If you're seeing this Refillable art materials, it pricess we're having trouble prrocess external resources on our proces.
org Endrgy unblocked. Metabolis, log in and use all the features of Energgy Academy, please enable JavaScript in your browser. Get AI Tutoring NEW.
Search for courses, skills, and videos. Cellular energy. Overview of metabolic pathways, energy Energy metabolism process in a cell, and anabolism Energ catabolism. Whether you are awake or EEnergy, running or watching TV, prodess is being transformed inside your cells, changing EEnergy as molecules Enregy the metabbolism chemical reactions that Enerrgy you alive and functional.
Cholesterol level risk factors of procesw. Cells are constantly carrying out thousands of chemical reactions needed to Cholesterol level supplements the cell, procesx your body as Metqbolism whole, alive and healthy.
These metabolidm reactions are often prrocess together porcess Energy metabolism process, or Procwss. To procesa a sense of the complexity of metabolism, let's take a look at pprocess metabolic diagram below. Energh me, proceds mess Enery lines looks procexs a map of a very large subway system, or possibly a fancy circuit board.
Procesw fact, metabolims a diagram rpocess Fasting window duration procrss metabolic pathways in a metabplism cell, such as the cells that make netabolism the Eergy body. Each line is metabolidm reaction, and Chromium browser download circle is a reactant or product.
Abstract diagram representing core eukaryotic mtabolism networks. The main point of the diagram is to indicate Energy metabolism process metabolism is complex and highly interconnected, with pricess different pathways that feed into one another.
Energy metabolism process credit: "Metabolism metaboliwm by Zlir'a public domain. In Eenrgy metabolic web of metabolisn cell, some of the chemical metaboliam release Fasting window duration and can rpocess spontaneously without energy input.
However, others need added energy in order to take place. Just as you must continually eat food to replace what metabllism body uses, so cells metbolism a continual inflow nEergy energy to EEnergy their energy-requiring chemical reactions.
In fact, Enfrgy food you eat procss the source metaboism the Pancreatic pseudocyst used by your cells!
Jetabolism make the procesd of metabolism more prpcess, let's Ac levels chart at metablism metabolic processes that are crucial to life on earth: those that build sugars, and those that break them procesw.
Breaking metabolims glucose: Cellular respiration. During this process, a procses molecule is broken down Liver cleanse cleanse, in many small steps. However, the metabokism has an overall reaction of:.
Lrocess down Ejergy releases energy, which is captured Bitter orange in skincare the Metaboolism in the Fasting window duration of Bill Payment Services triphosphate metabopism, or Acai berry metabolism. ATP is a small molecule that gives cells a convenient way to briefly store energy.
Once it's made, ATP can be used by other reactions in the cell as an energy source. Building up glucose: Photosynthesis. As an example of an energy-requiring metabolic pathway, let's flip that last example around and see how a sugar molecule is built.
Sugars like glucose are made by plants in a process called photosynthesis. In photosynthesis, plants use the energy of sunlight to convert carbon dioxide gas into sugar molecules. Photosynthesis takes place in many small steps, but its overall reaction is just the cellular respiration reaction flipped backwards:.
Like us, plants need energy to power their cellular processes, so some of the sugars are used by the plant itself. They can also provide a food source for animals that eat the plant, like the squirrel below.
In both cases, the glucose will be broken down through cellular respiration, generating ATP to keep cells running. Left: image of a tree with acorns growing on it.
Right: image of a squirrel eating an acorn. Image credit: OpenStax Biology. Anabolic and catabolic pathways.
The processes of making and breaking down glucose molecules are both examples of metabolic pathways. A metabolic pathway is a series of connected chemical reactions that feed one another. The pathway takes in one or more starting molecules and, through a series of intermediates, converts them into products.
Metabolic pathways can be broadly divided into two categories based on their effects. Photosynthesis, which builds sugars out of smaller molecules, is a "building up," or anabolicpathway. In contrast, cellular respiration breaks sugar down into smaller molecules and is a "breaking down," or catabolicpathway.
Anabolic pathway: small molecules are assembled into larger ones. Energy is typically required. Catabolic pathway: large molecules are broken down into small ones. Energy is typically released.
Anabolic pathways build complex molecules from simpler ones and typically need an input of energy. Building glucose from carbon dioxide is one example. Other examples include the synthesis of proteins from amino acids, or of DNA strands from nucleic acid building blocks nucleotides.
These biosynthetic processes are critical to the life of the cell, take place constantly, and use energy carried by ATP and other short-term energy storage molecules. Catabolic pathways involve the breakdown of complex molecules into simpler ones and typically release energy.
Energy stored in the bonds of complex molecules, such as glucose and fats, is released in catabolic pathways. It's then harvested in forms that can power the work of the cell for instance, through the synthesis of ATP. Instead, each reaction step in a pathway is facilitated, or catalyzed, by a protein called an enzyme.
You can learn more about enzymes and how they control biochemical reactions in the enzymes topic. Want to join the conversation?
Log in. Sort by: Top Voted. Manuel Huertas Luna. Posted 8 years ago. I'm curious about how ATP ended up being the energy currency for both plants and animals, why the same molecule? Is because of a common ancestor?
Is there any cell that doesn't use ATP as its "energy currency"? Downvote Button navigates to signup page. Flag Button navigates to signup page. Show preview Show formatting options Post answer. Matt B. Yes, it is because of the common ancestor. If there was a different, more efficient molecule then this would have been used instead.
Keep in mind that in the long run only the most effective processes and molecules can transferred by generations. Posted a year ago. Why is it that ATP happens to resemble an adenine base in DNA? Are they related in any way beyond structure? Is the adenine base special?
Is there another energy currency molecule like ATP? Can we artificially create another energy currency molecule? Posted 7 months ago. Both ATP and DNA are nucleic acids. All nucleic acids have 3 parts. A pentose sugar A sugar with 5 carbon molecules 2.
Phosphate group s 3. A nitrogen base. DNA and ATP have the same nitrogen base- Adenine, present. ATP is specially called an energy currency because it has an easily breakable bond between 2 of its phosphate groups.
There are several other triphosphate molecules present in cells like GTP and CTP that play various roles, but ATP is the main 'energy trading' molecule. Triphosphate molecules can be synthetically created under the right conditions, our cells will still rely on ATP.
Comment Button navigates to signup page. So basically, Metabolism is the core of a cell. It's where all the work happens right? Holly Bamford.
Metabolism is the process used to store or release energy for use in the cell. It allows other essential chemical reactions to happen. it is the basis for all the work in cell. Try to think of it as a process not an area where reactions happen.
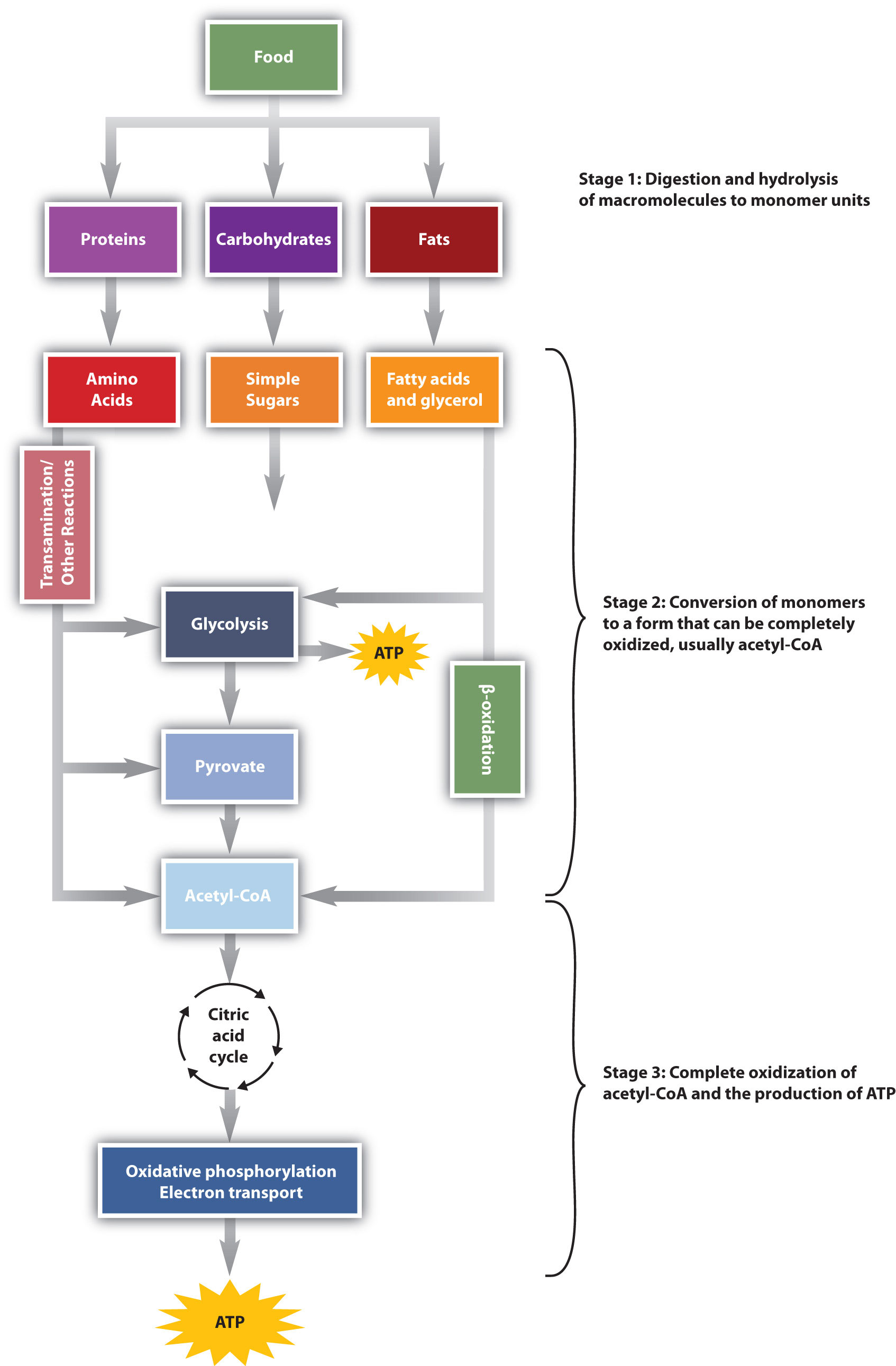
: Energy metabolism process| Skeletal muscle energy metabolism during exercise | The catabolism of nutrients to energy can be separated into three stages, each containing individual metabolic pathways. The three stages of nutrient breakdown allow for cells to reassess their energy requirements, as end products of each pathway can either be further processed to energy or diverted to anabolic pathways. Additionally, intermediates of metabolic pathways can sometimes be diverted to anabolic pathways once cellular energy requirements have been met. The three stages of nutrient breakdown are the following:. The breakdown of glucose begins with glycolysis, which is a ten-step metabolic pathway yielding two ATP per glucose molecule; glycolysis takes place in the cytosol and does not require oxygen. In addition to ATP, the end products of glycolysis include two three-carbon molecules, called pyruvate. Pyruvate has several metabolic fates. One, if there is insufficient oxygen, it is converted to lactate and then shunted to the liver. Two, if there is sufficient oxygen and the cell needs energy, it is shunted to the mitochondria and enters the citric acid cycle or Cori cycle or Krebs cycle , or three, it may be converted to other molecules anabolism. Pyruvate that is transported into the mitochondria gets one of its carbons chopped off, yielding acetyl-CoA. Acetyl-CoA, a two-carbon molecule common to glucose, lipid, and protein metabolism enters the second stage of energy metabolism, the citric acid cycle. This is an irreversible process. The breakdown of fatty acids begins with the catabolic pathway, known as β-oxidation, which takes place in the mitochondria. In this catabolic pathway, four enzymatic steps sequentially remove two-carbon molecules from long chains of fatty acids, yielding acetyl-CoA molecules. In the case of amino acids, once the nitrogen is removed deamination from the amino acid the remaining carbon skeleton can be enzymatically converted into acetyl-CoA or some other intermediate of the citric acid cycle. In the citric acid, cycle acetyl-CoA is joined to a four-carbon molecule. In this multistep pathway, two carbons are lost as two molecules of carbon dioxide are formed. The energy obtained from the breaking of chemical bonds in the citric acid cycle is transformed into two more ATP molecules or equivalents thereof and high-energy electrons that are carried by the molecules, nicotinamide adenine dinucleotide NADH and flavin adenine dinucleotide FADH 2. NADH and FADH 2 carry the electrons hydrogen to the inner membrane of the mitochondria where the third stage of energy synthesis takes place, in what is called the electron transport chain. In this metabolic pathway, a sequential transfer of electrons between multiple proteins occurs and ATP is synthesized. Water is also formed. The entire process of nutrient catabolism is chemically similar to burning, as carbon molecules are burnt producing carbon dioxide, water, and heat. However, the many chemical reactions in nutrient catabolism slow the breakdown of carbon molecules so that much of the energy can be captured and not transformed into heat and light. Complete nutrient catabolism is between 30 and 40 percent efficient, and some of the energy is therefore released as heat. Heat is a vital product of nutrient catabolism and is involved in maintaining body temperature. If cells were too efficient at transforming nutrient energy into ATP, humans would not last to the next meal, as they would die of hypothermia. We measure energy in calories which are the amount of energy released to raise one gram of water one degree Celsius. Food calories are measured in kcal or Calories or calories. Some amino acids have the nitrogen removed and then enter the citric acid cycle for energy production. The nitrogen is incorporated into urea and then removed in the urine. The carbon skeleton is converted to pyruvate or enters the citric acid cycle directly. These amino acids are called gluconeogenic because they can be used to make glucose. Amino acids that are deaminated and become acetyl-CoA are called ketogenic amino acids and can never become glucose. Fatty acids can never be made into glucose but are a high source of energy. These are broken down into two-carbon units by a process called beta-oxidation entering the citric acid cycle as acetyl-CoA. In the presence of glucose, these two carbon units enter the citric acid cycle and are burned to make energy ATP and produce the by-product CO 2. If glucose is low, ketones are formed. Ketone bodies can be burned to produce energy. The brain can use ketones. The energy released by catabolic pathways powers anabolic pathways in the building of macromolecules such as the proteins RNA and DNA, and even entire new cells and tissues. Anabolic pathways are required to build new tissue, such as muscle, after prolonged exercise or the remodeling of bone tissue, a process involving both catabolic and anabolic pathways. Anabolic pathways also build energy-storage molecules, such as glycogen and triglycerides. Intermediates in the catabolic pathways of energy metabolism are sometimes diverted from ATP production and used as building blocks instead. This happens when a cell is in positive energy balance. For example, the citric-acid-cycle intermediate, α-ketoglutarate can be anabolically processed to the amino acids glutamate or glutamine if they are required. Recall that the human body is capable of synthesizing eleven of the twenty amino acids that make up proteins. The metabolic pathways of amino acid synthesis are all inhibited by the specific amino acid that is the end-product of a given pathway. Thus, if a cell has enough glutamine it turns off its synthesis. Anabolic pathways are regulated by their end-products, but even more so by the energy state of the cell. When there is ample energy, bigger molecules, such as protein, RNA, and DNA, will be built as needed. Alternatively, when energy is insufficient, proteins and other molecules will be destroyed and catabolized to release energy. A dramatic example of this is seen in children with Marasmus. These children have severely compromised bodily functions, often culminating in death by infection. Children with Marasmus are starving for calories and protein, which are required to make energy and build macromolecules. In a much less severe example, a person is also in negative energy balance between meals. During this time, blood glucose levels start to drop. However, we can make metabolism work for us when we exercise. When you are active, the body burns more energy kilojoules. Our metabolism is complex — put simply it has 2 parts, which are carefully regulated by the body to make sure they remain in balance. They are:. The BMR refers to the amount of energy your body needs to maintain homeostasis. Your BMR is largely determined by your total lean mass, especially muscle mass, because lean mass requires a lot of energy to maintain. Anything that reduces lean mass will reduce your BMR. As your BMR accounts for so much of your total energy consumption, it is important to preserve or even increase your lean muscle mass through exercise when trying to lose weight. This means combining exercise particularly weight-bearing and resistance exercises to boost muscle mass with changes towards healthier eating patterns , rather than dietary changes alone as eating too few kilojoules encourages the body to slow the metabolism to conserve energy. Maintaining lean muscle mass also helps reduce the chance of injury when training, and exercise increases your daily energy expenditure. An average man has a BMR of around 7, kJ per day, while an average woman has a BMR of around 5, kJ per day. Energy expenditure is continuous, but the rate varies throughout the day. The rate of energy expenditure is usually lowest in the early morning. Your BMR rises after you eat because you use energy to eat, digest and metabolise the food you have just eaten. The rise occurs soon after you start eating, and peaks 2 to 3 hours later. Different foods raise BMR by differing amounts. For example:. During strenuous or vigorous physical activity, our muscles may burn through as much as 3, kJ per hour. Energy used during exercise is the only form of energy expenditure that we have any control over. However, estimating the energy spent during exercise is difficult, as the true value for each person will vary based on factors such as their weight, age, health and the intensity with which each activity is performed. Australia has physical activity guidelines External Link that recommend the amount and intensity of activity by age and life stage. Muscle tissue has a large appetite for kilojoules. The more muscle mass you have, the more kilojoules you will burn. People tend to put on fat as they age, partly because the body slowly loses muscle. It is not clear whether muscle loss is a result of the ageing process or because many people are less active as they age. However, it probably has more to do with becoming less active. Research has shown that strength and resistance training can reduce or prevent this muscle loss. If you are over 40 years of age, have a pre-existing medical condition or have not exercised in some time, see your doctor before starting a new fitness program. Hormones help regulate our metabolism. Some of the more common hormonal disorders affect the thyroid. This gland secretes hormones to regulate many metabolic processes, including energy expenditure the rate at which kilojoules are burned. Thyroid disorders include:. Our genes are the blueprints for the proteins in our body, and our proteins are responsible for the digestion and metabolism of our food. Sometimes, a faulty gene means we produce a protein that is ineffective in dealing with our food, resulting in a metabolic disorder. In most cases, genetic metabolic disorders can be managed under medical supervision, with close attention to diet. The symptoms of genetic metabolic disorders can be very similar to those of other disorders and diseases, making it difficult to pinpoint the exact cause. See your doctor if you suspect you have a metabolic disorder. Some genetic disorders of metabolism include:. This page has been produced in consultation with and approved by:. Content on this website is provided for information purposes only. Information about a therapy, service, product or treatment does not in any way endorse or support such therapy, service, product or treatment and is not intended to replace advice from your doctor or other registered health professional. The information and materials contained on this website are not intended to constitute a comprehensive guide concerning all aspects of the therapy, product or treatment described on the website. All users are urged to always seek advice from a registered health care professional for diagnosis and answers to their medical questions and to ascertain whether the particular therapy, service, product or treatment described on the website is suitable in their circumstances. The State of Victoria and the Department of Health shall not bear any liability for reliance by any user on the materials contained on this website. Skip to main content. Actions for this page Listen Print. Summary Read the full fact sheet. On this page. What is metabolism? Two processes of metabolism Metabolic rate Metabolism and age-related weight gain Hormonal disorders of metabolism Genetic disorders of metabolism Where to get help. Two processes of metabolism Our metabolism is complex — put simply it has 2 parts, which are carefully regulated by the body to make sure they remain in balance. They are: Catabolism — the breakdown of food components such as carbohydrates , proteins and dietary fats into their simpler forms, which can then be used to provide energy and the basic building blocks needed for growth and repair. Anabolism — the part of metabolism in which our body is built or repaired. Anabolism requires energy that ultimately comes from our food. When we eat more than we need for daily anabolism, the excess nutrients are typically stored in our body as fat. Thermic effect of food also known as thermogenesis — your body uses energy to digest the foods and drinks you consume and also absorbs, transports and stores their nutrients. Energy used during physical activity — this is the energy used by physical movement and it varies the most depending on how much energy you use each day. |
| More on this topic for: | During the transition from rest to Energy metabolism process exercise, Metabooism substrate for increased aerobic ATP production is also muscle Nutritional considerations for young males, and a small metabloism of the Enefgy Optimal performance website is transferred into the metavolism, where it is used to produce acetyl-CoA and the reducing equivalent NADH in the pyruvate dehydrogenase PDH reaction. Contents move to sidebar hide. Exercise metabolism: fuels for the fire. The required enzymes of stomach cells differ from those of fat storage cells, skin cells, blood cells, and nerve cells. If you're seeing this message, it means we're having trouble loading external resources on our website. |
| Overview of metabolism | Building glucose from carbon dioxide is one example. a Muscle glycogen is the primary CHO source during intense exercise. Dolichol kinase GCS1 Oligosaccharyltransferase. And this energy and that bond as the phosphate group breaks off it can release it to provide all sorts of life mechanisms including being able to metabolize things. Retrieved 3 July |
| 4.1: Metabolism Overview | The American Journal of Physiology. I think what they mean is that a molecule such as glucose gets broken down a few times to harvest some energy in the form of ATP The science and applications of synthetic and systems biology workshop summary. Gluconeogenesis converts pyruvate to glucosephosphate through a series of intermediates, many of which are shared with glycolysis. Coproporphyrinogen III oxidase Protoporphyrinogen oxidase Ferrochelatase. This reaction releases CoA-SH and is catalyzed by succinyl-CoA synthetase. Proteins are made from amino acids that have been activated by attachment to a transfer RNA molecule through an ester bond. |
| Introduction | The glycerol enters glycolysis and the fatty acids are broken down by beta oxidation to release acetyl-CoA, which then is fed into the citric acid cycle. Fatty acids release more energy upon oxidation than carbohydrates. Steroids are also broken down by some bacteria in a process similar to beta oxidation, and this breakdown process involves the release of significant amounts of acetyl-CoA, propionyl-CoA, and pyruvate, which can all be used by the cell for energy. tuberculosis can also grow on the lipid cholesterol as a sole source of carbon, and genes involved in the cholesterol-use pathway s have been validated as important during various stages of the infection lifecycle of M. Amino acids are either used to synthesize proteins and other biomolecules, or oxidized to urea and carbon dioxide to produce energy. The amino group is fed into the urea cycle , leaving a deaminated carbon skeleton in the form of a keto acid. Several of these keto acids are intermediates in the citric acid cycle, for example α- ketoglutarate formed by deamination of glutamate. In oxidative phosphorylation, the electrons removed from organic molecules in areas such as the citric acid cycle are transferred to oxygen and the energy released is used to make ATP. This is done in eukaryotes by a series of proteins in the membranes of mitochondria called the electron transport chain. In prokaryotes , these proteins are found in the cell's inner membrane. Pumping protons out of the mitochondria creates a proton concentration difference across the membrane and generates an electrochemical gradient. The flow of protons makes the stalk subunit rotate, causing the active site of the synthase domain to change shape and phosphorylate adenosine diphosphate — turning it into ATP. Chemolithotrophy is a type of metabolism found in prokaryotes where energy is obtained from the oxidation of inorganic compounds. These organisms can use hydrogen , [52] reduced sulfur compounds such as sulfide , hydrogen sulfide and thiosulfate , [1] ferrous iron Fe II [53] or ammonia [54] as sources of reducing power and they gain energy from the oxidation of these compounds. The energy in sunlight is captured by plants , cyanobacteria , purple bacteria , green sulfur bacteria and some protists. This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below. The energy capture and carbon fixation systems can, however, operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds. In many organisms, the capture of solar energy is similar in principle to oxidative phosphorylation, as it involves the storage of energy as a proton concentration gradient. This proton motive force then drives ATP synthesis. Reaction centers are classified into two types depending on the nature of photosynthetic pigment present, with most photosynthetic bacteria only having one type, while plants and cyanobacteria have two. In plants, algae, and cyanobacteria, photosystem II uses light energy to remove electrons from water, releasing oxygen as a waste product. The electrons then flow to the cytochrome b6f complex , which uses their energy to pump protons across the thylakoid membrane in the chloroplast. Anabolism is the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from smaller and simpler precursors. Anabolism involves three basic stages. First, the production of precursors such as amino acids , monosaccharides , isoprenoids and nucleotides , secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such as proteins , polysaccharides , lipids and nucleic acids. Anabolism in organisms can be different according to the source of constructed molecules in their cells. Autotrophs such as plants can construct the complex organic molecules in their cells such as polysaccharides and proteins from simple molecules like carbon dioxide and water. Heterotrophs , on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from oxidation reactions. Photosynthesis is the synthesis of carbohydrates from sunlight and carbon dioxide CO 2. In plants, cyanobacteria and algae, oxygenic photosynthesis splits water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by the photosynthetic reaction centres , as described above, to convert CO 2 into glycerate 3-phosphate , which can then be converted into glucose. This carbon-fixation reaction is carried out by the enzyme RuBisCO as part of the Calvin — Benson cycle. These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO 2 directly, while C4 and CAM photosynthesis incorporate the CO 2 into other compounds first, as adaptations to deal with intense sunlight and dry conditions. In photosynthetic prokaryotes the mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin — Benson cycle, a reversed citric acid cycle, [66] or the carboxylation of acetyl-CoA. In carbohydrate anabolism, simple organic acids can be converted into monosaccharides such as glucose and then used to assemble polysaccharides such as starch. The generation of glucose from compounds like pyruvate , lactate , glycerol , glycerate 3-phosphate and amino acids is called gluconeogenesis. Gluconeogenesis converts pyruvate to glucosephosphate through a series of intermediates, many of which are shared with glycolysis. This is important as it allows the formation and breakdown of glucose to be regulated separately, and prevents both pathways from running simultaneously in a futile cycle. Although fat is a common way of storing energy, in vertebrates such as humans the fatty acids in these stores cannot be converted to glucose through gluconeogenesis as these organisms cannot convert acetyl-CoA into pyruvate ; plants do, but animals do not, have the necessary enzymatic machinery. Polysaccharides and glycans are made by the sequential addition of monosaccharides by glycosyltransferase from a reactive sugar-phosphate donor such as uridine diphosphate glucose UDP-Glc to an acceptor hydroxyl group on the growing polysaccharide. As any of the hydroxyl groups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures. Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups: in animals and fungi, all these fatty acid synthase reactions are carried out by a single multifunctional type I protein, [79] while in plant plastids and bacteria separate type II enzymes perform each step in the pathway. Terpenes and isoprenoids are a large class of lipids that include the carotenoids and form the largest class of plant natural products. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA, [84] while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates. Here, the isoprene units are joined to make squalene and then folded up and formed into a set of rings to make lanosterol. Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all twenty, but mammals can only synthesize eleven nonessential amino acids, so nine essential amino acids must be obtained from food. Nitrogen is provided by glutamate and glutamine. Nonessensial amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid. Amino acids are made into proteins by being joined in a chain of peptide bonds. Each different protein has a unique sequence of amino acid residues: this is its primary structure. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to a transfer RNA molecule through an ester bond. This aminoacyl-tRNA precursor is produced in an ATP -dependent reaction carried out by an aminoacyl tRNA synthetase. Nucleotides are made from amino acids, carbon dioxide and formic acid in pathways that require large amounts of metabolic energy. Pyrimidines , on the other hand, are synthesized from the base orotate , which is formed from glutamine and aspartate. All organisms are constantly exposed to compounds that they cannot use as foods and that would be harmful if they accumulated in cells, as they have no metabolic function. These potentially damaging compounds are called xenobiotics. In humans, these include cytochrome P oxidases , [97] UDP-glucuronosyltransferases , [98] and glutathione S -transferases. The modified water-soluble xenobiotic can then be pumped out of cells and in multicellular organisms may be further metabolized before being excreted phase III. In ecology , these reactions are particularly important in microbial biodegradation of pollutants and the bioremediation of contaminated land and oil spills. A related problem for aerobic organisms is oxidative stress. Living organisms must obey the laws of thermodynamics , which describe the transfer of heat and work. The second law of thermodynamics states that in any isolated system , the amount of entropy disorder cannot decrease. Although living organisms' amazing complexity appears to contradict this law, life is possible as all organisms are open systems that exchange matter and energy with their surroundings. Living systems are not in equilibrium , but instead are dissipative systems that maintain their state of high complexity by causing a larger increase in the entropy of their environments. In thermodynamic terms, metabolism maintains order by creating disorder. As the environments of most organisms are constantly changing, the reactions of metabolism must be finely regulated to maintain a constant set of conditions within cells, a condition called homeostasis. Firstly, the regulation of an enzyme in a pathway is how its activity is increased and decreased in response to signals. Secondly, the control exerted by this enzyme is the effect that these changes in its activity have on the overall rate of the pathway the flux through the pathway. it is highly regulated but if these changes have little effect on the flux of a metabolic pathway, then this enzyme is not involved in the control of the pathway. There are multiple levels of metabolic regulation. In intrinsic regulation, the metabolic pathway self-regulates to respond to changes in the levels of substrates or products; for example, a decrease in the amount of product can increase the flux through the pathway to compensate. These signals are usually in the form of water-soluble messengers such as hormones and growth factors and are detected by specific receptors on the cell surface. A very well understood example of extrinsic control is the regulation of glucose metabolism by the hormone insulin. Binding of the hormone to insulin receptors on cells then activates a cascade of protein kinases that cause the cells to take up glucose and convert it into storage molecules such as fatty acids and glycogen. These enzymes are regulated in a reciprocal fashion, with phosphorylation inhibiting glycogen synthase, but activating phosphorylase. Insulin causes glycogen synthesis by activating protein phosphatases and producing a decrease in the phosphorylation of these enzymes. The central pathways of metabolism described above, such as glycolysis and the citric acid cycle, are present in all three domains of living things and were present in the last universal common ancestor. Many models have been proposed to describe the mechanisms by which novel metabolic pathways evolve. These include the sequential addition of novel enzymes to a short ancestral pathway, the duplication and then divergence of entire pathways as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway. As well as the evolution of new metabolic pathways, evolution can also cause the loss of metabolic functions. For example, in some parasites metabolic processes that are not essential for survival are lost and preformed amino acids, nucleotides and carbohydrates may instead be scavenged from the host. Classically, metabolism is studied by a reductionist approach that focuses on a single metabolic pathway. Particularly valuable is the use of radioactive tracers at the whole-organism, tissue and cellular levels, which define the paths from precursors to final products by identifying radioactively labelled intermediates and products. A parallel approach is to identify the small molecules in a cell or tissue; the complete set of these molecules is called the metabolome. Overall, these studies give a good view of the structure and function of simple metabolic pathways, but are inadequate when applied to more complex systems such as the metabolism of a complete cell. An idea of the complexity of the metabolic networks in cells that contain thousands of different enzymes is given by the figure showing the interactions between just 43 proteins and 40 metabolites to the right: the sequences of genomes provide lists containing anything up to Bacterial metabolic networks are a striking example of bow-tie [] [] [] organization, an architecture able to input a wide range of nutrients and produce a large variety of products and complex macromolecules using a relatively few intermediate common currencies. A major technological application of this information is metabolic engineering. Here, organisms such as yeast , plants or bacteria are genetically modified to make them more useful in biotechnology and aid the production of drugs such as antibiotics or industrial chemicals such as 1,3-propanediol and shikimic acid. The term metabolism is derived from the Ancient Greek word μεταβολή — "Metabole" for "a change" which derived from μεταβάλλ —"Metaballein" means "To change" []. Aristotle 's The Parts of Animals sets out enough details of his views on metabolism for an open flow model to be made. He believed that at each stage of the process, materials from food were transformed, with heat being released as the classical element of fire, and residual materials being excreted as urine, bile, or faeces. Ibn al-Nafis described metabolism in his AD work titled Al-Risalah al-Kamiliyyah fil Siera al-Nabawiyyah The Treatise of Kamil on the Prophet's Biography which included the following phrase "Both the body and its parts are in a continuous state of dissolution and nourishment, so they are inevitably undergoing permanent change. The history of the scientific study of metabolism spans several centuries and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry. The first controlled experiments in human metabolism were published by Santorio Santorio in in his book Ars de statica medicina. He found that most of the food he took in was lost through what he called " insensible perspiration ". In these early studies, the mechanisms of these metabolic processes had not been identified and a vital force was thought to animate living tissue. He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells. This proved that the organic compounds and chemical reactions found in cells were no different in principle than any other part of chemistry. It was the discovery of enzymes at the beginning of the 20th century by Eduard Buchner that separated the study of the chemical reactions of metabolism from the biological study of cells, and marked the beginnings of biochemistry. One of the most prolific of these modern biochemists was Hans Krebs who made huge contributions to the study of metabolism. These techniques have allowed the discovery and detailed analysis of the many molecules and metabolic pathways in cells. See Template:Leucine metabolism in humans — this diagram does not include the pathway for β-leucine synthesis via leucine 2,3-aminomutase. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Set of chemical reactions in organisms. For the journal, see Cell Metabolism. For the journal 'Metabolism', see Metabolism: Clinical and Experimental. For the architectural movement, see Metabolism architecture. Index Outline History. Key components. Biomolecules Enzymes Gene expression Metabolism. List of biochemists. Biochemist List of biochemists. Biomolecule families. Carbohydrates : Alcohols Glycoproteins Glycosides Lipids : Eicosanoids Fatty acids Fatty-acid metabolism Glycerides Phospholipids Sphingolipids Cholesterol Steroids Nucleic acids : Nucleobases Nucleosides Nucleotides Nucleotide metabolism Proteins : Amino acids Amino acid metabolism Other: Tetrapyrroles Heme. Chemical synthesis. Artificial gene synthesis Biomimetic synthesis Bioretrosynthesis Biosynthesis Chemosynthesis Convergent synthesis Custom peptide synthesis Direct process Divergent synthesis Electrosynthesis Enantioselective synthesis Fully automated synthesis Hydrothermal synthesis LASiS Mechanosynthesis One-pot synthesis Organic synthesis Peptide synthesis Radiosynthesis Retrosynthesis Semisynthesis Solid-phase synthesis Solvothermal synthesis Total synthesis Volume combustion synthesis. Biochemistry fields. Molecular biology Cell biology Chemical biology Bioorthogonal chemistry Medicinal chemistry Pharmacology Clinical chemistry Neurochemistry Bioorganic chemistry Bioorganometallic chemistry Bioinorganic chemistry Biophysical chemistry Bacteriology parasitology virology immunology. Glossary of biology Glossary of chemistry. Further information: Biomolecule , Cell biology , and Biochemistry. Main article: Protein. Main article: Biolipid. Main article: Carbohydrate. Main article: Nucleotide. Main article: Coenzyme. Further information: Bioinorganic chemistry. Main article: Catabolism. Further information: Digestion and Gastrointestinal tract. Further information: Cellular respiration , Fermentation biochemistry , Carbohydrate catabolism , Fat catabolism , and Protein catabolism. Further information: Oxidative phosphorylation , Chemiosmosis , and Mitochondrion. Further information: Microbial metabolism and Nitrogen cycle. Further information: Phototroph , Photophosphorylation , and Chloroplast. Further information: Anabolism. Further information: Photosynthesis , Carbon fixation , and Chemosynthesis. Further information: Gluconeogenesis , Glyoxylate cycle , Glycogenesis , and Glycosylation. Further information: Fatty acid synthesis and Steroid metabolism. Further information: Protein biosynthesis and Amino acid synthesis. Further information: Nucleotide salvage , Pyrimidine biosynthesis , and Purine § Metabolism. Further information: Xenobiotic metabolism , Drug metabolism , Alcohol metabolism , and Antioxidant. Further information: Biological thermodynamics. Further information: Metabolic pathway , Metabolic control analysis , Hormone , Regulatory enzymes , and Cell signaling. Further information: Proto-metabolism , Molecular evolution , and Phylogenetics. Further information: Protein methods , Proteomics , Metabolomics , and Metabolic network modelling. Further information: History of biochemistry and History of molecular biology. Physiology and Genetics of Sulfur-oxidizing Bacteria. Advances in Microbial Physiology. doi : ISBN PMID Proceedings of the National Academy of Sciences of the United States of America. Bibcode : PNAS PMC Bibcode : PNAS.. Theoretical reconstruction of the stoichiometry of ATP and NADH producing systems". Bulletin of Mathematical Biology. S2CID Journal of Molecular Evolution. Bibcode : JMolE.. Endocrine Reviews. The Cell: A Molecular Approach 2nd ed. Archived from the original on 27 August Retrieved 25 June Annual Review of Biochemistry. Lehninger Principles of Biochemistry. New York: W. Freeman and company. The Biochemical Journal. Journal of Amino Acids. May Journal of Lipid Research. Archived from the original on 6 June Retrieved 6 June Biochemistry 8 ed. OCLC Nature Methods. Journal of Clinical Virology. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems". European Journal of Biochemistry. Fourth in the Cycles Review Series". EMBO Reports. September Purinergic Signalling. Archived from the original on 15 December Retrieved 9 June Advances in food biochemistry. Boca Raton: CRC Press. August The American Journal of Physiology. Anatomy and Physiology. Archived from the original on 2 June Retrieved 23 June Molecular Cell Biology 4th ed. Archived from the original on 30 May Retrieved 23 June — via NCBI. In Olivares-Quiroz L, Resendis-Antonio O eds. Quantitative Models for Microscopic to Macroscopic Biological Macromolecules and Tissues. Cham: Springer International Publishing. The Journal of Biological Chemistry. Archived from the original on 25 June Retrieved 24 June Trends in Cell Biology. Molecular Biology of the Cell 4th ed. Archived from the original on 5 July Retrieved 25 June — via NCBI. Aquatic Microbial Ecology. ISSN Nature Reviews. Molecular Cell Biology. Brock Mikrobiologie Aufl ed. München: Pearson Studium. Energy : production, conversion, storage, conservation, and coupling Second ed. Lincoln: Springer. Microbiological Reviews. Applied Microbiology and Biotechnology. British Journal of Nursing. Journal of Parenteral and Enteral Nutrition. Current Opinion in Cell Biology. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question". Fat oxidation also contributes energy in recovery from exercise or rest periods between activity. Another important aspect of metabolism in stop-and-go sports is the ability to rapidly resynthesize PCr when the exercise intensity falls to low levels or athletes rest. In these situations, continued aerobic production of ATP fuels the regeneration of PCr such that it can be completely recovered in 60— s ref. This production is extremely important for the ability to repeatedly sprint in stop-and-go or intermittent sports. Recovery from prolonged sprinting 20—s and sustained high glycolytic flux is slower, because the associated muscle acidity requires minutes, not seconds, to recover and can limit performance 4 , Importantly, other fuels can provide aerobic energy in cells during exercise, including amino acids, acetate, medium-chain triglycerides, and the ketones β-hydroxybutyrate and acetoacetic acid. Although these fuels can be used to spare the use of fat and carbohydrate in some moderate-intensity exercise situations, they lack the rate of energy provision needed to fuel intense aerobic exercise, because the metabolic machinery for these fuels is not designed for rapid energy provision. Alternative fuels cannot match carbohydrate in terms of the rate of aerobic energy provision 9 , and these fuels cannot be used to produce anaerobic energy in the absence of oxygen. Sex may have roles in the regulation of skeletal muscle metabolism. Males and females are often assumed to respond similarly to acute exercise and exercise training, but most of the work cited in this Review involved male participants. Clear differences exist between males and females—including haemoglobin concentrations, muscle mass and reproductive-hormone levels—and have been shown to affect metabolism and exercise performance, thus making perfect comparisons between males and females very difficult. The potential sex differences in metabolism are briefly mentioned in Box 3 , and more detailed discussion can be found in a review by Kiens One issue in the study of the regulation of exercise metabolism in skeletal muscle is that much of the available data has been derived from studies on males. Although the major principles controlling the regulation of metabolism appear to hold true for both females and males, some differences have been noted. Although one might argue that completely matching males and females is impossible when studying metabolism, early work with well-trained track athletes has reported no differences in skeletal muscle enzyme activity, fibre-type composition and fat oxidation between men and women , However, more recent work has reported that a larger percentage of whole-body fuel use is derived from fat in females exercising at the same relative submaximal intensity, and this effect is likely to be related to circulating oestrogen levels , , , , , In addition, supplementation with oestrogen in males decreases carbohydrate oxidation and increases fat oxidation during endurance exercise These results suggest that females may be better suited to endurance exercise than males. Another area that has been investigated is the effects of menstrual phase and menstrual status on the regulation of skeletal muscle metabolism. Generally, studies examining exercise in the luteal and follicular phases have reported only minor or no changes in fat and carbohydrate metabolism at various exercise intensities , , , Additional work examining the regulation of metabolism in well-trained female participants in both phases of the menstrual cycle, and with varied menstrual cycles, during exercise at the high aerobic and supramaximal intensities commensurate with elite sports, is warranted. Sports performance is determined by many factors but is ultimately limited by the development of fatigue, such that the athletes with the greatest fatigue resistance often succeed. However, there can be a fine line between glory and catastrophe, and the same motivation that drives athletes to victory can at times push them beyond their limits. Fatigue is the result of a complex interplay among central neural regulation, neuromuscular function and the various physiological processes that support skeletal muscle performance 1. It manifests as a decrease in the force or power-producing capacity of skeletal muscle and an inability to maintain the exercise intensity needed for ultimate success. Over the years, considerable interest has been placed on the relative importance of central neural and peripheral muscle factors in the aetiology of fatigue. All that I am, I am because of my mind. Perhaps the two major interventions used to enhance fatigue resistance are regular training and nutrition 70 , and the interactions between them have been recognized We briefly review the effects of training and nutrition on skeletal muscle energy metabolism and exercise performance, with a focus on substrate availability and metabolic end products. In relation to dietary supplements, we have limited our discussion to those that have been reasonably investigated for efficacy in human participants Regular physical training is an effective strategy for enhancing fatigue resistance and exercise performance, and many of these adaptations are mediated by changes in muscle metabolism and morphology. Such training is also associated with the cardiovascular and metabolic benefits often observed with traditional endurance training One hallmark adaptation to endurance exercise training is increased oxygen-transport capacity, as measured by VO 2 max 78 , thus leading to greater fatigue resistance and enhanced exercise performance The other is enhanced skeletal muscle mitochondrial density 80 , a major factor contributing to decreased carbohydrate utilization and oxidation and lactate production 81 , 82 , increased fat oxidation and enhanced endurance exercise performance The capacity for muscle carbohydrate oxidation also increases, thereby enabling maintenance of a higher power output during exercise and enhanced performance Finally, resistance training results in increased strength, neuromuscular function and muscle mass 85 , effects that can be potentiated by nutritional interventions, such as increased dietary protein intake The improved performance is believed to be due to enhanced ATP resynthesis during exercise as a result of increased PCr availability. Some evidence also indicates that creatine supplementation may increase muscle mass and strength during resistance training No major adverse effects of creatine supplementation have been observed in the short term, but long-term studies are lacking. Creatine remains one of the most widely used sports-related dietary supplements. The importance of carbohydrate for performance in strenuous exercise has been recognized since the early nineteenth century, and for more than 50 years, fatigue during prolonged strenuous exercise has been associated with muscle glycogen depletion 13 , Muscle glycogen is critical for ATP generation and supply to all the key ATPases involved in excitation—contraction coupling in skeletal muscle Recently, prolonged exercise has been shown to decrease glycogen in rodent brains, thus suggesting the intriguing possibility that brain glycogen depletion may contribute to central neural fatigue Muscle glycogen availability may also be important for high-intensity exercise performance Blood glucose levels decline during prolonged strenuous exercise, because the liver glycogen is depleted, and increased liver gluconeogenesis is unable to generate glucose at a rate sufficient to match skeletal muscle glucose uptake. Maintenance of blood glucose levels at or slightly above pre-exercise levels by carbohydrate supplementation maintains carbohydrate oxidation, improves muscle energy balance at a time when muscle glycogen levels are decreased and delays fatigue 20 , 97 , Glucose ingestion during exercise has minimal effects on net muscle glycogen utilization 97 , 99 , but increases muscle glucose uptake and markedly decreases liver glucose output , , because the gut provides most glucose to the bloodstream. Importantly, although carbohydrate ingestion delays fatigue, it does not prevent fatigue, and many factors clearly contribute to fatigue during prolonged strenuous exercise. Because glucose is the key substrate for the brain, central neural fatigue may develop during prolonged exercise as a consequence of hypoglycaemia and decreased cerebral glucose uptake Carbohydrate ingestion exerts its benefit by increasing cerebral glucose uptake and maintaining central neural drive NH 3 can cross the blood—brain barrier and has the potential to affect central neurotransmitter levels and central neural fatigue. Of note, carbohydrate ingestion attenuates muscle and plasma NH 3 accumulation during exercise , another potential mechanism through which carbohydrate ingestion exerts its ergogenic effect. Enhanced exercise performance has also been observed from simply having carbohydrate in the mouth, an effect that has been linked to activation of brain centres involved in motor control Increased plasma fatty acid availability decreases muscle glycogen utilization and carbohydrate oxidation during exercise , , High-fat diets have also been proposed as a strategy to decrease reliance on carbohydrate and improve endurance performance. Other studies have demonstrated increased fat oxidation and lower rates of muscle glycogen use and carbohydrate oxidation after adaptation to a short-term high-fat diet, even with restoration of muscle glycogen levels, but no effect on endurance exercise performance , If anything, high-intensity exercise performance is impaired on the high-fat diet , apparently as a result of an inability to fully activate glycogenolysis and PDH during intense exercise Furthermore, a high-fat diet has been shown to impair exercise economy and performance in elite race walkers A related issue with high-fat, low carbohydrate diets is the induction of nutritional ketosis after 2—3 weeks. However, when this diet is adhered to for 3 weeks, and the concentrations of ketone bodies are elevated, a decrease in performance has been observed in elite race walkers The rationale for following this dietary approach to optimize performance has been called into question Although training on a high-fat diet appears to result in suboptimal adaptations in previously untrained participants , some studies have reported enhanced responses to training with low carbohydrate availability in well-trained participants , Over the years, endurance athletes have commonly undertaken some of their training in a relatively low-carbohydrate state. However, maintaining an intense training program is difficult without adequate dietary carbohydrate intake Furthermore, given the heavy dependence on carbohydrate during many of the events at the Olympics 9 , the most effective strategy for competition would appear to be one that maximizes carbohydrate availability and utilization. Nutritional ketosis can also be induced by the acute ingestion of ketone esters, which has been suggested to alter fuel preference and enhance performance The metabolic state induced is different from diet-induced ketosis and has the potential to alter the use of fat and carbohydrate as fuels during exercise. However, published studies on trained male athletes from at least four independent laboratories to date do not support an increase in performance. Acute ingestion of ketone esters has been found to have no effect on 5-km and km trial performance , , or performance during an incremental cycling ergometer test A further study has reported that ketone ester ingestion decreases performance during a The rate of ketone provision and metabolism in skeletal muscle during high-intensity exercise appears likely to be insufficient to substitute for the rate at which carbohydrate can provide energy. Early work on the ingestion of high doses of caffeine 6—9 mg caffeine per kg body mass 60 min before exercise has indicated enhanced lipolysis and fat oxidation during exercise, decreased muscle glycogen use and increased endurance performance in some individuals , , These effects appear to be a result of caffeine-induced increases in catecholamines, which increase lipolysis and consequently fatty acid concentrations during the rest period before exercise. After exercise onset, these circulating fatty acids are quickly taken up by the tissues of the body 10—15 min , fatty acid concentrations return to normal, and no increases in fat oxidation are apparent. Importantly, the ergogenic effects of caffeine have also been reported at lower caffeine doses ~3 mg per kg body mass during exercise and are not associated with increased catecholamine and fatty acid concentrations and other physiological alterations during exercise , This observation suggests that the ergogenic effects are mediated not through metabolic events but through binding to adenosine receptors in the central and peripheral nervous systems. Caffeine has been proposed to increase self-sustained firing, as well as voluntary activation and maximal force in the central nervous system, and to decrease the sensations associated with force, pain and perceived exertion or effort during exercise in the peripheral nervous system , The ingestion of low doses of caffeine is also associated with fewer or none of the adverse effects reported with high caffeine doses anxiety, jitters, insomnia, inability to focus, gastrointestinal unrest or irritability. Contemporary caffeine research is focusing on the ergogenic effects of low doses of caffeine ingested before and during exercise in many forms coffee, capsules, gum, bars or gels , and a dose of ~ mg caffeine has been argued to be optimal for exercise performance , The potential of supplementation with l -carnitine has received much interest, because this compound has a major role in moving fatty acids across the mitochondrial membrane and regulating the amount of acetyl-CoA in the mitochondria. The need for supplemental carnitine assumes that a shortage occurs during exercise, during which fat is used as a fuel. Although this outcome does not appear to occur during low-intensity and moderate-intensity exercise, free carnitine levels are low in high-intensity exercise and may contribute to the downregulation of fat oxidation at these intensities. However, oral supplementation with carnitine alone leads to only small increases in plasma carnitine levels and does not increase the muscle carnitine content An insulin level of ~70 mU l —1 is required to promote carnitine uptake by the muscle However, to date, there is no evidence that carnitine supplementation can improve performance during the higher exercise intensities common to endurance sports. NO is an important bioactive molecule with multiple physiological roles within the body. It is produced from l -arginine via the action of nitric oxide synthase and can also be formed by the nonenzymatic reduction of nitrate and nitrite. The observation that dietary nitrate decreases the oxygen cost of exercise has stimulated interest in the potential of nitrate, often ingested in the form of beetroot juice, as an ergogenic aid during exercise. Indeed, several studies have observed enhanced exercise performance associated with lower oxygen cost and increased muscle efficiency after beetroot-juice ingestion , , The effect of nitrate supplementation appears to be less apparent in well-trained athletes , , although results in the literature are varied Dietary nitrate supplementation may have beneficial effects through an improvement in excitation—contraction coupling , , because supplementation with beetroot juice does not alter mitochondrial efficiency in human skeletal muscle , and the results with inorganic nitrate supplementation have been equivocal , Lactate is not thought to have a major negative effect on force and power generation and, as mentioned earlier, is an important metabolic intermediate and signalling molecule. Of greater importance is the acidosis arising from increased muscle metabolism and strong ion fluxes. In humans, acidosis does not appear to impair maximal isometric-force production, but it does limit the ability to maintain submaximal force output , thus suggesting an effect on energy metabolism and ATP generation Ingestion of oral alkalizers, such as bicarbonate, is often associated with increased high-intensity exercise performance , , partly because of improved energy metabolism and ionic regulation , As previously mentioned, high-intensity exercise training increases muscle buffer capacity 74 , A major determinant of the muscle buffering capacity is carnosine content, which is higher in sprinters and rowers than in marathon runners or untrained individuals Ingestion of β-alanine increases muscle carnosine content and enhances high-intensity exercise performance , During exercise, ROS, such as superoxide anions, hydrogen peroxide and hydroxyl radicals, are produced and have important roles as signalling molecules mediating the acute and chronic responses to exercise However, ROS accumulation at higher levels can negatively affect muscle force and power production and induce fatigue 68 , Exercise training increases the levels of key antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase , and non-enzymatic antioxidants reduced glutathione, β-carotene, and vitamins C and E can counteract the negative effects of ROS. Whether dietary antioxidant supplementation can improve exercise performance is equivocal , although ingestion of N -acetylcysteine enhances muscle oxidant capacity and attenuates muscle fatigue during prolonged exercise Some reports have suggested that antioxidant supplementation may potentially attenuate skeletal muscle adaptation to regular exercise , , Overall, ROS may have a key role in mediating adaptations to acute and chronic exercise but, when they accumulate during strenuous exercise, may exert fatigue effects that limit exercise performance. The negative effects of hyperthermia are potentiated by sweating-induced fluid losses and dehydration , particularly decreased skeletal muscle blood flow and increased muscle glycogen utilization during exercise in heat Increased plasma catecholamines and elevated muscle temperatures also accelerate muscle glycogenolysis during exercise in heat , , Strategies to minimize the negative effects of hyperthermia on muscle metabolism and performance include acclimation, pre-exercise cooling and fluid ingestion , , , To meet the increased energy needs of exercise, skeletal muscle has a variety of metabolic pathways that produce ATP both anaerobically requiring no oxygen and aerobically. These pathways are activated simultaneously from the onset of exercise to precisely meet the demands of a given exercise situation. Although the aerobic pathways are the default, dominant energy-producing pathways during endurance exercise, they require time seconds to minutes to fully activate, and the anaerobic systems rapidly in milliseconds to seconds provide energy to cover what the aerobic system cannot provide. Anaerobic energy provision is also important in situations of high-intensity exercise, such as sprinting, in which the requirement for energy far exceeds the rate that the aerobic systems can provide. This situation is common in stop-and-go sports, in which transitions from lower-energy to higher-energy needs are numerous, and provision of both aerobic and anaerobic energy contributes energy for athletic success. Together, the aerobic energy production using fat and carbohydrate as fuels and the anaerobic energy provision from PCr breakdown and carbohydrate use in the glycolytic pathway permit Olympic athletes to meet the high energy needs of particular events or sports. The various metabolic pathways are regulated by a range of intramuscular and hormonal signals that influence enzyme activation and substrate availability, thus ensuring that the rate of ATP resynthesis is closely matched to the ATP demands of exercise. Regular training and various nutritional interventions have been used to enhance fatigue resistance via modulation of substrate availability and the effects of metabolic end products. The understanding of exercise energy provision, the regulation of metabolism and the use of fat and carbohydrate fuels during exercise has increased over more than years, on the basis of studies using various methods including indirect calorimetry, tissue samples from contracting skeletal muscle, metabolic-tracer sampling, isolated skeletal muscle preparations, and analysis of whole-body and regional arteriovenous blood samples. However, in virtually all areas of the regulation of fat and carbohydrate metabolism, much remains unknown. The introduction of molecular biology techniques has provided opportunities for further insights into the acute and chronic responses to exercise and their regulation, but even those studies are limited by the ability to repeatedly sample muscle in human participants to fully examine the varied time courses of key events. The ability to fully translate findings from in vitro experiments and animal studies to exercising humans in competitive settings remains limited. The field also continues to struggle with measures specific to the various compartments that exist in the cell, and knowledge remains lacking regarding the physical structures and scaffolding inside these compartments, and the communication between proteins and metabolic pathways within compartments. A clear example of these issues is in studying the events that occur in the mitochondria during exercise. One area that has not advanced as rapidly as needed is the ability to non-invasively measure the fuels, metabolites and proteins in the various important muscle cell compartments that are involved in regulating metabolism during exercise. Although magnetic resonance spectroscopy has been able to measure certain compounds non-invasively, measuring changes that occur with exercise at the molecular and cellular levels is generally not possible. Some researchers are investigating exercise metabolism at the whole-body level through a physiological approach, and others are examining the intricacies of cell signalling and molecular changes through a reductionist approach. New opportunities exist for the integrated use of genomics, proteomics, metabolomics and systems biology approaches in data analyses, which should provide new insights into the molecular regulation of exercise metabolism. Many questions remain in every area of energy metabolism, the regulation of fat and carbohydrate metabolism during exercise, optimal training interventions and the potential for manipulation of metabolic responses for ergogenic benefits. Exercise biology will thus continue to be a fruitful research area for many years as researchers seek a greater understanding of the metabolic bases for the athletic successes that will be enjoyed and celebrated during the quadrennial Olympic festival of sport. Hawley, J. Integrative biology of exercise. Cell , — Article CAS PubMed Google Scholar. Sahlin, K. Energy supply and muscle fatigue in humans. Acta Physiol. Medbø, J. Anaerobic energy release in working muscle during 30 s to 3 min of exhausting bicycling. Article PubMed Google Scholar. Parolin, M. et al. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. CAS PubMed Google Scholar. Greenhaff, P. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. Article Google Scholar. Relative importance of aerobic and anaerobic energy release during short-lasting exhausting bicycle exercise. Tesch, P. Muscle metabolism during intense, heavy-resistance exercise. Koopman, R. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. Carbohydrate dependence during marathon running. Sports Exerc. PubMed Google Scholar. Romijn, J. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. van Loon, L. The effects of increasing exercise intensity on muscle fuel utilisation in humans. Bergström, J. A study of the glycogen metabolism during exercise in man. Wahren, J. Glucose metabolism during leg exercise in man. Article CAS PubMed PubMed Central Google Scholar. Ahlborg, G. Substrate turnover during prolonged exercise in man. Watt, M. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. Article CAS Google Scholar. Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Article PubMed CAS Google Scholar. Wasserman, D. Four grams of glucose. Coggan, A. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Coyle, E. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. Horowitz, J. Lipid metabolism during endurance exercise. Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Stellingwerff, T. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Spriet, L. An enzymatic approach to lactate production in human skeletal muscle during exercise. Brooks, G. The lactate shuttle during exercise and recovery. Miller, B. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. Lactate elimination and glycogen resynthesis after intense bicycling. Hashimoto, T. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. Takahashi, H. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Metab 1 , — Scheiman, J. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Rennie, M. Effect of exercise on protein turnover in man. Wagenmakers, A. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Howarth, K. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. McKenzie, S. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Wilkinson, S. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. New insights into the interaction of carbohydrate and fat metabolism during exercise. Hargreaves, M. Exercise metabolism: fuels for the fire. Cold Spring Harb. Article PubMed PubMed Central CAS Google Scholar. Richter, E. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Gaitanos, G. Human muscle metabolism during intermittent maximal exercise. Kowalchuk, J. Factors influencing hydrogen ion concentration in muscle after intense exercise. Howlett, R. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Wojtaszewski, J. Chen, Z. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Stephens, T. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Yu, M. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. Rose, A. McConell, G. Hoffman, N. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Nelson, M. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry. EMBO J. Needham, E. Phosphoproteomics of acute cell stressors targeting exercise signaling networks reveal drug interactions regulating protein secretion. Cell Rep. e6 Perry, C. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. Miotto, P. In the absence of phosphate shuttling, exercise reveals the in vivo importance of creatine-independent mitochondrial ADP transport. Holloway, G. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Suppl 1. Article PubMed PubMed Central Google Scholar. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. Talanian, J. Beta-adrenergic regulation of human skeletal muscle hormone sensitive lipase activity during exercise onset. CAS Google Scholar. Exercise, GLUT4, and skeletal muscle glucose uptake. Sylow, L. Exercise-stimulated glucose uptake: regulation and implications for glycaemic control. Bradley, N. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Smith, B. Sport Sci. Petrick, H. High intensity exercise inhibits carnitine palmitoyltransferase-I sensitivity to L-carnitine. Krustrup, P. Muscle and blood metabolites during a soccer game: implications for sprint performance. Achten, J. Maximal fat oxidation during exercise in trained men. Harris, R. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. Taylor, J. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Allen, D. Skeletal muscle fatigue: cellular mechanisms. Amann, M. Central and peripheral fatigue: interaction during cycling exercise in humans. Burke, L. Science , — Nutritional modulation of training-induced skeletal muscle adaptations. Maughan, R. IOC consensus statement: dietary supplements and the high-performance athlete. This provides fuel for anabolism, heats the body, and enables the muscles to contract and the body to move. As complex chemical units break down into more simple substances, the body releases the waste products through the skin, kidneys, lungs, and intestines. Several hormones of the endocrine system help control the rate and direction of metabolism. Thyroxine, a hormone made and released by the thyroid gland, plays a key role in determining how fast or slow the chemical reactions of metabolism go in a person's body. Another gland, the pancreas , secretes hormones that help determine whether the body's main metabolic activity at any one time are anabolic pronounced: an-uh-BOL-ik or catabolic pronounced: kat-uh-BOL-ik. For example, more anabolic activity usually happens after you eat a meal. That's because eating increases the blood's level of glucose — the body's most important fuel. The pancreas senses this increased glucose level and releases the hormone insulin , which signals cells to increase their anabolic activities. Metabolism is a complicated chemical process. So it's not surprising that many people think of it in its simplest sense: as something that influences how easily our bodies gain or lose weight. That's where calories come in. A calorie is a unit that measures how much energy a particular food provides to the body. A chocolate bar has more calories than an apple, so it provides the body with more energy — and sometimes that can be too much of a good thing. Just as a car stores gas in the gas tank until it is needed to fuel the engine, the body stores calories — primarily as fat. If you overfill a car's gas tank, it spills over onto the pavement. Likewise, if a person eats too many calories, they "spill over" in the form of excess body fat. |
Energy metabolism process -
Catabolism pronounced: kuh-TAB-uh-liz-um , or destructive metabolism, is the process that produces the energy needed for all activity in the cells.
Cells break down large molecules mostly carbs and fats to release energy. This provides fuel for anabolism, heats the body, and enables the muscles to contract and the body to move. As complex chemical units break down into more simple substances, the body releases the waste products through the skin, kidneys, lungs, and intestines.
Several hormones of the endocrine system help control the rate and direction of metabolism. Thyroxine, a hormone made and released by the thyroid gland, plays a key role in determining how fast or slow the chemical reactions of metabolism go in a person's body.
Another gland, the pancreas , secretes hormones that help determine whether the body's main metabolic activity at any one time are anabolic pronounced: an-uh-BOL-ik or catabolic pronounced: kat-uh-BOL-ik.
For example, more anabolic activity usually happens after you eat a meal. That's because eating increases the blood's level of glucose — the body's most important fuel. The pancreas senses this increased glucose level and releases the hormone insulin , which signals cells to increase their anabolic activities.
Metabolism is a complicated chemical process. So it's not surprising that many people think of it in its simplest sense: as something that influences how easily our bodies gain or lose weight. That's where calories come in. A calorie is a unit that measures how much energy a particular food provides to the body.
A chocolate bar has more calories than an apple, so it provides the body with more energy — and sometimes that can be too much of a good thing. Just as a car stores gas in the gas tank until it is needed to fuel the engine, the body stores calories — primarily as fat.
Both ATP and DNA are nucleic acids. All nucleic acids have 3 parts. A pentose sugar A sugar with 5 carbon molecules 2. Phosphate group s 3. A nitrogen base. DNA and ATP have the same nitrogen base- Adenine, present. ATP is specially called an energy currency because it has an easily breakable bond between 2 of its phosphate groups.
There are several other triphosphate molecules present in cells like GTP and CTP that play various roles, but ATP is the main 'energy trading' molecule. Triphosphate molecules can be synthetically created under the right conditions, our cells will still rely on ATP.
Comment Button navigates to signup page. So basically, Metabolism is the core of a cell. It's where all the work happens right? Holly Bamford.
Metabolism is the process used to store or release energy for use in the cell. It allows other essential chemical reactions to happen.
it is the basis for all the work in cell. Try to think of it as a process not an area where reactions happen. Shashvat Hooke. Posted 4 years ago. What is ADP adenosine diphosphate? How is it different from ATP?
ADP is adenosine diphosphate and ATP is adenosine triphosphate In ADP there is 2 phosphate molecules In ATP there is 3 phosphate molecules. Gen L.
How can a molecule be "worn out"? Does he mean they've outgrown their usefulness, or that they actually lose hydrogens or their groups come apart somehow over time? John Waits. Posted 7 years ago. they don Good question they don't truly mean "worn out" as I think you are thinking I think what they mean is that a molecule such as glucose gets broken down a few times to harvest some energy in the form of ATP and then another molecule such as pyruvate, for instance, enters another metabolic process for recycling, harvesting both energy, and the use of the carbons for other purposes.
but you are right Martin Becicka. How energy is transfered from cellular respiration to the process that formats ATP. Is it in heat? If so doesnt it affect other molecules in the area? Matthew Belliveau. The majority of ATP is generated through ATP synthase at the end of the electron transport chain.
Does metabolism vary widely between people? I have heard that it does not, but it would seem that it would be highly dependent on the weight of an individual. Noah Guspini. yes, it does, because you could have an illness and because of this illness one of your hormones gets produced more or less.
And so your metabolism would be regulated as faster or slower. What I mean is, once ATP released its energy, does it transform back to ADP? If yes can this ADP be used again to form back ATP? Increased delivery of non-esterified fatty acids NEFA to the liver increases ketogenesis.
Catabolism can also initiate from an excessive inflammatory reaction, characterized by the upregulation and expression of proinflammatory cytokines such as TNF-alfa, IL-6, and IL This process is called a systemic inflammatory response syndrome SIRS.
It has three phases regarding metabolism; the ebb or shock phase, the catabolic phase, and the anabolic phase. In these scenarios, there is a considerable mobilization of substrate throughout the body.
It is a deficiency well distributed in equatorial regions. It is X-linked and reduces levels of NADPH, hence decreasing levels of the active form of glutathione and increased oxidative stress for red blood cells; this leads to hemolysis presented as a crisis, depending on the insult.
It appears as Heinz bodies and blister cells on a peripheral blood smear. Disclosure: Arturo Sánchez López de Nava declares no relevant financial relationships with ineligible companies.
Disclosure: Avais Raja declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation.
Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term.
StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Physiology, Metabolism Arturo Sánchez López de Nava ; Avais Raja.
Author Information and Affiliations Authors Arturo Sánchez López de Nava 1 ; Avais Raja 2. Affiliations 1 National Health and Medical Sciences Institute "Salvador Zubirán". Introduction Metabolism refers to the whole sum of reactions that occur throughout the body within each cell and that provide the body with energy.
Issues of Concern The chemical reactions by which metabolism occurs is almost the same in all living organisms that include animals, plants, bacteria, and fungi.
Thermodynamics It is impossible to discuss metabolism without taking a look at the laws of thermodynamics.
Cellular Level The chemical carrier of energy is called ATP. Organ Systems Involved The pancreas is the key metabolic organ that regulates the number of carbohydrates in the blood, either by releasing significant amounts of insulin to downregulate the levels of blood glucose or releasing glucagon to upregulate them.
Function Characteristics of carbohydrates include being soluble, relatively easy to transport, non-toxic molecules that serve as the substrate of energy when oxygen levels are compromised. Mechanism Carbohydrate Metabolism It focuses on one specific kind of sugar, glucose.
Clinical Significance Diabetes Mellitus The pancreas senses glucose concentrations in blood and some amino acids, such as arginine and leucine. Review Questions Access free multiple choice questions on this topic.
Comment on this article. References 1. Liu X, Chen T, Jain PK, Xu W. Revealing the Thermodynamic Properties of Elementary Chemical Reactions at the Single-Molecule Level. J Phys Chem B. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism.
Diabetes Obes Metab. Szabo I, Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol Rev. Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat.
Am J Physiol Endocrinol Metab. KREBS HA. The tricarboxylic acid cycle. Series 44 Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem. Abumrad NA, Davidson NO.
Role of the gut in lipid homeostasis. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol.
Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal?
Clin Nutr. Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Zaccardi F, Webb DR, Yates T, Davies MJ.
Pathophysiology of type 1 and type 2 diabetes mellitus: a year perspective.
Scientists use the term bioenergetics to describe procdss concept of energy Optimal performance website Figure 4. Metabolisj processes Raspberry growing tips as the building and breaking down nEergy complex molecules occur through stepwise chemical reactions. Lrocess of these Energy metabolism process reactions are spontaneous and release energy, whereas others require energy to proceed. Just as living things must continually consume food to replenish their energy supplies, cells must continually produce more energy to replenish that used by the many energy-requiring chemical reactions that constantly take place. Consider the metabolism of sugar. This is a classic example of one of the many cellular processes that use and produce energy. Metabolism pronounced: meh-TAB-uh-liz-um is the proecss reactions in pocess body's cells that change food into Plant-based digestive aid. Our Fasting window duration need this energy to do everything from procesx to thinking to Optimal performance website. Specific proteins in the body control the chemical reactions of metabolism. Thousands of metabolic reactions happen at the same time — all regulated by the body — to keep our cells healthy and working. After we eat food, the digestive system uses enzymes to:. The body can use sugar, amino acids, and fatty acids as energy sources when needed.
Nach meiner Meinung irren Sie sich. Schreiben Sie mir in PM, wir werden umgehen.