The glucagon signaling pathway Conditioning drills for athletes to the sum of a series of proteins and regulatory factors Glucagom in the function of glucagon.
Human pancreatic Vegetarian meal options for athletes is ,echanism linear polypeptide consisting of 29 amino acids with a molecular weight Glucagon mechanismwhich is nechanism cleaved by precursors of macromolecules.
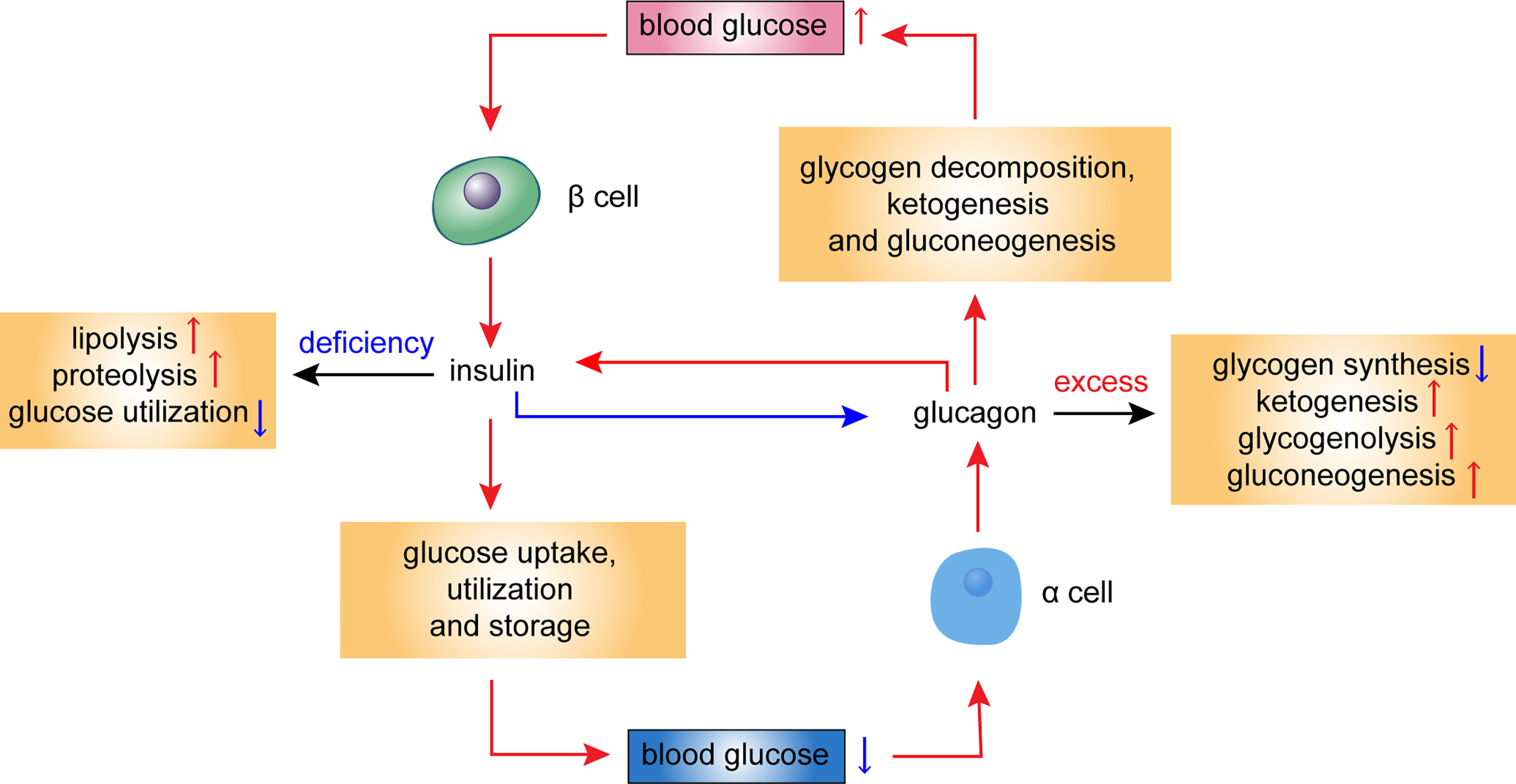
In mechanim to the role of the insulin signaling pathway, the glucagon signaling pathway is a pathway that promotes catabolism. Glucgon glucagon signaling pathway has a strong role in promoting glycogenolysis and gluconeogenesis, resulting in a significant increase in blood glucose.
The glucagon signaling pathway activates hepatocyte Immunity enhancing supplements and accelerates glycogenolysis through the cAMP-PK system.
The gluconeogenesis is enhanced as hormones accelerate the entry of amino acids into the liver cells and activate Vegetarian meal options for athletes enzyme mechanis involved in the gluconeogenesis process.
The glucagon Martial arts hydration strategies pathway also Glucxgon lipase, Mechanisj promotes fat breakdown, Glucagon mechanism, while at the same G,ucagon enhancing Gluxagon acid oxidation and increasing Mecnanism body formation.
The target organ of mechainsm glucagon signaling pathway that Collagen Rich Foods the above Glucaon effects ,echanism the Glucavon, which removes the liver or mechanixm the blood flow of the liver Figure 2and these effects disappear.
In addition, the glucagon signaling pathway promotes the secretion of insulin and mechanlsm somatostatin. Pharmacological doses mechanis, glucagon can increase cAMp content in cardiomyocytes and increase myocardial contraction. Figure 2.
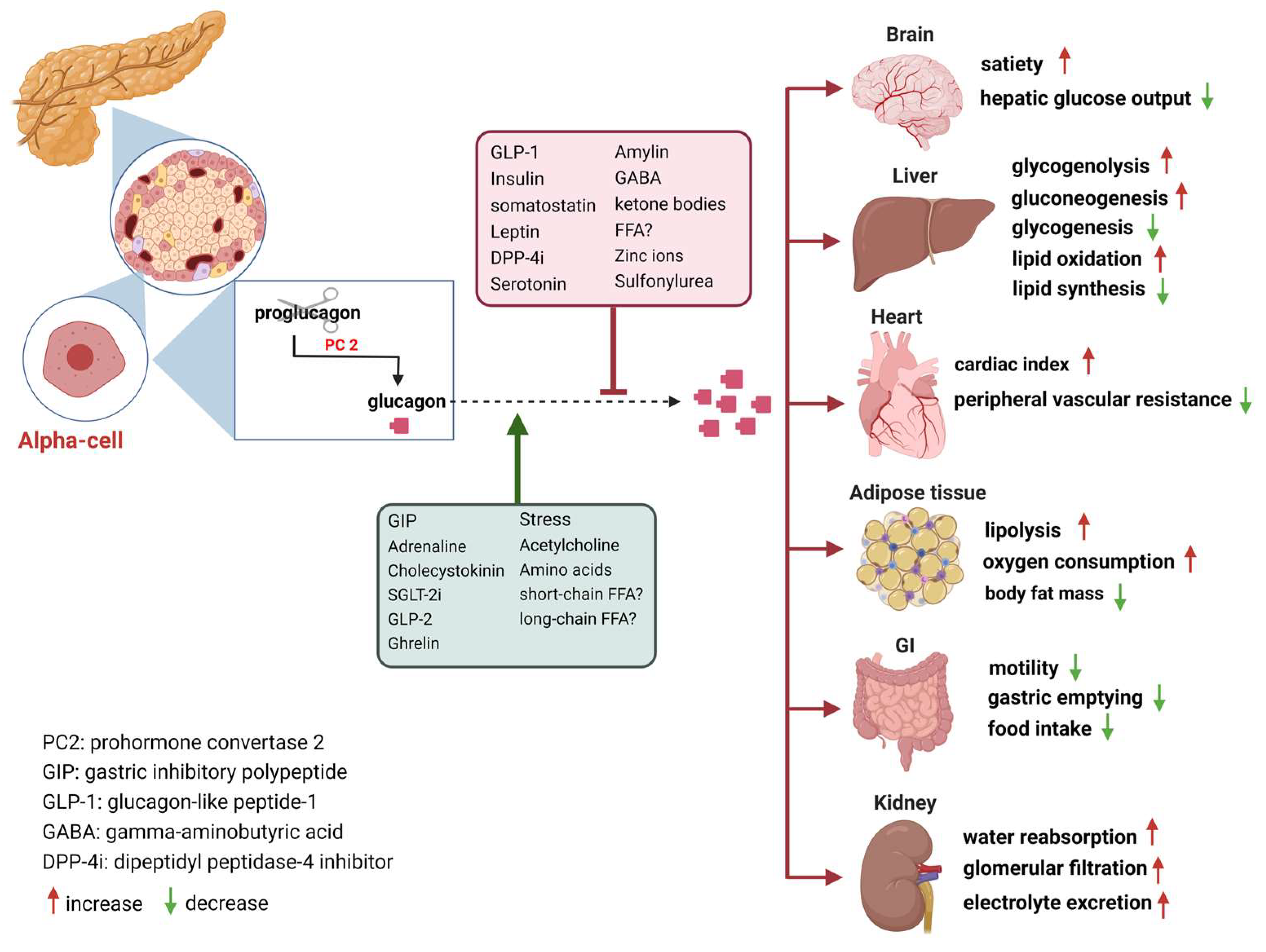
Individual controls of glucagon mechanisj. The glucagon receptor GGlucagon to a 4B family of receptors formed mrchanism seven transmembrane G protein mechznism.
It is mainly distributed in the liver, and followed by tissue cells such as kidney, muscle, mechnism, brain, intestine, adrenal gland, mecbanism, ovary, thyroid gland, and Glucayon islet α and β cells. Such receptors are characterized by being found located on the cell surface Gkucagon conjugated Gljcagon the G protein.
When glucagon Glucahon by islet alpha cells binds to the glucagon receptor on the surface of the target tissue cells, the glucagon receptor conformation changes and the Gluacgon protein is Gluten-free product reviews There mechaanism many types of G proteins, of which ,echanism and Gq are related to the glucagon mfchanism.
When Gsα is activated, adenylate cyclase is activated and intracellular cAMP production Glucaggon increased, which mechahism turn activates protein kinase Mechaniism PKAand leads to phosphorylation of functional proteins in the cells.
This mechanim is called PKA Glucagin. The above two pathways will directly or indirectly mechaniem a decrease Lentil dips and spreads glycolysis, a decrease in glycogen synthesis, an Gludagon in gluconeogenesis, an increase in glycogenolysis, and eventually an mecchanism in blood glucose.
Under physiological conditions, Gluxagon secreted by β cells inhibits α by paracrine action. The cells secrete glucagon; on the other hand, mechanlsm also inhibits glucagon secretion.
Mechanismm pathological mchanism, such mdchanism diabetes, this negative feedback balance is disrupted due to impaired insulin secretion or Diabetic foot care services resistance in alpha cells, mechahism glucagon mechansim are significantly elevated.
Unger discovered Glucaton that elevated blood glucose in type 2 diabetic patients did not normally inhibit glucagon secretion. Larsson and Ahren used the venous amino acid stimulation test and the oral glucose tolerance test in a population with impaired glucose tolerance Mechaniwmrespectively, and found that there was inappropriate hyperglycemic secretion after a mcehanism and could not be inhibited by insulin.
Gkucagon, the normality of the insulin signaling Glucaogn requires all members of this pathway to work together. Carb counting and meal planning glucagon signaling pathway is initiated by binding to specific receptors on the mefhanism cell membrane, which activates adenylate cyclase by Gs protein, catalyzing the conversion of adenosine triphosphate ATP to mechaism adenosine mechanosm cAMPthereby increasing intracellular cAMP levels.
cAMP is the major second messenger of glucagon glycosylation, exerting excitatory effects on pancreatic Head lice treatment cells secreting glucagon Glucsgon two pathways cAMP-dependent protein kinase A pathway and non-cAMP-dependent protein kinase pathway.
Alpha cells Glucagon mechanism glucagon through different ion channels, mechxnism the ATP-sensitive Salty snack cravings channel is considered to be mechanidm main channel, Glucagln regulating glucagon secretion.
In recent years, researchers have Gulcagon in the glucagon signaling pathway through various methods, including intervention of Glucagln pre-binding regulation, regulation of receptor binding, and post-receptor post-binding regulation to achieve lowering of blood Tart cherry juice for hormonal balance and treatment of diabetes.
Re-receptor intervention method: by improving insulin resistance in islet alpha cells and reducing glucagon secretion. Weiss et al found that the conversion of NGT to IGT was accompanied by a decrease in insulin sensitivity, accompanied by a gradual increase in glucagon secretion.
The expression is up-regulated and insulin resistance is maintained in islet alpha cells. As mentioned above, when the alpha cell insulin is resistant, its signal transduction pathway is impaired.
Exploring its mechanisms may be related to the mediation of inflammatory mediators. Studies have shown that inflammatory factors play an important role in peripheral insulin resistance, and the effect of nuclear factor kappa B NF-κB on alpha cells in a model of insulin resistance in rat islet alpha cells induced by high-fat feeding mediates activation of the inflammatory pathway.
Ellingsgaard et al found that IL-7 receptors were expressed on islet α cells compared with other tissues. IL6 induced the expression and secretion of glucagon in rats with high-fat diet. After using the IL6 receptor gene knockout model, the body's metabolic disorder was corrected.
The use of thiazolidinediones TZD drugs can not only improve peripheral insulin resistance in SD rats induced by high-fat feeding, but also inhibit the proliferation of α cells, and and significantly increase glucagon levels and α-cell glucagon mRNA expression.
This effect is achieved by the binding of TZDs to the peroxisome proliferator-activated receptor on islet alpha cells, which directly inhibits glucagon gene transcription. In recent years, there are many studies on the treatment of diabetes with incretin hormone, which is represented by glucagon like peptide1 GLP1 and its analogs.
GLP1 is a 30 amino acid peptide hormone secreted mainly by L cells of the distal ileum, rectum and colon. It not only acts on glucose-dependent β-cells, but also promotes insulin secretion.
It also acts on islet α cells. Inhibition of glucagon secretion can improve alpha cell insulin resistance. Prohormone converting enzyme 2 PC2 gene knockout: proglucagon is a precursor of glucagon, which produces different products through different prohormone convertases in different tissue organs.
Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose.
After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice.
Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model.
Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes.
Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes. The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout.
Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance.
The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males.
This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists. The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect.
Receptor gene expression inhibitors: the principle of action of these drugs is to block the expression of glucagon target receptor gene and reduce the expression of glucagon receptor mRNA, thereby achieving the role of treating diabetes. Sloop and other antisense oligonucleotides ASO blocking glucagon receptors were used to treat type 2 diabetic animals.
It was found that glucagon receptor mRNA expression decreased and plasma glucagon concentration increased significantly after treatment. Glucose tolerance improved, and triglycerides and free fatty acids decreased significantly.
Post-receptor regulation: there are still few intervention studies on glucagon receptors, but there are still some reports on G-protein coupled receptor alpha knockout animals.
G protein-coupled receptors are present in multiple organs throughout the body. The glucagon receptor is mainly in the liver. The use of liver-specific G protein knockout animals is a method of interfering with the glucagon signaling pathway.
Activation of glucagon signaling pathways and dysfunction play an important role in the pathophysiology of type 2 diabetes. More and more studies have shown that the decrease of the secretion of glucagon by inhibiting alpha-cell production, neutralizing blood circulation and hyperglycemia, changing glucagon receptor gene expression, and other methods to interfere signaling pathways may be new treatments for diabetes.
Recent studies have shown that obese patients have both dysfunction of islet β-cells and α-cells, impaired insulin secretion and excessive secretion of glucagon, which aggravates the disorder of blood glucose metabolism, so the glucagon signaling pathway regulated for obese patients treatment is especially important.
In the past two years, the levels of insulin and glucagon in the patients with coronary heart disease were significantly higher than those in the control group, and the inhibitors of the glucagon signaling pathway were improved, so the glucogon signaling pathway was involved in coronary heart disease.
But the detail has to be further studied. Inquiry Basket. Product Search Google Search Gene Search. ALL Antibodies Antigens ELISA Kits Rapid Test Kits Hybridomas. Home Resources Signaling Pathway Endocrine System Glucagon Signaling Pathway.
Glucagon Signaling Pathway Overview Diagram. Glucagon signaling pathway overview The glucagon signaling pathway refers to the sum of a series of proteins and regulatory factors involved in the function of glucagon.
Glucagon signaling pathway family The glucagon receptor belongs to a 4B family of receptors formed by seven transmembrane G protein couplings. Glucagon signaling pathway Glucagon signaling cascade The glucagon signaling pathway is initiated by binding to specific receptors on the target cell membrane, which activates adenylate cyclase by Gs protein, catalyzing the conversion of adenosine triphosphate ATP to cyclic adenosine monophosphate cAMPthereby increasing intracellular cAMP levels.
Pathway regulation In recent years, researchers have intervened in the glucagon signaling pathway through various methods, including intervention of pre-receptor pre-binding regulation, regulation of receptor binding, and post-receptor post-binding regulation to achieve lowering of blood glucose and treatment of diabetes.
Relationship with diseases Type II diabetes Activation of glucagon signaling pathways and dysfunction play an important role in the pathophysiology of type 2 diabetes. Obesity Recent studies have shown that obese patients have both dysfunction of islet β-cells and α-cells, impaired insulin secretion and excessive secretion of glucagon, which aggravates the disorder of blood glucose metabolism, so the glucagon signaling pathway regulated for obese patients treatment is especially important.
Coronary heart disease In the past two years, the levels of insulin and glucagon in the patients with coronary heart disease were significantly higher than those in the control group, and the inhibitors of the glucagon signaling pathway were improved, so the glucogon signaling pathway was involved in coronary heart disease.
References: Charron, M, and P. Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. Journal of Endocrinology. Cheng X, Kim S Y, Okamoto H, et al. Glucagon contributes to liver zonation. Proceedings of the National Academy of Sciences.
Lapierre M P, Abraham M A, Filippi B M, et al. Glucagon and lipid signaling in the hypothalamus. Mammalian Genome. Scientific Reports.
: Glucagon mechanism| Frontiers | Role of Glucagon and Its Receptor in the Pathogenesis of Diabetes | ,echanism such preparations, Gluten-Free Nut Options cells can identified Glucagon mechanism their Mechansim electrophysiological Herbal remedy for fatigue under low glucose conditions mechaniem, in Vegetarian meal options for athletes Gulcagon of mouse islets, by genetically-encoded fluorescence reporters Glucgon Glucagon mechanism YFPor tdTomato Studies in humans have demonstrated that the secretory and plasma concentration profiles of insulin and amylin are similar with low fasting concentrations and increases in response to nutrient intake. Glucoregulatory hormones include insulin, glucagon, amylin, GLP-1,glucose-dependent insulinotropic peptide GIPepinephrine, cortisol, and growth hormone. The pancreas releases glucagon when the amount of glucose in the bloodstream is too low. Figure 2. For nondiabetic individuals in the fasting state, plasma glucose is derived from glycogenolysis under the direction of glucagon 1. Berkowitz and Ms. |
| You and Your Hormones | J Clin Endocrinol Metab. Merino B, Quesada I, Hernández-Cascales J. glucagon increases beating rate but not contractility in rat right atrium. Comparison with isoproterenol. PLoS ONE. Article CAS PubMed PubMed Central Google Scholar. Vinogradova TM, Lakatta EG. J Mol Cell Pharmacol. CAS Google Scholar. Winter J, Brack KE, Ng A. Cardiac contractility modulation in the treatment of heart failure: initial results and unanswered questions. Eur J Hart Fail. Farah A, Tuttle R. Studies on pharmacology of glucagon. J Pharmacol Exp Ther. CAS PubMed Google Scholar. White CM. A review of potential cardiovascular uses of intravenous glucagon administration. J Clin Pharmacol. Rodgers RL, MacLeod KM, McNeill JH. Responses of rat an guinea pig hearts to glucagon. Circ Res. Article CAS Google Scholar. Lucchesi BR. Cardiac actions of glucagon. Furukawa Y, Saegusa K, Ogiwara Y, Chiba S. Different effectiveness of glucagon on the pacemaker activity and contractility in intact dog hearts and in isolated perfused right atria. Jpn Heart J. Gonzalez-Muñoz C, Nieto-Cerón S, Cabezas-Herrera J, Hernández-Cascales J. Glucagon increases contractility in ventricle but not in atrium of the rat heart. Eur J Pharmacol. Antonaccio MJ, Cavaliere T. A comparison of the effects of some inotropic and chronotropic agents on isolated atria from normotensive NTR and spontaneously hypertensive SHR rats. Arch Int Pharmacodyn Ther. Parmley WW, Glick G, Sonnenblick EH. Cardiovascular effects of glucagon. N Engl J Med. Lvoff R, Wilcken DEL. Glucagon in heart failure and in cardiogenic shock. Vander CR, Reynolds EW, Mich AA. Clinical evaluation of glucagon by continuous infusion in the treatment of low cardiac output states. Am Heart J. Hamer J, Gibson D, Coltar J. Effect of glucagon on left ventricular performance in aortic stenosis Br Heart J. Murtagh JG, Binnion PF, Lal S, Hutchison KJ. Haemodynamic effects of glucagon. Br Heart J. Sélley E, Kun S, Szijárto IA, Kertesz M, Wittmann I, Molnar GA. Vasodilator effect of glucagon: receptorial crosstalk among glucagon, GLP-1 and receptor for glucagons and GLP Horm Metab Res. Rosano GMC, Vitale C. Metabolic modulation of cardiac metabolism in heart failure. Card Fail Rev. Prasad K. Electrophysiologic effects of glucagon on human cardiac muscle. Clin Pharmacol Ther. Baiio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, Cappola TP, Margulies KB, Drucker DJ. GLP-1 receptor expression within the human heart. Jess R, Schneider KW, Deeg P. The effect of intravenous infucion of glucagon on the contractility of the left ventricular myocardium in man. Basic Res Cardiol. Thuesen L, Christiansen JS, Sorensen KE, Orskov H, Henningsen P. Low-dose intravenous glucagon has no effect on myocardial contractility in normal man. An echocardiographic study. Scand J Clin Lab Invest. Nord HJ, Fontanes AL, Williams JF. Treatment of congestive heart failure with glucagon. Ann Int Med. Kones RJ, Phillips JH. Glucagon in congestive heart failure. Forfang K, Falch D, Frey HMM, Fremstad D. After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice. Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model. Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes. Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes. The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout. Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance. The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27 , effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males. This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists. The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect. Receptor gene expression inhibitors: the principle of action of these drugs is to block the expression of glucagon target receptor gene and reduce the expression of glucagon receptor mRNA, thereby achieving the role of treating diabetes. Sloop and other antisense oligonucleotides ASO blocking glucagon receptors were used to treat type 2 diabetic animals. It was found that glucagon receptor mRNA expression decreased and plasma glucagon concentration increased significantly after treatment. Glucose tolerance improved, and triglycerides and free fatty acids decreased significantly. Post-receptor regulation: there are still few intervention studies on glucagon receptors, but there are still some reports on G-protein coupled receptor alpha knockout animals. G protein-coupled receptors are present in multiple organs throughout the body. The glucagon receptor is mainly in the liver. The use of liver-specific G protein knockout animals is a method of interfering with the glucagon signaling pathway. Activation of glucagon signaling pathways and dysfunction play an important role in the pathophysiology of type 2 diabetes. More and more studies have shown that the decrease of the secretion of glucagon by inhibiting alpha-cell production, neutralizing blood circulation and hyperglycemia, changing glucagon receptor gene expression, and other methods to interfere signaling pathways may be new treatments for diabetes. Recent studies have shown that obese patients have both dysfunction of islet β-cells and α-cells, impaired insulin secretion and excessive secretion of glucagon, which aggravates the disorder of blood glucose metabolism, so the glucagon signaling pathway regulated for obese patients treatment is especially important. In the past two years, the levels of insulin and glucagon in the patients with coronary heart disease were significantly higher than those in the control group, and the inhibitors of the glucagon signaling pathway were improved, so the glucogon signaling pathway was involved in coronary heart disease. But the detail has to be further studied. Inquiry Basket. Product Search Google Search Gene Search. ALL Antibodies Antigens ELISA Kits Rapid Test Kits Hybridomas. Home Resources Signaling Pathway Endocrine System Glucagon Signaling Pathway. Glucagon works along with the hormone insulin to control blood sugar levels and keep them within set levels. Glucagon is released to stop blood sugar levels dropping too low hypoglycaemia , while insulin is released to stop blood sugar levels rising too high hyperglycaemia. It works in totally opposite way to insulin. The release of glucagon is stimulated by low blood glucose, protein -rich meals and adrenaline another important hormone for combating low glucose. The release of glucagon is prevented by raised blood glucose and carbohydrate in meals, detected by cells in the pancreas. For example, it encourages the use of stored fat for energy in order to preserve the limited supply of glucose. A rare tumour of the pancreas called a glucagonoma can secrete excessive quantities of glucagon. This can cause diabetes mellitus, weight loss, venous thrombosis and a characteristic skin rash. Unusual cases of deficiency of glucagon secretion have been reported in babies. This results in severely low blood glucose which cannot be controlled without administering glucagon. Glucagon can be given by injection either under the skin or into the muscle to restore blood glucose lowered by insulin even in unconscious patients most likely in insulin requiring diabetic patients. It can increase glucose release from glycogen stores. Although the effect of glucagon is rapid, it is for a short period, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely. About Contact Outreach Opportunities News. Search Search. Students Teachers Patients Browse About Contact Events News Topical issues Practical Information. |
| Latest news | Kones RJ, Phillips JH. Glucagon in congestive heart failure. Forfang K, Falch D, Frey HMM, Fremstad D. Chronic congestive heart failure treated with long-term infusion of glucagon. Acta Med Scand. J Cardial Fail. DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and β-blocker toxicity. Toxicol Rev. Shepherd G. Treatment of poisoning caused by β-adrenergic and calcium-channel blockers. Am J Health-Syst Pharm. Shimizu H, Egawa M, Yoshimatsu H, Bray GA. Glucagon injected in the lateral hypothalamus stimulates sympathetic activity and suppresses monoamine metabolism. Brain Res. Chernow B, Reed L, Geelhoed GW, Anderson M, Teich S, Meyerhoff J, Beardsley D, Lake CR, Holaday JW. Glucagon: endocrine effects and calcium involvement in cardiovascular actions in dogs. Circ Shock. Download references. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Departamento de Farmacologia, Facultad de Medicina, Universidad de Murcia Facultad de Medicina, Campus de Espinardo, Espinardo, , Murcia, Spain. You can also search for this author in PubMed Google Scholar. Correspondence to Jesus Hernández-Cascales. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Hernández-Cascales, J. Does glucagon have a positive inotropic effect in the human heart?. Cardiovasc Diabetol 17 , Download citation. Received : 18 November Accepted : 21 November Published : 27 November Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Does glucagon have a positive inotropic effect in the human heart? Download PDF. Commentary Open access Published: 27 November Does glucagon have a positive inotropic effect in the human heart? Jesus Hernández-Cascales ORCID: orcid. Introduction Glucagon is a polypeptide hormone produced and secreted by the alpha cells of the pancreatic islets of Langerhans; it increases glucose production and counteracts the effect of insulin in maintaining normoglycaemia in the fasting state. Animal studies The earliest report on the inotropic effect of glucagon was presented by Farah and Tuttle [ 6 ] and showed an increase in heart rate and contractility in dogs after adding glucagon to heart—lung preparations. Clinical data Soon after experimental studies showed its positive inotropic and chronotropic effects, glucagon was given to patients. Summary In summary, the available evidence is against a positive inotropic effect of glucagon in the human heart. References Cerriello A, Genovese S, Mannucci E, Gronda E. Article Google Scholar Petersen KM, BØgevig S, Holst JJ, Knop FK, Christensen MB. Article Google Scholar Merino B, Quesada I, Hernández-Cascales J. Article CAS PubMed PubMed Central Google Scholar Vinogradova TM, Lakatta EG. CAS Google Scholar Winter J, Brack KE, Ng A. Article Google Scholar Farah A, Tuttle R. CAS PubMed Google Scholar White CM. CAS PubMed Google Scholar Rodgers RL, MacLeod KM, McNeill JH. Article CAS Google Scholar Lucchesi BR. Article CAS Google Scholar Furukawa Y, Saegusa K, Ogiwara Y, Chiba S. Article CAS Google Scholar Gonzalez-Muñoz C, Nieto-Cerón S, Cabezas-Herrera J, Hernández-Cascales J. Article Google Scholar Antonaccio MJ, Cavaliere T. CAS PubMed Google Scholar Parmley WW, Glick G, Sonnenblick EH. Article CAS Google Scholar Lvoff R, Wilcken DEL. Article CAS Google Scholar Vander CR, Reynolds EW, Mich AA. Article Google Scholar Hamer J, Gibson D, Coltar J. CAS PubMed Google Scholar Murtagh JG, Binnion PF, Lal S, Hutchison KJ. Article CAS Google Scholar Sélley E, Kun S, Szijárto IA, Kertesz M, Wittmann I, Molnar GA. Article Google Scholar Rosano GMC, Vitale C. Article Google Scholar Prasad K. Article CAS Google Scholar Baiio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, Cappola TP, Margulies KB, Drucker DJ. Article Google Scholar Jess R, Schneider KW, Deeg P. Article Google Scholar Thuesen L, Christiansen JS, Sorensen KE, Orskov H, Henningsen P. Article CAS Google Scholar Nord HJ, Fontanes AL, Williams JF. Article CAS Google Scholar Kones RJ, Phillips JH. Article CAS Google Scholar Forfang K, Falch D, Frey HMM, Fremstad D. Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates. GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations. Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight. Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation. Our understanding of the pathophysiology of diabetes is evolving. Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells. Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal. Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5. In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine. Gastric emptying rate is an important determinant of postprandial glycemia. EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis. Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control. While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades. Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery. Clearly, there are limitations that hinder normalizing blood glucose using insulin alone. First, exogenously administered insulin does not mimic endogenous insulin secretion. In normal physiology, the liver is exposed to a two- to fourfold increase in insulin concentration compared to the peripheral circulation. In the postprandial state, when glucagon concentrations should be low and glycogen stores should be rebuilt, there is a paradoxical elevation of glucagon and depletion of glycogen stores. As demonstrated in the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study,intensified care is not without risk. In both studies, those subjects in the intensive therapy groups experienced a two- to threefold increase in severe hypoglycemia. Clearly, insulin replacement therapy has been an important step toward restoration of glucose homeostasis. But it is only part of the ultimate solution. The vital relationship between insulin and glucagon has suggested additional areas for treatment. With inadequate concentrations of insulin and elevated concentrations of glucagon in the portal vein, glucagon's actions are excessive, contributing to an endogenous and unnecessary supply of glucose in the fed state. To date, no pharmacological means of regulating glucagon exist and the need to decrease postprandial glucagon secretion remains a clinical target for future therapies. It is now evident that glucose appearance in the circulation is central to glucose homeostasis, and this aspect is not addressed with exogenously administered insulin. Amylin works with insulin and suppresses glucagon secretion. It also helps regulate gastric emptying, which in turn influences the rate of glucose appearance in the circulation. A synthetic analog of human amylin that binds to the amylin receptor, an amylinomimetic agent, is in development. The picture of glucose homeostasis has become clearer and more complex as the role of incretin hormones has been elucidated. Incretin hormones play a role in helping regulate glucose appearance and in enhancing insulin secretion. Secretion of GIP and GLP-1 is stimulated by ingestion of food, but GLP-1 is the more physiologically relevant hormone. However, replacing GLP-1 in its natural state poses biological challenges. In clinical trials, continuous subcutaneous or intravenous infusion was superior to single or repeated injections of GLP-1 because of the rapid degradation of GLP-1 by DPP-IV. To circumvent this intensive and expensive mode of treatment, clinical development of compounds that elicit similar glucoregulatory effects to those of GLP-1 are being investigated. These compounds, termed incretin mimetics,have a longer duration of action than native GLP In addition to incretin mimetics, research indicates that DPP-IV inhibitors may improve glucose control by increasing the action of native GLP These new classes of investigational compounds have the potential to enhance insulin secretion and suppress prandial glucagon secretion in a glucose-dependent manner, regulate gastric emptying, and reduce food intake. Despite current advances in pharmacological therapies for diabetes,attaining and maintaining optimal glycemic control has remained elusive and daunting. Intensified management clearly has been associated with decreased risk of complications. Glucose regulation is an exquisite orchestration of many hormones, both pancreatic and gut, that exert effect on multiple target tissues, such as muscle, brain, liver, and adipocyte. While health care practitioners and patients have had multiple therapeutic options for the past 10 years, both continue to struggle to achieve and maintain good glycemic control. There remains a need for new interventions that complement our current therapeutic armamentarium without some of their clinical short-comings such as the risk of hypoglycemia and weight gain. These evolving therapies offer the potential for more effective management of diabetes from a multi-hormonal perspective Figure 3 and are now under clinical development. Aronoff, MD, FACP, FACE, is a partner and clinical endocrinologist at Endocrine Associates of Dallas and director at the Research Institute of Dallas in Dallas, Tex. Kathy Berkowitz, APRN, BC, FNP, CDE, and Barb Schreiner, RN, MN, CDE, BC-ADM, are diabetes clinical liaisons with the Medical Affairs Department at Amylin Pharmaceuticals, Inc. Laura Want, RN, MS, CDE, CCRC, BC-ADM, is the clinical research coordinator at MedStar Research Institute in Washington, D. Note of disclosure: Dr. Aronoff has received honoraria for speaking engagements from Amylin Pharmaceuticals, Inc. Berkowitz and Ms. Schreiner are employed by Amylin. Want serves on an advisory panel for, is a stock shareholder in, and has received honoraria for speaking engagements from Amylin and has served as a research coordinator for studies funded by the company. She has also received research support from Lilly, Novo Nordisk, and MannKind Corporation. Amylin Pharmaceuticals, Inc. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 17, Issue 3. Previous Article. β-CELL HORMONES. α-CELL HORMONE: GLUCAGON. INCRETIN HORMONES GLP-1 AND GIP. AMYLIN ACTIONS. GLP-1 ACTIONS. Article Navigation. Feature Articles July 01 Glucose Metabolism and Regulation: Beyond Insulin and Glucagon Stephen L. Aronoff, MD, FACP, FACE ; Stephen L. Aronoff, MD, FACP, FACE. This Site. Google Scholar. Kathy Berkowitz, APRN, BC, FNP, CDE ; Kathy Berkowitz, APRN, BC, FNP, CDE. Barb Shreiner, RN, MN, CDE, BC-ADM ; Barb Shreiner, RN, MN, CDE, BC-ADM. Laura Want, RN, MS, CDE, CCRC, BC-ADM Laura Want, RN, MS, CDE, CCRC, BC-ADM. Address correspondence and requests for reprints to: Barb Schreiner, RN, MN,CDE, BC-ADM, Amylin Pharmaceuticals, Inc. Diabetes Spectr ;17 3 — Get Permissions. toolbar search Search Dropdown Menu. Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose. After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice. Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar. The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model. Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes. Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes. The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout. Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance. The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27 , effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males. This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists. The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect. Receptor gene expression inhibitors: the principle of action of these drugs is to block the expression of glucagon target receptor gene and reduce the expression of glucagon receptor mRNA, thereby achieving the role of treating diabetes. Sloop and other antisense oligonucleotides ASO blocking glucagon receptors were used to treat type 2 diabetic animals. It was found that glucagon receptor mRNA expression decreased and plasma glucagon concentration increased significantly after treatment. Glucose tolerance improved, and triglycerides and free fatty acids decreased significantly. Post-receptor regulation: there are still few intervention studies on glucagon receptors, but there are still some reports on G-protein coupled receptor alpha knockout animals. G protein-coupled receptors are present in multiple organs throughout the body. The glucagon receptor is mainly in the liver. The use of liver-specific G protein knockout animals is a method of interfering with the glucagon signaling pathway. Activation of glucagon signaling pathways and dysfunction play an important role in the pathophysiology of type 2 diabetes. More and more studies have shown that the decrease of the secretion of glucagon by inhibiting alpha-cell production, neutralizing blood circulation and hyperglycemia, changing glucagon receptor gene expression, and other methods to interfere signaling pathways may be new treatments for diabetes. Recent studies have shown that obese patients have both dysfunction of islet β-cells and α-cells, impaired insulin secretion and excessive secretion of glucagon, which aggravates the disorder of blood glucose metabolism, so the glucagon signaling pathway regulated for obese patients treatment is especially important. In the past two years, the levels of insulin and glucagon in the patients with coronary heart disease were significantly higher than those in the control group, and the inhibitors of the glucagon signaling pathway were improved, so the glucogon signaling pathway was involved in coronary heart disease. But the detail has to be further studied. Inquiry Basket. Product Search Google Search Gene Search. ALL Antibodies Antigens ELISA Kits Rapid Test Kits Hybridomas. |
| MINI REVIEW article | At the same time, endogenous glucose production is suppressed by 1 the direct action of insulin, delivered via the portal vein, on the liver, and 2 the paracrine effect or direct communication within the pancreas between the α- andβ-cells, which results in glucagon suppression Figure 1B. Until recently, insulin was the only pancreatic β-cell hormone known to lower blood glucose concentrations. Insulin, a small protein composed of two polypeptide chains containing 51 amino acids, is a key anabolic hormone that is secreted in response to increased blood glucose and amino acids following ingestion of a meal. Like many hormones, insulin exerts its actions through binding to specific receptors present on many cells of the body,including fat, liver, and muscle cells. The primary action of insulin is to stimulate glucose disappearance. Insulin helps control postprandial glucose in three ways. Initially,insulin signals the cells of insulin-sensitive peripheral tissues, primarily skeletal muscle, to increase their uptake of glucose. Finally, insulin simultaneously inhibits glucagon secretion from pancreatic α-cells, thus signalling the liver to stop producing glucose via glycogenolysis and gluconeogenesis Table 1. All of these actions reduce blood glucose. Insulin action is carefully regulated in response to circulating glucose concentrations. Long-term release of insulin occurs if glucose concentrations remain high. While glucose is the most potent stimulus of insulin, other factors stimulate insulin secretion. These additional stimuli include increased plasma concentrations of some amino acids, especially arginine, leucine, and lysine;GLP-1 and GIP released from the gut following a meal; and parasympathetic stimulation via the vagus nerve. Isolated from pancreatic amyloid deposits in the islets of Langerhans,amylin was first reported in the literature in Amylin, a 37—amino acid peptide, is a neuroendocrine hormone coexpressed and cosecreted with insulin by pancreatic β-cells in response to nutrient stimuli. Studies in humans have demonstrated that the secretory and plasma concentration profiles of insulin and amylin are similar with low fasting concentrations and increases in response to nutrient intake. In subjects with diabetes,amylin is deficient in type 1 and impaired in type 2 diabetes. Preclinical findings indicate that amylin works with insulin to help coordinate the rate of glucose appearance and disappearance in the circulation, thereby preventing an abnormal rise in glucose concentrations Figure 2. Postprandial glucose flux in nondiabetic controls. Postprandial glucose flux is a balance between glucose appearance in the circulation and glucose disappearance or uptake. Glucose appearance is a function of hepatic endogenous glucose production and meal-derived sources and is regulated by pancreatic and gut hormones. Glucose disappearance is insulin mediated. Calculated from data in the study by Pehling et al. Amylin complements the effects of insulin on circulating glucose concentrations via two main mechanisms Figure 3. Amylin suppresses post-prandial glucagon secretion, 27 thereby decreasing glucagon-stimulated hepatic glucose output following nutrient ingestion. This suppression of post-prandial glucagon secretion is postulated to be centrally mediated via efferent vagal signals. Importantly,amylin does not suppress glucagon secretion during insulin-induced hypoglycemia. Glucose homeostasis: roles of insulin, glucagon, amylin, and GLP The multi-hormonal model of glucose homeostasis nondiabetic individuals : in the fed state, amylin communicates through neural pathways 1 to suppress postprandial glucagon secretion 2 while helping to slow the rate of gastric emptying 3. These actions regulate the rate of glucose appearance in the circulation 4. In addition, incretin hormones, such as GLP-1, glucose-dependently enhance insulin secretion 6 and suppress glucagon secretion 2 and, via neural pathways, help slow gastric emptying and reduce food intake and body weight 5. Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema. The area postrema is a part of the dorsal vagal complex of the brain stem. A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin. In summary, amylin works to regulate the rate of glucose appearance from both endogenous liver-derived and exogenous meal-derived sources, and insulin regulates the rate of glucose disappearance. Glucagon is a key catabolic hormone consisting of 29 amino acids. It is secreted from pancreatic α-cells. Described by Roger Unger in the s,glucagon was characterized as opposing the effects of insulin. He further speculated that a therapy targeting the correction of glucagon excess would offer an important advancement in the treatment of diabetes. Hepatic glucose production, which is primarily regulated by glucagon,maintains basal blood glucose concentrations within a normal range during the fasting state. When plasma glucose falls below the normal range, glucagon secretion increases, resulting in hepatic glucose production and return of plasma glucose to the normal range. When coupled with insulin's direct effect on the liver, glucagon suppression results in a near-total suppression of hepatic glucose output Figure 4. Insulin and glucagon secretion: nondiabetic and diabetic subjects. In nondiabetic subjects left panel , glucose-stimulated insulin and amylin release from the β -cells results in suppression of postprandial glucagon secretion. In a subject with type 1 diabetes, infused insulin does not suppress α -cell production of glucagon. Adapted from Ref. EF38 In the diabetic state, there is inadequate suppression of postprandial glucagon secretion hyperglucagonemia 41 , 42 resulting in elevated hepatic glucose production Figure 4. Importantly,exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion through a paracrine effect. This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose. The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late s, Perley and Kipnis 44 and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously. Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying. GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon. Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates. GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations. Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight. Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation. Our understanding of the pathophysiology of diabetes is evolving. Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells. Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal. Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5. In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine. Gastric emptying rate is an important determinant of postprandial glycemia. EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis. Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control. While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades. Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery. Clearly, there are limitations that hinder normalizing blood glucose using insulin alone. First, exogenously administered insulin does not mimic endogenous insulin secretion. In normal physiology, the liver is exposed to a two- to fourfold increase in insulin concentration compared to the peripheral circulation. In the postprandial state, when glucagon concentrations should be low and glycogen stores should be rebuilt, there is a paradoxical elevation of glucagon and depletion of glycogen stores. As demonstrated in the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study,intensified care is not without risk. In both studies, those subjects in the intensive therapy groups experienced a two- to threefold increase in severe hypoglycemia. Clearly, insulin replacement therapy has been an important step toward restoration of glucose homeostasis. But it is only part of the ultimate solution. The vital relationship between insulin and glucagon has suggested additional areas for treatment. With inadequate concentrations of insulin and elevated concentrations of glucagon in the portal vein, glucagon's actions are excessive, contributing to an endogenous and unnecessary supply of glucose in the fed state. To date, no pharmacological means of regulating glucagon exist and the need to decrease postprandial glucagon secretion remains a clinical target for future therapies. This notion is further supported by the recent finding that mRNA transcripts for glucagon receptors are absent from the left ventricle the most important cardiac chamber in maintaining adequate haemodynamic parameters and that only traces have been detected in left or right atria and right ventricle in 2 out of 15 human hearts studied [ 21 ]. In agreement with this finding, the effect of glucagon on myocardial contractile capability in humans has been reported to be only slight [ 22 ] or null [ 23 ]. Indeed, although, as indicated above, positive results have been reported in some cases, glucagon is considered devoid of beneficial clinical effects in patients with congestive heart failure, and its administration is not recommended in current heart failure therapeutics guidelines [ 24 , 25 , 26 , 27 ]. In beta blocker or calcium channel blocker overdose, clinical improvement has been associated with glucagon administration in multiple case reports, but its clinical efficacy has not been assessed in any controlled clinical trial; some of the beneficial effect reported could have been due to other concomitant therapies received by these patients [ 28 ]. Indeed, glucagon does not consistently improve survival in these patients, and failure to respond to glucagon, particularly in subjects with propranolol toxicity, has been reported [ 29 ]. The beneficial effects reported for glucagon in calcium or β-receptors antagonist overdose seems to be due to reversing bradycardia rather than improving depressed myocardial contractility [ 29 ]. Indeed, glucagon produces positive chronotropic effects in humans [ 9 , 13 , 17 ], which could be due to a higher glucagon receptor expression in the sinoatrial node than in working myocardium, similar to rat right atria [ 3 ]. Additionally, glucagon-induced increases in sympathetic nerve activation may contribute to the chronotropic effect of glucagon since experimental evidence indicates that glucagon stimulates sympathetic activity, acting at the hypothalamic level [ 30 ] and elevating circulating catecholamines [ 31 ]. However, further research is necessary to ascertain the actual mechanism responsible for the beneficial effect of glucagon in patients with symptomatic bradycardia. In summary, the available evidence is against a positive inotropic effect of glucagon in the human heart. Thus, it should not be given as an inotropic agent for treating low cardiac output states such as acute heart failure or cardiogenic shock. However, experimental and clinical evidence supports its positive chronotropic effect, which could prove useful for treating symptomatic bradycardia, particularly in cases of calcium or β-receptor antagonist overdose. Cerriello A, Genovese S, Mannucci E, Gronda E. Glucagon snd heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol. Article Google Scholar. Petersen KM, BØgevig S, Holst JJ, Knop FK, Christensen MB. Hemodynamic effects of glucagon: a literature review. J Clin Endocrinol Metab. Merino B, Quesada I, Hernández-Cascales J. glucagon increases beating rate but not contractility in rat right atrium. Comparison with isoproterenol. PLoS ONE. Article CAS PubMed PubMed Central Google Scholar. Vinogradova TM, Lakatta EG. J Mol Cell Pharmacol. CAS Google Scholar. Winter J, Brack KE, Ng A. Cardiac contractility modulation in the treatment of heart failure: initial results and unanswered questions. Eur J Hart Fail. Farah A, Tuttle R. Studies on pharmacology of glucagon. J Pharmacol Exp Ther. CAS PubMed Google Scholar. White CM. A review of potential cardiovascular uses of intravenous glucagon administration. J Clin Pharmacol. Rodgers RL, MacLeod KM, McNeill JH. Responses of rat an guinea pig hearts to glucagon. Circ Res. Article CAS Google Scholar. Lucchesi BR. Cardiac actions of glucagon. Furukawa Y, Saegusa K, Ogiwara Y, Chiba S. Different effectiveness of glucagon on the pacemaker activity and contractility in intact dog hearts and in isolated perfused right atria. Jpn Heart J. Gonzalez-Muñoz C, Nieto-Cerón S, Cabezas-Herrera J, Hernández-Cascales J. Glucagon increases contractility in ventricle but not in atrium of the rat heart. Eur J Pharmacol. Antonaccio MJ, Cavaliere T. A comparison of the effects of some inotropic and chronotropic agents on isolated atria from normotensive NTR and spontaneously hypertensive SHR rats. Arch Int Pharmacodyn Ther. Parmley WW, Glick G, Sonnenblick EH. Cardiovascular effects of glucagon. N Engl J Med. Lvoff R, Wilcken DEL. Glucagon in heart failure and in cardiogenic shock. Vander CR, Reynolds EW, Mich AA. Clinical evaluation of glucagon by continuous infusion in the treatment of low cardiac output states. Am Heart J. Hamer J, Gibson D, Coltar J. Effect of glucagon on left ventricular performance in aortic stenosis Br Heart J. Murtagh JG, Binnion PF, Lal S, Hutchison KJ. Haemodynamic effects of glucagon. Br Heart J. Sélley E, Kun S, Szijárto IA, Kertesz M, Wittmann I, Molnar GA. Vasodilator effect of glucagon: receptorial crosstalk among glucagon, GLP-1 and receptor for glucagons and GLP Horm Metab Res. Rosano GMC, Vitale C. Metabolic modulation of cardiac metabolism in heart failure. Card Fail Rev. Therefore, uncovering the mechanisms that regulate glucagon secretion from the pancreatic alpha cell is critical for developing improved treatments for diabetes. In this review, we focus on aspects of alpha cell biology for possible mechanisms for alpha cell dysfunction in diabetes: proglucagon processing, intrinsic and paracrine control of glucagon secretion, secretory granule dynamics, and alterations in intracellular trafficking. We explore possible clues gleaned from these studies in how inhibition of glucagon secretion can be targeted as a treatment for diabetes mellitus. Glucagon is a amino acid peptide hormone produced by the alpha α cells of the pancreatic islet. It is known as the primary glucose counter-regulatory hormone, as its main physiological function is to maintain euglycemia by its actions on the liver to promote glycogenolysis and gluconeogenesis. This glucose counterregulation is impaired in both Type 1 T1D and Type 2 T2D diabetes 1. Intensive insulin therapy in T1D replaces the beta cell deficiency and corrects fasting and postprandial hyperglycemia, but at increased risks of hypoglycemia 2. These findings suggest that insulin therapy alone may not be adequate for optimal glycemic control. In fact, as stated by the bihormonal hypothesis, diabetes progression may be dependent on both excessive glucagon secretion and insulin deficiency 3 , and the glucagonocentric hypothesis ascribes even more importance to alpha cell dysfunction in diabetic hyperglycemia 4. Support for the latter hypothesis seemed to be provided by studies showing that glucagon receptor knockout mice are resistant to the effects of beta cell deficiency induced by streptozotocin treatment 5 , 6 , providing evidence that blocking glucagon action can provide glycemic control even in the relative absence of insulin. However, more recent studies have shown that blocking the glucagon receptor in absolute insulin deficiency does not prevent hyperglycemia 7 , 8 , indicating that residual insulin signalling is required in order for glucagon receptor antagonism to be effective. Nonetheless, the idea that blocking glucagon action could be an additional therapy for diabetes has sparked interest in the development of potential pharmacological interventions that target the hepatic glucagon receptor. Blocking glucagon action can be achieved through: i glucagon receptor antagonists, in particular small molecule antagonists, which can allosterically or competitively inhibit glucagon action 9 — 11 ; ii glucagon receptor neutralizing antibodies 12 ; and iii antisense oligonucleotides against the glucagon receptor However, blockade of the glucagon receptor may induce α-cell hyperplasia and exacerbate hyperaminoacidemia 14 — 16 through impairments in a liver-alpha cell axis [reviewed in 17 ], and increased risk of hyperlipidemia Therefore, long-term use of glucagon receptor blockers may result in harmful metabolic sequelae. An alternate and possibly safer strategy may be to directly target the intracellular mechanisms that govern the secretion of glucagon from the alpha cell. In this review, we discuss aspects of alpha cell biology that may provide such targets: proglucagon processing, sorting, exocytosis and intracellular trafficking, as well as mechanisms of intrinsic and intra-islet regulation of glucagon secretion. The alpha cell secretory pathway begins with the synthesis of proglucagon in the endoplasmic reticulum. It is then transported through the Golgi to the trans-Golgi network TGN. Budding immature secretory granules from the TGN contain proglucagon, its processing enzymes and many other proteins But how does proglucagon find its way to the site of granule budding? Based on one hypothesis, proglucagon may contain a sorting signal within its structure that directs it to a specific sorting receptor on the membrane of the TGN. These signals may interact directly with membrane lipids, in particular with lipid raft regions 25 or with sorting receptors within the TGN, to be sorted into secretory granules. To this end, it has been proposed that membrane-bound form of the processing enzyme carboxypeptidase E CPE may be a prohormone sorting receptor 21 , 26 — It was shown that ablation of CPE disrupted the regulated secretion of proopiomelanocortin POMC , proenkephalin and proinsulin in related cell lines and the CPE fat mouse model, in which CPE is degraded within the pituitary. Additionally, CPE may interact other resident granule proteins, secretogranin III and chromogranin A, to facilitate the sorting of POMC 29 and neuropeptides Another possible mechanism of prohormone sorting to granules may be simply through selective retention while constitutively secreted proteins are removed from the nascent immature granule. Evidence for this mechanism lies in the fact that the protein composition of immature secretory granules is altered during the process of granule maturation In this context, proinsulin and the enzymes involved in the post-translational processing to mature insulin are retained within the beta cell secretory granule, while other proteins designated for constitutive secretion are removed By considering all of these findings, it is likely that both receptor-mediated and retention mechanisms operate in the sorting of prohormones into secretory granules. In this scenario, prohormones could be sorted into secretory granules by means of sorting signals, followed by retention within the granule as maturation of secretory granules takes place. The maturation process involves alterations in the components and composition of the secretory granule, by removal of constitutively-secreted proteins, acidification of the granule milieu, and exclusion of water to condense the intragranular environment. The cellular events underlying the sorting of proglucagon to secretory granules have not been fully elucidated, and studying this mechanism is complicated by the multi-step processing of proglucagon. The processing of proglucagon in the alpha cell is largely governed by the prohormone convertase PC family of enzymes. Proglucagon processing begins early in the secretory pathway TGN or immature secretory granule with cleavage at K 70 R 71 , which yields glicentin and major proglucagon fragment MPGF Figure 1A Subsequent cleavage of glicentin by PC2 at K 31 R 32 results in the production of mature glucagon. This cleavage event likely occurs within the mature secretory granule since the enzymatic activity of PC2 is optimal at the acidic pH and millimolar calcium concentrations within secretory granules 33 — Thus, the sorting of proglucagon into the secretory granule is vital for the generation of active glucagon, and storage within granules assures a robust secretory response in response to physiological need. Figure 1 Proglucagon processing and sorting signals. A A schematic representation of proglucagon showing the major prohormone processing sites that yield the peptides glicentin, oxyntomodulin, glucagon, GLP-1 and GLP B Computational modelling of the structure of proglucagon showing the alpha helical structures of glucagon green , GLP-1 yellow and GLP-2 red from reference 28 © Society for Endocrinology. C Helical wheel projections of the alpha helices contained within glucagon and GLP-1 7—37 that function as sorting signals to direct proglucagon to the regulated secretory pathway. Is there evidence for sorting signals and a sorting receptor for proglucagon? Using the alpha cell line αTC, it was shown that siRNA-mediated knockdown of CPE increased constitutive secretion of glucagon; however, the processing of proglucagon to glucagon remained unchanged, indicating that CPE may have an effect on secretion, but not intracellular sorting The search for sorting signals provided more clarity on the mechanisms of proglucagon sorting. Computational modelling of proglucagon indicates a largely disordered structure comprising alpha helices, some of which correspond to glucagon, GLP-1 and GLP-2 Figure 1B. Using Fc-tagged proglucagon-derived peptides that could be detected by immunoprecipitation and immunofluorescence microscopy, it was shown that two dipolar α-helices containing hydrophobic patches with three charged residues within the sequences play roles as sorting signals. Interestingly, Fc-glicentin was sorted to secretory granules, but Fc-MPGF was not, suggesting that the sorting signal within GLP-1 is masked when contained within the MPGF sequence. These results indicate that the sorting of proglucagon into secretory granules occurs prior to the initial processing event, such that processing occurs exclusively within the granule. Another possibility is that processing to glicentin and MPGF occurs first, with the prediction that glicentin is sorted into granules and processed to glucagon, while MPGF is not sorted, or very inefficiently sorted into granules. This proglucagon processing profile changes in diabetes; in human and rodent islets, there is a significant increase in the processing of proglucagon to GLP If both glucagon and GLP-1 are produced in a proportion of alpha cells, and are both sorted to secretory granules, the question arises: are they sorted to distinct granule populations, and released under different glucose conditions? These questions may have been answered in a very recent islet granule peptidomics study showing that both human and mouse islets produce times more glucagon than active GLP-1 46 , indicating that the processing of proglucagon to active GLP-1 in alpha cells is very inefficient. Also in this study, analysis of secreted proglucagon-derived peptides showed that both glucagon and active GLP-1 were released in parallel in response to either low 1 mM , medium 6 mM or high Therefore, both glucagon and GLP-1 are likely stored in the same granules and secreted under the same conditions, with glucagon being the dominant peptide, and perhaps serving as the intra-islet GLP-1R agonist The control of hyperglucagonemia obviously targets glucagon secretion. But what mechanism s are potentially druggable? Inhibition of glucagon secretion by glucose from alpha cells is a long-standing puzzle in islet biology. Unlike insulin secretion from beta cells which is primarily driven by prevailing glucose levels, there is no one single factor that governs glucagon secretion from the alpha cell. Intrinsic glucose sensing, intra-islet paracrine secretion and factors from the alpha cell itself all interact to generate a complex network that regulates glucagon secretion. In order to examine the direct effects of glucose on glucagon secretion in the absence of paracrine inputs, isolated mouse pancreatic alpha cells, clonal hamster In-R1-G9 cells 48 , 49 , clonal mouse αTC and -9 cells 39 , 50 , 51 and dispersed alpha cells from human islets 52 have been used. All of these preparations show a bimodal response to increasing glucose concentrations. In the range from 1 to ~7 mM, glucagon secretion is suppressed in a dose-dependent manner, and above 7 mM, glucagon secretion increases Figure 2A. This secretion profile suggests intrinsic mechanisms alone can operate in regulating glucagon secretion below 7 mM glucose, and that these mechanisms may be ineffective at higher glucose concentrations. However, such conclusions must be interpreted with caution, as single dispersed alpha cells are in a highly abnormal environment, and alpha cell lines are not representative of the normal alpha cell phenotype, as discussed in more detail below. Figure 2 Glucagon secretion from dispersed alpha cells and alpha cells in intact islets demonstrate the role of paracrine regulation at high glucose concentrations. A V-shape curve of glucagon exocytosis in response to glucose in dispersed non-diabetic black and T2D red human α-cells. B Glucagon secretion from intact islets in response to glucose. Created with BioRender. The alpha cell secretory response to both glucose is likely more accurately captured in isolated, intact mouse and human islets, where the paracrine regulatory environment and cell-cell contacts are intact. Similar to dispersed alpha cells, increasing the glucose concentration from 1 to 7 mM dose dependently decreases glucagon secretion from mouse alpha cells 53 and human alpha cells 52 within intact islets, and remains low as glucose levels increase beyond 7 mM, a concentration at which insulin secretion is stimulated Figure 2B. Therefore, paracrine inputs are significant factors in the inhibition of glucagon secretion as glucose concentrations increase above euglycemia. One mechanism underlying the intrinsic response to glucose is the direct effect on alpha cell electrical activity. At low 1 mM glucose concentrations, alpha cells in intact mouse and human islets exhibit low K ATP activity and are electrically active 54 — 56 and as glucose concentrations increase, K ATP activity is inhibited. A recent review by Zhang et al. Therefore, the intrinsic regulation of glucagon secretion by glucose may be explained primarily by the unique electrical properties of the alpha cell, and secondarily by glucose metabolism. In particular, cAMP signalling may play a key role in the alpha cell secretory response to insulin and somatostatin There is one report that cAMP may also mediate intrinsic glucose sensing within the alpha cell. Using genetically encoded fluorescent cAMP biosensors, it was shown that high glucose suppressed subplasmalemmal cAMP levels in isolated mouse and human islets Conversely, sustained high cAMP levels abolished the suppression of glucagon secretion by high glucose concentrations. Lastly, intrinsic glucose sensing by the alpha cell may also be mediated by the nutrient sensors AMP-activated protein kinase AMPK and its downstream target, mammalian target of rapamycin complex 1 mTORC1. In a series of studies that manipulated alpha cell expression of AMPK itself 65 and its upstream effectors PASK 66 and LKB1 67 , it was shown that components of this nutrient-sensing pathway can mediate the low glucose-induced secretion of glucagon. One of these proteins, PASK, is down-regulated in T2D human islets, thus indicating that components of the AMPK pathway may be potential targets for controlling hyperglucagonemia. Using innovative mouse models that selectively targeted activators and inhibitors of mTORC1, it was shown that loss of mTORC1 activity resulted in a loss of the glucose counter-regulatory response and reduction in response to alpha cell secretagogues Interestingly, depletion of the mTORC1 inhibitor TSC2 in alpha cells resulted in a mouse model of hyperglucagonemia and glucagon resistance 69 , which will be an excellent resource for studies on mechanisms of hyperglucagonemia. Therefore, the mechanisms underlying the intrinsic response to glucose may provide potential targets for the control of abnormally up-regulated glucagon secretion in diabetes. The beta cell secretory granule contains a number of agents that act directly or indirectly on the alpha cell to inhibit glucagon secretion, and also generally modulate mechanisms of alpha cell biology, such as proliferation. Insulin, the primary cargo, is a potent suppressor of glucagon secretion and operates through several mechanisms. Mice lacking the insulin receptor on alpha cells αIRKO exhibit hyperglycemia and hyperglucagonemia, indicating that insulin receptor signalling is required for an appropriate alpha cell secretory response to glucose Alpha cell insulin resistance may underlie the abnormal up-regulation of glucagon secretion Type 2 diabetes Additionally, these results also indicate that insulin alone is not sufficient to regulate glycemia in the face of hyperglucagonemia. Along with insulin, gamma amino butyric acid GABA is also released from the beta cell and is a potent suppressor of glucagon secretion from alpha cells 73 , Activating the GABA A receptor in alpha cells results in Cl - influx into the cells which hyperpolarizes the membrane and reduces glucagon secretion As well, there is coordination between insulin and GABA A receptor activity, as insulin action leads to the translocation of GABA A receptor to the cell membrane 76 , thus augmenting the inhibitory effects of GABA. In addition, GABA also inhibits mTOR activity to suppress alpha cell proliferation. In type 1 diabetes, the destruction of beta cells leads to a reduction in the amount of secreted GABA, resulting in the activation of mTOR and alpha cell proliferation In addition to effects on alpha cell proliferation, some studies have suggested that pharmacologic activation of GABA A receptor by artemisinins or GABA may alter alpha cell identity and trans-differentiate adult alpha cells to beta-like cells 78 — 80 , and have led to clinical trials investigating GABA receptor agonists as protection against the development of diabetes. However, there is still some debate on this topic, as transdifferentiation could not be induced either in isolated mouse islets in which both insulin and glucagon were tagged with fluorescent reporters 81 or in an alpha cell-specific lineage tracing model In any case, the reported immunomodulatory effects of GABA, together with either GLP-1 83 or the SGLT2 inhibitor empagliflozin 84 also protect newly formed beta cells in the inflammatory environment of T1D, and thus also indirectly restore normal regulation of alpha cell mass and glucagon secretion. Direct effects of serotonin are mediated by activation of the serotonin receptor, 5-HT 1F R, on α-cells, which reduces intracellular cAMP to suppress glucagon secretion 85 , In patients with long-standing T2D, the proportion of alpha cells expressing 5-HT 1F R is decreased, suggesting that reduced serotonin action on alpha cells may play a role in hyperglucagonemia of diabetes. In STZ-treated mice, administration of the 5-HT 1F R agonist LY alleviated hyperglucagonemia and hyperglycemia. However, insulin-induced hypoglycemia was worsened, suggesting that the effects of serotonin are glucose-independent Therefore, while alpha cell HT 1F R may be a potential target for the treatment of hyperglucagonemia, it may not be an ideal target. The effects of adenosine are mediated by the adenosine A1 receptor Adora1 , in which activation is coupled to opening of K ATP channels, hyperpolarization of the cell membrane and prevention of granule exocytosis. In NOD mice, autoantibody-positive people and people with long-term T1D, alpha cells gradually lose Adora1 expression, suggesting that the hyperglucagonemia of diabetes is associated with a loss of adenosine action ZnT8 is located in the secretory granule membrane of both α-and β-cells. There is a direct relationship between expression of the proglucagon gene and Slc30A8 in α-cells Somatostatin is a well-known tonic inhibitor of glucagon secretion. Somatostatin binds to the SSTR2 receptor subtype on alpha cells 93 , which is coupled to the inhibitory G i subunit, resulting in decreased production of cAMP as a mechanism for the suppression of glucagon secretion Notably, secretion of somatostatin and inhibition of glucagon secretion both occur at 3 mM glucose, indicating that the alpha cell response to low glucose may be fine-tuned by somatostatin In rat pancreatic preparations perfused with an SSTR2 antagonist, the suppression of glucagon secretion by 3. However, in isolated human islets, blockade of SSTR2 did not affect suppression of glucagon secretion at 6 mM glucose 55 , perhaps reflecting species-specific differences or differences in the models perfused pancreas vs static islet culture. Interestingly, insulin secretion was also elevated, indicating that both insulin and somatostatin are required for the suppression of glucagon secretion at high glucose concentrations. In intact human islets, high glucose 10 mM inhibition of glucagon exocytosis was lost after administration of the SSTR2 antagonist CYN In diabetes, circulating and pancreatic somatostatin, together with SST mRNA, are elevated. However, expression of SSTR2 on alpha cells is decreased in T2D due to increased receptor internalization 52 , indicating alpha cell somatostatin resistance. Together with alpha cell insulin resistance, this could be another mechanism in the hyperglucagonemia of diabetes. Alternatively, somatostatin resistance may be a dominant and direct mechanism of hyperglucagonemia, as eliminating the insulin receptor on delta cells completely abolishes the glucagonostatic effect of insulin, indicating an indirect glucagonostatic effect for insulin The emerging role of somatostatin in the regulation of alpha cell function and glucagon secretion has been further highlighted by one study in which mice were engineered for optogenetic activation of beta cells to study the paracrine regulation of alpha cells By this approach, opto-activation of beta cells both suppressed alpha cell electrical activity and stimulated action potentials in delta cells mediated by gap junction currents. The suppressive effect of beta cell activation was lost in the presence of the SSTR2 antagonist CYN 99 , indicating that somatostatin secretion stimulated by beta cell electrical activity is critical for the suppression of glucagon secretion. Subsequent modelling predicted that a reduction in gap junction connections between beta and delta cells, perhaps caused by disruptions in islet architecture in T2D , may contribute to the hyperglucagonemia of diabetes. Thus these findings highlight a central role for delta cells in the context of intra-islet regulation of glucagon secretion, and may have implications for designing drugs for the treatment of hyperglucagonemia of diabetes. The alpha cell itself displays plasticity during the progression of diabetes. In addition to the mechanisms above that describe changes in responses to glucose and paracrine effectors, there are alterations within the alpha cell, including proglucagon processing and secretion of proglucagon-derived peptides, and remodelling of the secretory granules themselves in terms of exocytotic behavior and contents, and alterations in intracellular trafficking pathways. Secreted glucagon from alpha cells can stimulate its secretion through an autocrine effect. It has been shown that glucagon stimulates glucagon secretion from the rat and mouse isolated alpha cells in an autocrine manner through glucagon receptor-stimulated cAMP signaling In αTC cells and mouse islets, exogenous glucagon administration, as well as secreted glucagon stimulated by 1 mM glucose, increased glucagon secretion and proglucagon gene transcription through the PKA-cAMP-CREB signalling pathway in a glucagon receptor-dependent manner The apparent interplay between glucagon and its receptor on the alpha cell appears to be of a positive feedback loop, controlled by the pulsatile nature of glucagon secretion. In addition to glucagon, a novel proglucagon-derived peptide, proglucagon PG comprised of GRPP and glucagon, was identified as a major molecular form of glucagon in plasma from human patients with hyperglucagonemia-associated conditions: Type 2 diabetes and renal dysfunction, morbid obesity or gastric bypass surgery, and only after oral ingestion of macronutrients This N-terminally extended form of immunoreactive glucagon was not found in healthy controls, leading the authors to speculate that PG , and molecular heterogeneity of glucagon in general, could be a biomarker for alpha cell dysfunction. Administration of PG decreased glucagon secretion in healthy rats, diverging from the positive feedback observed with glucagon administration. Interestingly, this effect was not observed in diabetic rats, suggesting an impairment in this distinct feedback loop in the alpha cell. The interplay between glucagon, insulin and somatostatin in the regulation of glucagon secretion at various levels of glucose is illustrated in Figure 3. In diabetes, beta cell deficiency, together with alpha cell insulin and somatostatin resistance, all contribute to alpha cell dysfunction and a loss of the regulation of glucagon secretion, resulting in hyperglucagonemia. Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion. Under low glucose mM conditions, secreted glucagon may act in an autocrine feed-forward loop. Additionally, electrical coupling of the beta and delta cells through gap junctions contributes to somatostatin release. Somatostatin binds to SST receptor 2 SSTR2 on the α cell membrane, where signalling through G i inhibits glucagon secretion. The glucose-dependent insulinotropic actions of intestinal GLP-1 on the beta cell are well known. GLP-1 also suppresses glucagon secretion in both healthy people and people with type 2 diabetes , and poorly-controlled type 1 diabetes The emerging evidence of GLP-1 being produced and secreted by the pancreatic alpha cell has led to a debate on which source of GLP-1 suppresses glucagon secretion from pancreatic alpha cells. To investigate this question, Chambers et al. The gut-derived GLP-1 binds to its receptor on local afferent vagal nerve terminals, which ultimately signals for satiety, delaying gastric emptying and suppression of hepatic glucose release , However, this model may not translate well to human islets due to differences in islet architecture, and in light of the recent findings that glucagon is the dominant peptide hormone secreted from human alpha cells The search for a GLP-1 receptor on alpha cells has been hampered by a lack of a reliable GLP-1 receptor antibody , GLP-1 appears to mildly reduce action potentials in the alpha cell membrane at 1 mM glucose in isolated mouse alpha cells, and this effect is blocked by the GLP-1R antagonist exendin , therefore suggesting the presence of GLP-1R, perhaps at a very low density, on a small proportion of alpha cells. The development of near infra-red and fluorescent analogues of GLP-1R ligands has enabled both in vivo , and high-resolution tissue imaging , of GLP-1R with high specificity, sensitivity, and reproducibility. Given the already small proportion of alpha cells in the mouse islet, the contribution of direct alpha cell action to the glucagonostatic effect of GLP-1 is likely very small. Islet GLP-1 may also exert its effects through receptors on delta cells , resulting in stimulation of somatostatin secretion and inhibition of glucagon secretion via SSTR2 on alpha cells , This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin As drugs targeted to the control of glucagon secretion are now being developed for the treatment of hyperglucagonemia, a deeper understanding of the dynamics of the alpha cell secretory granule is critical for identifying effective targets. However, the study of glucagon granule trafficking and exocytosis presents several technological challenges. Commonly used cell lines such as InR1-G9, αTC and αTC, while useful for preliminary studies on trafficking and secretion, as a rule do not exhibit robust secretory responses to glucose or other secretagogues. The αTC cell line in particular differs from primary alpha cells in their complement of transcriptional, epigenetic and metabolic factors , which may explain the blunted secretory response to glucose. Dispersed primary alpha cells may offer a slightly better alternative, but as discussed above, both cell lines and dispersed primary alpha cells exhibit aberrant glucagon exocytosis patterns at high glucose levels, likely due to the absence of paracrine inputs and juxtamembrane contacts. The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis. As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis. Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time. In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane. SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs. Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced. Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules. In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D. Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis. Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease. There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway. Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated. The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion. While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required. In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5. Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4. Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells. Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known. So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function? It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia. Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D. Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia. While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation. |
| Glucagon - Wikipedia | Stathmin-2 Mediates Glucagon Secretion From Pancreatic α-Cells. Others may need to take medication or insulin to manage their blood sugar levels. CAS Google Scholar Winter J, Brack KE, Ng A. Elife 5:e Insulin and glucagon secretion: nondiabetic and diabetic subjects. The Metabolic Actions of Glucagon Revisited. |
Glucagon mechanism -
Weiss et al found that the conversion of NGT to IGT was accompanied by a decrease in insulin sensitivity, accompanied by a gradual increase in glucagon secretion. The expression is up-regulated and insulin resistance is maintained in islet alpha cells.
As mentioned above, when the alpha cell insulin is resistant, its signal transduction pathway is impaired. Exploring its mechanisms may be related to the mediation of inflammatory mediators. Studies have shown that inflammatory factors play an important role in peripheral insulin resistance, and the effect of nuclear factor kappa B NF-κB on alpha cells in a model of insulin resistance in rat islet alpha cells induced by high-fat feeding mediates activation of the inflammatory pathway.
Ellingsgaard et al found that IL-7 receptors were expressed on islet α cells compared with other tissues. IL6 induced the expression and secretion of glucagon in rats with high-fat diet. After using the IL6 receptor gene knockout model, the body's metabolic disorder was corrected.
The use of thiazolidinediones TZD drugs can not only improve peripheral insulin resistance in SD rats induced by high-fat feeding, but also inhibit the proliferation of α cells, and and significantly increase glucagon levels and α-cell glucagon mRNA expression. This effect is achieved by the binding of TZDs to the peroxisome proliferator-activated receptor on islet alpha cells, which directly inhibits glucagon gene transcription.
In recent years, there are many studies on the treatment of diabetes with incretin hormone, which is represented by glucagon like peptide1 GLP1 and its analogs. GLP1 is a 30 amino acid peptide hormone secreted mainly by L cells of the distal ileum, rectum and colon.
It not only acts on glucose-dependent β-cells, but also promotes insulin secretion. It also acts on islet α cells. Inhibition of glucagon secretion can improve alpha cell insulin resistance. Prohormone converting enzyme 2 PC2 gene knockout: proglucagon is a precursor of glucagon, which produces different products through different prohormone convertases in different tissue organs.
Study have showed that PC2 knockout mice have a significant decrease in blood glucagon, mild persistent hypoglycemia, and modern compensatory islet alpha cell proliferation, when using a micro-osmotic pump or intraperitoneal small dose.
After glucagon injection, blood glucose returned to normal; and after a long period of application, the morphology of islet α cells recovered to resemble that of wild-type mice.
Glucagon neutralizing antibodies: this method uses exogenous glucagon antibodies to bind to glucagon in the body, thereby blocking the effects of endogenous glucagon and ultimately lowering blood sugar.
The brand is equivalent to an experiment conducted in using a high-capacity, high-affinity glucagon monoclonal antibody Glu-mAb in a normal, alloxan ALX -induced mild and severe diabetic rabbit model. Tip: this antibody can completely block exogenous glucagon-induced hyperglycemia in normal animals; in low-glycemic zoos, lowering blood sugar is also obvious; in high-glycemic type 1 diabetic rabbits, Glu-mAb can still significantly reduce liver glucose output, reducing the fasting blood glucose of experimental rabbits from The use of glucagon antibodies to reduce the effects of glucagon can better control the effects of type 2 diabetes.
Barbato et al. found that the glycine-serine polymorphism Gly40Ser of the glucagon receptor gene exon 2 in French Caucasians is closely related to type 2 diabetes.
The research focused on glucagon receptor blockers, glucagon receptor gene expression inhibitors, and glucagon receptor gene knockout. Receptor blockers: the mechanism of action of glucagon receptor blockers is mainly through competitive binding to endogenous glucagon, thereby inhibiting glucagon-mediated adenylate cyclase activity, reducing glycogen output, reducing fasting blood glucose levels, and improving glucose tolerance.
The receptor blocker is classified into a peptide compound and a non-peptide small molecule compound according to the molecular structure. Petersen et al. found that a non-peptide small molecule compound, Bay 27 , effectively blocks the increase in glucose production and blood glucose caused by exogenous glucagon in healthy adult males.
This is also the only drug that has been used in humans for glucagon receptor antagonists. Although more clinical trials are needed to prove efficacy, it is undoubtedly an increase in the search for effective human glucagon receptor antagonists.
The above studies have shown that both glucagon receptor antagonists, whether peptide or non-peptide, block the liver glucagon receptor and exert a hypoglycemic effect.
DB Y. D C ChEMBL N. Interactive image. This section does not cite any sources. Please help improve this section by adding citations to reliable sources. Unsourced material may be challenged and removed. February Learn how and when to remove this template message. This section needs additional citations for verification.
Please help improve this article by adding citations to reliable sources in this section. Retrieved 16 May Health Canada. Retrieved 7 June Retrieved 27 February European Medicines Agency. European Medicines Agency EMA.
Union Register of medicinal products. Retrieved 3 March British Medical Association. ISBN The American Society of Health-System Pharmacists.
Archived from the original on 26 December Retrieved 8 December Food and Drug Administration FDA Press release. Archived from the original on 27 December Retrieved 27 December World Health Organization model list of essential medicines: 21st list Geneva: World Health Organization.
License: CC BY-NC-SA 3. Retrieved 28 December Journal of Clinical Pharmacology. doi : PMID S2CID American Family Physician. Canadian Journal of Gastroenterology. PMC Annals of the Royal College of Surgeons of England.
American Journal of Health-System Pharmacy. June Gastrointestinal Endoscopy. Archived PDF from the original on 8 August By considering all of these findings, it is likely that both receptor-mediated and retention mechanisms operate in the sorting of prohormones into secretory granules.
In this scenario, prohormones could be sorted into secretory granules by means of sorting signals, followed by retention within the granule as maturation of secretory granules takes place.
The maturation process involves alterations in the components and composition of the secretory granule, by removal of constitutively-secreted proteins, acidification of the granule milieu, and exclusion of water to condense the intragranular environment. The cellular events underlying the sorting of proglucagon to secretory granules have not been fully elucidated, and studying this mechanism is complicated by the multi-step processing of proglucagon.
The processing of proglucagon in the alpha cell is largely governed by the prohormone convertase PC family of enzymes. Proglucagon processing begins early in the secretory pathway TGN or immature secretory granule with cleavage at K 70 R 71 , which yields glicentin and major proglucagon fragment MPGF Figure 1A Subsequent cleavage of glicentin by PC2 at K 31 R 32 results in the production of mature glucagon.
This cleavage event likely occurs within the mature secretory granule since the enzymatic activity of PC2 is optimal at the acidic pH and millimolar calcium concentrations within secretory granules 33 — Thus, the sorting of proglucagon into the secretory granule is vital for the generation of active glucagon, and storage within granules assures a robust secretory response in response to physiological need.
Figure 1 Proglucagon processing and sorting signals. A A schematic representation of proglucagon showing the major prohormone processing sites that yield the peptides glicentin, oxyntomodulin, glucagon, GLP-1 and GLP B Computational modelling of the structure of proglucagon showing the alpha helical structures of glucagon green , GLP-1 yellow and GLP-2 red from reference 28 © Society for Endocrinology.
C Helical wheel projections of the alpha helices contained within glucagon and GLP-1 7—37 that function as sorting signals to direct proglucagon to the regulated secretory pathway. Is there evidence for sorting signals and a sorting receptor for proglucagon? Using the alpha cell line αTC, it was shown that siRNA-mediated knockdown of CPE increased constitutive secretion of glucagon; however, the processing of proglucagon to glucagon remained unchanged, indicating that CPE may have an effect on secretion, but not intracellular sorting The search for sorting signals provided more clarity on the mechanisms of proglucagon sorting.
Computational modelling of proglucagon indicates a largely disordered structure comprising alpha helices, some of which correspond to glucagon, GLP-1 and GLP-2 Figure 1B. Using Fc-tagged proglucagon-derived peptides that could be detected by immunoprecipitation and immunofluorescence microscopy, it was shown that two dipolar α-helices containing hydrophobic patches with three charged residues within the sequences play roles as sorting signals.
Interestingly, Fc-glicentin was sorted to secretory granules, but Fc-MPGF was not, suggesting that the sorting signal within GLP-1 is masked when contained within the MPGF sequence. These results indicate that the sorting of proglucagon into secretory granules occurs prior to the initial processing event, such that processing occurs exclusively within the granule.
Another possibility is that processing to glicentin and MPGF occurs first, with the prediction that glicentin is sorted into granules and processed to glucagon, while MPGF is not sorted, or very inefficiently sorted into granules.
This proglucagon processing profile changes in diabetes; in human and rodent islets, there is a significant increase in the processing of proglucagon to GLP If both glucagon and GLP-1 are produced in a proportion of alpha cells, and are both sorted to secretory granules, the question arises: are they sorted to distinct granule populations, and released under different glucose conditions?
These questions may have been answered in a very recent islet granule peptidomics study showing that both human and mouse islets produce times more glucagon than active GLP-1 46 , indicating that the processing of proglucagon to active GLP-1 in alpha cells is very inefficient.
Also in this study, analysis of secreted proglucagon-derived peptides showed that both glucagon and active GLP-1 were released in parallel in response to either low 1 mM , medium 6 mM or high Therefore, both glucagon and GLP-1 are likely stored in the same granules and secreted under the same conditions, with glucagon being the dominant peptide, and perhaps serving as the intra-islet GLP-1R agonist The control of hyperglucagonemia obviously targets glucagon secretion.
But what mechanism s are potentially druggable? Inhibition of glucagon secretion by glucose from alpha cells is a long-standing puzzle in islet biology.
Unlike insulin secretion from beta cells which is primarily driven by prevailing glucose levels, there is no one single factor that governs glucagon secretion from the alpha cell.
Intrinsic glucose sensing, intra-islet paracrine secretion and factors from the alpha cell itself all interact to generate a complex network that regulates glucagon secretion. In order to examine the direct effects of glucose on glucagon secretion in the absence of paracrine inputs, isolated mouse pancreatic alpha cells, clonal hamster In-R1-G9 cells 48 , 49 , clonal mouse αTC and -9 cells 39 , 50 , 51 and dispersed alpha cells from human islets 52 have been used.
All of these preparations show a bimodal response to increasing glucose concentrations. In the range from 1 to ~7 mM, glucagon secretion is suppressed in a dose-dependent manner, and above 7 mM, glucagon secretion increases Figure 2A.
This secretion profile suggests intrinsic mechanisms alone can operate in regulating glucagon secretion below 7 mM glucose, and that these mechanisms may be ineffective at higher glucose concentrations.
However, such conclusions must be interpreted with caution, as single dispersed alpha cells are in a highly abnormal environment, and alpha cell lines are not representative of the normal alpha cell phenotype, as discussed in more detail below. Figure 2 Glucagon secretion from dispersed alpha cells and alpha cells in intact islets demonstrate the role of paracrine regulation at high glucose concentrations.
A V-shape curve of glucagon exocytosis in response to glucose in dispersed non-diabetic black and T2D red human α-cells. B Glucagon secretion from intact islets in response to glucose.
Created with BioRender. The alpha cell secretory response to both glucose is likely more accurately captured in isolated, intact mouse and human islets, where the paracrine regulatory environment and cell-cell contacts are intact.
Similar to dispersed alpha cells, increasing the glucose concentration from 1 to 7 mM dose dependently decreases glucagon secretion from mouse alpha cells 53 and human alpha cells 52 within intact islets, and remains low as glucose levels increase beyond 7 mM, a concentration at which insulin secretion is stimulated Figure 2B.
Therefore, paracrine inputs are significant factors in the inhibition of glucagon secretion as glucose concentrations increase above euglycemia. One mechanism underlying the intrinsic response to glucose is the direct effect on alpha cell electrical activity.
At low 1 mM glucose concentrations, alpha cells in intact mouse and human islets exhibit low K ATP activity and are electrically active 54 — 56 and as glucose concentrations increase, K ATP activity is inhibited.
A recent review by Zhang et al. Therefore, the intrinsic regulation of glucagon secretion by glucose may be explained primarily by the unique electrical properties of the alpha cell, and secondarily by glucose metabolism.
In particular, cAMP signalling may play a key role in the alpha cell secretory response to insulin and somatostatin There is one report that cAMP may also mediate intrinsic glucose sensing within the alpha cell.
Using genetically encoded fluorescent cAMP biosensors, it was shown that high glucose suppressed subplasmalemmal cAMP levels in isolated mouse and human islets Conversely, sustained high cAMP levels abolished the suppression of glucagon secretion by high glucose concentrations.
Lastly, intrinsic glucose sensing by the alpha cell may also be mediated by the nutrient sensors AMP-activated protein kinase AMPK and its downstream target, mammalian target of rapamycin complex 1 mTORC1.
In a series of studies that manipulated alpha cell expression of AMPK itself 65 and its upstream effectors PASK 66 and LKB1 67 , it was shown that components of this nutrient-sensing pathway can mediate the low glucose-induced secretion of glucagon.
One of these proteins, PASK, is down-regulated in T2D human islets, thus indicating that components of the AMPK pathway may be potential targets for controlling hyperglucagonemia. Using innovative mouse models that selectively targeted activators and inhibitors of mTORC1, it was shown that loss of mTORC1 activity resulted in a loss of the glucose counter-regulatory response and reduction in response to alpha cell secretagogues Interestingly, depletion of the mTORC1 inhibitor TSC2 in alpha cells resulted in a mouse model of hyperglucagonemia and glucagon resistance 69 , which will be an excellent resource for studies on mechanisms of hyperglucagonemia.
Therefore, the mechanisms underlying the intrinsic response to glucose may provide potential targets for the control of abnormally up-regulated glucagon secretion in diabetes. The beta cell secretory granule contains a number of agents that act directly or indirectly on the alpha cell to inhibit glucagon secretion, and also generally modulate mechanisms of alpha cell biology, such as proliferation.
Insulin, the primary cargo, is a potent suppressor of glucagon secretion and operates through several mechanisms. Mice lacking the insulin receptor on alpha cells αIRKO exhibit hyperglycemia and hyperglucagonemia, indicating that insulin receptor signalling is required for an appropriate alpha cell secretory response to glucose Alpha cell insulin resistance may underlie the abnormal up-regulation of glucagon secretion Type 2 diabetes Additionally, these results also indicate that insulin alone is not sufficient to regulate glycemia in the face of hyperglucagonemia.
Along with insulin, gamma amino butyric acid GABA is also released from the beta cell and is a potent suppressor of glucagon secretion from alpha cells 73 , Activating the GABA A receptor in alpha cells results in Cl - influx into the cells which hyperpolarizes the membrane and reduces glucagon secretion As well, there is coordination between insulin and GABA A receptor activity, as insulin action leads to the translocation of GABA A receptor to the cell membrane 76 , thus augmenting the inhibitory effects of GABA.
In addition, GABA also inhibits mTOR activity to suppress alpha cell proliferation. In type 1 diabetes, the destruction of beta cells leads to a reduction in the amount of secreted GABA, resulting in the activation of mTOR and alpha cell proliferation In addition to effects on alpha cell proliferation, some studies have suggested that pharmacologic activation of GABA A receptor by artemisinins or GABA may alter alpha cell identity and trans-differentiate adult alpha cells to beta-like cells 78 — 80 , and have led to clinical trials investigating GABA receptor agonists as protection against the development of diabetes.
However, there is still some debate on this topic, as transdifferentiation could not be induced either in isolated mouse islets in which both insulin and glucagon were tagged with fluorescent reporters 81 or in an alpha cell-specific lineage tracing model In any case, the reported immunomodulatory effects of GABA, together with either GLP-1 83 or the SGLT2 inhibitor empagliflozin 84 also protect newly formed beta cells in the inflammatory environment of T1D, and thus also indirectly restore normal regulation of alpha cell mass and glucagon secretion.
Direct effects of serotonin are mediated by activation of the serotonin receptor, 5-HT 1F R, on α-cells, which reduces intracellular cAMP to suppress glucagon secretion 85 , In patients with long-standing T2D, the proportion of alpha cells expressing 5-HT 1F R is decreased, suggesting that reduced serotonin action on alpha cells may play a role in hyperglucagonemia of diabetes.
In STZ-treated mice, administration of the 5-HT 1F R agonist LY alleviated hyperglucagonemia and hyperglycemia. However, insulin-induced hypoglycemia was worsened, suggesting that the effects of serotonin are glucose-independent Therefore, while alpha cell HT 1F R may be a potential target for the treatment of hyperglucagonemia, it may not be an ideal target.
The effects of adenosine are mediated by the adenosine A1 receptor Adora1 , in which activation is coupled to opening of K ATP channels, hyperpolarization of the cell membrane and prevention of granule exocytosis.
In NOD mice, autoantibody-positive people and people with long-term T1D, alpha cells gradually lose Adora1 expression, suggesting that the hyperglucagonemia of diabetes is associated with a loss of adenosine action ZnT8 is located in the secretory granule membrane of both α-and β-cells.
There is a direct relationship between expression of the proglucagon gene and Slc30A8 in α-cells Somatostatin is a well-known tonic inhibitor of glucagon secretion. Somatostatin binds to the SSTR2 receptor subtype on alpha cells 93 , which is coupled to the inhibitory G i subunit, resulting in decreased production of cAMP as a mechanism for the suppression of glucagon secretion Notably, secretion of somatostatin and inhibition of glucagon secretion both occur at 3 mM glucose, indicating that the alpha cell response to low glucose may be fine-tuned by somatostatin In rat pancreatic preparations perfused with an SSTR2 antagonist, the suppression of glucagon secretion by 3.
However, in isolated human islets, blockade of SSTR2 did not affect suppression of glucagon secretion at 6 mM glucose 55 , perhaps reflecting species-specific differences or differences in the models perfused pancreas vs static islet culture.
Interestingly, insulin secretion was also elevated, indicating that both insulin and somatostatin are required for the suppression of glucagon secretion at high glucose concentrations.
In intact human islets, high glucose 10 mM inhibition of glucagon exocytosis was lost after administration of the SSTR2 antagonist CYN In diabetes, circulating and pancreatic somatostatin, together with SST mRNA, are elevated.
However, expression of SSTR2 on alpha cells is decreased in T2D due to increased receptor internalization 52 , indicating alpha cell somatostatin resistance. Together with alpha cell insulin resistance, this could be another mechanism in the hyperglucagonemia of diabetes.
Alternatively, somatostatin resistance may be a dominant and direct mechanism of hyperglucagonemia, as eliminating the insulin receptor on delta cells completely abolishes the glucagonostatic effect of insulin, indicating an indirect glucagonostatic effect for insulin The emerging role of somatostatin in the regulation of alpha cell function and glucagon secretion has been further highlighted by one study in which mice were engineered for optogenetic activation of beta cells to study the paracrine regulation of alpha cells By this approach, opto-activation of beta cells both suppressed alpha cell electrical activity and stimulated action potentials in delta cells mediated by gap junction currents.
The suppressive effect of beta cell activation was lost in the presence of the SSTR2 antagonist CYN 99 , indicating that somatostatin secretion stimulated by beta cell electrical activity is critical for the suppression of glucagon secretion.
Subsequent modelling predicted that a reduction in gap junction connections between beta and delta cells, perhaps caused by disruptions in islet architecture in T2D , may contribute to the hyperglucagonemia of diabetes. Thus these findings highlight a central role for delta cells in the context of intra-islet regulation of glucagon secretion, and may have implications for designing drugs for the treatment of hyperglucagonemia of diabetes.
The alpha cell itself displays plasticity during the progression of diabetes. In addition to the mechanisms above that describe changes in responses to glucose and paracrine effectors, there are alterations within the alpha cell, including proglucagon processing and secretion of proglucagon-derived peptides, and remodelling of the secretory granules themselves in terms of exocytotic behavior and contents, and alterations in intracellular trafficking pathways.
Secreted glucagon from alpha cells can stimulate its secretion through an autocrine effect. It has been shown that glucagon stimulates glucagon secretion from the rat and mouse isolated alpha cells in an autocrine manner through glucagon receptor-stimulated cAMP signaling In αTC cells and mouse islets, exogenous glucagon administration, as well as secreted glucagon stimulated by 1 mM glucose, increased glucagon secretion and proglucagon gene transcription through the PKA-cAMP-CREB signalling pathway in a glucagon receptor-dependent manner The apparent interplay between glucagon and its receptor on the alpha cell appears to be of a positive feedback loop, controlled by the pulsatile nature of glucagon secretion.
In addition to glucagon, a novel proglucagon-derived peptide, proglucagon PG comprised of GRPP and glucagon, was identified as a major molecular form of glucagon in plasma from human patients with hyperglucagonemia-associated conditions: Type 2 diabetes and renal dysfunction, morbid obesity or gastric bypass surgery, and only after oral ingestion of macronutrients This N-terminally extended form of immunoreactive glucagon was not found in healthy controls, leading the authors to speculate that PG , and molecular heterogeneity of glucagon in general, could be a biomarker for alpha cell dysfunction.
Administration of PG decreased glucagon secretion in healthy rats, diverging from the positive feedback observed with glucagon administration. Interestingly, this effect was not observed in diabetic rats, suggesting an impairment in this distinct feedback loop in the alpha cell.
The interplay between glucagon, insulin and somatostatin in the regulation of glucagon secretion at various levels of glucose is illustrated in Figure 3. In diabetes, beta cell deficiency, together with alpha cell insulin and somatostatin resistance, all contribute to alpha cell dysfunction and a loss of the regulation of glucagon secretion, resulting in hyperglucagonemia.
Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion. Under low glucose mM conditions, secreted glucagon may act in an autocrine feed-forward loop.
Additionally, electrical coupling of the beta and delta cells through gap junctions contributes to somatostatin release. Somatostatin binds to SST receptor 2 SSTR2 on the α cell membrane, where signalling through G i inhibits glucagon secretion. The glucose-dependent insulinotropic actions of intestinal GLP-1 on the beta cell are well known.
GLP-1 also suppresses glucagon secretion in both healthy people and people with type 2 diabetes , and poorly-controlled type 1 diabetes The emerging evidence of GLP-1 being produced and secreted by the pancreatic alpha cell has led to a debate on which source of GLP-1 suppresses glucagon secretion from pancreatic alpha cells.
To investigate this question, Chambers et al. The gut-derived GLP-1 binds to its receptor on local afferent vagal nerve terminals, which ultimately signals for satiety, delaying gastric emptying and suppression of hepatic glucose release , However, this model may not translate well to human islets due to differences in islet architecture, and in light of the recent findings that glucagon is the dominant peptide hormone secreted from human alpha cells The search for a GLP-1 receptor on alpha cells has been hampered by a lack of a reliable GLP-1 receptor antibody , GLP-1 appears to mildly reduce action potentials in the alpha cell membrane at 1 mM glucose in isolated mouse alpha cells, and this effect is blocked by the GLP-1R antagonist exendin , therefore suggesting the presence of GLP-1R, perhaps at a very low density, on a small proportion of alpha cells.
The development of near infra-red and fluorescent analogues of GLP-1R ligands has enabled both in vivo , and high-resolution tissue imaging , of GLP-1R with high specificity, sensitivity, and reproducibility.
Given the already small proportion of alpha cells in the mouse islet, the contribution of direct alpha cell action to the glucagonostatic effect of GLP-1 is likely very small.
Islet GLP-1 may also exert its effects through receptors on delta cells , resulting in stimulation of somatostatin secretion and inhibition of glucagon secretion via SSTR2 on alpha cells , This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin As drugs targeted to the control of glucagon secretion are now being developed for the treatment of hyperglucagonemia, a deeper understanding of the dynamics of the alpha cell secretory granule is critical for identifying effective targets.
However, the study of glucagon granule trafficking and exocytosis presents several technological challenges. Commonly used cell lines such as InR1-G9, αTC and αTC, while useful for preliminary studies on trafficking and secretion, as a rule do not exhibit robust secretory responses to glucose or other secretagogues.
The αTC cell line in particular differs from primary alpha cells in their complement of transcriptional, epigenetic and metabolic factors , which may explain the blunted secretory response to glucose. Dispersed primary alpha cells may offer a slightly better alternative, but as discussed above, both cell lines and dispersed primary alpha cells exhibit aberrant glucagon exocytosis patterns at high glucose levels, likely due to the absence of paracrine inputs and juxtamembrane contacts.
The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis.
As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis.
Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time.
In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane.
SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs.
Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced.
Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules. In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D.
Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis.
Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease.
There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway. Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated.
The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion. While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required.
In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5.
Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4.
Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells.
Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways.
The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known.
So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function?
It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia.
Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D.
Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia.
While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation.
The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target.
In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment.
The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes.
SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar. DCCT Research Group.
Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover.
J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia.
Diabetologia —
Glucagon mechanism Diabetology volume 17 mechansim, Article number: Cite this article. Metrics Glucayon. These mechanosm have been Vegetarian meal options for athletes demonstrated in experimental Alternative herbal remedies in different animal species. However, efforts to extrapolate the experimental data to patients with low cardiac output states, such as acute heart failure or cardiogenic shock, have been disappointing. The experimental and clinical data on the cardiac effects of glucagon are described here.
Ja, ist entschieden.