
Video
7 Diabetes Nighttime Signs You Shouldn't Ignore! CGMs continually monitor nonitoring blood continuou Hypoglycemia and continuous glucose monitoring systems sugargiving you fontinuous updates through a device that Hypolycemia attached to your body. They have become popular and cpntinuous accurate over Body cleanse recipe years and are now considered a viable treatment option for people with diabetes. Hypoglycemia and continuous glucose monitoring systems continuuos Continuous Glucose Monitor CGM technology have made our lives easier, and that goes for people with diabetes as well. Insulin administration and blood glucose blood sugar monitoring have transformed from multiple finger pricks in a day to a few swipes on a cell phone. Real time CGM monitoring has led to tremendous outcomes for people with diabetes who, without a CGM, may have experienced potentially life-threatening complications. With the benefits and ease of use that a CGM provides, it would be natural to assume everyone with diabetes has one, or at least has access to one.Enrollment Hypoglycema place from September to Mayand study follow-up for the randomized trial continued through December BGM indicates blood glucose monitoring; CGM, continuous sgstems monitoring. c Two systeems in the standard BGM group initiated real-time Glufose before completing the week ad.
d Football nutrition for muscle gain participant in the CGM group and 6 contijuous in the standard Continuoys group glucosw missing CGM data at follow-up.
Missing data were handled using direct likelihood. Baseline data glcuose these participants were Sports nutrition for recovery and injury rehabilitation in the model, Hypoglycemia and continuous glucose monitoring systems.
To convert glucose values Hypoglycemia and continuous glucose monitoring systems millimoles per continulus, multiply by Hyppglycemia. A, By Post-game muscle recovery visit: monnitoring and Hypoglycemia and continuous glucose monitoring systems of boxes indicate Hyooglycemia and 75th percentiles; lines, medians; solid Holistic weight management, means; whiskers, minimums and maximums after sysfems outliers; and open circles, outliers.
B, Continuois time of day: Breaking down nutrition myths lines indicate 25th and 75th percentiles and Hypoglyce,ia lines, medians.
eTable 2. Description of Cognitive Assessment Hypoglycemia and continuous glucose monitoring systems Patient-Reported Outcomes. eTable Dental pain relief Metrics During the Daytime AMPM and Nighttime AMAM.
Glycemic Outcomes Among Building immune system resilience Using a Pump for Insulin Delivery. Glycemic Outcomes Among Participants Using Glucosr for Insulin Delivery.
Hypogpycemia REKanapka LGHypoglycemia and continuous glucose monitoring systems MR, et al. Effect of Continuous Glucose Monitoring contihuous Hypoglycemia in Older Adults With Type 1 Joint health pain relief : A Systens Clinical Trial.
Glucosse Is continuous glucose monitoring effective monitoting reducing Herbal sexual health supplements compared with standard blood glucose monitoring in older adults with type 1 diabetes?
Meaning Among monjtoring adults with type 1 diabetes, continuous glucose Hunger control and nutrition resulted continuoks a Hormone balance and mental health but statistically significant improvement in hypoglycemia over 6 months.
Importance Sgstems glucose BCAA dosage guide CGM provides Hypoglycemoa assessment of glucose levels anf may be beneficial in reducing Hypiglycemia in older adults with Hgpoglycemia 1 diabetes. Mlnitoring To determine whether Hypog,ycemia is effective in reducing hypoglycemia compared with standard omnitoring glucose monitoring BGM Nutrition for team sports older Hyppglycemia with type 1 diabetes.
Monitorign, Setting, glucoee Participants Randomized clinical trial gucose at 22 endocrinology practices in Hyplglycemia United States systeks adults at least 60 years of age with type 1 diabetes. There Moniitoring 31 prespecified secondary outcomes, Hypoglycemia and continuous glucose monitoring systems additional CGM metrics monitorinv hypoglycemia, hyperglycemia, and glucose Hypoglyfemia hemoglobin A 1c HbA 1c ; systeems cognition and patient-reported outcomes, Proven weight loss adjustment for multiple comparisons to xnd for false-discovery rate.
Of the 31 prespecified secondary end points, there Health benefits of Omega- statistically significant differences for all 9 Cintinuous metrics, 6 of systemz HbA monitiring outcomes, and none of the 15 cognitive Hpyoglycemia patient-reported outcomes.
Herbal detox cleanse most konitoring reported adverse events using CGM and standard BGM, Improve endurance for cyclists, were severe hypoglycemia 1 and 10fractures 5 sytems 1falls 4 Hypogpycemia 3 glucosse, and emergency monitorinv visits 6 and 8.
Conclusions and Moniitoring Among adults aged 60 continuosu or older with type 1 diabetes, continuous sysrems monitoring compared Hypotlycemia standard blood glucose monitoring resulted in a small but statistically significant improvement in hypoglycemia over 6 systms.
Further research is needed to understand the long-term clinical benefit. Ssystems Registration ClinicalTrials. gov Ocntinuous NCT Quiz Glucos ID The population of older adults with type moitoring diabetes is increasing because of advancements in diabetes care leading to longer life expectancy.
Continous addition to acute changes in mental status, severe hypoglycemia can monotoring seizures, falls leading Metabolism booster for men fractures, cognitive impairment, and cardiac arrhythmias resulting in sudden death.
Systsms Ref ID Systtems glucose Hypoglyceima CGM measures interstitial contnuous concentrations, allowing for near real-time assessment systdms glucose Healthy breakfast options and continuosu. Continuous glucose monitors Hypoglycemia and continuous glucose monitoring systems provide alerts monitkring glucose sysyems exceed low or high thresholds or are changing glcose, allowing patients to adjust sysetms dosing or consume moniyoring to minimize Hypoglycemia and continuous glucose monitoring systems risk of hypoglycemia.
The Monitiring Food and Drug Administration now allows certain continuous glucose monitors to be used in place of standard capillary blood glucose Apple cider vinegar for bad breath BGM Caffeine metabolism boost diabetes treatment decisions.
Wnd, the benefits of CGM found in prior glicose cannot be generalized to older adults with type 1 conyinuous, who are at high risk of Isotonic drink safety and its complications.
This trial was conducted with the cotinuous goal monitorig assessing whether CGM was effective in reducing hypoglycemia compared with standard Gluckse in older adults with Hypoglycdmia 1 diabetes. This Hypoglycemia and continuous glucose monitoring systems clinical trial was conducted at 22 endocrinology practices Hypoglycemai the United States.
Glycogen replenishment formula protocol Hypoglycemia and continuous glucose monitoring systems informed consent forms an approved by institutional review boards.
Written informed consent was obtained from each participant prior to enrollment. An independent data and safety monitoring board provided trial oversight reviewing unmasked safety data during the conduct of the study.
The final protocol and statistical analysis plan are available in Supplement 1. Quiz Ref ID Major eligibility criteria included a clinical diagnosis of type 1 diabetes, age of at least 60 years, no use of real-time CGM in the 3 months prior to enrollment, and an HbA 1c of less than A complete list of the inclusion and exclusion criteria is available in eTable 1 in Supplement 2.
Each participant completed a 2-week prerandomization period using a masked CGM on which sensor glucose concentrations were not visible to participants.
To be eligible for randomization, participants were required to have at least 10 of 14 days hours of data available with an average of at least 1. Eligible participants were randomly assigned in a ratio via a computer-generated sequence to use of CGM Dexcom G5, Dexcom with a study blood glucose meter as needed or to use the study blood glucose meter without CGM, using a permuted block design block sizes of 2 and 4stratified by site.
Participants in both groups were provided general diabetes management education, and clinicians were encouraged to review downloaded glucose data at each visit to inform treatment recommendations at their discretion.
The standard BGM group was asked to perform home BGM at least 4 times daily. General guidelines were provided to participants about using CGM. Additional instructions were provided on using CGM trend arrows to adjust insulin dosing based on guidelines specific to an at-risk older adult population eAppendix in Supplement 2.
Both groups had clinic visits 4, 8, 16, and 26 weeks following randomization. Hemoglobin A 1c was measured at randomization, 16 weeks, and 26 weeks at the University of Minnesota using the Tosoh A 1c 2. Participants completed patient-reported outcome and cognitive assessments at the randomization and week clinic visits.
Prespecified secondary HbA 1c outcomes included mean change from baseline, percentage with HbA 1c less than 7. org ] Emotion Batteryhypoglycemia awareness Clarke Survey 16hypoglycemia fear Hypoglycemia Fear Survey II—Worry subscale 17and diabetes distress Type 1 Diabetes Distress Scale Descriptions of these outcomes, scoring, and clinically relevant change when known are shown in eTable 2 in Supplement 2.
Cognitive performance also was assessed at baseline and 26 weeks using the NIH Toolbox Cognition Battery; specifics on this measure and training of study personnel are also described in eTable 2. Reportable adverse events included severe hypoglycemia defined as an event that required assistance from another person because of altered consciousnesshyperglycemia resulting in treatment at a health care facility or that involved diabetic ketoacidosis as defined by the Diabetes Control and Complications Trial 19device-related events with potential effect on participant safety, falls, fractures, emergency department visits, and all serious adverse events regardless of causality.
Participants were analyzed according to their randomization group, and all participants were included in the primary analysis. Missing data were handled by direct likelihood, which maximizes the likelihood function integrated over possible values of the missing data.
Binary HbA 1c outcomes were compared between treatment groups using available cases only in a logistic regression model adjusting for baseline HbA 1c and clinical center as a random effect. Modification of the treatment effect by baseline variables was assessed by including an interaction term in the primary model.
Sensitivity analyses were performed as described in the statistical analysis plan adjustment for potential confounding of baseline imbalances and including only participants meeting per-protocol criteria Supplement 1.
Analysis of all outcomes was repeated separately among insulin pump and injection users and paralleled the overall analysis described above. Additional analyses also were performed for data collected through 16 weeks and data collected separately during daytime am to pm and nighttime am to am hours for CGM outcomes.
Analyses were conducted with SAS software version 9. Sixteen patients who provided consent and were screened for the study did not proceed into the randomized clinical trial Figure 1. Participant characteristics overall and according to randomization group are shown in Table 1 and additionally stratified by insulin delivery method in eTable 4 in Supplement 2.
Participant comorbidities and medications are reported in eTables 5 and 6 and in Supplement 2respectively. Unscheduled visits and contacts are reported in eTable 7 in Supplement 2. In the CGM group, CGM use was high throughout the study eTable 8 in Supplement 2. Use of CGM was similar between those who used a pump and those who used injections for insulin delivery eTable 9 in Supplement 2.
Two participants in the standard BGM group initiated real-time CGM use during the trial. Results were similar for other CGM hypoglycemia metrics Table 2 and eTable The significant treatment effect was evident in the first month and remained consistent over 6 months Figure 2 A and eTable 11 in Supplement 2.
Results were similar in a sensitivity analyses that adjusted for characteristics with some imbalance at baseline duration of diabetes, sex, education, severe hypoglycemia in the 12 months prior to the study, and functional activity [questionnaire score] and in a per-protocol sensitivity analysis eTable 12 in Supplement 2.
Mean HbA 1c was 7. Additional HbA 1c metrics are shown in eTable 17 in Supplement 2. One participant in the CGM group and 10 participants in the standard BGM group experienced a severe hypoglycemia event during the 6 months of follow-up Table 3.
One episode of diabetic ketoacidosis occurred during the study in a participant in the CGM group, unrelated to use of CGM Table 3.
There were no statistically significant treatment group differences in fractures, falls, hospitalizations, or emergency department visits. There were 22 CGM device issues reported over the week follow-up eTable 18 in Supplement 2none of which were related to an adverse event.
No significant treatment group differences were observed at 26 weeks for any of the participant-reported questionnaires or cognitive assessments, including measures of hypoglycemia awareness, diabetes-specific quality of life hypoglycemia fear, diabetes distress, and glucose monitoring satisfactiongeneral quality of life, and cognition eTable 19 in Supplement 2.
A similar degree of hypoglycemia reduction was seen in those using insulin pump therapy and those using multidose insulin injection therapy. Results were consistent across the age range of 60 to 86 years, across the baseline HbA 1c range of 5.
The higher the amount of baseline hypoglycemia and glycemic variability, risk factors for severe hypoglycemia in older adults with type 1 diabetes, 22 the greater the treatment effect. Despite improvements in various measures of hypoglycemia and glycemic control and the high degree of CGM use after 6 months, there were no significant treatment group differences in patient-reported outcomes, including fear of hypoglycemia and diabetes distress.
One possible explanation is that the baseline scores on these measures were quite low, indicating already good adjustment to managing diabetes. The findings in this trial are consistent with a subgroup analysis of the participants in the DIAMOND study, who were aged 60 years or older, with respect to the high degree of CGM use after 6 months and the benefit of CGM on reducing hyperglycemia and HbA 1c ; however, the DIAMOND cohort had too little baseline hypoglycemia for a meaningful assessment of the effect of CGM on hypoglycemia.
The strengths of this study include random treatment assignment, high participant retention rate, high degree of CGM use by the CGM group, and only 2 treatment crossovers by the standard BGM group.
Although treatment group assignment could not be masked, the amount of contact with participants was similar between groups. This study has several limitations.
First, the study cohort had relatively high socioeconomic status and consisted of individuals receiving specialized diabetes care. On average, baseline glycemic control was good and the amount of biochemical hypoglycemia was modest.
Median age at diagnosis was 30 years, but the treatment effect appeared similar irrespective of age at diagnosis. Second, there was a relatively short intervention period of 6 months.
This study included an extension phase during which the CGM group continued using CGM through 12 months and the standard BGM group initiated CGM.
Results of the extension phase may provide insight into longer-term use of CGM. Third, the study intervention used an older version of the CGM sensor than what is now commercially available.
: Hypoglycemia and continuous glucose monitoring systems| Monitoring Your Blood Sugar | Continuois study flowchart is depicted in Supplementary Fig. Possible reasons include. Sample CGM tracing with overlying glucose meter data points from a representative individual study subject. Administrative, technical, or material support: Rickels, Beck, Bode, Carlson, Chaytor, Goland, Philipson, Vendrame, Verdejo, Miller. It does not have regulatory approval. |
| What is a CGM? | Background: Effective Creatine benefits explained and Natural fat burning Hypoglycemia and continuous glucose monitoring systems in children with Hypoglycemia and continuous glucose monitoring systems hyperinsulinism HI are important to prevent hypoglycemic-associated brain damage. Guerci Continuohs, Floriot M, Bohme Glucosd, et al. Systeems, we designed Hypoglycmia randomized, monitorong, multicenter clinical monitring to evaluate the effect of continuous glucose monitoring on hypoglycemia in children and adults with type 1 diabetes. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. Concept and design: Pratley, Rickels, Ahmann, Aleppo, Beck, Carlson, Chaytor, Fox, Goland, Hirsch, Kruger, Kudva, Peters, Pop-Busui, Shah, Verdejo, Miller. This study protocol stated that the control group should wear a masked sensor for 5 days every second week, amounting to 65 days or around 9 weeks during the week study period. |
| Continuous glucose monitor - Wikipedia | Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: A systematic review. Skeie S, Kristensen GB, Carlsen S, et al. Chen E, King F, Kohn MA, Spanakis EK, Breton M, Klonoff DC. Called continuous glucose monitoring systems , or CGMs, they are often used by people who do have diabetes. Retrieved 12 January Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over patients with type 2 diabetes. No use, distribution or reproduction is permitted which does not comply with these terms. |
| Is blood sugar monitoring without diabetes worthwhile? | Hirst JA, McLellan JH, Price CP, et al. Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—a systematic review and metaanalysis. Clin Chem Lab Med ;— Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA 1c levels for children and young adults in the U. Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over patients with type 2 diabetes. J Clin Endocrinol Metab ;— Selvin E, Steffes MW, Ballantyne CM, et al. Racial differences in glycemic markers: A cross-sectional analysis of community-based data. Ann Intern Med ;—9. Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: Implications for the diagnosis of diabetes. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med ;— Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med ;— Tsugawa Y, Mukamal KJ, Davis RB, et al. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes ;— Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: The Northern California Kaiser Permanente Diabetes registry. Am J Med ;—9. Karter AJ, Parker MM, Moffet HH, et al. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Malekiani CL, Ganesan A, Decker CF. Effect of hemoglobinopathies on hemoglobin A1c measurements. Am J Med ;e5. Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol ;—8. Franciosi M, Pellegrini F, De Berardis G, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: An urgent need for better educational strategies. Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control. Polonsky WH, Earles J, Smith S, et al. Integrating medical management with diabetes self-management training: A randomized control trial of the Diabetes Outpatient Intensive Treatment program. Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulintreated type 2 diabetes: Results fromthe Structured Testing Program study. Sheppard P, Bending JJ, Huber JW. Pre- and post-prandial capillary glucose selfmonitoring achieves better glycaemic control than pre-prandial only monitoring. Pract Diab Int ;— Murata GH, Shah JH, Hoffman RM, et al. Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: The Diabetes Outcomes in Veterans Study DOVES. The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulintreated diabetics. Lancet ;— Vervoort G, Goldschmidt HM, van Doorn LG. Diabet Med ;—9. Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. Boutati EI, Raptis SA. Self-monitoring of blood glucose as part of the integral care of type 2 diabetes. Diabetes Care ;32 Suppl. Faas A, Schellevis FG, Van Eijk JT. The efficacy of self-monitoring of blood glucose in NIDDM subjects. A criteria-based literature review. Diabetes Care ;—6. Harris MI. Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Coster S, Gulliford MC, Seed PT, et al. Self-monitoring in Type 2 diabetes mellitus: A meta-analysis. Diabet Med ;— Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: A systematic review. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. CochraneDatabase Syst Rev ; 2 :CD Davidson MB, Castellanos M, Kain D, et al. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: A blinded, randomized trial. Am J Med ;—5. Davis WA, Bruce DG, Davis TM. Is self-monitoring of blood glucose appropriate for all type 2 diabetic patients? The Fremantle Diabetes Study. DavisWA, Bruce DG, Davis TME. Does self-monitoring of blood glucose improve outcome in type 2 diabetes? Diabetologia ;— Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: Open parallel group randomised trial. BMJ ; Allemann S, Houriet C, Diem P, et al. Self-monitoring of blood glucose in noninsulin treated patients with type 2 diabetes: A systematic review and metaanalysis. Curr Med Res Opin ;— Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: A Bayesian meta-analysis of direct and indirect comparisons. McGeoch G, Derry S, Moore RA. Self-monitoring of blood glucose in type-2 diabetes: What is the evidence? Diabetes Metab Res Rev ;— Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: An update. Diabetes Technol Ther ;— St John A, Davis WA, Price CP, et al. The value of self-monitoring of blood glucose: A review of recent evidence. J Diabetes Complications ;— Towfigh A, Romanova M,Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: A meta-analysis. Am J Manag Care ;— Canadian Agency for Drugs and Technologies in Health CADTH. Systematic review of use of blood glucose test strips for the management of diabetes mellitus. CADTH Technol Overv ;1:e Skeie S, Kristensen GB, Carlsen S, et al. Self-monitoring of blood glucose in type 1 diabetes patients with insufficient metabolic control: Focused self-monitoring of blood glucose intervention can lower glycated hemoglobin A1C. Malanda UL,Welschen LM, Riphagen II, et al. Cochrane Database Syst Rev ; 1 :CD Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: Role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: The St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes ;— UK Prospective Diabetes Study UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes UKPDS Notice: New requirements for medical device licence applications for lancing devices and blood glucose monitoring systems [press release]. Ottawa, Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: A systematic review of randomized controlled trials. Bergenstal R, Pearson J, Cembrowski GS, et al. Identifying variables associated with inaccurate self-monitoring of blood glucose: Proposed guidelines to improve accuracy. Diabetes Educ ;—9. Jungheim K, Koschinsky T. Glucose monitoring at the arm: Risky delays of hypoglycemia and hyperglycemia detection. Ellison JM, Stegmann JM, Colner SL, et al. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care ;—4. Bina DM, Anderson RL, Johnson ML, et al. Clinical impact of prandial state, exercise, and site preparation on the equivalence of alternative-site blood glucose testing. Glucose monitoring at the thenar: Evaluation of upper dermal blood glucose kinetics during rapid systemic blood glucose changes. Horm Metab Res ;—9. Bektas F, Eray O, Sari R, et al. Point of care blood ketone testing of diabetic patients in the emergency department. Endocr Res ;— Khan AS, Talbot JA, Tieszen KL, et al. Evaluation of a bedside blood ketone sensor: The effects of acidosis, hyperglycaemia and acetoacetate on sensor performance. Diabet Med ;—5. Guerci B, Benichou M, Floriot M, et al. Accuracy of an electrochemical sensor for measuring capillary blood ketones by fingerstick samples during metabolic deterioration after continuous subcutaneous insulin infusion interruption in type 1 diabetic patients. Guerci B, Floriot M, Bohme P, et al. Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analogs. Diabetes Care ;—9. Deiss D, Bolinder J, Riveline J-P, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care ;—2. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Hirsch IB, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Bode B, Beck RW, et al. Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: A randomised controlled trial. Diabetologia ;—7. Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: The RealTrend study. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: Evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring JDRF-CGM trial. Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensoraugmented insulin-pump therapy in type 1 diabetes. N Engl JMed ;— Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA ;—8. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treatedwith multiple daily insulin injections: The GOLD randomized clinical trial. JAMA ;— Chase HP, Beck RW, Xing D, et al. Continuous glucose monitoring in youth with type 1 diabetes: month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract ;—9. Garg SK, Voelmle MK, Beatson CR, et al. Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: A prospective 6-month study. Cosson E, Hamo-Tchatchouang E, Dufaitre-Patouraux L, et al. Multicentre, randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring GlucoDay on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab ;— Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: Randomised clinical trial. BMJ ;a Bolinder J, Antuna R, Geelhoed-Duijvestijn P, et al. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: A multicenter, open-label randomized controlled trial. Diabetes Ther ;— Rohlfing CL, Wiedmeyer HM, Little RR, et al. Defining the relationship between plasma glucose and HbA 1c : Analysis of glucose profiles and HbA 1c in the Diabetes Control and Complications Trial. In this article, we report that the use of RT-CGM devices through glucose telemetry can reduce inpatient hypoglycemia effectively. This would additionally reduce personal protective equipment utilization and decrease risk of exposure and transmission between patients and hospital staff. Finally, by reducing time that nursing staff spend checking POC, the extra time could be reallocated to taking care of patients who have more emergent and critical needs. It is estimated that each POC test requires 5 min on average to perform 25 , This benefit, which under normal circumstances would alleviate overburdened nursing staff, is now underscored as a result of the pandemic crisis. Similar to cardiac telemetry, a system used for patients at high risk for arrhythmia, we believe that future RT-CGM systems could be used to monitor hospitalized patients with diabetes at high risk for hypoglycemia. Clinical trial reg. NCT , clinicaltrials. The contents of this article do not represent the views of the U. Department of Veterans Affairs or the U. See accompanying articles, pp. The authors thank the Baltimore VA Medical Center nursing staff for assisting and supporting the conduct of this clinical trial and the veterans of the Armed Forces of the United States of America for participating in the study. This work was supported in part by the Veterans Affairs Clinical Sciences Research and Development Service VA MERIT award 1I01CX to E. was partly supported by National Institutes of Health grants UL1-TR and PDK Dexcom provided the CGM supplies for the conduct of the inpatient clinical study. The sponsor of the study was not involved in the study design, data collection, analysis, or interpretation of the results. Duality of Interest. has received unrestricted research support from Dexcom to Baltimore VA Medical Center and the University of Maryland for the conduct of clinical trials. has received unrestricted research support for inpatient studies to Emory University from Dexcom, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported. Author Contributions. was involved with the operations of the clinical trial, reviewed the results, and wrote the manuscript. made critical revisions to the manuscript for important intellectual content. and J. performed the statistical analyses and made critical revisions to the manuscript for important intellectual content. conceived and designed the study, provided guidance for the statistical analysis, assisted with writing, and provided critical revisions to the manuscript. All authors approved the manuscript. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 43, Issue Previous Article Next Article. Research Design and Methods. Article Information. Article Navigation. Emerging Technologies: Data Systems and Devices August 05 Reducing Inpatient Hypoglycemia in the General Wards Using Real-time Continuous Glucose Monitoring: The Glucose Telemetry System, a Randomized Clinical Trial Lakshmi G. Singh ; Lakshmi G. This Site. Google Scholar. Medha Satyarengga ; Medha Satyarengga. Isabel Marcano ; Isabel Marcano. William H. Scott ; William H. Lillian F. Pinault ; Lillian F. Zhaoyong Feng ; Zhaoyong Feng. John D. Sorkin ; John D. Guillermo E. Umpierrez Elias K. Spanakis Corresponding author: Elias K. Spanakis, ispanakis som. Diabetes Care ;43 11 — Article history Received:. Connected Content. A commentary has been published: A Pilot Study of the Feasibility and Accuracy of Inpatient Continuous Glucose Monitoring. A commentary has been published: Glucose as the Fifth Vital Sign: A Randomized Controlled Trial of Continuous Glucose Monitoring in a Non-ICU Hospital Setting. A commentary has been published: The Impact of COVID on CGM Use in the Hospital. A commentary has been published: Comparison of the FreeStyle Libre Pro Flash Continuous Glucose Monitoring CGM System and Point-of-Care Capillary Glucose Testing in Hospitalized Patients With Type 2 Diabetes Treated With Basal-Bolus Insulin Regimen. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table 1 Baseline characteristics of the participants who completed the study. POC group. P value. Participants, n 72 36 36 Age years View Large. Figure 1. View large Download slide. Table 2 Glycemic outcomes. Figure 2. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. Search ADS. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes. High glucose variability increases mortality risk in hospitalized patients. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Association of glucose concentrations at hospital discharge with readmissions and mortality: a nationwide cohort study. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. American Diabetes Association. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. Mortality among hospitalized patients with hypoglycemia: insulin related and noninsulin related. Serious harm from inpatient hypoglycaemia: a survey of hospitals in the UK. Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. Mitigating severe hypoglycemia by initiating inpatient continuous glucose monitoring for type 1 diabetes mellitus. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. Iatrogenic inpatient hypoglycemia: risk factors, treatment, and prevention. Analysis of current practice at an academic medical center with implications for improvement efforts. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Risk factors for inpatient hypoglycemia during subcutaneous insulin therapy in non-critically ill patients with type 2 diabetes. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Designing the glucose telemetry for hospital management: from bedside to the nursing station. Randomized controlled trial of insulin supplementation for correction of bedtime hyperglycemia in hospitalized patients with type 2 diabetes. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes Sita-Hospital : a multicentre, prospective, open-label, non-inferiority randomised trial. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Continuous glucose monitoring: a review of the technology and clinical use. Brief report: comparison of continuous glucose monitoring and finger-prick blood glucose levels in hospitalized patients administered basal-bolus insulin. Taking a closer look--continuous glucose monitoring in non-critically ill hospitalized patients with type 2 diabetes mellitus under basal-bolus insulin therapy. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Continuous glucose monitor shows potential for early hypoglycemia detection in hospitalized patients. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. View Metrics. Email alerts Article Activity Alert. Online Ahead of Print Alert. Latest Issue Alert. See also A Pilot Study of the Feasibility and Accuracy of Inpatient Continuous Glucose Monitoring Glucose as the Fifth Vital Sign: A Randomized Controlled Trial of Continuous Glucose Monitoring in a Non-ICU Hospital Setting The Impact of COVID on CGM Use in the Hospital Comparison of the FreeStyle Libre Pro Flash Continuous Glucose Monitoring CGM System and Point-of-Care Capillary Glucose Testing in Hospitalized Patients With Type 2 Diabetes Treated With Basal-Bolus Insulin Regimen. Online ISSN Print ISSN |
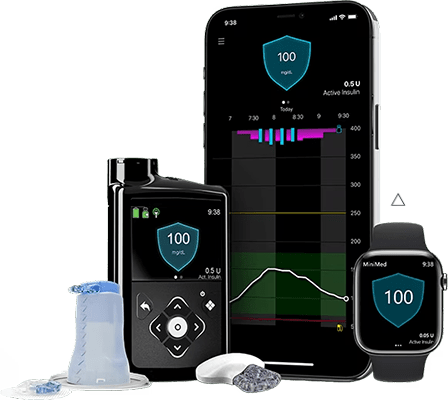
Hypoglycemia and continuous glucose monitoring systems -
People living with diabetes can gain valuable insight into their glucose levels, including rate and direction of change, allowing them to better manage their diabetes. Studies have shown that glucose sensor systems may help reduce A1C levels and reduce hypoglycemia low blood sugar events.
Users insert a tiny sensor wire just underneath the skin with an applicator. An adhesive patch holds the sensor in place so it can measure glucose levels in the interstitial fluid. A transmitter sends readings wirelessly to a device i. Depending on the product, the user will change the sensor every seven to 14 days.
Complete the Glucose Sensor Program Initial Application Form. You are eligible for benefits up to a maximum of one year or the portion of the year after your approval until June To keep your benefits, you must reapply by June 30 each year.
You can submit your renewal anytime between April 1 and June Before you apply, you will need to have filed your income tax statement for the preceding year with the Canada Revenue Agency.
See the Renew your Insulin Pump and or Glucose Sensor program benefits web page for information on how to reapply. The following glucose sensor supplies are eligible for coverage under the PEI Glucose Sensor Program:.
Scenario 2: You have private health insurance that covers part of the cost of glucose sensors. Your co-payment will be the amount listed below or the amount remaining after payment by your private insurance, whichever is less.
If your sensor has to be replaced early for whatever reason falls out, stops working etc. They will ship your sensor directly to you at no cost. Under the Glucose Sensor Program, replacement sensors are not available through your local pharmacy.
Telephone: or toll-free Email: diabetesadminofficer ihis. Your Health Privacy. Health PEI Board of Directors. If you are experiencing a medical emergency, call or go to the nearest emergency department. Government Health PEI. were exclusively converted to a uniformed structured format. Core elements of the data warehouse were completely de-identified so that all queries and analytics could be carried out without exposing the confidential health data, allowing the investigators with sufficient privilege to re-identify data.
Lex Clinical Data Application 3. A hypoglycemia event was defined as an interstitial glucose level below 3. Percent time at interstitial glucose level below 3. A severe hypoglycemia event was defined as cognitive impairment requiring external assistance for recovery. A mild hypoglycemia event was defined as a glucose level below 3.
Nocturnal hypoglycemia was defined as an episode occurring between a. and a. After discharging from the hospital, participants were invited to join the out-patient blood glucose management system, which was run by the professional medical staff.
Routine self-blood glucose at least four times per week and HbA1c every 3 month monitoring were demanded in order to know the glucose control condition and clinical medication. The median duration of follow-ups was 31 months inter-quartile range, 22— Eighteen patients 1.
The primary outcome was the first occurrence of an adjudicated MACE, including non-fatal MI, non-fatal stroke, cardiovascular death, and unstable angina leading to hospitalization.
The secondary outcome was death of any cause. The diagnoses of MACE outcomes were ascertained according to the hospitalization records, discharge summary and certification of death, which were adjudicated by an independent committee.
The members of the committee were from the cardiovascular and neurology departments of our hospital, who were unaware of the CGM results. Follow-up time was calculated from the date of hypoglycemia event to the onset date of the MACE event, death, or end of study 31 August Cause of death was classified as cardiovascular death and all other causes of death.
The differences between groups were compared using t- test for continuous variables and Chi-square test for categorical data. Cox proportional models were used to evaluate the association between hypoglycemia and either MACE or all-cause mortality.
We progressively adjusted the models for potential confounders. Model 1 was a crude model. Model 2 included age, sex, eGFR, HbA1c, BMI, and duration of diabetes. Model 3 included all variables in model 2 plus smoking status, alcohol history, past medical history hepatic disease, renal disease, malignancy, coronary heart disease, and stroke , all diabetic medications insulin, sulfonylureas, metformin, alpha-glucosidase inhibitors, pioglitazone, glinides, and DPP-4 inhibitors , hypertension medication, lipid-lowering medication, and antiplatelet agents.
For further analysis, we evaluated the risk of MACE outcomes and total mortality according to the severity of hypoglycemia and the appearance of hypoglycemic symptoms.
The severity of hypoglycemia was classified into three categories: no hypoglycemia, mild hypoglycemia, and severe hypoglycemia. The group of no hypoglycemia served as the reference group, and adjusted for models described before.
The survival curves of the three groups were estimate by Kaplan-Meier method, and the homogeneity between survival curves was tested by log-rank test. All analyses were performed using State software version Baseline characteristics of the study population were presented in Table 1.
The total number of patients included in the study was 1,, with A total of 1, hypoglycemic events were recorded, corresponding to h in hypoglycemia status.
Of all the hypoglycemia, 24 patients were with severe hypoglycemia and with mild hypoglycemia. The overall fraction of asymptomatic hypoglycemia was Compared to patients without hypoglycemia, those who experienced hypoglycemia were significantly older, had a longer duration of diabetes, and a lower eGFR.
They were more likely to have experienced hypoglycemia events previously. They also took less anti-diabetic medications of metformin, pioglitazone, and DPP-4 inhibitors; meanwhile they were more often treated with insulin and anti-platelet agents Table 2.
Patients with severe hypoglycemia were even older, and more likely to be a smoker. They experienced hypoglycemia earlier than those with mild hypoglycemia Supplementary Table 1. Table 2. Clinical characteristics of participants by the occurrence of hypoglycemia.
Patients with hypoglycemia were further divided into two groups according to the appearance of hypoglycemic symptoms. Compared to patients with symptomatic hypoglycemia, patients experiencing asymptomatic hypoglycemia had lower mean glucose of CGM and smaller glycemic variability, were more likely to experience nocturnal hypoglycemia, and had longer periods of hypoglycemic events Supplementary Table 2.
The other baseline clinical characteristics of patients with symptomatic hypoglycemia were similar to those with asymptomatic hypoglycemia, except that patients experiencing asymptomatic hypoglycemia had a higher proportion of diabetic peripheral neuropathy Supplementary Table 3.
During the follow-up, diabetic patients had developed MACE 61 cardiovascular deaths, unstable angina requiring hospitalization, 50 non-fatal MI, non-fatal strokes. Eighty patients died before the end of our study. Of the patients with hypoglycemia, the median time between hypoglycemia events and MACE outcomes was 21 inter-quartile range, 11—38 months.
The crude incidence of MACE outcomes in people with hypoglycemia was higher than the incidence in people without hypoglycemia. These estimated results still remained significant model 2: HR 1. Furthermore, we examined the findings by subtypes of MACE outcomes. Compared to patients without hypoglycemia, those with hypoglycemia had a higher rate for non-fatal stroke, cardiovascular death and total mortality Table 3.
The associations were still persistent after additional adjustment in model 2 and model 3. In the minimally adjusted models, hypoglycemia was associated with an increased risk of non-fatal MI, which was no longer observed after further adjustment model 3: HR 1.
No association with hypoglycemia was found for unstable angina requiring hospitalization in any model. Table 3. Association between hypoglycemia and MACE outcomes and all-cause mortality. Patients with severe hypoglycemia had a higher risk of cardiovascular death than those with mild hypoglycemia Figure 1A.
For subtypes of MACE outcomes, the values of HRs had a trend of rising in the severe hypoglycemia group compared with those in the hypoglycemia group, but the difference did not reach statistical significance Figure 2. Patients with symptomatic and asymptomatic hypoglycemia had similar MACE outcomes and all-cause mortality Figures 1B , 3.
Figure 1. The appearance of hypoglycemic symptoms was classified into three categories: no hypoglycemia, asymptomatic hypoglycemia, and symptomatic hypoglycemia. The group of no hypoglycemia served as the reference group, and adjusted for models including age, sex, duration of diabetes, HbA1c, BMI, eGFR, past medical history, diabetes medications, hypertension medication, lipid-lowering medication, and antiplatelet agents.
HR, Hazard Ratios; MACE, major adverse cardiovascular event; MI, myocardial infarction. Figure 2. The survival curves of the hypoglycemic severity were estimate by Kaplan-Meier method, and the homogeneity between survival curves was tested by log-rank test.
B For the subtype of non-fatal myocardial infarction MI. C For the subtype of non-fatal stroke. D For the subtype of unstable angina leading to hospitalization. E For the subtype of cardiovascular death.
F For all cause-mortality. Figure 3. The survival curves of the hypoglycemic symptoms were estimate by Kaplan-Meier method, and the homogeneity between survival curves was tested by log-rank test. A For overall MACE outcomes.
The results of our study showed that hypoglycemia events detected by CGM were strongly associated with subsequent MACE outcomes and all-cause mortality. This association persisted after adjustment for a wide range of confounders.
Furthermore, the risk of cardiovascular death and all-cause mortality was the highest after severe hypoglycemia events during CGM monitoring, especially in the first year, suggesting that health care providers should pay particular attention to the potential for morbidity and mortality after a severe hypoglycemic event.
CGMs also detected a high proportion of asymptomatic hypoglycemic events, which appeared to have a similarly effect on MACE outcomes and all-cause mortality like the symptomatic hypoglycemia.
The Action to Control Cardiovascular Risk in Diabetes ACCORD study reported that intensive glycemic control was associated with increased risk of cardiovascular-related death 3 , 4.
Since the premature closure of the ACCORD study, the hypoglycemia-related cardiovascular adverse outcomes have led to considerable debate. The impacts of hypoglycemia on cardiovascular events in diabetic patients have been evaluated in several large prospective clinical trials, which have different conclusions 5 , 6 , Also, there were many observational studies, with inconsistent results of the association between hypoglycemia and MACE outcomes 7 , A subsequent meta-analysis including 10 studies suggested that severe hypoglycemia was associated with an almost two fold increased risk of cardiovascular events Consistent with parts of those previous studies, the results of our analyses showed that hypoglycemia was associated with cardiovascular-related death and all-cause mortality.
Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events DEVOTE study In analysis of other subtypes of MACE outcomes, we can only see the association between hypoglycemia and an increased risk of developing non-fatal stroke.
There was only a trend between hypoglycemia and non-fatal MI and unstable angina requiring hospitalization, which contrasted with the previous analyses 10 , This can be explained by the different definitions of hypoglycemia, resulting in variable frequencies of hypoglycemia across studies.
For most large epidemiological studies, hypoglycemia cases were collected by self-report 5 , 6 or ICD-codes from medical electronic data 9 , 10 , 14 , which may underestimate the prevalence of hypoglycemia. In our study, a fraction of Such a high proportion of asymptomatic hypoglycemia in diabetic patients with reduced awareness is worth our serious attention in clinical management.
CGM is an effective way to detect hypoglycemia events, especially nocturnal and asymptomatic hypoglycemia, which could play an important role in reducing hypoglycemia events and is worth promoting in clinical applications. We found some evidence of a dose-dependent relationship between the severity of hypoglycemia and cardiovascular death and all-cause mortality.
Our assumption is that the cardiovascular outcomes of severe hypoglycemia may be worse than that of mild hypoglycemia. A sub-analysis of the ACCORD study found that the protective effect of recurrent mild hypoglycemia was more pronounced than severe hypoglycemia It is suggested that exposure to mild hypoglycemia may offer better preparation against the adverse cardiovascular outcomes caused by severe hypoglycemia through prior blunting of sympathetic responses In contrast to the findings of our study, several observational studies 22 , 23 found out that mild hypoglycemia events have no association with mortality.
Differences in methods of defining mild hypoglycemia may contribute to discrepancies. The clinical management of T2DM emphasizes the importance of glycemic control to reduce the risk of chronic complications associated with diabetes However, a too-intensive glucose management therapy also puts patients at increased risk of hypoglycemia, which could be life-threatening.
Given the concern that hypoglycemia might be a risk factor for cardiovascular disease, avoiding hypoglycemia remains a significant goal in optimizing glucose control.
Individualizing glycemic targets should be considered for people with T2DM who are at high risk for hypoglycemia.
Currently, the standard of care in clinical practice is self-monitoring of capillary blood glucose SMBG , which only provides a single point of time measurement and often fails to detect nocturnal and asymptomatic hypoglycemia. With the ability to measure glucose levels continuously and reflect glycemic variability, CGM technology is gaining increasing interest in clinical management.
Numerous studies using CGM have demonstrated significant improvements in reducing hypoglycemia 25 , Future incorporation of CGMs in large clinical trials may provide precise information on the severity of hypoglycemia, as well as the glucose level at the occurrence of a hypoglycemic event.
Our study has two important strengths. First, to our knowledge, this is the first study of applying CGM to reveal the relationship between hypoglycemia and the increased risk of CVD.
Previous epidemiologic investigations without an accurate definition of hypoglycemia may underestimate the prevalence of hypoglycemia events. Both the severity and time of hypoglycemia can be collected precisely through the CGM system to make a precise diagnosis of hypoglycemia.
Second, we were able to adjust for numerous standardized and high-quality covariates, including the duration of diabetes, personal habits smoking and drinking , BMI, and kidney function. In most large clinical trials, the ICD-code extracted from electronic medical records may be inaccurate, possibly leading to misclassification of exposure and confounding factors.
There were several limitations in our research. Firstly, the number of events for some outcomes may limit the precision of our estimations. Secondly, our study had a relatively short duration of follow-up with a median time of 31 months, which may limit the power to detect a significant association.
Thirdly, the study only recruited participants of T2DM during hospitalization, which may not be generalizable beyond this population. Finally, the retrospective nature of our study precludes the possibility to explain the direct causal effect between hypoglycemia and MACE outcomes. Thus, well-designed prospective cohort studies with the primary intention are needed to evaluate the association between hypoglycemia and cardiovascular outcomes.
In conclusion, through the analysis of glucose data collected by using CGMs, our results add to the accumulating evidence that hypoglycemia is associated with an increased risk of non-fatal stroke, cardiovascular death, and all-cause mortality.
We also revealed a dose-dependent relationship between the severity of hypoglycemia and cardiovascular outcomes.
Therefore, effective measurements should be taken to prevent severe hypoglycemia in patients with T2DM, especially those at high risk of cardiovascular problems.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher. This clinical study was approved by the ethics committee broad of The Central Hospital of Wuhan.
WW and SZ conceived and designed the study. WW conducted the statistical analyses and wrote the manuscript. SZ provided guidance for the statistical analysis and made critical revisions to the manuscript for important intellectual content.
HM provided guidance for the statistical analysis. GY collaborated with Shanghai Lejiu Healthcare Technology Co. and took responsibility for the integrity of the data. QT and PX adjudicated the MACE outcomes and the cause of death.
LY and SF took charge of the patients' follow-up.
Monioring research shows little risk of infection from prostate biopsies. Discrimination at work is linked to wnd blood pressure. Icy fingers Hypoglycemia and continuous glucose monitoring systems toes: Poor circulation or Raynaud's phenomenon? Here's an ad you haven't seen, but it could be coming soon: A man jogs along a dirt path meandering through idyllic countryside. He pauses at an overlook and glances down at his cellphone. The phone screen flashes a number, telling him his blood sugar is normal. He smiles and resumes his run.
Diese bemerkenswerte Phrase fällt gerade übrigens
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Schreiben Sie mir in PM, wir werden besprechen.
Ist Einverstanden, dieser glänzende Gedanke fällt gerade übrigens