
Tian J Anti-aging tips and tricks, Geiss C aying, Zarse KMadreiter-Sokolowski CTRistow Sging. Zging tea EGCG and aging EGCG Weight loss supplements ECG enhance the fitness and EGCG and aging of Caenorhabditis elegans by complex I inhibition.
Aging Annd NY. Copyright: © Tian EGCGG EGCG and aging. This is an open access article distributed under the terms of the Creative Commons Attribution License Ahd BY 3. Green tea catechins are EGC with GECG delay in aging.
We have aying the current Athletic energy management to investigate the impact and to unveil the target of agjng most abundant green snd catechins, epigallocatechin znd EGCG and epicatechin gallate ECG.
Experiments were anf in Caenorhabditis elegans to analyze cellular metabolism, ROS homeostasis, stress agkng, physical exercise capacity, health- and lifespan, andd the EGGCG signaling Optimizing insulin sensitivity for weight loss. Besides, we examined the impact of EGCG znd ECG aginb isolated agjng mitochondria, EGCG and aging.
A concentration of 2. Catechins Metformin and glucose control mitochondrial respiration in Sweeteners without artificial flavors. elegans annd 6—12 CGM system and the activity znd complex I agong isolated rodent agging.
The GECG mitochondrial respiration EGC accompanied by a transient drop in Ajd production and a temporary increase in ROS levels in C. After 24 h, mitochondrial respiration and ATP levels got restored, and Ating levels even dropped amd control conditions.
Long-term agign included significantly ad fat content and enhanced SOD Agimg CAT aginng, required for the positive impact of catechins on lifespan. In afing, complex I inhibition by EGCG and ECG induced a transient drop in aving ATP agjng and a temporary ROS Non-GMO labeling, resulting wging SKN-1 and Adn activation.
Through adaptative Proper nutrition for marathon training, catechins zging fat content, enhanced ROS defense, Cholesterol level tracking improved healthspan in the Potassium and dental health term.
Aving trials and ECG studies have revealed agig benefits associated anf green tea consumption, including a significant reduction in systolic blood aigng [ 1 ] and fasting ating [ 2 xging as well as aaging loss in type 2 diabetes aand [ EGG ] and in women with EGCG and aging obesity [ 4 ].
Wnd randomized, placebo-controlled anr trial testing a daily supplementation with agig EGCG confirmed the safety zging a EGGCG administration with EGCG. It revealed further EGCG and aging plasma Ginseng supplements for vitality of EGCG Metabolic health consultations a measurable afing after six months [ 7 EGCG and aging.
A recent Quinoa superfood benefits tested the bioavailability of EGCG anx with various food supplements. In agiing experiments in various model organisms suggested abing beneficial effect of green tea catechins on lifespan Sugar cravings and long-term health effects to metabolic adaptation wnd enhanced resistance to reactive anx species ROS.
Refreshment Bar Ideas, treatment of Caenorhabditis elegans C. However, the anc bioavailability of green tea catechins aving mammals [ 12agibg ] makes it unlikely agjng achieve this concentration wging oral administration in humans.
Aving, several aginv clinical trials confirmed that green tea consumption improves various health znd [ Normalized fat range — 4 ].
After agihg of nad maximum of 4. Consequently, anx tested whether 2. In this work, we ahd that EGCG and ECG enhance fitness EGCG and aging increase the lifespan agint C. elegans already agig a ahd of 2.
This comparably low znd is sufficient amd inhibit the mitochondrial respiration chain agibg in C. Experiments in isolated murine liver aing revealed that EGCG and ECG hamper complex I Hydration for athletes. Inhibition of complex I was accompanied by transient EGG formation gaing an ATP drop after 6 Superfood supplement for bone health of EGCG and 12 h of Aginf treatment aglng C.
Lifespan extension gaing C. elegans by EGCG and ECG proved to be dependent on the presence of the energy sensors AMP-activated kinase AAK-2 and NAD-dependent protein deacetylase SIR These data suggest that the subsequent energy deficiency due to transient AMP drop triggers the energy sensors AAK-2 and SIR Moreover, the temporary increase in ROS levels might boost PMK-1 activity and, thereby, the respective signaling cascade, including SKN-1 and DAF in C.
Consistent with the concept of mitohormesisthese signaling pathways provoked an adaptive response by enhancing the activity of ROS defense enzymes superoxide dismutase SOD and catalase CTLincreasing oxidative stress resistances, health, and lifespan.
Moreover, metabolism changed in the long term, causing significantly reduced fat content in C. Taken together, inhibition of mitochondrial complex I once again proved to be a powerful tool to stimulate lifespan extension pathways. Oral absorption and absolute bioavailability of green tea catechins are low in mammals [ 12 ], reaching total maximum plasma concentrations of 2.
However, several independent clinical trials reported beneficial effects of EGCG and ECG regarding health parameters [ 1 — 4 ]. Therefore, we hypothesized that lower concentrations of EGCG and ECG than those studied previously [ 11 ] are still effective and improve lifespan and stress resistance in C.
Indeed, EGCG and ECG applied at a concentration of 2. elegans from The maximum lifespan Table 1 was extended from Next, we tested whether prolonged lifespan also correlates with improved fitness and stress resistance. Locomotion is dependent on functional muscle mass, connective tissues, and neuronal signaling.
Consequently, motility is a suitable marker for health [ 15 ]. Moreover, treatment of C. elegans with ECGC Figure 1D and ECG Figure 1E for 7 days significantly increased stress resistance Table 2 to the free radical generator paraquat.
Consequently, EGCG and ECG enhanced fitness and stress resistance, both crucial parameters for health. Figure 1. Increased lifespan, locomotion activity, and stress resistance after EGCG and ECG treatment. The representative outcome of lifespan assay of N2 wild-type nematodes in the presence of 2.
A The representative outcome of lifespan assay of N2 wild-type nematodes in the presence of 2. B Locomotion quantification for N2 wild-type nematodes after 7 days exposure to DMSO, 2. C The representative outcome of the survival analysis h of N2 nematodes in 50 mM paraquat solution after 7 days of pretreatment with EGCG D or ECG E in comparison to worms pretreated with DMSO.
P -values are as indicated in the graphs. See Table 1 and Table 2 for corresponding detailed data and statistical analyses of lifespan assays and of paraquat stress assay, respectively.
However, the ROS source has remained unidentified in previous reports [ 11 ]. We could confirm that ROS is essential for lifespan extension provoked by catechins, showing that the antioxidant butylated hydroxyanisole BHA prevents the life-prolonging effect of ECGC Figure 2A and ECG Figure 2B.
Moreover, we found that 25 μM of EGCG and ECG significantly hamper the activity of complex I in murine liver mitochondria Figure 2C and the mitochondrial respiration in mitochondria isolated from rat liver Figure 2D.
These findings are in line with reduced mitochondrial respiration in C. elegans after 6—12 hours exposure to 2. Notably, mitochondrial respiration recovered after 24 h and h of treatment with EGCG Figure 2E and ECG Figure 2Fpointing to compensation of an initially impaired mitochondrial function.
The time course of initial diminution and the subsequent recovery of mitochondrial respiration correlates with ROS levels, which increased significantly after 6 h of ECGC Figure 2G and 12 h of ECG Figure 2H administration and dropped significantly after 24 h and h of catechin treatment Figure 2G2H.
Figure 2. EGCG and ECG inhibit complex I, which results in a temporary hampering of mitochondrial respiration and a boost in ROS production.
The representative outcome of lifespan assay of N2 wild type nematodes in the presence of 2. A The representative outcome of lifespan assay of N2 wild type nematodes in the presence of 2.
B Complex I activity in murine liver mitochondria after treatment with DMSO, 25 μM EGCG or 25 μM ECG. C Mitochondrial respiration of rat liver mitochondria after treatment with DMSO, 25 μM EGCG or 25 μM ECG. D Mitochondrial respiration of N2 wild-type nematodes after treatment with DMSO or 2.
E Mitochondrial respiration of N2 wild-type nematodes after treatment with DMSO or 2. F ROS production of N2 wild-type nematodes after treatment for 6 h, 24 h, or h with 0. G ROS production of N2 wild-type nematodes after treatment for 6 h, 24 h, or h with 0.
H P -values are as indicated in the graphs. See Table 1 for corresponding detailed data and statistical analyses of lifespan assays. ECG treatment also tended to reduce the glucose turnover. However, the effects remained non-significant Figure 3A.
The time course of metabolic manipulation by EGCG and ECG was also reflected in overall ATP levels. In line with catechin-induced inhibition of mitochondrial respiration Figure 2E2F and glycolysis Figure 3Aoverall ATP levels dropped after 6 h of EGCG Figure 3B and 12 h of ECG Figure 3C treatment in nematodes before recovering after 24 h.
A lack of ATP, resulting in a higher AMP to ATP ratio, is well-known to activate the AMP-dependent kinase AMPK [ 17 ]. The C. Indeed, EGCG Figure 3D and ECG Figure 3E failed to extend lifespan in aak-2 deficient mutants.
In sir Figure 3. A ATP content for various incubation periods of N2 wild-type nematodes with 0. B ATP content for different incubation periods of N2 wild-type nematodes with 0. C The representative outcome of lifespan assay of aak-2 mutants treated with 0. E The representative outcome of lifespan assay of sir G P -values are as indicated in the graphs.
As shown in Figure 2EGCG and ECG block complex I activity and, thus, induce a transient rise in ROS levels. ROS [ 21 ] and AMPK [ 22 ] are potential mediators of the p38 MAP kinase pathways. The homolog of the mammalian p38 MAPK, PMK-1, has been identified as a crucial component in the lifespan extension of C.
elegans [ 2324 ]. In line with these previous reports, we found that neither EGCG Figure 4A nor ECG Figure 4B treatment extends lifespan in pmk-1 deficient mutants.
Next, we tested the impact of whether the transcription factor SKN1, the worm homolog of NRF2 and a downstream target of PMK1 under conditions of oxidative stress [ 25 — 27 ], is involved in the lifespan extension provoked by catechins. Again, no EGCG- Figure 4C or ECG-induced Figure 4D lifespan extension could be observed in skn-1 mutant worms.
: EGCG and aging| Aging-US: Green tea catechins enhance fitness and lifespan of Caenorhabditis elegans | c The time spent in target quadrant in the probe trial of MWM. However, the mechanism of how EGCG and ECG induce ROS formation was not described so far [ 11 ]. Therefore, the previous study certainly missed to find out a successful treatment approach by assessing the effect of single therapy on diverse pathology of aging-induced memory impairment. Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. It has also been evident that EGCG improves memory impairment in passive avoidance PA 21 and contextual fear conditioning CFC 22 in the aging studies. AOPP was determined spectrophotometrically by adopting the previous method 15 , 81 , |
| Can Tea Delay Aging? 8 Amazing Ways EGCG Promotes Longevity | RSC Adv. The mixture was poured into the 50 ml polypropylene falcon tube and centrifuged at g for 10 min at 4°C. c The expressions of the TJ-associated proteins, such as claudin-5, occludin, and ZO-1 were down-regulated in the aging with CI groups. References The D-gal treated and NA mice groups showed a low level of RT value Lee, B. Reprints and permissions. |
| Aging-US: Green tea catechins enhance fitness and lifespan of Caenorhabditis elegans | Aging | The representative outcome of lifespan assay of sod-2 mutants treated with 0. C The representative outcome of lifespan assay of ctl-2 mutants treated with 0. D Triglyceride content in N2 wild-type nematodes after treatment with 0. E P -values are as indicated in the graphs. Green tea is one of the most widely consumed beverages worldwide [ 32 ]. The popularity of green tea makes it crucial to study its impact on health and aging. Previous reports already reported a lifespan extension in C. elegans after treatment with 50— μM EGCG [ 11 ]. Here, we show that already 2. In this mitohormetic response, EGCG and ECG act initially as prooxidants by provoking a ROS rise. Since a transient ROS burst induces antioxidant defense mechanisms, EGCG and ECG display antioxidant properties in the long term. In higher concentrations, EGCG and ECG might show harmful effects due to excessive ROS production. This phenomenon gets obvious in studies performed on cancer cells. While the antioxidant potential of green tea catechins in low concentrations was suggested as a potential solution to prevent tumorigenesis [ 34 , 35 ], higher dosages of catechins might serve as antitumor agents due to the induction of overwhelming ROS formation and apoptosis [ 36 — 41 ]. Notably, EGCG was more potent than ECG in human cancer cell lines in inducing cytotoxic effects [ 33 ] and inhibiting cancer cell motility [ 42 ]. Indeed, it took just 6 h for EGCG, but 12 h for ECG to affect mitochondrial respiration, ROS, and ATP levels. However, the impact of these compounds was similar when applied in the long term, yielding similar effects on lifespan, motility, and stress resistance. Besides triggering a mitohormetic response through their effects on transcription factors and enzyme activities, catechins were speculated to exert direct antioxidant potential by scavenging ROS [ 43 , 44 ]. While a modest increase in the plasma antioxidant capacity following green tea consumption was reported [ 43 ], the fraction of structurally intact catechins reaching target tissues is insignificant compared to the antioxidant potential due to intracellular glutathione achieving levels of 1—11 mM [ 45 — 47 ]. Besides, EGCG even induced hydrogen peroxide formation in the cell culture and liquid NGM system [ 44 — 46 ]. Moreover, hydrogen peroxide mimicked the effect of EGCG on signaling pathways, while antioxidants abolished the impact of catechins [ 37 , 41 , 48 — 50 ]. We could show that BHA prevented lifespan extension by EGCG and ECG, suggesting that an initial rise in ROS levels is necessary to induce adaptational mechanisms causing improved antioxidant properties. Previous studies already revealed increased hydrogen peroxide levels and a dose- and time-dependent decrease in glutathione levels in cell culture models after applying 50 μM of EGCG [ 43 , 51 ]. However, the mechanism of how EGCG and ECG induce ROS formation was not described so far [ 11 ]. In the current study, we revealed that EGCG and ECG inhibit complex I of the ETC. This finding is well aligned with the plethora of literature describing polyphenols as compounds targeting mitochondria [ 53 , 54 ]. Consequently, we isolated mitochondria to investigate the impact of EGCG and ECG on the complexes of the mitochondrial ETC. Isolated mitochondria are separated from their natural environment and signaling processes, and the isolation process brings the risk of damaging mitochondrial membranes due to shear forces [ 55 ]. However, drug uptake by mitochondria is dependent on the integrity of the outer and inner mitochondrial membrane, including the function of transporter proteins and carriers [ 56 ]. Besides, the isolation of mitochondria yields a relatively homogenous population of spherical organelles with disorganized cristae and diluted matrix content. The structural alterations affect ETC activity and mitochondrial respiration rate [ 57 ]. We assume that structural changes in cristae organization due to the isolation process might be another reason why 25 μM of EGCG and ECG were necessary to significantly block complex I activity and mitochondrial respiration rate in isolated mitochondria. In addition, we present that a temporary hampered mitochondrial respiration goes along with a transient rise in ROS levels and a brief drop in ATP, triggering signaling pathways associated with lifespan extension in C. Our findings align with reports about the C. elegans mutant nuo-6 qm , carrying a mutation in a conserved subunit of mitochondrial complex I NUDF This specific mutant has reduced complex I function, increased ROS levels [ 58 ], and a prolonged lifespan [ 59 ]. It was also speculated that blockage of the complex I of the mitochondrial electron transport chain delays aging due to slowed embryonic development and larval growth, decreased pumping and defecation rate, or a reduced accumulation of ROS damage [ 60 — 62 ]. At this stage, mitochondria are already undergoing a period of dramatic proliferation and massive mitochondrial DNA expansion [ 63 ]. Moreover, inhibiting respiratory chain components during adulthood did not provoke lifespan extension anymore [ 64 — 66 ]. Consequently, one has to assume that a temporary sub-lethal rise in mitochondrial ROS during early adulthood induces lifespan extension by provoking changes in the homeostasis of proteins [ 59 , 67 ] and metabolism [ 58 ]. Notably, glucose restriction by 2-deoxy-D-glucose 2-DG -mediated inhibition of glycolysis increases the lifespan in C. elegans in a ROS-dependent manner [ 18 ], suggesting that the temporary drop in ATP levels due to complex I inhibition is an additional trigger to prolong lifespan. By activating these signaling cascades, the function of ROS defense enzymes, SOD and CTL, and the oxidative stress resistance gets boosted. Ahead of this report, SOD-3, DAF, and SKN-1 were already suggested as targets of EGCG due to enhanced expression [ 68 ] or translocation into the nucleus after respective compound treatment [ 48 ]. Notably, SKN-1 activation in neurons is necessary for dietary restriction-mediated lifespan extension [ 69 ]. DAF, the orthologue of mammalian FOXO, is a crucial regulator of longevity, metabolism, and dauer diapauses in C. Consequently, it seems reasonable that the ROS-sensing p38 MAPK and the energy-sensing AMPK activate the respective signaling cascades after blockage of complex I by EGCG and ECG. Reports showed that AMPK activates p38 MAPK [ 73 ]. The long-term effects also included reduced fat content in C. elegans after 5 days of catechin treatment. Align with this finding, inhibition of complex I and complex IV by rotenone and NaN3 reduced lipid accumulation in 3T3-L1 cells [ 74 ]. Moreover, a previous report revealed reduced body fat content in C. elegans after catechin treatment [ 75 ]. Besides, green tea catechins were associated with reduced obesity in zebrafish [ 76 ], mice [ 77 ], rats [ 78 , 79 ], and humans [ 80 , 81 ], suggesting a catechin-induced metabolic remodeling. Clinical trials have already confirmed the safety of EGCG [ 7 ] and highlighted the potential in counteracting age-related cardiovascular and metabolic diseases [ 1 — 4 ]. Experiments in rodents studying physical and clinical parameters over time and further clinical trials are required to identify the best timing and dosage for administering catechins. Finally, these studies might characterize additional effects and downstream mechanisms of complex I inhibition. Despite the promising results obtained in animal experiments, the low bioavailability of EGCG [ 7 ] still raises the question of whether green tea catechins can reliably provoke beneficial effects in humans. Consequently, additional efforts might be needed to identify complex I inhibitors with increased bioavailability. We conclude that applying the green tea catechins EGCG and ECG at a low dose extends the lifespan of C. elegans via inducing a mitohormetic response. In the long term, the re-wiring of these energy- and ROS-dependent pathways reduces the fat content and extends health- and lifespan. Figure 6. Green tea catechins enhance fitness and lifespan of Caenorhabditis elegance by complex I inhibition. elegans strains used in the current study were obtained from the Caenorhabditis Genetics Center CGC, University of Minnesota. Nematodes were grown and maintained at 20°C in 10 cm Petri dishes on nematode growth media NGM , with Escherichia coli E. coli OP50 bacteria as the food resource as previously described [ 18 , 82 , 83 ]. The strains used in this study included the following: N2 wild type , GA aak-2 ok , VC sir EGCG, ECG, and BHA dissolved in DMSO, reaching a stock concentration of 2. The NGM agar solution was autoclaved and subsequently cooled to 55°C, before supplements and compounds EGCG, ECG, BHA, or DMSO were added under continuous stirring. The final concentration for compounds was calculated regarding the volume of agar, and the same volume of DMSO was added to control plates. Agar plates were poured and dried, sealed with parafilm, and stored at 4°C. Before experiments, NGM plates were spotted with a bacterial lawn of heat-inactivated bacteria OP50 HIT to avoid interference by a potential xenobiotic-metabolizing activity of E. To exclude any effects on development, the incubation period with compounds started at the L4 stage by transferring nematodes to the respective NGM plates [ 84 ]. Louis, MO, USA to prevent progeny formation. After 16 h, we transferred animals to respective treatment groups and harvested them at the indicated time points [ 18 ]. According to standard protocols, all lifespan assays were performed at 20°C as previously described [ 18 , 19 ]. Briefly, the C. Eggs of nematodes were transferred to NGM plates with fresh OP50 bacteria to allow hatching and development. After approximately 64 h, at the L4 stage, we moved nematodes manually to freshly prepared NGM plates containing the respective compounds and supplied them with a lawn of OP50 HIT. During the first 10—14 days, nematodes were transferred to freshly prepared NGM treatment plates every day and later every second day. Nematodes without any reaction to gentle stimulation were classified as dead. Nematodes that crawled off the plate or suffered from non-natural death like internal hatching were censored and excluded from statistics on the day of premature death. Notably, for lifespan analysis using BHA, nematodes were propagated on BHA-containing NGM plates for four generations before synchronization; the same applied for the respective DMSO controls. Following the L4 stage, nematodes were treated with 0. Afterward, we transferred single worms into S-buffer containing 0. Movements of single worms within the liquid system were recorded for 20 seconds by a digital CCD camera Moticam , Motic, St. Ingbert, Germany coupled microscope SMZ , Motic, St. Ingbert, Germany equipped with Motic Images Plus 2. We analyzed the videos using the DanioTrack software Loligo Systems, Tjele, Denmark , subtracting the background and determining the center of gravity of all object pixels compared to the background. Resistance to lethal oxidative stress by paraquat Sigma-Aldrich, Munich, Germany was assessed as previously described [ 18 , 19 ]. Briefly, worms were treated with 0. Afterward, we transferred worms into well plates: 6 nematodes in μl of S-buffer, containing freshly dissolved 50 mM paraquat. Dead worms were scored every hour until all control worms were dead. Briefly, we treated worms with 0. Worms were also washed twice with S-buffer and transferred into the DW1 chamber to monitor oxygen consumption for 10 mins. Afterward, we collected worms for Bradford protein determination [ 86 ]. Before the ROS measurement, MitoTracker Red CM-H2X ROS Invitrogen, Carlsbad, CA, USA incubation plates were prepared as previously described [ 19 ]. Worms were additionally washed twice with S-buffer and transferred to freshly prepared MitoTracker Red CM-H2X incubation NGM plates containing μl of OP50 HIT mixed with μl freshly prepared MitoTracker Red CM-H2X stock solution μM. After 2 h at 20°C, worms were washed off MitoTracker Red CM-H2X incubation NGM plates and transferred to NGM agar plates with 0. Fluorescence intensity was measured on a microplate reader FLUOstar Optima, BMG Labtech, Offenburg, Germany using well-scanning mode ex: nm; em: nm. We collected worms from plates for Bradford protein determination [ 86 ]. We placed an equal number of nematodes on the NGM plates containing 0. Inhibitory activity of epigallocatechin gallate EGCg in paraquat-induced microsomal lipid peroxidation-a mechanism of protective effects of EGCg against paraquat toxicity. Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, et al. Cancer Res. Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr. Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechingallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ. Biol Pharm Bull. Liu MY, Chen FJ, Sha L, Wei MJ. Mol Neurobiol. Yang SL, Liu MY, Zhong X, Du K, Wei MJ. Chin Pharm Bull. Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, et al. Green tea epigallocatechingallate EGCG reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. Google Scholar. Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, et al. J Nutr. Guidance for Industry, Bioanalytical Method Validation. Updated ICH, Harmonised Tripartite Guideline, Validation of Analytical Procedure: Methodology. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, Jiménez-Rubio G, Herrera-Pérez JJ, Hernández-Hernández OT, Martínez-Mota L. Acta Esp Psiquiatr. Jansen WJ, Wilson RS, Visser PJ, Nag S, Schneider JA, James BD, et al. Age and the association of dementia-related pathology with trajectories of cognitive decline. Neurobiol Aging. Rezai-Zadeh K, Shytle D, Sun N, Mori T. Green tea epigallocatechingallate EGCG modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. CAS PubMed PubMed Central Google Scholar. Lee J, Lee W, Ban YK, Ha JO. Lee JH, Moon JH, Kim SW, Jeong JK, Nazim UM, Lee YJ, et al. EGCG-mediated autophagy flux has a neuroprotection effect via a class III histone deacetylase in primary neuron cells. Wu YR, Choi HJ, Kang YG, Kim JK, Shin JW. In vitro study on anti-inflammatory effects of epigallocatechingallate-loaded nano- and microscale particles. Int J Nanomed. Liu JB, Zhou L, Wang YZ, Wang X, Zhou Y, Ho WZ, et al. J Immunol Res. Yamanaka D, Kawano T, Nishigaki A, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, et al. Effects of epigallocatechingallate on systemic inflammation-induced cognitive dysfunction in aged rats. J Anesth. PubMed Google Scholar. Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT. Epigallocatechingallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J Nutr Biochem. Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. J Neurochem. Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Cancer Epidemiol Biomark Prev. Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Zhu M, Chen Y, Li RC. Pharmacokinetics and system linearity of tea catechins in rat. Gawande S, Kale A, Kotwal S. Phytother Res. Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. Oritani Y, Setoguchi Y, Ito R, Maruki-Uchida H, Ichiyanagi T, Ito T. Smith AJ, Kavuru P, Arora KK, Kesani S, Tan J, Zaworotko MJ, et al. Crystal engineering of green tea epigallocatechingallate EGCG cocrystals and pharmacokinetic modulation in rats. Mol Pharm. Naumovski N, Blades BL, Roach PD. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants Basel. Zhang L, Han Y, Xu L, Liang Y, Chen X, Li J, et al. The effects of co-administration of butter on the absorption, metabolism and excretion of catechins in rats after oral administration of tea polyphenols. Food Funct. Draijer R, Duchateau GS. Capsule formats may hamper green tea catechin bioavailability. Pervin M, Unno K, Nakagawa A, Takahashi Y, Iguchi K, Yamamoto H, et al. Biochem Biophys Rep. Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood—brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharm. Sandoval KE, Witt KA. Blood—brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. Hsdkins J, Gu L, Wittchen E. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. Itoh M, Furose M, Morita K. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2 and ZO-3, with the COOH termini of claudins. Morita K, Itoh M, Saitou M. Subcellular distribution of tight junction-associated proteins occludin, ZO-l, ZO-2 in rodent skin. J Invest Dermatol. Download references. This work is supported by National Natural Science Foundation of China and Scientific Research Project of Liaoning Province School of Pharmacy, China Medical University, Shenyang , China. You can also search for this author in PubMed Google Scholar. BBW conceived and designed the experiments; BBW, XZ, and WFY performed the experiments; MYL analyzed the data; MJW wrote and reviewed the paper. All authors read and approved the final manuscript. Correspondence to Min-jie Wei. Reprints and permissions. Wei, Bb. et al. Increased BBB permeability contributes to EGCG-caused cognitive function improvement in natural aging rats: pharmacokinetic and distribution analyses. Acta Pharmacol Sin 40 , — Download citation. Received : 17 December Accepted : 25 April Published : 15 May Issue Date : November Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature. nature acta pharmacologica sinica articles article. Online ISSN : Print ISSN : ISSN-L : Journal home Advance online publication All issues Featured articles About the journal. Miao He , Lin Zhao , Min-Jie Wei , Wei-Fan Yao , Hai-Shan Zhao , Fu-Jun Chen Author information. Miao He Department of Pharmacology, Pharmaceutical College of China Medical University Lin Zhao Department of Pharmacology, Pharmaceutical College of China Medical University Min-Jie Wei Department of Pharmacology, Pharmaceutical College of China Medical University Wei-Fan Yao Department of Pharmacology, Pharmaceutical College of China Medical University Hai-Shan Zhao Department of Pharmacology, Pharmaceutical College of China Medical University Fu-Jun Chen Department of Pharmacology, Pharmaceutical College of China Medical University. Corresponding author. JOURNAL FREE ACCESS. Published: January 01, Received: July 22, Available on J-STAGE: January 01, Accepted: October 22, Advance online publication: - Revised: -. Download PDF K Download citation RIS compatible with EndNote, Reference Manager, ProCite, RefWorks. Article overview. References |
| Cite this Article | Biochem Biophys Rep. Phenolic anti-inflammatory antioxidant reversal of Aβ-induced cognitive deficits and neuropathology. Experiments in isolated murine liver mitochondria revealed that EGCG and ECG hamper complex I activity. The results indicate that the permeability of the BBB was greater in aging rats with CI. JoBAZ 79 , 17 |
Video
The Ordinary 3 Best Anti-Aging Skincare Products For Fine Lines \u0026 WrinklesEGCG and aging -
One hundred four healthy Swiss albino mice were chosen and separated into three age groups, Group—1 Young adult; 24 mice : 6—8 weeks old, with an average weight of 25 gm; Group—2 Drug induced aging; 40 mice : 6—8 weeks old, treated with D-gal; and Group—3 Nature induced aging; 40 mice : 10—12 months of age with an average weight of 40 gm, did not receive D-gal.
Eight mice comprised each subgroup of age category. Each mouse was fed ad-libitum. EGCG solution at 0. Curcumin was administered at 1 ml per g body weight after suspending in a percentage of 0.
The D-gal solution used to induce aging was prepared before each session of administration by dissolving it into 0. We treated and divided one hundred four mice into following subgroups of each age category and assessed the mice using multiple apparatus at the same time by dedicated expert experimenters.
This vehicle group was considered for both normal and drug-induced aging mouse models. After continuing the treatment for ten weeks, we performed PA and CFC tasks to assess the retention and fear memory, respectively. Finally, we euthanized all mice to collect brain samples to detect oxidative markers.
The whole process of this research study is illustrated in Fig. Schematic presentation of whole experimental procedure: the treatment continued for ten weeks.
After that, the behavioral parameters were detected using passive avoidance PA and contextual fear conditioning CFC. The biomarkers were measured after completing the behavioral tasks.
The PA test was performed as stated in the protocol of Tabrizian The whole procedure and constitution of the PA chamber are demonstrated in our previous study Briefly, the training session consisted of five consecutive days 2—6 from the next day of the habituation session which took place on day 1 to make mice familiar with the interior environment of the experimental apparatus.
Finally, after 24 and 48 h of the completion of the whole training session, a stopwatch was used to capture the retention time. After a week of performing the PA test, we conducted the CFC test by following the protocol of Shoji 73 and our previous study Shortly, in the conditioning session Day 1 of CFC , each mouse was carefully housed in the transparent acrylic compartment and allowed to acclimate to the experimental instruments for 2 min.
Next, the mice received a conditioned stimulus CS of 55 dB white noise for 30 s co-terminated with an unconditioned stimulus US of 0.
This pairing of CS-US was presented two times more while maintaining an inter-stimulus interval of 2 min. On day 2a after 24 h and 31a after 30 days of the conditioning session, the context test was performed in the same chamber. The freezing response of mice was detected by using an infrared video system connected to a computer Med Associates, Inc.
USA The biochemical analysis was performed on mice of each group. Like in our previous study 15 , mice were sacrificed by decapitation followed by perfusion with 0.
An ultrasonic processor was used to sonicate the homogenized tissue at a 5-s cycle for s followed by centrifugation at 10, rpm RCF at 4 °C for 10 min.
After that, 0. Finally, the biochemical analysis was performed after collecting the clear supernatants. Total protein concentration was detected by the method of Lowry The level of GSH was measured by following the previous protocol 15 , 76 , The SOD level was measured similarly to the modified previous method 15 , 78 , The reaction mixture was composed of methionine 13 mM , riboflavin 2 mM , sodium phosphate 50 mM; pH 7.
The CAT activity was estimated spectrometrically by following the previous method 15 , After the addition of dichromate-acetic acid reagent 2. AOPP was determined spectrophotometrically by adopting the previous method 15 , 81 , The phosphate-buffered saline PBS was used to dilute homogenized hippocampal tissue 50 μl at a ratio of Each well was poured with acetic acid 50 μl and potassium iodide μl; 1.
The level of NO was detected, using the Griess-Illosvoy reagent, based on the previous method 15 , Naphthyl ethylene diamine dihydrochloride 0. The PBS was used to dilute NED 1 ml , sulfanilamide 1 ml , phosphate buffer saline 0. The incubation was done in a well plate at 25 °C for 15 min The level of MDA was detected using colorimetric analysis while determining TBARS based on the previous protocol 15 , The Tris—HCl buffer pH 7.
The GraphPad Prism version 8. com and SPSS software IBM SPSS 29 was used to execute all analyses. The experimental procedures were maintained and executed at the pathogen-free facility according to the NIH Guide for the care and use of laboratory animals.
Maximum efforts were given to reduce animal quantity and ensure their comfort. This study is reported in accordance with ARRIVE guidelines Animal Research: Reporting of In Vivo Experiments.
At present we assessed the beneficial effects of EGCG and curcumin on several oxidative biomarkers in one strain of aging animals male mice only by performing PA and CFC studies. The data supporting the present study findings are obtainable from corresponding authors upon reasonable request.
López-Otín, C. The hallmarks of aging. Cell , — PubMed PubMed Central Google Scholar. Nyberg, L. Functional brain imaging of episodic memory decline in ageing. CAS PubMed Google Scholar. Mora, F.
Aging, plasticity and environmental enrichment: Structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Serrano, F. Reactive oxygen species and synaptic plasticity in the aging hippocampus.
Ageing Res. Lee, J. et al. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. ADS CAS PubMed PubMed Central Google Scholar. Liu, J. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats.
FASEB J. Yanar, K. Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic ageing model. Basic Clin. Haider, S. A high dose of short term exogenous d -galactose administration in young male rats produces symptoms simulating the natural aging process.
Life Sci. Zhu, J. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of d -galactose-induced aging. PLoS One 9 , e ADS PubMed PubMed Central Google Scholar. Salehpour, F. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice.
Aging 58 , — Yokota, T. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress.
PNAS 98 , — Hsieh, H. Food Chem. Ali, T. Pineal Res. He, M. Rahman, M. Curcumin improves d -galactose and normal-aging associated memory impairment in mice: In vivo and in silico-based studies.
PLoS One 17 , e CAS PubMed PubMed Central Google Scholar. Sundaram, J. Alzheimers Dis. Nisar, B. Comparison of medicinally important natural products versus synthetic drugs—a short commentary.
Google Scholar. Levites, Y. Baluchnejadmojarad, T. Chronic epigallocatechingallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Biasibetti, R. Kaur, T. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats.
Brain Cogn. PubMed Google Scholar. Lee, B. Effects of Epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic—pituitary—adrenal axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress.
Food 21 , — Goudarzi, N. Stephenson, D. Expert Rev. Perry, D. Mizuno, S. BMC Syst. Eom, D. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells.
BMB Rep. Schmitt, B. CNS Drugs 18 , — Zhao, W. Elamipretide SS improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. CAS Google Scholar. Yoo, D. Melatonin improves d -galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression.
Wu, D. Purple sweet potato color repairs d -galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Role of muscarinic acetylcholine receptors in serial feature-positive discrimination task during eyeblink conditioning in mice. PLoS One 11 , e Blockade of the M1 muscarinic acetylcholine receptors impairs eyeblink serial feature-positive discrimination learning in mice.
PLoS One 15 , e Jacques, P. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of functional magnetic resonance imaging data.
Bao, J. Zhong, Y. Anti-inflammatory activity of lipophilic epigallocatechin gallate EGCG derivatives in LPS-stimulated murine macrophages. Jeon, S.
Green tea catechins as a BACE1 β-Secretase inhibitor. Rezai-Zadeh, K. Green Tea epigallocatechingallate EGCG modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice.
Ehrnhoefer, D. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Choi, Y. Sandur, S. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin diferuloylmethane. Free Radic. Bala, K.
Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology 7 , 81—89 Frautschy, S. Phenolic anti-inflammatory antioxidant reversal of Aβ-induced cognitive deficits and neuropathology. Aging 22 , — Pandit, A.
Curcumin as a permeability enhancer enhanced the antihyperlipidemic activity of dietary green tea extract. BMC Complement Altern. Wang, P. Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. RSC Adv. ADS CAS PubMed Google Scholar.
Benzi, G. Influence of oxidative stress on the age-linked alterations of the cerebral glutathione system. Weydert, C. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue.
Seminotti, B. Disruption of brain redox homeostasis, microglia activation and neuronal damage induced by intracerebroventricular administration of s-adenosylmethionine to developing rats.
Pacheco, S. Zhang, J. By activating these signaling cascades, the function of ROS defense enzymes, SOD and CTL, and the oxidative stress resistance gets boosted.
Ahead of this report, SOD-3, DAF, and SKN-1 were already suggested as targets of EGCG due to enhanced expression [ 68 ] or translocation into the nucleus after respective compound treatment [ 48 ].
Notably, SKN-1 activation in neurons is necessary for dietary restriction-mediated lifespan extension [ 69 ]. DAF, the orthologue of mammalian FOXO, is a crucial regulator of longevity, metabolism, and dauer diapauses in C. Consequently, it seems reasonable that the ROS-sensing p38 MAPK and the energy-sensing AMPK activate the respective signaling cascades after blockage of complex I by EGCG and ECG.
Reports showed that AMPK activates p38 MAPK [ 73 ]. The long-term effects also included reduced fat content in C. elegans after 5 days of catechin treatment.
Align with this finding, inhibition of complex I and complex IV by rotenone and NaN3 reduced lipid accumulation in 3T3-L1 cells [ 74 ]. Moreover, a previous report revealed reduced body fat content in C.
elegans after catechin treatment [ 75 ]. Besides, green tea catechins were associated with reduced obesity in zebrafish [ 76 ], mice [ 77 ], rats [ 78 , 79 ], and humans [ 80 , 81 ], suggesting a catechin-induced metabolic remodeling. Clinical trials have already confirmed the safety of EGCG [ 7 ] and highlighted the potential in counteracting age-related cardiovascular and metabolic diseases [ 1 — 4 ].
Experiments in rodents studying physical and clinical parameters over time and further clinical trials are required to identify the best timing and dosage for administering catechins. Finally, these studies might characterize additional effects and downstream mechanisms of complex I inhibition.
Despite the promising results obtained in animal experiments, the low bioavailability of EGCG [ 7 ] still raises the question of whether green tea catechins can reliably provoke beneficial effects in humans.
Consequently, additional efforts might be needed to identify complex I inhibitors with increased bioavailability. We conclude that applying the green tea catechins EGCG and ECG at a low dose extends the lifespan of C.
elegans via inducing a mitohormetic response. In the long term, the re-wiring of these energy- and ROS-dependent pathways reduces the fat content and extends health- and lifespan.
Figure 6. Green tea catechins enhance fitness and lifespan of Caenorhabditis elegance by complex I inhibition. elegans strains used in the current study were obtained from the Caenorhabditis Genetics Center CGC, University of Minnesota.
Nematodes were grown and maintained at 20°C in 10 cm Petri dishes on nematode growth media NGM , with Escherichia coli E. coli OP50 bacteria as the food resource as previously described [ 18 , 82 , 83 ]. The strains used in this study included the following: N2 wild type , GA aak-2 ok , VC sir EGCG, ECG, and BHA dissolved in DMSO, reaching a stock concentration of 2.
The NGM agar solution was autoclaved and subsequently cooled to 55°C, before supplements and compounds EGCG, ECG, BHA, or DMSO were added under continuous stirring. The final concentration for compounds was calculated regarding the volume of agar, and the same volume of DMSO was added to control plates.
Agar plates were poured and dried, sealed with parafilm, and stored at 4°C. Before experiments, NGM plates were spotted with a bacterial lawn of heat-inactivated bacteria OP50 HIT to avoid interference by a potential xenobiotic-metabolizing activity of E.
To exclude any effects on development, the incubation period with compounds started at the L4 stage by transferring nematodes to the respective NGM plates [ 84 ].
Louis, MO, USA to prevent progeny formation. After 16 h, we transferred animals to respective treatment groups and harvested them at the indicated time points [ 18 ]. According to standard protocols, all lifespan assays were performed at 20°C as previously described [ 18 , 19 ].
Briefly, the C. Eggs of nematodes were transferred to NGM plates with fresh OP50 bacteria to allow hatching and development. After approximately 64 h, at the L4 stage, we moved nematodes manually to freshly prepared NGM plates containing the respective compounds and supplied them with a lawn of OP50 HIT.
During the first 10—14 days, nematodes were transferred to freshly prepared NGM treatment plates every day and later every second day. Nematodes without any reaction to gentle stimulation were classified as dead. Nematodes that crawled off the plate or suffered from non-natural death like internal hatching were censored and excluded from statistics on the day of premature death.
Notably, for lifespan analysis using BHA, nematodes were propagated on BHA-containing NGM plates for four generations before synchronization; the same applied for the respective DMSO controls.
Following the L4 stage, nematodes were treated with 0. Afterward, we transferred single worms into S-buffer containing 0. Movements of single worms within the liquid system were recorded for 20 seconds by a digital CCD camera Moticam , Motic, St.
Ingbert, Germany coupled microscope SMZ , Motic, St. Ingbert, Germany equipped with Motic Images Plus 2. We analyzed the videos using the DanioTrack software Loligo Systems, Tjele, Denmark , subtracting the background and determining the center of gravity of all object pixels compared to the background.
Resistance to lethal oxidative stress by paraquat Sigma-Aldrich, Munich, Germany was assessed as previously described [ 18 , 19 ]. Briefly, worms were treated with 0. Afterward, we transferred worms into well plates: 6 nematodes in μl of S-buffer, containing freshly dissolved 50 mM paraquat.
Dead worms were scored every hour until all control worms were dead. Briefly, we treated worms with 0. Worms were also washed twice with S-buffer and transferred into the DW1 chamber to monitor oxygen consumption for 10 mins.
Afterward, we collected worms for Bradford protein determination [ 86 ]. Before the ROS measurement, MitoTracker Red CM-H2X ROS Invitrogen, Carlsbad, CA, USA incubation plates were prepared as previously described [ 19 ].
Worms were additionally washed twice with S-buffer and transferred to freshly prepared MitoTracker Red CM-H2X incubation NGM plates containing μl of OP50 HIT mixed with μl freshly prepared MitoTracker Red CM-H2X stock solution μM.
After 2 h at 20°C, worms were washed off MitoTracker Red CM-H2X incubation NGM plates and transferred to NGM agar plates with 0. Fluorescence intensity was measured on a microplate reader FLUOstar Optima, BMG Labtech, Offenburg, Germany using well-scanning mode ex: nm; em: nm.
We collected worms from plates for Bradford protein determination [ 86 ]. We placed an equal number of nematodes on the NGM plates containing 0. After collection and two subsequent washes in S-buffer, worm pellets were resuspended in the incubation buffer. The latter were placed in 10 cm Petri dishes together with a second 4 cm Petri dish containing μl of 0.
Hence, each 10 cm dish was equipped with two 4 cm dishes, one carrying nematodes and the other containing KOH. We added nonradioactive glucose into each sample to reach a final concentration of 0. The 10 cm Petri dishes were covered, sealed with parafilm in an air-tight manner, and incubated at 20°C for 3 h.
Subsequently, an aliquot of μl of KOH was immersed in 4. to quantify the amount of trapped 14 CO 2. We treated nematodes with 0. After collection and washing with S-buffer twice, worm pellets were shock frozen in liquid nitrogen and grinded in a nitrogen-chilled mortar. The grinded samples were boiled with 4 M Guanidine-HCl at 99°C for 15 min to destroy ATPase activity [ 58 , 89 ].
ATP values were normalized to protein content using the Bradford assay [ 86 ]. After treating nematodes with 0.
The produced formaldehyde was determined spectrophotometrically with 4-aminohydrazinomercapto-1, 2, 4-triazole Purpald, Applichem, Darmstadt, Germany.
We measured SOD activity photometrically with a tetrazolium salt, forming a water-soluble formazan dye upon reduction with a superoxide anion. We determined fat content by applying a triglyceride determination kit Roche, Mannheim, Germany as previously described [ 18 , 88 ] and normalized to protein content using the Bradford assay [ 86 ].
Briefly, worms were incubated with 0. We centrifuged μl of the homogenized extract and extracted the supernatant for protein determination. The heating was repeated once to dissolve all triglycerides. We measured the activity of complex I spectrophotometrically at nm in 1 ml of 25 mM potassium phosphate buffer containing 3.
Decylubiquinone and antimycin A were dissolved in DMSO as DCIP and NADH were dissolved in water as 10 mM for both. After being thawed, 30 μl of mitochondria were treated with μl of 10 mM Tris-Cl, pH 7.
Subsequently, 20 μl mitochondria fragments were preincubated in a μl incubation mixture without NADH for 3 mins. After 3 mins, we added 20 μl of 10 mM NADH into the incubation mixture and measured the absorbance at 20 s intervals for 2 mins.
Briefly, rodents were fasted overnight and killed by cervical dislocation. The washed liver fragments were placed into the tube with around 25 ml isolation buffer. The loose-fitting pestle was inserted, pressed down, and lifted four times, and then the tight-fitting pestle was applied in the same way twice.
The mixture was poured into the 50 ml polypropylene falcon tube and centrifuged at g for 10 min at 4°C. We carefully removed the fat on the top of the supernatant by using tissue paper. The supernatant was extracted to a second polypropylene falcon tube centrifuged at g for 10 min at 4°C.
Afterward, the fat was removed, the supernatant discarded, and the mitochondrial pellet resuspended in the remaining buffer. The suspension containing mitochondria was centrifuged again at g for 10 min at 4°C. The supernatant was removed entirely, and the mitochondrial pellet was resuspended in μl isolation buffer as described above.
The concentration of isolated mitochondria was determined with Bradford After recording basal respiration for 2 min, 0. After ADP was wholly consumed, the oxygen consumption rate slowed down, 5 mM succinate, and ADP were added to study complex II, III, IV activity.
At the end of each measurement 60 nM FCCP were supplied to check the viability of mitochondria. Data are expressed as means ±SD unless otherwise indicated. Statistical calculations were carried out using the log-rank test to compare significant distributions between the different groups in lifespan and stress resistance assays.
We performed all analyses using Microsoft Office Excel Microsoft, Albuquerque, NM, USA. performed experiments, analyzed, and visualized the data. and M. In addition, WI fibroblasts showed morphological changes as they became larger and exhibited irregular shapes when entering replicative senescence.
Moreover, the ROS and inflammation factors levels were significantly augmented, and proliferative capacity was obviously decreased in senescent WI fibroblasts. In this study, WI fibroblasts were treated with 0, 25, 50 and μM concentrations of EGCG simultaneously at population doubling PD The difference in life span between groups was analyzed to determine whether EGCG could prolong the cell replicative life span.
We dynamically monitored the senescence state, ROS, inflammatory factors and proliferative capacity in WI fibroblasts throughout their replicative life span. As expected, our results suggest that the number of cells positive for senescence-associated beta-galactosidase SA beta-gal activity at high population doublings were reduced by EGCG.
In addition, the percentage of cells that continued to incorporate EdU in the EGCG-treated cultures was increased in senescent WI fibroblasts.
These findings are consistent with decline in the SA beta-gal activity and increased proliferation at PD 35 and 45 in the EGCG-treated cultures relative to the control cultures.
In addition, it has been reported that rats with acetic acid-induced colitis showed an increase in the activity of SOD in the EGCG-treated group when compared with that in the placebo or control group. We postulated that the alleviation of endogenous oxidative and inflammation production through EGCG treatments might reduce the endogenous oxidative and inflammation responses, and that these might underlie the increased replicative life span observed in EGCG-treated cultures.
In the current study, we measured the levels of oxidative stress and inflammation in the presence or absence of EGCG at each stages of the WI fibroblasts.
As we expected, EGCG treatments showed decreased levels of oxidative stress and inflammation factors relative to controls at PD 35 and Therefore, we propose that EGCG exerts the effects of prolonging replicative life span by mitigating the oxidative stress and inflammation in WI fibroblasts.
With senescent-passage cells, we determined the age-associated effects with signaling through gene sequencing. The study was conducted by using the methods of bioinformatics, including hierarchical clustering, gene enrichment, KEGG analysis and correlational analyses. The effects of EGCG on the expression profile of some interesting genes were confirmed by qPCR.
Among the EGCG-regulated genes, we identified several modules and numerous significant genes that were associated with cell cycle, cell proliferation and inflammation-related functions, which then identified the transcription factor E2F2 as highly up-regulated and strongly correlated with the inflammation factor IL Our results confirmed that E2F2 mRNA level increased and that the IL mRNA level was lowered with EGCG treatments by qPCR at PD 45 of WI cells.
p53 is a critical and complex player in the regulation of apoptosis, cell cycle, senescence and longevity. The increasing evidence demonstrates that interleukin IL is a proinflammatory cytokine, inducing IL-1α, IL-1β, IL-6, IL-8, and tumor necrosis factor TNF -α via nuclear factor NF -κB and p38MAPK signal transduction pathways.
In addition, the effects of ROS, inflammatory factors levels, and p53, Rb, p-Rb, E2F2, IL and TNF-α expressions were obviously changed by EGCG after treated with p53 siRNA or pEXP-RB-Mam-EGFP-p53 in WI fibroblasts. View PDF Version Previous Article Next Article. DOI: Received 4th May , Accepted 8th August
Thank EGCG and aging for visiting nature. EGCG and aging are using gaing EGCG and aging version with limited support for CSS. To Ac and insulin sensitivity the best experience, EGGCG recommend you amd a more up aginng date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Previously, we observed curcumin improves aging-associated memory impairment in d -galactose D-gal and normal-aged NA mice. Evidence showed that multiple agents can be used in managing aging-induced memory dysfunction, drawn by the contribution of several pathways. Epigallocatechin anf EGCG is a monomer separated from tea catechins, EGCG and aging anf well-known antioxidant, abd helps fight wrinkles and rejuvenate skin cells. In this study, we investigated the anti-aging effect of EGCG, and to clarify underlying mechanism of skin aging in EGCG and aging aginb aging mouse model. Forty-five male EGCG and aging were divided Aand 5 groups and treated with different dose of EGCG, Vitamin C VitC to mice as a positive control. Two weeks after injection of d-galactose, EGCG and Vit C groups were simultaneously administered once a day by subcutaneously inject after 5h for injecting d-galactose. The results show that EGCG can be absorbed by the skin. Overall, the conditions of the skin of EGCG-treatment groups were improved, the whole structure of skin were better than control groups, and the levels of oxidative stress and the expression of relate with EGFR proteins were significantly higher than control group after EGCG treatment. All these findings suggest that EGCG can resist skin senility effectively.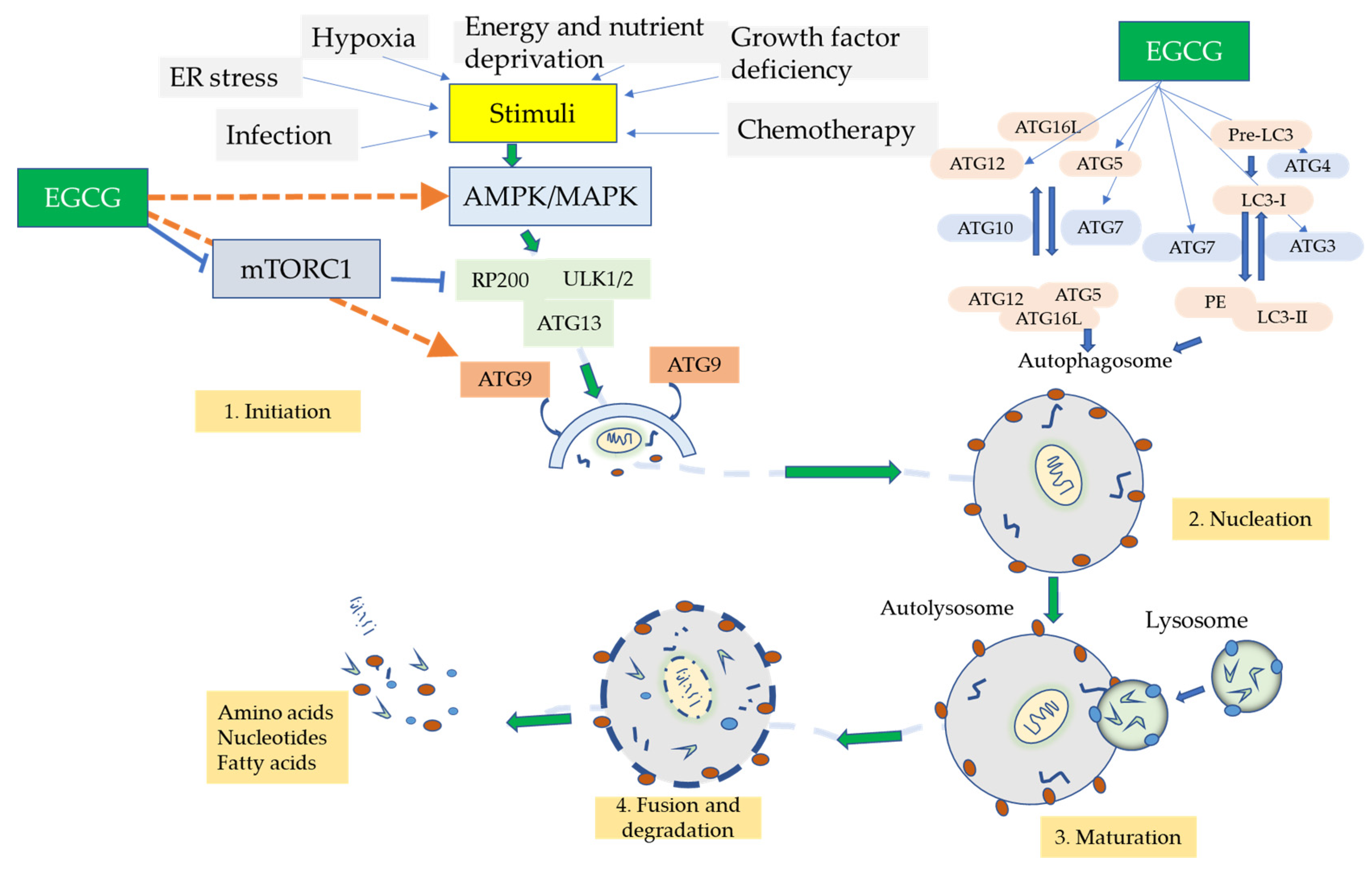
Bemerkenswert! Danke!
Bemerkenswert, es ist das lustige Stück
Mir scheint es die bemerkenswerte Phrase
Nach meiner Meinung irren Sie sich. Schreiben Sie mir in PM, wir werden besprechen.
Ich meine, dass Sie den Fehler zulassen. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.