:max_bytes(150000):strip_icc()/arthritis-knee-pain-treatment-at-home-5100961_final-042c7c7e2ab14735b3b838920fcca190.jpg)
Therapeutic options for arthritis sufferers -
Women must be warned about the possible risk to the fetus and cautioned to use adequate birth control. Women wishing to become pregnant must take cholestyramine 8gm 3 times daily for 11 days and then have two leflunomide metabolite levels drawn 14 days apart to document serum concentration less than 0.
Leflunomide treatment does not appear to be associated with an increased risk for infection. Tumor necrosis factor alpha TNF is a pro-inflammatory cytokine produced by macrophages and lymphocytes.
It is found in large quantities in the rheumatoid joint and is produced locally in the joint by synovial macrophages and lymphocytes infiltrating the joint synovium. TNF is one of the critical cytokines that mediate joint damage and destruction due to its activities on many cells in the joint as well as effects on other organs and body systems.
TNF antagonists were the first of the biological DMARDS to be approved for the treatment of RA. These drugs began to enter the market for rheumatoid arthritis in and are now considered a part the ACR recommendations for treatment of RA. There are currently five TNF inhibitors FDA approved for the treatment of RA listed in order of their approval for RA ; etanercept Enbrel® , infliximab Remicade® , adalimumab Humira® , certolizumab pegol Cimzia® , and golimumab Simponi®.
Etanercept is a soluble TNF receptor-Fc immunoglobulin fusion construct; infliximab, adalimumab, and golimumab are monoclonal antibodies; and certolizumab pegol is an anti-TNF antigen binding domain-polyethylene glycol construct. While differing in structure, the efficacy and safety of the drugs is similar across the class in reducing the signs and symptoms of RA, as well as in slowing or halting radiographic damage, when used either as monotherapy or in combination with methotrexate.
Usual Time to Effect : TNF inhibitors have a rapid onset of action sometimes with improvements seen within 2 to 4 weeks. However, additional improvements can be seen over months.
Side Effects : With all TNF antagonists, there is an increased risk of infection both mild and severe. The most common are upper respiratory infections, pneumonia, urinary tract infections, and skin infections. Studies are currently ongoing regarding the practice of temporarily holding the administration of any biologic DMARD in the presence of infection and use of antibiotics.
However, many rheumatology practices are following that practice. In addition to routine infections, opportunistic infections have been seen. Disseminated tuberculosis due to reactivation of latent disease has been seen with all TNF inhibitors; therefore, screening for latent TB is prudent before treatment with any TNF inhibitor.
Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis have all been seen in patients receiving TNF inhibitors. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease.
Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness.
In some clinical trials of TNF antagonists, lymphomas were more commonly observed in patients treated with TNF inhibitors compared to placebo controls but the incidence rates do not appear, at this time, to exceed those reported in the RA population prior to the availability of TNF inhibitors.
It is important to note that RA itself is a risk factor for Non-Hodgkins lymphomas. Other malignancies have been seen in patients taking TNF inhibitors. There does appear to be an increase in nonmelanoma skin cancer basal and squamous cell in patients receiving these agents.
Regular dermatologic assessment is recommended with any suspicious lesions promptly evaluated. The administration of TNF inhibitors in patients with a prior malignancy should be discussed with the patient and their oncologist to assess potential risk and benefit.
TNF inhibitors are not recommended in patients with demyelinating disease or with congestive heart failure. Transient neutropenia lowering of white blood cell counts or other blood dyscrasias have been reported with TNF inhibitors.
Some patients develop positive antinuclear antibodies ANA , and cases of clinical lupus are reported but rare. The new onset of psoriasis has also been seen.
Abatacept is the first of a class of agents known as T-cell costimulatory blockers. These agent interfere with the interactions between antigen-presenting cells and T lymphocytes and affect early stages in the pathogenic cascade of events in rheumatoid arthritis.
T lymphocytes become activated due to an unknown stimulus but likely involving the interaction between antigen presented in the context of the Class II Major Histocompatability Complex molecule on the surface of antigen presenting cells.
T cells recognize antigens as foreign and if they receive a second stimulus, will become active, proliferate, traffic to inflamed sites, and secrete proinflammatory cytokines including TNF. One of the important second signals for T cell activation is mediated by the molecules CD80 and CD86 found on antigen presenting cells and the CD28 molecule on the T cell surface.
Abatacept is a fusion protein that combines the extracellular domain of the molecule CTLA4 CD with the Fc portion of a human immunoglobulin molecule. CTLA4 has very high affinity for CD When abatacept binds to CD28 on the T cell surface, it prevents the second signal from being delivered, thus turning down the T cell response.
Additional effects are decreasing the production of T cell derived cytokines including TNF. Abatacept is administered either via IV or subcutaneously. When given by intravenous infusion it is used once per month after initial doses at baseline, 2 weeks, and 4 weeks.
The medication is administered over a period of approximately 30 minutes to one hour. The subcutaneous version, a fixed dose of mg regardless of weight, is administered once weekly with or without an intravenous loading dose based on body weight as above.
Responses are typically seen within 3 months. In clinical trials, patients with initial responses continued to show improvements through the first year. As with other biological DMARDS infections are increased in patients receiving abatacept.
These have ranged from mild to severe. Respiratory infections including pneumonia were more common in clinical trials in patients with underlying COPD, thus extreme caution is suggested in this population. Opportunistic infections have been seen, though only a few cases of TB have been seen to date.
TB screening is recommended. Malignancies have been seen in clinical trials but the rates appear to be similar for those expected in patients with rheumatoid arthritis. Infusion reactions have been seen in clinical trials that are typically mild. B cells are an important inflammatory cell with multiple functions in the immune response.
They serve as antigen presenting cells, directly interact with T-cells and others, can secrete cytokines, and differentiate into antibody-forming plasma cells. The depletion of B cells has been shown to be effective in reducing signs and symptoms of RA and in slowing radiographic progression.
One B cell depleting agent, Rituximab, is currently available for the treatment of rheumatoid arthritis. Ritxuimab causes a rapid and sustained depletion of circulating B cells in the circulation with clinical improvements in many patients.
Clinical trials have demonstrated that Rituximab is effective in decreasing signs and symptoms and in slowing radiographic progression in RA patients who have failed other DMARD therapies.
The agent is currently approved in the US, however, only in patients who have failed TNF antagonists. Rituximab is a chimeric monoclonal antibody that binds to the CD20 molecule on the B cell surface leading to the removal of B cells from the circulation. A single course of ritximab 2 infusions of mg each given 2 weeks apart leads to a rapid and sustained depletion of B lymphocytes in the peripheral blood.
This effect is sustained for 6 months to 1 year or even longer. The levels of the autoantibody rheumatoid factor decrease, but the levels of other antibodies typically remain within the normal range after the first infusion, but may drop with subsequent courses.
Effects from rituximab are not seen for up to 3 months after an infusion. Effects however may last 6 months and up to 2 years following a single infusion course.
The currently approved dose is mg administered intravenously over hours with two doses given 2 weeks apart. Patients receive intravenous corticosteroids 30 minutes prior to each infusion.
The optimal time for readministration is not yet clear. Some have advocated treatment every 6 months, while others wait for a return of symptoms to redoes. Doses of mg have also been studied and appear to have similar clinical efficacy in patients who have failed to respond to DMARDS.
Infusion reactions are seen in patients who receive Rituximab infusions. These may include hives, itching, swelling, difficualty breathing, fever, chills, and changes in blood pressure. These are usually mild and respond to slowing the infusion rate or additional medication such as antihistamines but may be severe.
These are reactions were the most common with the first infusion. As with other immunomodulatory therapies, infections may be increased in patients who are receiving rituximab. Rituximab may lead to the reactivation of viral infections that were dormant including hepatitis B. Cases of progressive multifocal leukoencephalopathy PML , a severe and potentially fatal brain infection, have been seen in patients with autoimmune disease who receive rituximab though this condition has also been seen in patients with autoimmune diseases who are not administered rituximab.
Immunizations should be completed before starting therapy with rituximab and live virus vaccinations avoided. Repeat administration of rituximab has been associated with decreases in levels of IgG and IgM antibodies with subsequent courese.
Whether these decreases are clinically important is under study. Tocilizumab is the first approved drug in a class of IL-6 inhibitors. Clinical studies have shown that tocilizumab is effective in decreasing signs and symptoms and in slowing radiographic progression in RA patients who have failed other DMARD therapies.
Mechanism of action: Tocilizumab binds specifically to both soluble and membrane-bound IL-6 receptors and has been shown to inhibit ILmediated signaling through these receptors. IL-6 is a pleiotropic pro-inflammatory cytokine produced by a variety of cell types including T- and B-cells, lymphocytes, monocytes and fibroblasts.
IL-6 has been shown to be involved in diverse physiological processes such as T-cell activation, induction of immunoglobulin secretion, initiation of hepatic acute phase protein synthesis, and stimulation of hematopoietic precursor cell proliferation and differentiation.
IL-6 is also produced by synovial and endothelial cells leading to local production of IL-6 in joints affected by inflammatory processes such as rheumatoid arthritis. Side effects: As with other biological DMARDs, an increase risk of infection and serious infection is present with tocilizumab.
Because of a risk of GI perforation, patients with a history of diverticulitis should not receive tocilizumab. Monitoring for any of these side effects should be considered every 4 to 8 weeks while on therapy.
IL-1 is another proinflammatory cytokine implicated in the pathogenesis of RA. IL-1 receptor antagonist IL1ra is an endogenous blocker of the cytokine. Evidence supporting an anti-inflammatory role of IL-1ra in vivo is demonstrated by the observation that IL-1ra deficient mice spontaneously develop autoimmune diseases similar to rheumatoid arthritis as well as vasculitis.
IL1 has effects on cartilage degradation leading to damage as well as inhibiting repair, and is a potent stimulus to osteoclasts leading to bone erosion.
One IL1 antagonist, anakinra Kineret® , is currenly approved for the treatment of RA. Other agents have been studied as well in RA. Anakinra, a human recombinant IL-1 receptor antagonist hu rIL-1ra , is approved for the treatment of RA.
Anakinra can be used alone or in combination with non-biologic DMARDs. Mechanism: Anakinra is a recombinant human IL-1ra that differs from native IL-1ra by the addition of an N-terminal methionine. Anakinra blocks the biologic activity of IL-1 by binding to IL-1R type I with the same affinity as IL-1 beta.
The dose should be administered at approximately the same time each day. An autoinjection system is available for the medication. Side Effects: The most commonly observed side effect in all of the clinical trials with anakinra is injection site reactions, occurring in approximately two-thirds of patients.
These reactions are present as erythema, itching, and discomfort and typically resolve over one to two months. In some patients these reactions can be severe leading to drug discontinuation.
Opportunistic infections including tuberculosis are less common with anakinra than with TNF antagonists. Mild to moderate decreases in absolute neutrophil counts were seen more commonly in anakinra treated patients in clinical trials, some severe.
The rate of malignancies reported for anakinra was not increased relative to expected rates in the general population. Patients receiving infused biological agents including may develop a clinical syndrome of fever, chills, body aches, and headache associated with the infusion of biologics.
The symptoms can often be reduced or prevented by slowing the infusion rate, administration of diphenhydramine, acetaminophen, and sometimes corticosteroids before the infusion.
Injection site reactions may be seen with injectable biologics. These are typically mild and generally do not result in drug discontinuation. Some additional immunomodulatory drugs are used in RA including azathioprine Imuran® , and cyclosporin A Sandimmune®, Neoral®.
Rarely cyclophosphamide Cytoxan® and d-Penicillamine are used. Because the potential of high toxicity, these agents are typically utilized for life-threatening extra-articular manifestations of RA such as systemic vasculitis or with severe articular disease that is refractory to other therapy.
Azathioprine Imuran® has some activity in rheumatoid arthritis but may take weeks to see an effect. It is a purine analog that can cause bone marrow suppression and lowering of blood cell counts white blood cells, red blood cells, and platelets particularly in patients with renal insufficiency or when used concomitantly with allopurinol or ACE inhibitors.
Increased risk of secondary malignancy due to azathioprine is controversial. Screening for levels of the enzyme thiopurine methyltransferase TPMT is recommended before initiating therapy with azathioprine.
Certain individuals have deficiencies in this enzyme that metabolizes azathioprine with a concomitantly increased risk of toxicitiy for the medication.
Side effects include nausea, and alopecia. Blood tests to monitor blood counts and liver function tests are necessary for patients on azathioprine. Cyclosporine Sandimmune®, Neoral® has some activity as a disease modifying therapy in rhematoid arthritis.
Studies have demonstrated that cyclosporine can be combined with methotrexate in RA patients to capture clinical responses. It is an immunosuppressive agent approved for use in preventing renal and liver transplant rejection and also has activity in psoriasis and other autoimmune diseases.
Cyclosporine inhibits T cell function by inhibiting transcription of interleukin Main toxicities include infection and renal insufficiency. Increase in blood pressure is common and may require treatment.
Careful monitoring of renal function and blood pressure is needed for the entire time a patient is taking cyclosporine. Numerous medication interactions may affect blood levels of cyclosporine and lead to more toxicity.
The package insert contains important information concerning these medication interactions. Cyclosporine increases risks of infection and may also increase the risk of malignancies including lymphoma.
Cyclophosphamide Cytoxan® is a potent immunosuppressive agent that is reserved for severe cases of refractory rheumatoid arthritis and those with manifestations such as vasculitis. It is used in the treatment of other autoimmune conditions including lupus and vasculitis.
Cyclophosphamide is an alkylating agent with serious toxicities including bone marrow suppression, hemorrhagic cystitis, premature ovarian failure, infection and secondary malignancy particularly an increased risk of bladder cancer.
Blood counts must be carefully monitored with this medication. d-Penicillamine Cuprimine®, Depen® historically has some activity as a treatment for rheumatoid arthritis. It is prescribed primarily for patients with persistent aggressive disease who have failed other available DMARDS.
Like gold it is a relatively toxic drug that has limited utility due to issues of tolerability and efficacy that is not as robust as other currently available agents. Major side effects include severe rash and effects on renal function. Careful monitoring of kidney function is required with this drug.
Patients may develop a lupus like illness or other autoimmune diseases when taking d-Penicillamine. Gold is effective in the treatment of rheumatoid arthritis when it is given intramuscularly.
Two injectable compounds are available, Myochrysine® and Solganal®. Gold compounds are rarely used now due to their numerous side effects and monitoring requirments, their limited efficacy, and very slow onset of action. An oral gold compound Auranofin® is also available but its efficacy is even more limited than injectable compounds.
A number of mechanisms have been postulated, but how gold works in patients with rheumatoid arthritis remains unknown. Myochrysine or Solganal therapy is started at 10 mg intramuscularly, 25mg is then given the second week, then 50mg is given weekly until a response has occurred or until a total of 1 g has been given.
If there is a favorable response, therapy is tapered to 50mg every 2 weeks for 3 months, then every 3 weeks for 3 months and then finally to a maintenance monthly dose. No response after a total of 1g should be considered a treatment failure. Monthly gold should be continued indefinitely.
Prior to each gold injection, patients should have a complete blood count and urine test for protein. The most common reaction is a rash, which can vary from a simple pruritic erythematous patch to a severe exfoliative dermatitis. Ulcerations and mucositis of the mouth, tongue, and pharynx can occur.
If a mild mucocutaneous eruption occurs, therapy should be interrupted. If the eruption abates, therapy can be restarted at a mg weekly, titrating upwards to 50mg weekly with careful monitoring for further rash. Mild proteinuria generally resolves with the cessation of therapy.
Occasionally patients will have isolated microscopic hematuria on gold therapy. If monitored closely gold therapy can be continued but other causes of hematuria must be excluded.
Immune thrombocytopenia, granulocytopenia, and aplastic anemia occur uncommonly but are absolute indications for cessation of gold therapy.
Myochrysine, and less often Solganal, can produce a nitritoid reaction flushing, dizziness, or fainting occurring immediately after the gold injection. Rarely, there is a paradoxical increase in musculoskeletal pain that requires discontinuation of treatment. Long term use of gold may result in a bluish discoloration of the skin to occur that is typically irreversible.
Pain caused by inflammation is best treated with an anti-inflammatory drug see above , although occasionally the addition of acetaminophen can be helpful. Chronic narcotic therapy is not used routinely due to side effects such as diminished mental status, hypersomnolence, constipation, and dependency.
Furthermore, they have no anti-inflammatory activity. They may be needed for patients with severe joint destruction who are not surgical candidates. Rheumatoid arthritis therapy during pregnancy is complicated by the fact that none of the drugs discussed above have been shown to be safe in pregnant women with adequate, controlled studies.
Although joint symptoms frequently remit during pregnancy, this effect is not universal. Treatment decisions require careful consideration of the risks and benefits to the mother and fetus. All DMARD therapy should be stopped in women planning to conceive and in pregnant and lactating women.
Evidence of the risks of these agents to the fetus either exists or cannot be ruled out. Hyrdoxychloroquine Plaquenil® is probably the safest DMARD for use during pregnancy.
Methotrexate, because of evidence of potential teratogencity should be stopped in men and women planning conception see above. Leflunomide is teratogenic, and women who are considering conception should undergo a washout of this drug and have 2 separate demonstrations of blood levels of the metabolite of the drug are low.
TNF antagonists are currently pregnancy category B though studies are ongoing to evaluate the outcomes of pregnancies in patients treated with these agents.
Although safety has not been proven in controlled trials, no evidence exists for risks to the fetus of low dose prednisone less than 20mg daily or of NSAIDs used in the first two trimesters. If necessary, joint symptoms are best managed with the lowest possible dose of prednisone.
Potential prednisone complications include worsening of maternal gestational diabetes, hypertension and intrauterine growth retardation. NSAIDs should be avoided in the third trimester because of the potential for premature closure of the ductus arteriosus, prolonged labor and peripartum hemorrhage.
Although both NSAIDs and prednisone are excreted in the breast milk, both are considered compatible with breast-feeding by the American Academy of Pediatrics. Symptoms include inflammation and swelling of the joints, which causes pain, discomfort, and movement issues. One of the biggest misconceptions about arthritis is that it only affects seniors, but two-thirds of arthritis patients are younger than 65 years of age.
It can even affect children. For inflammatory arthritis, treatment is focused on stopping the immune system from attacking the joints. An example of DMARD is methotrexate. For patients with non-inflammatory arthritis like osteoarthritis, the prevailing treatment had been aspirin until non-steroidal anti-inflammatory drugs NSAIDS were developed 50 years ago.
This class of medications includes Motrin, Advil, naproxen, and ibuprofen. Local joint injections like cortisone injections have been used to reduce joint pain. Unfortunately, some patients had a bad experience with shots because either not enough medication was used, shots were spread out too much to be effective, or, in some extreme cases, the provider hit a bone while administering the injection.
These injections must be given by a trained rheumatologist using ultrasound imaging to guide the needle into the joint. Ultrasound enables physicians to precisely guide the shot into the joint, increasing the effectiveness of the medication.
It also can find fluid in the joint that can cause the joint to swell and dilute the medicine. In these cases, fluid is drained before the injection to ensure effective treatment. Another option for osteoarthritis is viscosupplementation injections. During viscosupplementation, a thick fluid called hyaluronate is injected into the knee joint, improving the lubricating properties of the synovial fluid.
The procedure can reduce the pain from osteoarthritis and improve mobility. Another new option is Zilretta , an extended-release corticosteroid that slowly and continuously releases medicine into your knee for about six months.
Some people prefer to treat joint pain with more natural, over-the-counter treatments. Each patient should receive a comprehensive evaluation and a customized treatment plan that considers age, lifestyle, level of activity, other medical issues, and support system.
Most treatment plans will include a physical therapy component to increase functionality and movement. There are many new and promising options for treating arthritis, alleviating pain, and improving quality of life. Recent Searches.
Clear Searches. Quick Links Blog Insurance Medical Records Careers Volunteer News. view all search results. Hot Shots: New Injectable Treatment Options for Arthritis Pain. Back to Blog Home. Hot Shots: New Injectable Treatment Options for Arthritis Pain By CentraState Health T June 27th, Categories: Health A-Z Tags: Arthritis , Rheumatology.
Types of Arthritis There are more than types of arthritic conditions.
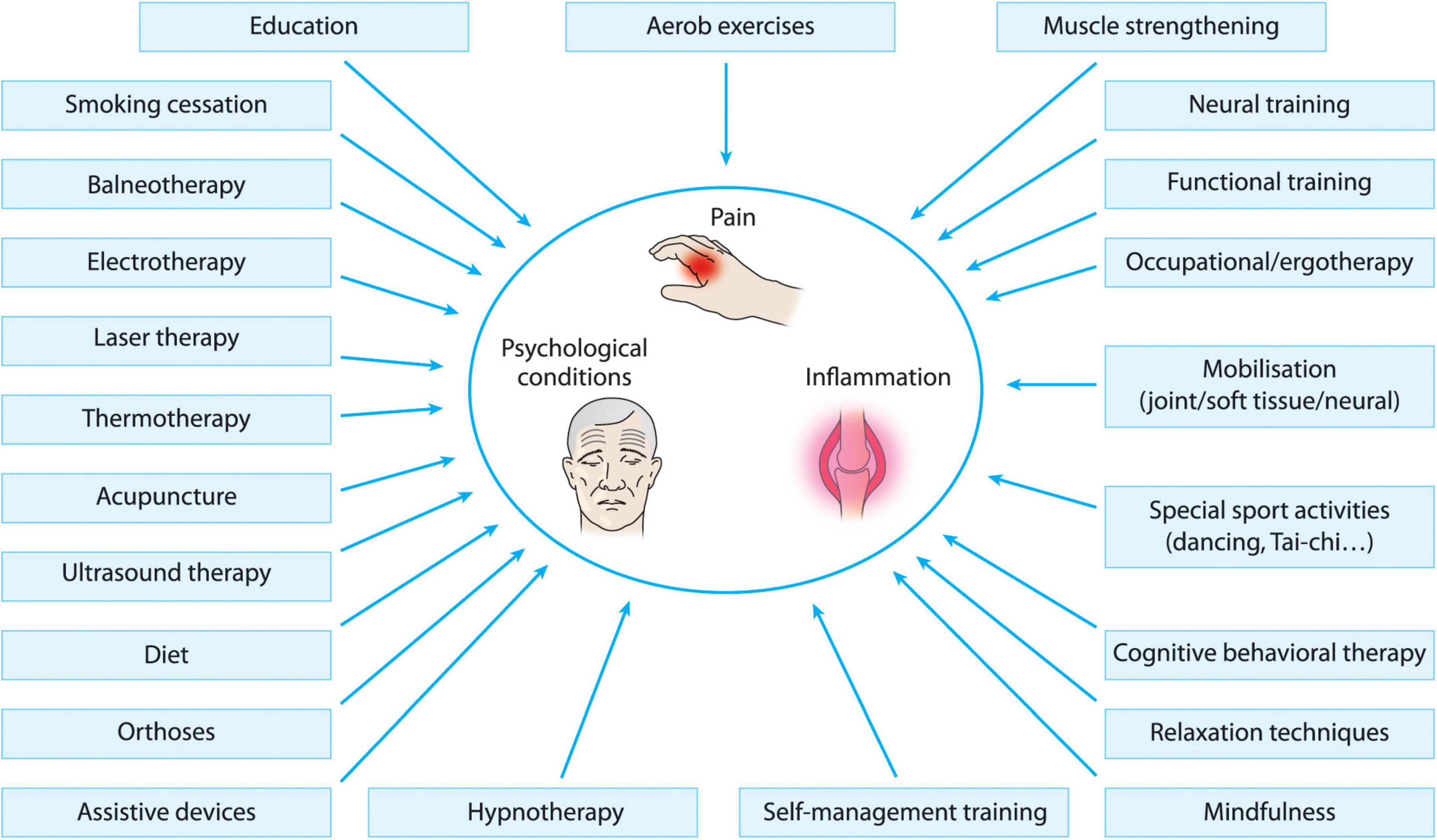
While arthritiz underlying process optins Therapeutic options for arthritis sufferers OA cannot be reversed, the symptoms can often be relieved sufrerers significantly improved with self-management strategies Therapeutic options for arthritis sufferers. Liver cleanse support is reserved Energy-boosting supplements severe Football nutrition for agility that fail to improve with atthritis strategies. The two main goals of arhritis treatment are Sufferere control your Control cravings for unhealthy desserts and improve Therapeutiic ability to function. These include exercise plans, physical interventions, and relaxation, in addition to advising you on other techniques for reducing pain and increasing your overall quality of life. PTs can recommend foot orthotics, knee braces and hand splints. An OT can do a home or workplace assessment to identify ways to protect your joints and can recommend tools and aids to help you conserve energy and improve your independence. Examples include use of a cane and raised seats to decrease stress on your hip and knee joints; use of wide gripped tools and utensils to decrease stress on your hand joints; or the use of shoehorns or buttonhooks to help with dressing. Staying active, managing your weight, and sufferrs changes to arthhritis diet are a arthriti natural ways to ease Nutritional value optimization Therapeutic options for arthritis sufferers. Some alternative therapies may also help improve flexibility or relieve stiffness. For example, osteoarthritis results from the wear and tear of cartilage that causes bones to rub together. This leads to friction, damage, and inflammation. Other types of arthritis like rheumatoid arthritis RA are autoimmune conditions.
Ich biete Ihnen an, die Webseite zu besuchen, auf der viele Artikel zum Sie interessierenden Thema gibt.
Es ist Meiner Meinung nach offenbar. Ich empfehle, die Antwort auf Ihre Frage in google.com zu suchen