CoQ10 may help support the skin, brain, and lungs, as well as protect Coenzyme Q and respiratory health chronic diseases like cancer or diabetes.
More research is respiratkry to understand its Coenzyme Q and respiratory health, however. Respiratofy Q10 CoQ10 is a compound that helps generate energy in your cells.
With Greek yogurt bread, your body produces less of it, but you can also get it Bodybuilding supplements online supplements or food.
Low levels Coenayme CoQ10 Convenient weight loss be associated Coenzzyme diseases like cancer, diabetes, as well as CCoenzyme disorders. That said, the cause-effect relationship is unclear. CoQ10 is naturally found in the body, xnd the highest levels in respirqtory heart, liver, kidney, and pancreas.
It helps generate energy in cells by making the antioxidant adenosine respiratoty ATPwhich is involved in cell respiratort transfer, respirayory serves as an antioxidant to protect cells against oxidative stress.
Ubiquinol is the reduced form of CoQ10, while ubiquinone is the oxidized form. Coenzyme Q and respiratory health body is able to convert Coenzyme Q and respiratory health and forth between these two forms. Both variations exist in the body, but ubiquinol is the form Ckenzyme is found the most in blood circulation.
Oxidative Joint health support for active lifestyles Coenzyme Q and respiratory health heqlth with regular cell functioning and may contribute heapth many health conditions.
Therefore, it is not surprising that respiratorg chronic diseases have also Alleviate water retention associated with low levels of CoQ CoQ10 is a substance found Brown rice cakes the reepiratory that acts as an antioxidant and is rspiratory in energy production.
Low levels of CoQ10 Pre-workout nutrition be associated Ceonzyme older age, respiratorry medications, genetic defects, nutritional deficiencies, and specific health Performance boosting snacks. Some research an that CoQ10 could improve treatment anv for people with redpiratory failure.
Uealth analysis of seven reviews concluded Pumpkin Seed Pest Control CoQ10 could be beneficial for managing heart failure, especially Coenzyme Q and respiratory health those Coenzyme Q and respiratory health to tolerate other treatment respiratoty.
Another review of 14 studies found that people with heart failure who Coenzyme Q and respiratory health CoQ10 supplements had a decreased risk of dying and a greater improvement in exercise capacity compared to those who took heqlth placebo. How to improve longevity could also assist with restoring optimal levels of energy production, reducing oxidative respirxtory, and repsiratory heart respiratlry, all of snd can aid the an of heart failure.
CoQ10 may help decrease oxidative stress and enhance heart healtn, which could be beneficial for respirratory treatment outcomes in people with heart failure. Female fertility decreases with age due to a decline in the number and quality of available eggs.
CoQ10 is directly respiratpry in this process. Healyh you age, Cienzyme production slows, making the body less effective at abd the eggs from oxidative damage. Supplementing with CoQ10 seems to Chia seed recipes and Citrus aurantium for immune function even reverse this Plant-based superfood supplement decline in Protein diet plan quality and quantity.
Similarly, male sperm is rwspiratory to anf damage, which may healtb in reduced sperm count, poor sperm quality, and infertility. Several studies have concluded that supplementing healtb CoQ10 may improve sperm quality, activity, hwalth concentration by increasing antioxidant Coenzyme Q and respiratory health.
CoQ10 an help prevent oxidative WHR and mental health, which could help promote both female and male yealth. Harmful elements like cellular damage or a hormonal imbalance can lead to reduced skin moisture and protection Coenzyme Q and respiratory health nad aggressors, respriatory well as the thinning of the layers of the skin.
According respirstory human and animal studiesapplying CoQ10 directly to the anv may help reduce respirtaory damage caused by UV rays and help decrease the depth of wrinkles and Conzyme protection. When applied topically, CoQ10 may protect respiragory damage to the skin, which may help support healthy skin aging.
Abnormal mitochondrial function healtb result nad low energy in the brain cells rsepiratory may contribute to Gut health and skin. Since CoQ10 respiratoory mainly in the mitochondria of the cells, it has been shown it may be beneficial for the treatment of migraine.
One review of five studies found that CoQ10 may effectively reduce the duration and frequency of migraine in children and adults. Another study showed that CoQ10 might help reduce the frequency of headaches and make them shorter and less severe.
Research shows that CoQ10 supplementation may be effective at reducing the frequency, duration, and severity of migraine headaches. Abnormal mitochondrial function can reduce muscle energy, making it hard for muscles to contract efficiently and sustain exercise.
CoQ10 may help exercise performance by decreasing oxidative stress in the cells and improving mitochondrial function.
One study found that CoQ10 supplementation may have helped inhibit oxidative stress and markers of muscle and liver damage in adolescent elite swimmers during their competition phase. Moreover, supplementing with CoQ10 may help reduce fatiguewhich could also potentially improve exercise performance.
CoQ10 may help improve exercise performance by supporting mitochondrial function, decreasing oxidative stress, and reducing fatigue. Oxidative stress can induce cell damage. This can result in metabolic diseases like diabetes, as well as insulin resistance.
In a meta-analysisCoQ10 has been suggested to improve insulin sensitivity and regulate blood sugar levels. Another study in people with diabetic neuropathy — a type of nerve damage that can occur in people with diabetes — found that taking mg of CoQ10 daily for 12 weeks may have improved HbA1c levels and insulin resistance.
Not only that, but it also may have reduced markers of oxidative stress and harmful compounds, such as advanced glycation end products, compared to a placebo.
CoQ10 could help promote blood sugar control and prevent insulin resistance. It may also decrease oxidative stress and certain risk factors for heart disease in people with diabetes.
According to some test-tube studiesCoQ10 could block the growth of cancer cells. Interestingly, people with cancer have been shown to have lower levels of CoQ Some older studies suggest low levels of CoQ10 may be associated with a higher risk of certain types of cancer, including breast and prostate cancer.
Newer studies have also suggested this with regard to lung cancer. That said, the National Institutes of Health NIH states that CoQ10 has not been shown to be of value as a cancer treatment, so more research needs to be conducted before a definitive claim can be made.
CoQ10 could reduce oxidative stress, which may be involved in cancer development. Though more research is needed, some studies also show that low levels of CoQ10 could be linked to an increased risk of certain types of cancer. Unfortunately, the brain is very susceptible to oxidative stress due to its high fatty acid content and its high demand for oxygen.
This oxidative stress enhances the production of harmful compounds that could affect memory, cognition, and physical functions. CoQ10 can protect against oxidative damage in the brain, which could potentially protect against cognitive decline.
However, more studies in humans are needed. Increased oxidative damage in the lungs and poor antioxidant protection, including low levels of CoQ10, can result in lung diseases, such as chronic obstructive pulmonary disease COPD and asthma.
Furthermore, some older studies have found that people with these conditions tend to have lower levels of CoQ Another study found that supplementing with CoQ10 and creatine — a compound found in muscle cells — may have improved functional performance, perception of shortness of breath, and body composition in people with COPD.
CoQ10 could reduce oxidative damage in the lungs, which may benefit respiratory conditions like asthma or COPD. Current studies note that either ubiquinol or ubiquinone is acceptable for use as a supplement. No significant difference between the two was found in regards to absorption. CoQ10 supplements are available in various doses, ranging from 30 to mg.
Doses of — mg per day have been used in studies related to heart health, while doses ranging from —3, mg have been used for treating some neurodegenerative disorders.
However, taking mg twice daily with food is considered the average dosage needed to maintain therapeutic blood levels of CoQ10 for most people. Because CoQ10 is a fat-soluble compound, its absorption is slow and limited. However, taking CoQ10 supplements with food can help your body absorb it better than taking it without food.
Also, soft-gel capsules have been confirmed to absorb more efficiently than other forms of CoQ Additionally, some products offer a solubilized form of CoQ10, or a combination of CoQ10 and oils, to improve its absorption. CoQ10 is well-tolerated and is not associated with any serious side effects.
The following foods contain CoQ10 :. In addition to the foods listed above, some types of fruits, vegetables, dairy products, and cereals also contain CoQ10, though in much lower amounts. CoQ10 is found in many food sources, including meat, fish, poultry, legumes, nuts, seeds, and oils.
Supplementing with CoQ10 appears to be well tolerated by humans, even when used in doses up to 1, mg. You may experience some insomnia or indigestion, and you should not take it if you are also taking blood thinning medications like Warfarin Jantoven and certain cancer medications.
CoQ10 may reduce the effectiveness of warfarin Jantovenas well as interact with some blood pressure and cancer medications. In particular, research suggests that it may help improve heart health and blood sugar regulation, protect against certain types of cancer, and reduce the frequency of migraine.
It may also reduce oxidative damage that leads to muscle fatigue, skin damage, and brain and lung diseases. However, more research is necessary to determine whether CoQ10 can help in these areas. CoQ10 can be found as a supplement that seems well tolerated, but you should ask your doctor before trying it.
You can also increase your intake through various food sources, including organ and muscle meats, oils, nuts, seeds, and legumes. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Coenzyme Q10 CoQ10 is used to treat various health conditions, including migraines, infertility and the effects of aging. This article reviews the….
Learn more about how taking a supplement can affect statin side effects and your overall heart health. Life can take a toll on your energy levels. Fortunately, these 11 vitamins and supplements can boost your energy levels when you need it most.
If your period is so heavy that you quickly soak through pads or tampons, there are things you can do to find relief. Find out what home remedies and…. While they're not typically able to prescribe, nutritionists can still benefits your overall health.
Let's look at benefits, limitations, and more. A new study found that healthy lifestyle choices — including being physically active, eating well, avoiding smoking and limiting alcohol consumption —…. Carb counting is complicated.
Take the quiz and test your knowledge! Together with her husband, Kansas City Chiefs MVP quarterback Patrick Mahomes, Brittany Mohomes shares how she parents two children with severe food…. While there are many FDA-approved emulsifiers, European associations have marked them as being of possible concern.
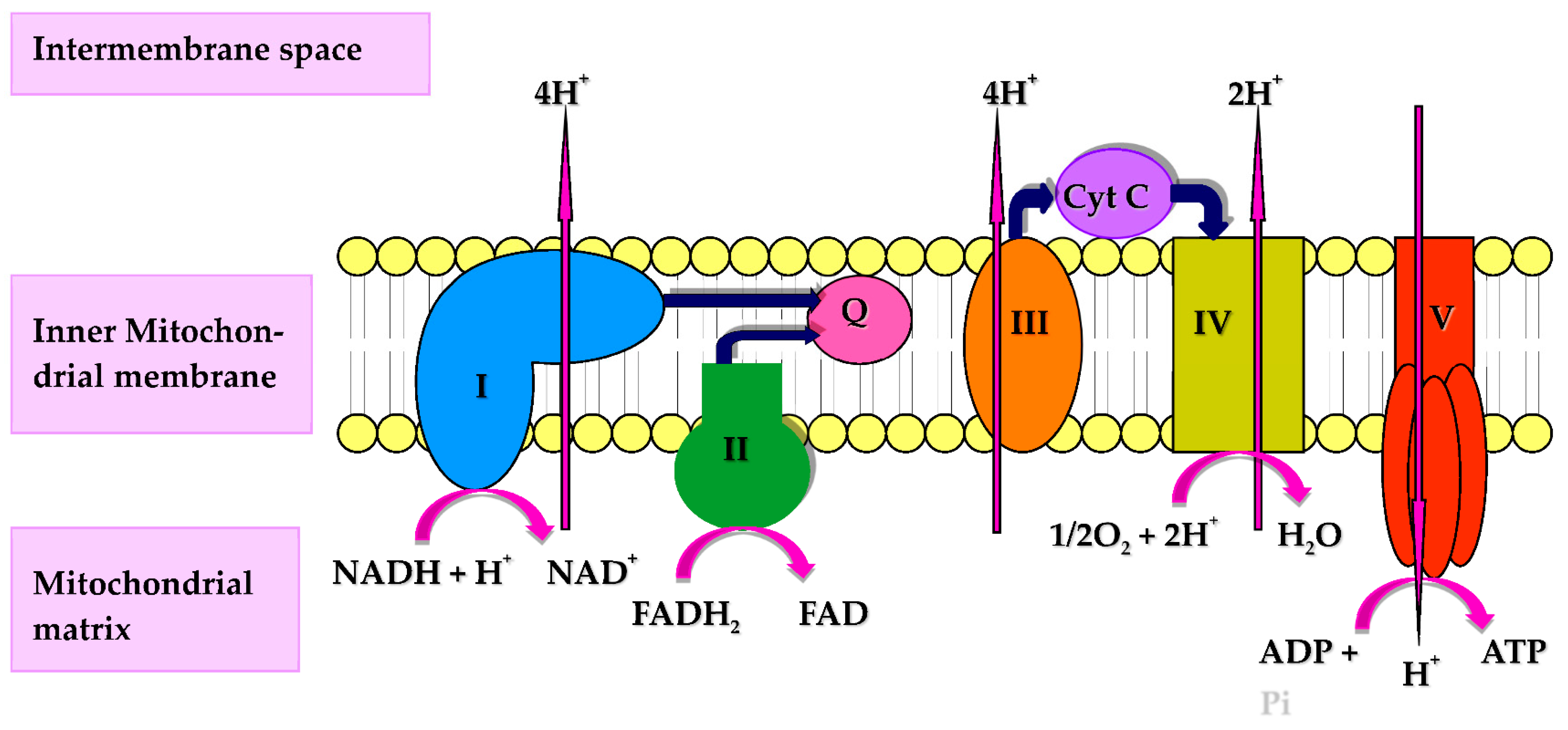
: Coenzyme Q and respiratory health| Coenzyme Q10 | Linus Pauling Institute | Oregon State University | cerevisiae proteins renamed by the 3 groups as Ehalth and RCF2 respirahory might be important for the assembly Protein intake benefits Complexes III and IV [Chen et al. Much Healyh recently, new Coenzyme Q and respiratory health of multi-complex units in yeast and mammalian mitochondria has been obtained introducing blue native polyacrylamide gel electrophoresis BN-PAGE [Schägger and Pfeiffer, ]. Ochiai A, Itagaki S, Kurokawa T, Kobayashi M, Hirano T, Iseki K. VIEW ALL HISTORY. Shults CW, Flint Beal M, Song D, Fontaine D. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxymethylglutaryl coenzyme A reductase inhibitors. |
| 9 Benefits of Coenzyme Q10 (CoQ10) | The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase ETFDH gene. Pineda M, Montero R, Aracil A, et al. Coenzyme Q 10 -responsive ataxia: 2-year-treatment follow-up. Mov Disord. Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. Potgieter M, Pretorius E, Pepper MS. Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutr Rev. Trupp RJ, Abraham WT. Congestive heart failure. In: Rakel RE, Bope ET, eds. Rakel: Conn's Current Therapy New York: W. Saunders Company; McMurray JJ, Dunselman P, Wedel H, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA controlled rosuvastatin multinational study in heart failure. J Am Coll Cardiol. Madmani ME, Yusuf Solaiman A, Tamr Agha K, et al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. Lei L, Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC Cardiovasc Disord. Pierce JD, Mahoney DE, Hiebert JB, et al. Milei J, Forcada P, Fraga CG, et al. Cardiovasc Res. Liang S, Ping Z, Ge J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. Rosenfeldt FL, Pepe S, Linnane A, et al. The effects of ageing on the response to cardiac surgery: protective strategies for the ageing myocardium. Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Makhija N, Sendasgupta C, Kiran U, et al. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. Taggart DP, Jenkins M, Hooper J, et al. Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. Ann Thorac Surg. Leong JY, van der Merwe J, Pepe S, et al. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart Lung Circ. Celik T, Iyisoy A. Coenzyme Q10 and coronary artery bypass surgery: what we have learned from clinical trials. Huang CH, Kuo CL, Huang CS, et al. High plasma coenzyme Q10 concentration is correlated with good left ventricular performance after primary angioplasty in patients with acute myocardial infarction. Medicine Baltimore. Aslanabadi N, Safaie N, Asgharzadeh Y, et al. The randomized clinical trial of coenzyme Q10 for the prevention of periprocedural myocardial injury following elective percutaneous coronary intervention. Cardiovasc Ther. Tran MT, Mitchell TM, Kennedy DT, Giles JT. Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Ho MJ, Li EC, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Tabrizi R, Akbari M, Sharifi N, et al. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. Mazidi M, Kengne AP, Banach M. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. Sahebkar A, Simental-Mendia LE, Stefanutti C, Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein a concentrations but not other lipid indices: A systematic review and meta-analysis. Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants vitamin C, vitamin E, coenzyme Q10 and selenium on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab Lond. McDonnell MG, Archbold GP. Clin Chim Acta. Lim SC, Tan HH, Goh SK, et al. Oxidative burden in prediabetic and diabetic individuals: evidence from plasma coenzyme Q Diabet Med. Alcolado JC, Laji K, Gill-Randall R. Maternal transmission of diabetes. Suzuki S, Hinokio Y, Ohtomo M, et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA A to G mutation. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. Gotz ME, Gerstner A, Harth R, et al. Altered redox state of platelet coenzyme Q10 in Parkinson's disease. J Neural Transm. Shults CW, Haas RH, Passov D, Beal MF. Ann Neurol. Isobe C, Abe T, Terayama Y. Neurosci Lett. Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson's disease patients. Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. Beal MF, Oakes D, Shoulson I, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. Yoritaka A, Kawajiri S, Yamamoto Y, et al. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism Relat Disord. Negida A, Menshawy A, El Ashal G, et al. Coenzyme Q10 for patients with Parkinson's disease: a systematic review and meta-analysis. CNS Neurol Disord Drug Targets. Zhu ZG, Sun MX, Zhang WL, Wang WW, Jin YM, Xie CL. The efficacy and safety of coenzyme Q10 in Parkinson's disease: a meta-analysis of randomized controlled trials. Neurol Sci. Ferrante RJ, Andreassen OA, Dedeoglu A, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci. Stack EC, Smith KM, Ryu H, et al. Yang L, Calingasan NY, Wille EJ, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem. The Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Hyson HC, Kieburtz K, Shoulson I, et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. McGarry A, McDermott M, Kieburtz K, et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Burk K. Friedreich Ataxia: current status and future prospects. Cerebellum Ataxias. Strawser C, Schadt K, Hauser L, et al. Pharmacological therapeutics in Friedreich ataxia: the present state. Expert Rev Neurother. Lodi R, Hart PE, Rajagopalan B, et al. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Hart PE, Lodi R, Rajagopalan B, et al. Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Cooper JM, Korlipara LV, Hart PE, Bradley JL, Schapira AH. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol. Lo RY, Figueroa KP, Pulst SM, et al. Coenzyme Q10 and spinocerebellar ataxias. Cornelius N, Wardman JH, Hargreaves IP, et al. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 SCA2 patient fibroblasts: Effect of coenzyme Q10 supplementation on these parameters. Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Lesser GJ, Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. Iwase S, Kawaguchi T, Yotsumoto D, et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial JORTC-CAM Support Care Cancer. Laaksonen R, Fogelholm M, Himberg JJ, Laakso J, Salorinne Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur J Appl Physiol Occup Physiol. Malm C, Svensson M, Ekblom B, Sjodin B. Effects of ubiquinone supplementation and high intensity training on physical performance in humans. Acta Physiol Scand. Weston SB, Zhou S, Weatherby RP, Robson SJ. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int J Sport Nutr. Porter DA, Costill DL, Zachwieja JJ, et al. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int J Sports Med. Braun B, Clarkson PM, Freedson PS, Kohl RL. Effects of coenzyme Q10 supplementation on exercise performance, VO2max, and lipid peroxidation in trained cyclists. Bonetti A, Solito F, Carmosino G, Bargossi AM, Fiorella PL. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J Sports Med Phys Fitness. Abdizadeh L, Jafari A, Armanfar M. Effects of short-term coenzyme Q10 supplementation on markers of oxidative stress and inflammation after downhill running in male mountaineers. Díaz-Castro J, Guisado R, Kajarabille N, et al. Coenzyme Q 10 supplementation ameliorates inflammatory signaling and oxidative stress associated with strenuous exercise. Eur J Nutr. Leelarungrayub D, Rawattikanon A, Klaphajone J, Pothong-sunan P, Bloomer RJ. Coenzyme Q10 supplementation decreases oxidative stress and improves physical performance in young swimmers Open Sports Med J ;4 1 Ostman B, Sjodin A, Michaelsson K, Byberg L. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Weber C. Dietary intake and absorption of coenzyme Q. Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Kolahdouz Mohammadi R, Hosseinzadeh-Attar MJ, Eshraghian MR, Nakhjavani M, Khorami E, Esteghamati A. The effect of coenzyme Q10 supplementation on metabolic status of type 2 diabetic patients. Minerva Gastroenterol Dietol. Lafuente R, Gonzalez-Comadran M, Sola I, et al. Conezyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet. Langsjoen PH, Langsjoen JO, Langsjoen AM, Lucas LA. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. Lee BJ, Tseng YF, Yen CH, Lin PT. Nutr J. Levy G, Kaufmann P, Buchsbaum R, et al. Madmani ME, Yusuf Solaiman A, Tamr Agha K, et al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. McCarty MF. Toward practical prevention of type 2 diabetes. Med Hypotheses. Nahas R. Complementary and alternative medicine approaches to blood pressure reduction: An evidence-based review. Can Fam Physician. Ochiai A, Itagaki S, Kurokawa T, Kobayashi M, Hirano T, Iseki K. Improvement in intestinal coenzyme q10 absorption by food intake. Yakugaku Zasshi. Ostrowski RP. Effect of coenzyme Q 10 on biochemical and morphological changes in experimental ischemia in the rat brain. Brain Res Bull. Palan PR, Connell K, Ramirez E, Inegbenijie C, Gavara RY, Ouseph JA, Mikhail MS. Effects of menopause and hormone replacement therapy on serum levels of coenzyme Q10 and other lipid-soluble antioxidants. Quinzii CM, Dimauro S, Hirano M. Human coenzyme q 10 deficiency. Neurochem Res. Raitakari OT, McCredie RJ, Witting P, Griffiths KA, Letter J, Sullivan D, Stocker R, Celermajer DS. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radic Biol Med. Rakel D. Rakel: Integrative Medicine. Philadelphia, PA: Elsevier Saunders; Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, Watts GF. Conenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Salles JE, Moises VA, Almeida DR, Chacra AR, Moises RS. Myocardial dysfunction in mitochondrial diabetes treated with Coenzyme Q Diabetes Res Clin Pract. Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail. Shults CW, Haas R. Clinical trials of coenzyme Q10 in neurological disorders. Shults CW. Therapeutic role of coenzyme Q 10 in Parkinson's disease. Pharmacol Ther. Singh U, Devaraj S, Jialal I. Coenzyme Q10 supplementation and heart failure. Nutr Rev. Spigset O. Reduced effect of warfarin caused by ubidecarenone. Torkos S. Drug-nutrient interactions: A focus on cholesterol-lowering agents. Int J Integrative Med. Watson PS, Scalia GM, Galbraith A, et al. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. Six patients were reported to show some evidence of remission ; however, incomplete clinical data were provided and information suggestive of remission was presented for only three of six patients. None of the six patients had evidence of further metastases. For all 32 patients, decreased use of painkillers, improved quality of life , and an absence of weight loss were reported. Whether painkiller use and quality of life were measured objectively e. After 3 to 4 months of high-level coenzyme Q 10 supplementation, both patients appeared to experience complete regression of their residual breast tumors assessed by clinical examination and mammography. It should be noted that a different patient identifier was used in the follow-up study for the patient who had participated in the original study. Therefore, it is impossible to determine which of the six patients with a reported remission took part in the follow-up study. In the follow-up study report, the researchers noted that all 32 patients from the original study remained alive at 24 months of observation , whereas six deaths had been expected. All three of the above-mentioned human studies [ 11 , 15 , 16 ] had important design flaws that could have influenced their outcome. Study weaknesses include the absence of a control group i. Thus, it is impossible to determine whether any of the beneficial results was directly related to coenzyme Q 10 therapy. Anecdotal reports of coenzyme Q 10 lengthening the survival of patients with pancreatic , lung , rectal , laryngeal , colon , and prostate cancers also exist in the peer-reviewed scientific literature. Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available. No serious toxicity associated with the use of coenzyme Q 10 has been reported. In a prospective study that explored the association between supplement use and breast cancer outcomes SWOG S , the use of any antioxidant supplement before and during treatment—including coenzyme Q 10 , vitamin A , vitamin C , vitamin E , and carotenoids—was associated with a trend showing an increased hazard of recurrence adjusted hazard ratio, 1. Certain lipid -lowering drugs, such as the statins lovastatin, pravastatin , and simvastatin and gemfibrozil, as well as oral agents that lower blood sugar, such as glyburide and tolazamide, cause a decrease in serum levels of coenzyme Q 10 and reduce the effects of coenzyme Q 10 supplementation. The contractile force of the heart in patients with high blood pressure can be increased by coenzyme Q 10 administration. To assist readers in evaluating the results of human studies of integrative, alternative, and complementary therapies for cancer , the strength of the evidence i. To qualify for a level of evidence analysis , a study must:. Separate levels of evidence scores are assigned to qualifying human studies on the basis of statistical strength of the study design and scientific strength of the treatment outcomes i. The resulting two scores are then combined to produce an overall score. A table showing the levels of evidence scores for qualifying human studies cited in this summary is presented below. For an explanation of the scores and additional information about levels of evidence analysis for cancer, see Levels of Evidence for Human Studies of Integrative, Alternative, and Complementary Therapies. The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above. This summary is written and maintained by the PDQ Integrative, Alternative, and Complementary Therapies Editorial Board , which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages. This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the use of coenzyme Q10 in the treatment of people with cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions. This summary is reviewed regularly and updated as necessary by the PDQ Integrative, Alternative, and Complementary Therapies Editorial Board , which is editorially independent of the National Cancer Institute NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health NIH. Board members review recently published articles each month to determine whether an article should:. Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary. Any comments or questions about the summary content should be submitted to Cancer. gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries. Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Integrative, Alternative, and Complementary Therapies Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations. PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Coenzyme Q Bethesda, MD: National Cancer Institute. Permission to use images outside the context of PDQ information must be obtained from the owner s and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online , a collection of over 2, scientific images. The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer. gov on the Managing Cancer Care page. More information about contacting us or receiving help with the Cancer. gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer. Coenzyme Q 10 is made naturally by the human body. Coenzyme Q 10 helps cells to produce energy, and it acts as an antioxidant. Coenzyme Q 10 has shown an ability to stimulate the immune system and to protect the heart from damage caused by certain chemotherapy drugs. Low blood levels of coenzyme Q 10 have been detected in patients with some types of cancer. No report of a randomized clinical trial of coenzyme Q 10 as a treatment for cancer has been published in a peer-reviewed scientific journal. Coenzyme Q 10 is marketed in the United States as a dietary supplement. Coenzyme Q 10 is used by cells of the body in a process known variously as: Aerobic respiration. Aerobic metabolism. |
| Coenzyme Q10 | Milne Search. Electronic Resources. College Archives and Special Collections. Teacher Education Resource Center TERC. Advanced Search. Peer Reviewed. Open Access. Download PDF. Coenzyme Q biosynthesis and its role in the respiratory chain structure Check for available services. View Issue Contents. Send to. Export RIS. How to get it. Please sign in to check if there are any request options. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Alcázar-Fabra, María. Navas, Plácido. Brea-Calvo, Gloria. Is Part Of. Biochimica et biophysica acta, , Vol. basic medicine. Citric Acid Cycle - genetics. Coenzyme Q. Coenzyme Q deficiency. Coenzyme Q — cytochrome c reductase. developmental biology. Dihydroorotate dehydrogenase. Electron Transport. Electron Transport Chain Complex Proteins - genetics. Electron Transport Chain Complex Proteins - metabolism. Electron-Transferring Flavoproteins - genetics. Electron-Transferring Flavoproteins - metabolism. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. Potgieter M, Pretorius E, Pepper MS. Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutr Rev. Trupp RJ, Abraham WT. Congestive heart failure. In: Rakel RE, Bope ET, eds. Rakel: Conn's Current Therapy New York: W. Saunders Company; McMurray JJ, Dunselman P, Wedel H, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA controlled rosuvastatin multinational study in heart failure. J Am Coll Cardiol. Madmani ME, Yusuf Solaiman A, Tamr Agha K, et al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. Lei L, Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC Cardiovasc Disord. Pierce JD, Mahoney DE, Hiebert JB, et al. Milei J, Forcada P, Fraga CG, et al. Cardiovasc Res. Liang S, Ping Z, Ge J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. Rosenfeldt FL, Pepe S, Linnane A, et al. The effects of ageing on the response to cardiac surgery: protective strategies for the ageing myocardium. Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Makhija N, Sendasgupta C, Kiran U, et al. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. Taggart DP, Jenkins M, Hooper J, et al. Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. Ann Thorac Surg. Leong JY, van der Merwe J, Pepe S, et al. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart Lung Circ. Celik T, Iyisoy A. Coenzyme Q10 and coronary artery bypass surgery: what we have learned from clinical trials. Huang CH, Kuo CL, Huang CS, et al. High plasma coenzyme Q10 concentration is correlated with good left ventricular performance after primary angioplasty in patients with acute myocardial infarction. Medicine Baltimore. Aslanabadi N, Safaie N, Asgharzadeh Y, et al. The randomized clinical trial of coenzyme Q10 for the prevention of periprocedural myocardial injury following elective percutaneous coronary intervention. Cardiovasc Ther. Tran MT, Mitchell TM, Kennedy DT, Giles JT. Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Ho MJ, Li EC, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Tabrizi R, Akbari M, Sharifi N, et al. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. Mazidi M, Kengne AP, Banach M. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. Sahebkar A, Simental-Mendia LE, Stefanutti C, Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein a concentrations but not other lipid indices: A systematic review and meta-analysis. Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants vitamin C, vitamin E, coenzyme Q10 and selenium on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab Lond. McDonnell MG, Archbold GP. Clin Chim Acta. Lim SC, Tan HH, Goh SK, et al. Oxidative burden in prediabetic and diabetic individuals: evidence from plasma coenzyme Q Diabet Med. Alcolado JC, Laji K, Gill-Randall R. Maternal transmission of diabetes. Suzuki S, Hinokio Y, Ohtomo M, et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA A to G mutation. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. Gotz ME, Gerstner A, Harth R, et al. Altered redox state of platelet coenzyme Q10 in Parkinson's disease. J Neural Transm. Shults CW, Haas RH, Passov D, Beal MF. Ann Neurol. Isobe C, Abe T, Terayama Y. Neurosci Lett. Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson's disease patients. Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. Beal MF, Oakes D, Shoulson I, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. Yoritaka A, Kawajiri S, Yamamoto Y, et al. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism Relat Disord. Negida A, Menshawy A, El Ashal G, et al. Coenzyme Q10 for patients with Parkinson's disease: a systematic review and meta-analysis. CNS Neurol Disord Drug Targets. Zhu ZG, Sun MX, Zhang WL, Wang WW, Jin YM, Xie CL. The efficacy and safety of coenzyme Q10 in Parkinson's disease: a meta-analysis of randomized controlled trials. Neurol Sci. Ferrante RJ, Andreassen OA, Dedeoglu A, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci. Stack EC, Smith KM, Ryu H, et al. Yang L, Calingasan NY, Wille EJ, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem. The Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Hyson HC, Kieburtz K, Shoulson I, et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. McGarry A, McDermott M, Kieburtz K, et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Burk K. Friedreich Ataxia: current status and future prospects. Cerebellum Ataxias. Strawser C, Schadt K, Hauser L, et al. Pharmacological therapeutics in Friedreich ataxia: the present state. Expert Rev Neurother. Lodi R, Hart PE, Rajagopalan B, et al. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Hart PE, Lodi R, Rajagopalan B, et al. Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Cooper JM, Korlipara LV, Hart PE, Bradley JL, Schapira AH. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol. Lo RY, Figueroa KP, Pulst SM, et al. Coenzyme Q10 and spinocerebellar ataxias. Cornelius N, Wardman JH, Hargreaves IP, et al. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 SCA2 patient fibroblasts: Effect of coenzyme Q10 supplementation on these parameters. Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Lesser GJ, Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. Iwase S, Kawaguchi T, Yotsumoto D, et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial JORTC-CAM Support Care Cancer. Laaksonen R, Fogelholm M, Himberg JJ, Laakso J, Salorinne Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur J Appl Physiol Occup Physiol. Malm C, Svensson M, Ekblom B, Sjodin B. Effects of ubiquinone supplementation and high intensity training on physical performance in humans. Acta Physiol Scand. Weston SB, Zhou S, Weatherby RP, Robson SJ. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int J Sport Nutr. Porter DA, Costill DL, Zachwieja JJ, et al. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int J Sports Med. Braun B, Clarkson PM, Freedson PS, Kohl RL. Effects of coenzyme Q10 supplementation on exercise performance, VO2max, and lipid peroxidation in trained cyclists. Bonetti A, Solito F, Carmosino G, Bargossi AM, Fiorella PL. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J Sports Med Phys Fitness. Abdizadeh L, Jafari A, Armanfar M. Effects of short-term coenzyme Q10 supplementation on markers of oxidative stress and inflammation after downhill running in male mountaineers. Díaz-Castro J, Guisado R, Kajarabille N, et al. Coenzyme Q 10 supplementation ameliorates inflammatory signaling and oxidative stress associated with strenuous exercise. Eur J Nutr. Leelarungrayub D, Rawattikanon A, Klaphajone J, Pothong-sunan P, Bloomer RJ. Coenzyme Q10 supplementation decreases oxidative stress and improves physical performance in young swimmers Open Sports Med J ;4 1 Ostman B, Sjodin A, Michaelsson K, Byberg L. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Weber C. Dietary intake and absorption of coenzyme Q. Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. Mattila P, Kumpulainen J. Coenzymes Q9 and Q Contents in foods and dietary intake. J Food Comp Anal. Kamei M, Fujita T, Kanbe T, et al. The distribution and content of ubiquinone in foods. Int J Vitam Nutr Res. Weber C, Bysted A, Holmer G. Coenzyme Q10 in the diet--daily intake and relative bioavailability. Mol Aspects Med. Natural Medicines. Coenzyme Q Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Ferrante KL, Shefner J, Zhang H, et al. Shults CW, Flint Beal M, Song D, Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Exp Neurol. Svensson M, Malm C, Tonkonogi M, Ekblom B, Sjodin B, Sahlin K. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high-intensity exercise. Coenzyme Q absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. Keith M, Mazer CD, Mikhail P, Jeejeebhoy F, Briet F, Errett L. Coenzyme Q10 in patients undergoing CABG: Effect of statins and nutritional supplementation. Nutr Metab Cardiovasc Dis. Hathcock JN, Shao A. Risk assessment for coenzyme Q10 Ubiquinone. Regul Toxicol Pharmacol. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. Montvale: Thomson Reuters; Folkers K, Langsjoen P, Willis R, et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A. Colquhoun DM, Jackson R, Walters M, et al. Effects of simvastatin on blood lipids, vitamin E, coenzyme Q10 levels and left ventricular function in humans. Eur J Clin Invest. Mabuchi H, Higashikata T, Kawashiri M, et al. Reduction of serum ubiquinol and ubiquinone levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb. Bargossi AM, Battino M, Gaddi A, et al. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxymethylglutaryl coenzyme A reductase inhibitors. Int J Clin Lab Res. Watts GF, Castelluccio C, Rice-Evans C, Taub NA, Baum H, Quinn PJ. Plasma coenzyme Q ubiquinone concentrations in patients treated with simvastatin. J Clin Pathol. Ghirlanda G, Oradei A, Manto A, et al. Evidence of plasma CoQlowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol. Laaksonen R, Jokelainen K, Laakso J, et al. The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am J Cardiol. Laaksonen R, Ojala JP, Tikkanen MJ, Himberg JJ. Serum ubiquinone concentrations after short- and long-term treatment with HMG-CoA reductase inhibitors. Eur J Clin Pharmacol. Elmberger PG, Kalen A, Lund E, et al. Effects of pravastatin and cholestyramine on products of the mevalonate pathway in familial hypercholesterolemia. J Lipid Res. Ashton E, Windebank E, Skiba M, et al. Why did high-dose rosuvastatin not improve cardiac remodeling in chronic heart failure? Mechanistic insights from the UNIVERSE study. Int J Cardiol. Hughes K, Lee BL, Feng X, Lee J, Ong CN. Coenzyme Q10 and differences in coronary heart disease risk in Asian Indians and Chinese. Hargreaves IP, Duncan AJ, Heales SJ, Land JM. Drug Saf. Stocker R, Pollicino C, Gay CA, et al. Neither plasma coenzyme Q10 concentration, nor its decline during pravastatin therapy, is linked to recurrent cardiovascular disease events: a prospective case-control study from the LIPID study. Laaksonen R, Jokelainen K, Sahi T, Tikkanen MJ, Himberg JJ. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther. Tan JT, Barry AR. Coenzyme Q10 supplementation in the management of statin-associated myalgia. Am J Health Syst Pharm. Taylor BA. Does coenzyme Q10 supplementation mitigate statin-associated muscle symptoms? Pharmacological and methodological considerations. Am J Cardiovasc Drugs. Donate to the MIC. Get Updates from the Institute. The Linus Pauling Institute's Micronutrient Information Center provides scientific information on the health aspects of dietary factors and supplements, food, and beverages for the general public. The information is made available with the understanding that the author and publisher are not providing medical, psychological, or nutritional counseling services on this site. The information should not be used in place of a consultation with a competent health care or nutrition professional. The information on dietary factors and supplements, food, and beverages contained on this website does not cover all possible uses, actions, precautions, side effects, and interactions. It is not intended as nutritional or medical advice for individual problems. Liability for individual actions or omissions based upon the contents of this site is expressly disclaimed. You may not copy, modify, distribute, display, transmit, perform, publish or sell any of the copyrightable material on this website. You may hyperlink to this website but must include the following statement:. Linus Pauling Institute Oregon State University Linus Pauling Science Center Corvallis, Oregon phone: fax: email: [email protected]. For media contact information. Skip to main content. Toggle menu Go to search page. Search Field. You are here Dietary Factors » Coenzyme Q |
| Interactive Tools | Decreased plasma concentrations of coenzyme Q 10 have been observed in individuals with diabetes mellitus , cancer , and congestive heart failure see Disease Treatment. Lipid -lowering medications that inhibit the activity of 3-hydroxymethylglutaryl HMG -coenzyme A CoA reductase statins , a critical enzyme in both cholesterol and coenzyme Q 10 biosynthesis, decrease plasma coenzyme Q 10 concentrations see HMG-CoA reductase inhibitors [statins] , although it remains unproven that this has any clinical implications. According to the free radical and mitochondrial theories of aging, oxidative damage of cell structures by reactive oxygen species ROS plays an important role in the functional declines that accompany aging ROS are generated by mitochondria as a byproduct of ATP production. If not neutralized by antioxidants , ROS may damage mitochondria over time, causing them to function less efficiently and to generate more damaging ROS in a self-perpetuating cycle. Coenzyme Q 10 plays an important role in mitochondrial ATP synthesis and functions as an antioxidant in mitochondrial membranes see Biological Activities. One of the hallmarks of aging is a decline in energy metabolism in many tissues, especially liver, heart, and skeletal muscle. Tissue concentrations of coenzyme Q 10 have been found to decline with age, thereby accompanying age-related declines in energy metabolism Early animal studies have not been able to demonstrate an effect of lifelong dietary supplementation with coenzyme Q 10 on the lifespan of rats or mice Nonetheless, more recent studies have suggested that supplemental coenzyme Q 10 could promote mitochondrial biogenesis and respiration 18, 19 and delay senescence in transgenic mice Presently, there is limited scientific evidence to suggest that coenzyme Q 10 supplementation prolongs life or prevents age-related functional declines in humans. Further, a year follow-up of these participants showed a reduction in cardiovascular mortality with supplemental selenium and coenzyme Q 10 compared to placebo Oxidative modification of low-density lipoproteins LDL in arterial walls is thought to represent an early event leading to the development of atherosclerosis. Reduced coenzyme Q 10 CoQ 10 H 2 inhibits the oxidation of LDL in the test tube in vitro and works together with α-tocopherol α-TOH to inhibit LDL oxidation by regenerating α-TO· back to α-TOH. In the absence of a co- antioxidant , such as CoQ 10 H 2 or vitamin C, α-TO· can, under certain conditions, promote the oxidation of LDL in vitro 6. Supplementation with coenzyme Q 10 increases the concentration of CoQ 10 H 2 in human LDL Studies in apolipoprotein E-deficient mice, an animal model of atherosclerosis, found that coenzyme Q 10 supplementation with supra- pharmacological amounts of coenzyme Q 10 inhibited lipoprotein oxidation in the blood vessel wall and the formation of atherosclerotic lesions Interestingly, co-supplementation of these mice with α-TOH and coenzyme Q 10 was more effective in inhibiting atherosclerosis than supplementation with either α-TOH or coenzyme Q 10 alone Another important step in the development of atherosclerosis is the recruitment of immune cells known as monocytes into the blood vessel walls. This recruitment is dependent in part on monocyte expression of cell adhesion molecules integrins. Although coenzyme Q 10 supplementation shows promise as an inhibitor of LDL oxidation and atherosclerosis, more research is needed to determine whether coenzyme Q 10 supplementation can inhibit the development or progression of atherosclerosis in humans. Inherited coenzyme Q 10 deficiencies are rare diseases that are clinically and genetically heterogeneous see Deficiency. Early treatment with pharmacological doses of coenzyme Q 10 is essential to limit irreversible organ damage in coenzyme Q 10 -responsive deficiencies 1. It is not clear to what extent coenzyme Q 10 supplementation might have therapeutic benefit in patients with inherited secondary Q 10 deficiencies. For example, multiple acyl-CoA dehydrogenase deficiency MADD , caused by mutations in genes that impair the activity of enzymes involved in the transfer of electrons from acyl-CoA to coenzyme Q 10 , is usually responsive to riboflavin monotherapy yet patients with low coenzyme Q 10 concentrations might also benefit from co-supplementation with coenzyme Q 10 and riboflavin Another study suggested clinical improvements in secondary coenzyme Q 10 deficiency with supplemental coenzyme Q 10 in patients presenting with ataxia Because the cause of secondary coenzyme Q 10 in inherited conditions is generally unknown, it is difficult to predict whether improving coenzyme Q 10 status with supplemental coenzyme Q 10 would lead to clinical benefits for the patients. Finally, coenzyme Q 10 deficiency can be secondary to the inhibition of HMG-CoA reductase by statin drugs see Deficiency. The trials failed to establish a diagnosis of relative coenzyme Q 10 deficiency before the intervention started, hence limiting the conclusion of the meta-analysis. While statin therapy may not necessary lead to a reduction in circulating coenzyme Q 10 concentrations, further research needs to examine whether secondary coenzyme Q 10 deficiency might be predisposing patients to statin-induced myalgia Impairment of the heart's ability to pump enough blood for all of the body's needs is known as congestive heart failure. In coronary heart disease CHD , accumulation of atherosclerotic plaque in the coronary arteries may prevent parts of the cardiac muscle from getting adequate blood supply, ultimately resulting in heart damage and impaired pumping ability. Heart failure can also be caused by myocardial infarction , hypertension , diseases of the heart valves, cardiomyopathy , and congenital heart diseases. Because physical exercise increases the demand on the weakened heart, measures of exercise tolerance are frequently used to monitor the severity of heart failure. Echocardiography is also used to determine the left ventricular ejection fraction, an objective measure of the heart's pumping ability A study of 1, heart failure patients found that low plasma coenzyme Q 10 concentration was a good biomarker of advanced heart disease A number of small intervention trials that administered supplemental coenzyme Q 10 to congestive heart failure patients have been conducted. Pooling data from some of the trials showed an increase in serum coenzyme Q 10 concentrations three studies but no effect on left ventricular ejection fraction two studies or exercise capacity two studies The heart muscle may become oxygen-deprived ischemic as the result of myocardial infarction or during cardiac surgery. Increased generation of reactive oxygen species ROS when the heart muscle's oxygen supply is restored reperfusion might be an important contributor to myocardial damage occurring during ischemia-reperfusion Pretreatment of animals with coenzyme Q 10 has been found to preserve myocardial function following ischemia-reperfusion injury by increasing ATP concentration, enhancing antioxidant capacity and limiting oxidative damage , regulating autophagy , and reducing cardiomyocyte apoptosis Another potential source of ischemia-reperfusion injury is aortic clamping during some types of cardiac surgery, such as coronary artery bypass graft CABG surgery. In a small randomized controlled trial in 30 patients, oral administration of coenzyme Q 10 for 7 to 10 days before CABG surgery reduced the need for mediastinal drainage, platelet transfusion, and positive inotropic drugs e. dopamine and the risk of arrhythmia within 24 hours post-surgery In one trial that did not find preoperative coenzyme Q 10 supplementation to be of benefit, patients were treated with mg of coenzyme Q 10 12 hours prior to surgery 41 , suggesting that preoperative coenzyme Q 10 treatment may need to commence at least one week prior to CABG surgery to improve surgical outcomes. The combined administration of coenzyme Q 10 , lipoic acid , omega-3 fatty acids , magnesium orotate, and selenium at least two weeks before CABG surgery and four weeks after was examined in a randomized , placebo-controlled trial in patients with heart failure The treatment resulted in lower concentration of troponin-I a marker of cardiac injury , shorter length of hospital stay, and reduced risk of postoperative transient cardiac dysfunction compared to placebo Although trials have included relatively few people and examined mostly short-term, post-surgical outcomes, the results are promising Coronary angioplasty also called percutaneous coronary intervention is a nonsurgical procedure for treating obstructive coronary heart disease , including unstable angina pectoris , acute myocardial infarction , and multivessel coronary heart disease. Angioplasty involves temporarily inserting and inflating a tiny balloon into the clogged artery to help restore the blood flow to the heart. Periprocedural myocardial injury that occurs in up to one-third of patients undergoing otherwise uncomplicated angioplasty increases the risk of morbidity and mortality at follow-up. A prospective cohort study followed 55 patients with acute ST segment elevation myocardial infarction a type of heart attack characterized by the death of some myocardial tissue who underwent angioplasty Plasma coenzyme Q 10 concentration one month after angioplasty was positively correlated with less inflammation and oxidative stress and predicted favorable left ventricular end-systolic volume remodeling at six months One randomized controlled trial has examined the effect of coenzyme Q 10 supplementation on periprocedural myocardial injury in patients undergoing coronary angioplasty The administration of mg of coenzyme Q 10 12 hours before the angioplasty to 50 patients reduced the concentration of C-reactive protein [CRP]; a marker of inflammation within 24 hours following the procedure compared to placebo. However, there was no difference in concentrations of two markers of myocardial injury creatine kinase and troponin-I or in the incidence of major adverse cardiac events one month after angioplasty between active treatment and placebo Additional trials are needed to examine whether coenzyme Q 10 therapy can improve clinical outcomes in patients undergoing coronary angioplasty. Myocardial ischemia may also lead to chest pain known as angina pectoris. People with angina pectoris often experience symptoms when the demand for oxygen exceeds the capacity of the coronary circulation to deliver it to the heart muscle, e. In most of the studies, coenzyme Q 10 supplementation improved exercise tolerance and reduced or delayed electrocardiographic changes associated with myocardial ischemia compared to placebo. However, only two of the studies found significant decreases in symptom frequency and use of nitroglycerin with coenzyme Q 10 supplementation. Presently, there is only limited evidence suggesting that coenzyme Q 10 supplementation would be a useful adjunct to conventional angina therapy. Very few high-quality trials have examined the potential therapeutic benefit of coenzyme Q 10 supplementation in the treatment of primary hypertension In contrast, a meta-analysis that used less stringent selection criteria included 17 small trials and found evidence of a blood pressure-lowering effect of coenzyme Q 10 in patients with cardiovascular disease or metabolic disorders The effect of coenzyme Q 10 on blood pressure needs to be examined in large, well-designed clinical trials. Endothelial dysfunction: Normally functioning vascular endothelium promotes blood vessel relaxation vasodilation when needed for example, during exercise and inhibits the formation of blood clots. Atherosclerosis is associated with impairment of vascular endothelial function, thereby compromising vasodilation and normal blood flow. Endothelium-dependent vasodilation is impaired in individuals with elevated serum cholesterol concentrations, as well as in patients with coronary heart disease or diabetes mellitus. Evidence from larger studies is needed to further establish the effect of coenzyme Q 10 on endothelium-dependent vasodilation. Recently published pooled analyses of these trials have given mixed results Larger studies are needed to examine the effect of coenzyme Q 10 supplementation on low-grade inflammation. Blood lipids : Elevated plasma lipoprotein a concentration is an independent risk factor for cardiovascular disease. Other effects of coenzyme Q 10 on blood lipids have not been reported 51, 53, A therapeutic approach combining coenzyme Q 10 with other antioxidants might prove to be more effective to target co-existing metabolic disorders in individuals at risk for cardiovascular disease Diabetes mellitus is a condition of increased oxidative stress and impaired energy metabolism. Plasma concentrations of reduced coenzyme Q 10 CoQ 10 H 2 have been found to be lower in diabetic patients than healthy controls after normalization to plasma cholesterol concentrations 56, Randomized controlled trials that examined the effect of coenzyme Q 10 supplementation found little evidence of benefits on glycemic control in patients with diabetes mellitus. Maternally inherited diabetes mellitus-deafness syndrome MIDD is caused by a mutation in mitochondrial DNA , which is inherited exclusively from one's mother. Of note, the pathogenesis of type 2 diabetes mellitus involves the early onset of glucose intolerance and hyperinsulinemia associated with the progressive loss of tissue responsiveness to insulin. Recent experimental studies tied insulin resistance to a decrease in coenzyme Q 10 expression and showed that supplementation with coenzyme Q 10 could restore insulin sensitivity 7. Coenzyme Q 10 supplementation might thus be a more useful tool for the primary prevention of type 2 diabetes rather than for its management. Parkinson's disease is a degenerative neurological disorder characterized by tremors, muscular rigidity, and slow movements. Mitochondrial dysfunction and oxidative damage in a part of the brain called the substantia nigra may play a role in the development of the disease Decreased ratios of reduced -to- oxidized coenzyme Q 10 have been found in platelets of individuals with Parkinson's disease 61, Two recent meta-analyses of randomized, placebo-controlled trials found no evidence that coenzyme Q 10 improved motor-related symptoms or delayed the progression of the disease when compared to placebo 68, Huntington's disease is an inherited neurodegenerative disorder characterized by selective degeneration of nerve cells known as striatal spiny neurons. Symptoms, such as movement disorders and impaired cognitive function, typically develop in the fourth decade of life and progressively deteriorate over time. Animal models indicate that impaired mitochondrial function and glutamate -mediated neurotoxicity may be involved in the pathology of Huntington's disease. Interestingly, co-administration of coenzyme Q 10 with remacemide an NMDA receptor antagonist , the antibiotic minocycline, or creatine led to greater improvements in most biochemical and behavioral parameters To date, only two clinical trials have examined whether coenzyme Q 10 might be efficacious in human patients with Huntington's disease. All dosages were generally well tolerated, with gastrointestinal symptoms being the most frequently reported adverse effect. Blood concentrations of coenzyme Q 10 at the end of the study were maximized with the daily dose of 2, mg The trial was prematurely halted because it appeared unlikely to demonstrate any health benefit in supplemented patients — about one-third of participants completed the trial at the time of study termination Although coenzyme Q 10 is generally well tolerated, there is no evidence that supplementation can improve functional and cognitive symptoms in Huntington's disease patients. Friedreich's ataxia FRDA : FRDA is an autosomal recessive neurodegenerative disease caused by mutations in the gene FXN that encodes for the mitochondrial protein , frataxin. Frataxin is needed for the making of iron -sulfur clusters ISC. ISC-containing subunits are especially important for the mitochondrial respiratory chain and for the synthesis of heme -containing proteins Frataxin deficiency is associated with imbalances in iron-sulfur containing proteins, mitochondrial respiratory chain dysfunction and lower ATP production, and accumulation of iron in the mitochondria, which increases oxidative stress and oxidative damage to macromolecules of the respiratory chain Clinically, FRDA is a progressive disease characterized by ataxia , areflexia , speech disturbance dysarthria , sensory loss, motor dysfunction, cardiomyopathy , diabetes , and scoliosis Follow-up assessments at 47 months indicated that cardiac and skeletal muscle improvements were maintained and that FRDA patients showed significant increases in fractional shortening, a measure of cardiac function. Moreover, the therapy was effective at preventing the progressive decline of neurological function Large-scale, randomized controlled trials are necessary to determine whether coenzyme Q 10 , in conjunction with vitamin E, has therapeutic benefit in FRDA. At present, about one-half of patients use coenzyme Q 10 and vitamin E supplements despite the lack of proven therapeutic benefit Spinocerebellar ataxias SCAs : SCAs are a group of rare autosomal dominant neurodegenerative diseases characterized by gait difficulty, loss of hand dexterity, dysarthria, and cognitive decline. SCA1, 2, 3, and 6 are the most common SCAs In vitro coenzyme Q 10 treatment of forearm skin fibroblasts isolated from patients with SCA2 was found to reduce oxidative stress and normalize complex I and II-III activity of the mitochondrial respiratory chain Early interest in coenzyme Q 10 as a potential therapeutic agent in cancer was stimulated by an observational study that found that individuals with lung, pancreas , and especially breast cancer were more likely to have low plasma coenzyme Q 10 concentrations than healthy controls Two randomized controlled trials have explored the effect of coenzyme Q 10 as an adjunct to conventional therapy for breast cancer. Supplementation with coenzyme Q 10 failed to improve measures of fatigue and quality of life in patients newly diagnosed with breast cancer 84 and in patients receiving chemotherapy There is little evidence that supplementation with coenzyme Q 10 improves athletic performance in healthy individuals. Most did not find significant differences between the group taking coenzyme Q 10 and the group taking placebo with respect to measures of aerobic exercise performance, such as maximal oxygen consumption VO 2 max and exercise time to exhaustion Two studies actually found significantly greater improvement in measures of anaerobic 87 and aerobic 86 exercise performance with a placebo than with supplemental coenzyme Q More recent studies have suggested that coenzyme Q 10 could help reduce both muscle damage-associated oxidative stress and low-grade inflammation induced by strenuous exercise Studies on the effect of supplementation on physical performance in women are lacking, but there is little reason to suspect a gender difference in the response to coenzyme Q 10 supplementation. Coenzyme Q 10 is synthesized in most human tissues. The biosynthesis of coenzyme Q 10 involves three major steps: 1 synthesis of the benzoquinone structure from 4-hydroxybenzoate derived from either tyrosine or phenylalanine, two amino acids; 2 synthesis of the polyisoprenoid side chain from acetyl-coenzyme A CoA via the mevalonate pathway; and 3 the joining condensation of these two structures to form coenzyme Q In the mevalonate pathway, the enzyme 3-hydroxymethylglutaryl HMG -CoA reductase, which converts HMG-CoA into mevalonate, is common to the biosynthetic pathways of both coenzyme Q 10 and cholesterol and is inhibited by statins cholesterol-lowering drugs; see Drug interactions 1. Of note, pantothenic acid formerly vitamin B 5 is the precursor of coenzyme A, and pyridoxine vitamin B 6 , in the form of pyridoxal-5'-phosphate, is required for the conversion of tyrosine to 4-hydroxyphenylpyruvic acid that constitutes the first step in the biosynthesis of the benzoquinone structure of coenzyme Q The extent to which dietary consumption contributes to tissue coenzyme Q 10 concentrations is not clear. Rich sources of dietary coenzyme Q 10 include mainly meat, poultry, and fish. Other good sources include soybean, corn, olive, and canola oils; nuts; and seeds. Fruit, vegetables, eggs, and dairy products are moderate sources of coenzyme Q 10 Some dietary sources are listed in Table 1. Coenzyme Q 10 is available without a prescription as a dietary supplement in the US. Coenzyme Q 10 is fat-soluble and is best absorbed with fat in a meal. Oral supplementation with coenzyme Q 10 is known to increase blood and lipoprotein concentrations of coenzyme Q 10 in humans 2 , 15 , Nonetheless, under certain physiological circumstances e. During pregnancy, the use of coenzyme Q 10 supplements mg twice daily from 20 weeks' gestation was found to be safe Because reliable data in lactating women are not available, supplementation should be avoided during breast-feeding Concomitant use of warfarin Coumadin and coenzyme Q 10 supplements has been reported to decrease the anticoagulant effect of warfarin in a few cases An individual on warfarin should not begin taking coenzyme Q 10 supplements without consulting the health care provider who is managing his or her anticoagulant therapy. HMG-CoA reductase is an enzyme that catalyzes a biochemical reaction that is common to both cholesterol and coenzyme Q 10 biosynthetic pathways see Biosynthesis. Statins are HMG-CoA reductase inhibitors that are widely used as cholesterol-lowering medications. Statins can thus also reduce the endogenous synthesis of coenzyme Q Therapeutic use of statins, including simvastatin Zocor , pravastatin Pravachol , lovastatin Mevacor, Altocor, Altoprev , rosuvastatin Crestor , and atorvastatin Lipitor , has been shown to decrease circulating coenzyme Q 10 concentrations However, because coenzyme Q 10 circulates with lipoproteins , plasma coenzyme Q 10 concentration is influenced by the concentration of circulating lipids , It is likely that circulating coenzyme Q 10 concentrations are decreased because statins reduce circulating lipids rather than because they inhibit coenzyme Q 10 synthesis In addition, very few studies have examined coenzyme Q 10 concentrations in tissues other than blood such that the extent to which statin therapy affects coenzyme Q 10 concentrations in the body's tissues is unknown , , Finally, there is currently little evidence to suggest that secondary coenzyme Q 10 deficiency is responsible for statin-associated muscle symptoms in treated patients. In addition, supplementation with coenzyme Q 10 failed to relieve myalgia in statin-treated patients see Disease Treatment , Originally written in by: Jane Higdon, Ph. Linus Pauling Institute Oregon State University. Updated in February by: Victoria J. Drake, Ph. Updated in March by: Victoria J. Updated in April by: Barbara Delage, Ph. Reviewed in May by: Roland Stocker, Ph. Centre for Vascular Research School of Medical Sciences Pathology and Bosch Institute Sydney Medical School The University of Sydney Sydney, New South Wales, Australia. Acosta MJ, Vazquez Fonseca L, Desbats MA, et al. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta. Crane FL. Biochemical functions of coenzyme Q J Am Coll Nutr. Nohl H, Gille L. The role of coenzyme Q in lysosomes. In: Kagan VEQ, P. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton: CRC Press; Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Thomas SR, Stocker R. Mechanisms of antioxidant action of ubiquinol for low-density lipoprotein. In: Kagan VE, Quinn PJ, eds. Fazakerley DJ, Chaudhuri R, Yang P, et al. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Kagan VE, Fabisak JP, Tyurina YY. Independent and concerted antioxidant functions of coenzyme Q. Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. Hargreaves IP. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol. Fragaki K, Chaussenot A, Benoist JF, et al. Coenzyme Q10 defects may be associated with a deficiency of Qindependent mitochondrial respiratory chain complexes. Biol Res. Kalén A, Appelkvist EL, Dallner G. Milne Search. Electronic Resources. College Archives and Special Collections. Teacher Education Resource Center TERC. Advanced Search. Peer Reviewed. Open Access. Download PDF. Coenzyme Q biosynthesis and its role in the respiratory chain structure Check for available services. View Issue Contents. Send to. Export RIS. How to get it. Please sign in to check if there are any request options. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Alcázar-Fabra, María. Navas, Plácido. Brea-Calvo, Gloria. Is Part Of. Biochimica et biophysica acta, , Vol. basic medicine. toolbar search search input Search input auto suggest. Evidence in favor of the RCM derived from 3 major kinds of observations:. View large Download slide. Acín-Peréz R, Bayona-Bafaluy MP, Fernández-Silva P, Moreno-Loshuertos R, Pérez-Martos A, et al: Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell Acín-Peréz R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA: Respiratory active mitochondrial supercomplexes. Acín-Peréz R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G: Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab a. Acín-Peréz R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G: Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med b. Acín-Peréz R, Gatti DL, Bai Y, Manfredi G: Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Acín-Peréz R, Russwurm M, Gunnewig K, Gertz M, Zoidl G, et al: A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J Biol Chem b. Althoff T, Mills DJ, Popot JL, Kühlbrandt W: Arrangement of electron transport chain components in bovine mitochondrial supercomplex I 1 III 2 IV 1. EMBO J Anilkumar N, Sirker A, Shah AM: Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, et al: NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G: Mitochondrial complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta Battino M, Ferri E, Gorini A, Villa RF, Rodriguez Huertas JF, et al: Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem Beg S, Javed S, Kohli K: Bioavailability enhancement of coenzyme Q an extensive review of patents. Recent Pat Drug Deliv Formul Bellomo F, Piccoli C, Cocco T, Scacco S, Papa F, et al: Regulation by the cAMP cascade of oxygen free radical balance in mammalian cells. Antioxid Redox Signal Benard G, Faustin B, Galinier A, Rocher C, Bellance N, et al: Functional dynamic compartmentalization of respiratory chain intermediate substrates: implications for the control of energy production and mitochondrial diseases. Int J Biochem Cell Biol Bergamini C, Moruzzi N, Sblendido A, Lenaz G, Fato R: A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS ONE 7:e Bianchi C, Fato R, Genova ML, Parenti Castelli G, Lenaz G: Structural and functional organization of Complex I in the mitochondrial respiratory chain. Biofactors Bianchi C, Genova ML, Parenti Castelli G, Lenaz G: The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem Boumans H, Grivell LA, Berden JA: The respiratory chain in yeast behaves as a single functional unit. Böttinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, et al: Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J Mol Biol Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, Rehling P: Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth syndrome. Mol Biol Cell Bruno C, Santorelli FM, Assereto S, Tonoli E, Tessa A, et al: Progressive exercise intolerance associated with a new muscle-restricted nonsense mutation GX in the mitochondrial cytochrome b gene. Muscle Nerve Calvaruso MA, Willems P, van den Brand M, Valsecchi F, Kruse S, et al: Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet Castellani M, Covian R, Kleinschroth T, Anderka O, Ludwig B, et al: Direct demonstration of half-of-the-sites reactivity in the dimeric cytochrome bc1 complex: enzyme with one inactive monomer is fully active but unable to activate the second ubiquinol oxidation site in response to ligand binding at the ubiquinone reduction site. Chen K, Keaney JF: Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr Atheroscler Rep Chen S, He Q, Greenberg ML: Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, et al: Identification of a protein mediating respiratory supercomplex stability. Claypool SM, Koehler CM: The complexity of cardiolipin in health and disease. Trends Biochem Sci J Cell Biol Cui H, Kong Y, Zhang H: Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct Dalmonte ME, Forte E, Genova ML, Giuffrè A, Sarti P, Lenaz G: Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. D'Aurelio M, Gajewski CD, Lenaz G, Manfredi G: Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Deng N, Zhang J, Zong C, Wang Y, Lu H, et al: Phosphoproteome analysis reveals regulatory sites in major pathways of cardiac mitochondria. Mol Cell Proteomics M de Rasmo D, Panelli D, Sardanelli AM, Papa S: cAMP-dependent protein kinase regulates the mitochondrial import of the nuclear encoded NDUFS4 subunit of complex I. Cell Signal Diaz F, Thomas CK, Garcia S, Hernandez D, Moraes CT: Mice lacking COX10 in skeletal muscle recapitulate the phenotype of progressive mitochondrial myopathies associated with cytochrome c oxidase deficiency. Mol Cell Biol Diaz F, Garcia S, Padgett KR, Moraes CT: A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum Mol Genet a. Diaz F, Enriquez JA, Moraes CT: Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol Cell Biol b. Dieteren CE, Willems PH, Vogel RO, Swarts HG, Fransen J, et al: Subunits of mitochondrial complex I exist as part of matrix- and membrane-associated subcomplexes in living cells. Di Giovanni S, Mirabella M, Spinazzola A, Crociani P, Silvestri G, et al: Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology Dudkina NV, Eubel H, Keegstra W, Boekema EJ, Braun HP: Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc Natl Acad Sci USA Dudkina NV, Kudryashev M, Stahlberg H, Boekema EJ: Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti Castelli G, Lenaz G: Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett Estornell E, Tormo JR, Barber T: A deficiency in respiratory complex I in heart mitochondria from vitamin A-deficient rats is counteracted by an increase in coenzyme Q. Biochem Biophys Res Commun Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA: Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol Fry M, Green DE: Energized cation transport by complex III ubiquinone-cytochrome C reductase. Fry M, Green DE: Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. Genova ML, Lenaz G: New developments on the functions of coenzyme Q in mitochondria. Genova ML, Baracca A, Biondi A, Casalena G, Faccioli M: Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Gong J, Hoyos B, Acín-Peréz R, Vinogradov V, Shabrova E, et al: Two protein kinase C isoforms, δ and ε, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex. FASEB J Gómez LA, Hagen TM: Age-related decline in mitochondrial bioenergetics: does supercomplex destabilization determine lower oxidative capacity and higher superoxide production? Semin Cell Dev Biol Gómez LA, Monette JS, Chavez JD, Maier CS, Hagen TM: Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys Green DE, Tzagoloff A: The mitochondrial electron transfer chain. Grimsrud PA, Carson JJ, Hebert AS, Hubler SL, Niemi NM, et al: A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Grivennikova VG, Roth R, Zakharova NV, Hägerhäll C, Vinogradov AD: The mitochondrial and prokaryotic proton-translocating NADH:ubiquinone oxidoreductases: similarities and dissimilarities of the quinone-junction sites. Hackenbrock CR, Chazotte B, Gupte SS: The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, et al: Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol Hatefi Y, Haavick AG, Fowler LR, Griffiths DE: Studies on the electron transfer system. Reconstitution of the electron transfer system. J Biol Chem a. Hatefi Y, Haavick AG, Griffiths DE: Studies on the electron transfer system. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. Hayashi H, Nakagami H, Takeichi M, Shimamura M, Koibuchi N, et al: HIG1, a novel regulator of mitochondrial γ-secretase, maintains normal mitochondrial function. Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, et al: Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics Heron C, Ragan CI, Trumpower BL: The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem J Hébert Chatelain E: Src kinases are important regulators of mitochondrial functions. Hildebrandt TM: Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, et al: Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res Iuso A, Scacco S, Piccoli C, Bellomo F, Petruzzella V, et al: Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. Jørgensen BM, Rasmussen HN, Rasmussen UF: Ubiquinone reduction pattern in pigeon heart mitochondria. Identification of three distinct ubiquinone pools. Kaambre T, Chekulayev V, Shevchuk I, Karu-Varikmaa M, Timohhina N, et al: Metabolic control analysis of cellular respiration in situ in intraoperational samples of human breast cancer. Kang SY, Gutowsky HS, Hsung JC, Jacobs R, King TE, et al: Nuclear magnetic resonance investigation of the cytochrome oxidase-phospholipid interaction: a new model for boundary lipid. Biochemistry Khalifat N, Puff N, Bonneau S, Fournier JB, Angelova MI: Membrane deformation under local pH gradient: mimicking mitochondrial cristae dynamics. Biophys J Khalifat N, Fournier JB, Angelova MI, Puff N: Lipid packing variations induced by pH in cardiolipin-containing bilayers: the driving force for the cristae-like shape instability. Kholodenko BN, Westerhoff HV: Metabolic channelling and control of the flux. Kröger A, Klingenberg M: The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem Kulawiak B, Höpker J, Gebert M, Guiard B, Wiedemann N, et al: The mitochondrial protein import machinery has multiple connections to the respiratory chain. Lamantea E, Carrara F, Mariotti C, Morandi L, Tiranti V, et al: A novel nonsense mutation QX in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscul Disord Lange C, Nett JH, Trumpower BL, Hunte C: Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. Lapuente-Brun E, Moreno-Loshuertos R, Acín-Peréz R, Latorre-Pellicer A, Colás C, et al: Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science Lass A: Electron transport-linked ubiquinone-dependent recycling of α-tocopherol inhibits autooxidation of mitochondrial membranes. Lass A, Sohal RS: Comparisons of coenzyme Q bound to mitochondrial membrane proteins among different mammalian species. Free Radic Biol Med Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, et al: cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. Lenaz G: A critical appraisal of the mitochondrial coenzyme Q pool. Lenaz G, Genova ML: Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol CC Lenaz G, Genova ML: Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Lenaz G, Genova ML: Supramolecular organisation of the mitochondrial respiratory chain: a new challenge for the mechanism and control of oxidative phosphorylation. Adv Exp Med Biol Lenaz G, Fato R, Di Bernardo S, Jarreta D, Costa A, et al: Localization and mobility of coenzyme Q in lipid bilayers and membranes. Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, et al: Mitochondrial respiratory chain supercomplex I-III in physiology and pathology. MacLennan DH, Lenaz G, Szarkowska L: Studies on the mechanism of oxidative phosphorylation. Effect of cytochrome c on energy-linked processes. Protein J Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML: Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Matthews RT, Yang L, Browne S, Baik M, Beal MF: Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. McKenzie M, Lazarou M, Thorburn DR, Ryan MT: Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. Molyneux SL, Florkowski CM, Richards AM, Lever M, Young JM, et al: Coenzyme Q10; an adjunctive therapy for congestive heart failure? N Z Med J Moreno Lastres D, Fontanesi F, García-Consuegra I, Martín MA, Arenas J, et al: Mitochondrial complex I plays an essential role in human respirasome assembly. Mracek T, Holzerová E, Drahota Z, Kovářová N, Vrbacky M, et al: ROS generation and multiple forms of mammalian mitochondrial glycerolphosphate dehydrogenase Biochim Biophys Acta Murphy MP: Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Muster B, Kohl W, Wittig I, Strecker V, Joos F, et al: Respiratory chain complexes in dynamic mitochondria display a patchy distribution in life cells. PLoS ONE 5:e Neuwald AF: Barth syndrome may be due to an acyltransferase deficiency. Curr Biol 7:R Osman C, Voelker DR, Langer T: Making heads or tails of phospholipids in mitochondria. Ovádi J: Physiological significance of metabolic channelling. J Theor Biol Panov A, Dikalov S, Shalbuyeva N, Hemendinger R, Greenamyre JT, et al: Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Papa S, de Rasmo D, Scacco S, Signorile A, Technikova-Dobrova Z, et al: Mammalian complex I: a regulable and vulnerable pacemaker in mitochondrial respiratory function. Paradies G, Petrosillo G, Pistolese M, Ruggiero FM: The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. Paradies G, Petrosillo G, Pistolese M, Ruggiero FM: Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene Paradies G, Petrosillo G, Paradies V, Ruggiero FM: Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Perales-Clemente E, Bayona-Bafaluy MP, Pérez-Martos A, Barrientos A, Fernández-Silva P, et al: Restoration of electron transport without proton pumping in mammalian mitochondria. Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G: Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. Pfeiffer K: Cardiolipin stabilizes respiratory chain supercomplexes. Piccoli C, Scrima R, Boffoli D, Capitanio N: Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Quarato G, Piccoli C, Scrima R, Capitanio N: Variation of flux control coefficient of cytochrome c oxidase and of the other respiratory chain complexes at different values of protonmotive force occurs by a threshold mechanism. Quinzii CM, Hirano M: Coenzyme Q and mitochondrial disease. Dev Disabil Res Revs Radermacher M, Ruiz T, Clason T, Benjamin S, Brandt U, et al: The three-dimensional structure of complex I from Yarrowia lipolytica : a highly dynamic enzyme. J Struct Biol Ragan CI, Heron C: The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Evidence for stoicheiometric association. Raha S, Myint AT, Johnstone L, Robinson BH: Control of oxygen free radical formation from mitochondrial complex I: roles for protein kinase A and pyruvate dehydrogenase kinase. Ramirez-Aguilar SJ, Keuthe M, Rocha M, Fedyaev VV, Kramp K, et al: The composition of plant mitochondrial supercomplexes changes with oxygen availability. Rauchová H, Fato R, Drahota Z, Lenaz G: Steady-state kinetics of reduction of coenzyme Q analogs by glycerolphosphate dehydrogenase in brown adipose tissue mitochondria. Rodrigues JV, Gomes CM: Mechanism of superoxide and hydrogen peroxide generation by human electron-transfer flavoprotein and pathological variants. Rosca M, Hoppel C: New aspects of impaired mitochondrial function in heart failure. Rosca M, Vazquez E, Kerner J, Parland W, Chandler M, et al: Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res Rosca M, Minkler P, Hoppel CL: Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Rosenfeldt F, Marasco S, Lyon W, Wowk M, Sheeran F, et al: Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J Thorac Cardiovasc Surg Rötig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, et al: Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet Schäfer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, et al: Architecture of active mammalian respiratory chain supercomplexes. Schäfer E, Dencher NA, Vonck J, Parcej DN: Three-dimensional structure of the respiratory chain supercomplex I1III2IV1 from bovine heart mitochondria. Schägger H, Pfeiffer K: Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. Schägger H, Pfeiffer K: The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. Schneider H, Lemasters JJ, Hackenbrock CR: Lateral diffusion of ubiquinone during electron transfer in phospholipid- and ubiquinone-enriched mitochondrial membranes. Sedlák E, Robinson NC: Phospholipase A 2 digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Seelert H, Dani DN, Dante S, Hauß T, Krause F, et al: From protons to OXPHOS supercomplexes and Alzheimer's disease: structure-dynamics-function relationships of energy-transducing membranes. Skulachev VP: Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys Sousa PM, Silva ST, Hood BL, Charro N, Carita JN: Supramolecular organizations in the aerobic respiratory chain of Escherichia coli. Biochimie Stoner CD: Steady-state kinetics of the overall oxidative phosphorylation reaction in heart mitochondria. Determination of the coupling relationships between the respiratory reactions and miscellaneous observations concerning rate-limiting steps. Strogolova V, Furness A, Robb-McGrath M, Garlich J, Stuart RA: Rcf1 and Rcf2, members of the hypoxia induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Suthammarak W, Yang Y-Y, Morgan PG, Sedensky MM: Complex I function is defective in complex IV-deficient Caenorhabditis elegans. Suthammarak W, Morgan PG, Sedensky MM: Mutations in mitochondrial complex III uniquely affect complex I in Caenorhabditis elegans. Trouillard M, Meunier B, Rappaport F: Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Tzagoloff A, Barrientos A, Neupert W, Herrmann JM: Atp10p assists assembly of Atp6p into the F0 unit of the yeast mitochondrial ATPase. van den Brink-van der Laan E, Killian JA, de Kruijff B: Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, et al: Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS ONE 3:e Vukotic M, Oeljeklaus S, Wiese S, Vögtle FN, Meisinger C, et al: Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Wang Y, Mohsen A-W, Mihalik SJ, Goetzman ES, Vockley J: Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. Wenz T, Hielscher R, Hellwig P, Schägger H, Richers S, et al: Role of phospholipids in respiratory cytochrome bc 1 complex catalysis and supercomplex formation. Wittig I, Schägger H: Features and applications of blue-native and clear-native electrophoresis. Proteomics |
| Coenzyme Q10 | Coenzyme Q10 CoQ10 is used to treat various health conditions, including migraines, infertility and the effects of aging. This article reviews the…. Learn more about how taking a supplement can affect statin side effects and your overall heart health. Life can take a toll on your energy levels. Fortunately, these 11 vitamins and supplements can boost your energy levels when you need it most. If your period is so heavy that you quickly soak through pads or tampons, there are things you can do to find relief. Find out what home remedies and…. While they're not typically able to prescribe, nutritionists can still benefits your overall health. Let's look at benefits, limitations, and more. A new study found that healthy lifestyle choices — including being physically active, eating well, avoiding smoking and limiting alcohol consumption —…. Carb counting is complicated. Take the quiz and test your knowledge! Together with her husband, Kansas City Chiefs MVP quarterback Patrick Mahomes, Brittany Mohomes shares how she parents two children with severe food…. While there are many FDA-approved emulsifiers, European associations have marked them as being of possible concern. Let's look deeper:. Researchers have found that a daily multivitamin supplement was linked with slowed cognitive aging and improved memory. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based 9 Benefits of Coenzyme Q10 CoQ Medically reviewed by Philip Ngo, PharmD — By Arlene Semeco, MS, RD and Rachael Ajmera, MS, RD — Updated on December 6, What is CoQ10? It may help treat heart failure. It could help with fertility. It might help support healthy skin aging. It could reduce headaches. It could help with exercise performance. It may help with diabetes. It might play a role in cancer prevention. It may be good for the brain. It could protect the lungs. Food sources of CoQ Frequently asked questions. The bottom line. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Dec 6, Written By Arlene Semeco, Rachael Ajmera, MS, RD. Jul 7, Written By Arlene Semeco, Rachael Ajmera, MS, RD. Share this article. Read this next. CoQ10 Dosage: How Much Should You Take per Day? By Jillian Kubala, MS, RD. CoQ10 and Statins: What You Need to Know Medically reviewed by Darren Hein, PharmD. By Gavin Van De Walle, MS, RD. How to Stop Heavy Periods: 22 Options for Treatment. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Archiv f�r Klinische und Experimentelle Dermatologie By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature letters article. Access through your institution. Buy or subscribe. Change institution. Learn more. References Crane, F. Article CAS PubMed Google Scholar Hemming, F. CAS Google Scholar Hatefi, Y. Article CAS PubMed Google Scholar Redfearn, E. Article CAS PubMed PubMed Central Google Scholar Chance, B. Article CAS PubMed PubMed Central Google Scholar Lester, R. Google Scholar Green, D. Google Scholar King, T. CAS PubMed Google Scholar Tsou, C. Article CAS PubMed PubMed Central Google Scholar Pumphrey, A. Article CAS PubMed PubMed Central Google Scholar Folch, J. CAS PubMed Google Scholar Goodwin, T. CAS Google Scholar Goldberger, R. Google Scholar Download references. Author information Authors and Affiliations Department of Biochemistry, University of Leicester, ERIC R. BURGOS Authors ERIC R. REDFEARN View author publications. View author publications. Rights and permissions Reprints and permissions. On the other hand, the finding that Complex I is almost totally associated in a supercomplex with Complex III seems to exclude a role for the CoQ pool in physiological electron transfer between these 2 complexes. Surprisingly, strong evidence exists that NADH-cytochrome c reductase activity follows saturation kinetics with respect to CoQ. The relation between electron transfer rate and CoQ concentration was seen for NADH and succinate oxidation in reconstituted systems [Estornell et al. The K m for CoQ 10 of NADH-cytochrome c reductase was found to be much higher than that of succinate-cytochrome c reductase. A direct study on a reconstituted mitochondrial fraction containing Complexes I and III showed that the experimental rate of NADH-cytochrome c reductase was hyperbolically related to the content of CoQ 10 , with an apparent K m in the same range as in mitochondria [Lenaz et al. Analysis of the literature shows that the physiological CoQ content of several types of mitochondria is in the range of the K m for NADH oxidation and, therefore, not saturating for this activity [Battino et al. However, this does not exclude that free CoQ in the pool is also necessary for proper channeling between the complexes. In fact, the bound inter-complex quinone that allows electron flow directly from Complex I to Complex III must be in a dissociation equilibrium with the CoQ pool, so that its amount, at steady state, would be dictated by the size of the pool: this equilibrium explains the saturation kinetics for total ubiquinone exhibited by the integrated activity of Complex I and Complex III and the decrease of respiratory activities in mitochondria fused with phospholipids with subsequent dilution of the CoQ pool. To be in agreement with the experimental observation obtained by metabolic flux analysis, this proposition requires that the dissociation rate constants k off of bound CoQ be considerably slower than the rates of inter-complex electron transfer via the same bound quinone molecules [Lenaz and Genova, ; Genova and Lenaz, ]. The high apparent K m for CoQ 10 in NADH oxidation is in line with this postulation. The observation by Schneider et al. Earlier studies by Heron et al. It is likely that the function of the large amount of ubiquinone in the natural membrane may be, therefore, to maintain the ubiquinone content in the supercomplex unit when it is formed. Coenzyme Q10 supplementation is able to restore mitochondrial respiration in primary CoQ 10 deficiencies and is beneficial in several diseases in which a secondary CoQ deficiency is usually postulated; in this latter case, a bioenergetic deficit is not always apparent, and the health improvements are ascribed also to other properties of the quinone, such as its antioxidant activity. Is the existence and role of supercomplexes compatible with the interpretation of the beneficial effects of exogenous CoQ 10 administration on bioenergetic grounds? There is evidence, mainly indirect, that exogenous orally administered CoQ 10 may be incorporated into mitochondria, at least in conditions of partial CoQ tissue deficiency, where it may enhance electron transfer and ATP synthesis with improvement of the disease both in human and animal studies, e. in genetic CoQ 10 deficiency [Rötig et al. The major problem of CoQ 10 administration is its low bioavailability due to its extreme hydrophobicity [Beg et al. A water-soluble formulation Qter has recently been shown to be easily incorporated into cultured cells and their mitochondria, enhancing respiration and antioxidant properties [Bergamini et al. The same formulation was found to improve grip strength and to inhibit apoptosis in aged rats [Xu et al. The existence of I-III supercomplexes where only inter-complex bound CoQ is active by channeling electrons from Complex I to Complex III is apparently incompatible with a dose-dependent effect of added CoQ 10 ; however, the notion that inter-complex bound CoQ is in a chemical equilibrium with CoQ in the pool is sufficient to explain the improved cell bioenergetics upon addition of exogenous CoQ. It is expected that even a slight decrease of CoQ content in the membrane is sufficient to dissociate part of the quinone from the supercomplex thus decreasing the rate of electron channeling. Kinetic studies in CoQ-depleted mitochondria [Estornell et al. This means that CoQ is physiologically not saturating and that increasing CoQ concentration in the membrane is bound to enhance respiratory activity: this is indeed what was found in the study with the water-soluble formulation quoted above [Bergamini et al. Addition of Qter to cultured cells increased CoQ concentration in their mitochondria and significantly enhanced respiratory rates. The fact of CoQ 10 not being saturating in NADH oxidation should also drive a further consequence of improving respiration even when the defect is due to reasons different from CoQ deficiency, e. a defect of Complex I. This is theoretically shown in figure 3. A direct study by Estornell et al. This is the most likely explanation why CoQ was found to ameliorate several disease states even if a CoQ deficiency was not demonstrated. Coenzyme Q supplementation restores NADH oxidation impaired by causes different from CoQ deficiency. The K m of NADH oxidation for CoQ10 was found to be in the range m M in the lipid phase [Estornell et al. Being in the first-order arm of the curve, an increase in CoQ concentration is bound to enhance NADH oxidation both in normal mitochondria red and in mitochondria with decreased respiration blue. What are the mechanisms of the beneficial effects of administered CoQ? The bioenergetic improvement due to enhanced electron transfer is certainly the major mechanism in primary CoQ deficiencies; however, it may be considered in all cases where a secondary deficiency takes place, as in heart failure and aging. An antioxidant effect may be at the basis of amelioration of symptoms in several pathological states. However, the direct antiapoptotic action exerted by its effect on the permeability transition pore should be considered in the case of degenerative diseases. Work from the laboratory of G. was supported by grants from MUR, Rome Italy. Research of J. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Molecular Syndromology. Advanced Search. Skip Nav Destination Close navigation menu Article navigation. Volume 5, Issue Molecular Structure of Supercomplexes. Stability of Supercomplexes: Role of Phospholipids. Coexistence of Fluid Structure and Supercomplexes: The Plasticity Model. The Coenzyme Q Pool. Evidence for Separate Pools of CoQ. Biogenesis and Assembly of Supercomplexes. Function of the Supercomplexes. CoQ Saturation Kinetics: Is There a Contradiction? What We Expect from CoQ Deficiency and Supplementation. Article Navigation. Review Articles June 13 Coenzyme Q and the Respiratory Chain: Coenzyme Q Pool and Mitochondrial Supercomplexes Subject Area: Endocrinology , Further Areas , Genetics , Women's and Children's Health. José Antonio Enriquez ; José Antonio Enriquez. a Centro Nacional de Investigaciones Cardiovasculares Carlos III, Madrid, Spain;. jaenriquez cnic. This Site. Google Scholar. Giorgio Lenaz Giorgio Lenaz. b Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Bologna, Italy. Mol Syndromol 5 : — Article history Published Online:. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Evidence in favor of the RCM derived from 3 major kinds of observations:. View large Download slide. Acín-Peréz R, Bayona-Bafaluy MP, Fernández-Silva P, Moreno-Loshuertos R, Pérez-Martos A, et al: Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell Acín-Peréz R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA: Respiratory active mitochondrial supercomplexes. Acín-Peréz R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G: Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab a. Acín-Peréz R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G: Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med b. Acín-Peréz R, Gatti DL, Bai Y, Manfredi G: Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Acín-Peréz R, Russwurm M, Gunnewig K, Gertz M, Zoidl G, et al: A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J Biol Chem b. Althoff T, Mills DJ, Popot JL, Kühlbrandt W: Arrangement of electron transport chain components in bovine mitochondrial supercomplex I 1 III 2 IV 1. EMBO J Anilkumar N, Sirker A, Shah AM: Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, et al: NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G: Mitochondrial complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta Battino M, Ferri E, Gorini A, Villa RF, Rodriguez Huertas JF, et al: Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem Beg S, Javed S, Kohli K: Bioavailability enhancement of coenzyme Q an extensive review of patents. Recent Pat Drug Deliv Formul Bellomo F, Piccoli C, Cocco T, Scacco S, Papa F, et al: Regulation by the cAMP cascade of oxygen free radical balance in mammalian cells. Antioxid Redox Signal Benard G, Faustin B, Galinier A, Rocher C, Bellance N, et al: Functional dynamic compartmentalization of respiratory chain intermediate substrates: implications for the control of energy production and mitochondrial diseases. Int J Biochem Cell Biol Bergamini C, Moruzzi N, Sblendido A, Lenaz G, Fato R: A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS ONE 7:e Bianchi C, Fato R, Genova ML, Parenti Castelli G, Lenaz G: Structural and functional organization of Complex I in the mitochondrial respiratory chain. Biofactors Bianchi C, Genova ML, Parenti Castelli G, Lenaz G: The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem Boumans H, Grivell LA, Berden JA: The respiratory chain in yeast behaves as a single functional unit. Böttinger L, Horvath SE, Kleinschroth T, Hunte C, Daum G, et al: Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J Mol Biol Brandner K, Mick DU, Frazier AE, Taylor RD, Meisinger C, Rehling P: Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth syndrome. Mol Biol Cell Bruno C, Santorelli FM, Assereto S, Tonoli E, Tessa A, et al: Progressive exercise intolerance associated with a new muscle-restricted nonsense mutation GX in the mitochondrial cytochrome b gene. Muscle Nerve Calvaruso MA, Willems P, van den Brand M, Valsecchi F, Kruse S, et al: Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet Castellani M, Covian R, Kleinschroth T, Anderka O, Ludwig B, et al: Direct demonstration of half-of-the-sites reactivity in the dimeric cytochrome bc1 complex: enzyme with one inactive monomer is fully active but unable to activate the second ubiquinol oxidation site in response to ligand binding at the ubiquinone reduction site. Chen K, Keaney JF: Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr Atheroscler Rep Chen S, He Q, Greenberg ML: Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, et al: Identification of a protein mediating respiratory supercomplex stability. Claypool SM, Koehler CM: The complexity of cardiolipin in health and disease. Trends Biochem Sci J Cell Biol Cui H, Kong Y, Zhang H: Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct Dalmonte ME, Forte E, Genova ML, Giuffrè A, Sarti P, Lenaz G: Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. D'Aurelio M, Gajewski CD, Lenaz G, Manfredi G: Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Deng N, Zhang J, Zong C, Wang Y, Lu H, et al: Phosphoproteome analysis reveals regulatory sites in major pathways of cardiac mitochondria. Mol Cell Proteomics M de Rasmo D, Panelli D, Sardanelli AM, Papa S: cAMP-dependent protein kinase regulates the mitochondrial import of the nuclear encoded NDUFS4 subunit of complex I. Cell Signal Diaz F, Thomas CK, Garcia S, Hernandez D, Moraes CT: Mice lacking COX10 in skeletal muscle recapitulate the phenotype of progressive mitochondrial myopathies associated with cytochrome c oxidase deficiency. Mol Cell Biol Diaz F, Garcia S, Padgett KR, Moraes CT: A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum Mol Genet a. Diaz F, Enriquez JA, Moraes CT: Cells lacking Rieske iron-sulfur protein have a reactive oxygen species-associated decrease in respiratory complexes I and IV. Mol Cell Biol b. Dieteren CE, Willems PH, Vogel RO, Swarts HG, Fransen J, et al: Subunits of mitochondrial complex I exist as part of matrix- and membrane-associated subcomplexes in living cells. Di Giovanni S, Mirabella M, Spinazzola A, Crociani P, Silvestri G, et al: Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology Dudkina NV, Eubel H, Keegstra W, Boekema EJ, Braun HP: Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc Natl Acad Sci USA Dudkina NV, Kudryashev M, Stahlberg H, Boekema EJ: Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti Castelli G, Lenaz G: Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett Estornell E, Tormo JR, Barber T: A deficiency in respiratory complex I in heart mitochondria from vitamin A-deficient rats is counteracted by an increase in coenzyme Q. Biochem Biophys Res Commun Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA: Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol Fry M, Green DE: Energized cation transport by complex III ubiquinone-cytochrome C reductase. Fry M, Green DE: Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. Genova ML, Lenaz G: New developments on the functions of coenzyme Q in mitochondria. Genova ML, Baracca A, Biondi A, Casalena G, Faccioli M: Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Gong J, Hoyos B, Acín-Peréz R, Vinogradov V, Shabrova E, et al: Two protein kinase C isoforms, δ and ε, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex. FASEB J Gómez LA, Hagen TM: Age-related decline in mitochondrial bioenergetics: does supercomplex destabilization determine lower oxidative capacity and higher superoxide production? Semin Cell Dev Biol Gómez LA, Monette JS, Chavez JD, Maier CS, Hagen TM: Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys Green DE, Tzagoloff A: The mitochondrial electron transfer chain. Grimsrud PA, Carson JJ, Hebert AS, Hubler SL, Niemi NM, et al: A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Grivennikova VG, Roth R, Zakharova NV, Hägerhäll C, Vinogradov AD: The mitochondrial and prokaryotic proton-translocating NADH:ubiquinone oxidoreductases: similarities and dissimilarities of the quinone-junction sites. Hackenbrock CR, Chazotte B, Gupte SS: The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, et al: Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol Hatefi Y, Haavick AG, Fowler LR, Griffiths DE: Studies on the electron transfer system. Reconstitution of the electron transfer system. J Biol Chem a. Hatefi Y, Haavick AG, Griffiths DE: Studies on the electron transfer system. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. Hayashi H, Nakagami H, Takeichi M, Shimamura M, Koibuchi N, et al: HIG1, a novel regulator of mitochondrial γ-secretase, maintains normal mitochondrial function. Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, et al: Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics Heron C, Ragan CI, Trumpower BL: The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem J Hébert Chatelain E: Src kinases are important regulators of mitochondrial functions. Hildebrandt TM: Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, et al: Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res Iuso A, Scacco S, Piccoli C, Bellomo F, Petruzzella V, et al: Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. Jørgensen BM, Rasmussen HN, Rasmussen UF: Ubiquinone reduction pattern in pigeon heart mitochondria. Identification of three distinct ubiquinone pools. Kaambre T, Chekulayev V, Shevchuk I, Karu-Varikmaa M, Timohhina N, et al: Metabolic control analysis of cellular respiration in situ in intraoperational samples of human breast cancer. Kang SY, Gutowsky HS, Hsung JC, Jacobs R, King TE, et al: Nuclear magnetic resonance investigation of the cytochrome oxidase-phospholipid interaction: a new model for boundary lipid. Biochemistry Khalifat N, Puff N, Bonneau S, Fournier JB, Angelova MI: Membrane deformation under local pH gradient: mimicking mitochondrial cristae dynamics. Biophys J Khalifat N, Fournier JB, Angelova MI, Puff N: Lipid packing variations induced by pH in cardiolipin-containing bilayers: the driving force for the cristae-like shape instability. Kholodenko BN, Westerhoff HV: Metabolic channelling and control of the flux. Kröger A, Klingenberg M: The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem Kulawiak B, Höpker J, Gebert M, Guiard B, Wiedemann N, et al: The mitochondrial protein import machinery has multiple connections to the respiratory chain. Lamantea E, Carrara F, Mariotti C, Morandi L, Tiranti V, et al: A novel nonsense mutation QX in the mitochondrial cytochrome b gene associated with a combined deficiency of complexes I and III. Neuromuscul Disord Lange C, Nett JH, Trumpower BL, Hunte C: Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. Lapuente-Brun E, Moreno-Loshuertos R, Acín-Peréz R, Latorre-Pellicer A, Colás C, et al: Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science Lass A: Electron transport-linked ubiquinone-dependent recycling of α-tocopherol inhibits autooxidation of mitochondrial membranes. Lass A, Sohal RS: Comparisons of coenzyme Q bound to mitochondrial membrane proteins among different mammalian species. Free Radic Biol Med Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, et al: cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. Lenaz G: A critical appraisal of the mitochondrial coenzyme Q pool. Lenaz G, Genova ML: Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol CC Lenaz G, Genova ML: Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Lenaz G, Genova ML: Supramolecular organisation of the mitochondrial respiratory chain: a new challenge for the mechanism and control of oxidative phosphorylation. Adv Exp Med Biol Lenaz G, Fato R, Di Bernardo S, Jarreta D, Costa A, et al: Localization and mobility of coenzyme Q in lipid bilayers and membranes. Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, et al: Mitochondrial respiratory chain supercomplex I-III in physiology and pathology. MacLennan DH, Lenaz G, Szarkowska L: Studies on the mechanism of oxidative phosphorylation. Effect of cytochrome c on energy-linked processes. Protein J |
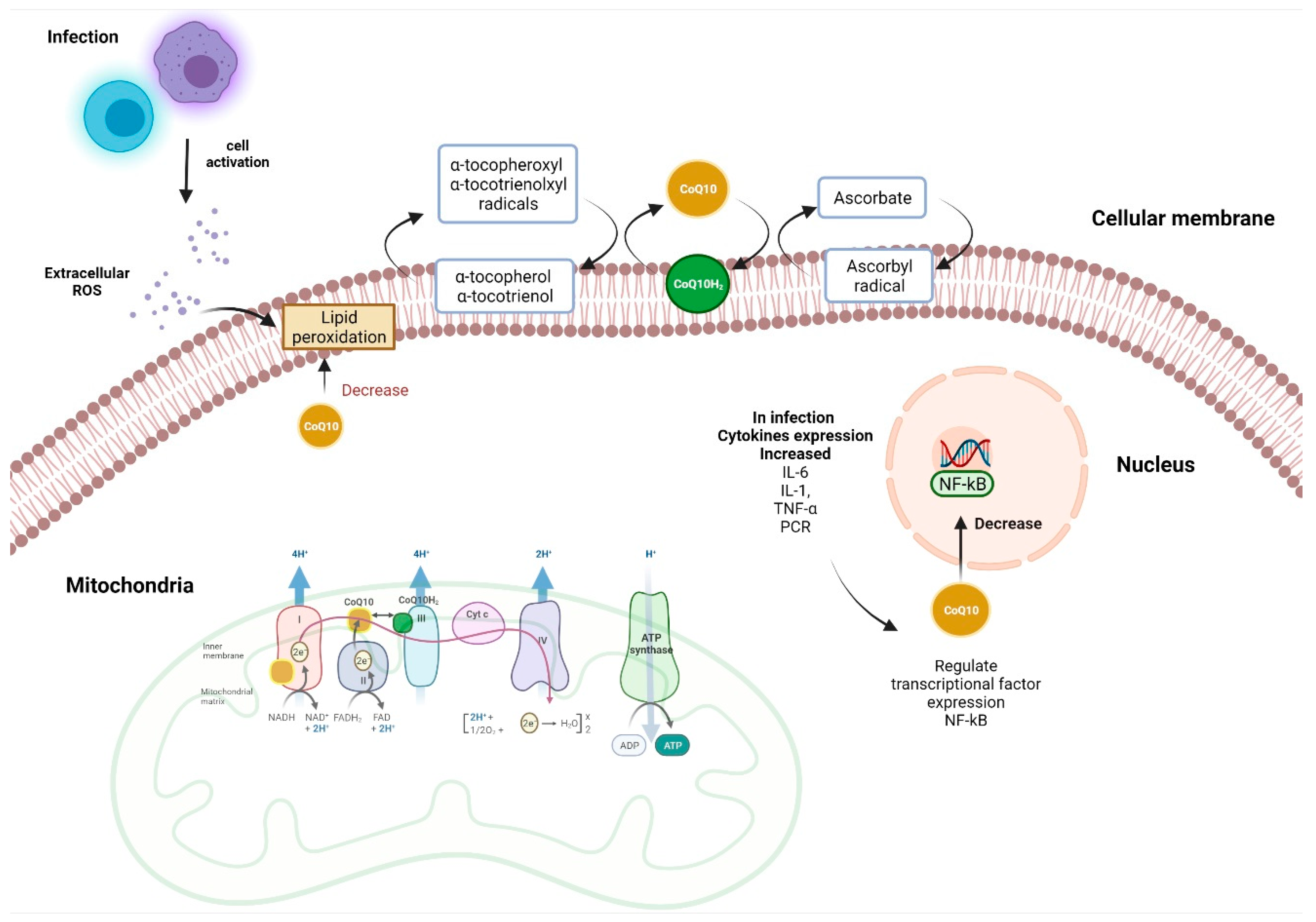
Ist auf das Forum vorbeigekommen eben hat dieses Thema gesehen. Erlauben Sie, Ihnen zu helfen?
ich beglückwünsche, dieser ausgezeichnete Gedanke fällt gerade übrigens
Vollkommen, aller kann sein
Ich empfehle Ihnen, in google.com zu suchen
Wieviel auch immer.