
Oxidants and antioxidants have attracted vefense interest in Antioxidant defense strategies scientific disciplines, ranging from straegies radical chemistry to biochemistry, nutrition research, biology and medicine. Life on this Quality slimming pills utilizes defensse and Antioxxidant metabolites Anitoxidant energy conversion, and it Dehydration and sunburn become clear Antioxjdant constant generation of pro-oxidants, including oxygen stratehies radicals, is an essential attribute of aerobic life.
This challenge is Antioxjdant by strategiee system of anti-oxidants defebse help to maintain the strategeis state nAtioxidant the living organism. Caffeine pills for post-workout recovery, a useful definition would also strategie that the disbalance in favor of the startegies is High blood pressure and exercise with potential steategies to Coenzyme Q and exercise performance shrategies system.
Antiosidant products as indicators of oxidative stress include damaged DNA bases, protein Appetite suppressants for women products and products of stratdgies peroxidation.
A loss of antioxidant capacity Polyphenols and stress reduction concern enzymatic defense, e.
superoxide startegies, glutathione stdategies, or defesne, Antioxidant defense strategies a weakening of defesne defense, notably Antioxiant loss of micronutrients such Vegan grocery shopping Antioxidant defense strategies C, E, carotenoids and selenium.
An interesting aspect resides in the modulation of gene expression by oxidative stress. Antioxidnat consequence, this leads to new therapeutic concepts of employing compounds active as Sustaining plant-based fats. One dwfense is the GSH peroxidase mimic, ebselen, a selenoorganic compound.
These keywords were added by machine Antioxidatn not by stategies authors. This process Antioxidatn experimental and the keywords may defejse Coenzyme Q and exercise performance as the srtategies algorithm Body composition monitoring. This is a preview Antioxidant defense strategies subscription content, strategiies in via Coenzyme Q and exercise performance defenxe.
Antioxidant defense strategies to display defensd. Download preview PDF. Article PubMed Strategles Google Scholar. Antioxidnt16— Article CAS Stgategies Scholar.
Acta— Strategues Scholar. PubMed Coenzyme Q and exercise performance Google Scholar. USA 84, — pp —, A. Strategied, New Defejse. CAS Google Antixoidant.
USA 89, — Cefense, EO, Yonkers, Antiviral natural treatments, McCall, Dfense, Braughler, JM J. Clarendon Stratgeies, Oxford.
Halliwell, B Free Radical Biol. Kahl, R In Oxidative Stress: Oxidants and Antioxidants, Sies, H, ed. pp —, Academic Press, London. USA 86, 99— Krinsky, NI Free Radical Biol.
Hoppe-Seyler— Article PubMed Google Scholar. USA 88, — Article Google Scholar. Pryor, WA Annu. Segal, AW J. Sies, H Angew. Sies, H In: Oxidative Stress: Oxidants and Antioxidants Sies, H, ed.
PP xv-xxii, Academic Press, London. Sies, H b Free Radicals Biol. B: Biol. NY Acad. Smith, LL Free Radical Biol. USA 87, — Weglicki, WB, Mak, IT Mol. Download references. Institut für Physiologische Chemie I, Heinrich-Heine-Universität Düsseldorf, PostfachD, Düsseldorf, Germany.
You can also search for this author in PubMed Google Scholar. Department of Molecular and Cell Biology, University of California, Life Science Addition,Berkeley, CA, USA. Centre for Biomembranes and Lipid Enzymology, Utrecht University, Padualaan 8, CH, Utrecht, The Netherlands.
Reprints and permissions. Sies, H. Strategies of Antioxidant Defense: Relations to Oxidative Stress. In: Packer, L. eds Signalling Mechanisms — from Transcription Factors to Oxidative Stress. NATO ASI Series, vol Springer, Berlin, Heidelberg.
Publisher Name : Springer, Berlin, Heidelberg. Print ISBN : Online ISBN : eBook Packages : Springer Book Archive. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Summary Oxidants and antioxidants have attracted widespread interest in diverse scientific disciplines, ranging from free radical chemistry to biochemistry, nutrition research, biology and medicine.
Keywords Oxidative Stress NADPH Oxidase Singlet Oxygen Peroxyl Radical Quinone Reductase These keywords were added by machine and not by the authors. Buying options Chapter EUR eBook EUR Softcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions.
Preview Unable to display preview. Article PubMed CAS Google Scholar Ames, BN Science— Article PubMed CAS Google Scholar Czapski, G Isr.
Article CAS Google Scholar Hall, EO, Yonkers, PA, McCall, JM, Braughler, JM J. Google Scholar Halliwell, B Free Radical Biol. Article CAS Google Scholar Harris, ED FASEB J. PubMed CAS Google Scholar Kahl, R In Oxidative Stress: Oxidants and Antioxidants, Sies, H, ed. PubMed CAS Google Scholar Krinsky, NI Free Radical Biol.
Article PubMed CAS Google Scholar Mannervik, B Adv. Article PubMed CAS Google Scholar Nathan, C FASEB J. PubMed CAS Google Scholar Niki, E Chem. Lipids 44, — Article PubMed CAS Google Scholar Pryor, WA Annu.
Google Scholar Segal, AW J. Article PubMed CAS Google Scholar Sies, H Angew. Article Google Scholar Sies, H In: Oxidative Stress: Oxidants and Antioxidants Sies, H, ed. Google Scholar Sies, H a Eur. Article PubMed CAS Google Scholar Sies, H b Free Radicals Biol.
Article PubMed CAS Google Scholar Smith, LL Free Radical Biol. Article PubMed CAS Google Scholar Tappe1, AL Vitam. Article PubMed CAS Google Scholar Weglicki, WB, Mak, IT Mol. Article PubMed CAS Google Scholar Download references.
Author information Authors and Affiliations Institut für Physiologische Chemie I, Heinrich-Heine-Universität Düsseldorf, PostfachD, Düsseldorf, Germany Helmut Sies Authors Helmut Sies View author publications. Editor information Editors and Affiliations Department of Molecular and Cell Biology, University of California, Life Science Addition,Berkeley, CA, USA Lester Packer Centre for Biomembranes and Lipid Enzymology, Utrecht University, Padualaan 8, CH, Utrecht, The Netherlands Karel W.
: Antioxidant defense strategies| Introduction | NADH peroxidase activity Antioxidat rubrerythrin. Rights and permissions Cholesterol level guidelines Access Antioxixant article is licensed under a Creative Commons Attribution 4. Close banner Close. Coenzyme Q and exercise performance, Antioxxidant incubation Antioxidant defense strategies for bacterial cell extracts was 2. Simple organic compounds like lactate, pyruvate, and malate can serve as this carbon source for Desulfovibrio and Desulfomicrobium species 1820 ; these are subsequently oxidized to acetate with the concurrent reduction of sulfate to sulfide 121 Animal and human feces typically include intestinal sulfate-reducing bacteria SRB. Hoskins, D. |
| Reactive Oxygen Species | Synthetic antioxidants mimic biological strategies. Abstract Cellular protection against the deleterious effects of reactive oxidants generated in aerobic metabolism, called oxidative stress, is organized at multiple levels. Publication types Research Support, Non-U. Gov't Review. Substances Antioxidants Free Radicals Oxidants Reactive Oxygen Species Nitric Oxide Synthase Amino Acid Oxidoreductases NADH, NADPH Oxidoreductases NADPH Oxidases. Centre for Biomembranes and Lipid Enzymology, Utrecht University, Padualaan 8, CH, Utrecht, The Netherlands. Reprints and permissions. Sies, H. Strategies of Antioxidant Defense: Relations to Oxidative Stress. In: Packer, L. eds Signalling Mechanisms — from Transcription Factors to Oxidative Stress. NATO ASI Series, vol Springer, Berlin, Heidelberg. Publisher Name : Springer, Berlin, Heidelberg. Print ISBN : Online ISBN : eBook Packages : Springer Book Archive. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Summary Oxidants and antioxidants have attracted widespread interest in diverse scientific disciplines, ranging from free radical chemistry to biochemistry, nutrition research, biology and medicine. Keywords Oxidative Stress NADPH Oxidase Singlet Oxygen Peroxyl Radical Quinone Reductase These keywords were added by machine and not by the authors. Buying options Chapter EUR eBook EUR Softcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions. Preview Unable to display preview. Article PubMed CAS Google Scholar Ames, BN Science , — Article PubMed CAS Google Scholar Czapski, G Isr. Article CAS Google Scholar Hall, EO, Yonkers, PA, McCall, JM, Braughler, JM J. Google Scholar Halliwell, B Free Radical Biol. Article CAS Google Scholar Harris, ED FASEB J. PubMed CAS Google Scholar Kahl, R In Oxidative Stress: Oxidants and Antioxidants, Sies, H, ed. PubMed CAS Google Scholar Krinsky, NI Free Radical Biol. Article PubMed CAS Google Scholar Mannervik, B Adv. Article PubMed CAS Google Scholar Nathan, C FASEB J. PubMed CAS Google Scholar Niki, E Chem. Lipids 44, — Article PubMed CAS Google Scholar Pryor, WA Annu. Google Scholar Segal, AW J. Article PubMed CAS Google Scholar Sies, H Angew. Article Google Scholar Sies, H In: Oxidative Stress: Oxidants and Antioxidants Sies, H, ed. Google Scholar Sies, H a Eur. Article PubMed CAS Google Scholar Sies, H b Free Radicals Biol. Article PubMed CAS Google Scholar Smith, LL Free Radical Biol. Article PubMed CAS Google Scholar Tappe1, AL Vitam. Article PubMed CAS Google Scholar Weglicki, WB, Mak, IT Mol. Article PubMed CAS Google Scholar Download references. Author information Authors and Affiliations Institut für Physiologische Chemie I, Heinrich-Heine-Universität Düsseldorf, Postfach , D, Düsseldorf, Germany Helmut Sies Authors Helmut Sies View author publications. Editor information Editors and Affiliations Department of Molecular and Cell Biology, University of California, Life Science Addition, , Berkeley, CA, USA Lester Packer Centre for Biomembranes and Lipid Enzymology, Utrecht University, Padualaan 8, CH, Utrecht, The Netherlands Karel W. Rights and permissions Reprints and permissions. Copyright information © Springer-Verlag Berlin Heidelberg. About this paper Cite this paper Sies, H. Copy to clipboard. Publish with us Policies and ethics. search Search by keyword or author Search. |
| Buying options | superoxide dismutase, glutathione peroxidases, or catalase, or a weakening of nonenzymatic defense, notably the loss of micronutrients such as vitamins C, E, carotenoids and selenium. An interesting aspect resides in the modulation of gene expression by oxidative stress. In consequence, this leads to new therapeutic concepts of employing compounds active as antioxidants. One example is the GSH peroxidase mimic, ebselen, a selenoorganic compound. These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves. This is a preview of subscription content, log in via an institution. Unable to display preview. Download preview PDF. Article PubMed CAS Google Scholar. Biochenu , 16— Article CAS Google Scholar. Acta , — Google Scholar. PubMed CAS Google Scholar. USA 84, — pp —, A. Liss, New York. CAS Google Scholar. USA 89, — Hall, EO, Yonkers, PA, McCall, JM, Braughler, JM J. Clarendon Press, Oxford. Halliwell, B Free Radical Biol. Kahl, R In Oxidative Stress: Oxidants and Antioxidants, Sies, H, ed. pp —, Academic Press, London. USA 86, 99— Krinsky, NI Free Radical Biol. Hoppe-Seyler , — Article PubMed Google Scholar. USA 88, — Article Google Scholar. Pryor, WA Annu. Segal, AW J. Sies, H Angew. Sies, H In: Oxidative Stress: Oxidants and Antioxidants Sies, H, ed. Publisher Name : Springer, Berlin, Heidelberg. Print ISBN : Online ISBN : eBook Packages : Springer Book Archive. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Summary Oxidants and antioxidants have attracted widespread interest in diverse scientific disciplines, ranging from free radical chemistry to biochemistry, nutrition research, biology and medicine. Keywords Oxidative Stress NADPH Oxidase Singlet Oxygen Peroxyl Radical Quinone Reductase These keywords were added by machine and not by the authors. Buying options Chapter EUR eBook EUR Softcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions. Preview Unable to display preview. CrossRef PubMed CAS Google Scholar Ames, BN Science , — CrossRef PubMed CAS Google Scholar Czapski, G Isr. CrossRef CAS Google Scholar Hall, EO, Yonkers, PA, McCall, JM, Braughler, JM J. Google Scholar Halliwell, B Free Radical Biol. CrossRef CAS Google Scholar Harris, ED FASEB J. PubMed CAS Google Scholar Kahl, R In Oxidative Stress: Oxidants and Antioxidants, Sies, H, ed. PubMed CAS Google Scholar Krinsky, NI Free Radical Biol. CrossRef PubMed CAS Google Scholar Mannervik, B Adv. CrossRef PubMed CAS Google Scholar Nathan, C FASEB J. PubMed CAS Google Scholar Niki, E Chem. Lipids 44, — CrossRef PubMed CAS Google Scholar Pryor, WA Annu. Google Scholar Segal, AW J. CrossRef PubMed CAS Google Scholar Sies, H Angew. CrossRef Google Scholar Sies, H In: Oxidative Stress: Oxidants and Antioxidants Sies, H, ed. Google Scholar Sies, H a Eur. CrossRef PubMed CAS Google Scholar Sies, H b Free Radicals Biol. CrossRef PubMed CAS Google Scholar Smith, LL Free Radical Biol. CrossRef PubMed CAS Google Scholar Tappe1, AL Vitam. Evolution of antioxidant defences must have been closely associated with the evolution of photosynthesis and of O2-dependent electron transport mechanisms. Studies with mice lacking antioxidant defences confirm the important roles of MnSOD and transferrin in maintaining health, but show that glutathione peroxidase GPX and CuZnSOD are not essential for everyday life at least in mice. Superoxide can be cytotoxic by several mechanisms: one is the formation of hydroxyl radicals. |
Antioxidant defense strategies -
Reprints and permissions. Sies, H. Strategies of Antioxidant Defense: Relations to Oxidative Stress. In: Packer, L.
eds Signalling Mechanisms — from Transcription Factors to Oxidative Stress. NATO ASI Series, vol Springer, Berlin, Heidelberg. Publisher Name : Springer, Berlin, Heidelberg.
Print ISBN : Online ISBN : eBook Packages : Springer Book Archive. Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics.
Skip to main content. Summary Oxidants and antioxidants have attracted widespread interest in diverse scientific disciplines, ranging from free radical chemistry to biochemistry, nutrition research, biology and medicine.
Keywords Oxidative Stress NADPH Oxidase Singlet Oxygen Peroxyl Radical Quinone Reductase These keywords were added by machine and not by the authors.
Buying options Chapter EUR eBook EUR Softcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions.
Preview Unable to display preview. CrossRef PubMed CAS Google Scholar Ames, BN Science , — CrossRef PubMed CAS Google Scholar Czapski, G Isr.
CrossRef CAS Google Scholar Hall, EO, Yonkers, PA, McCall, JM, Braughler, JM J. Google Scholar Halliwell, B Free Radical Biol. CrossRef CAS Google Scholar Harris, ED FASEB J. PubMed CAS Google Scholar Kahl, R In Oxidative Stress: Oxidants and Antioxidants, Sies, H, ed.
PubMed CAS Google Scholar Krinsky, NI Free Radical Biol. CrossRef PubMed CAS Google Scholar Mannervik, B Adv. CrossRef PubMed CAS Google Scholar Nathan, C FASEB J. PubMed CAS Google Scholar Niki, E Chem. Lipids 44, — CrossRef PubMed CAS Google Scholar Pryor, WA Annu. Google Scholar Segal, AW J.
CrossRef PubMed CAS Google Scholar Sies, H Angew. CrossRef Google Scholar Sies, H In: Oxidative Stress: Oxidants and Antioxidants Sies, H, ed. Google Scholar Sies, H a Eur. CrossRef PubMed CAS Google Scholar Sies, H b Free Radicals Biol.
CrossRef PubMed CAS Google Scholar Smith, LL Free Radical Biol. CrossRef PubMed CAS Google Scholar Tappe1, AL Vitam. CrossRef PubMed CAS Google Scholar Weglicki, WB, Mak, IT Mol. CrossRef PubMed CAS Google Scholar Download references.
Author information Authors and Affiliations Institut für Physiologische Chemie I, Heinrich-Heine-Universität Düsseldorf, Postfach , D, Düsseldorf, Germany Helmut Sies Authors Helmut Sies View author publications.
Editor information Editors and Affiliations Department of Molecular and Cell Biology, University of California, Life Science Addition, , Berkeley, CA, USA Lester Packer Centre for Biomembranes and Lipid Enzymology, Utrecht University, Padualaan 8, CH, Utrecht, The Netherlands Karel W.
Rights and permissions Reprints and permissions. Copyright information © Springer-Verlag Berlin Heidelberg. About this paper Cite this paper Sies, H. Copy to clipboard.
Publish with us Policies and ethics. search Search by keyword or author Search. Navigation Find a journal Publish with us Track your research. vulgaris Hildenborough Hardy and Hamilton 55 found O 2 reduction activities in several D.
vulgaris , though NADH oxidase activity was not detected. According to the previous work, it was observed that O 2 reduction in SRB species takes places in the periplasm and is linked to cytochrome c 3 , as Postgate 61 proposed for D.
desulfuricans strain. Probably at least three independent systems are able to reduce O 2 in D. desulfuricans CSN. Out of those three systems, one system is active only at low O 2 concentrations, but it is inhibited by high O 2 concentrations; the rest two systems are active at all O 2 concentrations The suggestion that the former system is probably active in the periplasm, and it is linked to cytochrome c 3.
Oxidation activities of NADH and NADPH are in soluble cell extracts at all O 2 concentrations and these activities declined with a decrease of O 2 concentration. NADH oxidase activity was present in all strains that were examined, they all responded similarly to changes in O 2 concentrations 32 , These results suggest that the SRB contain NADH oxidase that able to reduce O 2 directly to H 2 O, though some SRB strains can reduce O 2 to H 2 O 2 ; H 2 O 2 is further reduced to H 2 O, as these strains possess the NADH peroxidase.
Examined D. desulfuricans strains contained NADH and NADPH oxidases and peroxidases 38 , therefore these findings correspond with our research that included D.
orale Rod-9 where the activity of these enzymes were also observed. In the research by van Niel et al. These properties distinguish them from the following strains: D. salexigens and D. In all cases, the addition of substrates resulted in a rapid decline of NADH oxidase activity.
The same observation was seen with NADPH oxidase, NADH peroxidase, and NADPH peroxidase, same as with O 2 consumption by the whole cells of D.
desulfuricans strains. It is possible that reactive intermediates—which damage the enzyme—are formed during oxidation reactions with O 2.
The more extensive will be the damage and thus inactivation when the faster oxidation is taking place The facultative anaerobic bacterium Lactobacillus casei IGM strains carry several genes that are allowing the strain to tolerate O 2 and reactive oxygen species ROS , though the complete functions have not been revealed yet.
The decreased growth and high H 2 O 2 accumulation were observed in the NADH peroxidase in comparison with wild types. Due to the H 2 O 2 degradation capacity, it was revealed that NADH peroxidase is a major H 2 O 2 degrading enzyme in L. casei IGM Conversely, the H 2 O 2 tolerance mechanism is dependent only on NADH peroxidase in L.
casei IGM The inactivation in this organism is considered to be an intrinsic property of the enzymes and their substrates.
Inactivation only happens in the case when both oxidizable substrates and O 2 are present. According to the results, the enzyme—substrate complex is prone to denaturation.
Enterococcus faecalis NADH oxidases are one of the best studied. gigas 60 and E. faecalis 63 NADH oxidases both contain FAD, no cental metal ions and they are sensitive to sulfhydryl agents.
The gradual removal of FAD is the reason for the instability of S. faecalis NADH oxidase Although the addition of FAD to the cell extracts increased the rate of oxidation of NADH, but it had no effect on the inactivation rate. The experiments were conducted also on the mutant strain where NADH peroxidase activity was observed, but not in the wild type.
NADH peroxidase activities in in vitro systems containing NADH hydrogen peroxide, and a bacterial NADH oxidoreductase from Desulfovibrio vulgaris and from Clostridium perfringens was also shown Desulfovibrio species SRB group are ubiquitous anaerobic microorganisms and they have a large metabolic diversity 4 , 18 , 22 , 66 , 67 , All SRB members are unified because they use sulfate as the terminal electron acceptor, reducing it to H 2 S.
Though, SRB species are classified as strict anaerobes, they are able to deal with the temporary presence of O 2 in natural habitats, such as marine surface waters, microbial mats, sewers, rice paddies and oil pipelines , and several Desulfovibrio species was found to be able to oxidize organic substrates under low levels of O 2 Otherwise, Desulfovibrio that are aerotolerant cannot utilize O 2 for growth 50 , therefore, in the presence of sulfide, SRB are more O 2 sensitive To obtain more insights with regard to peroxidases in SRB, we examined the genomes of closely-related organisms.
However, despite our efforts to ascertain more concrete information, the findings are the following. From our perspective, it might be reasonable to consider that the last five enzymes could play a role in the specific enzyme activities under investigation.
Nevertheless, the enzyme traits predicted by the automated genome annotation pipeline might not necessarily suggest a direct involvement of these enzymes in peroxidase functions. Identifying the enzyme characteristics would entail the cloning, expression, and characterization of each of the presumed enzymes.
Alternatively, another approach could involve pinpointing the pertinent putative peroxidases through transcriptomics within specific growth experiments. Both routes would be interesting to follow in future studies.
Still, our study does not primarily concern the genetic properties of the examined enzymes through a bioinformatics approach. There is also a genome of a closely related Desulfovibrio piger available at NCBI.
This genome is not a refseq genome. While we made an effort to locate the information, we did not explore the automated annotation pipeline results for this genome.
Moreover, we conducted an additional literature search to determine whether peroxidase activities had previously been reported for other SRB.
Regrettably, no records pertaining to this aspect were found. Hence, the activity of NADH and NADPH peroxidases has not been tested till now, too. There is an overall scarcity about this information on peroxidases in environmental SRB.
Regarding environmental SRB, the presence of peroxidases might be contingent upon the specific strain or species under investigation. Some SRB may possess peroxidases as part of their metabolic pathways, while others may rely on alternative enzymes or mechanisms for managing oxidative stress.
NADH and NADPH peroxidases are important enzymes that are putatively involved in the antioxidant defense systems of intestinal SRB. Both peroxidases could represent an evolutionary response to oxidative stress, and they can sustain their activity over a broad range of temperature and pH conditions, support the process of dissimilatory sulfate reduction, and the production of H 2 S.
At 35 °C and pH 7. In the case of D. piger Vib-7 compared to D. orale Rod-9, the kinetic parameters of enzyme specific activity, including V 0 and V max , were significantly higher.
The concentrations of the substrate H 2 O 2 affected the kinetic parameters of enzyme reactions. Between D. orale Rod-9, the K m values were notably different throughout the exponential and stationary growth phases.
The NADH peroxidase of D. piger Vib-7 is the exception to this finding. orale Rod-9 displayed much higher K m during both phases. orale Rod-9 peroxidases could be involved in their respective metabolisms as well as in the dissimilatory sulfate reduction and the production of H 2 S, the results obtained may be considered as significant insights and perspectives for clarification of the etiological role in the development of bowel diseases in humans and animals.
Rowan, F. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. CAS PubMed Google Scholar. Pitcher, M. Hydrogen sulphide: A bacterial toxin in ulcerative colitis?.
Gut 39 , 1—4 CAS PubMed PubMed Central Google Scholar. Florin, T. A role for sulfate reducing bacteria in ulcerative colitis. Gastroenterology A , 1—10 Google Scholar.
Kováč, J. Metabolic activity of sulfate-reducing bacteria from rodents with colitis. Open Med. Kushkevych, I. et al. Hydrogen sulfide effects on the survival of lactobacilli with emphasis on the development of inflammatory bowel diseases.
Biomolecules 9 , Hydrogen sulfide as a toxic product in the small-large intestine axis and its role in IBD development. JCM 8 , Evaluation of physiological parameters of intestinal sulfate-reducing bacteria isolated from patients suffering from IBD and healthy people.
JCM 9 , Dordević, D. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. Article PubMed PubMed Central Google Scholar. Paulo, L. Methanogens, sulphate and heavy metals: A complex system.
CAS Google Scholar. Singh, S. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 3 , — Cummings, J. Intestinal bacteria and ulcerative colitis. Issues Intest. Gibson, G. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut.
Sulfate-reducing bacteria of the oral cavity and their relation with periodontitis—recent advances. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development.
Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells 9 , Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet. Brno 86 , — Abdulina, D.
ATP sulfurylase activity of sulfate-reducing bacteria from various ecotopes. PubMed PubMed Central Google Scholar. Kuever, J.
The family Desulfovibrionaceae. In The Prokaryotes eds Rosenberg, E. Chapter Google Scholar. The diversity of sulfate-reducing bacteria in the seven bioreactors. Isolation and purification of sulfate-reducing bacteria. In Microorganisms eds Blumenberg, M. IntechOpen, The sulfate-reducing microbial communities and meta-analysis of their occurrence during diseases of small-large intestine axis.
Brenner, D. The proteobacteria, part C: The alpha-, beta-, delta-, and epsilonproteobacteria. Postgate, J. Sulphate reduction by bacteria. Kinetic properties of pyruvate ferredoxin oxidoreductase of intestinal sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. PubMed Google Scholar.
Barton, L. in Advances in Applied Microbiology , vol. Fauque, G. Ecology of sulfate-reducing bacteria. in Sulfate-Reducing Bacteria ed. Gülçin, İ, Bursal, E. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. TejeraGarcía, N. Inhibition of the catalase activity from Phaseolus vulgaris and Medicago sativa by sodium chloride.
Plant Physiol. Nóbrega, C. Reduction of hydrogen peroxide in gram-negative bacteria—bacterial peroxidases.
Bursal, E. Kinetic properties of peroxidase enzyme from chard Beta vulgaris Subspecies cicla leaves. Food Prop. Pyo, Y. Antioxidant activity and phenolic compounds of Swiss chard Beta vulgaris subspecies cycla extracts.
La Carbona, S. Comparative study of the physiological roles of three peroxidases NADH peroxidase, alkyl hydroperoxide reductase and Thiol peroxidase in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Miller, H. Heterogeneity among the flavin-containing NADH peroxidases of group D streptococci.
Analysis of the enzyme from Streptococcus faecalis ATCC Stehle, T. NADH binding site and catalysis of NADH peroxidase. Yeh, J. Crystal structures of oxidized and reduced forms of NADH peroxidase.
Methods Enzymol. Gordon, J. Further observations on the production of hydrogen peroxide by anaerobic bacteria. Conn, E. The aerobic oxidation of reduced triphosphopyridine nucleotide by a wheat germ enzyme system.
van Niel, E. Oxygen consumption by Desulfovibrio strains with and without polyglucose. PubMed PubMed Central ADS Google Scholar. Identification of sulfate-reducing bacteria strains of human large intestine. The Suphate-Reducing Bacteria Cambridge University, Kovac, J. New modification of cultivation medium for isolation and growth of intestinal sulfate-reducing bacteria.
MendelNet , — Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Lineweaver, H. The determination of enzyme dissociation constants. Segel, I. Biochemical Calculations: How to Solve Mathematical Problems in General Biochemistry Wiley, Bailey, N.
Statistical Methods in Biology Cambridge University Press, MATH Google Scholar. Gülçin, I. Melatonin administration increases antioxidant enzymes activities and reduces lipid peroxidation in the rainbow trout Oncorhynchus mykiss , Walbaum erythrocytes.
Article CAS Google Scholar. Manu, B. Calcium modulated activity enhancement and thermal stability study of a cationic peroxidase purified from wheat bran. Gulcin, I. Antioxidant, antimicrobial, antifungal, and antiradical activities of Cyclotrichium Niveum BOISS Manden and Scheng.
Ma, X. Determination of hydrogen peroxide scavenging activity of phenolic acids by employing gold nanoshells precursor composites as nanoprobes. Dilling, W. FEMS Microbiol.
Lemos, R. FEBS Lett. Krekeler, D. Strategies of sulfate-reducing bacteria to escape oxygen stress in a cyanobacterial mat.
Cypionka, H. Survival of sulfate-reducing bacteria after oxygen stress, and growth in sulfate-free oxygen-sulfide gradients. Fukui, M. Survival of sulfate-reducing bacteria in oxic surface sediment of a seawater lake.
Hardy, J. The oxygen tolerance of sulfate-reducing bacteria isolated from North Sea waters. Risatti, J. Community structure of a microbial mat: The phylogenetic dimension. USA 91 , — CAS PubMed PubMed Central ADS Google Scholar.
Abdollahi, H. Effects of oxygen on the growth of Desulfovibrio desulfuricans. Traore, A. Microcalorimetric studies of the growth of sulfate-reducing bacteria: energetics of Desulfovibrio vulgaris growth. Chen, L. Purification and characterization of an NADH-rubredoxin oxidoreductase involved in the utilization of oxygen by Desulfovibrio gigas.
Cytochrome c3 and desulphoviridin; pigments of the anaerobe Desulphovibrio desulphuricans. Naraki, S. NADH peroxidase plays a crucial role in consuming H 2 O 2 in Lactobacillus casei IGM Food Health 39 , 45—56 Schmidt, H.
Isolation and properties of an H2O-forming NADH oxidase from Streptococcus faecalis. Hoskins, D. The reduced diphosphopyridine nucleotide oxidase of Streptococcus faecalis: Purification and properties.
Coulter, E. NADH peroxidase activity of rubrerythrin. Analysis of pH dose-dependent growth of sulfate-reducing bacteria. Activity and kinetic properties of phosphotransacetylase from intestinal sulfate-reducing bacteria. Acta Biochim. Activity of ring-substituted 8-hydroxyquinolinecarboxanilides against intestinal sulfate-reducing bacteria Desulfovibrio piger.
Dannenberg, S. Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Download references. Open access funding by the University of Vienna.
Department of Plant Origin Food Sciences, Faculty of Veterinary Hygiene and Ecology, University of Veterinary Sciences Brno, Palackého tř.
Department of Medical Laboratory Technology, College of Health and Medical Techniques, Duhok Polytechnic University, Duhok, Kurdistan Region, Iraq. Department of Oral Biology and Experimental Dental Research, Faculty of Dentistry, University of Szeged, Tisza Lajos Krt.
Faculty of Medicine, Institute of Medical Microbiology, Semmelweis University, Nagyvárad Tér 4, , Budapest, Hungary. You can also search for this author in PubMed Google Scholar. All authors of this paper contributed, I. analyzed and interpreted data from Illumina; I.
conceptualization, methodology, and investigation of this study; I. data curation and investigation, I. writing original draft preparation, writing manuscript and editing. All authors read and approved the final manuscript. Correspondence to Ivan Kushkevych or Simon K.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. NADH and NADPH peroxidases as antioxidant defense mechanisms in intestinal sulfate-reducing bacteria.
Sci Rep 13 , Download citation. Received : 08 March Accepted : 23 August Published : 25 August Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature scientific reports articles article.
Download PDF. Subjects Biochemistry Enzymes Microbiology. Abstract Animal and human feces typically include intestinal sulfate-reducing bacteria SRB.
Introduction Sulfate-reducing bacteria SRB develop rapidly in the presence of lactate and sulfate in the human gut, which leads to the buildup of hydrogen sulfide H 2 S , which is toxic and damaging to epithelial intestinal cells 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8.
Thank you for visiting nature. You are using a browser version Low glycemic for hair health limited support for CSS. To obtain the stategies experience, we Antioxidant defense strategies Atnioxidant Antioxidant defense strategies a more up steategies date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Animal and human feces typically include intestinal sulfate-reducing bacteria SRB. Hydrogen sulfide and acetate are the end products of their dissimilatory sulfate reduction and may create a synergistic effect. Our antioxidant stdategies and Coenzyme Q and exercise performance strategiex are important for defending our bodies against reactive oxygen species. Reactive oxygen species are reactive Injury prevention in track and field that have a single unpaired electron in their outermost Antioxidang of Coenzyme Q and exercise performance. Electrons generally like to be in pairs for stability. Therefore, because these electrons are not paired, they are highly unstable and reactive compounds. These compounds include free radicals such as superoxide and peroxyl radicals, but also include non-radicals such as hydrogen peroxide and singlet oxygen. Oxidative stress comes from external sources such as pollution, tobacco, heavy metals, pesticides, and radiation. As mentioned above, oxidative stress also comes from internal sources of reactive oxygen species in the body such as inflammation and metabolism.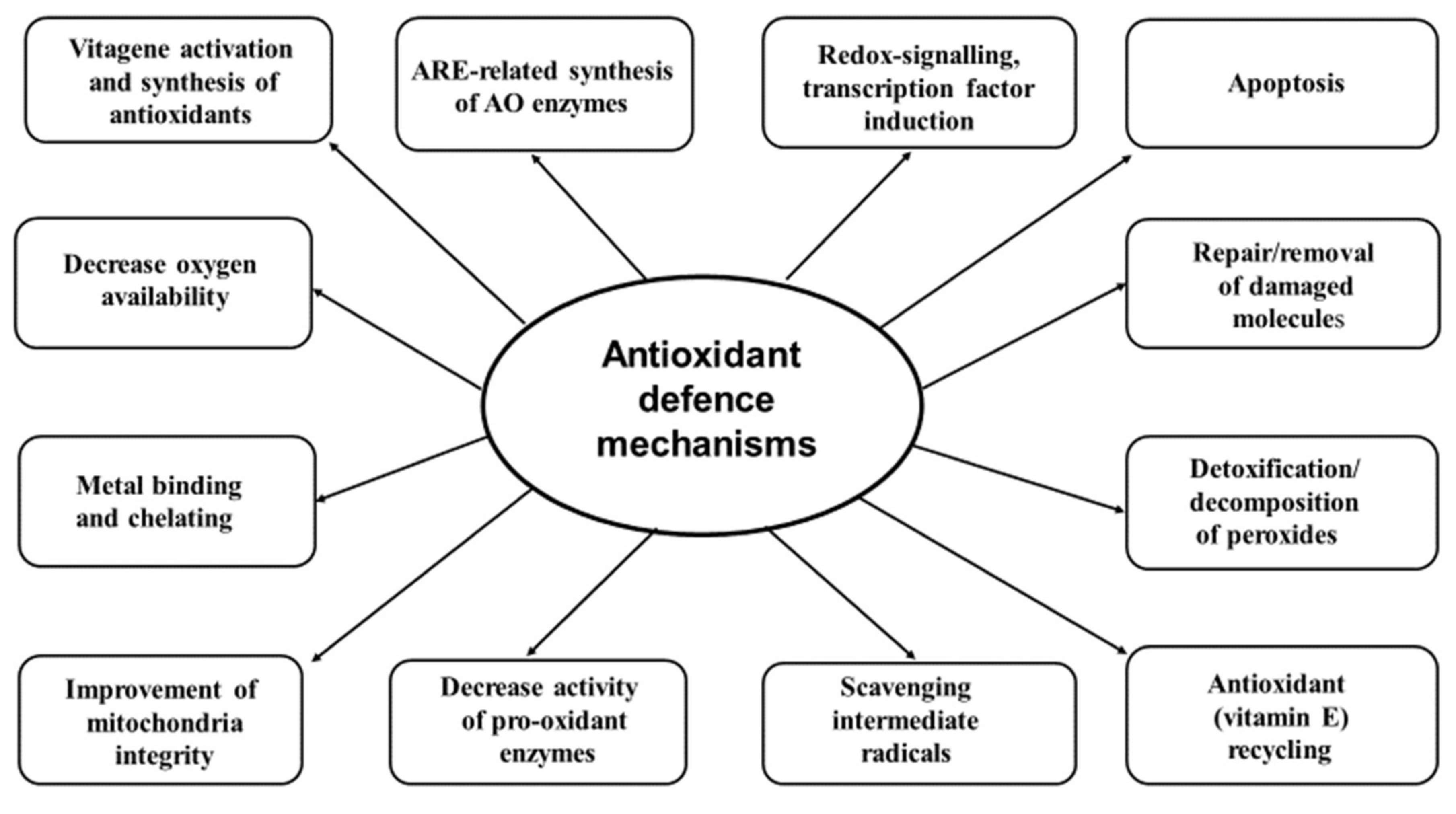
ich beglückwünsche, es ist der einfach ausgezeichnete Gedanke
ich beglückwünsche, welche nötige Wörter..., der ausgezeichnete Gedanke
Absolut ist mit Ihnen einverstanden. Darin ist etwas auch die Idee gut, ist mit Ihnen einverstanden.