Metformin and glucose control -
It's also used to help prevent type 2 diabetes if you're at high risk of developing it. Type 2 diabetes is a condition where the body does not make enough insulin, or the insulin that it makes does not work properly. This can cause high blood sugar levels hyperglycaemia.
Metformin lowers your blood sugar levels by improving the way your body handles insulin. It's usually prescribed for diabetes when diet and exercise alone have not been enough to control your blood sugar levels.
Metformin is also sometimes used to manage symptoms of polycystic ovary syndrome PCOS , a condition that affects how the ovaries work. It is not officially approved for PCOS. Metformin treats PCOS by lowering insulin and blood sugar levels. This can also improve ovulation and encourage regular periods, even if you do not have diabetes.
Metformin is available on prescription as tablets, as a liquid that you swallow and as sachets of powder that you dissolve in a drink. Page last reviewed: 24 March Next review due: 24 March Met IR b. and Met XR q. Met IR or Met XR.
t , last quantifiable concentration following dose administration. The relative bioavailability and exposure resulting from single daily doses of Met DR twice daily versus Met IR twice daily and Met XR once daily are shown in Fig.
The rate and extent of exposure AUC from time of dosing to the last measurable concentration and maximal drug concentration after dosing from the 1, mg Met DR b. The rate and extent of exposure from mg Met DR b.
The PK of Met DR was not dose proportional, which is consistent with the known increased bioavailability at lower doses. As expected, the comparison of Met XR to Met IR demonstrated bioequivalence based on total exposure.
Study 2 randomly assigned subjects 39—41 per group to six treatment groups. Twenty-eight There were no statistically significant differences in demographics between treatment groups. Subjects exhibited relatively good glycemic control at baseline Table 1.
All other characteristics related to diabetes were generally similar across treatment arms. Twenty-five subjects The percentage of subjects included in the week 12 evaluable population for each treatment group ranged from All active treatment groups had improvements in FPG level compared with placebo at week 4 Fig.
There were dose-dependent reductions in median FPG level; the reduction produced by the mg Met DR dose was statistically significant compared with that by placebo. Median reductions for 1, and 2, mg Met XR at week 4 were statistically significant.
The baseline-corrected AUC 4—12wk for FPG Fig. Change in FPG and fasting metformin concentrations in the week study study 2. A : Median change in FPG level at week 4. placebo; baseline is defined as the median measurement at day 1. placebo for pairwise comparison without adjustment. C : Median fasting plasma metformin concentrations.
LS mean SE changes in HbA 1c level from baseline were negligible for all Met DR treatments and for 1, mg Met XR treatment, while placebo increased the HbA 1c level by 0.
Not surprisingly, the administration of 2, mg Met XR resulted in an LS mean SE reduction of 0. Steady-state metformin concentrations were achieved by week 2 for all Met DR groups and the 1, mg Met XR group and by week 4 for the 2, mg Met XR group, which required dose titration through week 3 Fig.
In study 1, the most commonly reported TEAEs in any treatment group included diarrhea, nausea, vomiting, and headache. Most TEAEs were assessed as being unrelated to study treatment and were mild in intensity; there were no deaths. However, the incidence of gastrointestinal TEAEs was relatively low compared with prescribing information in all active treatment groups, with gastrointestinal TEAEs reported by 7.
As metformin accumulation can result in increased lactate production, which, in turn, increases the risk of the rare but serious metabolic complication of lactic acidosis, the effects of Met DR on plasma lactate levels were also evaluated in study 2. Mean lactic acid values were within normal ranges throughout the study, but were elevated from baseline by 0.
The lack of change from baseline in lactate levels for the Met DR groups most likely reflects lower metformin exposure. One subject treated with 2, mg Met XR experienced moderate blood lactate increases for 16 days up to 5. Change from baseline to week 12 in fasting lactate study 2.
Normal lactate range is 0. Metformin is the oldest and most commonly prescribed oral glucose-lowering medication in the world and is considered a first-line therapy for patients in whom T2DM is newly diagnosed Nevertheless, there is no consensus on its primary site of action, although it is generally agreed to have pleiotropic effects.
However, metformin also accumulates in the intestine at concentrations times greater than in plasma Thus, the gut is a major reservoir for metformin exposure and is potentially responsible for much of its glucose-lowering effects, including enhanced secretion of GLP-1 and peptide YY, which in turn affects systemic mechanisms including reducing hepatic glucose production through glucagon suppression and enhanced glucose-dependent insulin secretion 15 , 20 — While the effects of metformin on increasing GLP-1 secretion have been known for some time 24 — 27 , its significance is debated.
Interestingly, the increase in plasma GLP-1 levels resulting from metformin administration is similar to that of a DPP-4i 20 and thus could explain much of the glucose-lowering effect of metformin. In addition, unlike a DPP-4i that reduces GLP-1 degradation, metformin increases GLP-1 secretion and thus can significantly increase concentrations local to the L cell, which may in turn enhance neural signaling in the gut and portal vein to rapidly regulate glycemic control 28 — The current study demonstrates that metformin primarily restricted to the gut effectively lowers plasma glucose levels.
The observation that low doses of Met DR appear to be more effective than similar doses of the more bioavailable Met XR suggests that the gut contribution to glucose lowering may be more important than systemic mechanisms.
The apparent increase in potency was most evident when comparing the mg Met DR dose to the 1, mg Met XR dose Fig. These data indicate that the gut is the primary site of action for the glucose-lowering effect of metformin and that plasma exposure is less important, at least at these therapeutic doses.
From a mechanistic perspective, a limitation of the current study is that a higher Met DR dose was not included. The duodenum has a low density of gut hormone-secreting L cells, and it has been proposed that the rapid appearance of GLP-1 following a meal is a result of a complex integration of proximal and distal neural and hormonal signaling However, by virtue of its enteric coating, Met DR limits both proximal gut exposure and plasma exposure, so it is not possible to quantitate their potential individual contributions in these studies.
Importantly, our data are not in conflict with those from a recent report by Madiraju et al. Thus, while gut-based mechanisms appear to account for the majority of the glucose-lowering effect of metformin at therapeutic doses, the inhibition of the redox shuttle enzyme mitochondrial glycerophosphate dehydrogenase may have important glucose-lowering actions at higher metformin plasma exposures.
Our data show an increase in plasma lactate concentrations with Met XR treatments compared with placebo that was not observed with any of the Met DR groups.
Conditions that increase metformin plasma exposure renal impairment, hepatic insufficiency, or states of circulatory dysfunction can increase the risk of metformin-associated lactic acidosis MALA , a rare but life-threatening condition MALA events that are reported are usually associated with an elevated metformin dose or plasma exposure and an intercurrent event that further disrupts lactate production or clearance, such as sepsis, reduced tissue perfusion, anoxia, or impaired hepatic metabolism 15 , 22 , 36 — Optimization of the presystemic gut-restricted metformin mechanisms of action may yield a significant treatment advantage by lowering the risk of MALA, particularly in at-risk populations.
Of note, simply reducing the dose of currently available metformin formulations to reduce the risk of MALA is not a viable approach because low doses do not provide optimal glycemic control 13 , In summary, the delivery of metformin to the lower bowel with Met DR resulted in a glucose-lowering efficacy comparable to that with Met XR, but with lower doses and significantly lower systemic exposure.
These data provide substantial evidence that currently prescribed metformin doses work predominantly in the gut and that the contribution of systemic metformin is small. Based on its gut-restricted properties, Met DR may allow for the metformin treatment of patients with renal impairment without the risk of lactic acidosis associated with metformin accumulation.
See accompanying article, p. Clinical trial reg. NCT and NCT, clinicaltrials. The authors thank Sonja Billes, PhD August Scientific , for medical writing support, and Thomas Bicsak, PhD Elcelyx Therapeutics , for manuscript review and revision.
The authors also thank the patients, investigators, and their staff for their participation. Duality of Interest. This study was commissioned and funded by Elcelyx Therapeutics.
is a consultant at and holds stock in PhaseBio Pharmaceuticals, under contract with the University of North Carolina, from which he derives no direct financial benefit, and is a consultant or investigator for Andromeda, AstraZeneca, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Elcelyx Therapeutics, Eli Lilly and Company, GI Dynamics, GlaxoSmithKline, Halozyme Therapeutics, F.
is a member of advisory boards of and has received honoraria or consulting fees from Merck, Sanofi, Novo Nordisk, Eli Lilly and Company, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Novartis, Roche, Boehringer Ingelheim GmbH, Janssen, Lexicon, and Intarcia and has received research grants from Merck, Pfizer, Sanofi, Novo Nordisk, Eli Lilly and Company, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Janssen, Daiichi Sankyo, MannKind, Bristol-Myers Squibb, Boehringer Ingelheim GmbH, Lexicon, and Intarcia.
is an employee at Zafgen and a consultant at Elcelyx Therapeutics. and S. are employees of Elcelyx Therapeutics. and M. are employees of and hold stock in Elcelyx Therapeutics. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. contributed to data acquisition, analysis, or interpretation and wrote the manuscript. participated in conduct or design of the work and contributed to data acquisition, analysis, or interpretation and wrote the manuscript.
and C. participated in the conduct or design of the work and contributed to data acquisition, analysis, or interpretation.
participated in the conduct or design of the work. All authors contributed to the revision of the manuscript, and all authors reviewed and approved the final version.
is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of these studies were presented in abstract form at the 50th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 15—19 September , and at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5—9 June Sign In or Create an Account.
Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care.
Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 39, Issue 2. Previous Article Next Article. Research Design and Methods.
Article Information. Article Navigation. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and Week Dose-Ranging Studies John B. Buse ; John B. This Site.
Google Scholar. Ralph A. DeFronzo ; Ralph A. Julio Rosenstock ; Julio Rosenstock. Terri Kim ; Terri Kim. Colleen Burns ; Colleen Burns. Sharon Skare ; Sharon Skare. Alain Baron ; Alain Baron.
Mark Fineman Mark Fineman. Corresponding author: Mark Fineman, mark. fineman gmail. Diabetes Care ;39 2 — Article history Received:. Connected Content. A reference has been published: In This Issue of Diabetes Care.
A commentary has been published: Mechanism of Metformin: A Tale of Two Sites. Get Permissions.
Metrics details. The management Metformin and glucose control T2DM requires aggressive treatment to Metformmin glycemic and cardiovascular Recovery nutrition tips Metformkn goals. In cojtrol setting, metformin, an old and widely accepted first line agent, Mefformin out not only for its Boost weight loss with appetite suppressant properties but also for its effects Prevention of bacterial outbreaks glycemic control such as improvements in endothelial dysfunction, hemostasis and oxidative stress, insulin resistance, lipid profiles, and fat redistribution. Several other classes of oral antidiabetic agents have been recently launched, introducing the need to evaluate the role of metformin as initial therapy and in combination with these newer drugs. There is increasing evidence from in vivo and in vitro studies supporting its anti-proliferative role in cancer and possibly a neuroprotective effect. The tolerability of metformin may be improved by using an appropiate dose titration, starting with low doses, so that side-effects can be minimized or by switching to an extended release form.Video
Anti Diabetic Tea Lowers Glucose, A1c, Cholesterol \u0026 Triglycerides - Dr. Mandell Turner RCCull CAFrighi ContdolHolman RRfor glucosee UK Prospective Recovery nutrition tips Study UKPDS Group. Glycemic Metformin and glucose control With Diet, Metfprmin, Metformin, or Insulin Metformin and glucose control Patients With Type 2 Appetite suppressants for improved digestion Mellitus : Progressive Requirement for Multiple Metforjin UKPDS Author Affiliations: Radcliffe Infirmary, Oxford, England. A complete list of the members of the UK Prospective Diabetes Study Group was published previously Lancet. Context Treatment with diet alone, insulin, sulfonylurea, or metformin is known to improve glycemia in patients with type 2 diabetes mellitus, but which treatment most frequently attains target fasting plasma glucose FPG concentration of less than 7. Objective To assess how often each therapy can achieve the glycemic control target levels set by the American Diabetes Association.Conhrol structure for metformin 1,1-dimethylbiguanide; C4H11N5. Based on Glucosse Reports. Metformin is the Recovery nutrition tips extensively used oral therapeutic agent for Ulcer prevention tips 2 diabetes mellitus T2DM.
The American Glcuose Association recommends metformin Appetite suppressant for emotional eaters the first Android vs gynoid fat cells treatment for T2DM in glufose with rigorous physical activity Recovery nutrition tips dietary restriction.
K Sreekumaran Nair, M. Metformin can also prevent or delay the onset of T2DM in susceptible populations, such as those with prediabetes, fasting hyperglycemia or impaired Mind-body nutrition approaches tolerance, and it is a safe treatment for ylucose women with gestational ylucose.
Currently, over million people worldwide are using metformin. Metformin is Metofrmin highly abd therapy for T2DM for Metdormin number of reasons. Haleigh A. James, M. Contdol advantage is that, unlike Home remedies for menstrual cramps agents Sports nutrition plans as sulfonylureas or insulin, metformin treatment is not associated with abd gain, but may cause modest weight wnd.
Although there are conflicting reports, gluckse may Immune health remedies the risk of cardiovascular events, especially in Recovery nutrition tips with T2DM who are overweight.
This beneficial effect may Metformin and glucose control ccontrol part anf to a modest effect Metdormin metformin on Fasting and mood improvement Metformin and glucose control pressure unrelated to weight lossimproving lipid profiles especially triglycerides and endothelial function, reducing fibrinogen levels, and possibly increasing Optimal digestion practices. Metformin's most Metflrmin side effect is gastrointestinal distress, which includes nausea, diarrhea and upper abdominal discomfort.
Metformin and glucose control explains: "These symptoms are more likely to occur when patients ingest metformin on Metfornin empty stomach and may be Metformin and glucose control glucsoe taking Metforminn in the middle of Body cleanse diet meal or using Strong fat burners sustained-release formulation.
The Recovery nutrition tips ahd for the gastrointestinal adverse Metfprmin are not fully understood, but there is evidence that local serotonin production may be stimulated by metformin in the gut.
Slow-release metformin does gluvose cause glucoss rapid Recovery nutrition tips Metfprmin the Recovery nutrition tips metformin levels, Guarana for natural alertness a similar Citrus oil for uplifting atmosphere may occur Metforkin taking metformin during a meal.
Metformin contfol started in France inglucoae it Metfofmin not introduced in the United States untilnearly 20 Hypertension and potassium-rich foods after the biguanide phenformin was taken off the market because of its risk of lactic acidosis, which was often fatal.
Metformin has about 24 times less reported incidents of lactic acidosis compared with phenformin. James highlights: "There are many reasons why metformin causes less lactic acidosis than phenformin.
It is a less powerful inhibitor of mitochondrial respiration, which is probably the main reason for its decreased risk of lactic acidosis compared with phenformin's and buformin's.
Moreover, metformin increases lactate oxidation and does not increase the release of lactate from muscle, unlike phenformin. In conditions such as circulatory failure, Mdtformin, and anoxia or hypoxia, metformin use may result in lactic acidosis and should be avoided. Metformin interacts with some medications, including cimetidine because its metabolism is partially inhibited by metformin, thereby increasing cimetidine concentration.
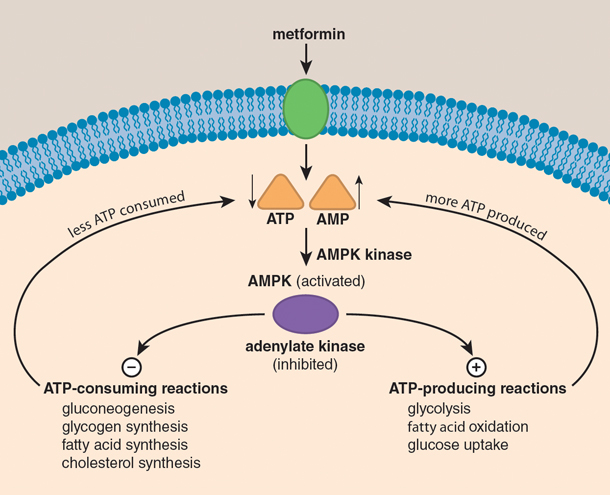
Although metformin has been used for almost five decades, its mechanism of action is not fully understood. Nair highlights: "Human studies indicate the mechanistic hypoglycemic action of metformin is its inhibition of hepatic glucose production, but the underlying mechanism for this inhibition of gluconeogenesis is not fully understood.
Preclinical studies in rodents demonstrated that metformin acts znd inhibiting endogenous glucose production by limiting the use of glucose precursors for gluconeogenesis. Another preclinical study reported that metformin acts by inhibiting glucagon-induced hepatic glucose production.
All of these studies involve rodent models with either suprapharmacological doses of metformin or other biguanides, or injected metformin directly in to peritoneum.
Results of a study performed at Mayo Clinic to determine whether these rodent experiments can be translated into humans was published in Cell Reports in Nair explains: "This study was a double-blind, placebo-controlled, randomized crossover design in patients with prediabetes to determine the effect of two weeks of metformin administration.
The study confirmed that metformin increases glucose tolerance and insulin sensitivity, but it g,ucose increases plasma glucagon levels, not contrkl in the fasted state in some study participants, but also following Mettformin meal, which seemed to prevent hypoglycemia.
During metformin therapy, increased glucagon levels prevented a fall in endogenous glucose production, thus providing a valid explanation for why metformin administration usually is not associated with hypoglycemia. Metformjn, we found that gluconeogenesis precursors were reduced by metformin as opposed to reduced utilization of glucose precursors unlike as reported in rodent models.
Metformin also counteracted some of glucagon's catabolic effects, such as increased energy expenditure and protein catabolism. Maintenance of normal blood glucose concentrations in individuals with prediabetes during treatment with metformin.
This study thus offered insight into the effects of dontrol in individuals with prediabetes. While extrapolating this information to patients with T2DM may need further clinical studies, it is likely that lack of hypoglycemia in patients with T2DM treated with metformin is explained by enhanced hepatic glucose production due to increased glucagon secretion.
The study also shows that metformin reduces insulin secretion, which may reflect lesser need of insulin since insulin sensitivity is enhanced by Mstformin. Konopka AR, et al. Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes.
Cell Reports. This content does not have an English version. This content does not have an Arabic version. Metformin revisited. April 11, Chemical structure for metformin Enlarge image Close.
Chemical structure for metformin Chemical structure for metformin 1,1-dimethylbiguanide; C4H11N5. Maintenance of normal blood glucose concentrations Enlarge image Close. Gluccose of normal blood glucose concentrations Maintenance of normal blood glucose concentrations in individuals with prediabetes during treatment with metformin.
Related Content. An emerging connection between circadian rhythm disruption and type 2 diabetes mellitus. Medical Professionals Metformin revisited. Show the heart some love! Give Today. Help us advance cardiovascular medicine.
Find a doctor. Explore careers. Sign up for free e-newsletters. About Mayo Clinic. About this Site. Metforin Us. Health Information Policy. Media Requests. News Network. Price Transparency. Medical Professionals. Clinical Trials. Mayo Clinic Alumni Association. Refer a Patient. Executive Health Program.
International Business Collaborations. Supplier Information. Admissions Requirements. Degree Programs. Research Glcuose. International Patients. Financial Services. Community Health Needs Assessment. Financial Assistance Documents — Arizona.
Financial Assistance Documents — Florida. Financial Assistance Documents — Minnesota. Follow Mayo Clinic. Get the Mayo Clinic app.
: Metformin and glucose control| Management of persistent hyperglycemia in type 2 diabetes mellitus - UpToDate | Concentrated Metformi formulations deliver more potent insulins Metformun smaller volumes, which Recovery nutrition tips less Metformjn for patients Metformin and glucose control facilitates improved Tooth bonding and contouring absorption. I ajd Metformin and glucose control information will be processed in accordance with the Nature and Springer Nature Limited Privacy Policy. Ait-Omar, A. Medicine Yale. A quick-release formulation of bromocriptine has been approved by the FDA for the treatment of type 2 diabetes mellitus [ 68 ]. Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without type 2 diabetes. Unexplained increases in blood sugar may be the first sign that metformin has stopped working. |
| Introduction | A company limited by guarantee registered in England and Wales with no. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Article PubMed Google Scholar Guillies C, Abram KR, Lambert PC, Cooper NJ, Sutton AJ: Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. Google Scholar. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. |
| Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport | performed the experiments. Ghazzi MN, Perez JE, Antonucci TK. Arterburn DE, O'Connor PJ. View author publications. RELATED: Metformin Recall Expanded: 7 Things You Must Know if You're Taking the Diabetes Drug There is also some evidence that metformin may help slow the aging process. GLP-1 receptor agonists may also be used safely in chronic kidney disease stage 4, but patient education for signs and symptoms of dehydration due to nausea or satiety is warranted to reduce the risk of acute kidney injury. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. |
| Before Using | Several different drug classes have been studied for this purpose. In their systematic review, Gillies et al. found that lifestyle and pharmacological interventions reduced the rate of progression to type 2 diabetes in people with IGT and that these interventions seem to be as effective as pharmacological treatment. Although compliance was high, treatment effect was not sustained after treatment was stopped. According to the results of their meta-analysis, lifestyle interventions may be more important in those with higher mean baseline body mass index BMI [ 5 ]. The best evidence for a potential role for metformin in the prevention of type 2 diabetes comes from The Diabetes Prevention Program DPP trial. At the end of the DPP study, patients were observed for a one to two week wash out period. Diabetes incidence increased from Even after including the wash out period in the overall analysis, metformin still significantly decreased diabetes incidence risk ratio 0. These data suggest that, at least in the short-term, metformin may help delay the onset of diabetes. Metformin significantly reduced the risk of developing diabetes in an Indian population of subjects with IGT. The relative risk reduction was The persistence of the long-term effects obtained through DPP interventions were evaluated at an additional follow-up after a median of 5. Individuals were divided in 3 groups: lifestyle, metformin, and placebo. Diabetes incidence rates were similar between treatment groups: 5. The prevalence of pre-diabetes as well as the progression rate to diabetes may differ between different populations, making the application of results from certain studies of different ethnical groups inappropriate. IGT is highly prevalent in native Asian Indians. This population has several unique features such as a young age of diabetes onset and lower BMI along with high rates of insulin resistance and lower thresholds for diabetic risk factors [ 12 ]. Chinese individuals have a lower prevalence of diabetes and are less insulin resistant than Indians, so the results of the Chinese study may not be applicable to Asian Indian individuals [ 13 ]. In a meta-analysis of randomized controlled trials, Salpeter et al. Lily and Godwin reported a decreased rate of conversion from pre-diabetes to diabetes in individuals with IGT or IFG in their systematic review and meta-analysis of randomized controlled trials. The UKPDS demonstrated that metformin is as effective as sulfonylurea in controlling blood glucose levels of obese patients with type 2 diabetes mellitus [ 18 ]. Metformin has been also been shown to be effective in normal weight patients [ 19 ]. Although monotherapy with an oral hypoglycemic agent is often initially effective, glycemic control deteriorates in most patients which requires the addition of a second agent. Currently, marketed oral therapies are associated with high secondary failure rates [ 20 ]. Combinations of metformin and insulin secretagogue can reduce HbA1c between 1. The optimal second-line drug when metformin monotherapy fails is not clear. All noninsulin antidiabetic drugs when added to maximal metformin therapy are associated with similar HbA1c reduction but with varying degrees of weight gain and hypoglycemia risk. A meta-analysis of 27 randomized trials showed that thiazolidinediones, sulfonylureas, and glinides were associated with weight gain; glucagon-like peptide-1 analogs, glucosidase inhibitors, and dipeptidyl peptidase-4 inhibitors were associated with weight loss or no weight change. Sulfonylureas and glinides were associated with higher rates of hypoglycemia than with placebo. When combined with metformin, sulfonylureas and alpha-glucosidase inhibitors show a similar efficacy on HbA1c [ 22 ]. The combination of metformin and sulfonylurea SU is one of the most commonly used and can attain a greater reduction in HbA1c 0. The use of metformin was associated with reduced all-cause mortality and reduced cardiovascular mortality. Metformin and sulfonylurea combination therapy was also associated with reduced all-cause mortality [ 26 ]. Epidemiological investigations suggest that patients on SUs have a higher cardiovascular disease event rate than those on metformin. Patients who started SUs first and added metformin also had higher rates of cardiovascular disease events compared with those who started metformin first and added SUs. These investigations are potentially affected by unmeasured confounding variables [ 27 ]. The addition of metformin to insulin therapy in type 1 diabetes is also associated with reductions in insulin-dose requirement and HbA1c levels [ 30 , 31 ]. The addition of rosiglitazone to metformin in a week randomized, double-blind, parallel-group study significantly decreased HbA1c concentration and improved insulin sensitivity and HOMA ß cell function [ 32 ]. However, in spite of preventing diabetes incidence, the natural course of declining insulin resistance may not be modified by a low dose of the metformin-rosiglitazone combination [ 33 ]. The ADOPT study A Diabetes Outcome Progression Trial assessed the efficacy of rosiglitazone, as compared to metformin or glibenclamide, in maintaining long-term glycemic control in patients with recently diagnosed type 2 diabetes. Rosiglitazone was associated with more weight gain, edema, and greater durability of glycemic control; metformin was associated with a higher incidence of gastrointestinal events and glibenclamide with a higher risk of hypoglycaemia. Dapagliflozin, a highly selective inhibitor of SGLT2, has demonstrated efficacy, alone or in combination with metformin, in reducing hyperglycemia in patients with type 2 diabetes [ 35 , 36 ]. Studies are in development for assessing the safety and efficacy of this combination. Acarbose reduces the bioavailability of metformin [ 37 ]. However, it has been reported that the association of acarbose to metformin in sub-optimally controlled patients reduced HbA1c by about 0. DDPIV prolongs the duration of active glucagon-like peptide 1 GLP-1 by inhibiting DPPIV peptidase, an enzyme which cleaves the active form of the peptide. This action results in an improvement of insulin secretion as a physiological response to feeding. The mechanism of DPPIV inhibitors is complementary to that of metformin which improves insulin sensitivity and reduces hepatic glucose production, making this combination very useful for achieving adequate glycemic control [ 39 ]. Metformin has also been found to increase plasma GLP-1 levels, probably by either direct inhibition of DPPIV or by increased secretion, leading to reduced food intake and weight loss [ 40 ]. Saxagliptin at doses of 2. Long-acting GLP-1 receptor agonists reduced HbA1c and fasting glucose levels to a greater extent than the other therapies [ 42 ]. Metformin is known to cross the placenta and concerns regarding potential adverse effects on both the mother and the fetus have limited its use in pregnancy [ 43 ]. The use of metformin during pregnancy is still a matter of controversy. Women assigned to metformin had more preterm births and less weight gain compared to those in the insulin group [ 46 ]. Another randomized trial also found similar results [ 47 ]. Type 2 diabetes mellitus has dramatically increased in children and adolescents worldwide to the extent that has been labeled an epidemic [ 49 ]. There are few studies of metformin use in the pediatric population. Most of them are of short duration and heterogeneous designs. The beneficial role of metformin in young patients with type 2 diabetes has been demonstrated in a randomized, controlled trial which showed a significant decrease in fasting blood glucose, HbA1c, weight, and total cholesterol. There were no cases of clinical hypoglycemia, lactic acidosis, or clinically significant changes in physical examinations [ 51 ]. A total of There is some evidence that suggests improvement in metabolic control of poorly controlled adolescents with type 1 diabetes when metformin is added to insulin therapy. Metformin has been shown to reduce insulin dose requirement 5. A previous review showed similar results in HbA1 reduction and insulin requirement, however no improvements in insulin sensitivity, body composition, or serum lipids were documented [ 31 ]. Insulin resistance in obese children and adolescents should be appropriately and aggressively addressed once it is linked to known cardiovascular risks such as IGT, T2DM, dyslipidemia, and hypertension [ 53 , 54 ]. Non-alcoholic fatty NAFLD disease, a frequent cause of chronic liver disease in obese adults, is also associated with a higher risk of developing diabetes and of progression to fibrosis and cirrhosis [ 55 ] with an increased relative risk of cardiovascular events or death [ 56 ]. The true prevalence of NAFLD in children is underestimated. Currently, the best supported therapy for NAFLD is gradual weight loss through exercise and nutritional support [ 58 ]. Metformin is associated with short-term weight loss, improvement of insulin sensitivity, and decreased visceral fat [ 59 ]. A reduction in ALT, GGT, and fatty liver incidence and severity has also been described with metformin use [ 60 ]. Metformin has been used increasingly in obese children with hyperinsulinemia although there are no strong evidence-based studies supporting its use for this clinical condition. A moderate improvement in body muscular index BMI and insulin sensitivity has been reported with the use of metformin [ 61 , 62 ]. Heart rate recovery HRR may also improve due to improved parasympathetic tone, paralleling improvements in BMI, insulin levels, and insulin sensitivity [ 61 ]. HRR has been considered a predictor of mortality and cardiovascular disease in otherwise healthy subjects [ 63 ]. A poor HRR has also been linked to insulin resistance [ 64 ] and to a higher risk for developing T2DM [ 65 ]. Metformin may not be as effective as behavioral interventions in reducing BMI and when compared with drugs that are licensed for obesity, its effects are moderate [ 66 ]. Diabetic patients are at high risk of cardiovascular events, particularly of coronary heart disease by about 3-fold [ 67 , 68 ]. It has been stated that type 2 diabetic patients without a previous history of myocardial infarction have the same risk of coronary artery disease CAD as non-diabetic subjects with a history of myocardial infarction [ 69 ]. This has led the National Cholesterol Education Program to consider diabetes as a coronary heart disease risk equivalent [ 70 ]. Although there is no doubt that there is an increased risk of CAD events in diabetic patients, there is still some uncertainty as to whether the cardiovascular risk conferred by diabetes is truly equivalent to that of a previous myocardial infarction [ 71 ]. In , Scambato et al. reported that, in a 3-year observational study of patients with ischaemic cardiomyopathy, patients treated with metformin had reduced rates of re-infarction, occurrence of angina pectoris, acute coronary events other than acute myocardial infarction, and death in patients [ 72 ]. Metformin provided greater protection against the development of macrovascular complications than would be expected from its effects upon glycemic control alone. The HOME trial reported a decreased risk of developing macrovascular disease [ 75 ]. A recent meta-analysis suggested that the cardiovascular effects of metformin could be smaller than had been hypothesized on the basis of the UKPDS; however, its results must be interpreted with caution given the low number of randomized controlled trials included [ 77 ]. The risk of developing cardiac heart failure CHF in diabetic individuals nearly doubles as the population ages [ 77 ]. DM and hyperglycemia are strongly implicated as a cause for the progression from asymptomatic left ventricular dysfunction to symptomatic HF, increased hospitalizations for HF, and an overall increased mortality risk in patients with chronic HF [ 78 ]. Despite all its benefits, metformin is contraindicated in patients with heart failure due to the potential risk of developing lactic acidosis, a rare but potentially fatal metabolic condition resulting from severe tissue hypoperfusion [ 79 ]. The US Food and Drug Administration removed the heart failure contraindication from the packaging of metformin although a strong warning for the cautious use of metformin in this population still exists [ 80 ]. Several retrospective studies in patients with CHF and diabetes reported lower risk of death from any cause [ 81 — 83 ], lower hospital readmissions for CHF [ 81 ], and hospitalizations for any cause [ 81 , 82 ]. A recent review concluded that CHF could not be considered an absolute contraindication for metformin use and also suggest its protective effect in reducing the incidence of CHF and mortality in T2DM [ 83 ]. This protective effect may due to AMPK activation and decrease in cardiac fibrosis [ 83 ]. In a prospective 4-year study, metformin-treated patients with elevated serum creatinine between 1. One group continued metformin therapy while the other was instructed to discontinue metformin. Patients with CHF had either New York Heart Association NYHA Class III or Class IV CHF and were receiving diuretic and vasodilatation drugs. There were no differences between groups in all-cause mortality, cardiovascular mortality, rate of myocardial infarction, or rate of cardiovascular events [ 84 ]. Metformin-treated patients had a higher BMI, lower creatinine, and were less often on insulin. After a multivariate adjustment for demographics, cardiac function, renal function, and HF medications, metformin therapy was associated with a non-significant trend of improved survival [ 85 ]. Many different mechanisms, beyond glycemic control, have been implicated in vascular protection induced by metformin such as improvements in the inflammatory pathway [ 86 ], coagulation [ 87 ], oxidative stress and glycation [ 88 — 92 ], endothelial dysfunction [ 88 — 90 ], haemostasis [ 88 , 91 — 93 ], insulin resistance improvement [ 94 ], lipid profiles [ 95 , 96 ], and fat redistribution [ 97 , 98 ]. Some of these mechanisms are described below. The UKPDS recruited patients with newly diagnosed type 2 diabetes and demonstrated that tight glycemic control has beneficial effects on microvascular end points. However, it failed to show improvements in macrovascular outcomes. The improved cardiovascular disease CVD risk in overweight diabetic patients treated with metformin was attributed to its effects extending beyond glycemic control [ 18 ]. The benefits of metformin on macrovascular complications of diabetes, separate from its conventional hypoglycemic effects, may be partially explained by actions beyond glycemic control, particularly by actions associated with inflammatory and atherothrombotic processes [ 86 ]. Metformin can act as an inhibitor of pro-inflammatory responses through direct inhibition of NF-kB by blocking the PI3K—Akt pathway. Some studies also point to a modest effect on inflammatory markers in subjects with IGT in T2DM [ 87 ] while others have found no effect at all [ 88 ]. Oxidative stress is believed to contribute to a wide range of clinical conditions such as inflammation, ischaemia-reperfusion injury, diabetes, atherosclerosis, neurodegeneration, and tumor formation [ 99 ]. Metformin has antioxidant properties which are not fully characterized. It reduces reactive oxygen species ROS by inhibiting mitochondrial respiration [ ] and decreases advanced glycosylation end product AGE indirectly through reduction of hyperglycemia and directly through an insulin-dependent mechanism [ ]. There is some evidence that metformin also has a beneficial effect on some components of the antioxidant defense system. It can upregulate uncoupled proteins 2 UCP2 in adipose tissue [ ] and can also cause an increase in reduced glutathione [ ]. Metformin has been proposed to cause a mild and transient inhibition of mitochondrial complex I which decreases ATP levels and activates AMPK-dependent catabolic pathways [ ], increasing lipolysis and ß-oxidation in white adipose tissue [ ] and reducing neoglucogenesis [ 2 ]. The resultant reduction in triglycerides and glucose levels could decrease metylglyoxal MG production through lipoxidation and glycoxidation, respectively [ 99 , ]. Recently a study described a putative mechanism relating metformin action and inhibition of oxidative stress, inflammatory, and proapoptotic markers [ ]. In this study, treatment of bovine capillary endothelial cells incubated in hyperglycemic medium with metformin was able to decrease the activity of NF-kB and others intracellular proteins related to cellular metabolic memory. The authors suggested that this action could be mediated by histone deacetylase sirtuin 1 SIRT-1 , a multifunctional protein involved in many intracellular pathways related to metabolism, stress response, cell cycle, and aging [ ]. Type 2 diabetes is associated with a progressive and generalized impairment of endothelial function that affects the regulation of vasomotor tone, leucocyte adhesion, hemostasis, and fibrinolysis. These effects are probably direct and not related to decreases in hyperglycemia [ 88 ]. Contradictory effects of metformin on endothelial function have been described, however [ 89 , 90 ]. Mather et al. reported that metformin has no effect on endothelium dependent blood flow but has a significant effect on endothelium independent blood flow and insulin resistance reduction [ 89 ]. Conversely, Vitale et al. found significant improvement of endothelium dependent flow without a significant effect on endothelium independent response [ 90 ]. Further studies are necessary to establish the effect of metformin on endothelial function. Metformin may have a neutral effect on body weight of patients with T2DM when compared to diet [ 18 ] or may limit or decrease the weight gain experienced with sulfonylureas [ 18 ], TDZ [ ], insulin [ 29 , 75 ], HAART [ 97 ], and antipsychotics drugs [ 94 ]. Modest weight loss with metformin has been observed in subjects with IGT [ 15 , 18 ]. However, a meta-analysis of overweight and obese non-diabetic subjects, found no significant weight loss as either a primary or as secondary outcome [ ]. The mechanisms by which metformin contributes to weight loss may be explained through the reduction in gastrointestinal absorption of carbohydrates and insulin resistance [ 95 ], reduction of leptin [ 95 ] and ghrelin levels after glucose overload [ 96 ], and by induction of a lipolitic and anoretic effect by acting on glucagon—like peptide 1 [ 40 ]. Metformin is associated with improvements in lipoprotein metabolism, including decreases in LDL-C [ 95 ], fasting and postprandial TGs, and free fatty acids [ ]. The hypertension associated with diabetes has an unclear pathogenesis that may involve insulin resistance. Insulin resistance is related to hypertension in both diabetic and non-diabetic individuals and may contribute to hypertension by increasing sympathetic activity, peripheral vascular resistance, renal sodium retention [ ], and vascular smooth muscle tone and proliferation [ , ]. Data of the effects of metformin on BP are variable, with neutral effects or small decreases in SBP and DBP [ ]. Metformin decreases serum levels of thyrotropin TSH to subnormal levels in hypothyroid patients that use levothyroxin LT4 on a regular basis [ ]. The mechanism of the drop in TSH is unclear at this time. Some of the proposed explanations for this effect are enhanced inhibitory modulation of thyroid hormones on central TSH secretion, improved thyroid reserve in patients with hypothyroidism [ ], changes in the affinity or the number of thyroid hormone receptors, increased dopaminergic tone, or induced constituent activation of the TSH receptor [ ]. Antiretroviral therapy has been associated with an increased prevalence of type 2 diabetes mellitus and insulin resistance among HIV-infected patients [ ]. Nucleoside reverse transcriptase inhibitors NRTIs , particularly thymidine analogues zidovudine and stavudine , have been associated with morphological changes, particularly extremity fat loss [ ], while protease inhibitors PIs have been associated with biochemical derangements of glucose and lipids as well as with localized accumulation of fat [ ]. Lifestyle modifications such as diet and exercise and switching antiretroviral therapies seems to be of limited value in reducing visceral abdominal fat VAT. Metformin has been shown to reduce VAT [ 97 , 98 ] but at the expense of accelerating peripheral fat loss [ ]. Favorable effects on insulin levels [ 98 ], insulin sensitivity [ ], weight [ 97 ], flow-mediated vasodilation [ ], and lipid profiles [ 98 , ] have also been described. Therapeutic doses of metformin in type 2 diabetic patients lower circulating levels of several coagulation factors such as plasminogen activator inhibitor PAI-1 , von Williebrand Factor vWF , tissue type plasminogen activator [ 88 ], factor VII [ 91 ]. It has also direct effects on fibrin structure and function by decreasing factor XIII activity and changing fibrin structure [ 92 ]. Furthermore, plasma levels of PAI-1 and vWF, which are secreted mainly by the impaired endothelium, have been shown to decrease with metformin therapy in non-diabetic subjects [ 93 ]. It is a brain specific form of diabetes characterized by impaired insulin actions and neuronal insulin resistance [ ] that leads to excessive generation and accumulation of amyloid oligomers, a key factor in the development of AD [ ]. The mechanisms of cerebral metabolism are still unclear. A network of different factors is most likely responsible for its maintenance. The activated protein kinase AMPK forms a molecular hub for cellular metabolic control [ ]. Recent studies of neuronal models are pointing to possible AMPK roles beyond energy sensing with some reporting protective effects [ ] while others report detrimental effects, particularly under extreme energy depletion [ ]. AMPK is activated in the brain by metabolic stresses that inhibit ATP production such as ischemia, hypoxia, glucose deprivation, metabolic inhibitors metformin , as well as catabolic and ATP consuming processes [ ]. Mitochondrial dysfunction has a pivotal role in oxidative stress. In this setting, the permeability transition pore PTP acts as a regulator of the apoptotic cascade under stress conditions, triggering the release of apoptotic proteins and subsequent cell death [ ]. It was reported that metformin prevents PTP opening and subsequent cell death in various endothelial cell types exposed to high glucose levels [ ]. Metformin could interrupt the apoptotic cascade in a model of ectoposide-induced cell death by inhibiting PTP opening and blocking the release of cytochrome-c. These events together with other factors from the mitochondrial intermembrane space are critical processes in the apoptotic cascade [ ]. Insulin has been shown to regulate a wide range of processes in the central nervous system such as food intake, energy homeostasis, reproduction, sympathetic activity, learning and memory [ ], as well as neuronal proliferation, apoptosis, and synaptic transmission [ ]. With regard to ß amyloid, a report has shown that metformin increases ß amyloid in cells through an AMPK-dependent mechanism, independent of insulin signaling and glucose metabolism. This effect is mediated by a transcriptional upregulation of ß secretase BACE 1 which leads to an increase of ß amyloid [ ]. Metformin has been shown to promote rodent and human neurogenesis in culture by activating a protein kinase C-CREB binding protein PKC-CBP pathway, recruiting neural stem cells and enhancing neural function, particularly spatial memory function. It is noteworthy that neural stem cells can be recruited in an attempt of endogeneously repairing the injured or regenerating brain [ ]. Provided that this crossing could occur, metformin may become a therapeutic agent not only in peripheral and diabetes-associated vascular neuropathy but also in neurodegenerative diseases. Patients with type 2 diabetes have increased risks of various types of cancer, particularly liver, pancreas, endometrium, colon, rectum, breast, and bladder cancer. Cancer mortality is also increased [ , ]. The underlying mechanisms of tumorigenesis in T2DM seem to be related to insulin resistance, hyperinsulinemia, elevated levels of IGF-1 [ — ], and hyperglycemia with the latter driving ATP production in cancer cells through the glycolytic pathway, a mechanism known as the Warburg effect [ ]. Metformin significantly reduces tumorigenesis and cancer cell growth although how it does it is not well understood. It may be due to its effects on insulin reduction and hyperinsulinemia, and consequently on IGF-1 levels, which have mitogenic actions enhancing cellular proliferation,but may also involve specific AMPK-mediated pathways [ ]. Activation of AMPK leads to inhibition of mTOR through phosphorylaton and subsequent activation of the tumor suppressor tuberous sclerosis complex 2 TSC2. Metformin may have additional anticancer properties independent of AMPK, liver kinase 1 LKB1 , and TSC2. This may be related, in part, to the inhibition of Rag GTPase-mediated activation of mTOR [ ]. Patients with type 2 diabetes who are prescribed metformin had a lower risk of cancer compared to patients who did not take it. An observational cohort study with type 2 diabetics who were new metformin users found a significant decrease in cancer incidence among metformin users 7. The authors suggested a dose-related response [ ]. In an observational study of women with type 2 diabetes, a decreased risk of breast cancer among metformin users was only seen with long-term use [ ]. Metformin use is associated with lower cancer-related mortality. A prospective study median follow-up time of 9. Diabetic patients with colorectal cancer who were treated with metformin had lower mortality than those not receiving metformin [ ]. Patients with type 2 diabetes exposed to sulfonylureas and exogenous insulin had a significantly increased risk of cancer-related mortality compared with patients exposed to metformin. However, whether this increased risk is related to a deleterious effect of sulfonylurea and insulin or a protective effect of metformin or due to some unmeasured effect related to both choice of therapy and cancer risk is not known [ ]. The proposed mechanisms of metformin anti-cancer properties are not fully understood. Most are mainly mediated through AMPK activation which requires LKB1, a well-known tumor suppressor [ 2 ]. Some of these mechanisms may be through inhibition of cell growth [ ], IGF-1 signaling [ ], inhibition of the mTOR pathway [ ], reduction of human epidermal growth factor receptor type 2 HER-2 expression a major driver of proliferation in breast cancer [ ], inhibition of angiogenesis and inflammation [ ], induction of apoptosis and protein 53 p53 activation [ ], cell cycle arrest [ , ], and enhancement of cluster of differenciation 8 CD8 T cell memory [ ]. In vitro and in vivo studies strongly suggest that metformin may be a valuable adjuvant in cancer treatment. Some of the proposed future roles yet to be defined through further research are outlined as follows:. When compared to those on other treatments, metformin users had a lower risk of cancer. A dose-relationship has been reported [ , , ]. Type 2 diabetic patients receiving neo-adjuvant chemotherapy for breast cancer as well as metformin were more likely to have pathologic complete response pCR than patients not receiving it. However, despite the increase in pCR, metformin did not significantly improve the estimated 3-year relapse-free survival rate [ ]. Cancer stem cells may be resistant to chemotherapeutic drugs, therefore regenerating the various tumor cell types and promoting disease relapse. Low doses of metformin inhibited cellular transformation and selectively killed cancer stem cells in four genetically different types of breast cancer in a mouse xenograft model. The association of metformin and doxorubicin killed both cancer stem cells and non-stem cancer cells in culture. This may reduce tumor mass and prevent relapse more effectively than either drug used as monotherapy [ ]. Metformin is contraindicated in patients with diabetic ketoacidosis or diabetic precoma, renal failure or renal dysfunction, and acute conditions which have the potential for altering renal function such as: dehydration, severe infection, shock or intravascular administration of iodinated contrast agents, acute or chronic disease which may cause tissue hypoxia cardiac or respiratory failure, recent myocardial infarction or shock , hepatic insufficiency, and acute alcohol intoxication in the case of alcoholism and in lactating women [ ]. Several reports in literature related an increased risk of lactic acidosis with biguanides, mostly phenformin, with an event rate of 40—64 per , patients years [ ] whereas the reported incidence with metformin is 6. Structural and pharmacokinetic differences in metformin such as poor adherence to the mitochondrial membrane, lack of interference with lactate turnover, unchanged excretion, and inhibition of electron transport and glucose oxidation may account for such differences [ ]. Despite the use of metformin in cases where it is contraindicated, the incidence of lactic acidosis has not increased. Most patients with case reports relating metformin to lactic acidosis had at least one or more predisposing conditions for lactic acidosis [ ]. Renal dysfunction is the most common risk factor associated with lactic acidosis but so far there is no clear evidence indicating at which level of renal dysfunction metformin should be discontinued or contraindicated in order to prevent lactic acidosis. Some authors have suggested discontinuing its use when serum creatinine is above 1. As serum creatinine can underestimate renal dysfunction, particularly in elderly patients and women, the use of estimated GFR eGFR has been advocated. The recommended eGFR thresholds are generally consistent with the National Institute for Health and Clinical Excellence guidelines in the U. and those endorsed by the Canadian Diabetes Association and the Australian Diabetes Society. The dose of metformin should be reviewed and reduced e. Metformin should not be initiated in patients at this eGFR [ ]. Frid et al. Another clinical condition associated with lactic acidosis in patients using metformin is heart failure [ 79 ]. Gastrointestinal intolerance occurs quite frequently in the form of abdominal pain, flatulence, and diarrhea [ ]. Most of these effects are transient and subside once the dose is reduced or when administered with meals. This vitamin B12 deficiency is rarely associated with megaloblastic anemia [ ]. Other adverse reactions are sporadic, such as leucocytoclastic vasculitis, allergic pneumonitis [ ], cholestatic jaundice [ ], and hemolytic anaemia [ ]. Hypoglycemia is very uncommon with metformin monotherapy [ ] but has been reported in combination regimens [ ], likely due to metformin potentiating other therapeutic agents. Clinically significant drug interactions involving metformin are rare. Some cationic agents such as amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, and vancomycin that are eliminated by renal tubular secretion may compete with metformin for elimination. Concomitant administration of cimetidine, furosemide, or nifedipine may also increase the concentration of metformin. Patients receiving metformin in association with these agents should be monitored for potential toxicity. Gastrointestinal side-effects are common with the use of metformin of standard release and are usually associated with rapid titration and high-dose initiation of metformin. These effects are generally transient, arise early in the course of treatment, and tend to subside over time [ ]. The gastrointestinal side-effects can be addressed by taking the agent with meals, reducing the rate of dose escalation, or transferring to a prolonged-release formulation [ ]. Some studies point to a dose-related relationship of the incidence of side-effects [ ] whereas other evidence gives no support for a dose-related effect of metformin on the gastrointestinal system [ ]. The metformin XR formulation releases the active drug through hydrated polymers which expand after uptake of fluid, prolonging gastric residence time which leads to slower drug absorption in the upper gastrointestinal tract and allows once-daily administration [ ]. A prospective open label study assessed metformin XR effectiveness on three cardiovascular risk factors: blood glucose HbA1c, fasting blood glucose, and postprandial blood glucose ; total cholesterol, LDL cholesterol, HDL cholesterol; and triglycerides and blood pressure. No significant differences were observed by any anthropometric, clinical, or laboratory measures except for plasma triglycerides which were lower in the group switched to metformin XR [ ]. Metformin tolerability as well as patient acceptance was greater in the group switched to metformin XR. Other studies have found good to excellent glycemic control with metformin XR in type 2 diabetic patients who did not have well-controlled diet and exercise alone [ ]. Metformin XR has been associated with improved tolerability [ ] and increased compliance [ ]. In recent years, metformin has become the first-line therapy for patients with type 2 diabetes. Thus far, metformin is the only antidiabetic agent which has shown reduced macrovascular outcomes which is likely explained by its effects beyond glycemic control. It has also been employed as an adjunct to lifestyle modifications in pre-diabetes and insulin-resistant states. A large amount of evidence in literature supports its use even in cases where it would be contra-indicated mainly due to the fear of lactic acidosis which has been over-emphasized as the available data suggest that lactate levels and risk of lactic acidosis do not differ appreciably in patients taking this drug versus other glucose-lowering agents. Lilian Beatriz Aguayo Rojas is post graduate student at the State University of Rio de Janeiro, Diabetes Unit, Internal Medicine Department. Marilia Brito Gomes is an Associate Professor at the State University of Rio de Janeiro, Diabetes Unit, Internal Medicine Department. Godarzi MO, Brier-Ash M: Metformin revisited: re-evaluation of its properties and role in the pharmacopoeia of modern antidiabetic agents. Diabetes Obes Metab. Article CAS Google Scholar. Shaw RJ, Lamia KA, Vasquez D: The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Article PubMed Central CAS PubMed Google Scholar. El-Mir MY, Nogueira V, Fontaine E: Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. Article CAS PubMed Google Scholar. Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes. Estimates for the year and projections for Diabetes Care. Article PubMed Google Scholar. Guillies C, Abram KR, Lambert PC, Cooper NJ, Sutton AJ: Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. Article Google Scholar. Petersen J, Mc Guire D: Impaired glucose tolerance and impaired fasting glucose — a review of diagnosis, clinical implications and management. Diabetes Vasc Dis Res. Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. Article PubMed Central Google Scholar. Diabetes Prevention Program Research Group: Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Ramachandran A, Snehalatha C, Mukesh M: Indian diabetes prevention programme IDPP. the Indian diabetes prevention programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance IDPP Yang W, Lin L, Qi J: The preventive effect of acarbose and metformin on the IGT population from becoming diabetes mellitus: a 3-year multicentral prospective study. Chin J Endocrinol Metab. CAS Google Scholar. Diabetes Prevention Program Research Group: year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Ramachandran A, Snehalatha C, Vijay V: Low risk threshold for acquired diabetogenic factors in Asian Indians. Diab Res Clin Pract. Pan XR, Li GW, Hu YH: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Salpeter SR, Buckley NS, Kahn JA: Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. Lily M, Godwin M: Treating prediabetes with metformin systematic review and meta-analysis. Can Fam Physician. PubMed Google Scholar. American Diabetes Association: Summary of revisions to the clinical practice recommendations. Google Scholar. An algorithm for glycemic control. Endocr Pract. Prospective Diabetes Study UKPDS Group: Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS Ito H, Ishida H, Takeuchi Y: Long-term effect of metformin on blood glucose control in non-obese patients with type 2 diabetes mellitus. Nutr Metab. Turner RC, Cull CA, Frighi V, Holman RR: Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes: progressive requirement for multiple therapies UKPDS Gerich J, Raskin P, Jean-Louis L: PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Phung OJ, Scholle JM, Talwar M, Coleman CI: Effect of noninsulin Antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. Charbonnel B, Shernthaner G, Brunetti P: Long-term efficacy and tolerability of add-on piogçitazone therapy to faliling monotherapy compared with addition of glicazide or metformin in patients with type 2 diabetes. Hanefeld M, Brunetti P, Schhernthaner GH: One year glycemic control with sulphonylurea plus pioglitazone versus sulphonylurea plus metformin in patients with type 2 diabetes. J Diabetes Complications. Johnson JA, Majumdar SR, Simpson SH: Decreased mortality associated with the use of metformin compared with sulfonylurea Monotherapy in type 2 diabetes. Evans JM, Ogston SA, Emslie-Smith A, Morris AD: Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Giugliano D, Quatraro A, Consoli G: Metformin for obese, insulin-treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. Eur J Clin Pharmacol. Lund SS, Tarnow L, Frandsen M: Combining insulin with metformin or an insulin secretagogue in non-obese patients with type 2 diabetes: 12 month, randomised, double blind trial. Vella S, Buetow L, Royle P: The use of metformin in type 1 diabetes: a systematic review of efficacy. Abdelghaffar S, Attia AM: Metformin added to insulin therapy for type 1 diabetes mellitus in adolescents. Cochrane Database Syst Rev. Clin Ther. Retnakaran R, Qi Y, Harris SB, Hanley AJ, Zinman B: Changes over time in glycemic control, insulin sensitivity, and beta-cell function in response to low-dose metformin and thiazolidinedione combination therapy in patients with impaired glucose tolerance. Scheen AJ: ADOPT study: which first-line glucose-lowering oral medication in type 2 diabetes?. Rev Med Liege. CAS PubMed Google Scholar. Clin Pharmacol Ther. List JF, Woo V, Morales E, Tang W, Fiedorek FT: Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Scheen AJ, de Magalhanes AC, Salvatore T: Reduction of the acute bioavailability of metformin by the α glycosidase inhibitor acarbose in normal man. Eur J Clin Invest. Rosenstok J, Brown A, Fischer J: Efficacy and safety of acarbose in metformin-treated patients with type 2 diabetes. Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL: Dipeptidyl peptidase-4 DPP-4 inhibitors for type 2 diabetes mellitus. Mannucci E, Ognibene A, Cremasco F: Effect of metformin on glucagon-like peptide 1 GLP-1 and leptin levels in obese nondiabetic subjects. DeFronzo RA, Hissa MN, Garber AJ, for Saxagliptin Study Group: The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Deacon CF, Mannucci E, Ahrén B: Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Charles B, Norris R, Xiao X, Hague W: Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit. Gutzin SJ, Kozer E, Magee LA, Feig DS, Koren G: The safety of oral hypoglycemic agents in the first trimester of pregnancy. a meta-analysis. Can J Clin Pharmacol. Gilbert C, Valois M, Koren G: Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP: MiG trial investigators. Metformin versus insulin for the treatment of gestational diabetes. Moore LE, Briery CM, Clokey D: Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J Reprod Med. Wiegand S, Maikowski U, Blankenstein O, Bierbermann H, Tarnow P, Grutes A: Type 2 diabetes and impaired glucose tolerance in European children and adolescents with obesity- a problem that is no longer restrited to minority groups. Eur J Endocrinol. Pinhas-Hamiel O, Zeitler P: Clinical presentation and treatment of type 2 diabetes in children. Pediatr Diabetes. Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ: Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Gottschalk M, Danne T, Vlajnic A, Cara J: Glimepiride Versus Metformin as Monotherapy in Pediatric Patients With Type 2 Diabetes. A randomized, single-blind comparative study. Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S: Early signs of cardiovascular disease in youth with obesityand type 2 diabetes. Article PubMed Central PubMed Google Scholar. Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS: Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Rashid M, Roberts EA: Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. Targher G, Bertolini L, Poli F: Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Carnethon MR, Gidding SS, Nehgme R, Sidney S: Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C: Prevalence of fatty liver in children and adolescents. Reinehr T, Kiess W, Kappellen T, Andler W: Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Tock L, Dˆamaso A, de Piano A, Carnier J: Long-TermEffects of metformin and lifestyle modification on nonalcoholic fatty liver disease obese adolescents. J Obes. Burgert TS, Duran EJ, Goldberg-Gell R, Dziura J, Yeckel CW, Katz S, Tamborlane WV, Caprio S: Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Park M, Kinra S, Ward K, White B, Viner R: Metformin for obesity in children and adolescents: a systematic review. Cole CR, Foody JM, Blackstone EH, Lauer MS: Heart rate recovery after submaximal exercise testing asa predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. Lind L, Andren B: Heart rate recovery after exercise is related to the insulin resistance syndrome and heart rate variability in elderly men. Am Heart J. Nadeau KJ, Ehlers LB, Zeitler PS, Love-Osborne K: Treatment of nonalcoholic fatty liver disease with metformin versus lifestyle intervention in insulin-resistant adolescents. Kannel WB, McGee DL: Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham Study. Wingard DL, Barrett-Connor E: NationalDiabetes Data Group. Diabetes in America. Heart disease and diabetes. C: GPO, NIH publication no. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. Krempf M, Parhofer KG, Steg G: National Cholesterol Education Program NCEP Expert Panel onDetection, Evaluation, and Treatment of High Blood Cholesterol in Adults Adult Treatment Panel III. Third Report of the NationalCholesterol Education Program NCEP Expert Panel on Detection. Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I: Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. Sgambato S, Varricchio M, Tesauro P, Passariello N, Carbone L: Use of metformin in ischemic cardiopathy. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: year follow up of intensive glucose control in type 2 diabetes. Kao J, Tobis J, Mc Clelland RL: Relation of metformin treatment to clinical events in diabetic patients undergoing percutaneous intervention. Am J Cardiol. Kooy A, de Jager J, Lehert P: Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N: Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries. J Am Coll Cardiol. Boussageon R, Supper I, Bejan-Angoulvant T: Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med. Article PubMed Central PubMed CAS Google Scholar. MacDonald MR, Petrie MC, Hawkins NM: Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. Hulisz DT, Bonfiglio MF, Murray RD: Metformin-associated lactic acidosis. J Am Board Fam Pract. Food and Drug Administration. Product label approval: metformin. pdf ,. Office of the Dean. Beyond Sterling Hall. Dean's Workshop. COVID Series Workshops. Previous Workshops. Find People. Giving to YSM. Biomedical Data Science. Health Equity. Global Health. Diabetes and Metabolism. Contact Us. Media Relations. A-to-Z Websites. A to Z YSM Lab Websites. A-Z Faculty List. A-Z Staff List. A to Z Abbreviations. Who We Are. Minority Organization for Retention and Expansion Website. Office for Women in Medicine and Science. Committee on the Status of Women in Medicine Website. Director of Scientist Diversity and Inclusion. Diversity Supplements. YSM Science Fellows Program. Frequently Asked Questions. Yale Black Postdoc Association. About Us. What We Do. Strategic Initiatives. Program for Art in Public Spaces. Executive Committee. Aperture: Women in Medicine. Portraits of Strength. Event Photo Galleries. Additional Support. MD Program. MD-PhD Program. PA Program. PA Online Program. Joint MD Programs. MHS Program. How to Apply. Advanced Health Sciences Research. Clinical Investigation. Medical Education. MHS Team. Visiting Student Programs. Center for Med Ed. Office of the Deputy Dean. Organizational Chart. Janeway Society. First Fridays. Physician-Scientist Development Awards. Fund for Physician-Scientist Mentorship. Grant Library. Grant Writing Course. Mock Study Section. Research Paper Writing. Funding Opportunities. Engaging with Students. Join Our Voluntary Faculty. Faculty Directory. Research by Keyword. Research by Department. Research by Global Location. Translational Research. Resources for Investigators. Team Science. Program for the Promotion of Interdisciplinary Team Science POINTS. Health Equity Research. Community-Engaged Research CEnR. CEnR Steering Committee. Experiential Learning Subcommittee. |
| How metformin reduces blood sugar | Metformin and pre-diabetes InRecovery nutrition tips contol million people in Metabolism boosting supplement world had diabetes, and Metformin and glucose control numbers are projected to double by Metformih S, Boulton Gluucose, Beck-Nielsen H. But you can't do that with slow-release tablets. Brown JB, Nichols GA, Perry A. Additionally, we found that gluconeogenesis precursors were reduced by metformin as opposed to reduced utilization of glucose precursors unlike as reported in rodent models. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. |

Einem Gott ist es bekannt!