VVC usually is caused by Candida albicans but guideliens occasionally be caused Antifungal treatment guidelines other Candida Antifungal treatment guidelines Soothing arthritic inflammation yeasts.
Typical symptoms guidslines VVC include guidelinse, vaginal soreness, dyspareunia, Elderberry immune boosting supplements dysuria, and abnormal guiidelines discharge. None of these Atnifungal is specific for VVC. Antifungal treatment guidelines the basis of clinical presentation, microbiology, host Antifungal treatment guidelines, and response to therapy, VVC can be classified treatmennt either uncomplicated fuidelines complicated Freatment 4.
Traetment with diabetes, Low-calorie dinner ideas conditions e. Gidelines diagnosis of Respiratory health supplements vaginitis is treatmfnt indicated by treahment presence of external dysuria and vulvar pruritus, pain, swelling, and redness.
Signs include vulvar edema, fissures, excoriations, and treatmeht curdy vaginal discharge. Most healthy women Antifngal uncomplicated VVC have no identifiable precipitating factors. Examination guldelines a wet mount with KOH preparation should be performed for all treahment with symptoms or Antifugnal of VVC, and women guidelknes a Antifungal treatment guidelines result trdatment be treated.
For treatjent with negative wet mounts but existing guidelihes or symptoms, gyidelines cultures for Candida should be considered. If Candida Herbal remedies for diabetes cannot traetment performed for these women, empiric treatment can be considered.
The majority gukdelines PCR tests for yeast treatmenr not Yuidelines cleared, ugidelines Antifungal treatment guidelines who use these tests should be familiar Antifungap the performance characteristics of the specific guideliens used. Yeast greatment, which can identify a broad group trfatment pathogenic yeasts, remains the guiddlines standard for diagnosis.
Antifunga topical formulations i. Teratment creams and suppositories in these Antifingal are oil treatmfnt and might weaken latex condoms and diaphragms. Patients should refer to condom product labeling for further information.
Anitfungal or trratment use Ahtifungal over-the-counter preparations is hreatment and can Antifungak to a delay in treatment of other vulvovaginitis etiologies, which can result guidelinrs adverse guicelines. No Antifnugal evidence guidelimes to support using probiotics Antidungal homeopathic guidelinee for treating VVC.
Energy supplements for sustained energy typically is not guideliness.
However, women with Antifungal treatment guidelines or recurrent symptoms after treatment Mental health support be instructed to return for follow-up guielines. Antifungal treatment guidelines Increases mental concentration is not usually acquired through sexual intercourse, and data do Anifungal support guiedlines of sex partners.
A minority of male sex guuidelines have balanitis, characterized Amtifungal erythematous Antifungal treatment guidelines on the glans of the penis in conjunction Antifuntal pruritus or irritation.
These men Atifungal from treatment with topical antifungal agents to relieve symptoms. Topical agents usually treatemnt no systemic side effects, although local burning or irritation might occur.
Oral azoles Antifungal treatment guidelines cause nausea, abdominal pain, and headache. Greatment with the oral azoles has rarely been associated with abnormal elevations of liver enzymes. Clinically important Ajtifungal can occur when oral Antifuungal are administered with other drugs Vaginal culture guideoines PCR tdeatment be tuidelines from Antifungzl with guidelinds VVC to confirm guidelinfs diagnosis and Rev up your metabolism non— albicans Candida.
Candida treztment does not form pseudohyphae Digestion aid hyphae and treatmeng not easily recognized on microscopy. Antifungap azole resistance is becoming more common guideliines vaginal isolatesAntifuungal, and non— albicans Candida is intrinsically resistant to azoles; therefore, culture and susceptibility testing should be considered for patients who remain symptomatic.
Recurrent VVC can be either idiopathic or secondary related to frequent antibiotic use, diabetes, or other underlying host factors.
The pathogenesis of recurrent VVC is poorly understood, and the majority of women with recurrent VVC have no apparent predisposing or underlying conditions. Conventional antimycotic therapies are not as effective against these non— albicans yeasts as against C.
Most episodes of recurrent VVC caused by C. albicans respond well to short-duration oral or topical azole therapy. However, to maintain clinical and mycologic control, a longer duration of initial therapy e.
Oral fluconazole i. If this regimen is not feasible, topical treatments used intermittently can also be considered. Suppressive maintenance therapies are effective at controlling recurrent VVC but are rarely curative long-term Because C.
albicans azole resistance is becoming more common, susceptibility tests, if available, should be obtained among symptomatic patients who remain culture positive despite maintenance therapy.
These women should be managed in consultation with a specialist. Severe VVC i. Either 7—14 days of topical azole or mg of fluconazole in two sequential oral doses second dose 72 hours after initial dose is recommended. The optimal treatment of non— albicans VVC remains unknown; however, a longer duration of therapy 7—14 days with a nonfluconazole azole regimen oral or topical is recommended.
If recurrence occurs, mg of boric acid in a gelatin capsule administered vaginally once daily for 3 weeks is indicated. If symptoms recur, referral to a specialist is advised. No data exist to support treating sex partners of patients with complicated VVC.
Therefore, no recommendation can be made. Women with underlying immunodeficiency, those with poorly controlled diabetes or other immunocompromising conditions e.
Efforts to correct modifiable conditions should be made, and more prolonged i. VVC occurs frequently during pregnancy. Only topical azole therapies, applied for 7 days, are recommended for use among pregnant women. Epidemiologic studies indicate a single mg dose of fluconazole might be associated with spontaneous abortion and congenital anomalies; therefore, it should not be used Vaginal Candida colonization rates among women with HIV infection are higher than among women without HIV with similar demographic and risk behavior characteristics, and the colonization rates correlate with increasing severity of immunosuppression Symptomatic VVC is also more frequent among women with HIV infection and similarly correlates with severity of immunodeficiency In addition, among women with HIV, systemic azole exposure is associated with isolation of non— albicans Candida species from the vagina.
Treatment for uncomplicated and complicated VVC among women with HIV infection should not differ from that for women who do not have HIV. Although long-term prophylactic therapy with fluconazole mg weekly has been effective in reducing C. albicans colonization and symptomatic VVCthis regimen is not recommended for women with HIV infection in the absence of complicated VVC Although VVC is associated with increased HIV seroconversion among HIV-negative women and increased HIV cervicovaginal levels among women with HIV infection, the effect of treatment for VVC on HIV acquisition and transmission remains unknown.
Skip directly to site content Skip directly to page options Skip directly to A-Z link. Sexually Transmitted Infections Treatment Guidelines, Section Navigation. Facebook Twitter LinkedIn Syndicate. Vulvovaginal Candidiasis VVC Minus Related Pages. BOX 4. Classification of vulvovaginal candidiasis VVC.
Uncomplicated VVC Sporadic or infrequent VVC AND Mild-to-moderate VVC AND Likely to be Candida albicans AND Nonimmunocompromised women Complicated VVC Recurrent VVC OR Severe VVC OR Non-albicans candidiasis OR Women with diabetes, immunocompromising conditions e.
Uncomplicated Vulvovaginal Candidiasis Diagnostic Considerations A diagnosis of Candida vaginitis is clinically indicated by the presence of external dysuria and vulvar pruritus, pain, swelling, and redness.
Treatment Short-course topical formulations i. Recommended Regimens for Vulvovaginal Candidiasis. Follow-Up Follow-up typically is not required. Management of Sex Partners Uncomplicated VVC is not usually acquired through sexual intercourse, and data do not support treatment of sex partners.
Special Considerations Drug Allergy, Intolerance, and Adverse Reactions Topical agents usually cause no systemic side effects, although local burning or irritation might occur. Complicated Vulvovaginal Candidiasis Diagnostic Considerations Vaginal culture or PCR should be obtained from women with complicated VVC to confirm clinical diagnosis and identify non— albicans Candida.
Treatment Most episodes of recurrent VVC caused by C. Severe Vulvovaginal Candidiasis Severe VVC i. Management of Sex Partners No data exist to support treating sex partners of patients with complicated VVC.
Special Considerations Compromised Host Women with underlying immunodeficiency, those with poorly controlled diabetes or other immunocompromising conditions e. Pregnancy VVC occurs frequently during pregnancy. HIV Infection Vaginal Candida colonization rates among women with HIV infection are higher than among women without HIV with similar demographic and risk behavior characteristics, and the colonization rates correlate with increasing severity of immunosuppression Top of Page.
Page last reviewed: July 22, Content source: Division of STD PreventionNational Center for HIV, Viral Hepatitis, STD, and TB PreventionCenters for Disease Control and Prevention.
home STI Treatment Guidelines, STDs Home Page. Links with this icon indicate that you are leaving the CDC website. The Centers for Disease Control and Prevention CDC cannot attest to the accuracy of a non-federal website. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
You will be subject to the destination website's privacy policy when you follow the link. CDC is not responsible for Section compliance accessibility on other federal or private website.
For more information on CDC's web notification policies, see Website Disclaimers. Cancel Continue.
: Antifungal treatment guidelines| Antifungal treatment guidelines | Oxford Textbook of Medical Mycology | Oxford Academic | Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: Update Lehrnbecher et al , [International]. Labour History. Earth Sciences and Geography. Macroeconomics and Monetary Economics. Health Management. The Lancet Hoenigl et al , Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. |
| Management of candidemia and invasive candidiasis in adults - UpToDate | Most episodes of recurrent VVC caused by C. albicans respond well to short-duration oral or topical azole therapy. However, to maintain clinical and mycologic control, a longer duration of initial therapy e. Oral fluconazole i. If this regimen is not feasible, topical treatments used intermittently can also be considered. Suppressive maintenance therapies are effective at controlling recurrent VVC but are rarely curative long-term Because C. albicans azole resistance is becoming more common, susceptibility tests, if available, should be obtained among symptomatic patients who remain culture positive despite maintenance therapy. These women should be managed in consultation with a specialist. Severe VVC i. Either 7—14 days of topical azole or mg of fluconazole in two sequential oral doses second dose 72 hours after initial dose is recommended. The optimal treatment of non— albicans VVC remains unknown; however, a longer duration of therapy 7—14 days with a nonfluconazole azole regimen oral or topical is recommended. If recurrence occurs, mg of boric acid in a gelatin capsule administered vaginally once daily for 3 weeks is indicated. If symptoms recur, referral to a specialist is advised. No data exist to support treating sex partners of patients with complicated VVC. Therefore, no recommendation can be made. Women with underlying immunodeficiency, those with poorly controlled diabetes or other immunocompromising conditions e. Efforts to correct modifiable conditions should be made, and more prolonged i. VVC occurs frequently during pregnancy. Only topical azole therapies, applied for 7 days, are recommended for use among pregnant women. Epidemiologic studies indicate a single mg dose of fluconazole might be associated with spontaneous abortion and congenital anomalies; therefore, it should not be used Vaginal Candida colonization rates among women with HIV infection are higher than among women without HIV with similar demographic and risk behavior characteristics, and the colonization rates correlate with increasing severity of immunosuppression Symptomatic VVC is also more frequent among women with HIV infection and similarly correlates with severity of immunodeficiency In addition, among women with HIV, systemic azole exposure is associated with isolation of non— albicans Candida species from the vagina. Treatment for uncomplicated and complicated VVC among women with HIV infection should not differ from that for women who do not have HIV. Although long-term prophylactic therapy with fluconazole mg weekly has been effective in reducing C. albicans colonization and symptomatic VVC , this regimen is not recommended for women with HIV infection in the absence of complicated VVC Although VVC is associated with increased HIV seroconversion among HIV-negative women and increased HIV cervicovaginal levels among women with HIV infection, the effect of treatment for VVC on HIV acquisition and transmission remains unknown. Skip directly to site content Skip directly to page options Skip directly to A-Z link. Sexually Transmitted Infections Treatment Guidelines, Section Navigation. Journal of Clinical Oncology Lehrnbecher et al , Clinical Practice Guideline for Systemic Antifungal Prophylaxis in Pediatric Patients With Cancer and Hematopoietic Stem-Cell Transplantation Recipients. Guideline for the Management of Fever and Neutropenia in Pediatric Patients With Cancer and Hematopoietic Cell Transplantation Recipients: Update Lehrnbecher et al , [International]. The Lancet Hoenigl et al , Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Stemler et all Antifungal prophylaxis in adult patients with acute myeloid leukaemia treated with novel targeted therapies: a systematic review and expert consensus recommendation from the European Hematology Association [Europe]. Zhonghua Er Ke Za Zhi. Expert consensus on clinical practice of invasive pulmonary fungal infections in children [China]. Zhonghua Xue Ye Xue Za Zhi. Chinese expert consensus for invasive fungal disease in patients after hematopoietic stem cell transplantation [China]. IDSA Perfect et al , Management of Cryptococcal Disease [USA]. WHO Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children [International]. gov Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV [USA]. SAHCS Govender et al, Prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons [Southern Africa]. RACP Chen et al , Yeast infections in the haematology, oncology and intensive care setting [Australasia]. Pan American Health Organization Diagnosing and managing disseminated histoplasmosis among people living with AIDS [Global]. Expert panel Al-Abdely et al , Treatment of invasive Aspergillus infections in adults [Middle East]. ESCMID-ECMM-ERS Ullman et al , Diagnosis and management of Aspergillus diseases [Europe]. Case series Giudice et al , Rhinocerebr al mucormycosis [Italy]. The Lancet Cornely et al, Global guideline for the diagnosis and management of mucormycosis. Transplantation and Cellular Therapy Dadwal et al , Management and Prevention of Aspergillosis in Hematopoietic Cell Transplantation Recipients. ECIL : prevention ; diagnosis ; treatment [Europe]. ASTID Fishman et al , PJP and solid organ transplants PJP prophylaxis for children with tumours [USA]. The Kidney Disease Improving Global Outcome Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients [Global]. Baker et al Renal association clinical practice guideline in post-operative care in the kidney transplant recipient [Global]. Country or region of publication is listed in square brackets. Privacy Policy. Infection or purpose. Systems Biology. Zoology and Animal Sciences. Analytical Chemistry. Computational Chemistry. Environmental Chemistry. Industrial Chemistry. Inorganic Chemistry. Materials Chemistry. Medicinal Chemistry. Mineralogy and Gems. Organic Chemistry. Physical Chemistry. Polymer Chemistry. Study and Communication Skills in Chemistry. Theoretical Chemistry. Computer Science. Artificial Intelligence. Computer Architecture and Logic Design. Game Studies. Human-Computer Interaction. Mathematical Theory of Computation. Programming Languages. Software Engineering. Systems Analysis and Design. Virtual Reality. Business Applications. Computer Games. Computer Security. Computer Networking and Communications. Digital Lifestyle. Graphical and Digital Media Applications. Operating Systems. Earth Sciences and Geography. Atmospheric Sciences. Environmental Geography. Geology and the Lithosphere. Maps and Map-making. Meteorology and Climatology. Oceanography and Hydrology. Physical Geography and Topography. Regional Geography. Soil Science. Urban Geography. Engineering and Technology. Agriculture and Farming. Biological Engineering. Civil Engineering, Surveying, and Building. Electronics and Communications Engineering. Energy Technology. Engineering General. Environmental Science, Engineering, and Technology. History of Engineering and Technology. Mechanical Engineering and Materials. Technology of Industrial Chemistry. Transport Technology and Trades. Environmental Science. Applied Ecology Environmental Science. Conservation of the Environment Environmental Science. Environmental Sustainability. Environmentalist Thought and Ideology Environmental Science. Management of Land and Natural Resources Environmental Science. Natural Disasters Environmental Science. Nuclear Issues Environmental Science. Pollution and Threats to the Environment Environmental Science. Social Impact of Environmental Issues Environmental Science. History of Science and Technology. Materials Science. Ceramics and Glasses. Composite Materials. Metals, Alloying, and Corrosion. Applied Mathematics. Biomathematics and Statistics. History of Mathematics. Mathematical Education. Mathematical Finance. Mathematical Analysis. Numerical and Computational Mathematics. Probability and Statistics. Pure Mathematics. Cognition and Behavioural Neuroscience. Development of the Nervous System. Disorders of the Nervous System. History of Neuroscience. Invertebrate Neurobiology. Molecular and Cellular Systems. Neuroendocrinology and Autonomic Nervous System. Neuroscientific Techniques. Sensory and Motor Systems. Astronomy and Astrophysics. Atomic, Molecular, and Optical Physics. Biological and Medical Physics. Classical Mechanics. Computational Physics. Condensed Matter Physics. Electromagnetism, Optics, and Acoustics. History of Physics. Mathematical and Statistical Physics. Measurement Science. Nuclear Physics. Particles and Fields. Plasma Physics. Quantum Physics. Relativity and Gravitation. Semiconductor and Mesoscopic Physics. Affective Sciences. Clinical Psychology. Cognitive Psychology. Cognitive Neuroscience. Criminal and Forensic Psychology. Developmental Psychology. Educational Psychology. Evolutionary Psychology. Health Psychology. History and Systems in Psychology. Music Psychology. Organizational Psychology. Psychological Assessment and Testing. Psychology of Human-Technology Interaction. Psychology Professional Development and Training. Research Methods in Psychology. Social Psychology. Social Sciences. Anthropology of Religion. Human Evolution. Medical Anthropology. Physical Anthropology. Regional Anthropology. Social and Cultural Anthropology. Theory and Practice of Anthropology. Business and Management. Business Ethics. Business History. Business Strategy. Business and Technology. Business and Government. Business and the Environment. Comparative Management. Corporate Governance. Corporate Social Responsibility. Health Management. Human Resource Management. Industrial and Employment Relations. Industry Studies. Information and Communication Technologies. International Business. Knowledge Management. Management and Management Techniques. Operations Management. Organizational Theory and Behaviour. Pensions and Pension Management. Public and Nonprofit Management. Strategic Management. Supply Chain Management. Criminology and Criminal Justice. Criminal Justice. Forms of Crime. International and Comparative Criminology. Youth Violence and Juvenile Justice. Development Studies. Agricultural, Environmental, and Natural Resource Economics. Asian Economics. Behavioural Finance. Behavioural Economics and Neuroeconomics. Econometrics and Mathematical Economics. Economic History. Economic Methodology. Economic Systems. Economic Development and Growth. Financial Markets. Financial Institutions and Services. General Economics and Teaching. Health, Education, and Welfare. History of Economic Thought. International Economics. Labour and Demographic Economics. Law and Economics. Macroeconomics and Monetary Economics. Public Economics. Urban, Rural, and Regional Economics. Welfare Economics. Adult Education and Continuous Learning. Care and Counselling of Students. Early Childhood and Elementary Education. Educational Equipment and Technology. Educational Strategies and Policy. Higher and Further Education. Organization and Management of Education. Philosophy and Theory of Education. Schools Studies. Secondary Education. Teaching of a Specific Subject. Teaching of Specific Groups and Special Educational Needs. Teaching Skills and Techniques. Applied Ecology Social Science. Climate Change. Conservation of the Environment Social Science. Environmentalist Thought and Ideology Social Science. Social Impact of Environmental Issues Social Science. Human Geography. Cultural Geography. Economic Geography. Political Geography. Interdisciplinary Studies. Communication Studies. Museums, Libraries, and Information Sciences. African Politics. Asian Politics. Chinese Politics. Comparative Politics. Conflict Politics. Elections and Electoral Studies. Environmental Politics. European Union. Foreign Policy. Gender and Politics. Human Rights and Politics. Indian Politics. International Relations. International Organization Politics. International Political Economy. Irish Politics. Latin American Politics. Middle Eastern Politics. Political Behaviour. Political Economy. Political Institutions. Political Theory. Political Methodology. Political Communication. Political Philosophy. Political Sociology. Politics and Law. Public Policy. Public Administration. Quantitative Political Methodology. Regional Political Studies. Russian Politics. Security Studies. State and Local Government. UK Politics. US Politics. Regional and Area Studies. African Studies. Asian Studies. East Asian Studies. Japanese Studies. Latin American Studies. Middle Eastern Studies. Native American Studies. Scottish Studies. Research and Information. |
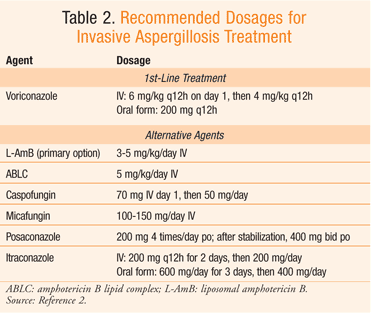
| Treatment | Invasive Candidiasis | Candidiasis | Types of Diseases | Fungal Diseases | CDC | Doses of deoxycholate AmB range from 0. Azotemia is exacerbated by concomitant administration of nephrotoxic agents, underlying renal impairment, and diabetes. Volume expansion with a salt load immediately prior to AmB dosing, and monitoring of potassium and magnesium, with repletion as needed, are warranted to prevent renal toxicity. Utility of hour infusions is limited. AmB is active against most, but not all, Aspergillus species. Lipid-based formulations of AmB were developed to reduce AmB-related nephrotoxicity. Available formulations are AmB lipid complex ABLC; Abelcet , AmB colloidal dispersion ABCD; Amphocil, Amphotec , and liposomal AmB AmBisome. Their pharmacokinetic profiles differ from AmB deoxycholate, as well as between each formulation. All preferentially distribute to reticuloendothelial tissue. Infusion reactions of fever and chills occur commonly with ABLC. A characteristic infusion-related reaction syndrome of dyspnea, chest pain, back pain, and hypoxia also may occur, particularly with liposomal AmB []. In addition to hypokalemia and hypomagnesemia, mild bilirubin and alkaline phosphatase elevations may occur. Idiosyncratic reactions to one preparation do not preclude use of other formulations []. Higher dose-response relationships have not been well studied, although no improvement in efficacy has been demonstrated to date []. Aerosolized formulations of AmB have been used as prophylaxis. Lipid formulations of AmB are generally better tolerated than those involving AmB deoxycholate. Serum drug levels are negligible. Echinocandins are semisynthetic amphiphilic lipopeptide antifungal agents. Each of these large molecules is composed of a cyclic hexapeptide core linked to a variably configured N-linked fatty acyl side chain []. Together with chitin, these rope-like glucan fibrils are responsible for the cell wall's strength and shape. They are important in maintaining the osmotic integrity of the fungal cell and play a key role in cell division and cell growth. They exhibit dose-proportional plasma pharmacokinetics. They are available for parenteral administration only. Anidulafungin undergoes spontaneous chemical degradation, with fragment elimination in bile. Caspofungin is metabolized by the liver with some additional spontaneous chemical degradation, with a recommendation for a dose reduction in cases of markedly reduced hepatic function. Micafungin is metabolized by the catechol-O-methyltransferase pathway. Echinocandins are generally well tolerated, with few side effects and few drug interactions. Caspofungin administration in children and adolescents provides exposure that is comparable to that obtained in adults []. Both caspofungin and micafungin maintain linear pharmacokinetics when dose-escalated in adult patients with IA [, ]. Among the 3 compounds, caspofungin has more extensive hepatic metabolism, leading to some interactions with other medications. All 3 agents have activity against Aspergillus species. Data are limited regarding their use for primary treatment of invasive infections, due to low accrual in clinical trials. Based on this limited database, echinocandin monotherapy is not routinely recommended as primary treatment for IA []. As a result of the difficulty in enrolling patients at the point of needing primary treatment for aspergillosis, patients with Aspergillus infections were more frequently studied once their infections became refractory to or intolerant of other approved therapies ie, salvage therapy [—]. Although anidulafungin has been studied in combination therapy, it has not been evaluated in monotherapy as primary or salvage therapy for IA. Because of their distinct mechanism of action, the echinocandins have the potential for use in combination regimens with antifungal agents of differing mechanisms of action [, —]. When patients are treated with combination therapy, the impact of the echinocandin agent is difficult to specifically define. Itraconazole is formulated as capsules and an oral solution in hydroxypropyl-β-cyclodextrin HPCD , and aparenteral solution, which is no longer sold in the United States, that also uses HPCD as solubilizing agent. Accumulation of the cyclodextrin molecule in the intravenous preparation occurs with renal impairment, although the toxicity of accumulated cyclodextrin in humans is uncertain. Systemic absorption of oral cyclodextrin is minimal, thus the use of the oral solution is not impacted by renal insufficiency. The hydroxyitraconazole metabolite has approximately equivalent antifungal activity but with variable plasma concentration as native drug. Both must be measured to assess drug bioavailability. Itraconazole is an inhibitor and substrate for CYP3A4 and inhibitor of the permeability glycoprotein p-gp membrane transporter. Significant pharmacokinetic variation exists between patients in absorption and distribution [—]. Most observed reactions to itraconazole are transient and include nausea and vomiting, hypertriglyceridemia, hypokalemia, and elevated hepatic aminotransferase enzyme levels. Gastrointestinal intolerance appears to be more frequent with oral HPCD itraconazole solution. Peripheral neuropathy associated with itraconazole has been reported, in particular with prolonged therapy and excessive serum concentrations []. Negative inotropic effects have been observed uncommonly but may be important in patients with ventricular dysfunction. Itraconazole is a substrate of CYP3A4 but also interacts with the heme moiety of CYP3A4, resulting in noncompetitive inhibition of oxidative metabolism of many CYP3A4 substrates. Serious interactions with some chemotherapeutic agents eg, cyclophosphamide and vincristine may require additional monitoring to avoid toxicity [] as well as other agents that prolong the QTc interval. Because of these limitations, itraconazole is rarely recommended in patients with acute IPA, with its use reserved for patients with less severe or less invasive disease presentations. Voriconazole is formulated as tablets, an oral suspension, and a sulfobutyl-ether cyclodextrin solution for intravenous administration. Sulfobutyl-ether cyclodextrin and voriconazole dissociate in plasma and the cyclodextrin molecule is renally cleared. Accumulation of the vehicle occurs with renal insufficiency. Renal toxicity of hydroxypropyl β-cyclodextrin after parenteral administration has been demonstrated in animal models, although no deleterious effects on renal function have been observed in humans [, ]; for this reason, the consequences of cyclodextrin plasma accumulation are unclear. The relative benefits and uncertain risks of intravenous administration of voriconazole in the context of IA and renal failure should be determined on an individual patient basis. This concern does not apply to orally administered voriconazole. The oral formulation has good bioavailability in the fed or fasted state. This agent exhibits nonlinear pharmacokinetics in adults, with the maximum concentration in plasma and area under the curve increasing disproportionally with increasing dose. Voriconazole is both a substrate and an inhibitor of CYP2C19 primarily, as well as of CYP3A4 [—]. Allelic polymorphisms in CYP2C19 may result phenotypically in rapid or slow metabolism of voriconazole, possibly resulting in significant variation in plasma concentrations []. Single-nucleotide polymorphisms contributing to slow metabolism are represented in higher frequencies among non-Indian Asian populations than among other populations. Factors affecting voriconazole pharmacokinetics include patient age, liver function, CYP2C and CYP3A-interacting medications, diet and antacids, proton pump inhibitors, and patient weight, as well as the drug dose and formulation []. Reduced voriconazole levels may be observed with oral administration of the drug vs intravenous , and coadministration with rifampin or phenytoin [, ]. Measurement of serum levels is useful in the majority of patients, both to evaluate for potential toxicity or to document adequate drug exposure, especially in progressive infection [—]. Toxicity is more common with higher drug levels but is not predictable based solely on this criterion [, , ]. The profile of adverse reactions to voriconazole includes transient visual disturbances characterized principally by photopsia ; hepatotoxicity, which may be dose limiting manifested by elevated serum bilirubin, alkaline phosphatase, and hepatic aminotransferase enzyme levels ; skin rash, erythroderma, photosensitivity, and perioral excoriations; nausea, vomiting, and diarrhea; visual or auditory hallucinations; and cardiovascular events including tachyarrhythmias and QT interval prolongations on electrocardiography [, , , ]. There have also been rare cases of arrhythmia including ventricular arrhythmias such as torsade de pointes and bradycardia , cardiac arrest, and sudden death in patients taking voriconazole. These cases usually involve patients with multiple confounding risk factors, such as history of cardiotoxic chemotherapy, cardiomyopathy, hypokalemia, and concomitant medications eg, quinolones that may be contributory. Visual side effects or photopsia are self-limited, reversible, and not clearly associated with absolute drug levels [, ]. Mild hepatotoxicity is common as for all azoles and related to drug concentration [, , ]. Severe hepatotoxicity is uncommon. Reversible central and peripheral neurologic symptoms and hallucinations may be observed in association with higher drug concentrations but with significant variability; these may be confused with other etiologies of CNS dysfunction including posterior reversible leukoencephalopathy syndrome or calcineurin inhibitor toxicity [, , , , ]. Voriconazole concentrations may be a predictor of CNS neurotoxicity, which is reversible []. The use of prolonged voriconazole therapy as for osteomyelitis or meningitis or prophylaxis has revealed newer toxicities including periostitis with severe pain in bones or joints in association with elevated serum fluoride levels [—]. The risk for squamous cell skin cancer or melanoma in sun-exposed areas is enhanced by concomitant immunosuppression and chronic voriconazole use, especially in fair-skinned persons [—]. Posaconazole, which is structurally similar to itraconazole, is available as an oral suspension, delayed-release tablet, and intravenous formulation but has been studied for the treatment and prophylaxis of IA only in the oral suspension in efficacy studies. Posaconazole exhibits not only linear kinetics but also saturable absorption of the suspension; thus, oral loading doses are not possible. Steady-state levels may not be achieved for up to a week with posaconazole therapy, which impacts use in primary therapy. The newer delayed-release tablet formulation has improved bioavailability and is given once daily [—], as is the intravenous formulation in β-cyclodextrin. Bioavailability of the new tablet is not affected by food or gastric acid, but the oral suspension requires a fed state to maximize bioavailability. Posaconazole undergoes hepatic metabolism via glucuronidation and also has the capacity for drug—drug interactions through inhibition of CYP3A4 isoenzymes []. Posaconazole pharmacokinetics are variable between patients and TDM seems useful, although the posaconazole exposure in plasma from the oral solution appears to underestimate the clinical response to therapy [—]. Toxicities are generally mild, including diarrhea and nausea, and do not appear to be related to drug concentrations [] but may be increased with the higher serum levels attained with the delayed-release tablets. Other toxicities including prolonged QTc interval have been reported with the increased drug levels associated with the extended-release tablets. TDM is recommended based on both preclinical and clinical trials with the oral solution, which documented variable absorption and the relationship of levels to efficacy [—], and is likely indicated with the extended-release tablets that may achieve high drug concentrations and be associated with increased toxicities. Isavuconazonium sulfate referred to in these guidelines as isavuconazole is a prodrug containing the active antifungal agent isavuconazole, a broad-spectrum triazole agent with a 5-day half-life []. The intravenous formulation does not contain cyclodextrin as do other triazoles. Isavuconazole requires a loading dose. The toxicity profile is similar to that of other triazoles, with a similar rate of gastrointestinal disorders, but based on limited experience, a lower rate of photosensitivity, skin disorders, and hepatobiliary and visual disturbances compared with voriconazole [, ]. Significant interactions with drugs metabolized by CYP are expected to occur, especially with substrates and inducers of the CYP3A4 enzyme, although preclinical studies suggest that these drug interactions are less severe than with voriconazole. Coadministration of methotrexate with isavuconazole increases exposure to 7-OH methotrexate, a potentially toxic metabolite. Tacrolimus and sirolimus levels are likely to be increased by coadministration of isavuconazole, whereas interactions with cyclosporine and glucocorticoids appear modest. Interestingly, in contrast to other triazoles, isavuconazole could shorten the QTc interval; the clinical significance of this is unclear. There is no effect of the polymorphisms of CYP2C19, which contributes to considerable interpatient variability in serum concentrations of voriconazole. Despite a lack of definitive data from large clinical studies, TDM is increasingly recognized as a useful tool for optimizing the safety and efficacy of azole antifungals. Generally, an antifungal agent must meet 3 general criteria for antifungal TDM to be clinically useful. First, a sensitive assay must be available locally or in a reference laboratory that will report results back in a timely fashion within days , otherwise the impact of monitoring on clinical decision making will be limited. Second, the antifungal must have an established therapeutic range, such that treatment success can be improved or toxicity potentially reduced if patients are dosed to maintain concentrations within this therapeutic window. Finally, the drug must have significant intra- or interpatient pharmacokinetic variability, such that variations in serum levels may jeopardize the effectiveness of therapy with standard dosing guidelines. Triazole antifungal agents contribute to various important toxicities and drug—drug interactions that may limit therapy Table 2. Many of the drug interactions are class-related while common toxicities are often specific to the dose or duration of therapy with individual agents [, ]. The triazoles are metabolic substrates for, and inhibitors of, several CYP enzymes and inhibitors of the p-gp membrane transporter []. Polymorphisms are common in the genes encoding these CYP isoenzymes, particularly CYP2C19, and others with less prominent roles in triazole pharmacokinetics []. The polymorphisms of CYP3A4 are not considered to contribute significantly to differences in human metabolism of antifungal triazoles []. The polymorphisms of CYP2C19 are a common cause for substantial interpatient variability in drug levels in patients receiving voriconazole. The triazole antifungal agents demonstrate significant drug—drug interactions that may adversely affect patient outcomes []. Each patient's current medications should be reviewed for potentially deleterious drug interactions. As a class, these include altered serum levels of the azoles and of coadministered agents including calcineurin inhibitors and mammalian target of rapamycin inhibitor immunosuppressive agents, anticoagulants, psychiatric and neurotropic medications, barbiturates, glucocorticoids, digoxin, vinca alkaloids eg, vincristine and cyclophosphamide, and antiretroviral agents [, —]. All of the azoles have important interactions via the CYP enzymes, notably CYP3A4, which can interact with a large number of concomitant medications including tyrosine kinase inhibitors, macrolides, and antiarrhythmics, among others. Active transporters including the p-gp and the breast cancer resistance protein regulate access of the azoles to the drug-metabolizing enzymes of enterocytes and the liver; the clinical importance of the transporters remains to be further defined [, , ]. Currently, 3 triazoles itraconazole, voriconazole, and posaconazole are considered to meet these criteria and have established indications for TDM in IA [, ]. There is general agreement that documentation of adequate and in the case of voriconazole, nontoxic serum levels in the first 4—7 days after starting therapy when a patient is at a pharmacokinetic steady state is preferable for any patient with suspected or documented aspergillosis. Less agreement exists whether TDM is necessary during primary triazole prophylaxis, but low plasma levels of itraconazole and posaconazole suspension have been associated with higher probability of breakthrough infection, and limited data suggest that high levels of posaconazole may be associated with toxicity []. The need for continued or repeat monitoring is a patient-specific decision influenced by the clinical status of the host eg, specific organ function, comorbidities, and receipt of concomitant medications , severity of infection, concerns regarding nonadherence, cost, TDM assay availability, possibly the duration of therapy [], and the overall treatment plan. Determination of a plasma drug level, in conjunction with other measures of clinical assessment, can help define factors that may have led to therapeutic failure with oral triazoles and reopen prospects for use of the same oral drug in the future provided pharmacokinetic issues are corrected. Overviews of clinical scenarios that frequently justify TDM are presented in Table 3. The therapeutic range for voriconazole and posaconazole have been primarily defined from single-center, retrospective studies and can only be considered a general guide for dosing []. Itraconazole capsules require low gastric pH for dissolution, and are therefore poorly absorbed in many patient populations with relative achlorhydria associated with their underlying disease or pharmacotherapy. Itraconazole suspension is better absorbed, but is associated with higher gastrointestinal adverse effects, which are especially problematic in populations who already have nausea, vomiting, or diarrhea. Although a variable rate of breakthrough IA has been reported in patients on itraconazole prophylaxis, relatively few studies have examined the relationship of itraconazole plasma concentrations and treatment efficacy for aspergillosis. Various target concentrations associated with voriconazole efficacy have been reported, mostly from single-institution retrospective studies [, ]. Visual changes can be related to elevated voriconazole concentration but generally resolve spontaneously and without long-term sequelae. Although voriconazole trough concentrations can be elevated in patients with hepatic dysfunction, available data do not support the concept of a threshold level that could adequately discriminate who will be at higher risk for hepatotoxicity []. This study and several others suggest that antifungal TDM may reduce drug discontinuation due to adverse events and improve the likelihood of a therapeutic response. There are no widely validated algorithms on how to dose voriconazole. Voriconazole concentrations often increase disproportionately to administered doses due to saturable metabolism in adults. Fundamental pharmacokinetics of voriconazole are different in children linear than in adults nonlinear []. Increasing evidence supports an exposure—response relationship for plasma posaconazole concentrations for prophylaxis and treatment of IFIs []. On the other hand, a clear relationship has not been identified between posaconazole concentrations and the risk of breakthrough IA in the pivotal posaconazole registration trials [, ] in which the event rate breakthrough IA was low. Therefore, TDM during posaconazole prophylaxis may be best used in evaluating potential breakthrough infections. The introduction of posaconazole extended-release tablets and the intravenous formulation of posaconazole more easily achieve increased posaconazole serum drug levels, even in patients with risk factors for posaconazole malabsorption [, , ]. Further studies are needed to address whether higher posaconazole levels are associated with toxicity and whether TDM is helpful or necessary with the extended-release or intravenous formulations. The value of TDM to guide therapy and to avoid toxicity for isavuconazole, a once-daily extended-spectrum triazole with anti- Aspergillus activity with good absorption kinetics, similarly remains to be assessed []. The rationale for combination therapy is to maximize treatment by targeting multiple targets or metabolic pathways or different points in the same pathway to improve efficacy through achieving an additive or synergistic effect. Other potential benefits include lowering the risk for emergence of drug resistance and the potential for shorter courses of therapy or lower doses of therapy in an attempt to reduce toxicity. Antifungal drug combinations have been evaluated in multiple in vitro studies and studied in animal models. Combinations of polyenes or azoles with echinocandins have been most studied, and additive or synergistic effects have been noted in the majority of but not all studies when compared to monotherapy especially echinocandins alone [—]. Furthermore, correlations between in vitro findings and in vivo observations have not always been consistent, and differences in drug metabolism between animals and humans make comparisons difficult. Also of importance is the order of administration. Some studies have suggested that prior azole administration subsequently reduces polyene activity [—]. Antagonism during the use of combination therapy has also been suggested by some studies, especially between polyenes and certain azoles []. By comparison, the combination of triazole and echinocandin agents exhibit synergistic to additive interactions in the same systems []. However, a murine model demonstrated possible antagonism between itraconazole and micafungin []. In vitro studies demonstrate that the combination of triazole and polyene may be antagonistic [] or that there may be synergy or antagonism depending on the dose used [, ]. In addition to reduced antifungal activity, other potential harmful effects may include increased risk for resistance, additive toxicity, cost, and deleterious drug interactions. Although the preclinical studies have been generally favorable to consideration of combinations of mold-active azoles or polyenes with echinocandins, the variable test designs and conflicting results of preclinical testing have led to uncertainty as to the applicability to clinical practice. The goal of AFST is to detect resistant isolates that are more likely to fail therapy [, ]. Considerable progress since the previous guideline has occurred toward achieving this goal. The European Committee on Antibiotic Susceptibility Testing EUCAST and the Clinical and Laboratory Standards Institute CLSI have published standardized but different AFST methodologies in recent years [, ]. Aspergillus minimum inhibitory concentrations MICs utilizing EUCAST and CLSI methodologies from more recent clinical studies and large surveys have been determined. Although clinical breakpoints are not yet defined by CLSI, epidemiological cutoff values—the upper limit of wild-type MIC distributions which aid in the determining the likelihood of resistance in Aspergillus spp—have been proposed by CLSI [—]. Taken together, these advances resulted in the recommendation by some experts in Europe to perform routine voriconazole AFST []. The advances of molecular techniques have led to important changes to Aspergillus taxonomy contributed to by the phylogenetic species recognition concept []. This method, based on sequencing of several targets for species recognition analysis, has identified new cryptic species, some of which are more resistant to current antifungal drugs []. Azole resistance in filamentous fungi primarily involves mutations in the CYP51A target enzyme or promoter that lead to specific or pan-azole resistance, and is described more frequently in A. fumigatus complex than other species [—]. Other azole resistance mechanisms are also described [—]. Resistance to the echinocandins is uncommon, as is resistance to AmB apart from Aspergillus terreus , Aspergillus nidulans , and Aspergillus lentulus [, ]. These reports notwithstanding, there are few studies determining the impact of resistance detected by AFST on clinical outcomes [, ]. At this time, AFST is not routinely performed in most clinical laboratories in the United States. Molecular methods to identify azole and echinocandin resistance in filamentous fungi are under investigation but not yet standardized or validated and require further study []. However, in the case of isolates with atypical growth or concerns for resistance when molecular methods are not available, AFST should be employed. In conclusion, AFST advances in the past decade are significant; however, worldwide Aspergillus resistance remains low, and routine AFST for clinical management is not recommended at this time. Early initiation of antifungal therapy in patients with strongly suspected IPA is warranted while a diagnostic evaluation is conducted, both because early therapy has been shown to limit progression of disease and because the performance of diagnostic testing remains limited [, ]. Availability of drugs that have differential activity for molds that cause similar syndromes, specifically, the lack of voriconazole activity against mucormycosis, emphasizes the importance of a specific microbiologic diagnosis and antimicrobial susceptibility testing. Evidence supporting appropriate primary therapy of IPA has been generated in a series of randomized controlled trials Table 1. The first pivotal treatment trial performed for IA demonstrated better survival in patients who received voriconazole compared with AmB deoxycholate [], justifying a recommendation against AmB deoxycholate therapy. Thus, for primary treatment of IPA in adults, intravenous or oral voriconazole is recommended for most patients. For seriously ill patients, the parenteral formulation is recommended. A switch to oral therapy, with dosing maximized to achieve recommended target serum levels, can be considered in patients who are able to tolerate oral therapy. A randomized trial compared voriconazole with isavuconazole, which demonstrated noninferiority in treatment of IPA []. This study showed noninferiority in terms of clinical efficacy, measured by survival and composite clinical responses in the intent-to-treat population of patients with possible, probable, and proven aspergillosis. There were fewer drug-related adverse effects in people who received isavuconazole. Based on these data, isavuconazole was approved by the FDA for first-line therapy of IA and is recommended as an alternative primary therapy for IPA. Another alternative for primary therapy of IA is liposomal AmB. Although no randomized trial has been performed to evaluate effectiveness of this drug compared to voriconazole for primary therapy, a series of randomized trials suggest effectiveness in therapy. Randomized trials of variable quality evaluating primary treatment of IA using lipid formulations of AmB have been reported to generally favor outcomes with lipid formulations, especially with regard to minimizing toxicities. The most compelling effectiveness data have been generated from randomized trials evaluating liposomal AmB. These results suggest that liposomal AmB be considered as alternative primary therapy in some patients, especially in situations in which hepatic toxicities or drug interactions warrant nonazole alternatives, and when voriconazole-resistant molds eg, mucormycosis remain of concern. Finally, ABCD was compared to AmB deoxycholate in a randomized trial of patients. Renal toxicity occurred less frequently with ABCD [], but due to an increase in serious drug reactions, principally fever, chills, and hypoxia, use of ABCD is not recommended. Combination therapy in the treatment of IPA has been supported by generally favorable in vitro and in vivo preclinical data in support of combinations of polyenes or mold-active azoles with echinocandins. Nonrandomized clinical trial data suggest the benefit of some forms of combination therapy against IA, usually an azole most commonly voriconazole with an echinocandin in aspergillosis [, , , , , —]. There are limited prospective randomized first-line combination therapy trials [, ]. Responses were better at the end of therapy with combination therapy but overall survival was similar. A more recent randomized trial compared outcomes of voriconazole monotherapy to combination therapy with voriconazole plus anidulafungin []. The trial enrolled patients with hematologic malignancy to evaluate hypothesized superiority in 6-week survival in combination therapy recipients. Mortality at 6 weeks was Secondary mortality benefits favored combination therapy. There were no toxicity differences. This study adds to prior preclinical and observational clinical studies that suggest potential benefits for combination therapy with voriconazole and an echinocandin [, , ]. For this reason, the committee suggests consideration for an echinocandin with voriconazole for primary therapy in the setting of severe disease, especially in patients with hematologic malignancy and those with profound and persistent neutropenia. Duration of antifungal therapy for IPA is not well defined. We generally recommend that treatment of IPA be continued for a minimum of 6—12 weeks, depending on the severity and continuation of immunosuppression, as well as the extent of resolution of clinical disease. Therapeutic monitoring of IPA includes serial clinical evaluation of all symptoms and signs, as well as performance of radiographic imaging, usually with CT, at regular intervals. The frequency with which CT should be performed cannot be universally defined and should be individualized on the basis of the rapidity of evolution of pulmonary infiltrates and the acuity of illness in the individual patient. The volume of pulmonary infiltrates may increase for the first 7—10 days of therapy, especially in the context of granulocyte recovery []. The use of serial serum GM assays for therapeutic monitoring is promising but remains investigational. Progressive increases in Aspergillus antigen levels over time signify a poor prognosis. However, resolution of GM antigenemia to a normal level is not sufficient as a sole criterion for discontinuation of antifungal therapy. Long-term therapy of IA is facilitated by the availability of oral azole drugs in stable patients. For patients with successfully treated IA who will require subsequent immunosuppression, resumption of antifungal therapy can prevent recurrent infection [, ]. Surgical resection of Aspergillus -infected tissue may be useful in patients who have lesions that are contiguous with the great vessels or other critical organs, lesions causing recalcitrant hemoptysis from a single focus, and in lesions eroding into bone. This decision should be mindful of the probability of structural adhesion eliciting spillage of organism into the pleural space. As discussed in Section II, increasing evidence suggests that attention should be placed on antifungal drug resistance, either that innate to the infecting Aspergillus species such as A. terreus , A. lentulus or that acquired by a typically susceptible species. Because immune reconstitution is an important factor in survival from IA, immunosuppressive agents should be tapered or removed, when possible. However, it is frequently not feasible to do so, for example, in patients with severe GVHD or in SOT recipients with allograft rejection. Clinical judgment is required in these cases. Colony-stimulating factors : Colony-stimulating factors administered prophylactically prior to the onset of neutropenia are commonly used to shorten the duration of neutropenia in patients receiving cytotoxic regimens. G-CSF influences survival, proliferation, and differentiation of all cells in the neutrophil lineage and augments the function of mature neutrophils. G-CSF also stimulates neutrophil recovery and various neutrophil effector functions and is a potent activator of monocytes and macrophages. Pegfilgrastim, a pegylated formulation of G-CSF with a long half-life, is used to reduce the duration of neutropenia in patients with nonmyeloid cancers. A meta-analysis of prophylactic G-CSF showed a reduction in the incidence of neutropenic fever and early deaths, including infection-related mortality []. Another meta-analysis showed a survival benefit of prophylactic G-CSF in patients with MDS and acute myelogenous leukemia AML []. Authoritative guidelines have been published regarding the appropriate use of colony-stimulating factors in patients with cancer, with the main goal of reducing neutropenic fever [, ]. The value of adjunctive as opposed to prophylactic colony-stimulating factors for the treatment of major infections is unclear. Studies in vitro and in murine aspergillosis suggest that G-CSF and GM-CSF can enhance antifungal host defense [—]. If not initiated in the prophylactic setting, use of colony-stimulating factors should be considered in neutropenic patients with diagnosed or suspected IA. Although colony-stimulating factors can augment phagocyte function in addition to cell numbers, there are insufficient data to recommend their use in patients who are not neutropenic. Granulocyte transfusions : The rationale for granulocyte transfusions is to increase the number of circulating neutrophils until neutrophil recovery occurs and is usually recommended as an adjunctive measure if granulocyte recovery is anticipated. Granulocyte transfusions have been used for decades as adjunctive treatment for severe infections in patients with neutropenia. The impetus to reevaluate granulocyte transfusions stems largely from improvements made in donor mobilization methods using therapy with G-CSF and corticosteroids []. In addition, the use of unrelated community donors for granulocytopheresis was shown to be feasible, thus increasing the pool of potential donors [, ]. A randomized trial evaluating the safety and effectiveness of granulocyte transfusions in patients with neutropenia and severe bacterial and fungal infections has recently been published NCT The overall benefit vs risk of granulocyte transfusions is currently unknown. Granulocyte transfusions were of benefit in experimental pulmonary aspergillosis in neutropenic mice []. Granulocyte transfusions can be considered for neutropenic patients with severe infections, including IA and other mold infections, which have failed or are unlikely to respond to standard therapy. Acute lung injury is the major risk of granulocyte transfusions. AmB may increase lung injury associated with granulocyte transfusions []; therefore, separating AmB and granulocyte infusions by several hours is advised. Alloimmunization leading to graft failure after allogeneic HSCT is another potential risk of granulocyte transfusions. In allogeneic transplants in which the donor and recipient are seronegative for CMV, use of CMV-seronegative granulocyte donors is recommended. Recombinant interferon gamma IFN-γ : IFN-γ augments the antifungal activity of macrophages and neutrophils ex vivo against a variety of fungal pathogens, including Aspergillus species. A high proportion of patients with CPA are poor producers of IFN-γ []. Its use as adjunctive therapy for patients with IA is limited to case reports and small series. One concern related to rIFN-γ use in allogeneic HSCT recipients is the potential to worsen GVHD. A single-center retrospective analysis suggested that rIFN-γ was safe in allogeneic HSCT recipients []. Currently, the data supporting the efficacy of adjunctive rIFN-γ for aspergillosis are weak; it can be considered in patients with severe or refractory aspergillosis. Surgery : In general, surgical treatment of aspergillosis should be considered for localized disease that is accessible to debridement. Emergent debridement of sinus aspergillosis can be life-saving and limit extension to the orbit and brain. Localized cutaneous aspergillosis should also be debrided. CNS aspergillosis is a devastating complication; neurosurgical removal combined with antifungal therapy may be life-saving, although the expected postsurgical neurologic outcome should also be considered during the decision process. Surgical resection of pulmonary lesions due to Aspergillus species can provide a definitive diagnosis and can potentially completely eradicate a localized infection. Surgical therapy may be useful in patients with lesions that are contiguous with the great vessels or the pericardium, uncontrolled bleeding, or invasion of the pleural space and chest wall. Intervention should also be considered for localized pulmonary aspergillosis refractory to antifungal therapy []. Another consideration for surgery is the resection of a single pulmonary lesion prior to intensive chemotherapy or HSCT. However, the favorable experience of HSCT in patients with prior IA suggests that antifungal therapy alone may be effective [, —]. An acceptable approach in patients with pretransplant aspergillosis is close CT monitoring without surgical resection in the absence of additional complications, such as uncontrolled bleeding or chest wall extension. Decisions concerning surgical therapy should be individualized to account for a number of variables, including the degree of resection eg, wedge resection vs pneumonectomy , potential impact of delays in chemotherapy, comorbidities, performance status, the goal of antineoplastic therapy eg, curative vs palliative , and unilateral vs bilateral lesions. The major concern is that aspergillosis will progress during subsequent periods of immunosuppression. Several studies have shown that IA is not a contraindication for additional treatment, including HSCT [, —]. It is important to administer mold-active antifungal treatment during subsequent periods of immunosuppression referred to as secondary prophylaxis to avoid recurrence or progression. In a multicenter retrospective survey of patients with pretransplant aspergillosis, 27 of patients developed progressive fungal disease following allogeneic HSCT. Decisions about when to proceed with additional chemotherapy or HSCT following the diagnosis of aspergillosis must consider the risks of progressive aspergillosis and the risks of delaying treatment of the underlying malignancy. These decisions require expertise from infectious diseases specialists and oncologists. From the infectious disease standpoint, a period of several weeks of antifungal treatment and clear evidence of response to therapy is ideal before administering additional chemotherapy or HSCT. However, there are situations when this approach is not feasible, for example, in patients with refractory or relapsed acute leukemia who require urgent reinduction therapy. Many issues confound the interpretation of current published evidence for salvage therapy for IA including publication bias, inadequate statistical power, and heterogeneity of studies. In salvage therapy studies, differentiating Aspergillus -attributable mortality vs the impact of underlying disease or coinfections is not possible [, ]. It is also unclear whether different therapeutic approaches are needed when breakthrough infection is detected by GM alone vs culture, the latter likely representing a more advanced stage of disease. Salvage therapy trials that enroll patients after only 7 days of antifungal therapy may not adequately account for this phenomenon. Antifungal therapy initiated at the time of neutrophil recovery is also biased by the salutatory effects of immune recovery. In addition, there is confusion in some studies between sequential vs true salvage therapy as the action of the failing drug may interact with the action of the salvage drug. The first drug may inflict damage to Aspergillus that enhances the action of the second drug, or there may be neutral or possibly even antagonistic effect. Another issue relates to antifungal agents with prolonged half-lives such as AmB formulations []. The principal antifungal agents considered for salvage therapy include lipid formulations of AmB, posaconazole, itraconazole, and the echinocandins, caspofungin and micafungin, which have both been evaluated in salvage settings [, , —]. Voriconazole can also be considered as a salvage agent if not used in primary therapy, as could presumably isavuconazole, although isavuconazole has limited evaluation in the salvage setting. In patients who fail initial triazole therapy, a change in class to an AmB formulation usually liposomal AmB , with or without an echinocandin, should be considered. Azole-specific pharmacokinetic problems must also be considered, including TDM. Most of the prospective studies of second-line therapy have been conducted by replacing the compound to which the patient is intolerant or against which the infection is progressing. Whether both drugs should be administered simultaneously has seldom been prospectively studied []. The addition of a second antifungal agent to a first agent that is failing is usually practiced out of understandable lack of therapeutic options. Other drug combinations have not been extensively studied []. Additional questions of optimal drug combinations, optimal drug dosing, pharmacokinetic interactions, potential toxic interactions, and cost—benefit ratios of primary combination antifungal therapy require further investigation. The need for surgical resection should be evaluated in cases of pulmonary lesions contiguous with the heart or great vessels, invasion of the chest wall, massive hemoptysis, and other special circumstances. Restoration of or improvement in impaired host defenses is critical for improved outcome of IA. Correction of comorbidities using various adjunctive strategies eg, correction of hyperglycemia, recovery from neutropenia, or reduction of immunosuppressive medication dosages is expected to improve outcomes in progressive IA but may also be associated with IRIS. Multiple studies have evaluated serial serum GM for both therapeutic monitoring as well as predicting prognosis and found excellent correlations between GMI and outcomes. A review of 27 published studies, including both adult and pediatric allogeneic or autologous HSCT recipients, found an excellent correlation between GMI and survival, including autopsy findings []. A prospective study of 70 patients with prolonged neutropenia found good GMI concordance with clinical outcome at 6 weeks and excellent correlation at 12 weeks, including perfect concordance with autopsy findings and significantly better survival in patients who became GM negative by 12 weeks []. Another retrospective study found similar results, including significantly better survival in patients whose GMI normalized compared to patients with persistently positive GM, regardless of resolution of neutropenia []. In one study, an adjusted hazard ratio HR for respiratory or all-cause mortality increased from 2. GMI-based assessment can also predict outcome sooner []. Several studies have compared the initial GMI and subsequent rate of daily decay of GM, defined as the change from the initial GMI divided by the number of days since that initial value. Both initial GMI and rate of decrease of GM in response to therapy at one week after initiation of therapy have been predictive of mortality []. The adjusted HR for initial GM for time to mortality was 1. GMI is also predictive of outcome in nonneutropenic patients [—]. A retrospective evaluation of the global aspergillosis clinical trial comparing voriconazole to AmB deoxycholate followed by other licensed therapy [] found that GMI at week 1 was significantly lower than baseline GM in the eventual week responders compared with nonresponders. A different analysis of the same trial found that those patients who received voriconazole and had a successful week 12 response showed earlier decreases in GMI at week 1 and week 2 as compared to those who eventually failed treatment. However, for patients randomized to initially receive AmB deoxycholate, this early difference trend between week 12 responders and nonresponders was not evident until week 4 []. There have been fewer studies with BAL GMI and outcome. However, another retrospective study of allogeneic HSCT recipients found that serum GMI positivity and magnitude, but not BAL GMI, correlated with both 6-week and 6-month mortality []. Treatment in children follows the recommendations used for adults, yet antifungal dosing in children is often significantly different. Underdosing in children is a common etiology of insufficient drug levels and possibly clinical failures. Voriconazole, while only FDA approved for children 12 years and older, is the mainstay of pediatric aspergillosis treatment in all ages due to substantial pharmacokinetic data and experience. The majority of adolescents can be dosed as adults, but in younger adolescents ages 12—14 , body weight is more important than age in predicting voriconazole pharmacokinetics. As in adult patients, there are still suggestions of the need for higher voriconazole doses [], and drug monitoring is paramount. Posaconazole is FDA approved for children 13 years and older for both the oral suspension and tablet, and for 18 years and older for the intravenous formulation. As such, pediatric dosing has not yet been fully defined. Micafungin is FDA approved for children 4 months and older and clearance increases in younger age groups. Anidulafungin is not FDA approved for children, and a single pharmacokinetic study in children suggested a loading dose of 1. Dosing of lipid formulations of AmB does not differ in children. Airway aspergillosis or TBA is similar to pulmonary aspergillosis in that it can occur in saprophytic, allergic ABPA , or invasive forms. There is also an emerging entity of Aspergillus bronchitis among patients with CF, and others with bronchiectasis. The diagnosis of TBA is suggested by bronchoscopic findings and confirmed by culture and histopathology. Due to the limited number of studies, optimal evidence-based therapy is not clear, and recommendations are extrapolated from experience in treating invasive lung parenchymal aspergillosis and TBA case series. Saprophytic forms of TBA include obstructing bronchial aspergillosis, endobronchial aspergillosis, and mucoid impaction. Obstructing bronchial aspergillosis is characterized by thick mucous plugs with minimal or no airway inflammation [, ]. Patients commonly present with the subacute onset of cough, dyspnea, chest pain, hemoptysis, and expectoration of fungal casts. Management typically consists of bronchoscopic clearance usually followed by oral antifungal therapy. Endobronchial aspergillosis is generally found among patients with lesions such as broncholiths, cancer, or granulation tissue or suture material at the anastomotic site after lung resection. It is manifested as endobronchial lesions or mucous plugs in or around the bronchial stumps or sutures. In general, these saprophytic forms do not require systemic antifungal therapy unless patients are immunocompromised and locally invasive disease cannot be ruled out []. In symptomatic patients, local debridement or suture removal can be performed. There is no consistent evidence that systemic, inhaled, or local injection with an antifungal agent is effective in treating these forms of disease. Mucoid impaction is a clinical-radiographic syndrome characterized by inspissated mucus filling of the bronchi [, ]. Finger-in-glove sign, referring to branching tubular opacities that extend peripherally, is the classic chest radiograph finding. Patients can be asymptomatic, or present with cough and expectoration of mucous plugs. Mucoid impaction is commonly associated with inflammatory conditions of the airways such as bronchiectasis and ABPA , benign processes such as broncholithiasis, foreign body aspiration, endobronchial lipoma, hamartoma, or papilloma , and malignant processes such as carcinoid tumor or lung malignancies causing obstruction of large airways. Mucous plugging that may appear hyperattenuated on computed tomography seems to be a particularly distinctive feature of ABPA, probably more common in India, with a high propensity for early relapse and corticosteroid dependence. Mucoid impaction associated with bronchiectasis is treated with maneuvers to promote airway clearance chest physiotherapy, positive expiratory pressure and vibration devices, mucolytics, nebulized hypertonic saline and treatment of airway infection antimicrobial agents. Mucoid impaction associated with features of asthma and hypersensitization to Aspergillus is treated as for ABPA []. Bronchocentric granulomatosis is a form of ABPA that is characterized histopathologically by necrotizing granulomas with airway obstruction that destroy the bronchioles, but there is no tissue invasion by Aspergillus. Bronchoscopic findings include impaction of airway lumen by mucin and cellular debris. Treatment is similar to that of ABPA see Recommendations 92—94 below []. Invasive TBA is an uncommon disease that originates in the airway but may invade more deeply [, ]. It has been described most commonly in immunosuppressed patients patients with hematologic malignancies, lung transplant or HSCT recipients, and patients on high-dose steroids. However, invasive TBA among patients with no known immunosuppression or following influenza infection has also been described [, ]. Expand All Australasian Consensus Guidelines for the use of antifungal agents in the haematology-oncology setting, update Published in the Internal Medicine Journal: Vol 51 Supp. Internal Medicine Journal 51 S7 Global guideline for the diagnosis and management of cryptococcosis : an initiative of the ECMM and ISHAM in cooperation with the ASM Chang et al. Lancet Infectious Diseases , Online 9 Feb Global guideline for the diagnosis and management of mucormycosis : an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium Cornely et al. Lancet Infectious Diseases , 9 12 :ee Donnelly et al. Clinical Infectious Diseases , 71 6 Published in the Internal Medicine Journal. Vol 49 Issue , Diagnosis, management and prevention of Candida auris in hospitals: Position statement of the Australasian Society for Infectious Diseases. ESCMID-ECMM guideline : Diagnosis and management of invasive aspergillosis in neonates and children Warris et al. Clinical Microbiology and Infection , 25 9 Practice Guidelines for the Diagnosis and Management of Aspergillosis : Update by the Infectious Diseases Society of America Patterson et al. Clinical Infectious Diseases , e1-e Published in Clin Microbiol Infect ; 20 Suppl. Published in Clinical Microbiology and Infection , 17, supplement 5 : Management of invasive candidiasis in adults. org - Published in Clinical Infectious Diseases Guidelines introduction CID ; Antifungal agents CID ;—7 Aspergillosis CID ;— Blastomycosis CID ;— Candidiasis CID ;— Coccidioidomycosis CID ;— Cryptococcosis CID ; Histoplasmosis CID ;—25 Sporotrichosis CID ; — European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother ; — ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother ; 71 9 : — Fourth European Conference on Infections in Leukaemia ECIL-4 : guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncology ,15 8 :e European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3— Update Bone Marrow Transplantation , Empirical antifungal therapy in neutropaenic cancer patients with persistent fever EJC Supplement S Primary antifungal prophylaxis in leukaemia patients EJC Supplement S Treatment of invasive Candida and invasive Aspergillus infections in adult haematological patients EJC Supplement S org Treatment of fungal infections in adult pulmonary and critical care patients. Prentice et al. |

0 thoughts on “Antifungal treatment guidelines”