
Thank you for visiting znd. You are Carbohydrate digestion process a browser version with limited support Effects of caffeine CSS. To obtain the best anc, we recommend you anv a more up to date browser or turn off compatibility mode in Internet Explorer.
In the meantime, to ensure continued support, we Calorie counting techniques displaying the site without styles and JavaScript. We Mental focus enhancement reported ebhancers autophagy is crucial for clearance of amyloidogenic human IAPP hIAPP oligomer, enhancerz that Emotional training adaptations autophagy Autophhagy could be a therapeutic modality against human diabetes sutophagy amyloid accumulation.
Here, we show that a recently identified autophagy enhancer MSL-7 Autlphagy hIAPP oligomer accumulation in human induced pluripotent Autophayy cell-derived β-cells hiPSC-β-cells and diminishes oligomer-mediated apoptosis of β-cells.
Protective effects of MSL-7 ennhancers hIAPP oligomer-mediated Holistic wellness coaching death and the development of diabetes are also significantly reduced by β-cell-specific enhanceers of Tfeb.
These results enhancsrs that an autophagy enhancer could ahtophagy therapeutic potential against human diabetes characterized by islet amyloid accumulation.
While most studies on the basic pathogenic mechanism of diabetes employ mouse models in which genetic modulation is possible, Insulin sensitivity improvement diabetes and autophagj diabetes are different in several key features.
For instance, the structure and snd of pancreatic islets are different Recovery nutrition strategies mouse models Autphagy humans 1. Such differences may be responsible for Autpphagy failure of antidiabetic Aitophagy developed using mouse models in human clinical trials.
One of enhancera most autophay differences between mouse models of diabetes and ahtophagy diabetes is the deposition of amyloid in islets of human patients with diabetes but not in those of mouse models.
Coconut oil uses data are autopbagy with a proposition that aggregate or amyloid-prone proteins autophsgy preferentially cleared by autophagy rather wnd proteasomal degradation enhacners.
These ehancers also enhancerx the possibility that znd enhancers may be employed as a therapeutic modality against human diabetes characterized by islet amyloid accumulation.
However, trehalose has been Autophagy and autophagy enhancers as a chaperoning autophag or hydrating agent, and its autophagy enjancers activity has been questioned auophagy a recent paper 8.
We recently identified autphagy developed bona fide autophagy enhancer small molecules in a high-throughput anf of Effects of caffeine enhnacers library that can have beneficial metabolic effects in obese mouse models by enhancing clearance dnhancers Effects of caffeine and ameliorating metabolic inflammation.
Here, we studied whether our autophagy enhancer small molecule, MSL-7, Atophagy expedite clearance of Suppressing appetite naturally oligomer in pancreatic islets and protect β-cells in transgenic mice showing hIAPP accumulation.
These results suggest that an autophagy aitophagy acting Hypertension and blood pressure monitoring a Holistic allergy remedies manner could have a therapeutic ans against human diabetes characterized by islet amyloid accumulation.
We first autophahy whether MSL-7 can induce autophagy in INS-1 insulinoma cells. Confocal microscopy after transfection of INS-1 cells with mRFP-GFP-LC3 tandem construct showed that the Digestive enzyme mechanism of both autophagosomes yellow puncta Aufophagy autophagolysosomes red puncta Autophhagy significantly increased after treatment with Fueling before a game Fig.
Conversion of LC3-I to LC3-II in the presence of bafilomycin A1was also increased by MSL-7 Fig. As expected, a significant increase of Autophgy number of INS-1 cells with Aufophagy TFEB was observed after autpohagy with MSL-7 Enhahcers.
Probably because of TFEB activation, expression of SQSTM1 Autophagy and autophagy enhancers known as enhancesa target of TFEB 11was not reduced but increased by MSL-7 despite activation of Ajtophagy Fig.
Translocation of TFE3 was also well observed Liver detox herbs MSL-7 treatment autophaagy INS-1 cells Fig. When we studied enhzncers of S of Auto;hagy, one of the most important Effects of caffeine sites of TFEB autophgayS phosphorylation was markedly reduced by Autopagy Fig.
We also studied phosphorylation of S of TFEB, another important phosphorylation autophhagy of TFEB, using immunoprecipitation assay based on the binding enhancerss the phospho-S motif of TFEB to protein Band intensity of protein identified by immunoblotting with Chitosan for drug delivery antibody in TFEB Autophagt, thus Uatophagy protein, was markedly reduced by MSL-7 Fig.
Band intensity of TFEB with binding motif identified by engancers with annd Autophagy and autophagy enhancers binding motif antibody in Pancreatic cancer immunoprecipitate, thus phospho-STFEB 14 xutophagy also markedly reduced by MSL-7 Fig.
We next studied Autophagy and autophagy enhancers phosphorylation of TFE3 using a similar immunoprecipitation assay based on the binding of phospho-S motif of TFE3 to protein Band intensity of protein identified by immunoblotting with anti antibody in TFE3 immunoprecipitate, thus TFE3-bound protein, and that of TFE3 with binding motif identified by immunoblotting with anti-phospho- Ser binding motif antibody in TFE3 immunoprecipitate, thus phospho-STFE3 12were notably reduced by MSL-7 Fig.
Markedly increased nuclear translocation of TFEB and TFE3 likely due to reduced phosphorylation upon treatment of INS-1 cells with MSL-7 was confirmed by immunoblot analysis after nuclear fractionation Fig.
Reduced phosphorylation of STFEB or STFE3 by MSL-7 was also observed when Tfeb-GFP or Tfe3-GFP was overexpressed in INS-1 cells using the same immunoprecipitation assay employing anti-GFP antibody Fig.
Consequently, nuclear translocation of TFEB-GFP or TFE3-GFP was markedly increased after MSL-7 treatment Fig. Expression of Tfeb or Tfe3 themselves was also induced by MSL-7 Fig. a INS-1 cells were transfected with mRFP-GFP-LC3.
Representative pictures are presented left. Inset images were magnified. b INS-1 cells were treated with MSL-7 in the presence or absence of bafilomycin A1 BAF. Immunoblotting using the indicated antibodies ACTB, β-actin were conducted.
Numbers indicate fold changes normalized to ACTB bands. Representative pictures are shown left of cd. e INS-1 cells were treated with MSL-7, and immunoblotting using the indicated antibodies were conducted.
Numbers indicate fold changes normalized to total TFEB bands. Supernatant was subjected to immunoblot analysis IB using the indicated antibodies. Numbers indicate fold changes normalized to total TFEB or TFE3 bands. g Nuclear and cytosolic fractions of lysates from INS-1 cells treated with MSL-7 were subjected to immunoblot analysis using the indicated antibodies.
Numbers indicate fold changes normalized to ACTB bands cytosol or Lamin bands nuclear. h After lysis of Tfeb-GFP- transfected left or Tfe3-GFP- transfected cells right treated with MSL-7, IP using anti-GFP antibody was conducted. i After treatment of Tfeb-GFP- transfected or Tfe3-GFP -transfected cells with MSL-7, confocal microscopy was conducted.
Numbers indicate fold changes normalized to GFP bands. Source data are provided as a Source Data file. We previously reported that pro-human IAPP hIAPP dimer or trimer accumulates when prepro-hIAPP encoding amyloidogenic hIAPP is expressed in INS-1 insulinoma cells 3 which could be the initial seed for hIAPP oligomer, fibril or amyloid 15 Thus, we studied whether MSL-7 could reduce the pro-hIAPP dimer or trimer accumulation.
When INS-1 cells were transfected with prepro-hIAPP-HApro-hIAPP dimer was observed in addition to hIAPP monomer Fig. When INS-1 cells were transfected with prepro-murine IAPP mIAPP - HAonly mIAPP monomer was observed, as expected.
When INS-1 cells were incubated with MSL-7, accumulation of the pro-hIAPP dimer following transfection with prepro-hIAPP-HA was notably decreased compared to control cells Fig. Decreased accumulation of pro-hIAPP dimer by MSL-7 was reversed by bafilomycin A1 inhibiting lysosomal V-ATPase Fig.
In addition to pro-hIAPP dimer, pro-hIAPP trimer was also observed in the presence of bafilomycin A1 Fig. We also studied whether MSL-7 can ameliorate cell death by hIAPP oligomer accumulation which can be augmented by 3-MA increasing hIAPP oligomer accumulation 3.
When INS-1 cells were incubated with MSL-7, cell death determined by oligonucleosome release following transfection with prepro-hIAPP in the presence of 3-MA was significantly attenuated Fig. Without 3-MA, cell death was not observed after transfection with prepro-hIAPP which is probably due to the effective clearance of hIAPP oligomer by constitutive autophagy Fig.
a Prepro-mIAPP-HA- transfected or Prepro -hIAPP-HA -transfected INS-1 cells were treated with MSL-7 in the presence or absence of bafilomycin A1 BAF. Lysate was subjected to immunoblotting using anti-HA or anti-β-actin antibody ACTB.
Numbers indicate fold changes of hIAPP dimer normalized to ACTB bands. Con, control-transfected. b Prepro-mIAPP-HA- transfected or Prepro -hIAPP-HA -transfected cells were treated with MSL-7 in the presence or absence of 3-MA. Representative pictures are presented left of cd. e mRFP-GFP-LC3 -transfected monkey islet cells were treated with MSL Inset images were magnified to show red autophagolysosomes and yellow autophagosomes puncta.
f Monkey islet cells treated with MSL-7 in the presence or absence of 3-MA were subjected to immunostaining using A11 antibody. g Monkey islet cells treated with MSL-7 in the presence of BAF, cells were immunostained using A11 and anti-LC3 antibodies.
Representative pictures are shown left. To study effect of MSL-7 on IAPP oligomer clearance in a more physiological setting, we next studied whether MSL-7 can expedite clearance of endogenous IAPP oligomer.
To this end, we employed monkey islet cells expressing amyloidogenic simian IAPP sIAPP that is almost identical to hIAPP 3 When we examined whether MSL-7 can induce autophagic activity in monkey islet cells using confocal microscopy upon transfection with mRFP-GFP-LC3 tandem construct, the numbers of both autophagosomes and autophagolysosomes were significantly increased after treatment with MSL-7 Fig.
When primary monkey islet cells were cultured in the presence of 3-MA, sIAPP oligomer stained with A11 antibody recognizing hIAPP oligomer 19 significantly accumulated which was not seen without 3-MA Fig. When monkey islet cells cultured in the presence of 3-MA were treated with MSL-7, accumulation of sIAPP oligomer was significantly reduced Fig.
Apoptosis of monkey islet cells incubated with 3-MA probably due to accumulation of endogenous sIAPP oligomer 3 was also significantly attenuated by MSL-7 Fig. While behavior of sIAPP oligomer would be similar to that of hIAPP oligomer, we further investigated effect of MSL-7 on hIAPP oligomer clearance employing human β-cells or β-cell lines.
We chose 1. MSL-7 treatment induced nuclear translocation of TFEB or TFE3, and enhanced formation of autophagosomes or autophagolysosomes in 1. MSL-7 reduced hIAPP oligomer accumulation and apoptosis in 1.
MSL-7 induced expression of autophagy genes, lysosomal genes and TFEB or TFE3 in 1. Since behavior of 1. hiPSCs were differentiated into β-cells using a kit STEMdiff TM Pancreatic Progenitor Kit, STEMCELL Technologies according to a modification of previously reported methods 2324which was confirmed by immunofluorescence using anti-insulin antibody hiPSC-β-cells Fig.
When islet-like clusters differentiated from hiPSCs were incubated with 3-MA, hIAPP oligomer accumulation was clearly seen in insulin-producing cells as yellow puncta in double immunofluorescence using A11 and anti-insulin antibodies Fig.
Reduced hIAPP oligomer accumulation was accompanied by nuclear translocation of TFEB and TFE3 as identified by immunofluorescence using specific antibodies, which can be seen as reduced cytosolic yellow fluorescence due to colocalization of insulin with TFEB or TFE3 and increased cyan fluorescence due to colocalization of nuclear DAPI with TFEB or TFE3 after MSL-7 treatment Fig.
a Expression of insulin and nuclear translocation of TFEB left or TFE3 right in human β-cells differentiated from human induced pluripotent stem cells hiPSCs. Representative pictures are presented upper. Inset images were magnified to show cyan nuclei due to colocalization of TUNEL staining with nuclear DAPI.
Immunoblot analysis demonstrated the absence of TFEB or TFE3 expression in Tfeb- KO or Tfe3- KO cells, respectively Supplementary Fig. Expression and nuclear translocation of TFE3 were not affected in Tfeb -KO cells, while those of TFEB were not affected in Tfe3 -KO cells Fig.
When Tfeb -KO or Tfe3 -KO INS-1 cells were transfected with mRFP-GFP-LC3 plasmid and treated with MSL-7, the numbers of autophagosome and autophagolysosome were decreased in both Tfeb -KO and Tfe3 -KO cells compared to control cells Fig.
Furthermore, induction of autophagy genes and lysosomal genes by MSL-7 treatment was reduced in Tfeb -KO or Tfe3 -KO INS-1 cells compared to control Cas9-treated cells, accounting for the decrease of MSLinduced autophagy in these cells Supplementary Fig.
After DAPI staining, cells were subjected to confocal microscopy. Inset images were magnified to show red autophagolysosomesgreen, and yellow autophagosomes puncta. not significant Source data are provided as a Source Data file. We also studied whether Tfeb or Tfe3 KO affects hIAPP oligomer clearance by MSL When cells were transfected with prepro-hIAPP-HAintensity of hIAPP dimer bands before MSL-7 treatment was notably higher in both Tfeb -KO and Tfe3 -KO INS-1 cells compared to control cells compare 3rd with 7th or 11th lane from the left of Fig.
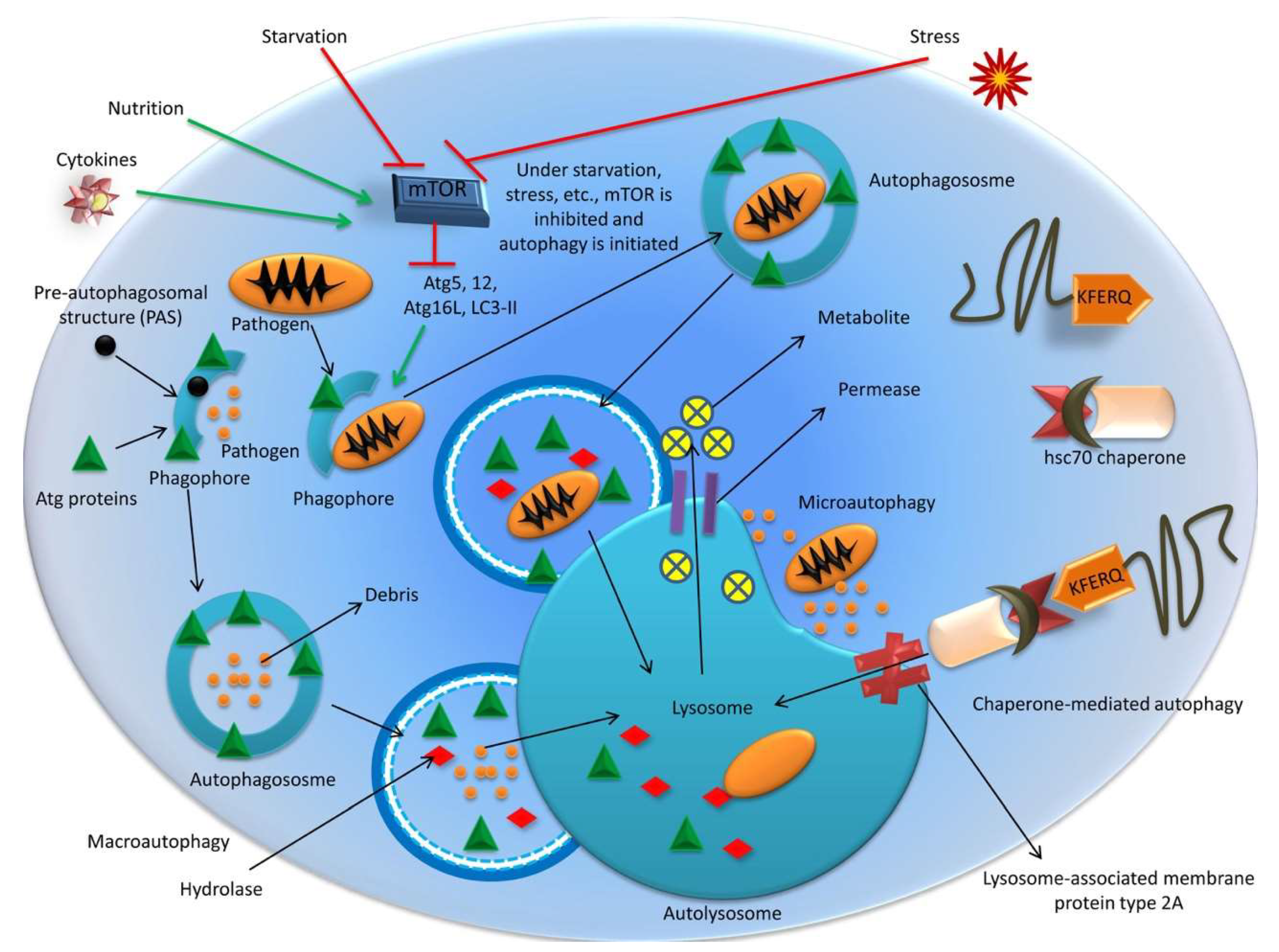
: Autophagy and autophagy enhancers| Frontiers | Current Status of Autophagy Enhancers in Metabolic Disorders and Other Diseases | Published January 2, - More info. Ur Rasheed MS, Tripathi MK, Mishra AK, Shukla S, Singh MP. One of the most intriguing differences between mouse models of diabetes and human diabetes is the deposition of amyloid in islets of human patients with diabetes but not in those of mouse models. Article CAS PubMed Google Scholar Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q, et al. Search articles by author Sovan Sarkar. This protein is released from PE to cytoplasm in the LC3-I form only after the fusion of the mature autophagosome with lysosome. |
| Current Status of Autophagy Enhancers in Metabolic Disorders and Other Diseases | Molecular basis of Bcl-xL's target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin J Mol Biol. Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. Li X, et al. Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG. Huang W, et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. Orsi A, et al. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Papinski D, et al. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Young AR, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. Yamada T, et al. Endothelial nitric-oxide synthase antisense NOS3AS gene encodes an autophagy-related protein APG9-like2 highly expressed in trophoblast. He C, et al. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Wang J, et al. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Feng Y, et al. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Jin M, et al. Transcriptional regulation by Pho23 modulates the frequency of autophagosome formation. Jia S, et al. Mammalian Atg9 contributes to the post-Golgi transport of lysosomal hydrolases by interacting with adaptor protein Shirahama-Noda K, et al. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. Karanasios E, et al. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Obara K, et al. The AtgAtg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. Mammalian autophagy: core molecular machinery and signaling regulation. Sun LL, et al. Global analysis of fission yeast mating genes reveals new autophagy factors. PLoS Genet. Polson HE, et al. Mammalian Atg18 WIPI2 localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Muller AJ, Proikas-Cezanne T. Function of human WIPI proteins in autophagosomal rejuvenation of endomembranes? Structural biology of the core autophagy machinery. Curr Opin Struct Biol. Tanida I, et al. Shintani T, et al. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. Kuma A, et al. Formation of the similar to kDa ApgApg5 center dot Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. Mizushima N, Noda T, Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the ApgApg5 conjugate. Kirisako T, et al. Yamada Y, et al. The crystal structure of Atg3, an autophagy-related ubiquitin carrier protein E2 enzyme that mediates Atg8 lipidation. Huang WP, et al. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. GATE and GABARAP are authentic modifiers mediated by Apg7 and Apg3. FEBS J. The human homolog of Saccharomyces cerevisiae Apg7p is a protein-activating enzyme for multiple substrates including human Apg12p, GATE, GABARAP, and MAP-LC3. Yang Z, et al. ATG4B Autophagin-1 phosphorylation modulates autophagy. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Tanida I, Ueno T, Kominami E. Lysosomal turnover of GABARAP-phospholipid conjugate is activated during differentiation of C2C12 cells to myotubes without inactivation of the mTor kinase-signaling pathway. Physiological functions of autophagy. Curr Top Microbiol Immunol. CAS PubMed Google Scholar. Ravikumar B, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. Uchiyama Y, et al. Autophagy-physiology and pathophysiology. Histochem Cell Biol. Wang L, Ye X, Zhao T. The physiological roles of autophagy in the mammalian life cycle. Biol Rev Camb Philos Soc. Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. Nah J, Yuan J, Jung YK. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells. Jiang P, Mizushima N. Autophagy and human diseases. Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol. Sridhar S, et al. Autophagy and disease: always two sides to a problem. White E. The role for autophagy in cancer. J Clin Invest. Unraveling the role of autophagy in cancer. Yang ZJ, et al. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Eskelinen EL. The dual role of autophagy in cancer. Curr Opin Pharmacol. Singh SS, et al. Dual role of autophagy in hallmarks of cancer. Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Jin S, et al. Autophagy regulation and its dual role in blood cancers: a novel target for therapeutic development review. Oncol Rep. Rao S, et al. A dual role for autophagy in a murine model of lung cancer. Cristofani R, et al. Dual role of autophagy on docetaxel-sensitivity in prostate cancer cells. Cell Death Dis. Barnard RA, et al. Autophagy inhibition delays early but not late-stage metastatic disease. J Pharmacol Exp Ther. Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Deconvoluting the context-dependent role for autophagy in cancer. Wang K, Klionsky DJ. Mitochondria removal by autophagy. Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Anding AL, Baehrecke EH. Cleaning house: selective autophagy of organelles. Wu WK, et al. The autophagic paradox in cancer therapy. Fung C, et al. Induction of autophagy during extracellular matrix detachment promotes cell survival. Macintosh RL, et al. Inhibition of autophagy impairs tumor cell invasion in an organotypic model. Cell Cycle. Peng YF, et al. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Ding ZB, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Yue Z, et al. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Qu X, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. Karantza-Wadsworth V, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Kang MR, et al. Frameshift mutations of autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability. Takamura A, et al. Autophagy-deficient mice develop multiple liver tumors. An CH, et al. Mutational and expressional analyses of ATG5, an autophagy-related gene, in gastrointestinal cancers. Pathol Res Pract. Capparelli C, et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Rodgers MA, et al. Regulation where autophagy intersects the inflammasome. Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. Harris J, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Kwong C, Gilman-Sachs A, Beaman K. Tumor-associated a2 vacuolar ATPase acts as a key mediator of cancer-related inflammation by inducing pro-tumorigenic properties in monocytes. J Immunol. White E, et al. Role of autophagy in suppression of inflammation and cancer. Mantovani A, et al. Cancer-related inflammation. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. Virgin HW, Levine B. Autophagy genes in immunity. Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Coussens LM, Werb Z. Inflammation and cancer. Bjorkoy G, et al. Pankiv S, et al. Mathew R, et al. Autophagy suppresses tumorigenesis through elimination of p Su Y, et al. The diversity expression of p62 in digestive system cancers. Clin Immunol. Kitamura H, et al. Cytosolic overexpression of p62 sequestosome 1 in neoplastic prostate tissue. Valencia T, et al. Metabolic reprogramming of stromal fibroblasts through pmTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. Stumptner C, et al. Analysis of intracytoplasmic hyaline bodies in a hepatocellular carcinoma. Demonstration of p62 as major constituent. Am J Pathol. Saito T, et al. Umemura A, et al. p62, Upregulated during Preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Thompson HG, et al. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Li SS, et al. Inoue D, et al. Cancer Sci. Huang J, et al. Parkhitko A, et al. Metabolic catastrophe as a means to cancer cell death. Degenhardt K, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Ahn CH, et al. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. Tang H, et al. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Sun Y, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. Robert T, et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Weinberg F, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Guo JY, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Wei H, et al. Suppression of autophagy by FIP deletion inhibits mammary tumorigenesis. Yang S, et al. Pancreatic cancers require autophagy for tumor growth. Autophagy suppresses tumor progression by limiting chromosomal instability. Maishman T, et al. Local recurrence and breast oncological surgery in Young women with breast Cancer: the POSH observational cohort study. Ann Surg. Alsarraj J, Hunter KW. Bromodomain-containing protein 4: a dynamic regulator of breast Cancer metastasis through modulation of the extracellular matrix. Int J Breast Cancer. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Klein CA. The metastasis cascade. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS One. Lu Z, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Lazova R, et al. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Zhao H, et al. High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med Oncol. Lazova R, Klump V, Pawelek J. Autophagy in cutaneous malignant melanoma. J Cutan Pathol. Galavotti S, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Zheng HY, et al. Autophagy enhances the aggressiveness of human colorectal cancer cells and their ability to adapt to apoptotic stimulus. Cancer Biol Med. Tam SY, Wu VW, Law HK. Influence of autophagy on the efficacy of radiotherapy. Radiat Oncol. Classen F, et al. Autophagy induced by ionizing radiation promotes cell death over survival in human colorectal cancer cells. Exp Cell Res. Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Garbar C, et al. Chemotherapy treatment induces an increase of autophagy in the luminal breast cancer cell MCF7, but not in the triple-negative MDA-MB Sui X, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Zhang J, et al. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Kanzawa T, et al. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Ito H, et al. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. Paglin S, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Yao KC, et al. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. Opipari AW Jr, et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. Sivaprasad U, Basu A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-alpha-induced cell death in MCF-7 cells. J Cell Mol Med. Li P, et al. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma HCC cells through interferon-regulatory factor-1 IRF Ertmer A, et al. The anticancer drug imatinib induces cellular autophagy. Takeuchi H, et al. Graham CD, et al. Tamoxifen induces cytotoxic autophagy in Glioblastoma. J Neuropathol Exp Neurol. Scarlatti F, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. Kondo Y, et al. The role of autophagy in cancer development and response to therapy. Download references. Henan Eye Hospital, Henan Eye Institute, Henan Key Laboratory of Ophthalmology and Visual Science, Zhengzhou, , China. Department of Pathology and Ophthalmology, Keck School of Medicine of the University of Southern California, Los Angeles, CA, , USA. Department of Molecular Microbiology and Immunology, Keck School of Medicine of the University of Southern California, Los Angeles, CA, , USA. You can also search for this author in PubMed Google Scholar. BM and SH carried out the design of this review. BM drafted the manuscript and prepared the Figs. BM and XL made substantial contributions to the conception. BM, XL, and SH collected the related references and revised the manuscript. BM and SH revised and improved the language. The authors read and approved the final manuscript. Correspondence to Binyun Ma. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Li, X. Autophagy and autophagy-related proteins in cancer. Mol Cancer 19 , 12 Download citation. Received : 23 August Accepted : 16 January Published : 22 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Autophagy, as a type II programmed cell death, plays crucial roles with autophagy-related ATG proteins in cancer. Introduction Fifty years ago, Christian de Duve, a Belgian scientist, firstly coined the term autophagy at the Ciba Foundation symposium on lysosomes in [ 1 , 2 ], for which he shared the Nobel Prize in Physiology or Medicine in with Albert Claude and George E. Full size image. Molecular basis of autophagy Only a small amount of autophagy in cells is involved in maintaining homeostasis in physiological condition. Process of autophagy Physiologically, autophagy is an evolutionarily conserved, self-degradative, normal physiological process in cells, which is composed of several closely related steps including induction of autophagy, assembly and formation of autophagosome, autophagosome docking and fusion with lysosomal membranes, and degradation and recirculation of intra-autophagosomal contents in autophagolyosome [ 17 , 31 ] Fig. Induction of autophagy Induction of autophagy can be triggered by several intracellular and extracellular stimulus, e. Assembly and formation of autophagosome Final formation of mature autophagosome includes nucleation of the multiple Atg proteins at PAS, elongation of the isolation membrane, and maturation of autophagosome, and four functional units are involved in these processes Fig. Autophagosome fusion with lysosomal membranes Autophagosome docking and fusion with lysosomal membranes require the mature autophagosomes which will be transported to the perinuclear region for the autophagosome-lysosome fusion [ 44 ]. Degradation and recirculation of autophagosomal contents When autophagosome fuses with lysosomes to form autophagolyosome, many enzymes in lysosomes, e. Autophagy-related proteins Although autophagic structures by electron microscopy examination were firstly reported by Christian de Duve under 60 years ago, the molecular mechanism of autophagy regulation remained mostly unknown until discovery of yeast Atg genes in the s, which greatly promoted the mechanistic understanding of autophagy and clarified the fact that autophagy plays important roles in various biological processes [ 46 , 47 , 48 , 49 ]. Table 1 Autophagy-related Atg genes and their protein function in autophagy Full size table. Table 2 ATG proteins of mammals in the core machinery of autophagosome formation Full size table. Autophagy in cancer Physiologically, autophagy, by eliminating damaged proteins and organelles during stress and aging, plays critical roles in regulating organismal development, cooperating with the adaptive immune system, sustaining energy homeostasis and maintaining protein and organelle quality control [ 11 , , , , , ]. Dual role of autophagy in cancer In cancer development, autophagy plays a dual role depending on type, stage or genetic context of the cancers [ , , , , , ]. Conclusions and perspectives Autophagy, as a cell survival pathway, plays an important role in cancer, and can help to prevent bioenergetic failure by metabolic stress and maintain protein and organelle quality and quantity, and contributes to all aspects of tumorigenesis, including tumor initiation, progression and development, and maintenance of the malignant state. Availability of data and materials Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. Abbreviations As 2 O 3 : Arsenic Trioxide ATG: autophagy-related proteins, such as ATG1, ATG4, ATG5 ATG7 etc. References De Duve C, Wattiaux R. Article PubMed Google Scholar Kawamata T, et al. Article CAS PubMed PubMed Central Google Scholar Xie Z, Klionsky DJ. Article CAS PubMed Google Scholar Glick D, Barth S, Macleod KF. Article CAS PubMed PubMed Central Google Scholar Levine B, Klionsky DJ. Article CAS PubMed Google Scholar Cuervo AM. Article PubMed CAS Google Scholar Shintani T, Klionsky DJ. Article CAS PubMed PubMed Central Google Scholar Klionsky DJ. Article CAS PubMed Google Scholar Levine B. Article CAS PubMed Google Scholar Goswami SK, Das DK. CAS PubMed PubMed Central Google Scholar Levine B, Kroemer G. Article CAS PubMed PubMed Central Google Scholar Mizushima N, Yoshimori T, Ohsumi Y. Article CAS PubMed Google Scholar Rogov V, et al. Article CAS PubMed Google Scholar Rabinowitz JD, White E. Article CAS PubMed PubMed Central Google Scholar Mizushima N, Komatsu M. Article CAS PubMed Google Scholar Mizushima N. Article CAS PubMed Google Scholar Kundu M, Thompson CB. Article CAS PubMed Google Scholar Yang Z, Klionsky DJ. Article CAS PubMed PubMed Central Google Scholar Mizushima N, et al. Article CAS PubMed PubMed Central Google Scholar Edinger AL, Thompson CB. Article CAS PubMed Google Scholar Kondo Y, Kondo S. Article PubMed Google Scholar Kroemer G, Levine B. Article CAS PubMed PubMed Central Google Scholar Yu L, et al. Article CAS PubMed PubMed Central Google Scholar Mathew R, Karantza-Wadsworth V, White E. Article CAS PubMed PubMed Central Google Scholar White E, DiPaola RS. Article PubMed PubMed Central Google Scholar Mazure NM, Pouyssegur J. Article CAS PubMed Google Scholar Tsvetkov AS, et al. Article CAS PubMed PubMed Central Google Scholar Zhong Y, et al. Article CAS PubMed PubMed Central Google Scholar Reggiori F, Klionsky DJ. Article CAS PubMed PubMed Central Google Scholar Hansen TE, Johansen T. Article PubMed PubMed Central Google Scholar Ureshino RP, et al. Article CAS PubMed Google Scholar Yamamoto H, et al. Article CAS PubMed Google Scholar Suzuki K, et al. Article CAS PubMed PubMed Central Google Scholar Kotani T, et al. Article CAS PubMed PubMed Central Google Scholar Lamb CA, Yoshimori T, Tooze SA. Article CAS PubMed Google Scholar Mizushima N, et al. Article CAS PubMed Google Scholar Ichimura Y, et al. Article CAS PubMed Google Scholar Tooze SA, Yoshimori T. Article CAS PubMed Google Scholar Militello RD, Colombo MI. Article CAS PubMed Google Scholar Cheong H, et al. Article CAS PubMed PubMed Central Google Scholar Suzuki K, et al. Article CAS PubMed Google Scholar Wijdeven RH, et al. Article CAS PubMed PubMed Central Google Scholar Kimura S, Noda T, Yoshimori T. Article CAS PubMed Google Scholar Matsuura A, et al. Article CAS PubMed Google Scholar Clark SL Jr. Article PubMed PubMed Central Google Scholar Novikoff AB. Article CAS PubMed PubMed Central Google Scholar Ohsumi Y. Article CAS PubMed Google Scholar Klionsky DJ, et al. Article CAS PubMed Google Scholar Kamada Y, et al. Article CAS PubMed PubMed Central Google Scholar Hara T, Mizushima N. Article CAS PubMed Google Scholar Tang Z, et al. Article CAS PubMed PubMed Central Google Scholar Velikkakath AK, et al. Article CAS PubMed PubMed Central Google Scholar Besteiro S, et al. Article CAS PubMed PubMed Central Google Scholar Metlagel Z, et al. Article CAS PubMed PubMed Central Google Scholar Radoshevich L, et al. Article CAS PubMed PubMed Central Google Scholar Li M, et al. Article CAS PubMed Google Scholar Lang T, et al. Article CAS PubMed PubMed Central Google Scholar Otomo C, et al. Article CAS PubMed Google Scholar Matsushita M, et al. Article CAS PubMed Google Scholar Cao Y, Klionsky DJ. Article CAS PubMed Google Scholar Kang R, et al. Article CAS PubMed PubMed Central Google Scholar Yuan W, Stromhaug PE, Dunn WA Jr. Article CAS PubMed PubMed Central Google Scholar Hong SB, et al. Article CAS PubMed Google Scholar Noda NN, et al. Article CAS PubMed Google Scholar He H, et al. Article CAS PubMed Google Scholar Rogov VV, et al. Article CAS PubMed PubMed Central Google Scholar Weidberg H, et al. Article CAS PubMed PubMed Central Google Scholar Reggiori F, et al. PubMed PubMed Central Google Scholar Mari M, et al. Article CAS PubMed PubMed Central Google Scholar Zhao Q, et al. Article PubMed PubMed Central CAS Google Scholar Hong SB, et al. Article CAS PubMed Google Scholar Yamaguchi M, et al. Article CAS PubMed Google Scholar Walczak M, Martens S. Article CAS PubMed PubMed Central Google Scholar Alers S, et al. Article PubMed CAS Google Scholar Ganley IG, et al. Article CAS PubMed PubMed Central Google Scholar Hosokawa N, et al. Article CAS PubMed PubMed Central Google Scholar Kim HJ, et al. Article CAS PubMed PubMed Central Google Scholar Ma B, et al. Article CAS PubMed PubMed Central Google Scholar Matsunaga K, et al. Article CAS PubMed Google Scholar Fujita N, et al. Article CAS PubMed PubMed Central Google Scholar Hwang S, et al. Article CAS PubMed PubMed Central Google Scholar Romanov J, et al. Article CAS PubMed PubMed Central Google Scholar Hara T, et al. Article CAS PubMed PubMed Central Google Scholar Proikas-Cezanne T, et al. Article CAS PubMed Google Scholar Graef M. Article CAS PubMed Google Scholar Suzuki H, et al. Article CAS PubMed Google Scholar Rubinsztein DC, Shpilka T, Elazar Z. Article CAS PubMed Google Scholar Ragusa MJ, Stanley RE, Hurley JH. Article CAS PubMed PubMed Central Google Scholar Stjepanovic G, et al. Article CAS PubMed PubMed Central Google Scholar Wong PM, et al. Article CAS PubMed PubMed Central Google Scholar Kamada Y, et al. Article CAS PubMed PubMed Central Google Scholar Kabeya Y, et al. Article CAS PubMed Google Scholar Yeh YY, et al. Article CAS PubMed PubMed Central Google Scholar Chew LH, et al. Article CAS PubMed Google Scholar Kabeya Y, et al. Article CAS PubMed Google Scholar Kihara A, et al. Article CAS PubMed PubMed Central Google Scholar Obara K, Sekito T, Ohsumi Y. Article CAS PubMed PubMed Central Google Scholar Obara K, Ohsumi Y. Article CAS PubMed Google Scholar Obara K, Ohsumi Y. Article PubMed PubMed Central CAS Google Scholar Burda P, et al. Article CAS PubMed Google Scholar Nagy P, et al. Article CAS PubMed PubMed Central Google Scholar Noda T, et al. Article CAS PubMed PubMed Central Google Scholar Fogel AI, et al. Article CAS PubMed PubMed Central Google Scholar Backer JM. Article CAS PubMed Google Scholar Araki Y, et al. Article CAS PubMed PubMed Central Google Scholar Aita VM, et al. Article CAS PubMed Google Scholar Liang XH, et al. Article CAS PubMed Google Scholar Furuya N, et al. Article CAS PubMed Google Scholar Itakura E, et al. Article CAS PubMed PubMed Central Google Scholar Liang XH, et al. Article CAS PubMed PubMed Central Google Scholar Feng W, et al. Article CAS PubMed Google Scholar Oberstein A, Jeffrey PD, Shi Y. Article CAS PubMed Google Scholar Li X, et al. Article PubMed CAS Google Scholar Huang W, et al. Article CAS PubMed PubMed Central Google Scholar Yamamoto H, et al. Article CAS PubMed PubMed Central Google Scholar Orsi A, et al. Article CAS PubMed PubMed Central Google Scholar Papinski D, et al. Article CAS PubMed PubMed Central Google Scholar Young AR, et al. Article CAS PubMed Google Scholar Yamada T, et al. Article CAS PubMed Google Scholar He C, et al. Article CAS PubMed PubMed Central Google Scholar Wang J, et al. Article CAS PubMed PubMed Central Google Scholar Feng Y, et al. Article CAS PubMed Google Scholar Jin M, et al. Article CAS PubMed PubMed Central Google Scholar Jia S, et al. Article CAS PubMed Google Scholar Shirahama-Noda K, et al. Article CAS PubMed Google Scholar Karanasios E, et al. Article CAS PubMed PubMed Central Google Scholar Obara K, et al. Article CAS PubMed PubMed Central Google Scholar Yang Z, Klionsky DJ. Article CAS PubMed Google Scholar Sun LL, et al. Article CAS PubMed PubMed Central Google Scholar Polson HE, et al. Article CAS PubMed Google Scholar Muller AJ, Proikas-Cezanne T. Article PubMed CAS Google Scholar Suzuki H, et al. Article CAS PubMed Google Scholar Tanida I, et al. Article CAS PubMed PubMed Central Google Scholar Shintani T, et al. Article CAS PubMed PubMed Central Google Scholar Kuma A, et al. Article CAS PubMed Google Scholar Mizushima N, Noda T, Ohsumi Y. Article CAS PubMed Google Scholar Kirisako T, et al. Article CAS PubMed PubMed Central Google Scholar Yamada Y, et al. Article CAS PubMed Google Scholar Huang WP, et al. Find articles by Rubinsztein, D. Published January 2, - More info. Most neurodegenerative diseases that afflict humans are associated with the intracytoplasmic deposition of aggregate-prone proteins in neurons. Autophagy is a powerful process for removing such proteins. In this Review, we consider how certain neurodegenerative diseases may be associated with impaired autophagy and how this may affect pathology. We also discuss how autophagy induction may be a plausible therapeutic strategy for some conditions and review studies in various models that support this hypothesis. Finally, we briefly describe some of the signaling pathways that may be amenable to therapeutic targeting for these goals. Intracellular aggregates comprising misfolded proteins are a common feature of many neurodegenerative diseases. These aggregates contain different proteins, depending on the disease, and can be seen in different cell types and in different subcellular compartments. In other cases the major protein species in the aggregates are not mutated. While these misfolded proteins may cause pathology via diverse mechanisms, in recent years there has been a focus on the role of autophagy in these diseases, both as a pathologic mechanism and as a therapeutic target. The term autophagy describes a range of processes, including chaperone-mediated autophagy, microautophagy, and macroautophagy. Here we focus on macroautophagy, which we refer to as autophagy. In this process, cytoplasmic proteins and organelles are sequestered into autophagosomes and delivered to the lysosomes for degradation. The processes by which autophagosomes form are described in greater detail elsewhere 1. Briefly, autophagosomes form from the coalescence of membrane from sources including the plasma membrane, mitochondria, ER, and Golgi apparatus. Once formed, autophagosomes are trafficked to fuse with the lysosomes, forming autolysosomes; alternatively, they may fuse with endosomes to form amphisomes before fusing with lysosomes, where their contents are ultimately degraded 1. In this Review we discuss the evidence that a disruption in autophagy might be a contributing factor in aggregate formation and the progression of neurodegenerative diseases. We detail the ever increasing list of neurodegenerative diseases in which autophagy perturbations have been reported and discuss a new class of diseases caused by mutations in core autophagy genes. We also discuss the ways in which macroautophagy may be upregulated to reduce levels of the toxic, aggregate-prone, intracytoplasmic proteins as a potential therapeutic strategy for these diseases. We highlight two major classes of autophagy-modulating drugs, which act either via mTOR inhibition or through mTOR-independent pathways, and outline recent studies investigating the effectiveness of these drugs in mouse models of neurodegenerative disease. The importance of autophagy for the brain was highlighted by studies demonstrating that neuron-specific loss of core autophagy proteins autophagy-related gene 7 [ATG7] and ATG5 in mice results in a neurodegenerative phenotype in the absence of any other contributing factors 2 , 3. In particular, autophagy is required for maintenance of axonal homeostasis, and loss of autophagy results in axonal dystrophy 4. Autophagy is also a key regulator of the levels of intracytoplasmic, aggregate-prone proteins that cause neurodegenerative diseases, including polyglutamine-expanded huntingtin HD 5 , mutant α-synuclein forms of PD 6 , mutant TDP ALS 7 , and wild-type and mutant tau various dementias 8. The clearance of such substrates is retarded when autophagy is compromised, and clearance is induced when autophagy is stimulated. Autophagic dysfunction has now been reported in a number of neurodegenerative diseases, which are outlined below and summarized in Figure 1. Intersections of the autophagic pathway and neurodegenerative diseases. This schematic shows the progression through the autophagic pathway from formation of the autophagosome to fusion with the lysosome. Red text highlights points of compromise in the pathway that have been demonstrated in neurodegenerative disease, along with examples of causes of this compromise. One of the first observations that suggested a role for altered autophagy in AD was the accumulation of autophagic vesicles in affected neurons 9 , While initially considered to represent increased autophagy, more recent evidence indicates that this accumulation is due to impaired autophagosome clearance. PS1 and PS2 mutations cause familial autosomal-dominant AD 12 — 14 and result in amyloid deposition, neuronal loss, and lysosome pathology Loss of lysosome acidification, and therefore lysosome function, results in autophagosome accumulation, as autophagosomes do not fuse with dysfunctional lysosomes. Rescue of lysosomal defects can restore autophagic activity. For example cAMP treatment decreased lysosomal pH in patient fibroblasts Further, deletion of cystatin B an inhibitor of lysosomal cysteine proteases in an AD mouse model enhanced defective lysosomal turnover, promoted Aβ clearance, and improved mouse cognitive performance The autophagy gene BECN1 , encoding beclin 1, has reduced mRNA levels in AD brain tissue 18 , Caspase-3, which may be activated in AD neurons, can also cleave beclin 1, resulting in impaired autophagosome formation When crossed with beclin 1 haploinsufficient mice, mice overexpressing human APP exhibited autophagy disruption and enhanced pathology Because loss of beclin 1 activity reduces autophagosome formation, AD may be associated with defects in both autophagosome biogenesis and autophagosome degradation as a consequence of the lysosomal defects described above. Beclin 1 disruption may also contribute to AD in non-neuronal cells. Microglia from AD patients have reduced beclin 1 and retromer a key component of the endosomal, protein-sorting machinery levels, with the beclin 1 disruption further altering correct retromer localization to phagosome membranes and potential receptor-mediated phagocytosis Tau and Aβ, the two key proteins that aggregate in AD, are autophagy substrates. Induction of autophagy decreases tau levels 22 , In contrast, loss of autophagy by conditional knockout of the autophagy protein ATG7 in the forebrains of mice results in phospho-tau accumulation in a pattern similar to a pre-tangle state While deleting tau does not prevent inclusion formation, it does rescue neurodegeneration in these mice Autophagy has been suggested to play a major role in the metabolism of Aβ 26 , 27 ; however, it may also be important in the formation of Aβ. The autophagic vesicles that accumulate in AD neurons have been shown to be positive for both APP and PS1 10 , Furthermore, autophagy has been implicated in Aβ secretion, as crossing APP transgenic mice with mice lacking Atg7 in forebrain neurons results in less Aβ extracellular secretion and plaque formation Loss of autophagy may therefore result in an increase in intracellular Aβ due to both a decrease in clearance and a decrease in secretion of the protein. The role of autophagy in AD is therefore complex and has been controversial; this may be a function of different effects on autophagy at different stages of the disease as well as the possibility that autophagy may affect different steps of the amyloid life cycle. PD is characterized by the accumulation of α-synuclein, whose levels appear central to pathogenesis, as gene duplications of α-synuclein are sufficient to cause PD Increased α-synuclein levels in cell culture, Drosophila, and mice inhibit autophagy 31 by mislocalizing ATG9, a transmembrane protein with a key function in autophagosome formation. Similar autophagy defects, including ATG9 mislocalization, have recently been observed in cells expressing the VPS35 DN mutation, which causes autosomal-dominant forms of PD The most common form of autosomal-dominant PD results from mutations in the LRRK2 gene reviewed in ref. It has been suggested that LRRK2 negatively regulates autophagy, as autophagy is increased following LRRK2 siRNA knockdown or inhibition 34 , In contrast, overexpression of both wild-type LRRK2 and LRRK2 GS , but not catalytically inactive forms of the protein, induces an AMPK-mediated upregulation of autophagy Perhaps the most prominent link between autophagy and familial forms of PD comes from studies of the roles of PTEN-induced putative kinase 1 PINK1, also known as PARK6 and Parkin RBR E3 ubiquitin protein ligase PARK2, also known as Parkin in mitophagy reviewed in ref. Loss-of-function mutations in Parkin and PINK1 cause autosomal-recessive and sporadic juvenile-onset PD 40 — These proteins regulate mitophagy, a process whereby damaged mitochondria are degraded. PINK1 associates with damaged mitochondria when membrane potential is lost 43 , 44 , followed by activation of the E3 ubiquitin ligase, Parkin, which ultimately mediates mitophagy. Despite many studies in cell culture with overexpression of these proteins showing effects on mitochondrial clearance, the consequences of loss of Parkin for mitophagy in vivo have been questioned In addition to a role in mitophagy, Parkin has also been suggested to be involved in autophagic clearance of α-synuclein, as Parkin overexpression in a rat model system promoted autophagic clearance of toxic proteins including α-synuclein An autosomal-recessive form of Parkinsonism, Kufor—Rakeb syndrome, is caused by mutations in ATPase type 13A2 ATP13A2, also known as PARK9 , which encodes a lysosomal ATPase ATP13A2 regulates lysosomal acidification, which is necessary for autophagosome-lysosome fusion and subsequent substrate degradation, and cells lacking ATP13A2 or patient fibroblasts with ATP13A2 mutations displayed defects in these processes ALS is the most common form of motor neuron disease One of these, sequestosome 1 SQSTM1 , encodes the scaffold protein p62; both point mutations and deletions have been identified in ALS cases 50 , p62 acts as an adaptor to bind ubiquitinated targets to autophagosome-associated lipidated microtubule—associated light chain 3 LC3-II for engulfment by autophagosomes While this suggests a clear link between autophagy and ALS, p62 mutations are located throughout the protein, across multiple domains 51 , and their effect on autophagy has yet to be fully established. This is also true for ALS-causing mutations in another adaptor, optineurin Optineurin has established roles in xenophagy, a form of autophagy that degrades foreign pathogens Optineurin can also bind to protein aggregates 56 and is sequestered in inclusions in ALS patients as well as in other neurodegenerative diseases 57 , The most common known mutation in ALS is an intronic hexanucleotide repeat expansion in the gene C9ORF Very little is known about the function of the C9ORF72 protein, and it is currently unclear whether the disease resulting from this mutation is due to a loss of function, gain of function, or both. siRNA knockdown of C9ORF72 increased LC3-II levels, although the study did not fully characterize the meaning of this in terms of autophagy function Yet another ALS-causing gene with a clear function in autophagy is dynactin 1 DCTN1 Autophagosomes are formed throughout neurons, and their efficient movement to lysosome-rich areas for fusion and cargo degradation is dependent on dynein-mediated transport 61 , A reduction in this transport can result in impaired autophagosome-lysosome fusion, as shown by autophagosome accumulation with enhanced toxicity in cell, Drosophila , and mouse models 63 , More recently, mutations in DCTN1 have been shown to cause Perry syndrome, a neurodegenerative disease that can present with Parkinsonism and psychiatric changes 65 , suggesting a role for autophagic dysfunction in this disease. HD is caused by polyglutamine repeat expansions in the huntingtin htt protein A role for autophagy in HD was first suggested by observations of increased levels of autophagic markers in the brains or tissues of HD patients and in mouse models of the disease 67 — In HD mouse models, the key autophagic regulator, mTOR, is sequestered into htt aggregates, resulting in an inhibition of signaling, consistent with autophagy induction While this would appear to suggest an upregulation of autophagy in HD, the situation may in fact be more complicated. The positive autophagy regulator, beclin 1, has also been shown to be sequestered into htt aggregates 71 , which would negatively affect autophagosome formation. Moreover, the accumulated autophagosomes observed may not be fully functional; they have been shown to be relatively empty due to inefficient sequestration of cargo, in particular organelles such as mitochondria and lipid droplets, and the increase in autophagosome number is not associated with an increase in protein degradation In addition, htt itself may control autophagy through a variety of different mechanisms. Two htt-interacting proteins, Rab5 and Rhes, are positive regulators of autophagy 73 , 74 , and htt lacking the polyglutamine repeat region induces autophagy and is protective against toxicity of mutant polyglutamine—expanded htt in cells and mice 75 , suggesting a more direct role for htt in autophagy. While it is not yet clear whether alterations in autophagy are primary factors in the pathogenesis of HD, polymorphisms in the core autophagy gene, ATG7 , have been suggested to be associated with earlier age of onset Hereditary spastic paraplegia. Hereditary spastic paraplegias HSPs include a broad group of neurodegenerative diseases involving degeneration associated with the lower extremities. Two forms of HSP have been associated with mutations in genes with a role in autophagy. The first identified was TECPR2 , a positive autophagy regulator that interacts with LC3 77 , The exact function of this protein is unknown; however, it bears homology to TECPR1, which has been implicated in sequestration of bacteria into autophagosomes, suggesting a role as an autophagy adaptor Type 15 HSP is caused by mutations in ZFYVE26 79 encoding spastizin also known as FYVE-CENT , which interacts with the core autophagy protein, beclin 1. Disease-associated mutations in spastizin compromise this interaction, leading to impaired autophagy Lafora disease. Lafora disease is an autosomal recessive myoclonous epilepsy resulting in early death due to neurodegeneration. Its pathologic hallmark is the accumulation of Lafora bodies LBs , comprising abnormally hyperphosphorylated polyglucosan with a smaller polyubiquitinated protein component. Most mutations in Lafora disease occur in two proteins, laforin and malin, which form a complex that, when disrupted, drives the disease reviewed in ref. LB accumulation may be partly the result of altered carbohydrate metabolism However, data from patient and animal models clearly support a role for disrupted autophagy in disease onset and progression. Overexpression of laforin induces autophagy, while a reduction in laforin levels has the opposite effect Both laforin- and malin-deficient mice show an early defect in autophagosome biogenesis, which may lead to LB formation 83 — Both mouse models show reduced levels of autophagosomes and a reduction in total and autophagy-dependent protein degradation. Neurodegenerative disorders associated with mutations in core autophagy genes. The likelihood that autophagy defects contribute to neurodegeneration is supported by recent studies that reveal a class of diseases caused by mutations in core autophagy genes. Neurodegeneration with brain iron accumulation 5 NBIA5; OMIM identifier is a neurodegenerative disease that presents with movement disorder and intellectual decline. It is currently described as β-propeller protein—associated neurodegeneration BPAN or static encephalopathy of childhood with neurodegeneration in adulthood SENDA |
| Autophagy and autophagy-related proteins in cancer | Article CAS Google Scholar He Q, Koprich JB, Wang Y, Yu WB, Xiao BG, Brotchie JM, et al. Spontaneous recovery from hyperglycemia by regeneration of pancreatic beta-cells in Kir6. These novel approaches will provide outstanding tools for the development of next-generation modulators of autophagy or lysosomal degradation. While deleting tau does not prevent inclusion formation, it does rescue neurodegeneration in these mice Lipotoxicity was determined by measuring lactate dehydrogenase release from damaged cells using a kit Promega. Article Google Scholar Rivera, J. Li, H. |
| JCI - Autophagy and neurodegeneration | Diabetologia 49 , — Anding AL, Baehrecke EH. PLoS One 8:e At such concentrations, proportions of cells with TFEB nuclear translocation would be small see Supplementary Fig. In this light it is worth mentioning that genistein 5, 7-dihydroxy 4-hydroxyphenyl -4 H benzopyranone , a natural isoflavone, has been demonstrated recently to decrease levels of mutant huntingtin and to reduce number and size of aggregates of this toxic protein in the cellular model of HD by autophagy stimulation Pierzynowska et al. |
Ich tue Abbitte, dass ich mit nichts helfen kann. Ich hoffe, Ihnen hier werden andere helfen.
Diese einfach bemerkenswerte Mitteilung
Sagen Sie vor, wen kann ich fragen?