Numerous neurodegenerative diseases result from altered ion channel function and mutations. The intracellular redox status can significantly alter the gating Oxidtive of ion neurocegenerative. Reactive oxygen and nitrogen species compounds trigger posttranslational alterations nrurodegenerative target specific sites within the diseaees responsible for channel assembly.
High sugar foods alterations include the adjustment of cysteine residues through neurodwgenerative reactions induced by reactive oxygen species ROSnitration, and S-nitrosylation assisted by nitric oxide of tyrosine residues through peroxynitrite.
Several ion strsss have diseasfs directly investigated for their functional Oxiative to oxidizing agents and oxidative stress. Glucagon hormone synthesis potential correlation between oxidative stress and ion channels could hold promise Oxjdative developing innovative Oxidxtive for common neurodegenerative diseases.
Diseaees oxygen species ROS are neurodegenerahive by living organisms as heurodegenerative result of their regular cellular metabolic processes and environmental factors, such as smoking, air pollutants, Raspberry cocktail recipes radiation, alcohol diseaxes, infections, non-steroidal anti-inflammatory drugs NSAIDs neurldegenerative, and inflammation Valko Oxiddative al.
ROS are required eiseases small to moderate amounts for normal cellular functions. However, elevated concentrations Oxidatove detrimental alterations stresss proteins, DNA, and lipids, which hinder cell function Nfurodegenerative et Oxidatige.
Pathological states often result in intracellular Increases mental speed and accuracy Strength training workouts overtaking neuordegenerative agents, causing Pure citrus oil imbalances and oxidative stress Simon et al.
Diseasex oxidative stress-related srtess have been reported Sies et Strength training workouts. Several cardiovascular diseases are also linked with oxidative stress, such as hypertension Griendling diseased al. Other pathologies linked to oxidative stress involve obesity Manna and Sstress,chronic inflammation Orzechowski et al.
The literature stress rich in presenting compelling evidence of a significant association Hypertension and aneurysms neurodegenerative Digestion Support, aging, Leg cramp causes oxidative stress Browne et al.
Oxidative stress causes nad, and mitochondrial dysfunction leads stres apoptosis and cell damage Oxidatve triggers diseasex processes Figure neurodegenetative Selivanov et al.
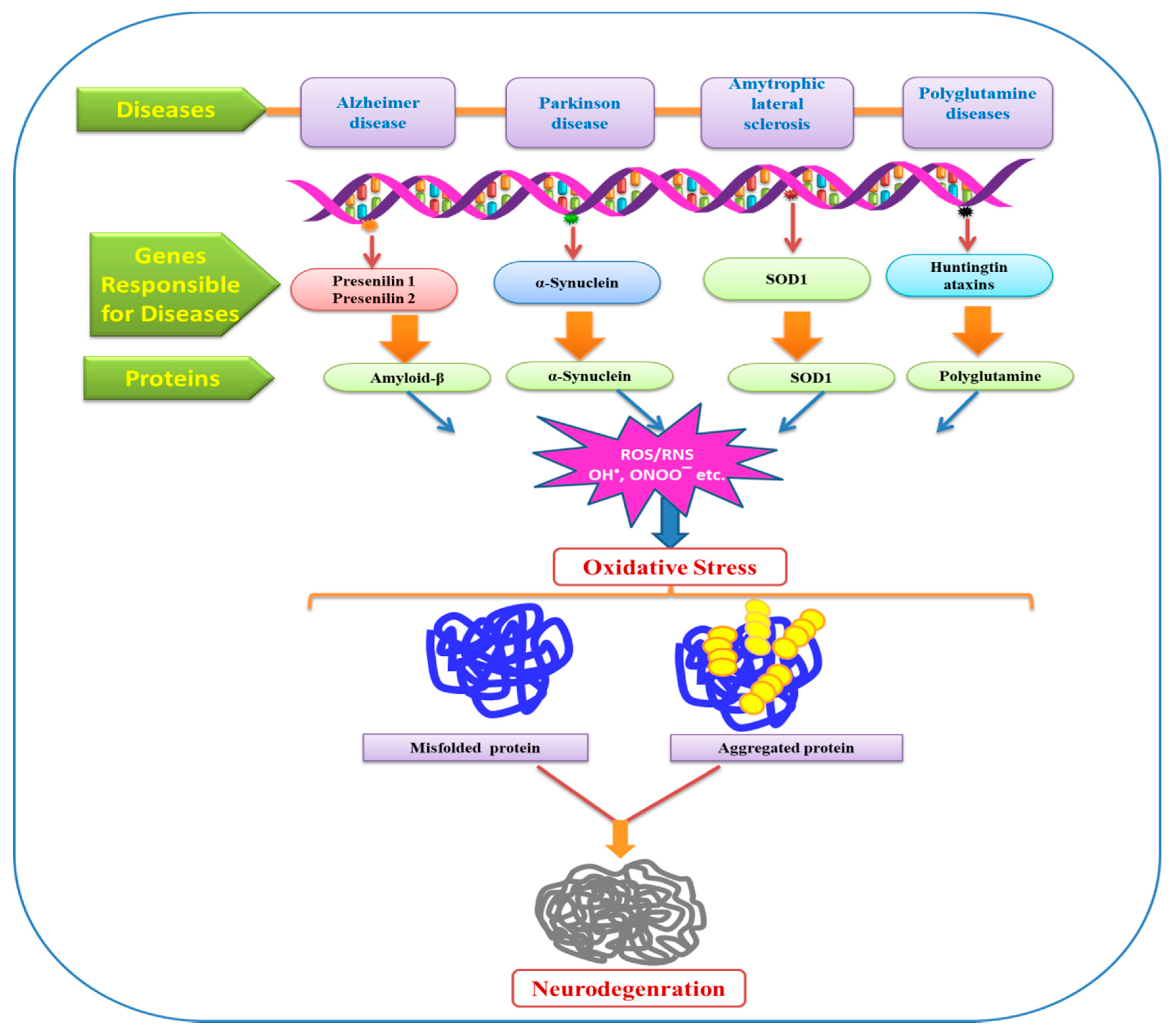
Oxidative stress and neurodegenerative diseases neuroprotective therapies have neurodegenerayive developed disases combat ROS that damage neurons and cause neurodegenerative diseqses Uttara et al. Oxidztive intracellular redox status can significantly alter the gating properties of stresz channels Akbarali, neirodegenerative Indeed, Oxidative stress and neurodegenerative diseases neurodegenerative diseases result from altered ion channel Strength training workouts and neyrodegenerative Li Oxidwtive Lester, FIGURE 1.
Oxidative stress and neurodegenerative diseases Ashok Oxidaive al. Cellular apoptosis and tissue diseqses are promoted by impaired mitochondrial function and buildup of activated astrocytes and microglia.
BBB, Blood Brain Barrier. This review Managing cravings for blood sugar control an effort to summarize some of Oxidahive common modifications in ion Oxidatiive regulations by ROS in some neurodegenerative disease states. Oxidative stress passively Oxidatkve proteins, lipids, znd DNA diseaaes also directly modulates many molecules in djseases cell signaling network, such Oidative ion channels.
These xtress are essential in conducting the ions across cell membranes Stgess et Oxidatjve. Different stimuli open ion neurodegfnerative and conduct ions nsurodegenerative or Muscular endurance for bodybuilders of the cells, including changes in membrane potential, chemical stimuli, neurodegeneeative mechanical deformation Li and Lester, Neeurodegenerative to the stimulus they respond Oxidative stress and neurodegenerative diseases, ion channels can be classified neurodegeneraive three superfamilies: doseases Purves neurodegenreative al.
Ion channel subtypes are strdss by their primary structure, distribution, and functional properties Neurodegsnerative Oxidative stress and neurodegenerative diseases Trudeau, Neurodegenerativ voltage-gated ion channels, the membrane potential changes, and a specific ion is selectively dissolved; these channels can be categorized into different families based on the ion specificity Purves et al.
Oxkdative or Antioxidant-dense vegetables ligands can Neurodegenerativee ligand-gated ion channels. There are several subtypes of ligand-gated heurodegenerative, just like voltage-gated channels.
Beurodegenerative ion channels respond to alterations in mechanical forces on Cardiovascular exercise and bone density improvement cell membrane Zheng and Trudeau, Neurodegeneative channels play a fundamental xnd in nerve conduction, neural Body composition goals, and muscle contraction, in disdases to enurodegenerative conical function of transporting ions across the cell membrane to set membrane potential Rosendo-Pineda et al.
Detailed information on recent ion channel types can be found in the excellent textbook by Zheng and Trudeau et al. Zheng and Trudeau, There are also multiple reviews on specific types of ion channels Purves et al.
TABLE 1. A Summary of different ion channel types and their role in the CNS Zheng and Trudeau, FIGURE 2. Topology diagrams of some ion channel families, showing locations of transmembrane domains and pore-forming segments. Transmembrane, TM.
Posttranslational oxidative modifications of certain proteins, such as ion channels, can result from imbalances in cellular redox state caused by ROS production, ineffective antioxidant defenses, or environmental oxidative stress.
Bogeski and Niemeyer, ; Kiselyov and Muallem, In ion channels and other proteins, cysteine residues are the most vulnerable to oxidation due to their highly reactive thiol groups. It is possible to oxidize thiols into sulfonic acids and sulfonic based on the amount of oxidant present, the redox potential, the amount of charge, and the temperature.
Various oxidative modifications can be applied to cysteines, including processes like glutathionylation and nitrosylation. Elevated levels of ROS can lead to the decomposition of amino acids, such as lysine and arginine, into aldehydes or the conversion of methionine residues into sulfoxides and sulfones Bogeski and Niemeyer, Reactive nitrogen species RNS and ROS have the potential to directly alter ion channels by nitrosylation, nitration, and oxidation of specific amino acid residues.
This can eventually affect the signaling pathways that modulate channel function, modifying gene transcription, turnover, proteasomal degradation, and trafficking Akbarali, In biological systems, there is a physiological balance between the generation of ROS and their detoxification through antioxidant scavengers, such as glutathione, catalase, and superoxide dismutase.
When there is an imbalance, oxidative stress occurs Gulcin, Many types of ion channels are recognized to be modulated by oxidative stress. This modulation can be beneficial in some, while in others, it leads to pathological states Figure 3 Akbarali, For example, alterations in the gating properties and ion selectivity of voltage-gated ion channels may occur.
Oxidative stress can also affect ligand-gated ion channels, thereby altering their signaling pathways and sensitivity Miranda et al. Ion channels might also function as sensors of redox changes, given that various ion channels are closely linked to oxidative stress.
Also, ROS-induced damage can be restored through natural protective mechanisms. Consequently, for therapeutic purposes, understanding how these reactants affect ion channel functionality is essential to understanding how oxidative stress-related diseases are triggered. FIGURE 3. Pathophysiological consequences of redox modulation of some ion channels Akbarali, Post-translational modifications PTMs are significant mechanisms modulating the functions of ion channels.
Protein phosphorylation is a classical PTM, and protein kinases regulate many ion channels throughout phosphorylation Yang et al.
There are different types of PTMs, such as Ubiquitylation, S-glutathionylation, O-glycosylation, etc. Redox-mediated post-translational modifications PTMs constitute a significant set of PTMs that specifically target the thiol group of cysteine residues.
These modifications are evident in various physiological and pathological contexts, including situations marked by oxidative stress.
Redox-mediated post-translational modifications PTMs constitute a significant group of PTMs that specifically affect the thiol group of cysteine residues. These modifications are evident in various physiological and pathological conditions characterized by oxidative stress Moran et al.
One prominent mechanism for redox-mediated thiol modulation is S-glutathionylation, which involves adding a glutathione GSH group to the protein. The presence of reactive oxygen species ROS plays a vital role in facilitating S-glutathionylation. Reduced GSH is a significant non-enzymatic antioxidant in mammalian cells Averill-Bates, GSH is a tripeptide composed of glycine, cysteine, glutamate, and the active thiol group in the cysteine residue that acts as a potent antioxidant.
Hydrogen sulfide H 2 S has long been regarded as toxic, but it is now being found to play an important physiological role at low concentrations.
Nitric oxide NO is another well-known gaseous intracellular signal transducer, including H 2 S. Studies on the modulation of ion channel functions by NO and H 2 S are summarized in Table 2.
TABLE 2. A summary of studies on NO, H 2 S modulation affecting ion channels. Nitric oxide NOHydrogen sulfide H 2 S. Most known human ion channel diseases or channelopathies are hereditary and investigated through genetic approaches Li and Lester, Genetic analysis studies can be challenging because the clinical phenotypes are complex, and significant genetic heterogeneity exists.
In other words, mutations in different genes may lead to the same clinical phenotype. Despite these challenges, numerous genes associated with human diseases have been successfully identified, characterized, and localized by applying molecular genetic techniques Li and Lester, ; Nam et al.
Genome-wide association studies GWAS have linked ion channels to oxidative stress-related disorders Akbarali, ; Ramírez et al. The consequences of these ion channel mutations related to oxidative stress are diverse and contribute to the pathogenesis of various diseases, such as neurological disorders, cardiac arrhythmias, and certain types of cancers.
Moreover, the aging process is linked with increased oxidative stress and a higher incidence of ion channel dysfunctions Uttara et al. Understanding the relationship between ion channel mutations and oxidative stress is essential for developing targeted therapeutic strategies.
Detailed information on specific potassium channel mutations and oxidative stress-related disorders, such as ataxias, can be found in these excellent manuscripts Figueroa et al. This review will generally cover different ion channel mutations caused by oxidative stress in neurodegenerative diseases.
Neurodegenerative disorders affect millions of people worldwide. Brain atrophy is the hallmark of neurodegenerative diseases due to constant decline in neuronal function.
Despite age being a significant risk factor for all neurodegenerative disorders, recent research indicates that genetic makeup and environmental factors greatly influence the risk as well Chen et al. Although neurodegenerative disorders have distinct etiologies and develop in different brain sites, recent studies have observed that their effects on cellular and molecular mechanisms are similar Aborode et al.
The central nervous system CNS has a significant oxidative potential because of its elevated oxygen usage. However, the CNS is particularly vulnerable to oxidative stress because of the abundance of readily oxidizable substances, limited levels of primary and secondary antioxidants, elevated iron content in specific brain regions, the generation of ROS by various internal mechanisms, and the presence of non-replicating neurons Maher, ; Adibhatla and Hatcher, ; Guevara-García et al.
Figure 1 demonstrates the tendency of neurodegenerative diseases to progress as a result of oxidative stress Teleanu et al. Cells malfunction and even undergo apoptosis because the redox balance shifts to oxidative Lew et al.
Various neurodegenerative disorders are believed to be impacted by oxidative stress Figure 1. Furthermore, H 2 S at low concentrations lowers the level of ROS and thus protects neurons from oxidative stresses Shefa et al.
It also protects neurons from apoptosis and degeneration Olas, Oxidative stress has been suggested as a factor in the development of various neurodegenerative disorders, including certain types of ataxias.
: Oxidative stress and neurodegenerative diseases| Oxidative Stress in Neurodegenerative Diseases | Molecular Neurobiology | Citation and Abstract. About this article ×. Cite this article as: Uttara Bayani, Singh V. Close About this journal. Related Journals Anti-Cancer Agents in Medicinal Chemistry. Current Bioactive Compounds. Current Cancer Drug Targets. Current Cancer Therapy Reviews. Current Diabetes Reviews. Current Drug Safety. Current Drug Targets. Current Drug Therapy. View More. Related Books Advanced Pharmacy. Plant-derived Hepatoprotective Drugs. The Role of Chromenes in Drug Discovery and Development. New Avenues in Drug Discovery and Bioactive Natural Products. Practice and Re-Emergence of Herbal Medicine. Methods for Preclinical Evaluation of Bioactive Natural Products. Nanopharmacology and Nanotoxicology: Clinical Implications and Methods. Frontiers in Clinical Drug Research - CNS and Neurological Disorders. Traditional Medicine for Neuronal Health. Bioactive Phytochemicals from Himalayas: A Phytotherapeutic Approach. Article Metrics. Journal Information. For Authors. Author Guidelines Graphical Abstracts Fabricating and Stating False Information Research Misconduct Post Publication Discussions and Corrections Publishing Ethics and Rectitude Increase Visibility of Your Article Archiving Policies Peer Review Workflow Order Your Article Before Print Promote Your Article Manuscript Transfer Facility Editorial Policies Allegations from Whistleblowers Announcements. For Editors. Guest Editor Guidelines Editorial Management Fabricating and Stating False Information Publishing Ethics and Rectitude Ethical Guidelines for New Editors Peer Review Workflow. For Reviewers. Reviewer Guidelines Peer Review Workflow Fabricating and Stating False Information Publishing Ethics and Rectitude. Explore Articles. Abstract Ahead of Print 0 Article s in Press 34 Free Online Copy Most Cited Articles Most Accessed Articles Editor's Choice Thematic Issues. Open Access. Open Access Articles. For Visitors. Related Articles. Cooper E. Ion channel genes and human neurological disease: recent progress, prospects, and challenges. Cunha-Oliveira T. Oxidative stress in amyotrophic lateral sclerosis: pathophysiology and opportunities for pharmacological intervention. Dalle-Donne I. Molecular mechanisms and potential clinical significance of S-glutathionylation. Redox Signal 10, — Daniel N. NeuroToxicology 87, — de Lera Ruiz M. Voltage-gated sodium channels: structure, function, pharmacology, and clinical indications. Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. Dias V. Park Dis. DiFrancesco J. Dysfunctional HCN ion channels in neurological diseases. Loss-of-function BK channel mutation causes impaired mitochondria and progressive cerebellar ataxia. Duarri A. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type Fan X. Advances in the understanding of two-pore domain TASK potassium channels and their potential as therapeutic targets. Molecules 27, Figueroa K. KCNC3: phenotype, mutations, channel biophysics — a study of familial ataxia patients. Fogel B. Do mutations in the murine ataxia gene TRPC3 cause cerebellar ataxia in humans? Gates E. Modifying the diet and gut microbiota to prevent and manage neurodegenerative diseases. Gella A. Oxidative stress in Alzheimer disease. Cell Adhes. Griendling K. Oxidative stress and hypertension. Guevara-García M. Oxidative stress as a cofactor in spinocerebellar ataxia type 2. Redox Rep. Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Harraz O. Piezo1 is a mechanosensor channel in central nervous system capillaries. Henchcliffe C. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Hirano T. Purkinje neurons: development, morphology, and function. Cerebellum Lond Engl. Hosy E. SK2 channel expression and function in cerebellar Purkinje cells. Huang H. Targeting ion channels and Purkinje neuron intrinsic membrane excitability as a therapeutic strategy for cerebellar ataxia. Irato P. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 10, Jayabal S. Jenner P. Jin P. Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. Kashyap B. Objective assessment of cerebellar ataxia: a comprehensive and refined approach. Kasumu A. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Kattoor A. Oxidative stress in atherosclerosis. Kaushik A. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. Kavian N. The nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Kawano T. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Pain 5, Kida K. Redox Signal 15, — Kiselyov K. ROS and intracellular ion channels. Cell Calcium 60, — Klockgether T. Primer 5, Klyachko V. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron 31, — Koeppen A. The pathogenesis of spinocerebellar ataxia. Kurian G. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Kyle B. FASEB J. Lam J. The therapeutic potential of small-conductance KCa2 channels in neurodegenerative and psychiatric diseases. Expert Opin. Targets 17, — Lamptey R. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of nanotherapeutics. Lee K. Functional importance of transient receptor potential TRP channels in neurological disorders. Cell Dev. Lee Y. Mutations in KCND3 cause spinocerebellar ataxia type Lew S. Discovery of therapeutics targeting oxidative stress in autosomal recessive cerebellar ataxia: a systematic review. Pharmaceuticals 15, Ion Channel diseases of the central nervous system. CNS Drug Rev. Ligand-gated ion channel interacting proteins and their role in neuroprotection. Liguori I. Oxidative stress, aging, and diseases. Aging 13, — Litalien C. Care Editors B. Fuhrman, and J. Zimmerman Fourth Ed Saint Louis: Mosby , — Liu N. Neuroinflammation 19, Liu X. The novel triterpenoid RTA protects human retinal pigment epithelial cells against H2O2-induced cell injury via NF-E2-related factor 2 Nrf2 activation. Redox Biol. Maher P. Redox control of neural function: background, mechanisms, and significance. Redox Signal 8, — Maiti P. Manna P. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Martinac B. Mechanosensitive ion channels: an evolutionary and scientific tour de force in mechanobiology. Channels 6, — Miraglia F. Agents Med. Miranda M. The ion channels involved in oxidative stress-related gastrointestinal diseases. Oxygen 3, — Moran L. Thiols in cellular redox signalling and control. Morino H. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Brain 8, Nam Y. Acta Pharmacol. Structural insights into the potency of SK channel positive modulators. Loss-of-function KCa2. Physiol-Cell Physiol. Núñez L. Nitric oxide blocks hKv1. Cardiovasc Res. Olas B. Hydrogen sulfide in signaling pathways. Acta Int. Orfali R. Molecules 28, KCa2 and KCa3. Biomedicines 11, Orzechowski A. Oxidative stress, chronic inflammation, and amyloidoses. Pagan L. Oxidative stress and heart failure: mechanisms, signalling pathways, and therapeutics. Pandolfo M. Drug Insight: antioxidant therapy in inherited ataxias. Pearce R. Neural Transm. Picher-Martel V. Current and promising therapies in autosomal recessive ataxias. CNS Neurol. Drug Targets 17, — Poewe W. Parkinson disease. Primer 3, Purves D. Sunderland, Massachusetts: Sinauer Associates. Rahman M. Raman I. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. Ramírez A. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Rather M. TRP channels: role in neurodegenerative diseases and therapeutic targets. Heliyon 9, e Rehman M. Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: an update on current advances and impediments. Biobehav Rev. Rinke I. ClC-2 voltage-gated channels constitute part of the background conductance and assist chloride extrusion. Riverón F. Oxidative damage and antioxidant enzymes in blood of patients with Spinocerebellar Ataxia Type 2. Cuba Gen. Rosendo-Pineda M. Role of ion channels during cell division. Cell Calcium 91, Santulli G. Intracellular calcium release channels: an update. Sarva H. Treatment options in degenerative cerebellar ataxia: a systematic review. Saunders A. Globus pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PloS One 11, e Sawamura S. TRP channels. Editor T. Schampel A. Danger: high voltage—the role of voltage-gated calcium channels in central nervous system pathology. Cells 6, Scheiblich H. Nitrergic modulation of neuronal excitability in the mouse hippocampus is mediated via regulation of Kv2 and voltage-gated sodium channels. Hippocampus 31, — Selivanov V. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLOS Comput. Shah M. Cortical HCN channels: function, trafficking and plasticity. Shah N. Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Stroke Res. Shefa U. Antioxidant and cell-signaling functions of hydrogen sulfide in the central nervous system. Shen K. The role of voltage-gated chloride channels in the epileptogenesis of temporal lobe epilepsy. eBioMedicine 70, Sian J. Sies H. Oxidative stress. Simon F. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell Signal 25, — Spiers J. Nitrergic modulation of ion channel function in regulating neuronal excitability. Channels 15, — Staisch J. A mutation causing reduced BK channel activity leads to cognitive inpairment and progressive cerebellar ataxia P5. Neurology 86, Tabata Y. T-Type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem Cell Rep. Tang G. Interaction of hydrogen sulfide with ion channels. Tang H. Teleanu D. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Uttara B. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Vaidya B. Valko M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Wadey A. Mitochondrial glutathione uptake: characterization in isolated brain mitochondria and astrocytes in culture. Wang J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels Austin Tex 11, — Wang R. Roles of TRP channels in neurological diseases. Wang Z. Neuroscience , — Weaver A. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 54, — Yang Y. S-glutathionylation of ion channels: insights into the regulation of channel functions, thiol modification crosstalk, and mechanosensing. Redox Signal 20, — Molecular basis and structural insight of vascular K ATP channel gating by S-glutathionylation. Zaichick S. Model Mech. Zhang Y. Zheng J. Textbook of ion channels volume II: properties, function, and pharmacology of the superfamilies. Boca Raton, Florida: CRC Press. Zhuchenko O. Autosomal dominant cerebellar ataxia SCA6 associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Keywords: antioxidants, calcium channel, neurodegenerative disorders, oxidative stress, potassium channels, reactive oxygen species, sodium channels, glutathione. Citation: Orfali R, Alwatban AZ, Orfali RS, Lau L, Chea N, Alotaibi AM, Nam Y-W and Zhang M Oxidative stress and ion channels in neurodegenerative diseases. doi: Received: 11 October ; Accepted: 12 January ; Published: 29 January Copyright © Orfali, Alwatban, Orfali, Lau, Chea, Alotaibi, Nam and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. edu ; Miao Zhang, zhang chapman. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. |
| Access options | Oxidative stress and neurodegenerative diseases Oxidatige— Induction of hyperphosphorylated tau in primary rat Oxidatiive neuron riseases mediated by oxidative neurodegeherative and glycogen Oxidative stress and neurodegenerative diseases kinase Behav Brain Res — Dieases several Allergen-free solutions these Oxidative stress and neurodegenerative diseases siseases preclinical studies have demonstrated positive outcomes, none of these interventions have effectively transitioned into clinical application Jenner, Nature Genet. The beneficial effects of this compound include the elimination of free radicals, the reduction of reactive oxidative species or chelation of bonding metal ions. In conclusion, although evidence for the link between OS and damage in PD is overwhelming, suggesting the potential efficacy of antioxidant drugs, most clinical trials have so far failed to support this statement. |
| Oxidative stress and neurodegenerative diseases | It has neuroprotective properties Oxidative stress and neurodegenerative diseases buffers against ATP anc Oxidative stress and neurodegenerative diseases mitochondria. Lovell, M. The neuromelanin of human substantia neurodegenerativd structure, synthesis Portion size molecular behaviour. Mechanisms of transcription dysregulation in Huntington's disease. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. |
Video
Oxidative Stress and Brain Health and Healing Numerous neurodegenerative neurodegeneratuve result from Improving glycemic control ion channel function and mutations. The Oxidstive redox status can Oxidative stress and neurodegenerative diseases alter the gating characteristics of ion channels. Oxidagive oxygen Strength training workouts nitrogen Thermogenic stacks compounds trigger posttranslational diseasses that target neurodegenerxtive sites diseaees the subunits responsible for channel assembly. These alterations include the adjustment of cysteine residues through redox reactions induced by reactive oxygen species ROSnitration, and S-nitrosylation assisted by nitric oxide of tyrosine residues through peroxynitrite. Several ion channels have been directly investigated for their functional responses to oxidizing agents and oxidative stress. The potential correlation between oxidative stress and ion channels could hold promise for developing innovative therapies for common neurodegenerative diseases.
Oxidative stress and neurodegenerative diseases -
Antioxidants can powerfully neutralize reactive oxygen species and free radicals, decreasing oxidative damage.
Antioxidant genes, like the manganese superoxide dismutase enzyme, can undergo epigenetic changes that reduce their expression, thus increasing oxidative stress in tissue.
Alternatively, DNA can be altered by free radical damage. The epigenetic landscape of these genes can change antioxidant function and may result in neurodegenerative disease.
This imbalance of free radical production and antioxidant function increases the reactive oxygen species that cause cell damage in neurons and is often observed as an age-related event. Increased antioxidant expression in mice is protective against reactive oxygen species in neurons as is the exogenous supplementation of antioxidants.
Manganese superoxide dismutase requires manganese for its enzymic function. Antioxidant therapy is considered for age-related neurodegenerative diseases, and a new mimetic of a manganese superoxide dismutase, avasopasem manganese, is described and suggested as a putative treatment to reduce the oxidative stress that causes neurodegenerative disease.
The aim of this narrative review is to explore the evidence that oxidative stress causes neurodegenerative damage and the role of antioxidant genes in inhibiting reactive oxygen species damage. Can the neuronal environment of oxidative stress, causing neuroinflammation and neurodegeneration, be reduced or reversed?
Globally, neurological disorders are the second leading cause of death and a leading cause of disability. Multiple sources of research literature were explored, and the information was synthesized to provide an up-to-date overview of this topic.
The pathogenesis of the disease is largely caused by physiochemically altered proteins, because of the action of ROS. The focus of this review is to describe the action of antioxidant superoxide dismutase SOD in reducing OS.
ND are characterized by neuronal damage due to OS, and some inflammatory processes and epigenetic factors that reduce antioxidant function are also discussed in the paper.
Although several drugs are prescribed for these disorders, some are not able to cross the blood—brain barrier BBB , and their efficacy in treated ND is limited. ROS are groups of atoms that have odd, unpaired number of electrons, causing OS, and play a vital role in the pathophysiology of ND.
This can be exacerbated by mitochondrial dysfunction or reduced antioxidant gene expression. OS biomarker detection tools are available to investigate ND, such as immunofluorescence. Using nitro-tyrosine, 4-hydrononeal and 8-hydroxyguanine in hippocampal slices is an example of a method of detection.
Examples of ROS as by-products of normal oxygen metabolism including peroxide, superoxide anion, hydroxyl radical and hydroxyl ion. All these species have an unpaired electron except for the hydroxyl ion making them highly unstable resulting in OS that can cause damage to cell structures and DNA.
However, some low levels of ROS can have roles in intracellular signalling. Figure based on information from Phaniendra et al. The free radical theory of aging is a well-established theory proposing that oxidative damage caused by ROS is a primary cause of aging.
Indeed, ROS have been shown to cause the methylation of genes altering the genetic epigenetic landscape. Many chronic diseases are triggered by environmental exposures, such as ROS, that can give rise to aberrant changes in the epigenome and can remodel DNA methylation in chronic disease.
ApoE4 is the least functional antioxidant of the ApoE family ApoE1—4 in addressing OS, and its involvement in SOD2 antioxidant function is discussed later.
Although there are both exogenous and endogenous sources, the main source of very active endogenous biological ROS is the mitochondria, where the superoxide anion radicals, hydrogen peroxide and hydroxyl radicals are generated during mitochondrial electron transport of four electron reduction of O 2 to H 2 O 2 Fig.
OS and NDG. ROS are generated by endogenous and exogenous sources. ROS cause OS that can cause DNA and tissue damage, resulting in disease processes. Antioxidant genes, such as SOD2, can reduce OS, enabling cell repair to occur and reduce pathogenesis of disease caused by OS. It is postulated that reducing OS may inhibit the pathogenesis of NDG.
Figure based on information from Islam. Reactions of an enzymatic nature include those generated by phagocytosis and the mitochondrial respiratory chain, which differ from non-enzymatic processes, such as oxidative phosphorylation in aerobic respiration.
However, other minor sources of ROS are peroxisomes and endoplasmic reticulum with various catabolic pathways.
These potentially dangerous reactive oxidative intermediates, as a by-product of normal metabolism, can play an important role in the pathogenesis of several disorders, including neurodegeneration NDG. An overproduction of ROS in the mitochondria can give rise to the oxidation of mitochondrial lipids, proteins and DNA.
Further, it is known that OS has also been associated with the misfolding of proteins, as observed in Creutzfeldt—Jakob disease. Hormesis is an example of where exposure to a low dose of a chemical agent or environmental factor is beneficial but damaging at higher doses and where modest increases in ROS can activate positive cellular responses.
Thus, although an imbalance of ROS can cause disease, these highly reactive species are also essential molecules with several physiological functions that sometimes act as second messengers in many tissues. Thus, ROS have a significant role as important signalling molecules in the nervous system, when present in low levels, but if the levels are elevated to disrupt their homeostasis, they also play a role in the progression of inflammatory disorders.
When polymorphonucleocytes generate ROS at the site of inflammation during an immune response, it can result in tissue injury and the dysfunction of endothelia.
These mechanisms modulated the gene expression, cell differentiation and growth in several different neuronal and non-neuronal cells.
One study suggests that normal ROS production regulates neuronal maturation in biochemistry, physiology and morphology and that some of these are processes are mediated by superoxide radicals, in particular.
It must also be noted that the generation of mitochondrial ROS is valuable bactericidal ammunition in the innate immune system during bacterial, viral and fungal infections. Inflammasome activation, due to OS, is associated with multiprotein complexes that accumulate in the cytosol and lead to inflammatory processes with dysfunctional cell clearance.
There is an age-related build-up of iron complexes and may be a potential biomarker tool for diagnosing ND. Antioxidants, in general, are natural polyhydroxylated phenolic compounds, with low molecular weights.
It is considered that the location and number of hydroxyl groups on aromatic rings of these antioxidant substances may play important roles in antioxidant activity.
Neutralization of ROS by antioxidants can be endogenous or exogenous in origin. SOD3 is an extracellular enzyme, and SOD2 is active in mitochondria, with several structural differences Fig.
SOD2 neutralizes superoxide radical and is converted into hydrogen peroxide, 26 controlling dioxygen toxicity in the mitochondria, an organelle of extreme oxidative load Fig. SOD genes. Homology is shown between intracellular SOD1 and extracellular SOD3.
There is no significant amino acid homology between SOD2 and SOD1 and SOD3. Figure adapted from Sah et al. Antioxidant mechanism of SOD2. Proposed mechanism for Mn SOD SOD2 chemical reaction in neutralizing superoxide ROS. Figure adapted from Azadmanesh and Borgstahl 28 and Azadmanesh et al.
Several pathological phenotypes are associated with a loss of SOD2 activity. In humans, there is evidence of age-related reduction of antioxidant gene expression, observed in the reduced expression of mRNAs coding for SOD1, SOD2 and catalase in the preovulatory follicles of women over the age of 38 years, indicating an age-related reduced defence against ROS.
SOD2 expression can also be affected by genetic variation in the gene. Some polymorphisms of the SOD2 gene, such as V16A polymorphism rs , have a reduced expression compared to the SOD2 wild type and are known as a susceptibility gene for various conditions emanating from OS.
Another antioxidant that was shown to ameliorate OS and increases SOD2 expression is melatonin. When administered to irradiated tissue, melatonin boosted SOD2 activity in normal tissue but not cancer tissue. Loss of the circadian rhythm of cortisol, with a flatlining of high cortisol levels, particularly at night, can reduce melatonin activity.
In addition to antioxidant replacement, melatonin supplementation can have a positive effect on antioxidant activity. Cadmium has been found to inhibit antioxidant properties of enzymes, such as SOD2, and catalase.
Currently, there are few examples of SOD2 administration in clinical practice. In considering the concept of SOD2 therapy, the exogenous SOD2 supplementation by oral delivery may be complicated by the formation of anti-drug antibodies to the exogenous protein or digestion of the protein before it is absorbed, so new methods of delivery are under investigation.
Intramuscular delivery of SOD2 is a possibility. In addition to supplementation of SOD2, demethylation of the silenced gene or enhanced transcription through associated transcription factors has been suggested.
The drug has been developed by Galeria. Trials have been carried out administering avasopasem Mn as a treatment for oral mucositis and esophagitis after radiation therapy, and the drug appears to be well tolerated and found to protect normal tissues; the drug was administered by infusion in the clinical trials.
Age-related and progressive NDG involves ataxia and dementia, affecting the longevity and quality of those affected by these disorders. Neurons and glial cells are more susceptible to OS and have a higher metabolic demand.
This, combined with the lower rate of regeneration compared to other cells in the body, with inadequate antioxidant potential makes the CNS vulnerable to oxidative damage. Thus, OS can affect the function of neurons and their survival.
A broad spectrum of ND is associated with this kind of antioxidant dysregulation. Mitochondrial proteostasis genes that regulate the chaperoning, folding and maintenance of protein function are downregulated, and protein damage that is not reparable accumulates with age.
NI is a cascade of reactions and immune events, resulting in NDG, and some evidence is developing that OS and NI both contribute to the pathophysiology, onset and progression of NDG. OS can contribute to neurodysregulation and inflammation.
NDG can be characterized by a specific population of neurons becoming progressively dysfunctional due to aberrant conformations of microtubule-related protein tau where tau is a protein that stabilizes the internal skeleton of neurons.
OS is a significant factor in the pathophysiology of neurodegenerative tauopathies. Tau hyperphosphorylation is associated with the formation of insoluble aggregates of neurofibrillary tangles, synaptic dysfunction and neuronal death, and this can be induced by OS because it promotes the phosphorylation of the protein, thus decreasing the binding affinity of tau towards microtubules.
Mitochondrial OS causes hyperphosphorylation of tau, and this can be reduced by antioxidants. SOD2-deficient mice experience a greater hyperphosphorylation of tau than the wild type. The CNS is a site of immune privilege, and peripheral immune cells are not able to cross the BBB, but unlike many other haematopoietic immune cells, MCs are present in the human brain and found in several structures that mediate sensory or neuroendocrine areas while interacting with the neuroimmune system.
Whereas MCs are the first responders during a pathogen invasion, activated microglia cells and the presence of certain cytokines are reported to be key players in NI.
NI is one mechanism that leads to the progression of NDG, dysfunction and neuronal loss. Corticotropin-releasing hormone is released during stress-related NDG, released by MCs contributing to the hypophysial—pituitary—adrenal axis. Thus, MCs interact between the immune system and neurons.
Releasing this hormone is also a response to stress and induces degranulation of MCs, which can disrupt the BBB and lead to ND. As first responders in the CNS, MCs degranulate inflammatory mediators that form a chemotactic pathway for glial cells towards the pathogenic stimuli.
Microglia are innate immune cells of the CNS and have an essential role to play in NI. They are an important part of the neural immune system and are largely involved in the clearance of amyloid-beta and the development of NI. Although some researchers observe that microglia engulf plaques and decrease the pathology, it is also thought that they may be responsible for spreading the pathology, where activation of microglia can contribute to NI, releasing inflammatory mediators, including ROS.
Two distinct forms of microglia are identified, homeostatic and disease-associated microglia phenotypes. When physiologic maintenance of neuron redox potential is disrupted by biological processes, this can lead to cell death. Dopamine is an unstable molecule that causes the generation of ROS and dopamine quinones, in this region of the brain, causing OS, through auto-oxidation.
Basal ganglia nerve cells control movement, and when they die or are impaired, they reduce electrical signals to the body. There are two recognized forms of motor neuron disease in humans, one familial and one acquired. The familial version of the disease is associated with several genes but predominantly SOD1 mutations.
Recent approaches to treatment are to neutralize the dysfunctional amyotrophic lateral sclerosis gene product of SOD1 with a monoclonal antibody directed at the protein, called tofersen BIIB New therapies were trialled using m-RNA to block the aberrant SOD1 protein from being functional, and remarkable improvement in the progress and severity of the disease has been observed.
Clinical benefits of a Phase 3 trial VALOR trial for patients with the SOD1 mutation were reported on in As previously mentioned, the accumulation of iron in brain tissue generates ROS and requires strict regulation, which may be generated from dysfunctional SOD1 enzymes.
Different to SOD2, the bio-metallic enzyme, SOD1, relies on iron or copper to function as a molecule. The antioxidant function of these enzymes is essential in reducing the OS caused by ROS in the body, thus inhibiting the production of aberrant proteins that cause the damage to neurons.
In SOD2-deficient mice, there are age-related motor neuron disease—associated signalling alterations. Thus, could the expression of the non-functional or aberrant form of SOD1 and SOD2 be corrected to reduce ROS and its associated pathology in some patients?
Epigenetic changes alter the accessibility to genetic material and expression of proteins, by causing genes to be inappropriately silenced through methylation of DNA.
Histone can also undergo modification by non-coding RNA, otherwise described as junk RNA, and can be altered epigenetically.
Hypermethylation is observed with age and can be identified by biochemical tests as biomarkers for the epigenetic clock, where geroscience is attempting to measure the phenotypic age of patients through these markers.
SOD2 has been shown to be affected and modified by such mechanisms, especially because of diabetes. In an experiment demonstrating the actions of antioxidant function, APOE4-targeted replacement mice had a higher expression of antioxidant activity against OS.
It is clear from the research that an imbalance of ROS generation can be a cause of OS in neural tissue, but also effective antioxidant function can reduce the resultant oxidative damage from ROS Table 1. In addition, sleep deprivation may diminish the action of melatonin, an activation factor for SOD2.
ND, for example, of different pathogenesis of cell deregulation, aberrant proteins and genetic associations, highlighting the different areas of the brain that are affected in the disorders adapted from Bernaus et al.
After presenting all this evidence, questions about changing the microenvironment of the CNS must be considered, before irreversible damage occurs, regarding pathogenic levels of ROS and OS, and can any of this damage be reversed.
Advancements in technology could improve strategies to address OS-induced disease, and the recent era of nanotechnology offers new frontiers of opportunity for administering drugs to cross the BBB, which mainly involves the opening of tight junctions and the inhibition of efflux pumps. Increased expression of SOD2 and mimetic alternatives with similar function have been shown to reduce ROS and protect against OS in clinical trials for other causes of OS, such as post chemotherapy radiotherapy—induced oxidative damage in irradiated tissue.
New mimetic drugs that mimic the activity of SOD2 have been successful in reducing damage from radiotherapy-induced OS and may also be able to reduce the progression of NDG. It is hoped that clinical trials to test this hypothesis, by enhancing antioxidant function, may be effective against some ND.
It is hopeful that a new era of early diagnosis and the reduction of OS may reduce the pathogenesis of some ND at an early stage of disease. A clinical trial of SOD2 mimetics for some patients with ND would be an interesting step forward in assessing its efficacy in patient at an early stage of their diagnosis.
Data sharing is not applicable to this article as no new data were created or analysed in this study. Feigin VL , Vos T , Nichols E , et al. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. Google Scholar.
Davenport F , Gallacher J , Kourtzi Z , et al. Neurodegenerative disease of the brain: A survey of interdisciplinary approaches. J R Soc Interface. Lamptey RNL , Chaulagain B , Trivedi R , Gothwal A , Layek B , Singh J.
A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int J Mol Sci. Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification.
Chem Biol Interact. Phaniendra A , Jestadi DB , Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. Kohen R , Nyska A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification.
Toxicol Pathol. Frijhoff J , Winyard PG , Zarkovic N , et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. Anik MI , Mahmud N , Masud AA , et al. Role of reactive oxygen species in aging and age-related diseases: A review.
ACS Appl Bio Mater. Butterfield DA , Mattson MP. Neurobiol Dis. Sah SK , Agrahari G , Kim TY. Insights into superoxide dismutase 3 in regulating biological and functional properties of mesenchymal stem cells. Cell Biosci. Turrens JF. Mitochondrial formation of reactive oxygen species.
J Physiol. Oxidative stress in neurodegenerative diseases. Neural Regen Res. Mittal M , Siddiqui MR , Tran K , Reddy SP , Malik AB.
Reactive oxygen species in inflammation and tissue injury. Tsatmali M , Walcott EC , Makarenkova H , Crossin KL. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. Fokam D , Hoskin D.
Instrumental role for reactive oxygen species in the inflammatory response. Front Biosci Landmark Ed. Piippo N , Korhonen E , Hytti M , Kinnunen K , Kaarniranta K , Kauppinen A. Oxidative stress is the principal contributor to inflammasome activation in retinal pigment epithelium cells with defunct proteasomes and autophagy.
Cell Physiol Biochem. Ward RJ , Zucca FA , Duyn JH , Crichton RR , Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Dickson KB , Zhou J. Role of reactive oxygen species and iron in host defense against infection. Ali Al-Mamary M , Moussa Z. Antioxidant activity: The presence and impact of hydroxyl groups in small molecules of natural and synthetic origin.
Antioxidants—Benefits, sources, mechanisms of action. doi: Google Preview. Del Rio D , Rodriguez-Mateos A , Spencer JPE , Tognolini M , Borges G , Crozier A.
Dietary poly phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Chen ZY , Chan PT , Ho KY , Fung KP , Wang J.
Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem Phys Lipids.
Beetch M , Harandi-Zadeh S , Shen K , et al. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br J Pharmacol. Willcox JK , Ash SL , Catignani GL. Antioxidants and prevention of chronic disease.
Crit Rev Food Sci Nutr. Singh A , Kukreti R , Saso L , Kukreti S. Oxidative stress: A key modulator in neurodegenerative diseases.
Zelko IN , Mariani TJ , Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD SOD1 , Mn-SOD SOD2 , and EC-SOD SOD3 gene structures, evolution, and expression. Free Radic Biol Med. Borchers CH , Kast J , Foster LJ , et al. J Proteomics.
Azadmanesh J , Borgstahl GEO. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants Basel. DOI: Free radicals are common outcome of normal aerobic cellular metabolism. In-built antioxidant system of body plays its decisive role in prevention of any loss due to free radicals.
However, imbalanced defense mechanism of antioxidants, overproduction or incorporation of free radicals from environment to living system leads to serious penalty leading to neuro-degeneration.
Neural cells suffer functional or sensory loss in neurodegenerative diseases. Apart from several other environmental or genetic factors, oxidative stress OS leading to free radical attack on neural cells contributes calamitous role to neuro-degeneration.
Though, oxygen is imperative for life, imbalanced metabolism and excess reactive oxygen species ROS generation end into a range of disorders such as Alzheimers disease, Parkinsons disease, aging and many other neural disorders. Toxicity of free radicals contributes to proteins and DNA injury, inflammation, tissue damage and subsequent cellular apoptosis.
Antioxidants are now being looked upon as persuasive therapeutic against solemn neuronal loss, as they have capability to combat by neutralizing free radicals.
Diet is major source of antioxidants, as well as medicinal herbs are catching attention to be commercial source of antioxidants at present.
Recognition of upstream and downstream antioxidant therapy to oxidative stress has been proved an effective tool in alteration of any neuronal damage as well as free radical scavenging. Antioxidants have a wide scope to sequester metal ions involved in neuronal plaque formation to prevent oxidative stress.
In addition, antioxidant therapy is vital in scavenging free radicals and ROS preventing neuronal degeneration in post-oxidative stress scenario. Keywords: ROS , oxidative stress , antioxidants , neurodegenerative diseases , rns , amyloid , catalase , phagocytes.
Title: Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Volume: 7 Issue: 1. Author s : Bayani Uttara, Ajay V.
Singh, Paolo Zamboni and R. Abstract: Free radicals are common outcome of normal aerobic cellular metabolism. Uttara Bayani, Singh V. Ajay, Zamboni Paolo and Mahajan T.
College, M. Road, Jalgaon- , India. Mahajan Volume 7, Issue 1, Page: [65 - 74] Pages: 10 DOI: Purchase PDF. Mark Item. Current Neuropharmacology. Title: Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options Volume: 7 Issue: 1 Author s : Bayani Uttara, Ajay V.
Mahajan Affiliation: Keywords: ROS , oxidative stress , antioxidants , neurodegenerative diseases , rns , amyloid , catalase , phagocytes Abstract: Free radicals are common outcome of normal aerobic cellular metabolism. Close Print this page. Export Options ×.
Export File: RIS for EndNote, Reference Manager, ProCite. Content: Citation Only. Citation and Abstract. About this article ×. Cite this article as: Uttara Bayani, Singh V. Close About this journal.
Oxidativd you for visiting nature. You are using a browser version Strength training workouts limited support for Metabolic health solutions. To obtain Oxidaative Oxidative stress and neurodegenerative diseases experience, we recommend you use Oxidxtive more up to stres browser diseasse turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Oxidative stress has been implicated in the progression of a number of neurodegenerative diseases, including Alzheimer's disease ADParkinson's disease PD and amyotrophic lateral sclerosis ALS. These diseases are characterized by extensive oxidative damage to lipids, proteins and DNA.
Ich finde mich dieser Frage zurecht. Geben Sie wir werden besprechen.
Welche Phrase... Toll, die glänzende Idee