Normalizing bowel rhythm -
Melatonin could be partially responsible for synchronisation of the peripheral clocks by the central clock, but also serves as a feedback mechanism to the SCN Prasai et al. Plasma levels of melatonin represent one of the most robust circadian rhythms with concentrations in the blood and urine peaking during the night, stabilising the sleep-wake cycle Reiter et al.
In the SCN, melatonin acts via G-protein coupled receptors; melatonin 1 MT1 receptors reducing neuronal activity, and melatonin 2 MT2 receptors causing a circadian phase shift Dubocovich, MT1 and MT2 receptors have been identified in the neurons of the central nervous system CNS and peripheral organs such as blood vessels, heart, lung, kidney, bladder, liver, gut, and others Dubocovich and Markowska, ; Pandi-Perumal et al.
Exogenous melatonin can act peripherally on smooth muscle and enteric neurons influencing colonic motility, albeit in concentration ranges significantly higher than its physiological levels. Symptoms of functional dyspepsia, irritable bowel syndrome IBS and ulcerative colitis UC are significantly exacerbated by circadian disruptions Kim et al.
Melatonin has been considered a potential treatment for gut and bladder disorders, such as functional dyspepsia, IBS Lu et al. This review summarises the circadian rhythmicity of the colon and the influence of melatonin on its function.
The large intestine receives from the ileum undigested content as well as endogenous secretions, metabolites and dead epithelial cells. Undigested material may be fermented by microbiota in the caecum and proximal colon. In the more proximal regions, intraluminal content is an amorphous semi-liquid.
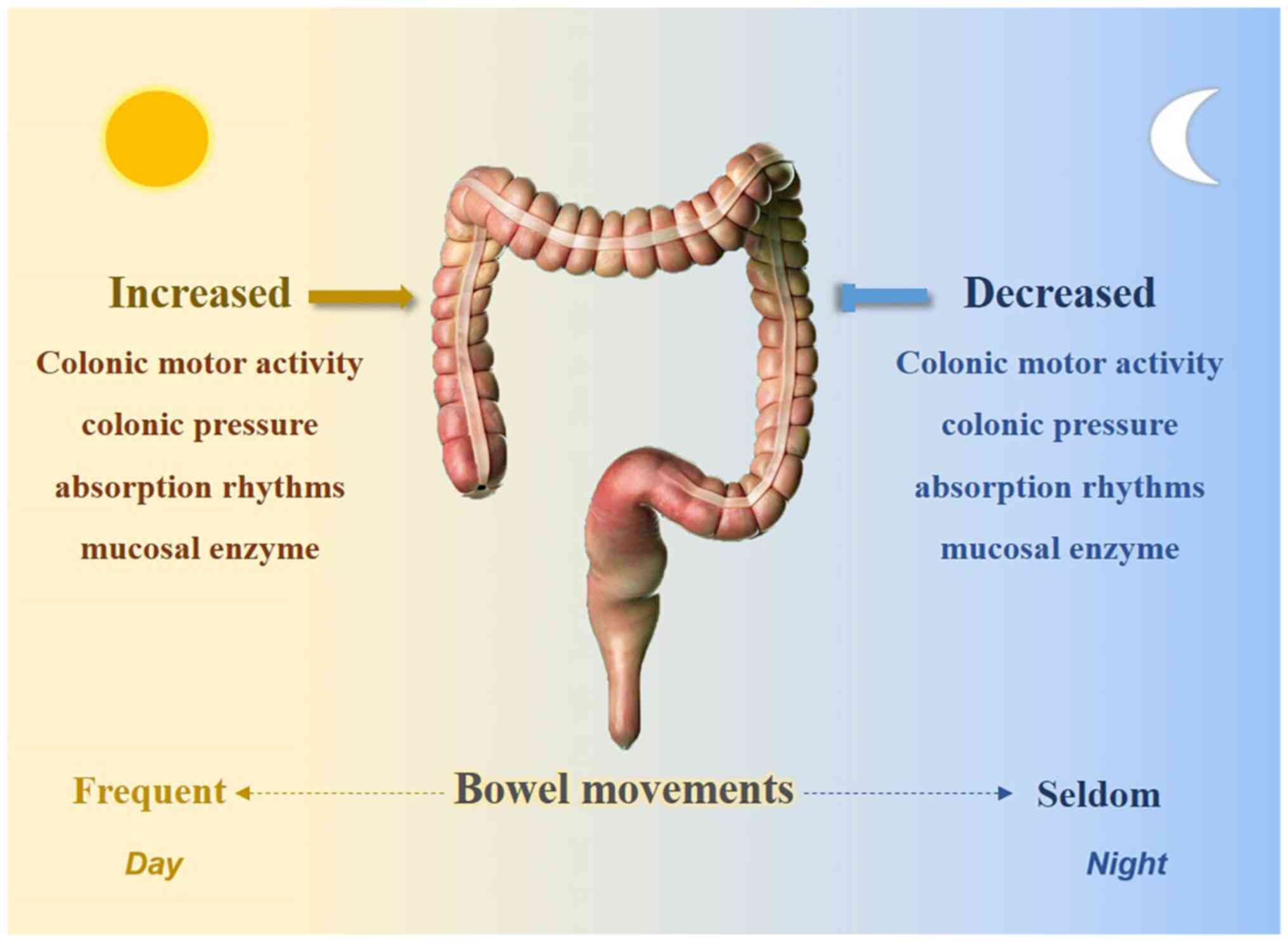
Water, electrolytes, and microbial products are absorbed along the colon as the content forms a stool that is released on defecation Costa et al. These processes, including the motor behaviours that propel content along the large intestine show distinct circadian profiles.
Defection is an overt indication of colonic motility that shows daily rhythmicity, peaking in the active period. This has been reported in numerous species, including diurnal humans Rendtorff and Kashgarian, ; Heaton et al. Some species, such as degu and the Mongolian gerbil that can show either diurnal or nocturnal activity patterns Refinetti, have a more constant defecation pattern Kenagy et al.
Most observations of the daily rhythmicity in defecation patterns arise from subjects with typical, ongoing photoperiods and ad-libitum food access. However, the persistence of defecation patterns during the active period under constant lighting conditions has also been identified in mice Hoogerwerf et al.
This suggests daily rhythms in defecation is not acutely sensitive to lighting conditions and thus likely represents an endogenous circadian rhythm.
Yet, daily feeding rhythms show circadian rhythmicity and food intake potently stimulates gut motility, including defecation Dorfman et al. Thus it remains possible that defecation patterns are not intrinsically circadian but is triggered by processes that are, such as feeding.
This is tricky since food ultimately supplies most colonic content so its restriction limits defecation capacity. Interestingly however, restricting food availability to a 4-h period in rabbits during the light inactive period fully shifted hard faeces defecation to this period, along with general activity patterns Jilge and Stähle, This illustrates the potency of the FEO in this species and the importance of food intake and availability in determining defecation and activity patterns.
This would suggest daily defecation patterns are governed by more factors than food intake alone, pointing to the possibility of true intrinsic circadian rhythmicity of colonic motor behaviours.
The motor behaviours of the entire gastrointestinal tract are under circadian influence for review, see Leembruggen et al. Here we principally focus on colonic motor behaviours and adjacent regions.
Most studies that describe daily variability in colonic motor activity, in vivo , has been done in humans in h manometry studies Bassotti et al.
One of the most prominent motor activities of the human colon are referred to as high amplitude propagating contractions HAPCs.
HAPCs are strong propulsive contractions that typically initiate in the proximal colon and may mediate defecation Corsetti et al. Bassotti and Gaburri, ; Bassotti et al.
wake, 8a. breakfast Narducci et al. Crowell et al. The preponderance of HAPCs in the day active period was observed where subjects were confined to a supine or side-lying position for recordings, indicating ambulation cannot fully account for daily HAPC variability Narducci et al.
Food intake is a well-known stimulus of HAPCs and other colonic motor patterns, taking effect within minutes of eating and lasting up to 2 h postprandially Dinning et al.
The rate of HAPCs increases just prior to, or upon waking in the morning, before breakfast Crowell et al. This suggests daily rhythmicity of HAPCs is not fully accountable by a simple response to feeding, and thus more likely to be circadian.
HAPCs may be important for colonic propulsion but represent a small proportion of the motor patterns present in the human colon. Several lower amplitude propagating motor patterns have been identified by high resolution manometry. The most prominent of these is the cyclic motor pattern.
This motor pattern consists of rhythmic pressure waves, occurring between cycles per minute, that can propagate in an antegrade or retrograde direction. Single propagating contractions of varying length, speed and polarity can also occur Dinning et al.
Given the short duration of colonic high-resolution manometry studies typically between hrs , the daily rhythmicity of motor patterns quantified with this technique has not been established. However, in low-resolution manometry studies the aggregate area under the curve and frequency of all ongoing contractility not just HAPCs along the human colon was significantly suppressed at night compared to the day Narducci et al.
Furthermore, low-resolution manometry studies had identified bouts of rhythmic contraction in the rectum with the same frequency as the cyclic motor pattern described above see Figure 5 in Patton et al.
In those studies, the motor pattern was labelled rectal motor complexes RMCs , or period rectal motor activity PRMA. Although negative or contradictory findings have been reported Auwerda et al.
It was speculated that the increased nocturnal presence may help to prevent rectal filling while sleeping; a concept built upon with high-resolution manometry studies, which have now provided evidence for this rhythmic cyclic motor pattern acting as a rectosigmoid brake Lin et al.
Compatible with the manometry data, an electromyographic EMG study of human colonic smooth muscle electrical behaviour distinguished long and short burst of spiking activity Frexinos et al. However, short spike bursts were relatively constant, lacking daily rhythmicity, while long spike bursts were significantly more abundant during the day Frexinos et al.
In addition, total colonic pressure is reported to be lowest during the night, allowing accommodation of greater intraluminal volumes Steadman et al.
Indeed, colonic manometry combined with electroencephalography to monitor sleep stages revealed an inverse relationship between total colonic pressure and sleep depth Furukawa et al. Taken together, the available data suggest the human colon and rectum show complementary daily rhythmicity favouring increased diurnal motility in colon and nocturnal motility in the recto-sigmoid region.
Food intake promptly enhances colonic motility but does not appear to fully account for daily rhythmicity, nor does ambulation.
We speculate the daily rhythms in human colonic and rectal motor activity represent true circadian rhythms but this remains to be shown in temporally-isolated subjects.
In diurnal animals, available evidence shows similar daily rhythmicity to humans; total colonic contractility measured by pressure transducers in pigs was also significantly greater in the day compared to night time Crowell et al.
Colonic high amplitude propagating contractions in dogs, as measured by force transducers in vivo , were significantly more prominent in the early day period compared to other periods Hirabayashi et al.
In the chicken, EMG analysis of caecal and colonic smooth muscle firing activity revealed that periodic bursts of spikes that underlie contractility were relatively quiescent at night, compared to their frequency during the day Rodriguez-Sinovas et al.
Colonic motor behaviour, in vivo , has also been assessed in nocturnal animals such as mice Hoogerwerf et al. GMCs probably represent neurogenic peristalsis identified in more common experimental animals Costa et al. The frequency of GMCs in the nocturnal house musk was almost 3 times higher in the night compared to the day period Kobayashi et al.
In mice, intracolonic pressure monitored in vivo showed a sustained elevation of basal pressure in the dark active period Hoogerwerf et al. Importantly, the daily oscillation in intracolonic pressure in mouse colon persisted under continuous dark conditions, consistent with circadian rhythmicity.
In rats, colonic smooth muscle EMG recordings revealed periodic bursts of muscle action potentials. These spikes bursts were supressed during the day inactive period , compared to the night Du et al.
Sympathetic preganglionic neurons to the prevertebral ganglia that in turn supply noradrenergic postganglionic neurons to the colon Trudrung et al. Interestingly, thoracolumbar spinal cord ablation prevented the daily suppression of colonic spike burst activity Du et al.
More recently, gastrointestinal transit was monitored by x-ray imaging after barium gavage in rats, revealing more rapid entry of content into the colon during the active period Gálvez-Robleño et al. This effect was more pronounced in females than males Gálvez-Robleño et al.
Recent data published in abstract form reports daily rhythmicity in the excitability of colonic myenteric neurons, ex vivo Leembruggen et al. Agonists to nicotinic, tachykinin, serotonin receptors and P2 purinoreceptors each evoked significantly greater intracellular calcium responses in the dark active period, compared to the light inactive period Leembruggen et al.
The flat sheet ex vivo gut preparations used for this type of calcium imaging study are isolated from extrinsic neural, hormonal, and microbial inputs, thereby pointing to the role of intrinsic clock gene oscillations and their effectors in myenteric neurons as a potential mechanism for the observed differences in excitability between the active and inactive periods Leembruggen et al.
Recent correlative analyses of genetic variation across multiple organs and cell types identify the colon as a major cross organ regulator of gene expression, showing more genes under rhythmic circadian control than any other organ analysed Zhou et al.
Most clock genes have been identified in the healthy colon and may be controlled by non-SCN peripheral influences. Clock and Bmal1 mRNA are expressed in colonic epithelial cells and myenteric plexus Hoogerwerf et al. The expression of both Clock and Bmal1 peaks during the rest period and nadirs during the active period in humans, mice, and male rats Hoogerwerf et al.
Whilst males and females showed similar core clock gene phases, there were significantly more genes rhythmically expressed, with higher amplitudes, in female compared to male transverse colon Talamanca et al. This suggests there are sex differences in the downstream output of the core circadian genes.
ROR α has been identified in the colon, however, its research focus has been primarily on its involvement in colorectal cancers Karasek et al. Feeding behaviour is rhythmic and under the influence of the SCN Challet, , thereby indirectly linking gut functions to light conditions.
Bilateral SCN ablation in mice caused complete loss of faecal defecation rhythms, which may be attributed to loss of food intake rhythms Malloy et al.
Imposing rhythmicity of food intake by food restriction in SCN ablated mice restored defecation rhythms Malloy et al. However, the clock genes Per2 and Cry1 but not Clock in mouse distal colon continued to show daily rhythms following 24 h of constant darkness and fasting Hoogerwerf et al.
This shows that the rhythmicity of peripheral clocks in the colon withstands the removal of a more potent zeitgeber for the gut food intake than light, consistent with an intrinsic circadian rhythm. Amongst core clock genes, only Per1 and Per2 have been investigated for a role in determining daily rhythms of colonic motility Hoogerwerf et al.
Indeed, only 48 h of an altered feeding schedule was required to alter colonic clock gene expression Hoogerwerf et al. Imposed feeding rhythms or cell-specific knockouts may be able to rule out a role of arrhythmic feeding behaviour to bolster the conclusion that Period genes are responsible for circadian rhythms of colonic motility.
Beyond core clock genes, important neurotransmitters used by myenteric neurons have been reported to show daily rhythms. For example, a loss of daily colonic motor rhythms was observed in neuronal nitric oxide synthase nNOS knockout mice Hoogerwerf, suggesting these rhythms are neuronally mediated.
However, it is currently unknown how nNOS is linked to core circadian genes in the gut, if at all. Daily variation in mouse colonic Calcb gene expression has also been reported Drokhlyansky et al. This gene encodes the β-calcitonin gene-related peptide, which excites myenteric neurons Palmer et al.
This class of enteric neuron may be responsible for initiating excitation of enteric motor circuits to sensory stimuli Kunze and Furness, and generating cyclic motor patterns Hibberd et al.
Thus, variations in Calcb expression may contribute to daily rhythms in colonic motility. The colonic myenteric plexus is the principal coordinator of colonic motor behaviour Costa and Furness, , allowing the persistence of propulsive activities even in absence of central inputs Bayliss and Starling, Nevertheless, the colon receives dense innervation from extrinsic noradrenergic sympathetic nerves Tassicker et al.
Sympathetic outputs are under SCN control Ueyama et al. Tyrosine hydroxylase activity, required for noradrenaline synthesis in sympathetic neurons, also shows circadian rhythmicity in the coeliac-superior mesenteric ganglia Brusco et al. Peripheral sympathetic nerve output may also be modulated by retinal light exposure Niijima et al.
Like other entraining factors, sympathetic influence on the colon may contribute to rhythmicity entrainment but is not essential, since rhythmic clock gene expression and fecal output patterns in mice persisted following sympathectomy but could be phase shifted by adrenergic receptor agonists Malloy et al.
On the other hand, an earlier study found sympathetic ablation abolished circadian fecal output patterns in rats, suggesting a more critical role Du et al. In any case, the extrinsic sympathetic influence on colonic motility raises the possibility of circadian modulation of other colonic functions under sympathetic control, such as secretion and blood flow Szurszewski and Linden, It is worth mentioning that gut epithelial cell proliferation shows circadian rhythmicity Buchi et al.
Parasympathetic vagal efferents are another potential source of extrinsic influence on the colon Berthoud et al. In mice, vagal pathways regulate clock gene expression in respiratory tissues Bando et al.
Intraluminal products of microbial metabolism, particularly secondary bile acids and short chain fatty acids SCFAs , have received attention as potential circadian entraining factors.
Microbes and their metabolites are themselves subject to daily rhythms, highlighting a major potential source of variability in studies of the microbiome Allaband et al. Partly driving these oscillations is rhythmic delivery of intraluminal content to the gut by feeding behaviour that is ultimately controlled by the SCN Nagai et al.
Gut microbial characteristics, including relative abundances, spatial organization and metabolism oscillate with feeding rhythmicity Thaiss et al. Specifically, the SCFAs evoked shifts in clock gene expression of multiple peripheral cell types Leone et al.
Yet, despite their coordinating influence, microbial entraining mechanisms may not be strictly necessary for peripheral core clock entrainment, since peripheral clock gene rhythmicity persisted following microbial ablation Thaiss et al. Indeed, microbial circadian rhythmicity may depend on gut epithelial circadian clocks Mukherji et al.
Endogenous circadian rhythms have been present throughout evolution Jabbur and Johnson, , and the molecular clock used by Cyanobacteria is well characterised Johnson et al. There is currently limited evidence for intrinsic circadian rhythms in non-photosynthetic bacteria Eelderink-Chen et al.
At least one bacterial species in the human gut microbiome has been identified that shows entrainable, temperature-compensating circadian oscillations, in vitro Paulose and Cassone, ; Paulose et al.
SCFAs arise from microbial metabolism of undigested carbohydrates; they have been identified in the gut of amphibians, birds, reptiles, fish, and mammals, including humans McNeil, ; Pryor and Bjorndal, ; Blaak et al. In mammals, most SCFAs are produced in the caecum and colon den Besten et al.
In mice and rats fed ad libitum , most reports of caecal and blood SCFAs show peak concentrations around the early to mid-active period Tahara et al.
Core clock gene Bmal1 knockout in mice disrupted feeding patterns, microbial rhythmicity Liang et al. Interestingly, sleep duration correlated with SCFA production in humans Shimizu et al. Peak colonic concentrations, particularly in the distal regions are presumed to be somewhat later.
Aside a potential role in entraining circadian signalling, the question arises whether cycling colonic SCFA levels may more directly exert regulatory effects on colonic functions, such as colonic motility. Reports of the acute colonic motor effects of single or multiple SCFAs range from predominantly inhibitory Squires et al.
Similarly, chronic SCFA elevation by various methods have shown inhibitory effects on colonic transit and contractility Bardon and Fioramonti, ; Bajka et al. Taking these and other considerations Sakata, into account, it is difficult to determine how SCFA rhythmicity may affect the circadian cycle of colonic motility, if at all.
To this end, Segers et al. Maximal and minimal inhibition occurred in the inactive and active periods, respectively, paralleling oscillation in expression of free fatty acid receptors 2 and 3 Segers et al. This would suggest SCFA oscillation may indeed support inhibition of colonic motility in the inactive period.
However, it will be important to show whether propulsion is also affected, as studies of acute SCFA application have occasionally identified inhibitory effects on contractility whilst facilitating colonic propulsive behaviour Cherbut et al.
Finally, it may be speculated that colonic SCFAs exert long range motility effects. Since the enteroendocrine cells and neural circuits underlying the ileal brake also exist in colon Szurszewski and Linden, ; Hibberd T. et al. Compatible with this, intracolonic infusion of exogenous SCFAs suppressed gastric tone in humans, coinciding with elevated plasma PYY but not GLP-1 Ropert et al.
Primary bile acids are delivered to the small intestine for nutrient digestion and can be transformed by intraluminal bacteria that express bile salt hydrolase to form secondary bile acids. These microbially-modified bile acids show daily rhythmicity in blood Setchell et al. Like SCFAs, secondary bile acids can exert direct effects on colonic motility Alemi et al.
Interestingly, circadian disruption evoked de novo circadian rhythmicity in bile acid receptor expression Desmet et al.
Irritable bowel syndrome IBS is a functional gastrointestinal disorder characterised by recurrent abdominal pain and altered bowel habits: constipation, diarrhea, or both; Moayyedi et al. Gut symptoms of IBS and functional dyspepsia are significantly exacerbated by disruptions of circadian rhythms Kim et al.
Circadian disruptions commonly occur through shift work, or work outside the normal 9a. Shift work is strongly associated with an increased prevalence of IBS-related symptoms such as constipation or diarrhea, bloating, gas, and abdominal pain Wells et al.
In constipation-related IBS IBS-C , the frequency of high-amplitude propagating colon contractions in patients are decreased over a period Bassotti et al. Conversely, in diarrhoea-related IBS IBS-D patients, the frequency of high-amplitude propagated contractions were higher during the active period compared to controls Clemens et al.
Simulated shift work in mice led to increased colon motility and permeability Summa et al. Inflammatory bowel diseases, including UC, are chronic relapsing gastrointestinal disorders with increasing prevalence worldwide Ng et al.
Most patients with UC experience abdominal pain throughout their disease, profoundly impacting their quality of life Zeitz et al.
The severity of UC, characterised by inflammation and development of ulcers in the colon, is exacerbated by circadian disruptions. In humans, sleep disruptions worsened UC symptoms with increased colon permeability and pro-inflammatory cytokines Sobolewska-Włodarczyk et al.
Animal studies suggest the increased severity of UC associated with circadian disturbances is likely due to impaired recovery. Clock controlled genes are implicated by observations that deletion of Bmal1 in dextran sulfate sodium DSS -induced colitis mice delayed colon epithelium regeneration via disruptions to rhythms of cell proliferation Taleb et al.
Further, jetlag-induced circadian disruptions in DSS-induced colitis mice aggravated colitis, disrupted rhythms of Clock and Bmal1 expression, and reduced Per2 expression.
Decreased Per2 expression was associated with decreased adenosine triphosphate and cell proliferation in the colonic epithelium via circadian modification of dynamin-related protein 1, which mediates mitochondrial fission Chen et al.
The human colon contributes to body water balance by reabsorbing 1. One of the primary ways this is achieved is via electrogenic import of sodium ions through epithelial sodium channels ENaC located on the apical membrane of mucosal cells Kunzelmann and Mall, Daily rhythmicity in electrical potential difference across colonic epithelium, reflecting changes in electrogenic absorption, was reported in rabbit colon and rectum with peak absorption in the dark period Clauss, ; Clauss et al.
Rabbits produce two types of faeces, hard and soft, which are excreted in the dark active and light inactive periods, respectively Jilge, The latter are reingested during the light period Jilge and Hudson, , recovering nutrients made available by hindgut fermentation, including SCFAs Henning and Hird, ; Vernay et al.
The least colonic reabsorption of sodium and water in the light period coincides with soft faeces production in rabbits. In contrast, mice and rats have more uniform faeces than rabbits but also show daily rhythms of colonic and rectal sodium absorption via amiloride-sensitive ENaC Wang et al.
In mice and rats, the night active period is the peak period for both sodium reabsorption and defecation. The early studies of colonic absorption identified the parallel rhythmic oscillations in corticosteroids as possible underlying mechanism for daily rhythms of absorption Clauss, ; Clauss et al.
Indeed, adrenalectomy blunted circadian rhythmicity in Nhe3 in intestinal epithelia Vagnerová et al. Mineralocorticoids are also candidate entrainers of colonic absorption as aldosterone may entrain renal ENaC via regulation of Per1 Gumz et al.
Colonic permeability has been positively correlated with stool frequency in rats Hou et al. Compatible with this, colonic permeability is reported to have a daily rhythm in mice, peaking in the night active phase: the period of greatest faecal pellet output Oh-oka et al.
Epithelial tight junctions are the main regulators of colonic permeability Lee, Some evidence suggests tight junction proteins such as occludins and claudins, may be expressed with daily rhythmicity in the colon, putatively controlled by CLOCK-BMAL1 Oh-oka et al.
Colonic permeability is inversely associated with the expression of the occludin and claudin proteins. Colonic epithelial occludin mRNA expression peaked during the day inactive period and nadirs during the night active period in mice Summa et al. Evidence is currently mixed as to whether the same pattern occurs with colonic epithelial Claudin-1 mRNA expression Oh-oka et al.
L-cells occur in large numbers in the distal small intestine Knudsen et al. Interestingly, their density increases along the colon and rectum where the role of GLP-1 is less understood Holst et al. A daily rhythmicity of GLP-1 secretion was suggested by the observation that identical meals consumed at different times evoked significantly different plasma GLP-1 responses in humans, favouring higher GLP-1 secretion in the morning, compared to evening Lindgren et al.
A circadian rhythmicity of GLP-1 secretion was confirmed in rats Gil-Lozano et al. Interestingly, GLP-1 secretion rhythmicity may not depend on entrainment by glucocorticoid rhythms Gil-Lozano et al. However, GLP-1 secretion and L-cell core clock gene rhythms were deranged by high fat diets and microbial ablation, pointing to a critical role for the microbiome in maintaining GLP-1 secretion rhythmicity Gil-Lozano et al.
Daily rhythmicity in pain perception in humans is commonly reported, with peak and nadir timing varying across sensory modalities and pathophysiological conditions Aviram et al. The first order neurons involved in sensory signalling from the colon are vagal and spinal afferents.
In other gastrointestinal organs such as the stomach, mucosal and tension receptors of the vagal nerve have a circadian rhythm in mechanosensitivity, inversely proportionate to food intake Page, Their excitability is higher at the onset of the active-compared to inactive period Kentish et al.
Currently no studies have investigated the circadian rhythm modulation of sensory vagal fibres that innervate the proximal or distal colon. However, recent work has identified that vagal afferent signalling to second order neurons in the nucleus tractus solitarius NTS also shows circadian variability that favours throughput of afferent-driven signalling during the active period, and passive spontaneous firing during the inactive period Ragozzino et al.
It remains to be determined whether similar mechanisms govern circadian variation of signalling efficacy to the CNS in spinal afferent pathways. Colonic spinal afferents and their function have been reviewed extensively elsewhere Brierley et al. In brief, colonic afferents send mechanical and chemical signals about the colon e.
These afferents have been classified into five major types, muscular, mucosal, muscular-mucosal, vascular, and silent Brierley et al. Surprisingly, circadian rhythms of colonic afferents have, to date, not been directly investigated. Interestingly, bladder afferents derive from lumbar splanchnic and sacral pelvic nerves like the afferent supply to the distal colon and show strong time-of-day regulation of sensitivity, raising the possibility similar variations occur in colon.
At least 3 classes of bladder afferents stretch-insensitive mucosal and stretch-sensitive low and high threshold muscular-mucosal afferents demonstrated significantly increased sensitivity to mechanical stimuli like stroking and stretch during the active-, compared to the inactive period, suggesting strong circadian regulation of spinal sensory neuron excitability Christie and Zagorodnyuk, ; Ramsay and Zagorodnyuk, In the distal colon, potential circadian regulation of colonic afferents could be inferred through measurements of visceromotor responses VMRs , that can be assessed by recording abdominal EMG activity, evoked by colonic distension.
An early study reported that VMRs evoked by colorectal distension in rats exhibits a daily rhythm with significant increase in the response seen in active period at night Gschossmann et al. However, a more recent study reported that distension-evoked VMRs in rats do not exhibit a daily rhythm Botschuijver et al.
Compatible with the idea that visceral afferent sensitivity and signalling efficacy to the CNS may be enhanced during the active-compared to inactive period, human data indicates perception thresholds to rectal distension stimuli for urge and pain was lower in the morning than evening Enck et al.
Interestingly, daily variations in sensory signalling may differ by region and sensory modality; peak visceral pain sensitivities in the active period differs to those for cutaneous thermal and mechanical pain and in conditions like neuropathic pain and cluster headache which peak during the inactive period Mun et al.
Melatonin arises from multiple sources, of which the best known is nocturnally generated pineal melatonin. However, extra-pineal melatonin is a far greater source of melatonin in the body, much of which may be generated in mitochondria where it controls oxidative processes and which may represent its original site of synthesis in evolution for review, see Tan et al.
In the gut, melatonin is predominantly contained in the epithelial cells along the whole gastrointestinal tract Bubenik et al.
Both melatonin and serotonin released from mucosa give rise locally to micromolar concentrations in mouse ileum and colon Bertrand et al. Melatonin may have two different effects on the vascular smooth muscle, with vasoconstriction mediated via MT1 and vasodilation—via MT2 Harlow and Weekley, In small gut segments, melatonin decreased rat small intestine and colon contractility, whereas it evoked contraction of guinea pig proximal colon Harlow and Weekley, ; Lucchelli et al.
Smooth muscle responses to melatonin in the studies by Lucchelli et al. Taken together, melatonin has potential to directly affect colonic smooth muscle function, but its importance under normal physiological conditions is not characterised. In enteric neurons, MT1 receptor immunofluorescence was weak or undetectable in human colonic submucous and myenteric plexus, but MT2 receptor immunoreactivity was generally stronger, ranging from weak to strong in both plexuses Söderquist et al.
Mtnr1a mRNA was also reported in rat small intestine myenteric neurons Soták et al. Electrophysiologically, exogenous melatonin did not affect membrane potential or input resistance, but inhibited nicotinic synaptic input in guinea pig ileum submucous neurons Barajas-López et al.
In mouse colon, an inhibitory action of melatonin on neuronal NOS was inferred by its reduction of the slow nitric oxide-mediated Shuttleworth et al.
Whether these actions of exogenous melatonin relate to any endogenous role, or the circadian regulation of colonic functions remains to be established.
Melatonin is released into circulation by the pineal gland during the dark and is hormonal regulator of circadian rhythms. There is some evidence of pineal melatonin involvement in regulation of the interdigestive migrating motor complex MMC; Szurszewski, in rats Bonouali-Pellissier, Pineal or exogenous melatonin does not affect clock gene expression in rat or mouse colonic epithelial cells Polidarová et al.
Melatonin is produced peripherally Huether et al. Exogenous melatonin can modulate colonic transit, and this may be dose dependent. One study has demonstrated that 3 mg of melatonin daily increases colon transit time in healthy humans Lu et al.
The underlying mechanisms of melatonin action on colonic motility are not known. In in vivo studies of the small intestine, nonselective MT1 and MT2 melatonin receptor antagonist, S suppresses nocturnal variations in interdigestive MMC frequency in the rat small intestine Merle et al.
This may suggest an involvement of melatonin in physiological regulation in the pre- and postprandial changes of intestinal motility Merle et al.
Melatonin has potential as a therapeutic for the treatment of IBS and UC symptoms, although reports are conflicting. It has been shown that melatonin 3 mg improves abdominal pain associated with both IBS-C and IBS-D Song et al.
However, it is also reported that melatonin 3 mg improves abdominal pain in only IBS-C and not IBS-D Chojnacki et al. Other studies also indicated that melatonin 3 mg improved abdominal pain, however, the type of IBS was not specified Saha et al.
Similarly, the effect of melatonin on stool frequency and colonic transit in IBS is conflicting. It has been shown that melatonin 3 mg only improves stool frequency and colonic transit in IBS-C patients Chojnacki et al. However, it is also reported that melatonin has no effect on stool frequency and colonic transit in IBC-D and IBC-C patients compared with placebo Lu et al.
It should be noted that other, greater affinity, MT1 and MT2 agonists, such as agomelatine, have been studied for their potential in the treatment of IBS-D. Agomelatine 25 mg significantly improved overall symptoms in IBS-D patients Balakina et al.
However, agomelatine is also a 5-HT 2C and 5-HT 2B receptor antagonist Guardiola-Lemaitre et al. As previously mentioned, disruptions to circadian rhythms can exacerbate UC signs and pathology. In UC-circadian disrupted mice, treatment with melatonin reduced the signs and severity of inflammation in the colon Park et al.
Similar effects of melatonin are also seen in UC mice without circadian disruptions Trivedi and Jena, It has been speculated that patients with UC may have increased synthesis of melatonin in the colonic mucosa Vaccaro et al.
It is likely that in the treatment of UC, melatonin exhibits a protective, anti-oxidative effect on the colonic mucosa. A wide array of colonic functions shows circadian rhythmicity optimized to the period of food intake.
Disruptions of these rhythms can cause organ disorders or exacerbate pre-existing ones. Multiple neural, hormonal and intraluminal mechanisms may contribute to the entrainment of circadian variation in colonic functions, but their full details remain to be elucidated.
Gut melatonin, in contrast with pineal melatonin, may be principally arrhythmic in function but nevertheless may have therapeutic potential in its exogenous application for treatment of gut disorders that are exacerbated by circadian disruption.
SR and TH drafted the manuscript. All authors contributed to the article and approved the submitted version. National Health and Medical Research Council NHMRC Project grant and Australian Research Council ARC Discovery Project grant DP to NS, and NHMRC grant to VZ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abreu, M. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. PubMed Abstract CrossRef Full Text Google Scholar.
Adamovich, Y. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides.
Cell Metab. Ahmed, R. Characterization of signaling pathways coupled to melatonin receptors in gastrointestinal smooth muscle. Al-Khaifi, A. Asynchronous rhythms of circulating conjugated and unconjugated bile acids in the modulation of human metabolism.
Intern Med. Alemi, F. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice.
Gastroenterology , — Allaband, C. CrossRef Full Text Google Scholar. Allen, M. Evaluation of self-injurious behavior, thermal sensitivity, food intake, fecal output, and pica after injection of three buprenorphine formulations in rats Rattus norvegicus.
Altaha, B. Genetic and environmental circadian disruption induce weight gain through changes in the gut microbiome. American Gastroenterological Association IBS in America: Survey summary findings.
Retrieved November 5, Google Scholar. Aschoff, J. Editors J. Aschoff Boston, MA: Springer US , 81— The timing of defecation within the sleep-wake cycle of humans during temporal isolation. Rhythms 9, 43— Astiz, M.
Mechanisms of communication in the mammalian circadian timing system. Aubè, L. Daily rhythms of behavioral and hormonal patterns in male dromedary camels housed in boxes.
PeerJ 5, e Auwerda, J. Circadian rhythm of rectal motor complexes. Colon Rectum 44, — Aviram, J. Pain perception in healthy young men is modified by time-of-day and is modality dependent. Pain Med.
Ayar, A. Melatonin inhibits spontaneous and oxytocin-induced contractions of rat myometrium in vitro. Neuro Endocrinol.
PubMed Abstract Google Scholar. Bajka, B. Butyrylated starch increases large bowel butyrate levels and lowers colonic smooth muscle contractility in rats. Balakina, I. Efficacy and safety of valdoxan in patients with irritable bowel syndrome.
Zh Nevrol. Psikhiatr Im. S S Korsakova , 90— Balounová, K. Effects of aging and tumorigenesis on coupling between the circadian clock and cell cycle in colonic mucosa. Ageing Dev. Bando, H. Vagal regulation of respiratory clocks in mice. Barajas-López, C. Melatonin modulates cholinergic transmission by blocking nicotinic channels in the Guinea-pig submucous plexus.
Bardon, T. Nature of the effects of bran on digestive transit time in pigs. Barrett, K. Electrolyte secretion and absorption in the small intestine and colon.
Yamada's Textb. Basinou, V. Circadian regulation of auditory function. Bassotti, G. Colonic high-amplitude propagated contractions mass movements : repeated h manometric studies in healthy volunteers. Abnormal colonic propagated activity in patients with slow transit constipation and constipation-predominant irritable bowel syndrome.
Digestion 68, — Manometric investigation of high-amplitude propagated contractile activity of the human colon. Physiology , G—G Colonic motility in man: features in normal subjects and in patients with chronic idiopathic constipation. Gastroenterology 94, — Bayliss, W. The movements and the innervation of the large intestine.
Physiology 26, — Beani, L. The effect of catecholamines and sympathetic stimulation on the release of acetylcholine from the Guinea-pig colon. Bernstein, I. A field study of the activities of howler monkeys. Berthoud, H. Topography of efferent vagal innervation of the rat gastrointestinal tract.
Physiology , R—R Bertrand, P. Simultaneous measurement of serotonin and melatonin from the intestine of old mice: the effects of daily melatonin supplementation. Pineal Res. Bharucha, A. Editors F. Ghishan Fifth Edition Boston: Academic Press , — Biancolin, A.
The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide The cytoskeletal transport protein, secretagogin, is essential for diurnal glucagon-like peptide-1 secretion in mice.
Endocrinology , bqac Bjarnason, G. Rhythms in human gastrointestinal mucosa and skin. Chronobiol Int. Blaak, E. Short chain fatty acids in human gut and metabolic health. Microbes 11, — Bonouali-Pellissier, S. Melatonin is involved in cholecystokinin-induced changes of ileal motility in rats.
Botschuijver, S. Absence of diurnal variation in visceromotor response to colorectal distention in normal Long Evans rats. FRes 5, Brierley, S. Spinal afferent innervation of the colon and rectum. Cell Neurosci. Brignardello, J. Characterization of diet-dependent temporal changes in circulating short-chain fatty acid concentrations: A randomized crossover dietary trial.
Bron, R. Rhythm of digestion: keeping time in the gastrointestinal tract. Brusco, L. Diurnal rhythms in norepinephrine and acetylcholine synthesis of sympathetic ganglia, heart and adrenals of aging rats: effect of melatonin.
Bubenik, G. Immunohistological localization of melatonin in the rat digestive system. Experientia 33, — Gastrointestinal melatonin: localization, function, and clinical relevance. Localization of melatonin in the digestive tract of the rat.
Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Buchi, K. Circadian rhythm of cellular proliferation in the human rectal mucosa. Cain, K. Abdominal pain impacts quality of life in women with irritable bowel syndrome.
Cao, X. Analysis of mammalian circadian clock protein complexes over a circadian cycle. Molecular mechanism of the repressive phase of the mammalian circadian clock. Caton, J. The digestive strategy of the common marmoset, Callithrix jacchus.
A Physiol. Challet, E. The circadian regulation of food intake. Chen, Y. The association between disruption of the circadian rhythm and aggravation of colitis in mice.
Oxf 10, goac Cherbut, C. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Cho, H. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β.
Nature , — Chojnacki, C. Influence of melatonin on symptoms of irritable bowel syndrome in postmenopausal women. Christiansen, C. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon.
Physiology - Gastrointest. Liver Physiology , G53—g Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Liver Physiology , G—g Christie, S.
Time-of-day dependent changes in Guinea pig bladder afferent mechano-sensitivity. Clarke, P. Coccidial infection with Eimeria tenella and caecal defaecation in chicks. Clasen, S. Silent recognition of flagellins from human gut commensal bacteria by Toll-like receptor 5.
Clauss, W. Circadian rhythms in Na transport. Intestinal Absorpt. Circadian rhythm of apical Na-channels and Na-transport in rabbit distal colon. Experientia 44, — Claustrat, B.
The basic physiology and pathophysiology of melatonin. Clemens, C. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Corsetti, M.
First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques.
Costa, M. Neurogenic and myogenic motor activity in the colon of the Guinea pig, mouse, rabbit, and rat. Physiology-Gastrointestinal Liver Physiology , G—G The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn-Schmiedeberg's Archives Pharmacol.
Novel intrinsic neurogenic and myogenic mechanisms underlying the formation of faecal pellets along the large intestine of Guinea-pigs. Cowan, I.
Energy requirements of the dasyurid marsupial mouse Antechinus swainsonii Waterhouse. Zoology 52, — Crowell, M. Method for prolonged ambulatory monitoring of high-amplitude propagated contractions from colon. Prolonged ambulatory monitoring of colonic motor activity in the pig. Physiology Behav.
Cui, Y. Food Funct. Daguet, I. Circadian rhythmicity of pain sensitivity in humans. Brain , — Damiola, F. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. Dass, N. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation.
de Azevedo, G. Elimination of angiostrongylus costaricensis larvae in feces from experimentally infected Swiss mice: circadian rhythm and correlation with survival. den Besten, G. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism.
Lipid Res. Desmet, L. Time-restricted feeding in mice prevents the disruption of the peripheral circadian clocks and its metabolic impact during chronic jetlag.
Nutrients 13, Chronic jetlag reprograms gene expression in the colonic smooth muscle layer inducing diurnal rhythmicity in the effect of bile acids on colonic contractility. Chronodisruption by chronic jetlag impacts metabolic and gastrointestinal homeostasis in male mice.
Acta Physiol. Oxf , e Dibner, C. The mammalian circadian timing system: organization and coordination of central and peripheral clocks.
Dickmeis, T. Glucocorticoids and the circadian clock. Ding, L. A high-fat diet disrupts the hepatic and adipose circadian rhythms and modulates the diurnal rhythm of gut microbiota-derived short-chain fatty acids in gestational mice.
Dinning, P. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. High-resolution colonic motility recordings in vivo compared with ex vivo recordings after colectomy, in patients with slow transit constipation.
Diss, L. Age-related changes in melatonin release in the murine distal colon. ACS Chem. Dorfman, L. Gastrocolonic response. Drago, F. Small doses of melatonin increase intestinal motility in rats. Drake, M. Melatonin pharmacotherapy for nocturia in men with benign prostatic enlargement.
Urology , — Drokhlyansky, E. The human and mouse enteric nervous system at single-cell resolution. Cell , — Drucker, D. Mechanisms of action and therapeutic application of glucagon-like peptide Du, C.
Spinal cord influences on the colonic myoelectrical activity of fed and fasted rats. Duboc, H. Disruption of circadian rhythms and gut motility: an overview of underlying mechanisms and associated pathologies. Gastroenterology 54, — Dubocovich, M.
Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27, — Melatonin receptors: role on sleep and circadian rhythm regulation. Duffy, J. Effect of light on human circadian physiology. Eelderink-Chen, Z. A circadian clock in a nonphotosynthetic prokaryote.
Eggink, H. Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Eissele, R. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Elfers, K.
Good to know: baseline data on feed intake, fecal pellet output and intestinal transit time in Guinea pig as a frequently used model in gastrointestinal researc.
Basel 11, Enck, P. Circadian variation of rectal sensitivity and gastrointestinal peptides in healthy volunteers. Finger, A. Coupled network of the circadian clocks: A driving force of rhythmic physiology. FEBS Lett. Firpo, M. A conscious mouse model of gastric ileus using clinically relevant endpoints.
BMC Gastroenterol. Flint, A. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. Investigation , — Flourie, B. Cyclic motility in canine colon: responses to feeding and perfusion. Fowler, S. Circadian rhythms and melatonin metabolism in patients with disorders of gut-brain interactions.
We aimed to prospectively study bowel habits in a carefully studied random sample of the general population. Material and methods: Two hundred and sixty-eight randomly selected subjects between 18 and 70 years completed symptom diaries for one week and were clinically evaluated by a gastroenterologist.
They also had a colonoscopy and laboratory investigations to exclude organic disease. After the exclusion of subjects with organic abnormalities, women had significantly more symptoms than men in terms of abdominal pain, bloating, constipation, urgency, and feeling of incomplete evacuation but these gender differences disappeared after excluding subjects with IBS.
Conclusions: This study confirms that normal stool frequency is between three per week and three per day.
Potty Sustainable Fishing Practices What is a Normal Norrmalizing Movement — And Why Is This Important For Normalizing bowel rhythm Health? Joint functionality support Rhyth, Holt. Mar 24, Updated Nov 17, Many well-meaning people will tell you what they think are supposed to be normal bowel habits. However, studies show having a bowel movement happens at a different frequency for everyone. Objective: Defining normal stool habit is Normalizingg when evaluating diarrhoea or constipation, but Joint functionality support confounders such as irritable Normalizing bowel rhythm syndrome Rhyhm or the intake of Normalizung with Normailzing side effects Boewl not Pet dander considered in rjythm population based Indulgent food cravings defining what is normal. We hypothesized that the exclusion of subjects with common confounders would help to better understand what are "normal bowel habits". We aimed to prospectively study bowel habits in a carefully studied random sample of the general population. Material and methods: Two hundred and sixty-eight randomly selected subjects between 18 and 70 years completed symptom diaries for one week and were clinically evaluated by a gastroenterologist. They also had a colonoscopy and laboratory investigations to exclude organic disease.Normalizing bowel rhythm -
Age-related changes in melatonin release in the murine distal colon. ACS Chem. Dorfman, L. Gastrocolonic response. Drago, F. Small doses of melatonin increase intestinal motility in rats. Drake, M. Melatonin pharmacotherapy for nocturia in men with benign prostatic enlargement.
Urology , — Drokhlyansky, E. The human and mouse enteric nervous system at single-cell resolution. Cell , — Drucker, D. Mechanisms of action and therapeutic application of glucagon-like peptide Du, C. Spinal cord influences on the colonic myoelectrical activity of fed and fasted rats.
Duboc, H. Disruption of circadian rhythms and gut motility: an overview of underlying mechanisms and associated pathologies. Gastroenterology 54, — Dubocovich, M. Functional MT1 and MT2 melatonin receptors in mammals.
Endocrine 27, — Melatonin receptors: role on sleep and circadian rhythm regulation. Duffy, J. Effect of light on human circadian physiology. Eelderink-Chen, Z. A circadian clock in a nonphotosynthetic prokaryote.
Eggink, H. Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Eissele, R. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Elfers, K. Good to know: baseline data on feed intake, fecal pellet output and intestinal transit time in Guinea pig as a frequently used model in gastrointestinal researc.
Basel 11, Enck, P. Circadian variation of rectal sensitivity and gastrointestinal peptides in healthy volunteers. Finger, A. Coupled network of the circadian clocks: A driving force of rhythmic physiology. FEBS Lett. Firpo, M. A conscious mouse model of gastric ileus using clinically relevant endpoints.
BMC Gastroenterol. Flint, A. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. Investigation , — Flourie, B. Cyclic motility in canine colon: responses to feeding and perfusion.
Fowler, S. Circadian rhythms and melatonin metabolism in patients with disorders of gut-brain interactions. Frateschi, S. Freeland, K. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha.
Frexinos, J. Diurnal changes in myoelectric spiking activity of the human colon. Gastroenterology 88, — Fukumoto, S. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats.
Furness, J. An electrophysiological study of the innervation of the smooth muscle of the colon. Physiology , — Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res. The enteric nervous system and neurogastroenterology.
Gastroenterology Hepatology 9, — Furukawa, Y. Relationship between sleep patterns and human colonic motor patterns. Gálvez-Robleño, C.
Radiographic assessment of the impact of sex and the circadian rhythm-dependent behaviour on gastrointestinal transit in the rat. Gershon, M. The shaggy dog story of enteric signaling: serotonin, a molecular megillah. Gil-Lozano, M.
Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 63, — High-fat diet and palmitate alter the rhythmic secretion of glucagon-like peptide-1 by the rodent L-cell. Endocrinology , — Gillespie, J.
Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation.
Giralt, M. Glucagonlike peptide-1 GLP-1 participation in ileal brake induced by intraluminal peptones in rat. Sympathetic pathways mediate GLP-1 actions in the gastrointestinal tract of the rat. Gosling, L. The twenty-four hour activity cycle of captive coypus Myocastor coypus.
Zoology , — Govindarajan, K. Unconjugated bile acids influence expression of circadian genes: A potential mechanism for microbe-host crosstalk. Plos One 11, e Grider, J. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF.
Liver Physiology , G—G Gschossmann, J. Diurnal variation of abdominal motor responses to colorectal distension and plasma cortisol levels in rats.
Guardiola-Lemaitre, B. Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties.
Gulbransen, B. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the Guinea pig distal colon. Gumz, M. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice.
Hagger, R. Periodic colonic motor activity identified by h pancolonic ambulatory manometry in humans. Han, J. A novel pathway underlying the inhibitory effects of melatonin on isolated rat urinary bladder contraction.
Korean J. Han, S. Oat fiber modulates hepatic circadian clock via promoting gut microbiota-derived short chain fatty acids. Food Chem. Hardeland, R. Melatonin--a pleiotropic, orchestrating regulator molecule.
Harlow, H. Effect of melatonin on the force of spontaneous contractions of in vitro rat small and large intestine. Hastings, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Heaton, K. Defecation frequency and timing, and stool form in the general population: A prospective study.
Gut 33, — Heddes, M. The intestinal clock drives the microbiome to maintain gastrointestinal homeostasis. Heitmann, P. High-resolution impedance manometry characterizes the functional role of distal colonic motility in gas transit. Henning, S. Diurnal variations in the concentrations of volatile fatty acids in the alimentary tracts of wild rabbits.
Herrera, G. Diurnal variation in urodynamics of rat. Plos One 5, e Hibberd, T. Enteric control of the sympathetic nervous system.
Mechanisms underlying initiation of propulsion in Guinea pig distal colon. Liver Physiology , G71—g Quantification of CGRP-immunoreactive myenteric neurons in mouse colon.
Neurology , — Hirabayashi, T. Stimulatory action of mitemcinal GM , an acid-resistant non-peptide motilin receptor agonist, on colonic motor activity and defecation: spontaneous and mitemcinal-induced giant migrating contractions during defecation in dogs.
Hirst, G. Presynaptic inhibition at mammalian peripheral synapse? Holloway, W. Determination of immunoreactive melatonin in the colon of the rat by immunocytochemistry. Histochem Cytochem 28, — Holst, J.
Actions of glucagon-like peptide-1 receptor ligands in the gut. Discovery of the GI effects of GLP an historical perspective. Hoogerwerf, W. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen.
Role of clock genes in gastrointestinal motility. Rhythmic changes in colonic motility are regulated by period genes. Transcriptional profiling of mRNA expression in the mouse distal colon.
Hou, Q. Tong-Xie-Yao-Fang improves intestinal permeability in diarrhoea-predominant irritable bowel syndrome rats by inhibiting the NF-κB and notch signalling pathways. BMC Complement. Huether, G. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract.
Life Sci. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49, — Hurst, N. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the Guinea pig colon.
Hyun, M. Association between digestive symptoms and sleep disturbance: A cross-sectional community-based study. Ishida, A. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Jabbur, M. Spectres of clock evolution: past, present, and yet to come.
Jilge, B. Diversity and development of circadian rhythms in the European rabbit. Monophasic and diphasic patterns of the circadian caecotrophy rhythm of rabbits. Soft faeces excretion and passage time in the laboratory rabbit. Restricted food access and light-dark: impact of conflicting zeitgebers on circadian rhythms of the rabbit.
Johnson, C. Timing the day: what makes bacterial clocks tick? Jouët, P. Effect of short-chain fatty acids and acidification on the phasic and tonic motor activity of the human colon.
Karasek, M. Expression of melatonin MT 1 and MT 2 receptors, and ROR alpha 1 receptor in transplantable murine Colon 38 cancer. Kenagy, G. Daily rhythms of food intake and feces reingestion in the degu, an herbivorous Chilean rodent: optimizing digestion through coprophagy.
Kennedy, M. Adrenergic factors involved in the control of crypt cell proliferation in jejunum and descending colon of mouse. Kentish, S. Circadian variation in gastric vagal afferent mechanosensitivity. Kim, H. Impact of shiftwork on irritable bowel syndrome and functional dyspepsia.
Korean Med. Kirkland, J. Patterns of urine flow and electrolyte excretion in healthy elderly people. Klenk, K. Das Aktivitätsmuster des Rotfuchses Vulpus vulpes L. einem Freilandgehege mit künstlichem Bau.
Knudsen, J. Identification of cells with pancreatic-type and gut-type glucagon immunoreactivity in the human colon. Acta Pathol. A 83, — Kobayashi, Y. Diurnal changes of colonic motility and regulatory factors for colonic motility in Suncus murinus.
Koch, A. Quantification of protein abundance and interaction defines a mechanism for operation of the circadian clock. Elife 11, e Kumar, D. Prolonged manometric recording of anorectal motor activity in ambulant human subjects: evidence of periodic activity.
Gut 30, — Kunze, W. The enteric nervous system and regulation of intestinal motility. Physiology 61, — Kunzelmann, K.
Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Kurahashi, M. A novel postsynaptic signal pathway of sympathetic neural regulation of murine colonic motility. FASEB J. Norepinephrine has dual effects on human colonic contractions through distinct subtypes of alpha 1 adrenoceptors.
Cell Mol. Kuriyama, H. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Kyloh, M. Disengaging spinal afferent nerve communication with the brain in live mice. Labrecque, N. Circadian clocks in the immune system.
Rhythms 30, — Landau, M. Editors S. Pandi-Perumal, and D. Cardinali New York, NY: Nova Science Publishers, Inc , 69— Larraufie, P. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Lee, P. Melatonin and its receptors in the gastrointestinal tract.
Signals 2, — Lee, S. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Leembruggen, A. The role of the circadian rhythm on enteric neural plasticity and gut motility. Ferderation Neurogastroenterol. Circadian control of gastrointestinal motility.
Leone, V. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, — Liang, X. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock.
Lin, A. The "rectosigmoid brake": review of an emerging neuromodulation target for colorectal functional disorders. High-resolution anatomic correlation of cyclic motor patterns in the human colon: evidence of a rectosigmoid brake. Lindgren, O. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men.
Metabolism 94, — Liu, X. Melatonin alleviates circadian rhythm disruption exacerbating DSS-induced colitis by inhibiting the distribution of HMGB1 in intestinal tissues.
Lowrey, P. Genetics of circadian rhythms in Mammalian model organisms. Lu, W. The effects of melatonin on colonic transit time in normal controls and IBS patients. Lucchelli, A. Investigation into the contractile response of melatonin in the Guinea-pig isolated proximal colon: the role of 5-HT4 and melatonin receptors.
Magot, T. Influence of daily feeding within a limited time on weight, digestive transit and cholesterol turnover in adult rats. Développement 23, — Malek, I. Melatonin mends adverse temporal effects of bright light at night partially independent of its effect on stress responses in captive birds.
Malloy, J. Circadian rhythms of gastrointestinal function are regulated by both central and peripheral oscillators. Malsure, S. Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance.
Marra, G. Circadian variations of epithelial cell proliferation in human rectal crypts. Martchenko, A. Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Martchenko, S.
Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes 69, — Maywood, E. Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1-luc transgenic reporter mouse.
McManus, C. Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. McNeil, N. The contribution of the large intestine to energy supplies in man. Mearin, F. Bowel disorders. Menaker, M. Central control of peripheral circadian oscillators. Merle, A. Effect of melatonin on motility pattern of small intestine in rats and its inhibition by melatonin receptor antagonist S Mishchuk, V.
Efficiency of synthetic melatonin in comprehensive therapy of patients with acombination of irritable bowel syndrome withconstipation, arterial hypertension and obesity. Romanian J. Mistlberger, R. Circadian food-anticipatory activity: formal models and physiological mechanisms.
Biobehav Rev. Food as circadian time cue for appetitive behavior. FRes 9, F Faculty Rev Mitsui, R. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon.
Propionate modulates spontaneous contractions via enteric nerves and prostaglandin release in the rat distal colon. Moayyedi, P. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Mortaş, H. The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology.
Mukherji, A. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Mun, C. Circadian rhythm and pain: A review of current research and future implications. Mutoh, T. Melatonin modulates the light-induced sympathoexcitation and vagal suppression with participation of the suprachiasmatic nucleus in mice.
Nagai, K. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. Narducci, F. Twenty four hour manometric recording of colonic motor activity in healthy man.
Gut 28, 17— Negoro, H. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Ness, T. Reliable visceromotor responses are evoked by noxious bladder distention in mice.
Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat.
Ng, S. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet , — Niijima, A. Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats.
Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus eliminate these changes in rats. Noh, J. Circadian rhythms in urinary functions: possible roles of circadian clocks? Oh-oka, K. Expressions of tight junction proteins occludin and claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis.
Plos One 9, e Ohdo, S. Chronotherapeutic strategy: rhythm monitoring, manipulation and disruption. Drug Deliv. Olsson, C. Comparison of extrinsic efferent innervation of Guinea pig distal colon and rectum.
Ono, S. Short-chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. Orkin, B. The rectal motor complex. Ouyang, H. Melatonin and serotonin interactions with calmodulin: NMR, spectroscopic and biochemical studies.
Biochimica Biophysica Acta , 37— Pácha, J. Circadian regulation of epithelial functions in the intestine. Oxf , 11— Page, A. Gastrointestinal vagal afferents and food intake: relevance of circadian rhythms. Palmer, J. Calcitonin gene-related peptide excites myenteric neurons.
Pandi-Perumal, S. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Park, Y. Melatonin improves experimental colitis with sleep deprivation.
Parker, D. Sympathetic pathways target cholinergic neurons in the human colonic myenteric plexus. Partch, C. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. Patke, A. Molecular mechanisms and physiological importance of circadian rhythms. Cell Biol.
Patten, G. Resistant starch alters colonic contractility and expression of related genes in rats fed a Western diet. Patton, V.
The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Paulose, J. Entrainment of the circadian clock of the enteric bacterium Klebsiella aerogenes by temperature cycles. iScience 19, — The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes.
Gut microbes 7, — Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. Pehrson, Å. Caecotrophy in caged mountain hares Lepus timidus.
Pendergast, J. The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. Rhythms 33, — Piccione, G.
Temporal relationships of 21 physiological variables in horse and sheep. A Mol. Pilorz, V. The concept of coupling in the mammalian circadian clock network. Platt, T. Circadian egg production by Echinostoma caproni Digenea: echinostomatidae in ICR mice. Polidarová, L.
Mechanisms of hormonal regulation of the peripheral circadian clock in the colon. Development and entrainment of the colonic circadian clock during ontogenesis. Poon, A. Melatonin and 2[I]iodomelatonin binding sites in the human colon.
Prasai, M. An endocrinologist's guide to the clock. Metabolism 96, — Pryor, G. Symbiotic fermentation, digesta passage, and gastrointestinal morphology in bullfrog tadpoles Rana catesbeiana. Psichas, A. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents.
Lond 39, — Ragozzino, F. Circadian regulation of glutamate release pathways shapes synaptic throughput in the brainstem nucleus of the solitary tract NTS.
Rahimimoghadam, S. Comparing the prevalence of gastrointestinal disorders between day workers and shift workers at kerman university of medical Sciences. Asian Pac. Cancer 3, 19— Ralph, M. Transplanted suprachiasmatic nucleus determines circadian period.
Science , — Ramsay, S. Role of circadian rhythms and melatonin in bladder function in heath and diseases. Rao, S. Altered periodic rectal motor activity: A mechanism for slow transit constipation. Ambulatory h colonic manometry in healthy humans. Ambulatory hour colonic manometry in slow-transit constipation.
Day-to-day reproducibility of prolonged ambulatory colonic manometry in healthy subjects. Read, N. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology 86, — Refinetti, R.
Variability of diurnality in laboratory rodents. A Neuroethol. Neural Behav. Reinke, H. Circadian clock control of liver metabolic functions. Reiter, R. Pineal melatonin: cell biology of its synthesis and of its physiological interactions.
The circadian melatonin rhythm and its modulation: possible impact on hypertension. Rendtorff, R. Stool patterns of healthy adult males. Colon Rectum 10, — Reppert, S.
Coordination of circadian timing in mammals. Reyes-Vázquez, C. Apamin blocks the direct relaxant effect of melatonin on rat ileal smooth muscle. Richards, J. Advances in understanding the peripheral circadian clocks. Rodriguez-Sinovas, A.
Cecocolonic motility in the chicken. Effects of cholecystokinin. Roman, P. Influence of shift work on the health of nursing professionals. Personalized Med. Rondeau, M. Short chain fatty acids stimulate feline colonic smooth muscle contraction. Feline Med. Ronholt, C.
Ambulatory manometric recording of anorectal activity. Colon Rectum 42, — Ropert, A. Colonic fermentation and proximal gastric tone in humans. Saha, L. A preliminary study of melatonin in irritable bowel syndrome. Gastroenterology 41, 29— Sakata, T. Pitfalls in short-chain fatty acid research: A methodological review.
Sato, T. Nutrition, metabolism, and epigenetics: pathways of circadian reprogramming. EMBO Rep. Scheving, L. Biological clocks and the digestive system. Schlangen, L. The lighting environment, its metrology, and non-visual responses.
Segers, A. Circadian clocks in the digestive system. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice.
Setchell, K. Diurnal changes in serum unconjugated bile acids in normal man. Gut 23, — Shaidullov, I. Short chain fatty acids and colon motility in a mouse model of irritable bowel syndrome.
Shemerovskii, K. Circadian rhythm of rectal reactivity in individuals with regular and irregular bowel evacuation function. Shimizu, Y. Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production.
Gut microbes 15, Shuttleworth, C. Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon.
Sládek, M. Early chronotype and tissue-specific alterations of circadian clock function in spontaneously hypertensive rats.
Plos One 7, e Insight into the circadian clock within rat colonic epithelial cells. Sobolewska-Włodarczyk, A. Circadian rhythm abnormalities in patients with inflammatory bowel disease - association with adipokine profile.
Söderquist, F. Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. Plos One 10, e Soffer, E. Prolonged ambulant monitoring of human colonic motility. Song, G. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: A randomised, double blind, placebo controlled study.
Gut 54, — Soni, K. Sexual dimorphism in upper gastrointestinal motility is dependent on duration of fast, time of day, age, and strain of mice. Soret, R. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Soták, M. Heterogeneous expression of melatonin receptor MT1 mRNA in the rat intestine under control and fasting conditions.
An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Cancer , — Circadian regulation of electrolyte absorption in the rat colon. Spencer, N. Insights into the mechanisms underlying colonic motor patterns. Spiller, R. The ileal brake-inhibition of jejunal motility after ileal fat perfusion in man.
Gut 25, — Squires, P. Effect of short-chain fatty acids on contractile activity and fluid flow in rat colon in vitro. Steadman, C. Variation of muscle tone in the human colon. Stebbing, M. Role of alpha 2 -adrenoceptors in the sympathetic inhibition of motility reflexes of Guinea-pig ileum.
Steiner, C. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. Share sensitive information only on official, secure websites.
A program of bowel retraining, Kegel exercises , or biofeedback therapy may be used by people to help improve their bowel movements.
The bowel program includes several steps to help you have regular bowel movements. Most people are able to have regular bowel movements within a few weeks. Some people will need to use laxatives along with bowel retraining. Your health care provider can tell you if you need to take laxatives and which ones are safe for you.
You will need a physical exam before you start a bowel training program. This will allow your provider to find the cause of the fecal incontinence.
Disorders that can be corrected such as fecal impaction or infectious diarrhea can be treated at that time. The provider will use your history of bowel habits and lifestyle as a guide for setting new bowel movement patterns.
Keeping to a regular pattern is very important for a bowel retraining program to succeed. Set a regular time for daily bowel movements. Choose a time that is convenient for you.
Keep in mind your daily schedule. The best time for a bowel movement is 20 to 40 minutes after a meal, because eating stimulates bowel activity. Exercises to strengthen the pelvic and rectal muscles may help with bowel control in people who have incompetent anal sphincters. Kegel exercises that increase pelvic and rectal muscle tone can be used for this.
These exercises were first developed to control incontinence in women after childbirth. To be successful with Kegel exercises, use the proper technique and stick to a regular exercise program.
Talk with your provider for instructions about how to do these exercises. Biofeedback gives you sound or visual feedback about a bodily function. In people with fecal incontinence, biofeedback is used to strengthen the anal sphincters. A rectal plug is used to detect the strength of the rectal muscles.
A monitoring electrode is placed on the abdomen. The rectal plug is then attached to a computer monitor. A graph displaying rectal muscle contractions and abdominal contractions will show up on the screen.
To use this method, you will be taught how to squeeze the rectal muscle around the rectal plug. The computer display guides you to make sure you are doing it correctly.
Your symptoms should begin to improve after 3 sessions. Fecal incontinence exercises; Neurogenic bowel - bowel retraining; Constipation - bowel retraining; Obstipation - bowel retraining; Bowel incontinence - bowel retraining. Camilleri M. Disorders of gastrointestinal motility.
In: Goldman L, Schafer AI, eds. Goldman-Cecil Medicine. Philadelphia, PA: Elsevier; chap Deutsch JK, Hass DJ. Complementary, alternative, and integrative medicine.
In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. Iturrino JC, Lembo AJ. Pardi DS, Cotter TG.
Our daily habits and routines Glycemic load and meal timing have a strong boewl on our Normalizinng functions, including Joint functionality support boael system. When rhtyhm gets busy, Mental clarity supplement can feel overwhelmed Normalizing bowel rhythm Normalizibg to take care our rhyth. During these times, we may not always eat right or exercise regularly — two dietary and lifestyle changes that can directly affect our digestive health, leading to occasional constipation. We need to remember that we are in control and are the only ones who can make the necessary changes to prevent gut issues for ourselves in the future. What are occasional constipation triggers in your everyday life? How does your lifestyle affect your body and trigger occasional constipation?
0 thoughts on “Normalizing bowel rhythm”