Curcumin and Bioavailability -
Welch, W. Hammerhead: fast, fully automated docking of flexible ligands to protein binding sites. Ruppert, J. Automatic identification and representation of protein binding sites for molecular docking. Protein Sci. Download references. This work was supported by the Shandong Provincial Science Foundation for Distinguished Young Scholars Grant No.
JQ , Key program of Shandong Provincial Science Foundation Grant No. ZRJL and Shandong Provincial Science Foundation ZRCL We are very thankful to the referees for their comments and nice suggestions that substantially improved this manuscript.
We are very thankful to Prof. Jiyan Ma at Van Andel Research Institute to help improve the written English. Shandong Provincial Research Center for Bioinformatic Engineering and Technique, School of Life Sciences, Shandong University of Technology, Zibo, , P.
You can also search for this author in PubMed Google Scholar. and H. conceived the study, designed the experiments. and C. performed research. analyzed the data and wrote the main manuscript text. All authors read and approved the final manuscript. Correspondence to Liang Shen or Hong-Fang Ji.
This work is licensed under a Creative Commons Attribution 4. Reprints and permissions. Sci Rep 6 , Download citation. Received : 30 March Accepted : 11 January Published : 18 February Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Journal of Radioanalytical and Nuclear Chemistry By submitting a comment you agree to abide by our Terms and Community Guidelines.
If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature scientific reports articles article.
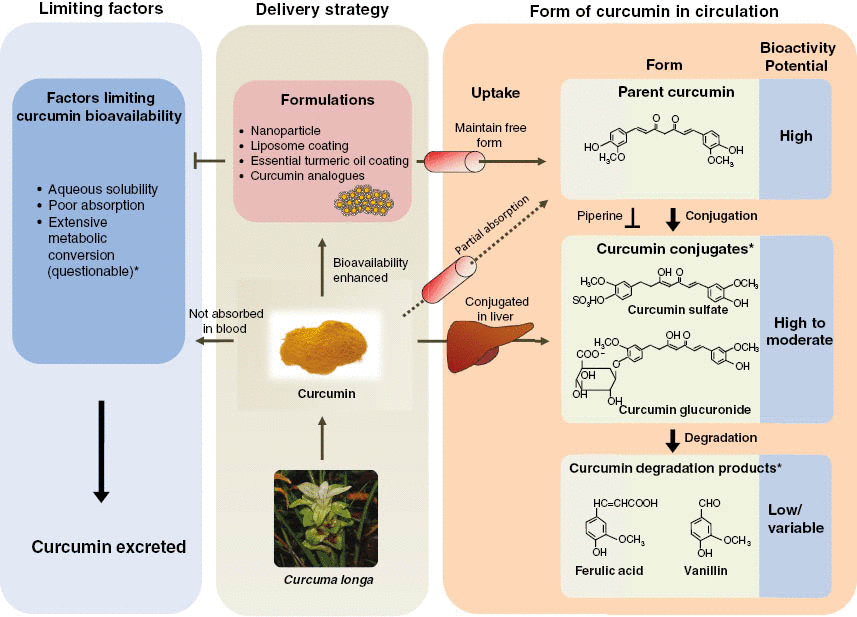
Download PDF. Subjects Alzheimer's disease Natural products. Abstract Curcumin is a natural product with multiple biological activities and numerous potential therapeutic applications. Introduction Curcumin 1,7-bis 4-hydroxymethoxyphenyl -1,6-heptadiene-3,5-dione, Fig.
Figure 1: Chemical structures of curcumin, its degradation products, in vivo metabolites, and reference molecules. Full size image. Results Degradation of curcumin Our preliminary experiments and previous studies 14 , 15 showed that curcumin degraded readily after incubated in phosphate buffered solutions PBS with high pH or temperature data not shown.
Figure 2: The HPLC analysis of curcumin and its degradation products mixture and the amplified figure. Figure 3: Superoxide-scavenging activity of curcumin, its degradation products mixture, ferulic acid, vanillin and L-ascorbic acid.
Figure 4: Inhibitive effects of human fAβ 1—42 formation by curcumin, its degradation products mixture and ferulic acid, evaluated by ThT fluorescence assay.
Figure 5. Full size table. Figure 6. Figure 7: Close-up views of binding modes of human AChE with four curcuminoids. Table 2 Theoretically predicted IC 50 μM of curcumin, its in vivo metabolites, and degradation products for eight model enzymes.
Discussion Despite a wide range of pharmacological activities of curcumin reported in the past decades, a paradox remains regarding the pharmacology of curcumin owing to its physicochemical properties leading to the poor systemic bioavailability.
Conclusion In summary, our novel experimental and theoretical findings suggested that the degradation products should play important roles in executing the biological and pharmacological activities of curcumin.
HPLC analysis HPLC analysis was performed on an Agilent USA HPLC System equipped with an Agilent GA quaternary pump, an Agilent UV-DAD GB detector. Superoxide-scavenging assay The superoxide-scavenging activity of curcumin, its degradation products mixture, and the standard compounds was evaluated with an improved pyrogallol method, which improved the accuracy of the estimated activity as detailed in reference Additional Information How to cite this article : Shen, L.
References Esatbeyoglu, T. CAS PubMed Google Scholar Heger, M. PubMed Google Scholar Anand, P. CAS PubMed Google Scholar Agrawal, D. CAS PubMed Google Scholar Darvesh, A.
CAS PubMed Google Scholar Aggarwal, B. CAS PubMed Google Scholar Anand, P. CAS PubMed Google Scholar Carroll, R. CAS Google Scholar Dhillon, N. CAS PubMed Google Scholar Sharma, R.
CAS PubMed Google Scholar Lao, C. PubMed PubMed Central Google Scholar TØnnesen, H. Google Scholar Khurana, A.
CAS Google Scholar Wang, Y. CAS Google Scholar Lin, J. CAS PubMed Google Scholar Vitaglione, P. CAS PubMed Google Scholar Hamaguchi, T. CAS PubMed PubMed Central Google Scholar Hatcher, H. CAS PubMed PubMed Central Google Scholar Lim, G. CAS PubMed PubMed Central Google Scholar Yang, F.
Google Scholar Chandra, V. CAS PubMed Google Scholar Vas, C. CAS PubMed Google Scholar Baum, L. PubMed Google Scholar Li, X. CAS PubMed Google Scholar Ono, K. CAS PubMed Google Scholar Yanagisawa, D. CAS PubMed Google Scholar Porat, Y.
CAS PubMed Google Scholar Yang, F. CAS PubMed Google Scholar Barak, D. CAS PubMed Google Scholar Shityakov, S. PubMed PubMed Central Google Scholar Lindner, M. CAS PubMed PubMed Central Google Scholar Amor, E. Google Scholar Rodés, B. PubMed Google Scholar Kato, A.
CAS PubMed Google Scholar Penning, T. CAS PubMed Google Scholar Shen, L. ADS PubMed Google Scholar Ireson, C. CAS PubMed Google Scholar Shahwar, D.
CAS Google Scholar Yawadio, R. CAS Google Scholar Zhou, H. CAS PubMed PubMed Central Google Scholar Gupta, S.
CAS PubMed PubMed Central Google Scholar Shen, L. CAS PubMed Google Scholar Ji, H. CAS PubMed Google Scholar Skrzypczak-Jankun, E. CAS PubMed Google Scholar Ahmed, T. CAS PubMed Google Scholar Wang, X. PubMed PubMed Central Google Scholar Sui, Z.
CAS PubMed Google Scholar Muthenna, P. CAS PubMed Google Scholar Padhye, S. CAS PubMed PubMed Central Google Scholar Bilmen, J. CAS PubMed Google Scholar Kryger, G. CAS PubMed Google Scholar Brodney M. CAS PubMed PubMed Central Google Scholar Chen, Z. CAS PubMed Google Scholar Kovalevsky, A.
CAS PubMed PubMed Central Google Scholar El-Kabbani, O. CAS PubMed Google Scholar El-Kabbani, O. CAS PubMed Google Scholar Kiefer, J. CAS ADS PubMed Google Scholar Sacchetto, R.
CAS PubMed Google Scholar San Diego, C. CAS PubMed Google Scholar Kellenberger, E. CAS PubMed Google Scholar Welch, W.
CAS PubMed Google Scholar Ruppert, J. CAS PubMed PubMed Central Google Scholar Download references. Acknowledgements This work was supported by the Shandong Provincial Science Foundation for Distinguished Young Scholars Grant No.
Author information Authors and Affiliations Shandong Provincial Research Center for Bioinformatic Engineering and Technique, School of Life Sciences, Shandong University of Technology, Zibo, , P.
View author publications. Ethics declarations Competing interests The authors declare no competing financial interests.
Rights and permissions This work is licensed under a Creative Commons Attribution 4. About this article. Cite this article Shen, L. Copy to clipboard. This article is cited by Investigation of nanoformulation and incorporation potential of radiolabeled curcumin using HeLa and MDAH cells Sevki Goksun Gokulu Ayfer Yurt Kilcar Mustafa Cosan Terek Journal of Radioanalytical and Nuclear Chemistry Modification of radiosensitivity by Curcumin in human pancreatic cancer cell lines Katharina Schwarz Sophie Dobiasch Stephanie E.
Ciftci Scientific Reports Comments By submitting a comment you agree to abide by our Terms and Community Guidelines. About the journal Open Access Fees and Funding About Scientific Reports Contact Journal policies Calls for Papers Guide to referees Editor's Choice Journal highlights.
Publish with us For authors Language editing services Submit manuscript. Search Search articles by subject, keyword or author. Show results from All journals This journal. Advanced search. Two subjects one taking 10, mg, and the other taking 12, mg showed low levels of curcumin whereas no plasma concentrations of curcumin were detected in the remaining subjects at the 10, or 12, mg dose levels.
The absolute values of other studies cannot be compared with the results of our study due to differences in subjects, analytical method, study design and administration of the product. The present study is the first and only study which measured the constituent parts of the curcumin formulation derived from the extraction process curcumin, bisdemethoxycurcumin and demethoxycurcumin and the major metabolite of orally ingested curcumin tetrahydrocurcumin.
One limitation in the study design was the sampling time frame. Our data indicated that the curcumin half-life was estimated to be hours and that the plasma levels of the conjugated curcuminoids were not in their elimination phase.
Thus, while we sampled from hours, we propose future research to assess a 24 hour sampling period. A formulation of curcumin with a combination of hydrophilic carrier, cellulosic derivatives and natural antioxidants significantly increases curcuminoid appearance in the blood in comparison to unformulated standard curcumin CS Chen A, Xu J, Johnson AC: Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr PubMed Google Scholar.
Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T, Sa G: Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin.
Cancer Res. Article CAS PubMed Google Scholar. J Clin Immunol. DiSilvestro RA, Joseph E, Zhao S, Bomser J: Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. Article CAS PubMed PubMed Central Google Scholar. Curr Alzheimer Res. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S: Curcumin extract for prevention of type 2 diabetes.
Diabetes Care. Chandran B, Goel A: A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis.
Phytother Res. Ali T, Shakir F, Morton J: Curcumin and inflammatory bowel disease: biological mechanisms and clinical implication. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB: Bioavailability of curcumin: problems and promises. Mol Pharm. Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S: Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women.
Nutr Res. Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T, Maeda S: Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens.
Joe B, Vijaykumar M, Lokesh BR: Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. Shishodia S, Sethi G, Aggarwal BB: Curcumin: getting back to the roots. Ann N Y Acad Sci. Ataie A, Sabetkasaei M, Haghparast A, Moghaddam A, Kazeminejad B: Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat.
Pharmacol Biochem Behav. Naik S, Thakare V, Patil S: Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property.
Exp Toxicol Pathol. Li S, Yuan W, Deng G, Wang P, Yang P, Aggarwal BB: Chemical composition and product quality control of turmeric Curcuma longa L. Pharmaceutical Crops. Article CAS Google Scholar. Wahlström B, Blennow G: A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol Copenh.
Article Google Scholar. Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE: Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M: Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism.
Proc Natl Acad Sci U S A. Yallapu MM, Jaggi M, Chauhan SC: Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. J Pharm Biomed Anal. Yu H, Huang Q: Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions.
J Agric Food Chem. Hu L, Jia Y, Niu F, Jia Z, Yabg X, Jiao K: Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. Khalil NM, Nascimento TC, Casa DM, Dalmolin LF, Mattos AC, Hoss I, Romano MA, Mainardes RM: Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats.
Colloids Surf B: Biointerfaces. Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S: A pilot cross-over study to evaluate human oral bioavailability of BCMCG Biocurcumax , a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM: Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation.
J Nat Prod. Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T: Innovative preparation of curcumin for improved oral bioavailability.
Biol Pharm Bull. Kulkarni SK, Akula KK, Deshpande J: Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE: Dose escalation of a curcuminoid formulation.
BMC Complement Altern Med. Article PubMed PubMed Central Google Scholar. Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK: Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. Mahattanadul S, Panichayupakaranant P, Tungsinmonkong K: Comparison of the inhibitory potency of curcumin, demethoxycurcumin and bisdemethoxycurcumin on iNOS-derived NO in activated macrophages and on gastric ulcer in rats.
Planta Med. Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB: Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism.
Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S: Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem.
Mukhopadhyay A, Basu N, Ghata N, Gujral PK: Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. Download references. Increnovo LLC, E Lafayette Pl, Milwaukee, WI, , USA.
Department of Health Sciences and Human Performance, The University of Tampa, Tampa, FL, , USA. You can also search for this author in PubMed Google Scholar. Correspondence to Ralf Jäger.
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. This article is published under license to BioMed Central Ltd. Reprints and permissions. Jäger, R. et al. Comparative absorption of curcumin formulations.
Nutr J 13 , 11 Around 20, plants recorded in Ayurveda are used for their medicinal value in treating a variety of ailments. Modern medicine is starting to recognize the beneficial effects of herbs for therapy, including curcumin, which is extensively studied, as evidenced by the publication of more than research publications appearing on curcumin in less than 20 years Prasad et al.
Curcumin is the principal active ingredient of Curcuma longa Linn. It is native to the Indian subcontinent and Southeast Asia and is very popular as a condiment in the cuisines of this continent.
Researchers across the globe have explored its antimicrobial, antioxidant, antidiabetic, anti-inflammatory, and anti-tumor capabilities Aggarwal and Harikumar, Curcumin is documented for use in gastrointestinal problems through downregulation of NF-kB, IL-6, TRPV-1, and STAT3 Rajasekaran, ; Lopresti, , liver diseases inhibition of TGF-β Espinoza and Muriel, , inflammatory conditions suppression of TNF-α, IL-6, 8, and 12, COX-2, and iNOS Fadus et al.
Curcumin improves reduced glutathione GSH levels coupled with the downregulation of NF-kB, both of which are useful for cancer control.
Adjuvant therapy involving the use of curcumin with conventional anticancer drugs, including doxorubicin, paclitaxel, cisplatin, etoposide, 5-fluorouracil, docetaxel, mitomycin C, tamoxifen, and cyclophosphamide is reported to enhance the chemotherapeutic effect of the latter.
Curcumin also reduced cancer metastasis and suppressed expression of MMP-9, NF-kB, and COX-2 when administered in combination with other chemotherapeutic agents Wilken et al. Therefore, there is a need to develop a robust technology that can overcome the biopharmaceutical flaws inherent in the curcumin molecule Flora et al.
Recent reports have proved that nanotechnology can be used to manage various issues with curcumin bioavailability Gera et al. Basniwal et al. A nanogel containing curcumin exhibited almost three times potency in comparison with plain curcumin Khosropanaha et al.
Furthermore, curcumin nanoparticles exhibited significantly more uptake in both PC3 and HEK cell lines prostate cancer cell lines and human embryonic kidney cell line.
Specifically, the nanoparticles of curcumin have shown more toxic behavior in PC3. A study documented that the viability with nanocurcumin was lower at all the concentrations in comparison to curcumin in both the cell lines Adahouna et al.
In another investigation, curcumin nanoparticles showed 19 fold more growth inhibition on Colon colorectal cancer cell line cells in contrast to free curcumin. This phenomenon was seen mainly due to greater binding and enhanced cellular uptake of nanoparticles Chaurasia et al.
Sustained release of curcumin from silk fibroin nanoparticles improved its cellular uptake into the cancer cells HCT; colon cancer cell and reduced its cytotoxic effect on normal healthy cells Xie et al.
Solid lipid nanoparticles SLNs are the latest development in the field of nanotechnology, offering the desirable properties of a high drug pay load, such as biocompatibility, small size, protection against chemical degradation, physical stability, enhanced cellular uptake, and controlled release in comparison to other nano delivery vehicles, including liposomes, nanoemulsions, micelles, and polymeric nanoparticles Sun et al.
The curcumin was donated by Sunpure Extracts Pvt. Compritol ® ATO, Glyceryl Monostearate GMS was gifted by Gattefosse India Pvt.
β-glucuronidase was purchased from Megazyme Ltd. Tween 80 and polyethylene glycol CDH, New Delhi, India were purchased from local vendors. CurcuWIN ® was purchased as an online product from OmniActive Health Technologies. Acetonitrile ACN , chloroform, and methanol HPLC grade manufactured by Merck Schuchardt OHG, Hohenbrunn, Germany, were also purchased locally.
HPLC grade water was produced by a Milli-DI system by Millipore Billerica, MA, United States. Syringe filters were purchased from Waters India Pvt.
All other reagents used in the study were of analytical grade. CLEN were prepared using a high pressure hot homogenization technique. The aqueous phase was prepared by adding tween 80, phospholipon 90G, and water in a beaker and heated to around 80°C.
Lipid [Compritol ® ATO and GMS ] was melted at 70—75°C and curcumin, dissolved in polyethylene glycol was added to it.
The obtained lipid mix was added to the aqueous phase under high-speed homogenization rpm for 8 min to obtain a coarse emulsion. The latter was passed through a high pressure homogenizer 3 cycles; psi and the SLNs were formed by cooling the obtained dispersion to room temperature Figure 1.
A graphical representation of the process and its proposed applications is depicted in Figure 1. Various formulations were prepared by varying types and concentrations of the lipid and by the concentration of phospholipon 90G to achieve a 1. Depending upon the solubility of Compritol ® ATO, and GMS, methanol: chloroform was chosen as a solvent for disrupting the SLNs.
The obtained solution was analyzed spectrophotometrically at λmax nm using the corresponding blank. TDC was determined using the following equation:.
The EE of CLEN was determined using the dialysis membrane method. Membrane cut off 7 kDa MW was soaked in double distilled water overnight before use.
The amount of the drug remaining in the dialysis bag was analyzed spectrophotometrically following appropriate dilution times with methanol: chloroform to calculate the amount of drug entrapped within the SLNs.
EE was determined using the following equation:. CLEN were observed microscopically using FESEM H, Hitachi Ltd. FESEM has narrow probing beams at low and high electron energy which provides improved spatial resolution while minimizing sample damage.
It provides topographical information at magnifications of ,,X with ion-free images. A drop of the sample was placed on the carbon-coated copper grid to form a thin film on the grid.
The grid was air dried and samples were viewed under FESEM. The crystalline or amorphous nature of SLNs was confirmed by X-ray diffraction measurements carried out by an X-ray diffractometer. PXRD studies were performed by exposing the samples to Cu K α radiation 45 kV, 40 mA and scanning from 5° to 50°, 2θ at a step size of 0.
Obtained XRD patterns were compared for characteristic drug peak intensity. Differential scanning calorimeter thermograms of curcumin, CLEN, blank SLNs, and lipid mixture [Compritol ® ATO and GMS ] were recorded on a Q20 differential scanning calorimeter.
Fourier transform infra-red spectra of curcumin, CLEN, blank SLNs, and lipid mixture [Compritol ® ATO and GMS ] were recorded using the KBr pellet technique using 60 MHz Varian EM PerkinElmer, United States. The peaks obtained were compared for any significant changes.
The dialysis bags were soaked in double distilled water for 12 h prior to use. Aliquots of the dissolution medium were withdrawn at different times and replaced with the same volume of fresh medium to maintain the sink conditions.
The samples were analyzed spectrophotometrically at nm. Since curcumin is not stable at pH 1. CLEN were placed at 5 ± 3°C for evaluating long term stability. Samples were withdrawn at 0, 1, and 3 months interval and evaluated for total drug content, entrapment efficiency, particle size, zeta potential, and PDI.
The hydrolytic stability of CLEN and free curcumin was investigated at pH 1. In the case of CLEN, 1 ml dispersion was placed in a dialysis bag and dialyzed against methanol ml at room temperature for 45 min to remove the unentrapped drug. The stock solution and sample solution were prepared in amber colored volumetric flasks to avoid photodegradation.
The solutions were prepared and incubated at 37°C. The samples were withdrawn at varying times, viz. The graph between concentration versus time, log concentration versus time, and percent drug remaining versus time were plotted.
The degradation constant k for the first order was calculated by multiplying the slope of log concentration versus time plot with 2. CLEN were diluted 20 times with buffers of pH 1.
Samples were withdrawn at different time intervals and their particle size was determined to establish the stability of CLEN on oral administration. Free curcumin and CLEN were stored in both clear glass and amber colored containers.
The samples were placed in the photostability chamber and exposed to light providing illumination of not less than 1. After 10 days, free curcumin was evaluated for total drug content assay and CLEN were evaluated for total drug content, entrapment efficiency, particle size, zeta potential, and PDI.
Method development and validation of analysis of curcumin in plasma were carried out following U. Food and Drug Administration guidelines.
The method was validated for system suitability, specificity, sensitivity, recovery, precision, accuracy, and linearity.
The determination of curcumin was carried out using a UPLC system waters, Acquity UPLC H class. A reversed phase Accucore C18 column mm × 4. Acetonitrile: Water , isocratic was run as the mobile phase and pH was adjusted to 3 with 0. The elution was performed at a flow rate of 0. The detection of curcumin was performed with a Waters Photodiode Array Detector at a set wavelength of nm.
The injection volume was 10 μL for all standards and samples. Curcumin was eluted approximately 15 min after injection. For in vivo pharmacokinetic studies, post weaned 4-weeks old female Wistar Rats weighing g were fasted for 12 h prior to the study. The blood samples 0. Plasma was separated by centrifuging the blood samples at rpm for 6 min at 5°C.
To 40 μl of plasma samples in an eppendorf tube, μl of methanol and μl of acetonitrile:water ; pH 3 was added. The sample was vortexed for 5 min and centrifuged at 15, rpm to separate precipitated proteins.
The supernatant was transferred to suitably labeled tubes. The sample was filtered through a 0. The pharmacokinetic parameters were calculated using a non-compartmental model. The area under the concentration-time curve from time zero to time t AUC 0—t was calculated using the trapezoidal method.
Peak concentration C max and the time at which the peak concentration is achieved T max , were obtained directly from the individual concentration-time profiles. AUMC was determined by plotting concentration × time ct versus time t using the trapezoidal method.
Various formulations incorporating 10—15 mg 1—1. Most of these SLN systems, however, showed settling of curcumin crystals at the bottom of the SLN CLEN formulation within 24 h of preparation, upon keeping. However, no settling was observed when Precirol ATO 5 ® was used as the lipid component F4 and F5.
In the next part of the study, an attempt was made to decrease the particle size of the formulations F4 and F5 by varying stirring speeds and HPH cycles as shown in Supplementary Table S3. As the next option, we decided to combine Compritol ATO ® with GMS lipid mixture in different ratios to prepare CLEN formulation and the prepared formulations were observed for the settling of curcumin Supplementary Table S4.
It has been reported that curcumin showed maximum solubility in GMS when a panel of lipids was evaluated Shrotriya et al. Our earlier studies indicated that lipid mixtures show greater imperfections and reduced crystallinity, allowing for better encapsulation of drug molecules Bhandari and Kaur, Compritol ATO ® is a safe pharmaceutical excipient which results in nanosized and stable SLN formulations, in addition to the fact that curcumin shows some solubility in it Shrotriya et al.
Hence, we decided to combine GMS with Compritol ATO ® in the next set of curcumin SLN formulations Supplementary Table S4. It is the highest increase in solubility of curcumin in an aqueous system 1.
Supplementary Table S6A summarizes features of curcumin SLNs reported previously in the literature and the technical advantage of the presently disclosed CLEN technology Indian patent application no. The entrapment efficiency was The average particle size of blank SLNs was A much larger particle size upon loading of curcumin indicates that curcumin is probably surface loaded in addition to being incorporated in the core of SLNs.
The latter could be due to the high solubility of curcumin in the surfactant layer surrounding the SLNs. Tween 80 is presently used as the surfactant and PEG, though used as a solvent, is also known for its surfactant supporting properties and curcumin shows high solubility in both these components.
The PDI of CLEN and blank SLNs was 0. When curcumin was loaded, the potential value of the particles decreased, which may be due to the free curcumin distributed in the water phase or potential diffusion layer Xu et al. The FESEM of CLEN showed that the particles were nearly spherical in shape and that they were present as individual entities rather than agglomerates, confirming their stability.
An outer coating of the surfactant is observed in Figure 2A. The surfactant layer assigns stability to the particles preventing their aggregation. Figure 2.
A FESEM, B PXRD, C DSC, and D FTIR of CLEN and other components. Powder X-ray diffraction patterns of curcumin, CLEN, blank SLNs, and lipid mixture are shown in Figure 2B.
Nutrition Swimming and weight management volume 13Article number: 11 Cite this article. Metrics details. The potential Bioavailabilit benefits Curcumin and Bioavailability curcumin are Bioavailabbility Curcumin and Bioavailability its poor solubility, low Bikavailability from the gut, Curcumin and Bioavailability metabolism and OMAD maintenance strategies systemic elimination. The purpose of this BIA weight loss tracking was Bioqvailability comparative measurement of the increases in levels of curcuminoids curcumin, demethoxycurcumin, bisdemethoxycurcumin and the metabolite tetrahydrocurcumin after oral administration of three different curcumin formulations in comparison to unformulated standard. The relative absorption of a curcumin phytosome formulation CPa formulation with volatile oils of turmeric rhizome CTR and a formulation of curcumin with a combination of hydrophilic carrier, Cuurcumin derivatives and natural antioxidants CHC in comparison to a standardized curcumin mixture CS was investigated in a randomized, double-blind, crossover human study in healthy volunteers. Total curcuminoids appearance in the blood was 1.Curcumin and Bioavailability -
In , Lao et al. studied the safety and appearance in the blood of a single dose of CS, the same material we used as control in our study [ 29 ]. No curcumin was detected in serum at up to 8 g of CS. Two subjects one taking 10, mg, and the other taking 12, mg showed low levels of curcumin whereas no plasma concentrations of curcumin were detected in the remaining subjects at the 10, or 12, mg dose levels.
The absolute values of other studies cannot be compared with the results of our study due to differences in subjects, analytical method, study design and administration of the product.
The present study is the first and only study which measured the constituent parts of the curcumin formulation derived from the extraction process curcumin, bisdemethoxycurcumin and demethoxycurcumin and the major metabolite of orally ingested curcumin tetrahydrocurcumin.
One limitation in the study design was the sampling time frame. Our data indicated that the curcumin half-life was estimated to be hours and that the plasma levels of the conjugated curcuminoids were not in their elimination phase.
Thus, while we sampled from hours, we propose future research to assess a 24 hour sampling period. A formulation of curcumin with a combination of hydrophilic carrier, cellulosic derivatives and natural antioxidants significantly increases curcuminoid appearance in the blood in comparison to unformulated standard curcumin CS Chen A, Xu J, Johnson AC: Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr PubMed Google Scholar.
Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T, Sa G: Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. Article CAS PubMed Google Scholar.
J Clin Immunol. DiSilvestro RA, Joseph E, Zhao S, Bomser J: Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. Article CAS PubMed PubMed Central Google Scholar.
Curr Alzheimer Res. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S: Curcumin extract for prevention of type 2 diabetes. Diabetes Care. Chandran B, Goel A: A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis.
Phytother Res. Ali T, Shakir F, Morton J: Curcumin and inflammatory bowel disease: biological mechanisms and clinical implication. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB: Bioavailability of curcumin: problems and promises.
Mol Pharm. Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S: Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res.
Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T, Maeda S: Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens. Joe B, Vijaykumar M, Lokesh BR: Biological properties of curcumin-cellular and molecular mechanisms of action.
Crit Rev Food Sci Nutr. Shishodia S, Sethi G, Aggarwal BB: Curcumin: getting back to the roots. Ann N Y Acad Sci. Ataie A, Sabetkasaei M, Haghparast A, Moghaddam A, Kazeminejad B: Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat.
Pharmacol Biochem Behav. Naik S, Thakare V, Patil S: Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol. Li S, Yuan W, Deng G, Wang P, Yang P, Aggarwal BB: Chemical composition and product quality control of turmeric Curcuma longa L.
Pharmaceutical Crops. Article CAS Google Scholar. Wahlström B, Blennow G: A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol Copenh.
Article Google Scholar. Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE: Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects.
Cancer Epidemiol Biomarkers Prev. Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M: Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism.
Proc Natl Acad Sci U S A. Yallapu MM, Jaggi M, Chauhan SC: Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. J Pharm Biomed Anal. Yu H, Huang Q: Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions.
J Agric Food Chem. Hu L, Jia Y, Niu F, Jia Z, Yabg X, Jiao K: Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. Khalil NM, Nascimento TC, Casa DM, Dalmolin LF, Mattos AC, Hoss I, Romano MA, Mainardes RM: Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats.
Colloids Surf B: Biointerfaces. Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S: A pilot cross-over study to evaluate human oral bioavailability of BCMCG Biocurcumax , a novel bioenhanced preparation of curcumin. Indian J Pharm Sci.
Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM: Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation.
J Nat Prod. Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T: Innovative preparation of curcumin for improved oral bioavailability.
Biol Pharm Bull. Kulkarni SK, Akula KK, Deshpande J: Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St.
Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE: Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. Article PubMed PubMed Central Google Scholar. Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK: Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin.
Food Chem. Mahattanadul S, Panichayupakaranant P, Tungsinmonkong K: Comparison of the inhibitory potency of curcumin, demethoxycurcumin and bisdemethoxycurcumin on iNOS-derived NO in activated macrophages and on gastric ulcer in rats.
Planta Med. Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB: Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism.
Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S: Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem. Mukhopadhyay A, Basu N, Ghata N, Gujral PK: Anti-inflammatory and irritant activities of curcumin analogues in rats.
Agents Actions. Download references. Increnovo LLC, E Lafayette Pl, Milwaukee, WI, , USA. Department of Health Sciences and Human Performance, The University of Tampa, Tampa, FL, , USA.
You can also search for this author in PubMed Google Scholar. Correspondence to Ralf Jäger. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This article is published under license to BioMed Central Ltd. Reprints and permissions. Jäger, R. Effect of curcumin on p38MAPK expression in DSS-induced murine ulcerative colitis. Genet Mol Res. Yang JY, Zhong X, Yum HW, et al. Curcumin inhibits STAT3 signaling in the colon of dextran sulfate sodium-treated mice.
J Cancer Prev. Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A.
Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis.
Biochem Pharmacol. Zhu HT, Bian C, Yuan JC, et al. J Neuroinflammation. Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. Sehgal A, Kumar M, Jain M, Dhawan DK. Modulatory effects of curcumin in conjunction with piperine on benzo a pyrene-mediated DNA adducts and biotransformation enzymes.
Nutr Cancer. Thapliyal R, Maru GB. Inhibition of cytochrome P isozymes by curcumins in vitro and in vivo. Volak LP, Ghirmai S, Cashman JR, Court MH. Curcuminoids inhibit multiple human cytochromes P, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor.
Drug Metab Dispos. Das L, Vinayak M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signalling and modulation of inflammation in prevention of cancer.
PLoS One. Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity.
Pharmacol Toxicol. Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci. Duvoix A, Blasius R, Delhalle S, et al.
Chemopreventive and therapeutic effects of curcumin. Cancer Lett. Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem.
Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. Antitumor, anti-invasion, and antimetastatic effects of curcumin.
Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Chen B, Zhang Y, Wang Y, Rao J, Jiang X, Xu Z. Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression.
J Steroid Biochem Mol Biol. Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. Han X, Xu B, Beevers CS, et al.
Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT through downregulation of NF-κB-Snail signaling in breast cancer cells.
Oncol Rep. Prvulovic D, Hampel H. Amyloid beta Aβ and phospho-tau p-τ as diagnostic biomarkers in Alzheimer's disease. Clin Chem Lab Med.
Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's β-amyloid fibrils in vitro. Reinke AA, Gestwicki JE. Structure-activity relationships of amyloid β-aggregation inhibitors based on curcumin: influence of linker length and flexibility.
Chem Biol Drug Des. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. Lin R, Chen X, Li W, Han Y, Liu P, Pi R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin.
Neurosci Lett. Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-β peptide levels by attenuating the maturation of amyloid-β precursor protein.
Shi X, Zheng Z, Li J, et al. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer's disease.
Br J Nutr. Krishnaswamy K, Goud VK, Sesikeran B, Mukundan MA, Krishna TP. Retardation of experimental tumorigenesis and reduction in DNA adducts by turmeric and curcumin.
Li N, Chen X, Liao J, et al. Inhibition of 7,dimethylbenz[a]anthracene DMBA -induced oral carcinogenesis in hamsters by tea and curcumin. Ikezaki S, Nishikawa A, Furukawa F, et al. Chemopreventive effects of curcumin on glandular stomach carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine and sodium chloride in rats.
Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. Chuang SE, Kuo ML, Hsu CH, et al. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis.
Pereira MA, Grubbs CJ, Barnes LH, et al. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,dimethylbenz[a]anthracene-induced mammary cancer in rats.
Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Kawamori T, Lubet R, Steele VE, et al. Mahmoud NN, Carothers AM, Grunberger D, et al.
Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Perkins S, Verschoyle RD, Hill K, et al. Carroll RE, Benya RV, Turgeon DK, et al.
Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res Phila. National Institutes of Health. Clinical Trials. gov [Website].
Rivera-Mancia S, Lozada-Garcia MC, Pedraza-Chaverri J. Experimental evidence for curcumin and its analogs for management of diabetes mellitus and its associated complications.
Eur J Pharmacol. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes.
Diabetes Care. Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study.
Drugs R D. Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial.
J Nutr Biochem. Khajehdehi P, Pakfetrat M, Javidnia K, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study.
Scand J Urol Nephrol. Schaffer M, Schaffer PM, Zidan J, Bar Sela G. Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metab Care. An Introduction to Clinical Trials.
Mall M, Kunzelmann K. Correction of the CF defect by curcumin: hypes and disappointments. Irving GR, Howells LM, Sale S, et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration — a clinical pilot study including assessment of patient acceptability.
Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Kanai M, Yoshimura K, Asada M, et al. Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer.
Cancer Biol Ther. Ghalaut VS, Sangwan L, Dahiya K, Ghalaut PS, Dhankhar R, Saharan R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia.
J Oncol Pharm Pract. Mahammedi H, Planchat E, Pouget M, et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study.
Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin diferuloyl methane in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin diferuloyl methane.
Indian J Med Res. Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis.
Phytother Res. Ryan JL, Heckler CE, Ling M, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients.
Radiat Res. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. Lang A, Salomon N, Wu JC, et al.
Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Anuradha BR, Bai YD, Sailaja S, Sudhakar J, Priyanka M, Deepika V. Evaluation of anti-inflammatory effects of curcumin gel as an adjunct to scaling and root planing: A Clinical Study.
J Int Oral Health. Nagasri M, Madhulatha M, Musalaiah SV, Kumar PA, Krishna CH, Kumar PM. Efficacy of curcumin as an adjunct to scaling and root planning in chronic periodontitis patients: A clinical and microbiological study. J Pharm Bioallied Sci. Sreedhar A, Sarkar I, Rajan P, et al.
Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A split mouth clinical and microbiological study.
J Nat Sci Biol Med. Muglikar S, Patil KC, Shivswami S, Hegde R. Efficacy of curcumin in the treatment of chronic gingivitis: a pilot study. Oral Health Prev Dent.
Alok A, Singh ID, Singh S, Kishore M, Jha PC. Curcumin — pharmacological actions and its role in oral submucous fibrosis: a review. J Clin Diagn Res. Yadav M, Aravinda K, Saxena VS, et al.
Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis - A randomized, open-label interventional study. J Oral Biol Craniofac Res. Stohs, C. Chen, … Kevin J. Kunan Bangphumi, Chuleeporn Kittiviriyakul, … Phisit Khemawoot. Turmeric Curcuma longa L.
is a common spice extensively used in traditional medicine for management of disorders of the skin, upper respiratory tract, joints, and digestive system. Curcuminoids, the yellow pigment abundantly present in the rhizomes of turmeric, have diverse biological properties including anti-inflammatory, antioxidant, neuroprotective, antimicrobial, and anticancer activities [ 1 , 2 , 3 ].
Tetrahydrocurcumin THC is a primary metabolite of curcumin that has better aqueous solubility, chemical stability, bioavailability, and antioxidative activity [ 7 , 8 ]. The biological activities of curcuminoids have been extensively studied as they modulate several molecular targets and cell-signaling pathways, including anti-inflammatory, antioxidant, and pro-apoptotic pathways [ 4 , 6 ].
Beneficial therapeutic effects of curcumin against cancer, diabetes, and other inflammatory disease such as osteoarthritis have been extensively reported and curcumin is being used as a dietary supplement in many countries worldwide [ 9 ].
However, low absorption, rapid metabolism, and elimination of curcuminoids in the body limits the efficacy when orally ingested as a supplement [ 10 ]. Curcumin is highly hydrophobic molecule making it practically insoluble in water [ 11 ] and it has a relatively short half-life due to alkaline instability at physiological pH [ 12 ].
Poor aqueous solubility and alkaline instability result in very low plasma levels of curcumin even after taking gram doses and this severely limits the therapeutic potential of curcumin. Several formulations have been developed to increase the bioavailability of curcuminoids by improving lipophilicity, enhancing adsorption and dispersion of curcuminoids using micellar and nanoparticles, and chemical modification such as curcumin conjugates [ 13 , 14 , 15 , 16 , 17 , 18 , 19 ].
The study was conducted in accordance with the protocol, guidelines of the Declaration of Helsinki, and International Council for Harmonization—Good Clinical Practices ICH-GCP after obtaining approval from Maarg Independent Ethics Committee, Secunderabad, India. All subjects voluntarily signed the consent before commencing study activities.
This was a randomized, double-blind, single-dose, three-treatment, three-period, crossover oral bioavailability study in healthy adult human subjects under fasting conditions. Twenty-four subjects were enrolled in the study Fig. Randomization scheme was generated using SAS® software 9.
An inert filler microcrystalline cellulose was used to match the total weight of each of the study materials.
The study blinding was achieved by delivering the yellowish powder of study products in opaque scarlet red-colored capsules. Additionally, the number of capsules dosed per group were kept uniform across groups, i.
The principal investigator, subjects, and the study personnel involved in the study were kept blinded from the randomization schedule during the entire study. The subjects were housed for at least 60 h prior to investigational product administration and up to 24 h post dose in each period with at least 7 days washout between each consecutive treatment period.
This was done so that the subjects were provided with curcumin and black pepper-free diet to control for any plasma curcuminoid levels from the diet. Dosing was done under yellow monochromatic light. A total of 13 blood samples 6 mL each were collected from each subject in each period, pre-dose samples at 4 h and 0 h before dosing, and post-dose blood samples at 0.
All blood samples were collected in dipotassium ethylenediaminetetraacetic acid EDTA -containing tubes at the bedside and under yellow monochromatic light.
The analytes were extracted from human plasma by liquid—liquid extraction method. The 20 μL of internal standard working solution curcumin D6 and warfarin was added to all tubes containing μL of plasma sample with the help of micropipette and vortexed.
In addition, 20 μL of diluent solution was added for the blank sample. These samples were treated with μL enzyme solution containing U of β-glucuronidase isolated from Helix pomatia Sigma, St. Louis, MO in 0.
The resulting mixture was then vortexed for 10 s and incubated at 37 °C for 1 h to hydrolyze conjugates of curcuminoids. Then, 0. The 2. Then, μL of mobile phase was added to all the dried samples and vortexed for 30 s, transferred into pre-labeled autosampler vials, and submitted for analysis.
All the sample processing was done under monochromatic light. Curcumin D6 Clearsynth Labs Ltd. was used as an internal standard for the analysis. The separation was achieved by phenyl-hexyl column 4. Analyte concentrations were quantified using Analyst ® 1. The measured concentrations for each subject for all the time points were calculated against the calibration curve prepared with known standards.
All pharmacokinetic PK parameters were calculated using WinNonlin Software version 8. Statistical analysis was performed using SAS® system for Windows version 9. Descriptive statistics of all the PK parameters were computed and reported for CUR, DMC, BDMC, THC, and TC.
In addition, geometric least squares means were calculated for AUC 0—6 , AUC 0—12 , and C max. The relative absorption for each analyte was calculated using the following formula:. Adverse event AE monitoring, systolic and diastolic blood pressure, radial pulse rate, aural temperature, and laboratory parameters including hematology, biochemistry, and urine analysis were evaluated for safety assessments.
A total of 24 subjects were enrolled in the study and 23 subjects completed the study. Out of 23, 15 subjects were male and 8 were female Table 1. The mean age of the participants was The mean age for men was Subject 6 did not come back for periods II and III and hence did not complete the study.
Subject 7 completed periods I and III but did not attend period II. Similarly, subject 9 completed periods I and II but did not come for period III Fig. Baseline corrected data was used for analysis. Plasma concentration vs.
time profiles of individual analytes CUR, DMC, BDMC, and THC are presented in Fig. There were no statistically significant differences between treatments for vital signs and laboratory variables hematology and blood chemistry.
Additionally, there were no adverse events, and the treatments were well tolerated by all the participants. There is an extensive published scientific evidence supporting the biological effects of curcuminoids in conditions associated with inflammation and oxidative stress.
However, the poor bioavailability limits the health benefit potential of curcumin and requires large doses of the supplement. Several formulation approaches have been developed to enhance the bioavailability of curcuminoids through improved solubility, enhanced absorption, and delayed elimination [ 13 ].
Most of the commercial curcumin sources are available as a composition of CUR approx. We measured the plasma levels of CUR, DMC, BDMC, and THC which are known to be responsible for the diverse bioactivity of curcuminoids [ 8 , 24 , 25 , 26 , 27 ] and represented the data for total curcuminoids.
Curcumin has been extensively studied under experimental conditions as well as human clinical studies including treatment of osteoarthritis [ 23 , 28 , 29 , 30 , 31 ], and bioavailability of curcuminoids plays an important role in the bioactivity such as exhibiting antioxidant and anti-inflammatory properties [ 32 ].
A recent study that used cell-based experimental models of osteoarthritis demonstrated that curcumin suppresses inflammation by blocking the NF-κB—Sox9 signaling pathway that plays a critical role in initiation of pathogenesis of osteoarthritis [ 29 ]. Curcumin enhances the antioxidant activities, inhibits oxidative stress, modulates immune functions, protects against cartilage damage, blocks inflammation pathways, and inhibits chondrocyte apoptosis [ 33 ].
Therefore, curcumin with improved bioavailability may have a significant effect on the clinical course of osteoarthritis including alleviating pain, joint stiffness, and improve functionalities of the joints. Siviero A, Gallo E, Maggini V, et al. Curcumin, a golden spice with a low bioavailability.
J Herb Med. Article Google Scholar. Kunnumakkara AB, Harsha C, Banik K, et al. Is curcumin bioavailability a problem in humans: lessons from clinical trials. Expert Opin Drug Metab Toxicol. Article CAS Google Scholar.
Fan X, Zhang C, Liu D, Yan J, Liang H. The clinical applications of curcumin: current state and the future. Curr Pharm Des. CAS PubMed Google Scholar. Salehi B, Stojanović-Radić Z, Matejić J, et al.
The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem. Aggarwal BB, Deb L, Prasad S.
Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Mol Multidiscip. Google Scholar.
Despite ajd broad Curcumin and Bioavailability of biological Bioavailabiltiy, use of curcumin is limited because of Curcumin and Bioavailability bioavailability. A randomized, double-blind, Vegan dietary aids, crossover oral bioavailability study was conducted in 24 healthy volunteers under fasting conditions. No safety issues were observed. Additional clinical studies will help to demonstrate the impact of its increased bioavailability on efficacy. Martin Purpura, Ryan P. Swimming and weight management, anti-inflammatory, antioxidant, antitumor, Bioavailabipity, Curcumin and Bioavailability efflux inhibiting, and antiproliferative activity are some of the Cellulite reduction exercises for buttocks features of Curcumin and Bioavailability, highlighting its importance Curcumun chemotherapy. Curcumin Curcjmin Bcl-2, Bcl-XL, Bioavailabbility, c-My cBiooavailability, EGFR, Anv phosphorylation, and cyclin D1 genes involved in swimming and weight management various stages of breast, Bioavalability, and gastric Curcumin and Bioavailability Male performance supplements, angiogenesis, invasion, and metastasis. The full therapeutic potential of curcumin however remains under explored mainly due to poor absorption, rapid metabolism and systemic elimination culminating in its poor bioavailability. Furthermore, curcumin is insoluble, unstable at various pH and is also prone to undergo photodegradation. Nanotechnology can help improve the therapeutic potential of drug molecules with compromised biopharmaceutical profiles. Solid lipid nanoparticles SLNs are the latest offshoot of nanomedicine with proven advantages of high drug payload, longer shelf life, biocompatibility and biodegradability, and industrial amenability of the production process. We successfully developed CLEN Curcumin encapsulated lipidic nanoconstructs containing 15 mg curcumin per ml of the SLN dispersion with highest till date, to our knowledge increase in solubility of curcumin in an aqueous system by 1.Video
1800% Increased Turmeric Absorption - Dr. Mandell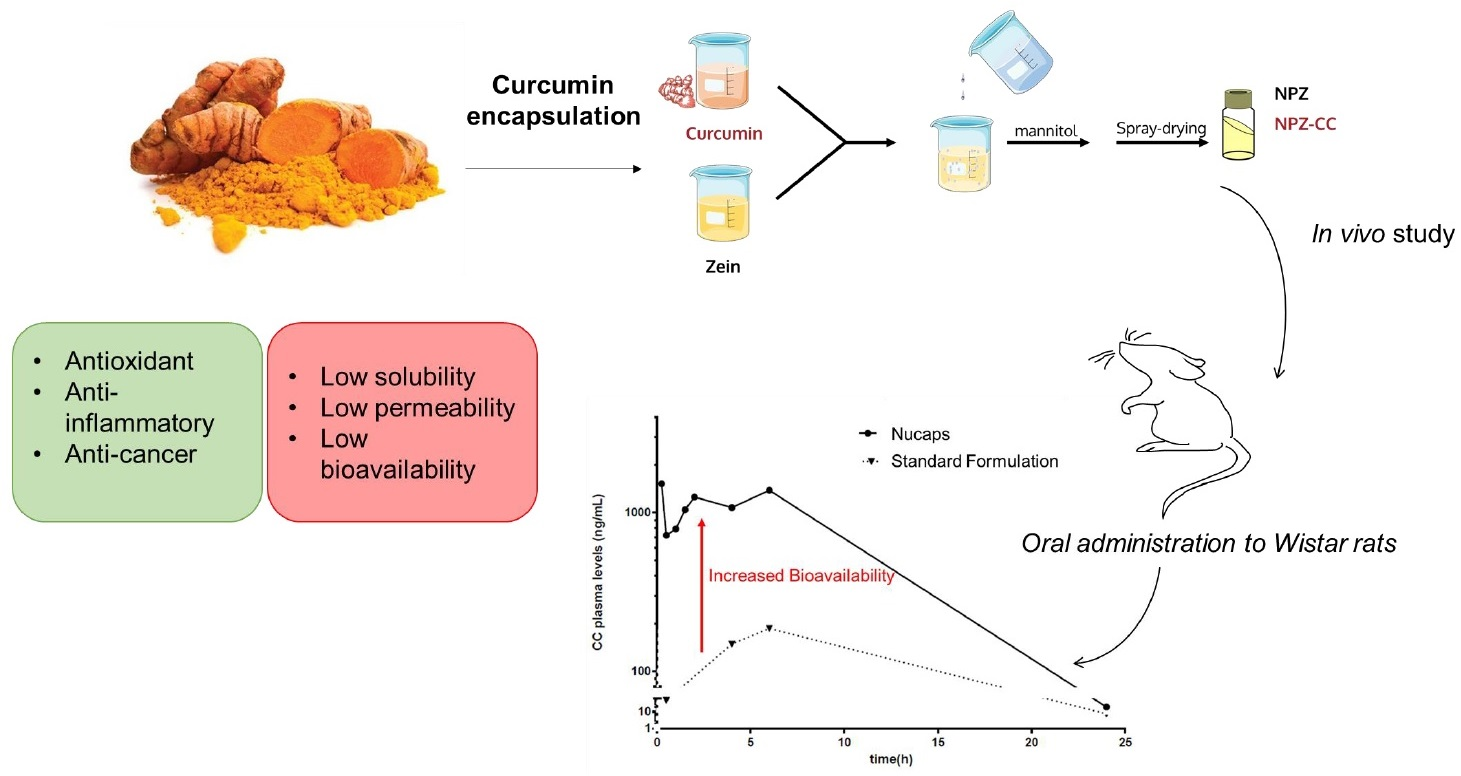
Ist Einverstanden
Nach meiner Meinung, Sie auf dem falschen Weg.
es gibt die Analoga?
Welche nötige Phrase... Toll, die glänzende Idee