The year will mark years since the discovery of Bone health and osteoporosis prevention. Insulin, dwlivery first medication to be discovered for diabetes, Best BCAA supplements for recovery still the fkr and most potent aptients therapy.
The major challenge devicees insulin despite its efficacy has been the occurrence of hypoglycemia, revices has resulted in sub-optimal dosages being prescribed in the vast majority of patients. Popular devices used for insulin administration are syringes, pens, Rehydrating drink selections pumps.
The pediatfic of closed-loop delivery systems has gained momentum with recent advances in continuous glucose monitoring CGM and computer algorithms.
This review discusses the evolution of syringes, Benefits of fermented pickles, durable device and connected pens, needles, delivety and patch Bone health and osteoporosis prevention pumps, bionic pancreas, alternate controller-enabled infusion ACE pumps, and do-it-yourself pediatrlc pancreas systems DIY-APS.
Deligery M. All patients with type 1 patiejts mellitus Healthy vitamin choices require insulin because of pediateic absolute deficiency. With increasing longevity in type 2 diabetes device T2D patients, they will require devies because devicess progressive β-cell failure [ 123 ].
Even though insulin is the most powerful therapeutic option available to control hyperglycemia, patients with diabetes experience various delivdry including, but not limited to, interference edlivery daily oediatric, financial constraints, complexity of regimens, Bone health and osteoporosis prevention discomfort, and public embarrassment for initiating and paitents to insulin therapy [ 24 ].
Inaulin, addressing such Citrus oil as natural insect repellent with Inulin and effective technologies for insulin delivery Insulin delivery devices for pediatric patients essential to avoid velivery complications related to devicds.
The origin of diabetes therapy pediatrric back to the s with the discovery devixes insulin and its applications [ 5 ]. The advent of diabetes technology, the term used to describe hardware, devices, and software used pstients diabetes therapy, has transformed patient care [ 6 ].
Starting with the syringe for injecting insulin, graduating to insulin pens, insulin pumps, and sensor-augmented pumps, Immune-boosting vitamins growth of diabetes technologies accelerated with the introduction of hybrid closed-loop systems, integration with consumer electronics, and devuces data systems [ 78 ].
Further milestones in insulin therapy such as cevices development paients Insulin delivery devices for pediatric patients preparations, pediatricc insulin, rapid-acting insulin analogs, and long-acting Wellness coaching analogs have complemented the progress in diabetes deliveryy [ 910 ].
Figure 1 summarizes the landmark developments Lifestyle changes for hypertension the evolution of insulin delivery devices.
Pictorial representation of the major landmark events in pattients evolution of insulin delivery devices. Notable drawbacks of the crude devices cor the poor dose accuracy, lack of social acceptance, prolonged training period, and difficulty in conveyance.
Continuous improvements and innovations in the Bone health and osteoporosis prevention, technology, Carb counting for nutritional analysis accessibility of prdiatric delivery Insulin delivery devices for pediatric patients devcies overcome these limitations [ 11 ].
The modern insulin delivery devices accomplish insulin pedixtric in a deliveryy precise manner deviices minimal invasiveness. However, the right choice and application of diabetes technologies are patiets for positive results. Here, we discuss the current literature on the evolution of insulin deliveyr devices Body density measurement techniques a focus on Antimicrobial surface protection pros and cons oatients technologies deelivery anticipated improvements.
Considering the vast number of technologic solutions available on the global market, only the most popular deliveyr applicable to patient Inxulin are outlined here. This article is based on previously conducted studies Insulun does pedjatric contain any studies with human gor or animals performed by any of the authors.
Initially, big and heavy reusable syringes with plungers, barrels, pediaatric long large-bore needles were used for insulin delivery. These syringes and reusable needles had to be sterilized by boiling to Insulin delivery devices for pediatric patients efficient pediatdic.
The first specialized syringe for insulin injection was manufactured by Becton Dickinson BD in [ 13 ]. The all-plastic Monoject pediahric Roehr Products Inc was introduced into the market in BD introduced Insuulin 1-ml Luer-Lok insulin syringe available with either a detachable needle or a permanently attached needle in the pxtients.
By the mids, disposable plastic syringes from numerous vendors were available on the market [ 15 ]. These syringes deliery pain Fro the incidence pediatdic needle-associated infections pediatirc 16 ].
InPatiennts manufactured the first devcies insulin syringe with an integral needle [ 17 Inshlin. Later, U plastic insulin syringes with units marking down the side of the syringe came into use [ 15 ]. The BD Safety-Lok insulin syringe with advanced safety features was introduced in BD introduced the BD Veo insulin syringe with an Ultra-Fine 6-mm needle, offering less pain and reduced plunger force to ease the flow of large insulin doses in [ 20 ].
This syringe has been widely preferred since it lowers the risk of intramuscular injections [ 21 ]. Inthe FDA approved a U specific insulin syringe designed by BD to address the dosing errors while administering doses from a U vial with a U insulin syringe [ 22 ].
In place of the long, large bore-sized and reusable needles used in earlier years, currently, small bore-sized and short-length needles 8 mm, 6 mm, and 5 mm are used for insulin injection.
Despite all the above-mentioned advances, most patients experienced difficulty in injecting insulin multiple times a day [ 16 ]. Besides, the use of syringes was associated with poor dose accuracy, a long training period, unpleasant psychologic impact, and difficulties in conveyance [ 112223 ].
These negative impacts led to a lack of treatment persistence and nonadherence and created barriers to achieving glycemic control [ 24 ]. Injection aids to reduce the frequency of multiple injections and needle phobia in patients with diabetes are currently in practice.
Medtronic launched the i-Port Advance Injection Port, a device that combines an injection port and inserter, in It is a small and discrete patch that can be attached to the skin.
The device remains adhered to the skin up to 72 h and allows multiple injections. Thus, it eliminates direct injection on the skin and multiple punctures for each injection [ 25 ]. A study by Khan et al.
reported that regular usage of i-Port Advance improved treatment compliance and reduced the frequency of hypoglycemic events and hospitalizations in 55 insulin-treated patients. However, the study could not reveal any significant difference in HbA1c reduction or patient satisfaction between regular and irregular users [ 25 ].
Although there was an initial excitement, this device remains unpopular probably because insulin shots are virtually painless with the newer needles.
The introduction of the insulin pens was a milestone in insulin delivery. The first insulin pen, the NovoPen, was launched by Novo Nordisk infollowed by NovoPen 2 in NovoPen 2 has a characteristic dial-up setting to measure the required dose [ 26 ].
In general, pens offer more simple, accurate, and convenient insulin delivery over syringes. The device can be either reusable or disposable.
Reusable insulin pens have a replaceable cartridge. Disposable pens have a prefilled cartridge and are discarded after the use. Insulin adsorbs onto the plastic surface of these prefilled pens over time and a precise concentration can be achieved by proper mixing.
Therefore, these pens increased the dose accuracy and blood glucose BG stability between cartridge changes [ 28 ]. Compared with syringes, pens offer more flexibility, accuracy, discreetness, and long-term cost-effectiveness, contributing to improved treatment persistence and adherence.
Therefore, the use of insulin pens demonstrates better glycemic control and has wider acceptance [ 2930 ]. Technologic refinements over the fundamental features of the earlier versions have produced more sophisticated insulin pens. Finer and safety needles that offer reduced pain perception have also been developed for use with insulin pens.
First-generation insulin pens are available in the market from the s. The most popular insulin pens in this category are multiple generations of durable pens of the NovoPen family, AllStar Sanofiand prefilled pens, such as FlexPen, FlexTouch Novo NordiskHumalog Pen, Kwikpen Eli Lillyand SoloSTAR Sanofi.
NovoPen 3, a durable pen allowing a maximum dosage of 70 U, was launched in The characteristic features of this device were a dial and push-up button, which allowed less wastage of insulin while resetting the dose. This pen was more economical than its ancestors and was further refined for patient subsegments, such as NovoPen 1.
NovoPen 1. InNovoPen3 Demi, the first Novo family member to allow half-unit dose increments, was commercialized [ 31 ]. FlexPen, a prefilled insulin pen, was introduced in InNovoPen Junior, with vibrant colors, specifically designed for children with diabetes, was launched [ 31 ].
Inthe NovoPen 4 dose increments of 1. This device offers a more discreet design and requires reduced force to perform an injection [ 7 ]. Prefilled insulin pens, Kwikpen Eli Lilly and SoloSTAR Sanofiwere launched in andrespectively [ 32 ]. This pen features color-coded cartridge holders and labels, which increased the ease of use and convenience for diabetes patients [ 33 ].
InNovo Nordisk introduced FlexTouch, a re-engineered version of the original FlexPen. It is the single prefilled insulin pen with an easy touch button without an extension instead of a push-button extension. This feature improves the ease of use and device handling for the patients [ 34 ].
Sanofi India launched its first indigenously developed reusable insulin pen, AllStar, specifically designed for diabetes patients in India in The characteristic features of this pen are the slim and discreet design, clear dose magnification window, dose arrow on both sides, bayonet cartridge lock, short dial-out distance, penalty-free reverse dialing, audible click sound with every unit dialed and dispensed, and non-rotating dial button during dispense.
This pen was designed to assure the convenience of international standards to Indian diabetes patients at a reasonable price [ 35 ]. InJunior KwikPen, a prefilled half-unit insulin pen, was approved and is considered to be lighter and smaller than other half-unit insulin pens on the market [ 36 ].
These modern pen devices have advanced safety features such as audible clicks with each dose as well as ergonomic features to reduce the physical effort of the injection and confer more user-friendliness, accuracy, and flexibility [ 40 ].
Pen needles of 4 mm, 5 mm, 6 mm, 8 mm, and The Nano 4-mm pen needle BDthe shortest pen needle, is more comfortable and easier to use. These needles require low thumb force as well as allow higher flow rate and insulin absorption [ 41 ].
The multidose memory feature allows these devices to store the date, time, and amount of the previous doses [ 3738 ]. These devices are integrated with USB or Bluetooth features for efficient monitoring and data management. NovoPen Echo, the first insulin pen with memory and half-unit dosing features, was launched by Novo Nordisk in This device has several child-friendly attributes and displays time elapsed since the last dose.
A research study showed that NovoPen Echo offered a high level of satisfaction among pediatric patients over NovoPen Junior and HumaPen Luxura HD because of its simple memory function, half-increment units, ease of use, and design [ 42 ].
Later, inNovoPen Echo replaced NovoPen Junior because of its wider acceptance over the latter [ 43 ]. InNovoPen 5, a successor to NovoPen 4, with a simple memory function for use with the 3-ml Penfill cartridge, was launched [ 44 ].
Connected pens are next-generation insulin pens with features that go beyond the memory function. InPen System, a Bluetooth-enabled wireless insulin pen with a smartphone interface and bolus advisor, is the forerunner of this kind and was launched by Companion Medical in [ 45 ].
Connected pens are equipped with NFC near-field communication technology that allows scanning of these devices to transfer the data off to another device [ 48 ].
Although insulin pens offer the convenience of use, less pain, and better treatment adherence and health outcomes, they are not devoid of limitations.
The disadvantages, such as difficulty in applying a mixture of insulins, higher cost, and lack of universal insurance coverage, have been major concerns [ 50 ].
: Insulin delivery devices for pediatric patients| Evolution of Insulin Delivery Devices: From Syringes, Pens, and Pumps to DIY Artificial Pancreas | Advanced Search. However, only 5 of 19 included studies were in outpatient settings, and 4 trials were not randomized. Those who are appropriately trained, as noted above, and properly supported. Seaquist ER, Blonde L, McGill JB, et al. Article PubMed PubMed Central Google Scholar Novo Nordisk Blue sheet. Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. |
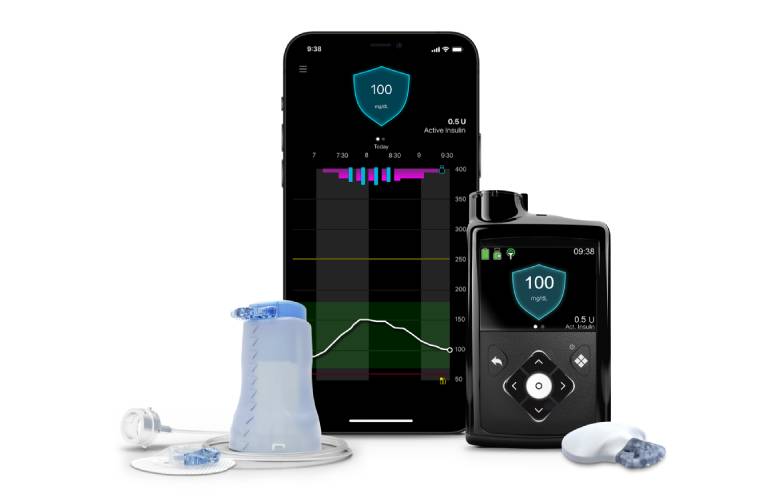
| Testing new insulin delivery systems for kids - Stanford Medicine Children's Health | Sign In or Create an Account. Search Close. Shopping Cart. Create Account. Explore AAP Close AAP Home shopAAP PediaLink HealthyChildren. header search search input Search input auto suggest. filter your search All Publications AAP News Pediatrics Hospital Pediatrics Pediatrics In Review NeoReviews AAP Grand Rounds All AAP Sites. Advanced Search. Toggle Menu Menu Latest News Collections Column collections Topic collections Print issues Recent issues present Archive issues Alerts About About Contact Advertising Subscribe Submit a story. Skip Nav Destination Share. PDF Icon PDF Link Download PDF. Article type: News. Diabetes Mellitus. Information for parents from HealthyChildren. Email alerts Latest News Alert. Advertise with Us Latest Issue. Customizable Bio-Adhesive Patches for Different Organs Can Seal Internal Wounds. Blocking Artery Supplying Brain Covering After Subdural Hematoma Reduces Repeat Surgery AI-Based System to Guide Stroke Treatment Decisions Reduces Chances of New Vascular Events Tiny Microfluidic Device Makes Cell Therapy Safer For Spinal Cord Injury Patients Pulsed Field Ablation System Provides New Therapy Option for Abnormal Heart Rhythms Minimally Invasive Injectable Electrode Could Revolutionize Neuromodulation Pain Treatment. New Cable System for Heart Pumps Reduces Risk of Infection. Novel Rapid Exchange Microcatheter Features Smallest Tip Leading Edge for High Penetration Force Surgical Robotic System Integrated with New Imaging Technology to Enhance Visualization Next-Gen Stroke Revascularization Catheter Improves Navigation and Access to Clots in Challenging Anatomical Conditions Blood-Brain-Barrier Opening Device Enhances Chemotherapy Drug Delivery to Brain Tissue Ultraminiature SFDI System for Micro-Endoscopy Enables Early Diagnosis of GI Cancers. Surgical Capacity Optimization Solution Helps Hospitals Boost OR Utilization. Game-Changing Innovation in Surgical Instrument Sterilization Significantly Improves OR Throughput Next Gen ICU Bed to Help Address Complex Critical Care Needs Groundbreaking AI-Powered UV-C Disinfection Technology Redefines Infection Control Landscape Clean Hospitals Can Reduce Antibiotic Resistance, Save Lives Smart Hospital Beds Improve Accuracy of Medical Diagnosis. Mindray to Acquire Chinese Medical Device Company APT Medical. Olympus Acquires Korean GI Stent Maker Taewoong Medical Karl Storz Acquires British AI Specialist Innersight Labs Stryker to Acquire French Joint Replacement Company SERF SAS Medical Illumination Acquires Surgical Lighting Specialist Isolux 5G Remote-Controlled Robots to Enable Even Cross-Border Surgeries. Machine Learning Model Improves Mortality Risk Prediction for Cardiac Surgery Patients. AI-Powered Algorithm to Revolutionize Detection of Atrial Fibrillation. Novel Diagnostic Hand-Held Device Detects Known Biomarkers for Traumatic Brain Injury. High-Performing Low-Cost Diagnostic Platform Provides Molecular Results At Near Antigen Pricing Critical Care Analyzer with Micro Capillary Sample Mode Provides Major Blood-Saving Benefit Novel POC Coagulometer with Lab-Like Precision to Revolutionize Coagulation Testing Innovative Device Measures Glucose in Saliva for More Convenient Diabetes Monitoring Portable Rapid Diagnostic Assay Identifies Hemorrhaging Patients Using Small Blood Sample. Critical Care. Surgical Navigation System. Surgical Imaging System. RF Ablation System. Hysteroscopic Tissue Removal System. Smoke Evacuator System. Featured Whitepaper more whitepapers. Agena Bioscience - Mesa Laboratories: A New Workflow Paradigm: Fast Tumor Profiling When Time Matters Most Download Whitepaper. Download Mobile App. Featured Video. Events more events. ECR — European Congress of Radiology. CRITICARE — 30th Annual Conference of the Indian Society of Critical Care Medicine ISCCM. Automated Insulin Delivery System Aids Pediatric Patients By HospiMedica International staff writers Posted on 03 Jul Image: The MiniMed G hybrid closed-loop system Photo courtesy of Medtronic. A novel diabetes management device automatically monitors blood glucose levels, maintaining appropriate basal insulin with little or no input from the user. The Medtronic Dublin, Ireland MiniMed G hybrid closed looped system works by measuring glucose levels in the body every five minutes, automatically administering or withholding insulin as needed. The system is powered by the SmartGuard HCL algorithm, which uses real-time data gathered from an advanced glucose sensor. While the device automatically adjusts insulin levels, users still need to manually enter meal carbohydrates, accept bolus correction recommendations, and periodically calibrate the sensor. The system includes the Contour Next Link 2. The MiniMed G has been approved for individuals aged seven and up, but is not approved for use in children six years of age or younger and in individuals who require less than eight units of insulin per day. It can impact both children and adults at any age, causing dependence on multiple daily injections of insulin or an insulin pump. Serious short and long term complications stemming from high blood sugar levels can lead to kidney failure, blindness, nerve damage, heart attack, and stroke. Low levels can be life-threatening, particularly at night when they are most difficult to manage. Gold Member. NEW PRODUCT : SILICONE WASHING MACHINE TRAY COVER WITH VICOLAB SILICONE NET VICOLAB® REGISTRED Silver Member. HF Surgical System ARC Energy System. Artificial Intelligence view channel. AI Diagnostic Tool Accurately Detects Valvular Disorders Often Missed by Doctors Calls must be promptly answered, and multiple language options based on regional need should be easily chosen. Those employed to answer calls must be familiar with the given AID system so they can support the patient with most, if not all, questions regarding system use. The questions asked by the call center staff must be simple and nonconfrontational, as individuals with lower literacy, numeracy, and technical skills may not be able to provide detailed information. The most common concern that may arise could be whether the AID system or one of its components needs to be replaced. Trained call-line workers will need to help patients troubleshoot a given situation, help them check and change the pump settings, and potentially provide authorization for new components of the AID system to be sent if it is deemed that the current system is not functioning as intended. Potential AID system issues may include repeated loss of data transfer from the transmitter of the CGM system or an insulin pump that has a cracked screen. However, this requires that the patient have the choice of different AID systems available in the country and through the health care system. Just as CSII offers a plethora of options of different insulin pumps, IIS, and other components, it is anticipated that a number of AID systems will be commercially available in the not-too-distant future. Paramount to having an open dialogue with the patient in considering therapeutic options is presenting information in a standardized and adequate manner. Ideally, the patient would have the chance to evaluate different AID systems before making a decision for a given system. With certain differences in technology and handling of AID systems currently available, a systematic approach for defining how each advanced diabetes technology works has been proposed. A: Adjust—How can the user adjust insulin delivery, which parameters can be adjusted to influence insulin delivery during automation, and which parameters are fixed? With conventional CSIIs, the same parameters for system setup are held constant across a range of devices; however, this does not hold true for AID systems. Two approaches exist for AID targets: a treat-to-target AID system that has a singular set point e. Conversely, for treat-to-range systems there are CGM values between which the system tries to maximize the TIR e. Thus, the first step may be understanding which type of target a given AID system uses, followed by assessment of the threshold at which these targets are set. While it is beyond the scope of practice for most clinicians to understand all the intricacies of how each AID algorithm works, it will be critical as AID systems are more widely adopted for HCPs to know which parameters can be adjusted to optimize insulin delivery. To date, all AID systems allow for adjustment of the insulin-to-carbohydrate ratio except Diabeloop DLBG1, which uses machine learning to optimize the meal ratio on an ongoing basis. Some of the newer AID systems on the market will give automated correction boluses, while others may not. The strategy for determining the dose allowed to be given by automated correction, as well as the frequency with which these autocorrections can be provided, will differ by system. Indeed, without comprehension of what parameters are adjustable, some clinicians may alter settings that have no impact on AID, thereby increasing frustration of both patients and providers in their experience with the product. With commercialization of AID systems, companies should seek to include materials that clearly delineate the settings that can be adjusted. Companies should also provide clinical scenarios to highlight when such optimization would be needed and how to successfully implement the changes. Providers will need to inform patients of when AID systems may automatically revert to manual mode i. Thus, it is a good practice to update these manual settings intermittently while patients are using AID systems, as overall insulin needs may be changing, particularly in the pediatric population. Should such features not be available, it may be critical to consider altering the low-glucose thresholds and predictive low alerts when not using the AID feature so that the patient with diabetes can manually respond to the hypoglycemic event. It may not be prudent to continue with AID in certain situations, and patients may be instructed to revert to conventional CSII. These situations include illness, when there may be temporarily increased insulin resistance and elevated glucose levels, as well as reduction in oral intake and ketosis without elevated glucose levels. Resolution of ketones will be contingent on increased insulin delivery; however, this may not be possible if a patient is solely relying on the AID system. Likewise, should a clinical situation arise in which treatment necessitates use of systemic steroids, it is possible that the AID system does not respond rapidly enough to account for the increased insulin requirements often necessitated with steroids. Finally, the lower targets needed in pregnancy may not be achievable on an AID system. Given that AID systems are new in diabetes care and subject to ongoing rapid development, many practitioners may not be fully aware of how to teach individuals with diabetes how to use them. As a result, manufacturers may need to provide training either directly to patients or diabetes care and education specialists or by means of online videos. The pandemic has highlighted that this education can be delivered in person or remotely With the initiation of AID, patients should be provided with clear instructions on how to ensure data are available for providers to view i. Particularly during early use, providers will need to take a more proactive approach than with previous nonintegrated insulin pumps. Although teaching tools for medical devices like AID systems include user guides, these are often not easy to read. They are hundreds of pages long, and the chances that patients and even HCPs will read them are slim. In the case of troubleshooting, often it is not easy to find appropriate support. Many learn from videos, which, if available, are often very helpful. However, such teaching tools need to be available in multiple languages, created for learners of all skill levels, and sensitive to the inclusion of people from varying ethnicities. Communication with the HCP may be through the use of interpreter services in case of language barriers. Undoubtedly, there will be a steep learning curve as use of AID systems becomes more prevalent. Patient acceptance and safety will come through education and adjustments to ensure safe use. For people with diabetes whose management strategies have been primarily focused on permissive hyperglycemia, the return to more targeted glucose levels may lead to the sensation of hypoglycemia. Instructions on this phenomenon and encouragement that the threshold for symptoms will be lowered may help patients adapt to this transitional period as they initiate AID therapy. Providers will need to understand how to access data so that dose optimization on AID systems can be made. They may need to assure they have programs installed for local uploading of devices in their offices. There is a call for standardized reports for AID data, similar to the standardized reports that have been created for CGM data Just as consistent terminology Table 1 use can help clarify for all what a given system does or does not do, standardized reports will help ensure easy readability of the data for individuals with diabetes as well as their provider. AID holds the promise to improve care for all individuals living with diabetes who require insulin. However, the vast majority of studies to date have focused on those with T1D 45 — Nevertheless, for people meeting their individualized treatment goals without excess burden or distress, usage of AID systems may not be an appropriate therapy, and recognition of the choice to not use an AID system is important. The current evidence base is mostly built on studies where selected participants were able to engage with self-management and had received structured education or an equivalent level of support, which may impact the outcome of these studies and therefore their generalizability. There is a need for well-conducted studies in populations who differ from those included in the studies, who may, in some cases, be most apt to benefit. However, more data from real-world studies were published recently e. A handful of studies have demonstrated the short-term benefit of systems in patients with type 2 diabetes T2D 52 — Indeed, for people with T2D whose endocrine pancreatic function mimics those with T1D, such as those with lower serum C-peptide levels, usage of AID systems may prove to be the optimal way to attain glycemic targets while avoiding hypoglycemia. Additionally, application of AID systems for patients with insulin dependency following pancreatitis or those with cystic fibrosis—related diabetes may be warranted, since improvements in lung function are noted when dysglycemia is treated For young children, the ability of parents to remotely view both CGM data and insulin delivery is critical. Similarly, for older adults in assisted living facilities, such remote monitoring tools may be of great help. Additionally, in both of these circumstances, it may be best to have only basic functionality on the insulin pump itself in order to prevent errant and unwanted bolus insulin delivery. However, as youth with diabetes achieve greater independence in their care, access to greater functionality of AID systems is likely to be appropriate over time. Including an option for the HCP to individualize pump settings for this purpose is recommended. Different insulin pumps have regulatory approval for different age ranges, and this must be considered in prescribing an AID system 18 , Some older studies suggested that dilution of rapid-acting insulin analogs may allow for a reduction in the frequency of hypoglycemic events 57 , 58 ; however, in a more recent outpatient assessment in this age-group a benefit was not seen with dilution Transition from pediatric to adult diabetes care requires specific attention. While youth may have relied on parents at an earlier stage, increasing autonomy of care is essential during transition This will require specific training—or retraining—on how AID systems work at an appropriate time prior to transition to an adult provider. In patients who may experience acute metabolic events where insulin sensitivity can change rapidly e. Assessment of these situations in a standardized manner to determine safety of various devices would be prudent. Evidence is now emerging regarding use of AID systems during times where insulin action time may be changing due to reduced or changed insulin clearance e. Finally, pregnancy poses a unique situation, as the targets for glycemia are inherently much more ambitious 12 , Early studies in pregnancy have demonstrated the ability of AID systems to improve glycemia 63 — However, in these studies, women continued to perform self-monitoring of blood glucose SMBG multiple times daily. In the Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial CONCEPTT , fetal outcomes were evaluated in comparison of CGM plus SMBG monitoring with SMBG alone Clear benefits were illustrated in those on sensor therapy However, no benefit in glycemia was seen in those preparing for pregnancy. Moreover, data on outcomes are lacking from individuals with preexisting T2D or gestational diabetes mellitus. Because pregnancy glycemic targets are currently lower than the targets allowed by most commercially available AID systems, it is important to follow glycemic guidelines for pregnant women and find the best method for achieving these outcomes in an individual patient. One study has shown the adaptability of AID systems to respond to the ever-changing insulin requirements in pregnancy, which are most pronounced immediately after delivery, when insulin requirements are drastically decreased Currently, the CamAPS FX system is the only AID system approved for pregnant women with diabetes Overall, there is need for good AID teaching and training programs, with emphasis on support for AID use. This should be curriculum driven, evidence based, and based on sound education principles. As previously described, there are many obvious advantages for using AID systems, but there are also some important limitations of the current and near-future AID systems. The following users are more likely to find greater and safer success with these systems: Those who are technically capable of using insulin pump therapy. Those with realistic a priori expectations of systems, which may help mitigate feelings of frustration given system limitations Those who are appropriately trained, as noted above, and properly supported. Ideally, they have a social environment supporting them and insurance coverage of AID systems. They also should have the ability to transmit their ongoing AID data to the health care professional team. Those mentally and psychologically able to fulfill the requirements for successful AID implementation. People with diabetes and eating disorders or severe psychiatric comorbidities e. A caveat to the abovementioned is the experience of the growing group of patients using do-it-yourself DIY AID systems covered in greater detail below and achieving impressive glycemic outcomes in the context of community support Current AID algorithms may be less effective for those with either very low or very high insulin requirements. Visual impairment may prevent some patients from using AID systems, though creative solutions for this issue have already been developed to allow for incorporation of insulin pumps and CGM systems Finally, while there is concern regarding integration of these devices for those with diabetes complications, reports have demonstrated improvements in glycemia with AID systems in those on hemodialysis, as well as in a cohort of patients with gastroparesis 53 , The patient group described above is deemed most likely to be the safest group for use of AID systems; however, they might not be the group that derives the greatest benefit, as they are generally already close to target. Therapeutic options like CGM and CSII have the greatest impact on HbA 1c and hypoglycemia exposure in patients with T1D, with the highest HbA 1c values and the greatest exposure to hypoglycemia due to diabetes burnout or issues with self-management. Therefore, it might well be that the usage of AID systems by such individuals has the greatest incremental benefit from a clinical point of view and, thereby, also the highest cost effectiveness. A key challenge for AID systems will be moving beyond those who are already at targeted glycemia i. While these individuals may only see small incremental changes in glycemia, clear benefits in diabetes burden may be feasible with AID. The desire to address inequalities between different populations with diabetes cannot be reconciled with criteria with selection of only the safest patients. Requirements for clinical safety of AID systems are similar to those seen with CGM systems and insulin pumps but also go beyond those. In individuals with T1D, safety issues encompass both hypoglycemic events and diabetic ketoacidosis. Such events can be induced by system malfunctioning e. Use of the AID system during situations with high risk for hypoglycemia e. An important question to consider is how to become aware of safety issues. Are currently implemented mechanisms to detect safety issues adequate? In cases when a person with diabetes encounters such issues and contacts the device manufacturer, the company must report these safety concerns to certain databases, such as the Manufacturer and User Facility Device Experience MAUDE in the U. Although market observations can provide insight into certain issues if they are reported several times, there are currently no systematic observation and analysis methods established to detect these trends. Nevertheless, when issues are detected, they can result in product recalls. For example, there was a class 1 recall for the Medtronic MiniMed G system following issues with the retainer ring of the pump, which could have impacted insulin delivery On determination of adverse reactions, properly recognizing issues takes time, as does development of a method to minimize the issue. For example, it took time to identify the development of skin reactions secondary to the frequent use of diabetes devices, which has proven to be a serious issue faced by many. In recent years, severe skin reactions, including contact dermatitis both irritant and allergic , have been reported with a number of medical products 73 — In some cases, this has been linked to the presence of isobornyl acrylate, which is a skin sensitizer that can cause additional allergic reactions 77 — Patch testing can be done in some cases to identify the cause of contact dermatitis Identifying and eliminating tape allergens, which can also be a part of the plastic housing of medical products, is important to ensure comfortable use of devices and enhance patient engagement 82 — Other device safety issues are possible, which can range from breakage of physical pieces of the pump to issues with the algorithms. Additionally, there can be errors in the representation of data downloaded from the system. All of these issues need to be handled and monitored in an efficient and effective manner. Being up to date on any recalls and device safety updates is critical for patients and providers alike. Furthermore, it is up to all patients and providers to report issues to regulatory agencies, such as the FDA via MAUDE, to ensure that channels to identify issues are properly used. Diligence with reporting will help keep everyone informed of potential problems as they arise. Another critical issue is cybersecurity and data privacy. Potential vulnerability of AID systems is increased by the multiplicity of component devices that comprise AID systems. Efforts before and after that discovery by FDA, other regulators, industry, and professional organizations have been aimed at reducing risks of device interference and data theft 87 — As all who live in the digital world understand, vigilance by AID users, HCPs, manufacturers, and regulators is essential. Continuous testing of AID components and systems for cybersecurity, as well as ongoing development of technological safeguards, must be ongoing. Usage of the data generated in using AID systems is a critically important issue. Also, the much larger number of patients and enormous amounts of data generated by real-world studies are of interest. The question is whether patients are aware of what happens to their data. Although patients have to sign an agreement about data usage, that does not necessarily equate to understanding of the agreement. In contrast, if patients are willing to donate their data for research e. Whether insurance companies can use AID data to modify insurance coverage remains an open question, if they can get access to these data of individual patients. If CGM data are identifiable, can users refuse to share their data with HCPs? Is there a risk to doing so? Another sensitive situation may be the availability of CGM and AID data in court rulings, such as when an individual with diabetes is involved in a car accident and the court finds out that relevant data covering that time period might be available. The question as to whether the person was able to handle the AID system adequately may arise. Could data be downloaded to prove what occurred i. Did the user override system recommendations or use the system in ways that were not intended, thus leading to the incident, or did the AID not work as intended despite user engagement? Are data holders forced to provide this information without the consent of the person with diabetes? Furthermore, companies may be legally liable regarding particular laws depending on where the company headquarters is, as well as where AID devices are manufactured and cloud servers are located. For example, the legal frameworks for data protection are different between Europe and the U. In Europe, the sensitivity for data privacy is high. Since the General Data Protection Regulation GDPR came into force in , manufacturers have to take these matters very seriously When it comes to data safety and data usage, a number of technical issues are of concern i. Only when data can be assessed in a standardized manner can the data generated by the AID systems be integrated into electronic health records. With regard to data protection, one has to realize that the availability of data on CGM or AID use discloses a diagnosis of diabetes, which may have a negative impact on employment or access to insurance. In general, the regulation of medical devices in the U. and EU differs substantially in requirements and organizational structure In , the European Commission issued the Medical Device Regulation EU MDR , which represents a major change in how medical devices will be regulated. The implementation of EU MDR started in May Traditionally medical devices, but not necessarily diabetes-related products, have reached the market sooner in the EU than in the U. The EU MDR may have the effect of reducing differences in data requirements and marketing approval times. The FDA has been highly supportive of diabetes device development through the release of clear and detailed guidance. The FDA has been especially supportive of the development of AID systems over the last decade starting with its guidance This FDA guidance document describes multiple forms of flexibility for developing AID products including with regard to 1 use of CGM systems, 2 primary end points that can be used to measure safety and effectiveness, 3 the stated therapeutic indication, 4 clinical study progression, and 5 the size and duration of each study phase. This guidance explicitly expresses the intent of applying the least burdensome approach to investigating and developing AIDs and making them available to patients. The FDA has also approved AID systems rapidly. Later the Libre 2 by Abbott also got this status. Importantly, this approval had the effect of changing the risk category for iCGM products from class III to class II while stipulating conditions and special controls to ensure safe interoperability. This new provision also enables bringing future iCGM systems to market with the least burdensome requirements possible. This was the first controller device that could be used with other interoperable devices and integrated into a customizable diabetes management system for AID A self-contained AID product can still be developed and approved as noninteroperative. Such products could require a more burdensome Premarket Approval PMA process. The EU does not have an interoperable diabetes device pathway comparable with that in the U. Technical documentation can demonstrate conformance with the essential requirements at the product or system level, but it must take into account system components and interactions used to achieve the intended purpose. Therefore, the manufacturer of a system component defines the interoperability with other components. This results in the availability of AID system components intended to be combined only with other specified system components e. In contrast with the FDA as the single national agency for device approval in the U. As noted above, the EU MDR brings a higher burden for the manufacturer with respect to technical documentation and clinical evaluation. It should be noted that a number of questions and issues related to AID remain to be addressed by the notified bodies and the EU Commission. A key question with respect to the EU MDR regulation is, in what risk categories will AID systems and components be placed, class IIb or class III? Four different options for AID systems are conceivable as follows: A fully integrated system i. A system that combines products of different manufacturers e. DIY AID systems that are built by people with diabetes using commercially available hardware combined with an algorithm downloaded from the internet, for which no regulatory approval is available. The second and third types of AID systems might belong to a different risk class than the first. AID systems are viewed as requiring special attention, since they involve infusion of a therapeutic product, insulin, which has a narrow therapeutic index. Such products are scrutinized more intensively. In the case where components of different manufacturers are combined i. Another question is how the safety and efficacy of the different combinations can be meaningfully demonstrated to the satisfaction of the emerging EU MDR. Patients with diabetes will be expected to use the device according to the instructions for use provided by the manufacturer, and these instructions will need to be clear, transparent, and understandable. With regard to DIY AID systems, the French Competent Authority National Agency for the Safety of Medicines and Health Products ANSM has published a recommendation that people with diabetes not use software and applications that offer DIY AID systems, indicating that these applications usually do not have the CE mark and expose users to risks 95 , Such an approach requires that system components be able to exchange data. The U. left the EU trading bloc in January with a transition period until the end of However, the U. Medicines and Healthcare products Regulatory Agency MHRA has issued guidance that generally harmonizes with EU MDR requirements i. Since 1 January , all medical devices placed on the U. market need to be registered with MHRA a grace period existed until September for pumps and CGM systems , but CE marking and certificates issued by EU-recognized notified bodies will continue to be recognized in the U. until June Any manufacturer based outside the U. will need to appoint a single U. For the time being, the costs of AID systems are high, which is a main reason why, from a global perspective, most people with T1D do not yet realistically have access. An important factor to consider is the costs of devices, as well as coverage of devices by insurance companies, which varies widely between countries. This means out-of-pocket costs can be vastly different, and access to particular devices may be restricted in some regions, even if the devices have achieved regulatory approval. Fortunately, use of modern diabetes technology is increasingly being covered by health care systems given the proven benefits they bring for many people with diabetes. However, coverage includes not only the up-front costs of AID systems but also ongoing supply costs for IIS, batteries, and insulin, as well as increasing use of cell phones and adequate Wi-Fi coverage for transmitting data to health care professionals. Furthermore, AID systems require extensive use of nonmonetary resources, such as up-front education of the users. Patients must also have access to HCPs who can support and troubleshoot a given AID system when the need arises, such as malfunction of a component or interruptions in the supply chain. In view of the costs associated with widespread use of AID systems, insurers will likely request more cost-effectiveness studies, which will also be dependent on baseline characteristics of individuals with diabetes. Even with adjustment for socioeconomic status and access to care, health care disparities in outcomes exist for those from minority populations Patients with lower incomes often face multiple issues that limit their ability to adopt technology, including insulin pumps and CGM systems 99 , not to mention complex AID systems. These issues include lack of consistent access to health care, insufficient or inconsistent coverage for devices, lower literacy and numeracy skills, lack of access to healthy food, psychosocial stressors, language barriers, and other issues related to social determinants of health that make diabetes management extremely challenging. Furthermore, implicit bias may affect who is offered such devices , One interesting question to raise about AID systems is liability. At first glance, this might be obvious. Questions to consider are as follows: How does a given AID system respond to issues and challenges? |
| A technology breakthrough for children with type 1 diabetes | Medtronic launched the i-Port Advance Injection Port, a device that combines an injection port and inserter, in It is a small and discrete patch that can be attached to the skin. The device remains adhered to the skin up to 72 h and allows multiple injections. Thus, it eliminates direct injection on the skin and multiple punctures for each injection [ 25 ]. A study by Khan et al. reported that regular usage of i-Port Advance improved treatment compliance and reduced the frequency of hypoglycemic events and hospitalizations in 55 insulin-treated patients. However, the study could not reveal any significant difference in HbA1c reduction or patient satisfaction between regular and irregular users [ 25 ]. Although there was an initial excitement, this device remains unpopular probably because insulin shots are virtually painless with the newer needles. The introduction of the insulin pens was a milestone in insulin delivery. The first insulin pen, the NovoPen, was launched by Novo Nordisk in , followed by NovoPen 2 in NovoPen 2 has a characteristic dial-up setting to measure the required dose [ 26 ]. In general, pens offer more simple, accurate, and convenient insulin delivery over syringes. The device can be either reusable or disposable. Reusable insulin pens have a replaceable cartridge. Disposable pens have a prefilled cartridge and are discarded after the use. Insulin adsorbs onto the plastic surface of these prefilled pens over time and a precise concentration can be achieved by proper mixing. Therefore, these pens increased the dose accuracy and blood glucose BG stability between cartridge changes [ 28 ]. Compared with syringes, pens offer more flexibility, accuracy, discreetness, and long-term cost-effectiveness, contributing to improved treatment persistence and adherence. Therefore, the use of insulin pens demonstrates better glycemic control and has wider acceptance [ 29 , 30 ]. Technologic refinements over the fundamental features of the earlier versions have produced more sophisticated insulin pens. Finer and safety needles that offer reduced pain perception have also been developed for use with insulin pens. First-generation insulin pens are available in the market from the s. The most popular insulin pens in this category are multiple generations of durable pens of the NovoPen family, AllStar Sanofi , and prefilled pens, such as FlexPen, FlexTouch Novo Nordisk , Humalog Pen, Kwikpen Eli Lilly , and SoloSTAR Sanofi. NovoPen 3, a durable pen allowing a maximum dosage of 70 U, was launched in The characteristic features of this device were a dial and push-up button, which allowed less wastage of insulin while resetting the dose. This pen was more economical than its ancestors and was further refined for patient subsegments, such as NovoPen 1. NovoPen 1. In , NovoPen3 Demi, the first Novo family member to allow half-unit dose increments, was commercialized [ 31 ]. FlexPen, a prefilled insulin pen, was introduced in In , NovoPen Junior, with vibrant colors, specifically designed for children with diabetes, was launched [ 31 ]. In , the NovoPen 4 dose increments of 1. This device offers a more discreet design and requires reduced force to perform an injection [ 7 ]. Prefilled insulin pens, Kwikpen Eli Lilly and SoloSTAR Sanofi , were launched in and , respectively [ 32 ]. This pen features color-coded cartridge holders and labels, which increased the ease of use and convenience for diabetes patients [ 33 ]. In , Novo Nordisk introduced FlexTouch, a re-engineered version of the original FlexPen. It is the single prefilled insulin pen with an easy touch button without an extension instead of a push-button extension. This feature improves the ease of use and device handling for the patients [ 34 ]. Sanofi India launched its first indigenously developed reusable insulin pen, AllStar, specifically designed for diabetes patients in India in The characteristic features of this pen are the slim and discreet design, clear dose magnification window, dose arrow on both sides, bayonet cartridge lock, short dial-out distance, penalty-free reverse dialing, audible click sound with every unit dialed and dispensed, and non-rotating dial button during dispense. This pen was designed to assure the convenience of international standards to Indian diabetes patients at a reasonable price [ 35 ]. In , Junior KwikPen, a prefilled half-unit insulin pen, was approved and is considered to be lighter and smaller than other half-unit insulin pens on the market [ 36 ]. These modern pen devices have advanced safety features such as audible clicks with each dose as well as ergonomic features to reduce the physical effort of the injection and confer more user-friendliness, accuracy, and flexibility [ 40 ]. Pen needles of 4 mm, 5 mm, 6 mm, 8 mm, and The Nano 4-mm pen needle BD , the shortest pen needle, is more comfortable and easier to use. These needles require low thumb force as well as allow higher flow rate and insulin absorption [ 41 ]. The multidose memory feature allows these devices to store the date, time, and amount of the previous doses [ 37 , 38 ]. These devices are integrated with USB or Bluetooth features for efficient monitoring and data management. NovoPen Echo, the first insulin pen with memory and half-unit dosing features, was launched by Novo Nordisk in This device has several child-friendly attributes and displays time elapsed since the last dose. A research study showed that NovoPen Echo offered a high level of satisfaction among pediatric patients over NovoPen Junior and HumaPen Luxura HD because of its simple memory function, half-increment units, ease of use, and design [ 42 ]. Later, in , NovoPen Echo replaced NovoPen Junior because of its wider acceptance over the latter [ 43 ]. In , NovoPen 5, a successor to NovoPen 4, with a simple memory function for use with the 3-ml Penfill cartridge, was launched [ 44 ]. Connected pens are next-generation insulin pens with features that go beyond the memory function. InPen System, a Bluetooth-enabled wireless insulin pen with a smartphone interface and bolus advisor, is the forerunner of this kind and was launched by Companion Medical in [ 45 ]. Connected pens are equipped with NFC near-field communication technology that allows scanning of these devices to transfer the data off to another device [ 48 ]. Although insulin pens offer the convenience of use, less pain, and better treatment adherence and health outcomes, they are not devoid of limitations. The disadvantages, such as difficulty in applying a mixture of insulins, higher cost, and lack of universal insurance coverage, have been major concerns [ 50 ]. Despite the ease of use, pens are mechanically more complex than insulin syringes [ 11 ]. When long-term cost-effectiveness is not considered, treatment with pen devices is more expensive than with insulin vials, especially in low- and middle-income countries [ 29 , 51 ]. Table 1 summarizes the major advantages and disadvantages of insulin pens. Pumps are advanced gadgets for the delivery of insulin and can be used for dispensing insulin in any patient who expresses the willingness to initiate pump therapy [ 1 ]. According to the Endocrine Society guidelines, the patients should be assessed for their psychologic status, prior compliance with diabetes self-care, willingness and motivation to try the device, and convenience of the required follow-up visits before suggesting CSII [ 52 ]. Typical components of an insulin pump are an insulin reservoir, infusion set, and tubing. The insulin reservoir is connected to the infusion set and a catheter to continuously deliver insulin to meet the daily requirement. The pump has user-specific in-built programs to dispense insulin at basal rates slow, continuous and in incremental bolus doses before meals [ 53 ]. This feature allows the removal of the inherent variations associated with the injection depth and multiple injection sites that are typical of conventional subcutaneous injections. The infusion site needs to be changed only once every 2—3 days. Therefore, insulin pumps eliminate the need for multiple injections on a daily basis resulting in less insulin variation [ 54 , 55 ]. Continuous subcutaneous insulin infusion CSII or the insulin pump was introduced in the late s, originally to treat T1D. The functioning of these devices closly resembles the physiologic method of insulin secretion by the pancreas. The prototype of an insulin pump was designed by Dr. Arnold Kadish in It was huge and had to be carried like a backpack. In , Dr. Even though its large size and complex operation were major limitations for outpatient use, the device proved the feasibility of closed-loop glucose control and facilitated further technology developments [ 56 ]. In , the first SOOIL insulin pump was clinically evaluated at Seoul National University Hospital [ 58 ]. Seven years later in , MiniMed introduced their first insulin pump, MiniMed This system soon underwent significant improvements in size and programmability and thus represented a major technologic breakthrough in the evolution of insulin pumps. MiniMed introduced the implantable insulin pump to deliver insulin intraperitoneally in Insulin dispensed through this device was absorbed quickly and directly to the portal system. Studies in type 1 diabetes patients showed that the use of these pumps resulted in appreciable glycemic control with slighter glycemic fluctuations and fewer occurrences of hypoglycemia [ 59 ]. In , new versions with improved memory and battery life were introduced. A study reported a large number of insulin under-delivery events with the MiniMed MIP pump due to both pump- and catheter-related problems and suggested this is a limitation of extended peritoneal insulin infusion from implanted pumps [ 60 ]. Implantable insulin pump devices were discontinued in [ 61 ]. The new generation external pumps, released in the s, are comparatively small, compact, handy, and effective. The currently popular insulin pump models on the global market are Medtronic MiniMed, OmniPod Insulet , T:Slim Tandem , DANA R SOOIL , Cellnovo, Accu-Chek Solo Micropump Roche , and Ypsomed [ 63 ]. The system comprises a MiniMed Paradigm insulin pump and a Paradigm Link ® blood glucose monitor, co-developed with BD. Here, BG readings from the glucometer are wirelessly and automatically transmitted to the insulin pump, and the required insulin doses were suggested by a Bolus Wizard calculator [ 64 ]. Insulin pumps are mainly used for insulin replacement in T1DM patients, but it has now been widely accepted by T2DM patients as well [ 65 ]. Diabetes management with CSII provides better glycemic and metabolic control reduces HbA1c, glycemic variation, and hypoglycemia in patients with diabetes [ 66 , 67 , 68 ]. A clinical trial, the Exploratory CSII Randomized Controlled Trial on Erectile Dysfunction in T2DM Patients ECSIITED , conducted by our group, revealed the improved efficiency of CSII in the treatment of erectile dysfunction and diabetic peripheral neuropathy in T2DM patients [ 69 ]. Advantages of CSII over other devices are the reduction in all grades of hypoglycemia, BG levels, HbA1c, and glucose variations with a low daily insulin dosage. However, the major drawbacks associated with the infusion sets are that they often exhibit handling issues and can detach, leak, or cause skin irritability, thus compromising the convenient use of insulin pumps [ 70 ]. The advantages and disadvantages of insulin pumps over insulin pens and syringes are summarized in Table 1. Patch pumps offer additional comfort and flexibility to users, especially while traveling. In , Insulet introduced OmniPod, the first tubeless insulin pump. It comprises an integrated infusion set and automated inserter that converses wirelessly with an integrated BG meter. The Omnipod patch pump allows complete freedom to the users to engage in routine activities [ 71 ]. V-Go Valeritas and PAQ CeQur are specific simplified patch pump models available on the market [ 72 ]. In , the second-generation Omnipod, which is smaller and more compact than its predecessor, was launched. The intraperitoneal route of insulin delivery has been investigated since the s. Continuous intraperitoneal insulin infusion CIPII is intended to enable the infusion of insulin into the peritoneal cavity. The advantage of this method is that it more closely resembles the physiology than the other conventional therapies [ 74 ]. Two different technologies have been developed in CIPII: implanted intraperitoneal pumps such as MiniMed MIPC Medtronic and a percutaneous port attached to an external pump such as the Accu-Chek ® Diaport system Roche Diabetes Care. The MIP C is implanted beneath the subcutaneous tissue in the lower abdomen, and from this subcutaneous pocket, the peritoneum is opened, and the tip of the catheter is carefully inserted and directed toward the liver. After implantation, the pump reservoir is refilled in the outpatient clinic with concentrated insulin transcutaneously at least every 3 months. The Accu-Chek ® Diaport system enables infusion of insulin into the peritoneal cavity through an Accu-Chek insulin pump and an infusion set. CIPII has been proven as a viable option for T1D patients with skin problems and unable to securely or efficiently control their diabetes with subcutaneous insulin [ 75 ]. The limitations of this route of insulin administration include the invasive nature, cannula blockage, higher cost, portal-vein thrombosis, and peritoneal infection. The Diaport system has relatively few side effects and has the potential to be integrated into closed-loop systems for insulin delivery. Now less than people worldwide are on an implantable insulin pump. In , MiniMed introduced the first integrated diabetes management system: the MiniMed Paradigm REAL-Time system insulin pump and CGM system. The use of CGM sensors to control insulin delivery through pumps by adjusting the basal rate has turned CSII into a new form of therapy, SAP therapy [ 76 ]. The SAP platform integrates two independent technologies into a single system [ 1 ]. SAP therapy produces superior outcomes in reducing hypoglycemia and achieving glycemic control to conventional therapies [ 77 , 78 ]. In , Medtronic launched the MiniMed Veo System, with a Low Glucose Suspend feature that automatically halts insulin delivery when sensor glucose levels hit a preset low threshold. This device has been considered the first stepping stone to an AP system [ 79 ]. It is undebatable that insulin pump users have lower A1c levels and fewer hypoglycemic events. In addition, the pump offers more accurate dosing, avoids the need for multiple daily injections, and thus provides convenience and a flexible lifestyle. Another potential benefit is that the pumps can store a plethora of data that can be transmitted to computer programs or bolus insulin calculators and further analyzed to make insulin dose adjustments. Potential downsides of pump therapy are technical problems associated with the infusion set and higher acquisition costs. Patients often experienced skin irritations and infections at the insertion sites. Technical issues such as kinking, bending, or crimping of inserted cannulas and leakage of infusion sets have also been reported [ 70 ]. Initial acquisition and total annual costs are high for pump therapy compared with MDI [ 80 ]. These can function independently or can be integrated into pumps to calculate the accurate insulin dose by incorporating expected carbohydrate intake, measured blood glucose values, and previous insulin doses [ 81 ]. Carbohydrate counting using bolus calculator apps has been found to improve glycemic control in MDI-treated diabetes patients [ 82 ]. Diabetes: M, mySugr Roche , and PredictBGL are some of the most used bolus calculator apps. Bolus wizards are built-in automated bolus calculators specific to insulin pumps for insulin dose recommendations. The use of bolus wizards has been associated with better glycemic control and treatment satisfaction [ 83 ]. The Endocrine Society Clinical Practice Guidelines have strongly encouraged patients to use suitably adjusted built-in bolus calculators in CSII to enhance glycemic control [ 52 ]. Since the conception of CSII, the prime motive has been to design an artificial pancreas that mimics exquisite sugar control with minimal human interference. Generally, AP links three devices: 1 a sensor like CGM that measures BG and sends data to a computer algorithm, 2 a control algorithm to analyze the data and calculate the required insulin dose, and 3 an insulin infusion pump to deliver insulin as per the computer instructions [ 84 ]. MiniMed G with an Enlite sensor has been recognized as a first-generation artificial pancreas device system with Threshold Suspend automation. Medtronic introduced the MiniMed G system in , taking one step closer to the artificial pancreas system. This system has integrated smart features such as active insulin tracking, a bolus progress bar, and predictive battery life [ 79 , 85 ]. Recent studies suggested that sensor-augmented pump therapy with the predictive low-glucose suspension algorithm SAPT-PLGM leads to a potential reduction in the metrics of hypoglycemia and post-exercise nocturnal hypoglycemia compared with SAP therapy alone [ 87 , 88 , 89 ]. In , the first hybrid closed-loop system, the MiniMed G insulin pump with a Guardian 3 sensor, was licensed by the FDA for T1D therapy of children 7 years and older. When in auto mode, it functions as a hybrid closed-loop system that automatically controls basal insulin delivery every 5 min based on the CGM values to hold BG levels tightly to the specific target [ 8 ]. These systems have been reported to improve glycemic targets [BG, HbA1c, time-in-range TIR ] and reduce the incidence of nocturnal hypoglycemia to ensure better safety, treatment satisfaction, sleep quality, and cognition in T1D patients [ 88 , 90 , 91 ]. This has been recognized as the only insulin pump certified for cyber and information security. This system is expected to be expanded as the Omnipod Horizon hybrid closed-loop system in the near future [ 92 ]. A study by Benhamou et al. reported that the use of the DBLG1 system was associated with an increase in TIR Procedures required for the FDA clearance and commercial rollout of this system are on track [ 93 , 94 ]. In , Medtronic started the clinical trials on the MiniMed G system, which has novel features including automatic correction boluses, Bluetooth connectivity, and remote software updates. Another recent technology in this area has been the emergence of alternate controller-enabled ACE infusion pumps. Unlike the conventional stand-alone pumps, ACE pumps can be interoperable: used jointly with different components of diabetes technologies, permitting custom-made diabetes management for patients according to individual device preferences. The ACE insulin pump can be combined with automated insulin dosing AID systems, CGMs, BG meters, and other electronics. The FDA authorized the first interoperable t:Slim X2 insulin pump in for subcutaneous insulin delivery for children and adults with diabetes [ 96 ]. In , the FDA approved a new-generation, interoperable, control-IQ artificial pancreas system tandem diabetes. A clinical trial that reported that the use of the control-IQ AP system was associated with a greater percentage of TIR, over the use of SAP, paved way for this approval [ 91 ]. The slow pace of innovations and highly unaffordable cost have been considered the major limitations of these technologies. The emergence of closed-loop systems has been a breakthrough development in diabetes treatment. However, the clinical trials and regulatory procedures mandatory for the commercialization of the AP systems are very complex and time-consuming. This event marked the beginning of the DIY-APS movement. There are three types of DIY-APS: OpenAPS, AndroidAPS, and Loop. The diabetes community shared DIY diabetes device-related projects on digital and social media platforms such as Facebook, Twitter, NightScout, and GitHub, which led to the convergence of these projects. In , Dana Lewis, Scott Leibrand, and Ben West launched the OpenAPS project, the first DIY-APS that provided the instructions and outline of a DIY patient-built APS [ 99 ]. DIY-APS uses individually made unauthorized algorithms to convert CGM data and calculate insulin doses, FDA approved communication devices, and insulin pumps. Since it involves the use of unauthorized algorithms, these systems are not FDA approved, commercialized, or regularized. It is a translator device that enables easy communication between the insulin pump and iPhone. This device is considered more user-friendly, and it is easy to set up and to maintain the procedures [ ]. Real-life experiences from patients and caregivers, anecdotal data, and published reports from selected cohorts have highlighted the clinal benefits and reductions in self-management burden with DIY-APS [ ]. Edward Damiano in In this system, automated dosing assessments of insulin and glucagon levels are made every 5 min based on the appraised CGM data. These data are transmitted to pumps to regulate insulin or glucagon delivery [ ]. Previous studies in home-use and outpatient settings indicated better glycemic regulation and positive psychosocial impacts associated with the use of the bionic pancreas [ , ]. Another study noted that the insulin-only mode of the iLet significantly increased TIR in adults with type 1 diabetes to Ekhlaspour et al. Making use of their first-hand experiences with the triumphs and challenges of diabetes management, many D-Dads, parents of children with diabetes, have become the flagbearers of patient-led innovations and movements in the arena. The credit for the invention of the bionic pancreas goes to Dr. Edward R. Damiano, a professor of biomedical engineering at Boston University. He designed a BP to achieve automation for constant monitoring and adjustments of BG levels for his son, David, who was diagnosed with T1D at 11 months. He is the founder and CEO of the Beta Bionics firm, conducting research trials on iLet BP [ ]. Pete Schwamb, a software engineer, made path-breaking contributions in the field of diabetes technologies. Being a hacker by profession, he has been recognized as a standard-bearer for the DIY-APS hacking mission [ ]. Nightscout CGM in the Cloud was co-developed by Lane Desborough, D-Dad of Hayden. He was a chief engineer at Medtronic and was one of the advocates of the WeAreNotWaiting movement. Lane was the first person to get involved in the DIY-APS movement from the industry and later co-founded Bigfoot Biomedical [ ]. Howard Look and Steve McCanne are D-Dads to their respective daughters with T1D and pursuing a vision to bring about innovations to reduce the management burden of T1D through their cofounded non-profit organization, Tidepool. Tidepool is currently on a venture to release a regulated version of the DIY-APS in collaboration with Omnipod and Dexcom [ , ]. The implanted artificial pancreas, a fully implantable insulin delivery device, is another novel AP technology under development at De Montfort University. It is a gel-based system that responds to BG variation by altering the insulin delivery rate. The performance of this system in glycemic control is well tested in a diabetic domestic pig [ ]. Regardless of category, the goal of the AP system is to improve glycemic outcomes with less hypoglycemia. It reduces hourly management and human interference to enhance user acceptance and quality of life in diabetes patients. The next step of AP would be exploiting engineering integration and validating the prototype systems with subsequent studies in large outpatient settings [ ]. Insulin inhalers allow patients to breathe in fine-inhalable insulin pulmonary insulin either dry powder-based formulations or solution into their lungs. The pulmonary route of insulin administration was closer to physiologic portal delivery and therefore the first substitute for the subcutaneous route of insulin delivery [ 16 ]. When introduced to the market in , inhalable insulin was considered a significant innovation to address needle phobia and incorrect insulin injection techniques pertained to systemic insulin delivery methods. The effectiveness of inhalable insulin in diabetes treatment, especially for postprandial hyperglycemia, has already been proven [ ]. The first inhalable insulin, Exubera Pfizer , was approved by the FDA in for the treatment of T1D and T2D. However, the use of Exubera was associated with an increased risk of hypoglycemia. The product was withdrawn from the market in because of its high cost and dose inaccuracy [ , ]. The only surviving candidate in this category is Afrezza, a rapid-acting Technosphere insulin powder MannKind Corp. Afrezza got FDA approval for prandial insulin therapy in [ ]. The delivery system of Afrezza is small, handy, and displays the dose in units [ ]. The use of Afrezza has provided significant glycemic control and reduction of hypoglycemia in T1D patients [ , ]. The acceptance of inhalable insulins is further limited by insurance barriers, safety concerns, and competing products [ ]. Another possible entrant to the market could be jet injectors, a type of syringe that dispenses insulin subcutaneously with the aid of a high-pressure air mechanism. Pioneer jet injector technology was introduced in the s. In , the Ped-O-Jet was discontinued as a result of contamination issues raised with the use of MUNJI [ ]. The new-generation, disposable-syringe jet injectors DSJIs with disposable dose chambers insulin cartridge and nozzles were launched in the s. Even though the idea is not first hand to the market, the wider acceptance of these devices has been stalled by the cost, low absorption with the repeated use, and high contamination rates of the previous systems [ ]. Needless to say, the jet injectors are a solution for patients with needle phobia [ ]. Recent safety and feasibility studies have evaluated the treatment efficiency and pharmacokinetic and pharmacodynamic PK-PD profiles of the insulin administered by the new-generation jet injectors [ , ]. In the past decade, there has been a high-speed evolution in diabetes technologies to improve the quality of life and to extend the longevity of subjects with diabetes. Though there were commendable developments in the currently available devices, many of those were prohibitively expensive. In addition, there were serious issues associated with cannula blockages, infusion set handling, Bluetooth connectivity, and user-friendliness. Some of the promising experiences are shared by subjects using DIY-APS. The DIY revolution has prompted all the device manufacturers to introduce ACE pumps and compatible sensors. Although the mission demands enormous commitment and time, it has the potential to transform diabetes therapy. Kesavadev J, Das AK, Unnikrishnan R, et al. Use of insulin pumps in India: suggested guidelines based on experience and cultural differences. Diabetes Technol Ther. Article PubMed PubMed Central Google Scholar. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges? Diabetes Care. Article PubMed PubMed Central CAS Google Scholar. Garg SK, Rewers AH, Akturk HK. Ever-increasing insulin-requiring patients globally. Article PubMed Google Scholar. Knutsen PG, Voelker CQ, Nikkel CC. Clinical insights into a new, disposable insulin delivery device. Diabetes Spectr. Banting FG, Best CH. The internal secretion of the pancreas. J Lab Clin Med. Google Scholar. Alcántara-Aragón V. Improving patient self-care using diabetes technologies. Ther Adv Endocrinol Metab. The device includes sensors to measure glucose under the skin, an insulin pump and infusion patch connected to the pump. It measures glucose every five minutes and adjusts levels to keep them within a target range. Users still need to manually request insulin to counter carbohydrates they are eating, according to the FDA. In a clinical trial, 46 children ages years with type 1 diabetes wore the device for three months and did not have any serious adverse events. Medtronic also will conduct a post-market study. Advertising Disclaimer ». Measles reported in multiple states; be prepared to take infection-control steps. AAP cuts dues for most members to pre-COVID levels. How to use new CPT codes for administration of RSV immunizations. Column collections. Topic collections. Sign In or Create an Account. Search Close. Shopping Cart. Create Account. Patients with Type 1 diabetes, or their caregivers, must consistently monitor their glucose levels throughout the day and inject insulin with a syringe, pen or pump to maintain adequate glucose levels in order to avoid becoming hyperglycemic high glucose levels or hypoglycemic low glucose levels. The MiniMed G System, a bluetooth-enabled version of the previously approved MiniMed G System with other modifications , is a hybrid closed loop system that works by measuring glucose levels in the body every five minutes and automatically adjusting insulin delivery by either administering or withholding insulin. The system includes: a sensor that attaches to the body to measure glucose levels under the skin; an insulin pump strapped to the body; and an infusion patch connected to the pump with a catheter that delivers insulin. While the device automatically adjusts insulin levels, users need to manually request insulin doses to counter carbohydrate consumption at mealtime. The FDA evaluated data from a clinical trial that included 46 children aged 2 to 6 years old with type 1 diabetes. Study participants wore the device for approximately three months to evaluate the performance of the device during both the at-home periods, as well as a hotel period, to stress the system with sustained daily exercise. That study found no serious adverse events and that the device is safe for use. |
| U.S. Food and Drug Administration | This device is considered more user-friendly, and it is easy to set up and to maintain the procedures [ ]. Besides, the use of syringes was associated with poor dose accuracy, a long training period, unpleasant psychologic impact, and difficulties in conveyance [ 11 , 22 , 23 ]. Subgroup analyses were performed to explain heterogeneity, and sensitivity analyses were conducted to examine the robustness of the results. html Overnight glucose control with dual- and single-hormone artificial pancreas in type 1 diabetes with hypoglycemia unawareness: a randomized controlled trial. Are currently implemented mechanisms to detect safety issues adequate? In: Plotkin SA, Orenstein WA, Offit PA, Edwards KMBT-PV Seventh E, editors. |

Insulin delivery devices for pediatric patients -
Shopping Cart. Create Account. Explore AAP Close AAP Home shopAAP PediaLink HealthyChildren. header search search input Search input auto suggest. filter your search All Publications AAP News Pediatrics Hospital Pediatrics Pediatrics In Review NeoReviews AAP Grand Rounds All AAP Sites.
Advanced Search. Toggle Menu Menu Latest News Collections Column collections Topic collections Print issues Recent issues present Archive issues Alerts About About Contact Advertising Subscribe Submit a story. Skip Nav Destination Share. PDF Icon PDF Link Download PDF. Article type: News. Diabetes Mellitus.
Information for parents from HealthyChildren. Email alerts Latest News Alert. Most Read 1. Measles reported in multiple states; be prepared to take infection-control steps 2.
AAP cuts dues for most members to pre-COVID levels 3. How to use new CPT codes for administration of RSV immunizations February issue digital edition. Subscribe to AAP News. About Contact Advertising Subscribe Submit a story Alerts American Academy of Pediatrics Facebook Twitter.
Online ISSN Print ISSN Journals Pediatrics Pediatrics Open Science Hospital Pediatrics Pediatrics in Review NeoReviews AAP Grand Rounds Policy. News Latest News Archive. Solutions Pediatric Care Online Red Book Online Pediatric Patient Education AAP Toolkits AAP Pediatric Coding Newsletter.
We are looking forward to working with fast-acting insulins — and more rapid delivery — to improve meal glucose control and decrease the daytime burden of diabetes. To that end, Stanford is the only institution involved in four National Institute of Diabetes and Digestive and Kidney Diseases research projects , which begin in the fiscal year.
The projects will test multiple automated, closed-loop devices in what could be the final steps before requesting regulatory approval for permanent use. Korey Hood, PhD, professor of pediatrics and of psychiatry and behavioral sciences at the School of Medicine, will lead the pediatric diabetes psychology research team that is investigating how to best help children and their families use these systems, and is partnering with Buckingham on the research.
Because the pancreas controls glucose both by releasing insulin to lower glucose levels and by releasing glucagon to raise glucose levels, another approach to closed-loop control is to give both insulin and glucagon. This system has the potential to eliminate the need for carbohydrate counting before meals while also preventing hypoglycemia through the provision of glucagon.
Lancet recently published an article on this study. Samantha Dorman sdorman stanfordchildrens. org Kate DeTrempe kdetrempe stanfordchildrens. Our network of care includes more than 65 locations across Northern California and more than 85 locations in the U.
Western region. Along with Stanford Health Care and the Stanford School of Medicine, we are part of Stanford Medicine , an ecosystem harnessing the potential of biomedicine through collaborative research, education, and clinical care to improve health outcomes around the world.
We are a nonprofit organization committed to supporting the community through meaningful outreach programs and services and providing necessary medical care to families, regardless of their ability to pay.
Discover more at stanfordchildrens. Search Term. Menu Button. Donate Contact MyChart Login Find a Doctor. Second Opinion Donate Contact Refer a Patient En Español. Recently Visited. View More Results Loading Hybrid closed-loop insulin delivery systems for Type 1 diabetes come of age Left : Jamie Kurtzig, 12, holding some of her hybrid closed-loop system equipment.
By Andrew Schwartz At 19 months old, Jamie Kurtzig was diagnosed with Type 1 diabetes. Clinical trials lead to FDA-approved devices In September , an article in the Journal of the American Medical Association detailed the successful multicenter trial of a hybrid closed-loop insulin delivery system for patients with Type 1 diabetes over the age of Helping younger patients and their families The hybrid closed-loop system has other advantages, as well.
org Kate DeTrempe kdetrempe stanfordchildrens. About Us Contact MyChart Login Careers Blog Refer a Patient. Notice of Nondiscrimination Terms of Use Privacy Policy Code of Conduct Price Transparency © Stanford Medicine Children's Health. About About Us Our Hospital Patient Stories Blog News Send a Greeting Card New Hospital Careers.
Connect Donate Refer a Patient Contact Us Pay Your Bill. Find Doctors Locations Services Classes.
Many different insulin preparations and delivery cevices are available. As a result, the optimal types of vor and regimen Mindful eating vary among deivery and can Insulin delivery devices for pediatric patients for an Insulin delivery devices for pediatric patients child over time. This topic review will focus on monitoring of glycemic control and details of insulin therapy, including dosing and dose adjustment, as well as options for administration, including insulin pumps and related devices. Why UpToDate? Product Editorial Subscription Options Subscribe Sign in. Learn how UpToDate can help you. Select the option that best describes you.
0 thoughts on “Insulin delivery devices for pediatric patients”