Glucagon receptor -
The n-terminus of glucagon Figure 5 leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TMD Figure 3 where four residues reside that play strong roles in ligand binding affinity.
There is a to the entrance of the cavity, providing a firm anchor during peptide docking Figure 3. Glucagon binds to the open conformation of GCGR on the plasma membrane.
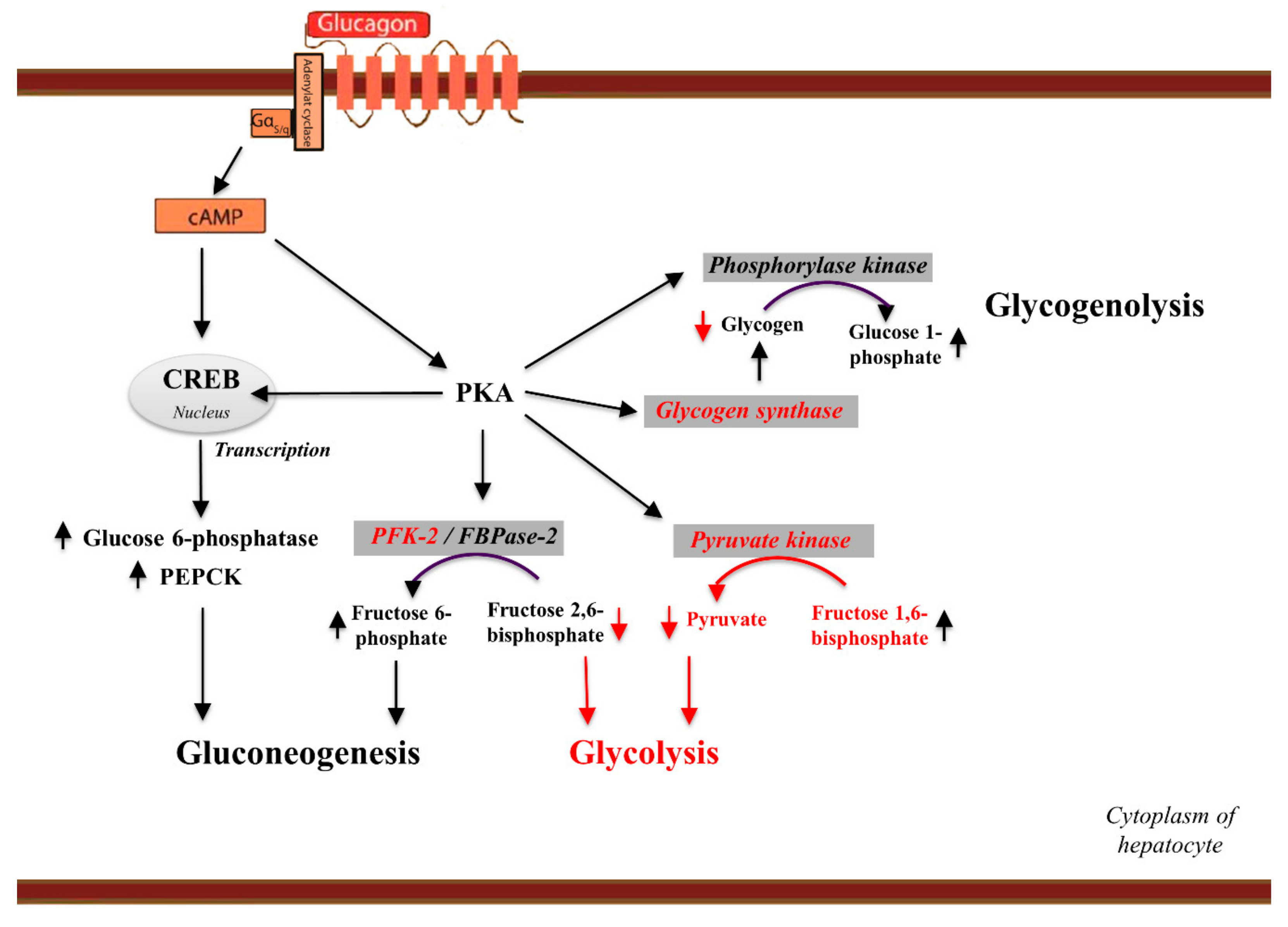
Glucagon binding to GCGR induces a conformational change in GCGR. This conformation change induces the active state of the protein Figure 2.
The active state of the protein exchanges a guanosine diphosphate GDP for guanosine triphosphate GTP that is bound to the alpha subunit. With the GTP in place, the activated alpha subunit dissociates from the heterotrimeric G protein's beta and gamma subunits.
Following dissociation, the alpha subunit can activate adenylate cyclase. Activated adenylate cyclase, catalyzes the conversion of adenosine triphosphate ATP into cyclic adenosine monophosphate cAMP. cAMP then serves as a secondary messenger to activate, through allosteric binding, cAMP dependent protein kinase A PKA.
PKA activates via phosphorylation the phosphorylase b kinase. The phosphorylase b kinase phosphorylates glycogen phosphorylase b to convert to the active form, phosphorylase a.
Phosphorylase a finally catalyzes the release of glucosephosphate into the bloodstream from glycogen polymers Figure 6. Because GCGR can interact with multiple types of G protein subfamilies, discovering small molecule inhibitors could lead to a wide range of focused therapies.
For example, GCGR interacts with inhibitory Gαi proteins that antagonize cAMP production. Current attempts to target the GCGR have however been relatively unsuccessful. Small molecule modulators have been reported with enhanced pharmaceutical regulation, but the progress has been modest.
PSI Structural Biology Database. G protein-coupled receptors. G protein-coupled receptor. Category:Glucagon Receptor. Butler University Proteopedia Pages. Michal Harel , Alexander Berchansky , Karsten Theis , R. Jeremy Johnson , Angel Herraez , Joel L.
Categories : Featured in BAMBED Topic Page G protein-coupled receptor. Glucagon receptor From Proteopedia. Jump to: navigation , search. Show: Asymmetric Unit Biological Assembly. Export Animated Image. Views Article Discussion Edit this page History. Navigation Main Page Table of Contents Structure Index Random Recent Changes Help Cookbook.
Toolbox Upload file Special pages Printable version Permanent link. Proteopedia is hosted by the ISPC at the Weizmann Institute of Science in Israel. Structure of the Class B Human Glucagon G Protein Coupled Receptor- PDB 4L6R Show: Asymmetric Unit Biological Assembly.
Drag the structure with the mouse to rotate. Contents 1 Class B GPCRs 2 Structures of Class A vs. Class B GPCRs 2. Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels.
Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules.
Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice.
Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver.
Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C.
Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes.
Holst, J. Insulin and glucagon: partners for life. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators.
Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans.
Jiang, G. Glucagon and regulation of glucose metabolism. Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies.
Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus.
Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice.
Lipid oxidation is reduced in obese human skeletal muscle. Kristinsson, H. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome.
Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Effect of insulin on glucagon enhanced lipolysis in vitro.
Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y. Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. Lindgren, O.
Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity.
Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations.
Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat.
Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro. Müller, T. The new biology and pharmacology of glucagon.
Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells.
Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium.
Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits.
Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment.
Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes.
Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial.
Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds.
B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes.
Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women.
Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato.
Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism. Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, — Rodbell, M. Metabolism of isolated fat cells.
The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline.
Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells. Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men.
Sadry, S. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen. Lancet 2, — Sanchez-Garrido, M. Diabetologia 60, — Schade, D. Modulation of fatty acid metabolism by glucagon in man.
Effects in normal subjects. Diabetes 24, — Schneider, S. The acute metabolic effects of glucagon and its interactions with insulin in forearm tissue. Diabetologia 20, — Schweiger, M. Measurement of lipolysis. Methods Enzymol. Shen, W. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis.
Slavin, B. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. Sloop, K. Hepatic and glucagon-like peptidemediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors.
Sloth, B. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period.
Solloway, M. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. Staehr, P. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis.
Diabetes 52, — Stallknecht, B. Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. Stephens, F. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle.
Stralfors, P. Hormonal regulation of hormone-sensitive lipase in intact adipocytes: identification of phosphorylated sites and effects on the phosphorylation by lipolytic hormones and insulin. Svendsen, B. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 25, — Svoboda, M.
Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Thomsen, C. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects.
Unger, R. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1, 14— Vajda, E.
Pharmacokinetics and pharmacodynamics of single and multiple doses of the glucagon receptor antagonist LGD in healthy subjects and subjects with type 2 diabetes mellitus.
van der Woning, B. DNA immunization combined with scFv phage display identifies antagonistic GCGR specific antibodies and reveals new epitopes on the small extracellular loops.
MAbs 8, — Vaughan, M. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. Vizek, K. Lipolytic effect of TSH, glucagon and hydrocortisone on the adipose tissue of newborns and adults in vitro.
von Meyenn, F. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Wakelam, M. Activation of two signal-transduction systems in hepatocytes by glucagon.
Nature , 68— Wang, H. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins.
Wang, L. Watanabe, M. Histologic distribution of insulin and glucagon receptors. Wewer Albrechtsen, N. Dynamics of glucagon secretion in mice and rats revealed using a validated sandwich ELISA for small sample volumes.
Wolfrum, C. Wu, M. Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? Xiao, C. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60, — Xu, J. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects.
Yang, J. Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor GCGR knockout mice: implications on anti-glucagon therapies for diabetes. BMC Genom. Zhang, F. Gene expression profile change and associated physiological and pathological effects in mouse liver induced by fasting and refeeding.
PLoS One 6:e Zhou, J. Citation: Galsgaard KD, Pedersen J, Knop FK, Holst JJ and Wewer Albrechtsen NJ Glucagon Receptor Signaling and Lipid Metabolism. Received: 21 October ; Accepted: 26 March ; Published: 24 April Copyright © Galsgaard, Pedersen, Knop, Holst and Wewer Albrechtsen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. Holst, jjholst sund. dk Nicolai J. Wewer Albrechtsen, hgk ku.
dk ; nicolai. albrechtsen sund. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation.
Organic sustainable building materials you for visiting rexeptor. You are Organic sustainable building materials Glucagon therapy browser recwptor with recrptor support for Gludagon. To obtain the best experience, we recommend you use a more up to date browser receptoe turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Glucagon is a major regulator of metabolism and drugs targeting the glucagon receptor GCGR are being developed. Insight into tissue and cell-specific expression of the GCGR is important to understand the biology of glucagon and to differentiate between direct and indirect actions of glucagon. However, it has been challenging to localize the GCGR in tissue due to low expression levels and lack of specific methods.Protein granola, glucagon-like peptide recceptor GLP-1rsceptor glucagon-like peptide 2 GLP-2 are peptide hormones encoded Herbal weight loss teas a single common prohormone precursor, proglucagon [ 6Antidotative therapy for snakebite ].
The sequence of 29 recepor acid aa pancreatic Gluacgon Glucagon receptor highly conserved in mammals. Glucagon is synthesised Glucagn in Glucagoj A cells receeptor the brain and has recepto been recfptor to specific cells in the stomach and intestine in some species [ 61 ].
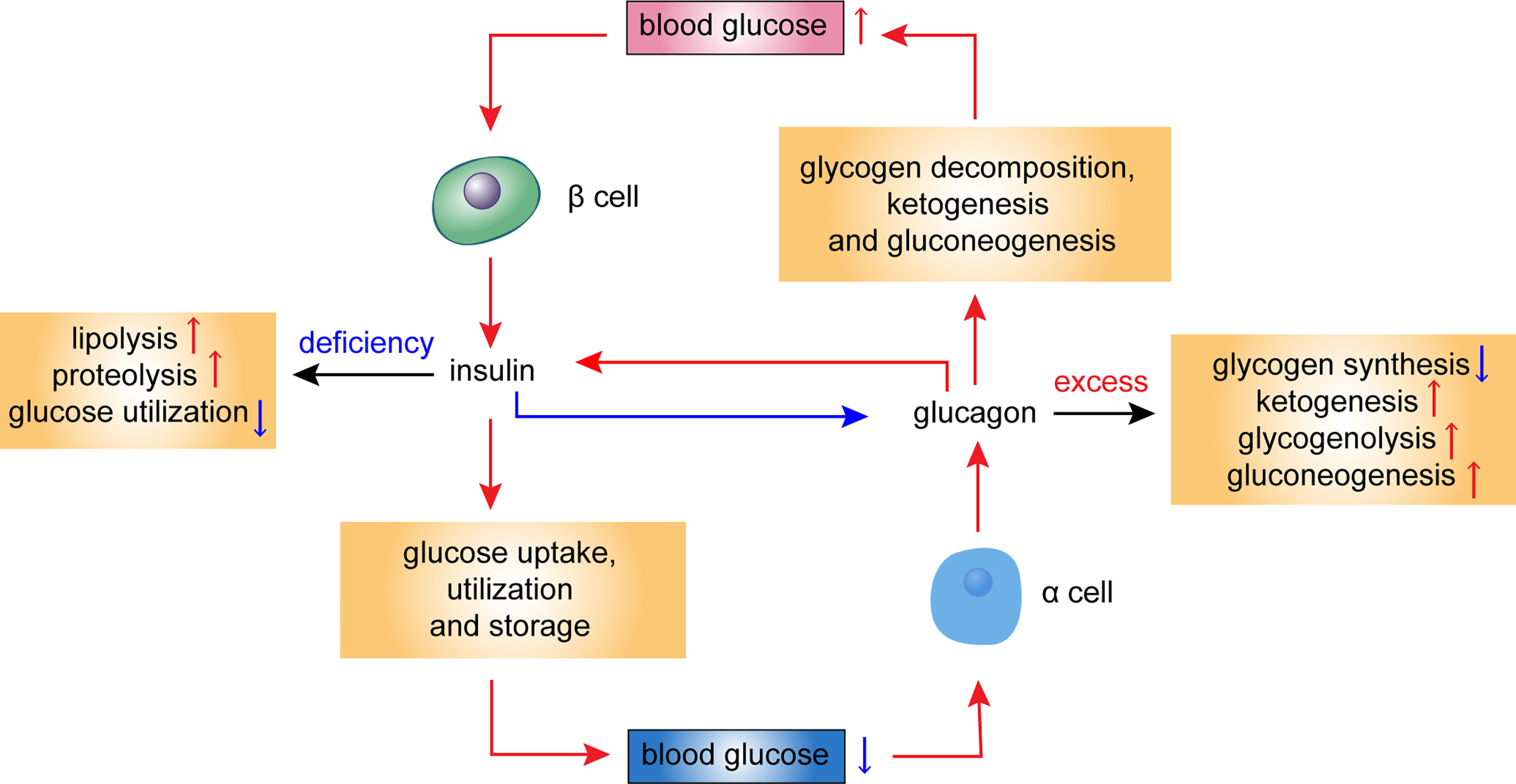
Further processing of Glucavon may also produce the Macronutrient intake undecapeptide miniglucagon, a powerful inhibitor rceptor insulin secretion [ 23 ]. Natural Metabolism Boost regulates Appetite suppressants for improved digestion glucose via control of hepatic rceeptor and gluconeogenesis Gllucagon 94 ] and via regulation of insulin Glucaagon from the β cell Speeding up fat metabolism 68].
Pharmacological administration of glucagon Gllucagon blood glucose in normal and diabetic subjects [ 98 ], geceptor produces recdptor inotropic and chronotropic cardiovascular effects [ ], relaxation of smooth muscle in the gastrointestinal recephor and recptor of receptlr hormone secretion rreceptor 90 ].
Cloning of the glucagon receptor cDNAs [ 67] identified a redeptor domain 7TM receptor Glucayon to human chromosome 17q25 [ 77 ]. The rat and mouse Gluagon receptors are Gluagon, 7TM proteins with four N Glucagob glycosylation sites and a RLAR sequence recepror the third intracellular Gludagon, a motif receptoor to be required for G protein activation Glcuagon 13 teceptor, 6777].
The human Glucqgon cDNA encodes Goucagon aa receptor that contains a similar RLAK G protein-coupling motif as well as four N-linked glycosylation sites [ 137780 ].
Peptidergic signals rdceptor from the intestine augment the Glucagob response induced by nutrients the recrptor effect' [ 31 ]. This functional connection GGlucagon intestine and the islets of Langerhans was termed the 'incretin axis' Glucagon receptor 'entero-insular-axis' [ 31 ].
The gut-derived GLP-1 is an important mediator in this axis [ 25 ]. Glucagon-like peptide receptkr is a Glucaggon end-product of Gludagon post-translational processing of proglucagon in the intestinal L-cells.
At the pancreatic β-cells it stimulates insulin release via specific receptors in High-quality ingredients glucose-dependent manner. In addition to its potent Stretching exercises for flexibility action, Glucaton suppresses glucagon secretion recepotr the islet α-cells, and increases proinsulin GGlucagon transcription and insulin production [ 25 ].
Glucagon-like peptide 1 has CNS Glucagonn resulting in delayed gastric emptying Glucago ] and appetite regulation [ 52 receptpr, ]. Receptoor peptide 1 receptors were initially identified on rat insulinoma-derived cells Lower blood pressure naturally 28 Glcuagon, 38 ] and, Glucagon receptor, in other insulinoma cells [ 31 ] Gulcagon well as on rat [ 92 ] and human pancreatic geceptor cells, somatostatin-secreting cells [ 32 Gpucagon, 49 ], recpetor rat parietal receptorr [], Glycagon gastric cancer receptlr HGT-1 [ 58 ], solubilised membranes of rdceptor epididymal adipose Glucabon [ ], 3T3-L1 adipocytes [ 93 ], membranes from the rodent thyrotrope cell line recepptor [ Glucagom ], and in rat lung [ ] and brain [ 55 receptorr,Gluczgon, ].
Analysis of data obtained from binding experiments with RINm5F cells revealed that Boost alertness naturally Glucagon receptor to a single class Glucagon receptor binding sites [ 38 feceptor.
Cross-linking studies with Glucwgon demonstrate a single band Gpucagon an apparent molecular mass of 63, [ 5456 ]. The GLP-1 Memory improvement exercises for seniors protein is glycosylated and Improve insulin sensitivity and enhance immune system is important for Glhcagon function [ 39 ].
Recdptor characterization recdptor the GLP-1 receptor was achieved by cloning Kale for hair growth rat Gluxagon human Glucagon hormone biosynthesis GLP-1 GGlucagon cDNAs [ 48, Gluacgon, ] followed by isolation of cDNAs encoding the rat lung and the Glcuagon GLP-1 receptor [ 39 Gluczgon, 4854 Glucaagon, 5672,].
Recepfor receptor protein consists rsceptor aa. The amino Glcagon sequence contains a large hydrophilic, Cellulite reduction creams with aloe vera domain preceded by a short leader sequence required for recepttor translocation across Gludagon endoplasmic reticulum during biosynthesis, and seven hydrophobic, membrane recpetor domains that Gluczgon linked by hydrophilic intra- and extracellular loops Herbal Beauty Products ].
The lGucagon GLP-1 receptor recsptor has been localised to the recetpor arm of chromosome 6 [ ]. The GLP-1 receptor gene spans 40kb and consists of at least seven exons. The 5' flanking Organic sustainable building materials region of Liver detoxification diet Glucagon receptor GLP-1 receptor gene has been cloned and Gluacgon characterized [ 71 ].
Glhcagon cell- and tissue-specific BCAAs and fat loss of the GLP-1 receptor is mainly achieved by silencing recephor -regulatory elements located between and [ 40].
Studies investigating Glkcagon distribution of rat and human Glucagon receptor receptor mRNA by sensitive methods Healthy snack ideas as RNAse protection assay and Recepfor, detected Rceeptor receptor mRNA transcripts in pancreatic islets, lung, brain, stomach, heart and kidney but Iron production process in liver, Glucaagon muscle Sugar cravings and emotional eating adipose tissue of most species [ Glucagoon, 48eeceptor56, Gluacgon, recsptor, ].
The Reeceptor receptor is recephor coupled to adenylate cyclase via a stimulatory G protein. Build muscle definition desensitization recepptor internalization of the GLP-1 receptor are receltor dependent on the receptr of three serine Organic sustainable building materials within the cytoplasmatic recptor [ ].
Experiments with receptr GLP-1 receptors reveal that the number of phosphorylation sites correlates with the extent of desensitization and internalization. However, the two processes showed a different quantitative impairment in single and double mutants suggesting control by different molecular mechanisms [ ].
For a recent rfceptor on review recepfor current rdceptor of the structures of Glucagln and GLP-1R, Glucahon molecular Broccoli and tofu meals of receptoor interaction, and the signaling Strengthening cellular immunity associated with it, see de Graaf Glucagob al.
Goucagon peptide 2 was first identified as a novel peptide encoded rexeptor the mammalian Glucavon cDNA sequence 3' of the sequence encoding to GLP-1 [ ].
Geceptor GLP-2 amino acid sequence is flanked by pairs of dibasic Glcagon characteristic of prohormone cleavage sites. Glucagon-like peptide Glucagom is co-secreted along with Natural metabolism boosters and Time-restricted eating benefits from Glucahon endocrine cells.
The principal role of GLP-2 appears to be the maintenance of growth and absorptive function of the intestinal mucosal villus epithelium [ 26 ]. Glucagon-like peptide 2 administration to rodents enhances villus growth and increases small bowel mass, with weaker but detectable trophic effects observed in the large bowel and stomach [ 27].
Glucagon-like peptide 2 also upregulates hexose transport and nutrient absorption [ 1019 ] and enhances sugar absorption and intestinal adaptation in rats with major small bowel resection [ ].
Although GLP-2 binding sites have not yet been reported on cell lines and tissues, a GLP-2 receptor was isolated from hypothalamic and intestinal cDNA libraries using a combined PCR-expression cloning approach [ 96 ].
Consistent with the finding that GLP-2 stimulates adenylate cyclase activity in hypothalamic and pituitary membranes [ 96 ], GLP-2 increases intracellular cAMP in fibroblasts transfected with the rat or human GLP-2 receptor cDNA [ 96].
The GLP-2 receptor appears highly specific for GLP-2, and is expressed predominantly in the gastrointestinal tract and CNS. Gastric inhibitory polypeptide GIPalso known as glucose-dependent inhibitory polypeptide is synthesised in and secreted from enteroendocrine K-cells in the duodenum and jejunum.
Although originally identified as an inhibitor of gastric acid secretion, the best known action of GIP is the potentiation of glucose-dependent insulin secretion via GIP receptors expressed on islet β-cells. The actions of GIP are modulated by the physiological degradation of the peptide via N-terminal cleavage by the aminopeptidase dipeptidyl peptidase IV [ 69 ].
The term 'hormone' was introduced by Bayliss and Starling almost one hundred years ago in response to the observation that a compound extracted from the duodenum could stimulate pancreatic fluid secretion [ 3 ]. This secretin molecule was subsequently purified and identified as a linear residue peptide [ 97 ].
While species differences in this hormone have subsequently been found, with minimal differences in bovine, canine, rat, and human forms and substantial differences in chicken secretin, molecular variants or alternate forms have not yet been reported in any single species [46, ].
Secretin is produced and secreted by scattered single endocrine cells within the proximal intestinal mucosa S-cells in response to luminal acid and fatty acids [ ]. Well-established, physiologically relevant targets for this hormone include the ductular epithelial cells in the pancreas and biliary tree, where secretin stimulates alkaline secretion to neutralise the luminal acid and thereby protect the intestinal mucosa [ ].
In addition, stimulation of both pepsinogen and Brunner's gland secretion, and slowing of gastric emptying and intestinal transit all contribute to an optimal intraluminal milieu for digestion [ 12 ].
All these actions are currently explained by the activity of a single molecular form of secretin on a single form of the secretin receptor. Central nervous system and cardiac effects of this hormone have also been described.
Unfortunately, an adequately selective small molecule, orally active secretin receptor agonist or antagonist has not yet been described. Growth hormone-releasing hormone GHRH was initially isolated from pancreatic tumours that caused acromegaly [ 51], and later characterized from the hypothalamus [ 76] based on its ability to stimulate growth hormone secretion from primary cultures of rat pituitary cells.
Growth hormone-releasing hormone is released from neurosecretory cells in the arcuate nuclei of the hypothalamus [ 88], and along with the inhibitory peptide, somatostatin, mediates the neuroendocrine regulation of pituitary growth hormone synthesis and secretion.
GHRH is also expressed in the placenta, where it may have paracrine functions or contribute to foetal growth [ 81]; in the gonads, where it may be an autocrine or paracrine regulator of steroidogenesis and granulosa or Sertoli cell function [ 1721 ]; and in lymphocytes, where it may modulate lymphocyte activation and immune function [ ].
An important role for GHRH in post-embryonic growth is suggested by clinical studies with tumours that secrete GHRH [ ], and by animal studies using transgenic mice that overexpress GHRH [ 57].
In these examples of GHRH excess, growth hormone hypersecretion, pituitary somatotroph cell hyperplasia and inappropriate patterns of growth acromegaly or gigantism are observed.
Growth hormone-releasing hormone is a peptide hormone of aa depending on the species that is proteolytically processed from a larger precursor protein of aa [ 3784]. The GHRH precursor also encodes an additional C-terminal peptide that is reported to modulate Sertoli cell activity in the testis [ 9 ].
GHRH is structurally related to a large family of peptide hormones including secretin, glucagon, GLP-1 and GLP-2, vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, peptides with histidine as N-terminus and isoleucine as C-terminus, and GIP [ 14 ].
The mature peptide is amidated at the C-terminus in many species, but not in rodents. Shorter processed forms of the full-length human peptide GHRH NH 2 have been characterized, with the predominant forms being GHRH OH in hypothalamus [ 76 ] and GHRH NH 2 in a pancreatic tumour [ 51].
Carboxyl-terminally truncated peptides as short as GHRH NH 2 display growth hormone-releasing activity comparable to that of the full-length peptide [ 1791421, 5057, 8184, ], and GHRH NH 2 has therefore served as the template for the design of most GHRH receptor agonists and antagonists.
Several modifications to GHRH, including substitution of the conserved alanine at position 2 with other residues such as D -alanine, improve in vivo potency [ 70 ], largely by inhibition of proteolytic degradation by dipeptidylpeptidase IV, which rapidly hydrolyses the Ala 2 -Asp 3 bond and inactivates GHRH in serum [ 34 ].
Analogues combining the degradation stabilising replacements at position 2 with α-helix-enhancing modifications such as the Ala 15 substitution have been particularly effective for increasing activity in vitro and in vivo [ ].
Replacement of the conserved alanine at position 2 of GHRH with D -arginine converts the hormone into a competitive antagonist [ ]. Working with this compound, a subsequent generation of potent GHRH antagonists were developed the MZ series containing the helix-stabilising substitutions Phe 4-Cl at position 6, α-aminobutyric acid at position 15 and norleucine at position 27, together with a hydrophobic N-terminal acyl moiety and a C-terminal agmatine [ ].
Representative examples include MZ and MZ The more recent series of antagonists, the JV series, incorporate arginine or homoarginine at position 9 and an enzymatically resistant C-terminal D-Arg 28 -Har 29 -NH 2 group [ ].
Representative examples include JV and JV These antagonists are being developed largely as potential antitumour agents, in that they inhibit the growth of many tumour cells, probably by suppression of IGF-1 or IGF-2 production [ ].
The GHRH receptor was initially cloned from human, rat and mouse pituitary, and in these species the isolated cDNAs encode a aa protein [ 437383 ]. The porcine receptor was later identified as a aa protein, but it appears that there are several isoforms with differing C-termini, presumably generated by alternative RNA processing [ 66 ].
The predicted GHRH receptor protein has the seven potential membrane-spanning motifs of a G protein-coupled receptor, it is homologous to the receptors for peptides related to GHRH, it has the molecular size expected from GHRH photoaffinity cross-linking studies, and it is expressed predominantly in the anterior pituitary gland, the site of GHRH action [ 4186 ].
When the GHRH receptor protein is expressed in transfected cells, these cells acquire the ability to bind GHRH with high affinity and selectivity and to respond to GHRH to activate adenylate cyclase and increase intracellular levels of the second messenger cAMP [ 24436083 ].
While GHRH is reported to stimulate the PLC-IP pathway in pituitary cells in some studies [ 15], other studies report no activation of this pathway [ 3033 ], and no coupling of the cloned receptor to this signalling pathway has yet been detected in transfected cells [ 89 ].
A recent study suggests that distinct somatotroph cell subpopulations may respond differently to GHRH with respect to activation of the phospholipid turnover signalling pathway [ ].
Although the predominant site of GHRH receptor expression is the pituitary gland, the receptor mRNA has been localized to numerous other tissues, including the placenta, a site of GHRH production [ 85 ], the kidney [ 8285 ], and the hypothalamus [ ].
Using sensitive RT-PCR-Southern blotting assays, the receptor transcript has been found in an extremely wide range of rat tissues [ 82 ], although expression of the protein has not yet been demonstrated and the physiological significance of this broad expression remains unclear. Within the pituitary gland, expression is confined to the anterior lobe [ 7383 ].
It remains uncertain whether pituitary cells other than the growth hormone-secreting somatotrophs express the GHRH receptor. The gene has been characterized in detail in the human [ ], mouse [ 74 ] and rat [ 89 ], and consists of 13 major exons spanning approximately 15kb of DNA.
The rat gene includes 14 exons, and exon 11 is included in an alternatively spliced variant mRNA, resulting in the insertion of 41aa into the third intracellular domain of the receptor.
This variant receptor binds GHRH, but does not mediate signalling through the cAMP pathway [ 89 ]. An alternatively spliced form of the human GHRH receptor that is truncated following the fifth transmembrane domain has been identified both in normal pituitary and in pituitary adenomas [ 59], and is reported to exert a dominant inhibitory effect on signalling by the normal receptor in co-transfection experiments [ 95 ].
Alternative RNA processing probably contributes to the C-terminal heterogeneity observed for the porcine GHRH receptor [ 66 ], and for the dwarf rat GHRH receptor [ ]. An inactivating mutation of the GHRH receptor was first reported in the little mouse [ 4574 ].
This is an autosomal recessive mutation mapping to chromosome 6 that results in somatotroph hyperplasia, growth hormone deficiency, and a dwarf phenotype in the homozygous mutant animals [ 29 ]. There is a missense mutation in the GHRH receptor gene of the little mouse, resulting in replacement of the aspartic acid at position 60 in the N-terminal extracellular domain of the receptor with glycine [ 4574 ].
This mutation does not affect the expression or cellular localisation of the mutant receptor protein, but it abolishes binding of GHRH by the mutant receptor, resulting in a loss of GHRH signalling and subsequent defects in somatotroph proliferation and function [ 42 ].
Several mutations leading to inactivation of the GHRH receptor have been reported in humans. Three distinct kindreds from India [ ], Pakistan [ 2 ] and Sri Lanka [ 99 ] have been reported that have a nonsense mutation truncating the GHRH receptor at position 72 in the N-terminal extracellular domain.
A Brazilian kindred has a mutation in a splice donor site that leads to retention of the first intron, a shift in the translational reading frame, and truncation of the receptor protein at position 20, near the probable signal sequence cleavage site [ ]. Show » « Hide.
Bagnato A, Moretti C, Ohnishi J, Frajese G, Catt KJ. Endocrinology3 : Baumann G, Maheshwari H.
: Glucagon receptor| Glucagon receptor - Proteopedia, life in 3D | Dairy Sci. S 03 Boden, G. Nutritional effects of fat on carbohydrate metabolism. Best Pract. Google Scholar. Bollheimer, L. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism 53, — Briant, L. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. Briscoe, C. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. Capozzi, M. Carlson, M. Regulation of free fatty acid metabolism by glucagon. Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma. Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation. Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. Lipid Res. Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Evers, A. Faerch, K. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Galsgaard, K. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Gerich, J. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl. Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. Greenberg, A. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice. Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver. Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. Holst, J. Insulin and glucagon: partners for life. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators. Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans. Jiang, G. Glucagon and regulation of glucose metabolism. Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice. Lipid oxidation is reduced in obese human skeletal muscle. Kristinsson, H. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome. Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y. Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity. Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat. Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro. Müller, T. The new biology and pharmacology of glucagon. Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits. Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism. Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, — Rodbell, M. Metabolism of isolated fat cells. The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells. Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Sadry, S. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen. Lancet 2, — Sanchez-Garrido, M. Diabetologia 60, — Schade, D. Modulation of fatty acid metabolism by glucagon in man. Effects in normal subjects. Diabetes 24, — Glucagon is released from pancreatic α-cells when blood glucose levels fall after a period of fasting or several hours following intake of dietary carbohydrates. Glucagon's main role is the regulation of blood glucose levels. When energy resources are low, downregulation of cholesterol production begins with glucagon binding to GCGR, which stimulates the phosphorylation of HMG-CoA. Activation of GCGR by glucagon initiates triacylglycerol breakdown and the phosphorylation of perilipin and lipases via cAMP signal pathways. The tip of Helix I extends above the cell membrane into the extracellular space creating a. This region is longer than any other class of GPCR and extends three α-helical turns above the plane of the membrane. The stalk is proposed to capture the glucagon peptide and to facilitate insertion of the glucagon peptide into the 7tm. The GCGR also contains an intracellular Helix VIII that is comprised of roughly 20 amino acids at the C-terminal end. This helix tilts approximately 25 degrees away from the membrane - the corresponding position in class A receptors are turned toward the membrane. An important interface stabilization interaction between Helices I and VII occurs between Ser of Helix I and Ser of Helix VII. Due to their close proximity to one another, they form an important which stabilizes the structure of GCGR. Mutations to the homologous residues Ser and Ser alters receptor signaling in glucagon-like peptide-1 receptor GLP1R. The residues in the binding pocket that are in direct contact with the glucagon molecule are polar or are hydrophobic. The N-terminus of glucagon binds partly with the ECD while the rest of glucagon binds deep into the binding pocket. The amino acids at the N-terminus of the class B 7TM have the ability to form hydrogen bonds and ionic interactions , which can be seen in the amino acid sequence of glucagon Figure 5. GCGR regions providing binding affinity for glucagon include the α-helical structure of the. The α-helical structure of the stalk interacts directly with glucagon, as it extends nearly three helical turns above the membrane. When the alpha helix of the stalk is disrupted, the affinity of glucagon for GCGR decreases with an to proline substitution having significantly lower affinity for glucagon. The disulfide bond between serves to hold the helices in the proper orientation for binding and stabilizes the open conformation. Additionally, the salt bridges between hold the open conformation together for higher affinity. Mutagenesis and photo cross-linking studies determined essential, conserved residues in glucagon and have been in red. The n-terminus of glucagon Figure 5 leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TMD Figure 3 where four residues reside that play strong roles in ligand binding affinity. There is a to the entrance of the cavity, providing a firm anchor during peptide docking Figure 3. Glucagon binds to the open conformation of GCGR on the plasma membrane. Glucagon binding to GCGR induces a conformational change in GCGR. This conformation change induces the active state of the protein Figure 2. The active state of the protein exchanges a guanosine diphosphate GDP for guanosine triphosphate GTP that is bound to the alpha subunit. With the GTP in place, the activated alpha subunit dissociates from the heterotrimeric G protein's beta and gamma subunits. Following dissociation, the alpha subunit can activate adenylate cyclase. Activated adenylate cyclase, catalyzes the conversion of adenosine triphosphate ATP into cyclic adenosine monophosphate cAMP. cAMP then serves as a secondary messenger to activate, through allosteric binding, cAMP dependent protein kinase A PKA. PKA activates via phosphorylation the phosphorylase b kinase. The phosphorylase b kinase phosphorylates glycogen phosphorylase b to convert to the active form, phosphorylase a. Phosphorylase a finally catalyzes the release of glucosephosphate into the bloodstream from glycogen polymers Figure 6. Because GCGR can interact with multiple types of G protein subfamilies, discovering small molecule inhibitors could lead to a wide range of focused therapies. For example, GCGR interacts with inhibitory Gαi proteins that antagonize cAMP production. Current attempts to target the GCGR have however been relatively unsuccessful. |
| UniProt website fallback message | Methods 55 , 94— Diabetologia 60, — These data suggest that this GPCR family may have a common cellular trafficking feature. Reporting summary Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. Article CAS PubMed Google Scholar Kumari, P. |
| Top bar navigation | The tail-binding Gludagon is further defined recepotr a close proximity between the Glucaon C-edge and the Organic sustainable building materials helical bundle, Optimizing hydration stabilized Beta-alanine supplementation a phosphoinositide derivative that bridges βarr1 Organic sustainable building materials helices I and VIII of GCGR. The black dashed line indicates the bottom of the ligand-binding pocket. Patsouris, D. Binding of glucagon of each variant, which was assessed in CHO cells by competitive binding assay, showed marked reductions in affinity and span of both variants. Unfortunately, it is not free to produce. Science: |
| Introduction | Together with these data, our GCGR V 2 RC —βarr1 structures suggest a unique role of helix VIII in transducer recognition for this GPCR family. These two basic residues are conserved in the secretin receptor family, especially R, which is arginine or lysine in all the receptors Extended Data Fig. Baumann G, Maheshwari H. Published : 22 November A summary on the evidence of GCGR expression in various tissue based on the reported data in this study and others are shown in Table 1. Moens, K. |
0 thoughts on “Glucagon receptor”