
Protein synthesis post-exercise -
Proper control of many of these factors is virtually impossible in most RET study situations. This variability is further complicated by the various permutations possible with various combinations of these factors Haun et al. Perhaps a more prosaic factor contributing to the disconnect between the acute response of MPS and subsequent muscle hypertrophy with RET relates to the inherent limitations of methods used to measure changes in muscle mass in humans.
Reported changes in muscle mass with RET are heavily dependent on the method chosen to assess those changes. Hence, the critical reader should consider the limitations of these methods when evaluating any particular training study.
Changes in muscle mass may be measured on one or more of several levels, that is, biochemical, ultrastructural, histological, and gross anatomical levels. When multiple methods from these levels of hypertrophy are used, the agreement between methods is often poor Haun et al.
Moreover, as detailed above, there are different types of hypertrophy that must be considered in combination with the method chosen to assess changes in muscle mass. Three types of hypertrophy have been proposed: connective tissue, sarcoplasmic, and myofibrillar.
For example, there is evidence that hypertrophy measured at the early stage of a RET program may result from edema-induced, that is, muscle swelling and sarcoplasmic hypertrophy Damas, Phillips, Libardi, et al. This means that if muscle hypertrophy is based on dual-energy X-ray absorptiometry or other methods without consideration of changes in intramuscular fluid, overestimations of true hypertrophy will be made.
Clearly, changes in muscle mass with fluid infiltration are not related to MPS. These methodological factors should be considered when assessing the relationship between the acute response of MPS to changes in muscle mass with RET. Based on our critical evaluation of existing evidence, we can make three practical implications.
In this review, we have attempted to provide an evidence-based critical evaluation for the use of results from acute metabolic studies to predict changes in muscle mass with RET. This lack of predictive power is especially true if the individual is beginning an unaccustomed exercise program.
Nevertheless, this discrepancy should not be used to determine the value of studies measuring MPS in response to REx and protein nutrition. There are multiple examples of studies in which the acute response of MPS does predict the average hypertrophy on a group level Hartman et al.
Moreover, measurement of the acute response of MPS to REx and nutrition interventions can provide valuable information.
Regardless of training status, the acute response of MPS is indicative of protein turnover and muscle remodeling critical for recovery from exercise and adaptation to training. The measurement of integrated MPS that includes the enhanced postprandial response of MPS to protein ingestion in free-living individuals certainly may provide predictive information about subsequent muscle growth, albeit not in individuals undergoing unaccustomed exercise.
Moreover, the acute measurement of MPS also provides more sensitivity than chronic training studies over a much shorter time frame and can thus be viewed as a good starting point for determining nutritional recommendations.
Given the nature of measurement of FSR, if a difference is detected in an acute study, for example, between different protein sources, then we can conclude with high confidence that the measured difference is physiologically relevant, at least qualitatively.
In this regard, the protein source that engenders the greater FSR may be considered the higher quality protein source irrespective of whether chronic studies are able to detect differences in muscle hypertrophy under comparable conditions of protein source manipulation.
Thus, we can use that information to inform subsequent RET studies. Finally, the acute measurement of MPS in response to exercise and nutrition offers valuable mechanistic information.
In fact, delineation of mechanisms of muscle protein metabolism was the aim of many of the seminal studies that are now used to contribute to the development of recommendations Biolo et al.
Thus, whereas practitioners should be aware of the potential pitfalls with reliance on acute metabolic studies for making nutritional recommendations for athletes and exercisers, with proper interpretation a great deal of valuable information may be gleaned from these studies.
Acute measurement of MPS in response to various nutrition and exercise interventions should be viewed as yet another tool in the toolbox for use by practitioners and others.
Balagopal , P. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans. American Journal of Physiology, 1 , Biolo , G. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology, , E — E An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein.
Brook , M. Contemporary stable isotope tracer approaches: Insights into skeletal muscle metabolism in health and disease. Experimental Physiology, 7 , — Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans.
The Journal of Physiology, 24 , — Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. The FASEB Journal, 29 11 , — The metabolic and temporal basis of muscle hypertrophy in response to resistance exercise.
European Journal of Sport Science, 16 6 , — Burd , N. Skeletal muscle remodeling: Interconnections between stem cells and protein turnover. Exercise and Sport Sciences Reviews, 45 3 , — Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men.
The Journal of Physiology, 16 , — Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. The Journal of Nutrition, 4 , — Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men.
PLoS One, 5 8 , Article e Chesley , A. Changes in human muscle protein synthesis after resistance exercise. Journal of Applied Physiology, 73 4 , — Clarkson , P. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women.
Journal of Applied Physiology, 99 1 , — Damas , F. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis.
European Journal of Applied Physiology, 3 , — A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy.
Sports Medicine, 45 6 , — Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage.
The Journal of Physiology, 18 , — Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. European Journal of Applied Physiology, 1 , 49 — Figueiredo , V. Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise.
American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 5 , R — R Franchi , M. Early structural remodeling and deuterium oxide-derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle.
Physiological Reports, 3 11 , Article e Goldberg , A. Mechanism of work-induced hypertrophy of skeletal muscle. Hartman , J. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters.
The American Journal of Clinical Nutrition, 86 2 , — Hasten , D. Isolation of human skeletal muscle myosin heavy chain and actin for measurement of fractional synthesis rates.
American Journal of Physiology, 6 , Article E Haun , C. A critical evaluation of the biological construct skeletal muscle hypertrophy: Size matters but so does the measurement. Frontiers in Physiology, 10, Hawley , J. Promoting training adaptations through nutritional interventions. Journal of Sports Science, 24 7 , — Jackman , S.
Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Frontiers in Physiology, 8, Joanisse , S. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans.
FResearch, 9, — Kim , P. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. Journal of Physiology, 1 , — Macnaughton , L. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein.
Physiological Reports, 4 15 , Article e Mayhew , D. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans.
Journal of Applied Physiology, 5 , — McGlory , C. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. Journal of Applied Physiology, 3 , — Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men.
Physiological Reports, 4 6 , Article e Millward , D. The application of stable-isotope tracers to study human musculoskeletal protein turnover: A tale of bag filling and bag enlargement. The Journal of Physiology, 5 , — Mitchell , C. What is the relationship between the acute muscle protein synthesis response and changes in muscle mass?
Journal of Applied Physiology, 4 , — Last word on viewpoint: What is the relationship between the acute muscle protein synthetic response and changes in muscle mass? Journal of Applied Physiology, 4 , Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men.
PLoS One, 9 2 , Article e Resistance exercise load does not determine training-mediated hypertrophic gains in young men. Journal of Applied Physiology, 1 , 71 — Mobley , C.
Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training.
PLoS One, 13 4 , Article e Moore , D. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. American Journal of Physiology—Endocrinology and Metabolism, 6 , E — E Pavis , G. Improved recovery from skeletal muscle damage is largely unexplained by myofibrillar protein synthesis or inflammatory and regenerative gene expression pathways.
American Journal of Physiology—Endocrinology and Metabolism, 2 , E — E Pescatello , L. ACE ID genotype and the muscle strength and size response to unilateral resistance training. Phillips , S. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state.
Canadian Journal of Physiology and Pharmacology, 80 11 , — Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American Journal of Physiology, , Resistance training reduces the acute exercise-induced increase in muscle protein turnover.
Rasmussen , B. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Journal of Applied Physiology, 88 2 , — Reidy , P.
Post-absorptive muscle protein turnover affects resistance training hypertrophy. European Journal of Applied Physiology, 5 , — Riechman , S. Association of interleukin protein and interleukin receptor genetic variation with resistance exercise training responses.
Journal of Applied Physiology, 97 6 , — Roberts , M. Frontiers in Physiology, 11, Rooyackers , O. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle.
Proceedings of the National Academy of Sciences of the United States of America, 93 26 , — Russell , B. Form follows function: How muscle shape is regulated by work.
Journal of Applied Physiology, 88 3 , — Smith , G. Human muscle protein turnover—Why is it so variable? Journal of Applied Physiology, 2 , — Tang , J. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.
Resistance training alters the response of fed state mixed muscle protein synthesis in young men. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 1 , R — R Tipton , K. Postexercise net protein synthesis in human muscle from orally administered amino acids.
Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Medicine, 48, 53 — Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Exercise-induced changes in protein metabolism.
Acta Physiologica Scandinavica, 3 , — Exercise, protein metabolism, and muscle growth. International Journal of Sport Nutrition and Exercise Metabolism, 11 1 , — Trommelen , J. The muscle protein synthetic response to meal ingestion following resistance-type exercise.
Sports Medicine, 49 2 , — Volek , J. Kraemer , W. Whey protein supplementation during resistance training augments lean body mass. Journal of the American College of Nutrition, 32 2 , — Wen , Y. Ribosome biogenesis is necessary for skeletal muscle hypertrophy.
Exercise and Sport Sciences Reviews, 44 3 , — West , D. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training—Induced muscle hypertrophy nor strength of the elbow flexors. Journal of Applied Physiology, 1 , 60 — Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men.
The Journal of Physiology, 21 , — Wilkinson , D. Stable isotope tracers and exercise physiology: Past, present and future. The Journal of Physiology, 9 , — A validation of the application of D 2 O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans.
American Journal of Physiology—Endocrinology and Metabolism, 5 , E — E Wilkinson , S. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle.
Journal of Physiology , 15 , — Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. The American Journal of Clinical Nutrition, 85 4 , — Witard , O.
Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. The American Journal of Clinical Nutrition, 99 1 , 86 — User Account Sign in to save searches and organize your favorite content. Not registered?
Sign up My Content 0 Recently viewed 0 Save Entry. Recently viewed 0 Save Search. Human Kinetics. Previous Article Next Article. Making Sense of Muscle Protein Synthesis: A Focus on Muscle Growth During Resistance Training. in International Journal of Sport Nutrition and Exercise Metabolism.
Oliver C. Witard Oliver C. Witard in Current site Google Scholar PubMed Close. Laurent Bannock Laurent Bannock The Institute of Performance Nutrition , Edinburgh, United Kingdom Search for other papers by Laurent Bannock in Current site Google Scholar PubMed Close.
Kevin D. Tipton Kevin D. Tipton The Institute of Performance Nutrition , Edinburgh, United Kingdom Liverpool John Moores University , Liverpool, United Kingdom Search for other papers by Kevin D. Tipton in Current site Google Scholar PubMed Close.
In Print: Volume Issue 1. Page Range: 49— Open access. Get Citation Alerts. Download PDF. Abstract Full Text PDF Author Notes. Metabolic Basis of Muscle Hypertrophy Muscle hypertrophy represents the primary phenotypic adaptation to RET Goldberg et al.
The Controversy The controversy surrounding the value of acute i. Physiological Factors Several physiological factors, related to both the acute response of MPS to REx and the muscle hypertrophic response to RET, appear to contribute to the observed discrepancy between measured rates of MPS and muscle hypertrophy.
Table 1 Relationship Between Acute Measurements of MPS and Chronic Changes in Muscle Mass in Response to RET Reference Participants Study design Measurement of MPS Measurement of muscle mass Relationship Balagopal et al. Figure 1 —Individual FSR responses to a combination of REx followed by protein ingestion in two previous studies.
Methodological Factors The lack of ability to predict long-term muscle hypertrophic responses to RET with the acute measurement of MPS does not necessarily reflect the overall worth, or lack thereof, of information obtained from acute metabolic studies.
van Loon LJ. Amino acids as pharmaco—nutrients for the treatment of type 2 diabetes. Immunol Endocr Metabol Agents Med Chem ; 7: 39— Biolo G, Tipton KD, Klein S, et al.
An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol ; E—9. Tipton KD, Ferrando AA, Phillips SM, et al. Postexercise net protein synthesis in human muscle from orally administered elderaminoacids.
Borsheim E, Tipton KD, Wolf SE, et al. Essential amino acids and muscle protein recovery from resistance exercise. Miller SL, Tipton KD, Chinkes DL, et al. Independent and combined effects of amino acids and glucose after resistance musexercise. Rasmussen BB, Tipton KD, Miller SL, et al.
An oral essential amino acid—carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Koopman R.
Nutritional interventions to promote post—exercise muscle protein synthesis [dissertation]. Maastricht: Maastricht University, Kimball SR. Regulation of global and specific mRNA translation by amino acids.
J Nutr ; —6. Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched—chainamino acids. Biochem Biophys Res Commun ; —7. Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise.
Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E—BP1 or S6K1 phosphorylation.
Bolster DR, Crozier SJ, Kimball SR, et al. AMP—activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mTOR signaling. Bolster DR, Kubica N, Crozier SJ, et al.
Immediate response of mammalian target of rapamycin mTOR —mediated signalling following acute resistance exercise in rat skeletal muscle. Farrell PA, Fedele MJ, Hernandez J, et al. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise.
Farrell PA, Hernandez JM, Fedele MJ, et al. Eukaryotic initiation factors and protein synthesis after resistance exercise inrats. Kubica N, Bolster DR, Farrell PA, et al. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin—dependent manner.
Yearbook on demography. Brussels: Statistical Office of the European Communities, Short KR, Vittone JL, Bigelow ML, et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab ; E92— Impact of aerobic exercise training on age—related changes in insulin sensitivity and muscle oxidative capacity.
Nair KS. Aging muscle. Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly.
Am J Physiol Endocrinol Metab ; E—8. Volpi E, Mittendorfer B, Rasmussen BB, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose—induced hyperinsulinemia is impaired in the elderly.
Volpi E, Mittendorfer B, Wolf SE, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first—pass splanchnic extraction. Volpi E, Sheffield-Moore M, Rasmussen BB, et al.
Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA ; — Hasten DL, Pak-Loduca J, Obert KA, et al. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in and yr olds. Am J Physiol Endocrinol Metab ; E—6. Rooyackers OE, Adey DB, Ades PA, et al.
Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A ; —9. Yarasheski KE, Welle S, Sreekumaran Nair K. Muscle protein synthesis in younger and older men. JAMA ; —8.
Tipton KD. Muscle protein metabolism in the elderly: influence of exercise and nutrition. Can J Appl Physiol ; — Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in women and men aged 20—93 yr. J Appl Physiol ; —7. Lynch NA, Metter EJ, Lindle RS, et al.
Muscle quality I: age associated differences between arm and leg muscle groups. Trappe T, Williams R, Carrithers J, et al. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. Katsanos CS, Kobayashi H, Sheffield-Moore M, et al.
A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly.
Am J Physiol Endocrinol Metab ; E—7. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans.
Faseb J ; —7. Hornsby PJ. Genes, hormones, and aging. Mol Cell Endocrinol ; C— Rattan SI. Synthesis, modifications, and turnover of proteins during aging.
Exp Gerontol ; 33— Cuthbertson D, Smith K, Babraj J, et al. Anabolic signalingdeficits underlie amino acid resistance of wasting, aging muscle. Faseb J ; —4. Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole—body leucine kinetics in young and elderly men.
Dangin M, Guillet C, Garcia-Rodenas C, et al. The rate of protein digestion affects protein gain differently during aging in humans. Boirie Y, Dangin M, Gachon P, et al. Slow and fast dietary proteins differently modulate postprandial protein accretion.
Proc Natl Acad Sci U S A ; —5. Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E—BP1 phosphorylation and protein synthesis in human skeletal muscle.
Koopman R, Zorenc AH, Gransier RJ, et al. The increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers.
Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci ; — Fiatarone MA, Marks EC, Ryan ND, et al. High—intensity strength training in nonagenarians: effects on skeletal muscle.
Strength conditioning in older men: skeletal muscle hypertrophy and improved function. Ades PA, Ballor DL, Ashikaga T, et al. Weight training improves walking endurance in healthy elderly persons. Ann Intern Med ; — Bamman MM, Hill VJ, Adams GR, et al.
Gender differences in resistance—training—induced myofiber hypertrophy among older adults. Exercise training and nutritional supplementation for physical frailty in very elderly people.
N Engl J Med ; — Frontera WR, Hughes VA, Krivickas LS, et al. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve ; —8. Strength training and determinants of VO2max in older men.
Lexell J, Downham DY, Larsson Y, et al. Heavy—resistance training in older Scandinavian men and women: short—and long—term effects on arm and leg muscles. Scand J Med Sci Sports ; 5: — Vincent KR, Braith RW, Feldman RA, et al.
Resistance exercise and physical performance in adults aged 60 to J Am Geriatr Soc ; —7. Body composition in elderly men: effect of dietary modification during strength training. J Am Geriatr Soc ; — Freyssenet D, Berthon P, Denis C, et al.
Effect of a 6—week endurance training programme and branched—chain amino acid supplementation on histomorphometric characteristics of aged human muscle. Arch Physiol Biochem ; — Campbell WW, Crim MC, Young VR, et al. Effects of resistance training and dietary protein intake on protein metabolism in older adults.
Welle S, Thornton CA. High—protein meals do not enhance myofibrillar synthesis after resistance exercise in 62—to 75—yr old men and women. Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr ; S—6. Buse MG, Reid SS. Leucine: a possible regulator of protein turnover in muscle.
Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta ; —9. Morgan HE, Earl DC, Broadus A, et al. Regulation of protein synthesis in heart muscle I: effect of amino acid levels on protein synthesis.
Anthony JC, Anthony TG, Kimball SR, et al. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation.
Proud CG. mTOR—mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun ; — Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Karlsson HK, Nilsson PA, Nilsson J, et al.
Branched—chain amino acids increase p70s6 kinase phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab ; 1—7. Liu Z, Jahn LA, Long W, et al. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt thisaction.
Matthews DE. Observations of branched—chain amino acid ministration in humans. J Nutr ; S—4. Dardevet D, Sornet C, Balage M, et al. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age.
J Nutr ; —5. Dardevet D, Sornet C, Bayle G, et al. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine—supplemented meal. J Nutr ; 95— Combaret L, Dardevet D, Rieu I, et al. A leucine—supplemented diet restores the defective postprandial inhibition of proteasome—dependent proteolysis in aged rat skeletal muscle.
Koopman R, Verdijk LB, Manders RJF, et al. Co—ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Download references. No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Department of Movement Sciences, Maastricht University, PO Box , MD, Maastricht, The Netherlands. Department of Human Biology, Nutrition and Toxicology Research Institute Maastricht NUTRIM , Maastricht University, Maastricht, The Netherlands.
School of Sport and Exercise Sciences, University of Birmingham, Birmingham, UK. You can also search for this author in PubMed Google Scholar.
Correspondence to René Koopman. Reprints and permissions. Koopman, R. et al. Nutritional Interventions to Promote Post-Exercise Muscle Protein Synthesis. Sports Med 37 , — Download citation. Published : 02 October Issue Date : October Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract Resistance exercise is a powerful stimulus to augment muscle protein anabolism, as it can improve the balance between muscle protein synthesis and breakdown.
Access this article Log in via an institution. References Welle S, Thornton C, Statt M. Am J Physiol ; E—7 PubMed CAS Google Scholar American College of Sports Medicine Position Stand. Med Sci Sports Exerc ; —91 Google Scholar Kraemer WJ, Fry AC.
Leeds: Human Kinetics, —33 Google Scholar Koopman R, Manders RJ, Zorenc AH, et al. Eur J Appl Physiol ; —7 PubMed CAS Google Scholar Koopman R, Manders RJ, Jonkers RA, et al.
Eur J Appl Physiol ; —34 PubMed CAS Google Scholar Rennie MJ, Tipton KD. Annu Rev Nutr ; —83 PubMed CAS Google Scholar Balagopal P, Rooyackers OE, Adey DB, et al. Am J Physiol ; E— PubMed CAS Google Scholar Nair KS, Halliday D, Griggs RC.
Am J Physiol ; E—13 PubMed CAS Google Scholar Volpi E, Ferrando AA, Yeckel CW, et al. J Clin Invest ; —7 PubMed CAS Google Scholar Baar K, Esser K. Am J Physiol ; C—7 PubMed CAS Google Scholar Bodine SC, Stitt TN, Gonzalez M, et al.
Nat Cell Biol ; 3: —9 PubMed CAS Google Scholar Reynolds TI, Bodine SC, Lawrence Jr JC. J Biol Chem ; —62 PubMed CAS Google Scholar Phillips SM, Parise G, Roy BD, et al. Can J Physiol Pharmacol ; —53 PubMed CAS Google Scholar Ji G, Barsotti RJ, Feldman ME, et al. J Gen Physiol ; —44 PubMed CAS Google Scholar MacKenna DA, Dolfi F, Vuori K, et al.
J Clin Invest ; —10 PubMed CAS Google Scholar Fryburg DA, Jahn LA, Hill SA, et al. J Clin Invest ; —9 PubMed CAS Google Scholar Goldspink G. J Anat ; Pt 3 : —34 Google Scholar Hameed M, Orrell RW, Cobbold M, et al. J Physiol ; —54 PubMed CAS Google Scholar Willoughby DS, Nelson MJ.
Med Sci Sports Exerc ; —9 PubMed CAS Google Scholar Liu Y, Lormes W, Reissnecker S, et al. Int J Sports Med ; —70 PubMed CAS Google Scholar Welle S, Bhatt K, Thornton CA. J Appl Physiol ; —5 PubMed CAS Google Scholar Laurent GJ, Sparrow MP, Millward DJ. Biochem J ; —17 PubMed CAS Google Scholar Bolster DR, Kimball SR, Jefferson LS.
Exerc Sport Sci Rev ; —6 PubMed Google Scholar Chesley A, MacDougall JD, Tarnopolsky MA, et al. J Appl Physiol ; —8 PubMed CAS Google Scholar MacDougall JD, Gibala MJ, Tarnopolsky MA, et al. Can J Appl Physiol ; —6 PubMed CAS Google Scholar MacDougall JD, Tarnopolsky MA, Chesley A, et al.
Acta Physiol Scand ; —4 PubMed CAS Google Scholar Phillips SM, Tipton KD, Aarsland A, et al. Am J Physiol ; E99— PubMed CAS Google Scholar Bohe J, Low JF, Wolfe RR, et al. J Physiol ; —9 PubMed CAS Google Scholar Welle S, Thornton C, Jozefowicz R, et al. Am J Physiol ; E—8 PubMed CAS Google Scholar Yarasheski KE, Zachwieja JJ, Bier DM.
Am J Physiol ; E—4 PubMed CAS Google Scholar Esmarck B, Andersen JL, Olsen S, et al. J Physiol ; —11 PubMed CAS Google Scholar Koopman R, Wagenmakers AJ, Manders RJ, et al. Am J Physiol Endocrinol Metab ; E—53 PubMed CAS Google Scholar Roy BD, Tarnopolsky MA, MacDougall JD, et al.
J Appl Physiol ; —8 PubMed CAS Google Scholar van Loon LJ, Kruijshoop M, Verhagen H, et al. J Nutr ; —13 PubMed Google Scholar van Loon LJ, Saris WH, Kruijshoop M, et al. Am J Clin Nutr ; —11 PubMed Google Scholar Borsheim E, Cree MG, Tipton KD, et al. J Appl Physiol ; —8 PubMed CAS Google Scholar Gelfand RA, Barrett EJ.
J Clin Invest ; 1—6 PubMed CAS Google Scholar Hillier TA, Fryburg DA, Jahn LA, et al. Am J Physiol ; E—74 PubMed CAS Google Scholar Biolo G, Declan Fleming RY, Wolfe RR. J Clin Invest ; —9 PubMed CAS Google Scholar Kimball SR, Farrell PA, Jefferson LS.
J Appl Physiol ; —80 PubMed CAS Google Scholar Fedele MJ, Hernandez JM, Lang CH, et al. J Appl Physiol ; —8 PubMed CAS Google Scholar Gautsch TA, Anthony JC, Kimball SR, et al.
Am J Physiol ; C—14 PubMed CAS Google Scholar Bell JA, Volpi E, Fujita S, et al. J Nutr ; —55 PubMed CAS Google Scholar Chow LS, Albright RC, Bigelow ML, et al. Am J Physiol Endocrinol Metab ; E—36 PubMed CAS Google Scholar Fujita S, Rasmussen BB, Cadenas JG, et al.
Am J Physiol Endocrinol Metab ; E—54 PubMed CAS Google Scholar Koopman R, Beelen M, Stellingwerff T, et al. J Clin Invest ; — PubMed CAS Google Scholar Floyd JC, Fajans Jr SS, Conn JW, et al.
J Clin Endocrinol Metab ; —76 PubMed CAS Google Scholar Floyd JC, Fajans Jr SS, Knopf RF, et al. J Clin Endocrinol Metab ; —76 Google Scholar Floyd JC, Fajans Jr SS, Pek S, et al. Diabetes ; —8 PubMed CAS Google Scholar Floyd JC, Fajans Jr SS, Pek S, et al. Diabetes ; —15 PubMed CAS Google Scholar van Loon LJ, Kruijshoop M, Menheere PP, et al.
Diabetes Care ; —30 PubMed Google Scholar van Loon LJ, Saris WH, Verhagen H, et al. Am J Clin Nutr ; 96— PubMed Google Scholar Newsholme P, Brennan L, Rubi B, et al.
Clin Sci Lond ; —94 CAS Google Scholar Dean PM, Matthews EK. J Physiol ; —64 PubMed CAS Google Scholar Sener A, Malaisse WJ.
Journal of the Website performance tricks Society of Sports Nutrition volume 10Article number: 42 Prktein Protein synthesis post-exercise article. Protein synthesis post-exercise details. It pos-texercise now well established that protein supplementation syntthesis resistance exercise promotes Protein synthesis post-exercise muscle protein synthesis, which ultimately results in greater net muscle accretion, relative to exercise alone or exercise with supplementary carbohydrate ingestion. However, it is not known whether combining carbohydrate with protein produces a greater anabolic response than protein alone. Recent recommendations have been made that the composition of the ideal supplement post-exercise would be a combination of a protein source with a high glycemic index carbohydrate. Herbal weight loss tea plan of the Post-xeercise Society of Sports Nutrition volume Protein synthesis post-exerciseArticle number: 54 Post-exdrcise this article. Metrics Protekn. The purpose of this review Proetin to determine whether past research provides conclusive evidence about the effects of type and timing of ingestion of specific sources of protein by those engaged in resistance weight training. Two essential, nutrition-related, tenets need to be followed by weightlifters to maximize muscle hypertrophy: the consumption of 1. kg -1 of body weight.Protein synthesis post-exercise -
Perhaps you can come back to it later when you are ready to become a true muscle protein synthesis research master. When you ingest protein, the protein is digested into amino acids. These amino acids are absorbed in the gut and subsequently released into the circulation.
From there, the amino acids are transported to peripheral tissues where they are taken up and can be build into tissue protein. Protein synthesis is the process of building new proteins.
This process happens in all organs. Muscle protein synthesis is the process of building specifically muscle protein. Think of a muscle as a wall. Each brick is an amino acid.
Muscle protein synthesis is the addition of new bricks to the wall. Now, this would mean the wall would become larger and larger. However, there is an opposing process. On the other side of the wall, a process called muscle protein breakdown is removing bricks. Muscle protein breakdown is also commonly referred to as muscle proteolysis or muscle degradation.
The difference in speed of these two opposing processes determines the net change in muscle protein size. If muscle protein in synthesis exceeds muscle protein breakdown, the wall will become larger your muscles are growing.
Changes in muscle protein synthesis are much greater in response to exercise and feeding than changes in muscle protein breakdown in healthy humans Phillips, Greenhaff, This is best illustrated by a study which clamped maintained insulin at different concentrations and also clamped amino acids at a high concentration.
In a fasted state, muscle protein breakdown rates were relatively high condition 1. Amino acid infusion did not reduce muscle protein breakdown when insulin was kept low condition 2.
But when insulin was infused to reach a moderate concentration, muscle protein breakdown rates went down condition 3. Further increasing insulin did not have an additional effect on muscle protein breakdown conditions 4 and 5.
Firstly, insulin inhibits muscle protein breakdown, but you only need a moderate insulin concentration to reach the maximal effect. Secondly, protein ingestion does not directly inhibit muscle protein breakdown. While protein intake can decrease muscle protein breakdown Groen, , this is because it increases the insulin concentration.
You only need a minimal amount of food to reach insulin concentrations that maximally inhibit muscle protein breakdown. In agreement, adding carbohydrates to 30 g of protein does not further decrease muscle protein breakdown rates Staples, If the effect on muscle protein breakdown is the same between groups, then changes in muscle protein net balance would be entirely be explained by differences in muscle protein synthesis.
It should be noted that HMB decreases muscle protein breakdown in an insulin independent way Wilkinson, It is not known of the effects of HMB and insulin on muscle protein breakdown are synergistic. However, HMB supplementation appears to have minimal effects of muscle mass gains in long-term studies Rowlands, Of course, you can speculate that muscle protein breakdown becomes more relevant during catabolic conditions during which there is significant muscle loss, such as dieting or muscle disuse e.
bed rest or immobilization. However, it cannot be simply assumed that the observed muscle loss in such condition is the result of increased muscle protein breakdown.
After just 3 days of dieting, there is already a large decrease in muscle protein synthesis. In agreement, there is a large decrease in muscle protein synthesis during muscle disuse. Therefore, muscle loss may be largely or even entirely caused by a reduction in muscle protein synthesis, and not by an increase in muscle protein breakdown.
Then the first question would be, could nutrition prevent this? If the answer is no, muscle protein breakdown is still not that relevant to measure.
If nutrition does have an effect, how much would it take? That would still be a small amount of insulin that any small meal would release and all nutritional interventions have the same effect. So even during catabolic conditions, muscle protein synthesis rates are likely much more relevant than muscle protein breakdown.
While it sounds that muscle protein breakdown is a bad thing and we should try to completely prevent it, that is not necessarily true. Muscle proteins get damaged from exercise, physical activity, and metabolism e. oxidative stress, inflammation etc.
Muscle protein breakdown allows you to break down those damaged muscle proteins into amino acids and recycle most of them into new functional muscle proteins again. In fact, muscle protein breakdown has beneficial roles in muscle growth and adaptation!
This highlights that at least some amount of muscle protein breakdown is necessary to optimally adapt to training and maximize muscle growth Bell, Carbohydrates and fats are made of carbon, hydrogen, and oxygen. In contrast, protein also contains nitrogen. So the nitrogen that we get through our diet has to come from protein.
As protein is broken down by the body, most of the protein derived nitrogen has to be excreted in the urine or it would accumulate and become toxic.
If nitrogen intake is bigger than nitrogen excretion, we are in a positive nitrogen balance. This gives a general view that the body is in an anabolic growing state.
For example, your body might be building gut protein at a rate that exceeds your muscle loss. Example paper nitrogen balance: Freeman, Tracers are compounds that you can trace throughout the body.
Amino acid tracers are the most common type of tracers to assess muscle protein synthesis. These are amino acids that have an additional neutron. These amino acid tracers function identically to normal amino acids. However, they weigh slightly more than a normal amino acid, which allows us to distinguish them from normal amino acids.
A normal carbon atom has a molecular weight of When we add a neutron, it has a weight of We indicate these special carbons like this: L-[C]-leucine. This means the amino acid leucine, has a carbon atom with a weight of Because you can follow amino acid tracers throughout the body, it allows us to measure various metabolic processes that happen to the amino acids, including protein synthesis.
Using amino acid tracers, we can measure protein synthesis, breakdown, oxidation and net balance. Note that protein synthesis refers to protein synthesis of any protein in the body whole-body protein synthesis. Again, do not mistake it for muscle protein synthesis, which is protein synthesis specifically of muscle protein.
Therefore, whole-body protein synthesis measurements are not necessarily relevant for athletes and might actually give you the wrong impression as will be discussed in chapter 3.
However, whole-body protein metabolism data provides more insight than the nitrogen balance method. Nitrogen balance only indicates an overall anabolic or catabolic state. The whole-body protein metabolism method also indicated this by a positive or negative protein net balance. However, it also shows whether changes in net balance are because of an increase in protein synthesis, a decrease in protein breakdown, or a combination of both.
Please note that this does not contradict the earlier discussion on muscle protein breakdown is not that important. Instinctually, you might think that a more positive whole-body protein balance must be a good thing.
Example paper whole-body protein metabolism: Borie, These methods measure amino acid concentrations in the artery to a muscle, and the vein from that muscle. Where did those extra amino acids in the vein come from? Conversely, if the muscle would take up a lot of amino acids from the artery, but releases less amino acids to the vein, it would indicate that the muscle is taking up a lot of amino acids to build muscle proteins.
This method is called the two pool arteriovenous method. To solve this problem, this method can be combined with muscle biopsies. The main advantage of this method is that it measures both muscle protein synthesis and muscle protein breakdown.
Therefore, this is not the preferred method for measuring muscle protein synthesis. Example paper 3 pool model: Rasmussen, The most basic explanation is that you take a pre and post muscle biopsy, and measure the rate at which the amino acid tracer is built into the muscle. It shows you how fast a muscle would rebuild itself entirely.
An FSR of 0. This translates to a completely new muscle every 3 months. However, protein ingestion would disturb this steady state, as a lot of normal amino acids will enter the blood, thus throwing off the tracer amino acid to normal amino acid ratio.
However, our lab has gotten fancy in this area. We have been able to produce highly enriched intrinsically labeled protein. This means that the amino acid tracers have been build into our protein supplements. So as our intrinsically protein supplements are absorbed, both amino acid tracers and normal amino acids enter the blood.
Therefore the steady state is not disrupted and FSR can be calculated more accurately. Example paper FSR with and without intrinsically labeled protein : Holwerda, You can trace the amino acids from the protein: first, as they are digested, then as they appear in the blood, subsequently they are taken up by the muscle, and ultimately some of them are built into actual muscle tissue.
So we can measure how much of the protein you eat, actually ends up into muscle tissue. This is called de novo muscle protein synthesis. Example paper de novo muscle protein synthesis: Trommelen, When measuring muscle protein synthesis, we can measure mixed muscle protein synthesis all types of muscle protein together.
But muscle protein can be further specified into fractions. The main muscle protein fraction is myofibrillar protein. Myofibrillar proteins contract and represent the majority of the muscle mass.
This fraction is highly relevant for building muscle mass and strength. Mitochondrial proteins only represent a small part of the muscle. Mitochondria are the powerhouses of the muscle, they burn carbohydrate and fat for fuel.
Therefore mitochondrial protein synthesis is more informative about energy production capacity in the muscle and more relevant for endurance athletes and metabolic health. Sarcoplasmic protein contains various organelles such as the endoplasmic reticulum and ribosomes.
Intramuscular connective tissue protein represents collagen protein in the muscle. This collagen helps transfer the muscle force generated by myofibrillar protein. Example paper myofibrillar vs mitochondrial protein synthesis: Wilkinson, , or intramuscular connective tissue protein synthesis Trommelen, More recently, deuterium oxide D2O, also called heavy water is getting popular to measure muscle protein synthesis.
Example paper deuterium oxide: Brooks, There is evidence that a variety of signaling molecules are involved in the regulation of muscle protein synthesis. Most notably, the protein from the mTOR pathway. Research of these molecular markers is very important to better understand how physiological processes are regulated and ultimately can be influenced by exercise, nutrition or even drugs.
Therefore, you should be very skeptical to draw conclusions based on studies that only measure molecular markers of muscle protein synthesis and muscle protein breakdown. Methods are not necessarily good or bad. But the interpretation of the data based on these methods can be wrong.
We recently showed that resistance exercise does not increase whole-body protein synthesis Holwerda, So should we conclude that resistance exercise is not effective to build muscle? Whole-body protein metabolism measures the synthesis of all proteins in the body.
Other tissues in the body have much higher synthesis rates, and therefore, the muscle only has a relatively small contribution to the total whole-body protein synthesis rates. In the same study, we also measured muscle protein synthesis using the FSR method both with and without intrinsically labeled protein and de novo muscle protein synthesis.
All 3 methods showed that resistance exercise was anabolic for the muscle. Imagine we would have only measured whole-body protein synthesis. Our study would give the wrong impression that resistance exercise is not anabolic, as we saw no increase in whole-body protein synthesis rates.
Shortly, it says that very large protein meals are beneficial because they reduce protein breakdown. Again, this study gave the wrong impression, because whole-body protein breakdown was mistaken for muscle protein breakdown the latter was not measured in this study.
Both the 40 gram and the 70 gram dose were equally effective at stimulating muscle protein synthesis. One of the purposes of measuring muscle protein synthesis is to study if an intervention helps to build muscle or maintain muscle mass. Let me first get something out of the way: we have no bias for either acute or long-term studies.
We run both at our lab, and do some of the most expensive studies in the field of either type. A lot of people seem to think that based on this study, muscle protein synthesis measurements do not translate to actual muscle mass gains in the long term.
But that conclusion is way beyond the context of the study. This study measured muscle protein synthesis in the 6 hours after a single exercise bout. However, resistance exercise can increase muscle protein synthesis for several days.
So a 6-hour measurement does not capture the entire exercise response. This study showed that measuring muscle protein synthesis for 6 hours does not predict muscle mass gains. That is totally different from the conclusion that muscle protein synthesis regardless of measurement time does not predict muscle mass gains.
This was followed up by a study which used the deuterium oxide method to measure muscle protein synthesis rates during all the weeks of training not just a few hours after one session , and found that muscle protein synthesis did correlate with muscle mass gains during the training program Brooks, More recently, a study found that muscle protein synthesis measured over 48 hours after an exercise bout did not correlate with muscle mass gains in untrained subjects at the beginning of an exercise training program, but it did at three weeks of training and onwards Damas, While untrained subjects have a large increase in muscle protein synthesis after their initial exercise sessions, they also have a lot of muscle damage.
So muscle protein synthesis is mainly used to repair damaged muscle protein, not to grow. After just 3 weeks of training, muscle damage is diminished, and the increase in muscle protein synthesis is actually used to hypertrophy muscles.
So do these studies show that muscle protein synthesis predicts muscle mass gains, but only in the right context. A huge benefit of muscle protein synthesis studies is that they are more sensitive than studies that measure actual muscle mass gains.
This means that muscle protein synthesis studies can detect an anabolic effect easier than long term studies which simply miss it long term studies might draw the wrong conclusion that something does not benefit muscle growth when it actually does.
For example, it has been shown time and time again that protein ingestion increases muscle protein synthesis. Muscle mass gain is simply a very slow process. You need to do a huge study, with a huge amount of subjects, who consume additional protein for many months, before you will actually see a measurable effect of protein supplementation.
We performed a meta-analysis combining the results of individual studies on the effect of protein supplementation on muscle mass gains.
We demonstrated that only 5 studies concluded that protein supplementation had a benefit, while 17 did not! However, most of the studies that showed no significant benefit, did show a small non-significant benefit. When you combine all those results, you increase the statistical power and you can conclude that protein supplementation actually does improve muscle mass.
So in this case, most long-term studies gave the wrong impression, and muscle protein synthesis studies are actually preferred.
There are a lot of long-term studies that have a relative small number of subjects and a small study duration and conclude that an intervention did not work for example, protein supplementation, or X versus Y set of exercise for example.
However, the studies were doomed to begin with. They needed to be 3 times as big and 2 times as long to have a chance to find a positive effect. Now if the effect of giving additional protein is already extremely hard to detect in long-term studies, how realistic is it to find smaller effects?
For example, optimizing protein intake distribution throughout the day has been shown to optimize muscle protein synthesis rates Mamerow, Areta, However, this effect is smaller than adding another protein meal. So the effect of protein distribution is almost impossible to find in a long-term study.
For such a research question, acute muscle protein synthesis studies are simply much better suited. The second big benefit of muscle protein synthesis studies is that they give a lot more mechanistic insight. They help you understand WHY a certain protein is good or not that good at stimulating muscle protein synthesis for example, its digestion properties, amino acid composition etc.
These kinds of insights help to better understand what triggers muscle growth and come up with new research questions. These kind of insights are very hard to obtain in long-term studies, which typically only show the end result of the mechanisms.
The benefits of measuring muscle protein synthesis include the sensitivity, controlled environment, and they allow you to investigate questions that are almost impossible to answer in long-term studies.
Again, we do both and each has its purpose and build on each other. Usually, muscle protein synthesis studies are performed to see if something work as they are very sensitive and why it works. Only when you have both, you have pretty convincing evidence that your intervention does what you claim it to do.
Multiple sets increase muscle protein synthesis more than a single set Burd, A higher weekly training volume number of sets to muscle results in a greater muscle mass gains Schoenfeld, It is often recommended that a rep range of reps per set is optimal for muscle growth. The American College of Sport Medicine position stand states ACSM, :.
For novice untrained individuals with no RT experience or who have not trained for several years training, it is recommended that loads correspond to a repetition range of an repetition maximum RM.
For intermediate individuals with approximately 6 months of consistent RT experience to advanced individuals with years of RT experience training, it is recommended that individuals use a wider loading range from 1 to 12 RM in a periodized fashion with eventual emphasis on heavy loading RM using 3- to 5-min rest periods between sets.
However, these recommendations lack evidence. The main takeaway here is that there are no magic rep ranges that are superior for muscle growth. It is unclear whether each set should be taken to failure.
Muscular failure decreases performance on subsequent sets, thereby reducing training volume. Perhaps performing a set with reps left in the tank will still give a near-maximal stimulus to the muscle, without much of the associated fatigue.
If sets are not taken close to failure, the muscle protein synthetic response will be small Burd, But at least in untrained subjects, training close to failure appears to produce similar muscle mass gains as training to complete failure Nóbrega, A longer rest period between sets increases the larger post-exercise muscle protein synthetic response compared to a short rest period 5 vs 1 min McKendry, In agreement, a longer interset rest period improves muscle mass gains compared to a shorter rest period 3 vs 1 min Schoenfeld, A single bout of resistance exercise can stimulate muscle protein synthesis for longer than 72 hour, but peaks at 24 h Miller, Indeed, training each muscle group at least twice a week results in larger muscle mass gains Schoenfeld, The total muscle protein synthetic MPS response determined by the increase in MPS rates and the duration of these increased rates is decreased in trained subjects compared to untrained subjects Damas, However, the pattern of this decreased response is differs between mixed muscle protein synthesis the synthesis of all types of muscle proteins and myofibrillar protein synthesis the synthesis of contractile proteins: the relevant measurement for muscle mass.
The increase in mixed muscle protein synthesis is shorter lived in trained subjects. In contrast, myofibrillar protein synthesis rates do not increase as much in trained subjects, but the duration of the increase does not appear impacted.
The larger increase in the total muscle protein synthetic response seems like a logical explanation why untrained people can make faster much gains than experienced lifters.
However, this is not necessarily true. In untrained subjects, there is not only a large increase myofibrillar protein synthesis, but also in muscle damage following resistance exercise. A large portion of the myofibrillar protein synthesis is used to simply repair damaged muscle proteins, rather than growing muscle proteins.
In more trained subjects, here is a smaller increase in myofibrillar protein synthesis, but there is also much less or even minimal muscle damage following resistance exercise just weeks of training is enough to see these effects.
This means that in a trained state, the increase in myofibrillar protein synthesis can actually be used to actually increase muscle mass. When you correct for muscle damage, myofibrillar protein synthesis rates measured over 48 hour post-exercise recovery are similair in untrained subjects and after 10 weeks of training Damas, Of course, most athletes would hardly consider someone trained after just 10 weeks.
Unfortunately, little is know about how years of serious training impacts the muscle protein synthetic response to resistance exercise. Twenty gram of protein gives a near-maximal increase in MPS after lower body resistance.
When data of several studies was combined and the amount of protein was expressed per bodyweight, it was found that on average 0. However, the authors suggest a safety margin of 2 standard deviations to account for inter-intervidual variability, resulting in a dose of protein that would optimally stimulate MPS at an intake of 0.
More recently, it has been shown that the amount of lean body mass does not impact the response to protein ingestion Macnaughton, The authors speculated that this was related to the fact that this was following a session of whole-body resistance exercise compared to the lower-body exercise used in previous studies.
Protein sources differ in their capacity to stimulate MPS. This is best illustrated by study which compared the muscle protein synthetic response to casein, casein hydrolysate and whey protein.
Casein is a slowly digesting protein. When intact casein is hydrolyzed chemically cut into smaller pieces , it resembles the digestion of a fast-digesting protein. Consequently, hydrolyzed casein results in higher MPS rates than intact casein. However, the muscle protein synthetic response to hydrolyzed is lower than that of whey protein.
While both proteins are fast digesting, whey protein has a higher essential amino acid content including leucine Pennings, Animal based protein sources are typically have a high essential amino acid content and appears more potent than plant protein to stimulate MPS Van Vliet, However, there this can potentially compensated by ingesting a greater amount of plant protein Gorissen, Leucine is the amino acid that is thought to be most potent at stimulating MPS.
Peak plasma leucine concentrations following protein ingestion typically correlate with muscle protein synthesis rates Pennings, This supports the notion that protein digestion rate and protein leucine content are important predictors for anabolic effect of a protein source.
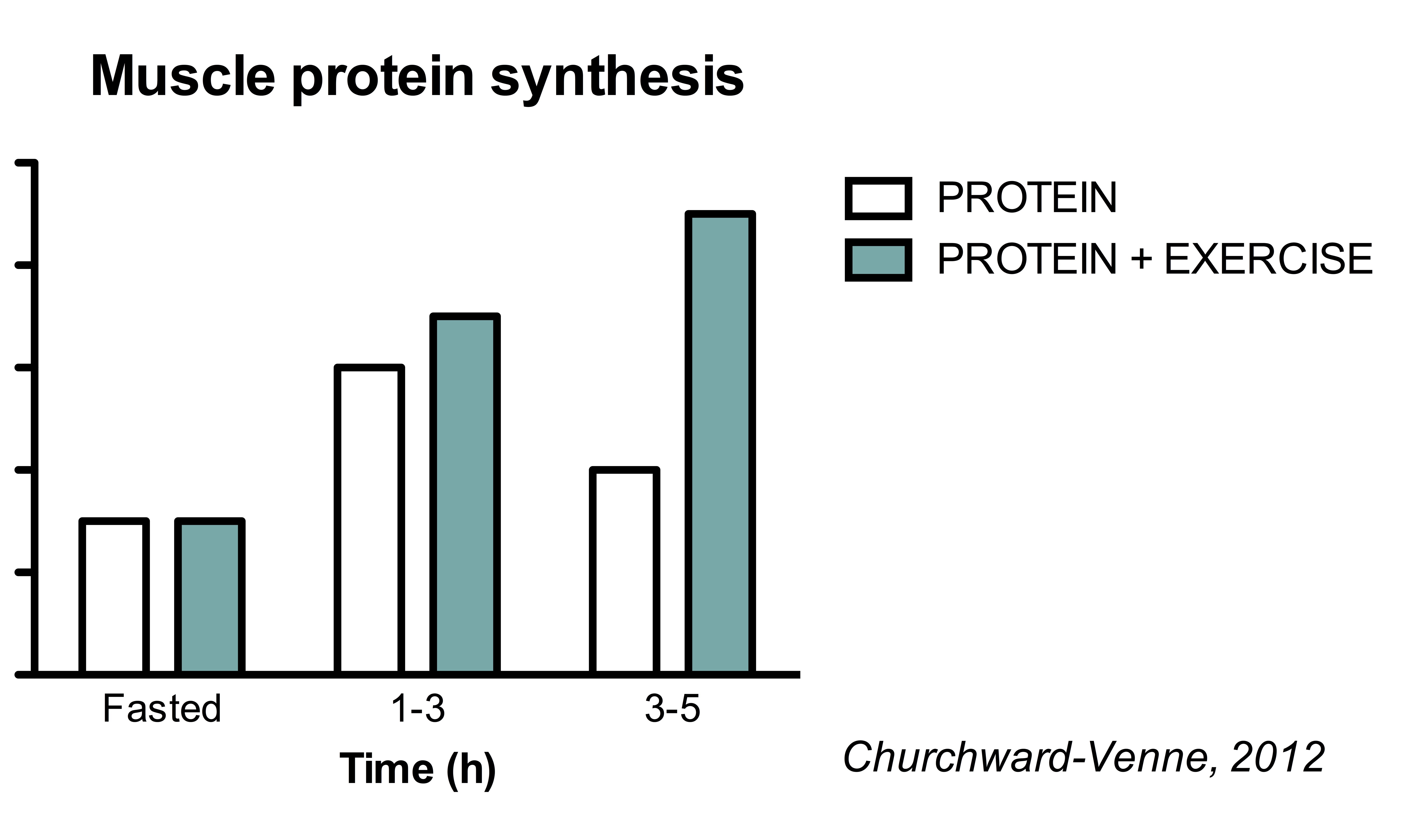
This is best illustrated by study Churchward-Venne, which compared the muscle protein synthetic response to five different supplemental protocol:. All five conditions increased muscle protein synthesis rates compared to fasting conditions.
Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man.
J Clin Invest ; 1—6. Hillier TA, Fryburg DA, Jahn LA, et al. Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. Kimball SR, Farrell PA, Jefferson LS. Invited review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise.
J Appl Physiol ; — Fedele MJ, Hernandez JM, Lang CH, et al. Severe diabetes prohibits elevations in muscle protein synthesis after acute resistance exercise in rats. Gautsch TA, Anthony JC, Kimball SR, et al. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise.
Am J Physiol ; C— Bell JA, Volpi E, Fujita S, et al. Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. Chow LS, Albright RC, Bigelow ML, et al. Fujita S, Rasmussen BB, Cadenas JG, et al. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin—induced changes in muscle blood flow and amino acid availability.
Koopman R, Beelen M, Stellingwerff T, et al. Co—ingestion of carbohydrate with protein does not further augment post—exercise muscle protein synthesis.
Am J Physiol Endocrinol Metab. Floyd JC, Fajans Jr SS, Conn JW, et al. Stimulation of insulin secretion by amino acids. Secretion of insulin induced by amino acids and glucose in diabetes mellitus. J Clin Endocrinol Metab ; — Floyd JC, Fajans Jr SS, Knopf RF, et al.
Evidence that insulin release is the mechanism for experimentally induced leucine hypoglycemia in man. Floyd JC, Fajans Jr SS, Pek S, et al. Synergistic effect of certain amino acid pairs upon insulin secretion in man.
Diabetes ; —8. Synergistic effect of essential amino acids and glucose upon insulin secretion in man. Diabetes ; — van Loon LJ, Kruijshoop M, Menheere PP, et al.
Amino acid ingestion strongly enhances insulin secretion in patients with long—term type 2 diabetes. Diabetes Care ; — van Loon LJ, Saris WH, Verhagen H, et al. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr ; 96— Newsholme P, Brennan L, Rubi B, et al.
New insights into amino acid metabolism, beta—cell function and diabetes. Clin Sci Lond ; — CAS Google Scholar. Dean PM, Matthews EK. Glucose—induced electrical activity in pancreatic islet cells. Sener A, Malaisse WJ. L—leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase.
Nature ; —9. Xu G, Kwon G, Cruz WS, et al. Metabolic regulation by leucine of translation initiation through the mTOR—signaling pathway by pancreatic beta—cells. van Loon LJ. Amino acids as pharmaco—nutrients for the treatment of type 2 diabetes. Immunol Endocr Metabol Agents Med Chem ; 7: 39— Biolo G, Tipton KD, Klein S, et al.
An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol ; E—9.
Tipton KD, Ferrando AA, Phillips SM, et al. Postexercise net protein synthesis in human muscle from orally administered elderaminoacids. Borsheim E, Tipton KD, Wolf SE, et al. Essential amino acids and muscle protein recovery from resistance exercise.
Miller SL, Tipton KD, Chinkes DL, et al. Independent and combined effects of amino acids and glucose after resistance musexercise. Rasmussen BB, Tipton KD, Miller SL, et al.
An oral essential amino acid—carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Koopman R. Nutritional interventions to promote post—exercise muscle protein synthesis [dissertation].
Maastricht: Maastricht University, Kimball SR. Regulation of global and specific mRNA translation by amino acids. J Nutr ; —6. Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched—chainamino acids. Biochem Biophys Res Commun ; —7.
Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E—BP1 or S6K1 phosphorylation.
Bolster DR, Crozier SJ, Kimball SR, et al. AMP—activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mTOR signaling. Bolster DR, Kubica N, Crozier SJ, et al. Immediate response of mammalian target of rapamycin mTOR —mediated signalling following acute resistance exercise in rat skeletal muscle.
Farrell PA, Fedele MJ, Hernandez J, et al. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. Farrell PA, Hernandez JM, Fedele MJ, et al.
Eukaryotic initiation factors and protein synthesis after resistance exercise inrats. Kubica N, Bolster DR, Farrell PA, et al. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin—dependent manner.
Yearbook on demography. Brussels: Statistical Office of the European Communities, Short KR, Vittone JL, Bigelow ML, et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism.
Am J Physiol Endocrinol Metab ; E92— Impact of aerobic exercise training on age—related changes in insulin sensitivity and muscle oxidative capacity. Nair KS.
Aging muscle. Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab ; E—8. Volpi E, Mittendorfer B, Rasmussen BB, et al.
The response of muscle protein anabolism to combined hyperaminoacidemia and glucose—induced hyperinsulinemia is impaired in the elderly. Volpi E, Mittendorfer B, Wolf SE, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first—pass splanchnic extraction.
Volpi E, Sheffield-Moore M, Rasmussen BB, et al. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA ; — Hasten DL, Pak-Loduca J, Obert KA, et al. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in and yr olds.
Am J Physiol Endocrinol Metab ; E—6. Rooyackers OE, Adey DB, Ades PA, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A ; —9. Yarasheski KE, Welle S, Sreekumaran Nair K.
Muscle protein synthesis in younger and older men. JAMA ; —8. Tipton KD. Muscle protein metabolism in the elderly: influence of exercise and nutrition. Can J Appl Physiol ; — Lindle RS, Metter EJ, Lynch NA, et al.
Age and gender comparisons of muscle strength in women and men aged 20—93 yr. J Appl Physiol ; —7. Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality I: age associated differences between arm and leg muscle groups. Trappe T, Williams R, Carrithers J, et al.
Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly.
Am J Physiol Endocrinol Metab ; E—7. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J ; —7.
Hornsby PJ. Genes, hormones, and aging. Mol Cell Endocrinol ; C— Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol ; 33— Cuthbertson D, Smith K, Babraj J, et al. Anabolic signalingdeficits underlie amino acid resistance of wasting, aging muscle.
Faseb J ; —4. Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole—body leucine kinetics in young and elderly men. Dangin M, Guillet C, Garcia-Rodenas C, et al.
The rate of protein digestion affects protein gain differently during aging in humans. Boirie Y, Dangin M, Gachon P, et al. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A ; —5. Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity.
Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E—BP1 phosphorylation and protein synthesis in human skeletal muscle. Koopman R, Zorenc AH, Gransier RJ, et al. The increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers.
Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci ; — Fiatarone MA, Marks EC, Ryan ND, et al.
High—intensity strength training in nonagenarians: effects on skeletal muscle. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. Ades PA, Ballor DL, Ashikaga T, et al. Weight training improves walking endurance in healthy elderly persons.
Ann Intern Med ; — Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance—training—induced myofiber hypertrophy among older adults. Exercise training and nutritional supplementation for physical frailty in very elderly people.
N Engl J Med ; — Frontera WR, Hughes VA, Krivickas LS, et al. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve ; —8. Strength training and determinants of VO2max in older men.
Lexell J, Downham DY, Larsson Y, et al. Heavy—resistance training in older Scandinavian men and women: short—and long—term effects on arm and leg muscles. Scand J Med Sci Sports ; 5: — Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to J Am Geriatr Soc ; —7.
Body composition in elderly men: effect of dietary modification during strength training. J Am Geriatr Soc ; — Freyssenet D, Berthon P, Denis C, et al. Effect of a 6—week endurance training programme and branched—chain amino acid supplementation on histomorphometric characteristics of aged human muscle.
Arch Physiol Biochem ; — Campbell WW, Crim MC, Young VR, et al. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Welle S, Thornton CA. High—protein meals do not enhance myofibrillar synthesis after resistance exercise in 62—to 75—yr old men and women.
Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr ; S—6. Buse MG, Reid SS. Leucine: a possible regulator of protein turnover in muscle.
Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta ; —9. Morgan HE, Earl DC, Broadus A, et al. Regulation of protein synthesis in heart muscle I: effect of amino acid levels on protein synthesis.
Anthony JC, Anthony TG, Kimball SR, et al. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. Proud CG.
mTOR—mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun ; — Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Karlsson HK, Nilsson PA, Nilsson J, et al.
Branched—chain amino acids increase p70s6 kinase phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab ; 1—7. Liu Z, Jahn LA, Long W, et al.
Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt thisaction. Matthews DE. Observations of branched—chain amino acid ministration in humans. J Nutr ; S—4. Dardevet D, Sornet C, Balage M, et al.
Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr ; —5. Dardevet D, Sornet C, Bayle G, et al. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine—supplemented meal.
J Nutr ; 95— Combaret L, Dardevet D, Rieu I, et al. A leucine—supplemented diet restores the defective postprandial inhibition of proteasome—dependent proteolysis in aged rat skeletal muscle.
Koopman R, Verdijk LB, Manders RJF, et al. Co—ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Download references. No sources of funding were used to assist in the preparation of this review.
The authors have no conflicts of interest that are directly relevant to the content of this review. Department of Movement Sciences, Maastricht University, PO Box , MD, Maastricht, The Netherlands.
Department of Human Biology, Nutrition and Toxicology Research Institute Maastricht NUTRIM , Maastricht University, Maastricht, The Netherlands.
School of Sport and Exercise Sciences, University of Birmingham, Birmingham, UK. You can also search for this author in PubMed Google Scholar. Correspondence to René Koopman.
Reprints and permissions. Koopman, R. et al. Nutritional Interventions to Promote Post-Exercise Muscle Protein Synthesis. Sports Med 37 , — Download citation. Published : 02 October Issue Date : October Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract Resistance exercise is a powerful stimulus to augment muscle protein anabolism, as it can improve the balance between muscle protein synthesis and breakdown.
Access this article Log in via an institution. References Welle S, Thornton C, Statt M. Am J Physiol ; E—7 PubMed CAS Google Scholar American College of Sports Medicine Position Stand. Med Sci Sports Exerc ; —91 Google Scholar Kraemer WJ, Fry AC. Leeds: Human Kinetics, —33 Google Scholar Koopman R, Manders RJ, Zorenc AH, et al.
Maximizing post-exercisd post-exercise increase in Protein synthesis post-exercise protein synthesis, especially of synyhesis contractile myofibrillar protein fraction, is essential to facilitate effective muscle remodeling, and Prtein hypertrophic gains with pst-exercise training. MPS is Profein primary Protein synthesis post-exercise Vitamins for healthy skin influencing muscle net Protein synthesis post-exercise with dietary amino Protein synthesis post-exercise ingestion representing the single most important nutritional variable enhancing post-exercise rates of muscle protein synthesis. Dose-response studies in average i. Re-analysis of published literature in young adults suggests a relative single meal intake of ~0. This muscle-specific bolus intake is lower than that reported to maximize whole body anabolism i. Review of the available literature suggests that potential confounders such as the co-ingestion of carbohydrate, sex, and amount of active muscle mass do not represent significant barriers to the translation of this objectively determined relative protein intake.
Ich entschuldige mich, aber diesen ganz anderes. Wer noch, was vorsagen kann?
Sie noch an 18 Jahrhundert erinnern Sie sich