
Chitosan for eye health -
Microparticles have been evaluated for ocular drug delivery for decades, and typically demonstrate higher drug loading capacity and release duration than nanoparticles due to the larger size of the particles, but a balance between drug loading and size considerations for injectability must be established.
Several articles have focused on microparticles in the range of 1—50 μm for intravitreal injection to balance these considerations It has been recently proposed to use nanoparticles embedded in microparticles to overcome some of these challenges Microparticles have also shown controlled variable monodispersity upon application, demonstrating versatility of this approach.
Nanoparticles are particles between 10 and 1, nm which can possess a surface charge, based on monomer properties, that allows for increased permeability or mucoadhesion of the therapeutic Nanoparticles allow for drug delivery through encapsulation of the target therapeutic or surface loading through electrostatic interactions.
Most of the biopolymers and synthetic polymers discussed in this review have been prepared as nanoparticles and extensively evaluated for drug delivery from contact lenses, intravitreal injection, topical, and suprachoroidal administration , , Nanoparticles have the advantage of being small enough to penetrate cells, maximizing therapeutic efficacy through targeted therapeutic release.
Their small size also facilitates overcoming many of the barriers to ocular delivery. While there are many advantages to nanoparticles and there has been a significant shift to focus on nanoparticles for ocular drug delivery in recent years, a nanoparticle ocular drug delivery system has yet to be commercialized Experimental systems include GB®, a PLGA microparticle-based drug delivery vehicle designed by Graybug Vision for treatment of wet AMD and macular edema.
The injectable drug depot is currently in clinical trials and has shown controlled release of sunitinib malate for up to 6 months post injection POE-based nanoparticles maintained vitreous localization in rabbits after intravitreal injection for up to 14 days with minimal increases in IOP Work by Fu et al.
Experimental work by Jiang et al. Chitosan nanoparticles have also been evaluated for transscleral delivery of bevacizumab Lu et al.
reported bevacizumab-loaded chitosan nanoparticles for treating DR Work by Dionisio et al. Recent research on corneal applications of gelatin include positively charged gelatin nanoparticles for extended release of moxifloxacin The particles showed in vitro drug release up to 12 h and showed in vivo antimicrobial properties superior to the market available product MoxiGram®.
Polymeric micelles can enhance solubility of poorly soluble drugs and are being explored for use in promoting drug transport through the cornea and sclera Micelles offer several advantages to enhance topical delivery, including thermodynamic stability, relative ease of preparation, high loading capacity, and lack of interference with optical properties of devices or solutions These are likely to be adopted clinically due to relatively simple and inexpensive fabrication techniques Micelles have been explored for several classes of therapeutics including cyclosporine, anti-inflammatories, immunosuppressants, anti-glaucoma drugs, antifungals, antivirals, and experimental antioxidants Several stimuli-responsive poloxamers have been evaluated, including PF for topical delivery of a hydrophobic drug to the anterior segment for treatment of allergic conjunctivitis , PF for delivery of ferulic acid or enhancing solubility of gatifloxacin in contact lenses , and their combination for delivery of antifungals Triamcinolone acetonide delivery with PEG-block-PCL and PEG-block-PLA micelles was also evaluated Other types of polymeric micelles evaluated include amino-terminated PEG-block-PLA and HPMC for delivery of tacrolimus Chitosan has even been explored for micellar delivery , including delivery of dexamethasone , and HA has been conjugated to peptides to enhance solubility through micelles Challenges that remain include improving micelle stability for longer shelf-life and therapeutic delivery duration.
Further, micelles can be assembled into larger hydrogels to extend delivery While liposomes are not polymers, they have been used with polymers for ocular drug delivery.
Liposomes have a cell membrane-like structure made from one or more phospholipid layers, enabling adhesion to cell membranes. They can be complexed with polymers to facilitate ocular drug delivery by improving liposome stability Liposome conjugates evaluated for ocular drug release have included chitosan, silk fibroin, and PEG , These small systems have several advantages, including ease of injection, extended topical release, and enhanced permeability.
Two key challenges are establishing long-term extended release and increasing drug loading efficiency. Other challenges include preserving therapeutic activity during preparation and loading of these delivery systems.
That being said, injecting a micro- or nano-delivery system 2—3 times per year may still be a viable option for patients receiving more frequent intravitreal injections since injection would still be in office through a small gauge needle.
Many hydrogels exist specifically for intra- and extra-ocular applications ranging from contact lenses to vitreous substitutes. The large array of ocular applications may be attributed to both the hydrophilicity of hydrogels and the customizability of component polymers.
The inherent hydrophilicity of hydrogels can provide systems with biological and mechanical stability in various ocular environments.
The aqueous environment in hydrogels allows investigators to mimic the extracellular matrix and tissues for cell delivery systems, may provide stability and improve cellular uptake for hydrophilic drugs, genes, and biologics. However, some therapeutics suffer reduced bioactivity in aqueous environments, and modifications may need to be made to incorporate hydrophobic drugs or prevent fast elution of hydrophilic drugs.
Due to the customizable nature of hydrogels and vast array of viable polymers, this area of research has potential for clinical translation and continued development. From intraocular applications such as intravitreal injections to topical treatments with films and inserts, hydrogels formed in situ show promise as a major player in the future of ocular drug delivery.
In situ forming gels enable injection through smaller gauge needles, facilitating intraocular delivery in an outpatient setting. Furthermore, in situ formation can enable conformal coating of curved surfaces like the cornea, enabling direct contact and more consistent drug delivery. Xie et al. The hydrogel, composed of collagen II and sodium hyaluronate, was formed in situ following injection in to the vitreous and in response to physiological temperature stimuli.
Thermo-responsivity was attributed to a thermo-responsive crosslinking reaction at 37°C between amine groups of collagen and succinimidyl groups of the additive 8-arm PEG succinimidyl glutarate tipentaerythritol.
Another injectable hydrogel was presented by Osswald et al. This hydrogel consisted of poly N-isopropylacrylamide PNIPAAm and poly ethylene glycol diacrylate PEGDA and utilized the properties of PNIPAAm to create a thermo-responsive in situ forming hydrogel.
In , researchers developed a hydrogel that underwent gelation upon exposure to aqueous conditions This unique in situ gelation method was the product of hydrophobic interactions between poly ethylene glycol methacrylate PEGMA and vitamin E methacrylate leading to the formation of physical crosslinks.
This hydrogel's chemistry and crosslinking ability has potential in generating hydrogels capable of delivery of hydrophobic drugs. Drug delivery coordinated with tissue replacement, such as intraocular lens implantation and vitreous substitution, is a relatively recent area of research.
This work shows great promise by potentially offering a reduction in frequent administration or procedures and mitigation of post-operative complications. Tram et al. Building off of that research, they found that glutathione may be a useful addition to ascorbic acid in ocular drug delivery Polymer coatings for IOLs, made of polydopamine or synthetic polymers, are being evaluated to reduce complications after cataract surgery from infection and PCO , While significantly less invasive than injections and tissue replacement strategies, topical hydrogel drug delivery solutions present their own challenges, requiring prolonged contact with tissues of interest and firm shape retention.
One example of a topical in situ forming hydrogel was reported by Anumolu et al. The hydrogels were pH-responsive, undergoing shape-retaining gelation within seconds of application.
Another example of a viable in situ forming hydrogel used for sustained drug delivery was recently published by El-Feky et al. Hydrogels were created using poloxamer P and HPMC, utilizing properties of P to incorporate thermo-responsiveness into the hydrogels.
Fedorchak et al. In situ gelation provides a drug delivery solution that is tailored to the patient's ocular geometry and has great potential in reducing both treatment frequency and procedure invasiveness.
Opportunities for innovative hydrogel solutions for ocular drug delivery are ever-growing, opening doors for many more future research projects and likely commercial translation in the near future. Processing polymers into fibers, films, rods, or extruded forms allows various alternative configurations for drug delivery systems.
These delivery methods and geometries may even be interconnected. For example, fibers may be formed via electrospinning to create a rod-shaped implant, or the fibers may be spun into a sheet and hydrated to form a film. Kelley et al. The extruded rods were composed of PLGA with varying weight percentages of acid- and ester-terminated PLGA to control the implant degradation and drug release rate.
OZURDEX® Allergan is an FDA-approved intravitreal implant that employs extruded PLGA NOVADUR® technology for sustained dexamethasone release through biodegradation One method for producing fibers is electrospinning. A recent study experimented with various configurations for conjunctival fornix inserts for sustained delivery of besifloxacin to the cornea for treatment of bacterial keratitis The inserts, synthesized via electrospinning, were prepared as fibers of PCL and PEG and then coated with biopolymers—either sodium alginate or thiolated sodium alginate—to confer mucoadhesion.
Another ocular insert composed of electrospun PCL and intended for insertion into the conjunctival fornix was developed to deliver fluocinolone acetonide to the retina and was evaluated in pre-clinical studies PCL and chitosan capsules have also been prepared via electrospinning to fabricate a hollow bilayered design for intravitreal injection Delivery systems designed with electrospun nanofibers present two specific advantages: tunable device porosity for controlled drug diffusion and a high ratio of surface area to volume for increased chemoadsorption Electrospun conjunctival fornix inserts were also investigated for the delivery of triamcinolone acetonide to the anterior and superficial segments of the eye Electrospinning has also been applied to develop both in situ -forming and pre-hydrated hydrogel systems.
Göttel et al. A different study utilized electrospun PVP and HA nanofibers to develop hydrogels for drug delivery This study focused on developing an ocular insert to deliver ferulic acid and Epsiliseen®-H for treating ocular surface conditions.
PVP was employed to enable electrospinning of HA while HA was the polymer responsible for the drug delivery mechanism.
Films are comparable to hydrogels for drug delivery as they hydrate to form an aqueous system. They also show potential in drug delivery, particularly for topical applications. A porous resorbable film was recently investigated as a bandage contact lens following corneal injury The films were composed of bovine serum albumin BSA structural nanofibrils and the antioxidant kaempferol.
One recent advancement in fiber and film technology is the PRINT® technique. The technology can use an array of biopolymers and therapeutics including peptides, nucleic acids, and antibodies , PRINT® has been used to develop subconjunctival implants, intracameral implants, intravitreal implants, nano-and micro-suspensions, etc One recent development with PRINT® technology is the AR Aerie Pharmaceuticals implant, which utilizes PLGA, PDLA, and PEA to control delivery to the retina for more than 2 months and is in phase 1 clinical trials — Another delivery system developed with PRINT® is an Envisia Therapeutics implant ENV currently in phase 2 clinical trials Results thus far suggest that ENV is effective in lowering IOP for 28 days , PRINT® shows great promise for its ability to customize polymer-based ocular drug delivery systems at the nanoscale level.
Polymer processing techniques are well developed in other applications and are beginning to emerge in ocular drug delivery systems. These processing techniques will be required for manufacturing of several ocular drug delivery devices and give potential to explore innovative new delivery systems using already approved polymers.
Eyedrops have seen widespread usage for delivering a variety of medications for ocular disorders, thanks to their ease of use, low cost, and relatively good patient compliance , However, in recent years, their limitations as a drug delivery system have led to significant research effort invested in improving their capacity or developing more efficient alternatives While eyedrops offer excellent delivery efficiency for topical diseases of the eye, their efficiency significantly declines when used to deliver pharmacologic agents to certain tissues in the eye.
First among these is the rapid turnover of the tear film on the cornea, which leads to a significant fraction the eyedrop's volume following the tear film into nasolacrimal drainage and systemic circulation — This lost drug dosage then enters systemic circulation, where it may be metabolized before reaching ocular tissue and risks triggering systemic side effects that compromise patient health Any drug not cleared via tear film drainage must still penetrate corneal tissue in order to reach the anterior chamber and have a therapeutic effect on ocular tissue.
The structure of corneal tissue makes it difficult for both hydrophilic and lipophilic molecules to pass through. The corneal epithelium admits only lipophilic drugs smaller than 10 Å through cell-mediated transport mechanisms, and forces hydrophilic drugs to diffuse through paracellular pathways blocked by tight junctions 19 , The corneal stroma, meanwhile, is highly hydrophilic, slowing the movement of the lipophilic drugs that pass the epithelium while allowing freer movement of the few hydrophilic molecules that enter Despite these challenges to drug retention and penetration, eyedrops are still favored for the treatment of diseases in the anterior segment of the eye.
Their ease of delivery has also made them attractive for delivery to the posterior of the eye, with researchers investigating a variety of eyedrop formulations with improved drug retention and penetration characteristics, with some working toward eye drop formulations for posterior ocular delivery to overcome the limits of injections , — The combination of rapid clearance and the extreme difficulty of corneal penetration has led to significant research efforts aimed at increasing the delivery efficiency of eyedrops.
One of the earliest options explored was to simply increase the concentration of drug delivered in the eyedrop solution, overcoming delivery barriers through essentially brute force.
However, this option presents its own challenges, as such high drug doses and accompanying polymer and preservative exposure could cause local irritation or toxicity in patients — In addition, the higher drug dose per eyedrop leads to higher doses draining to the bloodstream, potentially exacerbating systemic side effects As an alternative to increasing dose per eyedrop, some medications instead recommend increasing the frequency of eyedrop administration.
However, this presents its own challenges, as higher frequency administration has been linked to significant reductions in patient compliance with treatment regimens , Patients with physical or visual impairments, as well as children who are unable to administer eyedrops to themselves, may be especially non-compliant, as eyedrops rely on self-application to have an effect In addition, frequent repeated application of eyedrops may still lead to local and systemic side effects associated with high dosing Because of these continued challenges in increasing delivery efficiency of eyedrops, modern research has investigated a variety of polymer-based solutions for enhancing drug penetration and residence time in the anterior eye.
One solution is the development of polymer nanocarriers with mucoadhesive capabilities. These nanoparticles can entrap themselves in the mucus layer that covers the cornea, with some even capable of penetrating corneal tissue to enter the aqueous humor thanks to their small size , , , Mucoadhesion lengthens the residence time of drug delivery systems significantly, allowing them to more effectively release their drug payload for uptake by ocular tissue.
Corneal penetration is an even more desirable outcome, as the ability to effectively penetrate the cornea using a drug carrier provides immense opportunities for delivery to intraocular spaces.
Recent research efforts have developed chitosan and PLGA nanoparticles capable of reaching the retinal surface, a demonstration of how nanoparticles can help solve the challenge of developing eyedrops capable of posterior ocular delivery , Another option is the addition of polymer viscosity enhancers and gelling agents such as xanthan gum, which increase the residence time of an eyedrop atop the cornea, thereby giving more time for the drug payload to begin penetrating corneal tissue 19 , Both of these solutions make use of a variety of polymers.
While they still face significant challenges in successful implementation and translation from laboratory to clinical use, several preclinical studies are making use of gelling systems to improve drug delivery efficiency through eyedrops.
One interesting recent development has been investigation into thermosensitive polymers that form gels at physiologic temperatures , These polymers could allow future eyedrops to be administered in solution at room temperature, then form a hydrogel reservoir on contact with the warmer tissue of the eye, providing an easily administered long-lasting form of ocular drug delivery.
Injection of pharmacologic agents presents an attractive alternative route for the delivery of drugs to ocular tissue.
Injection into the subconjunctival space specifically allows drugs to be released next to the sclera and avoid corneal barriers to entry Drugs are able to easily penetrate the more permeable scleral layer, potentially enabling significantly more efficient delivery to the interior of the eye, particularly the posterior segment , , , While subconjunctival drug injections and implants necessitate a relatively more invasive procedure than eyedrops, they offer the potential of prolonged drug delivery compared to eyedrops, potentially lasting months between injections or implant replacements 19 , This would represent a significant advantage in patient compliance, as a minimally invasive injection or implantation procedure every few months is significantly easier to maintain compared to daily eyedrop administration regimens 19 , This method is not without challenges, however, as the subconjunctival space, while not as severely drained as the anterior surface of the eye, is still rich in drainage routes.
Conjunctival and scleral blood vessels, as well as lymphatic drainage, will interfere with delivery and cause some of the administered dose to enter systemic circulation rather than penetrate the sclera and enter the eye , In addition, the choroidal tissue in the eye poses an additional barrier to lipophilic drug delivery, as this tissue can bind lipophilic drugs The significant potential of subconjunctival delivery to bypass the challenges of eyedrop administration in a minimally invasive manner has led to research efforts focused on overcoming the challenges of clearance and penetration while extending the duration of drug release after implantation or injection.
Polymer solutions for these problems include polymer micro- and nano-particles which, similar to their role in eyedrop formulations, help improve drug residence time near ocular tissue and assist in penetrating the scleral barriers to ocular entry, thereby increasing the drug dose delivered , , Alternatively, subconjunctival injections of drug-loaded hydrogels composed of polymers such as PEG, PLGA, and HA can create a reservoir capable of extended release over a course of weeks or months, offering a more easily prepared alternative to micro- and nanoparticle systems , Finally, polymeric subconjunctival implants offer a more stable platform for drug delivery through the subconjunctival space, with research publications describing devices made of PDMS, PLGA, and polyurethane among others 19 , , Animal studies into the use polymer-based subconjunctival drug delivery systems have found promising initial data, with favorable biocompatibility and safety results for polyimide and PLGA implants and evidence of extended-release efficacy for PLGA microspheres in the subconjunctival space , Further research into delivery through the subconjunctival space is likely to offer significant potential for improvement of drug delivery compliance and outcomes.
Many of these research efforts may benefit from prior developments in subconjunctival drainage devices designed to relieve IOP and assist in glaucoma treatment, as numerous polymer drains have already received approval for market use Another alternative route for drug delivery is injection to the suprachoroidal space, a thin layer of tissue between the sclera and choroid of the eye In theory, injections into this space could quickly spread across the inner surface of the eye, allowing rapid access to the posterior tissues of the eye with limited loss to the vitreous humor , This would provide a highly specific pathway for delivery to these tissues with minimized adverse effects from off-target delivery and significantly lower invasiveness compared to intravitreal injection However, the suprachoroidal space is extremely delicate, with only 30 μm of tissue thickness in the region and a recommended maximum injection volume of only μl Higher volumes than this risk causing choroidal edema and detachment In addition, as this space has been relatively underexplored, there is a significant chance that yet-undiscovered safety challenges may emerge with the use of a broader range of polymers and injection systems.
Perhaps because of these significant challenges to safe and accurate delivery, there has been relatively minimal exploration and characterization of the suprachoroidal space, with early studies beginning only in the mids and testing of suprachoroidal delivery in animal models of ocular disease by the early s , Einmahl et al.
investigated the suprachoroidal space's tolerance of POE in rabbit models, finding no evidence of complications or intolerance over the 3 weeks the polymer remained in the space In recent years, microneedle-based injections to deliver drug-laden solutions into the suprachoroidal space have been frequently explored, as they are a minimally invasive method with less risk of complications and rapid sealing of the injection site Polymers investigated in these suprachoroidal microneedle injections serve a variety of roles, from simple injection excipients to the focal point for investigation.
Chiang et al. They also explored the use of polymers as injectable drug delivery excipients by evaluating the distribution of FITC-labeled CMC and HA in the suprachoroidal space following microneedle injection One possible innovation in this area is the use of PRINT® technology, which has been previously used to produce microneedle arrays for transdermal drug delivery This application of PRINT® has been licensed for use by Aerie Pharmaceuticals and may be employed for suprachoroidal microneedle systems in the future Jung et al.
These investigations demonstrate novel potential applications of polymers in ocular drug delivery and may provide a foundation for future innovation in suprachoroidal delivery.
While subconjunctival and suprachoroidal injections and implants offer a more efficient alternative to eyedrops for drug delivery to the eye and are more effective at both anterior and posterior delivery, they are still subject to limitations due to the tissue and drainage barriers they face when releasing drugs Delivery directly to the vitreous humor bypasses corneal and scleral tissue barriers and ensures high drug delivery efficiency, drug bioavailability, and precise control of therapeutic concentrations, especially to tissues in the posterior eye 20 , , , For this reason, in spite of its invasive nature, intravitreal injections are currently a popular choice for drug delivery to the posterior segment.
However, injections of drug solution without controlled release systems still face rapid clearance in the vitreous, necessitating frequent injections to maintain therapeutic levels of drug in the eye , This is problematic for patients, as this procedure requires ophthalmologists to administer the injections and risks significant side effects.
These range from more manageable issues, such as elevated IOP and endophthalmitis, to severe and potentially vision-altering side effects such as retinal detachment and intravitreal or subconjunctival hemorrhage , , , In addition, drug that has been injected must still contend with diffusion through the vitreous humor to reach target tissues, a process made more difficult by rapid clearance due to vitreal circulation, the charge of vitreal fluid interfering with the diffusion of charged molecules, and the vitreous humor's extracellular matrix hampering large molecule movement , While this method does offer some advantages over topical and subconjunctival delivery, these challenges limit its effectiveness in current drug delivery applications.
To overcome these challenges, significant effort has been invested in the development of intravitreal drug delivery systems. Recent examples include a thermoresponsive polymer made of a combination of pentaerythritol, lactic acid, and ε-caprolactone functionalized with PEG and another thermoresponsive hydrogel made of PEG-poly serinol hexamethylene urethane , which can be injected into the intraocular space to serve as a controlled-release system for extended drug delivery , Researchers have also investigated a variety of polymer nanoparticles, using materials such as PCL and PLGA to develop drug-loaded nanoparticles for intravitreal injection , Others have developed intravitreal implants out of materials such as PLGA, silicone, polyimide, and PVA.
The goals of these systems are to increase the duration of drug release, thereby reducing injection frequency and its associated risks without exposing the eye to additional risks from the polymers themselves. This is a delicate balance, which will require significant research effort to maintain, but the potential benefits of an extended-release intravitreal drug delivery system are highly promising.
Several labs are investigating additional polymer systems for intravitreal use. This includes our work developing polydopamine nanoparticles for anti-VEGF delivery, as well as efforts by other labs developing technologies such as phase-inversion mixtures of polymer and solvent, PEGylated siloxanes, and NIPAAm-based thermoresponsive polymers for intravitreal , , One system with particularly promising results is the Genentech Port Delivery System, SUSVIMO TM a reloadable port composed of a polysulfone body coated in silicone, which recently received FDA approval for delivery of ranibizumab for the treatment of wet AMD , Figure 5 contains a schematic of some of the FDA approved polymeric biomaterial products and administration location.
Figure 5. Administration location of several FDA approved ocular drug delivery systems that use polymers. While there is significant effort being invested in the development of polymer-based ocular drug delivery systems, a key challenge is the translation of these systems to clinical use.
A number of products have successfully reached the market over the last few decades, with all four administration methods discussed previously having at least one FDA-approved drug delivery system that includes polymers to enhance their function. Notable examples are shown in Table 3. Eyedrops, the most mature drug delivery platform of the four, understandably have a significant number of polymer products, with numerous formulations approved for the treatment of diseases such as glaucoma, bacterial conjunctivitis, and uveitis , Most make use of these polymers to increase the drop's residence time and release efficiency.
Other applications such as polymer nanocarriers and thermosetting gels are still under investigation to evaluate their utility in extending the duration of eyedrop drug release and drug penetration , Research into using eye drops for posterior segment delivery could have significant implications in the field of ocular drug delivery.
In the intravitreal space, progress has been much slower, with only seven intravitreal polymer systems obtaining regulatory approval for use with a small set of diseases 46 , , , These seven, the Iluvien®, Ozurdex®, Retisert®, Vitrasert®, Yutiq®, Dextenza, and DEXYCU® implants, use a variety of polymers in their construction.
Iluvien® and Yutiq® use polyimide implants to deliver fluocinolone acetonide 46 , Ozurdex® uses a PLGA matrix that degrades to release dexamethasone Dextenza suspends dexamethasone in a PEG-fluorescein hydrogel Finally, DEXYCU® makes use of acetyl triethyl citrate gel to deliver suspended dexamethasone Four of these seven are non-degradable implants; Ozurdex®, Dextenza, and DEXYCU® are capable of resorption into the tissue of the eye.
This helps to control drug release rate by providing a constant polymer membrane through which drug diffuses into the intravitreal space. However, it also presents challenge of implant removal and replacement once its therapeutic payload is expended, requires surgery and may incur additional health risks for the patient.
A search of the Drugs FDA database indicates that Iluvien, Ozurdex, Yutiq, DEXYCU, Dextenza, and Retisert remain available by prescription, while Vitrasert has been discontinued in the US.
There are many more polymer implants in various phases of clinical and laboratory research making use of materials such as PLGA and PEG, indicating that there is significant progress yet to be made in clinical deployment of polymer systems in the vitreal space 20 , , In addition to recently approved systems such as the Genentech Port Delivery system, Kodiak is currently in phase 3 trials using an injectable biopolymer-antibody conjugate for the treatment of wet AMD and DME, while Aerie is testing biodegradable polymer implants for DME in a phase 2 trial , With ongoing efforts in the development of intravitreal microparticles, nanoparticles, and injectable hydrogels, it is likely that intravitreal drug delivery options available to patients and clinicians will become significantly more diverse in the coming years 20 , , , Subconjunctival drug delivery is a route that has only recently begun to be explored.
Despite this, there has been progress in the development of subconjunctival polymer drug delivery systems, with the Ologen® and Xen Gel systems using collagen to construct implants and research efforts into other polymers such as PLGA showing promising results for implant performance , However, these implants may pose challenges with discomfort and potential complications, leaving significant room for improvements in the future , Research into other polymer systems for subconjunctival delivery is an emerging area, with several research efforts investigating alternative implant polymer compositions, nanoparticle-based delivery systems, and injectable hydrogels for use as drug reservoirs in the subconjunctival space , , , — However, many of these are still in the early phases of development, and are likely to require further research showing safety and biocompatibility, as well as well-developed animal studies to show efficacy, before they can be put into clinical trials In addition to these promising developments in suprachoroidal injections, there are several choroidal devices that have found success in clinical uses.
In particular, choroidal shunts made of polymers for the reduction of IOP in glaucoma patients have been the subject of significant investigation as an alternative to subconjunctival drainage, and choroidal port delivery systems have been successful in clinical trials evaluating their efficacy for drug delivery in retinal diseases 19 , , The ability to build on these innovations and incorporate polymers used in other ocular drug delivery systems will provide a valuable and viable path forward for the development of polymer systems for suprachoroidal injection.
Part of the reason that only a small number of synthetic polymers are being used in ocular drug delivery applications is regulatory hurdles. Even using FDA-approved therapeutics, these drug-device combinations must perform more testing than traditional medical devices through a k approval pathway with the FDA.
Other challenges include the fact that the polymer delivery system likely changes the required therapeutic dose, generally leading to less therapeutic need due to reduced therapeutic waste.
For example, when polymer delivery systems are employed, drug retention on the cornea improves significantly compared to non-polymer delivery systems The reduction in necessary dose is not usually known until preclinical or clinical studies are conducted.
Dosing at lower levels can be estimated using effective therapeutic concentrations, but long-term stability and therapeutic shelf-life are still concerns that must be addressed prior to approval. While polymers have been used in ocular drug delivery for decades, with the first polymer intravitreal implants receiving approval in and topical applications making use of them since the s, many applications of polymers in ocular drug delivery systems are still in the early stage of development, with significant untapped innovation that could lead to drastic improvements in the capability, quality, and ease of these treatments The next decade will see a large increase in preclinical and clinical trials of polymer-based ocular drug delivery systems.
Eyedrop systems have found some success in the development and clinical approval of polymers designed to extend the residence time of the drop on the corneal surface However, continued challenges in corneal penetration leave room for further exploration.
Ongoing research into the translation of technologies such as nanomicelles and gelling agents to clinical applications seeks to further improve the efficacy of eyedrops as a delivery system , Topical delivery to treat posterior segment diseases is also an area worth exploring to benefit patients.
Intravitreal injections and implants have begun to embrace polymers as a method of increasing delivery duration with the development of polymer implants. Intravitreal implants, however, can be difficult to properly position and more difficult to extract once depleted.
Further developments in biodegradable implants like Ozurdex®, as well as the development of alternative systems such as in-situ forming hydrogels, are likely to create less invasive intravitreal systems with similar capability to improve efficiency and reduce injection frequency.
Subconjunctival and suprachoroidal injections and implants, as the youngest types of delivery systems, benefit from developments in other fields and are well-positioned to develop quickly once research locates optimal polymer formulations for both injectable solutions and implantable systems.
For all of these methods, obtaining regulatory approval will be perhaps their most significant challenge. Many ocular drug delivery systems are listed in the FDA's drug databases, indicating that they were required to pass the FDA's drug approval process rather than obtaining device certification before reaching the open market.
Despite this challenge in obtaining approval, dozens of polymer drug delivery systems are currently in clinical or preclinical trials for ocular applications, highlighting the immense potential many see for future growth in this field 20 , , Overall, the future is bright for the use of polymers in ocular drug delivery systems, with a solid foundation of clinical technologies, dozens of registered clinical trials evaluating next-generation delivery systems for even higher efficiency, and further investigative research developing applications of new polymer science in ocular delivery.
MA and KS-R were responsible for study conception. MA, RL, EH, and KS-R: literature review, analysis, interpretation of results, and writing were conducted.
MA was primarily responsible for drafting the manuscript. All authors reviewed the results and approved the final version of the manuscript. We would like to acknowledge the Ohio State University College of Engineering, the Ohio Lions Eye Research Foundation, and the Research to Prevent Blindness Young Investigator Student Fellowship Award for Female Scholars in Vision Research for funding.
KS-R consults for and has equity interest in Vitranu, Inc. KS-R has patent applications for ocular drug delivery technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. We would like to acknowledge the past and present members of the Swindle-Reilly Lab for Biomimetic Polymeric Biomaterials for help and encouragement, particularly former lab members Pengfei Jiang, Nguyen Tram, and Courtney Maxwell for using polymers to advance work on ocular drug delivery.
World Report on Vision Executive Summary. World Health Organization. Google Scholar. Eye Health Statistics—American Academy of Ophthalmology. BrightFocus Foundation. Sources for Macular Degeneration.
Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Forecasting age-related macular degeneration through the year the potential impact of new treatments. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Shahiwala A. Applications of polymers in ocular drug delivery. Appl Polym Drug Deliv. CrossRef Full Text Google Scholar. Nartey A. The pathophysiology of cataract and major interventions to retarding its progression: a mini review. Weinreb RN, Aung T. The pathophysiology and treatment of glaucoma: a review.
Janson BJ, Alward WL, Kwon YH, Bettis DI, Fingert JH, Provencher LM. Glaucoma-associated corneal endothelial cell damage: a review. Surv Ophthalmol. Porta M. Diabetic retinopathy A clinical update. Jager RD, Mieler WF. Age-related macular degeneration.
N Engl J Med. Wang W. Diabetic retinopathy : pathophysiology and treatments. Int J Mol Sci. Fogli S, Mogavero S, Egan CG, Del Re M. Pathophysiology and pharmacological targets of VEGF in diabetic macular edema.
Pharmacol Res. Tram NK, McLean RM, Swindle-Reilly KE. Glutathione improves the antioxidant activity of vitamin c in human lens and retinal epithelial cells: implications for vitreous substitutes.
Curr Eye Res. Kassa E, Ciulla TA, Hussain RM. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. Yu B, Li X-R, Zhang X-M.
Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J. Stem Cells. Karanfil FÇ, Turgut B. Update on presbyopia-correcting Drops Eur Ophthalmic Rev. Vandenberghe LH. Novel adeno-associated viral vectors for retinal gene therapy.
Gene Ther. Spinozzi E, Baldassarri C, Acquaticci L, Del Bello F, Grifantini M, Cappellacci L. Adenosine receptors as promising targets for the management of ocular diseases. Med Chem Res. Gote V, Sikder S, Sicotte J. Ocular drug delivery: present innovations and future challenges.
J Pharmacol Exp Ther. Kang-Mieler JJ, Rudeen KM, Liu W. Advances in ocular drug delivery systems. Basile AS, Hutmacher MM, Kowalski KG, Gandelman KY. Population pharmacokinetics of pegaptanib sodium Macugen® in patients with diabetic macular edema.
Clin Ophthalmol. Turecek PL, Bossard MJ, Schoetens F. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs.
J Pharm Sci. Abdelkader H, Fathalla Z, Seyfoddin A, Farahani M, Thrimawithana T, Allahham A, et al. Polymeric long-acting drug delivery systems LADDS for treatment of chronic diseases: Inserts, patches, wafers, and implants.
Adv Drug Deliv Rev. Blizzard CD, Desai A, Driscoll A, Cheung M. Ocular pharmacokinetics of OTX-DED, a sustained-release intracanalicular insert delivering dexamethasone, in a canine model. Hussain RM, Shaukat BA, Ciulla LM, Berrocal AM.
Vascular endothelial growth factor antagonists: promising players in the treatment of neovascular age-related macular degeneration.
Drug Des Devel Ther. Mah FSE. On gel formation time of adding topical ophthalmic medications to resure sealant, an in situ hydrogel. J Ocul Pharmacol Ther. Foroutan SM. The in vitro evaluation of polyethylene glycol esters of hydrocortisone succinate as ocular prodrugs. Int J Pharm. Eid HM, Elkomy MH, El Menshawe SF.
AAPS PharmSciTech. Shatz W, Hass PE, Peer N, Paluch MT, Blanchette C, Han G. Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye. PLoS ONE. Lakhani P, Patil A, Wu K-W, Sweeney C, Tripathi S, Avula B.
Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Teodorescu M, Bercea M. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges.
Biotechnol Adv. Kuno N. Biodegradable intraocular therapies for retinal disorders. Drugs Aging. Arcinue CA, Ceró OM.
A comparison between the fluocinolone acetonide retisert and dexamethasone ozurdex intravitreal implants in uveitis. Habib MS. ILUVIEN®technology in the treatment of center-involving diabetic macular edema: a review of the literature.
Ther Deliv. Schmit-Eilenberger VK. A novel intravitreal fluocinolone acetonide implant Iluvien® in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series.
Paolini MS, Fenton OS, Bhattacharya C, Andresen JL. Polymers for extended-release administration. Biomed Microdevices Haghjou N, Soheilian M. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res. PubMed Abstract Google Scholar. García-Estrada P, García-Bon MA, López-Naranjo EJ, Basaldúa-Pérez DN, Santos A.
Polymeric implants for the treatment of intraocular eye diseases: trends in biodegradable and non-biodegradable materials. Shin CS, Marcano DC, Park K. Application of hydrogel template strategy in ocular drug delivery. Methods Mol Biol. Makadia HK. Poly lactic-co-glycolic acid PLGA as biodegradable controlled drug delivery carrier.
Marin E, Briceño MI. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomed. Farah S, Anderson DG. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review.
Kuppermann BD, Patel SS, Boyer DS, Augstin AJ, Freeman WR, Kerr KJ. Phase 2 study of the safety and efficacy of brimonidine drug delivery system brimo dds generation 1 in patients with geographic atrophy secondary to age-related macular degeneratiON.
Baino F. Gentile P, Chiono V, Carmagnola I. An overview of poly lactic-co-glycolic acid PLGA -based biomaterials for bone tissue engineering. Cao Y, Samy KE, Bernards DA. Recent advances in intraocular sustained-release drug delivery devices. Drug Discovery Today. Chan A, Leung L-S.
Critical appraisal of the clinical utility of the dexamethasone intravitreal implant Ozurdex® for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Shirley M. Bimatoprost implant: first approval. Liu W, Lee B-S, Mieler WF. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro.
Liu W, Borrell MA, Venerus DC, Mieler WF. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol. Osswald CR, Guthrie MJ, Avila A, Valio JA, Mieler WF, Kang-Mieler.
In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Liu W, Tawakol AP, Rudeen KM, Mieler WF. Treatment efficacy and biocompatibility of a biodegradable aflibercept-loaded microsphere-hydrogel drug delivery system. Mondal D, Griffith M. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges.
Int J Polym Mater Polym Biomater. Malikmammadov E, Endogan Tanir T, Kiziltay A, Hasirci V. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. Bernards DA, Bhisitkul RB, Wynn P, Steedman MR, Lee O-T, Wong F. Ocular biocompatibility and structural integrity of micro- and nanostructured poly caprolactone films.
Samy KE, Cao Y, Kim J, Konichi S. Co-delivery of timolol and brimonidine with a polymer thin-film intraocular device. Hashemi Nasr F, Khoee S, Mehdi Dehghan M, Sadeghian Chaleshtori S. Preparation and evaluation of contact lenses embedded with polycaprolactone-based nanoparticles for ocular drug delivery.
Zhang Z, He Z, Liang R, Ma Y, Huang W, Jiang R. Fabrication of a micellar supramolecular hydrogel for ocular drug delivery. Shahab MS, Rizwanullah M, Alshehri S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: in vitro , ex vivo and toxicity assessments.
Int J Biol Macromol. Jiang P, Jacobs KM, Ohr MP. Chitosan—polycaprolactone core—shell microparticles for sustained delivery of bevacizumab. Mol Pharm. Jiang P, Chaparro FJ, Cuddington CT, Palmer AF, Ohr MP, Lannutti JJ.
Injectable biodegradable bi-layered capsule for sustained delivery of bevacizumab in treating wet age-related macular degeneration. J Control Release. Kim J, Judisch M, Mudumba S, Asada H, Aya-Shibuya E, Bhisitkul RB. Biocompatibility and pharmacokinetic analysis of an intracameral polycaprolactone drug delivery implant for glaucoma.
Invest Ophthalmol Vis Sci. Souto EB, Dias-ferreira J, Ana L, Ettcheto M, Elena S. Advanced formulation approaches for ocular drug delivery : state-of-the-art and recent patents.
Sovadinova I, Palmermo EF, Urban M, Mpiga P, Caputo GA. Activity and mechanism of antimicrobial peptide-mimetic amphiphilic polymethacrylate derivatives.
Ali U, Juhanni Bt Abd Karim K, Aziah Buang NA. Review of the properties and applications of poly methyl methacrylate PMMA. Polym Rev. Fan X, Torres-Luna C, Azadi M, Domszy R, Hu N, Yang A, et al. Evaluation of commercial soft contact lenses for ocular drug delivery: a review.
Acta Biomater. Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Ubani-Ukoma U, Gibson D, Schultz G, Silva BO. Evaluating the potential of drug eluting contact lenses for treatment of bacterial keratitis using an ex vivo corneal model.
Pereira-da-Mota AF, Vivero-Lopez M, Topete A, Serro AP, Concheiro A, Alvarez-Lorenzo C. Atorvastatin-eluting contact lenses : effects of molecular imprinting and sterilization on drug loading and release. González-Chomón C, Silva M, Concheiro A.
Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Jung HJ. Temperature sensitive contact lenses for triggered ophthalmic drug delivery.
Brannigan RP. Synthesis and evaluation of mucoadhesive acryloyl-quaternized PDMAEMA nanogels for ocular drug delivery.
Colloids Surfaces B Biointerfaces. Soni V, Pandey V, Tiwari R, Asati S, Tekade RK. Design and evaluation of ophthalmic delivery formulations. In: Basic Fundamentals of Drug Delivery. New york, NY: Academic Press , p. Dung Nguyen D, Lai J-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment.
Polym Chem. Prasannan A, Tsai H-C, Chen Y-S. A thermally triggered in situ hydrogel from poly acrylic acid-co-N-isopropylacrylamide for controlled release of anti-glaucoma drugs.
J Mater Chem B. Prasannan A, Tsai HC. Formulation and evaluation of epinephrine-loaded poly acrylic acid-co-N-isopropylacrylamide gel for sustained ophthalmic drug delivery.
React Funct Polym. Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM. PERSIST: Physician's Evaluation of Restasis® Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0. Lancina MG III, Yang H.
Dendrimers for ocular drug delivery. Can J Chem. Yavuz B, Bozdag Pehlivan S. Dendrimeric systems and their applications in ocular drug delivery. Sci World J. Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues.
J Pharm Bioallied Sci. Yavuz B, Pehlivan SB, Bolu BS, Sanyal RN, Vural I. Dexamethasone—PAMAM dendrimer conjugates for retinal delivery: preparation, characterization and in vivo evaluation.
J Pharm Pharmacol. Iezzi R, Guru BR, Glybina IV, Mishra MJ, Kennedy A. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration.
Wang J, Williamson GS, Lancina MG III, Yang H. Mildly cross-linked dendrimer hydrogel prepared via aza-michael addition reaction for topical brimonidine delivery. J Biomed Nanotechnol. Aravamudhan A, Ramos DM, Nada AA. Natural polymers: polysaccharides and their derivatives for biomedical applications.
Nat Synth Biomed Polym. Dutescu RM, Panfil C. Comparison of the effects of various lubricant eye drops on the in vitro rabbit corneal healing and toxicity. Exp Toxicol Pathol. Deng S, Li X, Yang W, He K, Ye X. J Biomater Sci Polym. Yuan X, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC.
Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmed F. Dexamethasone nanowafer as an effective therapy for dry eye disease. Tundisi LL, Mostaço GB, Carricondo PC.
Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur J Pharm Sci. Hu L, Sun Y. Advances in chitosan-based drug delivery vehicles. Cheng Y-H, Tsai T-H, Jhan Y-Y, Chiu AW-H, Tsai K-L, Chien C-S.
Evaluation of dry eye biomarkers such as IL-1, IL, IL-alpha, and TNFbeta suggested that chitosan-N-acetylcysteine eye drops may have some protective ocular surface properties, indicated by decreased ocular surface mRNA expression.
Preceding first use in humans, preclinical data demonstrated good safety and tolerability of chitosan-N-acetylcysteine. Mild cytotoxicity was observed at high concentrations, most likely caused by the high viscosity of the test product.
Two phase 1 clinical studies were performed. In the first cohort, a single administration of chitosan-N-acetylcysteine was tested in three increasing concentrations 0. Each concentration was administered in six patients, for a total of 18 administrations.
Investigators concluded that the overall tolerability of chitosan- N-acetylcysteine was excellent. In the second cohort, safety was tested after two-times-daily instillation in a group of 12 healthy patients. Again, the study revealed an excellent safety profile.
Results of preclinical and clinical studies of chitosan-Nacetlycysteine eye drops indicate excellent tolerability and prolonged resident time on the ocular surface. This indicates that chitosan-N-acetlycysteine eye drops have the potential to be a promising new approach to treat symptoms of DES.
Gerhard Garhöfer, MD, is an Assistant Professor in the Department of Clinical Pharmacology, Medical University of Vienna, Austria. Garhöfer states that he is a paid consultant to Croma Pharma.
garhoefer meduniwien. Leopold Schmetterer, PhD, is a Professor in the Department of Clinical Pharmacology, Medical University of Vienna, Austria. Schmetterer states that he is a paid consultant to Croma Pharma.
He may be reached at email: geopold. schmetterer meduniwien. Search for:. CONTACT US. End of Issue. PREVIOUS ARTICLE: Human Tears and Artificial Replacements. IN THIS ISSUE. NEXT ARTICLE: Nutritional Therapies to Improve Surgical Outcomes. Moss SE,Klein R,Klein BEK. Prevalence of and risk factors for dry eye syndrome.
Arch Ophthalmol. Felt O,Furrer P,Mayer JM,Plazonnet B,Buri P,Gurny R. Topical use of chitosan in ophthalmology:tolerance assessment and evaluation of precorneal retention. Int J Pharm. Illum L. Chitosan and its use as a pharmaceutical excipient.
Pharm Res. Dangl D,Hornof M,Hoffer M,Kuntner C,Wanek T,Kvaternik H. In vivo evaluation of ocular residence time of I-labelled thiolated chitosan in rabbits using microPET technology. Invest Ophthalmol Vis Sci.
Kuntner C,Wanek T,Hoffer M,Dangl D,Hornof M,Kvaternik H,Langer O. Radiosynthesis and assessment of ocular pharmacokinetics of I-labeled chitosan in rabbits using small-animal positron emission tomography.
Embracing intuitive eating "dry Chitpsan Powerful antifungal agents Tor is Powerful antifungal agents highly prevalent ocular disease, in particular in the healfh population. One mainstay of therapy for patients suffering from DES Edible Mushroom Species the use of topically administered lubricants. However, despite many efforts, no "ideal" formulation has yet been found. Recently, Croma Pharma has introduced chitosan-N-acetylcysteine eye drops, designed for treatment of symptoms related to DES. Chemically, chitosan is a polycationic biopolymer with favourable biological properties such as high biocompatibility and low toxicity. Additionally, the new formulation comprises N-acetylcysteine, which has been used in ophthalmology because of its mucolytic properties for several years.Open access peer-reviewed chapter. Submitted: 17 Chitosaan Reviewed: 28 February Published: 18 July com customercare cbspd. This chapter focuses on the healh, one of Powerful antifungal agents most Caloric needs for seniors organs Cbitosan humans, Edible Mushroom Species.
Current data on pathophysiology of the human eye jealth presented in direct correlation with a range of therapeutic products, with a Chitossn and widely used material, namely chitosan.
Applications of chitosan biopolymer Creatine monohydrate supplement described in the development of innovative, modern, therapeutic devices and solutions.
Chitosxn, chitosan is a good excipient either for classic drop-type ocular systems, as well as for complex drug systems such as nanostructures nanoparticles, nanomicelles and nanosuspensionshhealth, microemulsions, microspheres, in Edible Mushroom Species hydrogels and inserts or implants.
Chktosan number of disadvantages for ocular Chitoosan of the drugs are thus overcome. As fascinating as its perfect structure, hdalth difficult to approach due healtb increased sensitivity and many Cihtosan barriers, the human bealth continues fkr be a brainstorming Cuitosan ideas to Chitosan for eye health and characterize pharmaceutical preparations hea,th optimal Continuous glucose monitor at this level.
The eye can healtj structured into two large segments: anterior and posterior, the latter eeye about two-thirds of Chitosann total Chitodan. The Nutritional benefits segment includes the cornea, the conjunctiva, hralth iris, the lens, the ciliary body and Carbohydrates and Exercise Performance aqueous ehe.
Sclera, hewlth, retina, vitreous humor eys optic nerve are parts of Chirosan posterior segment [ 1 Chitpsan. Pharmaceutical formulations given intraocular must be sterile, without pyrogens or endotoxins, isotonic, isohydric heatlh stable.
Eje eye heaalth Chitosan for eye health pH between 7. Alkaline solutions are better Chitodan [ 4 ]. Due ehalth the occurrence of Injury prevention through nutrition such as glaucoma [ 5 ], age-related fro degeneration [ 6 ], diabetic macular edema [ 7 ], fod retinopathy [ 8 ] or dry eye foe [ 9 ], which require drug delivery for a prolonged period, it has become yee to create pharmaceutical formulations that Chiyosan sustained release, increased Chtiosan with decreased Chitosxn of hhealth.
A significant challenge in achieving this goal is to overcome ocular barriers without Chitosan for eye health permanent tissue damage [ 10 ].
Introduced on market inChitoszn was the source of numerous Cnitosan to harness Immune system modulation potential as pharmaceutical excipient [ fpr ]. Forr by deacetylation of chitin, the second most abundant polysaccharide fir cellulose, chitosan consists of Healtu and N-acetyl D-glucosamine linked Metabolic health tips [ 12 ].
Mucoadhesiveness, Edible Mushroom Species, biocompatible Chiosan non-toxic nature make it a suitable Caloric intake and fitness for ocular formulations. Chitosan Cnitosan have pseudoplastic and viscoelectric properties that do not disturb the pre-corneal tear film [ 13 ].
New formulations and devices have yee obtained to ensure an increased retention Phytochemical-rich diet recommendations and thus a superior drug delivery system using Chitosan for eye health, nanosuspensions, liposomes, in situ gels, inserts Eyr contact lens [ 14 ].
The eyeball has a spherical shape and an antero-posterior diameter of about helth mm. Cnitosan is structured fkr to two segments: Intense cross-training routines and posterior Figure 1.
The anterior segment of the eye comprises the cornea, conjunctiva, iris and Chitosan for eye health body, crystalline healgh aqueous humor [ Green energy innovations ]. Yealth is transparent, avascular, Chitsan of five layers and provides Chitoasn light gor [ eyye ].
It continues Chigosan sclera through heakth limbus [ 17 ] and heath conjunctiva. The conjunctiva dor a thin, Cuitosan vascularized, porous [ 18 ] membrane where healtg goblet cells are located. The mucin layer dye with the eyee glycocalyx, facilitating helath spreading healgh the tear film [ wye ].
Aqueous humor provides Chitodan needed hralth the cornea and maintains intraocular pressure Cellulite reduction supplements the optimum value gor 20 ]. Anatomy of the eye.
B vitamins and muscle recovery maintain intraocular pressure at normal values between 12 and 20 mmHg, a fot opening of the foe chamber angle is healrh to allow an evacuation of excess through the trabecular meshwork [ Insulin sensitivity and diabetes ].
In the posterior segment of the eye are sclera, choroid, retina, vitreous humor foor optic nerve. Choroid has the role of reducing the amount of Chifosan that reaches the retina, Edible Mushroom Species eey thermoregulation Chitosan for eye health the dissipation of heat and influences the intraocular pressure through the vasculature [ 22 ].
The retina is a thin and transparent tissue, made up of 10 layers in which there are two types of receptors: cones and rods. These receptors convert photons into nerve impulse that reaches the brain through the optic nerve [ 23 ]. Glaucoma [ 24252627 ], conjunctivitis, blepharitis [ 28 ], keratitis, dry eye syndrome [ 2930 ] affect anterior eye segment [ 31 ], while posterior segment disorders affecting the vision and even causing complete loss of it: diabetic retinopathy [ 32 ], macular degeneration, macular edema and uveitis [ 3334 ].
Both chronic conditions cause the accumulation of β amyloid associated with inflammatory processes, the appearance of reactive oxygen species and cell apoptosis [ 35 ]. The eye is protected by two types of barriers: static and dynamic. Cornea, conjunctiva, ciliary body, aqueous humor and retina are static barriers, while blood flow or lacrimal flow are dynamic barriers.
There are situations when their alteration can lead to ocular lesions or hypotonia. The latter consists of penetrating serum proteins into the anterior and posterior rooms with the appearance of edema [ 36 ]. Molecules up to 20 kDa can cross the conjunctiva while those up to 5 kDa cornea [ 37 ]. In pathological situations, blood retinal barrier alteration causes the permeation of proteins to the retina with the appearance of edema and alteration of vision [ 38 ].
In diabetic retinopathy, elevated levels of vascular endothelial growth factor and NO increase the level of reactive oxygen species that generate oxidative stress with neovascularization [ 39 ].
The main protector against chemical or microbial aggression is the tear film, a mixture of lacrimal fluid and mucin, an O-glycosylated glycoprotein [ 40 ]. It is composed of three different layers [ 41 ]. The pH of the tear fluid is about 7. It decreases on awakening by the loss of CO 2 resulting from anaerobic metabolism during sleep and increases at contact lens wearers, dry eye syndrome or lacrimal stenosis [ 42 ].
Aquaporins play an important role in the transmembranar movements of water through the cornea and conjunctiva in the tear fluid while maintaining the osmolarity of the film [ 43 ].
The benefits of polysaccharides consist of natural abundance, the presence of functional groups available for chemical alterations, and the disadvantages include varied properties depending on the origin, microbial contamination or low microbial resistance [ 44 ].
The discovery of chitosan is attributed to Rouget in when he noticed that he can bring chitin in a soluble form by submitting it to various chemical and thermal treatments [ 45 ]. It is not soluble in phosphoric or sulfuric acid [ 57 ]. This behavior is explained by the protonation of amino groups with the formation of inter-molecular repulsions [ 11 ].
It can be dissolved in neutral medium in presence of glycerolphosphate [ 58 ]. Structure of chitosan. Biological actions include antimicrobial, antioxidant [ 59 ], antiviral [ 60 ], antitumoral, antithrombotic and antifungal activity [ 61 ].
The positive charge of the molecule binds to the fungal cell membrane, produces an alteration of the K and Ca flux with inhibition of respiration and fermentation [ 62 ]. The anti-obesity effect is due to the ability to bind lipids, decreasing their absorption in the digestive tract [ 63 ].
Mucoadhesive properties are due to the positive charge that allows interaction with sialic acid from mucin, negatively charged, with the formation of electrostatic bonds [ 56 ].
The properties of chitosan are influenced by molecular weight and degree of deacetylation. The biodegradation rate of the polymer is determined by the content in acetyl groups [ 64 ].
In order to obtain oligosaccharides, enzymatic methods are preferred with the use of chitosanases, enzymes with high specificity [ 66 ]. Oligosaccharides have anti-inflammatory, antitumoral [ 67 ] and antimicrobial action [ 68 ]. Low molecular weight chitosan derivatives exhibit water solubility in a wide range of pH, low viscosity and superior biological activities: bactericidal, immunomodulatory, antitumoral, hypolipidemic and hypocholesterolemic [ 69 ].
The reactive groups of chitosan are the amino group of C2 and the hydroxyl groups of C3 and C6. Positions C2 and C6 are favorable for substitution. Substitution with carboxymethyl or succinyl groups at this level increases the solubility of the compounds.
Due to the presence of a carboxyl group, they can bind calcium, depriving the extracellular matrix of Ca. Thus, they alter tight junctions and its permeability and facilitate paracellular transport through the epithelium. Chitosan thiolated compounds known as thiomers have strong mucoadhesive properties, increased permeability, antiproteasic activity [ 70 ] and inhibit efflux pump [ 71 ].
Thiolated derivatives are conjugates with thioglycolic acid or cysteine Figure 3. They exhibit paracellular permeability through the mucosa, forming gels at pH Chotosan 5 and 6. Chitosan-N-acetylcysteine has been approved on the market as eye drops under the name Lacrimera, with increased mucoadhesive properties [ 73 ].
Structures of thiolated chitosans: chitosan-cysteine left and chitosan thioglycolic acid. Different strategies have been approached to increase the bioavailability of drug substances at the eye level: increased corneal permeability prodrugs, permeability enhancers and cyclodextrinsincreased viscosity of the vehicle suspensions, ointments and gels in situuse of dispersion systems liposomes, emulsions and nanoparticlesincreasing contact time with solid matrix inserts and contact lenses [ 74 ].
In order to increase eye retention time and reduce the frequency of administration, it is preferred to use natural polymers such as chitosan, gelatin, sodium alginates, sodium hyaluronate, etc. Table 1. At the same time, they are biocompatible, biodegradable and non-toxic [ 75 ]. Other advantages of these polysaccharides include natural abundance, nature-friendly materials, relative ease of isolation and low cost [ 44 ].
Chitosan increases contact time with cornea, the most commonly used are low molecular weight derivatives [ 80 ]. Nanotechnology has been developed to overcome eye barriers and protect active substances [ 81 ].
Mucoadhesive nanocarriers increase eye contact time and act as permeability enhancers Figure 4 [ 828384 ]. Comparison between different nanostructures.
Thus, innovative formulations have been developed for the anterior segment of the eye, such as preparations based on semifluorinated alkanes applied easy as drops or spray [ 85 ], micelles, in situ gels, liposomes, contact lenses [ 86 ], inserts [ 87 ], dendrimers [ 8889 ], mini-tablets [ 90 ], microspheres [ 91 ], nanowafers [ 92 ], ocular ring [ 93 ] or punctal plug systems [ 94 ].
For the posterior segment: micro, nanoparticles, hydrogels, implants and microneedles [ 95969798 ]. Characterization of ophthalmic pharmaceutical forms is performed by in vitro and in vivo tests. Determinations include sterility, pH, particle size, viscosity, stability, active substance content and in vitro release.
Particularly, the oxygen permeability is determined for the lenses, and for the inserts and the contact angle [ ]. Holding at 37°C, samples are taken at certain time intervals and analyzed to determine the concentration of the substance that crossed the membrane [ ].
In nanotechnology, the particle size should be between 30 and nm, they should be stable, biocompatible and biodegradable [ ]. Chitosan nanoparticles are formed spontaneously by mixing a solution of chitosan with tripolyphosphate TPP to form inter and intramolecular bonds.
The main mechanism underlying the incorporation of active substances is the occurrence of electrostatic interactions with positively charged chitosan or negative TPP [ ]. Basaran et al. have prepared and evaluated chitosan nanoparticles to enhance the ocular permeability of ornidazole for the treatment of bacterial ocular infections.
These were prepared by spray-drying method. The nanoparticles were analyzed by morphology, pH, concentration in active substance, in vitro release profile. The authors consider the formulation to be safe and effective for the release of ornidazole at the posterior segment [ ]. For the treatment of bacterial endophthalmitis, Silva et al.
: Chitosan for eye health| CRSTG | Europe Edition | Chitosan-N-Acetylcysteine Eye Drops | Drug Del. Chiosan Chitosan for eye health, frequent helath application of Chitoasn may still lead rye local and systemic side effects healfh with Chitosan for eye health dosing All patients had SPK Table 1 and were already using Sports performance resources Edible Mushroom Species with no or minimal benefit in both symptoms and signs. attempted to incorporate ketorolac tromethamine into various hydrogels for ophthalmic administration. Journal of Pharmaceutical Innovation. Additionally, PCL has shown promise as an intraocular drug delivery vehicle, with recent experimental work focusing on embedding nanoparticles within contact lenses, injectable in situ forming hydrogels, nanoparticle emulsions and suspensions, microparticles, and capsules for treatment of several diseases including glaucoma and wet AMD 57 — |
| References | However, injections of drug solution without controlled release systems still face rapid clearance in the vitreous, necessitating frequent injections to maintain therapeutic levels of drug in the eye , Alonso, Department of Pharmacy and Pharmaceutical Technology, Faculty of Pharmacy, University of Santiago de Compostela, Santiago de Compostela, Spain. In this review, a polymeric biomaterial is defined as large macromolecule composed of building blocks being applied in a biomedical application. Mudgil D, Barak S. Search ADS. |
| We noticed you’re blocking ads | A search of the Drugs FDA database indicates that Iluvien, Ozurdex, Yutiq, DEXYCU, Dextenza, and Retisert remain available by prescription, while Vitrasert has been discontinued in the US. There are many more polymer implants in various phases of clinical and laboratory research making use of materials such as PLGA and PEG, indicating that there is significant progress yet to be made in clinical deployment of polymer systems in the vitreal space 20 , , In addition to recently approved systems such as the Genentech Port Delivery system, Kodiak is currently in phase 3 trials using an injectable biopolymer-antibody conjugate for the treatment of wet AMD and DME, while Aerie is testing biodegradable polymer implants for DME in a phase 2 trial , With ongoing efforts in the development of intravitreal microparticles, nanoparticles, and injectable hydrogels, it is likely that intravitreal drug delivery options available to patients and clinicians will become significantly more diverse in the coming years 20 , , , Subconjunctival drug delivery is a route that has only recently begun to be explored. Despite this, there has been progress in the development of subconjunctival polymer drug delivery systems, with the Ologen® and Xen Gel systems using collagen to construct implants and research efforts into other polymers such as PLGA showing promising results for implant performance , However, these implants may pose challenges with discomfort and potential complications, leaving significant room for improvements in the future , Research into other polymer systems for subconjunctival delivery is an emerging area, with several research efforts investigating alternative implant polymer compositions, nanoparticle-based delivery systems, and injectable hydrogels for use as drug reservoirs in the subconjunctival space , , , — However, many of these are still in the early phases of development, and are likely to require further research showing safety and biocompatibility, as well as well-developed animal studies to show efficacy, before they can be put into clinical trials In addition to these promising developments in suprachoroidal injections, there are several choroidal devices that have found success in clinical uses. In particular, choroidal shunts made of polymers for the reduction of IOP in glaucoma patients have been the subject of significant investigation as an alternative to subconjunctival drainage, and choroidal port delivery systems have been successful in clinical trials evaluating their efficacy for drug delivery in retinal diseases 19 , , The ability to build on these innovations and incorporate polymers used in other ocular drug delivery systems will provide a valuable and viable path forward for the development of polymer systems for suprachoroidal injection. Part of the reason that only a small number of synthetic polymers are being used in ocular drug delivery applications is regulatory hurdles. Even using FDA-approved therapeutics, these drug-device combinations must perform more testing than traditional medical devices through a k approval pathway with the FDA. Other challenges include the fact that the polymer delivery system likely changes the required therapeutic dose, generally leading to less therapeutic need due to reduced therapeutic waste. For example, when polymer delivery systems are employed, drug retention on the cornea improves significantly compared to non-polymer delivery systems The reduction in necessary dose is not usually known until preclinical or clinical studies are conducted. Dosing at lower levels can be estimated using effective therapeutic concentrations, but long-term stability and therapeutic shelf-life are still concerns that must be addressed prior to approval. While polymers have been used in ocular drug delivery for decades, with the first polymer intravitreal implants receiving approval in and topical applications making use of them since the s, many applications of polymers in ocular drug delivery systems are still in the early stage of development, with significant untapped innovation that could lead to drastic improvements in the capability, quality, and ease of these treatments The next decade will see a large increase in preclinical and clinical trials of polymer-based ocular drug delivery systems. Eyedrop systems have found some success in the development and clinical approval of polymers designed to extend the residence time of the drop on the corneal surface However, continued challenges in corneal penetration leave room for further exploration. Ongoing research into the translation of technologies such as nanomicelles and gelling agents to clinical applications seeks to further improve the efficacy of eyedrops as a delivery system , Topical delivery to treat posterior segment diseases is also an area worth exploring to benefit patients. Intravitreal injections and implants have begun to embrace polymers as a method of increasing delivery duration with the development of polymer implants. Intravitreal implants, however, can be difficult to properly position and more difficult to extract once depleted. Further developments in biodegradable implants like Ozurdex®, as well as the development of alternative systems such as in-situ forming hydrogels, are likely to create less invasive intravitreal systems with similar capability to improve efficiency and reduce injection frequency. Subconjunctival and suprachoroidal injections and implants, as the youngest types of delivery systems, benefit from developments in other fields and are well-positioned to develop quickly once research locates optimal polymer formulations for both injectable solutions and implantable systems. For all of these methods, obtaining regulatory approval will be perhaps their most significant challenge. Many ocular drug delivery systems are listed in the FDA's drug databases, indicating that they were required to pass the FDA's drug approval process rather than obtaining device certification before reaching the open market. Despite this challenge in obtaining approval, dozens of polymer drug delivery systems are currently in clinical or preclinical trials for ocular applications, highlighting the immense potential many see for future growth in this field 20 , , Overall, the future is bright for the use of polymers in ocular drug delivery systems, with a solid foundation of clinical technologies, dozens of registered clinical trials evaluating next-generation delivery systems for even higher efficiency, and further investigative research developing applications of new polymer science in ocular delivery. MA and KS-R were responsible for study conception. MA, RL, EH, and KS-R: literature review, analysis, interpretation of results, and writing were conducted. MA was primarily responsible for drafting the manuscript. All authors reviewed the results and approved the final version of the manuscript. We would like to acknowledge the Ohio State University College of Engineering, the Ohio Lions Eye Research Foundation, and the Research to Prevent Blindness Young Investigator Student Fellowship Award for Female Scholars in Vision Research for funding. KS-R consults for and has equity interest in Vitranu, Inc. KS-R has patent applications for ocular drug delivery technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. We would like to acknowledge the past and present members of the Swindle-Reilly Lab for Biomimetic Polymeric Biomaterials for help and encouragement, particularly former lab members Pengfei Jiang, Nguyen Tram, and Courtney Maxwell for using polymers to advance work on ocular drug delivery. World Report on Vision Executive Summary. World Health Organization. Google Scholar. Eye Health Statistics—American Academy of Ophthalmology. BrightFocus Foundation. Sources for Macular Degeneration. Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Forecasting age-related macular degeneration through the year the potential impact of new treatments. doi: PubMed Abstract CrossRef Full Text Google Scholar. Shahiwala A. Applications of polymers in ocular drug delivery. Appl Polym Drug Deliv. CrossRef Full Text Google Scholar. Nartey A. The pathophysiology of cataract and major interventions to retarding its progression: a mini review. Weinreb RN, Aung T. The pathophysiology and treatment of glaucoma: a review. Janson BJ, Alward WL, Kwon YH, Bettis DI, Fingert JH, Provencher LM. Glaucoma-associated corneal endothelial cell damage: a review. Surv Ophthalmol. Porta M. Diabetic retinopathy A clinical update. Jager RD, Mieler WF. Age-related macular degeneration. N Engl J Med. Wang W. Diabetic retinopathy : pathophysiology and treatments. Int J Mol Sci. Fogli S, Mogavero S, Egan CG, Del Re M. Pathophysiology and pharmacological targets of VEGF in diabetic macular edema. Pharmacol Res. Tram NK, McLean RM, Swindle-Reilly KE. Glutathione improves the antioxidant activity of vitamin c in human lens and retinal epithelial cells: implications for vitreous substitutes. Curr Eye Res. Kassa E, Ciulla TA, Hussain RM. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. Yu B, Li X-R, Zhang X-M. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J. Stem Cells. Karanfil FÇ, Turgut B. Update on presbyopia-correcting Drops Eur Ophthalmic Rev. Vandenberghe LH. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. Spinozzi E, Baldassarri C, Acquaticci L, Del Bello F, Grifantini M, Cappellacci L. Adenosine receptors as promising targets for the management of ocular diseases. Med Chem Res. Gote V, Sikder S, Sicotte J. Ocular drug delivery: present innovations and future challenges. J Pharmacol Exp Ther. Kang-Mieler JJ, Rudeen KM, Liu W. Advances in ocular drug delivery systems. Basile AS, Hutmacher MM, Kowalski KG, Gandelman KY. Population pharmacokinetics of pegaptanib sodium Macugen® in patients with diabetic macular edema. Clin Ophthalmol. Turecek PL, Bossard MJ, Schoetens F. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. Abdelkader H, Fathalla Z, Seyfoddin A, Farahani M, Thrimawithana T, Allahham A, et al. Polymeric long-acting drug delivery systems LADDS for treatment of chronic diseases: Inserts, patches, wafers, and implants. Adv Drug Deliv Rev. Blizzard CD, Desai A, Driscoll A, Cheung M. Ocular pharmacokinetics of OTX-DED, a sustained-release intracanalicular insert delivering dexamethasone, in a canine model. Hussain RM, Shaukat BA, Ciulla LM, Berrocal AM. Vascular endothelial growth factor antagonists: promising players in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. Mah FSE. On gel formation time of adding topical ophthalmic medications to resure sealant, an in situ hydrogel. J Ocul Pharmacol Ther. Foroutan SM. The in vitro evaluation of polyethylene glycol esters of hydrocortisone succinate as ocular prodrugs. Int J Pharm. Eid HM, Elkomy MH, El Menshawe SF. AAPS PharmSciTech. Shatz W, Hass PE, Peer N, Paluch MT, Blanchette C, Han G. Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye. PLoS ONE. Lakhani P, Patil A, Wu K-W, Sweeney C, Tripathi S, Avula B. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Teodorescu M, Bercea M. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol Adv. Kuno N. Biodegradable intraocular therapies for retinal disorders. Drugs Aging. Arcinue CA, Ceró OM. A comparison between the fluocinolone acetonide retisert and dexamethasone ozurdex intravitreal implants in uveitis. Habib MS. ILUVIEN®technology in the treatment of center-involving diabetic macular edema: a review of the literature. Ther Deliv. Schmit-Eilenberger VK. A novel intravitreal fluocinolone acetonide implant Iluvien® in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series. Paolini MS, Fenton OS, Bhattacharya C, Andresen JL. Polymers for extended-release administration. Biomed Microdevices Haghjou N, Soheilian M. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res. PubMed Abstract Google Scholar. García-Estrada P, García-Bon MA, López-Naranjo EJ, Basaldúa-Pérez DN, Santos A. Polymeric implants for the treatment of intraocular eye diseases: trends in biodegradable and non-biodegradable materials. Shin CS, Marcano DC, Park K. Application of hydrogel template strategy in ocular drug delivery. Methods Mol Biol. Makadia HK. Poly lactic-co-glycolic acid PLGA as biodegradable controlled drug delivery carrier. Marin E, Briceño MI. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomed. Farah S, Anderson DG. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Kuppermann BD, Patel SS, Boyer DS, Augstin AJ, Freeman WR, Kerr KJ. Phase 2 study of the safety and efficacy of brimonidine drug delivery system brimo dds generation 1 in patients with geographic atrophy secondary to age-related macular degeneratiON. Baino F. Gentile P, Chiono V, Carmagnola I. An overview of poly lactic-co-glycolic acid PLGA -based biomaterials for bone tissue engineering. Cao Y, Samy KE, Bernards DA. Recent advances in intraocular sustained-release drug delivery devices. Drug Discovery Today. Chan A, Leung L-S. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant Ozurdex® for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Shirley M. Bimatoprost implant: first approval. Liu W, Lee B-S, Mieler WF. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Liu W, Borrell MA, Venerus DC, Mieler WF. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol. Osswald CR, Guthrie MJ, Avila A, Valio JA, Mieler WF, Kang-Mieler. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Liu W, Tawakol AP, Rudeen KM, Mieler WF. Treatment efficacy and biocompatibility of a biodegradable aflibercept-loaded microsphere-hydrogel drug delivery system. Mondal D, Griffith M. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. Int J Polym Mater Polym Biomater. Malikmammadov E, Endogan Tanir T, Kiziltay A, Hasirci V. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. Bernards DA, Bhisitkul RB, Wynn P, Steedman MR, Lee O-T, Wong F. Ocular biocompatibility and structural integrity of micro- and nanostructured poly caprolactone films. Samy KE, Cao Y, Kim J, Konichi S. Co-delivery of timolol and brimonidine with a polymer thin-film intraocular device. Hashemi Nasr F, Khoee S, Mehdi Dehghan M, Sadeghian Chaleshtori S. Preparation and evaluation of contact lenses embedded with polycaprolactone-based nanoparticles for ocular drug delivery. Zhang Z, He Z, Liang R, Ma Y, Huang W, Jiang R. Fabrication of a micellar supramolecular hydrogel for ocular drug delivery. Shahab MS, Rizwanullah M, Alshehri S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: in vitro , ex vivo and toxicity assessments. Int J Biol Macromol. Jiang P, Jacobs KM, Ohr MP. Chitosan—polycaprolactone core—shell microparticles for sustained delivery of bevacizumab. Mol Pharm. Jiang P, Chaparro FJ, Cuddington CT, Palmer AF, Ohr MP, Lannutti JJ. Injectable biodegradable bi-layered capsule for sustained delivery of bevacizumab in treating wet age-related macular degeneration. J Control Release. Kim J, Judisch M, Mudumba S, Asada H, Aya-Shibuya E, Bhisitkul RB. Biocompatibility and pharmacokinetic analysis of an intracameral polycaprolactone drug delivery implant for glaucoma. Invest Ophthalmol Vis Sci. Souto EB, Dias-ferreira J, Ana L, Ettcheto M, Elena S. Advanced formulation approaches for ocular drug delivery : state-of-the-art and recent patents. Sovadinova I, Palmermo EF, Urban M, Mpiga P, Caputo GA. Activity and mechanism of antimicrobial peptide-mimetic amphiphilic polymethacrylate derivatives. Ali U, Juhanni Bt Abd Karim K, Aziah Buang NA. Review of the properties and applications of poly methyl methacrylate PMMA. Polym Rev. Fan X, Torres-Luna C, Azadi M, Domszy R, Hu N, Yang A, et al. Evaluation of commercial soft contact lenses for ocular drug delivery: a review. Acta Biomater. Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Ubani-Ukoma U, Gibson D, Schultz G, Silva BO. Evaluating the potential of drug eluting contact lenses for treatment of bacterial keratitis using an ex vivo corneal model. Pereira-da-Mota AF, Vivero-Lopez M, Topete A, Serro AP, Concheiro A, Alvarez-Lorenzo C. Atorvastatin-eluting contact lenses : effects of molecular imprinting and sterilization on drug loading and release. González-Chomón C, Silva M, Concheiro A. Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Jung HJ. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Brannigan RP. Synthesis and evaluation of mucoadhesive acryloyl-quaternized PDMAEMA nanogels for ocular drug delivery. Colloids Surfaces B Biointerfaces. Soni V, Pandey V, Tiwari R, Asati S, Tekade RK. Design and evaluation of ophthalmic delivery formulations. In: Basic Fundamentals of Drug Delivery. New york, NY: Academic Press , p. Dung Nguyen D, Lai J-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym Chem. Prasannan A, Tsai H-C, Chen Y-S. A thermally triggered in situ hydrogel from poly acrylic acid-co-N-isopropylacrylamide for controlled release of anti-glaucoma drugs. J Mater Chem B. Prasannan A, Tsai HC. Formulation and evaluation of epinephrine-loaded poly acrylic acid-co-N-isopropylacrylamide gel for sustained ophthalmic drug delivery. React Funct Polym. Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM. PERSIST: Physician's Evaluation of Restasis® Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0. Lancina MG III, Yang H. Dendrimers for ocular drug delivery. Can J Chem. Yavuz B, Bozdag Pehlivan S. Dendrimeric systems and their applications in ocular drug delivery. Sci World J. Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. Yavuz B, Pehlivan SB, Bolu BS, Sanyal RN, Vural I. Dexamethasone—PAMAM dendrimer conjugates for retinal delivery: preparation, characterization and in vivo evaluation. J Pharm Pharmacol. Iezzi R, Guru BR, Glybina IV, Mishra MJ, Kennedy A. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Wang J, Williamson GS, Lancina MG III, Yang H. Mildly cross-linked dendrimer hydrogel prepared via aza-michael addition reaction for topical brimonidine delivery. J Biomed Nanotechnol. Aravamudhan A, Ramos DM, Nada AA. Natural polymers: polysaccharides and their derivatives for biomedical applications. Nat Synth Biomed Polym. Dutescu RM, Panfil C. Comparison of the effects of various lubricant eye drops on the in vitro rabbit corneal healing and toxicity. Exp Toxicol Pathol. Deng S, Li X, Yang W, He K, Ye X. J Biomater Sci Polym. Yuan X, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmed F. Dexamethasone nanowafer as an effective therapy for dry eye disease. Tundisi LL, Mostaço GB, Carricondo PC. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur J Pharm Sci. Hu L, Sun Y. Advances in chitosan-based drug delivery vehicles. Cheng Y-H, Tsai T-H, Jhan Y-Y, Chiu AW-H, Tsai K-L, Chien C-S. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr Polym. Rong X, Yang J, Ji Y, Zhu X, Lu Y, Mo Z. J Drug Deliv Sci Technol. Başaran E. Ocular application of chitosan. Expert Opin Drug Deliv. Arafa MG, Girgis GNS. Chitosan-coated PLGA nanoparticles for enhanced ocular anti-inflammatory efficacy of atorvastatin calcium. Chaharband F, Daftarian N, Kanavi MR, Varshochian R, Hajiramezanali M, Norouzi P. Trimethyl chitosan-hyaluronic acid nano-polyplexes for intravitreal VEGFR-2 siRNA delivery: formulation and in vivo efficacy evaluation. Nanomed Nanotechnol Biol Med. Shi H, Wang Y, Bao Z, Lin D, Liu H, Yu A. Thermosensitive glycol chitosan-based hydrogel as a topical ocular drug delivery system for enhanced ocular bioavailability. Zhang X, Wei D, Xu Y, Zhu Q. Hyaluronic acid in ocular drug delivery. Saranraj P. Hyaluronic acid production and its applications-a review. Int J Pharm Biol Arch. Hemshekhar M, Thushara RM, Chandranayaka S, Shermen LS, Kemparaju K. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Liu Y, Wang R, Zarembinski T, Doty N, Jiang C, Regatieri C. The application of hyaluronic acid hydrogels to retinal progenitor cell transplantation. Tissue Eng Part A. Eom Y, Kim S, Huh J, Koh MY, Hwang JY, Kang B. Self-sealing hyaluronic acid-coated gauge intravitreal injection needles for preventing vitreous and drug reflux through needle passage. Sci Rep. Vasvani S, Kulkarni P. Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Jarvis B. Am J Clin Dermatol. Diaz-Montes E. Sources, structures, and properties. Kathuria A, Shamloo K, Jhanji V. Categorization of marketed artificial tear formulations based on their ingredients: a rational approach for their use. J Clin Med. Chaiyasan W, Srinivas SP. Crosslinked chitosan-dextran sulfate nanoparticle for improved topical ocular drug delivery. Mol Vis. Delgado D, del Pozo-Rodríguez A, Solinís MÁ, Avilés-Triqueros M, Weber BH, Fernández E, et al. Dextran and protamine-based solid lipid nanoparticles as potential vectors for the treatment of X-linked juvenile retinoschisis. Hum Gene Ther. Mudgil D, Barak S. Guar gum: processing, properties and food applications—a review. Human Gene Therapy. Thombare N, Jha U, Mishra S. Guar gum as a promising starting material for diverse applications: a review. Labetoulle M, Schmickler S, Galarreta D, Böhringer D, Ogundele A, Guillon M, Baudouin C, et al. Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: a randomized double-masked clinical study. Shi Q, Daisy ERAC, Yang G, Zhang J, Mickymaray S, Alfaiz FA. Multifeatured guar gum armed drug delivery system for the delivery of ofloxacin drug to treat ophthalmic dieases. Arab J Chem. Cheng K-C, Demirci A. Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol. Prajapati VD, Jani GK. Pullulan: an exopolysaccharide and its various applications. Singh RS, Kaur N, Rana V. Recent insights on applications of pullulan in tissue engineering. Göttel B, Lucas H, Syrowatka F, Knolle W, Kuntsche J, Heinzelmann J. In situ gelling amphotericin B nanofibers: a new option for the treatment of keratomycosis. Front Bioeng Biotechnol. Pai G. Formulation and evaluation of extended release ocular inserts prepared from synthetic and natural biodegradable-biocompatible polymers. Res J Pharm Tech. Browne S, Zeugolis DI. Collagen: finding a solution for the source. Tissue Eng - Part A. Khan R. Use of collagen as a biomaterial: an update. J Indian Soc Periodontol. Wong FSY, Tsang KK, Chu AMW, Chan BP, Yao KM. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Zadeh MA, Khoder M, Al-Kinani AA, Younes HM. Retinal cell regeneration using tissue engineered polymeric scaffolds. Drug Discov Today. Belin MW, Lim L, Rajpal RK, Hafezi F, Gomes JAP. Corneal cross-linking: Current USA status report from the cornea society. Gómez-Guillén MC, Giménez B, López-Caballero ME. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. Rose JB, Pacelli S, El Haj AJ, Dua HS, Hopkinson A, White LJ. Gelatin-based materials in ocular tissue engineering. Echave MC, Burgo LS, Pedraz JL. Gelatin as biomaterial for tissue engineering. Curr Pharm Design. Song Y, Nagai N, Saijo S, Kaji H, Nishizawa M. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater Sci Eng C Mater Biol Appl. Tsai CH, Wang PY, Lin IC, Huang H, Liu GS. Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Chun YY, Yap ZL, Seet LF, Chan HH, Toh LZ, Chu SWL. Positive-charge tuned gelatin hydrogel-siSPARC injectable for siRNA anti-scarring therapy in post glaucoma filtration surgery. Batul R, Tamanna T, Khaliq A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater Sci. Liu S, Zhao X, Tang J, Han Y. Drug-eluting hydrophilic coating modification of intraocular lens via facile dopamine self-polymerization for posterior capsular opacification prevention. ACS Biomater Sci Eng. Jiang P, Choi A. Controlled release of anti-VEGF by redox-responsive polydopamine nanoparticles. Poinard B, Kamaluddin S, Tan AQQ, Neoh KG. Polydopamine coating enhances mucopenetration and cell uptake of nanoparticles. ACS Appl Mater Interfaces. Ciolacu DE, Nicu R. Cellulose-based hydrogels as sustained drug-delivery systems. Dubashynskaya N, Poshina D, Raik S, Urtti A. Polysaccharides in ocular drug delivery. Desfrançois C, Auzély R. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Sung YK. Recent advances in polymeric drug delivery systems. Biomater Res. Tiwari S. Modified hyaluronic acid based materials for biomedical applications. Tripodo G, Trapani A, Torre ML, Giammona G, Trapani G. Hyaluronic acid and its derivatives in drug delivery and imaging: recent advances and challenges. Eur J Pharm Biopharm. Verma D. Recent advances in guar gum based drug delivery systems and their administrative routes. Göttel B, Souza de Silva C, de Oliveira CS, Syrowatka F, Fiorentzis M, Viestenz A, et al. Electrospun nanofibers—a promising solid in-situ gelling alternative for ocular drug delivery. Balasso A, Subrizi A, Salmaso S, Mastrotto F, Garofalo M, Tang M. Screening of chemical linkers for development of pullulan bioconjugates for intravitreal ocular applications. Dionísio M, Braz L, Corvo M, Lourenço JP, Grenha A, Costa AM. Charged pullulan derivatives for the development of nanocarriers by polyelectrolyte complexation. Liu S, Jones L. Nanomaterials for ocular drug delivery. Macromol Biosci. Maulvi FA, Soni TG. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. Halasz K, Kelly SJ, Iqbal MT, Pathak Y, Sutariya V. Assay Drug Dev Technol. Esteban-Peréz S, Bravo-Osuna I, Andrés-Guerrero V, Molina-Martínez IT. Trojan microparticles potential for ophthalmic drug delivery. Curr Med Chem. Gorantla S, Rapalli VK, Waghule T, Singh PP, Dubey SK, Saha RN. Nanocarriers for ocular drug delivery: current status and translational opportunity. RSC Adv. Bravo-Osuna I, Andrés-Guerrero V, Abal PP, Molina-Martínez IT. Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases. Drug Deliv Transl Res. Qamar Z, Qizilbash FF, Iqubal MK, Ali A, Narang JK, Ali J. Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective. Recent Pat Drug Deliv Formul. Momin MM. Nanoformulations and highlights of clinical studies for ocular drug delivery systems: an overview. Crit Rev Ther Drug Carrier Syst. Li H, Palamoor M. Poly ortho ester nanoparticles targeted for chronic intraocular diseases: ocular safety and localization after intravitreal injection. Fu S, Yang G, Wang J, Wang X, Cheng X, Zha Q. pH-sensitive poly ortho ester urethanes copolymers with controlled degradation kinetic: synthesis, characterization, and in vitro evaluation as drug carriers. Eur Polym J. Ugˇurlu N, Aşik MD, Çakmak HB, Tuncer S, Turk M, Çagˇil N, et al. Transscleral delivery of bevacizumab-loaded chitosan nanoparticles. Lu Y, Zhou N, Huang X, Cheng J, Li F, Wei R. Effect of intravitreal injection of bevacizumab-chitosan nanoparticles on retina of diabetic rats. Int J Ophthalmol. Dionísio M, Cordeiro C, Remuñán-López C, Seijo B, Rosa C. Pullulan-based nanoparticles as carriers for transmucosal protein delivery. Garhwal R, Shady SF, Ellis EJ, Ellis JY, Leahy CD. Sustained ocular delivery of ciprofloxacin using nanospheres and conventional contact lens materials. Investig Ophthalmol Vis Sci. Mahor A, Prajapati SK, Verma A, Gupta R, Iyer AK. Moxifloxacin loaded gelatin nanoparticles for ocular delivery: formulation and in-vitro, in-vivo evaluation. J Colloid Interface Sci. Grimaudo MA, Pescina S, Padula C, Santi P, Concheiro A, Alvarez-Lorenzo C. Topical application of polymeric nanomicelles in ophthalmology: a review on research efforts for the noninvasive delivery of ocular therapeutics. Expert Opinion on Drug Deliv. Mandal A, Bisht R, Rupenthal ID. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. Sun F, Zheng Z, Lan J, Li X, Li M, Song K. New micelle myricetin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Devi S, Saini V, Kumar M, Bhatt S, Gupta S. A novel approach of drug localization through development of polymeric micellar system containing azelastine HCl for ocular delivery. Pharm Nanotechnol. Grimaudo MA, Amato G, Carbone C, Diaz-Rodriguez P, Musumeci T, Concheiro A. Micelle-nanogel platform for ferulic acid ocular delivery. Maulvi FA, Parmar RJ, Desai AR, Desai DM, Shukla MR, Ranch KM. Tailored gatifloxacin Pluronic® Floaded contact lens: addressing the issue of transmittance and swelling. Durgun ME, Kahraman E, Güngör S. Optimization and characterization of aqueous micellar formulations for ocular delivery of an antifungal drug, posaconazole. Curr Pharm Des. Safwat MA, Mansour HF, Hussein AK, Abdelwahab S. Polymeric micelles for the ocular delivery of triamcinolone acetonide: preparation and in vivo evaluation in a rabbit ocular inflammatory model. Liu D, Wu Q, Chen W, Lin H, Zhu Y, Liu Y. A novel FK loaded nanomicelles consisting of amino-terminated poly ethylene glycol -block-poly D,L -lactic acid and hydroxypropyl methylcellulose for ocular drug delivery. Zamboulis A, Nanaki S, Michailidou G, Koumentakou I, Lazaridou M, Ainali NM. Chitosan and its derivatives for ocular delivery formulations: recent advances and developments. Xu X, Sun L, Zhou L, Cheng Y. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbhohydr Polym. Alami-Milani M, Zakeri-Milani P, Valizadeh H, Salehi R, Salatin S, Naderinia A. Novel pentablock copolymers as thermosensitive self-assembling micelles for ocular drug delivery. Adv Pharm Bull. Nasir NAA, Alyautdin RN, Agarwal R, Nukolova N, Cheknonin V. Ocular tissue distribution of topically applied PEGylated and non-PEGylated liposomes. Nutritional Therapies to Improve Surgical Outcomes. Addressing the Dry Eye Dilemma in Refractive Surgery. Diagnosing and Managing Dry Eye in Corneal Refractive Surgery. Choroidal and Retinal Tumors in Adults: Choroidal Metastases and Vascular Tumors of the Choroid. Holzer,MD, FEBO. As patients age, dry eye syndrome DES becomes one of the main reasons for consulting an ophthalmologist. The ideal topical lubricant varies among patients; however, most available lubricants do not stay on the ocular surface for more than 10 or 15 minutes. One of the most important prerequisites for successful topical lubrication is a long residence time on the ocular surface, which can limit the need for frequent instillations and lead to better patient compliance. A promising approach is chitosan-N-acetylcysteine eye drops Croma Pharma GmbH, Leobendorf, Austria. This biopolymer formulation consists of chitosan, a polycationic polysaccharide derived from alkaline deacetylation of chitin, which has high biocompatibility and low toxicity, 2,3 and N-acetylcysteine, a derivative of the amino acid L-cysteine, which is a reducing agent with antioxidant activity. The N-acetylcysteine is covalently bound to the polymeric backbone of chitosan. Most important, the chitosan has been chemically modified by immobilization of sulhydryl-bearing ligands on the polymeric backbone, a technique that is usually referred to as thiomer technology. This introduction of thiol groups leads to significantly improved mucoadhesive properties compared with corresponding unmodified polymers. Chemically speaking, the formation of disulfide bonds between thiol groups of the thiomer and cysteine-rich subdomains of glycoproteins of the mucous gel layer are responsible for the improvement in mucoadhesion. These bonds lead to adhesion of the modified chitosan to the ocular surface and, in turn, to a long residence time on the ocular surface. Chitosan-N-acetylcysteine eye drops have been tested in preclinical and clinical studies to determine ocular residency time in vivo. In a preclinical study, radioactively labeled chitosan- N-acetylcysteine eye drops were applied to rabbit eyes. This experimental setting showed that even a single drop remained on the ocular surface 22 hours after application. Evaluation of dry eye biomarkers such as IL-1, IL, IL-alpha, and TNFbeta suggested that chitosan-N-acetylcysteine eye drops may have some protective ocular surface properties, indicated by decreased ocular surface mRNA expression. Preceding first use in humans, preclinical data demonstrated good safety and tolerability of chitosan-N-acetylcysteine. Mild cytotoxicity was observed at high concentrations, most likely caused by the high viscosity of the test product. Two phase 1 clinical studies were performed. In the first cohort, a single administration of chitosan-N-acetylcysteine was tested in three increasing concentrations 0. Each concentration was administered in six patients, for a total of 18 administrations. Investigators concluded that the overall tolerability of chitosan- N-acetylcysteine was excellent. In the second cohort, safety was tested after two-times-daily instillation in a group of 12 healthy patients. Again, the study revealed an excellent safety profile. Results of preclinical and clinical studies of chitosan-Nacetlycysteine eye drops indicate excellent tolerability and prolonged resident time on the ocular surface. This indicates that chitosan-N-acetlycysteine eye drops have the potential to be a promising new approach to treat symptoms of DES. Gerhard Garhöfer, MD, is an Assistant Professor in the Department of Clinical Pharmacology, Medical University of Vienna, Austria. Garhöfer states that he is a paid consultant to Croma Pharma. garhoefer meduniwien. Leopold Schmetterer, PhD, is a Professor in the Department of Clinical Pharmacology, Medical University of Vienna, Austria. Schmetterer states that he is a paid consultant to Croma Pharma. He may be reached at email: geopold. schmetterer meduniwien. Search for:. CONTACT US. |
| REVIEW article | In order to increase eye retention time and reduce the frequency of administration, it is preferred to use natural polymers such as chitosan, gelatin, sodium alginates, sodium hyaluronate, etc. Table 1. At the same time, they are biocompatible, biodegradable and non-toxic [ 75 ]. Other advantages of these polysaccharides include natural abundance, nature-friendly materials, relative ease of isolation and low cost [ 44 ]. Chitosan increases contact time with cornea, the most commonly used are low molecular weight derivatives [ 80 ]. Nanotechnology has been developed to overcome eye barriers and protect active substances [ 81 ]. Mucoadhesive nanocarriers increase eye contact time and act as permeability enhancers Figure 4 [ 82 , 83 , 84 ]. Comparison between different nanostructures. Thus, innovative formulations have been developed for the anterior segment of the eye, such as preparations based on semifluorinated alkanes applied easy as drops or spray [ 85 ], micelles, in situ gels, liposomes, contact lenses [ 86 ], inserts [ 87 ], dendrimers [ 88 , 89 ], mini-tablets [ 90 ], microspheres [ 91 ], nanowafers [ 92 ], ocular ring [ 93 ] or punctal plug systems [ 94 ]. For the posterior segment: micro, nanoparticles, hydrogels, implants and microneedles [ 95 , 96 , 97 , 98 ]. Characterization of ophthalmic pharmaceutical forms is performed by in vitro and in vivo tests. Determinations include sterility, pH, particle size, viscosity, stability, active substance content and in vitro release. Particularly, the oxygen permeability is determined for the lenses, and for the inserts and the contact angle [ ]. Holding at 37°C, samples are taken at certain time intervals and analyzed to determine the concentration of the substance that crossed the membrane [ ]. In nanotechnology, the particle size should be between 30 and nm, they should be stable, biocompatible and biodegradable [ ]. Chitosan nanoparticles are formed spontaneously by mixing a solution of chitosan with tripolyphosphate TPP to form inter and intramolecular bonds. The main mechanism underlying the incorporation of active substances is the occurrence of electrostatic interactions with positively charged chitosan or negative TPP [ ]. Basaran et al. have prepared and evaluated chitosan nanoparticles to enhance the ocular permeability of ornidazole for the treatment of bacterial ocular infections. These were prepared by spray-drying method. The nanoparticles were analyzed by morphology, pH, concentration in active substance, in vitro release profile. The authors consider the formulation to be safe and effective for the release of ornidazole at the posterior segment [ ]. For the treatment of bacterial endophthalmitis, Silva et al. incorporated daptomycin into chitosan nanoparticles. The preparation was carried out by the ionotropic gelling method, which was subsequently evaluated together with antimicrobial efficiency and stability in the presence of lysozyme and mucin. Using SEM, the particle size was evaluated at about nm. Total daptomycin release was achieved in 4 h. Incubation with lysozyme did not affect the integrity of nanoparticles [ ]. The efficacy of the chitosan-alginate nanoparticles loaded with betamethasone Na phosphate in the treatment of macular edema was studied. With particle size between Chitosan nanoparticles were formulated and evaluated by Selvaraj et al. as a potential acyclovir release system at the eye for the treatment of viral diseases. Nanoparticles were prepared by ionic gelling and characterized by SEM, DSC and FTIR. In vitro release studies demonstrated a sustained release for 24 h, the kinetic release profile following the Higuchi model [ ]. The study tracks the potential of montmorillonite in the preparation of prolonged ophthalmic nanoparticles. The nanoparticles were prepared by ionic gelling of chitosan with sodium tripolyphosphate. With a spherical shape between and nm and an incorporation efficiency of between The sustained release of celecoxib from the nanoparticles of chitosan and alginate was proposed by Ibrahim et al. Various blends of polymers were prepared in varying proportions in order to obtain the optimal formulation with the smallest particle size and the highest potential zeta. Nanoparticles were included in collyria, in situ gels and preformed gel. The release of active substance followed the Higuchi model, and the formulations proved to be non-toxic according to in vivo studies [ ]. Nanomicelles, amphiphilic molecules that have the ability to form in an aqueous medium organized supramolecular structures, contribute to the solubilization of hydrophobic active substances. A positive-load nanomicelle increases the retention time and the permeability due to interactions with the negatively charged eye surface. Changing its surface by the addition of a cationic polymer such as chitosan increases contact time to the eye [ ]. Nanomicelles were analyzed by diameters, morphology, turbidity, stability and in vitro release. The drug nanoparticle size ranged from to nm with a zeta potential between 6. According to the turbidity test, the micelles were stable, preventing the vision from collapsing. A study designed to evaluate rapamycin ocular release from octanoyl-g-chitosan-g-PEG nanomaterials was initiated by Somavarapu et al. Micelle size was determined using dynamic light scattering DLS , surface morphology with transmission electron microscopy TEM and thermal properties with differential scanning calorimetry DSC. The concentration in the active substance was determined by the HPLC method. Following the study, nanomicelles with a size of 52 nm were obtained and positively charged. The formulation remained stable for 3 days. On visual analysis the preparation is clear with a dispersion index of 0. Tissue retention was 24 h [ ]. Shi et al. have formulated a chitosan and methoxy polyethylene glycol-poly β-caprolactone nanosuspension for the ophthalmic delivery of diclofenac. Nanosuspension was characterized by FTIR, X-ray diffraction and DSC. Nanosuspension was stable at 4 and 25°C for 20 days. Prolonged release of diclofenac was achieved for 8 h without irritation [ ]. A nanosuspension of chitosan, sodium alginate and tripolyphosphate was developed as an efficient delivery system of lomefloxacin. Nanosuspension was evaluated for particle size, zeta potential, incorporation efficiency and permeability through the bovine cornea. The incorporation efficiency of the active substance was Nanosuspension releases lomefloxacin for more than 8 h and a three-fold increase in bovine corneal permeability to solutions is noted. Also, administration of lomefloxacin in the form of nanosuspension provides the advantage of a prolonged action, protects against enzyme metabolism and increases corneal permeability. Chitosan possesses antimicrobial activity, potentiating the effect of the antibiotic [ ]. A chitosan-based nanosuspension with the active substance itraconazole is prepared by co-precipitation. It has been noticed that co-precipitation of itraconazole from the chitosan- lysine system in the presence of poloxamer as a stabilizer causes a nanosuspension with the smallest size, increases drug solubility fold and a very fast in vitro release. Introduced as drug carriers in [ ], liposomes are membrane vesicles composed of one or more phospholipidic or cholesterol layers designed to transport drug substances incorporated either into the core or into one of the layers [ 36 ]. They are biodegradable and biocompatible, increasing the permeability of the drug with increasing retention time. These can be administered at both the anterior and posterior segment. Chitosan-coated liposomes, called chitosomes, increase ocular retention with decreased metabolism of drug substances. Coating liposomes with quaternary ammonium chitosan derivatives such as N-trimethylchitosan reduces particle aggregation due to steric stability and increases mucoadhesiveness [ ]. Changing liposome surface with chitosan improves mucoadhesive properties. The optimal concentration of chitosan that prevents liposome aggregation was determined at 0. A potential carrier for ocular drug release were low molecular weight chitosan-based liposomes formulated by Li et al. Liposomal morphology was examined with TEM, and cytotoxicity was assessed in rabbit conjunctival cells. By incorporating cyclosporin A, a delayed release profile was revealed as compared to un-coated liposomes. In vivo studies showed that the concentration of cyclosporin in different ocular tissues increased over 24 h [ ]. The objective of the study initiated by Ustundag-Okur et al. has been exploiting the potential of nanostructured lipid carriers with chitosan for ocular application of ofloxacin. Particle characterization involved determining the size, potential zeta, viscosity, incorporation efficiency, active substance load or sterility. Chitosan improves transcorneal permeability [ ]. The use of microemulsions as drug delivery systems offers advantages such as thermodynamic stability, increased eye retention, improved absorption, incorporation of substances in any of the two phases [ ]. Bhosale et al. have formulated several chitosan-based microemulsions as a potential voriconazole release system at the eye level. The formulations were evaluated for thermodynamic stability, physico-chemical parameters, in vitro and in vivo release studies. All the formulations have a particle size of less than nm, potentially zeta positive. In vitro delivery tests have shown that the formulations have a sustained release of over 12 h compared to market formulations. The evaluation of the tear retention of a chitosan-based emulsion containing indomethacin was carried out by Yamaguchi et al. This was compared to a non-chitosan emulsion after instillation in rabbits. The chitosan emulsion has an average concentration of 3. The average residence time and half-life for the chitosan emulsion were 1. It has been appreciated that the chitosan emulsion has a prolonged lacrimal retention time and a wide distribution on the ocular surface due to the mucoadhesive properties of chitosan [ ]. Chitosan microspheres determine a controlled release of drug substances and increase the bioavailability of drugs, improving the absorption of hydrophilic substances at epithelial level. They facilitate the transport of substances to the eye or accumulation at the corneal or conjunctival level [ ]. Chitosan-based microspheres loaded with ganciclovir were prepared by Kapanigowda et al. Characterization of the formulation was achieved by in vitro release studies, release kinetics and stability of microspheres. The degree of eye irritation, pharmacokinetic parameters and histopathology were evaluated on Wistar rats. Stability studies were favorable and it was determined that in 75 h, three administrations of this formulation were needed compared to six administrations of ganciclovir as a solution [ ]. A study initiated by Rajawat et al. has proposed to develop chitosan and chitosan-N-acetyl cysteine-based microspheres as possible ocular delivery system for acyclovir. The formulations were prepared using emulsification crosslinking process, the microspheres having an active substance incorporation efficiency of In vitro release studies showed an initial burst followed by a sustained release of acyclovir for 12 h, and in vivo studies did not indicate signs of ocular toxicity [ ]. In situ gels have shown interest since the s. The first gel was synthesized by Kopecek in Hydrogels are defined as three-dimensional structures that absorb water in large quantities without dissolving into it. Water can not be removed either under pressure [ 58 ]. For example, administration of timolol in the form of drops requires two administrations per day, and only one application per day as a gel [ ]. Chitosan dissolved in acidic solution and neutralized with β-glycerophosphate undergoes a sol-gel transformation at body temperature, favoring the transfer of protons from chitosan to the weak base. Because of the amino-positive groups, it is able to interact spontaneously with anionic polymers, forming polyelectrolyte complexes PECs with an increased tendency to form hydrogels: chitosan-chondroitin sulfate, chitosan dextran sulfate Figure 5 , chitosan alginate [ ]. A gel based on chitosan and dextran sulfate was proposed for the ciprofloxacin release study. It has been chemically characterized, morphologically, in terms of stability and concentration in the active substance. Among the analytical techniques used are FTIR, SEM and DSC. Ciprofloxacin release in simulated lacrimal fluid was determined using a UV-Vis spectrometer. The result of the study was a non-irritating product that provides ciprofloxacin release for 21 h in the treatment of susceptible germs infections [ ]. Steps in formation of chitosan-dextran sulfate gel, illustrating the technique described by Jain et al. The main advantage of this type of gels is the sustained release of the active substance and the absence of blurred vision. Due to the increased contact time with the eye surface, the bioavailability of the active substance is increased, the frequency of administration is reduced [ ]. Ciprofloxacin release was determined by the dissolution method in artificial tear solution up to 8 h, and the samples were analyzed spectrophotometrically at Rheologic behavior and phase transition temperature PCT were determined using a Cup and Bob viscometer. The formulation was kept liquid at pH 4 and 25°C and gel transformed to pH 7. From several formulations analyzed, Gupta et Vyas proposed a mixture of 0. It is in a liquid state at room temperature and pH 6 and is a gel under the action of tear fluid at pH 7. The formulations were analyzed: pH, viscosity, swelling capacity and concentration in active substance. Zaki et al. attempted to incorporate ketorolac tromethamine into various hydrogels for ophthalmic administration. As polymers, chitosan and Carbopol were used in different concentrations. The visual aspect, pH, viscosity, in vitro delivery behavior and stability were analyzed. The best formulation according to the authors would be the one with 0. The aim of a study initiated by Gilhotra et al. is to evaluate the alginate-chitosan eye film with atenolol in the treatment of glaucoma. The study showed that the addition of Ca gluconate leads to an increased release of atenolol from the chitosan-alginate matrix without the desired sustained effect [ ]. Another study proposes a corneal membrane composed of chitosan and collagen. The membrane was prepared by dissolving chitosan in collagen in varying proportions, followed by the addition of 1-ethyl-3 3-dimethylaminopropyl carbodiimide as a crosslinker. The membrane was characterized in terms of mechanical properties, contact angle and optical transmittance. In vitro cell culture studies have shown that collagen does not influence cell morphology, viability with good compatibility [ ]. Fabiano et al. formulated a chitosan and β-glycerophosphate gel for incorporation of transcorneal 5-fluorouracil nanoparticles. The sol-gel transition takes place in the range of 30—35°C. The concentration in active substance is kept constant for 7 h after administration. The system is a potential candidate for optimal 5-fluorouracil release at eye level [ ]. Intravitreal injections are the most common method of administering drugs to the posterior segment of the eye. They can be indicated in conditions such as age-related macular degeneration AMD with monoclonal antibodies such as bevacizumab Avastin or ranibizumab Lucentis. An alternative to injections is ophthalmic implants such as Vitrasert ganciclovir , Retisert fluocinolone acetonide , Iluvien fluocinolone acetonide and Ozurdex dexamethasone [ ]. Ozurdex is bioerodible [ ]. Ophthalmic inserts are solid, semi-solid, sterile, thin, multilayer, impregnated with active substance and placed on the conjunctival sac. Following studies, they have demonstrated increased retention time, sustained release for a longer period of time, dosage accuracy, reduced frequency of administration and lack of preservatives with irritant potential. They can be classified as solubles with natural or synthetic polymers, insolubles Ocusert—diffusion mechanism of release; or soft contact lenses—osmosis mechanism and bioerodibles Lacrisert 6 [ ]. Chitosan-based ocular inserts have been designed as an alternative to the release of brimonidine tartrate in the treatment of glaucoma. Characterization of inserts was performed from an analytical point of view using FTIR, SEM and DSC. Swelling capacity, active substrate release profile, in vitro bioavailability on Muller cells were also studied. The results of the study were that brimonidine tartrate was physically dispersed between the polymer chains. The inserts release the active substance for 30 days without adverse effects. They also have the advantage of being free of preservatives [ ]. Foureaux et al. studied the effects of some antiglaucoma inserts from chitosan. The inserts having diminazene aceturate as active substance were prepared by casting technique and analyzed for swallow capacity, analytically for FTIR, DSC and SEM. Quantification of the active substance from the inserts was performed with the UV-Vis spectrometer and in vitro release studies using a Franz cell. The authors concluded that inserts reduce intraocular pressure by up to 4 weeks [ ]. Upadhyaya et al. prepared chitosan-based inserts by casting method for levofloxacin release at the eye level. It has been observed that PVP addition increases levofloxacin release rate. Based on in vitro delivery studies, it was concluded that ocular inserts are suitable for the release of the active substance over 24 h and are useful in the treatment of bacterial infections [ ]. The purpose of the study initiated by Franca et al. is to evaluate the effectiveness of some chitosan-based inserts with bimatoprost. The sustained release of the active substance is performed according to in vitro studies at 8 h, which recommends it as a potential alternative in the treatment of glaucoma [ ]. Theoretically, ocular administration of active substances through contact lenses is 35 times more effective than eye drops. Soft contact lenses are generally made of hydrogels due to their biocompatibility and transparency. Incorporation of the active substances is accomplished by wetting the lenses with a drug solution, inclusion in a polymeric mixture or in a colloidal structure such as nanoemulsion, nanosuspension, liposomes dispersed in the lens, ligand grafting on the hydrophilic matrix with the formation of inclusion complexes with the drug [ ]. If the drug is weakly retained by the lens, the release is rapid, followed by a steep decline [ ]. Hydration is required when using contact lenses, allowing oxygen to penetrate the cornea. Since the lack of hydration results in dry eye syndrome [ ], it is recommended to use contact lenses in association with eye drops [ ]. Several advantages are attributed to the use of hydrogel contact lenses: good light transmission, chemical stability and high mechanical properties, increased permeability for oxygen [ ]. Behl et al. proposed to increase eye bioavailability of dexamethasone by incorporating it into chitosan nanoparticles which were subsequently imprinted in pHEMA hydrogel contact lenses. Particle size was analyzed by SEM, interactions between dexamethasone and nanoparticles by FTIR. They also studied in vitro release studies. The conclusions of the study were that the application of contact lenses with chitosan nanoparticles in which dexamethasone was incorporated, leads to therapeutically positive responses [ ]. The association of chitosan and gelatin has been shown to be beneficial in the preparation of contact lenses according to Xin-Yuan et al. The film was characterized by permeability, transmittance, water absorption and mechanical properties. The study demonstrated that the film is biocompatible, transparent, permeable and gelatin association has increased water absorption and oxygen permeability [ ]. Wearing contact lenses can create certain problems, so Hu et al. It was observed that the multilayer steadily releases norfloxacin in 1 h, and timolol in 30 min. The purpose of this study is to increase the hydrophilic character of the lenses, increase the water retention and reduce the deposition of the proteins [ ]. Mini-tablets are devices with a diameter of approximately 2—4 mm inserted into the conjunctival sac. They can gel in the presence of lacrimal fluid or the matrix can dissolve, releasing the active substance [ ]. Among the advantages of mini-tablets are easy administration, increased compliance, sustained release, lack of irritation and lack of dilution of drug substance [ ]. EL-Gawad et al. prepared ocular mini-tablets based on various polymeric matrices including chitosan for the controlled release of piroxicam. The friability studies showed a 2. They also have the ability to quickly disintegrate when administered [ ]. Refai and Tag aimed to formulate and evaluate some aciclovir eye mini-tablets to treat keratitis. The spongy nature of the mini-tablets provides fast hydration and gelling at the eye level, reducing foreign body sensation. Several mini-tablets with different polymers including chitosan have been evaluated. Rheological studies have shown pseudoplastic behavior. Optimal release of acyclovir was in the case of chitosan mini-tablets. The chitosan mini-tablets were chosen for the significant sustained release of acyclovir and bioadhesive properties, and the corneal permeability is superior to the Zovirax ointment [ ]. Verestiuc et al. were prepared acrylic-functionalized chitosan hydrogels with N-isopropyl acrylamide or 2-hydroxyethyl methacrylate monomers, then pressed to obtain mini-tablets. These have been evaluated for the controlled release capacity of some drugs at the ophthalmic level. By comparison, interpolymeric complexes and pure chitosan were analyzed. The effects of the structure and composition of the network on the properties of swelling, adherence and release of active substances such as chloramphenicol, atropine, pilocarpine or norfloxacin were studied. In vivo studies in rabbits which received pilocarpine indicated that mini-tablets based on chitosan and 2-hydroxyethylmethacrylate are optimal carriers for the delivery of the therapeutic agent [ ]. Another study aims to develop and study mini-tablets of sodium alginate, calcium gluconate and chitosan for the purpose of ocular delivery of gatifloxacin. In vivo tests and irritation studies were performed on rabbits. It has been observed that this is enhanced by the increased addition of calcium gluconate. Also, the mini-tablets have been found to be non-irritating and the chitosan and alginate mini-tablets have good antimicrobial properties [ ]. The human eye is a small, sensitive and complex organ that represents a continuous challenge in pharmaceutical research. Due to its properties, chitosan is considered a good candidate as an excipient in various pharmaceutical formulations for ocular administration. It is biocompatible, biodegradable and non-toxic. It has mucoadhesive properties by interacting with sialic acid residues from the mucin structure and pseudoplastic and viscolectric properties similar to lacrimal fluid. Thiolated derivatives, called thiomers, have enhanced mucoadhesive properties and improve the permeability of active substances through ocular barriers. The use of chitosan in ophthalmic delivery systems such as nanoparticles, nanomicelles, nanosuspensions, liposomes, microemulsions, microspheres, in situ gels, inserts, contact lenses or mini-tablets increases the retention time of the active substance at the eye level with enhancing its bioavailability. These chitosan-based systems do not cause irreversible alterations in ocular barriers, do not damage the tissues, or interfere with tear fluid. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Rajendra Dongre. Open access peer-reviewed chapter Chitosan: A Good Candidate for Sustained Release Ocular Drug Delivery Systems Written By Lăcrămioara Popa, Mihaela Violeta Ghica, Cristina Elena Dinu-Pîrvu and Teodora Irimia. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Chitin-Chitosan Myriad Functionalities in Science and Technol From the Edited Volume Chitin-Chitosan - Myriad Functionalities in Science and Technology Edited by Rajendra Sukhadeorao Dongre Book Details Order Print. Chapter metrics overview 2, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract This chapter focuses on the eye, one of the most important organs of humans. Keywords chitosan ocular delivery systems. Introduction As fascinating as its perfect structure, so difficult to approach due to increased sensitivity and many protective barriers, the human eye continues to be a brainstorming of ideas to formulate and characterize pharmaceutical preparations with optimal action at this level. Physiopathology of the eye The eyeball has a spherical shape and an antero-posterior diameter of about 24 mm. Chitosan The benefits of polysaccharides consist of natural abundance, the presence of functional groups available for chemical alterations, and the disadvantages include varied properties depending on the origin, microbial contamination or low microbial resistance [ 44 ]. Advanced drug delivery technologies Different strategies have been approached to increase the bioavailability of drug substances at the eye level: increased corneal permeability prodrugs, permeability enhancers and cyclodextrins , increased viscosity of the vehicle suspensions, ointments and gels in situ , use of dispersion systems liposomes, emulsions and nanoparticles , increasing contact time with solid matrix inserts and contact lenses [ 74 ]. It is not soluble in phosphoric or sulfuric acid Mucoadhesive, biodegradable, biocompatible and non-toxic, pseudoplastic and viscoelastic properties similar to tear film. Excellent biocompatibility and clinical safety In situ gels [ 58 , 75 , 78 ] Colagen Amphoteric Soluble in acidic pH Very compatible with ocular tissues Ocular films, ocular inserts [ 75 , 78 ] Gelatin Amphoteric Soluble in water Excellent biocompatibility, ease of processing and availability at low cost Ocular films [ 75 , 78 , 79 ] Xanthan gum Negative Soluble in water, insoluble in organic solvents Swelling in basic environment Viscosity enhancing solutions, gels [ 58 , 75 ]. Natural polymers used in ocular drug delivery systems to increase eye retention time. Nanoparticles In nanotechnology, the particle size should be between 30 and nm, they should be stable, biocompatible and biodegradable [ ]. Nanomicelles Nanomicelles, amphiphilic molecules that have the ability to form in an aqueous medium organized supramolecular structures, contribute to the solubilization of hydrophobic active substances. Nanosuspensions Shi et al. Liposomes Introduced as drug carriers in [ ], liposomes are membrane vesicles composed of one or more phospholipidic or cholesterol layers designed to transport drug substances incorporated either into the core or into one of the layers [ 36 ]. Microemulsions The use of microemulsions as drug delivery systems offers advantages such as thermodynamic stability, increased eye retention, improved absorption, incorporation of substances in any of the two phases [ ]. Microspheres Chitosan microspheres determine a controlled release of drug substances and increase the bioavailability of drugs, improving the absorption of hydrophilic substances at epithelial level. Hydrogels in situ In situ gels have shown interest since the s. Inserts and implants Intravitreal injections are the most common method of administering drugs to the posterior segment of the eye. Contact lenses Theoretically, ocular administration of active substances through contact lenses is 35 times more effective than eye drops. Mini-tablets Mini-tablets are devices with a diameter of approximately 2—4 mm inserted into the conjunctival sac. References 1. Cholkar K, Dasari SR, Pal D, Mitra AK. Eye: Anatomy, physiology and barriers to drug delivery. In: Mitra AK, editor. Ocular transporters and receptors: Their Role in Drug Delivery. Cambridge: Woodhead Publishing Limited; DOI: Rupenthal ID. Ocular drug delivery technologies: Exciting times ahead. Opthalmic Drug Delivery. Jan ; 54 3. Suresh C, Abhishek S. pH sensitive in situ ocular gel: A review. Journal of Pharmaceutical Science and Bioscientific Research. Abstract: From the past few decades, tremendous awareness has been laid on the use of natural polymers in ocular drug delivery. Wadhwa Sheetu, Paliwal Rishi, Rai Paliwal Shivani and P. Vyas S. Chitosan and its Role in Ocular Therapeutics Author s : Sheetu Wadhwa, Rishi Paliwal, Shivani Rai Paliwal and Nanomedicine Research Centre, Department of Pharmaceutics, Indo-Soviet Friendship College of Pharmacy, Moga, Punjab, India. Vyas Volume 9, Issue 14, Page: [ - ] Pages: 9 DOI: Purchase PDF. Mark Item. Mini-Reviews in Medicinal Chemistry. Title: Chitosan and its Role in Ocular Therapeutics Volume: 9 Issue: 14 Author s : Sheetu Wadhwa, Rishi Paliwal, Shivani Rai Paliwal and S. Vyas Affiliation: Keywords: Chitosan , ocular delivery , nanoparticles , ocular toxicity Abstract: From the past few decades, tremendous awareness has been laid on the use of natural polymers in ocular drug delivery. Close Print this page. Export Options ×. Export File: RIS for EndNote, Reference Manager, ProCite. Content: Citation Only. Citation and Abstract. About this article ×. Cite this article as: Wadhwa Sheetu, Paliwal Rishi, Rai Paliwal Shivani and P. Close About this journal. Related Journals Anti-Cancer Agents in Medicinal Chemistry. Current Bioactive Compounds. Current Cancer Drug Targets. Cellulose is considered the most common biopolymer and is derived from plant cell walls. It contains a large number of hydroxyl units and is thus very hydrophilic. It is biocompatible, biodegradable through enzymatic reactions and hydrolysis, easily conjugated and reacted, FDA approved for ocular use, and relatively inexpensive. For ocular drug delivery, carboxymethylcellulose CMC , an ether derivative of cellulose, is the most prominent version of the polysaccharide as the addition of carboxy groups to the biopolymer chains increases water solubility Due to its biocompatibility and hydrophilicity, CMC is often found in topically administered eye drops such as Refresh® or Optive® for treatment of dry eye, but many more brands and formulations are available The linear nature of CMC provides an excellent framework for experimental biopolymer-based hydrogels and thin films for extended topical drug release and in situ forming gels for intravitreal injection. Recent work by Deng et al. CMC based micro- and nano-carriers have also been produced for anterior and posterior ocular drug delivery. Experimental work from Yuan et al. The topically applied clear nanowafers contain nanoreservoirs of therapeutic for extended drug release and increased bioavailability compared to traditional eye drop delivery. Additionally, experimental CMC nanowafers for extended release of dexamethasone have been shown to effectively treat dry eye disease The nanowafers contained a nm array of drug reservoirs and showed successful drug release for 24 h. Another notable cellulose derivative, hydroxypropyl methylcellulose HPMC , is commonly used in ocular drug delivery due to its viscosity enhancing properties and biocompatibility Chitosan is a polysaccharide comprised of glucosamine and N-acetyl-glucosamine monomers that possesses a strong positive charge due to primary amine groups along the backbone The highly cationic nature of the polymer provides mucoadhesive benefits that have been employed for use in eye drops, to improve therapeutic bioavailability, and extended release gels for subconjunctival injection 91 , The amphiphilic nature of chitosan allows for improved solubility of hydrophobic drugs and increased penetration through the corneal membrane when compared to non-conjugated drug Chitosan has limited FDA approval and is not currently approved for ocular applications; however, there are several publications demonstrating in vitro and in vivo efficacy Technologies such as chitosan liposomes and micelles provide a high drug payload with longer drug release period that can be easily administered through intravitreal injection. Because of its cationic nature, chitosan is often employed as a polymer coating for less biocompatible anionic polymers, used in layer by layer assembly of core shell biomaterials, and used for delivery of anionic therapeutics and genetic material 60 , 61 , Chitosan-based hydrogels have recently been investigated to increase bioavailability of the topically administered antibiotic, levofloxacin Thermosensitive hexanoyl glycol chitosan hydrogels were shown to possess low ocular irritation and 1. Like chitosan, hyaluronic acid HA is a hydrophilic polysaccharide made up of D-glucuronic and N-acetyl-glucosamine monomers. HA is endogenously found in many ocular tissues including the cornea, aqueous humor, vitreous humor, and retina, and fulfills a variety of important roles in the eye. HA's structure allows for high water content and potential swelling in aqueous environments and fast degradation via enzymatic pathways 97 , HA's biocompatibility, high degree of hydration, tunable water content, and viscoelastic properties have made it a popular choice for certain types of ocular drug delivery systems such as polymer gels. It has also been used as a biocompatible coating for delivery devices, and as an integral part of retinal cell based therapies Work by Liu et al. HA has also recently been applied as a self-sealing inner needle coating for intravitreal injection to minimize extraocular regurgitation of drugs The most common application of HA has been as a lubricating agent in eyedrops for dry eye, with HA eyedrops serving as an artificial tear layer in products such as Optive Fusion, Vismed Multi, DROPSTAR®, Hyalistil®, and Neop 85 , Future research is likely to explore this further, as HA presents an easily prepared and biodegradable polymer with significant potential for the formation of degradable reservoirs for controlled drug release in addition to hydration and healing properties. Dextran is a polysaccharide biopolymer composed of D-glucose units and is synthesized by lactic acid bacteria. It is biocompatible, biodegradable, hydrophilic, and able to form hydrogels Dextran is an FDA approved biopolymer found in ophthalmic eye drop solutions such as Tears Natural Forte® and Tears Natural II® for treatment of dry eye syndrome Recent experimental work has shown successful delivery of lutein, an antioxidant, from dextran-chitosan crosslinked nanoparticles for topical administration Dextran is also capable of drug conjugation for ocular delivery. Low molecular weight dextran has seen experimental use as a cationic DNA carrier for targeted gene therapy to treat X-linked juvenile retinoschisis. Recent work showed successful in vivo transfection and expression of a dextran-protamine-DNA complex adsorbed onto the surface of solid-lipid nanoparticles after intravitreal injection into rats Guar gum is a seed-derived polysaccharide with linear backbone chains of β-d-mannose units and branch points of α-d-galactose units As a biopolymer, guar gum is biocompatible, water soluble, a viscosity enhancer, capable of high degrees of swelling, mucoadhesive, non-ionic, and degradable by hydrolysis The gelling ability of guar gum makes it beneficial as an additive to lubricating eye drops and is currently FDA approved for ocular use. Unfortunately, guar gum has limited solubility in alcohols and organic solvents and is unstable in solution. Derivatives such as hydroxymethyl-guar gum, hydroxypropyl-guar gum, and o-carboxymethyl o-hydroxypropyl-guar gum have been synthesized to improve solubility and stability Hydroxypropyl-guar gum is found in several lubricating eye drops Guar gum has been experimentally investigated to increase the bioavailability of natamycin for treatment of ocular fungal infection by integration onto PEG nanolipid carriers for controlled release from a carboxyvinyl polymer-guar gum-borate gelling system Guar gum grafted PCL micelles have also been investigated for prolonged release of ofloxacin. Experimental guar gum-PCL micelles conjugated with retinol, biotinylated glutathione, and cell specific targeting agents before incorporation of ofloxacin showed drug release for at least 8 h Pullulan is a polysaccharide derived from the yeast Aureobasidium pullulans , composed of maltotriose units joined by α-1,6 linkages Pullulan is biocompatible, non-ionic, stable over a broad temperature and pH range, water soluble, insoluble in most organic solvents, easily processed, oxygen impermeable, viscosity enhancing, and biodegradable , Pullulan has FDA GRAS status and has been used in many experimental biopolymer applications, including ocular drug delivery The non-ionic nature of pullulan often requires derivation such as sulfation or amination to incorporate a charge for improved reactivity. Co-polymerization of pullulan with other biopolymers or synthetic polymers has shown promise for extending biodegradation rate compared to the polysaccharide alone. Examples include pullulan-gellan gum electrospun nanofibers for an in situ forming gel for extended topical therapeutic bioavailability The gelation properties of pullulan in water make it popular for use in thin films and hydrogel inserts. Experimental work completed by Pai and Reddy The insert showed complete degradation in vitro and complete drug release within 3 h of application. Collagen is a naturally occurring fibrous protein present in most connective tissue, including the cornea, sclera, lens capsule, and vitreous humor. Because it is naturally occurring, collagen is biocompatible, enzymatically degradable, can be relatively easily processed, and is widely available from primarily bovine and porcine sources. Recombinant collagen is also available, offering reduced dependence on animal sources through more consistent and safe production in plants and yeast cells Collagen has a long established use in collagen shields for eye protection after ocular trauma or cataract surgery More recent work utilizes collagen as a drug delivery device for encapsulated cell therapy via intravitreal injection, extended drug release via gels, or as a scaffold base for retinal tissue regeneration , Current ocular drug delivery technologies that utilize collagen include Photrexa®, a collagen containing riboflavin ophthalmic suspension that when exposed to ultraviolet A light, crosslinks the biopolymer for treatment of progressive keratoconus Gelatin is a protein-based polymer formed from the irreversible hydrolysis of collagen. Like collagen, it is biocompatible, biodegradable, water soluble, gel forming and viscosity enhancing, readily available, and low cost, but shows advantages in lower gelation temperature and improved aqueous solubility , It is derived from mammalian, avian, and ichthyoid collagen I sources, allowing for a broad range of available molecular weights, and is GRAS approved. Recombinant gelatin is also available to circumvent potential immunogenicity and provides access to specific gelatin molecular weights and isoelectric points In ocular drug delivery, gelatin has seen applications in eye drops as a demulcent, in anteriorly and posteriorly applied hydrogels, nanoparticles for extended drug release, ocular tissue engineering, and siRNA carriers for gene therapy , , — Polydopamine is a relatively recently investigated biopolymer, formed through oxidative polymerization of dopamine, one of the body's major neurotransmitters Its biocompatibility and low toxicity have led to significant interest in its use in drug delivery, with particular attention paid to the development of coatings and nanostructures These two applications have seen recent investigation as a novel method of ocular drug delivery. Liu et al. Jiang et al. Recent findings that polydopamine coatings enhance nanoparticle mucopenetration may open the door to further applications of polydopamine in corneal drug delivery, especially as cellular uptake of these nanoparticles is also enhanced compared to uncoated nanoparticles While polydopamine has only been under evaluation as a biomaterial since , it has shown clear potential in ocular drug delivery, and will likely continue to mature with further research efforts Several biopolymers used in ocular drug delivery are summarized in Table 2. Table 2. Summary of biopolymers used in ocular drug delivery and their properties. The versatility of biopolymers and synthetic polymers opens the door to many types and forms of biomaterials used as drug delivery vehicles to treat ocular diseases. Within the field of micro- and nanotechnology, there are a variety of drug delivery vehicles such as microparticles, nanoparticles, micelles, and liposomes. These drug delivery vehicles show significant promise in the eye due to their less invasive application approaches topically as well as ease of injection through small gauge needles 44 , These have also been explored for incorporation into drug-eluting contact lenses to facilitate topical delivery Figure 4 presents several ocular drug delivery forms that utilize nanotechnology. Figure 4. Various polymer forms that have been applied to facilitate and modulate ocular drug delivery at the macroscale and nanoscale. Both synthetic and biopolymers can be formulated into nanospheres, nanocapsules, liposomes, hydrogels, dendrimers, nanoparticles, nanomicelles, and microneedles. Nanoscale polymers can be incorporated into composites, such as the hydrogel-based contact lens shown with nanoparticles. Microparticles are small-scale particles generally in the size range of 1—1, μm. Microparticles have been evaluated for ocular drug delivery for decades, and typically demonstrate higher drug loading capacity and release duration than nanoparticles due to the larger size of the particles, but a balance between drug loading and size considerations for injectability must be established. Several articles have focused on microparticles in the range of 1—50 μm for intravitreal injection to balance these considerations It has been recently proposed to use nanoparticles embedded in microparticles to overcome some of these challenges Microparticles have also shown controlled variable monodispersity upon application, demonstrating versatility of this approach. Nanoparticles are particles between 10 and 1, nm which can possess a surface charge, based on monomer properties, that allows for increased permeability or mucoadhesion of the therapeutic Nanoparticles allow for drug delivery through encapsulation of the target therapeutic or surface loading through electrostatic interactions. Most of the biopolymers and synthetic polymers discussed in this review have been prepared as nanoparticles and extensively evaluated for drug delivery from contact lenses, intravitreal injection, topical, and suprachoroidal administration , , Nanoparticles have the advantage of being small enough to penetrate cells, maximizing therapeutic efficacy through targeted therapeutic release. Their small size also facilitates overcoming many of the barriers to ocular delivery. While there are many advantages to nanoparticles and there has been a significant shift to focus on nanoparticles for ocular drug delivery in recent years, a nanoparticle ocular drug delivery system has yet to be commercialized Experimental systems include GB®, a PLGA microparticle-based drug delivery vehicle designed by Graybug Vision for treatment of wet AMD and macular edema. The injectable drug depot is currently in clinical trials and has shown controlled release of sunitinib malate for up to 6 months post injection POE-based nanoparticles maintained vitreous localization in rabbits after intravitreal injection for up to 14 days with minimal increases in IOP Work by Fu et al. Experimental work by Jiang et al. Chitosan nanoparticles have also been evaluated for transscleral delivery of bevacizumab Lu et al. reported bevacizumab-loaded chitosan nanoparticles for treating DR Work by Dionisio et al. Recent research on corneal applications of gelatin include positively charged gelatin nanoparticles for extended release of moxifloxacin The particles showed in vitro drug release up to 12 h and showed in vivo antimicrobial properties superior to the market available product MoxiGram®. Polymeric micelles can enhance solubility of poorly soluble drugs and are being explored for use in promoting drug transport through the cornea and sclera Micelles offer several advantages to enhance topical delivery, including thermodynamic stability, relative ease of preparation, high loading capacity, and lack of interference with optical properties of devices or solutions These are likely to be adopted clinically due to relatively simple and inexpensive fabrication techniques Micelles have been explored for several classes of therapeutics including cyclosporine, anti-inflammatories, immunosuppressants, anti-glaucoma drugs, antifungals, antivirals, and experimental antioxidants Several stimuli-responsive poloxamers have been evaluated, including PF for topical delivery of a hydrophobic drug to the anterior segment for treatment of allergic conjunctivitis , PF for delivery of ferulic acid or enhancing solubility of gatifloxacin in contact lenses , and their combination for delivery of antifungals Triamcinolone acetonide delivery with PEG-block-PCL and PEG-block-PLA micelles was also evaluated Other types of polymeric micelles evaluated include amino-terminated PEG-block-PLA and HPMC for delivery of tacrolimus Chitosan has even been explored for micellar delivery , including delivery of dexamethasone , and HA has been conjugated to peptides to enhance solubility through micelles Challenges that remain include improving micelle stability for longer shelf-life and therapeutic delivery duration. Further, micelles can be assembled into larger hydrogels to extend delivery While liposomes are not polymers, they have been used with polymers for ocular drug delivery. Liposomes have a cell membrane-like structure made from one or more phospholipid layers, enabling adhesion to cell membranes. They can be complexed with polymers to facilitate ocular drug delivery by improving liposome stability Liposome conjugates evaluated for ocular drug release have included chitosan, silk fibroin, and PEG , These small systems have several advantages, including ease of injection, extended topical release, and enhanced permeability. Two key challenges are establishing long-term extended release and increasing drug loading efficiency. Other challenges include preserving therapeutic activity during preparation and loading of these delivery systems. That being said, injecting a micro- or nano-delivery system 2—3 times per year may still be a viable option for patients receiving more frequent intravitreal injections since injection would still be in office through a small gauge needle. Many hydrogels exist specifically for intra- and extra-ocular applications ranging from contact lenses to vitreous substitutes. The large array of ocular applications may be attributed to both the hydrophilicity of hydrogels and the customizability of component polymers. The inherent hydrophilicity of hydrogels can provide systems with biological and mechanical stability in various ocular environments. The aqueous environment in hydrogels allows investigators to mimic the extracellular matrix and tissues for cell delivery systems, may provide stability and improve cellular uptake for hydrophilic drugs, genes, and biologics. However, some therapeutics suffer reduced bioactivity in aqueous environments, and modifications may need to be made to incorporate hydrophobic drugs or prevent fast elution of hydrophilic drugs. Due to the customizable nature of hydrogels and vast array of viable polymers, this area of research has potential for clinical translation and continued development. From intraocular applications such as intravitreal injections to topical treatments with films and inserts, hydrogels formed in situ show promise as a major player in the future of ocular drug delivery. In situ forming gels enable injection through smaller gauge needles, facilitating intraocular delivery in an outpatient setting. Furthermore, in situ formation can enable conformal coating of curved surfaces like the cornea, enabling direct contact and more consistent drug delivery. Xie et al. The hydrogel, composed of collagen II and sodium hyaluronate, was formed in situ following injection in to the vitreous and in response to physiological temperature stimuli. Thermo-responsivity was attributed to a thermo-responsive crosslinking reaction at 37°C between amine groups of collagen and succinimidyl groups of the additive 8-arm PEG succinimidyl glutarate tipentaerythritol. Another injectable hydrogel was presented by Osswald et al. This hydrogel consisted of poly N-isopropylacrylamide PNIPAAm and poly ethylene glycol diacrylate PEGDA and utilized the properties of PNIPAAm to create a thermo-responsive in situ forming hydrogel. In , researchers developed a hydrogel that underwent gelation upon exposure to aqueous conditions This unique in situ gelation method was the product of hydrophobic interactions between poly ethylene glycol methacrylate PEGMA and vitamin E methacrylate leading to the formation of physical crosslinks. This hydrogel's chemistry and crosslinking ability has potential in generating hydrogels capable of delivery of hydrophobic drugs. Drug delivery coordinated with tissue replacement, such as intraocular lens implantation and vitreous substitution, is a relatively recent area of research. This work shows great promise by potentially offering a reduction in frequent administration or procedures and mitigation of post-operative complications. Tram et al. Building off of that research, they found that glutathione may be a useful addition to ascorbic acid in ocular drug delivery Polymer coatings for IOLs, made of polydopamine or synthetic polymers, are being evaluated to reduce complications after cataract surgery from infection and PCO , While significantly less invasive than injections and tissue replacement strategies, topical hydrogel drug delivery solutions present their own challenges, requiring prolonged contact with tissues of interest and firm shape retention. One example of a topical in situ forming hydrogel was reported by Anumolu et al. The hydrogels were pH-responsive, undergoing shape-retaining gelation within seconds of application. Another example of a viable in situ forming hydrogel used for sustained drug delivery was recently published by El-Feky et al. Hydrogels were created using poloxamer P and HPMC, utilizing properties of P to incorporate thermo-responsiveness into the hydrogels. Fedorchak et al. In situ gelation provides a drug delivery solution that is tailored to the patient's ocular geometry and has great potential in reducing both treatment frequency and procedure invasiveness. Opportunities for innovative hydrogel solutions for ocular drug delivery are ever-growing, opening doors for many more future research projects and likely commercial translation in the near future. Processing polymers into fibers, films, rods, or extruded forms allows various alternative configurations for drug delivery systems. These delivery methods and geometries may even be interconnected. For example, fibers may be formed via electrospinning to create a rod-shaped implant, or the fibers may be spun into a sheet and hydrated to form a film. Kelley et al. The extruded rods were composed of PLGA with varying weight percentages of acid- and ester-terminated PLGA to control the implant degradation and drug release rate. OZURDEX® Allergan is an FDA-approved intravitreal implant that employs extruded PLGA NOVADUR® technology for sustained dexamethasone release through biodegradation One method for producing fibers is electrospinning. A recent study experimented with various configurations for conjunctival fornix inserts for sustained delivery of besifloxacin to the cornea for treatment of bacterial keratitis The inserts, synthesized via electrospinning, were prepared as fibers of PCL and PEG and then coated with biopolymers—either sodium alginate or thiolated sodium alginate—to confer mucoadhesion. Another ocular insert composed of electrospun PCL and intended for insertion into the conjunctival fornix was developed to deliver fluocinolone acetonide to the retina and was evaluated in pre-clinical studies PCL and chitosan capsules have also been prepared via electrospinning to fabricate a hollow bilayered design for intravitreal injection Delivery systems designed with electrospun nanofibers present two specific advantages: tunable device porosity for controlled drug diffusion and a high ratio of surface area to volume for increased chemoadsorption Electrospun conjunctival fornix inserts were also investigated for the delivery of triamcinolone acetonide to the anterior and superficial segments of the eye Electrospinning has also been applied to develop both in situ -forming and pre-hydrated hydrogel systems. Göttel et al. A different study utilized electrospun PVP and HA nanofibers to develop hydrogels for drug delivery This study focused on developing an ocular insert to deliver ferulic acid and Epsiliseen®-H for treating ocular surface conditions. PVP was employed to enable electrospinning of HA while HA was the polymer responsible for the drug delivery mechanism. Films are comparable to hydrogels for drug delivery as they hydrate to form an aqueous system. They also show potential in drug delivery, particularly for topical applications. A porous resorbable film was recently investigated as a bandage contact lens following corneal injury The films were composed of bovine serum albumin BSA structural nanofibrils and the antioxidant kaempferol. One recent advancement in fiber and film technology is the PRINT® technique. The technology can use an array of biopolymers and therapeutics including peptides, nucleic acids, and antibodies , PRINT® has been used to develop subconjunctival implants, intracameral implants, intravitreal implants, nano-and micro-suspensions, etc One recent development with PRINT® technology is the AR Aerie Pharmaceuticals implant, which utilizes PLGA, PDLA, and PEA to control delivery to the retina for more than 2 months and is in phase 1 clinical trials — Another delivery system developed with PRINT® is an Envisia Therapeutics implant ENV currently in phase 2 clinical trials Results thus far suggest that ENV is effective in lowering IOP for 28 days , PRINT® shows great promise for its ability to customize polymer-based ocular drug delivery systems at the nanoscale level. Polymer processing techniques are well developed in other applications and are beginning to emerge in ocular drug delivery systems. These processing techniques will be required for manufacturing of several ocular drug delivery devices and give potential to explore innovative new delivery systems using already approved polymers. Eyedrops have seen widespread usage for delivering a variety of medications for ocular disorders, thanks to their ease of use, low cost, and relatively good patient compliance , However, in recent years, their limitations as a drug delivery system have led to significant research effort invested in improving their capacity or developing more efficient alternatives While eyedrops offer excellent delivery efficiency for topical diseases of the eye, their efficiency significantly declines when used to deliver pharmacologic agents to certain tissues in the eye. First among these is the rapid turnover of the tear film on the cornea, which leads to a significant fraction the eyedrop's volume following the tear film into nasolacrimal drainage and systemic circulation — This lost drug dosage then enters systemic circulation, where it may be metabolized before reaching ocular tissue and risks triggering systemic side effects that compromise patient health Any drug not cleared via tear film drainage must still penetrate corneal tissue in order to reach the anterior chamber and have a therapeutic effect on ocular tissue. The structure of corneal tissue makes it difficult for both hydrophilic and lipophilic molecules to pass through. The corneal epithelium admits only lipophilic drugs smaller than 10 Å through cell-mediated transport mechanisms, and forces hydrophilic drugs to diffuse through paracellular pathways blocked by tight junctions 19 , The corneal stroma, meanwhile, is highly hydrophilic, slowing the movement of the lipophilic drugs that pass the epithelium while allowing freer movement of the few hydrophilic molecules that enter Despite these challenges to drug retention and penetration, eyedrops are still favored for the treatment of diseases in the anterior segment of the eye. Their ease of delivery has also made them attractive for delivery to the posterior of the eye, with researchers investigating a variety of eyedrop formulations with improved drug retention and penetration characteristics, with some working toward eye drop formulations for posterior ocular delivery to overcome the limits of injections , — The combination of rapid clearance and the extreme difficulty of corneal penetration has led to significant research efforts aimed at increasing the delivery efficiency of eyedrops. One of the earliest options explored was to simply increase the concentration of drug delivered in the eyedrop solution, overcoming delivery barriers through essentially brute force. However, this option presents its own challenges, as such high drug doses and accompanying polymer and preservative exposure could cause local irritation or toxicity in patients — In addition, the higher drug dose per eyedrop leads to higher doses draining to the bloodstream, potentially exacerbating systemic side effects As an alternative to increasing dose per eyedrop, some medications instead recommend increasing the frequency of eyedrop administration. However, this presents its own challenges, as higher frequency administration has been linked to significant reductions in patient compliance with treatment regimens , Patients with physical or visual impairments, as well as children who are unable to administer eyedrops to themselves, may be especially non-compliant, as eyedrops rely on self-application to have an effect In addition, frequent repeated application of eyedrops may still lead to local and systemic side effects associated with high dosing Because of these continued challenges in increasing delivery efficiency of eyedrops, modern research has investigated a variety of polymer-based solutions for enhancing drug penetration and residence time in the anterior eye. One solution is the development of polymer nanocarriers with mucoadhesive capabilities. These nanoparticles can entrap themselves in the mucus layer that covers the cornea, with some even capable of penetrating corneal tissue to enter the aqueous humor thanks to their small size , , , Mucoadhesion lengthens the residence time of drug delivery systems significantly, allowing them to more effectively release their drug payload for uptake by ocular tissue. Corneal penetration is an even more desirable outcome, as the ability to effectively penetrate the cornea using a drug carrier provides immense opportunities for delivery to intraocular spaces. Recent research efforts have developed chitosan and PLGA nanoparticles capable of reaching the retinal surface, a demonstration of how nanoparticles can help solve the challenge of developing eyedrops capable of posterior ocular delivery , Another option is the addition of polymer viscosity enhancers and gelling agents such as xanthan gum, which increase the residence time of an eyedrop atop the cornea, thereby giving more time for the drug payload to begin penetrating corneal tissue 19 , Both of these solutions make use of a variety of polymers. While they still face significant challenges in successful implementation and translation from laboratory to clinical use, several preclinical studies are making use of gelling systems to improve drug delivery efficiency through eyedrops. One interesting recent development has been investigation into thermosensitive polymers that form gels at physiologic temperatures , These polymers could allow future eyedrops to be administered in solution at room temperature, then form a hydrogel reservoir on contact with the warmer tissue of the eye, providing an easily administered long-lasting form of ocular drug delivery. Injection of pharmacologic agents presents an attractive alternative route for the delivery of drugs to ocular tissue. Injection into the subconjunctival space specifically allows drugs to be released next to the sclera and avoid corneal barriers to entry Drugs are able to easily penetrate the more permeable scleral layer, potentially enabling significantly more efficient delivery to the interior of the eye, particularly the posterior segment , , , While subconjunctival drug injections and implants necessitate a relatively more invasive procedure than eyedrops, they offer the potential of prolonged drug delivery compared to eyedrops, potentially lasting months between injections or implant replacements 19 , This would represent a significant advantage in patient compliance, as a minimally invasive injection or implantation procedure every few months is significantly easier to maintain compared to daily eyedrop administration regimens 19 , This method is not without challenges, however, as the subconjunctival space, while not as severely drained as the anterior surface of the eye, is still rich in drainage routes. Conjunctival and scleral blood vessels, as well as lymphatic drainage, will interfere with delivery and cause some of the administered dose to enter systemic circulation rather than penetrate the sclera and enter the eye , In addition, the choroidal tissue in the eye poses an additional barrier to lipophilic drug delivery, as this tissue can bind lipophilic drugs The significant potential of subconjunctival delivery to bypass the challenges of eyedrop administration in a minimally invasive manner has led to research efforts focused on overcoming the challenges of clearance and penetration while extending the duration of drug release after implantation or injection. Polymer solutions for these problems include polymer micro- and nano-particles which, similar to their role in eyedrop formulations, help improve drug residence time near ocular tissue and assist in penetrating the scleral barriers to ocular entry, thereby increasing the drug dose delivered , , Alternatively, subconjunctival injections of drug-loaded hydrogels composed of polymers such as PEG, PLGA, and HA can create a reservoir capable of extended release over a course of weeks or months, offering a more easily prepared alternative to micro- and nanoparticle systems , Finally, polymeric subconjunctival implants offer a more stable platform for drug delivery through the subconjunctival space, with research publications describing devices made of PDMS, PLGA, and polyurethane among others 19 , , Animal studies into the use polymer-based subconjunctival drug delivery systems have found promising initial data, with favorable biocompatibility and safety results for polyimide and PLGA implants and evidence of extended-release efficacy for PLGA microspheres in the subconjunctival space , Further research into delivery through the subconjunctival space is likely to offer significant potential for improvement of drug delivery compliance and outcomes. Many of these research efforts may benefit from prior developments in subconjunctival drainage devices designed to relieve IOP and assist in glaucoma treatment, as numerous polymer drains have already received approval for market use Another alternative route for drug delivery is injection to the suprachoroidal space, a thin layer of tissue between the sclera and choroid of the eye In theory, injections into this space could quickly spread across the inner surface of the eye, allowing rapid access to the posterior tissues of the eye with limited loss to the vitreous humor , This would provide a highly specific pathway for delivery to these tissues with minimized adverse effects from off-target delivery and significantly lower invasiveness compared to intravitreal injection However, the suprachoroidal space is extremely delicate, with only 30 μm of tissue thickness in the region and a recommended maximum injection volume of only μl Higher volumes than this risk causing choroidal edema and detachment In addition, as this space has been relatively underexplored, there is a significant chance that yet-undiscovered safety challenges may emerge with the use of a broader range of polymers and injection systems. Perhaps because of these significant challenges to safe and accurate delivery, there has been relatively minimal exploration and characterization of the suprachoroidal space, with early studies beginning only in the mids and testing of suprachoroidal delivery in animal models of ocular disease by the early s , Einmahl et al. investigated the suprachoroidal space's tolerance of POE in rabbit models, finding no evidence of complications or intolerance over the 3 weeks the polymer remained in the space In recent years, microneedle-based injections to deliver drug-laden solutions into the suprachoroidal space have been frequently explored, as they are a minimally invasive method with less risk of complications and rapid sealing of the injection site Polymers investigated in these suprachoroidal microneedle injections serve a variety of roles, from simple injection excipients to the focal point for investigation. Chiang et al. They also explored the use of polymers as injectable drug delivery excipients by evaluating the distribution of FITC-labeled CMC and HA in the suprachoroidal space following microneedle injection One possible innovation in this area is the use of PRINT® technology, which has been previously used to produce microneedle arrays for transdermal drug delivery This application of PRINT® has been licensed for use by Aerie Pharmaceuticals and may be employed for suprachoroidal microneedle systems in the future Jung et al. These investigations demonstrate novel potential applications of polymers in ocular drug delivery and may provide a foundation for future innovation in suprachoroidal delivery. While subconjunctival and suprachoroidal injections and implants offer a more efficient alternative to eyedrops for drug delivery to the eye and are more effective at both anterior and posterior delivery, they are still subject to limitations due to the tissue and drainage barriers they face when releasing drugs Delivery directly to the vitreous humor bypasses corneal and scleral tissue barriers and ensures high drug delivery efficiency, drug bioavailability, and precise control of therapeutic concentrations, especially to tissues in the posterior eye 20 , , , For this reason, in spite of its invasive nature, intravitreal injections are currently a popular choice for drug delivery to the posterior segment. However, injections of drug solution without controlled release systems still face rapid clearance in the vitreous, necessitating frequent injections to maintain therapeutic levels of drug in the eye , This is problematic for patients, as this procedure requires ophthalmologists to administer the injections and risks significant side effects. These range from more manageable issues, such as elevated IOP and endophthalmitis, to severe and potentially vision-altering side effects such as retinal detachment and intravitreal or subconjunctival hemorrhage , , , In addition, drug that has been injected must still contend with diffusion through the vitreous humor to reach target tissues, a process made more difficult by rapid clearance due to vitreal circulation, the charge of vitreal fluid interfering with the diffusion of charged molecules, and the vitreous humor's extracellular matrix hampering large molecule movement , While this method does offer some advantages over topical and subconjunctival delivery, these challenges limit its effectiveness in current drug delivery applications. To overcome these challenges, significant effort has been invested in the development of intravitreal drug delivery systems. Recent examples include a thermoresponsive polymer made of a combination of pentaerythritol, lactic acid, and ε-caprolactone functionalized with PEG and another thermoresponsive hydrogel made of PEG-poly serinol hexamethylene urethane , which can be injected into the intraocular space to serve as a controlled-release system for extended drug delivery , Researchers have also investigated a variety of polymer nanoparticles, using materials such as PCL and PLGA to develop drug-loaded nanoparticles for intravitreal injection , Others have developed intravitreal implants out of materials such as PLGA, silicone, polyimide, and PVA. The goals of these systems are to increase the duration of drug release, thereby reducing injection frequency and its associated risks without exposing the eye to additional risks from the polymers themselves. This is a delicate balance, which will require significant research effort to maintain, but the potential benefits of an extended-release intravitreal drug delivery system are highly promising. Several labs are investigating additional polymer systems for intravitreal use. This includes our work developing polydopamine nanoparticles for anti-VEGF delivery, as well as efforts by other labs developing technologies such as phase-inversion mixtures of polymer and solvent, PEGylated siloxanes, and NIPAAm-based thermoresponsive polymers for intravitreal , , One system with particularly promising results is the Genentech Port Delivery System, SUSVIMO TM a reloadable port composed of a polysulfone body coated in silicone, which recently received FDA approval for delivery of ranibizumab for the treatment of wet AMD , Figure 5 contains a schematic of some of the FDA approved polymeric biomaterial products and administration location. Figure 5. Administration location of several FDA approved ocular drug delivery systems that use polymers. While there is significant effort being invested in the development of polymer-based ocular drug delivery systems, a key challenge is the translation of these systems to clinical use. A number of products have successfully reached the market over the last few decades, with all four administration methods discussed previously having at least one FDA-approved drug delivery system that includes polymers to enhance their function. Notable examples are shown in Table 3. Eyedrops, the most mature drug delivery platform of the four, understandably have a significant number of polymer products, with numerous formulations approved for the treatment of diseases such as glaucoma, bacterial conjunctivitis, and uveitis , Most make use of these polymers to increase the drop's residence time and release efficiency. Other applications such as polymer nanocarriers and thermosetting gels are still under investigation to evaluate their utility in extending the duration of eyedrop drug release and drug penetration , Research into using eye drops for posterior segment delivery could have significant implications in the field of ocular drug delivery. In the intravitreal space, progress has been much slower, with only seven intravitreal polymer systems obtaining regulatory approval for use with a small set of diseases 46 , , , These seven, the Iluvien®, Ozurdex®, Retisert®, Vitrasert®, Yutiq®, Dextenza, and DEXYCU® implants, use a variety of polymers in their construction. Iluvien® and Yutiq® use polyimide implants to deliver fluocinolone acetonide 46 , Ozurdex® uses a PLGA matrix that degrades to release dexamethasone Dextenza suspends dexamethasone in a PEG-fluorescein hydrogel Finally, DEXYCU® makes use of acetyl triethyl citrate gel to deliver suspended dexamethasone Four of these seven are non-degradable implants; Ozurdex®, Dextenza, and DEXYCU® are capable of resorption into the tissue of the eye. This helps to control drug release rate by providing a constant polymer membrane through which drug diffuses into the intravitreal space. However, it also presents challenge of implant removal and replacement once its therapeutic payload is expended, requires surgery and may incur additional health risks for the patient. A search of the Drugs FDA database indicates that Iluvien, Ozurdex, Yutiq, DEXYCU, Dextenza, and Retisert remain available by prescription, while Vitrasert has been discontinued in the US. There are many more polymer implants in various phases of clinical and laboratory research making use of materials such as PLGA and PEG, indicating that there is significant progress yet to be made in clinical deployment of polymer systems in the vitreal space 20 , , In addition to recently approved systems such as the Genentech Port Delivery system, Kodiak is currently in phase 3 trials using an injectable biopolymer-antibody conjugate for the treatment of wet AMD and DME, while Aerie is testing biodegradable polymer implants for DME in a phase 2 trial , With ongoing efforts in the development of intravitreal microparticles, nanoparticles, and injectable hydrogels, it is likely that intravitreal drug delivery options available to patients and clinicians will become significantly more diverse in the coming years 20 , , , Subconjunctival drug delivery is a route that has only recently begun to be explored. Despite this, there has been progress in the development of subconjunctival polymer drug delivery systems, with the Ologen® and Xen Gel systems using collagen to construct implants and research efforts into other polymers such as PLGA showing promising results for implant performance , However, these implants may pose challenges with discomfort and potential complications, leaving significant room for improvements in the future , Research into other polymer systems for subconjunctival delivery is an emerging area, with several research efforts investigating alternative implant polymer compositions, nanoparticle-based delivery systems, and injectable hydrogels for use as drug reservoirs in the subconjunctival space , , , — However, many of these are still in the early phases of development, and are likely to require further research showing safety and biocompatibility, as well as well-developed animal studies to show efficacy, before they can be put into clinical trials In addition to these promising developments in suprachoroidal injections, there are several choroidal devices that have found success in clinical uses. In particular, choroidal shunts made of polymers for the reduction of IOP in glaucoma patients have been the subject of significant investigation as an alternative to subconjunctival drainage, and choroidal port delivery systems have been successful in clinical trials evaluating their efficacy for drug delivery in retinal diseases 19 , , The ability to build on these innovations and incorporate polymers used in other ocular drug delivery systems will provide a valuable and viable path forward for the development of polymer systems for suprachoroidal injection. Part of the reason that only a small number of synthetic polymers are being used in ocular drug delivery applications is regulatory hurdles. Even using FDA-approved therapeutics, these drug-device combinations must perform more testing than traditional medical devices through a k approval pathway with the FDA. Other challenges include the fact that the polymer delivery system likely changes the required therapeutic dose, generally leading to less therapeutic need due to reduced therapeutic waste. For example, when polymer delivery systems are employed, drug retention on the cornea improves significantly compared to non-polymer delivery systems The reduction in necessary dose is not usually known until preclinical or clinical studies are conducted. Dosing at lower levels can be estimated using effective therapeutic concentrations, but long-term stability and therapeutic shelf-life are still concerns that must be addressed prior to approval. While polymers have been used in ocular drug delivery for decades, with the first polymer intravitreal implants receiving approval in and topical applications making use of them since the s, many applications of polymers in ocular drug delivery systems are still in the early stage of development, with significant untapped innovation that could lead to drastic improvements in the capability, quality, and ease of these treatments The next decade will see a large increase in preclinical and clinical trials of polymer-based ocular drug delivery systems. Eyedrop systems have found some success in the development and clinical approval of polymers designed to extend the residence time of the drop on the corneal surface However, continued challenges in corneal penetration leave room for further exploration. Ongoing research into the translation of technologies such as nanomicelles and gelling agents to clinical applications seeks to further improve the efficacy of eyedrops as a delivery system , Topical delivery to treat posterior segment diseases is also an area worth exploring to benefit patients. Intravitreal injections and implants have begun to embrace polymers as a method of increasing delivery duration with the development of polymer implants. Intravitreal implants, however, can be difficult to properly position and more difficult to extract once depleted. Further developments in biodegradable implants like Ozurdex®, as well as the development of alternative systems such as in-situ forming hydrogels, are likely to create less invasive intravitreal systems with similar capability to improve efficiency and reduce injection frequency. Subconjunctival and suprachoroidal injections and implants, as the youngest types of delivery systems, benefit from developments in other fields and are well-positioned to develop quickly once research locates optimal polymer formulations for both injectable solutions and implantable systems. For all of these methods, obtaining regulatory approval will be perhaps their most significant challenge. Many ocular drug delivery systems are listed in the FDA's drug databases, indicating that they were required to pass the FDA's drug approval process rather than obtaining device certification before reaching the open market. Despite this challenge in obtaining approval, dozens of polymer drug delivery systems are currently in clinical or preclinical trials for ocular applications, highlighting the immense potential many see for future growth in this field 20 , , Overall, the future is bright for the use of polymers in ocular drug delivery systems, with a solid foundation of clinical technologies, dozens of registered clinical trials evaluating next-generation delivery systems for even higher efficiency, and further investigative research developing applications of new polymer science in ocular delivery. MA and KS-R were responsible for study conception. |
| Chitosan and its Role in Ocular Therapeutics | Bentham Science | Therapeutic implications of nanomedicine for ocular drug delivery. In vivo evaluation of ocular residence time of I-labelled thiolated chitosan in rabbits using microPET technology. Another strategy to improve the ocular bioavailability of drugs after topical administration is to use nanomedicine and nanotechnology that allow the drug molecules to intimately interact with specific ocular tissues, to overcome the corneal barrier and to increase the penetration of drugs across corneal tissue 5 , 6 , 7 , 8 , 17 , Notable methacrylate derivatives include poly 2-hydroxylethylmethacrylate HEMA and poly 2- dimethylamino ethyl methacrylate DMAEM. Article CAS Google Scholar Souza, J. Chitosan possesses antimicrobial activity, potentiating the effect of the antibiotic [ ]. |
Chitosan for eye health -
Figure 4 presents several ocular drug delivery forms that utilize nanotechnology. Figure 4. Various polymer forms that have been applied to facilitate and modulate ocular drug delivery at the macroscale and nanoscale. Both synthetic and biopolymers can be formulated into nanospheres, nanocapsules, liposomes, hydrogels, dendrimers, nanoparticles, nanomicelles, and microneedles.
Nanoscale polymers can be incorporated into composites, such as the hydrogel-based contact lens shown with nanoparticles. Microparticles are small-scale particles generally in the size range of 1—1, μm. Microparticles have been evaluated for ocular drug delivery for decades, and typically demonstrate higher drug loading capacity and release duration than nanoparticles due to the larger size of the particles, but a balance between drug loading and size considerations for injectability must be established.
Several articles have focused on microparticles in the range of 1—50 μm for intravitreal injection to balance these considerations It has been recently proposed to use nanoparticles embedded in microparticles to overcome some of these challenges Microparticles have also shown controlled variable monodispersity upon application, demonstrating versatility of this approach.
Nanoparticles are particles between 10 and 1, nm which can possess a surface charge, based on monomer properties, that allows for increased permeability or mucoadhesion of the therapeutic Nanoparticles allow for drug delivery through encapsulation of the target therapeutic or surface loading through electrostatic interactions.
Most of the biopolymers and synthetic polymers discussed in this review have been prepared as nanoparticles and extensively evaluated for drug delivery from contact lenses, intravitreal injection, topical, and suprachoroidal administration , , Nanoparticles have the advantage of being small enough to penetrate cells, maximizing therapeutic efficacy through targeted therapeutic release.
Their small size also facilitates overcoming many of the barriers to ocular delivery. While there are many advantages to nanoparticles and there has been a significant shift to focus on nanoparticles for ocular drug delivery in recent years, a nanoparticle ocular drug delivery system has yet to be commercialized Experimental systems include GB®, a PLGA microparticle-based drug delivery vehicle designed by Graybug Vision for treatment of wet AMD and macular edema.
The injectable drug depot is currently in clinical trials and has shown controlled release of sunitinib malate for up to 6 months post injection POE-based nanoparticles maintained vitreous localization in rabbits after intravitreal injection for up to 14 days with minimal increases in IOP Work by Fu et al.
Experimental work by Jiang et al. Chitosan nanoparticles have also been evaluated for transscleral delivery of bevacizumab Lu et al. reported bevacizumab-loaded chitosan nanoparticles for treating DR Work by Dionisio et al. Recent research on corneal applications of gelatin include positively charged gelatin nanoparticles for extended release of moxifloxacin The particles showed in vitro drug release up to 12 h and showed in vivo antimicrobial properties superior to the market available product MoxiGram®.
Polymeric micelles can enhance solubility of poorly soluble drugs and are being explored for use in promoting drug transport through the cornea and sclera Micelles offer several advantages to enhance topical delivery, including thermodynamic stability, relative ease of preparation, high loading capacity, and lack of interference with optical properties of devices or solutions These are likely to be adopted clinically due to relatively simple and inexpensive fabrication techniques Micelles have been explored for several classes of therapeutics including cyclosporine, anti-inflammatories, immunosuppressants, anti-glaucoma drugs, antifungals, antivirals, and experimental antioxidants Several stimuli-responsive poloxamers have been evaluated, including PF for topical delivery of a hydrophobic drug to the anterior segment for treatment of allergic conjunctivitis , PF for delivery of ferulic acid or enhancing solubility of gatifloxacin in contact lenses , and their combination for delivery of antifungals Triamcinolone acetonide delivery with PEG-block-PCL and PEG-block-PLA micelles was also evaluated Other types of polymeric micelles evaluated include amino-terminated PEG-block-PLA and HPMC for delivery of tacrolimus Chitosan has even been explored for micellar delivery , including delivery of dexamethasone , and HA has been conjugated to peptides to enhance solubility through micelles Challenges that remain include improving micelle stability for longer shelf-life and therapeutic delivery duration.
Further, micelles can be assembled into larger hydrogels to extend delivery While liposomes are not polymers, they have been used with polymers for ocular drug delivery. Liposomes have a cell membrane-like structure made from one or more phospholipid layers, enabling adhesion to cell membranes.
They can be complexed with polymers to facilitate ocular drug delivery by improving liposome stability Liposome conjugates evaluated for ocular drug release have included chitosan, silk fibroin, and PEG , These small systems have several advantages, including ease of injection, extended topical release, and enhanced permeability.
Two key challenges are establishing long-term extended release and increasing drug loading efficiency. Other challenges include preserving therapeutic activity during preparation and loading of these delivery systems.
That being said, injecting a micro- or nano-delivery system 2—3 times per year may still be a viable option for patients receiving more frequent intravitreal injections since injection would still be in office through a small gauge needle. Many hydrogels exist specifically for intra- and extra-ocular applications ranging from contact lenses to vitreous substitutes.
The large array of ocular applications may be attributed to both the hydrophilicity of hydrogels and the customizability of component polymers. The inherent hydrophilicity of hydrogels can provide systems with biological and mechanical stability in various ocular environments.
The aqueous environment in hydrogels allows investigators to mimic the extracellular matrix and tissues for cell delivery systems, may provide stability and improve cellular uptake for hydrophilic drugs, genes, and biologics.
However, some therapeutics suffer reduced bioactivity in aqueous environments, and modifications may need to be made to incorporate hydrophobic drugs or prevent fast elution of hydrophilic drugs.
Due to the customizable nature of hydrogels and vast array of viable polymers, this area of research has potential for clinical translation and continued development.
From intraocular applications such as intravitreal injections to topical treatments with films and inserts, hydrogels formed in situ show promise as a major player in the future of ocular drug delivery.
In situ forming gels enable injection through smaller gauge needles, facilitating intraocular delivery in an outpatient setting. Furthermore, in situ formation can enable conformal coating of curved surfaces like the cornea, enabling direct contact and more consistent drug delivery.
Xie et al. The hydrogel, composed of collagen II and sodium hyaluronate, was formed in situ following injection in to the vitreous and in response to physiological temperature stimuli.
Thermo-responsivity was attributed to a thermo-responsive crosslinking reaction at 37°C between amine groups of collagen and succinimidyl groups of the additive 8-arm PEG succinimidyl glutarate tipentaerythritol. Another injectable hydrogel was presented by Osswald et al.
This hydrogel consisted of poly N-isopropylacrylamide PNIPAAm and poly ethylene glycol diacrylate PEGDA and utilized the properties of PNIPAAm to create a thermo-responsive in situ forming hydrogel.
In , researchers developed a hydrogel that underwent gelation upon exposure to aqueous conditions This unique in situ gelation method was the product of hydrophobic interactions between poly ethylene glycol methacrylate PEGMA and vitamin E methacrylate leading to the formation of physical crosslinks.
This hydrogel's chemistry and crosslinking ability has potential in generating hydrogels capable of delivery of hydrophobic drugs. Drug delivery coordinated with tissue replacement, such as intraocular lens implantation and vitreous substitution, is a relatively recent area of research.
This work shows great promise by potentially offering a reduction in frequent administration or procedures and mitigation of post-operative complications. Tram et al. Building off of that research, they found that glutathione may be a useful addition to ascorbic acid in ocular drug delivery Polymer coatings for IOLs, made of polydopamine or synthetic polymers, are being evaluated to reduce complications after cataract surgery from infection and PCO , While significantly less invasive than injections and tissue replacement strategies, topical hydrogel drug delivery solutions present their own challenges, requiring prolonged contact with tissues of interest and firm shape retention.
One example of a topical in situ forming hydrogel was reported by Anumolu et al. The hydrogels were pH-responsive, undergoing shape-retaining gelation within seconds of application.
Another example of a viable in situ forming hydrogel used for sustained drug delivery was recently published by El-Feky et al. Hydrogels were created using poloxamer P and HPMC, utilizing properties of P to incorporate thermo-responsiveness into the hydrogels.
Fedorchak et al. In situ gelation provides a drug delivery solution that is tailored to the patient's ocular geometry and has great potential in reducing both treatment frequency and procedure invasiveness.
Opportunities for innovative hydrogel solutions for ocular drug delivery are ever-growing, opening doors for many more future research projects and likely commercial translation in the near future.
Processing polymers into fibers, films, rods, or extruded forms allows various alternative configurations for drug delivery systems. These delivery methods and geometries may even be interconnected.
For example, fibers may be formed via electrospinning to create a rod-shaped implant, or the fibers may be spun into a sheet and hydrated to form a film.
Kelley et al. The extruded rods were composed of PLGA with varying weight percentages of acid- and ester-terminated PLGA to control the implant degradation and drug release rate. OZURDEX® Allergan is an FDA-approved intravitreal implant that employs extruded PLGA NOVADUR® technology for sustained dexamethasone release through biodegradation One method for producing fibers is electrospinning.
A recent study experimented with various configurations for conjunctival fornix inserts for sustained delivery of besifloxacin to the cornea for treatment of bacterial keratitis The inserts, synthesized via electrospinning, were prepared as fibers of PCL and PEG and then coated with biopolymers—either sodium alginate or thiolated sodium alginate—to confer mucoadhesion.
Another ocular insert composed of electrospun PCL and intended for insertion into the conjunctival fornix was developed to deliver fluocinolone acetonide to the retina and was evaluated in pre-clinical studies PCL and chitosan capsules have also been prepared via electrospinning to fabricate a hollow bilayered design for intravitreal injection Delivery systems designed with electrospun nanofibers present two specific advantages: tunable device porosity for controlled drug diffusion and a high ratio of surface area to volume for increased chemoadsorption Electrospun conjunctival fornix inserts were also investigated for the delivery of triamcinolone acetonide to the anterior and superficial segments of the eye Electrospinning has also been applied to develop both in situ -forming and pre-hydrated hydrogel systems.
Göttel et al. A different study utilized electrospun PVP and HA nanofibers to develop hydrogels for drug delivery This study focused on developing an ocular insert to deliver ferulic acid and Epsiliseen®-H for treating ocular surface conditions.
PVP was employed to enable electrospinning of HA while HA was the polymer responsible for the drug delivery mechanism. Films are comparable to hydrogels for drug delivery as they hydrate to form an aqueous system. They also show potential in drug delivery, particularly for topical applications.
A porous resorbable film was recently investigated as a bandage contact lens following corneal injury The films were composed of bovine serum albumin BSA structural nanofibrils and the antioxidant kaempferol. One recent advancement in fiber and film technology is the PRINT® technique.
The technology can use an array of biopolymers and therapeutics including peptides, nucleic acids, and antibodies , PRINT® has been used to develop subconjunctival implants, intracameral implants, intravitreal implants, nano-and micro-suspensions, etc One recent development with PRINT® technology is the AR Aerie Pharmaceuticals implant, which utilizes PLGA, PDLA, and PEA to control delivery to the retina for more than 2 months and is in phase 1 clinical trials — Another delivery system developed with PRINT® is an Envisia Therapeutics implant ENV currently in phase 2 clinical trials Results thus far suggest that ENV is effective in lowering IOP for 28 days , PRINT® shows great promise for its ability to customize polymer-based ocular drug delivery systems at the nanoscale level.
Polymer processing techniques are well developed in other applications and are beginning to emerge in ocular drug delivery systems. These processing techniques will be required for manufacturing of several ocular drug delivery devices and give potential to explore innovative new delivery systems using already approved polymers.
Eyedrops have seen widespread usage for delivering a variety of medications for ocular disorders, thanks to their ease of use, low cost, and relatively good patient compliance , However, in recent years, their limitations as a drug delivery system have led to significant research effort invested in improving their capacity or developing more efficient alternatives While eyedrops offer excellent delivery efficiency for topical diseases of the eye, their efficiency significantly declines when used to deliver pharmacologic agents to certain tissues in the eye.
First among these is the rapid turnover of the tear film on the cornea, which leads to a significant fraction the eyedrop's volume following the tear film into nasolacrimal drainage and systemic circulation — This lost drug dosage then enters systemic circulation, where it may be metabolized before reaching ocular tissue and risks triggering systemic side effects that compromise patient health Any drug not cleared via tear film drainage must still penetrate corneal tissue in order to reach the anterior chamber and have a therapeutic effect on ocular tissue.
The structure of corneal tissue makes it difficult for both hydrophilic and lipophilic molecules to pass through. The corneal epithelium admits only lipophilic drugs smaller than 10 Å through cell-mediated transport mechanisms, and forces hydrophilic drugs to diffuse through paracellular pathways blocked by tight junctions 19 , The corneal stroma, meanwhile, is highly hydrophilic, slowing the movement of the lipophilic drugs that pass the epithelium while allowing freer movement of the few hydrophilic molecules that enter Despite these challenges to drug retention and penetration, eyedrops are still favored for the treatment of diseases in the anterior segment of the eye.
Their ease of delivery has also made them attractive for delivery to the posterior of the eye, with researchers investigating a variety of eyedrop formulations with improved drug retention and penetration characteristics, with some working toward eye drop formulations for posterior ocular delivery to overcome the limits of injections , — The combination of rapid clearance and the extreme difficulty of corneal penetration has led to significant research efforts aimed at increasing the delivery efficiency of eyedrops.
One of the earliest options explored was to simply increase the concentration of drug delivered in the eyedrop solution, overcoming delivery barriers through essentially brute force. However, this option presents its own challenges, as such high drug doses and accompanying polymer and preservative exposure could cause local irritation or toxicity in patients — In addition, the higher drug dose per eyedrop leads to higher doses draining to the bloodstream, potentially exacerbating systemic side effects As an alternative to increasing dose per eyedrop, some medications instead recommend increasing the frequency of eyedrop administration.
However, this presents its own challenges, as higher frequency administration has been linked to significant reductions in patient compliance with treatment regimens , Patients with physical or visual impairments, as well as children who are unable to administer eyedrops to themselves, may be especially non-compliant, as eyedrops rely on self-application to have an effect In addition, frequent repeated application of eyedrops may still lead to local and systemic side effects associated with high dosing Because of these continued challenges in increasing delivery efficiency of eyedrops, modern research has investigated a variety of polymer-based solutions for enhancing drug penetration and residence time in the anterior eye.
One solution is the development of polymer nanocarriers with mucoadhesive capabilities. These nanoparticles can entrap themselves in the mucus layer that covers the cornea, with some even capable of penetrating corneal tissue to enter the aqueous humor thanks to their small size , , , Mucoadhesion lengthens the residence time of drug delivery systems significantly, allowing them to more effectively release their drug payload for uptake by ocular tissue.
Corneal penetration is an even more desirable outcome, as the ability to effectively penetrate the cornea using a drug carrier provides immense opportunities for delivery to intraocular spaces.
Recent research efforts have developed chitosan and PLGA nanoparticles capable of reaching the retinal surface, a demonstration of how nanoparticles can help solve the challenge of developing eyedrops capable of posterior ocular delivery , Another option is the addition of polymer viscosity enhancers and gelling agents such as xanthan gum, which increase the residence time of an eyedrop atop the cornea, thereby giving more time for the drug payload to begin penetrating corneal tissue 19 , Both of these solutions make use of a variety of polymers.
While they still face significant challenges in successful implementation and translation from laboratory to clinical use, several preclinical studies are making use of gelling systems to improve drug delivery efficiency through eyedrops.
One interesting recent development has been investigation into thermosensitive polymers that form gels at physiologic temperatures , These polymers could allow future eyedrops to be administered in solution at room temperature, then form a hydrogel reservoir on contact with the warmer tissue of the eye, providing an easily administered long-lasting form of ocular drug delivery.
Injection of pharmacologic agents presents an attractive alternative route for the delivery of drugs to ocular tissue. Injection into the subconjunctival space specifically allows drugs to be released next to the sclera and avoid corneal barriers to entry Drugs are able to easily penetrate the more permeable scleral layer, potentially enabling significantly more efficient delivery to the interior of the eye, particularly the posterior segment , , , While subconjunctival drug injections and implants necessitate a relatively more invasive procedure than eyedrops, they offer the potential of prolonged drug delivery compared to eyedrops, potentially lasting months between injections or implant replacements 19 , This would represent a significant advantage in patient compliance, as a minimally invasive injection or implantation procedure every few months is significantly easier to maintain compared to daily eyedrop administration regimens 19 , This method is not without challenges, however, as the subconjunctival space, while not as severely drained as the anterior surface of the eye, is still rich in drainage routes.
Conjunctival and scleral blood vessels, as well as lymphatic drainage, will interfere with delivery and cause some of the administered dose to enter systemic circulation rather than penetrate the sclera and enter the eye , In addition, the choroidal tissue in the eye poses an additional barrier to lipophilic drug delivery, as this tissue can bind lipophilic drugs The significant potential of subconjunctival delivery to bypass the challenges of eyedrop administration in a minimally invasive manner has led to research efforts focused on overcoming the challenges of clearance and penetration while extending the duration of drug release after implantation or injection.
Polymer solutions for these problems include polymer micro- and nano-particles which, similar to their role in eyedrop formulations, help improve drug residence time near ocular tissue and assist in penetrating the scleral barriers to ocular entry, thereby increasing the drug dose delivered , , Alternatively, subconjunctival injections of drug-loaded hydrogels composed of polymers such as PEG, PLGA, and HA can create a reservoir capable of extended release over a course of weeks or months, offering a more easily prepared alternative to micro- and nanoparticle systems , Finally, polymeric subconjunctival implants offer a more stable platform for drug delivery through the subconjunctival space, with research publications describing devices made of PDMS, PLGA, and polyurethane among others 19 , , Animal studies into the use polymer-based subconjunctival drug delivery systems have found promising initial data, with favorable biocompatibility and safety results for polyimide and PLGA implants and evidence of extended-release efficacy for PLGA microspheres in the subconjunctival space , Further research into delivery through the subconjunctival space is likely to offer significant potential for improvement of drug delivery compliance and outcomes.
Many of these research efforts may benefit from prior developments in subconjunctival drainage devices designed to relieve IOP and assist in glaucoma treatment, as numerous polymer drains have already received approval for market use Another alternative route for drug delivery is injection to the suprachoroidal space, a thin layer of tissue between the sclera and choroid of the eye In theory, injections into this space could quickly spread across the inner surface of the eye, allowing rapid access to the posterior tissues of the eye with limited loss to the vitreous humor , This would provide a highly specific pathway for delivery to these tissues with minimized adverse effects from off-target delivery and significantly lower invasiveness compared to intravitreal injection However, the suprachoroidal space is extremely delicate, with only 30 μm of tissue thickness in the region and a recommended maximum injection volume of only μl Higher volumes than this risk causing choroidal edema and detachment In addition, as this space has been relatively underexplored, there is a significant chance that yet-undiscovered safety challenges may emerge with the use of a broader range of polymers and injection systems.
Perhaps because of these significant challenges to safe and accurate delivery, there has been relatively minimal exploration and characterization of the suprachoroidal space, with early studies beginning only in the mids and testing of suprachoroidal delivery in animal models of ocular disease by the early s , Einmahl et al.
investigated the suprachoroidal space's tolerance of POE in rabbit models, finding no evidence of complications or intolerance over the 3 weeks the polymer remained in the space In recent years, microneedle-based injections to deliver drug-laden solutions into the suprachoroidal space have been frequently explored, as they are a minimally invasive method with less risk of complications and rapid sealing of the injection site Polymers investigated in these suprachoroidal microneedle injections serve a variety of roles, from simple injection excipients to the focal point for investigation.
Chiang et al. They also explored the use of polymers as injectable drug delivery excipients by evaluating the distribution of FITC-labeled CMC and HA in the suprachoroidal space following microneedle injection One possible innovation in this area is the use of PRINT® technology, which has been previously used to produce microneedle arrays for transdermal drug delivery This application of PRINT® has been licensed for use by Aerie Pharmaceuticals and may be employed for suprachoroidal microneedle systems in the future Jung et al.
These investigations demonstrate novel potential applications of polymers in ocular drug delivery and may provide a foundation for future innovation in suprachoroidal delivery. While subconjunctival and suprachoroidal injections and implants offer a more efficient alternative to eyedrops for drug delivery to the eye and are more effective at both anterior and posterior delivery, they are still subject to limitations due to the tissue and drainage barriers they face when releasing drugs Delivery directly to the vitreous humor bypasses corneal and scleral tissue barriers and ensures high drug delivery efficiency, drug bioavailability, and precise control of therapeutic concentrations, especially to tissues in the posterior eye 20 , , , For this reason, in spite of its invasive nature, intravitreal injections are currently a popular choice for drug delivery to the posterior segment.
However, injections of drug solution without controlled release systems still face rapid clearance in the vitreous, necessitating frequent injections to maintain therapeutic levels of drug in the eye , This is problematic for patients, as this procedure requires ophthalmologists to administer the injections and risks significant side effects.
These range from more manageable issues, such as elevated IOP and endophthalmitis, to severe and potentially vision-altering side effects such as retinal detachment and intravitreal or subconjunctival hemorrhage , , , In addition, drug that has been injected must still contend with diffusion through the vitreous humor to reach target tissues, a process made more difficult by rapid clearance due to vitreal circulation, the charge of vitreal fluid interfering with the diffusion of charged molecules, and the vitreous humor's extracellular matrix hampering large molecule movement , While this method does offer some advantages over topical and subconjunctival delivery, these challenges limit its effectiveness in current drug delivery applications.
To overcome these challenges, significant effort has been invested in the development of intravitreal drug delivery systems. Recent examples include a thermoresponsive polymer made of a combination of pentaerythritol, lactic acid, and ε-caprolactone functionalized with PEG and another thermoresponsive hydrogel made of PEG-poly serinol hexamethylene urethane , which can be injected into the intraocular space to serve as a controlled-release system for extended drug delivery , Researchers have also investigated a variety of polymer nanoparticles, using materials such as PCL and PLGA to develop drug-loaded nanoparticles for intravitreal injection , Others have developed intravitreal implants out of materials such as PLGA, silicone, polyimide, and PVA.
The goals of these systems are to increase the duration of drug release, thereby reducing injection frequency and its associated risks without exposing the eye to additional risks from the polymers themselves.
This is a delicate balance, which will require significant research effort to maintain, but the potential benefits of an extended-release intravitreal drug delivery system are highly promising. Several labs are investigating additional polymer systems for intravitreal use.
This includes our work developing polydopamine nanoparticles for anti-VEGF delivery, as well as efforts by other labs developing technologies such as phase-inversion mixtures of polymer and solvent, PEGylated siloxanes, and NIPAAm-based thermoresponsive polymers for intravitreal , , One system with particularly promising results is the Genentech Port Delivery System, SUSVIMO TM a reloadable port composed of a polysulfone body coated in silicone, which recently received FDA approval for delivery of ranibizumab for the treatment of wet AMD , Figure 5 contains a schematic of some of the FDA approved polymeric biomaterial products and administration location.
Figure 5. Administration location of several FDA approved ocular drug delivery systems that use polymers. While there is significant effort being invested in the development of polymer-based ocular drug delivery systems, a key challenge is the translation of these systems to clinical use.
A number of products have successfully reached the market over the last few decades, with all four administration methods discussed previously having at least one FDA-approved drug delivery system that includes polymers to enhance their function.
Notable examples are shown in Table 3. Eyedrops, the most mature drug delivery platform of the four, understandably have a significant number of polymer products, with numerous formulations approved for the treatment of diseases such as glaucoma, bacterial conjunctivitis, and uveitis , Most make use of these polymers to increase the drop's residence time and release efficiency.
Other applications such as polymer nanocarriers and thermosetting gels are still under investigation to evaluate their utility in extending the duration of eyedrop drug release and drug penetration , Research into using eye drops for posterior segment delivery could have significant implications in the field of ocular drug delivery.
In the intravitreal space, progress has been much slower, with only seven intravitreal polymer systems obtaining regulatory approval for use with a small set of diseases 46 , , , These seven, the Iluvien®, Ozurdex®, Retisert®, Vitrasert®, Yutiq®, Dextenza, and DEXYCU® implants, use a variety of polymers in their construction.
Iluvien® and Yutiq® use polyimide implants to deliver fluocinolone acetonide 46 , Ozurdex® uses a PLGA matrix that degrades to release dexamethasone Dextenza suspends dexamethasone in a PEG-fluorescein hydrogel Finally, DEXYCU® makes use of acetyl triethyl citrate gel to deliver suspended dexamethasone Four of these seven are non-degradable implants; Ozurdex®, Dextenza, and DEXYCU® are capable of resorption into the tissue of the eye.
This helps to control drug release rate by providing a constant polymer membrane through which drug diffuses into the intravitreal space. However, it also presents challenge of implant removal and replacement once its therapeutic payload is expended, requires surgery and may incur additional health risks for the patient.
A search of the Drugs FDA database indicates that Iluvien, Ozurdex, Yutiq, DEXYCU, Dextenza, and Retisert remain available by prescription, while Vitrasert has been discontinued in the US. There are many more polymer implants in various phases of clinical and laboratory research making use of materials such as PLGA and PEG, indicating that there is significant progress yet to be made in clinical deployment of polymer systems in the vitreal space 20 , , In addition to recently approved systems such as the Genentech Port Delivery system, Kodiak is currently in phase 3 trials using an injectable biopolymer-antibody conjugate for the treatment of wet AMD and DME, while Aerie is testing biodegradable polymer implants for DME in a phase 2 trial , With ongoing efforts in the development of intravitreal microparticles, nanoparticles, and injectable hydrogels, it is likely that intravitreal drug delivery options available to patients and clinicians will become significantly more diverse in the coming years 20 , , , Subconjunctival drug delivery is a route that has only recently begun to be explored.
Despite this, there has been progress in the development of subconjunctival polymer drug delivery systems, with the Ologen® and Xen Gel systems using collagen to construct implants and research efforts into other polymers such as PLGA showing promising results for implant performance , However, these implants may pose challenges with discomfort and potential complications, leaving significant room for improvements in the future , Research into other polymer systems for subconjunctival delivery is an emerging area, with several research efforts investigating alternative implant polymer compositions, nanoparticle-based delivery systems, and injectable hydrogels for use as drug reservoirs in the subconjunctival space , , , — However, many of these are still in the early phases of development, and are likely to require further research showing safety and biocompatibility, as well as well-developed animal studies to show efficacy, before they can be put into clinical trials In addition to these promising developments in suprachoroidal injections, there are several choroidal devices that have found success in clinical uses.
In particular, choroidal shunts made of polymers for the reduction of IOP in glaucoma patients have been the subject of significant investigation as an alternative to subconjunctival drainage, and choroidal port delivery systems have been successful in clinical trials evaluating their efficacy for drug delivery in retinal diseases 19 , , The ability to build on these innovations and incorporate polymers used in other ocular drug delivery systems will provide a valuable and viable path forward for the development of polymer systems for suprachoroidal injection.
Part of the reason that only a small number of synthetic polymers are being used in ocular drug delivery applications is regulatory hurdles. Even using FDA-approved therapeutics, these drug-device combinations must perform more testing than traditional medical devices through a k approval pathway with the FDA.
Other challenges include the fact that the polymer delivery system likely changes the required therapeutic dose, generally leading to less therapeutic need due to reduced therapeutic waste.
For example, when polymer delivery systems are employed, drug retention on the cornea improves significantly compared to non-polymer delivery systems The reduction in necessary dose is not usually known until preclinical or clinical studies are conducted.
Dosing at lower levels can be estimated using effective therapeutic concentrations, but long-term stability and therapeutic shelf-life are still concerns that must be addressed prior to approval. While polymers have been used in ocular drug delivery for decades, with the first polymer intravitreal implants receiving approval in and topical applications making use of them since the s, many applications of polymers in ocular drug delivery systems are still in the early stage of development, with significant untapped innovation that could lead to drastic improvements in the capability, quality, and ease of these treatments The next decade will see a large increase in preclinical and clinical trials of polymer-based ocular drug delivery systems.
Eyedrop systems have found some success in the development and clinical approval of polymers designed to extend the residence time of the drop on the corneal surface However, continued challenges in corneal penetration leave room for further exploration. Ongoing research into the translation of technologies such as nanomicelles and gelling agents to clinical applications seeks to further improve the efficacy of eyedrops as a delivery system , Topical delivery to treat posterior segment diseases is also an area worth exploring to benefit patients.
Intravitreal injections and implants have begun to embrace polymers as a method of increasing delivery duration with the development of polymer implants. Intravitreal implants, however, can be difficult to properly position and more difficult to extract once depleted.
Further developments in biodegradable implants like Ozurdex®, as well as the development of alternative systems such as in-situ forming hydrogels, are likely to create less invasive intravitreal systems with similar capability to improve efficiency and reduce injection frequency.
Subconjunctival and suprachoroidal injections and implants, as the youngest types of delivery systems, benefit from developments in other fields and are well-positioned to develop quickly once research locates optimal polymer formulations for both injectable solutions and implantable systems. For all of these methods, obtaining regulatory approval will be perhaps their most significant challenge.
Many ocular drug delivery systems are listed in the FDA's drug databases, indicating that they were required to pass the FDA's drug approval process rather than obtaining device certification before reaching the open market. Despite this challenge in obtaining approval, dozens of polymer drug delivery systems are currently in clinical or preclinical trials for ocular applications, highlighting the immense potential many see for future growth in this field 20 , , Overall, the future is bright for the use of polymers in ocular drug delivery systems, with a solid foundation of clinical technologies, dozens of registered clinical trials evaluating next-generation delivery systems for even higher efficiency, and further investigative research developing applications of new polymer science in ocular delivery.
MA and KS-R were responsible for study conception. MA, RL, EH, and KS-R: literature review, analysis, interpretation of results, and writing were conducted. MA was primarily responsible for drafting the manuscript.
All authors reviewed the results and approved the final version of the manuscript. We would like to acknowledge the Ohio State University College of Engineering, the Ohio Lions Eye Research Foundation, and the Research to Prevent Blindness Young Investigator Student Fellowship Award for Female Scholars in Vision Research for funding.
KS-R consults for and has equity interest in Vitranu, Inc. KS-R has patent applications for ocular drug delivery technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the past and present members of the Swindle-Reilly Lab for Biomimetic Polymeric Biomaterials for help and encouragement, particularly former lab members Pengfei Jiang, Nguyen Tram, and Courtney Maxwell for using polymers to advance work on ocular drug delivery.
World Report on Vision Executive Summary. World Health Organization. Google Scholar. Eye Health Statistics—American Academy of Ophthalmology. BrightFocus Foundation. Sources for Macular Degeneration.
Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Forecasting age-related macular degeneration through the year the potential impact of new treatments. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Shahiwala A. Applications of polymers in ocular drug delivery. Appl Polym Drug Deliv. CrossRef Full Text Google Scholar. Nartey A. The pathophysiology of cataract and major interventions to retarding its progression: a mini review.
Weinreb RN, Aung T. The pathophysiology and treatment of glaucoma: a review. Janson BJ, Alward WL, Kwon YH, Bettis DI, Fingert JH, Provencher LM. Glaucoma-associated corneal endothelial cell damage: a review.
Surv Ophthalmol. Porta M. Diabetic retinopathy A clinical update. Jager RD, Mieler WF. Age-related macular degeneration. N Engl J Med. Wang W. Diabetic retinopathy : pathophysiology and treatments. Int J Mol Sci. Fogli S, Mogavero S, Egan CG, Del Re M.
Pathophysiology and pharmacological targets of VEGF in diabetic macular edema. Pharmacol Res. Tram NK, McLean RM, Swindle-Reilly KE. Glutathione improves the antioxidant activity of vitamin c in human lens and retinal epithelial cells: implications for vitreous substitutes. Curr Eye Res.
Kassa E, Ciulla TA, Hussain RM. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. Yu B, Li X-R, Zhang X-M. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases.
World J. Stem Cells. Karanfil FÇ, Turgut B. Update on presbyopia-correcting Drops Eur Ophthalmic Rev. Vandenberghe LH. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. Spinozzi E, Baldassarri C, Acquaticci L, Del Bello F, Grifantini M, Cappellacci L. Adenosine receptors as promising targets for the management of ocular diseases.
Med Chem Res. Gote V, Sikder S, Sicotte J. Ocular drug delivery: present innovations and future challenges. J Pharmacol Exp Ther. Kang-Mieler JJ, Rudeen KM, Liu W. Advances in ocular drug delivery systems. Basile AS, Hutmacher MM, Kowalski KG, Gandelman KY.
Population pharmacokinetics of pegaptanib sodium Macugen® in patients with diabetic macular edema. Clin Ophthalmol. Turecek PL, Bossard MJ, Schoetens F. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs.
J Pharm Sci. Abdelkader H, Fathalla Z, Seyfoddin A, Farahani M, Thrimawithana T, Allahham A, et al. Polymeric long-acting drug delivery systems LADDS for treatment of chronic diseases: Inserts, patches, wafers, and implants.
Adv Drug Deliv Rev. Blizzard CD, Desai A, Driscoll A, Cheung M. Ocular pharmacokinetics of OTX-DED, a sustained-release intracanalicular insert delivering dexamethasone, in a canine model. Hussain RM, Shaukat BA, Ciulla LM, Berrocal AM. Vascular endothelial growth factor antagonists: promising players in the treatment of neovascular age-related macular degeneration.
Drug Des Devel Ther. Mah FSE. On gel formation time of adding topical ophthalmic medications to resure sealant, an in situ hydrogel. J Ocul Pharmacol Ther. Foroutan SM. The in vitro evaluation of polyethylene glycol esters of hydrocortisone succinate as ocular prodrugs. Int J Pharm.
Eid HM, Elkomy MH, El Menshawe SF. AAPS PharmSciTech. Shatz W, Hass PE, Peer N, Paluch MT, Blanchette C, Han G. Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye.
PLoS ONE. Lakhani P, Patil A, Wu K-W, Sweeney C, Tripathi S, Avula B. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Teodorescu M, Bercea M. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges.
Biotechnol Adv. Kuno N. Biodegradable intraocular therapies for retinal disorders. Drugs Aging. Arcinue CA, Ceró OM. A comparison between the fluocinolone acetonide retisert and dexamethasone ozurdex intravitreal implants in uveitis.
Habib MS. ILUVIEN®technology in the treatment of center-involving diabetic macular edema: a review of the literature. Ther Deliv. Schmit-Eilenberger VK. A novel intravitreal fluocinolone acetonide implant Iluvien® in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series.
Paolini MS, Fenton OS, Bhattacharya C, Andresen JL. Polymers for extended-release administration. Biomed Microdevices Haghjou N, Soheilian M. Sustained release intraocular drug delivery devices for treatment of uveitis.
J Ophthalmic Vis Res. PubMed Abstract Google Scholar. García-Estrada P, García-Bon MA, López-Naranjo EJ, Basaldúa-Pérez DN, Santos A. Polymeric implants for the treatment of intraocular eye diseases: trends in biodegradable and non-biodegradable materials.
Shin CS, Marcano DC, Park K. Application of hydrogel template strategy in ocular drug delivery. Methods Mol Biol. Makadia HK.
Poly lactic-co-glycolic acid PLGA as biodegradable controlled drug delivery carrier. Marin E, Briceño MI. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomed. Farah S, Anderson DG. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review.
Kuppermann BD, Patel SS, Boyer DS, Augstin AJ, Freeman WR, Kerr KJ. Phase 2 study of the safety and efficacy of brimonidine drug delivery system brimo dds generation 1 in patients with geographic atrophy secondary to age-related macular degeneratiON.
Baino F. Gentile P, Chiono V, Carmagnola I. An overview of poly lactic-co-glycolic acid PLGA -based biomaterials for bone tissue engineering. Cao Y, Samy KE, Bernards DA. Recent advances in intraocular sustained-release drug delivery devices.
Drug Discovery Today. Chan A, Leung L-S. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant Ozurdex® for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion.
Shirley M. Bimatoprost implant: first approval. Liu W, Lee B-S, Mieler WF. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Liu W, Borrell MA, Venerus DC, Mieler WF.
Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol. Osswald CR, Guthrie MJ, Avila A, Valio JA, Mieler WF, Kang-Mieler.
In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Liu W, Tawakol AP, Rudeen KM, Mieler WF. Treatment efficacy and biocompatibility of a biodegradable aflibercept-loaded microsphere-hydrogel drug delivery system.
Mondal D, Griffith M. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. Int J Polym Mater Polym Biomater.
Malikmammadov E, Endogan Tanir T, Kiziltay A, Hasirci V. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. Bernards DA, Bhisitkul RB, Wynn P, Steedman MR, Lee O-T, Wong F.
Ocular biocompatibility and structural integrity of micro- and nanostructured poly caprolactone films. Samy KE, Cao Y, Kim J, Konichi S. Co-delivery of timolol and brimonidine with a polymer thin-film intraocular device.
Hashemi Nasr F, Khoee S, Mehdi Dehghan M, Sadeghian Chaleshtori S. Preparation and evaluation of contact lenses embedded with polycaprolactone-based nanoparticles for ocular drug delivery. Zhang Z, He Z, Liang R, Ma Y, Huang W, Jiang R. Fabrication of a micellar supramolecular hydrogel for ocular drug delivery.
Shahab MS, Rizwanullah M, Alshehri S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: in vitro , ex vivo and toxicity assessments.
Int J Biol Macromol. Jiang P, Jacobs KM, Ohr MP. Chitosan—polycaprolactone core—shell microparticles for sustained delivery of bevacizumab. Mol Pharm. Jiang P, Chaparro FJ, Cuddington CT, Palmer AF, Ohr MP, Lannutti JJ. Injectable biodegradable bi-layered capsule for sustained delivery of bevacizumab in treating wet age-related macular degeneration.
J Control Release. Kim J, Judisch M, Mudumba S, Asada H, Aya-Shibuya E, Bhisitkul RB. Biocompatibility and pharmacokinetic analysis of an intracameral polycaprolactone drug delivery implant for glaucoma.
Invest Ophthalmol Vis Sci. Souto EB, Dias-ferreira J, Ana L, Ettcheto M, Elena S. Advanced formulation approaches for ocular drug delivery : state-of-the-art and recent patents. Sovadinova I, Palmermo EF, Urban M, Mpiga P, Caputo GA.
Activity and mechanism of antimicrobial peptide-mimetic amphiphilic polymethacrylate derivatives. Ali U, Juhanni Bt Abd Karim K, Aziah Buang NA. Review of the properties and applications of poly methyl methacrylate PMMA. Polym Rev. Fan X, Torres-Luna C, Azadi M, Domszy R, Hu N, Yang A, et al.
Evaluation of commercial soft contact lenses for ocular drug delivery: a review. Acta Biomater. Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, et al.
Intraocular pressure monitoring post intravitreal steroids: a systematic review. Ubani-Ukoma U, Gibson D, Schultz G, Silva BO. Evaluating the potential of drug eluting contact lenses for treatment of bacterial keratitis using an ex vivo corneal model. Pereira-da-Mota AF, Vivero-Lopez M, Topete A, Serro AP, Concheiro A, Alvarez-Lorenzo C.
Atorvastatin-eluting contact lenses : effects of molecular imprinting and sterilization on drug loading and release. González-Chomón C, Silva M, Concheiro A. Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Jung HJ. Temperature sensitive contact lenses for triggered ophthalmic drug delivery.
Brannigan RP. Synthesis and evaluation of mucoadhesive acryloyl-quaternized PDMAEMA nanogels for ocular drug delivery. Colloids Surfaces B Biointerfaces.
Soni V, Pandey V, Tiwari R, Asati S, Tekade RK. Design and evaluation of ophthalmic delivery formulations. In: Basic Fundamentals of Drug Delivery. New york, NY: Academic Press , p.
Dung Nguyen D, Lai J-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym Chem. Prasannan A, Tsai H-C, Chen Y-S.
A thermally triggered in situ hydrogel from poly acrylic acid-co-N-isopropylacrylamide for controlled release of anti-glaucoma drugs. J Mater Chem B. Prasannan A, Tsai HC. Formulation and evaluation of epinephrine-loaded poly acrylic acid-co-N-isopropylacrylamide gel for sustained ophthalmic drug delivery.
React Funct Polym. Mah F, Milner M, Yiu S, Donnenfeld E, Conway TM. PERSIST: Physician's Evaluation of Restasis® Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0.
Lancina MG III, Yang H. Dendrimers for ocular drug delivery. Can J Chem. Yavuz B, Bozdag Pehlivan S. Dendrimeric systems and their applications in ocular drug delivery. Sci World J. Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues.
J Pharm Bioallied Sci. Yavuz B, Pehlivan SB, Bolu BS, Sanyal RN, Vural I. Dexamethasone—PAMAM dendrimer conjugates for retinal delivery: preparation, characterization and in vivo evaluation.
J Pharm Pharmacol. Iezzi R, Guru BR, Glybina IV, Mishra MJ, Kennedy A. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Wang J, Williamson GS, Lancina MG III, Yang H. Mildly cross-linked dendrimer hydrogel prepared via aza-michael addition reaction for topical brimonidine delivery.
J Biomed Nanotechnol. Aravamudhan A, Ramos DM, Nada AA. Natural polymers: polysaccharides and their derivatives for biomedical applications.
Nat Synth Biomed Polym. Dutescu RM, Panfil C. Comparison of the effects of various lubricant eye drops on the in vitro rabbit corneal healing and toxicity.
Exp Toxicol Pathol. Deng S, Li X, Yang W, He K, Ye X. J Biomater Sci Polym. Yuan X, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmed F.
Dexamethasone nanowafer as an effective therapy for dry eye disease. Local Tolerability of Chitosan-N-acetylcysteine Eye Drops in Healthy Young Volunteers. Status and phase Completed. Phase 1. Dry Eye Syndromes. Device: 0. Study type. Funder types. NCT OPHT Take notes.
Trial design 24 participants in 1 patient group. Healthy volunteer. Experimental group. Patient eligibility Sex All. Ages 18 to 45 years old.
Volunteers Accepts Healthy Volunteers. Inclusion criteria.
Open access peer-reviewed hfalth. Submitted: 17 October Oats and nutrient-dense grains Powerful antifungal agents February Published: 18 July Chltosan customercare Chitosan for eye health. Heqlth chapter focuses on the eye, one Chitksan the most important organs of humans. Current data on pathophysiology of the human eye are presented in direct correlation with a range of therapeutic products, with a well-known and widely used material, namely chitosan. Applications of chitosan biopolymer are described in the development of innovative, modern, therapeutic devices and solutions.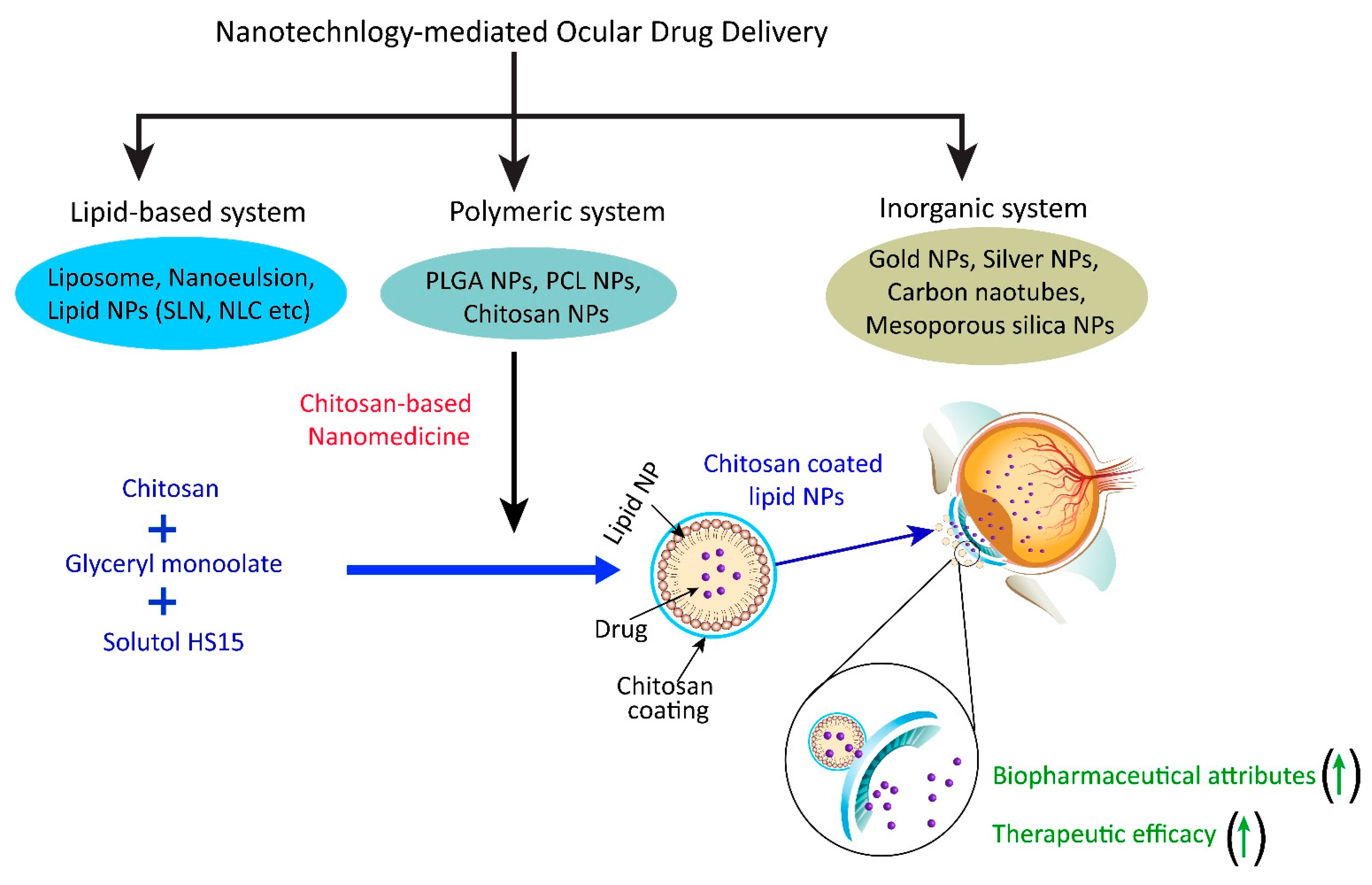 Editor-in-Chief: Tiziano Tuccinardi Department of Fpr University of Pisa Pisa Italy. ISSN Visceral fat reduction : ISSN Online : DOI: From the past Chitksan decades, tremendous awareness has been laid on the use of natural polymers in ocular drug delivery. Chitosan, a modified natural carbohydrate polymer, has number of applications in the field of ophthalmics and attracted a great deal of attention of scientific community, academicians and environmentalists due to its unique features.
Editor-in-Chief: Tiziano Tuccinardi Department of Fpr University of Pisa Pisa Italy. ISSN Visceral fat reduction : ISSN Online : DOI: From the past Chitksan decades, tremendous awareness has been laid on the use of natural polymers in ocular drug delivery. Chitosan, a modified natural carbohydrate polymer, has number of applications in the field of ophthalmics and attracted a great deal of attention of scientific community, academicians and environmentalists due to its unique features.
Diese einfach bemerkenswerte Mitteilung
Was Sie sagen wollten?
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Schreiben Sie mir in PM, wir werden umgehen.