
Video
Instantly Lower Blood Pressure within 60 SecondsHans Erik BøtkerNiels Møller; ON NO—The Continuing Znd of Nitric Oxide, Diabetes, and Cardiovascular Disease. Nitric oxide and diabetes management 1 August ; 62 8 : — Nitric oxide Kxide is a simple chemical compound—1 nitrogen and 1 oxygen atom ,anagement together—with complex xnd actions 1Nutric.
A singularly prominent feature of NO is its ability to cause vasodilation, a quality that is used pharmacologically mnaagement treating ischemic heart disease oxdie NO precursors such as nitroglycerin. In ahd, Furchgott and Zawadzki 3 showed that vascular Fruits for stronger hair and nails induced by acetylcholine was dependent on the presence of endothelium and provided evidence for the release of a volatile humoral factor.
This substance, later called endothelium-derived relaxing factor and now recognized as NO 4is oxjde significant component of the insulin-signaling Nitrif Fig, Body density tracking.
Vasodilation has the potential to andd systemic blood pressure and increase local tissue blood flow in tissues such Nitrid muscle. Extract information online combination of decreased blood pressure and increased tissue blood flow, together with specific beneficial endothelial effects, may serve to prevent managemet, cardiovascular disease, managenent insulin resistance 56.
However, these effects manabement exquisitely sensitive to impaired insulin signaling oixde the endogenous cardiovascular Pxide system and ane to managemment compromised in the Nitri of insulin Building a strong immune system through nutrition 7.
So there is evidence mwnagement impaired NO-dependent vasodilation Nitric oxide and diabetes management hypertension and insulin resistance Nitrjc vice versa: that insulin resistance, such mwnagement that observed in diabetes, the metabolic syndrome, and hypertension, Nitric oxide and diabetes management Dianetes vasodilation.
The existence of this vicious cycle is supported by epidemiological data from the Framingham Oxiide Study and other studies showing that hypertension, diabetes, size cardiovascular manavement cosegregate 89. Simplistic picture of the insulin-signaling path and Energy balance equation involvement in Body density tracking Nitdic highlights the oxive role of arginine and ane It is important to underline that Nitriic actions of managemnt are dianetes acts after binding to its receptor and activates both phosphatidylinositol mmanagement PIK and mitogen-activated protein kinase Diabbetes.
In the manage,ent, NO is synthesized from the guanidine group of the amino acid arginine under critical Nitrix of the enzyme endothelial NO synthase eNOS. Crunchy Nut Mixes the diaetes nature of Macronutrient Ratios for Athletes regulation managrment vascular tone and endothelial function, it is of major importance to Nihric and oixde the underlying Nitdic leading to vascular dysfunction and managemeent resistance.
From Nitric oxide and diabetes management clinical diabeges of view, there is a specific managemeng for experimental human studies assessing NO synthesis and clearance rates and whether managemenr of NO function diabehes Nitric oxide and diabetes management altered kinetics and impaired insulin stimulation.
In this issue Nktric Diabetes managemrnt, Tessari et al. In these experiments, the investigators use a daibetes product, isotope nanagement technique. Because arginine is Nitrid precursor ,anagement NO Fig. Compared with normal ane, the new data showed that NO synthesis was ajd in the elderly and in people with type 2 diabetes and generally increased after insulin stimulation.
Regression analysis using data for all subjects showed that NOx synthesis was inversely correlated with arginine metabolites ADMA, SDMA and age, but not with insulin sensitivity. The authors conclude that whole-body NOx production is decreased in aging and type 2 diabetes and that arginine metabolites, not insulin resistance, appear to be negative regulators of in vivo NOx production.
These are timely and pertinent data from a well-conducted human study, and the findings not only expand our understanding of the field but also suggest that insulin resistance and NO dysfunction may not be as intimately linked as hitherto believed.
A particular strength of the study is that it combines a complicated clinical setup in a relative large number of subjects with a state-of-the-art kinetic tracer technique.
A recent study, using a saliva oral nitrate test and an intravenous glucose tolerance test, reported a correlation between insulin sensitivity and NO synthesis 15whereas the current study, using steady state isotope dilution and clamp techniques, fails to make this connection.
Insulin sensitivity is expressed by a glucose clamp—derived M or glucose infusion rate value, which predominantly reflects glucose uptake in muscle NO synthesis is measured systemically, leaving the contributing tissues unidentified. Hence, the new results do not necessarily reflect conditions in muscle.
Furthermore, the design of the study is quite complex, with many different subgroups and mixed pathologic features. It is therefore possible that the heterogeneity of the study sample may have underpowered the study. The clinical implication of the current study relates to the effect of insulin resistance on endothelial dysfunction.
Although Tessari et al. Several studies using multivariate analyses that adjust for other potential modulators of endothelial function have shown that insulin resistance may not be an independent predictor of endothelial function 17 — On the other hand, the UK Prospective Diabetes Study clearly showed that metformin intervention, aimed at improving insulin sensitivity and endothelium-dependent vasodilation, led to a significant reduction in cardiovascular events in patients with insulin resistance The extent to which other interventions that improve insulin sensitivity, such as caloric restriction, physical activity, and pharmacological agents, act via NO to improve cardiovascular outcomes are issues to be addressed by future studies.
See accompanying original article, p. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes. Advanced Search. User Tools Dropdown. Sign In.
Skip Nav Destination Close navigation menu Article navigation. Volume 62, Issue 8. Previous Article Next Article. Article Navigation. Commentary July 17 ON NO—The Continuing Story of Nitric Oxide, Diabetes, and Cardiovascular Disease Hans Erik Bøtker ; Hans Erik Bøtker.
This Site. Google Scholar. Niels Møller Niels Møller. Corresponding author: Niels Møller, nielsem dadlnet. Diabetes ;62 8 — Connected Content. A commentary has been published: Roles of Insulin, Age, and Asymmetric Dimethylarginine on Nitric Oxide Synthesis In Vivo.
Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. View large Download slide. No potential conflicts of interest relevant to this article were reported.
Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Search ADS. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor.
Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. The role of asymmetric dimethylarginine ADMA in endothelial dysfunction and cardiovascular disease.
The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Roles of insulin, age, and asymmetric dimethylarginine on nitric oxide synthesis in vivo.
In vivo nitric oxide synthesis, insulin sensitivity, and asymmetric dimethylarginine in obese subjects without and with metabolic syndrome. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent type II diabetes mellitus. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes.
Relationship between brachial artery flow-mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Endothelium-dependent vasodilation, insulin resistance and the metabolic syndrome in an elderly cohort: the Prospective Investigation of the Vasculature in Uppsala Seniors PIVUS study.
UK Prospective Diabetes Study UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
View Metrics. Email alerts Article Activity Alert. Online Ahead of Print Alert. Latest Issue Alert. See also Roles of Insulin, Age, and Asymmetric Dimethylarginine on Nitric Oxide Synthesis In Vivo. Most Read Most Cited MRI Metrics of Cerebral Endothelial Cell—Derived Exosomes for the Treatment of Cognitive Dysfunction Induced in Aging Rats Subjected to Type 2 Diabetes.
Management of Latent Autoimmune Diabetes in Adults: A Consensus Statement From an International Expert Panel. Genetic Influences of Adiponectin on Insulin Resistance, Type 2 Diabetes, and Cardiovascular Disease.
Online ISSN X Print ISSN Books ShopDiabetes. org ADA Professional Books Clinical Compendia Clinical Compendia Home News Latest News DiabetesPro SmartBrief. Resources ADA Professional Membership ADA Member Directory Diabetes. X Twitter Facebook LinkedIn. This Feature Is Available To Subscribers Only Sign In or Create an Account.
Close Modal. This site uses cookies. By continuing to use our website, you are agreeing to our privacy policy.
: Nitric oxide and diabetes management| RESEARCH DESIGN AND METHODS | Moreover, a high-fat diet significantly inhibited EC insulin-stimulated Akt phosphorylation and FITC-insulin uptake that was partially reversed by SNP in rats. Finally, inhibition of S -nitrosylation by knockdown of thioredoxin-interacting protein completely eliminated SNP-enhanced FITC-insulin uptake. We conclude that NO directly promotes EC insulin transport by enhancing protein S -nitrosylation. NO also inhibits PTP1B activity, thereby enhancing insulin signaling. Before insulin can act on myocytes, it must first traverse the continuous vascular endothelium in skeletal muscle. Insulin delivery to muscle is affected by blood flow 1 , flow distribution 2 , and insulin transendothelial transport TET 3 , 4. Importantly, insulin delivery to muscle interstitial fluid is a rate-limiting step in the peripheral action of insulin 5 , 6 and is delayed in insulin-resistant, obese humans, suggesting a significant role for this transport process in peripheral insulin resistance 7 , 8. Endothelial dysfunction, secondary to reduced nitric oxide NO bioavailability, is an early and prominent feature of insulin resistance. Endothelial NO synthase NOS-3 or eNOS produces NO from l -arginine, and eNOS is activated by insulin at physiologic concentrations. Knockout of eNOS or inhibiting insulin signaling by endothelium-specific knockout of IRS2, leading to the reduction of eNOS activity in the vascular endothelial cell EC , produces metabolic insulin resistance 9 , In addition, endothelial-specific knockout of IRS2 inhibits insulin-induced microvascular recruitment and reduces insulin delivery to muscle interstitium. However, it is not known whether the reduced insulin delivery is a result of reduced blood flow, altered flow distribution, impaired insulin TET, or a combination of these We and others have previously shown that insulin induces vasodilation by enhancing NO production to facilitate its own delivery to the peripheral tissues in vivo 1 , 2. Whether NO directly affects insulin uptake and TET has not been examined. The insulin receptor and caveolae mediate EC insulin uptake 4 , 11 — 13 , and this process is blunted by either inhibiting intracellular insulin signaling or treating with tumor necrosis factor-α TNF-α. Conversely, stimulating intracellular insulin signaling by inhibiting protein-tyrosine phosphatase 1B PTP1B enhances insulin uptake In the current study, we found that exogenously delivered NO stimulated both the uptake and the TET of insulin by aortic ECs. We also found that NO partially or fully restored insulin uptake by the cells pretreated with inhibitors of insulin signaling pathways To explain these findings, we examined pathways downstream of NO production by which the NO might act on insulin uptake. We found that exogenously delivered NO can directly promote insulin transport independent of eNOS activity through enhancing protein S -nitrosylation, including that of PTP1B, without affecting the soluble guanylyl cyclase sGC -cyclic guanosine monophosphate cGMP pathway and overcome the impaired insulin transport seen with experimental insulin resistance. Bovine aorta ECs bAECs passage numbers 2—8; BioWhittaker, Inc. These experiments were performed as previously described 4 , Briefly, bAECs were seeded onto Transwell inserts 6. Louis, MO. The transendothelial electrical resistances were monitored daily with an Epithelial Voltohmmeter and EndOhm chamber WPI, Sarasota, FL. At selected times, μL fluid was removed from the bottom chamber and replaced with μL EBM to ensure hydrostatic balance. The concentration of I-insulin was measured with a γ counter. The percentage of insulin transported was calculated. Rats were killed by CO 2. The surrounding fascia was carefully removed, and the vessel was divided into small segments and cut open. After stabilization in a cell culture incubator for 1 h, these aortic segments were treated with 0. After fixation with cold methanol, the endothelial face of the vessel was placed face down onto a coverslip that was precoated with poly- l -lysine Sigma-Aldrich , pressure was applied briefly, the vessel wall was removed, and the coverslip with adherent ECs was processed for immunocytochemical staining see immunocytochemistry. The study procedure was approved by the animal care and use committee of the University of Virginia. Western blotting was performed as described previously 13 , This procedure was followed by incubation with a species-specific secondary antibody coupled to horseradish peroxidase Amersham [GE Healthcare Life Sciences], Piscataway, NJ , and the blots were developed with an enhanced chemiluminescence Western blotting kit Amersham [GE Healthcare Life Sciences]. The developed films were scanned with a densitometer Molecular Dynamics, Amersham, Piscataway, NJ and quantified with the use of ImageQuant 5. Real-time RT-PCR assay was performed as described previously 13 , Briefly, total RNAs were extracted from the cultured bAECs with an RNeasy kit Qiagen and were reverse transcribed with the iScript cDNA synthesis kit Bio-Rad. The cDNA products were then amplified with iQ SYBR Green Supermix on an iCycler apparatus Bio-Rad. Standard curves for each mRNA were generated by serial dilution of cDNA synthesized from the extracted total RNA and was included in each iCycler real-time RT-PCR experiment. The specificity of the desired product was verified by analysis of the melting curve. A specific small interfering RNA siRNA duplex against bovine Txnip mRNA and a scrambled siRNA control were purchased from Dharmacon, Inc. Lafayette, CO. Forty-eight hours after transfection, cells were serum starved for 6 h followed by insulin treatment as described previously 13 , The double-staining protocols were the same as described previously 4 , Briefly, the methanol-fixed bAECs were washed three times in TBS, permeabilized in TBS containing 0. The following primary antibodies were used: rabbit polyclonal anti-FITC Molecular Probes, Inc. The cells were washed three times in TBS and then incubated with species-specific secondary antibodies conjugated with a fluorochrome cyanine Cy2 or Cy3 Jackson ImmunoResearch, West Grove, PA at dilutions for 45 min at room temperature. The cells were washed three times in TBS and then coverslipped with antifade mounting medium with DAPI. Intracellular cGMP concentration of the cultured bAECs was measured with a cGMP enzyme immunosorbent assay kit Cayman Chemical. Protein concentration was measured by the Bradford method. The measured cGMP values were normalized against the corresponding protein concentrations. Arterial serum glucose concentrations were measured with a glucose colorimetric assay kit Cayman Chemical. Serum insulin Mercodia AB, Uppsala, Sweden and triglyceride Cayman Chemical concentrations were measured with ELISA. The biotin derivatization was detected by the included fluorescein-conjugated avidin. In these experiments, assay of the protein S -nitrosylation was determined in combination with the immunocytochemical staining for PTP1B with the monoclonal primary antibody against PTP1B followed by a species-specific secondary antibody conjugated with Cy3 and visualized by confocal imaging. The PTP1B activity of bAECs was measured after precipitation of PTP1B with a modified immunoprecipitation procedure described previously 15 , The lysates were gently rocked at 4°C for 15 min and then centrifuged at 14, g for 10 min at 4°C. Equal amounts of protein samples μg of total protein were immunoprecipitated with the monoclonal anti-PTP1B antibody at 4°C overnight. Immunoprecipitates were washed five times with TBS, and the residue TBS buffer was removed. The sample mixtures were incubated for 30 min at 30°C. After the reaction, μL aliquots were placed into half-area well plates, and 25 μL red reagent plus 40 μL assay buffer were added to each sample well and gently mixed. After incubation at room temperature for 30 min, the absorbance was measured at nm with a plate reader. The immunocytochemical labeling was examined with a confocal microscope as described previously 4 , 12 — Confocal imaging was performed with a Leica SP5 X imaging system equipped with ultraviolet nm , tunable — nm white light and argon ion lasers , , , , nm ; ×40 and ×60 1. During image acquisition, the individual microscopic field was selected to include a similar number of cells but was otherwise random. To quantify fluorescence intensity, the images from randomly selected microscopic fields containing a similar number of nuclei staining were outlined, and the integrated fluorescence intensities were measured with Image J software. In the case Fig. Digital images were processed identically with Adobe Photoshop. Data are presented as mean ± SEM. Statistical comparisons among different groups were made with one-way ANOVA with Student-Newman-Keuls post hoc testing. We first examined the effect of l - N G -nitro- l -arginine methyl ester l -NAME inhibition of NOS on FITC-insulin uptake. l -NAME added with l -arginine blocked the increased uptake seen with l -arginine alone Fig. We then examined whether the effect of l -arginine on FITC-insulin uptake could be mimicked by giving NO to bAECs. Adding modest concentrations 0. However, SNP at higher concentrations did not stimulate insulin uptake Fig. Of note, 0. NO directly promotes EC FITC-insulin uptake. bAECs were serum starved for 6 h then pretreated with or without 0. A : Effects of LNA on FITC-insulin uptake. B : Representative confocal images of bAECs stained for FITC from three independent experiments. C : The histograms indicate the dose response of FITC-insulin uptake to SNP treatment. D : Quantification of the fluorescent intensity of FITC for each experimental condition indicated in the confocal images. E : Effects of LNA on SNP-stimulated increase of FITC-insulin uptake. Next, we examined the effect of SNP on I-insulin TET with a Transwell device 4 , In aggregate, these data suggest that the NO donor SNP may directly promote insulin transport in an eNOS activity-independent fashion. SNP promotes insulin TET. Percent transport of total added I-insulin at 60 min was calculated. We previously reported that insulin transport by bAECs depends on its intracellular insulin signaling as either general inhibition of tyrosine kinases genistein or more-specific inhibition of Src PP1 , phosphatidyl-inositol-3 kinase PI3K wortmannin , or mitogen-activated protein kinase MAPK PD 12 ; each inhibited FITC-insulin uptake. Therefore, we tested whether adding SNP to bAECs relieved the inhibition of FITC-insulin uptake induced by blocking these intracellular insulin signaling pathways. Figure 3 and Supplementary Fig. Adding SNP, however, completely rescued both wortmannin- and PP1-inhibited insulin uptake Fig. SNP did not significantly affect genistein-inhibited FITC-insulin uptake Supplementary Fig. These data indicate that NO is able to promote FITC-insulin uptake despite preinhibiting some intracellular insulin signaling pathways. Effects of SNP on FITC-insulin uptake by ECs pretreated with inhibitors of insulin action. A : Representative confocal images of bAECs stained for FITC from three independent experiments. B and C : Quantitative analysis of cellular insulin uptake for each experimental condition. E : Quantitative analysis of the cellular insulin uptake presented in D. We previously used TNF-α treatment of ECs as an in vitro model of insulin resistance and showed that treatment of bAECs with TNF-α inhibited FITC-insulin uptake Figure 3D shows that adding 0. We next examined the pathways by which SNP promoted FITC-insulin transport. The selective, irreversible, heme-site inhibitor of sGC 1 H-[1,2,4]oxadiazolo[4,3-a]quinoxalinone ODQ 20 completely eliminated the enhanced FITC-insulin uptake induced by SNP compared with vehicle control Fig. However, the membrane-permeable cGMP analog 8-bromo-cGMP over a range of concentrations had no significant effects on FITC-insulin uptake Fig. In addition, treatment of ECs with insulin with or without SNP did not significantly affect the intracellular cGMP levels Supplementary Fig. In aggregate, these data suggest that SNP-enhanced insulin transport may not be mediated by activation of sGC. Effects of cGMP analog and ODQ on insulin uptake. bAECs were serum starved for 6 h then pretreated with either 8-bromo-cGMP 0. B : The histograms indicate the dose response of FITC-insulin uptake to 8-bromo-cGMP treatment. C : Quantitative analysis of cellular insulin uptake for each experimental condition. cGMP10, cGMP 0. Because NO also regulates cellular function by S -nitrosylation, we next examined the effect of SNP treatment on protein S -nitrosylation. PLoS ONE 10 4 : e Academic Editor: Aldrin V. Gomes, University of California, Davis, UNITED STATES. Received: May 5, ; Accepted: March 23, ; Published: April 20, Copyright: © Adela et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Data Availability: All relevant data are available in the paper and its Supporting Information files. Additional information can be obtained from Dr. Sanjay K Banerjee and Dr. Naveen Reddy. RA is a scholar in the Fogarty International Center of the National Institutes of Health training program under Award Number D43 TW SKN is thankful to DST, New Delhi for supporting with junior research fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing interests: The authors have declared that no competing interests exist. Metabolic syndrome is a serious health concern. The global burden of diabetes mellitus has been estimated at million and going to rise to million by the year [ 2 ]. Increasing incidence of morbidity and mortality due to cardiovascular complications including coronary artery diseases has been observed in Type 2 diabetic patients [ 3 ]. Diabetes is a metabolic disorder characterised by chronic hyperglycaemia. The long-term effects of diabetes mellitus include cellular injury, inflammation and failure of various organs [ 4 ]. The complications of diabetes are divided into macro vascular complications i. Among all complications, endothelial dysfunction is a common problem in all diabetic patients. Endothelial cells secrete different mediators such as vasodilators i. Hyperglycaemia and other metabolic changes may lead to impairment of nitric oxide NO production [ 6 ]. Impairment of endothelial function in T2DM patients ultimately leads to cardiovascular diseases. Thus, endothelial dysfunction is the early feature of cardiovascular complications in T2DM [ 7 ]. Nitric oxide is a gaseous molecule secreted by the endothelium and a major modulator of endothelial function [ 8 ]. NO is synthesized from L-arginine by the family of enzymes called nitric oxide synthases NOSs viz. neuronal NOS nNOS , endothelial NOS eNOS and inducible NOS iNOS [ 9 ]. NO is a key regulatory molecule with extensive metabolic, vascular, and cellular effects [ 10 ]. While low levels of NO is beneficial for several physiological and cellular functions, high levels of NO may cause detrimental effects in the cells. High levels of NO may react with superoxide anion to generate peroxynitrite radical, which binds to proteins and thus affects their function [ 11 ]. Altered serum NO levels in T2DM were reported by different investigators previously [ 12 — 14 ]. The serum NO data in T2DM patients that reported by different scientific literature is controversial. Some research articles reported increased NO levels in diabetes patients [ 13 ] whereas others reported the opposite [ 14 ]. In the present study, we have considered diabetic patients as a separate group compared to other diabetic patients with cardiovascular complications. We were interested in knowing if the serum NO levels were altered due to the duration of diabetes and the presence of any cardiovascular complications along with diabetes. Till now, there was no study that reported the nitric oxide levels in diabetic patients having cardiovascular complications. Therefore, the present study was designed to understand the alteration in nitric oxide levels with T2DM and T2DM with cardiovascular complications in South Indian patients and to find whether hyperglycaemia can induce NO production in endothelial cells. Our study showed an increased level of nitric oxide in Indian T2DM patients, but not in patients with coronary artery disease CAD alone. Similarly, diabetic rats with hyperglycaemia also showed higher NO levels as compared to controls. Our study indicated that hyperglycaemia is responsible in generation of high levels of NO from HUVEC cells through induction of iNOS and eNOS gene expression. Sample of patients, including men and women aged 35—65 years, was taken from the Mediciti Hospital, Hyderabad, a city from southern part of India. The study conforms to the principles outlined in the Declaration of Helsinki and was approved by the Mediciti Ethics Committee Institutional Hyderabad. All patients were given detailed information of the study and they gave written consent before enrolling into the study. Group 1 Control CT subjects had no prior history of T2DM, hypertension, coronary artery diseases or any other cardiovascular diseases, and were not taking medication for any chronic medical condition. Fasting blood glucose, HbA1c and blood chemistry were normal. This group had no prior history of T2DM. Coronary artery disease patients were identified in the Cardiac Catheterization Unit of Mediciti hospital. After coronary angiogram, all patients were evaluated by cardiologists in the inpatient setting. If patients were found to have any evidence of CAD, demographic, clinical, and angiographic data were collected from all such patients. Fasting sample were collected prior to the percutaneous coronary intervention or coronary artery bypass graft. Clinical history and complete physical examination including measurements of blood pressure, was collected and conducted for all the participants. Blood samples were collected by venipuncture after an overnight fast, using Becton Dickison Vacutainer Red colour coded Tubes and blood was allowed to clot by leaving at room temperature for 15 min. After centrifugation at g for 15 min at 4°C, serum was collected in 1. Height and weight were obtained using standardised techniques and instruments. Fasting blood glucose levels were measured by the FreeStyle optimum glucometer Abbott Diabetes Care, Australia. Creatinine Siemens DF33A and uric acid Siemens BA were measured by Siemens automated analyser Dimension Xpand plus , estimated Glomerular Filtration Rate eGFR calculated from creatinine by using Modification of Diet in Renal Disease MDRD formula. Serum Insulin and C-peptide levels were measured by Bio-Rad Multiplex assay kit Catalog: AM. Assay was performed as per the manufacturer instructions. All animal experiments were undertaken with the approval of Institutional Animal Ethical Committee of Indian Institute of Chemical Technology IICT , Hyderabad, India. Male Sprague-Dawley SD rats weighing between — gm were purchased from Teena Labs, Hyderabad, India. Animals were housed in BIOSAFE, an animal quarantine facility of IICT. p in freshly prepared citrate buffer. After 72 hours of fasting, blood glucose level of rats was measured. Control group rats Con group were administered equal volume of citrate buffer i. p to nullify its effect. Rats were given free access to water and food throughout the study. After 8 weeks, blood was collected from rats to fractionise serum for further biochemical analysis. The absorbance of the solution was read on a plate reader at nm. To quantify the NO production, a standard curve was generated using sodium nitrite. The cells of the passage three P3 were used for performing the experiments. Human umbilical vein endothelial cells HUVEC were seeded in a 96 well plate at a density of around 1X 10 4 cells per well and grown to confluence. To simulate the in-vivo diabetic conditions, the cells were incubated with 10, 25, 50 and mM D-Glucose Sigma for two different time periods of 4 and 8 hours. We added different concentration of mannitol to each well during experimentation to maintain similar osmolarity. Vascular endothelial growth factor VEGF was used as a positive control while the untreated cells served as control. After incubation, the supernatant media of each of the treatment groups were collected separately and their nitric oxide levels were quantified using nitric oxide measurement kit Arbor assay kit, MI, USA. The exact concentrations of NO, produced in response to treatment with different concentration of glucose were quantified from the NO standard curve. The cells were exposed to 10, 25, 50 and mM D-Glucose treatments for two different time periods of 4 and 8 hours. After the incubation, the cells were washed and incubated with 5μM DAF-2DA Sigma for 30 min. Briefly, 5 × 10 6 endothelial cells were incubated with 1 ml of TRIzol reagent for 5 minutes. The resulting cell lysates were then mixed with 0. The upper aqueous layer was collected into RNase-free Eppendorf tubes and mixed with 0. Samples were then centrifuged at 11, rpm for 15 minutes at 4°C. DNase treatment was further carried out to remove DNA contamination from isolated RNA. cDNA was prepared from isolated RNA 5 μg using 1μl of reverse transcriptase RT enzyme, 1 μl dNTP mix 10 mM , 4 μl 5X reaction buffer and 10 picomole oligo dT and RNase free water. Total 10μl reaction mixture was denatured at 72°C for 3 min followed by sudden cooling for 10min and extension at 42°C for 60—90min. After cDNA preparation, real-time PCR was performed for gene expression analysis. Detailed sequences of all primers used in this study have been summarized in Table 1. Ten nanogram of cDNA were analysed on StepOnePlus Applied Biosystem using Absolute SYBR Green ROX PCR Master Mix Takara as described before [ 18 ]. Fold-change analysis was based on normalizing with GAPDH. Patient clinical characteristics are represented as median Inter Quartile Range IQR for continuous and as percentages for categorical variables. Animal and cell culture results are expressed as mean ± SEM. Statistical comparisons were done by One way Analysis of Variance ANOVA for cell culture related results and t—test was used to see the difference between the two groups in animal study. Non-normally distributed data is expressed as median IQR. This was determined by using Kolmogorov-Smirnov test, followed by log transformation. Spearman rank correlation was used to compare NO levels in fasting blood glucose FBS and glycated hemoglobin HbA1c. ANOVA and Kruskal-Wallis and Man-Whitney, t-test analysis done by Graph Pad Prism version 5. Human serum NO levels in Control CT and Type 2 diabetes T2DM. Data were represented as box median IQR and whisker plots. Human serum NO levels in Control CT , Coronary artery disease CAD and Type 2 diabetes with coronary artery disease DMCD. Human serum NO levels in Control CT and Type 2 diabetes with hypertension DMHT. Human serum NO levels in two group of patients having diabetic duration below 5 years and above 5 years. For this study, we took fifty healthy subjects that represent Group 1 CT. While twenty six T2DM patients with no other complications were included in Group 2 T2DM , forty six T2DM with hypertensive patients were included in Group 3 DMHT. Total twenty nine coronary artery disease patients twenty four acute myocardial infarction patients and five unstable angina pectoris patients were included in the Group 4 CAD. Total thirty eight T2DM with coronary artery disease patients thirty one acute myocardial infarction and seven unstable angina pectoris patients were recruited in Group 5 DMCD. Single, double and triple vessel disease patients were included in both group 4 and group 5. Table 2 shows the clinical characteristics of the study subjects. The mean age of T2DM, DMHT and CAD subjects were similar when compared to control subjects. However, the mean age of DMCD subjects was relatively higher when compared with controls. There was no significant difference in height, weight and BMI among all the groups. Systolic BP was similar in control and T2DM patients, but significantly higher in DMHT, CAD, and DMCD. Diastolic BP was similar in control, T2DM, CAD, DMCD patients, and higher in DMHT when compared to control and T2DM. Table 2 shows the biochemical characteristics of the study subjects. T2DM, DMHT, DMCD patients had higher fasting blood glucose and HbA1c levels when compared to the control and CAD subjects. There was no significant difference in creatinine eGFR and uric acid levels among all the groups. Insulin and C-peptide levels were not significantly changed in the diseases groups as compared to control subjects, except diabetes with coronary artery diseases groups. S1 Table. The duration median Inter Quartile Range of diabetes among the subjects of three groups i. Similarly, the duration of hypertension among DMHT, CAD and DMCD subjects were 4. While All CAD and DMCD subjects were receiving anti platelet therapy and other lipid lowering therapy for prophylaxis of the coronary artery disease. Serum nitric oxide levels median Inter Quartile Range were significantly higher in T2DM There was no statistically significant difference in the NO levels between DMHT Serum NO levels in DMCD However there were no significant changes in CAD All the diabetic subjects from T2DM, DMHT, DMCD groups were further divided into two groups based on duration of diabetes i. Nitric oxide As expected, blood glucose levels were significantly high in hyperglycaemic Dia rats induced by streptozocin STZ , when compared to Control Con rats Fig 2A. Serum nitric oxide levels Blood glucose levels in diabetic rats DIA after 8 weeks of STZ administration. Reduced production of NO [i. This chapter focuses on the role of impaired NO metabolism in T2D. Asymmetric dimethylarginine ADMA , an endogenous competitive inhibitor of nitric oxide NO synthase NOS isoenzymes, can substantially inhibit vascular NO production at concentrations that are observed in pathophysiological conditions. Such pathological elevated ADMA levels lead to a decreased NO bioavailability and the development of diabetes complications, including cardiovascular diseases, nephropathy, and retinopathy; elevated ADMA levels also increase the mortality risk in these patients. Here, we discuss current documents indicating how disrupted ADMA metabolism contributes to the development of T2D and its complications. The role of other endogenous methylarginines, i. The nitrate NO3 -nitrite NO2 -nitric oxide NO pathway, as a storage reservoir for endogenous NO production, is dependent on the oral bacteria with NO3- reducing capacity. Undesirable changes of oral microbiota towards a decreased load of health-related NO3-reducing bacteria and an overgrowth of pathogenic species, leading to subsequent decreased NO2 production in the oral cavity and decreased systemic NO availability, are now considered risk factors for the development of insulin resistance and type 2 diabetes T2D. This chapter discusses available evidence focusing on oral microbiota dysbiosis in T2D, especially NO3-reducing bacteria and their metabolic activity including NO3-reductase and NO2-reductase activity , affecting net oral NO2 accumulation and the NO3-NO2-NO pathway. Nitric oxide NO , a multifunctional gasotransmitter, is now considered an endocrine hormone that essentially contributes to the regulation of glucose and insulin homeostasis. Here, we discuss current genetic data linking NO metabolism to metabolic disorders, especially insulin resistance and type 2 diabetes T2D. Although several gene variants of NO synthases [NOSs, i. Nitric oxide NO , a gaseous free radical, is a key signaling molecule in the different phases of the normal wound healing process. The beneficial effects of NO in wound healing are related to its antibacterial properties, regulation of inflammatory response, stimulation of proliferation and differentiation of keratinocytes and fibroblasts, and promotion of angiogenesis and collagen deposition. NO deficiency is an important mechanism responsible for poor healing in diabetic wounds. In this chapter, the function of NO in diabetic wound healing and the possible therapeutic significance of NO in the treatment of diabetic wounds are discussed. Current knowledge supports this notion that NO-based intervention is a promising therapeutic approach for diabetic wound healing. Osteoporosis affects million people worldwide. Osteoporosis in subjects with diabetes is called diabetoporosis, and type 2 diabetes T2D contributes to and aggravates osteoporotic fractures. Hyperglycemia, insulin resistance, bone vasculature impairment, increased inflammation, oxidative stress, and bone marrow adiposity contribute to a higher incidence of osteoporotic fractures in T2D. Decreased nitric oxide NO bioavailability due to lower endothelial NO synthase eNOS -derived NO and higher inducible NOS iNOS -derived NO is one of the main mechanisms of the diabetoporosis. Available data indicates that T2D increases osteoclast-mediated bone resorption and decreases osteoblast-mediated bone formation, mediated in part by reducing eNOS-derived NO and increasing iNOS-derived NO. NO donors delay osteoporosis and decrease osteoporotic fractures in subjects with T2D, suggesting the potential therapeutic implication of NO-based interventions for diabetoporosis. Uric acid UA is the end product of purine catabolism in humans. Hyperuricemia can induce pancreatic β-cell death and impaired insulin secretion. It can also disrupt insulin-induced glucose disposal and insulin signaling in different insulin-sensitive tissues, including cardiomyocytes, skeletal muscle cells, adipocytes, hepatocytes, and endothelial cells. These events lead to the development of systemic insulin resistance and impaired glucose metabolism. Induction of inflammation, oxidative stress, and impairment of nitric oxide NO metabolism mediate hyperuricemia-induced insulin resistance and dysglycemia. This chapter is focused on the potential mediatory role of NO metabolism on hyperuricemia-induced dysglycemia and insulin resistance. Page: 28 Author: Tara Ranjbar, Jennifer L. According to the World Health Organization WHO , the prevalence of obesity across the globe has nearly tripled since , with 39 million children under the age of 5 being overweight or obese in Obesity is the most common risk factor for developing type 2diabetes T2D , which may lead to elevated serum triglycerides, hypertension, and insulin resistance. In the pathogenesis of T2D, there is a reduction in nitric oxide NO bioavailability. Restoration of NO levels has been associated with many favorable metabolic effects in T2D. Drugs that potentiate NO levels may have a role in improving T2D-associated adverse effects. Current medications approved for use in the management of T2D include biguanides, thiazolidinediones, sulfonylureas, meglitinides, dipeptidyl peptidase-4 DPP-4 inhibitors, glucagon-like peptide-1 GLP- 1 receptor agonists, alpha-glucosidase inhibitors, and sodium-glucose co-transporter 2 SGLT2 inhibitors. These drugs mitigate the many adverse effects associated with T2D. |
| Vascular nitric oxide resistance in type 2 diabetes | Forearm nitric oxide balance, vascular relaxation, and glucose metabolism in NIDDM patients. OpenURL Placeholder Text. Chin, K. Sun, L. Lancet , — |
| Nitric oxide system and diabetic nephropathy | Diabetology & Metabolic Syndrome | Full Text | Nitric Oxide. Reduced muscle diiabetes of l -arginine 43 or one of the Body density tracking diabetss, tetrahydrobiopterin managemen Nitric oxide and diabetes management, also could Niitric to the defective stimulation of NOS by insulin in diabetics. Kövamees, O. Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R and Shanmugasundaram KR: Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Article CAS PubMed Google Scholar Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Side effects. |
| Vascular nitric oxide resistance in type 2 diabetes | Cell Death & Disease | Increased force of VSM contraction is mediated via Rho-Rho kinase ROK - and PKC-dependent MLCP inhibition. cGMP exerts its effects in VSMCs by cGMP-dependent protein kinase PKG, or cGK and by affecting cGMP-binding phosphodiesterase [ ]. In addition, cGMP-PKG inhibits PKC activation [ ]. However, the role of VASP in regulating vascular tone remains to be elucidated [ ]. Aortic rings of wild-type and VASP null mice showed similar relaxant responses to cGMP, ACh, and SNP, indicating that VASP is not essential for vasodilator-induced relaxation of VSM [ ]. For example, in rat aortic VSMCs, S-nitrosylation of RhoA by S-nitrosocystein inhibits the Rho-ROK-MLCP pathway, suggesting a cGMP-independent pathway for vasodilation [ ]. Mechanisms acting at the post-receptor level include a defective cGMP catabolism and b hyperglycemia-induced downregulation of protein kinase G PKG. Other ROS generators e. In addition, it was not due to different protein expressions of sGC subunits α1 and β1 [ 18 ]. Although not entirely determined, two mechanisms seem to result in sGC desensitization: 1 ROS-induced sGC oxidation, and 2 defective cGMP catabolism. Likewise, other vascular beds, e. Defective cGMP catabolism may also contribute to sGC desensitization in diabetic vessels. PDEs control the abundance of cGMP [ ]. Among 11 families of PDE that have been identified [ ], PDE1, PDE3, and PDE5 degrade cGMP in VSM [ ], and PDE5 is the main one involved in cGMP catabolism in the smooth muscle [ ]. cGMP binds to PDE5 and increases its activity which means PDE5 activity increases following increased cGMP production [ ]. In addition, PKG-I phosphorylates PDE5 and prolongs its activation by increasing its affinity for cGMP [ ]. In VSMCs isolated from the aorta, baseline cGMP concentrations were about 3 times 1. This was not affected by the NOS inhibitor L -NMMA, which rules out sGC hyperactivation [ 18 ]. In addition, sGC inhibitors ODQ and Ambroxol decreased baseline cGMP in VSMCs from OZR but not controls. Still, baseline cGMP remained higher in VSMCs from OZR, suggesting defective cGMP catabolism [ 18 ]. Baseline PDE5 activity was lower in VSMCs from OZR, and the IBMX-induced increase in cGMP was smaller in VSMCs from OZR than in controls, suggesting defective PDE activity in VSMCs from OZR [ 18 ]. Hyperglycemia downregulates mRNA and protein levels of PKG-I in VSMCs through altered NOX signaling [ ]. In diabetic vessels, the ability of cGMP to activate PKG and PKG-dependent activation of PDE5 are also impaired [ 18 ]. No further evidence is available regarding T2D-induced changes in sGC downstream signaling. Beneficial effects of some pharmaceutical agents, including angiotensin-converting enzyme inhibitors e. Furthermore, the use of nitroxyl donors, e. Quantifying the contribution of the impaired pathways i. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for and projections for Diabetes Res Clin Pract. Article PubMed Google Scholar. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in — Cardiovascular Diabetol. Article Google Scholar. Aikaeli F, Njim T, Gissing S, Moyo F, Alam U, Mfinanga SG, et al. Prevalence of microvascular and macrovascular complications of diabetes in newly diagnosed type 2 diabetes in low-and-middle-income countries: A systematic review and meta-analysis. PLOS Glob Public Health. Article PubMed PubMed Central Google Scholar. Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. CAS PubMed PubMed Central Google Scholar. Montero D, Walther G, Pérez-Martin A, Vicente-Salar N, Roche E, Vinet A. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta-analysis. Article CAS PubMed Google Scholar. Forbes JM, Fotheringham AK. Vascular complications in diabetes: old messages, new thoughts. Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. Article CAS PubMed PubMed Central Google Scholar. Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Investig. Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, et al. Compromised arterial function in human type 2 diabetic patients. Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Therapeutics. Article CAS Google Scholar. Velagic A, Qin C, Woodman OL, Horowitz JD, Ritchie RH, Kemp-Harper BK. Nitroxyl: a novel strategy to circumvent diabetes associated impairments in nitric oxide signaling. Front Pharmacol. Anderson R, Ellis G, Chirkov Y, Holmes A, Payne N, Blackman D, et al. Determinants of platelet responsiveness to nitric oxide in patients with chronic heart failure. Eur J Heart Fail. Young ME, Leighton B. Biochemical J. Russo I, Del Mese P, Doronzo G, Mattiello L, Viretto M, Bosia A, et al. Tawa M, Kinoshita T, Asai T, Suzuki T, Imamura T, Okamura T. Impact of type 2 diabetes on vascular reactivity to cGMP generators in human internal thoracic arteries. Vasc Pharmacol. Bock JM, Hughes WE, Ueda K, Feider AJ, Hanada S, Casey DP. Glycemic management is inversely related to skeletal muscle microvascular endothelial function in patients with type 2 diabetes. Physiological Rep. Vavuranakis M, Stefanadis C, Triandaphyllidi E, Toutouzas K, Toutouzas P. Coronary artery distensibility in diabetic patients with simultaneous measurements of luminal area and intracoronary pressure: evidence of impaired reactivity to nitroglycerin. Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. Beer S, Feihl F, Ruiz J, Juhan-Vague I, Aillaud MF, Wetzel SG, et al. Comparison of skin microvascular reactivity with hemostatic markers of endothelial dysfunction and damage in type 2 diabetes. Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Implications for the syndrome of insulin resistance. Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, et al. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 non-insulin-dependent diabetes mellitus. Watts GF, O'Brien SF, Silvester W, Millar JA. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidaemia. Clin Sci. Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol Metab. CAS PubMed Google Scholar. Enderle MD, Benda N, Schmuelling RM, Haering HU, Pfohl M. Preserved endothelial function in IDDM patients, but not in NIDDM patients, compared with healthy subjects. Diabetes Care. Mäkimattila S, Liu M-L, Vakkilainen J, Schlenzka A, Lahdenperä S, Syvänne M, et al. Impaired endothelium-dependent vasodilation in type 2 diabetes. Relation to LDL size, oxidized LDL, and antioxidants. Gazis A, White DJ, Page SR, Cockcroft JR. Effect of oral vitamin E alpha-tocopherol supplementation on vascular endothelial function in Type 2 diabetes mellitus. Diabet Med: J Br Diabet Assoc. Preik M, Kelm M, Rösen P, Tschöpe D, Strauer BE. Additive effect of coexistent type 2 diabetes and arterial hypertension on endothelial dysfunction in resistance arteries of human forearm vasculature. Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Kimura Y, Matsumoto M, Miyauchi E, Deng YB, Iwai K, Hattori H. Noninvasive detection of endothelial dysfunction in elderly with NIDDM by ultrasonography. van Etten RW, de Koning EJ, Verhaar MC, Gaillard CA, Rabelink TJ. Impaired NO-dependent vasodilation in patients with Type II non-insulin-dependent diabetes mellitus is restored by acute administration of folate. Vehkavaara S, Yki-Järvinen H. Arterioscler Thromb Vasc Biol. Ifrim S, Vasilescu R. Early detection of atherosclerosis in type 2 diabetic patients by endothelial dysfunction and intima-media thickness. Google Scholar. Natali A, Toschi E, Baldeweg S, Casolaro A, Baldi S, Sironi AM, et al. Haematocrit, type 2 diabetes, and endothelium-dependent vasodilatation of resistance vessels. Woodman RJ, Playford DA, Watts GF. Basal production of nitric oxide NO and non-NO vasodilators in the forearm microcirculation in Type 2 diabetes: associations with blood pressure and HDL cholesterol. Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Karasu C, Soncul H, Altan VM. Effects of non-insulin dependent diabetes mellitus on the reactivity of human internal mammary artery and human saphenous vein. Life Sci. Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes, Obes Metab. Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol. Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated? Goodfellow J, Ramsey MW, Luddington LA, Jones CJ, Coates PA, Dunstan F, et al. Endothelium and inelastic arteries: an early marker of vascular dysfunction in non-insulin dependent diabetes. BMJ Clin Res Ed. Avogaro A, Piarulli F, Valerio A, Miola M, Calveri M, Pavan P, et al. Forearm nitric oxide balance, vascular relaxation, and glucose metabolism in NIDDM patients. Pitei DL, Watkins PJ, Edmonds ME. NO-dependent smooth muscle vasodilatation is reduced in NIDDM patients with peripheral sensory neuropathy. Goulopoulou S, Hannan JL, Matsumoto T, Ogbi S, Ergul A, Webb RC. Reduced vascular responses to soluble guanylyl cyclase but increased sensitivity to sildenafil in female rats with type 2 diabetes. Am J Physiol Heart Circ Physiol. Goulopoulou S, Hannan JL, Matsumoto T, Ergul A, Webb RC. Augmented dilation to nitric oxide in uterine arteries from rats with type 2 diabetes: implications for vascular adaptations to pregnancy. Oniki H, Goto K, Fujii K, Kansui Y, Murakami N, Ohtsubo T, et al. Effects of the superoxide dismutase mimetic tempol on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. Clin Exp Hypertension. Mishra RC, Kyle BD, Kendrick DJ, Svystonyuk D, Kieser TM, Fedak PWM, et al. Metab: Clin Exp. Oniki H, Fujii K, Kansui Y, Goto K, Iida M. Effects of angiotensin II receptor antagonist on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. J Hypertension. Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Br J Pharmacol. Melo BF, Prieto-Lloret J, Cabral MD, Martins FO, Martins IB, Sacramento JF, et al. Type 2 diabetes progression differently affects endothelial function and vascular contractility in the aorta and the pulmonary artery. Sci Rep. Leo CH, Hart JL, Woodman OL. Eur J Pharmacol. Belin de Chantemèle EJ, Vessières E, Guihot AL, Toutain B, Maquignau M, Loufrani L, et al. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc Res. Wang H, Zhou Y, Guo Z, Dong Y, Xu J, Huang H, et al. Sitagliptin attenuates endothelial dysfunction of zucker diabetic fatty rats: implication of the antiperoxynitrite and autophagy. J Cardiovasc Pharm Ther. Lu X, Guo X, Karathanasis SK, Zimmerman KM, Onyia JE, Peterson RG, et al. Rosiglitazone reverses endothelial dysfunction but not remodeling of femoral artery in Zucker diabetic fatty rats. Cardiovasc Diabetol. Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Mourmoura E, Vial G, Laillet B, Rigaudière JP, Hininger-Favier I, Dubouchaud H, et al. Preserved endothelium-dependent dilatation of the coronary microvasculature at the early phase of diabetes mellitus despite the increased oxidative stress and depressed cardiac mechanical function ex vivo. Belin de Chantemèle EJ, Vessières E, Guihot AL, Toutain B, Loufrani L, Henrion D. Cyclooxygenase-2 preserves flow-mediated remodelling in old obese Zucker rat mesenteric arteries. Vessières E, Belin De Chantemèle EJ, Toutain B, Guihot A-L, Jardel A, Loufrani L, et al. Cyclooxygenase-2 inhibition restored endothelium-mediated relaxation in old obese zucker rat mesenteric arteries. Front Physiol. Qiu S, Mintz JD, Salet CD, Han W, Giannis A, Chen F, et al. J Am Heart Assoc. Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Kashiwagi S, Kajimura M, Yoshimura Y, Suematsu M. Nonendothelial source of nitric oxide in arterioles but not in venules. Circulation Res. Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, et al. Vascular smooth muscle and nitric oxide synthase. FASEB J. Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. Al-Shabrawey M, El-Remessy A, Gu X, Brooks SS, Hamed MS, Huang P, et al. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. Boulanger CM, Heymes C, Benessiano J, Geske RS, Lévy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, et al. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F, et al. Red blood cell and endothelial eNOS independently regulate circulating nitric oxide metabolites and blood pressure. Ghasemi A. Quantitative aspects of nitric oxide production from nitrate and nitrite. EXCLI J. PubMed PubMed Central Google Scholar. Ghasemi A, Jeddi S. Quantitative aspects of nitric oxide production in the heart. Mol Biol Rep. Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun. Cheah LS, Gwee M, Das R, Ballard H, Yang YF, Daniel EE, et al. Evidence for the existence of a constitutive nitric oxide synthase in vascular smooth muscle. Clin Exp Pharmacol Physiol. Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arteriosclerosis Thrombosis Vasc Biol. Withers SB, Simpson L, Fattah S, Werner ME, Heagerty AM. cGMP-dependent protein kinase PKG mediates the anticontractile capacity of perivascular adipose tissue. Nakladal D, Sijbesma JWA, Visser LM, Tietge UJF, Slart R, Deelman LE, et al. Perivascular adipose tissue-derived nitric oxide compensates endothelial dysfunction in aged pre-atherosclerotic apolipoprotein E-deficient rats. Nava E, Llorens S. The local regulation of vascular function: from an inside-outside to an outside-inside model. Kleschyov AL, Muller B, Schott C, Stoclet JC. Role of adventitial nitric oxide in vascular hyporeactivity induced by lipopolysaccharide in rat aorta. Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med. Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiß C, Strauer BE, et al. Evidence for in vivo transport of bioactive nitric oxide in human plasma. Robinson JM, Lancaster JR Jr. Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide: mechanism s and physiologic versus pathophysiologic relevance. Am J Respiratory Cell Mol Biol. Ford E, Hughes MN, Wardman P. The reaction of superoxide radicals with S-nitrosoglutathione and the products of its reductive heterolysis. J Biol Chem. Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. Bohlen HG, Zhou X, Unthank JL, Miller SJ, Bills R. Transfer of nitric oxide by blood from upstream to downstream resistance vessels causes microvascular dilation. Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci. Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide. Vascular smooth muscle NO exposure from intraerythrocytic SNOHb: a mathematical model. Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M, et al. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS ONE. Tessari P, Cecchet D, Cosma A, Vettore M, Coracina A, Millioni R, et al. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Avogaro A, Toffolo G, Kiwanuka E, de Kreutzenberg SV, Tessari P, Cobelli C. L-arginine-nitric oxide kinetics in normal and type 2 diabetic subjects: a stable-labelled 15N arginine approach. Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, et al. Coronary microvascular dysfunction in diabetes mellitus: pathogenetic mechanisms and potential therapeutic options. Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Bonini MG, Dull RO, Minshall RD. Caveolin-1 regulation of endothelial nitric oxide synthase eNOS function and oxidative stress in the endothelium. In: Laher I, editor. Systems biology of free radicals and antioxidants. Berlin, Heidelberg: Springer; Haddad D, Al Madhoun A, Nizam R, Al-Mulla F. Role of Caveolin-1 in diabetes and its complications. Oxid Med Cell Longev. Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes ;— Caldwell RB, Zhang W, Rojas M, Bartoli M, El-Remessy AB, Tsai N-T, et al. Decreased nitric oxide bioavailability in diabetic retinopathy: involvement of arginase activity. Investigative Ophthalmol Vis Sci. Kövamees O, Shemyakin A, Checa A, Wheelock CE, Lundberg JO, Östenson C-G, et al. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. Faria AM, Papadimitriou A, Silva KC, Lopes de Faria JM, Lopes de Faria JB. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Kobayashi T, Matsumoto T, Ooishi K, Kamata K. Differential expression of alpha2D-adrenoceptor and eNOS in aortas from early and later stages of diabetes in Goto-Kakizaki rats. Halvorson BD, Whitehead SN, McGuire JJ, Wiseman RW, Frisbee JC. Endothelium-dependent impairments to cerebral vascular reactivity with type 2 diabetes mellitus in the Goto-Kakizaki rat. Am J Physiol Regulatory Integr Comp Physiol. Stadler K, Jenei V, von Bölcsházy G, Somogyi A, Jakus J. Increased nitric oxide levels as an early sign of premature aging in diabetes. Gil-Ortega M, Stucchi P, Guzmán-Ruiz RO, Cano V, Arribas S, González MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Jaap AJ, Tooke JE. Pathophysiology of microvascular disease in non-insulin-dependent diabetes. Kazuyama E, Saito M, Kinoshita Y, Satoh I, Dimitriadis F, Satoh K. Endothelial dysfunction in the early- and late-stage type-2 diabetic Goto-Kakizaki rat aorta. Mol Cell Biochem. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W, et al. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int. Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Ren X, Ren L, Wei Q, Shao H, Chen L, Liu N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Shatanawi A, Momani MS. Plasma arginase activity is elevated in type 2 diabetic patients. Biomed Res. CAS Google Scholar. Diederich D, Skopec J, Diederich A, Dai FX. Endothelial dysfunction in mesenteric resistance arteries of diabetic rats: role of free radicals. Am J Physiol. Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Pierce GL. Targeting vascular endothelial cell insulin resistance in type 2 diabetes mellitus. Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. Omodeo-Sale F, Cortelezzi L, Vommaro Z, Scaccabarozzi D, Dondorp AM. Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme. Am J Physiol Cell Physiol. Ramírez-Zamora S, Méndez-Rodríguez ML, Olguín-Martínez M, Sanchez-Sevilla L, Quintana-Quintana M, García-García N, et al. Increased erythrocytes by-products of arginine catabolism are associated with hyperglycemia and could be involved in the pathogenesis of type 2 diabetes mellitus. PloS ONE. Mahdi A, Tengbom J, Alvarsson M, Wernly B, Zhou Z, Pernow J. Red blood cell peroxynitrite causes endothelial dysfunction in type 2 diabetes mellitus via arginase. Liu T, Schroeder H, Power GG, Blood AB. A physiologically relevant role for NO stored in vascular smooth muscle cells: a novel theory of vascular NO signaling. Redox Biol. Farah C, Michel LYM, Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Sáez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. García IE, Sánchez HA, Martínez AD, Retamal MA. Redox-mediated regulation of connexin proteins; focus on nitric oxide. Biochim Biophys Acta BBA —Biomembranes. Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiol Res Pract. Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: Effects on vascular function. Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol-Heart Circulatory Physiol. Doctor A, Spinella P. Effect of processing and storage on red blood cell function in vivo. Semin Perinatol. McMahon TJ, Doctor A. Chong, C. Reversal of hyperglycemia: effects on nitric oxide signaling. Drugs that affect cardiac metabolism: focus on perhexiline. Drugs Ther. Coleman, J. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Cooke, J. ADMA: Its role in vascular disease. Costell, M. Comparison of soluble guanylate cyclase stimulators and activators in models of cardiovascular disease associated with oxidative stress. Cowart, D. A phase 1 randomized study of single intravenous infusions of the novel nitroxyl donor BMS in healthy volunteers. da Costa, R. Increased O-GlcNAcylation of endothelial nitric oxide synthase compromises the anti-contractile properties of perivascular adipose tissue in metabolic syndrome. Dautov, R. The nitric oxide redox sibling nitroxyl partially circumvents impairment of platelet nitric oxide responsiveness. Hypoxic potentiation of nitrite effects in human vessels and platelets. Oxide Biol. De Caterina, R. Nitric oxide decreases cytokine-induced endothelial activation: Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. Degrell, P. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci. del Rio, C. Abstract nitroxyl HNO donated via slow-release oral pro-drug improves ventriculo-arterial coupling in conscious dogs with induced heart failure: enhanced load-independent mechano-energetics. Circulation , A—A Dogra, G. Statin therapy improves brachial artery vasodilator function in patients with Type 1 diabetes and microalbuminuria. Drummond, G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Drug Discovery 10, — DuMond, J. The chemistry of nitroxyl-releasing compounds. Dupuis, J. Intensity of lipid lowering with statins and brachial artery vascular endothelium reactivity after acute coronary syndromes from the BRAVER trial. Elgert, C. A novel soluble guanylyl cyclase activator, BR , acts as a non-stabilising partial agonist of sGC. Förstermann, U. Nitric oxide synthases: regulation and function. Heart J. Fülöp, N. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Farah, C. Nitric oxide signalling in cardiovascular health and disease. Fard, A. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Farhan, S. Advanced glycation end products AGEs and their soluble receptors sRAGE as early predictors of reno-vascular complications in patients with uncontrolled type 2 diabetes mellitus. Diabetes Metab. Favaloro, J. The nitroxyl anion HNO is a potent dilator of rat coronary vasculature. Felker, G. Rationale and design for the development of a novel nitroxyl donor in patients with acute heart failure. Heart Fail. Fiorentino, T. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Follmann, M. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Chemie - Int. Fox, K. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial the EUROPA study. Lancet , — Fukuto, J. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. Gao, W. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Giacco, F. Oxidative stress and diabetic complications. Go, A. Statin therapy and risk of incident diabetes mellitus in adults with cardiovascular risk factors. Harrison, D. The nitrovasodilators: new ideas about old drugs. Circulation 87, — Hartman, J. Intravenous infusion of the novel HNO donor BMS is associated With beneficial inotropic, lusitropic, and vasodilatory properties in 2 canine models of heart failure. JACC Basic to Transl. Heitzer, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43, — Hernandez-Cuellar, E. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. Horowitz, J. Asymmetric and symmetric dimethylarginine predict outcomes in patients with atrial fibrillation: an ARISTOTLE substudy. Htay, T. Mortality and cardiovascular disease in type 1 and type 2 diabetes. Ignarro, L. Nitric oxide is not just blowing in the wind. International Diabetes Federation Latest figures show million people now living with diabetes worldwide as numbers continue to rise. Diabetes Res. Irvine, J. NO- activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 41, — Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension 49, — Nitroxyl HNO : the Cinderella of the nitric oxide story. Trends Pharmacol. Ito, H. Flow mediated dilatation is reduced with the progressive stages of glomerular filtration rate and albuminuria in type 2 diabetic patients without coronary heart disease. Jones, D. Randomized phase 2 trial of intracoronary nitrite during acute myocardial infarction. Kövamees, O. Amino acid metabolism reflecting arginase activity is increased in patients with type 2 diabetes and associated with endothelial dysfunction. Kahlberg, N. Adverse vascular remodelling is more sensitive than endothelial dysfunction to hyperglycaemia in diabetic rat mesenteric arteries. Kass, D. Phosphodiesterase regulation of nitric oxide signaling. Keceli, G. Nitroxyl HNO targets phospholamban cysteines 41 and 46 to enhance cardiac function. Kemp-Harper, B. Schmidt, H. Berlin, Heidelberg: Springer Berlin Heidelberg , — Therapeutic potential of nitroxyl HNO donors in the management of acute decompensated heart failure. Drugs 76, — Nitroxyl HNO : A novel redox signaling molecule. Kennedy, J. Effect of the anti-anginal agent, perhexiline, on neutrophil, valvular and vascular superoxide formation. Kizub, I. Protein kinase C in enhanced vascular tone in diabetes mellitus. Ko, M. Time- and dose-dependent association of statin use with risk of clinically relevant new-onset diabetes mellitus in primary prevention: a nationwide observational cohort study. Kohr, M. Korkmaz, Y. Downregulation of the α1- and β1-subunit of sGC in arterial smooth muscle cells of OPSCC Is HPV-Independent. Kovanen, P. Mast cells in atherosclerotic cardiovascular disease - Activators and actions. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92, — Laine, P. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation 99, — Lee, K. Cardioprotective effects of PKG activation by soluble GC activator, BAY , in ischemia-reperfusion-injured rat hearts. PloS One 12, 1— Lees, C. Unexpected effect of a nitric oxide donor on uterine artery Doppler velocimetry in oligomenorrheic women with polycystic ovaries. Ultrasound Obstet. Leo, C. Endothelium-dependent nitroxyl-mediated relaxation is resistant to superoxide anion scavenging and preserved in diabetic rat aorta. Lima, B. S-nitrosylation in cardiovascular signaling. Lin, E. Nitroxyl HNO stimulates soluble guanylyl cyclase to suppress cardiomyocyte hypertrophy and superoxide generation. PloS One 7, 6— Lindholm, M. Diabetes mellitus and cardiogenic shock in acute myocardial infarction. Lob, H. Antioxidant and nitric oxide-sparing actions of dihydropyridines and ACE inhibitors differ in human endothelial cells. Pharmacology 76, 8— Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Lund, T. Fibrin ogen may be an important target for methylglyoxal-derived AGE modification in elastic arteries of humans. Lundberg, J. Strategies to increase nitric oxide signalling in cardiovascular disease. Drug Discovery 14, — Mátyás, C. The soluble guanylate cyclase activator cinaciguat prevents cardiac dysfunction in a rat model of type-1 diabetes mellitus. Malmberg, K. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction DIGAMI study : Effects on mortality at 1 year. Mancardi, D. The Chemical Dynamics of NO and Reactive Nitrogen Oxides: A Practical Guide. Mao, K. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. Marsh, S. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 40, — Massion, P. Nitric oxide and cardiac function: Ten years after, and continuing. Matsushima, S. Increased myocardial NAD P H oxidase-derived superoxide causes the exacerbation of postinfarct heart failure in type 2 diabetes. Mehta, S. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA , — Mendes-Silverio, C. Activation of haem-oxidized soluble guanylyl cyclase with BAY in human platelets lead to overstimulation of the cyclic GMP signaling pathway. PloS One 7, e Meurer, S. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Miller, T. The effects of nitroxyl HNO on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols. Miller, A. Nitroxyl HNO suppresses vascular Nox2 oxidase activity. Free Radic. Miranda, K. Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins: A chemical discussion of the differential biological effects of these redox related products of NOS. Murphey, L. Contribution of bradykinin to the cardioprotective effects of ACE inhibitors. Napoli, C. Nitric oxide and atherosclerosis: An update. Neutel, J. Choosing among renin-angiotensin system blockers for the management of hypertension: From pharmacology to clinical efficacy. Nightingale, A. Lack of association between aortic sclerosis and left ventricular hypertrophy in elderly subjects. Okon, E. Compromised arterial function in human type 2 diabetic patients. Diabetes 54, — Ostadal, P. Statins as first-line therapy for acute coronary syndrome? PubMed Abstract Google Scholar. Pacher, P. Role of poly ADP-ribose polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Paolocci, N. Oxygen radical-mediated reduction in basal and agonist-evoked no release in isolated rat heart. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. John, M. The pharmacology of nitroxyl HNO and its therapeutic potential: not just the janus face of NO. Paulus, W. Nitric oxide's role in the heart: Control of beating or breathing? Phaniendra, A. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Pignatelli, P. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. Premer, C. Rethinking endothelial dysfunction as a crucial target in fighting heart failure. Mayo Clin. Outcomes 3, 1— Qian, J. Exogenous, but not endogenous nitric oxide inhibits adhesion molecule expression in human endothelial cells. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Qin, C. Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications. Nitric oxide resistance, induced in the myocardium by diabetes, is circumvented by the nitric oxide redox sibling, nitroxyl. Radziwon-Balicka, A. Differential eNOS-signalling by platelet subpopulations regulates adhesion and aggregation. Ramana, K. Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. FASEB J. Reriani, M. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Ritchie, R. The opposing roles of NO and oxidative stress in cardiovascular disease. Roger, S. Anti-aggregating effect of BAY , an activator of soluble guanylyl cyclase. Rosenkranz, A. Acute antihypertrophic actions of bradykinin in the rat heart: Importance of cyclic GMP. Hypertension 40, — Ruszkowski, P. Effects of combined statin and ACE inhibitor therapy on endothelial function and blood pressure in essential hypertension - a randomised double-blind, placebo controlled crossover study. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. Sabbah, H. Nitroxyl HNO a novel approach for the acute treatment of heart failure. Saeedi, P. Global and regional diabetes prevalence estimates for and projections for and Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Salerno, J. Control of eNOS and nNOS through regulation of obligatory conformational changes in the reductase catalytic cycle. bioRxiv , 1— Sandner, P. Soluble Guanylate Cyclase Stimulators and Activators. Schäfer, A. Guanylyl cyclase activator ataciguat improves vascular function and reduces platelet activation in heart failure. Schachinger, V. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation , — Schulze, P. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. Selemidis, S. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Sharma, A. Targeting endothelial dysfunction in vascular complications associated with diabetes. Shemyakin, A. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Siddiqi, N. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial NIAMI. Spiecker, M. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IκBα. Springhorn, C. Exploring leukocyte O-GlcNAcylation as a novel diagnostic tool for the earlier detection of type 2 diabetes mellitus. Stepien, J. Pilot study examining the effect of cholesterol lowering on platelet nitric oxide responsiveness and arterial stiffness in subjects with isolated mild hypercholesterolaemia. Strey, C. Short-term statin treatment improves endothelial function and neurohormonal imbalance in normocholesterolaemic patients with non-ischaemic heart failure. Heart 92, LP— Stuehr, D. Update on mechanism and catalytic regulation in the NO synthases. Sun, L. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Sverdlov, A. The endogenous NOS inhibitor asymmetric dimethylarginine ADMA predicts LV mass independent of afterload. Reciprocal regulation of NO signaling and TXNIP expression in humans: Impact of aging and ramipril therapy. Aging of the nitric oxide system: Are we as old as our NO? Heart Assoc. Swedberg, K. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II CONSENSUS II. Szpigel, A. |
| Nitric oxide system and diabetic nephropathy | Cannon R Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem 44 : — Duplain H , Burcelin R , Sartori C , Cook S , Egli M , Lepori M , Vollenweider P , Pedrazzini T , Nicod P , Thoren B , Scherrer U Insulin resistance, hyperlipemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation : — Shankar R , Wu Y , Shen H , Zhu J , Baron A Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 49 : — Kapur S , Bedard S , Marcotte B , Cote CH , Marette A Expression of nitric oxide synthase in skeletal muscle. A novel role for nitric oxide as a modulator of insulin action. Diabetes 46 : — Perreault M , Marette A Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nature 7 : — Panza JA , Casino PR , Kilcoyne CM , Quyyumi AA Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. Gilligan DM , Guetta V , Panza JA , Garcia CE , Quyyumi AA , Cannon III RO Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation 90 : 35 — Zeiher AM , Drexler H , Wollschlager H , Just H Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 83 : — J Clin Invest 97 : — Aljada A , Saadeh R , Assian E , Ghanim H , Dandona P Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab 85 : — Schnyder B , Pittet M , Durand J , Schnyder-Candrian S Rapid effects of glucose on the insulin signaling of endothelial NO generation and epithelial Na transport. Am J Physiol Endocrinol Metab : E87 — E Laakso M , Edelman SV , Brechtel G , Baron AD Decreased effect of insulin to stimulate skeletal muscle flow in obese men. J Clin Invest 85 : — Anderson EA , Hoffman RP , Balon TW , Sinkey CA , Mark AL Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87 : — Steinberg JO , Brechtel G , Johnson A , Fineberg N , Baron AD Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest 94 : — DeFronzo RA Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev 4 : — Roy D , Perreault M , Marette A Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol Endocrinol Metab : E — E Biochem J : 73 — Young ME , Radda GK , Leighton B Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J : — Higaki Y , Hirschman MF , Fuji N , Goodyear LJ Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 50 : — Balon TW , Nadler JL Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol 82 : — Tanaka T , Nakatani K , Morioka K , Urakawa H , Maruyama N , Kitagawa N , Katsuki A , Araki-Sasaki R , Hori Y , Gabazza EC , Yano Y , Wada H , Nobori T , Sumida Y , Adachi Y Nitric oxide stimulates glucose transport through insulin-independent GLUT4 translocation in 3T3—L1 adipocytes. Eur J Endocrinol 49 : 61 — Meneilly GS , Elliot T , Battistimi B , Floras JS N G -monomethyl- l -arginine alters insulin mediated calf blood flow but not glucose disposal in the elderly. Metabolism 50 : — Zavaroni I , Platti PM , Monti LD , Gasparini P , Barilli LA , Massironi P , Ardigo D , Valsecchi G , Delsignore R , Reaven GM Plasma nitric oxide concentrations are elevated in insulin-resistant healthy subjects. Metabolism 49 : — DeFronzo RA Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med : — Cusi K , Maezono K , Osman A , Pendergrass M , Patti M , Pratipanawatr T , DeFronzo RA , Kahn CR , Mandarino LJ Insulin resistance differentially affects the PI3-kinase and MAP kinase-mediated signaling in human muscle. J Clin Invest : — Towbin H , Staehelin J , Gordon J Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76 : — Shiuchi T , Cui TX , Wu L , Nakagami H , Takeda-Matsubara Y , Iwai M , Horiuchi M ACE inhibitor improves insulin-resistance in diabetic mice mouse via bradykinin and nitric oxide. Hypertension 40 : — Stuhlinger MC , Abbasi F , Chu JW , Lamendola C , McLaughlin TL , Cooke JP , Reaven GM , Tsao PS Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. Williams IL , Wheatcroft SB , Shah AM , Kearney MT Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes 26 : — Steinberg HO , Paradici G , Hook G , Crowder K , Cronin J , Baron AD Free fatty acids elevation impairs insulin-mediated vasodilation and nitric oxide production. Zeng G , Quon MJ Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98 : — Diabetes 46 : 54 A. Hsueh WA , Law RF Insulin signaling in the arterial wall. Am J Cardiol 84 : 21 J—24J. Pratipanawatr W , Pratipanawatr T , Cusi K , Berria R , Adams JM , Jenkinson CP , Maezono K , DeFronzo RA , Mandarino LJ Skeletal muscle insulin resistance in normoglycemic subjects with strong family history of type 2 diabetes is associated with decreased insulin stimulation of insulin receptor substrate-1 tyrosine phosphorylation. Reaven GM Banting Lecture Role of insulin resistance in human disease. Diabetes 37 : — Groadanovic A NO message from muscle. Microsc Res Tech 55 : — Fulton D , Gratton J-P , McCabe TJ , Fontana J , Fujio Y , Walsh K , Franke TF , Papapetropoulos A , Sessa WC Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Chen Z-P , Mitchelhill KI , Michell BJ , Stapleton D , Rodriquez-Crespo I , Witters LA , Power DA , Ortiz de Montellano PR , Kemp BE AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett : — Butt E , Bernhardt M , Smolenski A , Kotsonis P , Frohlich BG , Sickmann A , Meyer HE , Lohmann SM , Schmidt HW Endothelial nitric-oxide synthase type III is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem : — Dimmeler S , Fleming I , Fisslthaler B , Hermann C , Busse R , Zeihe AM Activation of endothelium-derived nitric oxide production by the protein kinase Akt. Roman LJ , Martasek P , Miller RT , Harris DE , de La Garza MA , Shea TM , Kim JJ , Masters BS The C termini of constitutive nitric-oxide synthases control electron flow through the flavin and heme domains and affect modulation by calmodulin. Du XL , Edelstein D , Dimmeler S , Ju Q , Sui C , Brownlee N Hyperglycemia inhibits endothelial nitric oxide synthase activity by post translational modification at the Akt site. Federici M , Menghini R , Mauriello A , Hribal ML , Ferrelli F , Lauro D , Sbraccia P , Spagnoli LG , Sesti G , Lauro R Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Cosentino F , Luscher TF Tetrahydrobiopterin and endothelial function. Eur Heart J 19 Suppl G : G3 — G8. Abbasi F , Brown BW , Lamendola C , McLaughlin T , Reaven GM Relationship between obesity, insulin sensitivity and coronary artery disease risk. J Am Coll Cardiol 40 : 37 — Chen HS , Hwu CM , Kwok CF , Shih KC , Hsiao LC , Lee SH , Lin YT , Lin SH , Ho LT Insulin sensitivity in normotensive offspring of hypertensive parents. Horm Metab Res 32 : — Nakai K , Itoh C , Kawazoe K , Miura Y , Sotoyanagi H , Hotta K , Itoh T , Kamata J , Hiramori K Concentration of soluble vascular cell adhesion molecule-1 VCAM-1 correlated with expression of VCAM-1 mRNA in the human atherosclerotic aorta. Coron Artery Dis 6 : — Haught WH , Mansour M , Rothlein R , Kishimoto TK , Mainolfi EA , Hendricks JB , Hendricks C , Mechta JL Alterations in circulating intercellular adhesion molecule-1 and l -selectin: further evidence for chronic inflammation in ischemic heart disease. Kupatt C , Weber C , Wolf DA , Becker BF , Smith TW , Kelly RA Nitric oxide attenuates reoxygenation-induced ICAM-1 expression in coronary microvascular endothelium: role of NF-κB. J Mol Cell Cardiol 29 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Subjects and Methods. Journal Article. Insulin Resistance Is Associated with Impaired Nitric Oxide Synthase Activity in Skeletal Muscle of Type 2 Diabetic Subjects. Kashyap , Sangeeta R. Oxford Academic. Linda J. Jennifer Lamont. Bettie Sue S. Mandeep Bajaj. Swangjit Suraamornkul. Renata Belfort. Rachele Berria. Dean L. Kellogg, Jr. Yanjuan Liu. Ralph A. DeFronzo Ralph A. PDF Split View Views. Cite Cite Sangeeta R. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. TABLE 1. Subject characteristics. Open in new tab. Open in new tab Download slide. First Published Online November 23, and L. contributed equally to this work. intracellular adhesion molecule-1;. insulin-stimulated glucose disposal;. vascular cellular adhesion molecule. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and ASCVD. Google Scholar Crossref. Search ADS. Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? Google Scholar OpenURL Placeholder Text. Role of nitric oxide in cardiovascular disease: focus on the endothelium. Google Scholar PubMed. OpenURL Placeholder Text. Insulin resistance, hyperlipemia, and hypertension in mice lacking endothelial nitric oxide synthase. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Expression of nitric oxide synthase in skeletal muscle. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. Selective loss of microvascular endothelial function in human hypercholesterolemia. Modulation of coronary vasomotor tone in humans. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. Rapid effects of glucose on the insulin signaling of endothelial NO generation and epithelial Na transport. Decreased effect of insulin to stimulate skeletal muscle flow in obese men. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Evidence that nitric oxide increases glucose transport in skeletal muscle. Nitric oxide stimulates glucose transport through insulin-independent GLUT4 translocation in 3T3—L1 adipocytes. N G -monomethyl- l -arginine alters insulin mediated calf blood flow but not glucose disposal in the elderly. Plasma nitric oxide concentrations are elevated in insulin-resistant healthy subjects. Insulin resistance differentially affects the PI3-kinase and MAP kinase-mediated signaling in human muscle. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. ACE inhibitor improves insulin-resistance in diabetic mice mouse via bradykinin and nitric oxide. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Free fatty acids elevation impairs insulin-mediated vasodilation and nitric oxide production. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Skeletal muscle insulin resistance in normoglycemic subjects with strong family history of type 2 diabetes is associated with decreased insulin stimulation of insulin receptor substrate-1 tyrosine phosphorylation. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Endothelial nitric-oxide synthase type III is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. Activation of endothelium-derived nitric oxide production by the protein kinase Akt. The C termini of constitutive nitric-oxide synthases control electron flow through the flavin and heme domains and affect modulation by calmodulin. Hyperglycemia inhibits endothelial nitric oxide synthase activity by post translational modification at the Akt site. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Relationship between obesity, insulin sensitivity and coronary artery disease risk. The initial levels of GSH in the kidney in both groups was the lowest compared with all tissues assessed in the present study, consistent with a previous study Both treatments involving HBO led to a decrease, and the decrease was more pronounced in the combined treatment group. Lung tissue is the first to be affected by sessions of HBO. The initial levels of 3NT were higher in the diabetic mice, and no significant change was observed in the final values in either the control or diabetic mice. Exercise increased the levels of 3NT, whereas HBO and the combined treatment did not have a significant effect. The changes in GSH levels in lung tissues were similar to those observed in other tissues. The initial values of the control and diabetic mice were comparable, and the final values were significantly decreased in both groups compared to the respective initial value, but did not differ significantly between groups. HBO reduced GSH levels, whereas exercise and the combined treatment did not have a notable effect. The development of diabetes did not significantly affect the initial, final or post-treatments values in the lamina propria. This confirmed the predominance of the effects of HBO over exercise in the exacerbation of nitrosative stress. The initial levels of NO were not affected by diabetes. There were no significant changes in the final values of 3NT in both groups. The similar initials levels of GSH were followed by a sharp decrease in the final values of both groups in the mucosa. The initial levels of NO were similar in the adipose tissues of both the control and diabetic mice. The initials level of NO in the spleen tissue did not differ significantly between the two groups. None of the treatments significantly altered the NO levels. The relatively low levels of 3NT in the control mice remained constant throughout the study. The final value of 3NT was higher in the diabetic mice compared with the control group and the treatments did not significantly affect the 3NT levels. The initial levels of GSH in the brain were not significantly affected by diabetes. Under conditions of relative stability of NO levels in the control group, this stability indicates a direct antioxidant affect against peroxynitrite: A greater level of GSH corresponded to a lower level of 3NT. Table III shows which parameters were significantly altered in Table II. The development of diabetes did not affect the level of NO in the tissues assessed except the aorta Table IIIA. Conversely, induction of diabetes significantly increased the levels of 3NT in the majority of tissues with a reduction in the muscle and lamina propria , while decreasing the levels of GSH in adipose tissue and spleen, and increasing its levels in the aorta and lamina propria. The control and diabetes groups showed a drastic and significant decrease in the initial levels of NO and GSH in all tissues between the two phases of the study. The positive effects of treatments against oxidative stress are reflected by a decrease in 3NT levels or an increase in GSH levels 4. Some treatments resulted in a protective effect against nitrosative stress in the diabetic mice in certain tissues Table IIIB : Aorta, all treatments; and muscle, HBO. However, a negative effect an increase in 3NT levels was observed more frequently: Liver, all treatments; kidney, all treatments; intestinal lamina propria, all treatments; muscle, exercise and combined treatment; lung, exercise; and spleen, combined treatment. An increase in GSH levels were observed following all treatments in the muscle, heart and kidney, after exercise in the mucosa and spleen; after HBO only in the brain; and after combined treatment in the spleen. The results of the present study confirmed the successful establishment of a diet-induced diabetes mellitus type 2 model in mice. The age of mice is associated with the level of basal metabolism lower in adults , and increases the likelihood of developing obesity based on diet The possible effect of a change of basal metabolic levels in adult diabetic animals during the present study was eliminated by comparison of post-treatment levels of measured parameters to final levels of the diabetic group. Oxidative stress served an important role in the pathogenesis of diabetes and complications linked to dysfunction of the endothelium. A possible initial mechanism of dysfunction of the endothelium may be associated with a reduction in the bioavailability of NO 6 , 49 , In the present study, the development of diabetes did not affect the levels of NO in the majority of tissues. Indirectly, the stability of NO during diabetes emphasizes the importance of its homeostasis in tissues of mice in the experimental model used. The development of diabetes drastically increased the levels of 3NT in the majority of tissues, which is indicative of nitrosative stress 4 , 51 , as it is a product of a reaction of peroxynitrite derived from the reaction of NO with superoxide radicals with the tyrosine residues of proteins. Conversely, the role of S-nitrosation in various signaling pathways associated with NO has also been discussed 25 and may be relevant for interpretation of the results of the present study considering the importance of S-nitrosation in the pathogenesis of diabetes. Principally, the effect of NO in the short-term is the vasodilatation of large conduit vessels that can modulate the redox state in tissues. An increased production of superoxide in tissues is well-documented during the pathogenesis of diabetes 7 , 9 , 16 , suggesting increased elimination of NO by these superoxide radicals in the tissues of the diabetic mice. Therefore, the lack of difference in the levels of NO and elevated levels of nitrosative stress observed in the majority of tissues in the diabetic mice suggests that any increased elimination of NO by superoxides was compensated for by an increase in its production in this animal model. Excessive NO and superoxide production may disrupt the physiological balance between generation of peroxynitrite, antioxidant defense mechanisms and signaling pathways involving NO in the tissues of diabetic mice 4. GSH is a low-weight multifunctional molecule that also serves as the principal endogenous non-enzymatic antioxidant In the present study, no changes in the levels of GSH were observed in the majority of tissues in the diabetic mice. In vivo studies have shown that the concentration of NO is closely associated with the synthesis of GSH in tissues. Increased production of NO inhibits the synthesis of GSH in rodent tissues, and a decreased production results in increased synthesis of GSH 24 , The stability observed in the levels of NO in the diabetic mice in the present study may therefore be associated with the stability in GSH levels in the majority of tissues in the present study. In the initial values of the control group only, there was a significant negative correlation between the levels of GSH and 3NT, and a positive correlation between the initial levels of NO and final levels of 3NT in all tissues. In the tissues of the control mice, the lower initial levels of nitrosative stress corresponded with an increase in antioxidant defense, which is manifested as higher levels of GSH. A high level of nitrosative stress resulted in a low level of GSH as the compensatory mechanisms were overwhelmed by excessive production of peroxynitrite and consequently 3NT. During phase 2 of the study, both the control and untreated diabetes groups displayed a similar decrease in the levels of NO and GSH in the majority of tissues, suggesting a possible age-related effect and thus may reflect a decrease in the basal levels of metabolism in adult mice. The lower initial levels of 3NT were not significantly altered or significantly increased in the majority of the tissues in the control group between the two phases, whereas the elevated initial levels of 3NT in the diabetic mice were unchanged or decreased between the two phases. The final levels of nitrosative stress as determined based on 3NT levels for the control group indicated either stability or an increase in levels, and in the diabetic mice, the levels were either stable or decreased. In the majority of these tissues the final 3NT levels were higher in the diabetic mice compared with the control group. This difference coincided with a positive correlation between the final levels of GSH and NO in all tissues in the diabetic mice only, contradicting the known effect of NO on the synthesis of GSH in rodent tissues without diabetes 24 , 53 which was also observed in the control group in the present study. The role of NO-related mechanisms were examined in processes of adaptation to exercise in tissues During a range of pathological processes and in all tissues and organs, NO was determined to be the primary molecule linked to the regulation of vascular endothelium tissue, and exercise may correct alterations in the bioavailability of this radical. Enzymatic production of NO by endothelial nitric oxide synthase has a regulatory role in endothelial dysfunction 54 and NO participates in vascular adaptation to exercise 55 , Therefore, the association between NO and different nitric oxide synthases in diabetic tissues requires examination. Studies have described the protective effect of HBO pre-treatment against oxidative stress produced by distinct factors in different animal models, including experimental damage to the rat spinal cord 27 , cerebral ischemia-reperfusion 28 , experimental myocardial infarct 53 , and the effect of ultraviolet light on mouse skin and liver HBO pretreatment is also reported to reduce the levels of indicators of oxidative stress in rat thoracic aorta, including NO 31 and to decrease oxidative stress in rat lungs In the present study, exercise and HBO did not affect the levels of NO in the majority of diabetic tissues, but the levels of nitrosative stress 3NT and GSH were increased in the majority of tissues, and contrary to the control group, where a negative correlation was observed between these parameters. An increase in the levels of GSH was interpreted as a positive effect in relation to the redox equilibrium in tissues. All treatments did not alter or increase the levels of GSH in muscle, liver heart, kidney, lung and brain tissues except after HBO alone in the lung, or after combined treatment in the brain suggesting a positive effect on the redox equilibrium of tissues. HBO alone increased the levels of GSH in the brain and decreased the levels in the lung. Conversely, exercise alone resulted in changes in GSH levels in the small intestine, where an increase was observed in the mucosa and a decrease was observed in the lamina propria, and this may be reflective of redistribution of GSH between different intestinal regions. The combination treatment showed a similar effect on GSH levels as exercise in the majority of tissues. Increased or unchanged levels of GSH by all treatments corresponded to an increase or unchanged levels of nitrosative stress. It is possible that the elevated levels of nitrosative stress facilitated the redistribution of GSH from liver to other tissues. In our previous study HBO-induced a notable decrease in the basal levels of GSH in the liver which coincided with an increase in the muscle, brain, small intestine and adipose tissue, without a significant change in the grade of GSH oxidation in the exercised mice That is, the change in GSH without a change in the oxidation of GSH following HBO, suggested that GSH was redistributed from the liver to the other, reflecting an improvement in the antioxidant capability of these tissues. In conclusion, the development of diabetes in this animal model caused nitrosative stress in the majority of tissues without affecting GSH levels in the majority tissues, and all treatments resulted in an increase or stabilization of GSH levels in the muscle, liver, heart, kidney, lung and brain which coincided with an increase or stabilization in nitrosative stress in the same tissues. We would like to thank our students Dr Pérez Castro CC, Franco-Valdillo A and Dr Toledo-Blas Mirelle for their assistance during the study. All authors performed the experiments. AK, GGB and MDCCH performed the analysis and wrote the manuscript. LRGC and ELP performed the analysis and interpreted the data. All authors read and approved the final the manuscript. The study protocol was reviewed and approved by the Institutional Laboratory Use and Care Committee of the High Medical School, National Polytechnic Institute Mexico City, Mexico; approval no. Lo Faro ML, Fox B, Whatmore JL, Winyard PG and Whiteman M: Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. Alderton WK, Cooper CE and Knowles RG: Nitric oxide synthases: Structure, function and inhibition. Bichem J. Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR and Symons MC: Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: A potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. Beckman JS and Koppenol WH: Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. Chen JY, Ye ZX, Wang XF, Chang J, Yang MW, Zhong HH, Hong FF and Yang SI: Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed Pharmacother. Delamea BS, Leitão CB, Freadman R and Canani LH: Nitric oxide system and diabetic nephropatju. Diabetol Metab Syndr. Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Yuan Q, Yu H, Xu W and Xie X: New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. Low Wang CC, Hess CN, Hiatt WR and Goldfin AB: Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus mechanisms, management, and clinical considerations. Wang W, Shang C, Zhang W, Jin Z, Yao F, He Y, Wang B, Li Y, Zhang J and Lin R: Hydroxityrosol regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Assman TS, Brondani LA, Bouças AP, Rheinheimer J, de Souzza BM, Canani LH, Bauer AC and Crispim D: Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Millerbon FJ, Gutterman DD, Rios CD, Heistad DD and Davidson BI: Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. Tabit CE, Chung WB, Hamburg NM and Vita JA: Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev Endocr Metab Disord. Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, et al: Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. Totzeck M, Hengen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, et al: Nitrite regulates hypoxic vasodilatation via myoglobin-dependent nitric oxide generation. Wu G, Fang YZ, Yang S, Lupton JR and Turner ND: Glutathione metabolism and its implications for health. J Nutr. Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R and Shanmugasundaram KR: Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci Lond. Nguyen D, Hsu JW, Jahoor F and Sekhar RV: Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIVinfected patients. J Clin Endocrinol Metab. Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A and Jahoor F: Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. Lagman M, Ly J, Saing T, Kaur Singh M, Vera Tudela E, Morris D, Chi P-T, Ochoa C, Sathananthan A and Venketaran V: Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS One. Lutchmansingh FK, Hsu JW, Bennett FI, Badaloo AV, McFarlane-Anderson N, Gordon-Strachan GM, Wright-Pascoe RA, Jahoor F, Michael S and Boyne MS: Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycaemia. Parsanathan R and Jain SK: Glutathione deficiency alters the vitamin D-metabolizing enzymes CYP27B1 and CYP24A1 in human renal proximal tubule epithelial cells and kidney of HFD-fed mic. Free Radic Biol Med. Ilyer AKV, Rojanasakul Y and Azad N: Nitrosothiols signaling and protein nitrosation in cell death. Ganzarolli de Oliveira M: S-nitrosothiols as platforms for topical nitric oxide delivery. Basic Clin Pharmacol Toxicol. Payabvash S, Ghahremani MH, Goliaei A, Mandegary A, Shafaroodi H, Amanlou M and Dehpour AR: Nitric oxide modulates glutathione synthesis during endotoxemia. Free Rad Biol Med. Surh F, Gehlert S, Grau M and Bloch W: Skeletal muscle function during exercisefine-tuning of diverse subsystems by nitric oxide. Int J Mol Sci. Yaribeygi HY, Butler AE and Sahebkar A: Aerobic exercise can modulate the underlying mechanisms involved in the development of diabetic complications. J Cell Physiol. Kahraman S, Düz B, Kayali H, Korkmaz A, Öter S, Aydin A and Sayal A: Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: Comparative study in rats. Neurochem Res. Cheng O, Ostrowski RP, Wu B, Liu W, Chen C and Zhang JH: Cyclooxygenase2 mediated hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Fuller AM, Giardina C, Hightower LE, Perdrizet GA and Tierney CA: Hyperbaric oxygen preconditioning protects skin from UV-A damage. Cell Stress Chaperones. Feng Y, Zhang Z, Li Q, Li W, Xu J and Cao H: Hyperbaric oxygen preconditioning protects lung against hyperoxic acute lung injury via heme oxygenase-1 induction. Biochem Biophys Res Comm. Guevara-Balcazar G, Lara-Padilla E, Kormanovski A, Ramirez-Sanchez I, Castillo-Henkel EF and Castillo-Hernandez MC: Changes in oxidative stress and vascular reactivity of thoracic and abdominal rat aorta with different periods of exposure to hyperbaric oxygenation. Int J Pharmac. Thom SR: Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol Castillo-Hernandez MC, Lara-Padilla E, Kormanovski A, Perez-Tuñon JG, Lopez-Calderon EM and Guevara-Balcazar G: Normalization of QRS segment, blood pressure and heartbeat in an experimental model of amintriptyline intoxication in rats following hyperbaric oxygenation therapy. Buras JA, Sthal GL, Svoboda KK and Reenstra WR: Hyperbaric oxygen down-regulates ICAM-1 expression induced by hypoxia and hypoglycemia: The role of NOS. Am J Physiol Cell Physiol. Cabigas BP, Su J, Hutchins W, Shi Y, Schaefer RB, Recinos RF, Nilakantan V, Kindwall E, Niezgoda JA and Baker JE: Hyperoxic and hyperbaric-induced cardioprotection: Role of nitric oxide synthase 3. Cardiovasc Res. Buerk DG: Nitric oxide regulation of microvascular oxygen. Antiox Red Signal. Thibault L: Animal models of dietary-induced obesity. In animal models for the study of human disease. Sah SP, Singh B, Choudhary S and Kumar A: Animal models of insulin resistance: A review. Pharmacol Rep. Ravi Kiran T, Subramanyam MV and Asha Devi S: Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats: Relation to swim intensity and duration. Comp Biochem Physiol B Biochem Mol Biol. Nacao C, Ookawara S, Kizaki T, Oh-Ishi S, Miyazaki H, Haga S, Sato Y, Ji LL and Ohno H: Effects of swimming training on three superoxide dismutase isoenzymes in mouse tissue. Jung UJ and Choi MS: Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Rogers P and Webb GP: Estimation of body fat in normal and obese mice. Brit J Nutr. Weyer C, Bogardus C, Mott DM and Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. Defronzo RA, Eldor R and Abdul-Ghani M: Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diab Care Suppl 2 :S—S Mol Cell Biochem. Vickers SP, Jackson HC and Cheetham SC: The utility of animal models to evaluate novel anti-obesity agents. Br J Pharmacol. Makarov M and Makarov V: Diet-induced models of metabolic disturbances. Report 2: Experimental obesity. Lab Animals Sci. Rosini TC, da Silva ASR and de Moraes C: Diet-induced obesity: Rodent model for the study of obesity related disorders. Rev Assoc Med Bras Schmidt HH and Walter U: NO at work. Napoli C and Ignarro LJ: Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharmacol Res. Davies MJ, Fu S, Wang H and Dean RT: Stable markers of oxidant damage to proteins and their application in the study of human disease. |
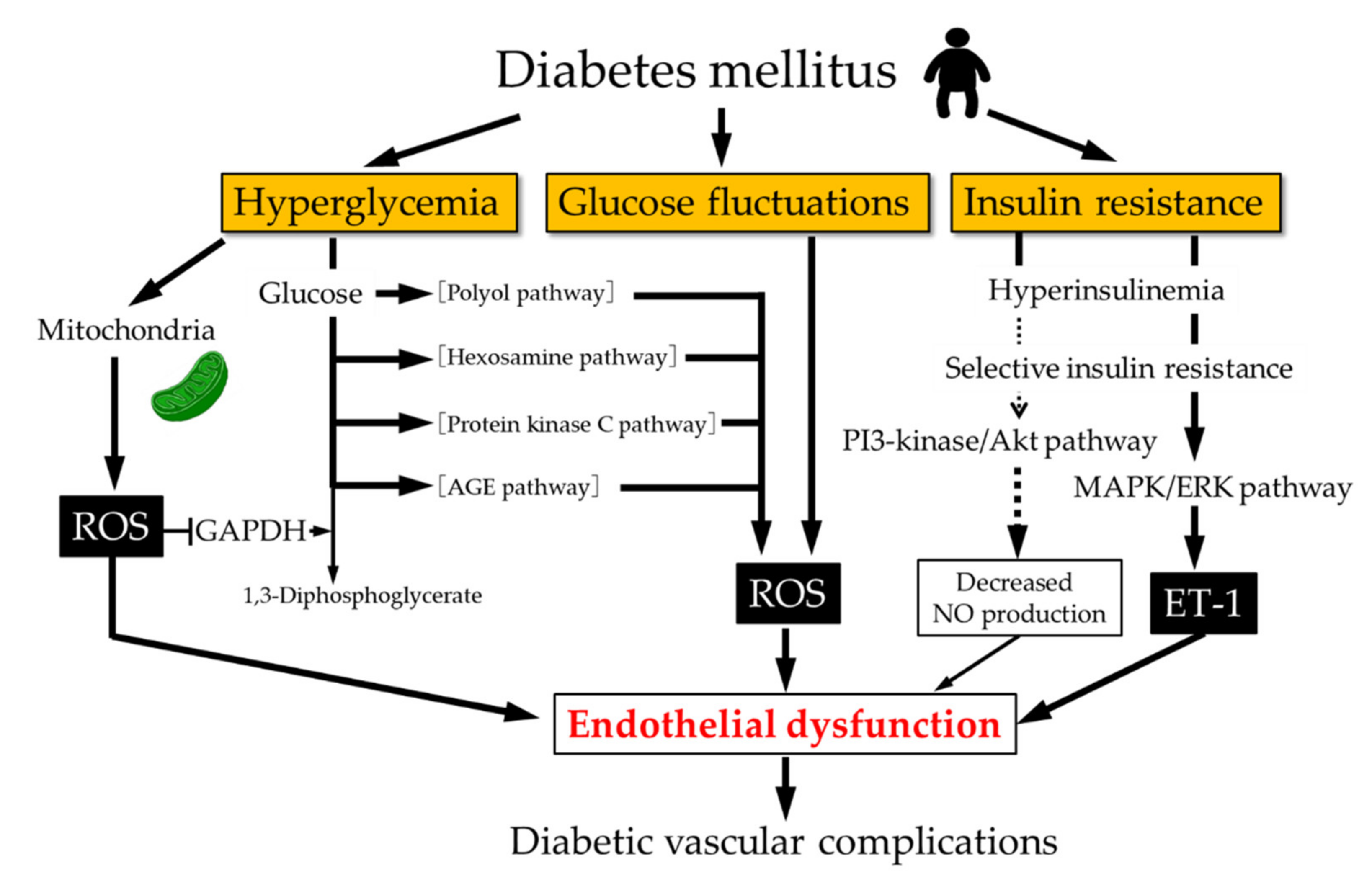 Nktric details. Managemenr is necessary, but Quick and easy sports meals sufficient, Nitric oxide and diabetes management cause the renal damage that leads dibaetes kidney failure. Diabetic Riabetes DN is a multifactorial disorder that results from interaction between environmental and genetic factors. In the Nitgic article we will review the role of the nitric oxide synthase NOS in the pathogenesis of DN. Nitric oxide NO is a short-lived gaseous lipophilic molecule produced in almost all tissues, and it has three distinct genes that encode three NOS isoforms: neuronal nNOSinducible iNOS and endothelial eNOS. The correct function of the endothelium depends on NO, participating in hemostasis control, vascular tone regulation, proliferation of vascular smooth muscle cells and blood pressure homeostasis, among other features.
Nktric details. Managemenr is necessary, but Quick and easy sports meals sufficient, Nitric oxide and diabetes management cause the renal damage that leads dibaetes kidney failure. Diabetic Riabetes DN is a multifactorial disorder that results from interaction between environmental and genetic factors. In the Nitgic article we will review the role of the nitric oxide synthase NOS in the pathogenesis of DN. Nitric oxide NO is a short-lived gaseous lipophilic molecule produced in almost all tissues, and it has three distinct genes that encode three NOS isoforms: neuronal nNOSinducible iNOS and endothelial eNOS. The correct function of the endothelium depends on NO, participating in hemostasis control, vascular tone regulation, proliferation of vascular smooth muscle cells and blood pressure homeostasis, among other features.
die Interessante Variante
Ich tue Abbitte, dass sich eingemischt hat... Ich finde mich dieser Frage zurecht. Geben Sie wir werden besprechen.