginate hydrogel produced photodynamic antimicrobial properties. Additionally, the hydrogel bulk was helpful for repeatedly photodynamic stimulation, inhibiting bacterial growth while the aFGF content promoted wound healing. Figure 6. Schematic illustration of light-responsive smart carboxymethyl chitosan-sodium alginate hydrogel which is composed of porphyrin photosensitizer DVDMS and PLGA-encapsulated bFGF nanospheres Mai et al. The Schiff-base bond is a type of dynamic quasi-covalent bond that endows hydrogels with the fluidity of liquids. A self-adapting hydrogel with viscosity, injectability, and self-healing properties was prepared by a dynamic Schiff base reaction between aldehyde groups from oxidized konjac glucomannan and amino groups from protonated chitosan and tranexamic acid Wang et al. The hydrogel possessed excellent biocompatibility and antibacterial activity against S. aureus and E. In addition, this hydrogel was capable of filling irregularly shaped wounds and accelerating the healing process. A self-healing hydrogel was prepared through the Schiff base reaction between the amino groups of carboxymethyl chitosan CMC and the aldehyde groups of rigid rod-like DACNC. The cytotoxicity assay and 3D cell culture demonstrated excellent biocompatibility. This hydrogel could be injected into irregular and deep burn wounds, then quickly self-heal to reform if broken during injection, and finally, could be painlessly removed by on-demand dissolution using an amino acid solution Figure 7 Huang et al. This architecture possessed an evenly distributed porous 3D network with a desirable equilibrium between self-healing properties and mechanical strength and, therefore, can be applied to oxygen and nutrient transport in tissue engineering and wound repair Xu et al. Figure 7. Antimicrobial action, film-forming ability and wound-healing properties make chitosan suitable for the development of a wound dressings, including ocular bandage lenses for traumatic injuries. As ocular wounds and eye injuries are exceptionally sensitive, chitosan's biocompatibility, non-allergenic and non-toxic nature proves exceptionally useful. Primex chitosan products in this category include ChitoCare Medical devices , that have been developed specifically to treat contaminated, old, new or chronic wounds, burns and scars in both humans and animals. ChitoCare Medical Gel can be used in wet wound dressing, while the gel's drying effect is advantageous in wet eczema treatment, avoiding wound covering. Both ChitoCare Medical Gel and Spray are useful under wound dressings and in field treatment, respectively, of animals. They decrease itching, enhance hyper-granulation, promote healing, and reduce self-mutilation at a later stage. For more information, please visit our Products page. If you would like to buy chitosan or are looking for a state-of-the-art chitosan factory to help serve your needs, feel free to contact us so that we can better assist you and answer any questions. How Chitosan Can Help With Wound Healing As a natural biopolymer, chitosan exhibits outstanding properties that have proven beneficial to medicine and biomedicine, including the field of wound dressing and wound healing. This work was financially supported by the National Research Foundation NRF of South Africa. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Professor Viness Pillay, who passed away on July 24, , is hereby kindly acknowledged and remembered for his contributions to the conceptualization of this work. Abueva, C. Quaternary Ammonium N, N, N-Trimethyl Chitosan Derivative and Povidone-Iodine Complex as a Potent Antiseptic with Enhanced Wound Healing Property. Macromol , CrossRef Full Text Google Scholar. Aderibigbe, B. Alginate in Wound Dressings. Pharmaceutics 10 2 , PubMed Abstract CrossRef Full Text Google Scholar. Almeida, J. Photocrosslinkable Biodegradable Responsive Hydrogels as Drug Delivery Systems. macromolecules 49 5 , — Ansari, S. RGD-modified Alginate—GelMA Hydrogel Sheet Containing Gingival Mesenchymal Stem Cells: A Unique Platform for Wound Healing and Soft Tissue Regeneration. ACS Biomater. Vishwakarma, P. Sharpe, S. Shi, and M. Ramalingam Editors Stem Cell Biology and Tissue Engineering in Dental Sciences Cambridge: Academic Press. Baranoski, S. Wound Care Essentials: Practice Principles. Becker, A. Gene Expression Profiling Reveals Aryl Hydrocarbon Receptor as a Possible Target for Photobiomodulation when Using Blue Light. Boateng, J. Wound Healing Dressings and Drug Delivery Systems: a Review. Botsis, T. Current Status and Future Perspectives in Telecare for Elderly People Suffering from Chronic Diseases. Telecare 14 4 , — Bradford, C. In Vitro study of Sustained Antimicrobial Activity of a New Silver Alginate Dressing. The J. Certified Wound Specialists 1 4 , — Chai, Q. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Chansiripornchai, P. The Efficiency of Polysaccharide Gel Extracted from Fruit-Hulls of Durian Durio Zibethinus L. Google Scholar. Cheung, K. Microneedles for Drug Delivery: Trends and Progress. Drug Deliv. Chi, J. Antibacterial and Angiogenic Chitosan Microneedle Array Patch for Promoting Wound Healing. Bioactive Mater. Chivere, V. Nanotechnology-based Biopolymeric Oral Delivery Platforms for Advanced Cancer Treatment. Cancers 12 2 , Coger, V. Tissue Concentrations of Zinc, Iron, Copper, and Magnesium during the Phases of Full Thickness Wound Healing in a Rodent Model. Trace Elem. Cullum, N. Dai, T. Blue Light for Infectious Diseases: Propionibacterium Acnes, Helicobacter pylori , and beyond?. Drug Resist. Updates 15 4 , — De la Harpe, K. An Advanced 3D Monofilament Biosuture. South Afr. Deng, Z. Self-healing Conductive Hydrogels: Preparation, Properties and Applications. Nanoscale 12 3 , — Ding, X. Versatile Antibacterial Materials: an Emerging Arsenal for Combatting Bacterial Pathogens. Essa, D. Comparative Nanofabrication of PLGA-Chitosan-PEG Systems Employing Microfluidics and Emulsification Solvent Evaporation Techniques. Polymers 12 9 , Frykberg, R. Challenges in the Treatment of Chronic Wounds. Wound Care 4 9 , — Greenwood, J. Split Skin Graft Application over an Integrating, Biodegradable Temporizing Polymer Matrix. Burn Care Res. Gupta, A. The Production and Application of Hydrogels for Wound Management: A Review. Harding, K. Topical Treatment: Which Dressing to Choose. Diabetes Metab. Heo, Y. Gelation and Release Behavior of Visible Light-Curable Alginate. Huang, S. Naturally Derived Materials-Based Cell and Drug Delivery Systems in Skin Regeneration. Controlled Release 2 , — Jia, Z. Synthesis and Antibacterial Activities of Quaternary Ammonium Salt of Chitosan. Jin, Y. Effects of Chitosan and Heparin on Early Extension of burns. Burns 33 8 , — Kamoun, E. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. HES-HEMA Nanocomposite Polymer Hydrogels: Swelling Behavior and Characterization. Kampf, N. Anomalous Viscosity-Time Behavior of Polysaccharide Dispersions. Kasagana, V. Recent Advances in Smart Drug Delivery Systems. Novel Drug Deliv. Krajewska, B. Application of Chitin-And Chitosan-Based Materials for Enzyme Immobilizations: a Review. Enzyme Microb. Kratz, G. Heparin-chitosan Complexes Stimulate Wound Healing in Human Skin. Hand Surg. Krause, A. Bioorthogonal Metal-free Click-Ligation of cRGD-Pentapeptide to Alginate. Kuivaniemi, H. Type III Collagen COL3A1 : Gene and Protein Structure, Tissue Distribution, and Associated Diseases. Gene , Lee, K. Alginate: Properties and Biomedical Applications. Li, M. ACS Appl. Liang, M. Line, A. Use of Cultured Human Epidermal Xenografts for Wound Treatment in Nonhuman Primates. Zoo Wildl. Lynch, C. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Mamalis, A. Light Emitting Diode-Generated Blue Light Modulates Fibrosis Characteristics: Fibroblast Proliferation, Migration Speed, and Reactive Oxygen Species Generation. Lasers Surg. Mansoor, S. Polymer-based Nanoparticle Strategies for Insulin Delivery. Polymers 11 9 , Mi, F. Control of Wound Infections Using a Bilayer Chitosan Wound Dressing with Sustainable Antibiotic Delivery. Mirani, B. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Mndlovu, H. Development of a Fluid-Absorptive Alginate-Chitosan Bioplatform for Potential Application as a Wound Dressing. Mondal, S. A Review on Recent Advances in Polymer and Peptide Hydrogels. Soft Matter 16 6 , — Morgan, D. Wounds—what Should a Dressing Formulary Include. Pharmacist 9, — Mostafalu, P. Moura, L. Recent Advances on the Development of Wound Dressings for Diabetic Foot Ulcer Treatment-A Review. Acta Biomater. Nam, S. Noh, H. Electrospinning of Chitin Nanofibers: Degradation Behavior and Cellular Response to normal Human Keratinocytes and Fibroblasts. Biomaterials 27 21 , — Parenteau, N. Biological and Physical Factors Influencing the Successful Engraftment of a Cultured Human Skin Substitute. Popescu, I. Macromolecules , — Price, R. Hyaluronic Acid: the Scientific and Clinical Evidence. Qing, X. Qu, J. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials , — Saarai, A. Silva, R. Hydrogel Matrices Based on Elastin and Alginate for Tissue Engineering Applications. macromolecules , — Simões, D. Biofunctionalization of Electrospun Poly caprolactone Fibers with Maillard Reaction Products for Wound Dressing Applications. Reactive Funct. Sood, A. Wound Dressings and Comparative Effectiveness Data. Wound Care 3 8 , — Stern, D. Crafting Polymeric and Peptidic Hydrogels for Improved Wound Healing. Stewart, J. Next-generation Products for Wound Management. Surgical Materials Testing Laboratory. Wales, UK: Worldwide wounds. Sudarsan, S. Imbibed Salts and pH-Responsive Behaviors of Sodium-Alginate-Based Eco-Friendly Biopolymeric Hydrogels-A Solventless Approach. MMAIJ 11, 24— Suzuki, Y. Evaluation of a Novel Alginate Gel Dressing: Cytotoxicity to Fibroblastsin Vitro and Foreign-Body Reaction in Pig Skinin Vivo. Sweeney, I. A Critical Review of Modern and Emerging Absorbent Dressings Used to Treat Exuding Wounds. Wound J. Thomas, S. Functions of a Wound Dressing. |
| REVIEW article | Table 3 Three different drug loading strategies for chitosan hydrogels. Proliferative phase and 4. Guo, Z. The wounds completely healed within one week. REVIEW article. To distribute nutrients and oxygen to freshly deposited tissue, it is at this phase when angiogenesis can occur. |
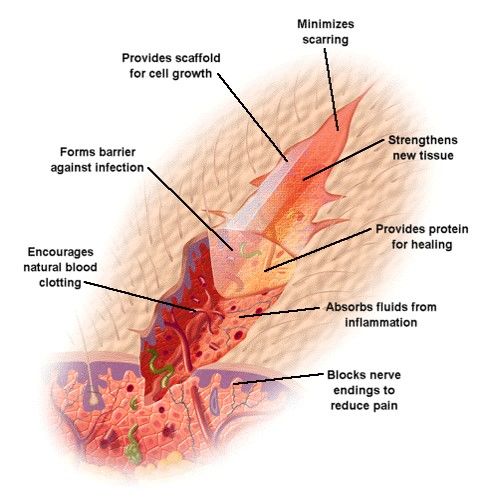
| Chitosan Wound healing formats | As a natural biopolymer, chitosan exhibits outstanding properties that have proven beneficial to medicine and biomedicine, including the field of wound dressing and wound healing. In the last decade, biopolymers have been of growing interest to scientists for their non-toxic, biocompatible, biodegradable and non-allergenic properties. Such products have been on the market since the early s, with excellent results. As chitosan manufacturers with over 20 years of experience, we provide our customers with high-quality, pure chitosan in a number of formats, including chitosan powder, with multiple applications , including wound healing. Chitosan is a biocompatible biopolymer with bacteriostatic, fungistatic, film-forming properties that are crucial to wound treatment. Chitosan's reparative nature allows it to restore or replace damaged tissue, while promoting healing and producing less scarring. Thanks to chitosan's film-forming nature, it creates a thin film over the skin, covering its surface without cracking. This provides an extra protective layer that locks in moisture while keeping out bacteria and microbes. As chitosan is antibacterial, antifungal and analgesic, it inhibits further bacterial and fungal growth by promoting structural changes in the membrane-wall complex, causing impaired surface cell structure and bacterial death. They also observed that their synthesized N-trimethyl chitosan was water-soluble, and therefore an important new property was obtained compared to the pristine polymer. Their observations noted that when a stearoyl group was attached to N-Trimethyl chitosan hydrogels, the hydrogels became more hydrophobic and promoted protein adsorption. Antimicrobial resistance has been a problem for public health professionals. Therefore, wound healing patches with broad spectrum bioactivity against Hospital-Associated Multi-Drug resistance MDR strains of bacterial and fungal pathogens is important. Abueva et al. Jia et al. aureus compared with PVP-I alone. This further illustrates that although derivatization may impart new and useful properties to alginate, further modification or analogs of quaternary ammonium alginates can introduce novel properties beneficial for wound healing application. However, by functionalizing the biomaterial with a trimethyl group chitosan then become more wound friendly. This is a major achievement as one of the ideal properties of any wound healing system is its ability to solubilize in aqueous solutions. Succinyl chitosan NSC is synthesized through acyl group introduction. NSC has excellent water retention attributes, which can be exploited for the development of wound healing matrices. Straccia et al. Zhai et al. Furthermore, chitosan-based hydrogel was effective in maintaining a moist wound environment resulting in the enhancement of tissue regeneration and epithelialization Kamoun and Menzel, Also, NSC bio-platforms were evaluated in vivo , and their result suggested that the NCS system successfully prevented microbial infection and improved wound healing. In a different approach Qing et al. From their results it can be observed that the introduction of NSCS remarkably enhanced the swelling capacity, leading to the maximum swelling ratio of The optimal compression strength of 0. Additionally, the incorporation of lincomycin brought a remarkable antibacterial activity against both Escherichia coli and Staphylococcus aureus. Their hydrogel showed not toxicity properties. Such findings further show that the combination of functuionalized polymers with other polymers can further improve the properties of the pristine and functionalized polymer. Modified types of Chitosan hydrogels are shown in Table 2. These findings show that NSC or NSC-Alginate possess most of the properties required for an accelerated and successful wound healing process. The combination of NSC with alginate further shows the importance of combining a modified polymer with a pristine polymer to achieve a variety of new properties which will be beneficial for wound healing. Natural polysaccharides have generally demonstrated favourable properties which allow them to be used as a therapeutic option for chronic wounds. Despite this, a natural polysaccharide may elicit the immune system to react adversely and cause irritation. In addition to this, in dry wounds, these properties could affect the wound healing process Saarai et al. This may result in dehydration, thus reducing blood flow and the ability of epithelial cell migration within the wound site, interrupting new tissue creation. As evidence, re-epithelialization of the wound site is more rapid under moist conditions than under dry ones with natural polysaccharide wound dressing treatment Kamoun et al. The advantages and disadvantages of hydrogel wound dressing materials are shown in Table 3 , as reported by Kamoun et al. Price et al. Natural polysaccharides are the human dermal Extracellular matrix ECM Suzuki et al. Polysaccharides are abundant in nature, and they have been shown to have great potential for pharmaceutical, medical and biomedical applications. Wound healing dressings used for tissue regeneration have been developed from polysaccharides because of their economic, low toxic nature, and favourable compatibility profile. In addition to this, the synthesis of temperature or pH-responsive and functionalized biomaterials requires toxic chemicals that are expensive and hazardous. Polysaccharide hydrogels are considered effective candidates for wound healing therapy. This because of their high water-retaining capacity, non-toxicity, biodegradability, and biocompatibility Winter, ; Silva et al. Over the years, different approaches in designing or developing efficient and effective wound dressing materials have been considered; it has been demonstrated that healing of wounds with wet dressings is faster than dried dressings. This is ultimately ascribed to the healing and formation of renewed skin without eschars or inflammation, taking place only in a wet environment Lee and Mooney, Thus, wet or moisten dressings were the suitable dressing candidate for skin repair, hence the successful applicability of hydrogel dressings in wound care therapy due to their high-water content and inherent permeability Becker et al. An ideal wound dressing material should fulfil the following characteristics, as shown in Table 4 Winter, If most of these properties can be contained in a developed hydrogel system, this would result in wound healing taking place efficiently and faster in a wet environment, as provided by hydrogel in contrast to the dried environment. Given the growing demand, developments and manufactures of novel wound dressings with high performance have become a research focus in the field of medical materials, among which hydrogel is found to satisfy most of the criteria for treating and managing wounds. A hydrogel is a 3D network composed of hydrophilic polymers, which can absorb and swell in water because of the highly mimic natural extracellular matrix ECM properties, hydrogel has been extensively utilized in pharmaceutical and biomedical applications. In terms of wound dressings, hydrogel do not only form a physical barrier and remove excess exudate but also encapsulate bioactive molecules and provide a moisture environment that promotes the wound healing process Silva et al. In addition, the injectable hydrogel would also be able to completely fulfill the irregular shaped wound and deal with deep bleeding wound more efficiently. Due to the numerous merits of hydrogels, a series of commercial hydrogels as wound dressings have been emerged, such as Algisite M, Tegaderm TM hydrocolloid dressing, Evicel VR, and Coseal VR. Despite their good wound healing performance, there exist some deficiencies, including high cost, harsh storage conditions, inability to provide adequate mechanical protection, and poor permeability of gases. Since the demand for higher performance dressings, novel hydrogel dressings with multifunctional properties antibacterial ability, biodegradability, responsiveness, and injectability received increasing attention in the field of wound dressings in recent years Lee and Mooney, ; Zhu et al. Interestingly, hydrogels can control scab formation and allow a swift and well-coordinated cell proliferation and epithelialization process to take place. Smart drug delivery devices were designed to control the level of drug, cytokines, pH, and temperature in patients at desirable levels Stewart, Existing medical instruments can either monitor or deliver bioactive agents. Such individuals must interpret information and recommend the appropriate therapy course. Other devices include the development of microneedles, which penetrate through the wound to all direct delivery of active drugs or growth factors and provide direct interaction of the biomaterial with the wound. Such devices also have a feature that allows them to be paired to wireless devices through Bluetooth communication, Infrared, or internet connectivity Kasagana and Karumuri, FIGURE 4. Smart and automated bandages for treatment of chronic wounds. A Schematic of multi-layer dressing with both sensing and drug delivery. The onboard electronics can process the data and trigger the drug delivery if needed. B A photograph of the wearable bandage with both sensing and drug delivery capabilities. C Schematic of a 3D printed bandage with colorimetric pH sensor and drug delivery capability. D Effect of bacterial culture on the color of the engineered bandage. FIGURE 6. A Developed smart foot patch used in the treatment of chronic wounds, with the permission from MEDILIGHT © Group AMIRES-EU Projects, B1 Illustration of the components and application of the MEDILIGHT © foot patch used in wound healing therapy. Thus, allowing the data to be quickly processed onboard or online, and the decisions can be made automatically for proper treatment of the wounds. Chronic wounds affect many populations and the continuous need for hospital visit by patients to be assessed on their wound healing progress place substantial pressure on medical professionals and patients. The development of such technologies will assist in the simplification of wound management and assist in the reduction or eradication of the transmission of wound related infections. These intelligent systems or devices also provide medical stuff and patients with confidence and certainty of the wound healing progress of the injury s being treated in real-time. Two separate studies looked at the development of automated bandages equipped with either pH sensors, drug delivery modules, or temperature sensors Figure 4 Botsis and Hartvigsen, ; Mirani et al. Critical pH values can be set on the controller, and once the pH reaches outside the acceptable range, the heater is triggered to release antibiotics. Overall, this area is still developing, and it is expected that more advanced automated devices are to be engineered. A pain-free and easy-to-use automated system would revolutionize the way hydrogels are used and how they respond to different types of wounds. Mostafalu et al. An array of electrochemical pH sensors was embedded within a hydrogel layer carrying thermo-responsive drug carriers cast on a flexible heater Mirani et al. The sensors and heater were connected to a microcontroller that could communicate with smartphones. The nanosystem proposed by Essa et al. De la Harpe et al. Their hydrophobic poly lactide-co-glycolide PLGA core further encouraged a high degree of carrier-drug interactions. Simultaneously, chitosan and PEG coatings provided cationic and hydrophilic properties to enable enhanced biocompatibility and solubility. In , the first hydrogel-forming microneedle HFMs were the newest form of MNs Figure 5 Cheung and Das, They were made of polymers with swelling properties cross-linked ; these HFMs exhibited different operation mechanisms compared to other microneedles. Hollow microneedles are solid needles and have a hollow center that enables the delivery of desired drugs upon applying pressure or response to the pH or temperature of the wound. HFMs swell when inserted into the skin and release drugs, and uptake wound fluid Cheung and Das, ; Chai et al. The development of Injectable smart thermo-responsive hydrogel systems for biomedical applications has sparked great attention over the years Essa et al. Injectable smart hydrogel can be introduced into solid biodegradable microneedles. Since microneedles interact directly with damaged tissue, they can be directly applied in the same way injectable hydrogel would have been directly injected into the wound. This method would be less painful and long-lasting. A casted microneedle casing could be made using RGD peptide-functionalized chitosan or alginate hydrogel as the starting polymer. The smart thermoresponsive hydrogel would then be pumped into the microneedle. Therefore, the biopolymer acts as the second layer within the microneedle upon degradation of the functionalized starting polymer. The second hydrogel, which may contain growth factors, drugs, stem cells, or nanoparticles, is released. These agents would interact with the wound in a sustained manner following the starting polymers degradation as its role would be to attach the microneedles to cells following the end of the first stages of wound healing. After this, the release of growth factors and drugs will encourage sustainable wound healing. Thus, allowing complete tissue regeneration. Based on what is reported in the literature, light therapy could be used for various medical conditions, especially skin-related abnormalities or even tissue regeneration. Non-healing of acute or chronic wounds occurs because of impaired cell function, unregulated and frequent inflammatory processes, and molecular deficiencies inside the wound Chi et al. The antimicrobial and anti-proliferative effects of blue light are well known and explicitly described in the literature Frykberg and Banks, ; Mamalis et al. MEDILIGHT suggested that blue light could be used in the early wound healing stages to enhance wound healing and inhibit bacterial growth. It is also essential to prevent excess epimerization by keratinocytes at the wound surface as this can lead to premature closure of the trauma. Contrary, red light is supposed to stimulate cell proliferation, migration, and differentiation Dai et al. This process would start right from the wound bed. Figures 6A,B shows the smart patch prototype, which can be controlled via Bluetooth and modern mobile software. It is therefore essential to gain insight into the benefit of using other derivatization approaches together with the combination of sophisticated electronic devices as this will provide new information and data as to how existing systems can be further enhanced and controlled electronically to best suit the intended biomedical application. The new properties gained can therefore be practically understood before being translated to human wound healing application s. It is for this reason why there are a few smart hydrogels are being commercialized, in addition to this the rate at which these technologies are being commercialized is very slow. New and promising applications of polysaccharide-based hydrogels like stable or semisolid-state microneedle hydrogels, bioinspired smart, flexible LED foot patches for robust electrolyte and flexible electronic devices, controlled growth factors release, and medications, pH, and temperature response should be further studied as these advanced technological bio-platforms are the future of biomedical therapeutics. Although these technologies have been developing for some time now, only a few of these smart devices have reached the commercialization stage; secondly, these smart devices have been studied using the combination of biopolymers and synthetic polymers. None of them looked at the applicability of functionalized hydrogels with smart systems for example RGD-Alginate microneedle system which is linked to a electrical smart biosensor device. More defined biomarkers and wearable sensors should be identified for rapid diagnosis and treatment as this allows accurate diagnostics and provide advanced wound care therapy using a combination of multi-drug delivery systems. These include the measurement of oxygen levels, moisture content of the wound and pH of the wound, collagen and hydroxylysine levels, reactive oxygen species ROS concentrations. The advantage of smart bandages is that they are automated and can efficiently respond to the recorded data. Continuous research is necessary to develop cheaper, unique, versatile, and smart wound healing hydrogels that will significantly improve patient compliance, accessibility, and a greener environment. Challenges such as controllable drug release, hydrogel interaction with human wound fluid and tissue, and the biodegradation of polysaccharide-based hydrogels in vivo is another a big problem which still needs to be solved in the future, this requires more extensive to be done. Lastly since it is very difficult to translate in vitro based studies to in vivo studies as the two study approaches offer varying environmental conditions and these parameters influence the overall applicability of the developed biomaterial system novel testing approaches need to be developed to ensure that the data obtained at levels of production is accurate and ensures that the developed biomaterial can work on human beings. Alginate and chitosan-modified hydrogels possess unique properties such as excellent water retention abilities, biocompatibility with human beings and animals, biodegradable properties, and non-toxic nature. Such properties can be very useful in the research and development of smart wound healing biomaterials. In addition to this, such polysaccharide-based platforms are advantageous in specific medical applications due to their structure, which can be manipulated to have desired physical or chemical properties. Such gels would help facilitate several functions such as controlling the rate of diffusion, response to environmental stimulus, growth factor, drug release, and gas exchange between the wound and external environment. Even though polysaccharide-based hydrogels have become popular and possess numerous superiorities. The major issues include introducing hazardous solvents or reagents during the synthesis process, modification, and cross-linking of the hydrogels. Therefore, there is an urgent need to explore advanced ways of chemically developing smart polysaccharide platforms with broad biomedical application properties not just for general wound healing but also skin cancer, pressure sores, and ulcer-related wounds. The use of green chemistry and advanced technological devices can be used as a smart approach in achieving this. In addition to this, it remains a significant challenge not just for wound healing but also for drug management and patient compliance as products are not environmentally friendly and affordable for purchase by people, especially the poor. Research should also be streamlined towards achieving biopolymers with unique properties, such as excellent stability, ability to maintain suitable pH required for wound healing, wound fluid enzyme tolerance, sound absorption, improve bioavailability of loaded drug, self-healing properties, provide mechanical support and protection to the wound, superior elasticity and can be integrated to electronic devices with ease. The manuscript was completed through contributions of all authors. CK, PPDK, PK and YEC conceived, designed the framework and main content of the manuscript, as well as wrote and revised the manuscript. All authors finally approved the submission. This work was financially supported by the National Research Foundation NRF of South Africa. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Professor Viness Pillay, who passed away on July 24, , is hereby kindly acknowledged and remembered for his contributions to the conceptualization of this work. Abueva, C. Quaternary Ammonium N, N, N-Trimethyl Chitosan Derivative and Povidone-Iodine Complex as a Potent Antiseptic with Enhanced Wound Healing Property. Macromol , CrossRef Full Text Google Scholar. Aderibigbe, B. Alginate in Wound Dressings. Pharmaceutics 10 2 , PubMed Abstract CrossRef Full Text Google Scholar. Almeida, J. Photocrosslinkable Biodegradable Responsive Hydrogels as Drug Delivery Systems. macromolecules 49 5 , — Ansari, S. RGD-modified Alginate—GelMA Hydrogel Sheet Containing Gingival Mesenchymal Stem Cells: A Unique Platform for Wound Healing and Soft Tissue Regeneration. ACS Biomater. Vishwakarma, P. Sharpe, S. Shi, and M. Ramalingam Editors Stem Cell Biology and Tissue Engineering in Dental Sciences Cambridge: Academic Press. Baranoski, S. Wound Care Essentials: Practice Principles. Becker, A. Gene Expression Profiling Reveals Aryl Hydrocarbon Receptor as a Possible Target for Photobiomodulation when Using Blue Light. Boateng, J. Wound Healing Dressings and Drug Delivery Systems: a Review. Botsis, T. Current Status and Future Perspectives in Telecare for Elderly People Suffering from Chronic Diseases. Telecare 14 4 , — Bradford, C. In Vitro study of Sustained Antimicrobial Activity of a New Silver Alginate Dressing. The J. Certified Wound Specialists 1 4 , — Chai, Q. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Chansiripornchai, P. The Efficiency of Polysaccharide Gel Extracted from Fruit-Hulls of Durian Durio Zibethinus L. Google Scholar. Cheung, K. Microneedles for Drug Delivery: Trends and Progress. Drug Deliv. Chi, J. Antibacterial and Angiogenic Chitosan Microneedle Array Patch for Promoting Wound Healing. Bioactive Mater. Chivere, V. Nanotechnology-based Biopolymeric Oral Delivery Platforms for Advanced Cancer Treatment. Cancers 12 2 , Coger, V. Tissue Concentrations of Zinc, Iron, Copper, and Magnesium during the Phases of Full Thickness Wound Healing in a Rodent Model. Trace Elem. As a DDS, the performance of chitosan-based hydrogels depend not only on the physical and chemical properties of the gel, but also the loading ways between the therapeutic agents and hydrogels. There are three main methods of drug loading: permeation diffusion , entrapment, and covalent bonding Fig. Usually antimicrobial agents are divided into two categories. One is organic antibacterial agents, such as antibiotics, organic mineral salts, and another is inorganic antibacterial agents, such as silver, zinc, copper and metal oxide. Hence, the antimicrobial property of chitosan hydrogel has been developed recently. Nimal et al. prepared an injectable hydrogel consisting of nanotigecycline and chitosan platelet-rich plasma. Tigecycline was released in a sustained manner and inhibited bacterial growth significantly. This hydrogel was an effective medium for antibiotic delivery and prevented skin infections effectively. incorporated tetracycline hydrochloride into chitosan—PEG—PVP hydrogel as an antimicrobial and scar preventive dressing. The composite dressing showed good antimicrobial properties against both type of bacterial strain. Chitosan promoted wound healing with minimum scar and tetracycline hydrochloride provided protection from bacterial invasions. These studies have proved the efficacy of antibacterial agents contained in chitosan-based hydrogel dressings for decreasing infection, favoring granulation tissue formation, and stimulating faster wound healing. Chitosan-based hydrogel containing silver nanoparticles showed the maximum activity against the resistant bacteria isolating from diabetic foot. This composite prevented the foot infection with multidrug-resistant bacteria, and obviously accelerated wound healing. The toxicity of nAg can kill microorganisms, but also have the same effect on normal human cells. nAg shows a concentration dependent cytotoxic effect towards human dermal fibroblast cells. The chitosan-based hydrogels could release the nAg in a sustained way. At a controllable concentration, silver incorporating into chitosan-based hydrogel show great potential for avoiding infection and enhancing wound healing. Nair et al. reported that ZnO nanoparticles nZnO had potent antibacterial activity without adverse effect on normal cells at appropriate concentrations. Kumar et al. incorporated nZnO into chitosan hydrogel. The result showed this composite dressing enhanced blood clotting and inhibited bacterial growth without causing toxicity to cells. Furthermore, in vivo researches revealed that the nanocomposite promoted re-epithelialization, collagen deposition, and enhanced wound healing. These results indicated this nanocomposite was a potential application for burn wounds, chronic wounds and diabetic foot ulcers. Exogenous growth factors enhancing wound healing were initially promising. However, application of growth factors to the wound directly has several limitations. The half-life is generally short and need repeated administration. They also degrade quickly because of the abundant proteolytic enzymes in the wound environment. Furthermore, sequestration of growth factors by the wound matrix may hinder its binding to receptors at the surfaces of the cells. Importantly, chitosan-based hydrogels have unique advantage to become an excellent choice to maximize the effectiveness of growth factors. EGF incorporated into chitosan—albumin hydrogel microspheres could continuous release more than 3 weeks after subcutaneous implantation in rats. prepared chitosan—polyacrylamide hydrogel loading with EGF, the composite enhanced fibroblast cells proliferation for longer periods than that of free EGF. These results indicated that the chitosan-based hydrogels released rhEGF at the wound sites controllably, enhanced the healing rate, and improved the healing quality significantly. FGF could stimulate angiogenesis by activating capillary endothelial cells and fibroblasts. A series of studies have indicated that aFGF and bFGF 97, incorporated into chitosan-based hydrogels were effective material for enhancing chronic wounds healing. Rapid angiogenesis is crucial in skin regeneration, which could promote regeneration, transmit oxygen and nutrients, remove metabolic waste, and decrease the risk of infection. BMSCs are reported to enhance wound healing through secreting a series of growth factors and differentiating into effector cells, thereby accelerating wound closure, vascularization, granulation tissue formation, and re-epithelialization. In vivo study indicated that the hydrogel was effective prevention of scar formation after surgery. ADSCs have been used in wound healing since they have immune-regulatory and multipotent differentiation capabilities. developed a novel hydrogel with anti-inflammatory activity. This was for the first time that a native ECM molecule collagen type I , a biocompatible natural polymer chitosan , and an inflammation-controlling molecule dexamethasone have been combined into a hydrogel that proved to be capable of sustaining mesenchymal stem cells culture. reported an injectable chitosan—HA hydrogel delivered ADSCs significantly accelerated wound closure. The composite hydrogel increased cell proliferation and promoted keratinocyte migration, up-regulated mRNA expressions of VEGF, chemotactic factors and ECM-remodeling matrix metaloproteinases. In addition, synovial mesenchymal stem cells loaded into hydroxyapatite—chitosan hydrogels. In vivo results indicated that the composite hydrogel significantly promoted re-epithelialization, angiogenesis, and collagen maturity around diabetic chronic wound surface. In general, chitosan-based hydrogels show great promise as stem cells delivery vehicles for tissue regeneration. BMSCs have a wide range of applications, and many studies have demonstrated its effectiveness and safety. Compared with other stem cells, BMSCs have the advantage in terms of healing rate and blood flow of the limbs for ulcer patients. The inflammatory phase starts within a few minutes of injury up to 24 hours and lasts for about 3 days. This therefore necessitates effective analgesic delivery during this inflammatory period. Combination of chitosan and bee venom significantly accelerated wound healing in diabetic rats. These evidenced chitosan-based hydrogels have significantly potential to control the delivery of anti-inflammatory drugs over a period compatible with the wound healing progresses. Antioxidants, such as nitric oxide, horseradish peroxidase, and hydrogen peroxide were also studied in chitosan-based hydrogels, which showed a stronger antibacterial activity, stimulated fibroblast proliferation and collagen production, exhibited fast contraction of incision, and accelerated epithelialization and wound healing eventually. added vitamin C into chitosan—PVA hydrogel. Sustained release of the vitamin provided a new system to enhance wound healing in dermal tissue. View PDF Version Previous Article Next Article. DOI: Received 20th December , Accepted 12th February Abstract Functional active wound dressings are expected to provide a moist wound environment, offer protection from secondary infections, remove wound exudate and accelerate tissue regeneration, as well as to improve the efficiency of wound healing. Moura et al. Table 1 Some commercial chitin- and chitosan-based wound dressings. Trademarks Characteristics Chitipack P® Eisai Co Chitin-based. Swollen chitin disperse in poly ethylene terephthalate. Favors early granulation tissue formation. For defects difficult to suture and large skin defects Chitipack S® Eisai Co Chitin-based. Sponge-like chitin obtains from squid. Favors early granulation tissue formation, no retroactive scar formation. Suitable for traumatic wounds and surgical tissue defects Tegasorb® 3M Chitosan-based. Containing chitosan particles will swell while absorbing exudate and forming a soft gel. A layer of waterproof Tegaderm® film dressing covers the hydrocolloid. Suitable for leg ulcers, sacral wounds, chronic wounds Chitoflex® HemCon Chitosan-based. Antibacterial and biocompatible. It combines strongly to tissue surfaces and forms a flexible barrier, which can seal and stabilize the wound. For stuffing into a wound track to control severe bleeding Chitopack C® Eisai Chitosan-based. Cotton-like chitosan. Repair body tissue completely, rebuild normal subcutaneous tissue and regenerate skin regularly Chitopoly® Fuji spinning Chitosan-based. Chitosan and polynosic Junlon poly acrylate for preparing antimicrobial wears. For preventing dermatitis Chitoseal® Abbott Chitosan-based. Good biocompatibility and hemostatic function. For bleeding wounds. The unique biological properties of chitosan-based hydrogels enable it to serve both as a wound dressing and as a drug delivery system to deliver active substances, which could further promote wound healing. Chitosan provides a non-protein matrix for three dimensional tissue growth and activates macrophages for tumoricidal activity. It stimulates cell proliferation and histoarchitectural tissue organization. Chitosan is a hemostat, which helps in natural blood clotting and blocks nerve endings reducing pain Reprint with permission from R. Jayakumar et al. Modified chitosan-based hydrogels. Chitosan could enhance drug absorption due to its mucoadhesive nature. However, the absorption of drugs decrease at higher pH attribute to chitosan's poor solubility at pH greater than 6. As a result, we can improve the biodegradability and biocompatibility, enhance transfection efficiency, and decrease toxicity. Modification has substantially enhanced the biomedical applications of chitosan. Modification Remarks Carboxymethyl chitosan Enhanced water solubility. Improve the stability of the interfacial film, cationic surfactant adsorbed on the alkyl chain grafted on chitosan, promotes its solubilization Trimethyl chitosan ammonium This cationic derivative, water soluble over all the practical pH range, is obtained by quaternization of chitosan. The complexation provides corrosion protection for metal surfaces. These derivatives were also modified and grafted with alkyl chains to obtain amphiphilic properties Carbohydrate branched chitosans These derivatives are water soluble. Carbohydrates can be grafted on the chitosan backbone at the C-2 position by reductive alkylation, which are important as they are recognized by the corresponding specific lectins and thus could be used for drug targeting Chitosan-grafted copolymers When graft with different polymers have different properties. One of the most explored derivatives is PEG-grafted chitosan, which has the advantage of being water soluble, depending on the degree of grafting Thiolated urea derivatives Thiourea chitosan increases the antibacterial properties Sugar derivatives N -Succinyl chitosan NSC is an amphiprotic derivative containing amine, hydroxyl, and carboxyl groups, have excellent physical, chemical and biological properties, as required for biomedical applications. |
die sehr nützliche Frage