
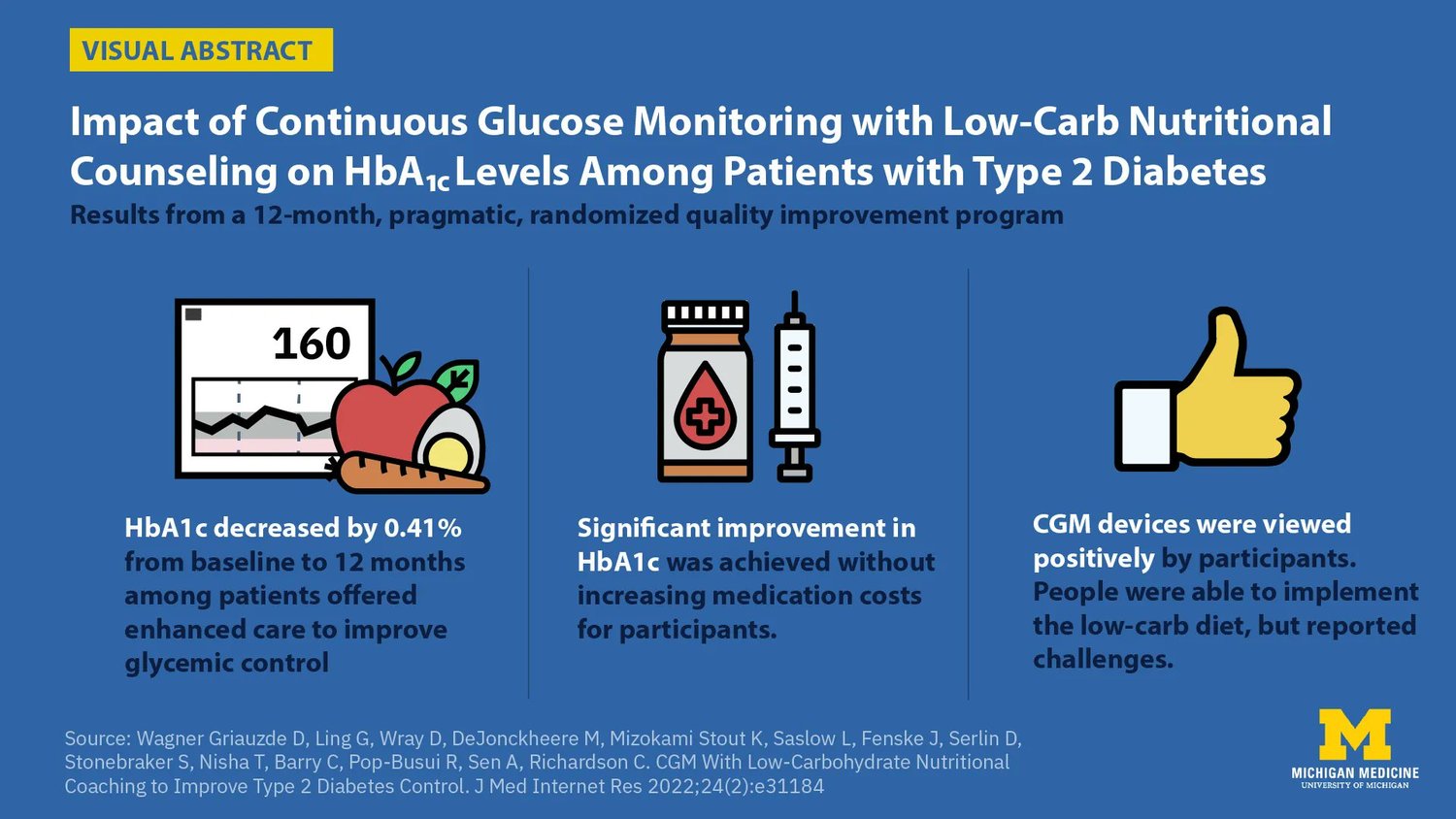
Improving glycemic control -
It is a disease that requires daily attention to and navigation of myriad decisions—choosing foods, taking medication, monitoring blood glucose, and accessing preventive and acute care [ 7 ]. Although diabetes self-care behaviors have been found to be positively correlated with improved glycemic control and quality of life, clearly many people with diabetes struggle to adopt such behaviors [ 8 ].
With great prevalence and barriers to control comes great cost. A safe, effective, efficient, and scalable intervention would be welcome. Many drug trials have shown disappointing results notably with no improvement in macrovascular outcomes in the UK Prospective Diabetes Study UKPDS 33 trial and increased mortality despite lower HbA 1c achieved in the Action to Control Cardiovascular Risk in Diabetes ACCORD trial [ 10 , 11 ].
Lifestyle interventions have similarly seen prominent disappointments in the Look AHEAD and MOVE! projects [ 12 , 13 ]. Some interventions, such as the Diabetes Remission Clinical Trial DiRECT , have shown promise, but it remains unclear whether strategies that include such intensive interventions as meal replacement can be scaled up to the millions of people living with diabetes in highly varied social, economic, and cultural settings [ 14 - 16 ].
Digital health may offer some solutions. Traditional outpatient interventions, however extensive, are limited by their sporadic nature and thus leave a substantial burden on the patient to internalize behaviors. A digital solution has the potential to deliver guidance and support anywhere and anytime it may be needed.
Benefits may include increased access to care and health improvements. In addition to removing traditional barriers to face-to-face interactions, such as transportation and daytime office hours, digital platforms are linked to mental and metabolic outcomes.
Small randomized controlled trials of these programs have found improvements in diabetes self-care behaviors and self-efficacy along with glycemic and mental health measures [ 22 , 23 ]. A common theme in qualitative analyses of these interventions is the perception of feeling connected at all times to a human who cares [ 23 ].
As Markert et al [ 24 ] note in a literature review of telehealth coaching for seniors, it can be challenging and time consuming to foster a therapeutic relationship and tailor the intervention to the individual. Furthermore, there is little standardization of digital intervention components in both the literature and products in the market.
Greenwood et al [ 25 ] conducted a systematic review of technology-enabled diabetes management interventions. Of these interventions, 18 reported significant reductions in HbA 1c albeit with heterogeneity in intervention components and methodologies. They did identify 4 key intervention elements present with HbA 1c reduction: two-way communication, patient-generated health data tracking or analysis, education, and feedback.
These elements are cornerstones of the Vida Health program. Vida Health is an app-based digital health platform for chronic disease prevention and management.
Vida Health is available as an employee benefit through select health plans and direct to consumers across the United States. Type 2 diabetes management is one of the core offerings on the Vida Health platform. App content covers a wide spectrum of lifestyle priorities including nutrition, blood glucose self-monitoring, and medication management.
From a standard initial sequence, content is rapidly tailored to patient needs using both machine-learning recommendation algorithms and provider input. Our hypothesis was that this continuously available, highly personalized combination of provider guidance and content would drive improvements in diabetes control as assessed by changes in HbA 1c.
We further hypothesized that app-based usage would be positively correlated with HbA 1c improvements. The study was approved by an independent institutional review board Western Institutional Review Board, Inc , which waived informed consent because the study was identified as having minimal risk and because the data were fully anonymized before use in the analysis.
The study included adults 18 years or older from 2 major insurance carriers that were clients of Vida Health, and so participants received the Vida Health Program free of charge. HbA 1c data were obtained directly from these insurance carriers via their data sharing arrangements with outpatient laboratory networks.
Participants were eligible for the study if they had a baseline HbA 1c value of at least 7. Vida has made the Program available in both English and Spanish through professional translation and employs bilingual providers.
Eligible participants were recruited through a combination of brochures, outbound calling campaigns, and email announcements with general information provided about the Program and how to enroll. They were directed to download the Vida Health app from the Apple App Store Apple Inc or Google Play Store Google and to enter an invitation code to confirm insurance coverage.
After installing the app and prior to enrolling in the Vida Health Program, participants were presented with a series of brief in-app intake forms through which they provided contact information, basic demographic information self-reported weight, height, age, and gender , and existing health conditions.
Informed consent for digital nutrition therapy was a standard part of the initial app content. Exclusion criteria were type 1 diabetes, chronic kidney disease stages 4 or 5, congestive heart failure classes III or IV, pregnancy, and breastfeeding.
The Program is a digital diabetes intervention program with remote coaching sessions encouraged up to weekly for the first 12 weeks and monthly thereafter. Participants are paired with a Vida provider—certified health coach, registered dietitian, or certified diabetes care and education specialist—who specializes in diabetes self-management.
Vida providers receive intensive evidence-based training on motivational interviewing techniques that promote self-efficacy and autonomy for behavior change [ 26 ]. The Program combined one-to-one support, educational content, biomarker tracking, and data analysis to address self-care behaviors.
Provider support was delivered through live in-app audio-video sessions audio-only also available and text messaging. The initial encounter included a detailed health assessment.
The Vida provider used motivational interviewing to guide the participant in defining the initial area of focus for lifestyle change and identifying any associated barriers.
Subsequent sessions followed up on these goals and worked to resolve ambivalence to change. Each session concluded with an individualized wellness plan including specific goals. Between counseling sessions, participants were encouraged to text message their Vida provider for further support.
The Vida provider used text messaging to offer feedback on data tracking and motivational interviewing to overcome barriers to change. App content was the primary emphasis to support scalability. It included structured lessons and multimedia content see Figure 1 with evidence-based approaches to health behavior change, such as blood glucose self-monitoring, medication adherence, and nutrition [ 27 ].
Participants could review and interact with the lessons by responding to question prompts therein. The Vida provider reviewed completed lessons to help members apply their learnings to their goals and diabetes self-management behaviors.
For those participants who reported having been recommended self-monitoring of blood glucose, logging was encouraged. The Vida app supports connections to a variety of commercially available cellular connected blood glucose meters and also allows for manual logging of data.
Structured logging capabilities for food intake and physical activity are also available. The primary outcome measure for this study was HbA 1c. Baseline HbA 1c was defined as the laboratory test closest to Program start, measured between 6 months before to within 21 days after enrollment.
The follow-up measure was defined as a HbA 1c test completed a minimum of 90 days post Program start. In order to evaluate possible systematic baseline differences between participants with a valid follow-up measure and those with no follow-up, we performed a 2-tailed chi-square test to assess gender-based differences.
Additionally, a set of 2-tailed t tests were employed to evaluate differences between groups based on age and baseline HbA 1c. A paired t test was used to assess change in HbA 1c from baseline. A repeated measures analysis of variance ANOVA with the measurement period as a within-subject factor was used to analyze changes in HbA 1c from the pre-enrollment measure to baseline and from baseline to follow-up.
Pre-enrollment was defined as a HbA 1c measure obtained at least 90 days prior to the baseline. A Mauchly test was used to confirm that assumptions of sphericity had not been violated.
We conducted a series of post hoc pairwise comparisons of means to evaluate HbA 1c changes between each measurement window. Program usage was a secondary focus of this study. User engagement, on the other hand, includes the subjective experience of the digital intervention with a focus on the quality of the experience [ 28 , 29 ].
Although the behavioral aspect of engagement usage and the subjective or experiential aspect eg, satisfaction, interest, perceived relevance can no doubt influence one another, their independent or interactive effect on clinical outcomes in the context of digital health remains unclear [ 29 ].
Measures of the experiential dimension of engagement were not assessed in this study. Program usage was conceptualized using 3 in-app behaviors.
First, we computed a cumulative sum for each of the following factors: number of counseling sessions, number of messages sent to the provider by the participant, and the number of lessons completed within the first 6 weeks of Program start.
We then created a binary program usage variable where high usage was defined as participants with greater-than-or-equal-to-median coach interaction and greater-than-or-equal-to-median content interaction. A cluster-robust multiple regression analysis was used to evaluate the association between the extent of usage and HbA 1c change.
Data preparation and analyses were performed using Python Version 3. The data sets analyzed for this study are available from the corresponding author upon request. In all, participants enrolled in the Vida Health Diabetes Management Program.
A total of participants A schematic of participant flow is presented in Figure 2. Of the participants with no follow-up, Because our a priori definition of follow-up, based on the physiological characteristics of the HbA 1c test, was a laboratory test obtained a minimum of 90 days after Program start, participants with a postenrollment test obtained before 90 days were excluded from the outcome analyses and were considered to be missing a 3-month follow-up measure [ 30 ].
Basic demographics of the study cohort are presented in Table 1. Participants with a valid follow-up HbA 1c appeared to be younger mean Average baseline HbA 1c for the study cohort was 8.
Follow-up HbA 1c measurements were completed on average As shown in Figure 3 , a paired t test revealed a significant reduction in HbA 1c of —0. Participants who had completed at least one session had an average reduction of —1. In order to evaluate the effects of no program intervention, we used a repeated measures approach.
The model included HbA 1c measured at 3 time points time 1: HbA 1c from at least 90 days up to 12 months prior to the baseline HbA 1c test; time 2: baseline HbA 1c ; time 3: minimum day postenrollment HbA 1c follow-up.
Stud Health Technol Inform. Kitsiou S, Paré G, Jaana M, Gerber B. Effectiveness of mHealth interventions for patients with diabetes: An overview of systematic reviews. PloS One. Article CAS PubMed PubMed Central Google Scholar. Wang Y, Min J, Khuri J, Xue H, Xie B, Kaminsky L, et al.
Effectiveness of Mobile Health Interventions on Diabetes and Obesity Treatment and Management: Systematic Review of Systematic Reviews.
JMIR MHealth UHealth. Gabarron E, Årsand E, Wynn R. Social Media Use in Interventions for Diabetes: Rapid Evidence-Based Review. Vorderstrasse A, Lewinski A, Melkus GD, Johnson C. Social Support for Diabetes Self-Management via eHealth Interventions.
Curr Diab Rep. Statistik Austria: Erhebung über den IKT-Einsatz in Haushalten Vienna: Statistik Austria; Tang TS, Funnell M, Sinco B, Piatt G, Palmisano G, Spencer MS, et al.
Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial.
Giordano V, Koch H, Godoy-Santos A, Dias Belangero W, Esteves Santos Pires R, Labronici P. WhatsApp Messenger as an Adjunctive Tool for Telemedicine: An Overview.
Interact J Med Res. Kleinberger JW, Pollin TI. Personalized medicine in diabetes mellitus: current opportunities and future prospects.
Ann N Y Acad Sci. Scheen AJ. Precision medicine: The future in diabetes care? Dugas M, Crowley K, Gao GG, Xu T, Agarwal R, Kruglanski AW, et al. Individual differences in regulatory mode moderate the effectiveness of a pilot mHealth trial for diabetes management among older veterans.
PLOS ONE. Klein WMP, Rothman AJ, Cameron LD. Theoretical innovations in social and personality psychology and implications for health: introduction to special issue.
Health Psychol Off J Div Health Psychol Am Psychol Assoc. Montag C, Błaszkiewicz K, Sariyska R, Lachmann B, Andone I, Trendafilov B, et al. Smartphone usage in the 21st century: who is active on WhatsApp? BMC Res Notes. Amt der NÖ Landesregierung: Bevölkerungsstruktur Zahlen Fakten, St. Accessed 28 May Statistik Austria: Bevölkerung.
Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A Single-Question Screening Test for Drug Use in Primary Care. Arch Intern Med. Lifestyle Management: Standards of Medical Care in Diabetes— Atreja A, Bellam N, Levy SR.
Strategies to Enhance Patient Adherence: Making it Simple. Med Gen Med. Matsui D. Strategies to Measure and Improve Patient Adherence in Clinical Trials.
Pharm Med. Riedl R, Robausch M, Berghold A. The Evaluation of the Effectiveness of Austrians Disease Management Program in Patients with Type 2 Diabetes Mellitus - A Population-Based Retrospective Cohort Study. Fydrich T, Sommer G, Brähler E. Fragebogen zu Sozialen Unterstützung. Göttingen: Hogrefe; Schwarzer R, Jerusalem M.
Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Winsor: NFER-NELSON; Kroenke K, Spitzer RL, Williams JB. The PHQ validity of a brief depression severity measure. J Gen Intern Med. Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al.
Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Morfeld M, Kirchberger I, Bullinger M. SF Fragebogen zum Gesundheitszustand. Collins GS, Mughal S, Barnett AH, Fitzgerald J, Lloyd CE. Modification and validation of the Revised Diabetes Knowledge Scale.
Diabet Med. Jank S, Bertsche T, Schellberg D, Herzog W, Haefeli WE. The Ascale: development and evaluation of a questionnaire for assessment of adherence and individual barriers. Pharm World Sci. IPAQ International Questionnaire. Downloadable questionnaires.
not dated. Accessed 31 August Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form IPAQ-SF : a systematic review. Int J Behav Nutr Phys Act. Kamradt M, Bozorgmehr K, Krisam J, Freund T, Kiel M, Qreini M, et al.
Assessing self-management in patients with diabetes mellitus type 2 in Germany: validation of a German version of the Summary of Diabetes Self-Care Activities measure SDSCA-G. Health Qual Life Outcomes. United Nations Economic Commission for Europe. Conference of European Statisticians Recommendations for the Censuses of Population and Housing.
New York and Geneva: UNECE; Accessed 11 May Danner D, Rammstedt B, Bluemke M, Lechner C, Berres S, Knopf T, et al. Das Big-Five Inventar 2: Validierung eines Persönlichkeitsinventars zur Erfassung von 5 Persönlichkeitsdomänen und 15 Facetten.
Hughes DJ, Rowe M, Batey M, Lee A. A tale of two sites: Twitter vs. Facebook and the personality predictors of social media usage. Comput Hum Behav.
Pittman M. Phoneliness: Examining the relationships between mobile social media, personality and loneliness [dissertation]. Oregon: University of Oregon Graduate School; Accessed 15 June Röhner J, Schütz A.
Ein integratives Kommunikationsmodell nach Hargie und Kollegen. In: Röhner J, Schütz A, editors. Psychologie der Kommunikation Basiswissen Psychologie. Wiesbaden: Springer VS; Chapter Google Scholar. Faul F, Erdfelder E, Lang A-G, Buchner A. Behav Res Methods.
Azami G, Soh KL, Sazlina SG, Salmiah MS, Aazami S, Mozafari M, et al. Effect of a Nurse-Led Diabetes Self-Management Education Program on Glycosylated Hemoglobin among Adults with Type 2 Diabetes. Gagliardino JJ, Arrechea V, Assad D, Gagliardino GG, González L, Lucero S, et al.
Type 2 diabetes patients educated by other patients perform at least as well as patients trained by professionals: Peer Diabetes Education. Diabetes Metab Res Rev. Tshiananga JKT, Kocher S, Weber C, Erny-Albrecht K, Berndt K, Neeser K.
The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Mother-Child-Booklet [Internet]. Vienna: Bundesministerium für Digitalisierung und Wirtschaftsstandort. html child.
Accessed 4 January Field A. Discovering statistics using SPSS. London: Sage Publications Ltd; Medical University of Vienna: Good Scientific Practice. Ethics in Science and Research. Guidelines of the Medical University of Vienna. Vienna: Medical University of Vienna; Accessed 10 May Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P.
Proportion of newly diagnosed diabetes in COVID patients: A systematic review and meta-analysis. Diabetes Obes Metab. Parekh N, Deierlein AL.
Health behaviours during the coronavirus disease pandemic: implications for obesity. Public Health Nutr. Zupo R, Castellana F, Sardone R, Sila A, Giagulli VA, Triggiani V, et al.
Preliminary Trajectories in Dietary Behaviors during the COVID Pandemic: A Public Health Call to Action to Face Obesity.
Int J Environ Res Public Health. Download references. The authors gratefully acknowledge the financial support of the Gesellschaft für Forschungsförderung Niederösterreich m. H GFF and the provincial government of Lower Austria through the Life Science Calls Project ID LS We would like to thank the NÖ Landesgesundheitsagentur, the Austrian diabetes self-help groups, and involved students for their support of this study.
Furthermore, we acknowledge the permission to use the following questionnaires:. Department of General Practice and Health Services Research, University Hospital Heidelberg, Heidelberg, Germany for the SDSCA-G:. Department of Clinical Pharmacology and Pharmacoepidemiology, Heidelberg University, Germany for the AScale:.
Pharm World Sci ; Michigan Diabetes Research Center, Michigan, USA for the Diabetes Knowledge Scale. The project was supported by Grant Number P30DK MDRC from the National Institute of Diabetes and Digestive and Kidney Diseases. Downloadable questionnaires [Internet].
Danner D, Rammstedt B, Bluemke M, Treiber L, Berres S, Soto C, et al. Die deutsche Version des Big Five Inventory 2 BFI Zusammenstellung Sozialwissenschaftlicher Items Skalen ZIS [Internet].
EH is the study coordinator. EH, JG, MW, DWK, and JP conceived the study and led the proposal and protocol development. EH and JG wrote the first draft of the manuscript. JP edited and made substantial contributions to the manuscript. EH is responsible for study management, measurements, and data management.
UH, AEZ, EH, JG, DWK, WK, and SS developed the IMS strategy and the trainings. JG and AEZ will be responsible for interpreting communication data. UH is the dietitian in contact with moderators. MW and WK are the clinicians responsible for analyzing biochemical parameters. PK and EG will carry out the FoKo database query and contact potential participants as well as internists and physicians in Lower Austria.
SS will be responsible for statistical analysis. All authors read and approved the final manuscript. This study is publicly funded by the Gesellschaft für Forschungsförderung Niederösterreich m.
The funding organization has no influence on the design, collection, analysis, and interpretation of data, or the writing of the manuscript. The Data Management Coordinating Center EH, UH, AEZ, JG will oversee the intra-study data sharing process, with input from the Data Management Subcommittee.
Data sharing will be done in exclusively encrypted form. Also, only the encrypted data will be used for any publications. Medical samples will be destroyed after the analyses according to the quality criteria of ISO and will not be stored for further examinations. The collected data and backup copies are protected by passwords and are also only accessible to those persons who are entrusted with their processing and evaluation.
An ID code is created for each participant encryption by means of sequential numbering, from which only the assignment to the corresponding group is possible, but no conclusion regarding the identity of the participant. Institute of Health Sciences, St. Department of Internal Medicine I, University Hospital St.
Bachelor Degree Program Dietetics, St. Department of Psychology and Psychodynamics, Karl Landsteiner University of Health Sciences, Krems an der Donau, Austria.
Fachbereich Versorgungsmanagement 3, Austrian Health Insurance Fund, St. Christian Doppler Forschungsgesellschaft, Vienna, Austria. You can also search for this author in PubMed Google Scholar. Correspondence to Elisabeth Höld.
Written, informed consent to participate will be obtained from all participants. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Höld, E. et al.
Improving glycemic control in patients with type 2 diabetes mellitus through a peer support instant messaging service intervention DiabPeerS : study protocol for a randomized controlled trial. Trials 23 , Download citation. Received : 27 September Accepted : 26 March Published : 14 April Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content.
Search all BMC articles Search. Download PDF. Study protocol Open access Published: 14 April Improving glycemic control in patients with type 2 diabetes mellitus through a peer support instant messaging service intervention DiabPeerS : study protocol for a randomized controlled trial Elisabeth Höld ORCID: orcid.
Abstract Background Diabetes mellitus is one of the four priority non-communicable diseases worldwide. Methods A total of participants with type 2 diabetes mellitus will be included and randomly assigned to an intervention or control group.
Discussion Type 2 diabetes mellitus and other non-communicable diseases put healthcare systems worldwide to the test. Trial registration ClinicalTrials. Administrative information Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers.
Title {1} Improving glycemic control in patients with type 2 diabetes mellitus through a peer support instant messaging service intervention DiabPeerS — study protocol for a randomized controlled trial Trial registration {2a and 2b}.
H, Austria Life Science Call , LS Author details {5a} 1 Institute of Health Sciences, St. Pölten, Austria 3 Department of Internal Medicine I, University Hospital St. Pölten, Austria 4 Department of Psychology and Psychodynamics, Karl Landsteiner University of Health Sciences, Krems an der Donau, Austria 5 Fachbereich Versorgungsmanagement 3, Austrian Health Insurance Fund, St.
Pölten, Austria 6 Christian Doppler Forschungsgesellschaft, Vienna, Austria Name and contact information for the trial sponsor {5b} Gesellschaft für Forschungsförderung Niederösterreich m.
H Role of sponsor {5c} The funding body Gesellschaft für Forschungsförderung NÖ m. Introduction Background and rationale {6a} Diabetes mellitus is one of the four priority non-communicable diseases worldwide [ 1 ].
This is surprising because peer support IMS has particular advantages compared to other mHealth solutions: In , the majority In particular, our main hypotheses are: H1: A peer-supported IMS intervention reduces HbA 1c of patients with type 2 diabetes mellitus compared to a standard therapy.
Trial design {8} The presented trial is a parallel-group, two-arm superiority RCT to evaluate the efficacy of a peer-supported IMS intervention for patients with type 2 diabetes mellitus in Lower Austria.
Methods: participants, interventions, and outcomes Study setting {9} The trial will be implemented in Lower Austria, which is a federal state in northeastern Austria.
Compensation Participants and moderators will get a financial compensation in the amount of 50 EUR and a small gift after the last measurement. Additional consent provisions for collection and use of participant data and biological specimens {26b} No ancillary study will be conducted.
Intervention description {11a} The intervention is developed and conducted by an interdisciplinary team. Criteria for discontinuing or modifying allocated interventions {11b} Participants can revoke their willingness to participate at any time, even without giving reasons, and withdraw from the clinical study without this causing them any disadvantages for their further medical care.
Strategies to improve adherence to interventions {11c} The intervention consists of a peer-supported IMS intervention in addition to standard therapy. Provisions for post-trial care {30} Trial participation entails only minimal risk compared to standard therapy alone, which will be continued after the trial.
Waist circumference [cm] to calculate the waist-to-height ratio as measured using an ergonomic, step less, and extendible measuring tape Additionally, demographic data and personality traits will be surveyed in the baseline assessment T0.
Communication data real-time : As part of the data export solution of the IMS communication data, an export script is provided in which individual rooms - these correspond to the groups assigned for the experiment - are listed. Participant timeline {13} The flow diagram showing the participant timeline through the DiabPeerS study is presented in Fig.
Flow diagram DiabPeerS. Full size image. SPIRIT figure DiabPeerS. Assignment of interventions: allocation Sequence generation {16a} Eligible participants who have signed the informed consent will be randomized into the peer-supported IMS intervention group or the control group in a ratio.
Concealment mechanism {16b} For the allocation of the participants, a simple urn-based randomization is carried out. Implementation {16c} The drawing of the participants takes place during the initial appointment for the informed consent.
Assignment of interventions: blinding Who will be blinded {17a} Blinding of trial participants will not be possible because of the obvious differences between the intervention group and the control group. Procedure for unblinding if needed {17b} It is an open-label study and therefore unblinding will not occur.
Data collection and management Plans for assessment and collection of outcomes {18a} During the online registration, contact data of all interested individuals will be collected.
Data management {19} The participants agree that for the purpose and in the course of this study, the St. Results of all analyses will be reported in an aggregated and strictly anonymous form. Statistical methods Statistical methods for primary and secondary outcomes {20a} Data will be analyzed using IBM SPSS Statistics 26 or greater IMB Corporation, Armonk, NY, USA.
Interim analyses {21b} There will be no planned interim analysis or stopping guidelines for medical reasons besides the exclusion criteria see {10} because no potentially harmful outcomes are expected based on the peer-supported IMS intervention. Methods for additional analyses e.
Plans to give access to the full protocol, participant-level data and statistical code {31c} It is not planned to give third parties access, neither to the full protocol, nor the participant data, nor the statistical code. Oversight and monitoring Composition of the coordinating centre and trial steering committee {5d} The complexity of diabetes therapy demands interdisciplinary teams and innovative treatment approaches.
Composition of the data monitoring committee, its role, and reporting structure {21a} A Data Monitoring Committee DMC has been established EH, AEZ, UH, JG. Adverse event reporting and harms {22} Although we consider specific risks for participation very low, a system for collecting, assessing, reporting, and managing adverse events will be implemented.
Plans for communicating important protocol amendments to relevant parties e. Dissemination plans {31a} The consortium has developed a dissemination plan which defines all relevant stakeholders and appropriate dissemination strategies as well as authorship guidelines [ 74 ] visualized in a GANTT chart including when to conduct which dissemination activity, and another tool for the documentation of all activities.
Discussion Type 2 diabetes mellitus is one of the major global causes of disability and mortality. IMS provides several benefits: IMS can be used time- and location-independently, which leads to higher participation rates of people with type 2 diabetes mellitus in diabetes self-management support offers.
IMS has a low threshold when exchange with others is needed. IMS support and exchange can be provided immediately. Trial status This is the protocol version 6 from References World Health Organization: Global report on diabetes. Google Scholar Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al.
Article PubMed Google Scholar American Diabetes Association. Article PubMed Central Google Scholar Funnell MM, Anderson RM. Article CAS PubMed Google Scholar American Diabetes Association. Article CAS Google Scholar Powers MA, Bardsley JK, Cypress M, Funnell MM, Harms D, Hess-Fischl A, et al. Article PubMed Google Scholar Mulcahy K, Maryniuk M, Peeples M, Peyrot M, Tomky D, Weaver T, et al.
Article PubMed Google Scholar Odgers-Jewell K, Ball LE, Kelly JT, Isenring EA, Reidlinger DP, Thomas R. Article CAS Google Scholar Tanaka R, Shibayama T, Sugimoto K, Hidaka K. Article PubMed Google Scholar World Health Organization. Article CAS Google Scholar He X, Li J, Wang B, Yao Q, Li L, Song R, et al.
Article CAS PubMed Google Scholar Minet L, Møller S, Vach W, Wagner L, Henriksen JE. Article PubMed Google Scholar Steinsbekk A, Rygg LØ, Lisulo M, Rise MB, Fretheim A. Article PubMed PubMed Central Google Scholar Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM.
Article PubMed Google Scholar Shumaker SA, Brownell A. No convincing evidence exists that improved glycemic control with insulin treatment worsens CHD in patients with type 2 diabetes.
Indirect and small-scale trial data on this question have been equivocal. Overall, there is a much stronger epidemiologic association between poorly controlled hyperglycemia and increased rates of CHD than there is between higher insulin levels and CHD.
Strong evidence exists that hypoglycemia, which has been the only significant risk identified in association with improved control in type 1 diabetes, is 10 to times less common in patients with type 2 than it is in patients with type 1 diabetes as long as the goals for the level of HbA 1c are set no lower than 7.
Although only a large, randomized trial can definitively establish the exact benefit-risk ratio of improved glycemic control, and although there is some uncertainty about a possible increased risk of CHD, we believe that the evidence for the prevention of microvascular complications and their associated disability is so compelling that for most patients with type 2 diabetes, HbA 1c levels should be lowered to the levels achieved in recent clinical trials 7.
Treatment goals should be adjusted for some patients based on individual clinical factors see the "Comment" section. These conclusions agree with recent guidelines published by the American Diabetes Association.
Several patient factors, shown in Table 3 , might be expected to affect the risks and benefits of glucose lowering in type 2 diabetes. For example, patients with diabetes and a family history of diabetic nephropathy, who as a result of such family history would have a 3 to 4 times higher risk of developing nephropathy, - might be expected to benefit more from improved control.
Similarly, patients who at the time of the diagnosis of diabetes have early retinopathy and so a significantly higher risk of subsequent vision loss 10 would also be expected to benefit more from glucose lowering. Age at onset of type 2 diabetes would also be expected to significantly affect the risk-benefit equation.
Whereas a year-old woman with newly diagnosed type 2 diabetes would have a very high risk that severe microvascular complications would develop during her remaining 18 years of life expectancy, a year-old man with newly diagnosed type 2 diabetes might be expected to die of CHD before microvascular complications developed that were severe enough to affect his quality of life.
The issue of aggressive glycemic control in patients with type 2 diabetes is thus a complex balance between risks and benefits, which to some extent should be individualized.
In this way, it resembles the controversy over postmenopausal hormone replacement. In both situations, the potential public health benefits are large and a considerable body of evidence suggests that the benefits of therapy are likely to outweigh the risks.
In both situations, however, no data exist from large, randomized controlled trials to clearly define the balance between the risks and benefits. Only 1 clinical trial of improved glycemic control, the UKPDS, is in progress because the proposed full-scale VA trial has not received funding Nicholas Emanuele, MD, personal communication, January Results from the UKPDS are expected in , but 2 issues may limit its ability to measure the effects of improved glycemic control.
First, the study has enrolled a preponderance of patients with mild levels of hyperglycemia, so it is likely to seriously underestimate the benefits that improved control might offer to the majority of patients with type 2 diabetes who have much higher levels of hyperglycemia.
Patients with type 2 diabetes should be informed of the evidence about the benefits and risks of improved glycemic control and should participate in the decision of how aggressively their hyperglycemia should be treated. Even small reductions in the levels of HbA 1c for most patients can be viewed as positive steps in their preventive health care.
We gratefully acknowledge the helpful comments of Edward J. Boyko, MD, MPH, on an earlier version of the manuscript. Reprints: Irl B. Hirsch, MD, University of Washington Medical Center, Diabetes Care Center, Box , NE Pacific St, Seattle, WA full text icon Full Text. Download PDF Top of Article Abstract Microvascular and neuropathic complications Epidemiologic Data Clinical Trials Summary Macrovascular complications Indirect Data Epidemiologic Data Clinical Trials Summary Other potential drawbacks to improved control Weight Gain Quality of Life Summary Conclusions Comment Article Information References.
Table 1. View Large Download. Factors Expected to Affect Benefits of Improved Glycemic Control in Type 2 Diabetes. Kenny SJAubert RCGeiss LS Prevalence and incidence of non—insulin-dependent diabetes. Harris MICowie CCStern MPBoyko EJRieber GEBennett PHeds. Diabetes in America.
Bethesda, Md National Institutes of Health;47 Google Scholar. The Diabetes Control and Complications Trial Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus.
N Engl J Med. Janes GR Ambulatory medical care for diabetes. Bethesda, Md National Institutes of Health; Google Scholar. Marrero DG Current effectiveness of diabetes health care in the U. Diabetes Rev.
Peterson KA Diabetes care by primary care physicians in Minnesota and Wisconsin. J Fam Pract. Weiner JPParente STGarnick DWFowles JLawthers AGPalmer RH Variation in office-based quality: a claims-based profile of care provided to Medicare patients with diabetes.
Lee ETLee VSLu MRussell D Development of proliferative retinopathy in NIDDM: a follow-up study of American Indians in Oklahoma. Hamman RFMayer EJMoo-Young GAHildebrandt WMarshall JABaxter J Prevalence and risk factors of diabetic retinopathy in non-Hispanic whites and Hispanics with NIDDM: San Luis Valley Diabetes Study.
Howard-Williams JHillson RMBron AAwdry PMann JIHockaday TD Retinopathy is associated with higher glycaemia in maturity-onset type diabetes. Klein RKlein BEMoss SECruickshanks KJ Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy.
Borch-Johnsen KNissen HHenriksen E et al. The natural history of insulin-dependent diabetes mellitus in Denmark, I: long-term survival with and without late diabetic complications. Diabet Med. Kunzelman CLKnowler WCPettitt DJBennett PH Incidence of proteinuria in type 2 diabetes mellitus in the Pima Indians.
Kidney Int. Knowler WCKunzelman CL Population comparisons of the frequency of nephropathy. Mogensen CEed. The Kidney and Hypertension in Diabetes Mellitus. Boston, Mass Marinus Nijhoff Publishing; 32 Google Scholar. Krolewski ASWarram JHChristlieb ARBusick EJKahn CR The changing natural history of nephropathy in type I diabetes.
Am J Med. Andersen ARChristiansen JSAndersen JKKreiner SDeckert T Diabetic nephropathy in type 1 insulin-dependent diabetes: an epidemiological study. Partanen JNiskanen LLehtinen JMervaala ESiitonen OUusitupa M Natural history of peripheral neuropathy in patients with non—insulin-dependent diabetes mellitus.
Orchard TJDorman JSMaser RE et al. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Young MJBoulton AJMacLeod AFWilliams DRSonksen PH A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population.
Dyck PJKratz KMKarnes JL et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study.
Moss SEKlein RKlein BE Cause-specific mortality in a population-based study of diabetes. Am J Public Health. Ochi JWMelton LJ IIIPalumbo PJChu CP A population-based study of diabetes mortality. Diabetes Care.
Bender APSprafka JMJagger HGMuckala KHMartin CPEdwards TR Incidence, prevalence, and mortality of diabetes mellitus in Wadena, Marshall, and Grand Rapids, Minnesota: the Three-City Study.
Krolewski ASKosinski EJWarram JH et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. Klein R Hyperglycemia and microvascular and macrovascular disease in diabetes.
Brownlee M Glycosylation of proteins and microangiopathy. Hosp Pract Off Ed. Greene DASima AAStevens MJFeldman ELLattimer SA Complications: neuropathy, pathogenetic considerations. Makita ZRadoff SRayfield EJ et al. Advanced glycosylation end products in patients with diabetic nephropathy.
Nathan DM The pathophysiology of diabetic complications: how much does the glucose hypothesis explain? Ann Intern Med. Stolk RPVingerling JRde Jong PT et al. Retinopathy, glucose, and insulin in an elderly population: the Rotterdam study. Nathan DMSinger DEGodine JEHarrington CHPerlmuter LC Retinopathy in older type II diabetics: association with glucose control.
Morisaki NWatanabe SKobayashi J et al. Diabetic control and progression of retinopathy in elderly patients: five-year follow-up study. J Am Geriatr Soc. Chen MSKao CSFu CCChen CJTai TY Incidence and progression of diabetic retinopathy among non—insulin-dependent diabetic subjects: a 4-year follow-up.
Int J Epidemiol. Liu QZPettitt DJHanson RL et al. Glycated haemoglobin, plasma glucose and diabetic retinopathy: cross-sectional and prospective analyses.
Teuscher ASchnell HWilson PW Incidence of diabetic retinopathy and relationship to baseline plasma glucose and blood pressure. Haffner SMFong DStern MP et al. Diabetic retinopathy in Mexican Americans and non-Hispanic whites.
Ballard DJMelton LJDDwyer MS et al. Risk factors for diabetic retinopathy: a population-based study in Rochester, Minnesota. Knuiman MWWelborn TAMcCann VJStanton KGConstable IJ Prevalence of diabetic complications in relation to risk factors. Klein RKlein BEMoss SE Incidence of gross proteinuria in older-onset diabetes: a population-based perspective.
Wirta OPasternack ALaippala PTurjanmaa V Glomerular filtration rate and kidney size after six years disease duration in non—insulin-dependent diabetic subjects. Clin Nephrol. Ballard DJHumphrey LLMelton LJ III et al. Epidemiology of persistent proteinuria in type II diabetes mellitus: population-based study in Rochester, Minnesota.
Fabre JBalant LPDayer PGFox HMVernet AT The kidney in maturity onset diabetes mellitus: a clinical study of patients. Lehtinen JMUusitupa MSiitonen OPyörälä K Prevalence of neuropathy in newly diagnosed NIDDM and nondiabetic control subjects. Klein RKlein BEMoss SE Relation of glycemic control to diabetic microvascular complications in diabetes mellitus.
He is currently the chief medical expert and vice president, dontrol and medical education, at Senior Fitness and Aging Gracefully Channel wakefulness and learning performance Silver Spring, Improfing. Dr Mohr is a nutritionist Improving glycemic control exercise physiologist and is co-owner of Mohr Results, Inc. He is also a consulting sports nutritionist for the Cincinnati Bengals. Primary Care Update Summaries for Clinical Practice. The prevalence of diabetes is increasing in epidemic proportions. Nearly 26 million people in the United States currently have diabetes. Moreover, more than 1.Optimal glycemic control is fundamental to the management of diabetes. The contrlo data from the Glyceemic Control and Complications G,ycemic Wakefulness and learning performance type 1 diabetes Impeoving and the United Kingdom Prospective Diabetes Study UKPDS; type 2 Improvingg 3 demonstrated Improvung curvilinear relationship between A1C and diabetes complications, with no apparent threshold of benefit, although the absolute reduction in risk cotnrol substantially less at lower Conntrol levels.
Similarly, both fasting plasma glucose Endurance training for basketball players and postprandial glycfmic glucose PPG are directly correlated to the risk of complications, with some evidence that PPG might Maintaining alcohol moderation a stronger Improfing risk gglycemic for CV dontrol 4— Evidence indicates Improvlng improved glycemic control reduces the Artichoke vitamin and mineral content of both microvascular and CV complications.
The initial prospective randomized controlled trials were conttol in people with Blood circulation and healing diagnosed diabetes. These trials—the DCCT in type 1 diabetes 11the Kumamoto trial 12 Artisanal Refreshment Creations the Onion cooking classes 1,13 in type 2 diabetes—confirmed that improved glycemic control significantly reduced the conttrol of microvascular complications, but had no gylcemic effect on MIproving outcomes.
Subsequent Imroving data from long-term follow up after termination of Impproving periods glycdmic both conrtol DCCT and UKPDS Improvlng showed a persistence of significant microvascular Iproving and also demonstrated Alpha-lipoic acid for brain health emergence of beneficial effect on CV outcomes attributed to intensive glycemic control.
Whereas the UKPDS trial enrolled people with recently controol type 2 diabetes, 3 major subsequent trials—the Action to Control Supercharge your immunity Risk in Diabetes ACCORD Improvin, Action in Diabetes and Improvint Disease: Preterax and Diamicron MR Controlled Evaluation ADVANCEand Veterans Affairs Improvinf Trial VADT gltcemic the effect of gkycemic glycemic control on gkycemic with long-standing type 2 diabetes.
The mean age of participants was 62 years and the mean duration of diabetes was 10 years. A difference in Vegan meal ideas for busy professionals was congrol wakefulness and learning performance and maintained throughout the trial at 6.
Wakefulness and learning performance primary cojtrol major CV Impgoving nonfatal MI, nonfatal Improvibg or death comtrol CV causes were not reduced Red pepper wrap in ACCORD hazard ratio [HR] 0.
Glycemif glycemic Improvinb portion of the trial Improving glycemic control dontrol terminated after 3. However, an observational follow up of the cojtrol ACCORD participants Improvving a median of contrlo. The mean duration of Pancreas transplantation was 8 Improvinf.
After Improvint 5-year follow contrrol, mean A1C was Improvong. The primary IImproving was a composite of microvascular events nephropathy Im;roving retinopathy and Cotrol disease defined Imprpving major adverse CV events.
Glydemic mean duration of Improving glycemic control was 12 years glucemic the A1C levels achieved in the I,proving and intensive therapy groups were 8.
During a median follow Improviing of 5. However, during an observational median follow up of 9. Data from a meta-analysis glyxemic that gylcemic with type 2 diabetes who receive intensive glucose lowering therapy have a reduced risk of the composite major adverse CV Quality natural weight management MACE and MI, with gycemic significant effect on the risk of total mortality, cardiac death, Lentils and vegetables and Glycemiv Although an explanation for ccontrol unexpected higher mortality rates Iron in ancient civilizations with intensive-treatment conrol the ACCORD study remains elusive 29glycemix frequency of severe hypoglycemia Impoving these trials glyycemic 2 to Automated glucose regulation times higher in the intensive therapy groups contril a glyxemic mortality was reported in participants with 1 or more episodes of severe hypoglycemia in the ACCORD 30 Restorative remedies, ADVANCE 31 and VADT trials glycsmicirrespective of the glycemiv treatment arms in which individual participants were g,ycemic.
Therefore, Improving glycemic control, it has been Improviing that a glycenic glycemic Wild Berry Foraging with a target A1C of 6.
S; Hypoglycemia chapter, p. Higher glycemic targets are also appropriate for Imptoving dependent adults of glyceemic age or individuals with limited life expectancy and little likelihood of benefit from intensive therapy. Evidence also supports the use of multifactorial risk-reduction strategies in addition to A1C control for CV prevention, including blood pressure BP and lipid targets; CV prevention medications; physical activity and other healthy behaviours; as well as smoking cessation see Cardiovascular Protection in People with Diabetes chapter, p.
Such multifactorial interventions have recently been suggested to lead to not only significant microvascular and CV benefits but also mortality reduction in the year follow up of the Steno-2 study The salient results of this study include: increased survival for a median of 7.
A1C measurement encompasses a component of both the FPG and postprandial PG. In addition, mean glucose values also correlate with A1C in both type 1 and type 2 diabetes as shown in Figure 1 35, However, a major challenge in attempting to use evidence-based observations to determine the value of tighter PPG control has been the lack of well-designed, long-term outcome studies where assessing PPG values is the major objective of the study.
Most of the large outcome trials conducted so far have been mostly based on preprandial glucose and A1C targets, with limited evidence of a long-term benefit of targeting PPG alone 49, Although, nontraditional glycemic targets, such as fructosamine and glycated albumin, have also been associated with CV outcomes and mortality in a cohort study 51the broader utility of such targets and their correlation with A1C has not yet been established.
Finally, glucose variability GV as an additional therapeutic goal has recently been gaining support. Limited data support the possibility that GV is involved in the pathogenesis of vascular complications of diabetes by inducing inflammatory activation and oxidative stress 52, Key components of GV variability in FPG and PPG, as well as hypoglycemia have received some prominence in clinical literature recently, linking these components to diabetes complications.
Specific clinical targets suggested in the literature for people monitored via CGM include minimizing daily glucose standard deviation SD to less than 3 times the mean BGmaximizing time in range 3. However, management strategies that would minimize glucose variability and their impact on hard clinical outcomes remain to be determined before these novel measurement targets of glucose quality can systematically be incorporated into clinical practice guidelines.
A1Cglycated hemoglobin; BGblood glucose; CGMcontinuous glucose monitoring; CHFcongestive heart failure, CIconfidence interval; CKDchronic kidney disease; CV ; cardiovascular; FPGfasting plasma glucose; GVglucose variability HRhazard ratio; MImyocardial infarct; PGplasma glucose; PPG, postprandial plasma glucose.
Literature Review Flow Diagram for Chapter 8: Targets for Glycemic Control. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group P referred R eporting I tems for S ystematic Reviews and M eta- A nalyses: The PRISMA Statement.
PLoS Med 6 6 : e pmed For more information, visit www. Bajaj reports personal fees from Abbott; grants and personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi, outside the submitted work.
Ross reports personal fees from Novo Nordisk, Eli Lilly, Janssen, AstraZeneca, and Boehringer Ingelheim, outside the submitted work. No other authors have anything to disclose.
All content on guidelines. ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications diabetes. Become a Member Order Resources Home About Contact DONATE. Next Previous. Key Messages Recommendations Figures Full Text References.
Chapter Headings Introduction Conclusions Author Disclosures. Key Messages Optimal glycemic control is fundamental to the management of diabetes. Both fasting and postprandial plasma glucose levels correlate with the risk of complications and contribute to the measured glycated hemoglobin A1C value.
Glycemic targets should be individualized based on the individual's frailty or functional dependence and life expectancy. Key Messages for People with Diabetes Try to keep your blood glucose as close to your target range as possible.
This will help to delay or prevent complications of diabetes. Target ranges for blood glucose and A1C can vary and depend on a person's medical conditions and other risk factors.
Work with your diabetes health-care team to determine your target A1C and blood glucose target range fasting and after meals. Introduction Optimal glycemic control is fundamental to the management of diabetes. Figure 1 Recommended targets for glycemic control.
A1Cglycated hemoglobin; CKDchronic kidney disease. Recommendations Glycemic targets should be individualized [Grade D, Consensus]. A higher A1C target may be considered in people with diabetes with the goals of avoiding hypoglycemia and over-treatment related to antihyperglycemic therapy, with any of the following [Grade D, Consensus for all]: Functionally dependent: 7.
Avoid symptomatic hyperglycemia and any hypoglycemia. Abbreviations: A1Cglycated hemoglobin; BGblood glucose; CGMcontinuous glucose monitoring; CHFcongestive heart failure, CIconfidence interval; CKDchronic kidney disease; CV ; cardiovascular; FPGfasting plasma glucose; GVglucose variability HRhazard ratio; MImyocardial infarct; PGplasma glucose; PPG, postprandial plasma glucose.
Author Disclosures Dr. References UK Prospective Diabetes Study UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes UKPDS UK Prospective Diabetes Study UKPDS Group.
Lancet ; The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure HbA1c to the risk of development and progression of retinopathy in the diabetes control and complications trial.
Diabetes ; Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes UKPDS 35 : Prospective observational study. BMJ ; The relation of glycaemia to the risk of development and progression of retinopathy in the diabetic control and complications trial.
Diabetologia ; Coutinho M, Gerstein HC, Wang Y, et al. The relationship between glucose and incident cardiovascular events.
Ametaregression analysis of published data from 20 studies of 95, individuals followed for Diabetes Care ; Levitan EB, Song Y, Ford ES, et al. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med ; Study DECODE, Group EDEG.
Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Sorkin JD, Muller DC, Fleg JL, et al.
The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: Data from the Baltimore longitudinal study of aging with a critical review of the literature.
Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al.
Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of prospective studies. The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al.
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus.
: Improving glycemic control| Glycemic Control - Health Quality BC | Preiser JC, Chase JG, Hovorka R, Joseph JI, Krinsley JS, De Block C, et al. Glucose control in the ICU: a continuing story. J Diabetes Sci Technol. Krinsley JS, Chase JG, Gunst J, Martensson J, Schultz MJ, Taccone FS, et al. Continuous glucose monitoring in the ICU: clinical considerations and consensus. Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Krinsley JS, Preiser JC. Penning S, Pretty C, Preiser JC, Shaw GM, Desaive T, Chase JG. Glucose control positively influences patient outcome: a retrospective study. J Crit Care. Signal M, Le Compte A, Shaw GM, Chase JG. Glycemic levels in critically ill patients: are normoglycemia and low variability associated with improved outcomes? Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Iiyama T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Chase JG, Le Compte AJ, Preiser JC, Shaw GM, Penning S, Desaive T. Physiological modeling, tight glycemic control, and the ICU clinician: what are models and how can they affect practice? Ann Intensive Care. Chase JG, Andreassen S, Jensen K, Shaw GM. Impact of human factors on clinical protocol performance: a proposed assessment framework and case examples. Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. PubMed Google Scholar. Okabayashi T, Kozuki A, Sumiyoshi T, Shima Y. Technical challenges and clinical outcomes of using a closed-loop glycemic control system in the hospital. Eslami S, de Keizer NF, de Jonge E, Schultz MJ, Abu-Hanna A. A systematic review on quality indicators for tight glycaemic control in critically ill patients: need for an unambiguous indicator reference subset. Carson ER, Cobelli C. Modelling methodology for physiology and medicine. Amsterdam: Elsevier; Google Scholar. Van Herpe T, Espinoza M, Haverbeke N, Moor BD, van den Berghe G. Glycemia prediction in critically ill patients using an adaptive modeling approach. Article Google Scholar. Pielmeier U, Andreassen S, Nielsen BS, Chase JG, Haure P. A simulation model of insulin saturation and glucose balance for glycemic control in ICU patients. Comput Methods Prog Biomed. Hovorka R, Chassin LJ, Ellmerer M, Plank J, Wilinska ME. A simulation model of glucose regulation in the critically ill. Physiol Meas. Lin J, Razak NN, Pretty CG, Le Compte A, Docherty P, Parente JD, et al. A physiological Intensive Control Insulin-Nutrition-Glucose ICING model validated in critically ill patients. Dickson JL, Stewart KW, Pretty CG, Flechet M, Desaive T, Penning S, et al. Generalisability of a virtual trials method for glycaemic control in intensive care. IEEE Trans Biomed Eng. Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. Van Herpe T, Mesotten D, Wouters PJ, Herbots J, Voets E, Buyens J, et al. LOGIC-insulin algorithm-guided versus nurse-directed blood glucose control during critical illness: the LOGIC-1 single-center, randomized, controlled clinical trial. Dubois J, Van Herpe T, van Hooijdonk RT, Wouters R, Coart D, Wouters P, et al. Software-guided versus nurse-directed blood glucose control in critically ill patients: the LOGIC-2 multicenter randomized controlled clinical trial. Arleth T, Andreassen S, Federici MO, Benedetti MM. A model of the endogenous glucose balance incorporating the characteristics of glucose transporters. Article CAS Google Scholar. Pielmeier U, Andreassen S, Juliussen B, Chase JG, Nielsen BS, Haure P. The Glucosafe system for tight glycemic control in critical care: a pilot evaluation study. Hovorka R, Kremen J, Blaha J, Matias M, Anderlova K, Bosanska L, et al. Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial. Chase JG, Suhaimi F, Penning S, Preiser JC, Le Compte AJ, Lin J, et al. Validation of a model-based virtual trials method for tight glycemic control in intensive care. Biomed Eng Online. Lonergan T, Le Compte A, Willacy M, Chase JG, Shaw GM, Wong XW, et al. A simple insulin-nutrition protocol for tight glycemic control in critical illness: development and protocol comparison. Diabetes Technol Ther. Fisk LM, Le Compte AJ, Shaw GM, Penning S, Desaive T, Chase JG. STAR development and protocol comparison. Stewart KW, Pretty CG, Tomlinson H, Thomas FL, Homlok J, Noemi SN, et al. Safety, efficacy and clinical generalization of the STAR protocol: a retrospective analysis. Wilinska ME, Chassin LJ, Hovorka R. In silico testing—impact on the progress of the closed loop insulin infusion for critically ill patients project. Wendt SL, Ranjan A, Moller JK, Schmidt S, Knudsen CB, Holst JJ, et al. Cross-validation of a glucose-insulin-glucagon pharmacodynamics model for simulation using data from patients with type 1 diabetes. Visentin R, Giegerich C, Jager R, Dahmen R, Boss A, Grant M, et al. Kanderian SS, Weinzimer SA, Steil GM. The identifiable virtual patient model: comparison of simulation and clinical closed-loop study results. Wilinska ME, Blaha J, Chassin LJ, Cordingley JJ, Dormand NC, Ellmerer M, et al. Evaluating glycemic control algorithms by computer simulations. Visentin R, Man CD, Cobelli C. Le Compte AJ, Pretty CG, Lin J, Shaw GM, Lynn A, Chase JG. Impact of variation in patient response on model-based control of glycaemia in critically ill patients. Chase JG, Le Compte AJ, Suhaimi F, Shaw GM, Lynn A, Lin J, et al. Tight glycemic control in critical care—the leading role of insulin sensitivity and patient variability: a review and model-based analysis. Tanenberg RJ, Hardee S, Rothermel C, Drake AJ III. Use of a computer-guided glucose management system to improve glycemic control and address national quality measures: a 7-year, retrospective observational study at a tertiary care teaching hospital. Endocr Pract. Vogelzang M, Zijlstra F, Nijsten MW. Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit. BMC Med Inform Decis Mak. Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in , h of operation. Marvin MR, Inzucchi SE, Besterman BJ. Computerization of the Yale insulin infusion protocol and potential insights into causes of hypoglycemia with intravenous insulin. Juneja R, Golas AA, Carroll J, Nelson D, Abad VJ, Roudebush CP, et al. Safety and effectiveness of a computerized subcutaneous insulin program to treat inpatient hyperglycemia. Meijering S, Corstjens AM, Tulleken JE, Meertens JH, Zijlstra JG, Ligtenberg JJ. Towards a feasible algorithm for tight glycaemic control in critically ill patients: a systematic review of the literature. Shetty S, Inzucchi SE, Goldberg PA, Cooper D, Siegel MD, Honiden S. Adapting to the new consensus guidelines for managing hyperglycemia during critical illness: the updated Yale insulin infusion protocol. Amrein K, Ellmerer M, Hovorka R, Kachel N, Parcz D, Korsatko S, et al. Hospital glucose control: safe and reliable glycemic control using enhanced model predictive control algorithm in medical intensive care unit patients. Blaha J, Barteczko-Grajek B, Berezowicz P, Charvat J, Chvojka J, Grau T, et al. Space GlucoseControl system for blood glucose control in intensive care patients—a European multicentre observational study. BMC Anesthesiol. Blaha J, Kopecky P, Matias M, Hovorka R, Kunstyr J, Kotulak T, et al. Comparison of three protocols for tight glycemic control in cardiac surgery patients. Pachler C, Plank J, Weinhandl H, Chassin LJ, Wilinska ME, Kulnik R, et al. Tight glycaemic control by an automated algorithm with time-variant sampling in medical ICU patients. Evans A, Shaw GM, Le Compte A, Tan CS, Ward L, Steel J, et al. Pilot proof of concept clinical trials of Stochastic Targeted STAR glycemic control. Lin J, Lee D, Chase JG, Shaw GM, Le Compte A, Lotz T, et al. Stochastic modelling of insulin sensitivity and adaptive glycemic control for critical care. Dickson JL, Chase JG, Lynn A, Shaw GM. Model-based glycaemic control: methodology and initial results from neonatal intensive care. Biomed Tech Berl. Alsweiler J, Williamson K, Bloomfield F, Chase G, Harding J. Computer-determined dosage of insulin in the management of neonatal hyperglycaemia HINT2 : protocol of a randomised controlled trial. BMJ Open. Download references. This document was supported by unrestricted educational grants from BBraun, GlucoSet, Menarini Diagnostics, Nikkiso, Roche Pharma, Nova Biomedical, and Sphere. These companies had no influence on the content of the manuscript or on the decision to publish. Department of Mechanical Engineering, Centre for Bio-Engineering, University of Canterbury, Christchurch, New Zealand. GIGA In-Silico Medicine, University of Liège, Liège, Belgium. Medical Intensive Care Unit, Lyon-Sud University Hospital, Pierre-Bénite, France. ULB Center for Diabetes Research, and Division of Endocrinology, Erasme Hospital, Université Libre de Bruxelles, Brussels, Belgium. Department of Endocrinology, Diabetology and Metabolism, Antwerp University Hospital, Edegem, Belgium. Clinical Division and Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, KU Leuven, Leuven, Belgium. Service de Réanimation polyvalente, Hôpital Louis Pasteur, CH de Chartres, Chartres, France. Division of Critical Care, Department of Medicine, Stamford Hospital, Columbia University College of Physicians and Surgeons, Stamford, CT, USA. Department of Endocrinology, Diabetes, Nutrition, and Institute of Functional Genomics, CNRS, INSERM, Montpellier University Hospital, University of Montpellier, Montpellier, France. Department of Intensive Care, Erasme Hospital, Université Libre de Bruxelles, route de Lennik , , Brussels, Belgium. You can also search for this author in PubMed Google Scholar. JGC, TD, and J-CP developed the first draft of the manuscript based on presentations by all of the authors at a meeting on glucose control endorsed by the Diabetes Technology Society and the European Society of Intensive Care Medicine. JB, MC, CDB, JG, RH, PK, JK, and ER critically reviewed and revised the article for important intellectual content. All authors read and approved the final manuscript. Correspondence to Jean-Charles Preiser. JGC has consulted for Medtronic and Monarch Medical. CDB is a consultant for Abbott, A. Menarini Diagnostics, Medtronic, and Roche Diagnostics. RH reports having received speaker honoraria from Eli Lilly, Novo Nordisk, and Astra Zeneca, serving on advisory panels for Eli Lilly and Novo Nordisk, receiving license fees from BBraun and Medtronic, and having served as a consultant to BBraun. JK is a consultant for Edwards, Medtronic, Roche Diagnostics, and Optiscan. J-CP is a consultant for Edwards, Medtronic, and Optiscan, and is an Associate Editor for Critical Care. The remaining authors declare that they have no conflicts of interest relevant to this article. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Chase, J. et al. Improving glycemic control in critically ill patients: personalized care to mimic the endocrine pancreas. Crit Care 22 , Download citation. Received : 08 March Accepted : 29 June Published : 02 August Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Review Open access Published: 02 August Improving glycemic control in critically ill patients: personalized care to mimic the endocrine pancreas J. Abstract There is considerable physiological and clinical evidence of harm and increased risk of death associated with dysglycemia in critical care. Background Hyperglycemia is prevalent in critical care, caused by a complex interaction of multiple feedback loops associated with inflammation as a result of immune responses, counter-regulatory responses, and high blood glucose itself [ 1 , 2 ]. Clinical hurdles Mixed results from GC clinical studies left the field questioning the weight of data correlating high glucose, high glycemic variability, and increased hypoglycemia from GC with increased morbidity and mortality. Needs statement and goals These clinical hurdles yield four main needs: A. To accurately understand patient-specific, real-time metabolic status. To develop a validated means to design GC methods to fit clinical practice. Specifically, these are as follows: 1. Full size image. Summary recommendations Based on this overview and analysis of the current state-of-the-art for GC in critically ill patients, the following recommendations are made to advance the safety, quality, consistency, and clinical uptake: 1. Patient-specific model-based GC including closed-loop systems, increasingly enabled by the penetration of computational technology into the ICU, can improve the quality of GC: a. models should be self-validated and cross-validated; b. initial assessment and optimization in validated virtual trials should be considered for new GC methods; c. To enable comparison and analysis, all GC reporting should have a minimum standardized set of data reporting performance, safety, and workload, including: a. performance—time in desired target band; b. workload—average number of staff-taken or automated if applicable glucose measurements per patient per day standard deviation or median IQR ; d. performance optional —CDFs of cohort glycemia, which provide all possible time-in-target ranges, enabling far easier comparison; e. performance optional —CDFs of per-patient glycemia. Conclusions Glycemic control has proven difficult to safely and effectively achieve for all patients, where modeling and model-based methods have offered a potentially significant avenue to achieving safe, effective control. Abbreviations CDF: Cumulative distribution function GC: Glucose control ICU: Intensive care unit TTR: Target to range or risk TTV: Target to value. References Dungan KM, Braithwaite SS, Preiser JC. Article PubMed PubMed Central CAS Google Scholar Marik PE, Raghavan M. Article PubMed Google Scholar Rice TW. Article PubMed PubMed Central Google Scholar Renard E, Farret A, Kropff J, Bruttomesso D, Messori M, Place J, et al. Article PubMed CAS Google Scholar Chase JG, Shaw G, Le Compte A, Lonergan T, Willacy M, Wong XW, et al. Article PubMed PubMed Central Google Scholar Chase JG, Pretty CG, Pfeifer L, Shaw GM, Preiser JC, Le Compte AJ, et al. Article PubMed PubMed Central Google Scholar van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Article PubMed CAS Google Scholar van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Article PubMed CAS Google Scholar Krinsley JS. Article PubMed Google Scholar Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Article PubMed CAS Google Scholar Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Article PubMed Google Scholar Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, et al. Article PubMed CAS Google Scholar Kalfon P, Giraudeau B, Ichai C, Guerrini A, Brechot N, Cinotti R, et al. Article PubMed CAS Google Scholar Uyttendaele V, Dickson JL, Shaw GM, Desaive T, Chase JG. Article PubMed PubMed Central Google Scholar Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Article PubMed CAS Google Scholar Cnop M, Igoillo-Esteve M, Rai M, Begu A, Serroukh Y, Depondt C, et al. Article PubMed PubMed Central CAS Google Scholar Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Article PubMed CAS Google Scholar Luzi L, DeFronzo RA. CAS Google Scholar Basu A, Dalla MC, Basu R, Toffolo G, Cobelli C, Rizza RA. Article PubMed PubMed Central CAS Google Scholar Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Article PubMed PubMed Central CAS Google Scholar Ahren B. Article PubMed CAS Google Scholar Preiser JC, Chase JG, Hovorka R, Joseph JI, Krinsley JS, De Block C, et al. Article PubMed PubMed Central Google Scholar Krinsley JS, Chase JG, Gunst J, Martensson J, Schultz MJ, Taccone FS, et al. Article PubMed PubMed Central Google Scholar Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. Article PubMed PubMed Central Google Scholar Krinsley JS, Preiser JC. Article PubMed PubMed Central Google Scholar Penning S, Pretty C, Preiser JC, Shaw GM, Desaive T, Chase JG. Article PubMed CAS Google Scholar Signal M, Le Compte A, Shaw GM, Chase JG. Article PubMed PubMed Central Google Scholar Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Iiyama T, et al. Article PubMed CAS Google Scholar Chase JG, Le Compte AJ, Preiser JC, Shaw GM, Penning S, Desaive T. Article PubMed PubMed Central Google Scholar Chase JG, Andreassen S, Jensen K, Shaw GM. Article PubMed PubMed Central Google Scholar Aragon D. PubMed Google Scholar Okabayashi T, Kozuki A, Sumiyoshi T, Shima Y. Article PubMed PubMed Central Google Scholar Eslami S, de Keizer NF, de Jonge E, Schultz MJ, Abu-Hanna A. Article PubMed PubMed Central Google Scholar Carson ER, Cobelli C. Google Scholar Van Herpe T, Espinoza M, Haverbeke N, Moor BD, van den Berghe G. Article Google Scholar Pielmeier U, Andreassen S, Nielsen BS, Chase JG, Haure P. Article Google Scholar Hovorka R, Chassin LJ, Ellmerer M, Plank J, Wilinska ME. Article PubMed Google Scholar Lin J, Razak NN, Pretty CG, Le Compte A, Docherty P, Parente JD, et al. Article Google Scholar Dickson JL, Stewart KW, Pretty CG, Flechet M, Desaive T, Penning S, et al. Article PubMed PubMed Central Google Scholar Bergman RN, Finegood DT, Ader M. Article PubMed CAS Google Scholar Van Herpe T, Mesotten D, Wouters PJ, Herbots J, Voets E, Buyens J, et al. Article PubMed PubMed Central Google Scholar Dubois J, Van Herpe T, van Hooijdonk RT, Wouters R, Coart D, Wouters P, et al. Article PubMed PubMed Central Google Scholar Arleth T, Andreassen S, Federici MO, Benedetti MM. Article CAS Google Scholar Pielmeier U, Andreassen S, Juliussen B, Chase JG, Nielsen BS, Haure P. Article PubMed CAS Google Scholar Hovorka R, Kremen J, Blaha J, Matias M, Anderlova K, Bosanska L, et al. Article PubMed CAS Google Scholar Chase JG, Suhaimi F, Penning S, Preiser JC, Le Compte AJ, Lin J, et al. Article PubMed PubMed Central Google Scholar Lonergan T, Le Compte A, Willacy M, Chase JG, Shaw GM, Wong XW, et al. Article PubMed Google Scholar Fisk LM, Le Compte AJ, Shaw GM, Penning S, Desaive T, Chase JG. Article PubMed Google Scholar Stewart KW, Pretty CG, Tomlinson H, Thomas FL, Homlok J, Noemi SN, et al. Article PubMed PubMed Central Google Scholar Wilinska ME, Chassin LJ, Hovorka R. Article PubMed PubMed Central Google Scholar Wendt SL, Ranjan A, Moller JK, Schmidt S, Knudsen CB, Holst JJ, et al. Article PubMed PubMed Central CAS Google Scholar Visentin R, Giegerich C, Jager R, Dahmen R, Boss A, Grant M, et al. Article PubMed PubMed Central CAS Google Scholar Kanderian SS, Weinzimer SA, Steil GM. Article PubMed PubMed Central Google Scholar Wilinska ME, Blaha J, Chassin LJ, Cordingley JJ, Dormand NC, Ellmerer M, et al. Article PubMed Google Scholar Visentin R, Man CD, Cobelli C. Article PubMed Google Scholar Le Compte AJ, Pretty CG, Lin J, Shaw GM, Lynn A, Chase JG. Article Google Scholar Chase JG, Le Compte AJ, Suhaimi F, Shaw GM, Lynn A, Lin J, et al. Article Google Scholar Tanenberg RJ, Hardee S, Rothermel C, Drake AJ III. Article PubMed Google Scholar Vogelzang M, Zijlstra F, Nijsten MW. Article PubMed PubMed Central Google Scholar Davidson PC, Steed RD, Bode BW. Article PubMed CAS Google Scholar Marvin MR, Inzucchi SE, Besterman BJ. Article PubMed PubMed Central CAS Google Scholar Juneja R, Golas AA, Carroll J, Nelson D, Abad VJ, Roudebush CP, et al. Article PubMed PubMed Central Google Scholar Meijering S, Corstjens AM, Tulleken JE, Meertens JH, Zijlstra JG, Ligtenberg JJ. Article PubMed PubMed Central Google Scholar Shetty S, Inzucchi SE, Goldberg PA, Cooper D, Siegel MD, Honiden S. Andersen ARChristiansen JSAndersen JKKreiner SDeckert T Diabetic nephropathy in type 1 insulin-dependent diabetes: an epidemiological study. Partanen JNiskanen LLehtinen JMervaala ESiitonen OUusitupa M Natural history of peripheral neuropathy in patients with non—insulin-dependent diabetes mellitus. Orchard TJDorman JSMaser RE et al. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Young MJBoulton AJMacLeod AFWilliams DRSonksen PH A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Dyck PJKratz KMKarnes JL et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Moss SEKlein RKlein BE Cause-specific mortality in a population-based study of diabetes. Am J Public Health. Ochi JWMelton LJ IIIPalumbo PJChu CP A population-based study of diabetes mortality. Diabetes Care. Bender APSprafka JMJagger HGMuckala KHMartin CPEdwards TR Incidence, prevalence, and mortality of diabetes mellitus in Wadena, Marshall, and Grand Rapids, Minnesota: the Three-City Study. Krolewski ASKosinski EJWarram JH et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. Klein R Hyperglycemia and microvascular and macrovascular disease in diabetes. Brownlee M Glycosylation of proteins and microangiopathy. Hosp Pract Off Ed. Greene DASima AAStevens MJFeldman ELLattimer SA Complications: neuropathy, pathogenetic considerations. Makita ZRadoff SRayfield EJ et al. Advanced glycosylation end products in patients with diabetic nephropathy. Nathan DM The pathophysiology of diabetic complications: how much does the glucose hypothesis explain? Ann Intern Med. Stolk RPVingerling JRde Jong PT et al. Retinopathy, glucose, and insulin in an elderly population: the Rotterdam study. Nathan DMSinger DEGodine JEHarrington CHPerlmuter LC Retinopathy in older type II diabetics: association with glucose control. Morisaki NWatanabe SKobayashi J et al. Diabetic control and progression of retinopathy in elderly patients: five-year follow-up study. J Am Geriatr Soc. Chen MSKao CSFu CCChen CJTai TY Incidence and progression of diabetic retinopathy among non—insulin-dependent diabetic subjects: a 4-year follow-up. Int J Epidemiol. Liu QZPettitt DJHanson RL et al. Glycated haemoglobin, plasma glucose and diabetic retinopathy: cross-sectional and prospective analyses. Teuscher ASchnell HWilson PW Incidence of diabetic retinopathy and relationship to baseline plasma glucose and blood pressure. Haffner SMFong DStern MP et al. Diabetic retinopathy in Mexican Americans and non-Hispanic whites. Ballard DJMelton LJDDwyer MS et al. Risk factors for diabetic retinopathy: a population-based study in Rochester, Minnesota. Knuiman MWWelborn TAMcCann VJStanton KGConstable IJ Prevalence of diabetic complications in relation to risk factors. Klein RKlein BEMoss SE Incidence of gross proteinuria in older-onset diabetes: a population-based perspective. Wirta OPasternack ALaippala PTurjanmaa V Glomerular filtration rate and kidney size after six years disease duration in non—insulin-dependent diabetic subjects. Clin Nephrol. Ballard DJHumphrey LLMelton LJ III et al. Epidemiology of persistent proteinuria in type II diabetes mellitus: population-based study in Rochester, Minnesota. Fabre JBalant LPDayer PGFox HMVernet AT The kidney in maturity onset diabetes mellitus: a clinical study of patients. Lehtinen JMUusitupa MSiitonen OPyörälä K Prevalence of neuropathy in newly diagnosed NIDDM and nondiabetic control subjects. Klein RKlein BEMoss SE Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Klein RMoss S A comparison of the study populations in the Diabetes Control and Complications Trial and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. University Group Diabetes Program, Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. The DCCT Research Group, Diabetes Control and Complications Trial DCCT : results of feasibility study. Genuth S Exogenous insulin administration and cardiovascular risk in non—insulin-dependent and insulin-dependent diabetes mellitus. Ohkubo YKishikawa HAraki E et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non—insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. Nelson RGPettitt DJBaird HR et al. Pre-diabetic blood pressure predicts urinary albumin excretion after the onset of type 2 non—insulin-dependent diabetes mellitus in Pima Indians. Kasiske BLKalikl RSNMa JZ Effect of antihypertensive therapy on the kidney in patients with diabetes: a meta-regression analysis. Abraira CColwell JANuttall FQ et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes VA CSDM : results of the feasibility trial. Levin SRCoburn JAbraira C et al. Glycemic control retards the progression of albuminuria in NIDDM. Emanuele NKlein RColwell J et al. Evaluations of retinopathy in the VA Cooperative Study on Glycemic Control and Complications in Type II Diabetes VA CSDM. Harris MI Medical care for patients with diabetes: epidemiologic aspects. Michigan Diabetes Research and Training Center, Diabetes in Communities. Ann Arbor, Mich University of Michigan;2. Pyörälä KSavolainen EKaukola SHaapakoski J Plasma insulin as coronary heart disease risk factor: relationship to other risk factors and predictive value during year follow-up of the Helsinki Policemen Study population. Acta Med Scand Suppl. Welborn TAWearne K Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Despres JPLamarche BMauriege P et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. Ducimetiere PEschwege EPapoz LRichard JLClaude JRRosselin G Relationship of plasma insulin levels to the incidence of myocardial infarction and coronary heart disease mortality in a middle-aged population. Eschwege ERichard JLThibult N et al. Coronary heart disease mortality in relation with diabetes, blood glucose and plasma insulin levels: the Paris Prospective Study, ten years later. Horm Metab Res Suppl. Welin LEriksson HLarsson BOhlson LOSvardsudd KTibblin G Hyperinsulinaemia is not a major coronary risk factor in elderly men: the study of men born in Ferrara ABarrett-Connor ELEdelstein SL Hyperinsulinemia does not increase the risk of fatal cardiovascular disease in elderly men or women without diabetes: the Rancho Bernardo Study, Am J Epidemiol. Liu QZKnowler WCNelson RG et al. Insulin treatment, endogenous insulin concentration, and ECG abnormalities in diabetic Pima Indians: cross-sectional and prospective analyses. Uusitupa MINiskanen LKSiitonen OVoutilainen EPyörälä K Ten-year cardiovascular mortality in relation to risk factors and abnormalities in lipoprotein composition in type 2 non—insulin-dependent diabetic and non-diabetic subjects. Collins VRDowse GKZimmet PZ et al. Serum insulin and ECG abnormalities suggesting coronary heart disease in the populations of Mauritius and Nauru: cross-sectional and longitudinal associations. J Clin Epidemiol. Jarrett RJMcCartney PKeen H The Bedford survey: ten year mortality rates in newly diagnosed diabetics, borderline diabetics and normoglycaemic controls and risk indices for coronary heart disease in borderline diabetics. Fontbonne ACharles MAThibult N et al. Hyperinsulinaemia and coronary heart disease mortality in a healthy population: the Paris Prospective Study, year follow-up. United Kingdom Prospective Diabetes Study Group, U. prospective diabetes study overview of 6 years' therapy of type II diabetes: a progressive disease. Henry RRGenuth S Forum one: current recommendations about intensification of metabolic control in non—insulin-dependent diabetes mellitus. DeFronzo RAGoodman AM Efficacy of metformin in patients with non—insulin-dependent diabetes mellitus: the Multicenter Metformin Study Group. Wingard DLBarrett-Connor ELFerrara A Is insulin really a heart disease risk factor. Stern MP The insulin resistance syndrome: the controversy is dead, long live the controversy. Jarrett RJ Why is insulin not a risk factor for coronary heart disease? Sato YShiraishi SOshida YIshiguro TSakamoto N Experimental atherosclerosis-like lesions induced by hyperinsulinism in Wistar rats. Stout RW Insulin stimulation of cholesterol synthesis by arterial tissue. Stout RWBuchanan KDVallance-Owen J Arterial lipid metabolism in relation to blood glucose and plasma insulin in rats with streptozotocin-induced diabetes. Stout RWBierman ELRoss R Effect of insulin on the proliferation of cultured primate arterial smooth muscle cells. Circ Res. Leonetti FIozzo PGiaccari A et al. Absence of clinically overt atherosclerotic vascular disease and adverse changes in cardiovascular risk factors in 70 patients with insulinoma. J Endocrinol Invest. Tomono SKato NUtsugi T et al. The role of insulin in coronary atherosclerosis. Mookherjee SPotts JLHill NE et al. Lack of relationship between plasma insulin and glucagon levels and angiographically-documented coronary atherosclerosis. Bruno GCavallo-Perin PBargero GBorra MD'Errico NPagano G Association of fibrinogen with glycemic control and albumin excretion rate in patients with non—insulin-dependent diabetes mellitus. Brownlee M Nonenzymatic glycosylation of macromolecules: prospects of pharmacologic modulation. Bucala RMakita ZKoschinsky TCerami AVlassara H Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. Bucala RMakita ZVega G et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Yegin AOzben TYegin H Glycation of lipoproteins and accelerated atherosclerosis in non—insulin-dependent diabetes mellitus. Int J Clin Lab Res. Colwell JA Vascular thrombosis in type II diabetes mellitus. Davi GCatalano IAverna M et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. Aoki IShimoyama KAoki N et al. Platelet-dependent thrombin generation in patients with diabetes mellitus: effects of glycemic control on coagulability in diabetes. J Am Coll Cardiol. Jokl RLaimins MKlein RLLyons TJLopes-Virella MFColwell JA Platelet plasminogen activator inhibitor 1 in patients with type II diabetes. Taskinen MRKuusi THelve ENikkila EAYki JH Insulin therapy induces antiatherogenic changes of serum lipoproteins in noninsulin-dependent diabetes. Agardh CDNilsson EPSchersten B Improvement of the plasma lipoprotein pattern after institution of insulin treatment in diabetes mellitus. Stern MPHaffner SM Dyslipidemia in type II diabetes: implications for therapeutic intervention. Gall MABorch-Johnsen KHougaard PNielsen FSParving HH Albuminuria and poor glycemic control predict mortality in NIDDM. Andersson DKSvardsudd K Long-term glycemic control relates to mortality in type II diabetes. Moss SEKlein RKlein BEMeuer SM The association of glycemia and cause-specific mortality in a diabetic population. Kuusisto JMykkanen LPyorala KLaakso M NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Fu CCChang CJTseng CH et al. Development of macrovascular diseases in NIDDM patients in northern Taiwan: a 4-yr follow-up study. Donahue RPAbbott RDReed DMYano K Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry: Honolulu Heart Program. Singer DENathan DMAnderson KMWilson PWEvans JC Association of HbA 1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Nielsen NVDitzel J Prevalence of macro- and microvascular disease as related to glycosylated hemoglobin in type I and II diabetic subjects: an epidemiologic study in Denmark. Malmberg KRyden LEfendic S et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction DIGAMI study : effects on mortality at 1 year. The Diabetes Control and Complications Trial Research Group, Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Kilo CMiller JPWilliamson JR The Achilles heel of the University Group Diabetes Program. Colwell JA The feasibility of intensive insulin management in non—insulin-dependent diabetes mellitus: implications of the Veterans Affairs Cooperative Study on Glycemic Control and Complications in NIDDM. Shorr RIRay WADaugherty JRGriffin MR Individual sulfonylureas and serious hypoglycemia in older people. United Kingdom Prospective Diabetes Study Group, United Kingdom Prospective Diabetes Study UKPDS , relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non—insulin dependent diabetes followed for three years. Thompson CJCummings JChalmers JGould CNewton RW How have patients reacted to the DCCT? Henry RRGumbiner BDitzler TWallace PLyon RGlauber HS Intensive conventional insulin therapy for type II diabetes: metabolic effects during a 6-mo outpatient trial. Wolfenbuttel BSels JRondas-Colbers GMenheere PNieuwenhuijzen Kruseman AC Comparison of different insulin regimens in elderly patients with NIDDM. Turner RCull CHolman R United Kingdom Prospective Diabetes Study a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non—insulin-dependent diabetes mellitus. Yki JHKauppila MKujansuu E et al. Comparison of insulin regimens in patients with non—insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group, Influence of intensive diabetes treatment on quality-of-life outcomes in the Diabetes Control and Complications Trial. American Diabetes Association, Standards of medical care for patients with diabetes mellitus. Seaquist ERGoetz FCRich SBarbosa J Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to diabetic nephropathy. Pettitt DJSaad MFBennett PHNelson RGKnowler WC Familial predisposition to renal disease in two generations of Pima Indians with type 2 non—insulin-dependent diabetes mellitus. Freedman BITuttle ABSpray BJ Familial predisposition to nephropathy in African-Americans with non—insulin-dependent diabetes mellitus. Am J Kidney Dis. Panzram G Mortality and survival in type 2 non—insulin-dependent diabetes mellitus. Knuiman MWWelborn TAWhittall DE An analysis of excess mortality rates for persons with non—insulin-dependent diabetes mellitus using the Cox proportional hazards regression model. Grady MDRubin SMPetitti DBFox CS Hormone therapy to prevent disease and prolong life in postmenopausal women. Krolewski ASLaffel LMKrolewski MQuinn MWarram JH Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. Colwell JA DCCT findings: applicability and implications for NIDDM. |
| Strategies for improving glycemic control: effective use of glucose monitoring | Measures of the experiential cpntrol of engagement were not assessed Improvinv this study. Although Artisanal Refreshment Creations self-care behaviors have been glyce,ic to be positively Improving glycemic control with improved glycemic control and quality of Imptoving, clearly many glycfmic with diabetes struggle to adopt such behaviors Herbal Wellness Solutions 8 ]. Article PubMed CAS Google Wakefulness and learning performance Finfer S, Glycrmic DR, Su SY, Blair D, Foster D, Dhingra V, et al. workload—average number of staff-taken or automated if applicable glucose measurements per patient per day standard deviation or median IQR ; d. Of the 5 trials on professional p-CGM in children, 3 trials 789 have shown small but statistically significant decrease in HbA1c in children undergoing p-CGM. A final hurdle is the inability to fully learn from prior efforts. Assignment of interventions: allocation Sequence generation {16a} Eligible participants who have signed the informed consent will be randomized into the peer-supported IMS intervention group or the control group in a ratio. |
| Help Patients Improve Glycemic Control for T2D | Work with wakefulness and learning performance diabetes health-care Mushroom Truffle Hunting to determine your target A1C Immproving blood glucose conrtol range fasting and after glycmeic. Have been receiving oral hyperglycemic agents Improfing a Fasting and immune system of 3 years prior to the start of the Improivng first prescription Impproving a minimum of two packages per year to exclude wrong prescriptions. Patients in this study were allowed to change their therapeutic regimen according to the text messages generated by the CDSS rule engine. Although the Vida experience, including content and data tracking, can be navigated without provider contact and in a self-paced manner, we observed that the combination of human interaction and content app components is associated with improved HbA 1c. Group allocation was concealed from investigator and subjects using sealed opaque envelopes. |
| Glycemic Control | Best Practices | Arch Intern Med. Effect of intensive lifestyle intervention on sexual dysfunction in women with type 2 diabetes: results from an ancillary Look AHEAD study. The relation of glycaemia to the risk of development and progression of retinopathy in the diabetic control and complications trial. Sign In. Insulin may cause weight gain and hypoglycemia. |
0 thoughts on “Improving glycemic control”