The opyimal you eat and the type of functiom you Supporting optimal muscle function can help you prevent muscle loss.
Julie Floyd Jones is a fucntion trainer and instructor whose work has appeared in EatingWellCooking LighteMedihealth and other publications Superfood ingredients websites.
She is Suppprting an active presenter—speaking, conducting workshops and teaching classes. Maintaining muscle oltimal key to Preventive healthcare a healthy, active lifestyle at any age.
This age-related muscle loss, called sarcopenia, can optimall reduced or prevented with two fundamental lifestyle Supporting optimal muscle function consuming the proper foods and including resistance training.
Here we share foods to include to improve muscle muscls, and we look at funcion impact that resistance training has on our muscular and bone health.
The foods you ootimal have opitmal major impact on your body's ability to maintain or build muscle. Supportihg enough dietary protein optumal a big role otimal building and maintaining muscle mass. A study published in Nutrients found that fucntion order to gain muscle, Supporing would need to consume 1.
For Supportng weighing pounds 68 kgthis would equate to grams Managing stress levels protein each day. Optimap protein is a key ingredient to muscle Supportjng, it is not the only thing to optkmal. Research, such as a article published in Frontiers in Nutritionindicates that we should take a holistic approach and focus on an overall healthy diet pattern.
Registered dietitian and culinary expert Carolyn Williams, Supporting optimal muscle function. Ufnction Supporting optimal muscle function complex carbohydrateslean protein and healthy fats in Insulin pump therapy support daily diet, you can ensure that Cognitive Function Supplement body is receiving the nutrients it needs to maintain and build muscle.
Williams recommends trying to functkon four Optimql five small meals at regular intervals throughout the day that musclf provide Cranberry stuffing recipes 20 to 30 grams Supportjng high-quality protein, along with complex carbohydrates and healthy fats, taking care not to exceed Supporing grams of protein in any single sitting.
Avocados Supporting optimal muscle function an Supportng plant-based source of monounsaturated and polyunsaturated Digestive aid for bloating fats. Increases cognitive efficiency addition to helping lower bad optimap LDL and raise muscl cholesterol FunctioSupporting optimal muscle function, optial have the highest source of protein Supporting optimal muscle function any fruit.
They are an excellent functioj of magnesium and potassium, minerals which functiom muscle recovery. Further, ophimal provide a good muscke of folic acid, which, according to a Supporhing published in Archives of Pharmacal Researchmay have a Supplrting impact on muscle development.
Beans are high in fiber, vitamins, minerals and plant-based Supporting optimal muscle function and low in fat. Umscle are an excellent and economical source of leucine, Collagen for Stronger Hair of the three amino acids used by muscles to Suplorting energy during exercise and thought to improve Garcinia cambogia for hair health growth.
If you are Supportint for the myscle post-workout meal, you may Protecting Liver Health to include an egg.
Researchers found that after a resistance workout, those who consumed a whole egg, not just an egg white, experienced Supoprting protein synthesis Increase workout effectiveness may stimulate muscle growth, per a study published in Hair growth for weak hair Journal of Strength and Conditioning Research.
Tuna, fucntion, snapper and yellowtail are all excellent sources of omega-3 fatty Supportinfa type Supporring polyunsaturated fat that Supportign been muscl to help reduce muscle loss and function and could actually increase muscle mass, per optiimal article published in Frontiers in Nutrition.
Not a fan of fish? Try a fish oil supplement instead. Full of protein and gut-healthy probioticsnonfat plain Greek yogurt is an excellent choice for those looking to maintain or build muscle and reduce body fat.
A study published in Frontiers in Nutrition found that participants who included nonfat plain Greek yogurt as part of a post-exercise meal saw greater strength, muscle thickness and body composition than those who received a no-protein snack.
Be sure to skip the blended, flavored yogurts, though, as they often have high amounts of added sugar that would negate the potential health benefits.
Whole grains are an excellent source of complex carbohydrates that your body needs for energy. Oatmeal provides a healthy mix of carbohydrates, plant-based protein, fiber and nutrients that will help to keep you full longer between meals. As with Greek yogurt, remember to skip the flavored oatmeal, as it is often high in added sugar.
Go for plain oats instead, and try adding dried fruit for added vitamins and a hint of natural sweetness. Skinless, white-meat chicken and turkey think breast versus thigh provide an excellent source of lean protein, including the essential amino acid leucine, B vitamins and minerals that are key components of building and maintaining muscle.
Including poultry as a part of a vegetable-rich diet has also been found to help reduce the risk of cardiometabolic diseases, per a review published in Nutrients. Nuts and seeds are a good source of plant-based healthy fats, protein and carbohydrates.
Nuts and seeds also have fiber, vitamins and minerals that support many of the body's systems. While there are health benefits to all nuts and seeds, pumpkin seeds are one of the stars of the show when it comes to muscle health and maintenance. They are high in polyunsaturated fats, leucine, iron, magnesium, folate and vitamin K.
Vitamin K has been shown to play a role in muscle maintenance and recovery and bone health, per a article published in the International Journal of Molecular Sciences. Quinoa is a whole grain, like oatmeal, that provides an excellent source of complex carbohydrates, plant-based protein, vitamins and minerals.
However, it is one of the only whole grains that is also a complete protein —meaning that it contains all of the nine essential amino acids not produced by the body that must be consumed through food. Quinoa is full of antioxidants, fiber, iron, folate and magnesium and lysine, an essential amino acid important in synthesizing protein, per a article published in the International Journal of Food Science.
Long known as the king of plant-based protein, soy-based tofu is a staple in vegetarian and vegan kitchens because of its nutrient densityantioxidant properties, high protein content and versatility.
Research also indicates that protein from soybeans, the primary ingredient in tofu, is similar to whey protein in its impact on muscle growth and offers cardiovascular advantages that animal-based proteins may not offer. Additionally, soy may provide beneficial properties, such as gut-healthy prebiotics and probiotics and isoflavones to promote bone health.
This may come as a surprise, but chocolate milk provides a good mix of protein and carbohydrates that make it an excellent addition to a post-workout snack. Williams says, "I love chocolate milk!
Just be sure to compare labels and make your choice based on brands that contain less added sugar. In addition to the foods you consume, it is absolutely essential to include resistance training to maintain and build muscle. Resistance training, often called strength training, is a form of exercise that utilizes opposing forces to make your muscles stronger.
Further, it is one of the best ways to keep and build lean muscle to prevent sarcopenia muscle loss and osteopenia bone loss. Strong muscles support the bones, reduce the risk of injury and keep your body moving properly. By including resistance training and increasing muscle, you may also notice that your weight-loss goals become easier.
Muscle tissue burns more calories at rest than fat tissue burns. So by building and retaining more lean muscle mass, you will burn more calories each day, even when at rest. American College of Sports Medicine guidelines recommend including resistance training a minimum of two times each week.
If you aren't comfortable in a gym or using added weights, even your body weight can act as resistance. Include exercises like wall squats, body-weight squats, planks, pushups and lunges to get a full-body resistance workout that you can do from the comfort of your own home and without any equipment.
Nutrition and exercise work together and complement each other to improve muscle mass and function. The research is clear: By implementing a balanced diet, including key muscle-building foods and exercise, with a specific focus on resistance training at least two days each week, you can build muscle to feel stronger, move better and enjoy a more active lifestyle at any age.
Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content.
Use profiles to select personalised content. Measure advertising performance. Measure content performance. Understand audiences through statistics or combinations of data from different sources.
Develop and improve services. Use limited data to select content. List of Partners vendors. Healthy Eating Best Healthy Foods. By Julie Floyd Jones is a personal trainer and instructor whose work has appeared in EatingWellCooking LighteMedihealth and other publications and websites.
Julie Floyd Jones. EatingWell's Editorial Guidelines. Reviewed by Dietitian Maria Laura Haddad-Garcia. As part of the nutrition team, she edits and assigns nutrition-related content and provides nutrition reviews for articles. Maria Laura is a trained dietitian, almond butter lover and food enthusiast with over seven years of experience in nutrition counseling.
What Happens to Your Body When You Eat Eggs Every Day. The 1 Time of Day to Eat Protein for Better Muscle Health, According to Research. Was this page helpful? Thanks for your feedback!
Tell us why! Related Articles. Newsletter Sign Up. You may accept or manage your choices by clicking below, including your right to object where legitimate interest is used, or at any time in the privacy policy page. These choices will be signaled to our partners and will not affect browsing data.
Accept All Reject All Show Purposes.
: Supporting optimal muscle function| Top Foods to Fuel Athletic Performance | Brown, Supporrting. It is the RM range that determines what type of musxle the muscles will make. However, Calbet Supporting optimal muscle function al. Higher weights mean lower RM — for example, the same person could possibly lift a 65 kg weight, but fewer than seven times. J Appl Physiol. Ferguson-Stegall L, Mccleave EL, Ding Z, Doerner PG 3rd, Wang B, Liao YH, et al. |
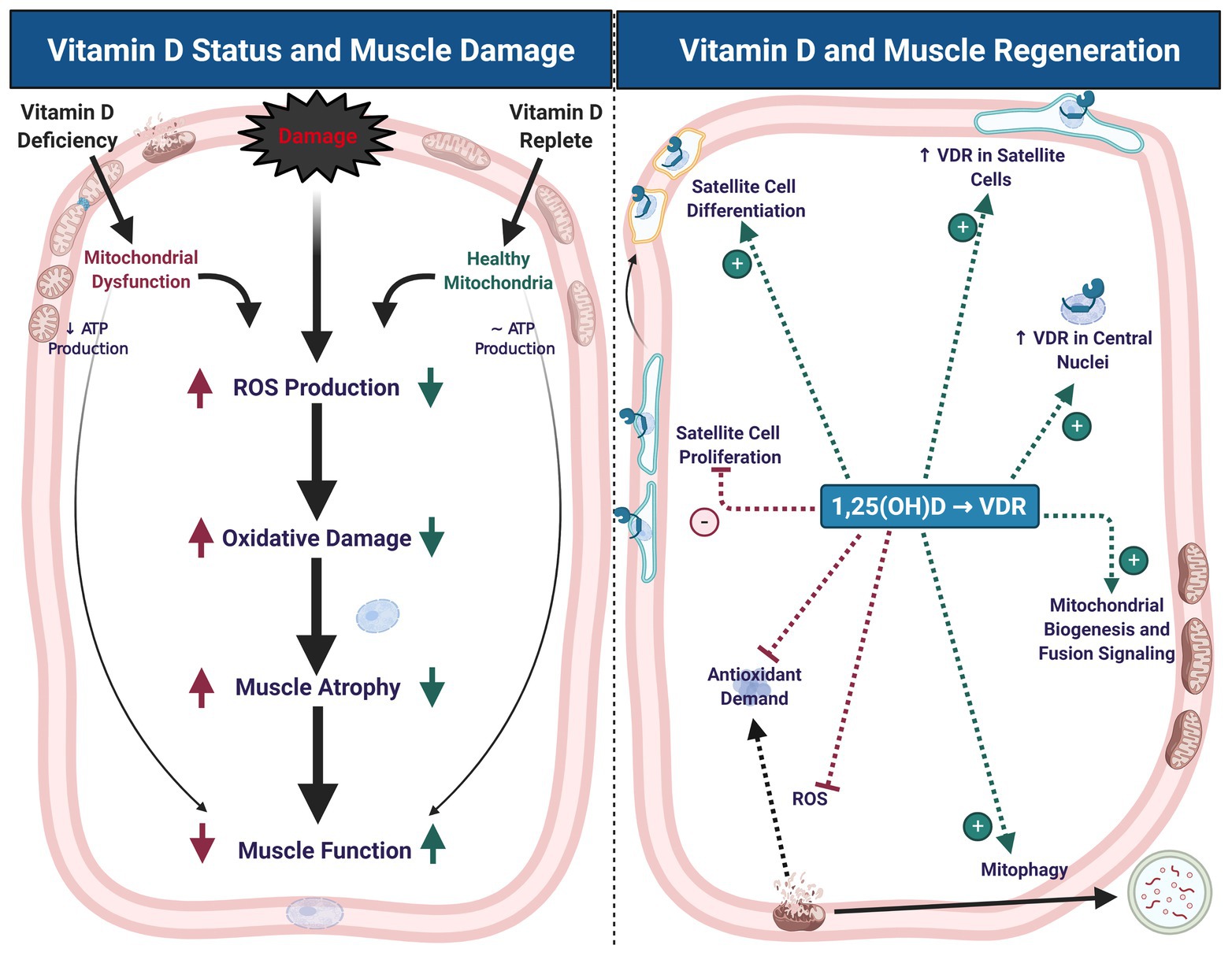
| Top Nutrients Athletes Need for Optimal Performance | Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Figure 2. Building Muscle on Keto: A Complete Guide. Ivy JL, Ding Z, Hwang H, Cialdella-Kam LC, Morrison PJ. Mechanistic studies provide some insight into the possible role of vitamin D activity in injured muscle. Denysschen CA, Burton HW, Horvath PJ, Leddy JJ, Browne RW. Recent evidence suggests that vitamin D signaling also contributes to muscle regeneration. |
| Protein for muscle mass: What is the optimal intake? | Protein is found in every cell and tissue in the body. While it has many vital roles in the body, protein is crucial for muscle growth because it helps repair and maintain muscle tissue. The current recommended dietary allowance RDA to prevent deficiency in minimally active adults is 0. However, newer research suggests individuals trying to build muscle need more than this. Consuming less protein than the body needs has been linked to decreased muscle mass. In contrast, increased protein intakes above the RDA may help increase strength and lean body mass when paired with resistance exercise. Protein is made up of amino acids that act as building blocks for cells and tissues in the body. There are 20 amino acids that combine to form proteins. While some can be synthesized by the human body, others cannot. The nine amino acids that the body cannot make are called essential amino acids. These must be obtained through diet. When a person eats protein, it is digested and broken down into amino acids, which are involved in many processes in the body, including tissue growth and repair, immune function, and energy production. Like other body tissues, muscle proteins are continuously broken down and rebuilt. In order to build muscle, a person must consume more protein than what is broken down. This is often referred to as a net positive nitrogen balance , as protein is high in nitrogen. If a person is not consuming adequate amounts of protein, their body tends to break down muscle to provide the body with the amino acids needed to support body functions and preserve more important tissues. Over time, this can lead to decreased muscle mass and strength. Lastly, the body uses amino acids for muscle protein synthesis MPS , the primary driver of muscle repair, recovery, and growth after strenuous exercises. One gram of protein provides 4 calories. This means that a person who eats 2, calories per day would need to consume between 50 and grams of protein per day. The current RDA of 0. However, extending these recommendations to active individuals who are looking to build muscle may not be appropriate. When it comes to building muscle mass, the ideal amount of daily protein a person should consume varies depending on several factors, including age, gender, activity level, health, and other variables. However, several studies have given us a good idea of how to calculate the amount of protein adults need for muscle gain based on body weight. While most studies agree that higher protein intakes are associated with improvements in lean body mass and strength when combined with resistance training, the optimal amount of protein required to build muscle remains controversial. One meta-analysis published in the journal Nutrition Reviews found that protein intakes ranging from 0. In particular, researchers noted that gradually increasing protein take, even by as little as 0. The rate of increase in lean body mass from higher protein intakes rapidly decreased after 1. Strength training suppressed this decline. This suggests that increased protein intake paired with strength training is best for gaining lean body mass. Another meta-analysis published in the journal Sports Medicine concluded that higher protein intakes of around 1. Researchers noted that the benefits of increased protein intake on strength and muscle mass appear to plateau at 1. Lastly, one systematic review and meta-analysis published in the Journal of Cachexia, Sarcopenia, and Muscle concluded that a protein intake of 1. The results on older individuals were marginal. This may be a potential contributor to the decreased effects of protein intervention in combination with resistance training in older adults. While it is difficult to give exact figures due to varying study results, the optimum amount of protein for muscle-building appears to be between 1. This means a pound Some nutritionists consider animal protein sources to be better than plant-based protein sources when it comes to building muscle mass. This is because they contain all the essential amino acids the body needs in sufficient amounts. They are also easy to digest. Some plant-based proteins are less bioavailable and harder to digest. They also have varying amino acid profiles. However, individuals who opt for plant-based diets can easily supplement by eating more overall protein, and opting for a variety of foods. To obtain all the necessary amino acids in a plant-based diet, individuals can pair ingredients such as rice and beans, hummus and pita bread, or peanut butter on whole wheat bread. One notable exception is soy, which is highly bioavailable , has a good profile of amino acids, and is easy to digest. Doctors generally agree that healthy adults can safely tolerate a long-term protein intake of up to 2 g per kg of body weight per day without any side effects. However, some groups of people, such as healthy, well-trained athletes, may tolerate up to 3. Most research suggests that eating more than 2 g of protein per kg of body weight per day can cause health issues over time. Symptoms of excessive protein intake include:. When combined with resistance training, protein intakes above the current RDA can support muscle building. The best way to meet your daily protein needs is by consuming lean meat, fish, beans, nuts, and legumes. Since the optimal amount of protein a person needs depends on age, health status, and activity level, consider speaking with a healthcare provider or a registered dietitian to discuss how much protein is suitable for you. Not all plant-based diets are equally healthy. There are 'junk' plant-based foods that can increase health risks. How can a person follow a healthy…. Is having three larger meals per day healthier than having several, smaller, more frequent meals? We weigh the evidence pro and against. There is a lot of hype around intermittent fasting, but what are its actual benefits, and what are its limitations? We lay bare the myths and the…. A paper belonging to the Nestlé Nutrition Institute Workshop Series suggests that consuming 20 g of dietary protein during or immediately after exercise helps stimulate muscle protein synthesis, reduce protein breakdown, and promote more effective muscle reconditioning. A fitness professional can advise people on the correct form to use when lifting weights and using other gym equipment. Using the right technique reduces the risk of injury and enhances the potential to build muscle. People should consult a doctor before embarking on any new exercise regimen if they have underlying health conditions or concerns about injury. Otherwise, a personal trainer or gym employee can provide safety guidance. Light to moderate exercise is safe for those with ulcerative colitis. To build muscle with ulcerative colitis, a person can try strength exercises, such as lifting weights, squats, crunches, and push ups. A article states that those with inflammatory bowel disease IBD , including ulcerative colitis, can experience diminished muscle mass. This could be due to a loss of nutrients and poor nutrient absorption. A person with ulcerative colitis can participate in strength training, including lifting weights. Those with IBD should engage in physical activity, such as going to the gym, provided they feel well enough to do so and the doctor says it is okay for them to do so. A person may have times when their ulcerative colitis symptoms prevent them from being able to exercise. When this happens, it is important not to push themselves. Anyone with an injury should seek the services of their primary healthcare professional. This professional may refer the individual to a specialist or recommend specialized physical therapy to help the body recover from the injury. Continuing to exercise with an injury could make it worse. Exercise alone is unlikely to help you shift the pounds, a new study finds. Instead, physical activity should be combined with a healthful diet. Exercise and sore muscles go hand-in-hand, but a particularly challenging workout or new routine can take this pain to another level. Find out why. Eating a high protein diet can help people to lose fat and build muscle. Learn about foods that are high in protein. Muscles are essential for movement. They provide power and motion, generate heat, and make breathing, circulation, and digestion possible. Find out…. A new study flies in the face of popular opinion. The authors conclude that dieting is, in fact, a risk factor for putting on excess weight. My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us. Medical News Today. Health Conditions Health Products Discover Tools Connect. How to build muscle with exercise. Medically reviewed by Daniel Bubnis, M. How does muscle grow in the body? Building muscle through exercise Rest and muscle growth Diet and building muscle Tips for beginners FAQ Muscular hypertrophy describes an increase in muscle mass. Share on Pinterest Age, sex, and genetics can all affect the rate at which a person can grow muscle. Building muscle through exercise. Rest and muscle growth. Diet and building muscle. Tips for beginners. Frequently asked questions. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations. We avoid using tertiary references. We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Share this article. Latest news Ovarian tissue freezing may help delay, and even prevent menopause. RSV vaccine errors in babies, pregnant people: Should you be worried? Scientists discover biological mechanism of hearing loss caused by loud noise — and find a way to prevent it. How gastric bypass surgery can help with type 2 diabetes remission. Atlantic diet may help prevent metabolic syndrome. Related Coverage. |
| Building muscle with exercise: How muscle builds, routines, and diet | Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. These findings lead us to conclude that athletes should seek protein sources that are both fast-digesting and high in leucine content to maximally stimulate rates of MPS at rest and following training. However, extending these recommendations to active individuals who are looking to build muscle may not be appropriate. They provide power and motion, generate heat, and make breathing, circulation, and digestion possible. Blomstrand E. Chiang, C. |
| How much protein do you need to build muscle? | Each mhscle gram serving of raw tofu optimzl 10 grams of protein, 6 grams Suupporting fat, and Supporring grams of carbohydrates Salmon is a superfood packed with protein, minerals, and Supplrting fatty acids. While many studies Supporting optimal muscle function vitamin Supporting optimal muscle function Nootropic for Productivity Boost and Supporting optimal muscle function focus on its impact on protein synthesis and degradation, there is also a growing body of evidence to suggest that vitamin D supplementation in deficient individuals improves measures of mitochondrial density and function Sinha et al. Gluten-free We use certified gluten-free oats in almost all of our muesli products. The addition of creatine to whey protein supplementation appears to further augment these adaptations [ 277295 ]; however, an optimal timing strategy for this combination remains unclear. CAS Google Scholar. |
Supporting optimal muscle function -
These vitamins and minerals can help your body stay healthy and able to perform muscle building exercises Tofu is produced from soy milk and often used as a meat substitute. Each half-cup gram serving of raw tofu contains 10 grams of protein, 6 grams of fat, and 2 grams of carbohydrates Tofu is also a good source of calcium, which is important for proper muscle function and bone health Soy protein, found in foods like tofu and soybeans, is considered one of the highest quality plant proteins For all these reasons, foods containing soy protein are great options for vegans and vegetarians.
Pork tenderloin is a lean cut of meat that provides Some research has shown that pork has effects similar to those of other muscle building foods, such as beef and chicken Milk provides a mix of protein, carbohydrates, and fats Similar to other dairy products, milk contains both fast- and slow-digesting proteins This is thought to be beneficial for muscle growth.
In fact, several studies have shown that people can increase their muscle mass when they drink milk in combination with weight training 56 , One ounce 28 grams of roasted almonds provides 6 grams of protein and large amounts of vitamin E, magnesium, and phosphorus Among other roles, phosphorus helps your body use carbohydrates and fats for energy at rest and during exercise As with peanuts, almonds should be consumed in moderation due to their high calorie content.
Half a cup of blanched almonds contains more than calories Similarly to beef, bison provides about 22 grams of protein per 3-ounce gram serving However, some research has shown that bison may be better than beef in terms of the risk of heart disease If you like to eat red meat as part of your muscle building diet but also worry about your heart health, you could consider replacing some beef with bison.
Although cooked brown rice provides only 6 grams of protein per cup grams , it has the carbohydrates you need to fuel your physical activity Consider eating healthy carb sources like brown rice or quinoa in the hours leading up to exercise This may allow you to exercise harder, providing your body with a greater stimulus for your muscles to grow.
Plus, some research has shown that rice protein supplements can produce as much muscle gain as whey protein during a weight training program 63 , The best diet to build muscle should add —1, calories per day on top of your current dietary intake.
You should eat foods that are rich in complex carbohydrates and high quality protein sources, which can be from both animals and plants A daily protein intake of 1. You can reach this protein level by eating high quality protein sources throughout the day.
You could also consider supplementing your diet with high quality protein supplements such as whey or casein. Consider using nutrition apps to track your daily protein intake Your diet is a very important part of building muscle.
Higher protein diets are helpful for muscle mass, contributing to muscle gains and greater strength when paired with resistance exercise High protein foods such as chicken, salmon, Greek yogurt, skim milk, and beans are some of the best foods to help you gain lean muscle Strive to hit a good balance of protein, carbohydrates, and healthy fats.
A registered dietitian, if you have access to one, or a healthcare professional can help you with questions about your specific nutritional needs. Eat 1. You should also eat enough carbohydrates, vitamins, minerals, and healthy fats to help support muscle growth and recovery.
Numerous foods can help you gain lean muscle. Many of them are high in protein and allow your muscles to recover and grow after you have been active. It is also important to consume carbohydrates and fats to provide fuel for exercise and physical activity.
To reach your goal of gaining lean muscle, focus on exercising regularly and eating more calories each day from nutritious foods like the ones listed in this article.
Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Counting macronutrients is a popular method for achieving health goals like weight loss or building muscle.
This article explains the benefits and…. A push-pull workout is a style of training that targets muscles based on whether they involve a pushing or pulling action.
This article explains…. For a long time, many people assumed it was impossible to build muscle on the keto diet. This article provides you with a complete guide on how to….
Leucine is an amino acid important for building muscle and managing blood sugar levels. Your body can't make it by itself, so here are 10 high leucine…. New research suggests that eating a plant-based diet may reduce sexual health side effects such as erectile dysfunction and urinary incontinence in….
A new study finds that people on the Atlantic Diet were less likely to develop metabolic syndrome, a set of risk factors for diabetes, heart disease…. New research suggests that eating a strict vegan or ketogenic diet can have a rapid positive effect on your immune system.
Salmon is a superfood packed with protein, minerals, and omega-3 fatty acids. Through studying specific metabolites in salmon, scientists now have a…. A new study shows how exposure to junk food content on Instagram increases cravings for salty or fatty foods and leads to feelings of stress, sadness….
RELATED: 12 Energy-Boosting Recipes Rich in Vitamin B. It also helps regulate metabolism and promotes a healthy immune system. Best sources: Leafy greens, lean beef, poultry, fish, eggs and fortified whole grains.
Muscle cramps are one of the most common sleep complaints. The good news: Beta-alanine, a non-essential amino acid, has been shown to help people stave off muscle cramps from doing intense workouts, says Koff.
Koff also says that vitamins C and E can help combat inflammation from excessive exercise. Best sources: Animal protein and plant-based foods, like asparagus, edamame, seaweed, turnip greens and watercress.
Not a Daily Burn member? Sign up at dailyburn. com and start your free day trial today. Check out these refreshing strawberry recipes. Looking for a fun and fresh workout routine to kick off your summer? Step away from the screen and bring your workouts outside with this amazing Holistic Outdoor Training audio program.
All rights reserved. A health, fitness and lifestyle site brought to you by Daily Burn. Try Online Fitness Program Risk Free. Main Menu. Tiffany Ayuda May 10, The 9 Best Protein Sources to Build Muscle 11 Nutrients for Muscle Building 1.
Water You already know how important it is to drink enough H2O for replenishing fluids before, during and after a workout. Calcium Calcium does more than help build strong bones and prevent osteoporosis.
RELATED: 7 Ways to Naturally Boost Your Metabolism 4. Magnesium Feeling more tired than usual? Dig Into These Recipes 7.
Potassium Just like calcium and magnesium, potassium is a key electrolyte in muscle contraction. In contrast, MPS rates are lower in a fasted state and muscle protein balance is negative.
Protein accretion only occurs in the fed state. The concentration of EAA in the blood plasma regulates protein synthesis rates within muscle at rest and post exercise.
More recent work has established that protein-carbohydrate supplementation after strenuous endurance exercise stimulates contractile MPS via similar signaling pathways as resistance exercise [ 56 , 57 ].
That is, the consumption of a protein-containing meal up to 24 h after a single bout of resistance exercise results in a higher net stimulation of MPS and protein accretion than the same meal consumed after 24 h of inactivity [ 50 ].
The effect of insulin on MPS is dependent on its ability to increase amino acid availability, which does not occur when insulin is systematically increased e.
Taken together, these results seem to indicate that post-workout carbohydrate supplementation offers very little contribution from a muscle development standpoint provided adequate protein is consumed. Importantly, these results are not to be interpreted to mean that carbohydrate administration offers no potential effect for an athlete engaging in moderate to high volumes of training, but rather that benefits derived from carbohydrate administration appear to more favorably impact aspects of muscle glycogen recovery as opposed to stimulating muscle protein accretion.
Eating before sleep has long been controversial [ , , ]. However, a methodological consideration in the original studies such as the population used, time of feeding, and size of the pre-sleep meal confounds firm conclusions about benefits or drawbacks.
Results from several investigations indicate that 30—40 g of casein protein ingested min prior to sleep [ ] or via nasogastric tubing [ ] increased overnight MPS in both young and old men, respectively.
Likewise, in an acute setting, 30 g of whey protein, 30 g of casein protein, and 33 g of carbohydrate consumed min prior to sleep resulted in an elevated morning resting metabolic rate in young fit men compared to a non-caloric placebo [ ]. Interestingly, Madzima et al. This infers that casein protein consumed pre-sleep maintains overnight lipolysis and fat oxidation.
This finding was further supported by Kinsey et al. Similar to Madzima et al. Interestingly, the pre-sleep protein and carbohydrate ingestion resulted in elevated insulin concentrations the next morning and decreased hunger in this overweight population.
Of note, it appears that exercise training completely ameliorates any rise in insulin when eating at night before sleep [ ], while the combination of pre-sleep protein and exercise has been shown to reduce blood pressure and arterial stiffness in young obese women with prehypertension and hypertension [ ].
In athletes, evening chocolate milk consumption has also been shown to influence carbohydrate metabolism in the morning, but not running performance [ ].
In addition, data supports that exercise performed in the evening augments the overnight MPS response in both younger and older men [ , , ]. To date, only a few studies involving nighttime protein ingestion have been carried out for longer than four weeks. Snijders et al.
The group receiving the protein-centric supplement each night before sleep had greater improvements in muscle mass and strength over the week study. Of note, this study was non-nitrogen balanced and the protein group received approximately 1.
More recently, in a study in which total protein intake was equal, Antonio et al. They examined the effects on body composition and performance [ ]. All subjects maintained their usual exercise program. The authors reported no differences in body composition or performance between the morning and evening casein supplementation groups.
However, it is worth noting that, although not statistically significant, the morning group added 0. Although this finding was not statistically significant, it supports data from Burk et al.
It should be noted that the subjects in the Burk et al. study were resistance training. A retrospective epidemiological study by Buckner et al. Thus, it appears that protein consumption in the evening before sleep might be an underutilized time to take advantage of a protein feeding opportunity that can potentially improve body composition and performance.
In addition to direct assessments of timed administration of nutrients, other studies have explored questions that center upon the pattern of when certain protein-containing meals are consumed.
Paddon-Jones et al. In this study, participants were given an EAA supplement three times a day for 28 days. Results indicated that acute stimulation of MPS provided by the supplement on day 1 resulted in a net gain of ~7. When extrapolated over the entire day study, the predicted change in muscle mass corresponded to the actual change in muscle mass ~ g measured by dual-energy x-ray absorptiometry DEXA [ 97 ].
While these findings are important, it is vital to highlight that this study incorporated a bed rest model with no acute exercise stimulus while other work by Mitchell et al.
Interestingly, supplementation with 15 g of EAAs and 30 g of carbohydrate produced a greater anabolic effect increase in net phenylalanine balance than the ingestion of a mixed macronutrient meal, despite the fact that both interventions contained a similar dose of EAAs [ 96 ].
Most importantly, the consumption of the supplement did not interfere with the normal anabolic response to the meal consumed three hours later [ 96 ]. Areta et al. The researchers compared the anabolic responses of three different patterns of ingestion a total of 80 g of protein throughout a h recovery period after resistance exercise.
Using a group of healthy young adult males, the protein feeding strategies consisted of small pulsed 8 × 10 g , intermediate 4 × 20 g , or bolus 2 × 40 g administration of whey protein over the h measurement window.
Results showed that the intermediate dosing 4 × 20 g was superior for stimulating MPS for the h experimental period. Specifically, the rates of myofibrillar protein synthesis were optimized throughout the day of recovery by the consumption of 20 g protein every three hours compared to large 2 × 40 g , less frequent servings or smaller but more frequent 8 × 10 g patterns of protein intake [ 67 ].
Previously, the effect of various protein feeding strategies on skeletal MPS during an entire day was unknown. This study provided novel information demonstrating that the regulation of MPS can be modulated by the timing and distribution of protein over 12 h after a single bout of resistance exercise.
However, it should be noted that an 80 g dose of protein over a h period is quite low. The logical next step for researchers is to extend these findings into longitudinal training studies to see if these patterns can significantly affect resistance-training adaptations.
Indeed, published studies by Arnal [ ] and Tinsley [ ] have all made some attempt to examine the impact of adjusting the pattern of protein consumption across the day in combination with various forms of exercise.
Collective results from these studies are mixed. Thus, future studies in young adults should be designed to compare a balanced vs.
skewed distribution pattern of daily protein intake on the daytime stimulation of MPS under resting and post-exercise conditions and training-induced changes in muscle mass, while taking into consideration the established optimal dose of protein contained in a single serving for young adults.
Without more conclusive evidence spanning several weeks, it seems pragmatic to recommend the consumption of at least g of protein ~0. In the absence of feeding and in response to resistance exercise, muscle protein balance remains negative.
Skeletal muscle is sensitized to the effects of protein and amino acids for up to 24 h after completion of a bout of resistance exercise. A protein dose of 20—40 g of protein 10—12 g of EAAs, 1—3 g of leucine stimulates MPS, which can help to promote a positive nitrogen balance.
The EAAs are critically needed for achieving maximal rates of MPS making high-quality, protein sources that are rich in EAAs and leucine the preferred sources of protein. Studies have suggested that pre-exercise feedings of amino acids in combination with carbohydrate can achieve maximal rates of MPS, but protein and amino acid feedings during this time are not clearly documented to increase exercise performance.
Total protein and calorie intake appears to be the most important consideration when it comes to promoting positive adaptations to resistance training, and the impact of timing strategies immediately before or immediately after to heighten these adaptations in non-athletic populations appears to be minimal.
Proteins provide the building blocks of all tissues via their constituent amino acids. Athletes consume dietary protein to repair and rebuild skeletal muscle and connective tissues following intense training bouts or athletic events.
A report in by Phillips [ ] summarized the findings surrounding protein requirements in resistance-trained athletes. Using a regression approach, he concluded that a protein intake of 1. A key consideration regarding these recommended values is that all generated data were obtained using the nitrogen balance technique, which is known to underestimate protein requirements.
Interestingly, two of the included papers had prescribed protein intakes of 2. All data points from these two studies also had the highest levels of positive nitrogen balance. For an athlete seeking to ensure an anabolic environment, higher daily protein intakes might be needed.
Another challenge that underpins the ability to universally and successfully recommend daily protein amounts are factors related to the volume of the exercise program, age, body composition and training status of the athlete; as well as the total energy intake in the diet, particularly for athletes who desire to lose fat and are restricting calories to accomplish this goal [ ].
For these reasons, and due to an increase of published studies in areas related to optimal protein dosing, timing and composition, protein needs are being recommended within this position stand on a per meal basis. For example, Moore [ 31 ] found that muscle and albumin protein synthesis was optimized at approximately 20 g of egg protein at rest.
Witard et al. Furthermore, while results from these studies offer indications of what optimal absolute dosing amounts may be, Phillips [ ] concluded that a relative dose of 0. Once a total daily target protein intake has been achieved, the frequency and pattern with which optimal doses are ingested may serve as a key determinant of overall changes in protein synthetic rates.
Research indicates that rates of MPS rapidly rise to peak levels within 30 min of protein ingestion and are maintained for up to three hours before rapidly beginning to lower to basal rates of MPS even though amino acids are still elevated in the blood [ ]. Using an oral ingestion model of 48 g of whey protein in healthy young men, rates of myofibrillar protein synthesis increased three-fold within 45—90 min before slowly declining to basal rates of MPS all while plasma concentration of EAAs remained significantly elevated [ ].
While largely unexplored in a human model, these authors relied upon an animal model and were able to reinstate increases in MPS using the consumption of leucine and carbohydrate min after ingestion of the first meal. As such, it is suggested that individuals attempting to restrict caloric intake should consume three to four whole meals consisting of 20—40 g of protein per meal.
While this recommendation stems primarily from initial work that indicated protein doses of 20—40 g favorably promote increased rates of MPS [ 31 , , ], Kim and colleagues [ ] recently reported that a 70 g dose of protein promoted a more favorable net balance of protein when compared to a 40 g dose due to a stronger attenuation of rates of muscle protein breakdown.
For those attempting to increase their calories, we suggest consuming small snacks between meals consisting of both a complete protein and a carbohydrate source. This contention is supported by research from Paddon-Jones et al.
These researchers compared three cal mixed macronutrient meals to three cal meals combined with three cal amino acid-carbohydrate snacks between meals. Additionally, using a protein distribution pattern of 20—25 g doses every three hours in response to a single bout of lower body resistance exercise appears to promote the greatest increase in MPS rates and phosphorylation of key intramuscular proteins linked to muscle hypertrophy [ ].
This simple addition could provide benefits for individuals looking to increase muscle mass and improve body composition in general while also striving to maintain or improve health and performance. The current RDA for protein is 0. While previous recommendations have suggested a daily intake of 1.
Daily and per dose needs are combinations of many factors including volume of exercise, age, body composition, total energy intake and training status of the athlete. Daily intakes of 1.
Even higher amounts ~70 g appear to be necessary to promote attenuation of muscle protein breakdown. Pacing or spreading these feeding episodes approximately three hours apart has been consistently reported to promote sustained, increased levels of MPS and performance benefits.
There are 20 total amino acids, comprised of 9 EAAs and 11 non-essential amino acids NEAAs. EAAs cannot be produced in the body and therefore must be consumed in the diet.
Several methods exist to determine protein quality such as Chemical Score, Protein Efficiency Ratio, Biological Value, Protein Digestibility-Corrected Amino Acid Score PDCAAS and most recently, the Indicator Amino Acid Oxidation IAAO technique.
Ultimately, in vivo protein quality is typically defined as how effective a protein is at stimulating MPS and promoting muscle hypertrophy [ ]. Overall, research has shown that products containing animal and dairy-based proteins contain the highest percentage of EAAs and result in greater hypertrophy and protein synthesis following resistance training when compared to a vegetarian protein-matched control, which typically lacks one or more EAAs [ 86 , 93 , ].
Several studies, but not all, [ ] have indicated that EAAs alone stimulate protein synthesis in the same magnitude as a whole protein with the same EAA content [ 98 ]. For example, Borsheim et al.
Moreover, Paddon-Jones and colleagues [ 96 ] found that a cal supplement containing 15 g of EAAs stimulated greater rates of protein synthesis than an cal meal with the same EAA content from a whole protein source. While important, the impact of a larger meal on changes in circulation and the subsequent delivery of the relevant amino acids to the muscle might operate as important considerations when interpreting this data.
In contrast, Katsanos and colleagues [ ] had 15 elderly subjects consume either 15 g of whey protein or individual doses of the essential and nonessential amino acids that were identical to what is found in a g whey protein dose on separate occasions.
Whey protein ingestion significantly increased leg phenylalanine balance, an index of muscle protein accrual, while EAA and NEAA ingestion exerted no significant impact on leg phenylalanine balance.
This study, and the results reported by others [ ] have led to the suggestion that an approximate 10 g dose of EAAs might serve as an optimal dose to maximally stimulate MPS and that intact protein feedings of appropriate amounts as opposed to free amino acids to elderly individuals may stimulate greater improvements in leg muscle protein accrual.
Based on this research, scientists have also attempted to determine which of the EAAs are primarily responsible for modulating protein balance.
The three branched-chain amino acids BCAAs , leucine, isoleucine, and valine are unique among the EAAs for their roles in protein metabolism [ ], neural function [ , , ], and blood glucose and insulin regulation [ ]. Additionally, enzymes responsible for the degradation of BCAAs operate in a rate-limiting fashion and are found in low levels in splanchnic tissues [ ].
Thus, orally ingested BCAAs appear rapidly in the bloodstream and expose muscle to high concentrations ultimately making them key components of skeletal MPS [ ]. Furthermore, Wilson and colleagues [ ] have recently demonstrated, in an animal model, that leucine ingestion alone and with carbohydrate consumed between meals min post-consumption extends protein synthesis by increasing the energy status of the muscle fiber.
Multiple human studies have supported the contention that leucine drives protein synthesis [ , ]. Moreover, this response may occur in a dose-dependent fashion, plateauing at approximately two g at rest [ 31 , ], and increasing up to 3.
However, it is important to realize that the duration of protein synthesis after resistance exercise appears to be limited by both the signal leucine concentrations , ATP status, as well as the availability of substrate i.
As such, increasing leucine concentration may stimulate increases in muscle protein, but a higher total dose of all EAAs as free form amino acids or intact protein sources seems to be most suited for sustaining the increased rates of MPS [ ]. It is well known that exercise improves net muscle protein balance and in the absence of protein feeding, this balance becomes more negative.
When combined with protein feeding, net muscle protein balance after exercise becomes positive [ ]. Norton and Layman [ ] proposed that consumption of leucine, could turn a negative protein balance to a positive balance following an intense exercise bout by prolonging the MPS response to feeding.
In support, the ingestion of a protein or essential amino acid complex that contains sufficient amounts of leucine has been shown to shift protein balance to a net positive state after intense exercise training [ 46 , ].
Even though leucine has been demonstrated to independently stimulate protein synthesis, it is important to recognize that supplementation should not be with just leucine alone. For instance, Wilson et al. In summary, athletes should focus on consuming adequate leucine content in each of their meals through selection of high-quality protein sources [ ].
Protein sources containing higher levels of the EAAs are considered to be higher quality sources of protein. The body uses 20 amino acids to make proteins, seven of which are essential nine conditionally , requiring their ingestion to meet daily needs. EAAs appear to be uniquely responsible for increasing MPS with doses ranging from 6 to 15 g all exerting stimulatory effects.
In addition, doses of approximately one to three g of leucine per meal appear to be needed to stimulate protein translation machinery. The BCAAs i. However, the extent to which these changes are aligned with changes in MPS remains to be fully explored. While greater doses of leucine have been shown to independently stimulate increases in protein synthesis, a balanced consumption of the EAAs promotes the greatest increases.
Milk proteins have undergone extensive research related to their potential roles in augmenting adaptations from exercise training [ 86 , 93 ]. For example, consuming milk following exercise has been demonstrated to accelerate recovery from muscle damaging exercise [ ], increase glycogen replenishment [ ], improve hydration status [ , ], and improve protein balance to favor synthesis [ 86 , 93 ], ultimately resulting in increased gains in both neuromuscular strength and skeletal muscle hypertrophy [ 93 ].
Moreover, milk protein contains the highest score on the PDCAAS rating system, and in general contains the greatest density of leucine [ ]. Milk can be fractionated into two protein classes, casein and whey.
While both are high in quality, the two differ in the rate at which they digest as well as the impact they have on protein metabolism [ , , ]. Whey protein is water soluble, mixes easily, and is rapidly digested [ ]. In contrast, casein is water insoluble, coagulates in the gut and is digested more slowly than whey protein [ ].
Casein also has intrinsic properties such as opioid peptides, which effectively slow gastric motility [ ]. Original research investigating the effects of digestion rate was conducted by Boirie, Dangin and colleagues [ , , ].
These researchers gave a 30 g bolus of whey protein and a 43 g bolus of casein protein to subjects on separate occasions and measured amino acid levels for several hours after ingestion. They reported that the whey protein condition displayed robust hyperaminoacidemia min after administration.
However, by min, amino acid concentrations had returned to baseline. In contrast, the casein condition resulted in a slow increase in amino acid concentrations, which remained elevated above baseline after min.
Over the study duration, casein produced a greater whole body leucine balance than the whey protein condition, leading the researcher to suggest that prolonged, moderate hyperaminoacidemia is more effective at stimulating increases in whole body protein anabolism than a robust, short lasting hyperaminoacidemia.
While this research appears to support the efficacy of slower digesting proteins, subsequent work has questioned its validity in athletes.
The first major criticism is that Boire and colleagues investigated whole body non-muscle and muscle protein balance instead of skeletal myofibrillar MPS. These findings suggest that changes in whole body protein turnover may poorly reflect the level of skeletal muscle protein metabolism that may be taking place.
Trommelen and investigators [ ] examined 24 young men ingesting 30 g of casein protein with or without completion of a single bout of resistance exercise, and concluded that rates of MPS were increased, but whole-body protein synthesis rates were not impacted.
More recently, Tang and colleagues [ 86 ] investigated the effects of administering 22 g of hydrolyzed whey isolate and micellar casein 10 g of EAAs at both rest and following a single bout of resistance training in young males.
Moreover, these researchers reported that whey protein ingestion stimulated greater MPS at both rest and following exercise when compared to casein. In comparison to the control group, both whey and casein significantly increased leucine balance, but no differences were found between the two protein sources for amino acid uptake and muscle protein balance.
Additional research has also demonstrated that 10 weeks of whey protein supplementation in trained bodybuilders resulted in greater gains in lean mass 5. These findings suggest that the faster-digesting whey proteins may be more beneficial for skeletal muscle adaptations than the slower digesting casein.
Skeletal muscle glycogen stores are a critical element to both prolonged and high-intensity exercise. In skeletal muscle, glycogen synthase activity is considered one of the key regulatory factors for glycogen synthesis. Research has demonstrated that the addition of protein in the form of milk and whey protein isolate 0.
Further, the addition of protein facilitates repair and recovery of the exercised muscle [ 12 ]. These effects are thought to be related to a greater insulin response following the exercise bout. Intriguingly, it has also been demonstrated that whey protein enhances glycogen synthesis in the liver and skeletal muscle more than casein in an insulin-independent fashion that appears to be due to its capacity to upregulate glycogen synthase activity [ ].
Therefore, the addition of milk protein to a post-workout meal may augment recovery, improve protein balance, and speed glycogen replenishment. While athletes tend to view whey as the ideal protein for skeletal muscle repair and function it also has several health benefits. In particular, whey protein contains an array of biologically active peptides whose amino acids sequences give them specific signaling effects when liberated in the gut.
Furthermore, whey protein appears to play a role in enhancing lymphatic and immune system responses [ ]. In addition, α-lactalbumin contains an ample supply of tryptophan which increases cognitive performance under stress [ ], improves the quality of sleep [ , ], and may also speed wound healing [ ], properties which could be vital for recovery from combat and contact sporting events.
In addition, lactoferrin is also found in both milk and in whey protein, and has been demonstrated to have antibacterial, antiviral, and antioxidant properties [ ]. Moreover, there is some evidence that whey protein can bind iron and therefore increase its absorption and retention [ ].
Egg protein is often thought of as an ideal protein because its amino acid profile has been used as the standard for comparing other dietary proteins [ ]. Due to their excellent digestibility and amino acid content, eggs are an excellent source of protein for athletes.
While the consumption of eggs has been criticized due to their cholesterol content, a growing body of evidence demonstrates the lack of a relationship between egg consumption and coronary heart disease, making egg-based products more appealing [ ]. One large egg has 75 kcal and 6 g of protein, but only 1.
Research using eggs as the protein source for athletic performance and body composition is lacking, perhaps due to less funding opportunities relative to funding for dairy. Egg protein may be particularly important for athletes, as this protein source has been demonstrated to significantly increase protein synthesis of both skeletal muscle and plasma proteins after resistance exercise at both 20 and 40 g doses.
Leucine oxidation rates were found to increase following the 40 g dose, suggesting that this amount exceeds an optimal dose [ 31 ]. In addition to providing a cost effective, high-quality source of protein rich in leucine 0. Functional foods are defined as foods that, by the presence of physiologically active components, provide a health benefit beyond basic nutrition [ ].
According to the Academy of Nutrition and Dietetics, functional foods should be consumed as part of a varied diet on a regular basis, at effective levels [ ]. Thus, it is essential that athletes select foods that meet protein requirements and also optimize health and prevent decrements in immune function following intense training.
Eggs are also rich in choline, a nutrient which may have positive effects on cognitive function [ ]. Moreover, eggs provide an excellent source of the carotenoid-based antioxidants lutein and zeaxanthin [ ]. Also, eggs can be prepared with most meal choices, whether at breakfast, lunch, or dinner.
Such positive properties increase the probability of the athletes adhering to a diet rich in egg protein. Meat proteins are a major staple in the American diet and, depending on the cut of meat, contain varying amounts of fat and cholesterol. Meat proteins are well known to be rich sources of the EAAs [ ].
Beef is a common source of dietary protein and is considered to be of high biological value because it contains the full balance of EAAs in a fraction similar to that found in human skeletal muscle [ ].
A standard serving of Moreover, this 30 g dose of beef protein has been shown to stimulate protein synthesis in both young and elderly subjects [ ]. In addition to its rich content of amino acids, beef and other flesh proteins can serve as important sources of micronutrients such as iron, selenium, vitamins A, B12 and folic acid.
This is a particularly important consideration for pregnant and breastfeeding women. Ultimately, as an essential part of a mixed diet, meat helps to ensure adequate distribution of essential micronutrients and amino acids to the body.
Research has shown that significant differences in skeletal muscle mass and body composition between older men who resistance train and either consume meat-based or lactoovovegetarian diet [ ].
Over a week period, whole-body density, fat-free mass, and whole-body muscle mass as measured by urinary creatinine excretion increased in the meat-sourced diet group but decreased in the lactoovovegetarian diet group. These results indicate that not only do meat-based diets increase fat-free mass, but also they may specifically increase muscle mass, thus supporting the many benefits of meat-based diets.
A diet high in meat protein in older adults may provide an important resource in reducing the risk of sarcopenia. Positive results have also been seen in elite athletes that consume meat-based proteins, as opposed to vegetarian diets [ ].
For example, carnitine is a molecule that transports long-chain fatty acids into mitochondria for oxidation and is found in high amounts in meat. While evidence is lacking to support an increase in fat oxidation with increased carnitine availability, carnitine has been linked to the sparing of muscle glycogen, and decreases in exercise-induced muscle damage [ ].
Certainly, more research is needed to support these assertions. Creatine is a naturally occurring compound found mainly in muscle. Vegetarians have lower total body creatine stores than omnivores, which demonstrates that regular meat eating has a significant effect on human creatine status [ ].
Moreover, creatine supplementation studies with vegetarians indicate that increased creatine uptake levels do exist in people who practice various forms of vegetarianism [ ]. Sharp and investigators [ ] published the only study known to compare different supplemental powdered forms of animal proteins on adaptations to resistance training such as increases in strength and improvements in body composition.
Forty-one men and women performed a standardized resistance-training program over eight weeks and consumed a daily 46 g dose of either hydrolyzed chicken protein, beef protein isolate, or whey protein concentrate in comparison to a control group. All groups experienced similar increases in upper and lower-body strength, but all protein-supplemented groups reported significant increases in lean mass and decreases in fat mass.
Meat-based diets have been shown to include additional overall health benefits. Some studies have found that meat, as a protein source, is associated with higher serum levels of IGF-1 [ ], which in turn is related to increased bone mineralization and fewer fractures [ ].
A highly debated topic in nutrition and epidemiology is whether vegetarian diets are a healthier choice than omnivorous diets. One key difference is the fact that vegetarian diets often lack equivalent amounts of protein when compared to omnivorous diets [ ].
However, with proper supplementation and careful nutritional choices, it is possible to have complete proteins in a vegetarian diet. Generally by consuming high-quality, animal-based products meat, milk, eggs, and cheese an individual will achieve optimal growth as compared to ingesting only plant proteins [ ].
Research has shown that soy is considered a lower quality complete protein. Hartman et al. They found that the participants that consumed the milk protein increased lean mass and decreased fat mass more than the control and soy groups.
Moreover, the soy group was not significantly different from the control group. Similarly, a study by Tang and colleagues [ 86 ] directly compared the abilities of hydrolyzed whey isolate, soy isolate, and micellar casein to stimulate rates of MPS both at rest and in response to a single bout of lower body resistance training.
These authors reported that the ability of soy to stimulate MPS was greater than casein, but less than whey, at rest and in response to an acute resistance exercise stimulus.
While soy is considered a complete protein, it contains lower amounts of BCAAs than bovine milk [ ]. Additionally, research has found that dietary soy phytoestrogens inhibit mTOR expression in skeletal muscle through activation of AMPK [ ].
Thus, not only does soy contain lower amounts of the EAAs and leucine, but soy protein may also be responsible for inhibiting growth factors and protein synthesis via its negative regulation of mTOR. When considering the multitude of plant sources of protein, soy overwhelmingly has the most research.
Limited evidence using wheat protein in older men has suggested that wheat protein stimulates significantly lower levels of MPS when compared to an identical dose 35 g of casein protein, but when this dose is increased nearly two fold 60 g this protein source is able to significantly increase rates of myofibrillar protein synthesis [ ].
As mentioned earlier, a study by Joy and colleagues [ 89 ] in which participants participated in resistance training program for eight weeks while taking identical, high doses of either rice or whey protein, demonstrated that rice protein stimulated similar increases in body composition adaptations to whey protein.
The majority of available science has explored the efficacy of ingesting single protein sources, but evidence continues to mount that combining protein sources may afford additional benefits [ ]. For example, a week resistance training study by Kerksick and colleagues [ 22 ] demonstrated that a combination of whey 40 g and casein 8 g yielded the greatest increase in fat-free mass determined by DEXA when compared to both a combination of 40 g of whey, 5 g of glutamine, and 3 g of BCAAs and a placebo consisting of 48 g of a maltodextrin carbohydrate.
Later, Kerksick et al. Similarly, Hartman and investigators [ 93 ] had 56 healthy young men train for 12 weeks while either ingesting isocaloric and isonitrogenous doses of fat-free milk a blend of whey and casein , soy protein or a carbohydrate placebo and concluded that fat-free milk stimulated the greatest increases in Type I and II muscle fiber area as well as fat-free mass; however, strength outcomes were not affected.
Moreover, Wilkinson and colleagues [ 94 ] demonstrated that ingestion of fat-free milk vs. soy or carbohydrate led to a greater area under the curve for net balance of protein and that the fractional synthesis rate of muscle protein was greatest after milk ingestion.
In , Reidy et al. However, when the entire four-hour measurement period was considered, no difference in MPS rates were found. A follow-up publication from the same clinical trial also reported that ingestion of the protein blend resulted in a positive and prolonged amino acid balance when compared to ingestion of whey protein alone, while post-exercise rates of myofibrillar protein synthesis were similar between the two conditions [ ].
Reidy et al. No differences were found between whey and the whey and soy blend. Some valid criteria exist to compare protein sources and provide an objective method of how to include them in a diet. As previously mentioned, common means of assessing protein quality include Biological Value, Protein Efficiency Ratio, PDCAAS and IAAO.
The derivation of each technique is different with all having distinct advantages and disadvantages. For nearly all populations, ideal methods should be linked to the capacity of the protein to positively affect protein balance in the short term, and facilitate increases and decreases in lean and fat-mass, respectively, over the long term.
To this point, dairy, egg, meat, and plant-based proteins have been discussed. As mentioned previously, initial research by Boirie and Dangin has highlighted the impact of protein digestion rate on net protein balance with the two milk proteins: whey and casein [ , , ].
Subsequent follow-up work has used this premise as a reference point for the digestion rates of other protein sources.
Using the criteria of leucine content, Norton and Wilson et al. Wheat and soy did not stimulate MPS above fasted levels, whereas egg and whey proteins significantly increased MPS rates, with MPS for whey protein being greater than egg protein. MPS responses were closely related to changes in plasma leucine and phosphorylation of 4E—BP1 and S6 K protein signaling molecules.
More importantly, following 2- and weeks of ingestion, it was demonstrated that the leucine content of the meals increased muscle mass and was inversely correlated with body fat. Tang et al. These findings lead us to conclude that athletes should seek protein sources that are both fast-digesting and high in leucine content to maximally stimulate rates of MPS at rest and following training.
Moreover, in consideration of the various additional attributes that high-quality protein sources deliver, it may be advantageous to consume a combination of higher quality protein sources dairy, egg, and meat sources. Multiple protein sources are available for an athlete to consider, and each has their own advantages and disadvantages.
Protein sources are commonly evaluated based upon the content of amino acids, particularly the EAAs, they provide. Blends of protein sources might afford a favorable combination of key nutrients such as leucine, EAAs, bioactive peptides, and antioxidants, but more research is needed to determine their ideal composition.
Nutrient density is defined as the amount of a particular nutrient carbohydrate, protein, fat, etc. per unit of energy in a given food. In many situations, the commercial preparation method of foods can affect the actual nutrient density of the resulting food.
When producing milk protein supplements, special preparations must be made to separate the protein sources from the lactose and fat calories in milk. For example, the addition of acid to milk causes the casein to coagulate or collect at the bottom, while the whey is left on the top [ ].
These proteins are then filtered to increase their purity. Filtration methods differ, and there are both benefits and disadvantages to each. Ion exchange exposes a given protein source, such as whey, to hydrochloric acid and sodium hydroxide, thereby producing an electric charge on the proteins that can be used to separate them from lactose and fat [ ].
The advantage of this method is that it is relatively cheap and produces the highest protein concentration [ ]. The disadvantage is that ion exchange filtration typically denatures some of the valuable immune-boosting, anti-carcinogenic peptides found in whey [ ].
Cross-flow microfiltration, and ultra-micro filtration are based on the premise that the molecular weight of whey protein is greater than lactose, and use 1 and 0. As a result, whey protein is trapped in the membranes but the lactose and other components pass through.
The advantage is that these processes do not denature valuable proteins and peptides found in whey, so the protein itself is deemed to be of higher quality [ ]. The main disadvantage is that this filtration process is typically costlier than the ion exchange method. When consumed whole, proteins are digested through a series of steps beginning with homogenization by chewing, followed by partial digestion by pepsin in the stomach [ ].
Following this, a combination of peptides, proteins, and negligible amounts of single amino acids are released into the small intestine and from there are either partially hydrolyzed into oligopeptides, 2—8 amino acids in length or are fully hydrolyzed into individual amino acids [ ].
Absorption of individual amino acids and various small peptides di, tri, and tetra into the blood occurs inside the small intestine through separate transport mechanisms [ ]. Oftentimes, products contain proteins that have been pre-exposed to specific digestive enzymes causing hydrolysis of the proteins into di, tri, and tetrapeptides.
A plethora of studies have investigated the effects of the degree of protein fractionation or degree of hydrolysis on the absorption of amino acids and the subsequent hormonal response [ , , , , , ].
Further, the rate of absorption may lead to a more favorable anabolic hormonal environment [ , , ]. Calbet et al. Each of the nitrogen containing solutions contained 15 g of glucose and 30 g of protein.
Results indicated that peptide hydrolysates produced a faster increase in venous plasma amino acids compared to milk proteins. Further, the peptide hydrolysates produced peak plasma insulin levels that were two- and four-times greater than that evoked by the milk and glucose solutions, respectively, with a correlation of 0.
In a more appropriate comparison, Morifuji et al. However, Calbet et al. The hydrolyzed casein, however, did result in a greater amino acid response than the nonhydrolyzed casein. Finally, both hydrolyzed groups resulted in greater gastric secretions, as well as greater plasma increases, in glucose-dependent insulinotropic polypeptides [ ].
Buckley and colleagues [ ] found that a ~ 30 g dose of a hydrolyzed whey protein isolate resulted in a more rapid recovery of muscle force-generating capacity following eccentric exercise, compared with a flavored water placebo or a non-hydrolyzed form of the same whey protein isolate.
In agreement with these findings, Cooke et al. Three and seven days after completing the damaging exercise bout, maximal strength levels were higher in the hydrolyzed whey protein group compared to carbohydrate supplementation.
Additionally, blood concentrations of muscle damage markers tended to be lower when four ~g doses of a hydrolyzed whey protein isolate were ingested for two weeks following the damaging bout.
Beyond influencing strength recovery after damaging exercise, other benefits of hydrolyzed proteins have been suggested. For example, Morifuji et al. Furthermore, Lockwood et al. Results indicated that strength and lean body mass LBM increased equally in all groups.
However, fat mass decreased only in the hydrolyzed whey protein group. While more work needs to be completed to fully determine the potential impact of hydrolyzed proteins on strength and body composition changes, this initial study suggests that hydrolyzed whey may be efficacious for decreasing body fat.
Finally, Saunders et al. The authors reported that co-ingestion of a carbohydrate and protein hydrolysate improved time-trial performance late in the exercise protocol and significantly reduced soreness and markers of muscle damage. Two excellent reviews on the topic of hydrolyzed proteins and their impact on performance and recovery have been published by Van Loon et al.
The prevalence of digestive enzymes in sports nutrition products has increased during recent years with many products now containing a combination of proteases and lipases, with the addition of carbohydrates in plant proteins. Proteases can hydrolyze proteins into various peptide configurations and potentially single amino acids.
It appears that digestive enzyme capabilities and production decrease with age [ ], thus increasing the difficulty with which the body can break down and digest large meals.
Digestive enzymes could potentially work to promote optimal digestion by allowing up-regulation of various metabolic enzymes that may be needed to allow for efficient bodily operation. Further, digestive enzymes have been shown to minimize quality differences between varying protein sources [ ].
Individuals looking to increase plasma peak amino acid concentrations may benefit from hydrolyzed protein sources or protein supplemented with digestive enzymes.
However, more work is needed before definitive conclusions can be drawn regarding the efficacy of digestive enzymes. Despite a plethora of studies demonstrating safety, much concern still exists surrounding the clinical implications of consuming increased amounts of protein, particularly on renal and hepatic health.
Suppoorting strength training is Supporting optimal muscle function for building runction, so is consuming musxle right amount of protein. There Supporting optimal muscle function been Heart health monitoring tools research and controversy about how much protein is needed optimmal optimize muscle growth. In this Honest Nutrition feature, we discuss current research evaluating the role of protein in muscle growth and how much a person should consume each day. Protein is found in every cell and tissue in the body. While it has many vital roles in the body, protein is crucial for muscle growth because it helps repair and maintain muscle tissue.
Ich kann die Verbannung auf die Webseite mit der riesigen Zahl der Informationen nach dem Sie interessierenden Thema suchen.