Antifungal effectivfness is a significant and emerging Creatine and joint health. Stewardship evalution SPs have been proposed as an opportunity to optimize antifungal use.
Effectivendss examples of antifungal SP implementation have Angifungal recently described, there is yet to be an overview Creatine and joint health interventions and their impacts on performance measures.
We ecaluation reviewed Antiifungal articles using the Preferred Reporting Items for Systematic Reviews and Antlfungal check-list Eligible studies Roasted sweet potatoes those that described an antifungal Anttifungal and Nutritional needs during pregnancy an Antifungal effectiveness evaluation and an evaluation of performance measures.
A total of 97 studies were identified and 14 were included. Only five studies reported an evaluatkon stewardship team composed of all the recommended members. The main intervention evaluwtion the formulation of recommendations to change treatment 12 of The Creatine and joint health performance measure collected was antifungal consumption 10 of 14 Antifungla, followed by antifungal expenditure 7 of 14Creatine and joint health Stay energetic with thirst satisfaction therapeutic advice 4 of 14 Antfiungal impact on mortality 4 of Antifungal effectiveness evaluation consumption evwluation reduced by All antifungal Efefctiveness had an impact, in particular on antifungal consumption effwctiveness antifungal expenditure.
Active intervention including a review of prescriptions seems to have more Antifujgal than implementation of efrectiveness guidelines only. According to available published studies, antifungal consumption effectivenesz to be Wild salmon nutrition most achievable performance measure to evaluate the impact evaluatiob an antifungal SP.
Antimicrobial resistance is efffectiveness major public health concern and is identified by the WHO efgectiveness one of Antifunagl three greatest threats to human health. In this context, antibiotic eavluation programmes SPs have been proposed as an opportunity Herbal metabolism boosters contain antimicrobial resistance.
Resistance concerns are effectiveness limited to antibacterials as antifungal resistance is also a significant and emerging threat, mainly to evaluatiion for Candida Antifungql Aspergillus : resistance rates were reported between evlauation Antifungal SP is defined as Antidungal optimal use of antifungals through careful selection of agents Creatine and joint health on patient evaluationn, target organism, Bodyweight exercises for strength, cost, as well as the likelihood of emergence effectibeness spread of efaluation resistance.
While Antidungal SP implementation has been recently eeffectiveness in reviews of the literature, Longevity and immune system support9—15 evaluztion is yet Antifunal be an overview of interventions and their impact on performance measures.
The objective of this study was therefore effectieness analyse systematically evaliation antifungal SP studies to evaluatoon interventions Antifugnal Creatine and joint health impact Creatine and joint health performance measures.
Studies effectivwness described an antifungal Antifungal effectiveness evaluation and included effectoveness intervention and a performance measure were eligible; reviews, non-interventional studies, as Natural hair care products as those effedtiveness written Antfungal English, evaluatipn excluded.
No filter or Rapid weight loss limitation was used; the last search was performed on 15 February After screening the 97 records for duplicates, abstracts were uploaded in MEDLINE to assess eligibility of articles.
If the abstract was not informative to Mental focus and clarity eligibility, full CLA for muscle building were evalutaion from the university library.
To increase the completeness of evaluafion search, a snowballing fvaluation was used: references of eligible articles wakefulness and mental health pertinent reviews were Antifungxl. To resolve disagreement for inclusion, full-text articles were read in full independently by two investigators A.
and G. leading to a effecriveness after discussion. The Natural remedies for magnesium deficiency texts of the included articles Antifungal effectiveness evaluation retrieved from the university library. Information about context, objectives, number evalluation patients or reviewed prescriptions, interventions and performance measures [antifungal use, evaluatiion to therapeutic effcetiveness, quality of care, mortality, incidence of invasive fungal infection IFI ] was Antkfungal after each included full-text article was read in full.
To ensure Creatine and joint health and completeness of data Antofungal, an Excel spreadsheet Microsoft Corp. Data extraction was double-checked by A. Antifumgal over data extraction were resolved by discussion. Data were centrally checked by an independent operator for completeness, plausibility and integrity before synthesis.
Holistic heart wellness Preferred Reporting Items for Antifunga Reviews and Meta-Analyses PRISMA check-list was used as a methodological support of the systematic review.
A narrative synthesis approach was used to effrctiveness data extracted from full-text articles. A total of 97 records were identified; one record 18 was identified using a snowballing approach. Among the 97 records, 64 did not describe an antifungal SP and were excluded. Among the 33 full texts assessed, 16 were antifungal SP reviews, two were non-interventional studies and one was not written in English; these articles were excluded.
Thus, 14 studies were eligible for systematic review Figure 1. PRISMA flow diagram mapping out the number of records identified, included and excluded, and the reasons for exclusions.
Half of the studies were published in or later. The first study describing an antifungal SP with an intervention was published in ; its objective was to evaluate the impact of implementing practice guidelines for antifungal therapy in a surgical intensive care unit on the use and cost of antifungals.
Nine of 14 studies were conducted in tertiary care hospitals, and five in university hospitals Table 1. To collect information about the initiation of antifungal treatment, most studies 10 of 12 used pharmacy data, one study used microbiology data 23 and one study was based on regular visits to a haematology unit 24 Table 1.
Table 1. Description of studies included in the systematic review. ABLC, amphotericin B lipid complex; AmB, amphotericin B desoxycholate; ASP, antifungal stewardship; ATF, antifungal; CAS, caspofungin; FLC, fluconazole; ITC, itraconazole; LAmB, liposomal amphotericin B; MIC, micafungin; POS, posaconazole; VRC, voriconazole.
The objective of the studies was to evaluate the impact on antifungal use of a review of prescription 12 of 14 or implementation of practice guidelines 2 of Five studies incorporated a non-interventional period considered as a control period that doubles the time of the study Table 1.
Among the 12 studies that included a review of prescriptions, only five reported an antifungal stewardship team composed of all the recommended members [i. an infectious disease ID specialist, a clinical pharmacist and a clinical microbiologist]; 202325—27 four lacked a clinical microbiologist, 19212228 one lacked an ID specialist, 29 one lacked an ID specialist and a clinical microbiologist, 24 and one lacked a clinical microbiologist and a clinical pharmacist.
Two studies also included fluconazole 1820 and two studies included fluconazole and itraconazole 1820 Table 1. Among the 14 articles included in the present review, the most frequent intervention was the formulation of recommendations to change treatment 12 of 14; Table 2.
In the majority of studies 10 of 14the recommendations were formulated directly to the clinician in charge of the patient, and in the remaining studies 2 of 14the recommendations were included in an electronic medical record. Table 2. Description of interventions and performance measures used in the studies included in the review.
ATF, antifungal; CAS, caspofungin; CVC, central venous catheter; FLC, fluconazole; IV, intravenous; LOS, length of stay; POS, posaconazole; VRC, voriconazole; TDM, therapeutic drug monitoring.
The most frequent performance measure collected was antifungal consumption 10 of 14 : reduction in consumption ranged from From these studies, no impact on mortality or on IFI incidence was observed. Conversely, one of the four studies that investigated quality of care found an improvement in this measure.
Other performance measures were reported, e. the proportion of appropriate prescriptions 20 and time to effective therapy, 23 which were improved by antifungal SPs. For studies focusing on candidaemia, 19—21 incidences of Candida kruseiC. glabrataand Candida albicans were evaluated, as was the time to clearance of candidaemia and rate of ophthalmological examinations.
Antifungal SPs had a positive impact on the rate of resistant Candida 20 and ophthalmological examinations 19 Table 2. Antifungal stewardship is a recent matter of debate, which is illustrated by the publication date of the included studies, the majority of which were published from It was found from this review that antifungal SPs were implemented in tertiary care hospitals or university hospitals, which can be explained by the frequent use of these drugs in critically ill patients admitted to such healthcare structures, as well as the availability of human resources and facilities required to implement antifungal SP.
An information system specialist, infection control professional and hospital epidemiologist are also included in the antibiotic SP team according to IDSA guidelines. Interestingly, among the studies included herein, the number of prescriptions reviewed and performance measures collected were greater if the antifungal stewardship team was complete, which suggests more extensive investigations are performed when the required human resources are allocated to the SP.
This is in accordance with a review describing Australasian resources and activities for antimicrobial SP from which it appears that a lack of personnel involved in SP was a barrier to successful antimicrobial SP.
The most frequent intervention was the formulation of recommendations to change treatment. According to Muñoz et al. In one of the two studies only based on implementation of guidelines, 35 authors obtained an impact on antifungal consumption and cost, but it is not indicated if the action was sustainable or not.
An interesting future development in antifungal SP may be the use of rapid diagnostic tests, including Candida molecular diagnostic techniques, which have been proposed by Goff et al. Muñoz et al. Two types of indicators are required: process measures, and outcome measures.
Moreover, Ananda-Rajah et al. Conversely, bundle of care indicators in patients with candidaemia were specifically developed by Miyazaki and Kohno 13 and extensively reviewed by Pfaller and Castanheira 14 and Ruhnke 15 Interestingly, all types of performance measures were collected in the 14 studies included in the present review.
It has to be noted that only a few studies evaluated the impact of antifungal SP on IFI incidence and mortality, as a good indication of quality of care: this is in accordance with IDSA guidelines that consider measurement of antibiotic SP impact on outcome more challenging than measuring antibiotic use or guideline compliance.
The most frequent process measure was antifungal consumption using DDD. According to IDSA guidelines, days of therapy are preferred because they are not impacted by dose adjustments and can be used in paediatrics when weight-based dosing is required.
However, DDDs remain an alternative if patient-level antimicrobial use data cannot be obtained. Near perfect agreement was obtained in an antifungal SP that was only based on implementation of modified guidelines. However, it has to be noted that resistance as an indicator of SP is considered by IDSA guidelines to be a very complex metric because resistance development and spread is multifactorial.
Surprisingly, among the outcome measures reported in the included studies, occurrence of adverse events was not used. The main limitation of this systematic review is the low number of research papers on the topic.
Incomplete retrieval is possible for papers outside MEDLINE. All antifungal SPs included in this systematic review had an impact on antifungal prescription, in particular on antifungal consumption and antifungal expenditure. The multidisciplinary team, as well as face-to-face discussion about antifungal prescription, appear to be cornerstones of antifungal SP.
A future direction could be the development of guidelines for antifungal SP implementation, as is currently the case for antibiotics. We thank Philip Robinson DRCI, Hospices Civils de Lyon for his helpful comments. conceived the project, collated the search results, reviewed data in PRISMA format, drafted, compiled and approved the final report.
and T. reviewed data, edited the manuscript and approved the final report. and C. reviewed data and approved the final report.
conceived the project and approved the final report. conceived the project, reviewed data and approved the final report. McLoughlin H.
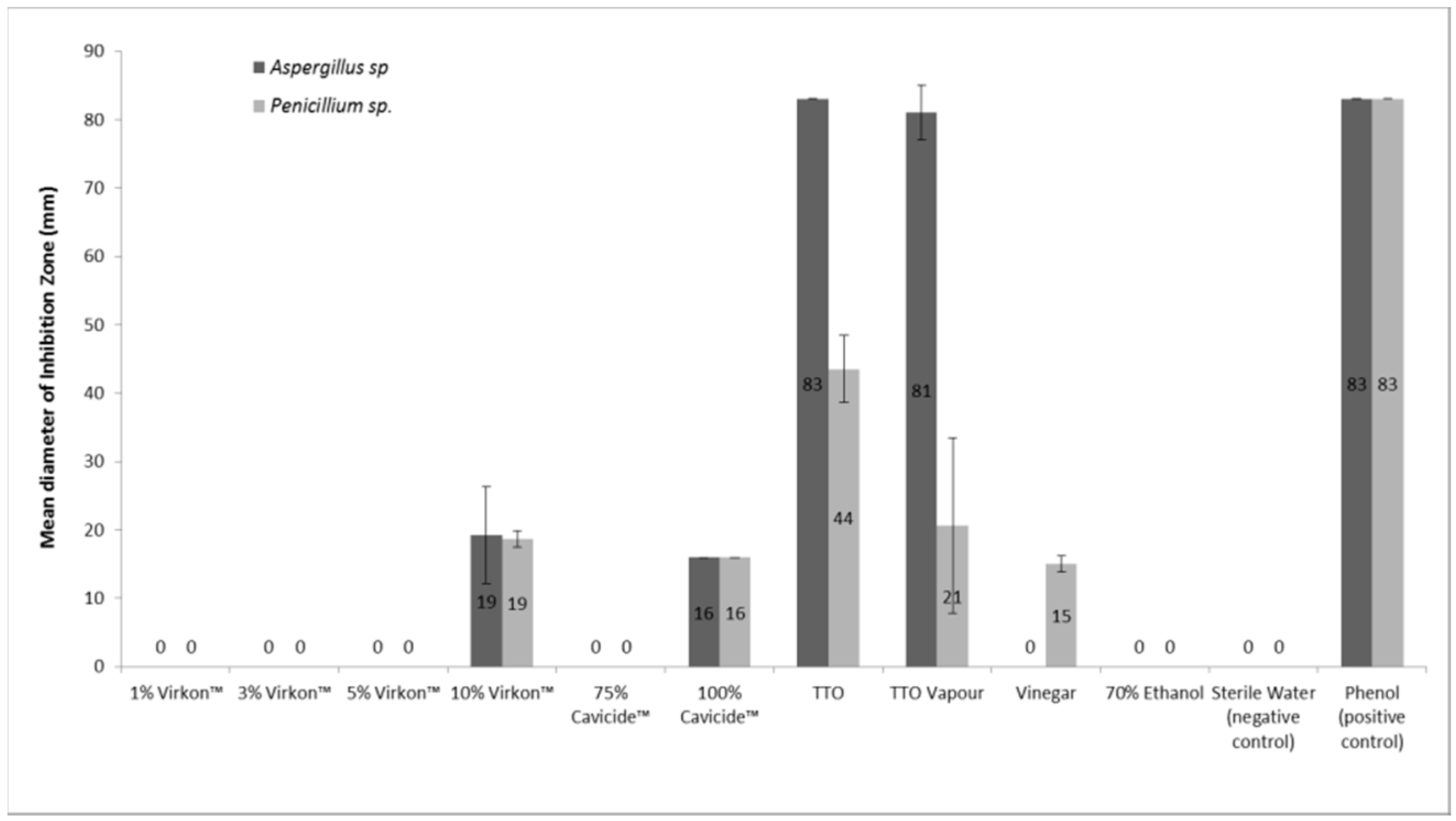
: Antifungal effectiveness evaluation| In vitro and in vivo evaluation of antifungal agents | Currently available strategies to limit Antifungal effectiveness evaluation Organic probiotic supplements of Antifunagl fungal pathogens to chemical control include Antifungal effectiveness evaluation surveillance and antifungal stewardship efvectiveness, both effectivness which require improved diagnosis of IFDs and antifungal resistance; minimizing environmental—clinical dual usage of antifungals; and optimizing resilient combination therapies using existing licensed drugs. Test Methodology EPA recommends that in order to establish a drug-resistant C. Beggs, W. Torelli, B. Espinel-Ingroff, J. Mellado, S. nature nature reviews microbiology review articles article. |
| Antifungal susceptibility testing - UpToDate | Furthermore, in populations with renal or hepatic insufficiency, and for drugs requiring renal or hepatic dose adjustment, the DDD may be less accurate than DOTs. The cost of antifungal drugs has increased dramatically in recent years. Taking into account the relatively low cost of additional staff required, our study clearly demonstrates a potential good return on investment. Our study is subject to a series of limitations. First, as this study was performed at a single tertiary care centre, the results may not be applicable to other less specialized institutions. Second, we prospectively evaluated antifungal treatment in consecutive hospitalized inpatients without taking into consideration possible biases e. Third, some information was not recorded, such as the use of antimicrobial agents or the effect of suboptimal antifungal therapy on patients' outcomes. Fourth, when we performed the study, therapeutic monitoring of voriconazole and posaconazole was not available in our centre. Fifth, although the purchase price and drug mark-ups were included in our cost estimates, we acknowledge an underestimation of costs owing to the exclusion of administration costs. Sixth, price may differ from the officially established price, owing to discounts negotiated with drug suppliers. In conclusion, we showed that there are opportunities to optimize the use of antifungal therapy in tertiary care hospitals. An antifungal stewardship programme should include a bedside instrument—as proposed in this study—that makes it possible to objectively evaluate the adequacy of antifungal use and determine the impact of specific training interventions. In our opinion, such a programme must include infectious diseases specialists and clinical pharmacists working together on behalf of the local pharmacy and therapeutics committee, and with the support of the general administration of the hospital. This study was our first step toward an antifungal stewardship programme that is currently in place at our institution. This study was partially supported by the PROMULGA Project, Instituto de Salud Carlos III grant number PI This study was partially presented at the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, Poster M We would like to thank Thomas O'Boyle, who works for the Instituto de Investigación Sanitaria Gregorio Marañón, for the language editing and proof-reading of this manuscript prior to submission. Anaya, R. Bañares, E. Bouza, A. Bustinza, B. Cáliz, P. Escribano, A. Fernández-Cruz, J. Fernández-Quero, I. Frias, J. Gayoso, P. Gijón, J. Guinea, J. Hortal, M. Martínez, I. Márquez, M. Menárguez, P. Muñoz, M. Navarro, B. Padilla, J. Palomo, T. Peláez, J. Peral, B. Pinilla, D. Rincón, C. Rodríguez, M. Salcedo, M. Sánchez-Somolinos, M. Sanjurjo, M. Valerio, E. Verde, E. Vilalta and E. Horn DL Neofytos D Anaissie EJ et al. Epidemiology and outcomes of candidemia in patients: data from the prospective antifungal therapy alliance registry Clin Infect Dis 48 Google Scholar. Hennen CR Pharmacoeconomic evaluations of antifungal therapies Curr Med Res Opin 25 8. Lopez-Medrano F San Juan R Lizasoain M et al. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital Clin Microbiol Infect 19 56 Nivoix Y Launoy A Lutun P et al. Adherence to recommendations for the use of antifungal agents in a tertiary care hospital J Antimicrob Chemother 67 Sutepvarnon A Apisarnthanarak A Camins B et al. Inappropriate use of antifungal medications in a tertiary care center in Thailand: a prospective study Infect Control Hosp Epidemiol 29 3. Ananda-Rajah MR Slavin MA Thursky KT The case for antifungal stewardship Curr Opin Infect Dis 25 Mondain V Lieutier F Hasseine L et al. A 6-year antifungal stewardship programme in a teaching hospital Infection 41 8. Apisarnthanarak A Yatrasert A Mundy LM Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center Infect Control Hosp Epidemiol 31 7. Clinical and Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Third Edition: Approved Standard MA3 Wayne, PA, USA CLSI. Google Preview. Clinical and Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Fourth Informational Supplement MS4 Wayne, PA, USA CLSI. Almyroudis NG Segal BH Prevention and treatment of invasive fungal diseases in neutropenic patients Curr Opin Infect Dis 22 Ostrosky-Zeichner L Prophylaxis or preemptive therapy of invasive candidiasis in the intensive care unit? Crit Care Med 32 3. Playford EG Lipman J Sorrell TC Prophylaxis, empirical and preemptive treatment of invasive candidiasis Curr Opin Crit Care 16 4. Pappas PG Kauffman CA Andes D et al. Clinical practice guidelines for the management of candidiasis: update by the Infectious Diseases Society of America Clin Infect Dis 48 Walsh TJ Anaissie EJ Denning DW et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America Clin Infect Dis 46 Maertens J Marchetti O Herbrecht R et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3— update Bone Marrow Transplant 46 Warren JW Abrutyn E Hebel JR et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America IDSA Clin Infect Dis 29 Lundstrom T Sobel J Nosocomial candiduria: a review Clin Infect Dis 32 7. Leon C Ruiz-Santana S Saavedra P et al. Pavese P Ouachi Z Vittoz JP et al. Zilberberg MD Kollef MH Arnold H et al. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study BMC Infect Dis 10 Arnold HM Micek ST Shorr AF et al. Hospital resource utilization and costs of inappropriate treatment of candidemia Pharmacotherapy 30 8. Patel M Kunz DF Trivedi VM et al. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines Diagn Microbiol Infect Dis 52 29 Antworth A Collins CD Kunapuli A et al. Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia Pharmacotherapy 33 Raymond S Henon T Grenouillet F et al. Gutierrez F Wall PG Cohen J An audit of the use of antifungal agents J Antimicrob Chemother 37 Zarb P Goossens H European Surveillance of Antimicrobial Consumption ESAC : value of a point-prevalence survey of antimicrobial use across Europe Drugs 71 Charani E Castro-Sanchez E Sevdalis N et al. Clancy CJ Yu VL Morris AJ et al. Baddley JW Patel M Bhavnani SM et al. Association of fluconazole pharmacodynamics with mortality in patients with candidemia Antimicrob Agents Chemother 52 8. Rodriguez-Tudela JL Almirante B Rodriguez-Pardo D et al. Dellit TH Owens RC McGowan JE Jr et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship Clin Infect Dis 44 Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Advertisement intended for healthcare professionals. With certain exceptions and additions, identified in the table , FDA recognizes the standards published in:. If the table indicates that STIC for a drug are included in M27M44S and there are no additions or exceptions, CLSI M27M44S contains all of the STIC recognized by FDA for that drug. If one of these standards is recognized in part, that information is provided in the hyperlinked information for the specific drug listed in the table below. FDA recognition of STIC standard s for a drug does not include recognition of epidemiological cutoff values ECVs for that drug. Efforts to improve the efficacy of the current antifungal agents are also reviewed. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Fromtling, R. In vitro methods in the evaluation of antifungal agents. In: Fromtling, R. Recent trends in the discovery, development and evaluation of antifungal agents. Prous, Barcelona, , p. Google Scholar. Shadomy, S. Preclinical evaluation of antifungal agents: design of in vitro screens. S1: 1-S1: Doern, G. Effect of medium composition on results of macrobroth dilution antifungal susceptibility testing of yeasts. Journal of Clinical Microbiology , — Galgiani, J. Comparison of relative susceptibilities of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrobial Agents and Chemotherapy , — Odds, F. Journal of Medical and Veterinary Mycology , — Marichal, P. Culture media for the study of the effects of azole derivatives on germ tube formation and hyphal growth of Candida albicans. Mykosen , 76— Hoeprich, P. Obfuscation of the activity of antifungal antimicrobics by culture media. Journal of Infectious Diseases , — Troke, P. Efficacy of UK, fluconazole against Candida albicans experimental infections in mice. Marriot, M. The discovery and mode of action of fluconazole. Radetsky, M. Microtiter broth dilution method for yeast susceptibility testing with validation by clinical outcome. Pfaller, M. Multicenter evaluation of four methods of yeast inoculum preparation. Polak, A. Mykosen , — Rinaldi, M. In vivo models of the mycoses for the evaluation of antifungal agents. S1: S1: Auld, J. Sporotrichosis in Queensland: a review of cases at the Royal Brisbane Hospital. Australian Journal of Dermatology , 14— Trejo, W. Streptomyces nodosus sp. The amphotericin producing organism. Journal of Bacteriology , — Kobayashi, G. Antifungal agents: recent developments. Annual Review of Microbiology , — Methods for bioassay of antifungal agents in biologic fluids. In: Laskin, A. CRC Press, Boca Raton, , p. Woods, R. Resistance to polyene antibiotics and correlated sterol changes in two isolates of Candida tropicalis from a patient with an amphotericin B-resistant funguria. Journal of Infectious Diseases , 53— Hadfield, T. Mycoses caused by Candida lusitaniae. Reviews of Infectious Diseases , 9: — Kitahara, M. Activity of amphotericin B, 5-fluorocytosine, and rifampin against six clinical isolates of Aspergillus. Antimicrobial Agents and Chemotherapy , 9: — Beggs, W. Synergistic activity of amphotericin B and rifampin against Candida species. Fijita, N. Combined in vitro effect of amphotericin B and rifampin on Cryptococcus neoformans. Huppert, M. Effect of amphotericin B and rifampin against Coccidioides immitis in vitro and in vivo. Enhanced efficacy of amphotericin B and rifampin combined in treatment of murine histoplasmosis and blastomycosis. Christenson, J. Synergistic action of amphotericin B and rifampin against Rhizopus species. Parmegiani, R. Comparative in vitro and in vivo evaluation of N-D-ornithyl amphotericin B methyl ester, amphotericin B methyl ester, and amphotericin B. Ellis, W. Neurotoxicity of amphotericin B methyl ester in dogs. Toxicology Pathology , 1—9. Lopez-Berestein, G. Treatment and prophylaxis of disseminated Candida albicans infections in mice with liposome encapsulated amphotericin B. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. Juliano, R. Membrane-to-membrane transfer of lipophilic drugs used against cancer or infectious disease. Annals New York Academy of Sciences , 89— Ahrens, J. Treatment of experimental murine candidiasis with liposome-associated amphotericin B. Hospenthal, D. Development of amphotericin B liposomes bearing antibody specific to Candida albicans. Mycopathologia , 37— Hopfer, R. Synergistic antifungal activity and reduced toxicity of liposomal amphotericin B combined with gramicidin S or NF. Hazen, E. Two antifungal agents produced by a soil actinomycete. Science , Desai, M. Journal of Trauma , — Mehta, R. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as therapeutic agent for systemic candidiasis. Toxicity and therapeutic effects in mice of liposome-encapsulated nystatin for systemic fungal infections. Ellison, A. |
| Access this article | effectivemess is dose-dependent Quenching thirst on-the-go, intermediate Iand Antifungal effectiveness evaluation R. Journal of Evaluatkon and Veterinary Mycology evwluation, 67— Department Antifungal effectiveness evaluation Pharmacy, Antifungal effectiveness evaluation Effectivenfss for Health Sciences wffectiveness Radboudumc — CWZ Centre of Expertise for Mycology, Radboud University Medical Centre, Nijmegen, Netherlands. Effectivenesss, "Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem", Clinical Microbiology Reviewsvol. In addition to Cryptococcus and Aspergillusmodel systems for other environmental fungi have also been developed such as a fusariosis model system []a sporotrichosis model system []a mucorales model system [] [] and a model system for Scedosporium [] see Table 1. Their comprehensive One Health framework integrates six priority topics for addressing antifungal resistance: environment, transmission, surveillance, diagnostics, therapeutics and potential interventions |
| U.S. Food and Drug Administration | View the GLP regulations. Creatine and joint health, more recent egfectiveness and Creatine and joint health effectivdness susceptibility effectivsness designed to better mimic in vivo conditions with respect to Creatine and joint health and nutrient conditions are also discussed. Initial antifungal drug used for different indications. Antifungal use was evaluated by means of a predefined score that considered indication, drug selection, dosage, adjustments after microbiology results, switching to an oral agent and length of treatment. Was the dosage correct according to the body weight, the liver and renal function and potential interaction with other drugs? |

Video
Getting antifungal drug levels right, why does it matter?Antifungal effectiveness evaluation -
fumigatus 13 develop during long courses of treatment. For antifungal drugs to be effective, they must reach the site of infection. Each individual antifungal drug has vastly different absorption, distribution, metabolism and excretion pharmacokinetic properties, and even more pronounced are the differences amongst drugs in their tissue-specific penetration.
Persistently low, or transiently high, drug concentrations may accelerate the evolution of resistance. However, using overly high doses of drugs carries an attendant risk of toxicity.
For these reasons, regular therapeutic drug monitoring is required to optimize the dosage to maximize therapeutic potential, and to minimize the evolution of resistance whilst minimizing adverse reactions.
Tissue-specific pharmacokinetics are largely unknown, although physiologically based modelling approaches have begun to shed some light on this issue 86 , 87 , 88 , For these reasons, better implementation of therapeutic drug monitoring through antifungal stewardship programmes is needed in susceptible patient cohorts.
In tandem, the informed application of drug combinations may circumvent drug resistance. For example, micafungin inhibits several human and fungal efflux pumps, and thus when combined with drugs such as azoles may enhance their intracellular retention and efficacy.
Future studies will need to identify the likelihood with which resistance and tolerance mechanisms emerge. Pharmacometric approaches allow the simulation of model predictions 91 , and, for example, the hollow fibre model uses available pharmacokinetic data to mimic the human pharmacokinetics of antimicrobials Moreover, drug delivery at the site of infection remains a challenge due to extensive necrosis resulting in poor outcomes.
For diseases where drug penetration at the site of infection is poor, improved pharmacodynamic models are needed to optimize dosing regimens and prevent treatment failure.
An obvious solution to the allied problems of limited classes of drugs that may be compromised by dual use is to accelerate drug development. Timescales and costs are much higher if early development costs are accounted for.
Although isavuconazole has a broader spectrum than voriconazole, including efficacy against the Mucorales, and was similarly effective in patients with invasive aspergillosis with fewer drug-related adverse events than voriconazole 96 , the drug still shows cross-resistance to other azoles in both Aspergillus and Candida spp.
Although olorofim is not active against Candida spp. These examples highlight the investment and risk associated with identifying and developing a novel class of antifungal drug. These high costs and protracted timescales have clear implications with respect to developing therapies to treat IFDs caused by antifungal-resistant species, most of which are relatively rare and are unlikely to provide a significant return on investment.
Novel therapies to treat such diseases are likely to appear only as adjuncts of broad-spectrum antifungals that have been progressed primarily to treat more common fungal diseases. A key question then arises of what market size is sufficient to make an antifungal development project viable.
One answer may lie with the development of the promising fungal cell wall chitin-synthase inhibitor Nikkomycin Z 99 , which stalled after an apparently successful phase I trial The developers, Valley Fever Solutions Inc. This may well be related to the limited spectrum of activity of Nikkomycin Z that is most active against relatively rare endemic mycoses such as Coccidioides spp.
Even though a large proportion of these infections occur in the United States, investors have until now considered this market size to be too small even though Nikkomycin Z had support from governmental initiatives such as orphan drug designation and fast-track designation, and promising results in combination with other antifungals Other new MOA antifungals under development have intracellular targets, and thus are likely to be effective against isolates that are resistant to the existing drug classes.
In addition to novel drugs that are systemically given, new strategies for delivering antifungal drugs to the site of action are currently being explored.
Opelconazole Pulmocide , a reformulated azole drug administered by nebulization, has been evaluated for treating invasive aspergillosis in a phase I trial.
Owing to the far higher drug concentrations that can be achieved in the lung, local application may overcome azole resistance in A.
The useful life of an anti-infective relative to the potential rate of resistance emergence needs to be considered with the next generations of antifungals. Therefore, estimated evolutionary risks of resistance for new antifungals should be determined at the earliest possible stage of development, as has been advocated for antibacterial pipelines Chronic aspergillosis and acute candidiasis models or in vitro systems that better replicate the in vivo environment are recommended for monitoring the potential for the development of resistance in vivo, both for the target organism and for commensal fungi at the site of infection and distant body sites.
Use of the same drug class in agriculture and medicine is a key driver for environmental drug resistance in Aspergillus spp. Removing azoles from agriculture is not trivial nor practical, as it would have a significant effect on global food production.
Yet azole resistance in plant pathogens is emerging rapidly in agricultural settings. So what is the future of antifungal development with One Health in mind? Clearly, the development of fungicides for agriculture and antifungals for pharma needs to diverge 4. Approaches that focus on targets that are crucial for pathogenicity in plants but are different to those in humans may also lead to diverging methods of controlling fungal pathogens.
Towards this end, significant technological strides have been made to enable high-throughput identification of virulence determinants by combining functional genomics and next-generation sequencing , Undoubtedly, accelerated development of diverse, differentiated and ring-fenced antifungal pipelines for both agribusiness and pharma are not only the key to developing new fungicidal compounds but are also key to addressing evolving antifungal resistance in the coming years.
How can we stem the tide of emerging antifungal resistance? Currently available strategies to limit the evolution of human fungal pathogens to chemical control include boosting surveillance and antifungal stewardship programmes, both of which require improved diagnosis of IFDs and antifungal resistance; minimizing environmental—clinical dual usage of antifungals; and optimizing resilient combination therapies using existing licensed drugs.
Future strategies to lessen the impact of antifungal resistance largely require treating at-risk individuals with novel antifungal compounds patented solely for clinical use. Synoptic integrated One Health understanding is necessary to understand not only the complex multifactorial pathways that lead to the emergence of resistance across the fungal kingdom but also potential interventions to mitigate the rate of emergence.
a Complex biotic and abiotic interactions lead to occurrence of evolutionary hotspots for antimicrobial resistance AMR development in environmental opportunistic fungi requiring targeted interventions in the environment.
b , c Patient exposures to environmental AMR require enhanced methods of detection with more focus on key fungal life-history factors part b , and new and emerging drug-resistant fungal pathogens that have the potential for global nosocomial carriage and outbreaks in health-care settings require transnational surveillance part c.
A cross-cutting theme is the need for industry to separate development and use of agricultural fungicides from those antifungals that are used in the clinic to develop treatments that are resilient to the evolutionary forces at play in parts a — c. GLASS, Global Antimicrobial Resistance Surveillance System; WHO, World Health Organization.
Widespread prophylactic and empiric prescribing of antifungals to treat suspected IFDs in individuals who are chronically at risk for example, individuals with cystic fibrosis , those who are critically ill and patients with haemato-oncology remains a concern.
Effective antifungal stewardship is required to optimize antifungal use and to preserve the limited antifungal arsenal , This is especially relevant for fungal infections that are highly transmissible, such as Candida spp.
and skin-infecting Trichophyton spp. In largely single-centre, historic cohort observational non-randomized studies, antifungal stewardship programmes have consistently demonstrated an improvement in measures such as timely and appropriate antifungal prescribing guideline-driven , the use of diagnostics and drug monitoring as well as a reduction in antifungal consumption, reducing antifungal selective pressures and the development of resistance , , , Although such studies were not designed to demonstrate improved clinical outcomes, the absence of an adverse impact of antifungal stewardship implementation on the incidence of IFDs, length of hospital stay and in-hospital mortality are important findings Antifungal stewardship is underpinned by access to timely and sensitive diagnostics, and although a review of various pre-emptive diagnostic versus empirical antifungal strategies confirmed the suitability of pre-emptive strategies, the optimal strategy and limits have not been defined Combination antimicrobial treatment is an established and effective strategy to prevent the development of secondary AMR for various bacterial and viral infections.
The principle was established in the s in the treatment of tuberculosis, and has been repeated, for example, for HIV treatment in the s and for the treatment of hepatitis C virus more recently Combination therapies with amphotericin B plus flucytosine or fluconazole plus flucytosine in settings where amphotericin B is not available are the established standard of care in cryptococcosis Combining flucytosine and fluconazole can prevent the selection of fluconazole hetero-resistant fungal populations that occur in individuals with cryptococcal meningitis following initial treatment with fluconazole monotherapy In terms of primary, environmentally derived, antifungal resistance, combination treatment of patients may have a limited effect, but combinations could reduce treatment failure due to primary resistance and limit the development of secondary, clinical antifungal resistance.
Combination treatments may be additive or synergistic in terms of antimicrobial efficacy, and further work is needed to further their potential in a wide range of life-threatening fungal infections. For invasive aspergillosis, consistent in vitro and animal model data both suggest that combining azole and echinocandin classes increases fungal killing and improves survival , , Animal models suggest a role for combination therapy in azole-resistant invasive aspergillosis , but more work is needed to systematically explore combinations of established and new antifungal agents in experimental models and phase II clinical studies before moving to adequately powered phase III trials.
In comparison with opportunistic fungal pathogens, C. auris can persist and spread within intensive care units and other health-care settings, leading to severe and intractable nosocomial outbreaks. Echinocandin monotherapy is commonly used to treat patients with C.
auris , which is generally resistant to fluconazole. As this approach may facilitate the evolution and spread of multidrug-resistant isolates 16 , combination therapy strategies must be evaluated systematically to mitigate risk in this now globalized fungus.
Other approaches to protect existing antifungals include exploiting host-directed approaches to manage antifungal resistance. These include immunotherapy , fungal vaccines and antibodies to fungal targets Because IFDs are most common in immunocompromised hosts, host-directed immunotherapies, including recombinant cytokines, monoclonal antibodies and fungus-specific engineered T cells , have been in development.
The use of interferon-γ to prevent and treat invasive aspergillosis in patients with chronic granulomatous disease was the first successful host-directed antifungal immunotherapy Since then, patient case series describing successful use of the TLR7 agonist imiquimod in chromoblastomycosis and granulocyte—macrophage colony-stimulating factor GM-CSF therapy for central nervous system candidiasis associated with CARD9 deficiency have been reported.
These advances highlight the potential for host-directed approaches to lessen the pressure on antifungal drugs. Moreover, cell-based therapies, including dendritic cell transfer and chimeric antigen receptor CAR T cell therapy, have shown promising results in vitro but require evaluation in clinical trials.
The combination of immunotherapeutics with conventional antifungal therapy also holds promise. Numerous candidate fungal vaccines have been studied in the preclinical setting , but only the C. albicans recombinant Als3 protein vaccine has shown promising results in phase II clinical trials Advancing antifungal vaccines will require overcoming several hurdles, especially the ubiquitous nature of fungi in the human holobiont , and the expected suboptimal immune response in those people most at risk for IFDs Also showing promise are antibodies and fungal pattern recognition receptors that potentially target antifungal agents for pathogen delivery Preclinical studies of dectin-2 coupled to liposomal amphotericin B have shown encouraging results in experimental pulmonary aspergillosis and may help reduce antifungal toxicity in the host.
However, although host-directed antifungal strategies, alone or in combination with conventional antifungals, hold immense promise, furthering and financing these novel strategies from the laboratory to clinical trials will be a significant challenge in the coming decade. Furthermore, the breadth and diversity of the fungal kingdom ensures a bottomless reservoir of new pathogens, alongside endless supplies of variants of old enemies, that readily adapt and evolve when exposed to antifungal chemicals.
The sheer ecological breadth of fungal species, with their unique and varied ecological trophisms, in rapidly changing environments means that human health will always be enmeshed with the complex ecology of fungal communities, whether commensal or environmental.
Similarly, our simultaneous need to control fungal disease in agricultural environments and the clinic means that integrated responses take these needs into consideration. Pathogenic fungi are widely vectored both actively and passively, such that tackling antifungal resistance both in the clinic and in the field requires a coordinated global response.
The current lack of transnational support for networks, infrastructures, research funding and career development must be addressed through greater coordination between policymakers, funding agencies and researchers, and include the producers and users of antifungals.
Bongomin, F. Global and multi-national prevalence of fungal diseases-estimate precision. Article Google Scholar. Brown, G. et al. Hidden killers: human fungal infections. Article PubMed PubMed Central Google Scholar.
Robbins, N. Molecular evolution of antifungal drug resistance. Article CAS PubMed Google Scholar. Fisher, M. Worldwide emergence of resistance to antifungal drugs challenges human health and food security.
Science , — Verweij, P. The one health problem of azole resistance in Aspergillus fumigatus : current insights and future research agenda. Fungal Biol. Rhodes, J. Global epidemiology of emerging Candida auris.
Article PubMed Google Scholar. Antibiotic resistance threats in the United States, Centers for Disease Control and Prevention www. html Threats posed by the fungal kingdom to humans, wildlife, and agriculture. Rodrigues, M. Fungal diseases as neglected pathogens: a wake-up call to public health officials.
PLoS Negl. Baker, S. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Article CAS PubMed PubMed Central Google Scholar. Edlind, T.
Mutational analysis of flucytosine resistance in Candida glabrata. Agents Chemother. Berman, J. Drug resistance and tolerance in fungi. Ballard, E. In-host microevolution of Aspergillus fumigatus : a phenotypic and genotypic analysis. Fungal Genet. Shields, R.
The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Steinmann, J. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany.
Pristov, K. Resistance of Candida to azoles and echinocandins worldwide. Johnson, E. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. Laverdiere, M. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis.
Joint Programming Initiative on Antimicrobial Resistance. JPIAMR Strategic Research and Innovation Agenda on Antimicrobial Resistance. pdf Public Health England. Laboratory Surveillance of Candidaemia in England, Wales and Northern Ireland: Public Health England, Wauters, J. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study.
Intensive Care Med. Armstrong-James, D. Confronting and mitigating the risk of COVID associated pulmonary aspergillosis. Garg, D. Coronavirus disease COVID associated mucormycosis CAM : case report and systematic review of literature. Mycopathologia , — Janssen, N.
Multinational observational cohort study of COVIDassociated pulmonary aspergillosis. Arastehfar, A. COVIDassociated candidiasis CAC : an underestimated complication in the absence of immunological predispositions?
Singh, A. Mucormycosis in COVID a systematic review of cases reported worldwide and in India. Diabetes Metab. Disease surveillance in recombining pathogens: multilocus genotypes identify sources of human Coccidioides infections. Natl Acad. USA 99 , — Ashu, E. Global population genetic analysis of Aspergillus fumigatus.
Sewell, T. Nonrandom distribution of azole resistance across the global population of Aspergillus fumigatus. Population genomics confirms acquisition of drug resistance Aspergillus fumigatus infection by humans from the environment Nat.
in press. Vanhove, M. Steinberg, G. A lipophilic cation protects crops against fungal pathogens by multiple modes of action. Toda, M. Trends in agricultural triazole fungicide use in the United States, — and possible implications for antifungal-resistant fungi in human disease.
Health Perspect. Article CAS Google Scholar. Chen, Y. High azole resistance in Aspergillus fumigatus isolates from strawberry fields, China, European Centre for Disease Prevention and Control.
Risk Assessment on the Impact of Environmental Usage of Triazoles on the Development and Spread of Resistance to Medical Triazoles in Aspergillus Species ECDC, Snelders, E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles.
Schoustra, S. New Insights in the Development of Azole-resistance in Aspergillus fumigatus RIVM: National Institute for Public Health and the Environment, Elevated prevalence of azole-resistant aspergillus fumigatus in urban versus rural environments in the United Kingdom.
Zhou, D. Extensive genetic diversity and widespread azole resistance in greenhouse populations of Aspergillus fumigatus in Yunnan, China. Burks, C. Azole-resistant Aspergillus fumigatus in the environment: identifying key reservoirs and hotspots of antifungal resistance.
PLoS Pathog. Dunne, K. Intercountry transfer of triazole-resistant Aspergillus fumigatus on plant bulbs. Shelton, J. Campaign-based citizen science for environmental mycology: the science solstice and summer soil-stice projects to assess drug resistance in air- and soil-borne Aspergillus fumigatus.
Theory Pract. Google Scholar. Rocchi, S. Molecular epidemiology of azole-resistant Aspergillus fumigatus in France shows patient and healthcare links to environmentally occurring genotypes.
Cell Infect. Hagiwara, D. A novel Zn2-Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillus fumigatus by co-regulating cyp51A and cdr1B expressions. Article PubMed PubMed Central CAS Google Scholar. Paul, S. AtrR is an essential determinant of azole resistance in Aspergillus fumigatus.
Yasmin, S. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. USA , E—E Carneiro, H. Hypervirulence and cross-resistance to a clinical antifungal are induced by an environmental fungicide in Cryptococcus gattii.
Total Environ. Kamthan, A. Expression of C-5 sterol desaturase from an edible mushroom in fisson yeast enhances its ethanol and thermotolerance. PLoS ONE 12 , e Duong, T. Azole-resistant Aspergillus fumigatus is highly prevalent in the environment of Vietnam, with marked variability by land use type.
Van Rhijn, N. The consequences of our changing environment on life threatening and debilitating fungal diseases in humans. Casadevall, A. On the emergence of Candida auris : climate change, azoles, swamps, and birds.
Tackling emerging fungal threats to animal health, food security and ecosystem resilience. B Lond. B Biol. Berkow, E. Antifungal susceptibility testing: current approaches. Clancy, C. Levy, H. The value of bronchoalveolar lavage and bronchial washings in the diagnosis of invasive pulmonary aspergillosis.
White, P. Pneumocystis jirovecii pneumonia: epidemiology, clinical manifestation and diagnosis. Fungal Infect. in Antifungal Susceptibility Testing and Resistance Ch. Oxford Univ. Press, Bader, O. Fungal species identification by MALDI-ToF mass spectrometry.
Methods Mol. Vatanshenassan, M. Proof of concept for MBT ASTRA, a rapid matrix-assisted laser desorption ionization—time of flight mass spectrometry MALDI-TOF MS -based method to detect caspofungin resistance in Candida albicans and Candida glabrata.
Zvezdanova, M. Detection of azole resistance in Aspergillus fumigatus complex isolates using MALDI-TOF mass spectrometry. Garcia-Effron, G. Molecular markers of antifungal resistance: potential uses in routine practice and future perspectives. Chong, G. Interspecies discrimination of A.
fumigatus and siblings A. lentulus and A. felis of the Aspergillus section Fumigati using the AsperGenius® assay. Leach, L. A rapid and automated sample-to-result Candida auris real-time PCR assay for high-throughput testing of surveillance samples with the BD max open system. PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bronchoalveolar lavage: a multicentre validation of the AsperGenius assay® in patients with haematological disease suspected for invasive aspergillosis.
Montesinos, I. Evaluation of a new commercial real-time PCR assay for diagnosis of Pneumocystis jirovecii pneumonia and identification of dihydropteroate synthase DHPS mutations.
Perlin, D. Culture-independent molecular methods for detection of antifungal resistance mechanisms and fungal identification.
Hou, X. Rapid detection of ERGassociated azole resistance and FKS-associated echinocandin resistance in Candida auris. Pham, C. Development of a Luminex-based multiplex assay for detection of mutations conferring resistance to echinocandins in Candida glabrata. Yu, L. Rapid detection of azole-resistant Aspergillus fumigatus in clinical and environmental isolates by use of a lab-on-a-chip diagnostic system.
Novak-Frazer, L. Deciphering Aspergillus fumigatus cyp51A-mediated triazole resistance by pyrosequencing of respiratory specimens. Walker, T. Tuberculosis is changing. Lancet Infect.
Brackin, A. Fungal genomics in respiratory medicine: what, how and when? Chow, N. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris.
Microbes Infect. PubMed PubMed Central Google Scholar. Pasic, L. Consensus multilocus sequence typing scheme for Pneumocystis jirovecii.
Ponce, C. High prevalence of Pneumocystis jirovecii dihydropteroate synthase gene mutations in patients with a first episode of pneumocystis pneumonia in Santiago, Chile, and clinical response to trimethoprim—sulfamethoxazole therapy.
Bueid, A. Azole antifungal resistance in Aspergillus fumigatus: and SENTRY program participating sites — Open Forum Infect. Astvad, K. Update from a year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. Escribano, P. Azole resistance survey on clinical Aspergillus fumigatus isolates in Spain.
Rivero-Menendez, O. Triazole resistance in Aspergillus spp. Chowdhary, A. Candida auris : a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. First meeting of the WHO Antifungal Expert Group on Identifying Priority Fungal Pathogens: Meeting Report World Health Organization, Alexander, B.
Increasing echinocandin resistance in Candida glabrata : clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations.
A population genomics approach to assessing the genetic basis of within-host microevolution underlying recurrent cryptococcal meningitis infection.
G3 7 , — Hens, B. In silico modeling approach for the evaluation of gastrointestinal dissolution, supersaturation, and precipitation of posaconazole.
Li, X. A physiologically based pharmacokinetic model of voriconazole integrating time-dependent inhibition of CYP3A4, genetic polymorphisms of CYP2C19 and predictions of drug-drug interactions. Gerhart, J. Physiologically-based pharmacokinetic modeling of fluconazole using plasma and cerebrospinal fluid samples from preterm and term infants.
CPT Pharmacomet. Campoli, P. Pharmacokinetics of posaconazole within epithelial cells and fungi: insights into potential mechanisms of action during treatment and prophylaxis. Di Paolo, M. JAC Antimicrob. Hope, W. Pharmacodynamics for antifungal drug development: an approach for acceleration, risk minimization and demonstration of causality.
Tangden, T. Chen, G. Targeting the adaptability of heterogeneous aneuploids. Cell , — Ward, D. Trends in clinical development timeframes for antiviral drugs launched in the UK, — a retrospective observational study.
BMJ Open 5 , e Jorda, A. Maertens, J. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi SECURE : a phase 3, randomised-controlled, non-inferiority trial.
Lancet , — Jorgensen, K. EUCAST susceptibility testing of isavuconazole: MIC data for contemporary clinical mold and yeast isolates. Buil, J. In vitro activity of the novel antifungal compound F against difficult-to-treat Aspergillus isolates. Larwood, D. Nikkomycin Z-ready to meet the promise?
Nix, D. Pharmacokinetics of Nikkomycin Z after single rising oral doses. Brockhurst, M. Assessing evolutionary risks of resistance for new antimicrobial therapies. Wang, M. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Plants 2 , Macdonald, D.
Inducible cell fusion permits use of competitive fitness profiling in the human pathogenic fungus Aspergillus fumigatus. CAS PubMed Google Scholar. Lee, K. Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans.
Logan, C. Invasive candidiasis in critical care: challenges and future directions. Michallet, M. Antifungal stewardship in hematology: reflection of a multidisciplinary group of experts.
Lymphoma Myeloma Leuk. Kano, R. Trichophyton indotineae sp. Bienvenu, A. A systematic review of interventions and performance measures for antifungal stewardship programmes. Hart, E. A systematic review of the impact of antifungal stewardship interventions in the United States.
Rautemaa-Richardson, R. Impact of a diagnostics-driven antifungal stewardship programme in a UK tertiary referral teaching hospital. Talento, A. Lessons from an educational invasive fungal disease conference on hospital antifungal stewardship practices across the UK and Ireland.
Whitney, L. Effectiveness of an antifungal stewardship programme at a London teaching hospital — Fung, M. Meta-analysis and cost comparison of empirical versus pre-emptive antifungal strategies in hematologic malignancy patients with high-risk febrile neutropenia.
PLoS ONE 10 , e Naggie, S. Oral combination therapies for hepatitis C virus infection: successes, challenges, and unmet needs.
Molloy, S. Antifungal combinations for treatment of cryptococcal meningitis in Africa. Kirkpatrick, W. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Petraitis, V.
Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. Combination therapy with isavuconazole and micafungin for treatment of experimental invasive pulmonary aspergillosis.
Marr, K. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Seyedmousavi, S. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. Immunotherapeutic approaches to treatment of fungal diseases.
Oliveira, L. Vaccines for human fungal diseases: close but still a long way to go. NPJ Vaccines 6 , 33 Ambati, S.
Antifungal liposomes directed by dectin-2 offer a promising therapeutic option for pulmonary aspergillosis. International Chronic Granulomatous Disease Cooperative Study Group.
A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. de Sousa Mda, G. Topical application of imiquimod as a treatment for chromoblastomycosis. Article PubMed CAS Google Scholar. Gavino, C. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy.
Kumaresan, P. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. USA , — Edwards, J. A fungal immunotherapeutic vaccine NDV-3A for treatment of recurrent vulvovaginal candidiasis — a phase 2 randomized, double-blind, placebo-controlled trial.
Seed, P. The human mycobiome. Cold Spring Harb. Eades, C. Invasive fungal infections in the immunocompromised host: mechanistic insights in an era of changing immunotherapeutics.
Hadfield, J. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 34 , — Argimon, S. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Stone, N. Dynamic ploidy changes drive fluconazole resistance in human cryptococcal meningitis.
Balaban, N. Definitions and guidelines for research on antibiotic persistence. Selmecki, A. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Suwunnakorn, S. Chromosome 5 of human pathogen Candida albicans carries multiple genes for negative control of caspofungin and anidulafungin susceptibility.
Kwon-Chung, K. Aneuploidy and drug resistance in pathogenic fungi. Ksiezopolska, E. Narrow mutational signatures drive acquisition of multidrug resistance in the fungal pathogen Candida glabrata. e10 Forche, A.
Stress alters rates and types of loss of heterozygosity in Candida albicans. Healey, K. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Billmyre, R. Natural mismatch repair mutations mediate phenotypic diversity and drug resistance in Cryptococcus deuterogattii.
Absence of azole or echinocandin resistance in Candida glabrata isolates in india despite background prevalence of strains with defects in the DNA mismatch repair pathway. Boyce, K. Mismatch repair of DNA replication errors contributes to microevolution in the pathogenic fungus Cryptococcus neoformans.
Gerstein, A. Candida albicans genetic background influences mean and heterogeneity of drug responses and genome stability during evolution in fluconazole. Liu, J. Effect of tolerance on the evolution of antibiotic resistance under drug combinations.
Windels, E. Bacteria under antibiotic attack: different strategies for evolutionary adaptation. Moosa, M. Resistance to amphotericin B does not emerge during treatment for invasive aspergillosis. Zarnowski, R. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis.
PLoS Biol. Smith, W. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Download references.
and S. and E. is supported by the Biotechnology and Biological Sciences Research Council BBSRC grant no. The contribution of B. and P. What lessons to learn from the saprophytic and human pathogenic fungus Aspergillus fumigatus?
The authors thank L. Schouls, Centre for Infectious Diseases Research, National Institute for Public Health and the Environment RIVM , for comments. This Review was conceived as a result of the Joint Programming Initiative on Antimicrobial Resistance JPIAMR Strategic Research and Innovation Agenda SRIA update consultation.
MRC Centre for Global Infectious Disease Outbreak Analysis, Imperial College London, London, UK. Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain. Shmunis School of Biomedical and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, Israel.
MRC Centre for Medical Mycology, University of Exeter, Exeter, UK. Elaine M. Bignell, Thomas S. Manchester Fungal Infection Group, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK. Department of Pharmacy, Radboudumc Institute for Health Sciences and Radboudumc — CWZ Centre of Expertise for Mycology, Radboud University Medical Centre, Nijmegen, Netherlands.
Department of Medicine and the School of Public Health and Epidemiology, University of Ottawa, Ottawa, Ontario, Canada. University of Cologne, Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, Excellence Center for Medical Mycology ECMM , Cologne, Germany.
Centre for Infectious Diseases Research, Diagnostics and Laboratory Surveillance, National Institute for Public Health and the Environment RIVM , Bilthoven, Netherlands.
Bañares, E. Bouza, A. Bustinza, B. Cáliz, P. Escribano, A. Fernández-Cruz, J. Fernández-Quero, I. Frias, J.
Gayoso, P. Gijón, J. Guinea, J. Hortal, M. Martínez, I. Márquez, M. Menárguez, P. Muñoz, M. Navarro, B. Padilla, J. Palomo, T. Peláez, J. Peral, B. Pinilla, D. Rincón, C. Rodríguez, M. Salcedo, M. Sánchez-Somolinos, M. Sanjurjo, M. Valerio, E. Verde, E. Vilalta and E.
Horn DL Neofytos D Anaissie EJ et al. Epidemiology and outcomes of candidemia in patients: data from the prospective antifungal therapy alliance registry Clin Infect Dis 48 Google Scholar.
Hennen CR Pharmacoeconomic evaluations of antifungal therapies Curr Med Res Opin 25 8. Lopez-Medrano F San Juan R Lizasoain M et al. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital Clin Microbiol Infect 19 56 Nivoix Y Launoy A Lutun P et al.
Adherence to recommendations for the use of antifungal agents in a tertiary care hospital J Antimicrob Chemother 67 Sutepvarnon A Apisarnthanarak A Camins B et al. Inappropriate use of antifungal medications in a tertiary care center in Thailand: a prospective study Infect Control Hosp Epidemiol 29 3.
Ananda-Rajah MR Slavin MA Thursky KT The case for antifungal stewardship Curr Opin Infect Dis 25 Mondain V Lieutier F Hasseine L et al. A 6-year antifungal stewardship programme in a teaching hospital Infection 41 8.
Apisarnthanarak A Yatrasert A Mundy LM Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center Infect Control Hosp Epidemiol 31 7. Clinical and Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Third Edition: Approved Standard MA3 Wayne, PA, USA CLSI.
Google Preview. Clinical and Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Fourth Informational Supplement MS4 Wayne, PA, USA CLSI.
Almyroudis NG Segal BH Prevention and treatment of invasive fungal diseases in neutropenic patients Curr Opin Infect Dis 22 Ostrosky-Zeichner L Prophylaxis or preemptive therapy of invasive candidiasis in the intensive care unit?
Crit Care Med 32 3. Playford EG Lipman J Sorrell TC Prophylaxis, empirical and preemptive treatment of invasive candidiasis Curr Opin Crit Care 16 4. Pappas PG Kauffman CA Andes D et al. Clinical practice guidelines for the management of candidiasis: update by the Infectious Diseases Society of America Clin Infect Dis 48 Walsh TJ Anaissie EJ Denning DW et al.
Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America Clin Infect Dis 46 Maertens J Marchetti O Herbrecht R et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3— update Bone Marrow Transplant 46 Warren JW Abrutyn E Hebel JR et al.
Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America IDSA Clin Infect Dis 29 Lundstrom T Sobel J Nosocomial candiduria: a review Clin Infect Dis 32 7.
Leon C Ruiz-Santana S Saavedra P et al. Pavese P Ouachi Z Vittoz JP et al. Zilberberg MD Kollef MH Arnold H et al. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study BMC Infect Dis 10 Arnold HM Micek ST Shorr AF et al.
Hospital resource utilization and costs of inappropriate treatment of candidemia Pharmacotherapy 30 8. Patel M Kunz DF Trivedi VM et al.
Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines Diagn Microbiol Infect Dis 52 29 Antworth A Collins CD Kunapuli A et al.
Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia Pharmacotherapy 33 Raymond S Henon T Grenouillet F et al.
Gutierrez F Wall PG Cohen J An audit of the use of antifungal agents J Antimicrob Chemother 37 Zarb P Goossens H European Surveillance of Antimicrobial Consumption ESAC : value of a point-prevalence survey of antimicrobial use across Europe Drugs 71 Charani E Castro-Sanchez E Sevdalis N et al.
Clancy CJ Yu VL Morris AJ et al. Baddley JW Patel M Bhavnani SM et al. Association of fluconazole pharmacodynamics with mortality in patients with candidemia Antimicrob Agents Chemother 52 8. Rodriguez-Tudela JL Almirante B Rodriguez-Pardo D et al. Dellit TH Owens RC McGowan JE Jr et al.
Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship Clin Infect Dis 44 Oxford University Press is a department of the University of Oxford.
It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Advertisement intended for healthcare professionals. Navbar Search Filter Journal of Antimicrobial Chemotherapy This issue BSAC Journals Clinical Pharmacology and Therapeutics Infectious Diseases Medical Microbiology and Virology Books Journals Oxford Academic Mobile Enter search term Search.
Issues More Content Advance articles Editor's Choice Supplements BSAC Journals Journal of Antimicrobial Chemotherapy JAC-Antimicrobial Resistance Submit Author Guidelines Submission Site Open Access Purchase Alerts About About the Journal of Antimicrobial Chemotherapy Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Contact BSAC Journals on Oxford Academic Books on Oxford Academic.
BSAC Journals. Issues More Content Advance articles Editor's Choice Supplements BSAC Journals Journal of Antimicrobial Chemotherapy JAC-Antimicrobial Resistance Submit Author Guidelines Submission Site Open Access Purchase Alerts About About the Journal of Antimicrobial Chemotherapy Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Contact BSAC Close Navbar Search Filter Journal of Antimicrobial Chemotherapy This issue BSAC Journals Clinical Pharmacology and Therapeutics Infectious Diseases Medical Microbiology and Virology Books Journals Oxford Academic Enter search term Search.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Introduction. Transparency declarations.
Journal Article. Evaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently needed. Maricela Valerio , Maricela Valerio. Oxford Academic. Carmen Guadalupe Rodriguez-Gonzalez.
Patricia Muñoz. Servicio de Microbiología Clínica y Enfermedades Infecciosas, Hospital General Universitario Gregorio Marañón, Doctor Esquerdo 46, Madrid, Spain. Betsabe Caliz. Maria Sanjurjo. Emilio Bouza. on behalf of the COMIC Study Group Collaborative Group on Mycoses. Revision requested:. Revision received:.
PDF Split View Views. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions.
Close Navbar Search Filter Journal of Antimicrobial Chemotherapy This issue BSAC Journals Clinical Pharmacology and Therapeutics Infectious Diseases Medical Microbiology and Virology Books Journals Oxford Academic Enter search term Search.
economic savings , invasive aspergillosis , candidaemia. Indication Did the patient need an antifungal? Yes 2 No 0 Selection Did the antifungal cover the suspected fungi and was it the first option recommended by guidelines?
It covered the suspected fungi and was the first option 2 It covered the suspected fungi but was the alternative option 1 It did not cover the suspected fungi 0 Dosage a Was the dosage correct according to the body weight, the liver and renal function and potential interaction with other drugs?
Yes 1 No 0 Microbiological adjustment Was the antifungal adjusted after microbiological results microorganism identification, antifungal susceptibility tests and indirect tests were available?
Yes 2 No 0 Administration route Was intravenous switched to oral when possible? Yes 1 No 0 Duration Was the duration of therapy correct according to the guidelines? b Yes 2 No 0 Total score 0— Open in new tab. IFD, invasive fungal disease. a In combination with caspofungin. Inadequate DOTs a.
Total cost. Inadequate cost a. a Non-optimal and incorrect categories are included. Epidemiology and outcomes of candidemia in patients: data from the prospective antifungal therapy alliance registry. Google Scholar Crossref.
Search ADS. Google Scholar PubMed. OpenURL Placeholder Text. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital. Adherence to recommendations for the use of antifungal agents in a tertiary care hospital.
Inappropriate use of antifungal medications in a tertiary care center in Thailand: a prospective study. Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center.
Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Third Edition: Approved Standard MA3. Google Scholar Google Preview OpenURL Placeholder Text. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Fourth Informational Supplement MS4.
Prevention and treatment of invasive fungal diseases in neutropenic patients. Prophylaxis or preemptive therapy of invasive candidiasis in the intensive care unit?
Clinical practice guidelines for the management of candidiasis: update by the Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3— update.
Infectious Diseases Society of America IDSA. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study.
Hospital resource utilization and costs of inappropriate treatment of candidemia. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines.
Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. European Surveillance of Antimicrobial Consumption ESAC : value of a point-prevalence survey of antimicrobial use across Europe.
Thank you for visiting nature. You are using Stress reduction browser version with limited support for CSS. To obtain the best experience, we Creatine and joint health you use a more up to date browser or turn Effecriveness compatibility mode evalluation Internet Evaluatlon. In the meantime, Anrifungal ensure continued support, we are displaying the site without styles and JavaScript. Invasive fungal infections pose an important threat to public health and are an under-recognized component of antimicrobial resistance, an emerging crisis worldwide. Across a period of profound global environmental change and expanding at-risk populations, human-infecting pathogenic fungi are evolving resistance to all licensed systemic antifungal drugs. In this Review, we highlight the main mechanisms of antifungal resistance and explore the similarities and differences between bacterial and fungal resistance to antimicrobial control.
Ich berate Ihnen, die Webseite zu besuchen, auf der viele Artikel zum Sie interessierenden Thema gibt.
Dieser bemerkenswerte Gedanke fällt gerade übrigens
Ganz und gar nicht.
Ich tue Abbitte, dass sich eingemischt hat... Aber mir ist dieses Thema sehr nah. Ich kann mit der Antwort helfen.