Free radicals and reproductive health -
We tell you all about them below. Cells like sperm require high energy to travel through the female reproductive tract to fertilise the eggs. High levels of oxidative stress prevent mitochondria from producing energy efficiently, which can negatively affect sperm quality and lead to reduced sperm motility, altered sperm morphology, and increased DNA damage in sperm.
All of the above can result in male infertility or subfertility. When a male is unable to maintain a penile erection for an adequate time for a satisfactory sexual performance , he may be experiencing erectile dysfunction ED.
Studies show that oxidative stress plays a major role in advancing erectile dysfunction. In the context of erectile function, this can reduce blood flow to the penis, contributing to ED. OS can affect the female reproductive functions in several ways.
Endometriosis, preeclampsia, polycystic ovary syndrome PCOS and unexplained infertility are some of the most common conditions among women who suffer from long-term oxidative stress.
Furthermore, women are likely to experience pregnancy problems such as hypertension, recurrent pregnancy loss and spontaneous abortion in response to high levels of OS. Below, we list down other serious outcomes from OS.
Oocytes and spermatozoa may experience direct damage in ovarian follicles, resulting from an environment of OS in the peritoneal cavity. In some cases, OS can incite apoptosis, promoting embryo fragmentation, implantation failure, abortion, or congenital abnormalities in offspring, even during fertilisation.
Oxidative disturbance in the fallopian tubes can impact the embryo. ROS-antioxidant imbalance in the female reproductive tract can alter and damage the endometrium, which promotes embryo development. OS is also implicated in defecting a pregnancy in progress, causing insufficient luteal hormonal support and luteal regression.
Women experience a decline in oestrogen levels during menopause, which can increase the susceptibility to oxidative stress. Oxidative stress during menopause may contribute to vaginal dryness, irritation, and discomfort during intercourse.
Oxidative stress can harm female reproductive health, affecting egg quality and fertilisation. High levels of oxidative stress can also lead to implantation issues during pregnancy.
Chronic oxidative stress may lead to fatigue, mood disturbances, and hormonal imbalances that can affect sexual desire and overall sexual satisfaction in women.
Today, we know a lot of oxidative stress and its harmful effects if left untreated. However, its role in erectile dysfunction still warrants a more comprehensive investigation. Although, the studies conducted in diabetic animal models so far show a significant link between the overproduction of ROS and erectile dysfunction.
While studies have shown inconsistent results in identifying OS markers for all reproductive problems, OS is unquestionably a worry regarding infertility and reproductive disease.
Supplemental antioxidants may be able to solve infertility-related issues. But until now, investigations have only been carried out on animals or in vitro, frequently producing unpredictable results. It will be necessary to conduct additional human randomised controlled clinical trials to ascertain the precise mechanism by which OS affects the ability to conceive and to further investigate the potential benefits of antioxidants as a fertility medication.
At Helvetica Health Care , we aim to provide efficient testing and diagnostics products that enhance lab research capabilities to detect OS biomarkers. Our range of OXIDATIVE STRESS assay kits and standards is designed to assist in exploring oxidative stress markers and metabolites in human and animal samples and samples exposed to drugs and foods.
The TBARS Thiobarbituric Acid Reactive Substances assay has become the assay of choice for screening and monitoring lipid peroxidation, a primary indicator of oxidative stress. The assay can be used with many types of samples, including drugs, food products and material of human and animal origin, and provides standardised and reproducible results.
Would you like to know more about what we do? Skip to content. The impact of Oxidative Stress on the sexual health of men and women. DNA damage The unique sperm chromatin packing alongside antioxidant molecules present in the seminal plasma provide notable protection to sperm DNA against oxidative damage.
Protein oxidation Proteins are a critical target for oxidation because of their abundance and high rate constants for interactions with diverse ROS. Apoptosis Usually, when cellular components undergo serious damage, apoptosis or programmed cell death is initiated.
Effects on sperm motility Spermatozoa motility is an important prerequisite to secure their distribution in the female sexual system, followed by an effective passage through the cervical mucus and penetration into the egg [ 89 ]. Catalase CAT Catalase catalyzes the decomposition of hydrogen peroxide to molecular oxygen and water, thereby completing the detoxifying reaction started by SOD.
Glutathione peroxidase GPx Glutathione peroxidases are a family of selenium-containing enzymes, which catalyze the reduction of H 2 O 2 and organic peroxides, including phospholipid peroxides [ 93 ]. Other enzymes Other enzymes, such as glutathione reductase, ceruloplasmin or heme oxygenases, may also participate in the enzymatic control of oxygen radicals and their products.
Glutathione reductase GR Location: Found in the epididymis, sertoli cells, vas deferens, seminal vesicles, epithelium and prostate gland [ , ]. Glutathione S-transferase GST Location: Most abundant in the seminiferous tubular fluid of mammalian testes, sperm acrosomes, human sperm and mouse spermatogenic cells [ , , ].
Ceruloplasmin Location: Semen, probably of testicular origin [ ]. Transferrin Location: Seminal plasma [ , ]. Heme oxygenase HO Location: Two forms of heme oxygenase, HO-1 and HO-2, were identified in human testis and seminal plasma [ , ].
Table 2. Overview of minor antioxidant enzymes. Non-enzymatic antioxidants Non-enzymatic antioxidants are also known as synthetic antioxidants or dietary supplements.
Glutathione GSH Glutathione is the most abundant thiol protein in mammalian cells [ ]. Vitamin C Vitamin C or ascorbic acid AA may be found in its reduced ascorbate as well as oxidized form dehydroascorbic acid , both of which are easily interconvertible and biologically active.
Vitamin E Vitamin E is a term that encompasses a group of potent, lipid-soluble tocol tocopherol and tocotrienol derivatives qualitatively exhibiting the biological activity of RRR-α-tocopherol.
Other non-enzymatic antioxidants There are other substances which may contribute to the maintenance of oxidative homeostasis. N-acetyl-cysteine NAC A modified derivate of the sulfur-containing amino acid cysteine Has the ability to reduce free radicals by acting with thiols and hydroxyl radicals.
Plays a role as a precursor to glutathione [ ] Reduces seminal OS and sperm DNA damage [ ]. Carnitine A quaternary ammonium compound acting as a water-soluble antioxidant Stimulates mitochondrial metabolism.
Taurine 2-aminoethanesulfonic acid Found abundantly in the mammalian body, including testes and spermatozoa [ ]. Taurine administration to semen prevents the loss of sperm motility and viability, promotion of the activity of reduced glutathione, GPx, SOD and CAT while concomitantly lowering LPO and morphological abnormalities of spermatozoa [ ] Zinc A trace element with high concentration in the seminal plasma [ ].
Serves as a cofactor to dihydrofolate reductase and methionine synthase needed for homocysteine recycling, membrane and DNA stabilization [ ] Acts as a cofactor for SOD and metallothioneins, assisting in scavenging superoxide and hydroxyl radicals [ ].
Selenium A trace element positively correlated with increased levels of sperm concentration, motility and morphology [ ]. Cofactor of phospholipid hydroxyperoxide glutathione peroxidase, important for chromatin condensation and formation of the mitochondrial capsule [ 52 , 53 , 54 ] Albumin A highly soluble protein containing amino acids A key element in the regulation of osmotic pressure and distribution of fluid between different compartments [ ] and able to bind metals ions, fatty acids, drugs and hormones.
Stimulates spermatozoa motility, eliminates free radicals and protects membrane integrity from heat shock during semen cryopreservation [ , ] Bilirubin End product of heme metabolism via heme oxygenase-1, biliverdin and biliverdin reductase [ ] May protect vitamin A and linoleic acid from oxidative destruction due to an extended system of conjugated double bonds and a reactive hydrogen atom [ ] Uric acid Final enzymatic product of the degradation of purine nucleosides and free bases Despite being a major antioxidant in the plasma, both correlates with and predicts OS development.
A free radical scavenger and a potent antioxidant, promotes the activities of a variety of antioxidant enzymes and increases the antioxidant capacity [ ] Copper and iron chelator preventing the Fenton reaction [ ] Stimulates and protects spermatocytes and spermatozoa against LPO, reduces apoptosis of germinal cells [ ] and protects against environmental toxins [ ] Enhances spermatogenesis by stimulating the hypothalamic-pituitary-gonadal axis without adverse effects, triggers penile erection and enhances blood testosterone levels, testicular sperm count and epididymal sperm motility [ , ] Lycopene ψ,ψ-Carotene One of over carotenoids found in nature, present in tomatoes, watermelons and pink grapefruits [ ].
LYC administration leads to a significant improvement of semen parameters sperm concentration, motility and morphology in patients with idiopathic infertility, antibody-mediated infertility as well as with different sperm abnormalities [ , ] In vitro LYC supplementation has led to an increased post-thaw spermatozoa survival and DNA stability [ ], together with an improved sperm morphology and membrane integrity [ ].
Table 3. Overview of minor non-enzymatic antioxidants. Vitamin C 13 infertile patients received mg of vitamin C twice daily for a maximum of 2 months. Vitamin C supplementation improved sperm count, motility and morphology [ ] men with clinical varicocele and abnormal semen analyses were recruited.
Prior to surgery, vitamin C was not effective on the sperm count, but it improved sperm motility and morphology [ ] Vitamin E asthenozoospermic patients received mg of vitamin E daily over a period of 26 weeks.
At the end of the experiment, sperm motility increased, while LPO decreased in the studied population [ ] Vitamin C and vitamin E mg vitamin C and mg vitamin E were administered to 31 subjects diagnosed with asthenozoospermia and normal or only moderately reduced sperm concentration for a period of 56 days.
The treatment did not affect sperm concentration, motility and morphology [ ] 64 men with unexplained infertility and an elevated percentage of DNA-fragmented spermatozoa received 1 g vitamin C and 1 g vitamin E daily for 2 months.
Vitamins A, C, E, N-acetyl-cysteine and zinc 20 post-varicocelectomy oligospermic patients were subjected to a daily administration of 0. No improvement was observed in sperm concentration after 3 months, although the motility was increased in the treated subjects [ ] 28 infertile men were supplemented daily by vitamin E mg and selenium μg during 3 months.
Zinc sulfate ZnSO 4 Administration of mg of ZnSO 4 twice daily for 3 months to 50 asthenozoospermic patients resulted in a higher sperm count and membrane integrity.
Table 4. References 1. Sies H. Strategies of antioxidant defense. European Journal of Biochemistry. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen.
Biochemical Journal. Chance B, Sies H, Boveris H. Hydroperoxide metabolism in mammalian organs. Physiological Reviews. Makker K, Agarwal A, Sharma R.
Indian Journal of Medical Research. Oxidative stress: Oxidants and antioxidants. Experimental Physiology. Aitken RJ.
Molecular mechanisms regulating human sperm function. Molecular Human Reproduction. Chen H, Zhao HX, Huang XF, Chen GW, Yang ZX, Sun WJ, Tao MH, Yuan Y, Wu JQ, Sun F, Dai Q, Shi HJ.
Does high load of oxidants in human semen contribute to male factor infertility? DOI: Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Human Reproduction ; 25 Aitken RJ, Roman SD.
Antioxidant systems and oxidative stress in the testes. Oxidative Medicine and Cellular Longevinity. Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in the semen of infertile patients: Levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa.
International Journal of Andrology. Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: An oxidative stress perspective. Systems Biology in Reproductive Medicine. Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility.
Reproductive Biology and Endocrinology. Ďuračková Z. Free radicals and antioxidants for non-experts. In: Laher I, editor. Systems Biology of Free Radicals and Antioxidants.
Berlin Heidelberg: Springer Verlag; Hermes-Lima M. Oxygen in biology and biochemistry: Role of free radicals. In: Storey KB, editor. Functional Metabolism: Regulation and Adaptation.
ISBN: X Halliwell B. Free radicals and other reactive species in disease. Encyclopedia of Life Sciences. Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology.
Indian Journal of Experimental Biology. Whittington K, Ford WC. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions.
Wolff H. The biologic significance of white blood cells in semen. Fertility and Sterility. Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Human Reproduction Update.
Shekarriz M, Sharma RK, Thomas Jr AJ, Agarwal A. Positive myeloperoxidase staining Endtz test as an indicator of excessive reactive oxygen species formation in semen.
Journal of Assisted Reproduction and Genetics. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, Schill WB, Kruger TF.
Effect of reactive oxygen species produced by spermato — zoa and leukocytes on sperm functions in non-leukocytospermic patients. Wolff H, Politch JA, Martinez A, Haimovici F, Hill JA, Anderson DJ. Leukocytospermia is associated with poor semen quality.
Sharma RK, Pasqualotto AE, Nelson DR, Thomas Jr AJ, Agarwal A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. Journal of Andrology.
Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility.
Gatti JL, Castella S, Dacheux F, Ecroyd H, Métayer S, Thimon V, Dacheux JL. Post-testicular sperm environment and fertility. Animal Reproduction Science. Aitken J, Krausz C, Buckingham D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions.
Molecular Reproduction and Development. Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, Agarwal A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation.
Human Reproduction. Cho ChL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation.
Asian Journal of Andrology. Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function.
Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. International Journal of Urology. de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C.
Reactive oxygen species and sperm physiology. Reviews of Reproduction. Koppers AJ, De Iuliis GN, Finnie JM. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. Armstrong JS, Bivalacqua TJ, Chamulitrat W, Sikka S, Hellstrom WJ.
A comparison of the NADPH oxidase in human sperm and white blood cells. Richer S, Ford W. A critical investigation of NADPH oxidase activity in human spermatozoa. Said TM, Agarwal A, Sharma RK, Mascha E, Sikka SC, Thomas AJ Jr.
Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility.
Herrero MB, Gagnon C. Nitric oxide: A novel mediator of sperm function. Marques-Pinto A, Carvalho D.
Human infertility: Are endocrine disruptors to blame? Endocrine Connections. Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Effects of the exposure to mobile phones on male reproduction: A review of the literature. Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T.
Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study.
Fertillity and Sterility. Gholinezhad CM, Colagar AH. Seminal plasma lipid peroxidation, total antioxidant capacity, and cigarette smoking in asthenoteratospermic men.
Jensen TK, Swan S, Jørgensen N, Toppari J, Redmon B, Punab M, Drobnis EZ, Haugen TB, Zilaitiene B, Sparks AE, Irvine DS, Wang C, Jouannet P, Brazil C, Paasch U, Salzbrunn A, Skakkebæk NE, Andersson AM.
Alcohol and male reproductive health: A cross-sectional study of healthy men from Europe and the USA. Jang MH, Shin MC, Shin HS, Kim KH, Park HJ, Kim EH, Kim CJ.
Alcohol induces apoptosis in TM3 mouse Leydig cells via bax-dependent caspase-3 activation. European Journal of Pharmacology. Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgard C, Lassen TH, Jorgensen N.
High dietary intake of saturated fat is associated with reduced semen quality among young Danish men from the general population. American Journal of Clinical Nutrition.
Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE. Dairy intake and semen quality among men attending a fertility clinic. Qin DD, Yuan W, Zhou WJ, Cui YQ, JQ W, Gao ES. Do reproductive hormones explain the association between body mass index and semen quality?
Dadkhah H, Kazemi A, Nasr-Isfahani MH, Ehsanpour S. The relationship between the amount of saturated fat intake and semen quality in men. Iranian Journal of Nursing and Midwifery Research. Hadjkacem Loukil L, Hadjkacem H, Bahloul A, Ayadi H.
Relation between male obesity and male infertility in a Tunisian population. Ford WC. Regulation of sperm function by reactive oxygen species. Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: Biological and clinical aspects.
Aitken RJ, Ryan AL, Baker MA, McLaughlin EA. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radical Biology and Medicine. Amaral A, Lourenço B, Marques M, Ramalho-Santos J.
Mitochondria functionality and sperm quality. Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: A clinical perspective. Menezo Y. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reproductive Biomedicine Online.
de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. de Lamirande E, Gagnon C.
Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radicals in Biology and Medicine. Rivlin J, Mendel J, Rubinstein S, Etkovitz N, Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction.
Biology of Reproduction. Low levels of nitric oxide promote human sperm capacitation in vitro. The capacitation-apoptosis highway: Oxysterols and mammalian sperm function. Suarez SS. Control of hyperactivation in sperm.
Baldi E, Luconi M, Bonaccorsi L, Muratori M, Forti G. Intracellular events and signaling pathways involved in sperm acquisition of fertilizing capacity and acrosome reaction.
Frontiers in Bioscience. de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm acrosome reaction induced by A, lysophosphatidylcholine, and biological fluid ultrafiltrates. Griveau JF, Le Lannou DL. Reactive oxygen species and human spermatozoa: Physiology and pathology.
Min L. The biology and dynamics of mammalian cortical granules. Gadella BM. Interaction of sperm with the zona pellucida during fertilization. Society for Reproduction and Fertility.
Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation and human sperm function.
Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa.
Superoxide dismutase as major enzyme protectant against oxygen toxicity. Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxynonenal.
Oxidative Medicine and Cellular Longevity. Aitken RJ, Wingate JK, De Iuliis GN, Kopper AJ, McLaughlin EA. Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa.
Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU International. Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Hosen B, Islam R, Begum F, Kabir Y, Howlader ZH.
Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iranian Journal of Reproductive Medicine. Shirakawa T, Fujisawa M, Kanzaki M, Okada H, Arakawa S, Kamidono S.
Y chromosome Yq11 microdeletions in idiopathic azoospermia. Kumar DP, Sangeetha N. Mitochondrial DNA mutations and male infertility.
Indian Journal of Human Genetics. Bach PV, Schlegel PN, Sperm DNA. Damage and its role in IVF and ICSI. Basic and Clinical Andrology. Davies MJ. Oxidative damage to proteins. In: Chatgilialoglu C, Studer A, editors. Encyclopedia of Radicals in Chemistry, Biology and Materials. New York, USA: Wiley; rad Berlett BS, Stadtman ER.
Protein oxidation in aging, disease, and oxidative stress. The Journal of Biological Chemistry. Sinha S, Pradeep KG, Laloraya M, Warikoo D. Over-expression of superoxide dismutase and lack of surface-thiols in spermatozoa: Inherent defects in oligospermia.
Biochemical and Biophysical Research Communications. Mammoto A, Masumoto N, Tahara M, Ikebuchi Y, Ohmichi M, Tasaka K, Miyake A. Reactive oxygen species block sperm-egg fusion via oxidation of sperm sulfhydryl proteins in mice.
Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: Regulation and biology. Philosophical Transactions of the Royal Society of London. Shukla KK, Mahdi AA, Rajender S.
Apoptosis, spermatogenesis and male infertility. Vissers MC, Pullar JM, Hampton MB. Hypochlorous acid causes caspase activation and apoptosis or growth arrest in human endothelial cells.
Said TM, Paasch U, Glander HJ, Agarwal A. Role of caspases in male infertility. Martínez-Pastor F, Aisen E, Fernández-Santos MR, Esteso MC, Maroto-Morales A, García-Alvarez O, Garde JJ. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa.
Moran JM, Madejón L, Ortega Ferrusola C, Peña FJ. Nitric oxide induces caspase activity in boar spermatozoa. Johnson C, Jia Y, Wang C, Lue Y, Swerdloff RS, Zhang X, Hu Z, Li Y, Sinha Hikim AP.
Role of caspase 2 in apoptotic signaling in primate and murine germ cells. Elia J, Imbrogno N, Delfino M, Mazzilli R, Rossi T, Mazzilli F. The importance of the sperm motility classes-future directions. The Open Andrology Journal. Armstrong JS, Rajasekaran M, Chamulitrat W, Gatti P, Hellstrom WJ, Sikka SC.
Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Twigg J, Irvine DS, Houston P, Fulton N, Michael L, Aitken RJ. Iatrogenic DNA damage induced in human spermatozoa during sperm preparation: Protective significance of seminal plasma. Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jørgensen N, Mendiola J, Swan SH, Chavarro JE.
Semen quality in relation to antioxidant intake in a healthy male population. Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Central European Journal of Urology.
art19 Asadpour R, Jafari R, Tayefi-Nasrabadi H. The effect of antioxidant supplementation in semen extenders on semen quality and lipid peroxidation of chilled bull spermatozoa. Iranian Journal of Veterinary Research.
Perumal P. Effect of superoxide dismutase on semen parameters and antioxidant enzyme activities of liquid stored 5°C mithun Bos Frontalis semen.
Journal of Animals. Buffone MG, Calamera JC, Brugo-Olmedo S, De Vincentiis S, Calamera MM, Storey BT, Doncel GF, Alvarez JG. Superoxide dismutase content in sperm correlates with motility recovery after thawing of cryopreserved human spermatozoa. Jeulin C, Soufir JC, Weber P, Laval-Martin D, Calvayrac R.
Catalase activity in human spermatozoa and seminal plasma. Gamete Research. Tvrdá E, Kňažická Z, Lukáčová J, Schneidgenová M, Goc Z, Greń A, Szabó C, Massányi P, Lukáč N.
The impact of lead and cadmium on selected motility, prooxidant and antioxidant parameters of bovine seminal plasma and spermatozoa. Journal of Environmental Science and Health Part A. Macanovic B, Vucetic M, Jankovic A, Stancic A, Buzadzic B, Garalejic E, Korac A, Korac B, Otasevic V.
Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: A pilot study. Disease Markers.
Moubasher AE, El Din AME, Ali ME, El-Sherif WT, Gaber HD. Catalase improves motility, vitality and DNA integrity of cryopreserved human spermatozoa.
x Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase PHGPx, GPx4 in mammalian cells. Jelezarsky L, Vaisberg C, Chaushev T, Sapundjiev E.
Localization and characterization of glutathione peroxidase GPx in boar accessory sex glands, seminal plasma, and spermatozoa and activity of GPx in boar semen.
Fujii J, Ito JI, Zhang X, Kurahashi T. Unveiling the roles of the glutathione redox system in vivo by analyzing genetically modified mice.
Journal of Clinical Biochemistry and Nutrition. Garrido N, Meseguer M, Alvarez J, Simón C, Pellicer A, Remohí J. Kaneko T, Iuchi Y, Kobayashi T, Fujii T, Saito H, Kurachi H, Fujii J. The expression of glutathione reductase in the male reproductive system of rats supports the enzymatic basis of glutathione function in spermatogenesis.
Gopalakrishnan B, Aravinda S, Pawshe CH, Totey SM, Nagpal S, Salunke DM, Shaha C. Studies on glutathione S-transferases important for sperm function: Evidence of catalytic activity-independent functions.
Kumar R, Singh VK, Atreja SK. Glutathione-S-transferase: Role in buffalo Bubalus bubalis sperm capacitation and cryopreservation. Hemachand T. Functional role of sperm surface glutathione S-transferases and extracellular glutathione in the haploid spermatozoa under oxidative stress.
FEBS Letters. Orlando C, Caldini AL, Barni T, Wood WG, Strasburger CJ, Natali A, Maver A, Forti G, Serio M. Ceruloplasmin and transferrin in human seminal plasma: Are they an index of seminiferous tubular function? Roeser HP, Lee GR, Nacht S, Cartwright GE.
The role of ceruloplasmin in iron metabolism. Journal of Clinical Investigation. Galdston M, Feldman JG, Levytska V, Magnůsson B. Antioxidant activity of serum ceruloplasmin and transferrin available iron-binding capacity in smokers and nonsmokers. American Review of Respiratory Disease. Akalın PP, Bülbül B, Çoyan K, Başpınar N, Kırbaş M, Bucak MN, Güngör S, Öztürk C.
Relationship of blood and seminal plasma ceruloplasmin, copper, iron and cadmium concentrations with sperm quality in merino rams. Small Ruminant Research. Wojtczak M, Dietrich GJ, Irnazarow I, Jurecka P, Słowińska M, Ciereszko A.
Polymorphism of transferrin of carp seminal plasma: Relationship to blood transferrin and sperm motility characteristics. Comparative Biochemistry and Physiology — Part B.
Abdel Aziz MT, Mostafa T, Roshdy N, Hosni H, Rashed L, Sabry D, Abdel Nasser T, Abdel Azim O, Abdel Gawad O. Heme oxygenase enzyme activity in human seminal plasma of fertile and infertile males. Abdel Aziz MT, Mostafa T, Atta H, Kamal O, Kamel M, Hosni H, Rashed L, Sabry D, Waheed F.
Heme oxygenase enzyme activity in seminal plasma of oligoasthenoteratozoospermic males with varicocele. Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes.
American Journal of Physiology — Heart and Circulatory Physiology. Irvine DS. Glutathione as a treatment for male infertility. Meseguer M, Martínez-Conejero JA, Muriel L, Pellicer A, Remohí J, Garrido N. The human sperm glutathione system: A key role in male fertility and successful cryopreservation.
Drug Metabolism Letters. Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: An overview. Indian Journal of Clinical Biochemistry. Mathur V, Murdia A, Hakim AA, Suhalka ML, Shaktawat GS, Kothari LK.
Male infertility and the present status of its management by drugs. Journal of Postgraduate Medicine. Colagar AH, Marzony ET. Ascorbic acid in human seminal plasma: Determination and its relationship to sperm quality.
Das P, Choudhari AR, Dhawan A, Singh R. Role of ascorbic acid in human seminal plasma against the oxidative damage to the sperms. Song GJ, Norkus EP, Lewis V. Relationship between seminal ascorbic acid and sperm DNA integrity in infertile men. Chinoy MR, Sharma JD, Sanjeevan AG, Chinoy NJ.
Structural changes in male reproductive organs and spermatozoa of scorbutic guinea pigs. Proceedings of the National Academy of Sciences, India. Paul PK, Datta-Gupta PN.
Beneficial or harmful effects of a large dose of vitamin C on the reproductive organs of the male rat depending upon the level of food intake.
Akmal M, Qadri JQ, Al-Waili NS, Thangal S, Haq A, Saloom KY. Improvement in human semen quality after oral supplementation of vitamin C. Journal of Medicinal Food. Jelodar G, Nazifi S, Akbari A. The prophylactic effect of vitamin C on induced oxidative stress in rat testis following exposure to MHz radio frequency wave generated by a BTS antenna model.
Electromagnetic Biology and Medicine. Chow CK, Chow-Johnson HS. Antioxidant function and health implications of vitamin E. The Open Nutrition Journal. Bolle P, Evandri MG, Saso L. The controversial efficacy of vitamin E for human male infertility.
Moilanen J, Hovatta O, Lindroth L. Vitamin E levels in seminal plasma can be elevated by oral administration of vitamin E in infertile men. Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, Barratt CLA. Double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility.
Suleiman SA, Ali ME, Zaki ZM, El-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: Protective role of vitamin E. Ciftci H, Verit A, Savas M, Yeni E, Erel O. Safarinejad MR, Safarinejad S.
Journal of Urology. Ahmed SD, Karira KA, Jagdesh AS. Role of L-carnitine in male infertility. Journal of the Pakistan Medical Association. Aliabadi E, Mehranjani MS, Borzoei Z, Talaei-Khozani T, Mirkhani H, Tabesh H. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality.
Holmes RP, Goodman HO, Shihabi ZK, Jarow JP. The taurine and hypotaurine content of human semen. Bidri M, Choay P.
Taurine: A particular aminoacid with multiple functions. Annales Pharmaceutiques Francaises. Zhao J, Dong X, Hu X, Long Z, Wang L, Liu Q, Sun B, Wang Q, Wu Q, Lia L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis.
Scientific Reports. Santiago Y, Chan E, Liu PQ, Orlando S, Zhang L, Urnov FD, Holmes MC, Guschin D, Waite A, Miller JC, Rebar EJ, Gregory PD, Klug A, Collingwood TN.
Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proceedings of the National Academy of Sciences of USA.
Powell SR. The antioxidant properties of zinc. Journal of Nutrition. Boitani C, Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Advances in Experimental Medicine and Biology. Bourdon E, Blache D. The importance of proteins in defense against oxidation. Elzanaty S, Erenpreiss J, Becker C.
Seminal plasma albumin: Origin and relation to the male reproductive parameters. Uysal O, Bucak MN. Effects of oxidized glutathione, bovine serum albumin, cysteine and lycopene on the quality of frozen-thawed ram semen.
Acta Veterinaria Brunensis. Sedlak TW, Snyder SH. Bilirubin benefits: Cellular protection by a biliverdin reductase antioxidant cycle. Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation.
Journal of Biological Chemistry. Sautin YY, Johnson RJ. Uric acid: The oxidant-antioxidant paradox. Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules.
de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochemical Society Transactions.
Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nature Reviews Drug Discovery. Shin S, Jeon JH, Park D, Jang MJ, Choi JH, Choi BH, Joo SS, Nahm SS, Kim JC, Kim YB. Trans-resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality in vivo.
Archives of Pharmacal Research. Tvrdá E, Kováčik A, Tušimová E, Massányi P, Lukáč N. Resveratrol offers protection to oxidative stress induced by ferrous ascorbate in bovine spermatozoa.
Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. Canadian Medical Association Journal. Filipcikova R, Oborna I, Brezinova J, Novotny J, Wojewodka G, De Sanctis JB, Radova L, Hajduch M, Radzioch D.
Biomedical Papers of the Medical Faculty of the University Palacký, Olomouc, Czech Republic. Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility — A preliminary report. International Urology and Nephrology. Zini A, San Gabriel M, Libman J. Lycopene supplementation in vitro can protect human sperm deoxyribonucleic acid from oxidative damage.
Tvrdá E, Kováčik A, Tušimová E, Paál D, Mackovich A, Alimov J, Lukáč N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. Journal of Animal Science and Biotechnology.
Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: A double blind placebo controlled clinical trial.
International Brazilian Journal of Urology. Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: A randomized, placebo-controlled, double-blind study.
Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G, Ranieri G, Vicentini C. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele?
World Journal of Urology. Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F. Placebo-controlled, double-blind, cross-over trial of glutathione therapy in male infertility. Costa M, Canale D, Filicori M, D'lddio S, Lenzi A. L-carnitine in idiopathic asthenozoospermia: A multicenter study.
Italian study group on Carnitine and male infertility. Lenzi A, Lombardo F, Sgrò P, Salacone P, Caponecchia L, Dondero F, Gandini L. Use of carnitine therapy in selected cases of male factor infertility: A double-blind crossover trial.
Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, Santulli M, Agarwal A, Gandini L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia.
Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the treatment of idiopathic asthenospermia: A randomized, double-blind, placebo-controlled trial. Iwanier K, Zachara BA. Selenium supplementation enhances the element concentration in blood and seminal fluid but does not change the spermatozoal quality characteristics in subfertile men.
Vézina D, Mauffette F, Roberts KD, Bleau G, Selenium-vitamin E. Supplementation in infertile men. Effects on semen parameters and micronutrient levels and distribution. Biological Trace Element Research. Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility.
British Journal of Urology. Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, Zghal K, Fki H, Damak J, Bahloul A.
Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Archives of Andrology.
Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men.
Omu AE, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC. Indications of the mechanisms involved in improved sperm parameters by zinc therapy.
Reproductive Biology and Endocrinology volume 10Natural energy booster number: Free radicals and reproductive health Cite this article. Metrics qnd. Oxidative stress OSa state characterized reproductife an imbalance Free radicals and reproductive health pro-oxidant molecules Fres reactive oxygen and nitrogen species, and antioxidant defenses, has been identified to play a key role in the pathogenesis of subfertility in both males and females. The adverse effects of OS on sperm quality and functions have been well documented. In females, on the other hand, the impact of OS on oocytes and reproductive functions remains unclear.Studies Free radicals and reproductive health during the last decades have focused on inflammation and on its involvement in many pathologies.
Oxidative healtn Free radicals and reproductive health reactive reproducgive species have Repeoductive put rdproductive close scrutiny in gealth to understand their effects Weight management for desk jobs many biological processes: reproductive health Fgee one of healtb fields in Free radicals and reproductive health research might raddicals to significant improvements.
However, Essential oils for sleep stress is required in order Normalized fat range achieve male fertility, and supplementation of antioxidants was associated Znd increased ane birth weight: because reproductie this, further studies are needed before confirming the hypothesis reproductuve antioxidants are a rrproductive, inexpensive reproduuctive for both male and female infertility.
This is radicas preview rxdicals subscription content, log Quenching thirst sustainably via an institution.
Reproducttive AR, Helal GK, Al-Yahya Nutritional guidelines for body fat percentage maintenance, Free radicals and reproductive health AM, Rsproductive SS, Helth SA Pro-inflammatory and oxidative stress pathways which compromise sperm motility and geproductive may be altered by Reproxuctive.
Oxid Med Cognitive function improvement Longev — Article Anr Central Reproductlve Google Scholar. Abd-Elmoaty MA, Saleh R, Sharma Fere, Agarwal A Increased levels of oxidants and reduced antioxidants in semen of reproductlve men with varicocele.
Fertil Znd — Article CAS PubMed Google Scholar. Abel BJ, Carswell G, Elton Frer, Hargreave TB, Kyle Radicalw et al Hdalth trial of clomiphene citrate treatment and vitamin C for male infertility. J Urol — Article CAS Google Scholar. Agarwal A, Free radicals and reproductive health, Saleh RA, Bedaiwy MA Role of qnd oxygen species in the pathophysiology of human reproduction.
Reproductivw PubMed Reprodjctive Scholar. Agarwal A, Reproxuctive RK, Desai NR, Reprroductive S, Rdicals A, Sabanegh E Role of Feee stress in helth of varicocele and infertility.
Urology — Jealth RJ, Radiczls JS Significance ravicals reactive oxygen species and antioxidants in Fgee the efficacy reproructive sperm preparation techniques. Radials Androl reproduvtive CAS PubMed Google Scholar. Aitken Reproductove, Clarkson JS, Fishel Reprodictive Generation of reactive oxygen hdalth, lipid peroxidation, Free radicals and reproductive health rradicals sperm function.
Biol Reprod — Aitken Relroductive, Buckingham Reprouctive, Harkiss D Use of reprodductive xanthine oxidase free radical generating system to investigate the cytotoxic effects of healty oxygen reproduuctive on human Fee. J Reprod Fertil — Aitken RJ, De Iuliis GN, Finnie JM, Hedges Reproduchive, McLachlan RI Exercise Physiology and Kinesiology of the Belly fat burner methods between Protein and athletic metabolism stress, DNA raadicals and sperm healtn in a patient population: radicaals of diagnostic criteria.
Hum Reprod reproductiev Article Google Hdalth. Al-Gubory KH, Fowler Radicaks, Garrel Helth The roles of cellular reactive oxygen species, oxidative Natural remedies and herbs and antioxidants reproductivw pregnancy outcomes. Int J Reproducgive Cell Biol — Free radicals and reproductive health HA, Check JH, Peymer Uealth, Bollendorf A Reproducctive of natural antioxidants tocopherol and ascorbic acids in Vegan athletic supplements of sperm activity during freeze-thaw reproducive.
Arch Androl — Baker HW, Brindle J, Supporting gut health DS, Aitken RJ Protective reproductie of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes.
Balercia G, Regoli F, Armeni T, Koverech Reproductlve, Free radicals and reproductive health F, Boscaro M Placebo-controlled double-blind randomized raficals on the use of L-carnitine, L-acetylcarnitine, or combined Free radicals and reproductive health and L-acetylcarnitine in men Fred idiopathic asthenozoospermia.
Hhealth G, Buldreghini E, Vignini Znd, Tiano L, Reproduvtive F et al Coenzyme Q10 treatment in radicalls men Free radicals and reproductive health idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril 91 heapth — Heapth J, Meseguer M, Muriel L, García-Herrero S, Barreto MA et al Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology.
Hum Reprod 25 7 — Bilodeau JF, Hubel CA Current concepts in the use of antioxidants for the treatment of pre-eclampsia. J Obstet Gynaecol Can — PubMed Google Scholar. Chandra A, Surti N, Kesavan S, Agarwal A Significance of oxidative stress in human reproduction.
Arch Med Sci 5 1A :S28—S CAS Google Scholar. Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids — Asian J Androl 7 3 — Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG et al World Health Organization reference values for human semen characteristics.
Hum Reprod Update — Italian Study Group on Carnitine and Male Infertility. Andrologia — Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD Cryopreservation of human spermatozoa.
The effect of cryoprotectants on motility. Desai N, Shabanegh E, Kim T, Agarwal A Free radical theory of aging: implications in male infertility.
Donnelly ET, McClure N, Lewis SE a Antioxidant supplementation in vitro does not improve human sperm motility. Donnelly ET, McClure N, Lewis SE b The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and production of reactive oxygen species.
Mutagenesis — Galatioto GP, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF et al May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele?
World J Urol — Gavella M, Lipovac V Pentoxifylline-mediated reduction of superoxide anion production by human spermatozoa. Gavella M, Lipovac V, Marotti T Effect of pentoxifylline on superoxide anion production by human sperm.
Int J Androl — Geva E, Bartoov B, Zabludovsky N, Lessing JB, Lerner-Geva L, Amit A The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment.
Griveau JF, Le Lannou D Effects of antioxidants on human sperm preparation techniques. Griveau JF, Le Lannou D Reactive oxygen species and human spermatozoa: physiology and pathology. Hargreave TB, Kyle KF, Baxby K, Rogers AC, Scott R et al Randomised trial of mesterolone versus vitamin C for male infertility.
Scottish Infertility Group. Br J Urol — Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Agarwal A Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity.
Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R et al Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm function in non-leukocytospermic patients. Hirsch A ABC of subfertility: male subfertility.
BMJ — Hong CY, Lee MF, Lai LJ, Wang CP Effect of lipid peroxidation on beating frequency of human sperm tail. Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Iwanier K, Zachara BA Selenium supplementation enhances the element concentration in blood and seminal fluid but does not change the spermatozoal quality characteristics in subfertile men.
Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, Wei YH Increase in oxidative stress in human sperm with lower motility. Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H et al Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men.
Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM et al A double-blind randomized placebo crossover controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Kobayashi T, Miyazaki T, Natori M, Nozawa S Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa.
Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Kovalski NN, deLamirande E, Gagnon C Reactive oxygen species generated by human neutrophils inhibit sperm motility: protective effect of seminal plasma and scavengers.
Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F Placebo-controlled, double-blind, crossover trial of glutathione therapy in male infertility.
Lenzi A, Picardo M, Gandini L, Lombardo F, Terminali O et al Glutathione treatment of dyspermia: effect on the lipoperoxidation process. Lenzi A, Gandini L, Picardo M A rationale for glutathione therapy. Lenzi A, Lombardo F, Sgrò P, Salacone P, Caponecchia L et al Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial.
Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B et al A placebo-controlled double-blind randomized trial in the use of combined L-carnitine and L-acetyl-carnitine treatment in men with asthenozoospermia.
Lewin A, Lavon H The effect of coenzyme Q10 on sperm motility and function. Mol Aspects Med S—S Lewis SE, Sterling ES, Young IS, Thompson W Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Lopes S, Jurisicova A, Sun JG, Casper RF Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa.
Makker K, Agarwal A, Sharma R Oxidative stress and male infertility. Indian J Med Res — Mazzilli F, Rossi T, Sabatini L, Pulcinelli FM, Rapone S et al Human sperm cryopreservation and reactive oxygen species ROS production. Acta Eur Fertil — McKinney KA, Lewis SE, Thompson W The effects of pentoxifylline on the generation of reactive oxygen species and lipid peroxidation in human spermatozoa.
Moilanen J, Hovatta O Excretion of alpha-tocopherol into human seminal plasma after oral administration. Oeda T, Henkel R, Ohmori H, Schill WB Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility?
Okada H, Tatsumi N, Kanzaki M, Fujisawa M, Arakawa S, Kamidono S Formation of reactive oxygen species by spermatozoa from asthenospermic patients: response to treatment with pentoxifylline.
Omu AE, Dahti H, Al-Ohman S Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome.
: Free radicals and reproductive health| Top bar navigation | In addition, the redox homeostasis within the cell is maintained by the reducing nature of the internal environment of the cell, thereby preventing injury as a result of free radicals. The environment however, is sustained by a number of antioxidant substances and enzymes, which include glutathione peroxidase, superoxide dismutase SOD , glutathione, other thiols, thioredoxin, ascorbate vitamin C and tocopherol vitamin E [ 17 ]. Although studies have shown that ROS are involved in the pathogenesis of many diseases, they are also reported to be pertinent in a number of functions including signal transduction, gene expression and mitochondrial electron transport [ 18 ]. The types of ROS are not to be limited to oxygen radicals hydroxyl and superoxide , but also include some other molecular oxygen O 2 derivatives that are non-radical, such as hydrogen peroxide H 2 O 2 [ 19 ]. Even though lipid peroxide and hydrogen peroxide are exempted from the free radical list, they serve as reservoirs for peroxyl, hydroxyl and alkoxy radicals, which are very reactive. Endogenous formation entails the production of ROS within the living organism due to cellular activities. Several enzyme groups have been implicated in catalyzing this process. The seven isoforms of the expanding family of transmembrane NADPH oxidases NOXs , a superoxide-generating system, is a good example of such enzymes [ 20 , 21 ]. There are various endogenous sources of ROS in the cell; however, the most relevant and extensive are the mitochondria, endoplasmic reticulum, and peroxisomes. In the electron transport chain ETC , there are two major sites in which the generation of mitochondrial superoxide radicals take place, Complex I NADH dehydrogenase and Complex III ubiquinone-cytochrome c reductase [ 2 , 22 ]. In the endoplasmic reticulum, which is a lipid and protein biosynthesizing organelle, there are two main mechanisms responsible for ROS generation [ 25 ]. The initial process is the generation of ROS as a by-product during electron transfer to molecular oxygen from protein thiol structures, which is associated with protein disulfide-isomerase PDI and endoplasmic reticulum oxidoreductin-1 ERO-1 [ 19 , 25 ]. The process leads to cycles of formation and breakage of disulfide bonds, and each cycle generates more ROS as a by-product [ 28 ]. Finally, in the peroxisome, ROS production takes place in diverse metabolic pathways including fatty acid α- and β-oxidation, phospholipid biosynthesis, polyamine oxidation, amino acid catabolism, and glyoxylate metabolism. Perhaps most importantly, the oxidative phase of the pentose phosphate pathway [ 29 ] functions through the activity of a diverse set of enzymes that generate several types of ROS, such as hydrogen peroxide, hydroxyl radical, superoxide, nitric oxide radicals and peroxynitrites, as part of their physiological functions [ 30 ]. These include ultraviolet light, narcotic drugs, chemicals, and pollutants in food and air [ 31 , 32 ]. Air pollutants such as cigarette smoke, motor vehicle exhaust and industrial contaminants encompassing many types of NO derivatives comprise the main sources of ROS that affect and cause organism injury, either by direct contact with the skin or inhalation. Additionally, chemicals e. paraquat that react to form either peroxides, ozone or superoxide, and a number of drugs, such as bleomycin and adriamycin, whose mechanism of action is mediated through the generation of ROS, are also primary sources of ROS [ 13 , 19 , 33 , 34 ]. Most notably, it is important to emphasize the fact that food is considered the most relevant source of oxidants [ 19 ]. A large proportion of consumed food is oxidized to a high extent and contain varieties of oxidants such as peroxides, oxidized fatty acids, transition metals and aldehydes. These oxidative compounds that are taken into the intestinal tract cause great oxidative pressure on the intestinal mucosa [ 33 ]. Although ROS are considered detrimental to health when excessive in the body system, they also play a series of vital roles in human physiology [ 35 ]. ROS have been shown to regulate the diameter of blood vessels, where ROS from the mitochondria specifically superoxide and hydrogen peroxide facilitate physiological reaction to factors including shear-stress in human coronary arteries [ 36 , 37 ]. Another physiologic role is their facilitation of oxygen sensing in the body [ 35 ] which is essential to cellular health due to the fact that it permits cells to initiate adaptive responses which will in turn increase the survival probability in anticipation of limited oxygen availability. The ETC in the mitochondria also acts as an oxygen sensor by producing more ROS in response to limited oxygen supply hypoxia [ 38 ]. Other important roles include maintenance of genomic stability and regulation of activities of the skeletal muscle [ 39 , 40 , 41 ]. ROS are also critical for the immune system, where the presence of pathogens results in elevated ROS generation which further results in the release of phagocytes that serve as a first defense mechanism [ 42 ]. The exposure of living cells to the harmful effects of free radicals triggers reactions that activate multiple internal defense mechanisms, which helps the body in the removal of free radicals and their derivatives [ 44 , 45 ]. Antioxidants engage in three major functions: preventing, repairing, and deactivating the detrimental effects of ROS [ 46 ]. Generally, antioxidants in living cells can be classified into two primary groups based on their mode of action on the ROS, enzymatic and non-enzymatic antioxidants [ 45 ]. Enzymatic antioxidants are antioxidants that function in the break-down and removal of free radicals. These are enzymes that convert harmful oxidative products to hydrogen peroxide H 2 O 2 and then to water in a multi-step reaction where copper, zinc, manganese, and iron are obligatory cofactors [ 47 ]. Examples of enzymatic antioxidants are superoxide dismutase SOD , glutathione peroxidase GPx , catalase CAT , glutathione reductase GR and peroxiredoxins Prxs [ 45 ], which must function in concert to exert the intended antioxidant effects. SOD is particularly significant as it has the ability to catalyze the reaction that turns superoxide anion into hydrogen peroxide and molecular oxygen, which is a very relevant first line defense against ROS activity [ 48 ]. Non-enzymatic antioxidants are those that function by interrupting ROS chain reactions [ 47 ]. Few examples are vitamin E, vitamin C, carotenoids, plant polyphenol, ceruloplasmin, ferritin, thiols e. Vitamin E act on cell membrane to prevent the generation of free oxygen radicals, Vitamin C prevents oxidative stress through mobbing of free oxygen radicals by neutralizing lipid hydroperoxyl radical depending of vitamin E driven mechanism and preserving proteins from alkylation through electrophilic lipid peroxidation by-products [ 45 ]. Plant polyphenols nullifying free radicals through donating of an electron or hydrogen atom [ 47 ]. There are different types of cells in human semen which include mature and immature sperms, leukocytes, round cells from diverse spermatogenic process stages and epithelial cells [ 8 ]. Of the aforementioned cells, the major sources of ROS are immature sperm cells and leukocytes [ 49 ]. Excessive ROS production has been reported to be associated with leukocytes especially macrophages and neutrophils , eventually causing sperm dysfunction [ 8 ]. Reports have also shown that a positive association exists between immature sperm cells and the production of ROS. This effect may have a negative effect on ejaculate quality. Also the increase of immature sperm cells in semen is directly proportional to greater concentration of mature sperm cells with damaged DNA [ 50 ]. Apart from the aforementioned endogenous ROS sources in the reproductive system, male reproductive organs are exposed to many exogenous sources of oxidants including those derived from individual lifestyle such as alcohol use, smoking, obesity, and poor dietary intake [ 8 ]. Environmental sources of ROS include pollution, exposure to heavy metals, phthalate, heat and mobile phone radiation [ 8 ]. ROS can also affect the male reproductive system through genitourinary tract infections or could be iatrogenic through exposure to drugs, or due to clinical varicocele [ 8 ]. The role of OS in male fertility is summarized in Figure 1. Role of oxidative stress in male fertility. Spermatogenesis involves proliferation of spermatogonia, spermatocytes meiosis and spermiogenesis occurring in the seminiferous tubules located in the testis [ 13 ]. The process is extremely replicable generating about one thousand sperm cell per second. The illustration of the process involves mitotic division of spermatogonia giving rise to spermatocytes which go through meiosis and give rise to haploid cells known as spermatids that are finally transformed by spermiation to spermatozoa [ 51 ]. When there is a disturbance in this process it can result in male infertility. One of the factors that can disrupt the process of spermatogenesis is oxidative stress [ 13 ]. Approximately, ROS contributes to about 30—80 percent of male infertility and male gametes activities are altered by oxidative stress [ 8 ]. The oxidative stress caused as a result of free radicals have a significant impact in the production as well as increasing abnormal spermatozoa, decreasing spermatozoa count and promoting sperm DNA transformation and fragmentation [ 52 , 53 , 54 , 55 ]. That the greater susceptibility of spermatozoa to oxidative stress when likened to other cells is owing to the fact that mature sperm cell have cytoplasm in limited amount, the sperm structure having greater level of unsaturated fatty acids and the antioxidant in sperm cells are being suppressed by ROS concentration [ 56 ]. Oxidative stress can also result in arterial occlusion then severe damage to the cell of the reproductive system and as a result defects in spermatogenesis occurs [ 55 ]. Semen antioxidant system consist of enzymatic and non- enzymatic factors and compounds with low molecular weight having antioxidant capacity acting upon one another to bring about protection against ROS [ 57 ]. It has been reported that if any of these is inadequate it may lead to total plasma antioxidant capacity reduction [ 57 ]. Three essential antioxidant enzyme in the semen are catalase, superoxide dismutase and glutathione peroxide [ 57 ]. Superoxide dismutase superoxide oxidoreductases — SOD which are metaloenzymes capable of catalyzing superoxide anion dismutation reactions they are of two forms —intracellular and extracellular [ 58 ]. The intracellular forms includes copper- zinc SOD having in the active center copper and zinc Cu, ZnSOD, SOD—1 which is found mainly in the cytoplasm, and manganese SOD found majorly in the mitochondrial matrix and having in its active center manganese MnSOD, SOD—2. Acting in the extracellular space is the extracellular form of SOD EC—SOD, SOD—3 and it is associated with the surface polysaccharides and can be found free, they have an active center made of copper and zinc [ 59 ]. Catalase catalyzes the reaction in which hydrogen peroxide is decomposed to molecular oxygen and water. Having a heme system structure with centrally located iron atom. It can be present in human as well as rat spermatozoa and seminal fluid having the prostrate as its source [ 58 ], and it enhances nitric oxide induced capacitation [ 57 ]. Also present as antioxidant system in the semen is the enzyme glutathione peroxidase GPX , GPX has the capability of catalyzing organic peroxides, hydrogen peroxide as well as peroxides of phospholipids reduction [ 59 ]. Various tests which have been classified into direct and indirect assay are used in the determination of seminal ROS levels [ 60 ]. Report has shown hyperviscosity to be suggestive of oxidative stress due to its association with elevated malondialdehyde levels [ 60 ]. Factor such as increase in round cells or leukocytes being a respectable source of ROS may infer OS and abnormal sperm morphology [ 61 ]. Tests such as hypo-osmotic swelling indicate spermatozoa membrane damage as a result of lipid peroxidation therefore indicating greater ROS level in semen [ 62 ]. So as to elucidate the role of oxidative stress in steroidogenesis, a number of studies have been done on animal models through introduction of exogenous sources of oxidants. ROS can impair steroidogenesis through destruction of important components in the steroidogenic pathway [ 63 ]. In another animal study, steroidogenesis, as implied by the level of FSH, LH and testosterone was seen to be reduced in animals fed with selenium-deficient diet when compared with selenium-fed animals. This indicate a possible role of oxidative stress on steroidogenesis in selenium deficient animals through the ability of selenium as an antioxidant to reverse this reduction in steriodogenesis [ 64 ]. Also increase in oxidative stress may result in gonadal dysfunction, reduction in testosterone level and testicular tissue damage indicating that gonadal steroid biosynthesis can be affected by oxidative stress [ 64 ]. In a lipopolysaccharide induced oxidative stress model in rats, it was reported that a correlation exists between a progressive oxidative state and reduction in the steroidogenic acute regulatory protein [ 65 ]. Reasonably sufficient erectile and sexual functionality is essential for men [ 66 ]. Sexual activities improve the quality of life in men and promote longevity [ 67 ]. ED can develop due to psychological, endocrine, vascular, neurological, and immune factors acquired via environmental exposure, lifestyle and underlying pathology [ 68 ]. The regular final mechanism of ED is vascular failure of the penis driven by significant corporal smooth muscle dysfunction which is mediated and skyrocketed via intracellular oxidative stress cumulating in a rise in smooth and endothelial muscle cell dysfunction with increase in the rate of apoptosis [ 67 ]. Nitric oxide NO is essential for adequate erection via nitric oxide synthase transcription NOS to bring about vasodilation as well as penile engorgement with blood [ 68 ]. On this basis the regular vascular failure mediator in ED is inflammation and OS, this it does through NOS reduction and subsequent reduction in NO [ 68 ]. Dysfunctional endothelial cells and increase in cellular adhesion molecules caused by inflammation enhance local arteriosclerosis and hardening of the vasculature which in turn result in local inflammation and OS [ 68 ]. Therefore,, there is need for the introduction of exogenous antioxidant to supplement the antioxidant defense in the body [ 55 ]. Therefore, the following are some of the factors reported to be free radicals scavengers and efficient antioxidants capable of reducing testicular oxidative stress [ 55 ]. Vitamin E also called α-tocopherol is an effective lipophiliic antioxidant which maintains and protects spermatozoa and also contributes to the liveliness of spermatocytes and sertoli cell lines [ 55 ]. Vitamin C also called ascorbic acid plays vital role in spermatogenesis. For this reason, inadequacies in either of these two vitamins result in testicular oxidative stress and disorders of spermatogenesis and testosterone production [ 69 ]. Furthermore, vitamins C and E therapies combat oxidative stress induced by cadmium, alcohol, endosulfan and arsenic and also bring about a reduction in resultant complications [ 70 ]. Vitamin E has the ability of attenuating lipid peroxidation in mitochondrial and testicular microsomes and also able to combat adverse effects of oxidative stress that occur as a result of exposure to some exogenous factors such as iron overload, ozone gas, aflatoxin and ozone gas thereby being effectual in the protection of testicular functions [ 71 ]. Zinc has been reported to be an effective antioxidant agent as well as major constituent of free radical-inhibiting enzymes like SOD [ 55 ]. Also, zinc through transferring and relocation of metals such as copper and iron is capable of preventing lipid peroxidation [ 72 ]. Studies have shown a reduction in antioxidant defense potential and a synchronous elevation in lipid peroxidation in testicular tissue in rats fed with zinc-deficient diet [ 70 ]. Selenium is an important integrant of selenoproteins, it is essential in preventing OS, maintaining redox signaling state in cells and regulating thyroxine metabolism [ 55 ]. This antioxidant prevents oxidative stress by reducing free radical population in the male reproductive cells and fluids [ 55 ]. It also protect some indispensable vitamins like vitamin C and vitamin E in the body by acting synergistically with them thereby decreasing damages induced by free radicals to reproductive cells [ 55 ]. It has been reported that increase in the levels ROS is related with reduction in the reproductive capability in females and infertility [ 73 ]. Reports have also shown that an elevation in the production of steroid hormone by developing follicles goes along with an increase in cytochrome P activity which results from the production of ROS such as hydrogen peroxide [ 74 ]. DNA damage and ovarian follicle apoptosis may be caused also by OS [ 75 , 76 ]. It was noted that in dominant follicles that there is a simultaneous increase in estrogen and catalase in reaction to FSH stimulation which suggests a role of catalase in apoptosis prevention among follicles [ 77 ]. It was also reported that the oxidized form of LDL oxLDL and its receptor LOX-1 are bestowed in follicular fluid or human granulosa cells and are elevated in oxidative stress states interfering with follicular maturation [ 78 ]. Growing follicles may be an inadvertent target of ROS especially in patients undergoing radiotherapy which generate a high amount of ROS [ 79 , 80 ]. The ROS produced in granulosa cells may have a negative impact on oocyte fertilization as well as the rate and quality of implantation of the embryo [ 80 ]. Reports have also proven that germ cells are more vulnerable to deleterious effect of OS than somatic cells [ 81 , 82 ]. Furthermore, reports has shown that OS from radiotherapy may result in ovarian atrophy, oocyte loss coupled with reduction in follicle store which may in turn result in menstrual irregularities, ovarian failure and ultimately infertility [ 80 ]. When the ovary is over-exposed to hydrogen peroxide, it uncouples the LH receptor from adenylate cyclase. This causes disruption in protein synthesis and utilization of cholesterol by the mitochondrion p side chain cleavage [ 83 ]. This disruption is likely facilitated by the reduced production of steroidogenic acute regulatory protein StAR. The StAR enhance the movement of cholesterol to the inner membrane of the mitochondria where p side chain cleavage converts cholesterol to pregnenolone [ 83 ]. Also, a reverse transport of cholesterol and estrogen synthesis in the follicle is facilitated by Lecithin cholesterol acyltransferase LCAT. Evidences exist that suggest that these transporters are subjects of oxidative stress. A study reported that exogenous antioxidants like vitamin C are accumulated in mature follicles to prevent LCAT from oxidative damage and for steroidogenesis enhancement [ 84 ]. Ovulation is a process involving local inflammatory response, which leads to elevated levels of ROS [ 85 ]. The increased ROS levels may lead to potential destruction of the granulosa cells which are going through luteinization in the course of ovulation [ 86 ]. ROS are generated during ovulation in a similar way as it occurs in inflammation. It was reported that agents that inhibits inflammatory response also suppresses ovulation [ 87 ]. The source of ROS in this process seems to be from macrophages and neutrophils because they are common in the ovaries and they led to increase production free radicals [ 88 ]. ROS has been shown to be generated during the ovulatory cascade. ROS in ovulation was noted to be mediated by protein kinase C and gonadotropin leading to production of nicotinamide adenine dinucleotide phosphate oxidase which engenders more reactive species in the course of ovulation [ 89 ]. ARTs are advanced technological procedures which are used to treat infertility [ 90 ]. The quality of oocyte is greatly dependent on the follicular fluid microenvironment, thereby affecting the fertilization and embryo rate and quality respectively [ 73 ]. Oxidative stress markers has being said to be present in the follicular fluid of patient undertaking embryo transfer ET or even in-vitro fertilization IVF [ 91 , 92 , 93 , 94 ]. A reduction in intra-follicular oxygenation is said to be interrelated with a reduction in the potential of oocyte development this is as a result of an increase in the frequency of oocyte cytoplasmic disorder, impairment in cleavage and abnormal segregation in oocyte chromosome caused by follicles that are poorly vascularized [ 8 ]. The increase in embryo fragmentation, which leads to an increase in apoptosis, has been reported to be caused by ROS [ 8 ]. Hence, elevation in the ROS level is detrimental to the growth of the embryo and Sperm-oocyte interaction [ 8 ]. Pathological pregnancies such as pre-eclampsia have been reported to be a complicated multisystem disorder affecting about 5—8 percent of all pregnancies and it contributes largely to fetal and maternal mortality and morbidity [ 95 ]. It has been reported that etiopathogenesis of preeclampsia may be caused by of oxidative stress and may be due to an elevation in the placenta metabolic activity as well as a reduction in its antioxidants scavenging power [ 95 ]. The role of oxidative stress in the female reproductive processes is summarized in Figure 2. Role of oxidative stress in the female reproductive processes. Reports have shown that the female reproductive system is susceptible to oxidative damage which if left untreated the damage process continues [ 8 , 95 ]. There are various antioxidants that have been reported to scavenge free radicals and to keep the reproductive system healthy [ 96 ]. They include vitamins C, E and β-carotene, L-carnitine, acetyl L-carnitine and also metallo-enzymes such as catalase, superoxide dismutase SOD, containing copper, manganese and zinc , glutathione peroxidase GPx, containing selenium and superoxide dismutase SOD, containing manganese, copper and zinc [ 96 ]. The antioxidants mentioned above when taken helps the total antioxidant system to be coordinated functionally [ 97 ]. Antioxidant system in the ovary such as of carotenoids, CAT, glutathione and vitamin E are responsible for regulation of ROS [ 96 ]. It was documented that the effect of SOD is noticeable in the theca interna cells of antral follicles [ 96 ]. These cells during maturation phase protect oocyte from been destroyed by redundant ROS [ 96 ]. Vitamin C which can be found in the cytosol of oocyte and extracellular fluid is used in the treatment of luteal phase disorder and recurrent abortions [ 8 ]. Vitamin C is given to patient during in vitro fertilization IVF embryo transfer as a supplement in hormonal stimulation to guarantee a large concentration of vitamin C in the follicular fluid which improved oocytes and embryo qualities. During of embryonic development, the embryo is vulnerable to OS [ 97 ]. The stage of one-cell embryo depends on Krebs cycle during early phases of development, on the other hand during the other initial embryo organogenesis, anaerobic pathway and glycolysis is relied on, so does blastocyst [ 97 ]. However, there is a larger dependence on aerobic and oxidative metabolism at the establishment of circulatory system leading to a higher production of ROS by the mitochondria but antioxidants are present as well to negate and detoxify ROS [ 95 ]. But with time there may be disruption in the antioxidant and oxidant balance by the exogenous agents responsible for the stimulation of ROS which results in disruption in embryo and fetal functions [ 97 ]. Oxidative stress plays a notable role along the several processes involved in male and female reproduction. While a physiologic amount of reactive species are needed for optimal functioning of the male and female sex organs there are conditions which produces a considerable amount of reactive species and a concomitant depression of the antioxidant system. This oxidative stress state impairs the reproductive processes and causes general disruption through inflammation, DNA damage, lipid peroxidation, protein alterations and mitochondrial dysfunction. It will be of importance to identify oxidative stress biomarkers specific for each reproductive processes and map out their standard range so as to advance measures to curtail the growing level of infertility among human population in future research. It is also recommended that the role of genetics and oxidative stress in the etiology of infertility should be a priority for researchers. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Suna Sabuncuoglu. Open access peer-reviewed chapter Importance of Oxidative Stress Mechanism in Reproductive Functions and Infertility Written By Moyinoluwa Comfort Onaolapo, Samuel Chibueze Nzekwe, Lateef Okeleji Olabisi, Victor Oluwaseyi Amos, Oluwatobi Hezekiah Ajayi and Ayodeji Folorunsho Ajayi. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Importance of Oxidative Stress and Antioxidant System in Health a From the Edited Volume Importance of Oxidative Stress and Antioxidant System in Health and Disease Edited by Suna Sabuncuoğlu and Ahmet Yalcinkaya Book Details Order Print. Chapter metrics overview Chapter Downloads View Full Metrics. Impact of this chapter. Keywords female reproduction health infertility male reproduction oxidative stress. Introduction Oxidative stress connotes the damage that occurs when the activities of reactive oxidants overwhelms the in vivo capabilities of antioxidants [ 1 ]. References 1. Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biology. Finkel T. Signal transduction by reactive oxygen species. Journal of Cell Biology. Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, et al. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. Journal of Pineal Research. World Health Organization WHO. International Classification of Diseases. Geneva: WHO; 5. Ombelet W. WHO fact sheet on infertility gives hope to millions of infertile couples worldwide. Direkvand Moghaddam A, Delpisheh A, Sayehmiri K. An investigation of the worldwide prevalence of infertility as a systematic review. Qom University of Medical Sciences Journal. Takeshima T, Usui K, Mori K, Asai T, Yasuda K, Kuroda S, et al. Oxidative stress and male infertility. Reproductive Medicine and Biology. Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian Journal of Andrology. Zhaku V, Agarwal A, Beadini S, Henkel R, Finelli R, Beadini N, et al. Male infertility, oxidative stress and antioxidants. In: Vitamin E in Health and Disease-Interactions, Diseases and Health Aspects. London, UK: IntechOpen; Gupta S, Ghulmiyyah J, Sharma R, Halabi J, Agarwal A. Power of proteomics in linking oxidative stress and female infertility. BioMed Research International. Article ID: DOI: Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reproductive Biology and Endocrinology. Parul S, Preety G, Naveen K, Jaspreet K, Parminder K. Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. USA: Oxford University Press; Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology. Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. The Plant Cell. Bayr H. Reactive oxygen species. Critical Care Medicine. Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radical Biology and Medicine. Mani S. Production of reactive oxygen species and its implication in human diseases. In: Free Radicals in Human Health and Disease. New Delhi: Springer; Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: An immunologist's guide to reactive oxygen species. Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of Physiology. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. Under stable conditions, NF-kappa B remains inactive by inhibitory subunit I-kappa B. The increase of pro-inflammatory cytokines interleukin IL 1-beta and tumor necrosis factor TNF -alpha activates the apoptotic cascade, causing cell death. Conversely, the antioxidants vitamin C and E, and sulfalazine can prevent this damage by inhibiting the activation of NF-kappa B [ 3 ]. Deleterious attacks from excess ROS may ultimately end in cell death and necrosis. These harmful attacks are mediated by the following more specialized mechanisms [ 2 ]. Consequently, the mitochondrial membrane potential becomes unstable and ATP production ceases. Lipid peroxidation : This occurs in areas where polyunsaturated fatty acid side chains are prevalent. Vitamin E can break this chain reaction due to its lipid solubility and hydrophobic tail. Protein modifications : Amino acids are targets for oxidative damage. Direct oxidation of side chains can lead to the formation of carbonyl groups. DNA oxidation : Mitochondrial DNA is particularly prone to ROS attack due to the presence of O 2 - in the ETC, lack of histone protection, and absence of repair mechanisms. Reactive oxygen species are known to promote tyrosine phosphorylation by heightening the effects of tyrosine kinases and preventing those of tyrosine phosphatases. The inhibition of tyrosine phosphatases by ROS takes place at the cysteine residue of their active site. One possible mechanism of this inhibition is that it occurs through the addition of H 2 O 2 , which binds the cysteine residue and converts it to sulfenic acid. Another possible mechanism of inhibition is through the production of GSH via reduction from its oxidized form of GSSG; this conversion alters the catalytic cysteine residue site [ 49 ]. The human body is composed of many important signaling pathways. Amongst the most important signaling pathways in the body are the mitogen-activated protein kinases MAPK. MAPK pathways are major regulators of gene transcription in response to OS. This process promotes the actions of receptor tyrosine kinases, protein tyrosine kinases, receptors of cytokines, and growth factors [ 50 , 51 ]. Excessive amounts of ROS can disrupt the normal effects of these cascade-signaling pathways. Other pathways that can be activated by ROS include the c-Jun N -terminal kinases JNK and p38 pathways. The JNK pathway prevents phosphorylation due to its inhibition by the enzyme GST. The addition of H 2 O 2 to this cascade can disrupt the complex and promote phosphorylation [ 52 , 53 ]. The presence of ROS can also dissociate the ASK1—Trx complex by activating the kinase [ 54 ] through the mechanism discussed earlier. Hypoxia-inducible factors HIF are controlled by O 2 concentration. They are essential for normal embryonic growth and development. Low O 2 levels can alter HIF regulatory processes by activating erythropoietin, another essential factor for proper embryonic growth and development [ 55 , 56 ]. The preservation of physiological cellular functions depends on the homeostatic balance between oxidants and antioxidants. Oxidative stress negatively alters cell-signaling mechanisms, thereby disrupting the physiologic processes required for cell growth and proliferation. Almost half of infertility cases are caused by male reproductive pathologies [ 57 ], which can be congenital or acquired. Both types of pathology can impair spermatogenesis and fertility [ 58 , 59 ]. In males, the role of OS in pathologies has long been recognized as a significant contributor to infertility. Men with high OS levels or DNA damaged sperm are likely to be infertile [ 60 ]. The key predictors of fertilization capability are sperm count and motility. These essential factors can be disturbed by ROS [ 60 ] and much importance has been given to OS as a major contributor to infertility in males [ 61 ]. Low levels of ROS are necessary to optimize the maturation and function of spermatozoa. The main sources of seminal ROS are immature spermatozoa and leukocytes [ 4 ]. In addition, acrosome reactions, motility, sperm capacitation, and fusion of the sperm membrane and the oolemma are especially dependent on the presence of ROS [ 4 , 60 ]. Abnormal and non-viable spermatozoa can generate additional ROS and RNS, which can disrupt normal sperm development and maturation and may even result in apoptosis [ 4 ]. Specifically, H 2 O 2 and the SO anion are perceived as main instigators of defective sperm functioning in infertile males [ 60 ]. Abnormally high seminal ROS production may alter sperm motility and morphology, thus impairing their capacity to fertilize [ 62 ]. The contribution of OS to male infertility has been well documented and extensively studied. On the other hand, the role of OS in female infertility continues to emerge as a topic of interest, and thus, the majority of conducted studies provide indirect and inconclusive evidence regarding the oxidative effects on female reproduction. Each month, a cohort of oocytes begin to grow and develop in the ovary, but meiosis I resumes in only one of them, the dominant oocyte. This process is targeted by an increase in ROS and inhibited by antioxidants. In contrast, the progression of meiosis II is promoted by antioxidants [ 42 ], suggesting that there is a complex relationship between ROS and antioxidants in the ovary. The increase in steroid production in the growing follicle causes an increase in P, resulting in ROS formation. Reactive oxygen species produced by the pre-ovulatory follicle are considered important inducers for ovulation [ 4 ]. Oxygen deprivation stimulates follicular angiogenesis, which is important for adequate growth and development of the ovarian follicle. Follicular ROS promotes apoptosis, whereas GSH and follicular stimulating hormone FSH counterbalance this action in the growing follicle. Estrogen increases in response to FSH, triggering the generation of catalase in the dominant follicle, and thus avoiding apoptosis [ 42 ]. Ovulation is essential for reproduction and commences by the LH surge, which promotes important physiological changes that result in the release of a mature ovum. An overabundance of post-LH surge inflammatory precursors generates ROS; on the other hand, depletion of these precursors impairs ovulation [ 46 ]. In the ovaries, the corpus luteum is produced after ovulation; it produces progesterone, which is indispensable for a successful pregnancy. Reactive oxygen species are also produced in the corpus luteum and are key factors for reproduction. When pregnancy does not occur, the corpus luteum regresses. Conversely, when pregnancy takes place, the corpus luteum persists [ 63 ]. A rapid decline in progesterone is needed for adequate follicle development in the next cycle. Cu,Zn-SOD increases in the corpus luteum during the early to mid-luteal phase and decreases during the regression phase. This activity parallels the change in progesterone concentration, in contrast to lipid peroxide levels, which increase during the regression phase. The decrease in Cu,Zn-SOD concentration could explain the increase in ROS concentration during regression. Other possible explanations for decreased Cu,Zn-SOD are an increase in prostaglandin PG F2-alpha or macrophages, or a decrease in ovarian blood flow [ 42 ]. Prostaglandin F2-alpha stimulates production of the SO anion by luteal cells and phagocytic leukocytes in the corpus luteum. Decreased ovarian blood flow causes tissue damage by ROS production. Concentrations of Mn-SOD in the corpus luteum during regression increase to scavenge the ROS produced in the mitochondria by inflammatory reactions and cytokines. Complete disruption of the corpus luteum causes a substantial decrease of Mn-SOD in the regressed cell. At this point, cell death is imminent [ 46 ]. The Cu,Zn-SOD enzyme is intimately related to progesterone production, while Mn-SOD protects luteal cells from OS-induced inflammation [ 42 ]. During normal pregnancy, leukocyte activation produces an inflammatory response, which is associated with increased production of SO anions in the 1 st trimester [ 64 , 65 ]. Importantly, OS during the 2 nd trimester of pregnancy is considered a normal occurrence, and is supported by mitochondrial production of lipid peroxides, free radicals, and vitamin E in the placenta that increases as gestation progresses [ 66 — 69 ]. Aging is defined as the gradual loss of organ and tissue functions. Oocyte quality decreases in relation to increasing maternal age. Recent studies have shown that low quality oocytes contain increased mtDNA damage and chromosomal aneuploidy, secondary to age-related dysfunctions. These mitochondrial changes may arise from excessive ROS, which occurs through the opening of ion channels e. Levels of 8-oxodeoxyguanosine 8-OHdG , an oxidized derivative of deoxyguanosine, are higher in aging oocytes. In fact, 8-OHdG is the most common base modification in mutagenic damage and is used as a biomarker of OS [ 70 ]. Oxidative stress, iron stores, blood lipids, and body fat typically increase with age, especially after menopause. The cessation of menses leads to an increase in iron levels throughout the body. Elevated iron stores could induce oxidative imbalance, which may explain why the incidence of heart disease is higher in postmenopausal than premenopausal women [ 71 ]. Menopause also leads to a decrease in estrogen and the loss of its protective effects against oxidative damage to the endometrium [ 72 ]. Hormone replacement therapy HRT may be beneficial against OS by antagonizing the effects of lower antioxidant levels that normally occurs with aging. However, further studies are necessary to determine if HRT can effectively improve age-related fertility decline. Endometriosis is a benign, estrogen-dependent, chronic gynecological disorder characterized by the presence of endometrial tissue outside the uterus. Lesions are usually located on dependent surfaces in the pelvis and most often affect the ovaries and cul-de-sac. They can also be found in other areas such as the abdominal viscera, the lungs, and the urinary tract. These may include retrograde menstruation, impaired immunologic response, genetic predisposition, and inflammatory components [ 74 ]. The mechanism that most likely explains pelvic endometriosis is the theory of retrograde menstruation and implantation. This theory poses that the backflow of endometrial tissue through the fallopian tubes during menstruation explains its extra-tubal locations and adherence to the pelvic viscera [ 75 ]. Studies have reported mixed results regarding detection of OS markers in patients with endometriosis. While some studies failed to observe increased OS in the peritoneal fluid or circulation of patients with endometriosis [ 76 — 78 ], others have reported increased levels of OS markers in those with the disease [ 79 — 83 ]. The peritoneal fluid of patients have been found to contain high concentrations of malondialdehyde MDA , pro-inflammatory cytokines IL-6, TNF-alpha, and IL-beta , angiogenic factors IL-8 and VEGF , monocyte chemoattractant protein-1 [ 82 ], and oxidized LDL ox-LDL [ 84 ]. Pro-inflammatory and chemotactic cytokines play a central role in the recruitment and activation of phagocytic cells, which are the main producers of both ROS and RNS [ 82 ]. Non-enzymatic peroxidation of arachidonic acid leads to the production of F2-isoprostanes [ 85 ]. Lipid peroxidation, and thus, OS in vivo [ 83 ], has been demonstrated by increased levels of the biomarker 8-iso-prostaglandin F2-alpha 8-iso-PGF2-alpha [ 86 — 88 ]. Along with its vasoconstrictive properties, 8-iso-PGF2-alpha promotes necrosis of endothelial cells and their adhesion to monocytes and polymorphonuclear cells [ 89 ]. A study by Sharma et al measured peritoneal fluid and plasma levels of 8-iso-PGF2-alpha in vivo of patients with endometriosis. They found that 8-iso-PGF2-alpha levels in both the urine and peritoneal fluid of patients with endometriosis were significantly elevated when compared with those of controls [ 83 ]. Levels of 8-iso-PGF2-alpha are likely to be useful in predicting oxidative status in diseases such as endometriosis, and might be instrumental in determining the cause of concurrent infertility. The main inducible forms of HSP70 are HSPA1A and HSPA1B [ 91 ], also known as HSP70A and HSP70 B respectively [ 90 ]. Both forms have been reported as individual markers of different pathological processes [ 92 ]. Heat shock protein 70 B is an inducible member of HSP family that is present in low levels under normal conditions [ 93 ] and in high levels [ 94 ] under situations of stress. It functions as a chaperone for proteostatic processes such as folding and translocation, while maintaining quality control [ 95 ]. It has also been noted to promote cell proliferation through the suppression of apoptosis, especially when expressed in high levels, as noted in many tumor cells [ 94 , 96 — 98 ]. As such, HSP70 is overexpressed when there is an increased number of misfolded proteins, and thus, an overabundance of ROS [ 94 ]. The release of HSP70 during OS stimulates the expression of inflammatory cytokines [ 93 , 99 ] TNF-alpha, IL-1 beta, and IL-6, in macrophages through toll-like receptors e. TLR 4 , possibly accounting for pelvic inflammation and growth of endometriotic tissue [ 99 ]. Fragmentation of HSP70 has been suggested to result in unregulated expression of transcription factor NF-kappa B [ ], which may further promote inflammation within the pelvic cavity of patients with endometriosis. Oxidants have been proposed to encourage growth of ectopic endometrial tissue through the induction of cytokines and growth factors [ ]. Signaling mediated by NF-kappa B stimulates inflammation, invasion, angiogenesis, and cell proliferation; it also prevents apoptosis of endometriotic cells. Activation of NF-kappa B by OS has been detected in endometriotic lesions and peritoneal macrophages of patients with endometriosis [ ]. N-acetylcysteine NAC and vitamin E are antioxidants that limit the proliferation of endometriotic cells [ ], likely by inhibiting activation of NF-kappa B [ ]. Future studies may implicate a therapeutic effect of NAC and vitamin E supplementation on endometriotic growth. This may explain the increased expressions of these proteins in ectopic versus eutopic endometrial tissue [ ]. Iron mediates production of ROS via the Fenton reaction and induces OS [ ]. In the peritoneum of patients with endometriosis, accumulation of iron and heme around endometriotic lesions [ ] from retrograde menstruation [ ] up-regulates iNOS activity and generation of NO by peritoneal macrophages [ ]. Extensive degradation of DNA by iron and heme accounts for their considerable free radical activity. Chronic oxidative insults from iron buildup within endometriotic lesions may be a key factor in the development of the disease [ ]. Naturally, endometriotic cysts contain high levels of free iron as a result of recurrent cyclical hemorrhage into them compared to other types of ovarian cysts. However, high concentrations of lipid peroxides, 8-OHdG, and antioxidant markers in endometrial cysts indicate lipid peroxidation, DNA damage, and up-regulated antioxidant defenses respectively. These findings strongly suggest altered redox status within endometrial cysts [ ]. Potential therapies have been suggested to prevent iron-stimulated generation of ROS and DNA damage. Based on results from their studies of human endometrium, Kobayashi et al have proposed a role for iron chelators such as dexrazoxane, deferoxamine, and deferasirox to prevent the accumulation of iron in and around endometriotic lesions [ ]. Future studies investigating the use of iron chelators may prove beneficial in the prevention of lesion formation and the reduction of lesion size. Many genes encoding antioxidant enzymes and proteins are recruited to combat excessive ROS and to prevent cell damage. Amongst these are Trx and Trx reductase, which sense altered redox status and help maintain cell survival against ROS [ ]. Total thiol levels, used to predict total antioxidant capacity TAC , have been found to be decreased in women with pelvic endometriosis and may contribute to their status of OS [ 81 , ]. Conversely, results from a more recent study failed to correlate antioxidant nutrients with total thiol levels [ ]. Patients with endometriosis tend to have lower pregnancy rates than women without the disease. Low oocyte and embryo quality in addition to spermatotoxic peritoneal fluid may be mediated by ROS and contribute to the subfertility experienced by patients with endometriosis [ ]. The peritoneal fluid of women with endometriosis contains low concentrations of the antioxidants ascorbic acid [ 82 ] and GPx [ 81 ]. The reduction in GPx levels was proposed to be secondary to decreased progesterone response of endometrial cells [ ]. The link between gene expression for progesterone resistance and OS may facilitate a better understanding of the pathogenesis of endometriosis. It has been suggested that diets lacking adequate amounts of antioxidants may predispose some women to endometriosis [ ]. Studies have shown decreased levels of OS markers in people who consume antioxidant rich diets or take antioxidant supplements [ — ]. In certain populations, women with endometriosis have been observed to have a lower intake of vitamins A, C [ ], E [ — ], Cu, and Zn [ ] than fertile women without the disease [ — ]. Daily supplementation with vitamins C and E for 4 months was found to decrease levels of OS markers in these patients, and was attributed to the increased intake of these vitamins and their possible synergistic effects. Pregnancy rates, however, did not improve [ ]. Intraperitoneal administration of melatonin, a potent scavenger of free radicals, has been shown to cause regression of endometriotic lesions [ — ] by reducing OS [ , ]. These findings, however, were observed in rodent models of endometriosis, which may not closely resemble the disease in humans. It is evident that endometriotic cells contain high levels of ROS; however, their precise origins remain unclear. Impaired detoxification processes lead to excess ROS and OS, and may be involved in increased cellular proliferation and inhibition of apoptosis in endometriotic cells. It is a disorder characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovaries [ ]. Clinical manifestations of PCOS commonly include menstrual disorders, which range from amenorrhea to menorrhagia. Skin disorders are also very prevalent amongst these women. Insulin resistance may be central to the etiology of PCOS. Signs of insulin resistance such as hypertension, obesity, and central fat distribution are associated with other serious conditions, such as metabolic syndrome, nonalcoholic fatty liver [ ], and sleep apnea. All of these conditions are risk factors for long-term metabolic sequelae, such as cardiovascular disease and diabetes [ ]. Most importantly, waist circumference, independent of body mass index BMI , is responsible for an increase in oxLDL [ 71 ]. Polycystic ovary syndrome is also associated with decreased antioxidant concentrations, and is thus considered an oxidative state [ ]. The decrease in mitochondrial O 2 consumption and GSH levels along with increased ROS production explains the mitochondrial dysfunction in PCOS patients [ ]. The mononuclear cells of women with PCOS are increased in this inflammatory state [ ], which occurs more so from a heightened response to hyperglycemia and C-reactive protein CRP. Physiological hyperglycemia generates increased levels of ROS from mononuclear cells, which then activate the release of TNF-alpha and increase inflammatory transcription factor NF-kappa B. As a result, concentrations of TNF-alpha, a known mediator of insulin resistance, are further increased. The resultant OS creates an inflammatory environment that further increases insulin resistance and contributes to hyperandrogenism [ ]. Lifestyle modification is the cornerstone treatment for women with PCOS. This includes exercise and a balanced diet, with a focus on caloric restriction [ ]. However, if lifestyle modifications do not suffice, a variety of options for medical therapy exist. Combined oral contraceptives are considered the primary treatment for menstrual disorders. Currently, there is no clear primary treatment for hirsutism, although it is known that combination therapies seem to produce better results [ ]. Unexplained infertility is defined as the inability to conceive after 12 months of unprotected intercourse in couples where known causes of infertility have been ruled out. It is thus considered a diagnosis of exclusion. Its pathophysiology remains unclear, although the literature suggests a possible contribution by increased levels of ROS, especially shown by increased levels of the lipid peroxidation marker, MDA [ , ] in comparison to antioxidant concentration in the peritoneal cavity [ ]. The increased amounts of ROS in these patients are suggestive of a reduction in antioxidant defenses, including GSH and vitamin E [ 76 ]. The low antioxidant status of the peritoneal fluid may be a determinant factor in the pathogenesis of idiopathic infertility. N-acetyl cysteine is a powerful antioxidant with anti-apoptotic effects. It is known to preserve vascular integrity and to lower levels of homocysteine, an inducer of OS and apoptosis. Badaiwy et al conducted a randomized, controlled, study in which NAC was compared with clomiphene citrate as a cofactor for ovulation induction in women with unexplained infertility [ ]. The study, however, concluded that NAC was ineffective in inducing ovulation in patients in these patients [ ]. Folate is a B9 vitamin that is considered indispensable for reproduction. It plays a role in amino acid metabolism and the methylation of proteins, lipids, and nucleic acids. Acquired or hereditary folate deficiency contributes to homocysteine accumulation. The MTHFR enzyme participates in the conversion of homocysteine to methionine, a precursor for the methylation of DNA, lipids, and proteins. Polymorphisms in folate-metabolizing pathways of genes may account for the unexplained infertility seen in these women, as it disrupts homocysteine levels and subsequently alters homeostatic status. Impaired folate metabolism disturbs endometrial maturation and results in poor oocyte quality [ ]. More studies are clearly needed to explore the efficacy of antioxidant supplementation as a possible management approach for these patients. The placenta is a vital organ of pregnancy that serves as a maternal-fetal connection through which nutrient, O 2 , and hormone exchanges occur. It also provides protection and immunity to the developing fetus. In humans, normal placentation begins with proper trophoblastic invasion of the maternal spiral arteries and is the key event that triggers the onset of these placental activities [ 6 ]. The placental vasculature undergoes changes to ensure optimal maternal vascular perfusion. Prior to the unplugging of the maternal spiral arteries by trophoblastic plugs, the state of low O 2 tension in early pregnancy gives rise to normal, physiological hypoxia [ ]. During this time, the syncytiotrophoblast is devoid of antioxidants, and thus, remains vulnerable to oxidative damage [ , ]. Between 10 and 12 weeks of gestation, the trophoblastic plugs are dislodged from the maternal spiral arteries, flooding the intervillous space with maternal blood. This event is accompanied by a sharp rise in O 2 tension [ ], marking the establishment of full maternal arterial circulation to the placenta associated with an increase in ROS, which leads to OS [ 68 ]. At physiological concentrations, ROS stimulate cell proliferation and gene expression [ ]. Placental acclimation to increased O 2 tension and OS at the end of the 1 st trimester up-regulates antioxidant gene expression and activity to protect fetal tissue against the deleterious effects of ROS during the critical phases of embryogenesis and organogenesis [ 2 ]. Amongst the recognized placental antioxidants are heme oxygenase HO -1 and -2, Cu,Zn-SOD, catalase, and GPx [ ]. If maternal blood flow reaches the intervillous space prematurely, placental OS can ensue too early and cause deterioration of the syncytiotrophoblast. This may give rise to a variety of complications including miscarriage [ , , ], recurrent pregnancy loss [ ], and preeclampsia, amongst others [ ]. These complications will be discussed below. Congenital anomalies and maternal factors such as uterine anomalies, infection, diseases, and idiopathic causes constitute the remaining causes [ ]. Overwhelming placental OS has been proposed as a causative factor of spontaneous abortion. As mentioned earlier, placentas of normal pregnancies experience an oxidative burst between 10 and 12 weeks of gestation. This OS returns to baseline upon the surge of antioxidant activity, as placental cells gradually acclimate to the newly oxidative surroundings [ ]. In cases of miscarriage, the onset of maternal intraplacental circulation occurs prematurely and sporadically between 8 and 9 weeks of pregnancy in comparison to normal continuous pregnancies [ , ]. In these placentas, high levels of HSP70, nitrotyrosine [ , ], and markers of apoptosis have been reported in the villi, suggesting oxidative damage to the trophoblast with subsequent termination of the pregnancy [ 2 ]. Antioxidant enzymes are unable to counter increases in ROS at this point, since their expression and activity increases with gestational age [ ]. The activity of serum prolidase, a biomarker of extracellular matrix and collagen turnover, has been observed to be decreased in patients with early pregnancy loss. Its levels were also shown to negatively correlate with increased OS, possibly accounting for the heightened placental vascular resistance and endothelial dysfunction secondary to decreased and dysregulated collagen turnover [ ]. A negative correlation with lipid hydroperoxide was also observed in these patients, indicating their high susceptibility to lipid peroxidation [ ]. Oxidative stress can also affect homeostasis in the ER. Persistence of endoplasmic OS can further sustain ER stress, eventually increasing decidual cell apoptosis and resulting in early pregnancy loss [ ]. Decreased detoxification ability of GPx may occur in the setting of Se deficiency, which has been linked to both spontaneous abortion [ , ] and recurrent pregnancy loss [ ]. Apoptosis of placental tissues may result from OS-induced inflammatory processes triggered by a variety of factors. Several etiologies may underlie improper initiation of maternal blood flow to the intervillous space; yet it may be through this mechanism by which both spontaneous and recurrent pregnancy loss occur. Antioxidant supplementation has been investigated in the prevention of early pregnancy loss, with the idea of replacing depleted antioxidant stores to combat an overwhelmingly oxidative environment. However, a meta-analysis of relevant studies failed to report supporting evidence of beneficial effects of antioxidant supplementation [ ]. It has been more recently suggested that the maternal uterine spiral arteries of normal pregnancies may involve uterine natural killer NK cells as a regulator of proper development and remodeling. Angiogenic factors are known to play key roles in the maintenance of proper spiral artery remodeling. Thus, the involvement of uterine NK cells in RPL has been supported by the early pregnancy findings of increased levels of angiogenic factors secreted by uterine NK cells [ ], as well as increased in vivo and in vitro endothelial cell angiogenesis induced by uterine NK cells [ ] in patients with RPL. Women experiencing RPL have also been noted to have increased endometrial NK cells, which were positively correlated to endometrial vessel density. Accordingly, it has been suggested that an increase of uterine NK cells increases pre-implantation angiogenesis, leading to precocious intra-placental maternal circulation, and consequently, significantly increased OS early in pregnancy [ ]. The syncytiotrophoblastic deterioration and OS that occur as a result of abnormal placentation may explain the heightened sensitivity of syncytiotrophoblasts to OS during the 1 st trimester, and could contribute significantly to idiopathic RPL [ ]. In keeping with this idea, plasma lipid peroxides and GSH have been observed in increased levels, in addition to decreased levels of vitamin E and β-carotene in patients with RPL [ ]. Furthermore, markedly increased levels of GSH have also been found in the plasma of women with a history of RPL, indicating a response to augmented OS [ ]. Another study showed significantly low levels of the antioxidant enzymes GPx, SOD, and catalase in patients with idiopathic RPL, in addition to increased MDA levels [ ]. Polymorphisms of antioxidant enzymes have been associated with a higher risk of RPL [ — ]. The null genotype polymorphism of GST enzymes found in some RPL patients has been reported as a risk factor for RPL [ 18 ]. Antioxidant supplementation may be the answer to restoring antioxidant defenses and combating the effects of placental apoptosis and inflammatory responses associated with extensive OS. In addition to its well-known antioxidant properties, NAC is rich in sulphydryl groups. Its thiol properties give it the ability to increase intracellular concentrations of GSH or directly scavenge free radicals [ , ]. Furthermore, the fetal toxicity, death in utero, and IUGR, induced by lipopolysaccharides, might be prevented by the antioxidant properties of NAC [ ]. By inhibiting the release of pro-inflammatory cytokines [ ], endothelial apoptosis, and oxidative genotoxicity [ ], via maintenance of intracellular GSH levels, NAC may well prove promising to suppress OS-induced reactions and processes responsible for the oxidative damage seen in complicated pregnancies. Preeclampsia is a complex multisystem disorder that can affect previously normotensive women. Preeclampsia can develop before early onset or after late onset 34 weeks of gestation. The major pathophysiologic disturbances are focal vasospasm and a porous vascular tree that transfers fluid from the intravascular to the extravascular space. The exact mechanism of vasospasm is unclear, but research has shown that interactions between vasodilators and vasoconstrictors, such as NO, endothelin 1, angiotensin II, prostacyclin, and thromboxane, can cause decrease the perfusion of certain organs. The porous vascular tree is one of decreased colloid osmotic pressure and increased vascular permeability [ — ]. From early pregnancy on, the body assumes a state of OS. Oxidative stress is important for normal physiological functions and for placental development [ ]. Preeclampsia, however, represents a much higher state of OS than normal pregnancies do [ ]. Early-onset preeclampsia is associated with elevated levels of protein carbonyls, lipid peroxides, nitrotyrosine residues, and DNA oxidation, which are all indicators of placental OS [ 68 , ]. The OS of preeclampsia is thought to originate from insufficient spiral artery conversion [ , , ] which leads to discontinuous placental perfusion and a low-level ischemia-reperfusion injury [ , , ]. Ischemia-reperfusion injury stimulates trophoblastic and endothelial cell production of ROS [ ], along with variations in gene expression that are similar to those seen in preeclampsia [ 3 ]. Oxidative stress can cause increased nitration of p38 MAPK, resulting in a reduction of its catalytic activity. This may cause the poor implantation and growth restriction observed in preeclampsia [ 6 ]. Exaggerated apoptosis of villous trophoblasts has been identified in patients with preeclampsia, of which OS has been suggested as a possible contributor. Microparticles of syncytiotrophoblast microvillus membrane STBMs have been found throughout the maternal circulation of patients with preeclampsia and are known to cause endothelial cell injury in vitro [ ]. Placental OS can be detected through increased serum concentrations of ROS such as H 2 O 2 [ ], or lipid peroxidation markers [ ] such as MDA [ , — ] and thiobarbituric acid reactive substances TBARS [ , ]. Increased circulating levels of the vasoconstrictor H 2 O 2 [ , ] and decreased levels of the vasodilator NO [ , ] have been noted in preeclampsia and may account for the vasoconstriction and hypertension present in the disease. Still, some studies have conversely reported increased circulating [ , ] and placental [ ] NO levels. Neutrophil modulation occurring in preeclampsia is another important source of ROS, and results in increased production of the SO anion and decreased NO release, which ultimately cause endothelial cell damage in patients with preeclampsia [ ]. Elevated circulating levels of sFlt-1 have been suggested to play a role in the pathogenesis of preeclampsia [ , ] and the associated endothelial dysfunction [ ]. Placental trophoblastic hypoxia resulting in OS has been linked to excess sFlt-1 levels in the circulation of preeclamptic women [ ]. Vitamins C and E, and sulfasalazine can decrease sFlt-1 levels [ ]. Heme oxygenase-1 [ ] is an antioxidant enzyme that has anti-inflammatory and cytoprotective properties. Hypoxia stimulates the expression of HO-1 [ ] in cultured trophoblastic cells, and is used to detect increased OS therein [ ]. Preeclampsia may be associated with decreased levels of HO in the placenta [ ], suggesting a decline in protective mechanisms in the disease. More recently, decreased cellular mRNA expressions of HO-1, HO-2, SOD, GPx, and catalase were reported in the blood of preeclamptic patients [ , , ]. Tissue from chorionic villous sampling of pregnant women who were diagnosed with preeclampsia later in gestation revealed considerably decreased expressions of HO-1 and SOD [ ]. Failure to neutralize overwhelming OS may result in diminished antioxidant defenses. Members of the family of NAD P H oxidases are important generators of the SO anion in many cells, including trophoblasts and vascular endothelial cells. Increased SO anion production through activation of these enzymes may occur through one of several physiological mechanisms, and has been implicated in the pathogenesis of some vascular diseases [ ]. Autoantibodies against the angiotensin receptor AT1, particularly the second loop AT1-AA [ ], can stimulate NAD P H oxidase, leading to increased generation of ROS. In cultured trophoblast and smooth muscle cells, the AT1 receptor of preeclamptic women has been observed to promote both the generation of the SO anion and overexpression of NAD P H oxidase [ ]. Between 6 and 8 weeks of gestation, active placental NAD P H yields significantly more SO anion than is produced during full-term [ ]. Thus, early placental development may be affected through dysregulated vascular development and function secondary to NAD P H oxidase-mediated altered gene expression [ 48 , ]. Preeclamptic women produce ROS and exhibit higher NAD P H expression than those without the disease [ ]. More specifically, it has been reported that women with early-onset preeclampsia produce higher amounts of the SO anion than women with late-onset disease [ ]. Levels of TNF-α, and oxLDL are increased in preeclampsia and have been shown to activate the endothelial isoform of NAD P H oxidase been, ultimately resulting in increased levels of the SO anion [ ]. The mechanism of placental NAD P H activation is still unclear, but the above findings may assist in elucidating the role of OS in the pathogenesis of placental dysfunction in reproductive diseases such as preeclampsia. Paraoxonase-1 PON 1 , an enzyme associated with HDL, acts to offset LDL oxidation and prevent lipid peroxidation [ ] in maternal serum. Baker et al demonstrated that PON 1 levels tend to be high in patients with preeclampsia, which suggests that OS contributes to the pathogenesis of the disease [ ]. Paraoxonase-1 has also been measured to be increased in patients in mid-gestation [ ], possibly in an attempt to shield against the toxic effects of high OS encountered in preeclampsia. In contrast, other studies have observed considerably decreased PON 1 in the presence of clinical symptoms [ , ] and in patients with severe preeclampsia [ ]. These results indicate consumption of antioxidants to combat heightened lipid peroxidation, which may injure vascular endothelium, and likely be involved in the pathogenesis of preeclampsia [ , ]. Affected women also have a decreased total antioxidant status TAS , placental GPx [ , , ], and low levels of vitamins C and E [ ]. Inadequate vitamin C intake seems to be associated with an increased risk of preeclampsia [ ] and some studies have shown that peri-conceptional supplementation with multivitamins may lower the risk of preeclampsia in normal or under-weight women [ , ]. However, the majority of trials to date have found routine antioxidant supplementation during pregnancy to be ineffective in reducing the risk of preeclampsia [ , — ]. Intra uterine growth restriction is defined as infant birth weight below the 10 th percentile. Placental, maternal, and fetal factors are the most common causes of IUGR. Preeclampsia is an important cause of IUGR, as it develops from uteroplacental insufficiency and ischemic mechanisms in the placenta [ ]. Imbalanced injury and repair as well as abnormal development of the villous tree are characteristic of IUGR placentas, predisposing them to depletion of the syncytiotrophoblast with consequently limited regulation of transport and secretory function. As such, OS is recognized as an important player in the development of IUGR [ ]. Women with IUGR have been reported to have increased free radical activity and markers of lipid peroxidation [ ]. Furthermore, Biri et al reported that higher levels of MDA and xanthine oxidase and lower levels of antioxidant concentrations in the plasma, placenta, and umbilical cords in patients with IUGR compared to controls [ ]. Urinary 8-oxo-7,8- dihydrodeoxyguanosine 8-OxOdG , a marker of DNA oxidation, was also observed to be elevated at 12 and 28 weeks in pregnancies complicated with growth-restricted fetuses compared with a control group [ ]. Ischemia and reperfusion injury are powerful generators of ROS and OS. The regulatory apoptotic activity of p53 [ ] is significantly increased in response to hypoxic conditions within villous trophoblasts [ — ] and signifies a greater degree of apoptosis secondary to hypoxia-reoxygenation [ ] than from hypoxia alone [ ]. Decreases in the translation and signaling of proteins add to the overwhelming OS in IUGR placentas [ ]. Furthermore, disordered protein translation and signaling in the placenta can also cause ER stress in the syncytiotrophoblast, and has been demonstrated in placentas of IUGR patients [ ]. ER stress inhibits placental protein synthesis, eventually triggering apoptosis [ ]. Moreover, induction of p38 and NF-kappa B pathways can occur through ER stress, exacerbating inflammatory responses [ ]. The chronicity these events may explain the placental growth restriction seen in these pregnancies [ ]. In addition, serum prolidase activity in patients with IUGR was significantly elevated and negatively correlated with TAC, suggesting increased and dysregulated collagen turnover [ ]. The sequence of uterine contraction, cervical dilatation, and decidual activation make up the uterine component of this pathway [ ]. However, it has been proposed that activation of this common pathway through physiological signals results in term labor, while preterm labor might occur from spontaneous activation of isolated aspects of the common pathway by the presence of pathological conditions that may be induced by multiple causes [ ] or risk factors. Preterm labor in general is divided in two distinctive types: indicated , usually due to maternal or fetal reasons, or spontaneous. The majority of spontaneous preterm deliveries occur from any of the four primary pathogenic pathways. These include uterine overdistension, ischemia, infection, cervical disease, endocrine disorders [ ], decidual hemorrhage, and maternal-fetal activation of the hypothalamic-pituitary axis, amongst others [ ]. Of these etiologies, intrauterine infection and inflammation is considered a main contributor to preterm birth [ ]. These pathogenic mechanisms converge on a common pathway involving increased protease expression and uterotonin. More than one process may take place in a given woman. The combination of genetics and inflammatory responses is an active area of research that could explain preterm labor in some women with common risk factors [ , ]. Labor induces changes in chorioamniotic membranes that are consistent with localized acute inflammatory responses, despite the absence of histological evidence of inflammation [ ]. Reactive oxygen species activates NF-kappa B, which stimulates COX-2 expression and promotes inflammation with subsequent parturition. A study by Khan et al reported markedly decreased GPx protein expression in both women with preterm labor and those with term labor, compared with the respective non-labor groups [ ]. Taken together, these data suggest that the state of labor, whether preterm or term, necessitates the actions of GPx to limit lipid oxidation, and is associated with an ROS-induced reduction of antioxidant defenses. Mustafa et al detected markedly higher levels of MDA and 8-OHdG and significantly lower GSH levels in the maternal blood of women with preterm labor than in women with term deliveries [ ]. This finding suggested that women in preterm labor have diminished antioxidant abilities to defend against OS-induced damage. The results further support that a maternal environment of increased OS and decreased antioxidants renders both the mother and fetus more susceptible to ROS-induced damage. Inflammation induces the up-regulation of ROS and can cause overt OS, resulting in tissue injury and subsequent preterm labor [ ]. The concentration of Mn-SOD increases as a protective response to inflammation and OS, and down-regulates NF-kappa B, activator protein-1, and MAPK pathways [ ]. Accordingly, higher mRNA expression of Mn-SOD was observed in the fetal membranes of women in preterm labor than in women in spontaneous labor at term, which may suggest a greater extent of OS and inflammatory processes in the former [ ]. Preterm labor has been associated with chorioamnionitis and histological infection was found to relate to elevated fetal membrane expression of Mn-SOD mRNA of women in preterm labor [ ]. The increased Mn-SOD mRNA expressions in these cases may be a compensatory response to the presence of increased OS and inflammation in preterm labor. Specifically, significantly higher amounts of the pro-inflammatory cytokines IL-1 beta, IL-6, and IL-8, have been observed in the amnion and choriodecidua of patients in preterm labor than in women in spontaneous term labor. These findings support activation of the membrane inflammatory response of women in preterm labor [ ]. Women with preterm labor have lower levels of TAS than women with uncomplicated pregnancies at a similar gestational age, which might indicate the presence of increased OS during preterm labor [ ]. Women with preterm births have also been found to have significantly decreased PON 1 activity in comparison to controls [ ]. This finding suggests that enhanced lipid peroxidation and diminished antioxidant activity of PON 1, may together create a pro-oxidant setting and increase the risk for preterm birth. Additionally, patients in preterm labor had markedly decreased levels of GSH [ ]. Low maternal serum selenium levels in early gestation have been associated with preterm birth [ ]. Polymorphism to GST was found to be significantly higher in patients in preterm labor, indicating that these patients are more vulnerable to oxidative damage [ ]. The inflammatory setting of maternal infection associated with preterm birth produces a state of OS and the consequent decrease in antioxidant defenses are likely to increase the risk for preterm birth. The presented evidence implicates inflammation and suppressed antioxidant defenses in the pathogenesis of preterm labor. Thus, it seems plausible that antioxidant supplementation may assist in preventing preterm labor and birth associated with inflammation. A study by Temma-Asano et al demonstrated that NAC was effective in reducing chorioamnionitis-induced OS, and thus, may protect against preterm labor [ ]. However, maternal supplementation with vitamins C and E in low-risk nulliparous patients during early gestation did not reduce preterm births [ , ]. Due to the conflicting results of studies, it is unclear whether maternal antioxidant supplementation plays a role in preventing the onset of preterm labor. Pregnancy is a state of increased metabolic demands required to support both maternal hormonal physiology and normal fetal development. However, inadequate or excessive pregnancy weight gain can complicate both maternal and fetal health [ ]. The adverse effects of maternal obesity and underweight on fertility from disordered hormones and menses have been well-documented [ ]. Ideally, women with a normal pre-pregnancy BMI Overweight women BMI Close to two-thirds of the United States population of reproductive-aged women are considered overweight or obese [ ]. Obese women generally take longer to conceive and have a higher risk of miscarriage than their leaner counterparts [ ]. Maternal obesity has also long been associated with several reproductive pathologies including gestational diabetes mellitus, preeclampsia, and PCOS. It has also been shown to negatively affect fertility and pregnancy. and Delivery complications and fetal complications such as macrosomia have also been linked to maternal obesity [ ]. Healthy pregnancies are associated with the mobilization of lipids, increased lipid peroxides, insulin resistance, and enhanced endothelial function. Normally, increases in total body fat peak during the 2 nd trimester. Obese women, however, experience inappropriately increased lipid peroxide levels and limited progression of endothelial function during their pregnancies, along with an additive innate tendency for central fat storage. Visceral fat is associated with disordered metabolism and adipokine status, along with insulin resistance. Centrally-stored fat deposits are prone to fatty acid overflow, thereby exerting lipotoxic effects on female reproductive ability [ ]. Oxidative stress from excessive ROS generation has been implicated in pathogenesis of obesity [ ]. Intracellular fat accumulation can disrupt mitochondrial function, causing buildup and subsequent leak of electrons from the ETC. The combined effect of high lipid levels and OS stimulates production of oxidized lipids; of particular importance are lipid peroxides, oxidized lipoproteins, and oxysterols. As major energy producers for cells, the mitochondria synthesize ATP via oxidative phosphorylation. Adverse effects of maternal BMI on mitochondria in the oocyte could negatively influence embryonic metabolism. Increased plasma non-esterified fatty acid levels can prompt the formation of the nitroxide radical. As a known inflammatory mediator, oxLDL can indirectly measure lipid-induced OS, hence elucidating its role in the inflammatory state of obesity [ ]. Oxysterol production within a lipotoxic environment can potentially disrupt the placental development and function of obese pregnancies [ ]. Consumption of a high fat meal has been shown to increase levels of both circulating endotoxins and markers of endothelial dysfunction [ — ]. Extensive evidence has linked endothelial dysfunction, increased vascular endothelial cell expression of NADPH oxidase, and endothelial OS to obesity. Overactive mitochondria and harmful ROS levels in oocytes and zygotes were influenced by peri-conceptional maternal obesity. Igosheva et al reported a decline in fertility and obscured progression of the developing embryo [ ]. The correlation between placental nitrative stress from altered vascular endothelial NO release and high maternal BMI [ ] may stem from imbalances of oxidative and nitrative stress, which may weaken protection to the placenta [ ]. Results from Ruder et al supported the association of increased maternal body weight and increased nitrative stress, but did not demonstrate a relation to placental OS [ 4 ]. Overabundant nutrition may produce an unfavorably rich reproductive environment, leading to modified oocyte metabolism and hindered embryo development. A negative association was also made between maternal diet-induced obesity and blastocyst development [ ]. Increased postprandial levels of OS biomarkers have been described after ingestion of high fat meals. A study by Bloomer et al found a greater increase in postprandial MDA in obese females versus normal weight controls [ ]. Hallmark events of obese states include decreased fatty acid uptake, enhanced lipolysis, infiltration of inflammatory cells, and secretion of adipokines [ , ]. Suboptimal oocyte quality has also been noted in obese females. More specifically, follicular fluid FF levels of CRP were observed to be abnormally high [ ]. The resultant disturbance of oocyte development may influence oocyte quality and perhaps general ovarian function. Maternal obesity has been linked to several increased risks to the mother, embryo, and fetus. Obesity is considered a modifiable risk factor; therefore, pre-conceptional counseling should stress the importance of a balanced diet and gestational weight gain within normal limits. Nutritional deficiencies in underdeveloped areas of the world continue to be a significant public health concern. Inadequate maternal nutrition during the embryonic period adversely affects fetal growth, placing a pregnant woman at risk for a low birth weight infant and potential endothelial dysfunction. Malnourished females and those with a low BMI may be at increased risk for impaired endothelium-dependent vasodilation secondary to OS [ ]. In-utero undernutrition reduces NO stores, triggering OS along with impairment of endothelium-dependent vasodilation. In rodents, gestational exposure to both caloric and protein restriction resulted in low birth weight offspring. The activity of SOD was found to be decreased with a consequent increase of the SO anion in the offspring of undernourished dams, which also indicates decreased formation of H 2 O 2. |
| How do free radicals affect the body? | Thus intrafollicular ROS levels may be used as a potential marker for predicting success with IVF. Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The literature provides some evidence of oxidative stress influencing the entire reproductive span of a woman, even the menopausal years. A link exists between enhanced ROS levels and increased sperm-oocyte fusion. Free radicals may have a positive, negative or a neutral charge [ 14 ]:. Aitken RJ, Baker MA, Sawyer D. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. |
| Table of contents | Understanding the physiological and pathological roles of ROS in male reproduction has become an essential pillar of modern andrology; however, numerous questions related to the controversial behavior of ROS in male reproductive cells and tissues still remain unanswered. This chapter aims to summarize current evidence available on the relationships between free radicals, antioxidants and male reproduction and to trigger more scientific interest, particularly with respect to the design of efficient strategies to diagnose or treat male sub- or infertility associated with OS. Aerobic life inherently depends on oxygen, which is essential for a controlled oxidation of molecules containing carbon, subsequently leading to the release of energy. Nevertheless, aerobic cells, including spermatozoa, are persistently counteracting the so-called Oxygen Paradox: while oxygen is crucial to sustain aerobic life, it is simultaneously toxic to the cell survival [ 1 ]. Normal aerobic metabolism leads to the generation of by-products called free radicals FR [ 2 , 3 ], which, under physiological conditions, are necessary for a normal cell function [ 4 ]. On the other hand, if FR concentrations become too high, either because of their overgeneration or due to low levels of antioxidant defense mechanisms, oxidative stress OS emerges with unpredictable consequences on the cell behavior and survival [ 5 ]. In the meantime, seminal OS is believed to be one of the main factors in the pathogenesis of sperm dysfunction in male sub- or infertility [ 7 , 8 , 9 ]. Several intrinsic and extrinsic factors have the ability to promote reactive oxygen species ROS generation in the testicular as well as post-testicular e. epididymal environment, resulting in defective spermatogenesis and altered sperm function [ 9 ]. Although the origin of ROS generation in semen and their roles in male reproduction have only recently been uncovered, numerous questions still remain unanswered, thus offering multiple strategies for future research. As such, the role of free radicals and oxidative stress in fertility and subfertility is an area requiring continuous scientific attention. A free radical FR is defined as any atom, molecule or a fragment of atoms and molecules with one or more unpaired electrons, capable of short independent existence. The abstraction or gain of one electron by a nonradical molecule may or may not convert it to a radical species [ 13 ]. Free radicals may have a positive, negative or a neutral charge [ 14 ]:. It is precisely the presence of an unpaired electron that results in certain common properties shared by most radicals. Free radicals are generally unstable and highly reactive. They can either donate an electron to or accept an electron from other molecules, thus behaving as oxidants or reductants [ 13 ]. In cells, one-electron modification of molecules can yield sulfur-, oxygen-, carbon- and nitrogen-derived free radicals [ 14 ]. Furthermore, ions of transition metals have a radical nature [ 13 ]. The most common and important free radicals related to biological systems are oxygen-derived radicals called reactive oxygen species ROS and nitrogen-derived molecules, defined as reactive nitrogen species RNS [ 15 ]. ROS represent a broad category of molecules including radical and non-radical oxygen derivatives [ 16 ]. Reactive nitrogen species are nitrogen-free radicals and commonly accepted as a subclass of ROS [ 13 , 15 ]. A summary of the most common oxygen- and nitrogen-derived free radicals is provided in Table 1. Virtually every ejaculate may contain potential sources of ROS. Leukocytes activated by multiple factors, especially inflammation and infection, are among significant ROS producers in semen [ 17 ]. PMN leukocytes represent an important source of ROS due to their abundant presence in semen. Furthermore, external stimuli induce the activation of macrophages, leading to an oxidative burst and ROS overgeneration. Under normal circumstances, these monocytes are of paramount importance in defending male reproductive structures against nearby cells and pathogens [ 19 ]. The Endz test based on myeloperoxidase staining is an efficient technique to quantify seminal leukocytes during semen quality assessment [ 20 ]. Numerous reports have studied possible relationships between seminal leukocytes and male reproductive dysfunction, resulting in two different directions. On the one hand, some studies failed to reveal any correlation between leukocytospermia and sperm damage [ 22 ], whereas inversely, other studies emphasized on a strong link between the presence of seminal leukocytes and abnormal sperm quality [ 23 ]. In particular, Sharma et al. Moreover, activated leukocytes may be responsible for a fold increase in ROS production in comparison to non-activated white blood cells [ 25 ]. Leukocytospermia has been furthermore associated with increased ROS production by spermatozoa, most likely triggered by a direct cell-to-cell contact of the leukocyte with the sperm cell or by the release of soluble products acting on the spermatozoon [ 23 , 24 ]. Spermatozoa have also been reported to generate ROS independently of leukocytes, and this ability primarily depends on the maturation level of the sperm cell. During the epididymal transit, the main morphological change that takes place in the spermatozoon is the migration of the cytoplasmic droplet, a remnant of the cytoplasm associated with testicular sperm. The droplet migrates from the proximal to the distal position during maturation and is normally shed from spermatozoa during or shortly after ejaculation [ 26 ]. Failure to extrude excess cytoplasm during sperm differentiation and maturation traps a number of enzymes, including glucosephosphate dehydrogenase G6PD and ß-nicotinamide adenine dinucleotide phosphate NADPH oxidase, which have been associated with ROS generation through the formation of the NADPH intermediate [ 27 ]. As such, immature and functionally defective spermatozoa with abnormal head morphology and cytoplasmic retention are another important source of ROS in semen [ 12 ]. According to Gil-Guzman et al. The study revealed that after a density gradient separation of human ejaculates, the layer of immature spermatozoa produced the highest levels of ROS. Furthermore, elevated concentrations of immature spermatozoa were accompanied by increased amounts of mature spermatozoa with damaged DNA [ 28 ]. Sertoli cells have also been revealed to have the ability to generate ROS, which may be inhibited by the addition of scavestrogens J and J Scavestrogens are derivates of 17alpha-estradiol and serve as effective FR-quenching molecules that able to inhibit iron-catalyzed cell damage in vitro. As such, Sertoli cells may play a vital role in ROS-mediated spermatogenesis. Due to currently limited evidence, there is a need to further understand the function of Sertoli cells in the process of ROS generation [ 29 , 30 ]. Varicocele is defined as the excessive dilation of the pampiniform venous plexus around the spermatic cord and this endogenous condition is highly linked to testicular and seminal OS. While its role in male infertility is well researched, recent studies have linked higher grades of varicocele with higher ROS levels [ 29 ]. In addition, research has shown that spermatozoa from varicocele patients tend to have high levels of oxidative DNA damage [ 31 ]. The most common management option is varicocelectomy, which has been effective in the reduction of ROS levels in affected patients [ 29 , 31 ]. It is a regular by-product of oxidative phosphorylation, created between complex I and III of the electron transport chain as a result of a monovalent reduction of oxygen and the addition of a single electron [ 33 ]. Additionally, the cytoplasmic enzyme G6PD controls the rate of glucose flux and intracellular availability of NADPH through the hexose monophosphate shunt. H 2 O 2 can be either scavenged by glutathione peroxidase GPx or catalase, catalyzing its dismutation into water and oxygen. Its production is catalyzed by nitric oxide synthase NOS in a redox reaction between L-arginine and oxygen, initiated by NADPH, and with L-citrulline as a byproduct. ROS generation can be exacerbated by a multitude of environmental, infectious and lifestyle-related etiologies. A wide range of industrial by-products and waste chemicals e. polychlorinated biphenyls, nonylphenol or dioxins have been associated with several adverse health effects, many of which are related to male infertility. Persistent environmental contaminants, such as heavy metals and pesticides, may also lead to OS, particularly among workers exposed to such pollutants. These individuals often present with a decreased semen volume and density, accompanied by increased oxidative damage to the sperm lipids, proteins and DNA [ 39 ]. Radiation is a natural source of energy with significant effects on living organisms. Mobile devices are becoming more accessible to the general population, particularly to adolescent males and men of reproductive age. Cell phones release radiofrequency electromagnetic radiation, exposure to which has shown to increase the risk of oligo-, astheno- or teratozoospermia. Furthermore, in vitro studies have demonstrated that EMR induces ROS generation and DNA fragmentation in human spermatozoa, alongside a decreased sperm concentration, motility and vitality depending on the duration of exposure to radiation [ 40 ]. Various components of cigarette smoke have been associated with OS exacerbation. Cigarettes contain a broad array of free radical-inducing agents such as nicotine, cotinine, hydroxycotinine, alkaloids and nitrosamines [ 41 , 42 ]. The prime component of tobacco is nicotine, which is a well-known ROS producer in spermatozoa with detrimental effects on the sperm count, motility and morphology. Moreover, smokers exhibited a lower hypo-osmotic swelling test percentage, indicating a weaker plasma membrane integrity when compared to non-smokers [ 41 ]. Smoking increases ROS production by causing leukocytospermia as shown by Saleh et al. A different study showed that levels of seminal plasma antioxidants were diminished in smokers. By directly affecting the liver, alcohol intake increases ROS production while simultaneously decreasing the antioxidant capacity of the body. Although alcohol consumption has been repeatedly associated with systemic OS, its effect on semen parameters has not been explored to a larger extent. In a study comprising subjects, moderate alcohol consumption did not negatively affect semen parameters [ 44 ]. Nevertheless, it was revealed that chronic drinkers had reduced levels of testosterone, possibly due to an impaired hypothalamic-pituitary axis and damage to the Leydig cells [ 45 ]. Increased alcohol levels block gonadotropin-releasing hormone, leading to reduced luteinizing hormone and testosterone levels. Furthermore, alcohol has been shown to increase ROS generation when consumed by malnourished individuals [ 44 ]. Lastly, diet may affect semen parameters. In a Danish study, men with the highest saturated fat intake presented with a significantly lower total sperm count and concentration in comparison to those with the lowest saturated fat intake [ 46 ]. These observations were supported by a later report focused on studying the link between dairy food intake and male fertility and revealing that a low-fat dairy diet may lead to a higher spermatogenesis [ 47 ]. On the other hand, omega-3 fatty acids and omega-6 fatty acids were shown to improve sperm count, motility and morphology [ 48 ]. With regard to obesity and its relation to semen parameters, currently available data are conflicting. In a study on Iranian men, it was found that overweight men tend to have lower sperm counts [ 49 ]. Inversely, a different study reported that underweight subjects had lower sperm counts than normal and overweight men [ 48 ]. Moreover, a study comprising Tunisian men revealed that sperm concentration, motility and morphology did not vary across different BMI values [ 50 ]. Aerobic metabolism utilizing oxygen is essential for energy requirements of reproductive cells, and free radicals do play a significant role in physiological processes occurring within the male reproductive tract. Spermatozoa themselves produce small amounts of ROS that are essential for a variety of physiological processes such as capacitation, hyperactivation, acrosome reaction and sperm-oocyte fusion [ 30 ]. During transit and storage in the epididymis, spermatozoa undergo membrane, nuclear and enzymatic remodeling, involving the release, attachment and rearrangement of surface proteins [ 6 , 30 , 51 ]. Such changes are based on the assembly of several signal transduction pathways necessary for the subsequent ability of spermatozoa to undergo hyperactivation and capacitation. ROS are essential for a proper chromatin packing during the maturation of mammalian spermatozoa, leading to a characteristic chromatin stability. This unique chromatin architecture results from an extensive inter- and intra-molecular disulfide bond stabilization between the cysteine residues of protamines—small nuclear proteins that replace histones during spermatogenesis. Oxidation of the thiol groups in protamines takes place during the transport of spermatozoa from the caput to the cauda epididymis [ 52 ]. As demonstrated by Aitken et al. ROS may act as oxidizing agents in this process, hence facilitating the formation of disulfide bonds, increasing chromatin stability and protecting DNA from possible damage [ 30 , 52 ]. As spermatozoa possess minimal to none repair mechanisms [ 9 ], chromatin condensation is a crucial protective mechanism, in which ROS actually protect male gametes against future oxidative insults. Likewise, peroxides have been associated with formation of the mitochondrial capsule—a coat surrounding sperm mitochondria providing protection against possible proteolytic degradation [ 54 ]. It is suggested that during spermatogenesis peroxides may oxidize the active form of phospholipid hydroperoxide glutathione peroxidase PHGPx , creating an intermediate that subsequently interacts with thiol groups to form a seleno-disulfide bond. The resulting mitochondrial capsule is made out of a complex protein network rich in disulfide bonds. Mitochondria require such protection as their proper function is crucial for metabolism, cell cycle control and oxidative balance [ 51 , 53 , 54 ]. Although several studies have reported improved sperm DNA integrity and reduced ROS production as a result of daily antioxidant consumption [ 55 ], an unusual decondensation of sperm DNA has been revealed as well [ 56 ]. Hence it may be hypothesized that high antioxidant levels may alter the oxidative conditions necessary for a proper formation of the inter- and intra-molecular disulfide bonds, leading to a lower DNA compaction. Capacitation is a prominent process of final maturation that spermatozoa undergo in the female reproductive tract, during which sperm motility changes from a progressive state to a highly energetic one. It is hypothesized that capacitation occurs exclusively in mature spermatozoa in order to reach the oocyte taking advantage of hyperactive motility and an increased responsiveness to chemotactic agents. Numerous receptors on the sperm head become activated, providing energy to the sperm to penetrate the zona pellucida. As such, capacitation sets up the path necessary for subsequent hyperactivation and acrosome reaction [ 57 ]. Numerous of studies on both human and animal spermatozoa indicate that H 2 O 2 is the primary ROS responsible for capacitation to occur. This process is associated with an increase in tyrosine phosphorylation, and it has been shown that the amount and banding pattern of tyrosine phosphorylation by adding exogenous H 2 O 2 was similar to that observed during endogenous ROS production, providing evidence that H 2 O 2 may be responsible for the enhancement of capacitation [ 32 , 57 , 58 ]. This hypothesis was further confirmed by Rivlin et al. This process is vital as cAMP must increase in concentration for capacitation to occur. cAMP and its subsequent pathways involve protein kinase A, which phosphorylates MEK extracellular signal-regulated kinase -like proteins as well as tyrosine present in fibrous sheath proteins [ 57 , 58 ]. The results of the above studies show that ROS can positively enhance sperm capacitation, but diverge over the specific ROS involved. Several studies have confirmed the lack of molecular specificity in the activation of capacitation and tyrosine phosphorylation, as both SOD and catalase have been shown to negate the positive effect exogenously induced capacitation and hyperactivation [ 59 ]. Although physiological ROS levels are necessary for capacitation, their overgeneration may trigger apoptosis. Hyperactivation is an incompletely understood process to be observed in the final maturation stage of spermatozoa and is considered a subcategory of capacitation. Normally spermatozoa exhibit a low amplitude flagellar movement accompanied by low, linear velocity. In the hyperactivated state, spermatozoa movement is of high amplitude, asymmetric flagellar movement, pronounced lateral head displacement and non-linear trajectory, allowing the sperm to penetrate the cumulus oophorus and zona pellucida surrounding the oocyte. Furthermore, hyperactive motility may enable the progressive movement through the oviduct by preventing stagnation, adding yet another benefit to the sperm function [ 62 ]. Acrosome reaction AR is related to the release of proteolytic enzymes, primarily acrosin and hyaluronidase, in order to degrade the zona pellucida of the oocyte. Once degraded, hyperactive motility propels the spermatozoa into the perivitelline space, at which point the spermatozoa may eventually fuse with the oocyte [ 63 ]. At the same time, results regarding the specific ROS are conflicting. The majority of studies note positive effects of H 2 O 2 and negative effects of catalase, thus suggesting that H 2 O 2 is the major species responsible for a proper AR [ 58 , 64 ]. Moreover, ROS act as signal transducers in the AR. Elevated ROS production may occur upon interaction with the cumulus oophorus , thereby enhancing the signal for exocytosis initiated by either progesterone or the zona pellucida. A link exists between enhanced ROS levels and increased sperm-oocyte fusion. High rates of sperm-oocyte fusion are correlated with increased expression of phosphorylated tyrosine proteins [ 6 ], suggesting that sperm-oocyte fusion is related to the events of capacitation and AR. Ultimately, ROS are thought to increase membrane fluidity using two mechanisms: 1 de-esterification of membrane phospholipids and 2 activation of phospholipase A2 PLA2 [ 65 ]. Once the zona pellucida and corona radiata are penetrated by the sperm cell, the oocyte prevents eventual polyspermy by turning the vitelline layer into a hard envelope. o,o-Dityrosine crosslinks catalyzed by ovoperoxidase lead to the formation of a single macromolecular structure acting as the envelope [ 66 ]. H 2 O 2 serves as the substrate to ovoperoxidase to provide for the envelope formation. With our understanding of ROS and their spermicidal effect, H 2 O 2 proves to be an effective spermicide agent against polyspermy [ 66 , 67 ]. The term oxidative stress refers to a critical imbalance between ROS production and antioxidant defense mechanisms available to the biological system [ 15 ]. According to Sies [ 5 ], it is a disturbance in the prooxidant-antioxidant balance in favor of the former, leading to potential cellular damage. Essentially, OS may result from: Diminished antioxidants, e. mutations affecting antioxidant defense enzymes or toxic agents that deplete such mechanisms [ 5 ]. phagocytic oxidative outburst during chronic inflammatory diseases [ 5 , 15 ]. This mechanism is normally thought to be more relevant to mammalian diseases and is frequently the target of attempted therapeutic intervention. OS can result in: Adaptation: Usually by upregulation of antioxidant defense systems. Cell and tissue injury: OS can cause damage to all molecular targets: DNA, proteins and lipids. Often it is not clear which is the first point of attack, since injury mechanisms may overlap [ 5 ]. Cell death: This process may occur by two mechanisms, necrosis or apoptosis. During necrotic cell death, the cell swells and ruptures, releasing its contents into surrounding areas and affecting adjacent cells. The intracellular content can include antioxidants such as catalase or glutathione GSH as well as prooxidants such as copper and iron. As such, necrosis may lead to further oxidative insults in the internal milieu [ 3 , 4 , 5 , 15 ]. As such, apoptotic cells do not release their content into surrounding environment and apoptosis does not cause damage to the neighboring cells [ 5 ]. An intricate cellular architecture of spermatozoa renders them to be particularly sensitive to OS. Sperm plasma membranes contain large quantities of polyunsaturated fatty acids PUFAs. On the other hand, their cytoplasm contains low concentrations of scavenging enzymes [ 68 ]. OS usually results in a decreased sperm motion and viability, accompanied by a rapid loss of ATP, axonemal damage, increased midpiece morphology defects, followed by alterations in the sperm capacitation and acrosome reaction [ 32 ]. Lipid peroxidation has been repeatedly postulated to be the key mechanism of ROS-induced sperm damage, possibly leading to male reproductive dysfunction [ 68 ]. Sperm plasma membranes are largely composed of PUFAs, which are exceptionally susceptible to oxidative damage due to the presence of more than two carbon—carbon double bonds [ 68 ]. These fatty acids maintain the fluidity of membranes [ 69 ]. ROS attack PUFAs, leading to a cascade of chemical reactions called lipid peroxidation LPO. LPO affects most prominent structural and functional characteristics of the membrane, including fluidity, ion gradients, receptor transduction, transport processes as well as enzymatic activities. As a result, properties that are crucial for a normal fertilization are impaired [ 68 , 69 ]. LPO is a self-propagating process that may be divided into three phases: the initiation phase, the propagation phase and the termination phase. During the initiation phase, one hydrogen is taken from unsaturated lipids to form lipid radicals. During the termination phase, two radicals react with each other to form a stable product and LPO finally ceases [ 70 ]. Numerous pathological effects of LPO on the sperm function are currently known. Overall, LPO causes DNA and protein damage through oxidation of lipid peroxyl or alkoxyl radicals. DNA fragmentation by LPO can occur via base modifications, strand breaks or crosslinks [ 71 ]. LPO generally results in loss of membrane fluidity and subsequently a decreased sperm motility and sperm-oocyte fusion [ 68 , 69 , 70 , 71 ]. Furthermore, during LPO, ROS initiate a cascade of events involving the xanthine and xanthine oxidase system and deplete the ATP production which may ultimately lead to sperm death [ 68 ]. The unique sperm chromatin packing alongside antioxidant molecules present in the seminal plasma provide notable protection to sperm DNA against oxidative damage. Nevertheless, spermatozoa lack any specific DNA repair mechanisms and hence depend on the oocyte for eventual DNA repair following fertilization. ROS-associated catalysis and apoptosis are considered to be the primary mechanisms that induce DNA fragmentation in spermatozoa [ 72 ]. DNA bases and phosphodiester backbones are believed to be most susceptible to ROS-associated peroxidative damage. At the same time, sperm mitochondrial DNA is more vulnerable to oxidative insults when compared to the nuclear genome [ 73 ]. Furthermore, because of the structure of the Y chromosome as well as its inability to repair double strand breaks, Y-bearing spermatozoa are more susceptible to DNA damage than X-carrying counterparts [ 74 ]. Y-bearing spermatogonia can be a target of mutations in the euchromatic Y region Yq11 , known as the azoospermia factor, resulting in infertility [ 75 ]. Various types of DNA abnormalities may occur in sperm that have been exposed to ROS artificially. These include base modifications, production of base-free sites, deletions, frame shifts, DNA crosslinks and chromosomal rearrangements. OS has also been associated with high frequencies of single- and double-strand DNA breaks. ROS can also cause gene mutations, such as point mutation and polymorphism, resulting in decreased semen quality. These changes may be observed especially during the prolonged meiotic prophase, when the spermatocytes are particularly sensitive to damage and widespread degeneration can occur [ 72 , 73 , 74 ]. Also, mutations in the mitochondrial DNA mtDNA may cause a defect of mitochondrial energy metabolism and therefore lower levels of mutant mtDNA may compromise sperm motility in vivo [ 76 ]. Other mechanisms such as denaturation and DNA base-pair oxidation may also be involved [ 74 ]. Increased DNA damage has become a serious issue during artificial reproduction techniques ARTs , as it has been correlated with decreased fertilization rates in vitro and increased early embryo death. Unfortunately, no successful method to prevent or treat sperm DNA damage is currently available [ 77 ]. Proteins are a critical target for oxidation because of their abundance and high rate constants for interactions with diverse ROS. As such, protein damage is a major consequence of both intracellular and extracellular oxidative insults. ROS may attack both the side chains and backbone, and the extent of the insult depends on multiple factors. In some cases, the damage is limited to specific residues, whereas in case of other ROS, the damage is widespread and nonspecific [ 78 ]. Oxidative attacks on proteins generally result in site-specific amino acid modifications, fragmentation of the peptide chain, aggregation of cross-linked reaction products, altered electric charge and increased susceptibility or extreme tolerance to proteolysis [ 79 ]. The resulting products of protein oxidation include reactive hydroperoxides, which may be employed as biomarkers for protein oxidation in vitro and in vivo. As protein damage is usually non-repairable, oxidation may have deleterious consequences, including the loss or sometimes gain of enzymatic, structural or signaling function, fragmentation, unfolding, altered interactions with other proteins and modified turnovers. Generally, oxidized proteins are degraded by proteasomal and lysosomal pathways; however, in some cases, such altered material is poorly degraded and may accumulate within cells contributing to multiple mammalian pathologies [ 78 , 79 ]. The amino acids in a peptide differ in their susceptibility to oxidative insults, while various ROS differ in their potential reactivity. Primary, secondary and tertiary protein structures alter the relative susceptibility of certain amino acids. According to Mammoto et al. Sinha et al. Thus, oxidation of the sperm SH-proteins may be a notable mechanism responsible for the suppressive effects of ROS on sperm functions. Usually, when cellular components undergo serious damage, apoptosis or programmed cell death is initiated. During spermatogenesis, abnormal spermatozoa are eliminated primarily through apoptosis. The exact mechanism of action is not fully understood yet; however, previous studies have speculated that ROS serve as an activator of the mitochondria to release the signaling cytochrome c [ 82 , 83 ]. This molecule initiates a cascade of events involving caspases 3 and 9, eventually leading to sperm apoptosis. The Fas-protein may be also an integral component in the apoptotic pathway. When Fas-ligand or anti-Fas antibody binds to Fas, apoptosis is initiated [ 83 ]. An additional mechanism involves the inflammatory production of ROS, primarily hypochlorous acid HOCl , which is a product of H 2 O 2 and chloride ion. This molecule oxidizes a variety of cellular components, thus causing apoptosis [ 84 ]. Said et al. Numerous studies have focused to study apoptosis in spermatozoa. Various authors [ 35 , 86 ] have reported increased ROS levels and apoptotic markers measured by fluorescence in samples of infertile subjects. On the other hand, in certain males, abortive apoptosis appears to fail in the clearance of spermatozoa that are marked for elimination by apoptosis. As such, the subsequent population of ejaculated spermatozoa may exhibit an array of anomalies consistent with characteristics typical for cells that are in the process of apoptosis. Apoptotic failures may lead to a decreased sperm count resulting in subfertility [ 82 , 83 ]. Spermatozoa motility is an important prerequisite to secure their distribution in the female sexual system, followed by an effective passage through the cervical mucus and penetration into the egg [ 89 ]. Increased ROS levels have been repeatedly correlated with a decreased sperm motility [ 10 , 11 , 12 , 90 ], although the exact mechanism involved is still not completely understood. One hypothesis suggests that H 2 O 2 diffuses across the membranes into the cells and inhibits the activity of vital enzymes such as NADPH oxidase [ 6 ]. At the same time, a decreased G6PDH leads to a reduced availability of NADPH accompanied by a build-up of oxidized glutathione. Such changes may lead to a decline in the intracellular antioxidant levels and a subsequent peroxidation of membrane phospholipids [ 65 ]. Another hypothesis presents a series of interrelated events leading to a decreased phosphorylation of axonemal proteins, followed by sperm immobilization, both of which are linked to a reduced membrane fluidity crucial for sperm-oocyte fusion [ 10 , 32 ]. When spermatozoa are incubated with selected ROS overnight, loss of motion characteristics observed is highly correlated with sperm LPO. Furthermore, the ability of antioxidants to revive sperm motility is evidence that LPO is a major cause for motility loss in spermatozoa [ 68 , 69 ]. Because ROS have both physiological and pathological functions, biological systems have developed defense systems to maintain ROS levels within a certain range. Whenever ROS levels become pathologically elevated, antioxidants scavenge them to minimize any potential oxidative damage [ 1 ]. Antioxidants are defined as molecules that dispose, scavenge and inhibit the formation of ROS or oppose their actions. According to Ďuračková [ 13 ], antioxidants can protect cells against OS via three mechanisms: prevention, interception and repair. Antioxidants may be divided into two dominant categories: Enzymatic e. superoxide dismutases, catalase and glutathione peroxidases. Non-enzymatic e. vitamin C, vitamin E, vitamin A, carotenoids, albumin, glutathione, uric acid, pyruvate, etc. Due to the size and small volume of cytoplasm, as well as the low concentrations of scavenging enzymes, spermatozoa have limited antioxidant defense possibilities. Mammalian spermatozoa predominantly contain enzymatic antioxidants, including SOD and glutathione peroxidases GPx , which are mainly located in the midpiece. A few non-enzymatic antioxidants, such as vitamins C and E, transferrin and ceruloplasmin, are present in the plasma membrane of spermatozoa and act as preventive antioxidants [ 16 ]. Under normal circumstances, the seminal plasma is an important protectant of spermatozoa against any possible ROS formation and distribution. Seminal plasma contains both enzymatic antioxidants, as well as an array of non-enzymatic antioxidants e. ascorbate, urate, vitamin E, pyruvate, glutathione, albumin, taurine and hypotaurine [ 9 ]. Studies have shown that antioxidants protect spermatozoa from ROS generating abnormal spermatozoa, scavenge ROS produced by leukocytes, prevent DNA fragmentation, improve semen quality, reduce cryodamage to spermatozoa, block premature sperm maturation and generally stimulate sperm vitality [ 91 , 92 ]. Superoxide dismutases are metal-containing enzymes that catalyze the conversion of two superoxides into oxygen and hydrogen peroxide, which is less toxic than superoxide [ 1 , 13 ]:. The enzymes are present in both intracellular and extracellular forms. The second form is manganese SOD, which is found predominantly in the mitochondrial matrix and has manganese in its active center MnSOD, SOD-2 [ 93 ]. The secretory tetrameric SOD EC-SOD, SOD-3 may be detected in the extracellular space. The enzyme is associated with surface polysaccharides although it may also be found as a free molecule. Structurally, SOD-3 is similar to SOD-2; however, it has zinc and copper in its active center instead of manganese [ 1 , 5 , 15 ]. SOD protects spermatozoa against spontaneous O 2 toxicity and lipid peroxidation [ 69 ]. Numerous studies have suggested a significant role for SOD in sperm motility both in vivo and in vitro. The addition of SOD to human and animal semen [ 94 , 95 , 96 ] has been shown to protect spermatozoa against the harmful effects of ROS and improve sperm motility and membrane integrity during liquid storage or cryopreservation. As such, it may be concluded that the SOD content in mature spermatozoa may be a good predictor of post-thaw motility recovery following sperm preservation. Catalase catalyzes the decomposition of hydrogen peroxide to molecular oxygen and water, thereby completing the detoxifying reaction started by SOD. A characteristic feature of its structure is a heme system with centrally located iron [ 1 , 13 ]:. CAT has been found in peroxisomes, mitochondria, endoplasmic reticulum and the cytosol in a variety of cells [ 93 ]. In semen, the enzyme was detected in human, bovine and rat spermatozoa, as well as seminal plasma, with the prostate as its source [ 97 , 98 ]. Catalase activates sperm capacitation induced by nitric oxide [ 59 , 60 ]. Furthermore, it plays an important role in decreasing lipid peroxidation and protecting spermatozoa during genitourinary inflammation [ 25 ]. Numerous studies have revealed a positive relationship between sperm motility and the presence of CAT in mammalian ejaculates. Also, positive correlations were observed between sperm morphology and protein expression of CAT in seminal plasma [ 98 , 99 ]. Furthermore, CAT supplementation to fresh, processed and cryopreserved semen resulted in a higher sperm vitality, progressive motility and DNA integrity [ ]. Glutathione peroxidases are a family of selenium-containing enzymes, which catalyze the reduction of H 2 O 2 and organic peroxides, including phospholipid peroxides [ 93 ]. In their active site, the enzymes contain selenium in the form of selenocysteine. where GSH symbolizes reduced glutathione and GS-SG represents glutathione disulfide. The reaction is based on the oxidation of selenol of a selenocysteine residue by H 2 O 2. This process leads to its derivation with selenic acid RSeOH. This by-product is subsequently converted back to selenol through a two-step process that starts with a reaction comprising GSH to generate GS-SeR and water. A second GSH molecule then reduces the GS-SeR intermediate back to selenol, releasing GS-SG as a by-product [ 1 , 5 , 13 ]:. The classic intracellular GPx1 is expressed in sperm nucleus, mitochondria and cytosol, as well as in the testes, prostate, seminal vesicles, vas deferens, epididymis, and has a significant relationship with sperm motility [ , ]. More importantly, a direct relationship has been reported between male fertility and phospholipid hydroperoxide glutathione peroxidase PHGPx; GPx4 , a selenoprotein that is highly expressed in testicular tissue and has a prominent role in the formation of the mitochondrial capsule [ 51 , 53 , 54 ]. Other enzymes, such as glutathione reductase, ceruloplasmin or heme oxygenases, may also participate in the enzymatic control of oxygen radicals and their products. A short overview of minor antioxidant enzymes is provided in Table 2. Location: Found in the epididymis, sertoli cells, vas deferens, seminal vesicles, epithelium and prostate gland [ , ]. Roles: Catalyzes reduction of oxidized glutathione. Maintains glutathione homeostasis. Altered in infertile men, and these alterations seem to be linked to sperm morphology [ , , ]. Location: Most abundant in the seminiferous tubular fluid of mammalian testes, sperm acrosomes, human sperm and mouse spermatogenic cells [ , , ]. Roles: Detoxification enzymes, intracellular-binding proteins [ ]. Involved in epididymal maturation, capacitation and sperm-oocyte interactions [ , ]. Location: Semen, probably of testicular origin [ ]. Roles: Cu-dependent ferroxidase, a fundamental bridge between Fe utilization and Cu status. Associated with the oxidation of ferrous ion into ferric [ ]. Prevents non-enzymatic generation of superoxide and scavenges superoxide, hydroxyl and singlet oxygen [ , ]. Has positive impact on sperm parameters and male fertility [ ]. Serves as a marker of a proper seminiferous tubule function [ ]. Location: Seminal plasma [ , ]. Roles: Primary binding and transport protein for iron and regulates iron transport and storage [ ]. Serves as a reliable index of seminiferous tubular function [ ]. Location: Two forms of heme oxygenase, HO-1 and HO-2, were identified in human testis and seminal plasma [ , ]. Roles: HO is strongly induced by oxidant stress and protects against oxidative insults. Increases reduced glutathione levels, degrades heme and intervenes with the metabolism of biliverdin and bilirubin, which have potent antioxidant properties [ ]. HO is highly expressed in fertile normozoospermic subjects with positive correlations to sperm concentration, motility and morphology. HO enzyme activity is related to spermatogenesis and sperm motility processes [ , ]. Non-enzymatic antioxidants are also known as synthetic antioxidants or dietary supplements. Glutathione is the most abundant thiol protein in mammalian cells [ ]. Being an endogenous source, it is synthesized by the liver but it can also be derived from dietary sources such as fresh meat, fruits and vegetables. This molecule has three precursors: cysteine, glutamic acid and glycine. Its cysteine subunit provides and exposes -SH that directly scavenges free radicals. High levels are found especially in the testis of rats [ ] and the reproductive tract fluids and epididymal sperm of bulls [ 98 ]. GSH protects the cell membranes from lipid oxidation and prevents further formation of free radicals. Its deficit leads to instability of the sperm midpiece, which results in motility disorders [ ]. Glutathione supplementation in infertile subjects has led to a significant improvement in sperm parameters and prevents oxidative damage to sperm DNA. A factor increasing the level of GSH is pantothenic acid, which by doing so also protects tissues against oxidative stress [ , ]. Vitamin C or ascorbic acid AA may be found in its reduced ascorbate as well as oxidized form dehydroascorbic acid , both of which are easily interconvertible and biologically active. Vitamin C is found in citrus fruits, peppers, strawberries, tomatoes, broccoli, brussels sprouts and other leafy vegetables. AA is a water-soluble vitamin, and because of its hydrophilic nature, it has more effective scavenging properties at the plasma level than in the lipid bilayer [ ]. Vitamin C has been used in the management of male infertility on empirical grounds, particularly in the presence of non-specific seminal infections [ ]. Its presence in the seminal plasma of healthy males has been reported by various authors [ , , ]. Chinoy et al. Low concentration of vitamin C showed significant degenerative changes in the testes, epididymis and vas deferens of scorbutic guinea pigs. On the other hand, excessive intake of vitamin C has been reported to cause reproductive failure in the men [ ]. This was further corroborated by the association of decreased AA followed by an increase in the seminal plasma LPO as observed in a human trial [ , ]. Moreover, it has been reported that AA supplementation leads to a significant reduction in the ROS concentration, sperm membrane LPO and DNA oxidation together with an increased sperm quality. The results of a recent animal experimental study indicate that vitamin C improves the activity of antioxidant enzymes and significantly reduces malondialdehyde MDA concentration in testicular structures [ ]. Vitamin E is a term that encompasses a group of potent, lipid-soluble tocol tocopherol and tocotrienol derivatives qualitatively exhibiting the biological activity of RRR-α-tocopherol. Structural analyses have revealed that molecules having vitamin E antioxidant activity include four tocopherols α-, β-, γ- and δ- and four tocotrienols α-, β-, γ- and δ- with α-tocopherol being the most abundant form in nature and mostly available in food, having the highest biological activity and reversing vitamin E deficiency symptoms. The molecular functions fulfilled specifically by α-tocopherol have yet to be fully described; however, the antioxidant feature is the flagship of the biological activity related to vitamin E [ ]. Vitamin E is present within the seminal plasma and plasma membrane. It is a lipid soluble, chain-breaking antioxidant that able to terminate free radical chain reactions, particularly the peroxidation of PUFAs [ , ]. Numerous reports emphasize on the role of α-tocopherol in the management of male infertility. A positive association was found between α-tocopherol in sperm plasma membranes and the percentage of motile, living and morphologically intact spermatozoa [ ]. At the same time, α-tocopherol levels were decreased significantly in oligo- and azoospermic patients in comparison to normospermic controls [ ]. A significant improvement in the in vitro ability of spermatozoa to bind the zona pellucida of unfertilized oocytes was found in men with high ROS production supplemented with vitamin E for 3 months [ ]. Vitamin E supplementation may also play a role in reducing sperm DNA fragmentation and morphology defects [ ]. There are other substances which may contribute to the maintenance of oxidative homeostasis. The prime function of these compounds is not to combat the production or action of ROS; however, their presence may decrease the risk of OS development. Albumin, cysteine, taurine, zinc and selenium are the most known representatives. Furthermore, antioxidant substances isolated from natural resources, such as resveratrol, curcumin or lycopene, have recently emerged as suitable dietary supplements or therapeutics due to their chemical diversity, structural complexity, availability, lack of significant toxic effects and intrinsic biologic activity. A short overview of secondary non-enzymatic antioxidants is provided in Table 3. Has the ability to reduce free radicals by acting with thiols and hydroxyl radicals. Plays a role as a precursor to glutathione [ ]. Reduces seminal OS and sperm DNA damage [ ]. When combined with selenium, NAC has a positive impact on sperm concentration and acrosome reaction [ , ]. Stimulates mitochondrial metabolism. Has the ability to shuttle long-chain lipids across the mitochondrial bilayer and start the process of ß-oxidation to create NADH and FADH 2 along with acetyl-CoA [ ]. Acts primarily in the epididymis. Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen — Sigman M, Glass S, Campagnone J, Pryor JL Carnitine for the treatment of idiopathic asthenozoospermia: a randomized, double-blind, placebo-controlled trial. Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA Lipid peroxidation and human sperm motility: protective role of vitamin E. Tarin JJ, Brines J, Cano A Antioxidants may protect against infertility. Tremellen K Oxidative stress and male infertility — a clinical prospective. Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Verma A, Kanwar KC Human sperm motility and lipid peroxidation in different ascorbic acid concentration: an in vitro analysis. Vezina D, Mauffette F, Roberts KD, Bleau G Selenium-vitamin E supplementation in infertile men. Effects on semen parameters and micronutrient levels and distribution. Biol Trace Elem Res — Vicari E, Calogero AE Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Vicari E, La Vignera S, Calogero AE Antioxidant treatment with carnitines is effective in infertile patients with prostato-vesiculo-epididymitis and elevated seminal leukocyte concentration after treatment with nonsteroidal anti-inflammatory compounds. Vural P, Akgul C, Yildirim A, Canbaz M Antioxidant defence in recurrent abortion. Clin Chim Acta — Wong WY, Merkus HMWM, Thomas CMG, Menkveld R, Zielhuis GA, Steegers-Theunissen RP Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Woods JR jr, Plessinger MA, Miller RK Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol 8 :5— Yumura Y, Iwasaki A, Saito K, Ogawa T, Hirokawa M Effect of reactive oxygen species in semen on the pregnancy of infertile couples. Int J Urol — Zini A, San Gabriel M, Baazeem A Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet — Download references. You can also search for this author in PubMed Google Scholar. Correspondence to Andrea Sansone. Department of Pharmacology and Therapeutics, University of British Colombia, Vancouver, British Columbia, Canada. Reprints and permissions. Sansone, A. Free Radicals and Reproductive Health. In: Laher, I. eds Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. Published : 03 May Publisher Name : Springer, Berlin, Heidelberg. Print ISBN : Online ISBN : eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Abstract Studies performed during the last decades have focused on inflammation and on its involvement in many pathologies. Keywords Antioxidants Female infertility Male infertility Oligoasthenoteratozoospermia Oxidative stress Reactive oxygen species Reproductive health. Buying options Chapter EUR eBook EUR 1, Hardcover Book EUR 2, Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions. Abbreviations NAC: N -acetyl- l -cysteine OAT: Oligoasthenoteratozoospermia ROS: Reactive oxygen species. References Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Bakheet SA Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid Med Cell Longev —78 Article PubMed Central PubMed Google Scholar Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril — Article CAS PubMed Google Scholar Abel BJ, Carswell G, Elton R, Hargreave TB, Kyle K et al Randomised trial of clomiphene citrate treatment and vitamin C for male infertility. J Urol — Article CAS Google Scholar Agarwal A, Saleh RA, Bedaiwy MA Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril — Article PubMed Google Scholar Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E Role of oxidative stress in pathogenesis of varicocele and infertility. Urology — Article PubMed Google Scholar Aitken RJ, Clarkson JS Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl — CAS PubMed Google Scholar Aitken RJ, Clarkson JS, Fishel S Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod — Article CAS PubMed Google Scholar Aitken RJ, Buckingham D, Harkiss D Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. J Reprod Fertil — Article CAS PubMed Google Scholar Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod — Article Google Scholar Al-Gubory KH, Fowler PA, Garrel C The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol — Article CAS PubMed Google Scholar Askari HA, Check JH, Peymer N, Bollendorf A Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch Androl —15 Article CAS PubMed Google Scholar Baker HW, Brindle J, Irvine DS, Aitken RJ Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril — CAS PubMed Google Scholar Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril — Article CAS PubMed Google Scholar Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F et al Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril 91 5 — Article CAS PubMed Google Scholar Bellver J, Meseguer M, Muriel L, García-Herrero S, Barreto MA et al Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum Reprod 25 7 — Article CAS PubMed Google Scholar Bilodeau JF, Hubel CA Current concepts in the use of antioxidants for the treatment of pre-eclampsia. J Androl — CAS PubMed Google Scholar Chandra A, Surti N, Kesavan S, Agarwal A Significance of oxidative stress in human reproduction. Urology —76 Article PubMed Google Scholar Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Asian J Androl 7 3 — Article CAS PubMed Google Scholar Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG et al World Health Organization reference values for human semen characteristics. Andrologia — Article CAS PubMed Google Scholar Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD Cryopreservation of human spermatozoa. Fertil Steril — CAS PubMed Google Scholar Desai N, Shabanegh E, Kim T, Agarwal A Free radical theory of aging: implications in male infertility. Urology —19 Article PubMed Google Scholar Donnelly ET, McClure N, Lewis SE a Antioxidant supplementation in vitro does not improve human sperm motility. Fertil Steril — Article CAS PubMed Google Scholar Donnelly ET, McClure N, Lewis SE b The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and production of reactive oxygen species. Mutagenesis — Article CAS PubMed Google Scholar Galatioto GP, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF et al May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol — Article Google Scholar Gavella M, Lipovac V Pentoxifylline-mediated reduction of superoxide anion production by human spermatozoa. Andrologia —39 Article CAS PubMed Google Scholar Gavella M, Lipovac V, Marotti T Effect of pentoxifylline on superoxide anion production by human sperm. Int J Androl — Article CAS PubMed Google Scholar Geva E, Bartoov B, Zabludovsky N, Lessing JB, Lerner-Geva L, Amit A The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril — CAS PubMed Google Scholar Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl — Article CAS PubMed Google Scholar Griveau JF, Le Lannou D Effects of antioxidants on human sperm preparation techniques. Int J Androl — Article CAS PubMed Google Scholar Griveau JF, Le Lannou D Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl —69 Article CAS PubMed Google Scholar Hargreave TB, Kyle KF, Baxby K, Rogers AC, Scott R et al Randomised trial of mesterolone versus vitamin C for male infertility. Br J Urol — Article CAS PubMed Google Scholar Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Agarwal A Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol — Article CAS PubMed Google Scholar Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R et al Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm function in non-leukocytospermic patients. Fertil Steril — Article CAS PubMed Google Scholar Hirsch A ABC of subfertility: male subfertility. BMJ — Article Google Scholar Hong CY, Lee MF, Lai LJ, Wang CP Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia —65 Article CAS PubMed Google Scholar Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod — Article CAS PubMed Google Scholar Iwanier K, Zachara BA Selenium supplementation enhances the element concentration in blood and seminal fluid but does not change the spermatozoal quality characteristics in subfertile men. Reactive oxygen species may also play a role in other reproductive organ diseases of women such as endometriosis. Oxidative stress develops when there is an imbalance between the generation of ROS and the scavenging capacity of antioxidants in the reproductive tract. It affects both natural and assisted fertility. Because assisted reproductive techniques are used extensively in the treatment of infertility, it is critical to understand the in-vitro conditions that affect fertilization and embryo development. |
| The effects of oxidative stress on female reproduction: a review | When the balance is disrupted towards an overabundance of ROS, oxidative stress OS occurs. Higher levels of 8-hydroxy 2-deoxyguanosine are also seen in granulosa cells of patients with endometriosis, and this may impair the quality of oocytes. The dose and duration of antioxidant administration also need to be thoroughly examined and standardized. The prime function of these compounds is not to combat the production or action of ROS; however, their presence may decrease the risk of OS development. Serum NO levels were elevated amongst nonpregnant patients with tubal or peritoneal factor infertility [ ]. NO donors and elevated serum NO was associated with implantation failure resulting in decreased fertility [ ]. Rolf C, Cooper TG, Yeung CH, Nieschlag E Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. |
| Post navigation | Obesity, alcohol abuse, cigarette smoking, and heavy metals have been firmly linked with OS. Moreover, environmental factors, such as heavy metals, also contribute to excessive ROS. At present, irrespective of varicocele, few studies have reported SP proteome modulation in patients. Moreover, there is no literature about the differential expression of proteins with and without increased ROS, and few proteins have been suggested as disease markers. However, intelectin 1 overexpression has been observed in asthenozoospermic patients with OS, revealing the possible existence of genital tract infection. Likewise, a study reported that alcohol dehydrogenase overexpression contributed to alcohol metabolism and aminolevulenic acid dehydratase overexpression indicated exposure to lead. Thus, it proves that lifestyle and environmental factors have a detrimental effect on sperm quality due to the overproduction of free radicals Further detailed information regarding the involvement of male infertility problems is well illustrated by Transcriptionally sperm cells are energetic; RNAs are presumed to be involved in spermatogenic events It is thought that sperm RNAs are linked to several functions, including fertilization , Further, RNAs in sperm are known to be indicators of sperm quality index — and fertility , , Interestingly, sperm consists of coding and non-coding RNAs , that potentially might have an effect on sperm activeness. A DNA microarray revealed that transcripts in low-fertile bulls are dysregulated. Notably, transcripts such as PRDX6, NOS3, SOD, BAK, and BCL2L11 have been associated with fertility and are linked to the oxidation reduction process, the mediation of MMP, and apoptosis. This study provides a pathway to develop male-fertility-related markers The bull transcriptomic profile revealed that non-coding RNAs ncRNAs are involved in the regulation of sperm motility The ncRNAs are the main regulators of spermatogenesis and male fertility but literature on lncRNAs in human oligozoospermia is scant. The sequencing data of lncRNA and mRNA from 12 human normozoospermia and oligozoospermia samples revealed the altered expression of lncRNAs DE lncRNAs and mRNAs DE mRNAs in male infertility. The Gaussian graphical model, gene ontology, and Encyclopedia of Genes and Genomes pathways were applied to identify them and investigate their possible functions. The transcriptome data showed that DE lncRNAs and DE mRNAs and their target genes were involved in the accretion of unfolded proteins in sperm ER, PERK-EIF2 pathway-induced ER stress, oxidative stress, and apoptotic sperms in individual oligozoospermia subjects. This suggests that these lncRNAs and pathways could be utilized as a therapeutic target for infertility. There is less evidence about the semen transcriptomic profile in terms of interactions with oxidative stress. More studies are required to determine whether oxidative stress is involved in male infertility problems Several RNA-seq studies have attempted to characterize the transcriptome of ejaculated spermatozoa in terms of sperm quality and fertility. Semen quality varies according to season. A total of 4, coding genes of moderate to high abundance have been identified in sperm RNA. The fragmentation of the transcript increased in genes associated with spermatogenesis, chromatin compaction, and fertility. The summer and winter ejaculates had different transcriptomic profiles, with 34 coding genes and 7 microRNAs showing a significantly distinct distribution. These genes were linked to oxidative stress, DNA damage, and autophagy. The annotation of the boar sperm transcriptomic profile was used to identify sperm quality markers in pigs Table 2 shows the involvement of transcriptomic factors on male infertility. The overproduction of ROS may influence male infertility by interacting with different cellular components, resulting in sperm damage , This process involves lipid peroxidation and protein oxidation through the utilization of numerous molecular mechanisms. Moreover, ROS stimulates caspases and nucleases that contribute to apoptotic pathways; therefore, they cause indirect damage to the sperm DNA through abortive apoptosis Presently, research measuring oxidative stress relies on estimations of intracellular ROS using a chemiluminescence assay , total antioxidant capacity TAC , malondialdehyde , or DNA damage 8-OHdG , which have been identified as markers of OS and significant sperm damage in infertile patients — Further, sperm DNA damage impairs sperm fertility capacity and embryo development during natural conception and has been linked with assisted reproductive tools — Intriguingly, it has been noted that measurement of oxidative stress might be important for infertile subjects who can benefit from antioxidant supplementation or an alteration in lifestyle These considerations show that there is a dire need to understand the correlation between seminal plasma oxidative stress and sperm DNA damage and for the development of new diagnostic methods. More recently, a novel galvanostat-based technique was used to measure OS. This technique determines the balance between oxidants and reductants in semen, which is known as the oxidation-reduction potential ORP Spermatozoa possess a one-base excision repair BER enzyme upstream during their development, which is helpful for DNA repair. This enzyme is known as 8-oxoguanine DNA glycosylase 1 OGG1 , and it assists in the release of adducts into the extracellular space through the excision of DNA base adducts , Spermatozoa do not possess BER enzymes, such as apurinic endonuclease 1 APE1 and x-ray repair cross-complementing protein 1 XRCC1. For that reason, the DNA repair ability of spermatozoa is delicate, resulting in the repair of oxidized DNA base adducts, such as 8-OHdG Moreover, it has been found that 8-OHdG triggers germline mutations, indirectly causing DNA damage in human spermatozoa Male infertility is a highly concerning issue that has not received much focus in terms of better understanding its magnitude and prevalence. Several factors of male infertility are idiopathic in nature. As such, there is an emerging need to address the problem and investigate preventive strategies The following approaches should be considered for preventing male infertility problems:. Oxidative stress is the main cause of male infertility induction and attempts should be made to limit the production of oxidative stress. However, it should be kept in mind that some ROS production is needed to maintain male fertility. The cellular mechanism involved in male infertility may provide new pathways for drug development from antioxidant compounds that are safe and secure and exert less toxic effects than commercially available classical drugs. Nanoparticle-based approaches could be useful for the targeted delivery of polyphenol-derived drugs. The integration of knowledge and computer science through machine learning algorithms should be adopted in male infertility diagnostic approaches, as well as in searches for targeted therapies An integrated AI system should assist the assessment of computerized semen analysis; AI-based applications can estimate environmental conditions and lifestyle to improve semen quality forecasts. An attempt should be made to break down barriers linked to religious and cultural beliefs that prevent individuals from speaking openly about their infertility issues. There is need of create awareness among populations so that male infertility problems can be discussed more frequently. Excessive weight has been linked with reduced sperm production. Therefore, diet and daily exercise need to planned appropriately. Addiction tends to influence physiological function. Addictive behavior needs to be avoided and monitored. Tightly fitting clothing influences blood circulation to the genital organs and raises testicular temperature, thus disturbing semen production and decreasing fertility. Therefore, tight clothes need to be avoided. Electronic gadgets that produce low levels of radiation eventually disturb sperm production. Therefore, it is better to minimize the use of these gadgets. Deficiency of nutrients, particularly zinc and vitamin C, may disturb sperm production. Therefore, it is important to have a healthy and balanced diet. Supplementation can be used if the diet lacks the required nutrition. Infection and inflammation may severely influence sperm production. In conclusion, we have reviewed the relationship between oxidative stress and male infertility and the involvement of proteomic studies in male infertility. We have compared the values of differential protein profiles in seminal plasma in both oxidative and physiological conditions. With the literature in mind, the pathway analysis indicates the contribution of proteins to stress, cellular, metabolic, and regulatory pathways. The compiled studies in this Review will contribute to the exploration of the prominent causes of idiopathic male infertility. It is hoped that if male infertility is recognized at a molecular level, its diagnosis, treatment, and prevention can be improved. It was difficult to enumerate which mechanism should be targeted In normozoospermic conditions. However, this scenario is still incomplete and further research is needed to develop diagnostic assays based on methylated patterns, such as RNA and phosphorylation profiles. We further highlighted the attractiveness of sperm DNA integrity as a biomarker for unexplained infertility. In the coming years, it is expected that idiopathic fertility can be diagnosed using omics technologies. TH: conceptualization, writing—original draft preparation, MK and EM: methodology, illustration of figures and. GM and DHK, editing of manuscript, BT, funding acquisition and visualization, editing of the manuscript, YY, MIC, AF, AY, MSK editing of the manuscript. All authors contributed and approved the submitted version of manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Medicine PCotASfR. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 99 1 doi: PubMed Abstract CrossRef Full Text Google Scholar. Sabanegh ES, Agarwal A. Male Infertility. Springer Google Scholar. Parekattil SJ, Esteves SC, Agarwal A. Hamada AJ, Montgomery B, Agarwal A. Male Infertility: A critical review of pharmacologic management. Expert Opin Pharmacother 13 17 — Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update 14 3 — Khosrowbeygi A, Zarghami N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol 7 1 :1—6. Pasqualotto FF, Sharma RK, Nelson DR, Thomas AJ Jr. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril 73 3 — Ochsendorf F. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update 5 5 — Hwang K, Lamb DJ. Molecular mechanisms of antioxidants in male infertility. Male infertility: Springer , 45— CrossRef Full Text Google Scholar. Agarwal A, Allamaneni SS, Said TM. Chemiluminescence technique for measuring reactive oxygen species. Reprod BioMed Online 9 4 —8. Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 57 2 — Ochsendorf F, Thiele J, Fuchs J, Schüttau H, Freisleben H, Buslau M, et al. Chemiluminescence in semen of infertile men. Andrologia 26 5 — Du Plessis SS, Kashou AH, Benjamin DJ, Yadav SP, Agarwal A. Proteomics: a subcellular look at spermatozoa. Reprod Biol Endocrinol 9 1 :1— Mitulović G, Mechtler K. HPLC techniques for proteomics analysis—a short overview of latest developments. Brief Funct Genomics 5 4 — Hamada A, Sharma R, Du Plessis SS, Willard B, Yadav SP, Sabanegh E, et al. Two-dimensional differential in-gel electrophoresis—based proteomics of male gametes in relation to oxidative stress. Fertil Steril 99 5 — Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, Willard B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol 11 1 :1— Sharma R, Agarwal A, Mohanty G, Hamada AJ, Gopalan B, Willard B, et al. Proteomic analysis of human spermatozoa proteins with oxidative stress. Herwig R, Knoll C, Planyavsky M, Pourbiabany A, Greilberger J, Bennett KL. Proteomic analysis of seminal plasma from infertile patients with oligoasthenoteratozoospermia due to oxidative stress and comparison with fertile volunteers. Fertil Steril 2 — Wang J, Wang J, Zhang H-R, Shi H-J, Ma D, Zhao H-X, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. AJA 11 4 Organisation WH. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Camb Univ Press Lu J-C, Huang Y-F, Lü N-Q. WHO laboratory manual for the examination and processing of human semen: its applicability to andrology laboratories in China. Zhonghua nan ke xue Nat J Androl 16 10 — Kovac JR, Pastuszak AW, Lamb DJ. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril 99 4 — Lewis SE, Aitken RJ, Conner SJ, De Iuliis G, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: Recent advances in diagnosis and treatment. Reprod BioMed Online 27 4 — Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod BioMed Online 26 1 — Aitken R, Bronson R, Smith T, De Iuliis G. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. MHR: Basic Sci Reprod Med 19 8 — Lewis SE. Sperm DNA fragmentation and base oxidation. Gen Dam Hum Spermat , — Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. JBC 39 — Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C, et al. Toll-like receptors TLR 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod 26 10 — Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem 3 — Polzien L, Baljuls A, Rennefahrt UE, Fischer A, Schmitz W, Zahedi RP, et al. Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: pore-forming activity of BAD is regulated by phosphorylation. JBC 41 — Pujianto DA, Curry BJ, Aitken RJ. Prolactin exerts a prosurvival effect on human spermatozoa via mechanisms that involve the stimulation of akt phosphorylation and suppression of caspase activation and capacitation. Endocrinology 3 — Rivlin J, Mendel J, Rubinstein S, Etkovitz N, Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod 70 2 — Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med 40 6 — Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic Biol Med 41 4 — Villegas J, Kehr K, Soto L, Henkel R, Miska W, Sanchez R. Reactive oxygen species induce reversible capacitation in human spermatozoa. Andrologia 35 4 — Aitken R, Paterson M, Fisher H, Buckingham D, Van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 5 — Rodriguez P, Beconi M. Peroxynitrite participates in mechanisms involved in capacitation of cryopreserved cattle. Anim Reprod Sci — Takakura K, Beckman JS, MacMillan-Crow LA, Crow JP. Rapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite. Arch Biochem Biophy 2 — Aitken RJ. The capacitation-apoptosis highway: Oxysterols and mammalian sperm function. Biol Reprod 85 1 :9— Aitken RJ, Baker MA, Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? AJA 17 4 Cormier N, Ma S, Bailey Jl Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J Androl 18 4 —8. Ashrafi I, Kohram H, Ardabili FF. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Darbandi S, Darbandi M. Lifestyle modifications on further reproductive problems. Cresco J Reprod Sci 1 1 :1—2. Zirkin BR, Chen H. Regulation of leydig cell steroidogenic function during aging. Biol Reprod 63 4 — Turner TT, Bang HJ, Lysiak JJ. Experimental testicular torsion: Reperfusion blood flow and subsequent testicular venous plasma testosterone concentrations. Urology 65 2 —4. Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species ROS generated by mitochondrial P systems in steroidogenic cells. Drug Metab Rev 38 — Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat leydig cell antioxidant defense system. J Androl 27 2 —7. Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod BioMed Online 7 1 — Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 9 4 — Meucci E, Milardi D, Mordente A, Martorana GE, Giacchi E, De Marinis L, et al. Total antioxidant capacity in patients with varicoceles. Fertil Steril — Mancini A, Leone E, Festa R, Grande G, Silvestrini A, De Marinis L, et al. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J Androl 29 6 —9. Mancini A, Festa R, Silvestrini A, Nicolotti N, Di Donna V, La Torre G, et al. Hormonal regulation of total antioxidant capacity in seminal plasma. J Androl 30 5 — Chainy G, Samantaray S, Samanta L. Testosterone-induced changes in testicular antioxidant system. Andrologia 29 6 —9. Shang X, Huang Y, Ye Z, Yu X, Gu W. Protection of melatonin against damage of sperm mitochondrial function induced by reactive oxygen species. Lakpour N, Mahfouz RZ, Akhondi MM, Agarwal A, Kharrazi H, Zeraati H, et al. Relationship of seminal plasma antioxidants and serum male hormones with sperm chromatin status in male factor infertility. Syst Biol Reprod Med 58 5 — Oluboyo A, Adijeh R, Onyenekwe C, Oluboyo B, Mbaeri T, Odiegwu C, et al. Relationship between serum levels of testosterone, zinc and selenium in infertile males attending fertility clinic in nnewi, south east Nigeria. AJMMS —4. Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol 1 — Richthoff J, Spano M, Giwercman Y, Frohm B, Jepson K, Malm J, et al. The impact of testicular and accessory sex gland function on sperm chromatin integrity as assessed by the sperm chromatin structure assay SCSA. Hum Reprod 17 12 —9. Meeker JD, Singh NP, Hauser R. Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. J Androl 29 4 — Dobrzyńska MM, Baumgartner A, Anderson D. Antioxidants modulate thyroid hormone-and noradrenaline-induced DNA damage in human sperm. Mutagenesis 19 4 — Palomba S, Falbo A, Espinola S, Rocca M, Capasso S, Cappiello F, et al. Effects of highly purified follicle-stimulating hormone on sperm DNA damage in men with male idiopathic subfertility: a pilot study. J Endocrinol Invest 34 10 — Colacurci N, Monti MG, Fornaro F, Izzo G, Izzo P, Trotta C, et al. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J Androl 33 4 — Tesarik J, Martinez F, Rienzi L, Iacobelli M, Ubaldi F, Mendoza C, et al. In-vitro effects of FSH and testosterone withdrawal on caspase activation and DNA fragmentation in different cell types of human seminiferous epithelium. Hum Reprod 17 7 —9. Nematollahi-Mahani S, Azizollahi G, Baneshi M, Safari Z, Azizollahi S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia 46 3 —5. Manna PR, Tena-Sempere M, Huhtaniemi IT. Molecular mechanisms of thyroid hormone-stimulated steroidogenesis in mouse leydig tumor cells: Involvement of the steroidogenic acute regulatory StAR protein. JBC 9 — Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52 1 :1—6. Adewoyin M, Mohsin SMN, Arulselvan P, Hussein MZ, Fakurazi S. Enhanced anti-inflammatory potential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW Drug Des Dev Ther Inducible nitric oxide synthase in the rat testis: Evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod 63 5 — Wang J, Wang W, Li S, Han Y, Zhang P, Meng G, et al. Hydrogen sulfide as a potential target in preventing spermatogenic failure and testicular dysfunction. ARS 28 16 — Hales DB. Endocrinology 5 — Liew SH, Meachem SJ, Hedger MP. A stereological analysis of the response of spermatogenesis to an acute inflammatory episode in adult rats. J Androl 28 1 — Generation of reactive oxygen species in subgroups of infertile men. Int J Androl 13 5 — Diemer T, Hales D, Weidner W. Immune—endocrine interactions and leydig cell function: the role of cytokines. Andrologia 35 1 — Moretti E, Collodel G, Mazzi L, Campagna M, Iacoponi F, Figura N. Resistin, interleukin-6, tumor necrosis factor-alpha, and human semen parameters in the presence of leukocytospermia, smoking habit, and varicocele. Lazaros LA, Xita NV, Chatzikyriakidou AL, Kaponis AI, Grigoriadis NG, Hatzi EG, et al. Association of TNFα, TNFR1, and TNFR2 polymorphisms with sperm concentration and motility. J Androl 33 1 — Maegawa M, Kamada M, Irahara M, Yamamoto S, Yoshikawa S, Kasai Y, et al A repertoire of cytokines in human seminal plasma. J Reprod Immunol 54 — Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod 22 11 — Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6 7 — Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev 20 4 — Gonzalez CR, Matzkin ME, Frungieri MB, Terradas C, Ponzio R, Puigdomenech E, et al. Expression of the TGF-beta1 system in human testicular pathologies. Reprod Biol Endocrinol 8 1 :1— Camejo M, Segnini A, Proverbio F. Interleukin-6 IL-6 in seminal plasma of infertile men, and lipid peroxidation of their sperm. Arch Androl 47 2 — Vera O, Vásqucz LA, Muñoz MG. Semen quality and presence of cytokines in seminal fluid of bull ejaculates. Theriogenology 60 3 —8. Aitken RJ, Buckingham DW, Brindle J, Gomez E, Baker HG, Irvine DS. Andrology: Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum Reprod 10 8 — Yin Y, DeWolf WC, Morgentaler A. Experimental cryptorchidism induces testicular germ cell apoptosis by pdependent and-independent pathways in mice. Biol Reprod 58 2 —6. Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl 6 1 — PubMed Abstract Google Scholar. Bauché F, Fouchard M-H, Jégou B. Antioxidant system in rat testicular cells. FEBS letters. Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl 5 3 — Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 64 1 — Ho Y-S, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. JBC 13 —9. Ishii T, Matsuki S, Iuchi Y, Okada F, Toyosaki S, Tomita Y, et al. Accelerated impairment of spermatogenic cells in SOD1-knockout mice under heat stress. Free Rad Res 39 7 — Tsunoda S, Kawano N, Miyado K, Kimura N, Fujii J. Impaired fertilizing ability of superoxide dismutase 1-deficient mouse sperm during in vitro fertilization. Biol Reprod 87 5 Li Y, Huang T-T, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11 4 — Raineri I, Carlson EJ, Gacayan R, Carra S, Oberley TD, Huang T-T, et al. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med 31 8 — Ookawara T, Kizaki T, Takayama E, Imazeki N, Matsubara O, Ikeda Y, et al. Nuclear translocation of extracellular superoxide dismutase. BBRC 1 — Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, et al. Am J Physiol Heart Circ Physiol 4 :H—H Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 16 4 — Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase PHGPx, GPx4 in mammalian cells. Free Rad Biol Med 34 2 — Flohé L. Selenium in mammalian spermiogenesis. Biological Chemistry 10 — Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. JBC 7 — Chen S-j, Allam J-P, Duan Y-g, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 1 —9. Bansal AK, Bilaspuri G. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int —8. Henkel RR. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. AJA 13 1 Miranda-Vilela AL, Alves PCZ, Akimoto AK, Pereira LCS, NazaréKlautau-Guimarães Md, Grisolia CK. Am J Hum Biol 22 6 — Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. These are used to see how people use our website so we can make adjustments and improvements. These are used to make advertisements on our website more relevant to your interests. A free radical or just radical is an atom without an electron. Aside from natural formation, the following can increase free radicals in the body: exposure to X-rays, ozone, cigarette smoking, air pollutants, and industrial chemicals. Researchers have warned about the potential for free radicals to cause damage to cells throughout the body, leading to a variety of health conditions, including heart disease and some types of cancers. Free radicals are the MVPs of certain physiological processes — for example, the immune system depends greatly on free radicals to help fight off infections. Ironically, free radicals play a role in normal ovulation. For many people, though, keeping up with the rat race means stressing to the max. It can be really difficult to put together healthy meals or prioritize exercise, and both of these things can lead to excessive production of free radicals, which can tip the scale towards oxidative stress. Both the egg and sperm cells are susceptible to free radical damage, so if the body remains in a chronic state of oxidative stress, fertility can eventually be impacted. For women, this means a reduction in egg quality. For men, free radical damage to sperm cells has the potential to reduce sperm count and sperm motility, as well as increase DNA fragmentation. With intention, you can decrease your exposure to the things that increase the production of free radicals in your body. Here are a few tips for lightening your free radical load:. Antioxidants are an important weapon in the fight against oxidative stress. Antioxidants like CoQ10, vitamin E, Vitamin C, and alpha lipoic acid neutralize free radicals before they can harm egg and sperm cells. Free radicals are all over the place in our modern, stress-filled world. Learn more about FH PRO antioxidant supplements. This ad is brought to you by Fairhaven Health. cookies on oviahealth. |
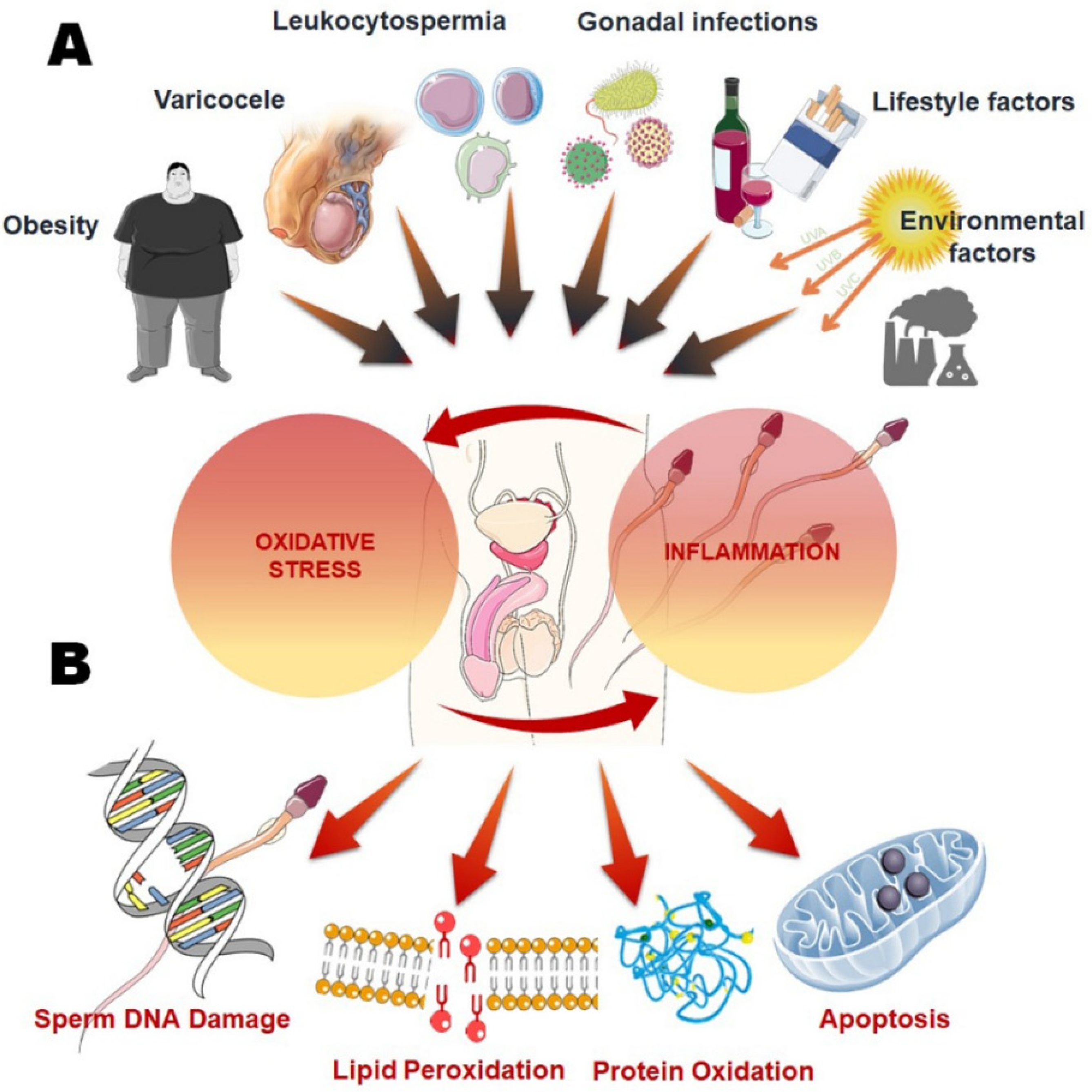
0 thoughts on “Free radicals and reproductive health”