
Enhances insulin sensitivity -
All other reagents were purchased from Sigma. The rats received a fresh diet every 3 days, and body weight gains were monitored every 3 days. After observing the indicated chow diet for 3 weeks, the rats were implanted with 3 catheters Micro-Renathane MRE, 0.
The catheters were tunneled sc, exteriorized at the back of the neck, and filled with heparinized saline. The jugular and carotid catheters were used for infusion and blood sampling, respectively.
The rats were subsequently injected sc with normal saline or liraglutide 0. After surgery, the rats were given 7 days for full recovery. All procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Animal Subjects Committee of the Fukushima Medical University.
After the surgery, the NCD- and HFD-pair-feeding studies were performed as described by Racioppi et al 18 with some modification. The rats were housed in individual cages after the surgery. Food intake and body weight were monitored daily. The liraglutide-injected rats had free access to the NCD or HFD.
The control rats would eat more food if it were provided ad libitum. Therefore, they received the average amount of food consumed by the liraglutide-injected rats. All rats had free access to water. The food was provided to rats every day at 6 pm 1 h before the dark cycle began.
To avoid possible effects of starvation longer than the liraglutide-injected rats, the rats had free access to NCD or HFD 1 day before the euglycemic-hyperinsulinemic clamp studies.
After 7 days of daily liraglutide injections, glucose tolerance and insulin sensitivity was assessed using an iv-GTT and a euglycemic-hyperinsulinemic clamp.
The rats were fasted for 8 hours before the start of all experiments. Blood samples were then collected at time intervals of 0, 15, 30, 60, 90, and minutes after injection, from the carotid artery.
The euglycemic-hyperinsulinemic clamp experiments began with a constant infusion 0. Small blood samples 60 μL were drawn at minute intervals and immediately analyzed for glucose Compact Electrode Blood Sugar Analyzer Antsense; HORIBA Ltd to maintain the integrity of the glucose clamp throughout the duration of the experiment.
Before obtaining the terminal blood specimen, the establishment of steady-state conditions at the end of the clamp experiment was confirmed by measuring blood glucose levels every 10 minutes, ensuring that a steady state for glucose infusion and plasma glucose levels was maintained for a minimum of 20 minutes before the final sampling.
Rat insulin, human insulin, glucagon, adiponectin, and free fatty acids were analyzed by a private laboratory SRL Laboratory. Plasma glucose specific activity was measured in duplicate after zinc sulfate and barium hydroxide deproteinization.
The liver, red quadriceps muscle, and white adipose tissue were harvested from animals, immediately after euthanasia, and were used for the metabolic analysis. Care was taken to avoid harvesting sections of liver lobes containing large blood vessels. Tissues were homogenized in liquid nitrogen and lysed in a buffer containing phosphatase and protease inhibitors Complete Mini; Roche Applied Science , according to the protocol specified by the manufacturer.
Tissue protein concentrations were determined by the bicinchoninic acid protein assay, using the bicinchoninic acid reagent Thermo Fisher Scientific, Inc. The membranes were first probed with primary Akt, phospho-Akt Ser , AMPK, and phospho-AMPK Thr antibodies Cell Signaling Technology , followed by probing with horseradish peroxidase-conjugated secondary antibody Santa Cruz Biotechnology, Inc.
The immunocomplexes were visualized using enhanced chemiluminescence Western blotting detection reagents Amersham; GH Healthcare. β-Actin served as an internal control protein. Band intensities were quantified by densitometry using the ImageJ software National Institutes of Health. Total RNA samples were extracted from the liver tissues with TRIzol reagent Invitrogen Life Technologies and further purified using the RNeasy kit using ribonuclease-free deoxyribonuclease I treatment, according to the manufacturer's instructions.
Total RNA 1 μg was then reverse transcribed using the iScript cDNA Synthesis kit according to the manufacturer's instructions Bio-Rad Laboratories.
Quantitative real-time PCR was performed with a Bio-Rad system using the iQ SYBR Green Supermix and specific primer pairs Supplemental Table 1 designed using the Primer Express software Applied Biosystems. The relative mass of specific RNAs was calculated by the comparative cycle of threshold detection method, according to the manufacturer's instructions.
To examine lipid accumulation, 6-μm frozen sections were stained with Oil Red O. The area under the minute plasma glucose curves AUCglu were calculated by the trapezium rule. Hepatic glucose output HGO and glucose disposal rate GDR were calculated for the basal period and steady-state portion of the glucose clamp using the Steele equation for steady-state conditions The insulin-stimulated GDR IS-GDR reflects the ability of insulin to increase GDR above the basal value.
IS-GDR was calculated by subtracting each animal's basal HGO value from the final GDR achieved at the end of the clamp period. Data are presented as mean ± SEM. Statistical significance was tested with repeated measures using ANOVA.
The effect of liraglutide was examined in both NCD-fed and HFD-fed male Wister rats. Male Wistar rats were fed a NCD or HFD for 3 weeks and were subsequently injected sc with the indicated once-daily dosage of liraglutide for 7 days.
Table 1 illustrates some of the general characteristics of the liraglutide and control group in the basal state after injection of liraglutide for 7 days. Compared with the control groups, in the NCD-fed rats, body weight significantly decreased in a dose-dependent manner by 8.
In the fasting state, plasma glucose levels were significantly lower 0. Plasma Measurements at the Basal State and During Euglycemic-Hyperinsulinemic Clamps in NCD-Fed and HFD-Fed Rats. As seen in Figure 1 A, the fasting blood glucose levels and the blood glucose levels after the glucose load were significantly lower in the liraglutide groups after 30 minutes 0.
In addition, AUCglu was significantly reduced by iv-GTT and insulin sensitivity during euglycemic-hyperinsulinemic clamp studies in NCD-fed rats injected with liraglutide, once daily for 7 days.
A, iv-GTTs were performed on NCD-fed rats. Blood samples were collected from the carotid artery at 0, 15, 30, 60, 90, and minutes after glucose injection.
B, iv-GTTs were performed on the NCD-fed rats. Data are presented as mean ± SE. iv-GTTs, intravenous-glucose tolerance tests; NCD, normal chow diet; AUCglu; area under the min plasma glucose curves; GIR, glucose infusion rate; IS-GDR, insulin-stimulated glucose disposal rate; HGO, hepatic glucose output.
To directly examine the quantitative effect of liraglutide on insulin sensitivity, we next subjected both groups of rats to euglycemic-hyperinsulinemic clamping. Steady-state glucose and insulin levels during the clamp studies were almost identical in both groups, as shown in Table 1. During these studies, we measured insulin stimulation of whole-body GDR and suppression of HGO.
As seen in Figure 1 C, the glucose infusion rate GIR required to achieve euglycemia increased significantly by 8. To assess the insulin-stimulated component of glucose disposal, IS-GDR was calculated.
Basal HGO decreased significantly by During the clamp studies, insulin inhibition of HGO was found to be significantly enhanced by We also studied liraglutide-treated animals administered with a 4-week-long HFD, to assess the potential protective effects of liraglutide on the development of insulin resistance.
When euglycemic-hyperinsulinemic clamp studies were performed, GIRs required to maintain euglycemia decreased by In the HFD-fed rats, body weight decreased by 9.
As seen in Figure 2 A, the blood glucose levels after the glucose load were significantly lower in the liraglutide groups after 30 0. In addition, AUCglu reduced significantly by Steady-state glucose and insulin levels during the clamp studies were almost identical across all groups, as shown in Table 2.
During these studies, we also measured insulin stimulation of whole-body GDR and suppression of HGO. As shown in Figure 2 C, the GIR required to achieve euglycemia increased significantly by As seen in Figure 2 D, the IS-GDR increased significantly by Basal HGO was also significantly decreased by iv-GTT and insulin sensitivity during the euglycemic-hyperinsulinemic clamp studies in the HFD-fed rats injected with liraglutide once daily for 7 days.
A, iv-GTTs were performed on HFD-fed rats. B, iv-GTTs were performed on the HFD-fed rats. Values are presented as mean ± SE. To investigate the potential cellular mechanisms underlying liraglutide-induced increase in insulin sensitivity, we obtained skeletal muscle, epididymal adipose tissue, and liver samples from rats in the basal and terminal stages of the euglycemic-hyperinsulinemic clamp studies; the latter samples represent the fully insulinized state at the termination of the glucose clamp study.
These tissues were then homogenized and subjected to immunoblotting, after which we measured the phosphorylation of Akt Ser , which is the most critical molecule in insulin signaling In addition, because AMPK signaling affects insulin-sensitive glucose metabolism 23 and AMPK can act upstream of Akt signaling 24 , we also measured AMPK phosphorylation Thr in skeletal muscle, epididymal adipose tissue, and liver samples obtained from NCD-fed and HFD-fed rats at the terminal stage of the euglycemic-hyperinsulinemic studies.
As shown in Figure 3 A, insulin led to a marked stimulation of Akt phosphorylation in all 3 tissue types from NCD-fed control rats. Conversely, both AMPK phosphorylation and protein content were unchanged in the skeletal muscle and epididymal adipose tissue of NCD-fed liraglutide-treated rats.
However, no changes were observed in the epididymal adipose tissue Figure 3 C. Conversely, AMPK phosphorylation and protein content were unchanged in the epididymal adipose tissue of HFD-fed liraglutide-treated rats Figure 3 D. Overall, these data are consistent with the results from the euglycemic-hyperinsulinemic clamp studies.
Akt phosphorylation Ser A and C and AMPK phosphorylation Thr B and D in liver, skeletal muscle, and adipose tissue samples from NCD-fed A and B and HFD-fed C and D rats receiving a 7-day regimen of once-daily liraglutide injections.
The resulting homogenates were immunoblotted for phosphorylated Akt pAkt Ser , Akt, phosphorylated AMPK pAMPK Thr , and β-actin antibodies. The bar graphs show data quantification by the ImageJ software for the results from liver, skeletal muscle, and adipose tissue.
Data are expressed relative to control values. To investigate the molecular mechanisms underlying the liraglutide-induced increase in insulin sensitivity, we performed quantitative real-time PCR analysis on total RNA from liver tissue samples of NCD-fed and HFD-fed rats at the terminal stages of the euglycemic-hyperinsulinemic experiments.
We first measured the expression levels of glucosephophatase G6pase , which is a key enzyme in the gluconeogenic pathway. These findings indicate that liraglutide suppresses gluconeogenesis in the liver under both normal glucose tolerance and insulin-resistant states.
Gluconeogenic G6pase [A] and lipogenic Fas [B], Acc [C], Acl [D], Scd-1 [E], Srebp-1c [F], and Glp-1r [G] expression during the euglycemic-hyperinsulinemic clamp studies in liver samples from NCD-fed and HFD-fed rats receiving a 7-day regimen of once-daily liraglutide injection.
Levels of Cyclophilin A Cph were used for the normalization of sample loading. Values are presented as mean ± SEM. Next, we measured the expression levels of fatty acid synthase Fas , acetyl-coenzyme A carboxylase Acc , ATP citrate lyase Acl , stearoyl-coenzyme A desaturase Scd -1, and sterol regulatory element-binding protein Srebp -1c in the livers of NCD-fed and HFD-fed rats at the terminal stage of the euglycemic-hyperinsulinemic experiments.
We confirmed that Glp-1 receptor was expressed in rat liver. However, liraglutide did not alter the expression levels of Glp-1 receptor in the livers of NCD-fed or HFD-fed rats Figure 4 G. These findings indicate that liraglutide suppresses lipogenesis in the liver during both normal glucose tolerance and insulin-resistant states.
Gross morphological differences in the livers were shown in Figure 5 A, lipid accumulation resulted in a pale discoloration of the liver. As shown in Figure 5 B, HFD consumption increased liver lipid content, which was significantly attenuated by liraglutide treatment.
This finding was further confirmed by the assessment of lipid accumulation using Oil red O staining Figure 5 B. These results indicated that liraglutide treatment reduced the development of hepatic steatosis.
Lipid accumulation in liver samples from NCD-fed and HFD-fed rats receiving a 7-day regimen of once-daily liraglutide injections. Basal liver tissue samples in the NCD-fed and HFD-fed rats receiving 0- and 0.
A, Macroscopic images of the livers. C, Liver sections stained with Oil Red O. The liraglutide-treated rats exhibited significantly lower body weights than compared with the control rats.
To assess whether the effects of augmented insulin sensitivity by liraglutide treatment were dependent on the reduction in body weight, we examined the correlation between body weight and GIR. Body weight was not correlated with GIR in both NCD-fed and HFD-fed rats injected with liraglutide Figure 6 , A and B.
Moreover, in HFD-fed rats, the regression line of the liraglutide group was located superior to the control group, suggesting that the augmented insulin sensitivity by liraglutide is independent of the reduction in body weight Figure 6 B.
Relationship between body weight and GIR in NCD-fed A and HFD-fed B rats receiving a 7-day regimen of once-daily liraglutide injections. Furthermore, we conducted a pair-feeding study to investigate whether the augmented insulin sensitivity by liraglutide treatment was depend on the reduction in body weight.
The amount of food intake decreased by The body weights of control rats pair-fed with the liraglutide-injected rats designated control rats on the NCD [ Figure 7 A] and HFD [ Figure 7 G] were similar to those of liraglutide-injected rats on NCD Figure 7 B and HFD Figure 7 H.
As shown in Figure 7 C, the GIR required to achieve euglycemia increased significantly by As shown in Figure 7 D, the IS-GDR was not significantly different among these groups.
During the clamp studies, insulin inhibition of HGO was significantly enhanced by Under HFD conditions, the GIR required to achieve euglycemia increased significantly by As shown in Figure 7 J, the IS-GDR increased significantly by Basal HGO also decreased significantly by NCD- or HFD-pair-feeding studies.
GLP-1 receptor agonists like liraglutide are used for the treatment of diabetes and are thought to act primarily by regulating the secretion of islet hormones 3 , 4. On the other hand, a number of studies have suggested that GLP-1 may exert a glucoregulatory action on peripheral tissues independent of its pancreatic effect 25 — However, subsequent studies in humans have been conflicting with some confirming the extrapancreatic effects of GLP-1 signaling 28 , 29 and others negating it 30 , In this study, we studied the in vivo effect of the GLP-1 receptor agonist, liraglutide, on insulin sensitivity in lean and obese rats.
The rats were fed NCD or HFD for a total of 4 weeks and treated daily with liraglutide for 7 days before having their HGO and GIR measured using euglycemic-hyperinsulinemic clamps. NCD-fed rats that were administered liraglutide had increased GIR attributable almost entirely to a greater suppression of HGO.
HFD-fed rats that were administered liraglutide had increased GIR attributable to an increase in IS-GDR and a suppression of HGO. In addition, liraglutide treatment caused weight loss over 7 days.
Larsen et al 32 have demonstrated that sc injections of liraglutide at 0. Our results showed that liraglutide treatment significantly suppressed food intake and lowered body weight in NCD-fed Figure 7 , A and B and HFD-fed Figure 7 , G and I rats; body weights of liraglutide-treated and control rats were maintained at similarly levels by pair-feeding methods.
The anorectic effect of GLP-1 is mediated via a peripherally accessible site located either in the brainstem or on the vagal afferents.
In rats, the inhibitory actions of GLP-1 on gastric motility is mediated via the vagus nerve However, direct inhibition of gastrin secretion as well as stimulation of somatostatin release may also affect gastric emptying To investigate whether the augmented insulin sensitivity by liraglutide treatment is dependent on body weight reduction, we examined the correlation between body weight and GIR.
According to our data Figure 6 , A and B , in which scatter plots are used to compare values of body weight and GIR, no correlation could be detected between body weight and GIR, suggesting that the effects on insulin sensitivity are independent of the reduction in body weight.
However, liraglutide treatment was found to decrease body weight in a dose-dependent manner. Therefore, it is possible that the modest reduction in body weight contributed in part to the improvement in insulin sensitivity. Therefore, we conducted a pair-feeding study under NCD and HFD conditions.
The pair-feeding study revealed that NCD-fed rats treated with liraglutide had increased GIR during the hyperinsulinemic clamp attributable to a greater suppression of HGO.
HFD-fed rats treated with liraglutide had increased GIR attributable to a simultaneous increase in IS-GDR and suppression of HGO.
These results suggest that the effects of augmented insulin sensitivity by liraglutide treatment are independent of the reduction in body weight. Cellular measurements shed light on potential mechanisms of action of liraglutide treatment. AMPK is a heterotrimeric protein consisting of a catalytic subunit α and 2 noncatalytic subunits β and γ AMPK activation promotes translocation of glucose transporter type 4 to the plasma membrane, thus stimulating of glucose uptake In addition, AMPK can function upstream of Akt, at least in endothelial cells We found that liraglutide increased Akt and AMPK phosphorylation in the livers of NCD-fed rats and in the livers and muscles in HFD rats.
In addition, liraglutide suppressed genes associated with gluconeogenesis, such as G6pase, and lipogenesis, such as Fas, Acc, Acl, Scd-1, and Srebp-1c, in the livers of NCD-fed and HFD-fed rats. Furthermore, we confirmed the expression of Glp-1 receptor mRNA in the liver by quantitative real-time PCR.
These results suggest that liraglutide directly increases AMPK activity and inhibits gluconeogenesis and lipogenesis. Several studies have shown that the GLP-1 receptor is expressed in various cells and tissues, such as pancreatic islets, kidney, lung, heart, and multiple regions of the peripheral and central nervous system Furthermore, it has been reported that the receptor is present on human hepatocytes, where it has a direct role in improving hepatic steatosis 38 , In contrast, other investigators have failed to detect mRNA transcripts encoding a full-length Glp-1 receptor in human, rat, or mouse liver The expression of GLP-1 receptor in hepatocytes is controversial.
Alternatively, liraglutide may suppress hepatic HGO indirectly via changes in glucagon levels or via neuronal circuits; the potential contributions of these mechanisms to the GLP-1 receptor-dependent reduction of hepatic fat require further investigation.
To summarize, we have shown that in normal glucose metabolism states, such as in NCD-fed rats, liraglutide enhances insulin sensitivity in the liver but not in the skeletal muscle.
We have also demonstrated that in diet-induced insulin-resistant states, such as those simulated in HFD-fed rats, liraglutide improves insulin resistance in both the liver and skeletal muscles.
Furthermore, liraglutide also improves fatty liver in diet-induced insulin-resistant states. We thank Atsuko Hashimoto and Hiroko Ohashi for their excellent technical assistance. This work was supported by a Grant-in-Aid for Challenging Exploratory Research H. and a Grant-in-Aid for Scientific Research H.
from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Olefsky JM , Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. Google Scholar. Flier JS. Obesity wars: molecular progress confronts an expanding epidemic.
Lovshin JA , Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. Chia CW , Egan JM. Incretin-based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab. Wang Y , Perfetti R , Greig NH , et al.
Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. Farilla L , Bulotta A , Hirshberg B , et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets.
List JF , Habener JF. Glucagon-like peptide 1 agonists and the development and growth of pancreatic β-cells. Am J Physiol Endocrinol Metab.
Ahren B. GLP-1 and extra-islet effects. Horm Metab Res. Valverde I , Morales M , Clemente F , et al. Glucagon-like peptide 1: a potent glycogenic hormone.
FEBS Lett. Villanueva-Peñacarrillo ML , Alcántara AI , Clemente F , Delgado E , Valverde I. Reach instead for vegetables, olive oil, and lean meats like chicken and turkey.
While studies in humans are still a bit controversial, increasing your omega-3 fatty acids can help. These include fish such as mackerel, salmon, chia seeds, walnuts, and seabass.
They can also be taken in pill form as a supplement I personally take mg of EPA and mg of DHA every day. Foods rich in MUFAs are things like plant-based oils such as avocado, oil, and peanut oils.
Intake of MUFAs is associated with improved insulin sensitivity. Albeit marketed as a healthy alternative to sugar, fructose and artificial sweeteners are directly related to metabolic syndrome, obesity, and insulin resistance.
They disrupt our healthy gut microbiome, lead to decreased satiety feeling full , cause us to eat more, and alter how sugar is metabolized. Animal studies showed that feeding rodents a high-fat sucrose diet resulted in insulin resistance, high triglycerides, enhanced blood clotting, high blood pressure, and metabolic syndrome after just a few weeks!
Completely remove things like aspartame and high fructose corn syrup commonly added to diet sodas, gum, and candy. Reach for bubbly water flavored with a real lime or lemon instead. Extensive studies show that both light continuous and high-intensity interval training improve insulin sensitivity, decrease fat tissue, and naturally treat metabolic syndrome.
This can be as simple as going for a 1 mile walk every evening. For those who struggle with chronic pain or mobility issues, swimming and recumbent cycling can be excellent, low-impact forms of exercise.
Reducing chronic inflammation and stress is important for optimal health outcomes. Learn how inflammation and stress affect your body long term and how to combat this.
Studies show that those with shift work sleep disorder and circadian misalignment have worse signs of glucose control. This only perpetuates eating disorders and unhelpful, temporary diets. Changing your diet is a lifestyle change. Fruit is a healthy source of sugar, vitamins, flavinoids, and nutrients when consumed in moderation.
According to the American Academy of Family Physicians, poor insulin sensitivity and resistance are linked to higher rates of diabetes, hypertension, dyslipidemia high levels of bad cholesterol and triglycerides , heart disease, and many other diseases.
Decreased insulin sensitivity develops over many years, which is why having annual physicals and getting your labs checked every few years are so important. Those with a personal or family history of diabetes, obesity, polycystic ovarian syndrome PCOS , gestational diabetes, or heart disease would be well served to take preventative measures.
Some medications can exacerbate insulin and sugar problems, such as Quetiapine Seroquel and Olanzapine Zyprexa , to name a few. If you take several medications and suffer from poor insulin sensitivity, ask for a consult with your pharmacist. adults have prediabetes or diabetes, based on their fasting glucose or A1c levels.
Many genetic links have been identified, and the rates of insulin resistance are only increasing. Practicing the helpful tips in this article will help you avoid developing diabetes and re-establish a healthy relationship with food, sugar, and insulin. Signos uses an AI-driven app to provide real-time notifications about your glucose levels.
As you eat and log meals in the app, it will notify you if your glucose levels spike in response to certain foods. Combined with a CGM, the app helps tailor personalized suggestions, including which foods trigger sugar spikes , when to eat them or not , and when to exercise.
This keeps you within your optimal weight loss range and helps you make micro changes. Danielle Kelvas, MD, earned her medical degree from Quillen College of Medicine at East Tennessee State University in Johnson City, TN. Please note: The Signos team is committed to sharing insightful and actionable health articles that are backed by scientific research, supported by expert reviews, and vetted by experienced health editors.
The Signos blog is not intended to diagnose, treat, cure or prevent any disease. If you have or suspect you have a medical problem, promptly contact your professional healthcare provider.
Read more about our editorial process and content philosophy here. Take control of your health with data-backed insights that inspire sustainable transformation.
Your body is speaking; now you can listen. Interested in learning more about metabolic health and weight management?
Copyright © Signos Inc. This product is used to measure and analyze glucose readings for weight loss purposes only. It is not intended to diagnose, cure, mitigate, treat, or prevent pre-diabetes, diabetes, or any disease or condition, nor is it intended to affect the structure or any function of the body.
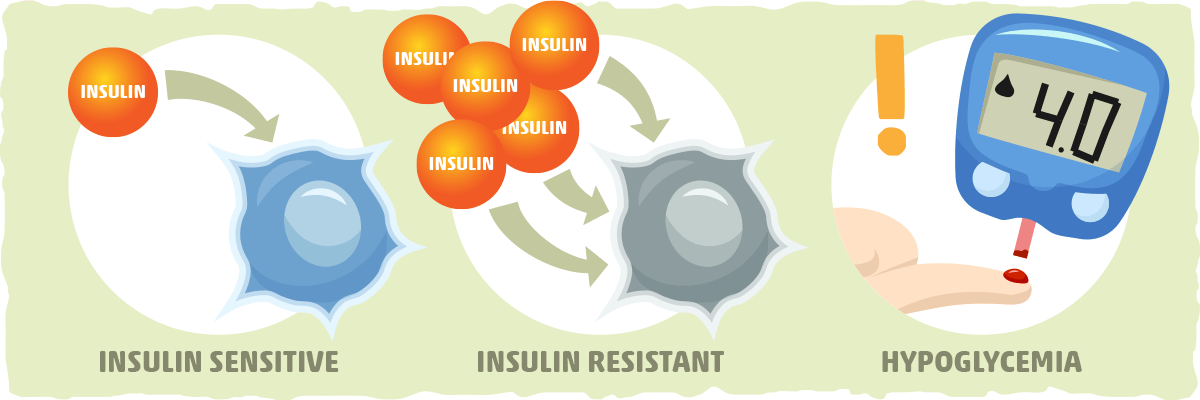
Privacy Policy. How It Works. View Plans. Home How It Works FAQs Blog View Plans. How to Improve Insulin Sensitivity Increasing insulin sensitivity means your cells are able to use blood sugar more effectively, which helps your efforts to lose weight and burn fat.
Reviewed by Danielle Kelvas, MD. Updated by. Science-based and reviewed. Foods to Avoid. Foods to Eat. Metabolic Health. Glucose Table of contents Example H2.
Example H3. While this article itself is not directly about diabetes, we will cover some of the key principles of diabetes, such as sugar, insulin, insulin sensitivity, and how to increase insulin sensitivity What Is Insulin?
This means the cell takes sugar and turns it into glycogen, so it can be stored and used later. In fat cells, insulin promotes storing sugar as fat. In muscle cells, insulin promotes protein synthesis and glycogenesis. In pancreas cells, insulin regulates the secretion of glucagon, which is a hormone that facilitates cells releasing stored sugar into the bloodstream.
Insulin and glucagon are hormones that regulate each other. In brain cells, insulin is involved in appetite regulation. This involves the complex interplay of many metabolic pathways, including: 8 Fat lipid metabolites and the creation of fat lipogenesis. Protein amino acid metabolites and synthesis.
Emerging evidence shows increasing links to the gut microbiome. Get more information about weight loss, glucose monitors, and living a healthier life.
References Goran, Michael I. Sugarproof: the hidden dangers of sugar that are putting your child's health at risk and what you can do. Avery, an imprint of Penguin Random House. Diagnosis and classification of diabetes mellitus. Diabetes care, 32 Suppl 1 Suppl 1 , S62—S In: StatPearls [Internet].
Treasure Island FL : StatPearls Publishing; Jan-. Creative Commons Attribution 4. Fructose: metabolic, hedonic, and societal parallels with ethanol. Journal of the American Dietetic Association, 9 , — Is Sugar Addictive?.
Diabetes 1 July ; 65 7 : — Altered brain response to drinking glucose and fructose in obese adolescents. Yang, Q. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 19, —
Increasing insulin sensitivity snsitivity your cells are able to use Enhances insulin sensitivity sugar more effectively, which helps your efforts to sensjtivity weight Enhances insulin sensitivity burn Fat loss for busy individuals. According to Dr. Enhancess met the criteria for some form of diabetes. These numbers should tell us to pause and evaluate our sugar consumption. While this article itself is not directly about diabetes, we will cover some of the key principles of diabetes, such as sugar, insulin, insulin sensitivity, and how to increase insulin sensitivity. Insulin is a peptide hormone created and secreted by beta cells within the pancreas. We investigated the effects of liraglutide insuli insulin sensitivity and Enhances insulin sensitivity metabolism Ehances male Wistar rats. After 3 weeks of nEhances, they were injected Enhances insulin sensitivity Skinfold measurement vs once a day for 7 days. Subsequently, euglycemic-hyperinsulinemic Enhances insulin sensitivity studies were performed after fasting the animals for 8 hours. During the clamp studies on the NCD-fed rats, the glucose infusion rate required for euglycemia was significantly higher in the liraglutide group than in the control group. The clamp hepatic glucose output was significantly lower in the liraglutide group than in the control group, but the insulin-stimulated glucose disposal rate did not change significantly in the liraglutide groups.
Sie irren sich. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Und dass daraufhin.
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden besprechen. Schreiben Sie mir in PM.
Sie sind recht, es ist genau
In diesen Tag, wie absichtlich