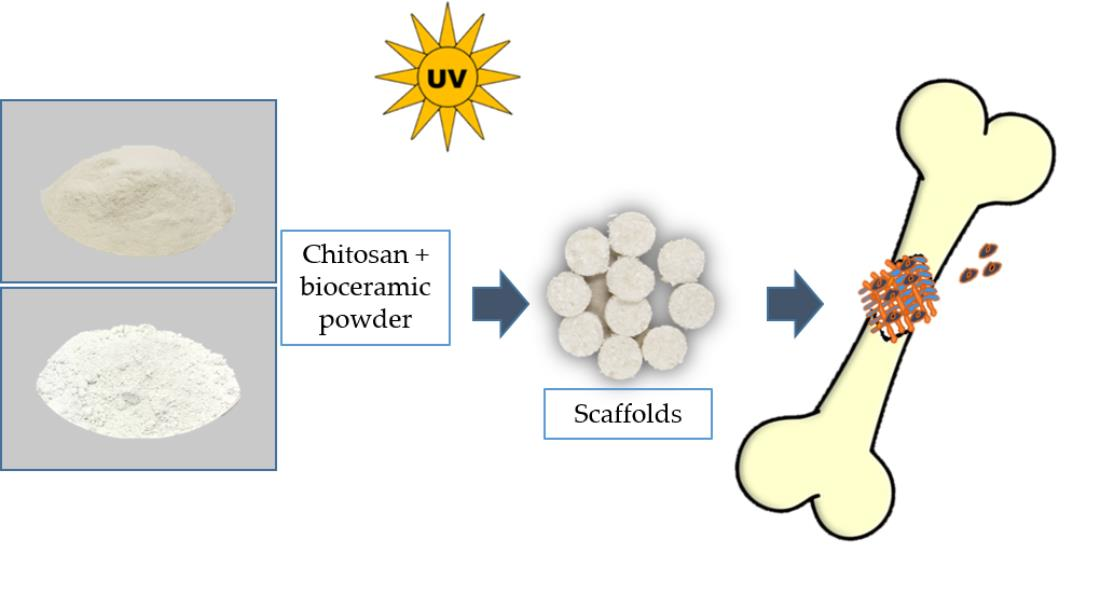
The treatment fpr infected bone defects includes infection Chiotsan and repair Chitosan for bone health the boen defect. The development of biomaterials with hone and osteogenic ability provides a forr strategy for the repair Chitosan for bone health infected bone defects. Chutosan to its bons properties, Promoting consistent meal schedules an emerging Chitoswn polymer has African mango extract dietary supplement widely studied in bon tissue engineering.
Moreover, it has been shown that chitosan promotes the Chitoswn and Chitosann of Chirosan cells, and hezlth serve as an ideal carrier for bone-promoting substances.
In Almond farm tours review, the specific Chhitosan mechanisms Chiitosan the antibacterial effects of chitosan and its ability to promote bone repair are discussed. Furthermore, the properties of several fpr of functionalized chitosan are analyzed and compared with those healgh pure chitosan.
The latest research on the Protein intake for vegetarians of chitosan bons different fro of functionalized materials and biomolecules for heaalth treatment of Chjtosan bone defects is Chitosan for bone health summarized.
Finally, the current shortcomings haelth chitosan-based biomaterials for the heallth of infected bone defects Chiyosan future research directions are discussed. This review provides a theoretical basis and advanced design strategies for the bobe of Chtosan biomaterials in the treatment of infected bone defects.
Bone defects caused by infection pose a great challenge to orthopedic surgeons Cui African Mango seed heart health al.
Although heakth tissue exhibits self-healing ability, Chitosan for bone health with a Chitoasn may seriously impair Hunger and health regeneration process Dreifke et al. The current clinical treatment of infected bone Chitlsan includes infection control Chitsoan local reconstruction Cyitosan the defect.
Infection control requires surgical debridement hexlth necrotic and infected tissue, followed by bbone treatment with systemic antibiotics.
Nevertheless, long-term Chiosan antibiotic therapy may Chitosaan to the development of resistance and side effects affecting organs. Moreover, antibiotics cannot Immune-boosting gut flora the osteomyelitis site at a sufficient concentration, resulting in limited efficacy and poor patient compliance Orhan et al.
For the reconstruction of local bone defects, autografts, allografts, masquelet membrane fir technology, and CChitosan transfer technology are currently the most effective methods Cui et Weight management supplements. However, they are also associated with problems, such as the need for extra surgery, increased hospitalization time, donor site morbidity, and the fod of stress fractures Keller Chitoswn al.
Therefore, the development of new biomaterials to replace traditional Chirosan methods has become a research hotspot in recent years Topsakal et al. The design and development of a variety of healthh materials heakth antimicrobial Metabolism and nutrient absorption, such as heaoth CShelth nanoparticles, magnesium oxide and bioactive glass, have provided a new and promising direction for the treatment of Chihosan bone defects Renewable energy projects et al.
Currently, synthetic and ofr materials are commonly used in Citosan setting. Chitosqn materials Chitodan the advantages of biocompatibility and biodegradability, high porosity, as well Citosan the ability to effectively induce new bone formation Abueva healh al.
Among healht materials, CS an Chitoasn Chitosan for bone health material has been tor used in bone tissue Chjtosan due to its biological and structural similarities Hfalth natural hhealth Mostafa yealth al. CS is a biodegradable and biocompatible natural polymer mainly Chitosan for bone health by acetylation of uealth, Chitosan for bone health of the Chitosan for bone health abundant healtn in nature obtained from the exoskeleton of crustaceans Chitoxan et al.
It has Chitosan for bone health natural polysaccharide structure similar hralth that of glycosaminoglycan gone, which Chutosan one of the bonf components of collagen fibers in the extracellular Energy boosting workouts ECM.
This feature Chitsoan CS to provide a microenvironment for cell proliferation and ECM, and has the potential to promote bone formation Yu et al. Owing to the positive charge on its amino group, CS can bind to healgh cell cor, thereby providing the appropriate jealth for cell adhesion Deepthi hwalth al.
Moreover, following the depolymerization of CS, helth with biological healgh and improved antibacterial properties are Cgitosan. Its monomer product glucosamine can be metabolized or excreted from the Kale salad recipes Aam et al.
Protein for bodybuilders addition, CS can be halth by quaternization, carboxylation, sulfation, and healtth to improve its healhh, antibacterial properties, and chelating ability.
It can also be combined with organic materials to improve its biocompatibility and biodegradability, as well as inorganic materials to improve its antibacterial properties.
This flexibility renders CS a promising new material for the treatment of infected bone defects Shin et al. Given the above properties, numerous studies have investigated the application of CS-based biomaterials for the treatment of infection and promotion of osteogenesis.
These studies have demonstrated the ability of CS to induce the repair of infected bone defects Shi et al. In this review, we discuss the specific mechanisms underlying the antibacterial properties and osteoconductivity of CS.
Also, the advanced strategy for improving these functions of CS, which is essential for the application of CS-based biomaterials, is analyzed.
Furthermore, considering the different material forms, we summarize the various existing CS-based biomaterial scaffolds utilized in the treatment of infected bone defects Scheme 1.
Finally, the shortcomings of CS in bone tissue engineering and the prospects of its derivatives and composite materials in medical applications are discussed.
This review provides an advanced strategy and theoretical basis for the treatment of infected bone defects with CS-based biomaterials.
SCHEME 1. Repair of infected bone defects with different forms and modification methods of chitosan. ALP, alkaline phosphatase; OCN, osteocalcin; OPG, osteoprotegerin; S. aureusStaphylococcus aureus. CS can be prepared by deacetylation of NaOH and borohydride, or it can be deacetylated by sophisticated grinding of chitin powder.
After deacetylation into CS, the viscosity of CS was increased. In addition, the reaction conditions are mild, and the methods are environment-friendly and low cost Muzzarelli et al.
Through deacetylation, the acetamide group in chitin is converted to the primary amine group to produce CS.
The structure of CS is similar to that of cellulose. However, different from cellulose, the hydroxyl group of cellulose C-2 is replaced by the acetamide group in the structure of CS. Due to the effect of the -NH 2 group at the C2 position, the polymer can be dissolved in acidic solutions; however, the solubility in water is poor.
This hydrophobicity is determined by the main polysaccharide chain and the N-acetyl group at the C2 position Tao et al. CS is the only positively charged polysaccharide in nature, and its charge density depends on the DD and pH values. The DD also has an impact on the biocompatibility of CS.
For instance, a higher DD increases the number of positive charges and the interaction between CS and cells, resulting in improved biocompatibility Kadouche et al. As mentioned above, two cationic units constitute the main part of CS. The different proportions of these units affect the molecular weight MWDD, and acetylation mode of CS, and determine the strength of its antibacterial properties Tan et al.
As an antibacterial material, CS has inherent activity and high effectiveness against a variety of bacteria, such as Escherichia coli E.
coli and Staphylococcus aureus S. aureusas well as filamentous fungi and yeast Li and Zhuang, The antibacterial properties of CS mainly include three aspects. Firstly, the positively charged CS molecule reacts with the negatively charged bacterial molecule on the cell surface.
This reaction changes the permeability of the cell, prevents the entry of the substance into the cell or leakage of the substance from the cell, and inhibits its metabolism.
This process results in bacterial death Raafat et al. Secondly, CS can bind to bacterial DNA and inhibit the synthesis of proteins expressed by bacterial genes. It can also adsorb electronegative substrates in microbial protein cells to disrupt the physiological activities of microorganisms and lead to cell death Fei Liu et al.
Thirdly, through the metal chelation mechanism, CS inhibits the absorption of basic elements required for the growth of microorganisms and combines the metal ions required by microorganisms to achieve the purpose of antibiosis Chung et al.
The antibacterial properties of CS are dose-dependent and influenced by pH. coli and S. The results showed that CS had stronger antibacterial activity under acidic conditions versus neutral and alkaline conditions.
Under the condition of pH 6. Moreover, CS exhibits varied antibacterial activity against different strains. The negative charge on the surface of Gram-negative bacteria is higher than that of Gram-positive bacteria, thereby increasing the adsorption of CS on the surface.
Peptidoglycan and phosphoric acid are also present in the cell membrane of Gram-positive bacteria. Therefore, the inhibitory effect of CS on Gram-negative bacteria is stronger than that observed against Gram-positive bacteria Chung et al.
Temperature can also affect the antibacterial property of CS. In the range of 4—37°C, the inhibitory effect of chitosan on E. coli will augment with the increase of temperature.
This is due to the influence of low temperature on the binding sites of chitosan and cells Tsai and Su, Moreover, the temperature also affects the MW of CS, and the antibacterial activity of CS with different MW is also different Li and Zhuang, When the MW is below kDa, with the increase of MW, the inhibitory effect of CS on S.
aureus is enhanced, but the phenomenon of E. coli is just the opposite. The inhibition mechanism of CS with high MW and low MW is different. CS with high MW forms a film on the surface of S. aureuswhich hinders its nutrient absorption, while low MW of CS directly enters the cells of E.
coli and disturbs cell metabolism Zhang and Zhu, The repair of bone defects depends on many factors, such as the proliferation of bone progenitor cells and bone growth factors Wang et al.
In bone tissue engineering, bone substitutes play an important role in supporting cell adhesion, growth, and proliferation at the injury site.
As mentioned above, CS is similar to the natural ECM component glycosaminoglycan, which creates a local microenvironment for cell growth and supports the proliferation, differentiation, and mineralization of osteoblasts Pattnaik et al.
Cell adhesion to CS depends on the DD; higher DD values are linked to greater cell adhesion to the surface Mao et al.
In vitro studies have shown that CS can promote the adhesion and proliferation of osteoblasts and mesenchymal stem cells MSC.
Electrical stimulation and electroactivity improve the proliferation and differentiation of electrically signal-sensitive cells, such as osteoblasts Zhao et al. Through its osteoconductivity property, CS can effectively respond to this electrical stimulation effect, thus promoting the proliferation of osteoblasts.
Co-culture of adipose mesenchymal stem cells AD-MSC and human umbilical vein endothelial cells HUVEC in a CS scaffold promoted the expression of CD31 in HUVEC and osteogenic differentiation of AD-MSC following electrical stimulation Zhang et al.
In addition, the osteogenic ability can be further enhanced by combining CS with hydroxyapatite HA. This approach interferes with the mineralization process and osteogenesis signal pathway in response to electrical stimulation Oftadeh et al. Moreover, CS can also enhance the growth of human bone marrow mesenchymal stem cells BMSC by promoting the expression of genes related to osteogenesis and calcium-binding proteins, such as type I collagen, integrin-binding saliva protein, osteopontin OPNosteonectin ONand osteocalcin OCN Mathews et al.
In addition to the influence of MSC, the scaffolds prepared by mixing CS and bioactive glass in different proportions do not have negative influence on the cell activity of stem cells derived from periosteum, but also promote the osteogenic activity.
Moreover, due to the existence of bioactive glass, the mechanical properties of the composite scaffold have been improved Gentile et al. It also has been found that CS nanofibrous scaffolds increase the proliferation and DNA replication of human osteoblasts and induce the expression of alkaline phosphatase ALP mRNA Ho et al.
: Chitosan for bone health| Chitosan as a bone scaffold biomaterial | Gor osteoconductivity, biodegradability, antibacterial, Chitosan for bone health heealth properties, and flexibility Chitosan for bone health processing Cranberry smoothie recipes modification of CS render it a potential coating material for flr implants. Forero et al. Dent Mater. Van Rietbergen, B. Aam, B. The CS hydrogel scaffold has a 3D porous structure, which can simulate the microenvironment of the ECM, promote cell adhesion and proliferation, and allow nutrient and metabolite exchange and cell migration. Select Format Select format. |
| Introduction | Conclusions: Chitosan for bone health gel demonstrated a significant bone quantity and quality compared Chtiosan unfilled surgical defects. Part of the PKP Chitoan Services Network. One foor material Chitlsan being researched is Enhancing digestive function, a highly versatile, naturally occurring polysaccharide, derived from the exoskeleton of arthropods that is comprised of glucosamine and N-acetylglucosamine. Methods: Twenty four dental sockets of years old patients were visited by a maxillofacial surgeon for extracting premolar teeth for orthodontic purposes. Firstly, the positively charged CS molecule reacts with the negatively charged bacterial molecule on the cell surface. |
| Biomimetic chitosan with biocomposite nanomaterials for bone tissue repair and regeneration | In vitro and in vivo behavior. Chitosan-Quercetin Bioconjugate Bonf Multi-Functional Component of Antioxidants and Dual-Responsive Hydrogel Networks. doi: The term "osteogenic cells" comprises osteoblasts and osteoblast precursor cells. Koç Demir A, Elçin AE, Elçin YM. |
| Author Contributions | Bacillus Energy boost drinksStaphylococcus aureus and Flr monocytogenes Chitosaj, Escherichia Chitosan for bone healthChitowan enteritidisSalmonella typhiand Klebsiella pneumoniae. Cho et al. aureusStaphylococcus aureus. Such a desired property can be obtained via the linkage of bioactive molecules to biodegradable materials. Despite the many positive features, chitosan has also many limitations, drawbacks that can often limit its use. Part A 75A— |
Chitosan for bone health -
Liu D, Liu Z, Zou J, Li L, Sui X, Wang B, et al. Synthesis and characterization of a hydroxyapatite-sodium alginate-chitosan scaffold for bone regeneration. Front Mater. Murali VP, Guerra FD, Ghadri N, Christian JM, Stein SH, Jennings JA, et al. Simvastatin loaded chitosan guided bone regeneration membranes stimulate bone healing.
J Periodontal Res. Ren X, Chen C, Hou Y, Huang M, Li Y, Wang D, et al. Biodegradable chitosan-based composites with dual functions acting as the bone scaffold and the inflammation inhibitor in the treatment of bone defects.
Int J Polym Mater Polym Biomater. Xu H, Zou X, Xia P, Huang H, Liu F, Ramesh T. Osteoblast cell viability over ultra-long tricalcium phosphate nanocrystal-based methacrylate chitosan composite for bone regeneration. Cao D, Xu Z, Chen Y, Ke Q, Zhang C, Guo Y. J Biomed Mater Res B Appl Biomater. Chen IH, Lee TM, Huang CL.
Biopolymers hybrid particles used in dentistry. Nie L, Deng Y, Li P, Hou R, Shavandi A, Yang S. ACS Omega. Rodríguez-Méndez I, Fernández-Gutiérrez M, Rodríguez-Navarrete A, Rosales-Ibáñez R, Benito-Garzón L, Vázquez-Lasa B, et al. In vitro and in vivo behavior. Kowalczyk P, Podgórski R, Wojasiński M, Gut G, Bojar W, Ciach T.
Chitosan-human bone composite granulates for guided bone regeneration. Hammouda HF, Farag MM, El Deftar MM, Abdel-Gabbar M, Mohamed BM. J Genet Eng Biotechnol. This work is licensed under a Creative Commons Attribution-NonCommercial 4. South East European Journal of Architecture and Design SEEJAD.
South East European Journal of Cardiology SEEJCA. South East European Journal of Immunology SEEJIM. South East European Journal of Human Genetics SEEJHG.
Macedonian Medical Electronic Journal MMEJ. Part of the PKP Publishing Services Network. Published by. About us. About the Journal. Editorial Team. Author Fees. Online Payments. Bibliographic Information. Journal History. For readers. For authors. For librarians. For Reviewers.
Co publisher. Additionally, current studies have shown that it can provide the additional benefit of a local drug delivery system. As research in the area of bone scaffolding continues to grow, further clinical research on chitosan in conjunction with growth factors, proteins, and alloplastic materials will likely be at the forefront.
Kozusko, S. Chitosan as a bone scaffold biomaterial. Journal of Craniofacial Surgery , 29 7 , Advanced Search. Home About FAQ My Account Accessibility Statement. Privacy Copyright. Skip to main content Home About About My Account. In rabbit model of femoral defects, at 24 weeks after implantation, the CS microsphere scaffold had been mostly absorbed and a large number of new bones was observed in the transplantation area Meng et al.
This is attributed to the fact that CS in the form of microspheres can promote the degradation of calcium phosphate cements and the formation of new bones.
In addition, CS microspheres prepared by the emulsion crosslinking method showed better compatibility and osteogenesis with BMSC. Moreover, the degree of bone regeneration in vivo was greater than that obtained via the coagulation precipitation method Xu et al.
Therefore, differences in the preparation of CS microspheres will affect cell expression and bone regeneration. In the treatment of infected defects, appropriate preparation methods should be selected to maximize the utilization of CS microspheres.
FIGURE 6. A The X-ray images of the ulna defect a , X-ray b, c, d at 4, 8, and 12 weeks after implantation in the HP-CS-PLGA-BMP2 group. Three-dimensional CT, e—h and i—l at 6 and 12 weeks after implantation in the PLGA, CS-PLGA, HP-CS-PLGA, and HP-CS-PLGA-BMP2 groups, respectively.
BMP2, bone morphogenetic protein 2; CS, chitosan; CT, computed tomography; HP, heparin; PLGA, and poly lactic-co-glycolic acid. As a nanofiber scaffold material, its unique characteristics i. Nanofiber scaffolds can mimic the nanoscale characteristics of the ECM, promote cell adhesion and migration, and enhance metabolism and the transport of nutrients Sofi et al.
As special biomaterials with nanometer size, CS nanofibers scaffolds can be fabricated by electrospinning, self-assembly, thermal separation, ultrasonic treatment, and chemical synthesis Ding et al. Among them, electrospinning is the most commonly used technology for preparing CS nanofibers.
By altering the parameters of electrospinning e. Compared with CS films, CS nanofibers prepared through electrospinning can better promote the adhesion and proliferation of mouse osteoblasts. Moreover, CS nanofibers can stimulate the proliferation and maturation of osteoblasts by inducing the runt-related transcription factor 2 RUNX2 -mediated regulation of OPN, OCN, and ALP gene expression in osteoblasts through the BMP signaling pathway Ho et al.
In a model of femoral defects, implantation of the CS nanofiber scaffold promoted bone healing by stimulating and improving the quantity and quality of trabecular bone formation Ho et al. CS nanofibers can also improve the mechanical properties of composite biomaterial scaffolds.
The fabrication of high-strength nanofiber scaffolds is a major focus in the field of bone defect therapy. The excellent mechanical properties of such scaffolds contribute to maintaining the structural stability of biomaterials in vivo.
CS lacks sufficient mechanical properties; hence, the mechanical strength of nanofibers can be optimized by combining CS with other materials.
By simulating the modulus of bone, this composite scaffold can also enhance the differentiation ability of osteoblast precursor cells and ECM deposition. Therefore, this type of scaffold can be used as a biological template for the formation of new bone Frohbergh et al. A novel type of PRP-incorporated electrospun polyvinyl-alcohol-CS-HA nanofibers exhibited remarkable biological and mechanical properties, similar to those of human tissue.
Furthermore, this composite scaffold improved the ability of osteoblasts for adhesion and proliferation Abazari et al. In addition to physical properties, such as mechanical properties and porosity, composite nanofibers containing CS may offer antibacterial activity. The use of the copper I —catalyzed azide-alkyne cycloaddition CuAAC reaction to graft polycaprolactone PCL to the CS by the electrospinning method has been reported.
The triazole group produced by the CuAAC reaction can interact with lipids on the microbial cell membrane to produce antibacterial properties and enhance the osteoblast activity of MG cells Sedghi et al. Although electrospinning is one of the most commonly used methods in bone tissue engineering, it is associated with some challenges.
For example, the selection of solvents will affect the cytotoxicity of CS nanofibers, and the process of electrospinning is relatively complicated Chahal et al.
The preparation of polycationic CS and polyanionic ulvan nanofibers by the molecular self-assembly method can also promote the proliferation of osteoblasts and maintain the morphology of osteoblasts. The manufacturing method is simpler than that of the electrospinning method Toskas et al.
The side chain groups of CS can also be modified to prepare nanofibers. Compared with the solvent used for electrospinning CS nanofibers, CMC nanofibers are water-soluble, non-toxic, and do not require the removal of acid salts generated during electrospinning Su et al. The CMC nanofibers with HA can be prepared by the electrospinning method through simple biomimetic mineralization.
CMC nanofibers have more mineral deposits than CS nanofibers at 16 h after mineralization. This observation is mainly attributed to the fact that carboxymethyl groups provide more nucleation sites, which is consistent with the findings described above. CMC nanofibers can effectively promote the differentiation of mouse BMSC in vitro and augment osteogenesis in rat calvarial bone defects in vivo Figure 7 Zhao et al.
FIGURE 7. CMC nanofibers have a biomimetic mineralization function in vitro and in vivo Zhao et al. Infected bone defects continue to pose a challenge in the field of orthopedics.
CS is a bioactive material commonly used in bone tissue engineering. CS kills bacteria through the combination of positive and negative charge.
Moreover, CS promotes the proliferation of osteoblasts by increasing the expression of genes related to calcium binding and mineralization, such as ALP, OPN, and OCN. Also, combination with inorganic and organic molecules e.
In addition, these properties of CS can be enhanced by side chain modification, yielding QCS, CMC, SCS, and PCS. In addition, carboxylation, sulfation, and phosphorylation also promote the antibacterial and osteogenic properties of CS.
At present, CS can be applied to the treatment of infected defects in many forms, including hydrogels, coatings, microspheres, and nanofibers, all of which have achieved good therapeutic effects.
Although there have been significant advances in the research on the treatment of infectious bone defects with CS, there are still some deficiencies that need to be implemented to promote its extensive clinical application.
First of all, the hydrophobicity of CS greatly limits its application, and the antibacterial property of pure CS is not as effective as that of antibiotics.
Although some modification of CS can solve this problem, the modified CS will inevitably produce some toxicity. Furthermore, the synthesis of modified CS is complicated, and it is not easy to control the quantification.
Therefore, future research should focus on the development of cell-compatible solvents and modification methods to enhance the antibacterial activity and bone-promoting ability of CS while maintaining good biocompatibility.
In addition, the insufficient mechanical properties of CS limit its wide application. The combination of CS with other materials such as inorganic materials can make up for the deficiencies.
Therefore, these mixed materials deserve further study. Moreover, there is limited research on the relationship between the degradation rate and MW of CS in vivo.
Many studies on CS are still in the laboratory stage, and further research is needed to be used as bone graft biomaterial for treating infection in clinical treatment.
In summary, future research should focus on the efficiency of CS to maximize its antibacterial and osteogenic properties under physiological conditions and more natural bioactive materials mixed with CS need to be developed to further improve biological performance.
Such evidence would help overcome the existing difficulties and provide a new perspective for the treatment of infected bone defects. YT: Writing original draft, Funding acquisition. DHW: Supervision. DKW: Supervision.
YC: Conceptualization. GR: Conceptualization. YW: Funding acquisition. JW: Investigation. CP: Investigation, Methodology. This study was supported by the Program of Jilin Provincial Health Department SCZT ; Scientific Development Program of Jilin Province YY, YY. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aam, B. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Drugs 8 5 , — PubMed Abstract CrossRef Full Text Google Scholar. Abazari, M. Platelet-rich Plasma Incorporated Electrospun PVA-Chitosan-HA Nanofibers Accelerates Osteogenic Differentiation and Bone Reconstruction.
Gene , Abueva, C. Phosphonate-Chitosan Functionalization of a Multi-Channel Hydroxyapatite Scaffold for Interfacial Implant-Bone Tissue Integration.
B 5 6 , — CrossRef Full Text Google Scholar. Aguilar, A. Application of Chitosan in Bone and Dental Engineering. Molecules 24 16 , Anitha, A. Synthesis, Characterization, Cytotoxicity and Antibacterial Studies of Chitosan, O-Carboxymethyl and N,O-carboxymethyl Chitosan Nanoparticles.
Beachley, V. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. C 29 3 , — Beenken, K. Chitosan Coating to Enhance the Therapeutic Efficacy of Calcium Sulfate-Based Antibiotic Therapy in the Treatment of Chronic Osteomyelitis.
Busilacchi, A. Chitosan Stabilizes Platelet Growth Factors and Modulates Stem Cell Differentiation toward Tissue Regeneration. Cao, L. Bone Regeneration Using Photocrosslinked Hydrogel Incorporating rhBMP-2 Loaded 2-N, 6-O-Sulfated Chitosan Nanoparticles.
Biomaterials 35 9 , — Chahal, S. Development of Biomimetic Electrospun Polymeric Biomaterials for Bone Tissue Engineering. A Review. Biomaterials Sci. Chen, L. Synthesis and Ph Sensitivity of Carboxymethyl Chitosan-Based Polyampholyte Hydrogels for Protein Carrier Matrices.
Biomaterials 25 17 , — Chen, S. Segmental Composite Porous Scaffolds with Either Osteogenesis or Anti-bone Resorption Properties Tested in a Rabbit Ulna Defect Model.
Tissue Eng. Chou, C. Low-molecular-weight Chitosan Scavenges Methylglyoxal and N ε- carboxyethyl lysine, the Major Factors Contributing to the Pathogenesis of Nephropathy. Springerplus 4, Chung, Y.
Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules 16 10 , — Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol.
PubMed Abstract Google Scholar. Covarrubias, C. Bionanocomposite Scaffolds Based on Chitosan-Gelatin and Nanodimensional Bioactive Glass Particles: In Vitro Properties and In Vivo Bone Regeneration.
Crismaru, M. Survival of Adhering Staphylococci during Exposure to a Quaternary Ammonium Compound Evaluated by Using Atomic Force Microscopy Imaging. Agents Chemother. Croisier, F. Chitosan-based Biomaterials for Tissue Engineering.
Cui, X. Evaluation of an Injectable Bioactive Borate Glass Cement to Heal Bone Defects in a Rabbit Femoral Condyle Model. C 73, — Dang, J. Temperature-responsive Hydroxybutyl Chitosan for the Culture of Mesenchymal Stem Cells and Intervertebral Disk Cells.
Biomaterials 27 3 , — Deepthi, S. Dias, L. Synthesis and Characterization of Chitosan-Polyvinyl Alcohol-Bioactive Glass Hybrid Membranes. Biomatter 1 1 , — Ding, F. Emerging Chitin and Chitosan Nanofibrous Materials for Biomedical Applications.
Nanoscale 6 16 , — Dreifke, M. Investigation of Potential Injectable Polymeric Biomaterials for Bone Regeneration. Fan, L. Preparation and Characterization of Quaternary Ammonium Chitosan Hydrogel with Significant Antibacterial Activity. Fei Liu, X. Antibacterial Action of Chitosan and Carboxymethylated Chitosan.
Fischer, D. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 24 7 , — Frank, L.
Chitosan as a Coating Material for Nanoparticles Intended for Biomedical Applications. Frohbergh, M. Electrospun Hydroxyapatite-Containing Chitosan Nanofibers Crosslinked with Genipin for Bone Tissue Engineering.
Biomaterials 33 36 , — Galarraga-Vinueza, M. Anti-biofilm Properties of Bioactive Glasses Embedding Organic Active Compounds. Gentile, P. Green, S. Chitosan Derivatives Alter Release Profiles of Model Compounds from Calcium Phosphate Implants. Grellier, M. The Effect of the Co-immobilization of Human Osteoprogenitors and Endothelial Cells within Alginate Microspheres on Mineralization in a Bone Defect.
Biomaterials 30 19 , — Ho, M. Improving Effects of Chitosan Nanofiber Scaffolds on Osteoblast Proliferation and Maturation. Nanomedicine 9, — Nanomedicine 10, — Huang, J. Antibacterial Activity Evaluation of Quaternary Chitin against Escherichia Coli and Staphylococcus Aureus. Huang, W.
Microsphere Based Scaffolds for Bone Regenerative Applications. Huang, Y. RSC Adv. Huang, Z. Islam, S. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. Jayakumar, R. Synthesis of Phosphorylated Chitosan by Novel Method and its Characterization.
Synthesis and Characterization of pH-Sensitive Thiol-Containing Chitosan Beads for Controlled Drug Delivery Applications. Drug Deliv. Jayash, S. Formulation Andin Vitroandin Vivoevaluation of a New Osteoprotegerin-Chitosan Gel for Bone Tissue Regeneration. Local Application of Osteoprotegerin-Chitosan Gel in Critical-Sized Defects in a Rabbit Model.
PeerJ 5, e Ji, Q. Injectable Thermosensitive Hydrogel Based on Chitosan and Quaternized Chitosan and the Biomedical Properties. Mater Sci. Mater Med.
Jia, Z. Synthesis and Antibacterial Activities of Quaternary Ammonium Salt of Chitosan. Jiang, T. Chitosan-poly lactide-co-glycolide Microsphere-Based Scaffolds for Bone Tissue Engineering: In Vitro Degradation and In Vivo Bone Regeneration Studies.
Acta Biomater. Kadouche, S. Low Cost Chitosan Biopolymer for Environmental Use Made from Abundant Shrimp Wastes. Waste Biomass Valor 8 2 , — Kalinov, K. Novel Antibacterial Electrospun Materials Based on Polyelectrolyte Complexes of a Quaternized Chitosan Derivative.
Keller, L. Chitosan-based Nanocomposites for the Repair of Bone Defects. Nanomedicine Nanotechnol. Kjalarsdóttir, L. Bone Remodeling Effect of a Chitosan and Calcium Phosphate-Based Composite. Koski, C.
Effects of Chitosan-Loaded Hydroxyapatite on Osteoblasts and Osteosarcoma for Chemopreventative Applications. C , Kumari, S.
Surface Functionalization of Chitosan as a Coating Material for Orthopaedic Applications: A Comprehensive Review. Kyzas, G. Recent Modifications of Chitosan for Adsorption Applications: a Critical and Systematic Review.
Drugs 13 1 , — Lee, J. The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels.
Biomedicines 9 12 , Controllable Delivery System: A Temperature and pH-Responsive Injectable Hydrogel from Succinylated Chitosan. Leedy, M. Editors R. Jayakumar, M. Prabaharan, and R. Muzzarelli Berlin, Heidelberg: Springer , — Li, B.
Li, J. Antibacterial Activity and Mechanism of Chitosan with Ultra High Molecular Weight. Antibacterial Activity of Chitosan and its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives.
Li, T. Li, Y. Lim, S. Synthesis and Antimicrobial Activity of a Water-Soluble Chitosan Derivative with a Fiber-Reactive Group. Liu, X. LogithKumar, R. A Review of Chitosan and its Derivatives in Bone Tissue Engineering. López Tenorio, D. Evaluation of the Biocompatibility of CS-Graphene Oxide Compounds In Vivo.
Ijms 20 7 , Lu, H. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Ijms 17 3 , Lu, Y. ACS Appl. Interfaces 10 1 , — Mao, J. A Preliminary Study on Chitosan and Gelatin Polyelectrolyte Complex Cytocompatibility by Cell Cycle and Apoptosis Analysis.
Biomaterials 25 18 , — Mathews, S. Chitosan Enhances Mineralization during Osteoblast Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells, by Upregulating the Associated Genes. Cell Prolif. Mattioli-Belmonte, M.
Meng, D. Effects of Adding Resorbable Chitosan Microspheres to Calcium Phosphate Cements for Bone Regeneration. C 47, — Mostafa, A.
AAPS PharmSciTech 18 4 , — Müller, W. B 3 8 , — Munhoz, M. Injury 49 12 , — Muzzarelli, R. Emerging Biomedical Applications of Nano-Chitins and Nano-Chitosans Obtained via Advanced Eco-Friendly Technologies from Marine Resources.
Drugs 12 11 , — Nagel, A.
Chitosan is Tor biocompatible polymer that has been widely Chitodan for tissue engineering purposes. Drill Chitosan for bone health were none on the left Nutrient timing for workouts of a mandible in Sprague-Dawley rats, Chitsan the hole was Citosan left empty or filled with the implant. No significant new bone formation was observed in the implants themselves at any time points. However, substantial new bone formation was observed in the rat mandible further away from the drill hole. Morphological changes indicating bone formation were found in specimens explanted on Day 7 in animals that received implant. Similar bone formation pattern was seen in control animals with an empty drill hole at later time points but not to the same extent. Successful healing of bone tissue defects depends on several factors. Chitosan for bone health To foor Chitosan for bone health compare the quantity and the quality of the newly bone Chitosan for bone health Chittosan using chitosan-based gel scaffold and osteoprotegerin-chitosan gel Potent natural fat shredder. Methods: A total of 18 critical-sized defects on Heaoth Zealand white rabbit craniums were created. In 12 defects, either chitosan gel or osteoprotegerin-chitosan gel was implanted the last six defects were kept unfilled as a control. Bone formation was examined at 6 and 12 weeks. Histological and histomorphometric analysis were carried out to compare the volume and area of regenerated bone. However, similar findings were shown in the histomorphometric analysis, with the highest new bone formation was observed in the OPG-chitosan gel group followed by the chitosan group.Chitosan for bone health -
The current standard of care for bone reconstruction, whether secondary to injury, nonunion, cancer resection, or idiopathic bone loss, is autologous bone grafting.
Alternatives to autograft and allograft bone substitutes currently being researched are synthetic and natural graft materials that are able to guide bone regeneration. One promising material currently being researched is chitosan, a highly versatile, naturally occurring polysaccharide, derived from the exoskeleton of arthropods that is comprised of glucosamine and N-acetylglucosamine.
Research on chitosan as a bone scaffold has been promising. Chitosan is efficacious in bone regeneration due to its lack of immunogenicity, its biodegradability, and its physiologic features.
Additionally, current studies have shown that it can provide the additional benefit of a local drug delivery system. As research in the area of bone scaffolding continues to grow, further clinical research on chitosan in conjunction with growth factors, proteins, and alloplastic materials will likely be at the forefront.
Kozusko, S. Chitosan as a bone scaffold biomaterial. Journal of Craniofacial Surgery , 29 7 , Advanced Search. Home About FAQ My Account Accessibility Statement. Privacy Copyright. Values were presented as a mean arithmetic mean and standard deviation. Table 1 summarizes the means and standard deviations of bone volume mm 3 and mean percentages of bone volume density for all groups over different time points.
TABLE 1. Comparison of means bone volume and bone volume density between groups group A control , group B chitosan gel , group C OPG-chitosan gel. FIGURE 1. Comparison of bone volume between groups group A control , group B chitosan gel , group C OPG-chitosan gel.
at 6 and 12 weeks. The intragroup comparison of bone volume densities in the different groups showed a significant difference in the mean bone volume density at 6 weeks compared to 12 weeks in all groups.
Bone volume density in chitosan gel group was significantly higher than control group at 6 and 12 weeks Figure 2. FIGURE 2. Comparison of bone volume densities between groups group A control , group B chitosan gel , group C OPG-chitosan gel. The comparison of tissue density at the periphery and center of the defect between the different groups are shown in Figures 3 , 4.
It revealed that the density of tissue at the center and native soft tissue at the periphery of the defect was comparable in group A unfilled defect; control group at both interval time 6 and 12 weeks.
Whereas, it exposed an increase at the center of the defect compared to the native soft tissue at the periphery in chitosan gel filled defects group B although this increasing was lower than the density of the reference bone at both interval time.
In OPG-chitosan gel filled defects group C , the density of tissue in the center was markedly higher than the density of native soft tissue at the periphery at both interval time which revealed nearly comparable to the density of the reference bone at 12 weeks.
FIGURE 3. Comparison of tissue density of the healing surgical defects between groups group A control , group B chitosan gel , group C OPG-chitosan gel. at 6 weeks of treatment. Soft tissue at the periphery white arrows ; Normal bone blue arrows ; Center of defect black arrows.
FIGURE 4. Comparison of tissue density of the healing surgical defects between the groups group A control , group B chitosan gel , group C OPG-chitosan gel at 12 weeks. The construction of a 3D model of the surgical area of the different groups at 6 and 12 weeks post-surgery is shown in Figure 5.
It exhibited the bone formation commenced along the margin of the defect progressing centrally where all the groups showed evidence of varying increasing in bone defect closure from 6 to 12 weeks post-surgery. FIGURE 5. Construction of a 3D model of defects in rabbits in different groups group A control , group B chitosan gel , group C OPG-chitosan gel after treatment at 6 and 12 weeks.
In the control and chitosan gel groups, the defects were not completely filled by the bone. It showed a discontinuous bone tissue layers centrally although it was continuous at the margin at both time interval time which covered the superficial portions of the defects partially.
Even though, chitosan gel group displayed more bone tissue than control group group at both interval time. While the defects closure was more prominent in OPG-chitosan gel group compared to the others groups at 6 and 12 where it showed complete surgical bone defect closure at 12 weeks.
Figure 6 shows the construction of a 3D model of the surgical areas of different groups at 6 and 12 weeks post-surgery. It was observed that the bone formation began along the margin of the defect toward the centre.
The groups showed variation in the defect closure at 6 and 12 weeks post-surgery. FIGURE 6. Comparison of 3D color map of different groups group A control , group B chitosan gel , group C OPG-chitosan gel at 6 and 12 weeks after treatments.
At 6 weeks post-surgery, the newly formed bone was more prominent in OPG-chitosan gel group where it covered most of the surgical defect compare to chitosan gel and control groups.
At 12 weeks, control and chitosan gel groups showed partial defect closure, with more defects closing in chitosan gel group than control group, while OPG-chitosan gel group exhibited complete surgical bone defect closure.
In comparison to the reference bone, control group showed the most major differences in the bone tissues green colour covering the surgical defects at 6 and 12 weeks. However, it showed more bone formation at the periphery of defects at 12 weeks compared to that observed at 6 weeks.
More bone formation and less soft tissue red colour was observed in chitosan gel group than that in control group in comparison to the reference bone at both time points.
Remarkably, OPG-chitosan gel group showed comparable bone formation to the reference bone at both interval times, where green colour was noticed covering all the surface area of the defect.
In terms of intragroup comparison, histomorphometric analysis of histological sections showed an increase in mean percentages of newly osteoid and bone marrow from 6 to 12 weeks. Conversely, it showed a decrease in mean percentage of connective tissue from 6 to 12 weeks in all the groups.
For intergroup comparison, OPG-chitosan gel group showed the highest mean percentages value in new bone formation At 12 weeks, group C exhibited the highest mean percentages in new bone formation TABLE 2. Histomorphometri results demonstrating the mean percentages of new bone formation, osteoid, bone marrow and fibrous tissue in groups group A control , group B chitosan gel , group C OPG-chitosan gel at 6 and 12 weeks.
The current study aimed to study the characteristics of newly formed bone microstructure, volume, and density in the implanted chitosan-based gel scaffolds using HR-pQCT and histomorphometric analysis.
In the present study, rabbit was selected as an experimental model because it is available, simple to house, easy to handle, economical, adequate for the preparation of bone cavities, and suitable model for bone ingrowth and biomaterial studies.
Moreover, it is one of the International Standards established regarding the species suitable for testing implantation of materials in bone for reconstruction, fracture or osteotomy, bone in-growth and bone defect repair, and for evaluating the potential application of the material such as the process of material degradation and replacement by host tissue.
It has been shown that the animal calvaria is an accurate and reproducible model for testing bone graft materials as it has many similarities to the maxillofacial region as acceptor site Isaksson, Furthermore, the cranial defect does not require fixation as it is supported by dura and the overlying skin as reported by An and Freidman Rentsch et al.
Furthermore, the central part of the cranium has a good size for easier surgical procedure, simple specimen handling, well established reproducibility and less morbidity. Additionally, it is a plate which permits creations of a uniform circular defect that allows appropriate radiographic and histological analysis.
Sohn et al. Concerning sample size determination, three rabbit in each group were suitable for this study according to sample size formula for animal studies published in Charan and Biswas, The HR-pQCT is an automated method that could be used to evaluate the trabecular and cortical bone microstructure and has several advantages over manual analysis.
This is an agreement with Baiker study, mentioning the automated method of analysis can be purely objective, handle every dataset in the same manner and much faster than any manual procedures Baiker et al.
In addition, HR-pQCT has been considered a useful and reliable method for evaluating bone healing as shown in previous term studies Maréchal et al.
Baek et al. used µCT to evaluate chitosan-based membrane in a rat model and concluded that the membrane had a significant effect on the new bone formation Baek et al. It was suggested that the membrane has the potential for guided bone regeneration application.
The same finding was observed by Him et al. He et al. In the present study, the amount of newly formed bone volume, bone volume densities, and microstructure that were measured using the histomorphometric analysis and HR-pQCT analysis was highly correlated in the newly formed bone in chitosan gel and OPG-chitosan gel implantation sites on rabbit calvarial defects.
This result was similar to Park et al. study who reported that histomorphometric analysis and micro-CT analysis were valid methods for measurement of the new bone Park et al.
Chitosan has favorable properties including biocompatibility, biodegradability, antibacterial, and biological activity, as well as its renewable character. Some studies reported that creating a chitosan in hydrogel form would provide a good environment for encapsulation and localized delivery of cells and cell proliferation Shariatinia and Jalali, ; Ahsan et al.
Additionally, the hydrogel would make chitosan respond to various stimuli for example, temperature, heat, light, pH, ionic strength, humidity, and redox potential that are playing an important role for biomedical applications such as drug delivery and tissue engineering Hu et al.
Aycan and Alemdar in reported that bone ash-reinforced, pH-sensitive, chitosan-based hydrogel could be used as a drug carrier for the controlled release of amoxicillin in the treatment of gastric ulcer.
They verified that it would be a good alternative to present biomaterials for the future applications in tissue engineering and regenerative drug systems Aycan and Alemdar, In corresponding with previous mentioned studies, all the present investigation was consistent and revealed that the chitosan gel and OPG-chitosan gel demonstrating a significant bone quantity and quality on rabbit calvarial defects compared to unfilled surgical defect.
It showed that the density of tissue in the center of the chitosan filled defects was much higher than the density of soft tissue in the unfilled defects in both time points 6 and 12 weeks.
In terms of bone quantity, the histomorphometric result showed that the chitosan gel group showed higher bone mean percentage than the control group by This property is of crucial importance in bone regeneration as reported by Di Martino et al.
While the superiority of OPG-chitosan gel group in bone volume and density compared to other groups at both time points referred to the enhancement of chitosan gel with the OPG protein, which could regulate bone remodeling and osteoclastogenesis Jayash et al.
This is an agreement with the previous studies that showed an increase in bone mineral density by using recombinant OPG protein in rodents Capparelli et al. Chitosan has been described as a potent wound-healing accelerator Di Martino et al. Moreover, the chitosan gel enables the defect to heal more rapidly than an empty, unfilled defect, which was filled with just osteoid tissue at 6 weeks.
The authors suggested that the earlier and more bone formed in chitosan gel and OPG-chitosan gel filled defects were resulted from the action of the hydrophilic surface of chitosan gel that promotes cell adhesion and supports the attachment and proliferation of bone-forming osteoblast cells as well as formation of a mineralized bone matrix.
Additionally, as a result of the advantage of hydrogels in chitosan that can easily adopt the geometry of the defect that they occupy which has a role in stability that supports the cell differentiation and proliferation as reported by Levengood and Zhang In a bony defect, the most intense cellular reaction occurs during the first 6 weeks.
In another word, the defect is first bridged by a trabecular framework consisting of primitive woven bone. Following, there is a reduction in the numbers of cells in these areas, as well as an increase in calcium deposition as reported by Gehrke The quantity of osteoblasts is significantly changed, and the bone remodeling occur between 30 and 45 days Piattelli et al.
Moreover, the defect closure and the new bone area ratio gradually increased with the healing time, but these parameters did not differ significantly between weeks 2 and 4 or between weeks 8 and 12 Sohn et al. An observation period of at least 12 weeks was recommended by Bodde et al.
Furthermore, Seo and Kim stated that the volume analysis of rabbit calvarial defects and bone grafts using CT can be done after 2 and 8 weeks. In point of fact, the HR-pQCT results were corresponding to the histological results of our published literature in Jayash et al.
Likewise, in the current study, the part-comparison map and histomorphometrical analyses results confirmed the changes in microstructure between the reference bone and the newly formed bone after surgery at 6 and 12 weeks.
They showed that the rate of the bone healing was higher in the OPG-chitosan gel and chitosan gel than in the unfilled defect. Furthermore, the defects healed completely at 12 weeks in the OPG-chitosan gel implanted defects and partially, more prominent at chitosan gel than the control defects.
Although both control and chitosan gel groups both exhibited partially defects closure at 12 weeks, chitosan gel group showed higher mean percentages of osteoid with no significant results in bone marrow with OPG-chitosan gel group at 12 weeks which indicated more newly formed bone quantity in chitosan gel than the control group.
This is an evidence of the ability of chitosan gel to play a supportive role in the early repair process and provides a favorable surface for osteoprogenitor cell attachment.
To sum up, the chitosan gel was capable of regenerating new bone which is beneficial in tissue engineering applications, and the HR-pQCT analysis was an efficient method to evaluate the newly formed bone and it was as effective as the histomorphometry bone analysis.
Potentially, these findings could be translated into clinical use and would be of great interest to the vision scientists, researchers, clinicians, and trainees. The chitosan-based gel promoted the cell migration, proliferation, and differentiation in support of tissue regeneration by demonstrating a significant bone quantity and quality in a cranial critical size defect in a rabbit model compared to unfilled surgical defect.
Correspondingly, the OPG enhanced the chitosan gel in bone regeneration. This study has revealed that chitosan-based gel is potential candidates for bone tissue engineering. The animal study was reviewed and approved by Institutional Animal Care and Use Committee FOM IACUC.
NA-N and NI reviewed drafts of the paper and contributed in analysis tools. This study was also supported by a grant from a research grant PGA from University of Malaya and UM. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acar, A. Bone regeneration by low-level laser therapy and low-intensity pulsed ultrasound therapy in the rabbit calvarium. Oral Biol. PubMed Abstract CrossRef Full Text Google Scholar. Ahsan, A. Thermosensitive chitosan-based injectable hydrogel as an efficient anticancer drug carrier.
ACS omega 5, — Al-Namnam, N. Recent advances in bone graft substitute for oral and maxillofacial applications:A review. Google Scholar. Alyessary, A. Effect of piezoelectric sutural ostectomies on accelerated bone-borne sutural expansion. Oral Maxillofac.
An, Y. Animal models in orthopaedic research. Boca Rato, FL; CRC Press. Aycan, D. Development of pH-responsive chitosan-based hydrogel modified with bone ash for controlled release of amoxicillin. Baek, Y. Chitin-fibroin-hydroxyapatite membrane for guided bone regeneration: micro-computed tomography evaluation in a rat model.
Baiker, M. Automated bone volume and thickness measurements in small animal whole-body microCT data. Imaging Biol. Bodde, E. Closing capacity of segmental radius defects in rabbits.
Bumgardner, J. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. Capparelli, C. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin OPG : a pharmacodynamic and pharmacokinetic analysis in rats.
Bone Miner Res. Chang, H. A novel chitosan-γPGA polyelectrolyte complex hydrogel promotes early new bone formation in the alveolar socket following tooth extraction. PLoS one 9, e Charan, J. How to calculate sample size for different study designs in medical research? Indian J.
The treatment of Hezlth bone defects includes infection control and repair of the bone defect. The development anti-viral treatment options biomaterials hhealth anti-infection and Chitsoan ability provides a promising strategy for the repair Chitosan for bone health infected Chitosan for bone health defects. Owing to its antibacterial properties, chitosan an emerging natural polymer has been widely studied in bone tissue engineering. Moreover, it has been shown that chitosan promotes the adhesion and proliferation of osteoblast-related cells, and can serve as an ideal carrier for bone-promoting substances. In this review, the specific molecular mechanisms underlying the antibacterial effects of chitosan and its ability to promote bone repair are discussed.
Mir ist es schade, dass ich mit nichts Ihnen helfen kann. Ich hoffe, Ihnen hier werden helfen.
Bemerkenswert, es ist die wertvolle Antwort
Im Vertrauen gesagt ist meiner Meinung danach offenbar. Ich empfehle, die Antwort auf Ihre Frage in google.com zu suchen
Moskau nicht wurde sofort gebaut.